Abstract
Background
Cerebrospinal fluid (CSF) β2-microglobulin (β2M) has been demonstrated as an important factor in β-amyloid (Aβ) neurotoxicity and a potential target for Alzheimer’s disease (AD). However, more investigation is required to ascertain the relationship between β2M and glial activities in AD pathogenesis.
Methods
In this study, 211 participants from the Alzheimer’s disease Neuroimaging Initiative (ADNI) with CSF and Plasma β2M, CSF glial fibrillary acidic protein (GFAP), soluble triggering receptor expressed on myeloid cells 2 (sTREM2), Aβ42, phosphorylated-tau (P-tau) and total tau (T-tau) were divided into four groups, stage 0, 1, 2, and suspected non-AD pathology (SNAP) based on the National Institute on Aging- Alzheimer’s Association (NIA-AA) criteria. Multiple linear regression, linear mixed effects models, and causal mediation analyses bootstrapped 10,000 iterations were used to investigate the underlying associations among β2M and CSF biomarkers at baseline and during a longitudinal visit.
Results
CSF β2M concentration decreased with amyloid in stage 1 compared with stage 0 and increased with tau pathology and neurodegeneration in stage 2 and SNAP compared with stage 1. Moreover, CSF β2M level was positively correlated with the Aβ42 (β = 0.230), P-tau (β = 0.564), T-tau (β = 0.603), GFAP (β = 0.552), and sTREM2 (β = 0.641) (all P < 0.001). CSF β2M was only longitudinally correlated with T-tau change. The correlation of CSF β2M with P-tau (proportion = 25.4%, P < 0.001) and T-tau (proportion = 26.7%, P < 0.001) was partially mediated by GFAP in total participants, reproduced in late-life individuals. Furthermore, the astrocyte cascade also partially mediated the pathological relationship between CSF β2M and tau pathology (β2M → GFAP → YKL-40 → P-tau/T-tau, IE: 0.424—0.435, all P < 0.001). Nevertheless, the mediation effects of sTREM2 were not significant. Additionally, there was no association between plasma β2M and CSF biomarkers.
Conclusions
CSF β2M is dynamic in AD pathology and associated with neuroinflammation. CSF GFAP might mediate the association between β2M and tau pathology, complementing the existing research on the effect of β2M in AD pathology and providing a new perspective on treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01665-8.
Keywords: Alzheimer’s disease, β2-Microglobulin, Tau, Microglia, Astrocyte
Background
In 2024, approximately 50 million people worldwide may be affected by dementias, notably an estimated 6.9 million Americans aged 65 and older who will have Alzheimer's disease (AD) [1]. The predominant pathological features of AD are β-amyloid (Aβ) plaques and tangles of microtubule-associated tau protein [2], while there are currently more studies exploring the impact of biomarkers on the underlying pathophysiology.
Recently, higher soluble β2M has been found in the cerebral spinal fluid (CSF) of patients with AD than in healthy controls [3]. Moreover, another study shows the connection between AD development and plasma β2M [4]. As a component of the major histocompatibility complex class I (MHC-I) molecule, β2M can be involved in the regulation of brain development, synaptic plasticity, and neurobehavior [5–7]. Notably, β2M has been demonstrated to play a significant role in Aβ-induced neurotoxicity and represents a promising target for AD therapy [8].
Meanwhile, the levels of CSF β2M have been suggested as a dependable indicator for various inflammatory or autoimmune disorders of the central nervous system (CNS) [9, 10]. In AD and neuroinflammation, microglia can contribute to synapse loss by engulfing synapses, worsening tau pathology, and releasing inflammatory factors that damage neurons or activate neurotoxic astrocytes [11, 12]. The triggering receptor expressed on myeloid cell 2 (TREM2) as a specific microglial surface receptor [13, 14], can be cleaved by metalloproteinases to release ectodomain via soluble TREM2 (sTREM2). CSF sTREM2 is also a promising microglia activity biomarker in AD and is associated with neuronal damage indicators [15]. Astrocytes may also exacerbate neurodegeneration when dysfunctional, resulting in cognitive decline in AD [16]. Exploring the correlation between β2M and microglia–astrocyte communication in AD would be of significant interest. First, CSF β2M is predominantly found in activated microglia [8], also involved in astrocyte response to inflammatory signaling such as interleukin, interferon, and tumor necrosis factor related pathways [17, 18]. Moreover, β2M may work itself or constitute inflammatory factors to participate in this interaction. Further, β2M was reported as a component of the glial fibrillary acidic protein (GFAP) [18], a signature protein of reactive astrocytes, impacting neuroinflammation, and is associated with AD pathology in the brain [19, 20]. Excitingly, in the 2023 Alzheimer's Association International Conference (AAIC), fluid GFAP is currently the sole biomarker of inflammatory (I) that has been introduced for AD prediction and staging [21, 22]. Although the above findings provide a possibility for studying the role of β2M in the microglia–astrocyte communication, it remains unclear whether CSF β2M triggers alterations in microglial activity or astrocyte function and phenotype in the human brain [8, 23]. The underlying mechanism among CSF β2M, GFAP and sTREM2 also remains to be studied.
To determine the intricate function of β2M in the pathogenesis development of AD and its unique relationship with glial cell activity, we intended to explore the relationship of CSF and plasma β2M levels with glial activation and AD biomarkers and ascertain their interrelationships. Therefore, we propose the hypothesis that CSF β2M may be associated with CSF GFAP or sTREM2, involved in the progression of AD pathology.
Materials and methods
Study participants
All data were from the ADNI database (https://adni.loni.usc.edu). The goal of the ADNI project is to identify biochemical, genetic, imaging, and clinical biomarkers that may be used to predict the early beginning of AD. Participants have been recruited from more than 50 sites in the US and Canada [24].
We included 211 individuals providing clinical conditions, CSF and plasma β2M, CSF GFAP, sTREM2, and AD biomarkers. All participants provided written informed consent according to the declaration of Helsinki before study enrollment. The institutional review boards of all participating institutions in ADNI approved the data used for this study.
Measurements of biomarkers
In the ADNI database, the CSF β2M (two peptides: VEHSDLSFSK, VNHVTLSQPK) GFAP and Chitinase-3-like protein 1 (YKL-40, three peptides: ILGQQVPYATK, SFTLASSETGVGAPISGPGIPGR, VTIDSSYDIAK) data were analyzed by mass spectrometry with multiple reaction monitoring (MRM) and then normalized [25]. CSF sTREM2 data was from “CSF soluble triggering receptor expressed on myeloid cells 2 (sTREM2) and progranulin (PGRN)” of ADNI file, which was tested by MSD platform-based assay [26]. CSF Aβ42, P-tau, and T-tau quantified by automated Roche Elecsys and cobas 601 immunoassay analyzer systems were obtained from the “ADNIMERGE-key ADNI tables merged into one table” [27]. Each CSF biomarker assay was duplicated and averaged. Building upon the previous study, we employed thresholds of Aβ42 < 976.6 pg/mL, P-tau > 21.8 pg/mL, and T-tau > 245 pg/mL in CSF to define abnormal levels [28].
The plasma β2M data were from “Biomarkers Consortium Plasma Proteomics Project RBM Multiplex Data and Primer”. Information on the biological preparation of ADNI samples and the analysis of the RBM Human Discovery MAP panel could be accessed on the ADNI websites (http://adni.loni.usc.edu/data-samples/biospecimen-data/) [29].
Group classification
According to the National Institute on Aging- Alzheimer’s Association (NIA-AA) criteria [30], participants with normal Aβ42, P-tau, and T-tau levels (A-T-N-) were classified as stage 0. Subsequent stages include stage 1 (A + TN-), stage 2 (A + TN +), and suspected non-AD pathology (SNAP) (A-TN +). Additional classifications were based on APOE ε4 allele statuses (non-carrier or carrier), mid-life (< 65 years) or late life (≥ 65 years), male or female, and education level (well-educated ≥ 7 years or ill-educated < 7 years).
Statistical analyses
Excessive values of CSF β2M, GFAP, sTREM2, AD biomarkers, and plasma β2M that fell outside of the 4 SD were not included. To attain or be near to a normal distribution, the values of each biomarker underwent log transformation and then standardized on the z-scale. One-way analysis of variance (ANOVA) or the Kruskal–Wallis test for continuous data and chi-square tests for categorical variables were used to examine the differences between the four AD stage groups. Then we further compared CSF β2M levels by one-way analysis of covariance (ANOCVA) while Fisher's LSD was employed for the post hoc test. Covariates included age, sex, education years, and Apolipoprotein E (APOE) ε4 carrier status. Spearman partial correlation analyses and multiple linear regression were used to examine the relationship between CSF or plasma β2M, CSF GFAP, sTREM2, and AD core biomarkers, taking into account the same variables. We performed mediation analyses using the "mediate" package of R software (version 4.2.1) to investigate whether CSF GFAP or sTREM2 could mediate the relationship between CSF β2M and CSF AD biomarkers, following the approach created by Baron and Kenny [31]. In the models, each path was adjusted for age, sex, education years, and APOE ε4 carrier status. In addition, we used interaction analysis to evaluate the effects of age, sex, education, and APOE ε4 status. Then we performed subgroup analyses according to the results of the interaction analysis. Besides, we used a linear mixed model to explore the relation between the levels of baseline CSF β2M and changes in AD biomarkers across time (Supplementary Table 1), while adjusting for follow-up duration, age, sex, education levels, and APOE ε4 status. Finally, the sensitivity analyses were conducted by (1) using CSF β2M-VEHSDLSFSK for main analyses then reproduced by CSF β2M-VNHVTLSQPK, (2) validating the relationship between CSF β2M and AD core biomarkers as well as GFAP and sTREM2 after screening of participants with diseases that may affect β2M concentrations, (3) selecting YKL-40 both as the secreted astrocyte cascade biomarker after GFAP to reproduce the findings of glial activity.
A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses and the creation of the diagrams were performed using the R Studio software, SPSS (version 26.0.0.0), and GraphPad Prism (version 9.4.2).
Results
Characteristics of participants
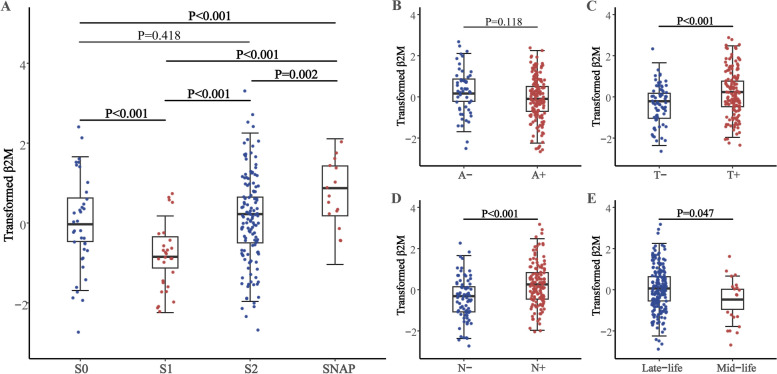
Table 1 shows the demographic, clinical, and biomarker features of 211 individuals (37 stage 0, 28 stage 1, 131 stage 2, and 15 SNAP). They had a mean age of 74.99 ± 7.16 years, an average education level of 15.75 ± 2.90 years, 80 females around 37.9% of proportion, and an APOE ε4 non-carrier proportion of 53.1%. In four groups, there were no differences in participants' gender, educated years, and plasma β2M levels. The proportion of APOE ε4 carriers and CSF biomarker levels (all P < 0.001) showed significant differences among the four stages. Using CSF β2M-VEHSDLSFSK for main analyses and age, sex, education years, and APOE ε4 status as covariates, participants in stage 1 had lower CSF β2M levels compared to stage 0, stage 2, and SNAP; participants in stage SNAP had higher CSF β2M levels compared to stage 0 (all P < 0.001) (Fig. 1A). Meanwhile, the T + and N + groups (both P < 0.001) had higher CSF β2M levels, but there was no difference between the A + and A- groups (Fig. 1B-D). The inference suggests that CSF β2M concentration declines during the pathological stage of amyloidosis, and subsequently rises with tau pathology during the downstream tau pathology and neurodegeneration even without considering amyloidosis. In comparative analysis between groups, it was also found that the levels of CSF β2M were significantly higher in the late-life (P = 0.047) (Fig. 1E), male (P < 0.001), Ill-educated (P = 0.009), T + (P < 0.001) and N + (P < 0.001) group, but not different based on APOE ε4 status classification (Supplementary Table 2). As age is the key risk factor for neuroinflammation and AD [32], also showed differences among the 4 stages in this study (P = 0.038, Table 1), we questioned whether levels of β2M and CSF AD biomarkers are related to normal aging. As expected, levels of CSF (VEHSDLSFSK, β = 0.050; VNHVTLSQPK, β = 0.054) and plasma β2M (β = 0.051), GFAP (β = 0.057), sTREM2 (β = 0.040) (all P < 0.001) increased significantly with age (Supplementary Table 3).
Table 1.
The demographic and clinical characteristics of participants
| Characteristics | Stage 0 | Stage 1 | Stage 2 | SNAP | P |
|---|---|---|---|---|---|
| Number | 37 | 28 | 131 | 15 | - |
| Age (years) | 74.42 (6.67) | 76.40 (4.98) | 74.34 (7.36) | 79.49 (8.54) | 0.038 |
| Female gender (N, %) | 13 (35.1) | 7 (25.0) | 55 (42.0) | 5 (33.3) | 0.367 |
| Education (years), | 15.38 (2.88) | 16.46 (2.71) | 15.77 (2.93) | 15.13 (2.92) | 0.394 |
| APOE ε4 carriers (N, %) | 2 (5.4) | 13 (46.4) | 94 (71.8) | 3 (20.0) | < 0.001 |
| CSF β2M-VEHSDLSFSK | 23.94 (0.41) | 23.57 (0.27) | 24.00 (0.46) | 24.33 (0.43) | < 0.001 |
| CSF β2M-VNHVTLSQPK | 28.50 (0.29) | 28.26 (0.20) | 28.56 (0.35) | 28.82 (0.34) | < 0.001 |
| Plasma β2M (ug/mL) | 0.32 (0.12) | 0.31 (0.15) | 0.29 (0.13) | 0.36 (0.15) | 0.212 |
| CSF Aβ42 (pg/ml), | 1417.84 (179.83) | 623.38 (209.25) | 590.22 (162.81) | 1235.79 (225.41) | < 0.001 |
| CSF P-tau (pg/ml), | 16.52 (2.77) | 15.90 (3.59) | 37.52 (11.30) | 34.48 (17.22) | < 0.001 |
| CSF T-tau (pg/ml), | 189.17 (28.43) | 174.21 (34.09) | 369.26 (100.69) | 359.70 (147.78) | < 0.001 |
| CSF GFAP (pg/ml), | 10.89 (0.44) | 10.92 (0.49) | 11.25 (0.51) | 11.39 (0.58) | < 0.001 |
| aCSF sTREM2 (pg/ml), | 4949.31 (2195.82) | 2973.32 (1276.08) | 4418.58 (1981.63) | 5396.33 (2017.27) | < 0.001 |
Categorical variables were reported as number and percentage; continuous variables were reported as means (SDs). P values were computed with the one-way ANOVA or kruskal–wallis test for continuous variables; with the χ2 test for categorical variables. Significant effects (P < 0.05) are shown in bold
Abbreviations: SNAP suspected non-Alzheimer’s pathophysiology, SD standard deviation, APOE Apolipoprotein E, CSF cerebrospinal fluid, β2M β2-microglobulin, Aβ42 amyloid-β1–42, P-tau phosphorylated-tau, T-tau total-tau, GFAP glial fibrillary acidic protein, sTREM2 soluble triggering receptor expressed on myeloid cells 2
aData were missing for CSF sTREM2 (n = 37)
Fig. 1.
Transformed baseline CSF β2M in participants classified according to the NIA-AA criteria (A, B, C, D) and age (E). Levels of transformed CSF β2M were significantly lower in S1, T-, N- and mid-life group. Notes: CSF β2M fitted the normal distribution after log10 transformation and then standardized by z-scale. Transformed plasma β2M was computed with the One-way ANCOVA for comparison of means while Fisher's LSD was employed for post hoc test. Models included age, gender, education, APOE ε4 status as covariates. Significant effects (P < 0.05) are shown in bold. Abbreviations: CSF, cerebrospinal fluid; β2M, β2-microglobulin; NIA-AA, National Institute on Aging- Alzheimer’s Association; S, stage; A, amyloidosis; T, tau pathology; N, neurodegeneration; SNAP, suspected non-Alzheimer’s pathophysiology; APOE, Apolipoprotein E
Association of baseline β2M with CSF biomarkers
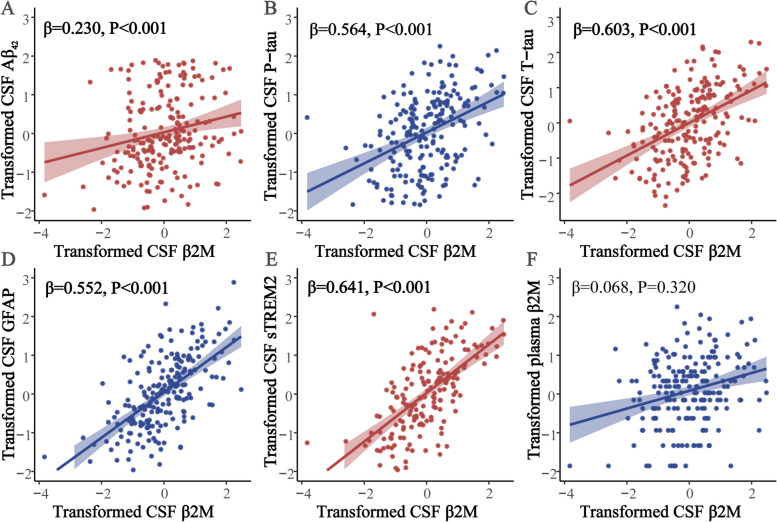
Supplementary Table 4 showed the results of multiple linear regression of baseline plasma and CSF β2M with CSF AD core biomarkers, GFAP, and sTREM2. We found that the elevated level of CSF β2M was correlated with the greater levels of Aβ42 (β = 0.230, P < 0.001), P-tau (β = 0.564, P < 0.001), and T-tau (β = 0.603, P < 0.001) (Fig. 2A-C). There was also a positive association between baseline CSF β2M and levels of GFAP (β = 0.552, P < 0.001) and sTREM2 (β = 0.641, P < 0.001) (Fig. 2D-E). Furthermore, CSF β2M was only longitudinally correlated with T-tau levels (β = -0.025, P = 0.025) (Supplementary Table 5). Nevertheless, plasma β2M was neither cross-sectionally nor longitudinally correlated with CSF biomarkers (Supplementary Table 4, 5).
Fig. 2.
Correlation between baseline CSF β2M and CSF biomarkers using multivariate linear regression analyses. CSF β2M level was positive correlated with the Aβ42 (A), P-tau (B) and T-tau (C), GFAP (D) and sTREM2 (E). There was no significant association between CSF and plasma β2M (F). Notes: The normalized regression coefficients (β) and P values computed by multiple linear regression after adjustment for age, gender, education, APOE ε4 status. Significant effects (P < 0.05) were shown in bold. Abbreviations: CSF, cerebrospinal fluid; β2M, β2-microglobulin; Aβ42, amyloid-β1–42; P-tau, phosphorylated-tau; T-tau, total-tau; GFAP, glial fibrillary acidic protein; sTREM2, soluble triggering receptor expressed on myeloid cells 2; APOE, Apolipoprotein E
The correlation between CSF β2M and tau pathology was mediated by CSF GFAP
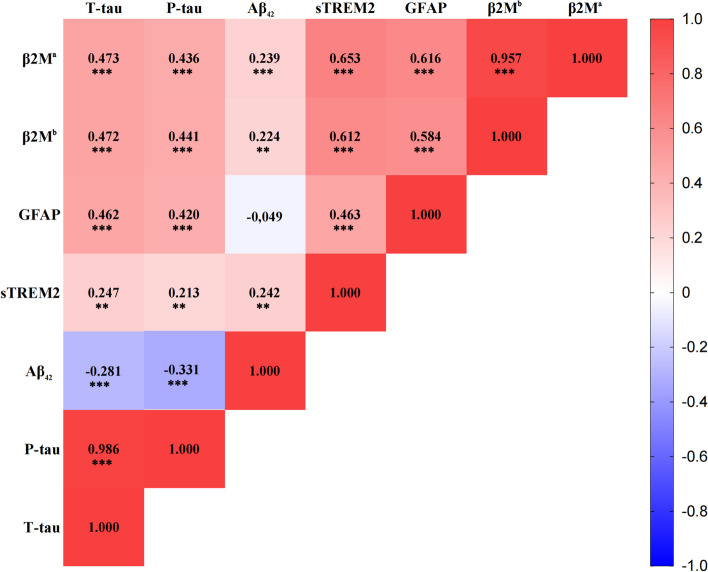
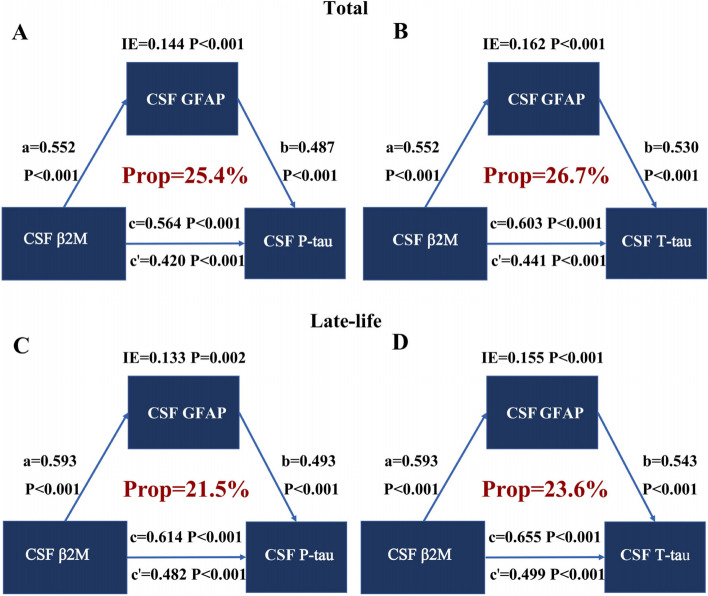
Except for CSF β2M, GFAP and sTREM2 exhibited a substantial association with P-tau and T-tau (Fig. 3). Then, we further explored whether the correlation between CSF β2M and AD pathology involved GFAP or sTREM2 in CSF. The results showed that CSF GFAP partially mediated the correlation between CSF β2M and CSF P-tau (proportion = 25.4%, IE = 0.144, P < 0.001) (Fig. 4A) as well as T-tau (proportion = 26.7%, IE = 0.162, P < 0.001) (Fig. 4B) in total participants. In addition, the mediation effects of sTREM2 were not significant (Supplementary Table 6). These results offered crucial human evidence for unraveling the interplay between CSF β2M, microglia-astrocyte communication, and AD pathogenesis, particularly in tau pathology. They supported our hypothesis that β2M could exert a pivotal role in tau pathology and AD-related neurodegeneration through cross-talk among glial cells.
Fig. 3.
CSF biomarker correlations. Red indicates positive correlation, and blue indicates negative correlation. Notes: The Spearman partial correlation coefficients (r) and P values are shown in each square after adjustment for age, gender, education, APOEε4 status. *P < 0.05; **P < 0.01; ***P < 0.001. a: VEHSDLSFSK, b: VNHVTLSQPK. Abbreviations: CSF, cerebrospinal fluid; β2M, β2-microglobulin; Aβ42, amyloid-β1–42; P-tau, phosphorylated-tau; T-tau, total-tau; GFAP, glial fibrillary acidic protein; sTREM2, soluble triggering receptor expressed on myeloid cells 2; APOE, Apolipoprotein E
Fig. 4.
Mediation analysis of CSF β2M, GFAP, and tau pathology. The association of CSF β2M with CSF P-tau (A) and T-tau (B) was partially mediated by CSF GFAP in total population. The association of CSF β2M with CSF P-tau (C) and T-tau (D) was also partially mediated by CSF GFAP in the late-life group. Notes: Adjusted for age, gender, educational years, APOE ε4 carrier status. Significant effects (P < 0.05) were shown in bold. Mediation analyses with 10,000 bootstrapped iterations were used to examine the mediation effects. a: effect of CSF β2M on CSF GFAP level. b: effects of CSF GFAP on P-tau or T-tau level. c: effect of CSF β2 on P-tau or T-tau level without mediation. c’: effect of CSF β2M on P-tau or T-tau level considering mediation. Abbreviations: CSF, cerebrospinal fluid; β2M, β2-microglobulin; P-tau, phosphorylated-tau; T-tau, total-tau; GFAP, glial fibrillary acidic protein; APOE, Apolipoprotein E
Interactions and stratified analyses
Interaction analyses revealed that the relationship of CSF β2M with Aβ42 (β = -0.018, P = 0.016), P-tau (β = 0.023, P = 0.001), T-tau (β = 0.022, P = 0.002), and GFAP (β = 0.015, P = 0.035) was affected by age (Supplementary Table 7). Then we performed additional stratified analyses (Supplementary Table 8, 9) and discovered that CSF β2M was only associated with CSF biomarkers in the late-life group. Besides, this connection was more substantial in APOE ε4 carriers, male, well-educated, A + , T + , and N + groups.
Performing further mediation analyses reproduced mediating effects in the late-life group that associations of CSF β2M with P-tau (proportion = 21.5%, IE = 0.133, P = 0.002) (Fig. 4C) and T-tau (proportion = 23.6%, IE = 0.155, P < 0.001) (Fig. 4D) were partially mediated by GFAP (Supplementary Table 10). There were similar results in A- (proportion: P-tau = 31.4%, T-tau = 33.9%), T + (proportion: T-tau = 17.1%), and N + (proportion: P-tau = 18.5%, T-tau = 22.1%) group, consistent with the findings of the previous group comparison (Supplementary Table 11–13).
Sensitivity analyses
Firstly, we performed sensitivity analyses with another peptide of CSF β2M-VNHVTLSQPK and found the relationship of CSF β2M with core biomarkers of AD, GFAP, and sTREM2 (Supplementary Table 14). Considering CSF β2M-VNHVTLSQPK was significantly correlated with CSF β2M-VEHSDLSFSK (β = 0.975, P < 0.001), CSF GFAP, and tau pathology (all P < 0.001), we reproduced the mediation analyses. CSF GFAP had similar mediation effects on the relationship between CSF β2M and tau pathology (proportion: P-tau = 24.4%, T-tau = 26.5%, both P < 0.001) (Supplementary Table 15). In addition, considering medical comorbidities, the correlation between CSF β2M-VEHSDLSFSK and AD core biomarkers, GFAP, and sTREM2 remained significant after the exclusion of 4 participants with any of the following diseases including diffuse large B-cell lymphoma, non-Hodgkin-lymphoma, mantle cell lymphoma, multiple myeloma, inflammatory bowel disease, chronic renal insufficiency and renal failure disease [33–38] (Supplementary Table 16). There were no significant associations between CSF β2M-VNHVTLSQPK and plasma β2M, as well as no association of CSF β2M-VNHVTLSQPK with longitudinal AD core biomarkers and sTREM2 (Supplementary Table 17). From the third perspective, another notable astrocyte cascade biomarker secreted after GFAP is YKL-40 [19], which has consistently been elevated in AD [39, 40]. The correlation of CSF YKL-40 (ILGQQVPYATK, SFTLASSETGVGAPISGPGIPGR, and VTIDSSYDIAK, 211 samples) with CSF biomarkers and plasma β2M (all P < 0.001) had consistent trend and significance with GFAP (Supplementary Table 18). As expected, CSF YKL-40 also mediated the association between CSF β2M and tau pathology (proportion: 16.5%—25.4%) (Supplementary Table 19–20). Given the strong correlation between YKL-40 and GFAP (β: 0.452—0.483, all P < 0.001), and the research which indicates that YKL-40 plays a role in AD pathology later downstream GFAP, we further performed the mediation analyses using the most significant YKL-40-VTIDSSYDIAK as the second mediator (Supplementary Fig. 1). The results showed that the astrocyte cascade mediated the pathological relationship between CSF β2M and tau pathology (β2M-VEHSDLSFSK → GFAP → YKL-40 → P-tau/T-tau, IE = 0.424 / IE = 0.435; β2M-VNHVTLSQPK → GFAP → YKL-40 → P-tau/T-tauIE = 0.432 / IE = 0.433, all P < 0.001).
Discussion
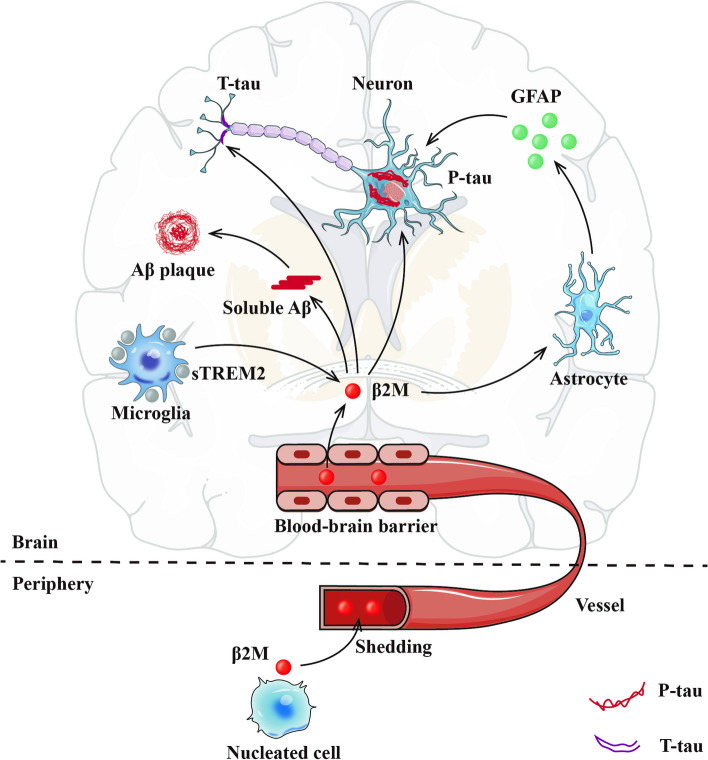
This is the first study to systematically reveal that CSF β2M has a positive correlation with CSF Aβ42, P-tau, T-tau, GFAP, and sTREM2 (Fig. 5). First, β2M is a key co-aggregating factor with Aβ in amyloid pathology and is also associated with the exacerbation of tau pathology. Second, β2M modulates microglial activation, leading to the secretion of sTREM2. In addition, β2M facilitates communication between microglia and astrocytes, promoting astrocyte reactivation and the secretion of GFAP. It is discovered that CSF GFAP rather than sTREM2 mediates the association of CSF β2M with P-tau and T-tau.
Fig. 5.
Schematic interlinking of the effect of β2M based microglia-astrocyte communication on AD pathology. CSF β2M is mainly derived from activated microglia (also secretes sTREM2) and peripheral β2M across the blood–brain barrier. β2M may be involved in Aβ aggregation. Findings in the present study suggest that CSF β2M also upregulates the secretion of GFAP by reactive astrocytes to promote the increase of P-tau and T-tau. Abbreviations: Alzheimer's disease, AD; CSF, cerebrospinal fluid; β2M, β2-microglobulin; Aβ, amyloid-β; P-tau, phosphorylated-tau; T-tau, total-tau; GFAP, glial fibrillary acidic protein; sTREM2, soluble triggering receptor expressed on myeloid cells 2
CSF β2M plays an important role in the glial cell crosstalk. For microglia, β2M and sTREM2 are expressed mainly on microglia [8], and have similar positive associations with AD biomarkers in our study [32]. Interestingly, males have higher β2M levels, and β2M only interacts with gender on sTREM2. That may reflect the consistency of β2M and microglia activities and the underlying sex differences [41]. Firstly, men generally have a slightly lower glomerular filtration rate (GFR) than women [42, 43], contributing to less β2M clearance. Secondly, sex hormones also influence β2M regulation: estrogen in women protects kidney function, while testosterone in men may increase β2M production through immune and metabolic effects [44, 45]. Furthermore, chronic conditions like inflammation, metabolic syndrome, kidney disease, and cardiovascular diseases, which are more common in men, also elevate β2M levels [46, 47]. Lastly, β2M may be involved in transferrin-bound iron regulation, possibly influenced by Y chromosome-linked genetic factors [48, 49]. What needs more attention is microglial β2M may change astrocyte functions and phenotypes, further affecting preserving the blood–brain barrier (BBB), CNS immunological homeostasis, synaptic plasticity, and regular neuronal communication [50, 51]. Reactive astrocytes overexpress GFAP which has also been discovered to have a role in the pathophysiology of tau and amyloid proteins in the brain [52, 53]. The main finding of our investigation is that CSF GFAP significantly mediates the relationship between CSF β2M and P-tau and T-tau, also found in the late-life population based on the interaction between age and CSF β2M. Besides positively associated with GFAP, CSF β2M levels are also increasing with age, suggesting that β2M may be a pro-aging substance [7]. In addition, β2M is not only as a component of the GFAP [18]. When MHC-I are unstable, higher levels of β2M over-activate reactive astrocytes leading to astrocyte proliferation and increased GFAP levels, which can cause neuronal dysfunction and increased neurotoxicity, which can further affect tau pathology [54–56]. knocking down MHC-I expression reduces astrogliosis, and β2M silencing causes astrocyte atrophy by reducing the expression of GFAP [17]. Meanwhile, enhanced MHC-I expression in astrocytes, driven by the GFAP promoter, significantly impairs mice's social behavior and recognition memory [57]. In our sensitivity analysis, we observed a high degree of consistency between YKL-40 and GFAP. Additionally, our mediation analysis revealed the pathway β2M → GFAP → YKL-40 → P-tau/T-tau, further supporting the hypothesis that β2M influences tau pathology through astrocyte reactivation. CSF YKL-40 concentrations and its pathological cascade after GFAP are primarily linked to tau pathology and associated neuronal injury, rather than Aβ [53, 58]. These findings provide additional clinical evidence for our proposed mechanism. Developing targeted therapeutics for AD would be aided by a thorough understanding of the mechanism behind the β2M-astrocyte-tau interaction.
Levels of CSF β2M alter dynamically in response to AD pathogenic processes. Although previous reports have shown the elevation of β2M in AD patients [3, 4, 59]. Our research shows more details in the AD continuum according to the ATN categories defined by CSF biomarkers. Reduced CSF β2M is related to positive Aβ pathology in individuals with T- and N- status, whereas increased CSF β2M is linked to positive tau pathology or neurodegeneration even in the absence of A + status [4]. In the early AD stages, two mechanisms might cause β2M to decrease. The formation process of amyloid plaques may consume CSF β2M to bind and local aggregation [8], then typically accompanied by early microglial activation for clearance of amyloid and inflammatory factors like β2M [60]. In later stages, tau pathology and neurodegeneration continue activating glial cells to secrete more β2M, damaging BBB and further increasing periphery β2M in the brain [61].
Meanwhile, β2M itself exacerbates AD pathology, but the mechanism between it and Aβ or tau pathology is complex and completely different. Significant correlation and uniformity between CSF β2M and CSF Aβ concentrations observed in our study further support the idea that the coaggregation of β2M with Aβ is a key factor in amyloid pathology toxicity, reported independent of MHC-I [8]. However, based on the results of our research, CSF β2M had a more significant association with tau pathology, supported by previous discovery that β2M knockdown notably mitigated tau pathologies in primary mouse neurons and the tau-P301S overexpression mouse model [62]. Moreover, there are two possible underlying mechanisms. Firstly, the reduction of tau pathology due to β2M deletion was found to be dependent on MHC expression [62]. Inhibiting the activation of antigen processing and presentation by MHC-I effectively ameliorates tau protein phosphorylation [41]. It has also been found that the APOE-MHC-I connection is the beginning of a causal chain driving tau pathology [63]. Secondly, another explanation is that soluble β2M-HFE mono chain (sHFE) forms a complex with β2M and associates with the transferrin receptor (TfR), disrupting the modulation of iron-regulated proteins and thereby affecting iron metabolism [64]. Iron accumulation, which is a well-documented consequence of aging and inflammation and a key factor in AD pathogenesis [65], has been linked to plaque, tangle pathology and activated microglia in the brain [32, 66]. CSF β2M possibly further influences AD pathology by affecting iron metabolism leading to microglia-astrocyte activation and phagocytosis dysfunction [67].
Moreover, β2M expressed in peripheral tissues [7], which persistently separates from MHC-I, enters the bloodstream and traverses the BBB. Finally reabsorbed and metabolized in the kidney [42], elevated levels of circulating β2M play a crucial part in the risk of AD and cognitive impairment associated with kidney disease and chronic hemodialysis [4, 68]. No significant associations between plasma β2M and CSF biomarkers have been found in our study. The small sample size may be one explanation, or although β2M can cross the BBB, there may be changes in concentration or structure [61] that contribute to different effects of CSF and blood β2M in neuroinflammation and neurodegeneration. In addition, CSF β2M promotes astrocytic inflammation, worsening tau pathology and compromising the BBB [69]. This disruption may allow peripheral β2M to enter the brain [70], creating a positive feedback loop that could accelerate AD pathology and neurodegeneration. Anti-β2M antibodies may be useful in reducing the harmful consequences of neuroinflammation on BBB while improving AD-associated neuropathology [8].
This study has several interesting strengths. It is the first to systematically examine the association of β2M with CSF GFAP, sTREM2, and AD core biomarkers by utilizing human population-based data. To further ensure the high caliber of the investigation, we have used AD diagnosis criteria to classify AD biomarkers from the NIA-AA study. The analyses of two β2M peptides, consideration of the concentration of β2M in comorbidity and validation of YKL-40 made our results more stable. Nonetheless, several considerations should be taken when interpreting the current findings. Firstly, this study is planned to be exploratory, to generate hypotheses and models. The cross-sectional results are not supposed to infer causality in lack of the longitudinal data of β2M. Experiments on animals and cells are required to validate the proposed hypothesis. Secondly, AD pathology in cerebrospinal fluid in our study has been used but not brain imaging data because of the small amount of β2M data, and more data are needed to harmonize with the brain imaging data to make the results more reliable. Thus, subsequent investigations need to corroborate our findings with extensive cohorts and highly sensitive CSF and plasma β2M assays. Besides, more secreted astrocytes reactive markers, especially in term of AD are also needed. Thirdly, the discussion of AD risk and cognition in the different fields requires further study.
Conclusion
There is a substantial association of CSF β2M with activated neuroinflammation and AD biomarkers. CSF β2M increases with age and changes dynamically at different AD stages. CSF β2M affects tau pathology through reactive astrocytes. β2M as a potential biomarker, warrants further investigation into its mechanisms in AD.
Supplementary Information
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hofmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Abbreviations
- CSF
Cerebrospinal fluid
- β2M
β2-Microglobulin
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s disease Neuroimaging Initiative
- GFAP
Glial fibrillary acidic protein
- sTREM2
Soluble triggering receptor expressed on myeloid cells 2
- YKL-40
Chitinase-3-like protein 1
- SNAP
Suspected non-Alzheimer’s pathophysiology
- P-tau
Phosphorylated tau181
- T-tau
Total tau
- NIA-AA
National Institute on Aging- Alzheimer’s Association
- MHC-I
Major histocompatibility complex class I
- BBB
Blood-brain barrier
- CNS
Central nervous system
- APOE
Apolipoprotein E
Authors' contributions
ZHS: study concept and design, data processing, statistical analysis, interpretation of the results, and writing the manuscript; LYW: statistical analysis and interpretation of the results and writing the manuscript; MC: chart design, critical revision of the manuscript; FXZ and SJW: statistical reproduce, interpretation of the results, and critical revision of the manuscript; SYL, JQS, LHC, YXC and SYC: critical revision of the manuscript; WHY and YL: study concept and design, interpretation of the results, and critical revision of the manuscript. All authors reviewed the manuscript.
Funding
This study was supported by grants from Chongqing Talent Plan (cstc2022ycjh-bgzxm0184), Key Project of Science and Technology Research Program of Chongqing Municipal Education Commission (KJZD-K202200405), Science Innovation Programs Led by the Academicians in Chongqing under Project (2022YSZX-JSX0002CSTB), STI2030-Major Projects (No. 2021ZD0201802) and Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0166).
Data availability
The datasets used and analyzed in the current study are available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
ADNI was approved by the Institutional Review Boards of all participating institutions. All participants provided written informed consent by the Declaration of Helsinki before study enrollment. All study participants, authorized representatives, and study partners have provided written informed consent, and each participating site of ADNI has obtained the necessary ethical permits. More details can be found at adni.loni.usc.edu.
ADNI study is conducted in compliance with the protocol, by GCP guidelines, and in full conformity with Regulations for the Protection of Human Subjects of Research codified in 45 CFR Part 46—Protection of Human Subjects, 21 CFR Part 50—Protection of Human Subjects, 21 CFR Part 56—IRBs, and/or the ICH E6, HIPAA, State and Federal regulations and all other applicable local regulatory requirements and laws. Study personnel involved in conducting this study will be qualified by education, training, and experience to perform their respective task(s) by GCP. Informed consent will be obtained by US 21 CFR 50.25, the Tri-Council Policy Statement: Ethical Conduct of Research Involving Humans and the Health Canada and ICH Good Clinical Practice. Applicable HIPAA privacy notifications will be implemented, and HIPAA authorizations signed before protocol procedures are carried out. Information should be given in both oral and written form as deemed appropriate by the site’s IRB.
All work complied with ethical regulations for working with human participants. Ethics approval was obtained from the institutional review boards of each institution involved: Oregon Health and Science University; University of Southern California; University of California—San Diego and so on.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Data used in preparation for this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zehu Sheng, Lanyang Wang and Ming Chen contributed equally and are co-first authors of the study.
Contributor Information
Weihua Yu, Email: yuweihua@cqmu.edu.cn.
Yang Lü, Email: yanglyu@hospital.cqmu.edu.cn.
References
- 1.Gustavsson A, Norton N, Fast T, Frölich L, Georges J, Holzapfel D, et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 2023;19(2):658–70. [DOI] [PubMed] [Google Scholar]
- 2.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118(1):5–36. [DOI] [PubMed] [Google Scholar]
- 3.Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P, et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics. 2003;3(8):1486–94. [DOI] [PubMed] [Google Scholar]
- 4.Huang YM, Ma YH, Gao PY, Wang ZB, Huang LY, Hou JH, et al. Plasma β(2)-microglobulin and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: the CABLE study. Alzheimer's Res Ther. 2023;15(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5(7):521–31. [DOI] [PubMed] [Google Scholar]
- 6.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science (New York, NY). 2000;290(5499):2155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21(8):932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Zheng Q, Hong Y, Gao Y, Hu J, Lang M, et al. β(2)-Microglobulin coaggregates with Aβ and contributes to amyloid pathology and cognitive deficits in Alzheimer’s disease model mice. Nat Neurosci. 2023;26(7):1170–84. [DOI] [PubMed] [Google Scholar]
- 9.van Westrhenen A, Smidt LC, Seute T, Nierkens S, Stork AC, Minnema MC, et al. Diagnostic markers for CNS lymphoma in blood and cerebrospinal fluid: a systematic review. Br J Haematol . 2018;182(3):384–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padovani A, Canale A, Schiavon L, Masciocchi S, Imarisio A, Risi B, et al. Is amyloid involved in acute neuroinflammation? A CSF analysis in encephalitis. Alzheimers Dement . 2022;18(11):2167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patani R, Hardingham GE, Liddelow SA. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol. 2023;19(7):395–409. [DOI] [PubMed] [Google Scholar]
- 12.Zhang PF, Hu H, Tan L, Yu JT. Microglia Biomarkers in Alzheimer’s Disease. Mol Neurobiol. 2021;58(7):3388–404. [DOI] [PubMed] [Google Scholar]
- 13.Kleinberger G, Yamanishi Y, Suárez-Calvet M, Czirr E, Lohmann E, Cuyvers E, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6(243):243ra86. [DOI] [PubMed] [Google Scholar]
- 14.Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci. 2016;17(4):201–7. [DOI] [PubMed] [Google Scholar]
- 15.Suárez-Calvet M, Kleinberger G, Araque Caballero M, Brendel M, Rominger A, Alcolea D, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med. 2016;8(5):466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin MS, Lim LW. Glial cells in Alzheimer’s disease: From neuropathological changes to therapeutic implications. Ageing Res Rev. 2022;78:101622. [DOI] [PubMed] [Google Scholar]
- 17.Bombeiro AL, Hell RC, Simões GF, Castro MV, Oliveira AL. Importance of major histocompatibility complex of class I (MHC-I) expression for astroglial reactivity and stability of neural circuits in vitro. Neurosci Lett. 2017;647:97–103. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro RS, Bombeiro AL, Chiarotto GB, Cartarozzi LP, Coser LO, Ghizoni E, et al. Interferon-beta induces major histocompatibility complex of class I (MHC-I) expression and a proinflammatory phenotype in cultivated human astrocytes. Differentiation. 2022;128:43–56. [DOI] [PubMed] [Google Scholar]
- 19.Carter SF, Herholz K, Rosa-Neto P, Pellerin L, Nordberg A, Zimmer ER. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol Med. 2019;25(2):77–95. [DOI] [PubMed] [Google Scholar]
- 20.Olsen M, Aguilar X, Sehlin D, Fang XT, Antoni G, Erlandsson A, et al. Astroglial Responses to Amyloid-Beta Progression in a Mouse Model of Alzheimer’s Disease. Mol Imag Biol. 2018;20(4):605–14. [DOI] [PubMed] [Google Scholar]
- 21.Carrillo MC, Masliah E, editors. NIA-AA Revised Clinical Criteria for Alzheimer's Disease. Alzheimer's Association International Conference: ALZ; 2023.
- 22.Guo Y, You J, Zhang Y, Liu WS, Huang YY, Zhang YR, et al. Plasma proteomic profiles predict future dementia in healthy adults. Nature Aging. 2024;4(2):247–60. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Liu TC, Duan R, Yang L. β2‐microglobulin: An essential coaggregation factor with β‐amyloid in amyloid pathology. Brain-X. 2023;1(4):49.
- 24.Aisen PS, Petersen RC, Donohue M, Weiner MW. Alzheimer’s Disease Neuroimaging Initiative 2 Clinical Core: Progress and plans. Alzheimers Dement. 2015;11(7):734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spellman DS, Wildsmith KR, Honigberg LA, Tuefferd M, Baker D, Raghavan N, et al. Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer’s Disease Neuroimaging Initiative (ADNI) CSF. Proteomics Clin Appl. 2015;9(7–8):715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suárez-Calvet M, Capell A, Araque Caballero MÁ, Morenas-Rodríguez E, Fellerer K, Franzmeier N, et al. CSF progranulin increases in the course of Alzheimer’s disease and is associated with sTREM 2, neurodegeneration and cognitive decline. EMBO Mol Med. 2018;10(12):e9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517–26. [DOI] [PubMed] [Google Scholar]
- 28.Suárez-Calvet M, Morenas-Rodríguez E, Kleinberger G, Schlepckow K, Araque Caballero M, Franzmeier N, et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol Neurodegener. 2019;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69(10):1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. [DOI] [PubMed] [Google Scholar]
- 32.Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. 2020;25(11):2932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo C, Yoon DH, Kim S, Huh J, Park CS, Park CJ, et al. Serum beta-2 microglobulin as a prognostic biomarker in patients with mantle cell lymphoma. Hematol Oncol. 2016;34(1):22–7. [DOI] [PubMed] [Google Scholar]
- 34.Seo S, Hong JY, Yoon S, Yoo C, Park JH, Lee JB, et al. Prognostic significance of serum beta-2 microglobulin in patients with diffuse large B-cell lymphoma in the rituximab era. Oncotarget. 2016;7(47):76934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muta T, Iida S, Matsue K, Sunami K, Isoda J, Harada N, et al. Predictive Significance of Serum Beta 2-Microglobulin Levels and M-Protein Velocity for Symptomatic Progression of Smoldering Multiple Myeloma. Blood. 2014;124:3379. [Google Scholar]
- 36.Wu L, Wang T, Gui W, Lin H, Xie K, Wang H, et al. Prognostic significance of serum beta-2 microglobulin in patients with non-Hodgkin lymphoma. Oncology. 2014;87(1):40–7. [DOI] [PubMed] [Google Scholar]
- 37.Yılmaz B, Köklü S, Yüksel O, Arslan S. Serum beta 2-microglobulin as a biomarker in inflammatory bowel disease. World J Gastroenterol. 2014;20(31):10916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sivanathan PC, Ooi KS, Mohammad Haniff MAS, Ahmadipour M, Dee CF, Mokhtar NM, et al. Lifting the Veil: Characteristics, Clinical Significance, and Application of β-2-Microglobulin as Biomarkers and Its Detection with Biosensors. ACS Biomater Sci Eng. 2022;8(8):3142–61. [DOI] [PubMed] [Google Scholar]
- 39.Kester MI, Teunissen CE, Sutphen C, Herries EM, Ladenson JH, Xiong C, et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimer's Res Ther. 2015;7(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordengen K, Kirsebom BE, Henjum K, Selnes P, Gísladóttir B, Wettergreen M, et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J Neuroinflammation. 2019;16(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fei Z, Pan B, Pei R, Ye S, Wang Z, Ma L, et al. Neuroprotective Effects of IVIG against Alzheimer’ s Disease via Regulation of Antigen Processing and Presentation by MHC Class I Molecules in 3xTg-AD Mice. J Prev Alzheimers Dis. 2023;10(3):581–94. [DOI] [PubMed] [Google Scholar]
- 42.Floege J, Ketteler M. beta2-microglobulin-derived amyloidosis: an update. Kidney Int Suppl. 2001;78:S164–71. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan MK, Lees JS, Rosales BM, Cutting R, Wyld ML, Woodward M, et al. Sex and the Relationship Between Cardiometabolic Risk Factors and Estimated GFR Decline: A Population-Based Cohort Study. Am J Kidney Dis. 2024;84(6):731-41.e1. [DOI] [PubMed] [Google Scholar]
- 44.Kitai Y, Toriu N, Yoshikawa T, Sahara Y, Kinjo S, Shimizu Y, et al. Female sex hormones inversely regulate acute kidney disease susceptibility throughout life. Kidney Int. 2024;S0085–2538:00712. [DOI] [PubMed] [Google Scholar]
- 45.Mauvais-Jarvis F, Lindsey SH. Metabolic benefits afforded by estradiol and testosterone in both sexes: clinical considerations. J Clin Invest. 2024;134(17):e180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garimella PS, Lee AK, Ambrosius WT, Bhatt U, Cheung AK, Chonchol M, et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J. 2019;40(42):3486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belarbia A, Nouira S, Sahtout W, Guedri Y, Achour A. Metabolic syndrome and chronic kidney disease. Saudi J Kidney Dis Transpl. 2015;26(5):931–40. [DOI] [PubMed]
- 48.Sproule TJ, Jazwinska EC, Britton RS, Bacon BR, Fleming RE, Sly WS, et al. Naturally variant autosomal and sex-linked loci determine the severity of iron overload in beta 2-microglobulin-deficient mice. Proc Natl Acad Sci USA. 2001;98(9):5170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waheed A, Grubb JH, Zhou XY, Tomatsu S, Fleming RE, Costaldi ME, et al. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci USA. 2002;99(5):3117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santello M, Toni N, Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci. 2019;22(2):154–66. [DOI] [PubMed] [Google Scholar]
- 52.Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F, et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021;78(12):1471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari-Souza JP, Ferreira PCL, Bellaver B, Tissot C, Wang YT, Leffa DT, et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer’s disease. Mol Psychiatry. 2022;27(11):4781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calsolaro V, Matthews PM, Donat CK, Livingston NR, Femminella GD, Guedes SS, et al. Astrocyte reactivity with late-onset cognitive impairment assessed in vivo using (11)C-BU99008 PET and its relationship with amyloid load. Mol Psychiatry. 2021;26(10):5848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc Natl Acad Sci U S A. 2021;118(33):e2102191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sánchez-Juan P, Valeriano-Lorenzo E, Ruiz-González A, Pastor AB, Rodrigo Lara H, López-González F, et al. Serum GFAP levels correlate with astrocyte reactivity, post-mortem brain atrophy and neurofibrillary tangles. Brain. 2024;147(5):1667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sobue A, Ito N, Nagai T, Shan W, Hada K, Nakajima A, et al. Astroglial major histocompatibility complex class I following immune activation leads to behavioral and neuropathological changes. Glia. 2018;66(5):1034–52. [DOI] [PubMed] [Google Scholar]
- 58.Milà-Alomà M, Salvadó G, Gispert JD, Vilor-Tejedor N, Grau-Rivera O, Sala-Vila A, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer’s continuum. Alzheimers Dement. 2020;16(10):1358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominici R, Finazzi D, Polito L, Oldoni E, Bugari G, Montanelli A, et al. Comparison of β 2-microglobulin serum level between Alzheimer’s patients, cognitive healthy and mild cognitive impaired individuals. Biomarkers. 2018;23(6):603–8. [DOI] [PubMed] [Google Scholar]
- 60.Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157–72. [DOI] [PubMed] [Google Scholar]
- 61.Wilkinson M, Gallardo RU, Martinez RM, Guthertz N, So M, Aubrey LD, et al. Disease-relevant β(2)-microglobulin variants share a common amyloid fold. Nat Commun. 2023;14(1):1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C, Liu TCY, Duan R, Yang L. β2-microglobulin: An essential coaggregation factor with β-amyloid in amyloid pathology. Brain-X. 2023;1(4):e49. [Google Scholar]
- 63.Zalocusky KA, Najm R, Taubes AL, Hao Y, Yoon SY, Koutsodendris N, et al. Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease. Nat Neurosci. 2021;24(6):786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laham N, Rotem-Yehudar R, Shechter C, Coligan JE, Ehrlich R. Transferrin [corrected] receptor association and endosomal localization of soluble HFE are not sufficient for regulation of cellular iron homeostasis. J Cell Biochem. 2004;91(6):1130–45. [DOI] [PubMed] [Google Scholar]
- 65.Ayton S, Portbury S, Kalinowski P, Agarwal P, Diouf I, Schneider JA, et al. Regional brain iron associated with deterioration in Alzheimer’s disease: A large cohort study and theoretical significance. Alzheimers Dement. 2021;17(7):1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenkhuis B, Somarakis A, de Haan L, Dzyubachyk O, ME IJ, de Miranda N, et al. Iron loading is a prominent feature of activated microglia in Alzheimer’s disease patients. Acta Neuropathol Commun. 2021;9(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217(2):459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis. 2008;15(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar Nelson V, Jha NK, Nuli MV, Gupta S, Kanna S, Gahtani RM, et al. Unveiling the impact of aging on BBB and Alzheimer’s disease: Factors and therapeutic implications. Ageing Res Rev. 2024;98: 102224. [DOI] [PubMed] [Google Scholar]
- 70.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124(1–2):83–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding authors upon reasonable request.