Abstract
Background
Fibrous dysplasia (FD) is a self-limiting benign disease with slow progression in which the normal bone is replaced by dysplastic fibrous tissue. The craniofacial skeleton is one of the most commonly affected areas, and it can create unique challenges in dental implant therapy. This case aims to report an unusual presentation of FD localized in the alveolar crest bone of the edentulous site, causing special obstacles to implant placement, and provide a diagnostic and treatment process that may be referenced.
Case presentation
A 32-year-old female patient presented with an abnormal expansion of the alveolar crest on the edentulous site of the mandibular first molar, which caused insufficient inter-arch space and pseudo-pocket in the adjacent teeth, previous medical history of residual root that existed for many years before tooth extraction was identified retrospectively. FD was diagnosed by the clinical, radiographic, and pathological examination, and the curettage was performed to remove abnormal bone tissue and obtain adequate restorative space for further implant therapy. This intervention was followed by histologically confirmed healing and successfully integrated implant placement.
Conclusion
We described an unreported pathogenic site of FD that occurred at the alveolar ridge of the edentulous site, which ended up with satisfactory oral implant rehabilitation through 5-year sequential diagnosis and treatment.
Keywords: Fibrous dysplasia, Dental implant, Alveolar crest, Bone remodeling, Curettage
Background
Fibrous dysplasia (FD) is a genetically based disease with mutations in the gene GNSA I, creating expansile fibro-osseous lesions filled with abnormally proliferating osteoprogenitor cells [1]. FD can occur in one bone (monostotic, MFD), several bones (polyostotic, PFD), and in combination with hyperfunctioning endocrinopathies and skin hyperpigmentation (McCune-Albright Syndrome) [2, 3]. The incidence of FD/MAS was 3.6 per 1 000 000 person-years, and most diagnoses were between the ages of 11 and 20 years [4]. The clinical presentation of FD varies from slow and benign-growing mass lesions and deformities to malignant bony matrix transformation [3]. The most common locations of FD are the rib, skull, and femur. This disease's inducing factors may be related to trauma, infection, endocrine dysfunction, or local blood circulation disorders caused by specific reasons [1, 3].
Histopathological features of FD are composed of abnormal fibrous tissue interspersed between dysplastic and disorganized bone trabecula [5]. A typical FD lesion appears as an area of radiolucent ground glass [6]. However, craniofacial FD typically demonstrates radiographic patterns virtually indistinguishable from other bone tissue [7], making it difficult for clinical diagnosis. The diagnosis of FD usually requires a combination of clinical, radiographic, and pathologic examination.
The latest WHO 2022 classification of Head and Neck Tumors advised new ideas for treating FD, including surgery and medication [8]. Bisphosphonates are classically used for drug treatment; studies showed that pamidronate or zoledronate could result in pain control [2, 9, 10], while a more aggressive method, like surgical excision of the entire affected area, is recommended when the lesion is extensive or unfavorable for subsequent prognosis [11, 12].
The adverse effects of FD on maxillofacial bones have been widely reported [10, 13]. Nevertheless, to our knowledge, literature reports have not so far documented how to conduct initial diagnosis and treatment when FD occurs at the alveolar crest of the edentulous site, requiring subsequent implant treatment to reconstruct occlusal function. The purpose of this case report is to provide an updated situation with the incriminated interocclusal space and periodontal diseases in the adjacent tooth, provide diagnosis and treatment modality for FD on the alveolar crest of the edentulous site in future implant placement, and discuss potential stimulating factors about this rare pathogenic site.
A written informed consent was obtained from the patient, which was approved by the Ethical Committee of the Affiliated Stomatology Hospital of Guangzhou Medical University (LCYJ2023017).
Case presentation
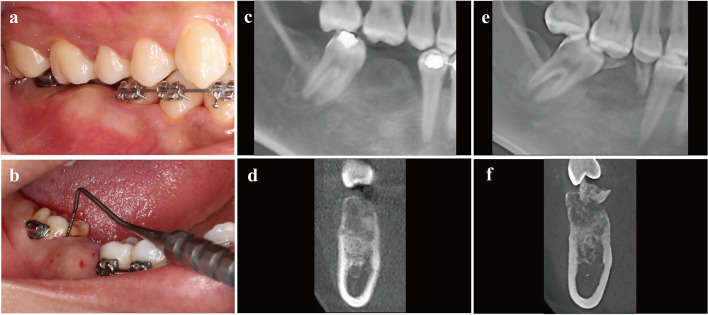
In November 2018, a 32-year-old female patient who was referred to the department of oral implantology by an orthodontist requesting implant therapy presented with abnormal expansion of the alveolar crest on the edentulous site of the mandibular first molar (Fig. 1a). The lesion was about 1 cm × 1 cm × 1 cm sitting on the buccal side of alveolar bone between teeth #45 and #47 (FDI tooth-numbering system). Concomitantly, the adjacent teeth #47 and #45 showed pseudo-pocket in the proximal region of the edentulous site, with a probing depth of approximately 12 mm and bleeding on probing (Fig. 1b). This situation caused inadequate restorative space and obstacles for future implant placement. The cone-beam computed tomography (CBCT) was performed to detect the disorder, which revealed an approximate 14 mm × 10 mm × 10 mm indistinct ground-glass lesion (Fig. 1c, d). The previous medical history can be traced back to orthodontic treatment in 2015, and #46 had a residual root that existed for many years before the request for tooth extraction by the orthodontist (Fig. 1e, f).
Fig. 1.
The clinical and radiographic views of the bone expansion in alveolar crest of edentulous site. a The buccal view presented with inadequate inter-arch space, which was caused by lesion bone extend, then the keratinized mucosa was normal. b The pseudo-pocket of the adjacent tooth and bleeding on probing of #47. c, d The radiographic views at initial diagnosis with the ground-glass bone of blurred boundaries. e, f The retrospective radiographic record of preoperative tooth extraction in 2015 with the residual root of #46 that existed for many years
In addition, this patient had no mental disease, other systemic disease, or family history of the similar disease.
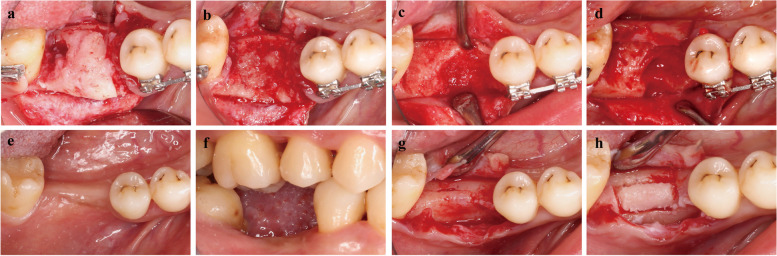
In January 2019, the patient was informed that a biopsy was needed to determine the final diagnosis, and written information was obtained. The biopsy was performed (Fig. 2a) under local anesthesia with 4% ArtiCaine (Prima Caine Adrenaline, Produits Dentaires Pierre Rolland, France). Pathological biopsy tissue obtained for the pathological evaluation was prepared by piezoelectric osteotomy at the alveolar crest of #46 (Fig. 2b). The pathological sections stained with hematoxylin–eosin staining (HE), histopathological features of FD could be seen which consist of fibrous tissue interspersed between bone trabeculae, the amount of bone within lesions was quite variable, trabeculae are dysplastic, non-stress oriented, and appear disorganized. HE sections, both in low and high power, showed irregular and discontinuous trabeculae within a fibrous stroma, demonstrating the unhealthy dysplastic bone (Fig. 3a, b). Finally, the FD was confirmed radiographically and pathologically.
Fig. 2.
Management of the FD in the alveolar crest of the edentulous site. a, b The occlusal view of the lesion area. The FD in the alveolar crest, the fibrous tissue can be seen inside the bone tissue, and pathological biopsy tissue was obtained by piezoelectric osteotomy. c, d The occlusal view of soft fibrotic bone tissue and curettage of pathological bone tissue using piezoelectric osteotomy. e, f The occlusal and buccal view of the healed lesion area. Uneventful healing and the recovery of interocclusal space. g, h The occlusal view of the healed lesion area. Intact and normal cortical bone texture
Fig. 3.
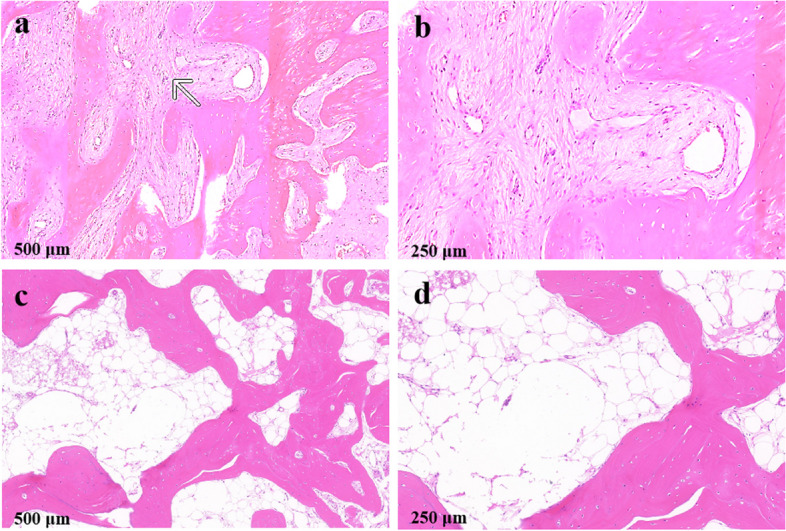
Hematoxylin–eosin staining of the bone tissue. a, b The first HE staining showed irregular, discontinuous trabeculae within fibrous trabeculae. c, d The second HE staining showed well-vascularized and normally structured bone without irregular fibrous tissue
In July 2019, the complete curettage by piezoelectric osteotomy was utilized to remove the pathological tissue of fibrous disorder represented by hypomineralized and soft bone (Fig. 2c, d). After a 1-year recovery, bone healing and healthy soft tissue were achieved around #46, providing suitable conditions and interocclusal space for successive implant treatment (Fig. 2e, f). The follow-up CBCT was uneventful, and the biopsy was performed again to confirm the healing state of the disease in August 2021 (Fig. 2g, h). Fortunately, the HE staining results of the restorative section showed the disappearance of abnormally fibrotic bone trabeculae and the appearance of vascularized bone marrow cavities of normal bone tissue (Fig. 3c, d).
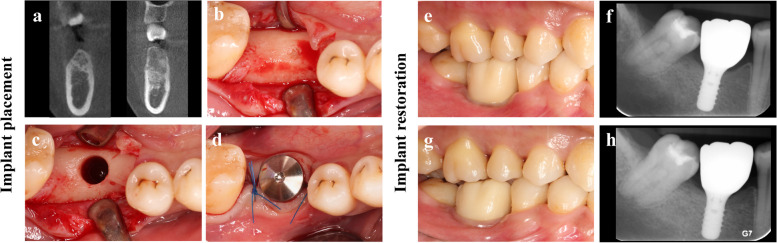
In May 2023, the CBCT re-examination was performed as part of the preoperative assessment for the implant treatment. The bone volume was adequate, and the bone density was classified as Type II. Then, a dental implant (4.8 mm in diameter, 10 mm in length, wide neck; Straumann, Institute Straumann AG, Basel, Switzerland) was placed in a site which was previously impossible. The implant achieved both well primary and secondary stability (Fig. 4b-d). Prosthesis delivery was completed three months after implant placement (Fig. 4e, f). At the one-year follow-up (Fig. 4g, h), the periapical X-ray confirmed stable marginal bone levels.
Fig. 4.
Implant placement and restoration. a the coronal cross-sectional CBCT slice through the center of the lesion area. Postoperative (a. left) versus preoperative (a. right) demonstrated the complete removal of previously abnormal bone fibrous tissue. b, c, d The occlusal view of the healed lesion area. After flap elevation, normal cortical bone provided decent primary stability of the implant. e The buccal view of the restoration. Illustration of the prosthetic delivery 3 months (2023-09-13) postoperative implant placement. f Periapical X-ray showed ideal osseointegration. g The buccal view of restoration. Illustration of the prosthetic delivery 1-year follow-up (2024-09-01). h Periapical X-ray showed stable marginal bone levels
Discussion
From the diagnosis of FD, removal of lesions, creation of restorative space, remodeling of bone tissue, to the successful osseointegration of implant, the following points are worth discussing in this case report.
Fibrous dysplasia is a rare complex disorder leading to fractures, deformities, pain, and functional impairment. Osseous changes in FD are characterized by the replacement and distortion of normal bone with poorly organized, structurally unsound, fibrous tissue [1]. FD can be classified as monostotic FD, polyostotic FD, and McCune-Albright syndrome associated with skin hyperpigmentation and endocrine hyperactivity [1, 3, 5]. A combination of clinical, radiographic, and pathologic evaluations is recommended for the diagnosis of FD. In this case, we confirmed the monostotic FD, which is localized in the alveolar crest bone.
According to existing studies, the lesion area of craniofacial FD usually occurs in the jawbone [14, 15]. However, only the edentulous alveolar crest bone was invaded in our case. Reviewing the patient's medical records 8 years before tooth extraction, we noted that the #46 residual roots had existed for many years. Based on the evidence in the present case, we hypothesize that long-term chronic infection or inflammation stimuli of residual roots in #46 may have plausibly resulted in the occurrence of FD localized in the alveolar crest bone in these special circumstances. This suggests that residual roots must be removed promptly to avoid long-term adverse stimuli leading to more serious consequences. Besides, what needs to be declared is that the etiopathogenesis and inducing factors of FD still need further research; a direct causal relationship between FD and inflammation stimuli by residual root can certainly not be established based on this solitary case report so far.
Currently, surgery and drug treatment are often used in clinical practice for common craniofacial FD [5, 8, 16]. However, unlike FD in the jawbone, which tended to adopt a conservative treatment strategy, the presence of FD on the alveolar crest bone directly affects implant treatment. The dental implant management of patients with craniofacial FD is challenging because the complication features of FD have been poorly characterized. Meanwhile, whether the osseointegration of the implant is affected and whether it’s easier to induce peri-implant inflammation by FD have not been verified. The cases where the lesion is asymptomatic and does not cause deformities or functional impairment should be monitored. Although successful cases had been reported to discuss the direct implant placement in the lesion area of FD [17], there was no clear evaluation and long-term follow-up on the healthy histological status of the osseointegration on the implant. It is necessary to mention that whether there is an increased risk of implant failure is unclear based on the limited literature. There is also the concern that osteomyelitis may occur in the setting of a failed implant [11]. In addition, it was demonstrated that the increased levels of interleukin-6 may be responsible for the increased bone resorption in FD of animal model [18]. The surgical indication is when the lesion is extensive, and the symptoms significantly affect the quality of life or when there is a risk of osteogenic sarcoma [19]. Some reports indicated that dentists choose to avoid or remove the FD lesion area before undergoing implant treatment in the clinic [11, 20].
Through literature review and comparison with clinical guidelines of common craniofacial FD [11] Previous studies shown that fibrous tissue would adversely stimulate the implant's long-term stability and should be obliterated [21]. Meanwhile, the healthy bone should be maintained for the following implant process. We chose a piezoelectric osteotomy, which can offer a safe,accurate, and minimally invasive method of performing alveolar crest bone osteotomy and cause fewer comminuted fractures than conventional osteotomy techniques [22, 23]. Meanwhile, the patient's alveolar bone underwent decent remodeling during the osteotomy surgery without bone augmentation. This is different from the previous study [24], which executed box osteotomy and simultaneous bone grafting of the alveolar bone in the craniofacial FD lesion area before implant surgery. Due to the positive outcomes observed over time in our case, using minimally invasive techniques to reshape the alveolar bone and thoroughly eliminate fibrous tissue may assist as a suitable pre-implant surgical approach for patients with craniofacial FD. However, additional cases and extended follow-up periods are required for justification.
In the present case, the patient healed with normal and vascularized bone before undergoing implant placement. Fortunately, due to the limited area of FD lesions that occurred on the alveolar crest bone, there was no recurrence after 1-year follow-up after curettage, and the implant therapy was successfully performed after 5-year clinical management. However, regular follow-up is needed, and further experimental mechanism study of this localized FD in the alveolar crest through molecular biology, animal experiments, and epidemiological investigations is limited in this case.
Conclusions
To our knowledge, this is the first case to describe a localized FD in alveolar crest bone of a complex situation where the periodontium of adjacent teeth and interocclusal space were all involved, providing a treatment modality including the curettage surgery by minimally invasive piezoelectric osteotomy with proof of recovery by clinical, radiographic and histological examination, followed by the ideal osseointegration of implant ultimately.
Acknowledgements
We would like to sincerely thank Danping Wen and Xiaojun Li for their support in taking pictures during the treatment process. We would like to acknowledge Prof. dr. Tong Xi from the Department of Oral and Maxillofacial Surgery, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands for his guidance in this research.
Abbreviations
- FD
Fibrous dysplasia
- CBCT
The cone-beam computed tomography
- WHO
World Health Organization
Authors' contributions
Shiyong Zhao: supervision, performed the operation, reviewed and edited the manuscript. Hao Chen: wrote and reviewed the manuscript, validation and data curation; Yuting Wen: sorted data and wrote the manuscript. Xueqi Guo, participate in the treatment. All the authors have read and approved the final manuscript.
Funding
This work was supported by a grant from the Traditional Chinese Medicine Research Project of Guangdong Province (NO.20212141), the Guangzhou Health Technology General Guidance Project (NO.2021A011102), and the Guangzhou Traditional Chinese Medicine and Integrated Traditional Chinese and Western Medicine Technology Project (NO.20242A010023). The funding body aided us financially in the histological examination of the case and publishing of the paper.
Data availability
The complete data and materials described in the case report are freely available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient, and all the treatment protocols of the case report were carried out according to relevant guidelines and regulations and approved by the Ethical Committee of the Affiliated Stomatology Hospital of Guangzhou Medical University (LCYJ2023017). The names of the participants were kept confidential, and only the data were available to the study investigators.
Consent for publication
Written informed consent for publication of clinical details and clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Chen and Yuting Wen contributed equally to this work.
References
- 1.Cohen MM, Howell RE. Etiology of fibrous dysplasia and McCune-Albright syndrome. Int J Oral Maxillofac Surg. 1999;28:366–71. [PubMed] [Google Scholar]
- 2.Burke A, Collins MT, Boyce AM. Fibrous Dysplasia of Bone: Craniofacial and Dental Implications. Oral Dis. 2017;23:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szymczuk V, Taylor J, Boyce AM. Craniofacial Fibrous Dysplasia: Clinical and Therapeutic Implications. Curr Osteoporos Rep. 2023;21:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier ME, Vágó E, Abrahamsen B, Dekkers OM, Horváth-Puhó E, Rejnmark L, et al. Incidence and Prevalence of Fibrous Dysplasia/McCune-Albright Syndrome: A Nationwide Registry-Based Study in Denmark. J Clin Endocrinol Metab. 2024;109:1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabali TM, Moshy JR, Owibingire SS, Sohal KS, Simon ENM. Craniofacial fibrous dysplasia associated with McCune-Albright syndrome: challenges in diagnosis and treatment: case reports. BMC Oral Health. 2019;19:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sontakke SA, Karjodkar FR, Umarji HR. Computed tomographic features of fibrous dysplasia of maxillofacial region. Imaging Sci Dent. 2011;41:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mete O, Wenig BM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head Neck Pathol. 2022;16:123–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simm PJ, Biggin A, Zacharin MR, Rodda CP, Tham E, Siafarikas A, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health. 2018;54:223–33. [DOI] [PubMed] [Google Scholar]
- 10.Chapurlat RD, Hugueny P, Delmas PD, Meunier PJ. Treatment of fibrous dysplasia of bone with intravenous pamidronate: long-term effectiveness and evaluation of predictors of response to treatment. Bone. 2004;35:235–42. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7 Suppl 1(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida K, Sakamoto Y, Kosugi K, Miwa T, Toda M. The Long-Term Natural History of Fibrous Dysplasia. J Craniofac Surg. 2024. 10.1097/SCS.0000000000010245. [DOI] [PubMed]
- 13.Florez H, Peris P, Guañabens N. Fibrous dysplasia. Clinical review and therapeutic management. Med Clin (Barc). 2016;147:547–53. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Li Y, Dong H, Zhao S, Yang P, Dai C, et al. Craniofacial Fibrous Dysplasia in Fronto-Orbital Region: A Single-Center Retrospective Study of 38 Cases. World Neurosurg. 2024;181:e1130–7. [DOI] [PubMed] [Google Scholar]
- 15.Pacino GA, Cocuzza S, Tonoli G, Boscolo Rizzo P, Tirelli G, Tofanelli M, et al. Jawbone fibrous dysplasia: retrospective evaluation in a cases series surgically treated and short review of the literature. Acta Biomed. 2020;92: e2021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikuta K, Sakai T, Koike H, Ito K, Imagama S, Nishida Y. Successful treatment with denosumab for pelvic fibrous dysplasia: A case report and review of the literature. Medicine (Baltimore). 2021;100:e28138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajwa MS, Ethunandan M, Flood TR. Oral rehabilitation with endosseous implants in a patient with fibrous dysplasia (McCune-Albright syndrome): a case report. J Oral Maxillofac Surg. 2008;66:2605–8. [DOI] [PubMed] [Google Scholar]
- 18.Motomura T, Kasayama S, Takagi M, Kurebayashi S, Matsui H, Hirose T, et al. Increased interleukin-6 production in mouse osteoblastic MC3T3-E1 cells expressing activating mutant of the stimulatory G protein. J Bone Miner Res. 1998;13:1084–91. [DOI] [PubMed] [Google Scholar]
- 19.Abd Elalim MM, Ettish AA, Elfeky WM, Abdelaal MM. Case Report Of Rib Polyostotic Fibrous Dysplasia. J Pak Med Assoc. 2023;73(Suppl 4):S334–6. [DOI] [PubMed] [Google Scholar]
- 20.Monje A, Monje F, Suarez F, González-García R, Villanueva-Alcojol L, Wang H-L. Oral Rehabilitation With Dental Implants for Teeth Involved in a Maxillary Fibrous Dysplasia. Clinical Advances in Periodontics. 2013;3:208–13. [Google Scholar]
- 21.Babu RSA, Ogle O. Tissue response: biomaterials, dental implants, and compromised osseous tissue. Dent Clin North Am. 2015;59:305–15. [DOI] [PubMed] [Google Scholar]
- 22.Sivolella S, Brunello G, Fistarol F, Stellini E, Bacci C. The bone lid technique in oral surgery: a case series study. Int J Oral Maxillofac Surg. 2017;46:1490–6. [DOI] [PubMed] [Google Scholar]
- 23.Schlabe J, Echlin K, Atherton D. A comparison of piezo surgery osteotomies with conventional internal osteotomies as performed by trainee surgeons: a cadaveric study. Ann R Coll Surg Engl. 2021;103:272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kablan F. Alveolar Bone Box Ostectomy Grafted with Particulate Bone Substitute with Subsequent Dental Implant Placement in a Case of Craniofacial Fibrous Dysplasia Involving the Posterior Maxilla: Case Report and Literature Review. J Clin Med. 2023;12:6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The complete data and materials described in the case report are freely available from the corresponding author on reasonable request.