Abstract
Background
To analyze the demographic characteristics of retinopathy of prematurity (ROP) in China, attempting to propose optimized screening criteria and hopefully providing valuable information for future updates to the ROP guideline.
Methods
A multicenter, retrospective-cohort study was conducted. The study included infants born between January 1, 2018, and July 31, 2023, who underwent ROP screening and were diagnosed with ROP at seven screening centers in China. Examinations were carried out in accordance with the ROP guidelines in 2014: infants with a gestational age (GA)<32 weeks and/or birth weight (BW)<2000 g, or infants who were suspected to be at risk of ROP. ROP treatment followed the recommendations of the Early Treatment for Retinopathy of Prematurity Cooperative Group. We utilized receiver operating characteristic (ROC) curves to determine the optimal predictive model, and conducted internal validation as well as compared the model to current standards.
Results
Among the 4770 infants diagnosed with ROP after fundus screening, 1330 (27.9%) infants received treatment. The mean GA at birth for all enrolled infants was 29.67 ± 2.45 weeks, with a mean BW of 1295.89 ± 403.64 g. This study proposed the optimization of guidelines to be ≤ 30 weeks of GA and ≤ 1600 g of BW, achieving a sensitivity of 99.4%, as high as the current standard, with an 18.0% reduction in screening requirements.
Conclusion
Considering the decrease in both GA and BW among the population requiring ROP treatment in China, it is imperative to contemplate updating the ROP screening guideline.
Keywords: Retinopathy of prematurity, Screening criteria, China
Introduction
Retinopathy of prematurity (ROP), a leading cause of blindness in premature infants, is characterized by pathological progression within immature retinal tissue and can ultimately lead to tractional retinal detachment [1]. Timing plays a crucial role in the successful treatment of ROP because the disease can advance very rapidly, and treatment-delayed ROP can lead to permanent blindness.
The optimal ROP screening guidelines are those that possess the highest sensitivity for detecting high-risk disease. Most screening guidelines have been based on birth weight (BW) and gestational age (GA) to identify infants in need of examination, as these factors have been acknowledged as major risk factors for ROP development. Compared with industrialized countries, developing countries exhibit a wider range of BWs and GAs among infants affected by severe ROP. In China, the ROP screening guidelines were recommended by the Ministry of health in 2004, specifying that infants meeting the following criteria should undergo ROP screening: GA of less than 32 weeks and/ or BW of less than 2000 g, or infants with an unstable clinical course [2, 3]. To sure that infants requiring treatment are not missed, the guidelines encompass a broader range of mature infants compared to the criteria in the UK and US [4, 5].
Recent studies have reported that only 11-27% of screened babies will develop ROP and 6.7-16.6% will require treatment [6–8]. Although current screening guidelines have been proven effective and highly sensitive [9], they result in excessive examinations. Some epidemiological studies have attempted to update the upper limits of GA and BW for screening by conducting population-based cohort studies of ROP to optimize screening guidelines in China [10–12]. However, most ROP studies are presently restricted to local regions.
The aims of this study are to report the demographic characteristics of ROP and to test the effectiveness of current China ROP screening guidelines based on examinations of high-risk, premature infants in seven screening centers in China. With the aspiration to offer some valuable foundations for future updated to the Chinese ROP screening guidelines, this study attempts to formulate optimized screening criteria based on GA and BW while validating their internal validity.
Methods
A multicenter, consecutive, retrospective-cohort study was conducted. The study was approved by the institutional ethics committee and was performed in accordance with the Declaration of Helsinki. It included infants born between January 1, 2018, and July 31, 2023, who underwent ROP screening and were diagnosed with ROP at seven screening centers in China. Infants with complete follow-up data and without the presence of other ocular diseases will be considered for inclusion in the study. Informed consent was obtained from guardians before each examination.
In the seven screening centers, examinations were conducted following the ROP guidelines recommended by the Chinese Ophthalmological Society in 2014: infants with a GA<32 weeks and/or BW<2000 g, or infants suspected to be at risk of ROP ( such as those with serious systemic diseases or infants who received long-term oxygen supplementation) [3]. The first screening was performed at 31 to 32 weeks postmenstrual age or 4 to 6 weeks after birth, depending on which came first. If ROP was identified, subsequent examinations were carried out weekly until the disease progressed to a stage requiring treatment or established ROP showed signs of regression.
Pupils were dilated using a combination of 0.5% tropicamide and 0.5% phenylephrine eye drops. The majority of fundus examinations were performed at the bedside by an experienced ophthalmologist, utilizing a 20-diopter lens and a binocular indirect ophthalmoscope. Additionally, some premature infants underwent examination with the RetCam Imaging System (Clarity Medical System, Pleasanton, CA, USA).
ROP was classified according to the international classification system [13, 14]. If the infants had different stages in their eyes, we recorded the more advanced stage as the patient’s stage. ROP was treated in accordance with the recommendations of the Early Treatment for Retinopathy of Prematurity Cooperative Group at the type 1 prethreshold stage [15]. Treatment was carried out within 72 h after type 1 ROP and aggressive ROP (AROP) were detected. Anti-vascular endothelial growth factor intravitreal injection was given, and eight hundred ten-nanometer diode laser surgery was performed for patients in the threshold and Type 1 prethreshold stages. Vitrectomy and/ or scleral buckling surgery were conducted for infants in stages 4 and 5.
We conducted statistical analysis using the Statistical Package for the Social Science (SPSS) program, version 22.0 (IBM Corporation, Armonk, New York, USA). Two-tailed probability levels of less than 0.05 were considered to indicate statistical significance. We performed a one-sample Kolmogorov-Smirnov test to examine whether the samples were normally distributed. Numerical data were presented as mean ± standard deviation (SD). We compared the infant characteristics between ROP cases with treatment and those without treatment. Univariate analysis of assumed risk factors was carried out using the χ2 test or the t-test. The distribution of GA and BW was analyzed for the entire study population and within each group. The ability of GA and BW to predict outcomes was measured using the are under the ROP curve (AUC) for their respective cut-off values. To establish the recommended cut-off points for GA and BW in ROP screening, Youden’s index was employed, maximizing the combined sensitivity and specificity. We generated four integrated values near the cut-point of the GA and BW pair, resulting in four screening models. These four models were internally validated and compared with the current screening criteria.
Results
From January 2018 to July 2023, a total of 5010 infants were diagnosed with ROP following fundus screening at seven screening centers in China. Among them, 240 were excluded due to death or lost to follow-up. As a result, the final study population comprised 4770 infants.
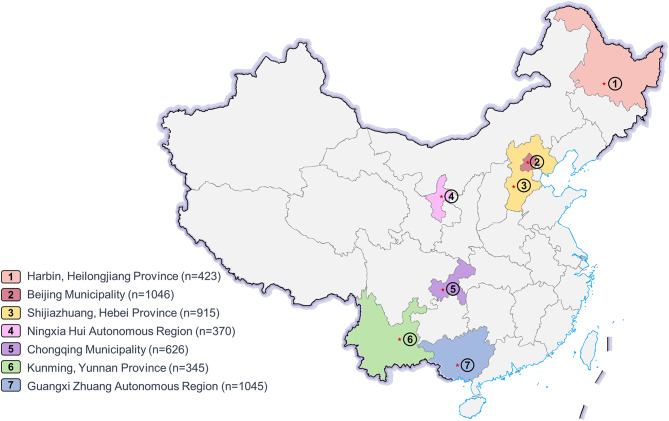
In this study, 2830 (59.3%) of infants were male. The data for GA and BW exhibited a normal distribution (Kolmogorov-Smirnov test, P>0.05). The mean GA at birth for all infants was 29.67 ± 2.45 weeks, and the mean BW was 1295.89 ± 403.64 g. Among all infants diagnosed with ROP, 1330 (27.9%) infants received treatment, while 3440 (72.1%) did not receive treatment. Baseline characteristics of the study cohort were analyzed (Table 1). Infants who receive treatment exhibited significantly lower GA and BW compared to those who did not receive treatment( p<0.001). The study cohort consisted of infants recruited from seven ROP screening centers across China, covering five geographic regions of Northeast, North, Northwest, Southwest, and South China (Fig. 1).
Table 1.
Demographic characteristics of the study population
| ROP with treatment | ROP without treatment | P value | ||
|---|---|---|---|---|
| Number (%) | 1330(27.9%) | 3440(72.1%) | ||
| Men (%) | 764(57.4%) | 2066(60.1%) | 0.099 * | |
| GA | Mean ± SD, w | 28.37 ± 2.13 | 30.17 ± 2.37 | <0.001† |
| Range | 20.57–36.86 | 24.43–36.86 | ||
| BW | Mean ± SD, g | 1093.73 ± 300.53 | 1374.04 ± 411.24 | <0.001† |
| Range | 450–2540 | 500–3600 | ||
*Estimated with Pearson χ2 test
†Estimated with independent-samples t-test
ROP, retinopathy of prematurity; GA, gestational age; BW, birth weight; w, weeks; g, grams
Fig. 1.
Distribution of retinopathy of prematurity screening centers in this study in China. This map is based on the standard map with the approval number GS (2016)2923 downloaded from China’s State Bureau of Surveying and Mapping website, and the base map has not been modified
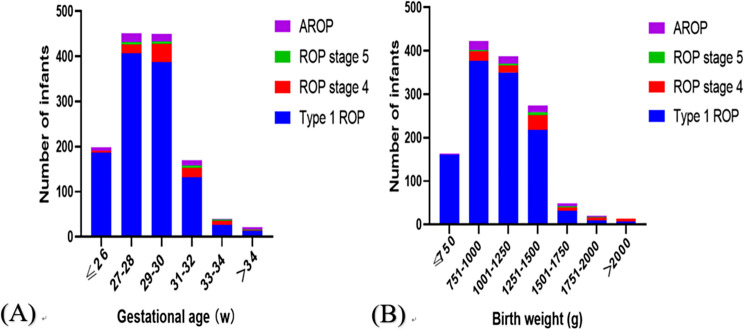
Treatment group for ROP was divided into four subgroups: Type 1 ROP, Stage 4 ROP, Stage 5 ROP, and AROP. Stratification was carried out based on BW and GA (Table 2). The association between the rate of ROP requiring treatment and BW, as well as GA, exhibited a negative correlation (p<0.001, with Kendall’s correlation coefficients of -0.905 and − 0.867, respectively). Among the 1330 cases of patients requiring treatment for ROP, 73.2% of patients had a BW of ≤ 1250 g, and 82.6% were born with a GA of ≤ 30 weeks. As BW and GA increased, there was a progressive decrease observed in the rate of ROP requiring treatment. Within the groups, those with a BW of 751–1000 g and a GA of 27–28 weeks exhibited the highest number of cases necessitating treatment for ROP. Furthermore, the highest count of severe ROP cases (including Stage 4, Stage 5, and AROP) was observed within the subgroup with a BW of 1251–1500 g and GA of 29–30 weeks (Fig. 2).
Table 2.
Numbers and proportions of infants developing different stages of ROP, according to BW and GA
| ROP with treatment, N (%) | ROP without treatment, N (%) |
Total, N |
|||||
|---|---|---|---|---|---|---|---|
| Type 1 ROP, N (%) |
ROP stage 4, N (%) |
ROP stage 5, N (%) |
AROP, N (%) |
Total, N (%) |
|||
| BW, g | |||||||
| ≤ 750 | 160 (72.7) | 1 (0.5) | 0 (0) | 3 (1.3) | 164 (74.5) | 56 (25.5) | 220 |
| 751–1000 | 377 (33.3) | 22 (1.9) | 3 (0.3) | 21 (1.9) | 423 (37.4) | 709 (62.6) | 1132 |
| 1001–1250 | 350 (29.7) | 17 (1.4) | 3 (0.3) | 17 (1.4) | 387 (32.8) | 790 (67.2) | 1177 |
| 1251–1500 | 218 (22.2) | 34 (3.5) | 7 (0.7) | 15 (1.5) | 274 (27.9) | 706 (72.1) | 980 |
| 1501–1750 | 32 (5.4) | 8 (1.4) | 2 (0.3) | 6 (1.0) | 48 (8.1) | 542 (91.9) | 590 |
| 1751–2000 | 10 (2.2) | 6 (1.3) | 2 (0.4) | 2 (0.4) | 20 (4.3) | 443 (95.7) | 463 |
| >2000 | 7 (3.3) | 6 (2.9) | 1 (0.5) | 0 (0) | 14 (6.7) | 194 (93.3) | 208 |
| Total | 1154 (24.2) | 94 (2.0) | 18 (0.4) | 64 (1.3) | 1330 (27.9) | 3440 (72.1) | 4770 |
| GA, w | |||||||
| ≤ 26 | 187 (60.7) | 3 (1.0) | 0 (0) | 8 (2.6) | 198 (64.3) | 110 (35.7) | 308 |
| 27–28 | 407 (36.7) | 19 (1.7) | 5 (0.5) | 20 (1.8) | 451 (40.7) | 657 (59.3) | 1108 |
| 29–30 | 387 (26.0) | 41 (2.8) | 5 (0.3) | 17 (1.2) | 450 (30.3) | 1037 (69.7) | 1487 |
| 31–32 | 132 (11.1) | 21 (1.8) | 5 (0.4) | 12 (1.0) | 170 (14.3) | 1014 (85.7) | 1184 |
| 33–34 | 27 (5.8) | 9 (2.0) | 2 (0.4) | 2 (0.4) | 40 (8.6) | 428 (91.4) | 468 |
| >34 | 14 (6.5) | 1 (0.5) | 1 (0.5) | 5 (2.3) | 21 (9.8) | 194 (90.2) | 215 |
| Total | 1154 (24.2) | 94 (2.0) | 18 (0.4) | 64 (1.3) | 1330 (27.9) | 3440 (72.1) | 4770 |
ROP, retinopathy of prematurity; AROP, aggressive retinopathy of prematurity; GA, gestational age; BW, birth weight; w, weeks; g, grams
Fig. 2.
Distribution of infants with retinopathy of prematurity requiring treatment according to birth weight and gestational age. The Y-axis represents the number of cases of retinopathy of prematurity (ROP) requiring treatment, categorized into aggressive retinopathy of prematurity (AROP), ROP Stage 5, ROP Stage 4, and Type 1 ROP groups, each represented by different colors. The X-axis shows stratification based on (A) gestational age and (B) birth weight
By calculating ROC curves based on the GA and BW data from this patient cohort, we identified recommended optimal cut-off points with maximum Youden index: 1502.50 g of BW (AUC 0.701) and 29.79 weeks of GA (AUC 0.720) (Fig. 3). At the 95% confidence level, the AUC range for BW was 0.685 to 0.717, while for GA, the AUC range was 0.705 to 0.736. The confidence intervals for both indicators were relatively narrow, indicating high precision of the estimates and stable results. The model demonstrated acceptable and robust discriminative ability. We synthesized four pairs of predictive models for ROP using integral values near the suggested GA and BW cut-off points for subsequent analysis. Assuming strict adherence to screening criteria, infants whose GA or BW surpasses the screening threshold, irrespective of other risk factors, are excluded from the screening process.
Fig. 3.
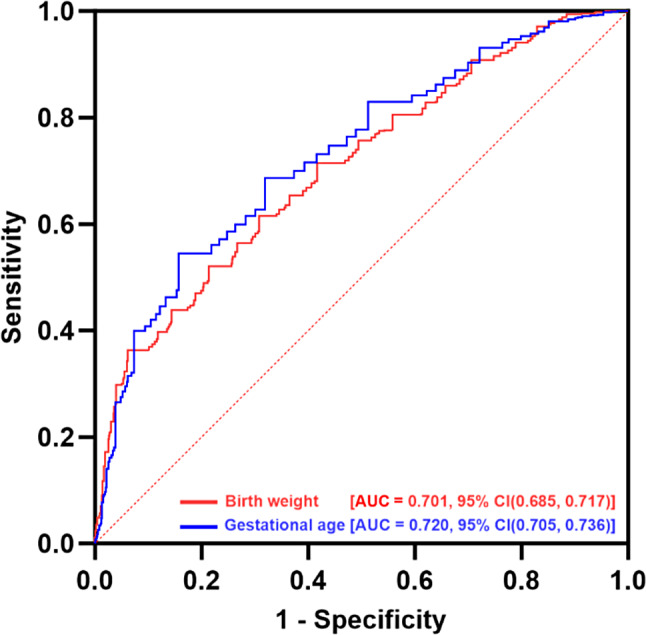
Receiver Operating Characteristic Curve Analysis of Birth weight and Gestational age. The Y-axis represents sensitivity and the X-axis represents 1-specificity. The red curve illustrates birth weight, with an area under the curve (AUC) of 0.701 and a 95% confidence interval (CI) of 0.685 to 0.717. The blue curve represents gestational age at birth, with an AUC of 0.720 and a 95% CIs of 0.705 to 0.736
Ensuring that no cases requiring treatment are missed is crucial in ROP screening, and sensitivity should therefore be prioritized when developing ROP screening guidelines. Although the Youden index seeks a balance between sensitivity and specificity, we will emphasize maintaining sensitivity in subsequent validations and the establishment of screening criteria. Our acceptable standard for sensitivity was set to be no lower than the existing screening criteria in China. In our study, the existing screening criteria accurately identified 1322 out of 1330 infants requiring treatment (sensitivity, 99.4%), as shown in Table 3. The remaining 8 unrecognized cases all exhibited an unstable clinical course, including concomitant systemic diseases. The United Kingdom standards achieve a sensitivity of 99.2% but may miss 11 cases requiring treatment for ROP, while the United states standards achieve a sensitivity of 98% but may miss 27 cases. Applying the most conservative model (GA ≤ 30 weeks or BW ≤ 1600 g) to the current cohort would have resulted in the diagnosis of 1322 out of 1330 infants requiring treatment (sensitivity, 99.4%), maintaining a sensitivity level as high as the current standard. Additionally, among the 4770 infants, the number requiring examinations would have decreased to 3913, resulting in a reduction of 857 examinations (18.0%). We also experimented with broader screening criteria. While these criteria maintained a sensitivity level comparable to the existing ones, they resulted in reduced specificity and did not significantly decrease the screening volume. Consequently, we identified the optimal screening criteria to be a GA ≤ 30 weeks or BW ≤ 1600 g.
Table 3.
Evaluation of different screening criteria for ROP
| Relevant criteria | Screening Criteria | Number of infants fulfilling criteria | Number of infants not meeting criteria | Number of infants requiring treatment | Sensitivity for ROP requiring treatment | |
|---|---|---|---|---|---|---|
| Number of infants fulfilling criteria | Number of infants not meeting criteria | |||||
| United Kingdom |
GA ≤ 32w or BW ≤ 1500 g |
4262 | 508 (12.0%) | 1319 | 11 | 99.2% |
| China |
GA < 32w or BW < 2000 g |
4550 | 220 (4.6%) | 1322 | 8 | 99.4% |
| Optimize criteria |
GA ≤ 29w or BW ≤ 1500 g |
3569 | 1201(25.2%) | 1266 | 64 | 95.2% |
| Optimize criteria |
GA ≤ 29w or BW ≤ 1600 g |
3828 | 942 (19.8%) | 1291 | 39 | 97.1% |
|
Optimize criteria /United States |
GA ≤ 30w or BW ≤ 1500 g |
3723 | 1047 (22.0%) | 1303 | 27 | 98.0% |
| Optimize criteria |
GA ≤ 30w or BW ≤ 1600 g |
3913 | 857 (18.0%) | 1322 | 8 | 99.4% |
| Broader criteria |
GA ≤ 30w or BW ≤ 1700 g |
4088 | 682(14.3%) | 1322 | 8 | 99.4% |
| Broader criteria |
GA ≤ 31w or BW ≤ 1600 g |
4075 | 695(14.6%) | 1322 | 8 | 99.4% |
ROP, retinopathy of prematurity; GA, gestational age; BW, birth weight; w, weeks; g, grams
Discussion
Since the issuance of government guidelines, awareness regarding ROP has increased, leading to a greater number of conducted ROP studies. A reduction in neonatal mortality has led to an increased population of babies at risk of ROP due to improvements in neonatal care. The reported rate of infants requiring treatment for ROP in our country ranges from 7.1 to 16.6% [6, 7, 10], whereas in developed countries, it ranges from 5.2 to 15.4% [16–18]. In Egypt, Aziz et al. reported that 12.4% of eyes exhibited high-risk ROP and required treatment [19]. A retrospective study conducted in 2022 on premature neonates from northern Iran yielded similar results [20], with the incidence of ROP requiring treatment estimated at approximately 13.4%. The result of our study was notably higher at 27.9%, surpassing most previous reports in our country. This discrepancy might be attributed to the fact that we exclusively included tertiary referral centers, which typically receive a larger number of referred patients, thereby resulting in an increased rate of ROP treatment. The percentage of ROP cases requiring treatment in this study was higher than that in other countries. This variation could be due to combined differences in neonatal care, distinct screening criteria, economic conditions, ethnicities, and other associated risk factors.
The mean GA and BW of ROP cases in this study were found to be similar to those reported in previous studies. However, the mean GA and BW in the ROP treatment group were lower than what has been previously documented [6, 10]. In this study, over half of the ROP requiring treatment had a GA of less than 30 weeks (82.6%) and a BW of less than 1250 g (73.2%). These proportions are higher than the 63.4% and 48.7% observed in a 2013 study [11]. A recent study conducted by Yang et al. also reported outcomes similar to those in our study [10]. The proportion of ROP requiring treatment occurring in infants with low GA and BW has increased, which may be associated with the improvement in medical care for neonates in China over the past few decades [21]. The workload of pediatric ophthalmologists for screening ROP is particularly burdensome due to the large population base in China. While numerous studies have confirmed the effectiveness of current guidelines, expansive screening standards seem to strain the healthcare system and lead to many unnecessary examinations. Fundoscopy for ROP screening has proven to be uncomfortable, especially for preterm babies [22]. A prospective study based in neonatal units across two tertiary-level hospitals in Shanghai indicates that by progressively narrowing the scope of screening, more cases of ROP would go undetected. However, the majority of these cases represent mild ROP that does not require treatment. It is suggested to optimize the screening criteria to include infants with a GA of ≤ 33 weeks and a BW of ≤ 1750 g, which could result in a 43% reduction in examinations [11]. Research findings from 2020 demonstrate that using an optimized model (GA < 32 weeks or BW < 1600 g) could spare examination for 2422 infants (43.2%), with only one case of Stage 1 ROP being missed (sensitivity of 98.41%) [10] A study from Hebei province proposed further narrowing the screening criteria to < 32 weeks of GA and < 1800 g of BW, resulting in a 21.6% reduction in screenings without missing severe cases [12]. Our study proposes an optimally refined screening standard of ≤ 30 weeks of GA and ≤ 1600 g of BW, which is narrower than those previously suggested in the literature.
In the internal validity verification, we observed that the sensitivity of the proposed optimized model (99.4%) is equally high compared to the sensitivity of the current screening standards, resulting in a reduction of 857 infant examination (18.0%). Although the optimized screening model may miss 8 cases, all of which are high-risk patients, these cases would also be missed by the existing screening criteria if relying solely on GA and BW. Among these 8 missed cases, 2 were Type 1 ROP, 5 were Stage 4 ROP, and 1 was Stage 5 ROP. All these patients underwent hyperbaric oxygen therapy. Among the 5 patients with Stage 4 ROP, 3 had concurrent cardiovascular diseases, and 2 had respiratory diseases. The patients with Stage 5 ROP also had a respiratory condition. Both cases of Type 1 ROP had cardiovascular diseases. However, according to the third criterion of the existing standards, which states that infants with an unstable clinical course should still be included in the screening, these 8 patients would not been missed since they present systemic high-risk factors.
The existing screening guidelines primarily rely on GA and BW. Although they demonstrate a high sensitivity in predicting severe ROP, it is crucial to acknowledge that their sensitivity is not 100%, as certain cases with higher GA and BW might also necessitate treatment. An ideal screening standard should minimize the number of screenings while ensuring that no sever ROP cases are missed. Therefore, it is essential to integrate “extra criteria” into ROP screening to improve both sensitivity and specificity. These criteria could include overall health condition and duration of oxygen therapy, and should be further refined based on high-risk factors specific in the Chinese population.
Developed countries have made significant advancements in the research of ROP screening models. In 2006, Sweden introduced the WINROP model [23], which was pioneering in incorporating postnatal factors such as Insulin-like Growth Factor 1 levels and postnatal weight gain into the ROP risk predictior. This significantly enhanced the sensitivity and specificity of screenings. Subsequently, new predictive models like the PINTROP [24], CHOPROP [25], and ROP Score models [26] were developed based on postnatal weight gain indicators. However, these models have showed inconsistent predictive performance and limited clinical utility during external validation across various countries, which has restricted their widespread application. In 2018, the United States introduced the G-ROP model [27], which was subjected to validation studies in multiple countries due to its scientific rigor, transparency, and practicality [28]. This model has been validated in developed regions like Japan and Taiwan, while its applicability might not be suitable for our country [29, 30]. Moreover, China’s research into ROP risk prediction models is still in its nascent stages. Further research should focus on conducting more in-depth prospective, multicenter studies to advance this field effectively.
This study represents the most extensive multicenter investigation conducted to date, encompassing a wide range of geographical regions in China. However, there are additional potential limitations to consider. The first limitation was the study design. Retrospective data collection can introduce bias into the study design. The loss of patients during follow-up in the retrospective cohort study resulted in data missingness, introducing potential bias into the analysis. Second, although this study encompasses diverse regions, it cannot be considered representative of entire Chinese population. As there are variations in demographic characteristics, economic level, and healthcare conditions across different regions in china, further population-based studies on premature infants in the broader community are essential.
Conclusion
The results of this study indicate a decrease in both GA and BW among the population requiring treatment for ROP, compared to previous data. However, the current guidelines remain relatively broad. Optimizing guidelines could lead to a more efficient ROP screening process. This study proposes the optimization of guidelines to be ≤ 30 weeks of GA and ≤ 1600 g of BW, resulting in a sensitivity of 99.4% and a reduction of 18.0% in screening requirements. For high-risk ROP cases with large GA and BW, comprehensive assessment in conjunction with other indicators is essential. Our future research plans include a multicenter, prospective study based on Chinese populations to explore high-risk factors for ROP and establish “extra screening criteria”. This effort aims to further optimize the ROP screening model and enhance its efficiency.
Acknowledgements
Not applicable.
Abbreviations
- ROP
retinopathy of prematurity
- BW
birth weight
- GA
gestational age
- ROC
receiver operating characteristic curve
- APROP
aggressive posterior retinopathy of prematurity
Author contributions
YC, JL and MZ conceived and designed the study. YZ and XL contributed important intellectual content to the study design, manuscript writing and revisions. YY, LH, NL, JL, YZ, RZ, YW, ZZ, XL, HY and MY orchestrated data collection, contributing to the gathering, organization, and validation of research data. YZ, YC, JL, XL, HY and MZ designed the analysis methods, analyzed data and contributed to manuscript writing.All authors reviewed the manuscript.
Funding
This work was supported by Peking University People’s Hospital Research And Development Funds (RDL2024-09), Beijing Science and technology project (grant no. Z201100005520078) and Beijing Bethune Charitable Foundation (grant no. 2018-Z-08). The funding organization had no role in the design or conduct of this research.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of Peking University People’s Hospital (2017PHB179-01) and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from guardians before each examination.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yusheng Zhong and Xiacheng Lin contributed equally to this work.
Contributor Information
Jianhong Liang, Email: drliangjianhong@126.com.
Yong Cheng, Email: raccocheng@126.com.
References
- 1.Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology. American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of Prematurity. Pediatrics. 2018;142(6):e20183061. 10.1542/peds.2018-3810. [DOI] [PubMed] [Google Scholar]
- 2.Li XX. Characteristics and screening guidelines of retinopathy of prematurity in China. Chin J Ocul Fundus Dis. 2004;20(6):384–6. [Google Scholar]
- 3.Li XX, Chinese Ophthalmological Society Fundus Diseases Group. Screening guidelines of retinopathy of prematurity in China 2014. Chin J Ophthalmol. 2014;50(12):933–5. [Google Scholar]
- 4.Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology. American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–95. 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson AR, Haines L, Head K, et al. UK retinopathy of prematurity guideline. Eye (Lond). 2009;23(11):2137–9. 10.1038/eye.2008.128. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Wang Z, Wang R, Tang H, Chen H, Feng Z. A prospective study of the incidence of retinopathy of Prematurity in China: evaluation of different screening criteria. J Ophthalmol. 2016;2016:5918736. 10.1155/2016/5918736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Gao Y, Chen W, Han M. Screening for retinopathy of prematurity in North China. BMC Ophthalmol. 2022;22(1):251. 10.1186/s12886-022-02470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bas AY, Demirel N, Koc E, et al. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol. 2018;102(12):1711–6. 10.1136/bjophthalmol-2017-311789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Feng J, Li F, Yin H, Liang J, Li X, ANALYSIS OF CHANGES, IN CHARACTERISTICS OF SEVERE RETINOPATHY OF PREMATURITY PATIENTS AFTER SCREENING GUIDELINES WERE ISSUED IN CHINA. Retina. 2015;35(8):1674–9. 10.1097/IAE.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Zhou X, Ni Y, et al. Optimised retinopathy of prematurity screening guideline in China based on a 5-year cohort study. Br J Ophthalmol. 2021;105(6):819–23. 10.1136/bjophthalmol-2020-316401. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Zhou X, Zhang Q, et al. Screening for retinopathy of prematurity in China: a neonatal units-based prospective study. Invest Ophthalmol Vis Sci. 2013;54(13):8229–36. 10.1167/iovs.13-12297. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Su M, Ren SG, Hua HL, Wang JC, Zheng W. Analysis of current status and strategies of retinopathy of Prematurity Screening during 6 years in local regions of China: implication and caution. J Ophthalmol. 2014;2014:756059. 10.1155/2014/756059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Committee for the Classification of Retinopathy of Prematurity. The International classification of retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9. 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 14.Chiang MF, Quinn GE, Fielder AR, et al. International classification of retinopathy of Prematurity, Third Edition. Ophthalmology. 2021;128(10):e51–68. 10.1016/j.ophtha.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15–23. 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 16.van Sorge AJ, Termote JU, Simonsz HJ, et al. Outcome and quality of screening in a nationwide survey on retinopathy of prematurity in the Netherlands. Br J Ophthalmol. 2014;98(8):1056–60. 10.1136/bjophthalmol-2013-304493. [DOI] [PubMed] [Google Scholar]
- 17.Tabarez-Carvajal AC, Montes-Cantillo M, Unkrich KH, Trivedi RH, Peterseim MMW. Retinopathy of prematurity: screening and treatment in Costa Rica. Br J Ophthalmol. 2017;101(12):1709–13. 10.1136/bjophthalmol-2016-310005. [DOI] [PubMed] [Google Scholar]
- 18.Holmström G, Tornqvist K, Al-Hawasi A, Nilsson Å, Wallin A, Hellström A. Increased frequency of retinopathy of prematurity over the last decade and significant regional differences. Acta Ophthalmol. 2018;96(2):142–8. 10.1111/aos.13549. [DOI] [PubMed] [Google Scholar]
- 19.Abdel Aziz I, Alsoda MF, Elmenofy TM, et al. Tailoring screening guidelines for retinopathy of Prematurity in Egypt: an exploratory Multicentric Study. Clin Ophthalmol. 2022;16:3625–30. 10.2147/OPTH.S383497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alizadeh Y, Behboudi H, Dourandeesh M, et al. Retinopathy of prematurity: applicability of international and national screening guidelines in the north of Iran. Turk J Pediatr. 2022;64(2):221–7. 10.24953/turkjped.2021.1943. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Yue G, Yu JL. Changes in perinatal care and predictors of in-hospital mortality for very low birth weight preterm infants. Iran J Pediatr. 2012;22(3):326–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen AM, Cook N, Harris MC, Ying GS, Binenbaum G. The pain response to mydriatic eyedrops in preterm infants. J Perinatol. 2013;33(6):462–5. 10.1038/jp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löfqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124(12):1711–8. 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 24.Binenbaum G, Ying GS, Quinn GE, et al. A clinical prediction model to stratify retinopathy of prematurity risk using postnatal weight gain. Pediatrics. 2011;127(3):e607–14. 10.1542/peds.2010-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binenbaum G, Ying GS, Quinn GE, et al. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch Ophthalmol. 2012;130(12):1560–5. 10.1001/archophthalmol.2012.2524. [DOI] [PubMed] [Google Scholar]
- 26.Eckert GU, Fortes Filho JB, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond). 2012;26(3):400–6. 10.1038/eye.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binenbaum G, Bell EF, Donohue P, et al. Development of modified screening criteria for retinopathy of Prematurity: primary results from the postnatal growth and retinopathy of Prematurity Study. JAMA Ophthalmol. 2018;136(9):1034–40. 10.1001/jamaophthalmol.2018.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binenbaum G, Tomlinson LA, de Alba Campomanes AG, et al. Validation of the postnatal growth and retinopathy of Prematurity Screening Criteria. JAMA Ophthalmol. 2020;138(1):31–7. 10.1001/jamaophthalmol.2019.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiraki A, Fukushima Y, Kawasaki R, et al. Retrospective validation of the postnatal growth and retinopathy of Prematurity (G-ROP) Criteria in a Japanese cohort. Am J Ophthalmol. 2019;205:50–3. 10.1016/j.ajo.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Huang CW, Yeh PT, Tsao PN, et al. Validation of the postnatal growth and retinopathy of Prematurity Screening Criteria in a Taiwanese cohort. Am J Ophthalmol. 2022;237:22–31. 10.1016/j.ajo.2021.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.