Abstract
Background
There is currently no definitive treatment for osteoarthritis. We examined the therapeutic effects and underlying mechanisms of platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells (ADSCs), individually or in combination, in a rat model of anterior cruciate ligament-induced degenerative osteoarthritis (OA) of the knee. This study seeks to advance clinical approaches to OA treatment.
Methods
Eight- to nine-week-old male Sprague-Dawley (SD) rats were randomly assigned to two groups: (1) a normal control group (Group A) and (2) a model group. The control group received no treatment. The model group underwent treatment and was further subdivided into six groups: Group B (an injury control group), Group C (high-dose ADSCs), Group D (PRP combined with high-dose ADSCs), Group E (low-dose ADSCs), Group F (PRP combined with low-dose ADSCs), and Group G (PRP alone). PRP and/or ADSCs were administered via intra-articular injection on Days 7, 37, and 67 post-surgery. Daily observations recorded activity levels and behavior, while weight changes were monitored weekly. Digital radiography (DR) was conducted on Days 30, 60, and 90 post-surgery to assess joint surface and contour alterations. Histopathological examination and inflammatory factor analysis were performed on cartilage and synovial tissue.
Results
No abnormal reactions were observed in any rats, and body weights increased as expected (P > 0.05). Significant differences in knee swelling rates and Wakitani scores were observed between Groups A and B (P < 0.01). Knee swelling rates also differed significantly between Group B and Groups C–G (P < 0.01). Wakitani scores decreased on Days 60 and 90 in Groups C–G. TNF-α and IL-1β expression levels were significantly higher in Group B compared to Group A (P < 0.05). Expression levels of these genes were significantly lower in Groups C–G than in Group B (P < 0.05).
Conclusions
Repeated intra-articular injections of PRP and ADSCs alleviated inflammation and pain, promoted tissue repair, and modulated immune responses in rats with surgically induced OA. The combination of PRP and ADSCs demonstrated enhanced therapeutic efficacy, suggesting its potential as a treatment option for OA.
Keywords: Osteoarthritis, Adipose-derived mesenchymal stem cells, Platelet-rich plasma, Treatment
Introduction
Osteoarthritis (OA) is a degenerative joint syndrome characterized by articular cartilage destruction, subchondral bone sclerosis, and cystic degeneration, leading to varying degrees of physical limitation and reduced quality of life [1–4]. Common symptoms include pain in peripheral joints, with the knee, hip, hand, and foot being most frequently affected [5, 6]. The pathogenesis of OA involves an imbalance between catabolic/proinflammatory and anabolic pathways. Reactive oxygen species (free radicals) induce chondrocyte senescence and upregulate inflammatory cytokines such as IL6, IL-1β, and TNFα, which activate additional cytokines and pathways implicated in disease progression [7, 8]. Current first-line treatments, including surgical and conservative approaches, primarily offer short-term symptomatic relief without modifying disease progression [9, 10]. Total knee replacement is the definitive treatment for refractory cases but results in persistent pain or functional impairment in up to 20% of patients after 12 months. Consequently, innovative strategies are required to repair cartilage, alleviate symptoms, and restore joint function. Biologics such as platelet-rich plasma (PRP), platelet lysate (PL), bone marrow aspirate concentrate (BMAC), growth factor concentrate (GFC), Wharton’s jelly (WJ), and mesenchymal stem cells (MSCs) have gained attention for regenerative applications in orthopaedics [11–19]. While these approaches are considered safe, their long-term efficacy requires further validation through extended preclinical and clinical trials.
MSCs, with high self-renewal, proliferative, and differentiation potential, can be derived from various sources, including skeletal muscle, synovium, and periosteum [20]. The most common MSCs sources are bone marrow and adipose tissue [21–25]. Adipose-derived MSCs (ADSCs) are preferred over bone marrow-derived counterparts due to their superior accessibility, higher proliferative and repair capacities, and lower donor site morbidity [26]. ADSCs exert paracrine [27], anti-inflammatory, and immunomodulatory effects depending on environmental conditions [28, 29]. Their therapeutic effects are likely mediated by paracrine signaling and the release of growth factors and cytokines that influence the intraarticular environment [30, 31]. ADSCs are known to polarize M0 macrophages and dendritic cells to an anti-inflammatory phenotype [32]. They have shown excellent safety and promising clinical efficacy in treating musculoskeletal disorders, especially rotator cuff tears and knee disorders [33, 34]. Preclinical studies demonstrate ADSCs’ positive effects on osteoarthritis (OA) treatment [35–37]. A systematic review revealed that removal of the cruciate ligament in animal models mimicked degenerative cartilage arthritis, with ligament reconstruction failing to prevent further degeneration [38]. Due to the lack of randomized double-blind trials and long-term follow-up data, further high-quality studies are needed to clarify the role and long-term safety of ADSCs in OA treatment [39, 40]. Platelets play a critical role in tissue healing and regeneration through mechanisms such as growth factor release, immune cell interactions, inflammation modulation, and angiogenesis promotion [41–43]. These findings support the development of platelet-based therapies. Platelet-rich plasma (PRP), a plasma product enriched with platelets, releases growth factors and cytokines upon degranulation [44]. Over the last decade, PRP therapy has gained widespread clinical use, particularly in spine, sports, and musculoskeletal medicine [45]. PRP injections have shown anti-inflammatory effects and pain relief in several studies [46–48], though no long-term improvement or complete pain relief has been reported. The effectiveness of PRP therapy is influenced by factors such as patient-specific characteristics and concurrent drug treatments, necessitating further research to optimize its use [49]. This study aims to investigate the effects of multiple intraarticular injections of PRP and ADSCs in alleviating OA symptoms in rats.
Materials and methods
This work was initiated after approval by the Ethics Committee.
Isolation and identification of ADSCs
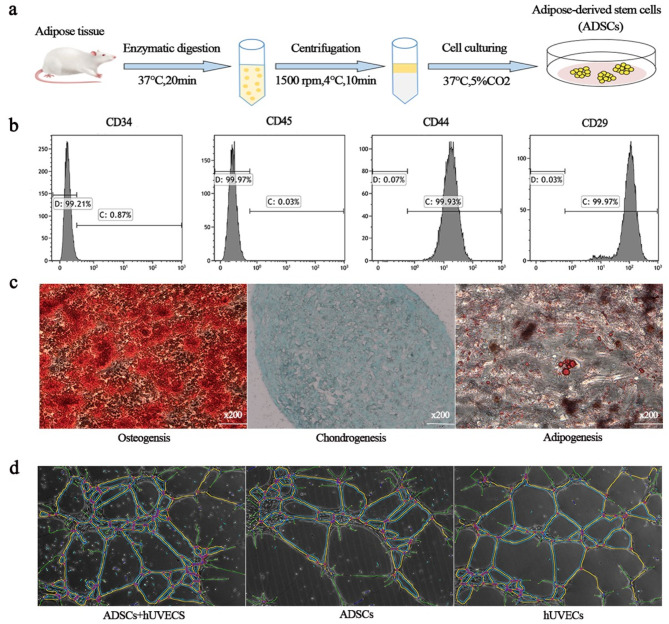
Inguinal subcutaneous adipose tissue was harvested under aseptic conditions. Fascial tissue and small blood vessels were removed, and the tissue was washed with 1× PBS (Biosharp, Cat# BL302A), minced with sterile scissors, and digested with 0.25% trypsin (Gibco, Cat# 25200-056) for 20 min at 37 °C in a water bath until the cell mixture became viscous. After centrifugation at 1500–2000 rpm, 4 °C for 10 min, the pellet was resuspended in 5 ml of αMEM (Pricella, Cat# PM150421). The mixture was filtered, centrifuged, and washed again. The pellet was resuspended in αMEM containing 15% FBS (αMEM, Pricella, Cat# PM150421; FBS, Gibco, Cat# 10099-141), transferred to a Petri dish, and incubated at 37 °C in 5% CO2. Cells were passaged at a 1:3 ratio when they reached 80–90% confluence (P1) (Fig. 1a). The third-generation cells (P3) were characterized by flow cytometry, and their differentiation potential (osteogenic, adipogenic, and chondrogenic) was assessed using induced differentiation kits (Solarbio, Cat# G1450, Cat# G1262, Cat# G2542).
Fig. 1.
Identification and function of ADSCs. a. Process of ADSC production. b. Flow cytometry analysis of surface markers. c. Multidirectional differentiation potential. The fat droplets were stained red with oil red O (left). The calcium nodules were stained orange with alizarin red S (middle). The cartilage was stained blue with alcian blue dye solution (right). d. Proangiogenic capacity of ADSCs
The angiogenic capacity of ADSCs
Inguinal subcutaneous adipose tissue was harvested under aseptic conditions. Fascial tissue and small blood vessels were removed, and the tissue was washed with 1× PBS (Biosharp, Cat# BL302A), minced with sterile scissors, and digested with 0.25% trypsin (Gibco, Cat# 25200-056) for 20 min at 37 °C in a water bath until the cell mixture became viscous. After centrifugation at 1500–2000 rpm, 4 °C for 10 min, the pellet was resuspended in 5 ml of αMEM (Pricella, Cat# PM150421). The mixture was filtered, centrifuged, and washed again. The pellet was resuspended in αMEM containing 15% FBS (αMEM, Pricella, Cat# PM150421; FBS, Gibco, Cat# 10099-141), transferred to a Petri dish, and incubated at 37 °C in 5% CO2. Cells were passaged at a 1:3 ratio when they reached 80–90% confluence (P1) (Fig. 1a). The third-generation cells (P3) were characterized by flow cytometry, and their differentiation potential (osteogenic, adipogenic, and chondrogenic) was assessed using induced differentiation kits (Solarbio, Cat# G1450, Cat# G1262, Cat# G2542).
Preparation of PRP
Blood was collected from the abdominal aorta using Ethylene Diamine Tetraacetic Acid (EDTA) anticoagulation tubes. After centrifugation at 300 × g for 5 min, the supernatant was collected and further centrifuged at 1200 × g for 7 min. The supernatant was discarded, and the platelets formed a white film. The mixture was shaken thoroughly and allowed to stand for 10 min to generate platelet-rich plasma (PRP).
Animal model of OA
Eight-to-nine-week-old SD rats (provided by Zhuhai BesTest Biotechnology Co., Ltd.) were randomly assigned to the following groups: a normal control group (Group A), an injury control group (Group B), and a model group (Groups C–G). In Groups C–G, the right knee joints were surgically exposed, the anterior cruciate ligament was severed using microscopic scissors, and half of the meniscus was removed. No treatment was administered to Group A. Each group consisted of 10 animals. The administered doses for each group are outlined in Table 1.
Table 1.
The administered doses in each group
| Group | Treatment | Injection |
|---|---|---|
| A | PBS | / |
| B | PBS | / |
| C | ADSCs | 1 × 106 |
| D | ADSCs + PRP | 1 × 106+ 2 ml |
| E | ADSCs | 1 × 105 |
| F | ADSCs + PRP | 1 × 105 +2 ml |
| G | PRP | 2 ml |
Specimen collection and processing
PRP and/or ADSCs were injected intraarticularly into the joints of Groups C–G on Days 7, 37, and 67 post-surgery. Activities and joint swelling were monitored. Digital radiographs (DRs) were taken at Days 30, 60, and 90 to assess changes in articular surfaces and contours. The rate of knee swelling was calculated. Finally, joint and synovial tissues were excised and fixed in 4% formaldehyde solution.Rate of knee swelling (%) = (Modelled knee circumference-Nonmodelled knee circumference) /Nonmodelled knee circumference × 100%.
Histopathological analysis
Knee cartilage from the femoral side of the model joint was dissected 90 days post-surgery. A 15 × 15 μm cartilage sample was cut, paraffin-embedded, sectioned, and stained with alizarin red, oil red O, and alcian blue. Synovial tissue was collected and fixed in 4% formaldehyde solution for Hematoxylin-Eosin (HE) staining. Cartilage Wakitani score.
Based on the staining results, the Wakitani score was determined by combining the cell morphology, matrix staining results, cartilage surface flatness, and cartilage thickness.
Inflammatory factor analysis
At 90 days post-surgery, knee joint tissue and synovial fluid were collected from the modeled position and fixed in 4% formaldehyde solution. IL-1β, TNF-α, and ADAMTS-4 expression levels were assessed by immunohistochemistry.Immunomodulation.
For the lymphocyte proliferation assay, ADSCs were plated at a density of 1 × 10^5 cells/ml in 6-well plates and allowed to adhere for 24 h. Mitomycin C (JSENB, Cat# ESM0503) was added at 10 µg/ml for 30 min to inhibit ADSC proliferation, after which the cells were washed with PBS. Carboxyfluorescein Succinimidyl Ester (CFSE) (Thermo, Cat# 65-0850-84)-labeled peripheral blood mononuclear cells (PBMCs) were stimulated with CD3/CD28 (Novoprotein, Cat# GMP-A018, Cat# GMP-A063) for 24 h and then plated. Three experimental groups were established: PBMC, Phytohaemagglutinin (PHA), and ADSC. Lymphocyte proliferation was assessed by flow cytometry after 72 h, and supernatants were collected for TNF-α measurement by enzyme-linked immunosorbent assay (ELISA).
Proliferation inhibition rate (%) = (division index of the control group-division index of the experimental group)/division index of the control group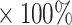
Immunomodulation of Th1/Th17 Subsets: Cells were divided into two groups: control (PBMC + CD3/CD28) and experimental (PBMC + CD3/CD28 + ADSC). The ratio of Th1/Th17 subpopulations was determined by flow cytometry after 48 h.
Immunomodulation of Tregs: The experimental cells were divided into control (PBMC + CD3/CD28 + IL2/IL15) (L2/IL15, Novoprotein, Cat# C013, Cat# C016) and experimental (PBMC + CD3/CD28 + IL2/IL15 + ADSC) groups. The proportions of Tregs were assessed by flow cytometry after 4 days of culture.
Results
Identification and function of ADSCs
Identification of ADSCs
Surface markers of ADSCs at P3 were analyzed by flow cytometry, and CD44 and CD29 were positively expressed at 99.93 and 99.97%, respectively. CD34 and CD45 were not expressed (Fig. 1b), which is consistent with the immunophenotypic characteristics of ADSCs.
Differentiation of ADSCs
ADSCs were incubated in lipogenic, osteogenic and chondrogenic induction media and then stained with oil red O, alizarin red and alizarin blue, respectively. Spherical lipid droplets within the cytoplasm are stained red by oil-red O staining. Alizarin red staining revealed reddish-stained calcium nodules. Endoacidic mucopolysaccharides in cartilage tissue were stained blue with alcian blue (Fig. 1c). These results suggest that rat ADSCs are stem cells with multidirectional differentiation potential.
Proangiogenic effects of ADSCs
Compared with those in the groups cultured alone, the number of crossing points and branch length of the vascular network was significantly greater in the group cocultured with ADSCs and HUVECs, suggesting that ADSCs can promote the formation of vascular networks in HUVECs (Fig. 1d).
Evaluation of growth and joint conditions
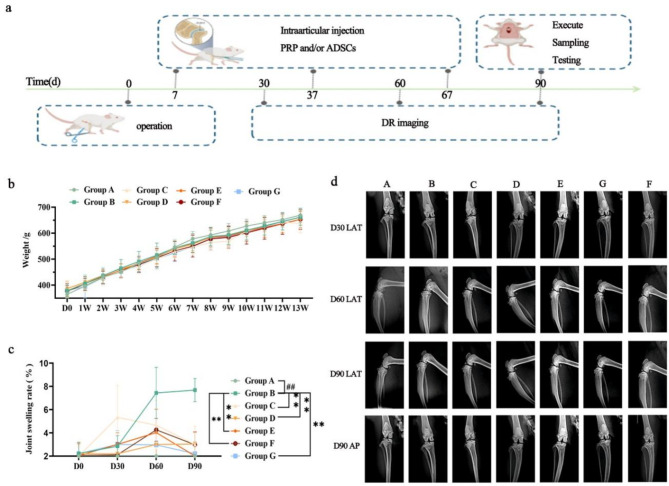
The processing time points of each group are shown in Fig. 2a.
Fig. 2.
Evaluation of growth and joint conditions. a. Processing time points for each group. b. Changes in the body weights of the rats. c. Joint swelling rates of the different groups of rats on days 30, 60 and 90 after treatment. d. DR was performed on days 30, 60 and 90 post surgery to analyze the changes in the articular surface and contour. N = 10, compared with Group A, “##”P < 0.01; compared with Group B, “**”P < 0.01
All groups of rats exhibited no abnormal activity and showed normal weight gain. There was no significant difference in body weight between Groups A and B at any time point (P > 0.05) (Fig. 2b). Joint analysis revealed that all rats in Group A had smooth articular surfaces and synovium. The fat pad beneath the synovium was elastic, and no joint effusion was observed. In contrast, rats in Group B exhibited rough cartilage on the articular surface, synovial fibrosis, and severe atrophy of the sub-synovial fat pad on the side of the resected meniscus, accompanied by joint effusion. Symptoms were alleviated to varying degrees in Groups C–G compared to Group B.
The rate of knee swelling in rats from Group B was significantly higher on Days 0, 30, 60, and 90 of treatment compared to Group A (P < 0.01). These findings confirm that anterior cruciate ligament resection effectively induces arthritis with concomitant joint swelling. Compared to Group B, the knee swelling rates in Groups C–G were significantly lower on Days 60 and 90 of treatment (P < 0.01) (Fig. 2c).
Radiological assessment results are shown in Fig. 2d. Lateral (LAT) radiographs taken on Day 30 showed that the articular surfaces of rats in Groups B–G were smooth, with clear borders and no narrowing or widening of the joint cavity. As the study progressed, no obvious arthritic symptoms were observed in any of the groups at this time. However, by Day 60, LAT radiographs revealed early signs of arthritis in Group B, including mild pitting of the articular surface, slight thinning of the cartilage layer, and early osteophyte formation. No significant differences were observed between Groups C–G and Group B at this time. By Day 90, LAT and anteroposterior (AP) radiographs showed that rats in Group B exhibited uneven joint surfaces, irregular joint space widths, a trend towards joint cavity narrowing, bone capillary formation, and deformation of the medial ankle joint surface, accompanied by cartilage thinning and other signs of arthritis. Compared to Group B, rats in Groups C–G showed slight improvements in articular surface unevenness and medial ankle joint deformation. Joint space narrowing was also significantly reduced in these groups.
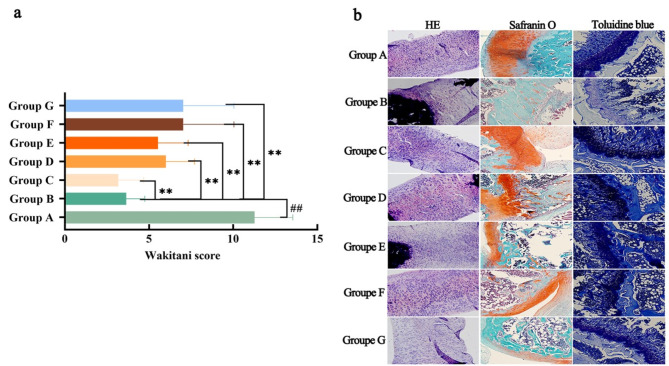
Histological analysis and cartilage Wakitani score
Compared to Group A, the Wakitani score for the knee joints in Group B was significantly higher (P < 0.01). In contrast, the Wakitani scores of knee joints in Groups C–G were significantly lower than those in Group B (P < 0.01) (Fig. 3a). Histological analysis via HE staining is shown in Fig. 3b. The synovium in Group A exhibited a normal histological structure, with no noticeable inflammatory cell infiltration. In Group B, the synovium had a low cell density and showed significant infiltration by inflammatory cells. Groups C and D displayed a normal cell density, with only a small number of infiltrating inflammatory cells. In Group E, the ratio of normal to inflammatory cells was approximately 50%, with a considerable presence of inflammatory cells. Group F had a higher number of normal cells than inflammatory cells, though moderate inflammatory cell infiltration was still present. Group G had fewer cells than Group B, and the majority of cells showed inflammatory infiltration. These results indicate a reduction in both inflammatory cell infiltration and the Wakitani score of degenerative arthritis with PRP, ADSCs, and their combination. However, the most significant improvements were observed in the ADSC and PRP groups.
Fig. 3.
Alleviation of cartilage symptoms in degenerative arthritis by PRP or/and ADSCs shown by pathological analysis. a. HE staining of the synovium (left) and toluidine blue (middle) and safranin O staining (right) of cartilage in different groups of rats. b. Wakitani scores were assigned to each group of rats based on the staining results, cell morphology, cartilage surface flatness, and cartilage thickness. N = 10, compared with Group A, “##”P < 0.01, compared with Group B, “**”P < 0.01
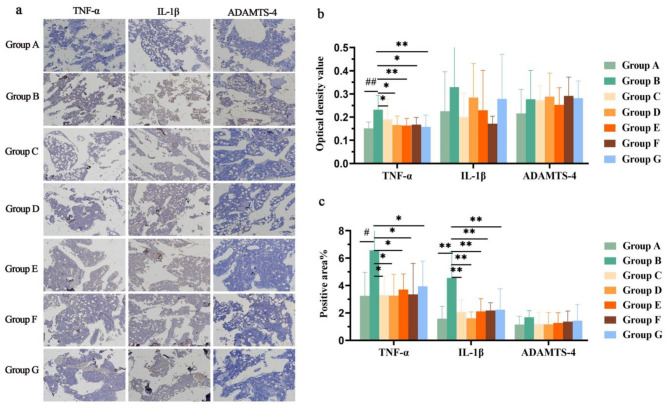
Anti-inflammatory effects
TNF-α, IL-1β and ADAMTS-4 are important inflammatory factors in knee joints. The protein expression in each group was determined by immunohistochemistry (Fig. 4a). Compared with those in Group A, TNF-α and IL-1β expression was significantly greater in the cartilage in Group B (P < 0.05), but there was no significant change in the expression of ADAMTS-4 (P > 0.05) (Fig. 4b and c). Compared with those in Group B, TNF-α and IL-1β expression was significantly lower in Groups C–G (P < 0.05), while there was no difference in the expression of ADAMTS-4 (P > 0.05). The above results indicate that Groups C–G had some anti-inflammatory effects, but Group D had the greatest effect.
Fig. 4.
Expression of the TNF-α, IL-1β and ADAMTS-4 proteins in rat knee joints cartilage. a. Protein expression of TNF-α, IL-1β and ADAMTS-4 in the cartilage of the knee joints of the rats in each group determined via immunohistochemistry. b. Optical density values of TNF-α, IL-1β, and ADAMTS-4 in each group. c. Areas positive for TNF-α, IL-1β and ADAMTS-4 in each group. N = 10, compared with Group A, “#”P < 0.05 and “##”P < 0.01, compared with Group B, “*”P < 0.05 and “**”P < 0.01
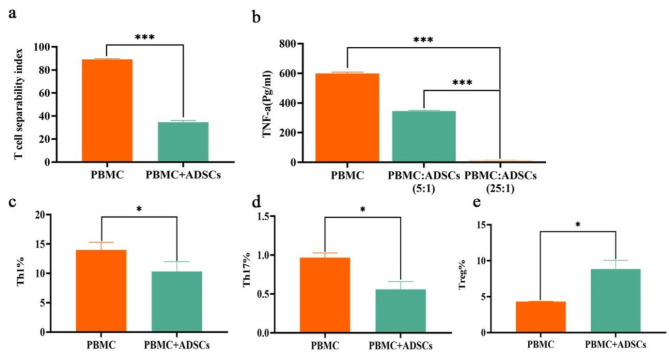
Immune regulation
We explored the effects of ADSCs on the inhibition of T lymphocyte proliferation, the inhibition of TNF-α secretion, and the proliferative capacity of various subpopulations (Fig. 5). When PBMCs were cocultured with ADSCs at a 5:1 ratio, the rate of ADSC-mediated inhibition of lymphocyte proliferation was 52.56% (Fig. 5a), and the rate of inhibition of lymphocyte secretion of TNF-α was 98.11% (Fig. 5b). In contrast, the percentage of TNF-α secreted by PBMCs cocultured with ADSCs at a 25:1 ratio was 42.44%. At a 5:1 ratio, the rates of ADSC-mediated inhibition of the proliferation of the Th1 and Th17 lymphocyte subpopulations were 18.20% and 42.05%, respectively (Fig. 5c and d). The proliferation of Tregs was promoted by 9.02% when PBMCs and ADSCs were cocultured at a 5:1 ratio (Fig. 5e).
Fig. 5.
Effects of ADSCs on T cells. a. Inhibition of T lymphocyte proliferation. b. Inhibition of TNF-α secretion. c-d. Proliferative capacity of Th1 and Th17 subpopulations. e. Treg proliferation assay. *P < 0.05, ***P < 0.001
Discussion
Previous animal studies and clinical trials involving intra-articular MSC injections for osteoarthritis (OA) have not consistently demonstrated definitive benefits [50, 51]. It has been noted that MSCs injected into the knee can be rapidly degraded or disrupted by macrophages, highlighting the need for effective delivery systems to enhance MSC translation. Variations in stem cell sources, injection frequencies, dosages, and animal models may all influence the therapeutic outcomes of MSCs in OA treatment [52]. MSCs may also be more effective when combined with other therapies, such as PRP, which has been shown to reduce pain and synovial thickness [45, 53].
This study examined the effects of PRP and/or ADSCs in treating surgery-induced OA. We observed significant alleviation of inflammation, immune cell infiltration, and arthritis symptoms, along with protection against synovial thickening in treated animals compared to the untreated group. Moreover, the treatment notably improved damaged articular cartilage. Changes in knee swelling rates confirmed that anterior cruciate ligament resection successfully induced arthritis with accompanying joint swelling, and PRP and/or ADSCs were effective in reducing knee swelling within 90 days. Radiological assessments at 90 days post-surgery revealed that rats in the model control group exhibited uneven joint surfaces, osteophyte formation, joint space narrowing, and joint surface deformation, along with cartilage thinning and other typical arthritic symptoms. In contrast, these symptoms were significantly alleviated in the treatment groups, particularly the joint space narrowing. OA-related metabolic imbalances lead to chondrocyte senescence, which is closely associated with the development of OA and the release of inflammatory cytokines. TNF-α and IL-1β are key negative regulators that are overexpressed in the cartilage and synovium of OA patients. These cytokines synergistically stimulate the release of matrix metalloproteinases from chondrocytes, promoting cartilage degradation [6, 7, 54]. TNF-α also induces ADAMTS-4, a metzincin protease primarily expressed in osteoarthritic cartilage [55], which plays a critical role in cleaving the extracellular matrix (ECM). A previous study demonstrated that ADAMTS-4 mRNA expression is significantly elevated in an experimentally induced cartilage degeneration model, and that silencing ADAMTS-4 with siRNA can slow degenerative changes in cartilage [56]. In the current study, we confirmed that PRP and/or ADSCs can reduce the protein expression of TNF-α and IL-1β in the cartilage of rats with OA. However, no significant difference was observed in the protein expression of ADAMTS-4 between the treatment and control groups.
Previous studies have shown that PRP or MSCs can downregulate the gene expression of inflammatory markers such as IL-1β, TNF-α, and IL-6 in chondrocytes, and that elevated expression of these markers stimulates catabolic activity and cartilage destruction [6, 7, 57]. Our findings are consistent with this body of work. Notably, the combination of PRP and MSCs provided greater therapeutic benefits than either treatment alone. Given that TNF-α induces ADAMTS-4 expression [58], the combined effects of PRP and MSCs may enhance the inhibition of cartilage degradation pathways.In the present study, despite the reduction in TNF-α protein expression, no corresponding effect on ADAMTS-4 expression was observed. TNF-α has been shown to regulate ADAMTS-4 expression in osteoarthritic chondrocytes at the transcriptional level in a time- and dose-dependent manner [59]. However, the changes in TNF-α levels in this study were not sufficient to modulate ADAMTS-4 expression. Future studies should focus on determining the optimal regulatory dose and investigating the underlying transcriptional mechanisms that govern this process. Increasing evidence suggests that synovitis and immune system involvement play critical roles in the progression of OA [60].T cells are the primary components of synovial infiltrates in osteoarthritis (OA) patients. Th1, Th17, and Treg cells are three distinct CD4 + T-cell subsets that play crucial roles in maintaining immune balance [61]. Th1 and Th17 cells accumulate in the synovium, with Th1 cells being more abundant than Th17 cells [62]. Despite their importance in regulating inflammation and autoimmune responses, Tregs are significantly less frequent at the site of inflammation in the synovial membrane [63]. In light of these findings, we investigated the effects of ADSCs on T lymphocyte proliferation, TNF-α secretion, and the proliferative capacity of different T-cell subpopulations. Interestingly, ADSCs not only inhibited the proliferation of Th1 and Th17 cells and TNF-α secretion but also promoted Treg proliferation, with higher doses yielding better results. Accumulating evidence suggests that ADSCs can reduce inflammation by secreting anti-inflammatory cytokines that suppress proinflammatory T cells and enhance the number of anti-inflammatory T cells.
Novel regenerative treatments, including MSC implantation, MSC-derived exosomes, PRP, autologous chondrocyte implantation, and gene therapy, have been extensively studied for OA treatment [38]. However, the optimal dosage, cell number, and injection interval, either individually or in combination, remain subjects of ongoing debate. Ensuring an adequate supply of MSCs for sustained therapeutic effects remains a significant challenge.
With multiple injections of PRP in combination with MSCs, we obtained unexpected results.
Conclusion
Our data demonstrate that multiple combined intraarticular injections of PRP and ADSCs significantly reduce inflammation, immune cell infiltration, and arthritic symptoms, while preventing synovial thickening compared to the untreated group. However, the long-term sustainability of these effects remains unclear and will be addressed in future clinical trials.
Acknowledgements
We are grateful to all who have worked on this project.
Abbreviations
- OA
Osteoarthritis
- ADSCs
Adipose-derived mesenchymal stem cells
- MSC
Mesenchymal stem cell
- PRP
Platelet-rich plasma
- HUVECs
Human umbilical vein endothelial cells
- TNF-α
Tumour necrosis factor
- IL-1β
Interleukin-1β
- IL6
Interleukin-6
- ADAMTS-4
Disintegrin-like metalloproteinase with thrombospondin type-1 motif-4
- EDTA
Ethylene diamine tetraacetic acid.
- HE
Staining Hematoxylin-Eosin staining
- ELISA
Enzyme-linked immunosorbent assays
- PBMC
Peripheral blood mononuclear cell
- CFSE
Carboxyfluorescein Succinimidyl Ester
- PHA
Phytohaemagglutinin
- FBS
Fetal bovine serum
- αMEM
Minimum Essential Medium-Alpha
Author contributions
All the authors contributed to conceptualizing and designing the study. Conceptualization, funding acquisition, material preparation, investigation, project administration, and data collection were performed by Weijie He, Jie Zhao, Jiafei Liu, and Fangxing Wang. Data analysis, visualization, writing, and editing of the manuscript were performed by Weijie He, Jie Zhao, Jiafei Liu, Fangxing Wang, Xueyi Qian and Zhenyu Xu. The first draft of the manuscript was written by Weijie He, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
This work was supported by the Key Project of Excellent Young Talents Support Foundation of Anhui Province University (gzyqZD2021143) and the Project of Development of Modern Medical and Pharmaceutical Industry of Anhui Province (2021/2022). The funder did not participate in the design of the study, the collection, analysis and interpretation of the data, or the writing of the report.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The Ethics Committee of Zhuhai BesTest Biotechnology Co., Ltd., approved the study (IAC 202105052).
Consent for publication
The manuscript does not contain any individual person data in any form.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018. 10.1016/j.joca.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramoff B, Caldera FE, Osteoarthritis. Pathology, diagnosis, and Treatment options. Med Clin North Am. 2020. 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and KneeOsteoarthritis: a review. JAMA. 2021. 10.1001/jama.2020.22171. [DOI] [PubMed] [Google Scholar]
- 4.Boffa A, Perucca Orfei C, Sourugeon Y, Laver L, Magalon J, Sánchez M, et al. The relationship between multisite peripheral joint pain and physical activity levels in older adults: a cross-sectional survey. Musculoskelet Care. 2022. 10.1002/msc.1593. [DOI] [PubMed] [Google Scholar]
- 5.Boffa A, Perucca Orfei C, Sourugeon Y, Laver L, Magalon J, Sánchez M, et al. Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 2: bone marrow-derived cell-based injectable therapies. Knee Surg Sports Traumatol Arthrosc. 2023. 10.1007/s00167-023-07320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katherine S, Norman,Adam P, Goode,Carolina Alvarez D, Hu,Steven Z, George,Todd A, Schwartz, et al. Association of Biomarkers with individual and multiple body sites of Pain: the Johnston County Osteoarthritis Project. J Pain Res. 2022. 10.2147/JPR.S365187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Roover A, Escribano-Núñez A, Monteagudo S, Lories R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthritis Cartilage. 2023. 10.1016/j.joca.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, et al. Cytokines and chemokines involved in Osteoarthritis Pathogenesis. Int J Mol Sci. 2021. 10.3390/ijms22179208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood G, Neilson J, Cottrell E, Hoole SP, Guideline Committee. Osteoarthritis in people over 16: diagnosis and management-updated summary of NICE guidance. BMJ. 2023. 10.1136/bmj.p24. [DOI] [PubMed] [Google Scholar]
- 10.Rossbach P. Arthrose [Osteoarthritis - therapy and management]. Ther Umsch. 2023. 10.1024/0040-5930/a001401. [DOI] [PubMed] [Google Scholar]
- 11.Migliorini F, Pilone M, Ascani J, Schäfer L, Jeyaraman M, Maffulli N. Management of knee osteoarthritis using bone marrow aspirate concentrate: a systematic review. Br Med Bull. 2024. 10.1093/bmb/ldae016. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Maffulli N. Platelet lysate and osteoarthritis of the knee: a review of current clinical evidence. Pain Ther. 2024. 10.1007/s40122-024-00661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta A, Maffulli N. Growth factor Concentrate (GFC) for the management of Osteoarthritis of the knee: a systematic review. Indian J Orthop. 2024. 10.1007/s43465-024-01172-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Potty AG, Maffulli N, Editorial. Regenerative biologics for musculoskeletal injuries. Front Pain Res (Lausanne). 2024. 10.3389/fpain.2024.1400548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aratikatla A, Maffulli N, Gupta M, Potti IA, Potty AG, Gupta A. Wharton’s jelly and osteoarthritis of the knee. Br Med Bull. 2024. 10.1093/bmb/ldad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta A, Potty AG, Maffulli N. Allogenic platelet-rich plasma for treatment of knee and hip osteoarthritis. Front Pain Res (Lausanne). 2023. 10.3389/fpain.2023.1216190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyaraman M, Maffulli N, Gupta A. Stromal vascular fraction in Osteoarthritis of the knee. Biomedicines. 2023. 10.3390/biomedicines11051460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pintore A, Notarfrancesco D, Zara A, et al. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: a prospective comparative clinical trial. J Orthop Surg Res. 2023. 10.1186/s13018-023-03841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Maffulli N. Allogenic umbilical cord tissue for treatment of knee osteoarthritis. Sports Med Arthrosc Rev. 2022. 10.1097/JSA.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Xue C, Wu H, Li C, Li S, Luo J, et al. Genetically Modified Mesenchymal Stromal Cells Cartil Regeneration Stem Cells Dev. 2023. 10.1089/scd.2022.0242. [DOI] [PubMed] [Google Scholar]
- 21.Tian Z, Yu T, Liu J, Wang T, Higuchi A. Introduction to stem cells. Prog Mol Biol Transl Sci. 2023. 10.1016/bs.pmbts.2023.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020. 10.1007/s00167-020-05859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie RH, Gong SG, Song J, Wu PP, Hu WL. Effect of mesenchymal stromal cells transplantation on the outcomes of patients with knee osteoarthritis: a systematic review and meta-analysis. J Orthop Res. 2024. 10.1002/jor.25724. [DOI] [PubMed] [Google Scholar]
- 24.Copp G, Robb KP, Viswanathan S. Culture-expanded mesenchymal stromal cell therapy: does it work in knee osteoarthritis? A pathway to clinical success. Cell Mol Immunol. 2023. 10.1038/s41423-023-01020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci. 2020. 10.1016/j.lfs.2020.118002. [DOI] [PubMed] [Google Scholar]
- 26.Freitag J, Wickham J, Shah K, Tenen A. Effect of autologous adipose-derived mesenchymal stem cell therapy in the treatment of an osteochondral lesionof the ankle. BMJ Case Rep. 2020. 10.1136/bcr-2020-234595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias de Oliveira FB, Antonioli E, Dias OFM, de Souza JG, Agarwal S, Chudzinski-Tavassi AM, et al. Comparative effects of Intra-articular versus Intravenous Mesenchymal stromal cells therapy in a rat model of Osteoarthritis by destabilization of medial Meniscus. Int J Mol Sci. 2023. 10.3390/ijms242115543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planat-Benard V, Varin A, Casteilla L. MSCs and inflammatory cells crosstalk in Regenerative Medicine: concerted actions for optimized resolution driven by Energy Metabolism. Front Immunol. 2021. 10.3389/fimmu.2021.626755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon DG, Kim MK, Jeon YS, Nam YC, Park JS, Ryu DJ. State of the art: the Immunomodulatory role of MSCs for Osteoarthritis. Int J Mol Sci. 2022. 10.3390/ijms23031618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei P, Bao R. Intra-articular mesenchymal stem cell injection for KneeOsteoarthritis: mechanisms and clinical evidence. Int J Mol Sci. 2022. 10.3390/ijms24010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuppa SS, Kim HK, Kang JY, Lee SC, Seon JK. Role of mesenchymal stem cells and their Paracrine mediators in Macrophage polarization: an Approach to reduce inflammation in Osteoarthritis. Int J Mol Sci. 2022. 10.3390/ijms232113016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, et al. Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother. 2019. 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]
- 33.Furia JP, Lundeen MA, Hurd JL, et al. Why and how to use the body’s own stem cells for regeneration in musculoskeletal disorders: a primer. J Orthop Surg Res. 2022. 10.1186/s13018-022-02918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurd JL, Facile TR, Weiss J, et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res. 2020. 10.1186/s13018-020-01631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz-Virumbrales M, Menta R, Pérez LM, Lucchesi O, Mancheño-Corvo P, Avivar-Valderas Á, et al. Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: role of IL-6 and PGE2.Stem. Cell Res Ther. 2020. 10.1186/s13287-020-01975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veronesi F, Berni M, Marchiori G, Cassiolas G, Muttini A, Barboni B, et al. Evaluation of cartilage biomechanics and knee joint microenvironment after different cell-based treatments in a sheep model of early osteoarthritis. Int Orthop. 2021. 10.1007/s00264-020-04701-y. [DOI] [PubMed] [Google Scholar]
- 37.Vasiliadis AV, Galanis N. Effectiveness of AD-MSCs injections for the treatment of knee osteoarthritis: analysis of the current literature. J Stem Cells Regen Med. 2020. 10.46582/jsrm.1601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perucca Orfei C, Boffa A, Sourugeon Y, Laver L, Magalon J, Sánchez M, et al. Cell-based therapies have disease-modifying effects on osteoarthritis in animal models. A systematic review by the ESSKA Orthobiologic Initiative. Part 1: adipose tissue-derived cell-based injectable therapies. Knee Surg Sports Traumatol Arthrosc. 2023. 10.1007/s00167-022-07063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andia I, Maffulli N. New biotechnologies for musculoskeletal injuries. Surgeon. 2019. 10.1016/j.surge.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Usuelli FG, D’Ambrosi R, Maccario C, Indino C, Manzi L, Maffulli N. Adipose-derived stem cells in orthopaedic pathologies. Br Med Bull. 2017. 10.1093/bmb/ldx030. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A, Jeyaraman M, Maffulli N. Common medications which should be stopped prior to platelet-rich plasma injection. Biomedicines. 2022. 10.3390/biomedicines10092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andia I, Maffulli N. Blood-derived products for tissue Repair/Regeneration. Int J Mol Sci. 2019. 10.3390/ijms20184581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andia I, Atilano L, Maffulli N. Biological targets of Multimolecular therapies in Middle-Age Osteoarthritis. Sports Med Arthrosc Rev. 2022. 10.1097/JSA.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 44.Xu Z, He Z, Shu L, Li X, Ma M, Ye C. Intra-articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is Superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving Pain and function. Arthroscopy. 2021. 10.1016/j.arthro.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Shen H, Wu Y, et al. Platelet-Rich plasma therapy enhances the beneficial effect of bone marrow stem cell transplant on endometrial regeneration. Front Cell Dev Biol. 2020. 10.3389/fcell.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barman A, Bandyopadhyay D, Mohakud S, Sahoo J, Maiti R, Mukherjee S et al. Comparison of clinical outcome, cartilage turnover, and inflammatory activity following either intra-articular or a combination of intra-articular with intraosseous platelet-rich plasma injections in osteoarthritis knee: a randomized, clinical trial.Injury. 2023; 10.1016/j.injury.2022.11.036 [DOI] [PubMed]
- 47.Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich plasma:New Performance understandings and therapeutic considerations in 2020. Int JMol Sci. 2020. 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of intra-articular platelet-rich plasma vs Placebo Injection on Pain and Medial Tibial cartilage volume in patients with knee osteoarthritis: the RESTORE Randomized Clinical Trial. JAMA. 2021. 10.1001/jama.2021.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andia I, Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen Med. 2018. 10.2217/rme-2018-0042. [DOI] [PubMed] [Google Scholar]
- 50.Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N et al. Shattering barriers toward clinically meaningful MSC therapies.Sci Adv. 2020; 10.1126/sciadv.aba6884 [DOI] [PMC free article] [PubMed]
- 51.Wang G, Xing D, Liu W, Zhu Y, Liu H, Yan L, et al. Preclinical studies and clinical trials on mesenchymal stem cell therapy for knee osteoarthritis: a systematic review on models and cell doses. Int J Rheum Dis. 2022. 10.1111/1756-185X.14306. [DOI] [PubMed] [Google Scholar]
- 52.Tan SHS, Kwan YT, Neo WJ, Chong JY, Kuek TYJ, See JZF, et al. Intra-articular injections of mesenchymal stem cells without adjuvant therapies for knee osteoarthritis: a systematic review and Meta-analysis. Am J Sports Med. 2021. 10.1177/0363546520981704. [DOI] [PubMed] [Google Scholar]
- 53.Lewis E, Merghani K, Robertson I, Mulford J, Prentice B, Mathew R, et al. The effectiveness of leucocyte-poor platelet-rich plasma injections on symptomatic early osteoarthritis of the knee: the PEAK randomized controlled trial.Bone. Joint J. 2022. 10.1302/0301-620X.104B6.BJJ-2021-1109.R2. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Zhang NZ, Li M, Zhang DF, Ma X, Zhou SL, et al. SappanoneA alleviated IL-1β-Induced inflammation in OA Chondrocytes through modulating the NF-κB and Nrf2/HO-1 pathways. Dis Markers. 2022. 10.1155/2022/2380879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Kalebota N, Salai G, Peric P, Hrkac S, Novak R, Durmis KK, et al. ADAMTS-4 as a possible distinguishing indicator between osteoarthritis and hemohaemophilic arthropathy. HemoHaemophilia. 2022. 10.1111/hae.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li T, Peng J, Li Q, Shu Y, Zhu P, Hao L. The mechanism and role of ADAMTS protein family in Osteoarthritis. Biomolecules. 2022. 10.3390/biom12070959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simental-Mendía M, Ortega-Mata D, Acosta-Olivo CA. Platelet-Rich plasma for knee osteoarthritis: what does the evidence say? Drugs Aging. 2023. 10.1007/s40266-023-01040-6. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Yang M, Zhang C, Huang F. The protective effects of dehydrocostus lactone against TNF-α-induced degeneration of extracellular matrix (ECM)in SW1353 cells. Aging. 2020. 10.18632/aging.103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue J, Wang J, Liu Q, Luo A. Tumor necrosis factor-α induces ADAMTS-4 expression in human osteoarthritis chondrocytes. Mol Med Rep. 2013. 10.3892/mmr.2013.1729. [DOI] [PubMed] [Google Scholar]
- 60.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012. 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 61.Ye X, Lu Q, Yang A, Rao J, Xie W, He C, et al. MiR-206 regulates the Th17/Treg ratio during osteoarthritis. Mol Med. 2021. 10.1186/s10020-021-00315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li YS, Luo W, Zhu SA, Lei GH. T cells in Osteoarthritis: alterations and Beyond. Front Immunol. 2017. 10.3389/fimmu.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007. 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.