Abstract
Objective
To analyze the independent associations of the Kidney Failure Risk Equation (KFRE) and neutrophil gelatinase-associated lipocalin (NGAL) with end-stage renal disease (ESRD) among patients with chronic kidney disease (CKD) stages 3–5 in China and evaluate their predictive values for ESRD.
Patients and Methods
A total of 716 patients with CKD stages 3–5 at the time of the initial renal medicine referral were retrospectively enrolled, and the study outcome was the observed incidence of ESRD at 2 years after the initial referral. Baseline characteristics were collected, and relevant laboratory indexes, including neutrophil gelatinase-associated lipocalin (NGAL), were detected. The binary logistic regression model was used to analyze the independent associations, and the receiver operating characteristic (ROC) curve was used to assess the predictive values.
Results
The 2-year incidence of ESRD was 20.5% (147/716). The 4-variable KFRE, 8-variable KFRE and NGAL were independently associated with ESRD after adjusting for potential confounding factors. The AUCs of the 4-variable KFRE, 8-variable KFRE and NGAL for predicting ESRD among patients with CKD stages 3–5 were 0.711 [standard error (SE): 0.026, 95% confidence interval (CI): 0.662–0.761], 0.725 (SE: 0.025, 95% CI: 0.677–0.774) and 0.736 (SE: 0.024, 95% CI: 0.686–0.785), respectively. The AUC of the 4-variable KFRE plus NGAL was significantly higher than those of the 4-variable KFRE and NGAL alone (0.900 vs 0.711, Z = 6.297, P < 0.001; 0.900 vs 0.736, Z = 5.795, P < 0.001), and the AUC of the 8-variable KFRE plus NGAL was also significantly higher than those of the 8-variable KFRE and NGAL alone (0.911 vs 0.725, Z = 6.491, P < 0.001; 0.911 vs 0.736, Z = 6.298, P < 0.001).
Conclusion
The KFRE was able to independently predict progression of CKD stage 3–5 to ESRD in Chinese population. The addition of NGAL to the KFRE was able to elevate the predictive value when applied in predicting 2-year ESRD.
Keywords: chronic kidney disease, end-stage renal disease, kidney failure risk equation, neutrophil gelatinase-associated lipocalin
Introduction
Chronic kidney disease (CKD) has become a major public health problem worldwide with rapidly increasing prevalence and mortality.1,2 Worsening kidney function is independently associated with progression to end-stage renal disease (ESRD) and increased risk of cardiovascular events in CKD patients.3–5 In China, the number of patients undergoing hemodialysis treatment has demonstrated a rapid growth trend, rising to 379.1 per million people in 2017 from 174.1 per million people in 2011.6 Therefore, accurate prediction for ESRD is critical for prognosis of CKD patients, which enables targeted treatment in high-risk patients including appropriate prioritisation of treatment pathways and supporting better risk communication with patients.7–9
The Kidney Failure Risk Equation (KFRE), developed in 2011, is an extensively validated model to predict the risk of progression to ESRD among patients with CKD stages 3–5.9,10 It utilizes laboratory data that can be obtained routinely in CKD patients to predict the risk of progression to ESRD among patients with CKD stages 3–5.8,10 The KFRE can be easily integrated into a clinic electronic medical record or laboratory information system prompts. The KFRE includes 4-variable and 8-variable equation. The 4-variable KFRE relies on sex, age, eGFR and urine albumin-to-creatinine ratio (ACR), whilst the 8-variable KFRE incorporates the additional laboratory data including serum albumin, phosphate, calcium and bicarbonate. To date, validation of the original KFRE equation has been carried out in many countries, demonstrating good-to-excellent performance in identifying patients at high-risk of ESRD.11–16 However, it has not been validated in China. Moreover, the area under the receiver operator characteristic curve (AUC) for prediction of ESRD is less than 0.800 in some study cohorts,17 and recalibration of the KFRE equation can increase the AUC significantly.18
Neutrophil gelatinase-associated lipocalin (NGAL), also named lipocalin, siderocalin or lipocalin-2 (LCN2), is one of the first molecules inducing development of the kidney. NGAL is associated with the formation of tubules and complete nephrons through transforming embryonic mesenchymal cells into epithelial cells.19,20 The main sources of NGAL include leucocytes, collecting ducts and loop of Henle in the body.21,22 NGAL is produced in response to tubulointerstitial damage, which frequently happens during the progression of kidney disease.23 It has been shown that urinary and plasma NGAL can reflect the severity of renal disease and can be applied in predicting progression of CKD even with adjustment for estimated glomerular filtration rate (eGFR).24
In this study, independent associations of the KFRE equation and NGAL with ESRD in CKD patients were first analyzed by binary logistic regression model, and their values of predicting ESRD individually or jointly were then evaluated by ROC curves. Therefore, we aimed to validate the KFRE in China and elevate the predictive value for ESRD in CKD patients through adding NGAL to the KFRE equation.
Patients and Methods
Patients
This retrospective cohort study was performed at Jiangjin Central Hospital between January 2020 and June 2022. Patients with CKD stages 3–5 at the time of the initial renal medicine referral were enrolled. The enrollment criteria included (1) patients with the age of ≥18 years and meeting the criteria of CKD stages 3–5; (2) patients having all measurements available for the 4-variable and 8-variable KFRE; and (3) patients were regularly followed up for at least 2 years in this department from as frequently as every half a month to a minimum of semiannually, which depended on the discretion of the nephrologist. The follow-up period was defined as the interval between the date of the initial referral and the date of last data collection or progressing to ESRD. The exclusion criteria were (1) patients had no records of baseline albuminuria or eGFR levels; and (2) kidney transplantation or renal replacement therapy had already been initiated at the initial referral. This study received the approval of the Ethical Committee of Jiangjin Central Hospital (JJ2019018036) and was conducted according to the guidelines of the Declaration of Helsinki. Informed consents were obtained from the study participants prior to the study commencement.
Definitions
CKD is defined as renal structure or function abnormality >3 months. In this study, individuals with one of the following indexes at least for 3 months were diagnosed as CKD: (1) eGFR < 60 mL/min/1.73 m2; (2) urine albumin excretion rate (ACR) ≥30 mg/24 h or albumin–creatinine ratio ≥30 mg/g; (3) urine sediment abnormality; (4) renal tubule-associated lesions; (5) renal histological abnormality; and (6) renal imaging abnormality.25 Kidney Disease Improving Global Outcomes guidelines were used to perform CKD staging,26 with stage 3 defined as an eGFR of 30–59 mL/min/1.73 m2, stage 4 defined as an eGFR of 16–29 mL/min/1.73 m2 and stage 5 defined as ≤15 mL/min/1.73 m2.
Study Outcome
The study outcome was the observed incidence of ESRD at 2 years after the initial referral. The definition of ESRD was reception of a pre-emptive renal transplantation or initiation of peritoneal dialysis or haemodialysis.
Detection of NGAL
Blood samples were collected within 6 h after the initial referral, centrifuged at 1820 × g and 4°C for 5 min, and the plasma was stored −80°C until detection. NGAL was detected by commercially available ELISA kit (Shanghai Kexing Biotechnology Company, China). Both the intra- and inter-assay variances were less than 15%.
Statistical Analysis
All data were statistically analyzed with SPSS version 20.0 (SPSS Inc., USA), and a P value of <0.05 was considered significant. For quantitative data, Kolmogorov–Smirnov test was first used to assess their normality, and then Student’s t test was used to conduct intergroup comparisons of the normally distributed ones between ESRD group and non-ESRD group, while Mann–Whitney U-test was used to conduct intergroup comparisons of the non-normally distributed ones. For qualitative data, chi-square test was used to conduct intergroup comparisons between ESRD group and non-ESRD group. The binary logistic regression model was used to conduct multivariate analysis for the variables with P <0.10 in univariate analysis, which could identify independent associations between the KFRE scores, NGAL levels and ESRD progression. The receiver operating characteristic (ROC) curve was used to assess the values of the KFRE scores, NGAL levels and their combination for the prediction of ESRD progression. Z test was used to conduct the comparison of the area under curve (AUC).
Results
General Information
A total of 716 patients meeting the inclusion criteria were included in this analysis, and the 2-year incidence of ESRD was 20.5% (147/716). The baseline characteristics of the whole cohort were shown in Table 1. The whole cohort included 429 (59.9%) males and 287 (40.1%) females with an average age of 67.3 ± 13.5 years. Among the cohort, the Han nationality accounted for 92.0% (659/716), while other nationalities accounted for 8.0% (57/716). The cohort had a median eGFR level of 32.1 mL/min/1.73 m2 (interquartile range [IQR] 28.2–36.0).
Table 1.
Baseline Characteristics of the Whole Cohort (N = 716)
| Characteristics | No.(%)/mean±SD/M(IQR) |
|---|---|
| Age(years) | 67.3±13.5 |
| Male | 429(59.9%) |
| BMI(Kg/m2) | 25.9±3.12 |
| Smoking | 95(13.3%) |
| Han nationality | 659(92.0%) |
| Diabetes mellitus | 432(60.3%) |
| Hypertension | 608(84.9%) |
| Atrial fibrillation | 42(5.9%) |
| Coronary artery disease | 168(23.5%) |
| Hyperlipidemia | 274(38.3%) |
| eGFR(mL/min/1.73 m2) | 32.1(28.2–36.0) |
| Urine ACR(mg/g) | 242(64–865) |
| Serum albumin(g/dL) | 3.47±0.93 |
| Serum calcium(mg/dL) | 9.28±2.17 |
| Serum phosphate(mg/dL) | 3.72±0.98 |
| Serum bicarbonate(mEq/L) | 22.93±4.25 |
| Serum potassium(mEq/L) | 4.82±1.37 |
| NGAL(ng/mL) | 5.16±1.82 |
| 4-variable KFRE(%) | 34.57±10.65 |
| 8-variable KFRE(%) | 30.79±9.74 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; ACR, albumin excretion rate; KFRE, Kidney Failure Risk Equation; NGAL, Neutrophil gelatinase-associated lipocalin, IQR, interquartile range; M, median; SD, standard deviation.
Independent Associations of the 4-Variable and 8-Variable KFRE with ESRD
In order to analyze the independent association of the 4-variable KFRE with ESRD, univariate analysis was first performed between ESRD group and non-ESRD group. The variables incorporated into the 4-variable KFRE, including sex, age, eGFR and urine ACR, were not compared again in univariate analysis. The results (Table 2) demonstrated that 4-variable KFRE, BMI, smoking, diabetes mellitus, hypertension, atrial fibrillation, serum albumin, phosphate, bicarbonate, potassium and NGAL were significantly different between ESRD group and non-ESRD group, and the rest variables were not significantly different. But coronary artery disease had a P value of <0.10. Multivariate analysis (Table 3) demonstrated that the 4-variable KFRE [odds ratio (OR): 1.139, 95% confidence interval (CI): 1.058–1.278], NGAL (OR: 1.209, 95% CI: 1.051–1.427), hypertension (OR: 1.485, 95% CI: 1.187–3.072), diabetes mellitus (OR: 1.514, 95% CI: 1.196–3.073), serum albumin (OR: 1.197, 95% CI: 1.078–1.428), phosphate (OR: 1.159, 95% CI: 1.070–1.412) and bicarbonate (OR: 0.673, 95% CI: 0.502–0.845) were independently associated with ESRD after adjusting for BMI, smoking, atrial fibrillation, coronary artery disease and serum potassium.
Table 2.
Univariate Analysis Results Between ESRD Group and Non-ESRD Group
| Variables | ESRD group (n1=147) | Non-ESRD group (n2=569) | χ2/Z/t | P |
|---|---|---|---|---|
| BMI(Kg/m2, mean±SD) | 26.3±2.89 | 25.8±3.18 | 2.197 | 0.031 |
| Smoking(n, %) | 29(19.7%) | 66(11.6%) | 6.707 | 0.010 |
| Han nationality(n, %) | 137(93.2%) | 522(91.7%) | 0.339 | 0.561 |
| Diabetes mellitus(n, %) | 102(69.4%) | 330(58.0%) | 6.334 | 0.012 |
| Hypertension(n, %) | 133(90.5%) | 475(83.5%) | 4.464 | 0.035 |
| Atrial fibrillation(n, %) | 14(9.5%) | 28(4.9%) | 4.482 | 0.034 |
| Coronary artery disease(n, %) | 43(29.3%) | 125(22.0%) | 3.451 | 0.063 |
| Hyperlipidemia(n, %) | 63(42.9%) | 211(37.1%) | 1.649 | 0.199 |
| Serum albumin(g/dL, mean±SD) | 3.26±0.85 | 3.52±0.95 | 3.225 | 0.001 |
| Serum calcium(mg/dL, mean±SD) | 9.04±2.35 | 9.34±2.12 | −1.407 | 0.166 |
| Serum phosphate(mg/dL, mean±SD) | 3.95±1.06 | 3.66±0.96 | 3.013 | 0.003 |
| Serum bicarbonate(mEq/L, mean±SD) | 21.84±3.92 | 23.21±4.34 | −3.693 | <0.001 |
| Serum potassium(mEq/L, mean±SD) | 4.53±1.56 | 4.89±1.32 | −2.570 | 0.011 |
| NGAL(ng/mL, mean±SD) | 6.03±2.04 | 4.94±1.76 | 5.933 | <0.001 |
| 4-variable KFRE(%, mean±SD) | 37.18±9.92 | 33.90±10.84 | 3.505 | <0.001 |
| 8-variable KFRE(%, mean±SD) | 34.26±9.12 | 29.89±9.90 | 5.087 | <0.001 |
Abbreviations: BMI, body mass index; KFRE, Kidney Failure Risk Equation; ESRD, end-stage renal disease; NGAL, Neutrophil gelatinase-associated lipocalin; SD, standard deviation.
Table 3.
Multivariate Analysis Results for Determining Independent Association of 4-Variable KFRE with ESRD
| Variables | β | SE | Wald χ2 | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| NGAL | 0.948 | 0.265 | 5.713 | 1.209 | 1.051–1.427 | <0.001 |
| Serum bicarbonate | −0.712 | 0.204 | 4.896 | 0.673 | 0.502–0.845 | <0.001 |
| 4-variable KFRE | 0.532 | 0.190 | 3.247 | 1.139 | 1.058–1.278 | 0.006 |
| Serum albumin | 0.453 | 0.171 | 3.008 | 1.197 | 1.078–1.428 | 0.010 |
| Serum phosphate | 0.349 | 0.106 | 2.747 | 1.159 | 1.070–1.412 | 0.021 |
| Diabetes mellitus | 0.317 | 0.088 | 2.195 | 1.514 | 1.196–3.073 | 0.039 |
| Hypertension | 0.286 | 0.062 | 2.083 | 1.485 | 1.187–3.072 | 0.042 |
| Smoking | 0.467 | 0.149 | 1.638 | 1.356 | 1.184–2.805 | 0.138 |
| Serum potassium | −0.445 | 0.138 | 1.543 | 0.729 | 0.594–1.226 | 0.160 |
| BMI | 0.308 | 0.130 | 1.356 | 1.108 | 0.720–1.223 | 0.254 |
| Atrial fibrillation | 0.296 | 0.127 | 1.328 | 1.257 | 0.713–1.514 | 0.273 |
| Coronary artery disease | 0.251 | 0.094 | 1.194 | 1.235 | 0.682–1.497 | 0.389 |
Abbreviations: KFRE, Kidney Failure Risk Equation; ESRD, end-stage renal disease; NGAL, Neutrophil gelatinase-associated lipocalin; BMI, body mass index; β, regression coefficient, SE, Standard error, OR, odds ratio, CI, Confidence interval.
For the analysis of independent association of 8-variable KFRE with ESRD, the variables incorporated into the KFRE, including sex, age, eGFR, urine ACR, serum albumin, phosphate, calcium and bicarbonate, were not compared again in univariate analysis. As shown in Table 2, 8-variable KFRE, BMI, smoking, diabetes mellitus, hypertension, atrial fibrillation, serum potassium and NGAL were significantly different between ESRD group and non-ESRD group, and coronary artery disease had a P value of <0.10. Multivariate analysis (Table 4) demonstrated that the 8-variable KFRE (OR: 1.143, 95% CI: 1.060–1.287), NGAL (OR: 1.211, 95% CI: 1.055–1.434), hypertension (OR: 1.490, 95% CI: 1.191–3.085) and diabetes mellitus (OR: 1.503, 95% CI: 1.187–3.028) were independently associated with ESRD after adjusting for BMI, smoking, atrial fibrillation, coronary artery disease and serum potassium.
Table 4.
Multivariate Analysis Results for Determining Independent Association of 8-Variable KFRE with ESRD
| Variables | β | SE | Wald χ2 | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| NGAL | 0.983 | 0.257 | 5.894 | 1.211 | 1.055–1.434 | <0.001 |
| 8-variable KFRE | 0.547 | 0.192 | 3.405 | 1.143 | 1.060–1.287 | 0.004 |
| Diabetes mellitus | 0.338 | 0.091 | 2.346 | 1.503 | 1.187–3.028 | 0.035 |
| Hypertension | 0.302 | 0.074 | 2.199 | 1.490 | 1.191–3.085 | 0.040 |
| Smoking | 0.449 | 0.145 | 1.542 | 1.348 | 1.177–2.795 | 0.163 |
| Serum potassium | −0.439 | 0.135 | 1.498 | 0.732 | 0.598–1.231 | 0.197 |
| BMI | 0.316 | 0.129 | 1.373 | 1.110 | 0.719–1.226 | 0.282 |
| Atrial fibrillation | 0.305 | 0.131 | 1.367 | 1.255 | 0.708–1.512 | 0.288 |
| Coronary artery disease | 0.274 | 0.089 | 1.206 | 1.241 | 0.693–1.503 | 0.381 |
Abbreviations: KFRE, Kidney Failure Risk Equation; ESRD, end-stage renal disease; NGAL, Neutrophil gelatinase-associated lipocalin; BMI, body mass index; β, regression coefficient; SE, Standard error, OR, odds ratio; CI, Confidence interval.
Individual Predictive Values
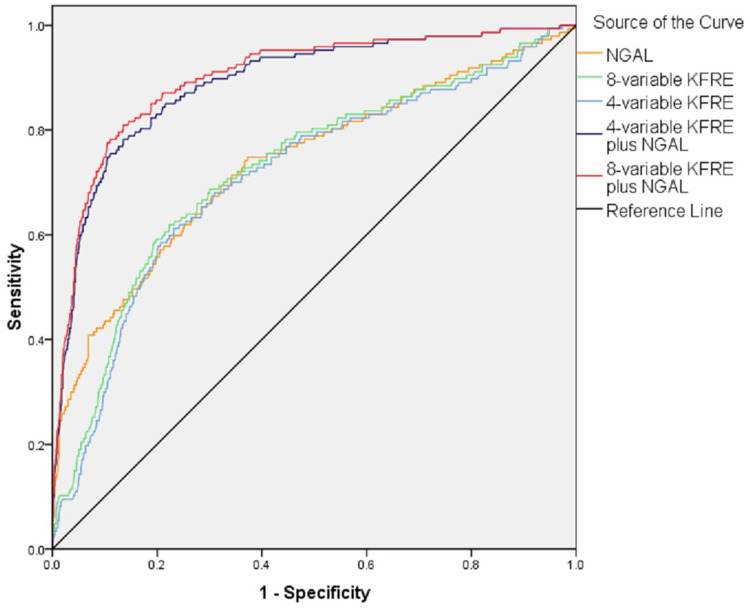
ROC curves were used to evaluate the values of the 4-variable KFRE, 8-variable KFRE and NGAL in individually predicting ESRD. As shown in Figure 1, the AUCs of the 4-variable KFRE, 8-variable KFRE and NGAL were 0.711 [standard error (SE): 0.026, 95% confidence interval (CI): 0.662–0.761], 0.725 (SE: 0.025, 95% CI: 0.677–0.774) and 0.736 (SE: 0.024, 95% CI: 0.686–0.785), respectively. Their predictive values were all moderate. Z test demonstrated that the AUC had no significant difference between the 4-variable KERE and 8-variable KFRE (0.711 vs 0.725, Z = −0.388, P = 0.697).
Figure 1.
ROC curves of 4-variable KFRE, 8-variable KFRE, NGAL, 4-variable KFRE plus NGAL and 8-variable KFRE plus NGAL in predicting ESRD.
Abbreviations: ROC: receiver operating characteristic, KFRE: Kidney Failure Risk Equation, NGAL: Neutrophil gelatinase-associated lipocalin, ESRD: end-stage renal disease.
Joint Predictive Values
In order to further elevate the predictive value for ESRD, the KFRE and NGAL were combined to predict ESRD. ROC curves (Figure 1) showed that the AUCs of the 4-variable KFRE plus NGAL and the 8-variable KFRE plus NGAL were 0.900 (SE: 0.015, 95% CI: 0.870–0.930) and 0.911 (SE: 0.014, 95% CI: 0.881–0.942), respectively. The AUC had no significant difference between the 4-variable KERE plus NGAL and 8-variable KFRE plus NGAL (Z = −0.536, P = 0.594). However, the AUC of the 4-variable KFRE plus NGAL was significantly higher than those of the 4-variable KFRE and NGAL alone (0.900 vs 0.711, Z = 6.297, P < 0.001; 0.900 vs 0.736, Z = 5.795, P < 0.001), and the AUC of the 8-variable KERE plus NGAL was also significantly higher than those of the 8-variable KERE and NGAL alone (0.911 vs 0.725, Z = 6.491, P < 0.001; 0.911 vs 0.736, Z = 6.298, P < 0.001). The clinical utility indexes of the 4-variable KERE plus NGAL and 8-variable KFRE plus NGAL were demonstrated in Table 5.
Table 5.
Clinical Utility Indexes of the 4-Variable KERE Plus NGAL and 8-Variable KFRE Plus NGAL in Predicting 2-Year ESRD Among Patients with CKD Stages 3–5
| Sensitivity | Specificity | Accuracy | FPR | FNR | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| 4-variable KERE plus NGAL | 93.9% | 88.9% | 89.9% | 31.3% | 1.7% | 68.7% | 98.3% |
| 8-variable KFRE plus NGAL | 95.9% | 88.6% | 90.1% | 31.6% | 1.2% | 68.4% | 98.8% |
Abbreviations: KFRE, Kidney Failure Risk Equation; ESRD, end-stage renal disease; NGAL, Neutrophil gelatinase-associated lipocalin; CKD, chronic kidney disease; FPR, false positive rate; FNR, false negative rate; PPV, positive predictive value; NPV, negative predictive value.
Discussion
To date, the KFRE has been validated in many countries. Kwek et al validated the KFRE in a multi-ethnic Singapore chronic kidney disease cohort with stages 3–5.27 The C-index of the 4-variable and 8-variable KFRE was 0.874 and 0.872, respectively, demonstrating excellent discrimination and predictive performance. Whitlock et al validated the KFRE among stage 3–5 CKD patients from the Diagnostic Services of Manitoba database in Canada.15 The AUC of the 4-variable KFRE for prognostic discrimination was 0.90 and significantly higher than that of eGFR alone (0.78). The KFRE threshold of 3% over 5 years yielded a specificity of 62% and a sensitivity of 97%, while 5% yielded a specificity of 80% and a sensitivity of 86%. Ibrahim et al validated the KFRE in patients in the Salford Kidney Study, which enrolled patients with non-dialysis CKD.17 The AUCs of the 4-variable KFRE for the prediction of ESRD at 5-years and 2-years were 0.773 and 0.796, respectively, demonstrating good discrimination. Moreover, there was good-to-excellent discrimination across disease aetiologies with the AUCs of ranging 0.713–0.850, including glomerulonephritis, hypertensive nephropathy, autosomal dominant polycystic kidney disease, diabetic nephropathy and other diseases. Wang et al validated the KFRE in CKD patients visiting primary care clinics in Singapore, recalibrated the KFRE for further elevation of predictive value, and determined optimally feasible KFRE thresholds for dialysis planning and nephrologist referral.18 The recalibrated KFRE demonstrated better predictive performance and discrimination than existing KFRE equations, the AUCs of the recalibrated KFRE were 0.96 at 2 years and 0.94 at 5 years, and the optimally feasible KFRE thresholds were >45% for 2-year dialysis planning and >10–16% for 5-year nephrologist referral.
Additionally, Ali et al validated the performance of the 4-variable and 8-variable KFRE in predicting the 5-year death-censored risk of graft failure among patients in the United Kingdom.28 The AUCs of the 4-variable and 8-variable KFRE were 0.743 and 0.751, respectively, demonstrating good discrimination. Salman et al evaluated the association of 2-year KFRE score categories with CKD care metrics, which demonstrated low-risk patients with a 2-year KFRE score of <3% had reduced odds of influenza vaccination, while high-risk patients with a 2-year KFRE score of ≥40% had elevated odds of completing advance directives.29 Hundemer et al assessed the influence of age on the predictive performance of the KFRE for kidney failure in patients with advanced CKD, which demonstrated that the KFRE overestimated the risk of kidney failure in elderly ones.30
In this study, the AUCs of the 4-variable and 8-variable KFRE in predicting 2-year ESRD among patients with CKD stages 3–5 were 0.711 and 0.725, respectively, showing moderate predictive performance. The predictive ability of the 8-variable KFRE was not significantly improved compared with that of the 4-variable KFRE. In order to further elevate the predictive value for ESRD in CKD patients, NGAL was added to the KFRE. NGAL level may be helpful in identifying high-risk patients with faster decline in kidney function. NGAL can activate the mitogenic function of epidermal growth factor receptor signaling, which leads to CKD progression and renal damage through stimulating hypoxia-inducible factor.31 The NGAL level in urine has been demonstrated to be an advisable predictive indicator for renal injury preceding detectable changes of eGFR.23,32 A study showed that the plasma NGAL level was inversely associated with eGFR in children with CKD stages 2–4.32 In addition, the NGAL levels in both plasma and urine have been demonstrated to have a good predictive performance for CKD progression. De Silva et al demonstrated that the increase of NGAL levels was correlated with detectable damage happening in distal convoluted tubule and loop of Henle.22
Our study showed that NGAL was independently associated with 2-year ESRD among patients with CKD stages 3–5, and had a moderate predictive performance with an AUC of 0.736. Excellent predictive performances were achieved after adding NGAL to the 4-variable and 8-variable KFRE with the AUCs of 0.900 and 0.911, respectively. Therefore, the KFRE could be applied in predicting 2-year ESRD among patients with CKD stages 3–5, and the addition of NGAL to the KFRE could elevate the predictive value for 2-year ESRD.
This study had two main limitations. The first was a small sample size of ESRD group, and the other was its retrospective characteristic.
Conclusion
The KFRE was independently associated ESRD among patients with CKD stages 3–5 in China and was able to be applied in predicting 2-year ESRD, and the addition of NGAL to the KFRE was able to elevate the predictive value for 2-year ESRD. The combination of the KFRE and NGAL provided a new tool for predicting 2-year ESRD and had clinical application potential.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All the authors do not have any conflict of interest.
References
- 1.Jha V, Garcia-Garcia G, Iseki K. et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015. Mortality and causes of death collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inker LA, Lambers Heerspink HJ, Mondal H, et al. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis. 2014;64(6):848–859. doi: 10.1053/j.ajkd.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 6.Zhao X, Niu Q, Gan L, et al. Baseline data report of the China Dialysis Outcomes and Practice Patterns Study (DOPPS). Sci Rep. 2021;11(1):873. doi: 10.1038/s41598-020-79531-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali I, Kalra P. Risk prediction in chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(6):513–518. doi: 10.1097/MNH.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 8.Grams ME, Coresh J. Predicting risk of RRT in patients with CKD. Clin J Am Soc Nephrol. 2017;12(1):3–4. doi: 10.2215/CJN.11841116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 10.Tangri N, Grams ME, Levey AS, et al.; CKD Prognosis Consortium. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: a Meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters MJ, van Zuilen AD, van den Brand JA, et al.; MASTERPLAN Study Group. Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant. 2013;28(7):1773–1779. doi: 10.1093/ndt/gft063. [DOI] [PubMed] [Google Scholar]
- 12.Kang MW, Tangri N, Kim YC, et al. An independent validation of the kidney failure risk equation in an Asian population. Sci Rep. 2020;10(1):12920. doi: 10.1038/s41598-020-69715-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L, Wang J, Deng Y, et al. External validation of the kidney failure risk equation among urban community-based Chinese patients with CKD. Kidney Med. 2024;6(5):100817. doi: 10.1016/j.xkme.2024.100817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lennartz CS, Pickering JW, Seiler-Mußler S, et al. External validation of the kidney failure risk equation and re-calibration with addition of ultrasound parameters. Clin J Am Soc Nephrol. 2016;11(4):609–615. doi: 10.2215/CJN.08110715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitlock RH, Chartier M, Komenda P, et al. Validation of the kidney failure risk equation in Manitoba. Can J Kidney Health Dis. 2017;4:2054358117705372. doi: 10.1177/2054358117705372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher F, Teece L, Major RW, et al. Using the kidney failure risk equation to predict end-stage kidney disease in CKD patients of South Asian ethnicity: an external validation study. Diagn Progn Res. 2023;7(1):22. doi: 10.1186/s41512-023-00157-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali I, Donne RL, Kalra PA. A validation study of the kidney failure risk equation in advanced chronic kidney disease according to disease aetiology with evaluation of discrimination, calibration and clinical utility. BMC Nephrol. 2021;22(1):194. doi: 10.1186/s12882-021-02402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Nguyen FNHL, Allen JC, et al. Validation of the kidney failure risk equation for end-stage kidney disease in Southeast Asia. BMC Nephrol. 2019;20(1):451. doi: 10.1186/s12882-019-1643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Blum A, Novak T, et al. An epithelial precursor is regulated by the ureteric bud and by the renal stroma. Dev Biol. 2002;246(2):296–310. doi: 10.1006/dbio.2002.0646 [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. doi: 10.1016/s1097-2765(02)00710-4 [DOI] [PubMed] [Google Scholar]
- 21.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin Chim Acta. 2015;438:350–357. doi: 10.1016/j.cca.2014.08.039 [DOI] [PubMed] [Google Scholar]
- 22.De Silva PM, Mohammed Abdul KS, Eakanayake EM, et al. Urinary biomarkers KIM-1 and NGAL for Detection of Chronic Kidney Disease of Uncertain etiology (CKDu) among agricultural communities in Sri Lanka. PLoS Negl Trop Dis. 2016;10(9):e0004979. doi: 10.1371/journal.pntd.0004979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuncio GS, Neilson EG, Haverty T. Mechanisms of tubulointerstitial fibrosis. Kidney Int. 1991;39(3):550–556. doi: 10.1038/ki.1991.63 [DOI] [PubMed] [Google Scholar]
- 24.Rysz J, Gluba-Brzózka A, Franczyk B, et al. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci. 2017;18(8):1702. doi: 10.3390/ijms18081702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Expert Group on Early Detection. Diagnosis and treatment system construction of chronic kidney disease in Shanghai. Guideline for screening, diagnosis, prevention and treatment of chronic kidney disease. Chin J Pract Internal Med. 2017;37(1):28–34. doi: 10.19538/j.nk2017010108. [DOI] [Google Scholar]
- 26.Major RW, Shepherd D, Medcalf JF, et al. The kidney failure risk equation for prediction of end stage renal disease in UK primary care: an external validation and clinical impact projection cohort study. PLoS Med. 2019;16(11):e1002955. doi: 10.1371/journal.pmed.1002955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwek JL, Pang HQJ, Li H, et al. Validation of the kidney failure risk equation in predicting the risk of progression to kidney failure in a multi-ethnic Singapore chronic kidney disease cohort. Singapore Med J. 2022;63(6):313–318. doi: 10.11622/smedj.2020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali I, Kalra PA. A validation study of the 4-variable and 8-variable kidney failure risk equation in transplant recipients in the United Kingdom. BMC Nephrol. 2021;22(1):57. doi: 10.1186/s12882-021-02259-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed S, Mothi SS, Sequist T, et al. The kidney failure risk equation score and CKD care delivery measures: a cross-sectional study. Kidney Med. 2021;4(1):100375. doi: 10.1016/j.xkme.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hundemer GL, Tangri N, Sood MM, et al. The Effect of Age on Performance of the Kidney Failure Risk Equation in Advanced CKD. Kidney Int Rep. 2021;6(12):2993–3001. doi: 10.1016/j.ekir.2021.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120(11):4065–4076. doi: 10.1172/JCI42004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22(1):101–108. doi: 10.1007/s00467-006-0244-x [DOI] [PubMed] [Google Scholar]