Abstract
Background
The challenge of dealing with isolated reactive treponemal chemiluminescence immunoassay (CIA) results in clinical practice has prompted the development of a more efficient algorithm for distinguishing true infection from false reactivity in isolated CIA sera.
Methods
A prospective cohort study was conducted at Wuhan Tongji Hospital, involving 119,002 individuals screened for syphilis using CIA from January 1, 2015, to January 6, 2017. Samples with reactive CIA results underwent simultaneous testing with the T. pallidum passive particle agglutination assay (TPPA) and the rapid plasma reagin test (RPR). Additionally, a subgroup of 189 individuals with differing TPPA statuses was selected for further analysis using Western blotting (WB) and a modified TPPA assay (titer, 1:20). To identify the optimal serological approach for distinguishing true from false reactivity in sera with isolated reactive CIAs (CIA+TPPA−RPR−), two distinct algorithms were developed and evaluated. The first algorithm involved reflexively testing CIA+TPPA−RPR− sera with the modified TPPA, followed by WB if nonreactive. The second algorithm began with WB, followed by the modified TPPA if nonreactive or indeterminate.
Results
WB demonstrated lower sensitivity compared to TPPA, but it identified six syphilis cases among the 89 CIA+TPPA− samples. Both WB and modified TPPA exhibited a specificity of 100%. The two supplementary confirmatory algorithms detected 12 additional syphilis cases, with the first algorithm being more cost-effective and labor-saving.
Conclusion
A combination of a modified TPPA (titer, 1:20) and WB can serve as a reliable algorithm for distinguishing true syphilis infection from false reactive signals in isolated reactive CIA sera.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10404-1.
Keywords: Isolated reactive chemiluminescence immunoassay; Modified TPPA (titer, 1:20); Syphilis serodiagnosis; Western blotting
Introduction
Syphilis, caused by the spirochete bacterium Treponema pallidum, is a sexually transmitted infection that is re-emerging as a global health concern, affecting millions of people worldwide [1–4]. While most infected individuals do not exhibit symptoms, without early diagnosis and proper treatment, the infection can progress to more severe stages and lead to serious complications [5].
The diagnosis of syphilis primarily relies on clinical history and serologic tests, including non-treponemal and treponemal tests. Treponemal tests detect antibodies specific to T. pallidum proteins, utilizing various techniques such as enzyme and chemiluminescence immunoassays (EIA/CIA), T. pallidum particle agglutination (TPPA), Western blotting (WB), and others. Treponemal antibodies generally remain reactive throughout a person’s life, regardless of treatment. However, it has been observed that up to 24% of syphilis patients treated in the early stage may revert to nonreactive years after therapy [6]. On the other hand, non-treponemal tests, such as rapid plasma reagin (RPR), venereal disease research laboratory (VDRL), and toluidine red unheated serum (TRUST) assays, are used to evaluate disease activity and monitor therapeutic response. These tests may yield nonreactive results in the early or late stages of syphilis, and their titers often decrease after adequate treatment, eventually becoming nonreactive over time. However, in some cases, despite an appropriate decline in non-treponemal titers, they may persistently remain reactive for a prolonged period [5].
In recent years, the implementation of various reverse syphilis screening algorithms (treponemal, then non-treponemal or another treponemal test) in high-volume laboratories has facilitated the identification of individuals previously treated for syphilis and those without treatment. However, this approach has also resulted in 11.3-20.8% of initially reactive EIA/CIA samples showing reactivity only on the EIA/CIA (referred to as isolated reactive EIA/CIA) [7, 8, 15]. The isolated reactive EIA/CIA result may occur during the incubation or early stage of syphilis, as the EIA/CIA test is generally more sensitive than other treponemal or non-treponemal tests. Additionally, it has been observed that isolated reactive CIA results can still occur even after seroreversion of traditional treponemal assays like TPPA and FTA Abs assays, indicating the presence of true T. pallidum infection [7]. Isolated reactive EIA/CIAs may indicate early syphilis, prior infection, or false reactivity, posing significant diagnostic and treatment challenges for clinicians, particularly in the absence of documented recent syphilis exposure or prior treatment. As a result, patients with isolated reactive EIA/CIA results may undergo unnecessary investigations, additional therapy, or require serologic follow-up. To address this issue, it is crucial to conduct further analysis of the isolated reactive EIA/CIA specimens using an additional confirmatory test. This will help improve our understanding and interpretation of discrepant results in specific situations and provide better guidance for patient management.
The dilemma arises regarding the optimal treponemal test for confirming or ruling out a suspicion of syphilis in cases with isolated treponemal EIA/CIAs results. The U.S. Centers for Disease Control and Prevention (CDC) once planned to conduct a study to characterize isolated reactive treponemal CIA sera through immunoblotting to define their reactivities with T. pallidum antigens and identify the causes of unconfirmed reactive treponemal tests [8]. The UK national guidelines recommend using an IgG immunoblot as an additional confirmatory test when reactive screening results are not confirmed by the standard confirmatory test [9]. Numerous other studies have also emphasized the necessity of using WB as the confirmation method for samples with inconsistent results [10–12]. However, the European guideline suggests that WB does not provide significant additional value compared to other treponemal tests [13]. In this study, our objective is to evaluate the performance of WB and assess the necessity of combining WB with a modified TPPA (titer, 1:20) for unconfirmed CIA sera in a low-prevalence population in China.
Materials and methods
Study design and population
We prospectively enrolled participants who underwent a treponemal CIA screening test (Abbott Diagnostics, Abbott Park, Illinois) at the Department of Laboratory Medicine, Tongji Hospital, between January 1, 2015, and January 6, 2017, in our study. All reactive CIA sera were tested simultaneously with RPR (KHB, Shanghai, China) and TPPA (Fujirebio, Tokyo, Japan) at a dilution of 1:80, as recommended by the manufacturer (hereafter referred to as TPPA), to confirm the true syphilis infection and disease activity. Additionally, sera from 189 individuals in the prospective component of the study were collected to evaluate the performance of a treponemal WB assay (EUROLineMaster Plus, Euroimmun Medizinische Labordiagostika AG, Germany). These included 61 samples reactive for both CIA and TPPA, 39 samples nonreactive for both CIA and TPPA, and 89 samples reactive for CIA but nonreactive for TPPA (including four cases reactive on RPR). The first two groups of sera were collected consecutively over a short period, while the last group—excluding the four CIA+TPPA−RPR+ sera—was collected consecutively over a more extended period. These sera represent the most commonly observed serological patterns for syphilis detection, facilitating a precise evaluation of the performance of the Western blot assay. Given that samples with CIA+TPPA−RPR+ results indicate a true syphilis infection [14], only 85 specimens with isolated reactive CIA results were retested using a modified TPPA assay at a titer of 1:20 (hereafter referred to as modified TPPA) to assess its complementary utility for cases with isolated reactive CIA results. The above-mentioned 39 samples that were nonreactive for both CIA and TPPA were also used to assess the specificity of the modified TPPA. Data regarding previous syphilis diagnoses, follow-up testing, and clinical management were retrospectively reviewed from electronic medical records for the recruited patients. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20231172).
Clinical diagnosis of syphilis
According to the guidelines outlined in the Sexually Transmitted Infections Treatment 2021 guidelines [14], a reactive result in the initial screening CIA, followed by a reactive result in either the RPR, TPPA, or WB tests, is considered indicative of syphilis infection. Results that are reactive on the initial CIA but are not confirmed by either RPR or TPPA should be interpreted within the clinical context. Cases with a reactive CIA but nonreactive RPR and TPPA results may still be considered as having syphilis if there is supporting prior or subsequent clinical or serological evidence.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism (version 5.0; GraphPad Software Inc., San Diego, CA, USA). The Kolmogorov–Smirnov test was used to assess whether or not a variable was normally distributed. Normally distributed data were analyzed using the unpaired t–test and expressed as means ± standard deviations. Non–normally distributed data were compared using the Mann–Whitney U test and expressed as medians and interquartile ranges (IQRs).
Results
Characteristics of the study population
A total of 119,002 participants aged from 0 to 102 years (median, 42.0; IQR: 28.0–56.0 years) were included in this hospital-based prospective study, with males accounting for 50.1% of the cohort. Among the participants, 2,385 (2.0%) were reactive on the screening CIA, and 20.0% of the CIA-reactive cases were demonstrated to be isolated CIA reactive when reflexively tested by TPPA and RPR.
Distribution of CIA signal/cutoff (S/CO) values across different serological patterns
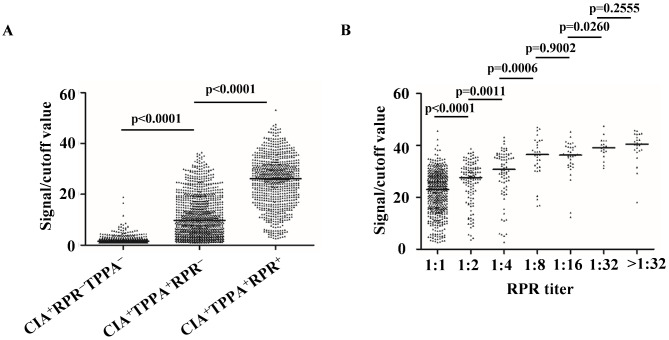
Among the 2,385 CIA-reactive sera, the median S/CO value of CIA+TPPA−RPR− sera (median = 1.52; IQR: 1.20–2.32) was significantly lower than that of the RPR-nonreactive confirmed-syphilis sera (CIA+TPPA+RPR−, median = 9.80; IQR: 4.41–17.9) (P < 0.0001). This indicates that isolated reactive CIAs were associated with lower S/CO ratios. Additionally, the S/CO value of RPR-reactive confirmed-syphilis sera (CIA+TPPA+RPR+, median = 26.2; IQR: 18.4–31.8) was significantly higher than that of CIA+TPPA+RPR− sera (P < 0.0001) (Fig. 1A). Among individuals with CIA+TPPA+RPR+, the CIA ratios increased gradually with higher RPR titers (Fig. 1B).
Fig. 1.
Distribution of CIA signal/cutoff values in different serum groups. (A) Signal/cutoff value distribution in CIA+TPPA−RPR− (n = 478), CIA+TPPA+RPR− (n = 1034) and CIA+TPPA+RPR+ (n = 791) groups. (B) RPR titers versus signal/cutoff values in CIA+TPPA+RPR+ sera. +, reactive; −, nonreactive; RPR (titer, 1:1), n = 477; RPR (titer, 1:2), n = 128; RPR (titer, 1:4), n = 76; RPR (titer, 1:8), n = 33; RPR (titer, 1:16), n = 34; RPR (titer, 1:32), n = 19; RPR (titer, > 1:32), n = 24. Abbreviations: CIA, chemiluminescence immunoassay; TPPA, Treponema pallidum particle agglutination assay; RPR, rapid plasma reagin test
WB compared to TPPA as a confirmatory test for syphilis
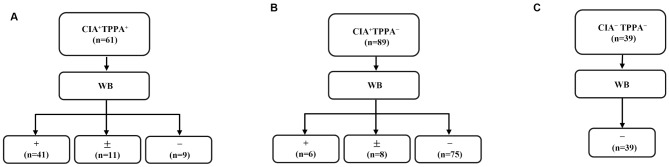
Among the 61 CIA+TPPA+ sera, WB was reactive in 41 samples (67.2%), indeterminate in 11 samples (18.0%), and nonreactive in nine samples (14.8%) (Fig. 2A). In the 89 CIA+TPPA− sera, six samples (6.74%) were reactive, eight samples (8.99%) were indeterminate, and 75 samples (84.3%) were nonreactive to WB (Fig. 2B). Among the six WB-reactive subjects in the CIA+TPPA− group, two (Patients 24–25) had reactive RPR results, one (Patient 26) had prior serological evidence of syphilis, and two (Patients 22–23) remained isolated CIA reactive during follow-up. The remaining subject (Patient 21) was not retested during the follow-up period (Supplementary Table S1). Additionally, among the eight CIA+TPPA−WB± cases, only one (Patient 28) exhibited evidence of prior syphilis. In the group of 75 cases with nonreactive WB results within the CIA+TPPA− category, two were RPR reactive while 73 remained nonreactive. Notably, five subjects (Patients 35, 37–40) among the CIA+TPPA−RPR−WB− subgroup had prior serological evidence of syphilis, one (Patient 46) had latent syphilis (subsequently seroconverted to TPPA reactive but remained persistently RPR nonreactive), and two (Patients 36, 45) had a documented history of syphilis (Table 1). Overall, 32.3% (61) of the 189 individuals evaluated were TPPA reactive, while only 24.9% (47) were WB reactive, suggesting that TPPA may have higher sensitivity than WB. The specificity of the WB test was evaluated against the TPPA test in a panel of 39 sera from individuals without evidence of syphilis (Fig. 2C), where both tests demonstrated a specificity of 100%. The overall agreement between TPPA and WB was 82.0%, with the details of discordant results provided in Supplementary Table S1.
Fig. 2.
TPPA compared to WB as a confirmatory test for syphilis. (A) WB results of samples with CIA+TPPA+ (n = 61). (B) WB results of samples with CIA+TPPA− (n = 89). (C) WB results of samples with CIA−TPPA− (n = 39). Abbreviations: TPPA, Treponema pallidum particle agglutination assay; WB, Western blotting; +, reactive; −, nonreactive; ±, indeterminate
Table 1.
Clinical and serological characteristics of 13 syphilis patients with isolated reactive CIA
| Sample No. | Gender | Age | CIA S/CO | TPPA (titer, 1:80) | WB | RPR | Modified TPPA (titer, 1:20) | Prior serological result (s) | Subsequent serological result (s) | History of prior syphilis | Treatment for syphilis | Diagnosis for syphilis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 | F | 33 y | 4.20 | Nonreactive | Nonreactive | Nonreactive | Reactive | CIA reactive, TPPA reactive, RPR nonreactive (1 mo) | None | Yes | None | Yes |
| 36 | M | 76 y | 3.24 | Nonreactive | Nonreactive | Nonreactive | Nonreactive | None | Isolated reactive CIA (10 mo); isolated reactive CIA (19 mo); CIA nonreactive (24 mo) | Yes | None | Yes |
| 37 | M | 9 m | 1.59 | Nonreactive | Nonreactive | Nonreactive | Reactive | CIA reactive, TPPA reactive, RPR reactive (9 mo); CIA reactive, TPPA reactive, RPR reactive (8 mo); CIA reactive, TPPA reactive, RPR nonreactive (5 mo); CIA reactive, TPPA reactive, RPR nonreactive (3 mo) | CIA nonreactive (4 mo) | Yes | None | Yes |
| 38 | M | 91 y | 4.13 | Nonreactive | Nonreactive | Nonreactive | Reactive | CIA reactive, TPPA reactive, RPR reactive (24 mo) | None | Yes | None | Yes |
| 39 | M | 52 y | 1.35 | Nonreactive | Nonreactive | Nonreactive | Reactive | CIA reactive, TPPA reactive, RPR reactive (40 mo) | Isolated reactive CIA (6 mo); CIA nonreactive (12 mo) | Yes | None | Yes |
| 40 | M | 65 y | 1.54 | Nonreactive | Nonreactive | Nonreactive | Reactive | CIA reactive, TPPA nonreactive, RPR nonreactive (38 mo); CIA reactive, TPPA reactive, RPR nonreactive (30 mo) | CIA reactive, TPPA indeterminate, RPR nonreactive (11 mo); CIA reactive, TPPA nonreactive, RPR nonreactive (26 mo) | Yes | None | Yes |
| 41 | M | 29 y | 3.19 | Nonreactive | Reactive | Nonreactive | Reactive | None | None | None | None | Yes |
| 42 | F | 60 y | 17.3 | Nonreactive | Reactive | Nonreactive | Nonreactive | Isolated reactive CIA (29 mo) | None | None | None | Yes |
| 43 | F | 27 y | 3.97 | Nonreactive | Reactive | Nonreactive | Nonreactive | None | Isolated reactive CIA (4 mo) | None | None | Yes |
| 44 | M | 61 y | 11.6 | Nonreactive | Reactive | Nonreactive | Reactive | CIA reactive, TPPA reactive, RPR nonreactive (2 mo) | None | Yes | None | Yes |
| 45 | M | 55 y | 1.35 | Nonreactive | Nonreactive | Nonreactive | Reactive | CIA reactive, TPPA nonreactive (60 mo) | Isolated reactive CIA (12 mo); isolated reactive CIA (30 mo) | Yes | Yes | Yes |
| 46 | F | 54 y | 1.15 | Nonreactive | Nonreactive | Nonreactive | Reactive | Isolated reactive CIA (1 mo) | CIA reactive, TPPA reactive, RPR nonreactive (2 mo); CIA reactive, TPPA reactive, RPR nonreactive (20 mo) | None | None | Yes |
| 47 | M | 92 y | 5.06 | Nonreactive | Indeterminate | Nonreactive | Reactive | CIA reactive, TPPA reactive, RPR nonreactive (54 mo); CIA reactive, TPPA indeterminate, RPR nonreactive (25 mo); CIA reactive, TPPA reactive, RPR nonreactive (13 mo) | CIA reactive, TPPA indeterminate, RPR nonreactive (12 mo); CIA reactive, TPPA nonreactive, RPR nonreactive (23 mo) | Yes | None | Yes |
Abbreviations: CIA, chemiluminescence immunoassay; S/CO, signal-to-cutoff ratio; TPPA, Treponema pallidum particle agglutination assay; RPR, rapid plasma reagin test; WB, western blotting
Modified TPPA combined with WB as a new supplementary confirmatory algorithm for isolated CIA results
Among the 85 cases with isolated reactive CIA serology, the majority were elderly individuals aged 60 years and over (n = 24), followed by patients with tumors (n = 17) and pregnant women (n = 11). Among them, nine patients had a documented history of syphilis, 43 had only one set of serological test results, and 42 underwent repeat serological testing for syphilis. Among those who underwent repeat testing, 23 exhibited persistently isolated CIA-reactive serology, while 13 ultimately converted to a CIA-nonreactive status during the follow-up period. Notably, one subject (Patient 46) became TPPA reactive while remaining nonreactive for RPR, despite lacking any prior history of syphilis infection or treatment. Additionally, three cases were confirmed as syphilis through WB testing. In summary, 15.3% (13 out of 85) of subjects with isolated reactive CIA results exhibited clinical or serological evidence of syphilis infection (Table 1).
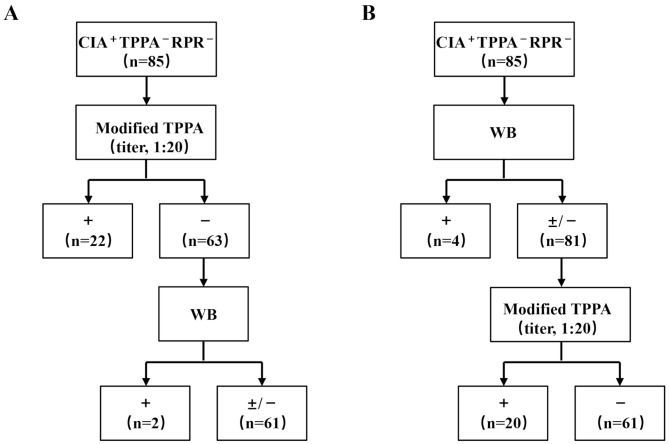
To address the management of isolated reactive CIA results, we developed two serological algorithms. The first algorithm employed the modified TPPA as the supplementary confirmatory test (Fig. 3A). Among the 22 cases that tested reactive for modified TPPA, 10 patients (45.5%) had clinical or serological evidence of syphilis, including seven (Patients 35, 37–40, 44, 47) with prior Treponema pallidum infections, one (Patient 46) with latent syphilis, one (Patient 45) with a documented history of syphilis, and one (Patient 41) with confirmed syphilis based on a reactive WB test. Further analysis of the 63 cases with nonreactive modified TPPA results revealed two additional syphilis cases confirmed by reactive WB tests (patients 42–43). Moreover, 39 sera that were nonreactive for CIA, TPPA, and RPR were also evaluated using the modified TPPA assay, all returning nonreactive results, indicating high specificity for syphilis diagnosis.
Fig. 3.
Working algorithms for isolated reactive CIA sera. (A) The algorithm starting with the modified TPPA (titer, 1:20). (B) The algorithm starting with WB. Abbreviations: TPPA, Treponema pallidum particle agglutination assay; RPR, rapid plasma reagin test; WB, Western blotting; +, reactive; −, nonreactive; ±, indeterminate
The second algorithm initiated with WB testing, which identified four confirmed syphilis cases based on reactive WB results (Patients 41–44) (Fig. 3B). Cases that were nonreactive or indeterminate on WB (WB±/−) were assessed using the modified TPPA. Among these WB±/− cases, 20 exhibited reactivity on the modified TPPA, and eight of these (Patients 35, 37–40, 45–47) presented evidence of previous or subsequent syphilis infection. However, one subject (Patient 36) with a documented history of syphilis was not identified by either algorithm and later showed seroreversion to nonreactive CIA serology two years later.
Discussion
In the current prospective study, 2.0% (2385/119002) of sera tested reactive by the treponemal screening CIA test, and among those, 20.0% (478/2385) showed isolated CIA reactivity in our low-prevalence population. The proportion of patients with isolated reactive CIA serology in our healthcare setting was higher compared to the rates reported by the five US laboratories (17.9%) [8] and another study from Australia (11.3%) [7], but similar to the proportion reported by Birmingham Whittall Street Clinic (20.8%) [15]. The observed discrepancies may be attributed to variations in the study population, the prevalence of Treponema pallidum, and the performance of both screening and confirmatory tests. However, additional studies are required to validate these findings.
Our study analyzed 2,385 CIA-reactive sera and identified significant differences in median CIA S/CO values across various serological patterns. Notably, a positive correlation was observed between CIA S/CO ratios and RPR titers in RPR-reactive individuals, suggesting that elevated S/CO ratios may indicate true Treponema pallidum infection and could correlate with syphilis stages and disease activity. However, it is important to emphasize that the current study lacks robust data from diverse clinical settings. Therefore, at present, S/CO ratios should not be regarded as direct surrogates for titers.
The implementation of the WB technique has been proposed to identify both false-reactive CIA results and likely false-nonreactive TPPA results [10, 12]. However, the lack of validated data on the diagnosis and management of syphilis limits its utility in clinical settings. In our study, among the recruited patients, 32.3% individuals were TPPA reactive compared to only 24.9% who were WB reactive. This finding suggests that WB is less sensitive than TPPA as a confirmatory test, which supports the current guideline recommending TPPA as the optimal confirmatory test for initial reactive treponemal CIA results [14]. However, we identified six WB-reactive cases among 89 subjects with CIA+TPPA− serology, including two with CIA+TPPA−RPR+, one with CIA+TPPA−RPR− (seroreverted from CIA+TPPA+ to CIA+TPPA−), and three with CIA+TPPA−RPR− serology. According to the European guideline, the six (6/89, 6.74%) TPPA-nonreactive but CIA-reactive and WB-reactive cases would be classified as true T. pallidum infection, and the TPPA-nonreactive results should be considered as false negative. This finding indicates that WB may be helpful in identifying syphilis in cases with conflicting serological results. Furthermore, previous studies have also demonstrated the utility of WB in discordant syphilis serological tests [11, 12]. Overall, the available evidence suggests that WB could be a valuable supplementary confirmatory test for discordant syphilis serological results. However, it should not be used as a substitute for TPPA.
In our study, we found that a modified TPPA with a titer of 1:20 demonstrated increased sensitivity compared to the original TPPA, while maintaining a high specificity. This was supported by the absence of false-reactive modified TPPA results in non-syphilis subjects. However, the analytical and clinical performance of the modified assay must be thoroughly validated in multicenter studies with large sample sizes to ensure reliable results in routine clinical testing.
To address the challenges in syphilis diagnosis and management related to isolated treponemal CIA results, we developed two distinct syphilis detection algorithms. In this research, based on the hypothesis that a reactive modified TPPA or WB indicates a true Treponema pallidum infection due to their high specificities, both algorithms successfully identified 24 additional syphilis cases among the 85 CIA+RPR−TPPA− cases, including 12 confirmed syphilis cases supported by clinical and/or serological evidence. However, the first algorithm, which commenced with the modified TPPA followed by WB, was considered more suitable for clinical practice due to its cost-effectiveness and labor-saving.
Based on the aforementioned findings, we recommend implementing a supplementary confirmatory algorithm for isolated reactive CIA sera. This algorithm should initially utilize a more sensitive modified TPPA test at a dilution of 1:20 for confirmation of isolated reactive CIA samples. If the modified TPPA results are nonreactive, a treponemal WB test should be conducted. In instances where sera are nonreactive for CIA, RPR, TPPA, modified TPPA, and WB, the likelihood of syphilis is minimal. However, clinical findings should always be considered when interpreting serological results, as a comprehensive diagnosis involves both clinical evaluation and laboratory data. This algorithm is currently suitable for populations with a low prevalence of syphilis. Further research is needed to develop appropriate strategies for populations in regions or settings with a high syphilis prevalence.
The significance of isolated reactive treponemal CIA results remains uncertain. A previous study by Hunter MG etc [7]. indicated that isolated reactive CIA specimens may represent true T. pallidum infection and were associated with “inactive” syphilis cases, as none of the patients developed clinical signs or symptoms of the disease. In our study, the majority of isolated reactive CIA results were observed in elderly patients, followed by individuals undergoing cancer therapies and pregnant women. This suggests that these populations may either have a historical syphilis infection from decades ago or may present with false-positive CIA results due to underlying medical conditions. Notably, we identified a patient with no previous history of syphilis or related treatment who was confirmed to have syphilis during follow-up testing, indicating the necessity for treatment [9, 13, 14]. Given the small sample size of our study, further longitudinal studies with a larger cohort over an extended period are necessary to fully elucidate the significance of isolated reactive treponemal CIA results. Nonetheless, distinguishing true infection from false reactivity among isolated treponemal CIA cases remains a critical priority, as it enables clinicians to make accurate diagnostic and treatment decisions.
Our study has several strengths. Most importantly, to the best of my knowledge, this is the first study to evaluate the performance of a modified TPPA in conjunction with WB for distinguishing true syphilis from false-reactive CIAs in individuals with isolated CIA results at a tertiary referral hospital. Our findings indicate that isolated treponemal CIA sera that present with either a reactive modified TPPA or a nonreactive modified TPPA accompanied by a reactive WB are indicative of a true Treponema pallidum infection. Conversely, a sample exhibiting CIA+RPR−TPPA−WB−modified TPPA− serology, in the absence of pertinent clinical history, is more likely to signify a false-positive CIA result. These novel findings provide a basis for future clinical management of isolated treponemal CIA sera. Additionally, participants in this study were followed up for up to 7.6 years, and individuals with reactive CIA sera were tested simultaneously with TPPA and RPR, ensuring the reliability of the conclusions.
However, we also acknowledge some study limitations. Firstly, the study focused on a population with a low prevalence of syphilis, limiting the generalizability of the findings to high-prevalence populations. Further studies are warranted to validate these findings in regions or settings with high syphilis prevalence. Secondly, there exists a potential for misclassification of patients with unrecognized prior syphilis history due to incomplete or missing documentation. Consequently, the true syphilis history of individuals with isolated reactive CIAs in our study remains uncertain. Thirdly, there was an insufficient clinical follow-up for patients with discordant results, leading to a significant rate of loss to follow-up. This may introduce bias in the results of subsequent testing and management, potentially favoring patients at higher risk. Lastly, it is important to acknowledge that the sample size in this study was limited. Therefore, further research with a larger sample size and extended follow-up is warranted to yield more robust and reliable findings.
Conclusions
In conclusion, isolated CIA results in low-risk individuals may not necessarily be false reactive and should be interpreted cautiously. It is advisable to further test sera with isolated treponemal CIA results using a modified TPPA test in combination with WB to confirm or rule out the suspicion of syphilis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the department of laboratory medicine, Tongji Hospital for providing instruments and reagents for sample testing.
Abbreviations
- EIA
Enzyme immunoassays
- CIA
Chemiluminescence immunoassay
- TPPA
T. pallidum passive particle agglutination assay
- RPR
Rapid plasma reagin test
- WB
Western blotting
- VDRL
Venereal disease research laboratory
- TRUST
Toluidine red unheated serum test
- EIA
Enzyme immunoassay
- IQR
Interquartile range
- CDC
Centers for Disease Control and Prevention
- S/CO
Signal/cutoff
Author contributions
Liming Cheng, Jing Peng and Ziyong Sun conceived and designed the study. Xia Luo, Hua Xiao, Weiming Gu and Jing Peng contributed to the statistical analysis, writing of the original draft, review and editing of the draft. Hua Xiao and Yanfang Lu were responsible for specimen collection and measurement, as well as the review of electronic medical records. Liming Cheng and Jing Peng had primary responsibility for final content. All authors were involved in critical revisions and approved the final version of the manuscript.
Funding
This research does not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Deidentified participant data from this study might be available after publication upon request to the corresponding authors (ppjj6@126.com; chengliming2015@163.com).
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20231172). The requirement for consent to participate was waived by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, as the study involved the use of de-identified medical records and residual serum for secondary analysis, with no direct participant intervention.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xia Luo and Hua Xiao contributed equally to this work.
Contributor Information
Jing Peng, Email: ppjj6@126.com.
Liming Cheng, Email: chengliming2015@163.com.
References
- 1.Moseley P, Bamford A, Eisen S, Lyall H, Kingston M, Thorne C, Pinera C, Rabie H, Prendergast AJ, Kadambari S. Resurgence of congenital syphilis: new strategies against an old foe. Lancet Infect Dis. 2024;24(1):e24–35. [DOI] [PubMed] [Google Scholar]
- 2.Peeling RW, Mabey D, Chen XS, Garcia PJ. Syphilis Lancet. 2023;402(10398):336–46. [DOI] [PubMed] [Google Scholar]
- 3.Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS, Syphilis. Nat Rev Dis Primers. 2017;3:17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. 2015;10(12):e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanem KG, Ram S, Rice PA. The modern epidemic of Syphilis. N Engl J Med. 2020;382(9):845–54. [DOI] [PubMed] [Google Scholar]
- 6.Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114(12):1005–9. [DOI] [PubMed] [Google Scholar]
- 7.Hunter MG, Robertson PW, Post JJ. Significance of isolated reactive treponemal chemiluminescence immunoassay results. J Infect Dis. 2013;207(9):1416–23. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease C, Prevention. Discordant results from reverse sequence syphilis screening–five laboratories, United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60(5):133–7. [PubMed] [Google Scholar]
- 9.Kingston M, French P, Higgins S, McQuillan O, Sukthankar A, Stott C, McBrien B, Tipple C, Turner A, Sullivan AK, et al. UK national guidelines on the management of syphilis 2015. Int J STD AIDS. 2016;27(6):421–46. [DOI] [PubMed] [Google Scholar]
- 10.Zhiyan L, Meiling W, Ping L, Jinhua D, Zhenlin Y, Zhenru F. Consistency between Treponema pallidum particle agglutination assay and architect chemiluminescent microparticle immunoassay and characterization of inconsistent samples. J Clin Lab Anal. 2015;29(4):281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, Li H, Wu X, Guo J, Zhang J, Hu X. Confirmation value of Western blotting in detecting anti-treponema pallidum specific antibodies with suspicious results. Eur J Med Res. 2022;27(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang YH, Liu H, Tang J, Wang YZ, Zheng XH, Gong Y, Xu XF, Gao X, Lu RQ, Ju SG, et al. Screening for syphilis with dual algorithms: analysis of discordant and concordant serology results in a population with a low prevalence of syphilis. J Eur Acad Dermatol Venereol. 2019;33(1):178–84. [DOI] [PubMed] [Google Scholar]
- 13.Janier M, Unemo M, Dupin N, Tiplica GS, Potocnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2021;35(3):574–88. [DOI] [PubMed] [Google Scholar]
- 14.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually transmitted infections Treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caswell RJ, Hathorn E, Manavi K. The significance of isolated reactive Treponemal enzyme Immunoassay in the diagnosis of early Syphilis. Sex Transm Dis. 2016;43(6):365–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data from this study might be available after publication upon request to the corresponding authors (ppjj6@126.com; chengliming2015@163.com).