Abstract
Background
Cardiovascular diseases (CVD) remain a significant global health burden, particularly in China, where kidney dysfunction (KD) is a key risk factor. This study analyzed trends in the burden of KD-induced CVD and subtypes among the working-age population (25–64 years) in China over the past 30 years and explored its association with age, period, and birth cohort.
Methods
This study extracted data from the Global Burden of Disease (GBD) 2021, focusing on deaths and disability-adjusted life years (DALYs) caused by KD-induced CVD and subtypes, including ischemic heart disease (IHD), stroke, and lower extremity peripheral artery disease (LEPAD) among 25–64 years globally and in China from 1992 to 2021. Trends in disease burden were described by calculating age-standardized mortality rates (ASMR) and age-standardized DALYs rates (ASDR). Additionally, an age-period-cohort (APC) model was employed to estimate the overall annual percentage change in mortality (net drift), the annual percentage change for specific age groups (local drift), the relative risks of period and cohort effects, and the age-specific rates adjusted for period bias (age effect).
Results
From 1992 to 2021, the number of deaths and DALYs caused by KD-induced IHD and LEPAD among 25–64 years globally and in China showed an upward trend, while the number caused by stroke decreased. However, the ASMR and ASDR demonstrated a declining trend, with the disease burden in China being lower than the global level. Notably, the ASMR for IHD and LEPAD in Chinese males showed an upward trend. The declines in ASMR and ASDR were more pronounced in females than in males. The net drift for CVD and subtypes showed a downward trend, with differing patterns between males and females. Mortality rates from stroke in males was increasingly affecting younger populations, while LEPAD was more prevalent in older individuals. Aside from male IHD, the relative risks for CVD and subtypes across cohort and period analyses showed a slight decline. Females exhibited higher relative risks in earlier periods, but their decline in both period and cohort analyses was faster than that of males. Mortality rates for IHD and stroke increased with age, with males exhibiting higher mortality rates across all age groups compared to females.
Conclusion
Our findings provide strong evidence that from 1992 to 2021, KD-induced CVD and subtypes still require attention among the working population in China. There were notable differences across subtypes, genders, and age groups, with males experiencing higher mortality rates and cohort-period risks than females. Our study highlights the need for China’s public health authorities to develop tailored guidelines targeting specific CVD subtypes, genders, and age groups to prevent the further escalation of the KD-induced CVD burden.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21183-4.
Keywords: Global burden of disease, Kidney dysfunction, Cardiovascular diseases, China, Death
Introduction
Cardiovascular diseases (CVD) are the leading cause of death globally, accounting for 33% of all deaths. They are the major contributor to premature mortality, rising healthcare costs, and a significant public health burden, drawing considerable attention from countries worldwide [1–3]. Similarly, CVD are the primary cause of death and long-term disability in China [4, 5]. Despite substantial improvements in healthcare accessibility and quality over the past 30 years, the number of incident cases and deaths of CVD in China continues to rise, driven by unhealthy lifestyles, a large at-risk population, and an aging demographic [6, 7]. According to the Global Burden of Disease (GBD) 2019 report, the prevalent cases of CVD in China reached 120 million in 2019 [9]. Recent research also showed that the direct economic burden of CVD in China has increased 5.3 times, with an annual growth rate surpassing that of gross domestic product [8]. CVD have become one of the most pressing health issues for Chinese residents, imposing a substantial economic strain on society.
Metabolic risk factors are the primary contributors to CVD [9, 10]. In 2019, 13.7 million CVD-related deaths globally were attributed to metabolic risk factors, accounting for 73.7% of all CVD-related deaths (18.6 million total). As societal development continues, understanding the impact of metabolic risk factors on the CVD burden has become increasingly important [11–14]. Kidney dysfunction (KD) is a key metabolic risk factor for CVD, characterized by impaired kidney function leading to the accumulation of metabolic waste products, including urea, creatinine, and blood urea nitrogen, as well as water, electrolyte, and acid-base imbalances. This dysfunction can further contribute to systemic diseases affecting the entire body [15, 16]. In individuals with KD, the risk of developing CVD is significantly elevated [17]. According to previous studies and the GBD 2019 report, the number of incident cases and deaths of KD-induced CVD in China has nearly doubled over the past 30 years [18]. As a developing country, China has experienced a sharp rise in the burden of KD amid rapid economic growth, industrialization, and urbanization, complicating efforts to control CVD incidence and mortality. The working-age population (25–64 years) plays a pivotal role in social and economic development, and its health is directly linked to productivity and economic growth. Studies have found that in recent years, while cardiovascular risk has been decreasing among older adults, it has been increasing among younger populations. This trend may be associated with rising rates of obesity, sedentary lifestyles, diabetes, and hypertension among young people [19–21]. Therefore, assessing the disease burden in this specific age group is critically important.
However, to date, no detailed analysis has been conducted on the burden of KD-induced CVD and subtypes in China’s 25–64 years population. To address this gap, we utilized data from the GBD 2021 study to examine the burden of KD-induced CVD over the past 30 years (1992–2021). Specifically, we calculated age-standardized mortality rates (ASMR) and age-standardized disability-adjusted life year rates (ASDR) for CVD and subtypes. Additionally, we estimated annual percentage change (EAPC) to identify trends in the burden of KD-induced CVD and subtypes in China between 1992 and 2021. An age-period-cohort (APC) model was also applied to analyze the effects of age, period, and cohort on mortality rates for CVD and subtypes. These findings may offer valuable insights for policymakers in developing cost-effective interventions and optimizing resource allocation.
Methods
Data source
The data for this study were obtained from GBD 2021, which provides epidemiological estimates for over 370 diseases, injuries, and risk factors across 204 countries and regions from 1990 to 2021. Detailed information on data collection, processing, synthesis, and modeling is available in GBD 2021 publications [22]. We used GBD 2021 data to analyze deaths and disability-adjusted life years (DALYs) due to KD-induced CVD and subtypes, including ischemic heart disease (IHD), stroke, and lower extremity peripheral artery disease (LEPAD), among individuals aged 25–64 years in China and globally from 1992 to 2021, with 95% uncertainty intervals (UI). As LEPAD primarily affects older populations, GBD 2021 did not provide data for individuals aged 25–39. Mortality is defined as the total number of deaths in a specific population during a specific period, while DALYs represent the sum of years of life lost due to premature death and years lived with disability [23]. The study did not include region-specific details within China. Since GBD utilizes de-identified public data, the Institutional Review Board of the University of Washington granted an exemption from informed consent requirements.
Overall CVD encompass rheumatic heart disease, IHD, stroke, hypertensive heart disease, non-rheumatic valvular heart disease, cardiomyopathy, myocarditis, atrial fibrillation and flutter, aortic aneurysm, LEPAD, endocarditis, and other cardiovascular and circulatory system diseases. In GBD 2021, the KD-induced CVD subtypes include IHD, stroke, and LEPAD. For further details, refer to the International Classification of Diseases, 10th Edition (ICD-10) codes in Supplementary Table 1. According to the GBD 2021 Risk Factors Study, KD is classified into four categories based on the albumin-to-creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR): (1) Stage 1 and 2 chronic kidney disease (CKD), with ACR > 30 mg/g and eGFR ≥ 60 ml/min/1.73 m²; (2) Stage 3 CKD, with eGFR between 30 and 59 ml/min/1.73 m²; (3) Stage 4 CKD, with eGFR between 15 and 29 ml/min/1.73 m²; and (4) Stage 5 CKD, with eGFR < 15 ml/min/1.73 m². None of these categories include individuals who have undergone renal replacement therapy [22].
Statistical analysis
We estimated ASMR and ASDR per 100,000 people for the total population, as well as for males and females. These rates were standardized according to the World Health Organization standard population to account for differences in age distribution over time. The calculation formula is as follows: ASR = 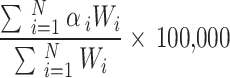 , where
, where 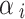 represents the age-specific rate for the
represents the age-specific rate for the 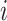 th age group,
th age group, 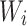 is the population of the corresponding
is the population of the corresponding 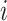 th age group in the standard population, and N is the number of age groups. To examine time trends in ASMR and ASDR from 1992 to 2021, we used log-linear regression to calculate the EAPC and its 95% confidence intervals (CI). The formula is:
th age group in the standard population, and N is the number of age groups. To examine time trends in ASMR and ASDR from 1992 to 2021, we used log-linear regression to calculate the EAPC and its 95% confidence intervals (CI). The formula is: 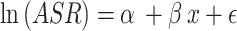 , where
, where 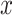 represents the year,
represents the year, 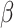 is the regression coefficient,
is the regression coefficient, 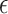 is the error term, and
is the error term, and 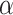 is the intercept. The formula for calculating the 95% CI is:
is the intercept. The formula for calculating the 95% CI is: 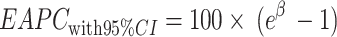 . If the lower bound of the 95% CI for the EAPC is positive, the ASR is considered to be increasing; conversely, if the upper bound of the 95% CI is negative, the ASR is considered to be decreasing [24, 25].
. If the lower bound of the 95% CI for the EAPC is positive, the ASR is considered to be increasing; conversely, if the upper bound of the 95% CI is negative, the ASR is considered to be decreasing [24, 25].
The population was divided into 5-year age groups: 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, and 65–69 years. We calculated the proportion of deaths for each age group across specific periods, serving as an indirect indicator of survival and allowing for an analysis of age distribution in mortality.
APC model analysis
We employed an APC model to analyze mortality trends related to KD-induced CVD and subtypes among individuals aged 25–64 in China. This model allows for the separate evaluation of the effects of age, period, and birth cohort on the burden of disease. Mortality rates of CVD and subtypes were treated as dependent variables, while age, period, and birth cohort were treated as independent variables. In the APC model, age effects capture the influence of different age groups on mortality rates; period effects reveal how societal changes or significant events between 1992 and 2021 impacted mortality rates; and cohort effects examine the long-term impact of birth cohorts on mortality trends.
The APC model requires equal intervals for both age groups and periods, meaning 5-year age groups correspond to 5-year calendar periods, which explains why this study starts from 1992. Given that the GBD 2021 age groups are divided into 5-year intervals, we used six 5-year periods to represent different time frames ([1994] 1992–1996, [1999] 1997–2001, … [2019] 2017–2021). Cohorts were calculated by subtracting participants’ ages from their respective years, resulting in 13 partially overlapping 10-year birth cohorts ([1932] 1928–1936, [1999] 1997–2001, … [1992] 1988–1996). Because birth cohorts are determined by the intervals of age groups and periods, their intervals are inherently consistent with those of the other dimensions and do not require separate equal interval specifications.
The main results of the APC model are as follows. Net drift shows the overall log-linear trend by calendar period and birth cohort, representing the annual percentage change in mortality. Local drift reflects the log-linear trends for different age groups by calendar period and birth cohort, indicating the annual percentage change for each group. The longitudinal age curve presents the fitted age-specific mortality rates in the reference cohort while accounting for period deviations. The period or cohort rate ratio represents the relative risk of a specific cohort or period compared to the reference cohort or period, after adjusting for age and nonlinear effects. A rate ratio greater than 1 indicates an increased risk of death, while a rate ratio less than 1 suggests a decreased risk [26].
In this study, the disease burden from 2002 to 2006 and the 1962 birth cohort were used as reference groups to estimate the relative risks of age, period, and cohort effects. All analyses and graphical representations were performed using R statistical software version 4.3.0.
Results
ASMR and ASDR trends on KD-induced CVD and subtypes among 25–64 years globally and in China
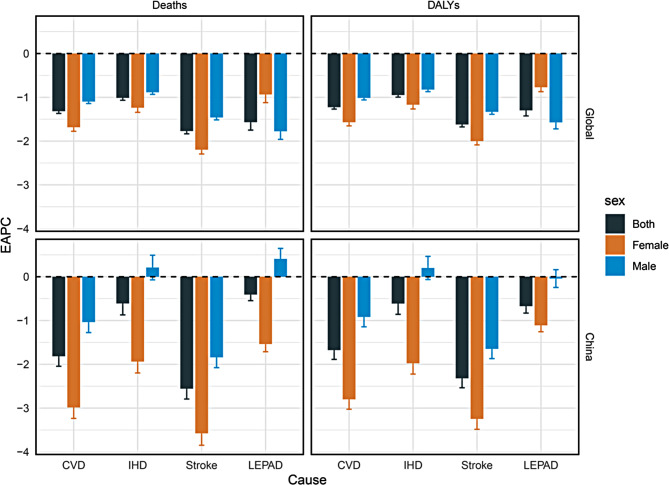
From 1992 to 2021, the global number of deaths and DALYs due to KD-induced CVD and subtypes increased across both males and females among individuals aged 25 to 64. However, the ASMR and ASDR showed declining trends in both sexes (EAPCs < 0). The global ASMR for KD-induced CVD per 100,000 population decreased from 14.66 in 1992 to 10.37 in 2021 (EAPC, -1.32 [95% CI, -1.37 to -1.27]), with IHD decreasing from 8.37 to 6.45 (EAPC, -1.01 [95% CI, -1.07 to -0.96]), stroke decreasing from 6.24 to 3.87 (EAPC, -1.77 [95% CI, -1.83 to -1.71]), and LEPAD decreasing from 0.06 to 0.04 (EAPC, -1.57 [95% CI, -1.75 to -1.39]) (Table 1; Fig. 1). Between 1992 and 2021, the global ASMR and ASDR of KD-induced IHD and stroke continued to decline, while LEPAD stabilized after slight fluctuations (Figure S1). Moreover, the EAPCs for IHD and stroke were higher in men than in women, whereas the EAPC for LEPAD was lower in men compared to women (Table 1; Fig. 1).
Table 1.
The disease burden of CVD and subtypes caused by kidney dysfunction among 25–64 years in globally and China in 1992 and 2021
| Deaths (95% UI) | DALYs (95% UI) | ASMR (95% UI, per 105 population) | ASDR (95% UI, per 105 population) | EAPC (95% Cl) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1992 | 2021 | 1992 | 2021 | 1992 | 2021 | 1992 | 2021 | ASMR | ASDR | |||
| Global | CVD | Both |
309,007 (245954 to 388446) |
421,366 (326601 to 543732) |
11,542,853 (9170832 to 14534095) |
15,889,762 (12313170 to 20472002) |
14.66 (11.68 to 18.41) |
10.37 (8.03 to 13.39) |
542.21 (431.50 to 681.78) |
392.70 (304.04 to 506.26) |
-1.32 (-1.37 to -1.27) |
-1.23 (-1.27 to -1.18) |
| Female |
114,135 (90910 to 142901) |
143,476 (111157 to 184298) |
4,294,947 (3410445 to 5385860) |
5,468,810 (4233187 to 6994877) |
10.83 (8.63 to 13.54) |
6.96 (5.39 to 8.95) |
404.25 (321.44 to 506.14) |
267.32 (206.69 to 342.19) |
-1.68 (-1.78 to -1.59) |
-1.57 (-1.65 to -1.49) |
||
| Male |
194,871 (153217 to 246965) |
277,890 (213242 to 361655) |
7,247,906 (5700619 to 9224985) |
10,420,952 (8010470 to 13581368) |
18.48 (14.55 to 23.4) |
13.85 (10.62 to 18.03) |
679.15 (535.12 to 862.98) |
520.28 (399.65 to 678.25) |
-1.10 (-1.14 to -1.05) |
-1.01 (-1.06 to -0.97) |
||
| IHD | Both |
176,973 (126311 to 237219) |
261,842 (184076 to 356830) |
6,519,033 (4641976 to 8782893) |
9,657,652 (6768890 to 13220680) |
8.37 (5.98 to 11.21) |
6.45 (4.54 to 8.80) |
304.83 (217.26 to 410.29) |
239.22 (167.59 to 327.55) |
-1.01 (-1.07 to -0.96) |
-0.95 (-1.00 to -0.90) |
|
| Female |
57,256 (40760 to 76430) |
80,688 (55888 to 109415) |
2,091,624 (1483865 to 2804748) |
2,945,453 (2028395 to 4016679) |
5.41 (3.85 to 7.21) |
3.93 (2.72 to 5.33) |
195.85 (139.12 to 262.21) |
144.41 (99.39 to 197.05) |
-1.24 (-1.34 to -1.13) |
-1.17 (-1.27 to -1.07) |
||
| Male |
119,717 (85425 to 162061) |
181,154 (126854 to 248553) |
4,427,409 (3153265 to 6021082) |
6,712,199 (4687964 to 9235448) |
11.31 (8.08 to 15.3) |
9.04 (6.33 to 12.4) |
412.80 (294.29 to 560.76) |
335.52 (234.24 to 461.68) |
-0.88 (-0.93 to -0.84) |
-0.82 (-0.87 to -0.77) |
||
| Stroke | Both |
130,856 (99771 to 169308) |
157,849 (118238 to 204533) |
4,973,381 (3763795 to 6430175) |
6,156,706 (4571846 to 8010140) |
6.24 (4.76 to 8.06) |
3.87 (2.90 to 5.02) |
234.96 (178.16 to 303.30) |
151.64 (112.51 to 197.49) |
-1.77 (-1.83 to -1.71) |
-1.62 (-1.68 to -1.56) |
|
| Female |
56,578 (43193 to 72304) |
62,269 (46203 to 81259) |
2,185,833 (1658828 to 2803309) |
2,493,354 (1834850 to 3256502) |
5.39 (4.12 to 6.88) |
3.01 (2.23 to 3.93) |
206.72 (157.21 to 264.71) |
121.46 (89.28 to 158.83) |
-2.20 (-2.29 to -2.10) |
-2.00 (-2.09 to -1.92) |
||
| Male |
74,278 (56056 to 96937) |
95,581 (71296 to 124515) |
2,787,548 (2092053 to 3636259) |
3,663,352 (2720661 to 4781964) |
7.09 (5.36 to 9.24) |
4.76 (3.55 to 6.20) |
263.16 (197.91 to 342.72) |
182.49 (135.48 to 238.39) |
-1.46 (-1.51 to -1.41) |
-1.33 (-1.39 to -1.28) |
||
| LEPAD | Both |
1177 (788 to 1620) |
1674 (1135 to 2300) |
50,438 (32221 to 70871) |
75,405 (48182 to 109780) |
0.06 (0.04 to 0.08) |
0.04 (0.03 to 0.06) |
2.43 (1.55 to 3.41) |
1.84 (1.18 to 2.69) |
-1.57 (-1.75 to -1.39) |
-1.30 (-1.43 to -1.17) |
|
| Female |
301 (206 to 411) |
520 (345 to 719) |
17,490 (10816 to 27085) |
30,003 (18479 to 46525) |
0.03 (0.02 to 0.04) |
0.02 (0.02 to 0.03) |
1.67 (1.04 to 2.59) |
1.44 (0.89 to 2.23) |
-0.94 (-1.12 to -0.75) |
-0.77 (-0.87 to -0.67) |
||
| Male |
876 (582 to 1213) |
1155 (782 to 1609) |
32,948 (21235 to 45670) |
45,402 (29392 to 64092) |
0.08 (0.06 to 0.12) |
0.06 (0.04 to 0.08) |
3.19 (2.05 to 4.42) |
2.26 (1.46 to 3.19) |
-1.78 (-1.96 to -1.59) |
-1.57 (-1.72 to -1.42) |
||
| China | CVD | Both |
54,551 (41704 to 71173) |
60,454 (43532 to 82810) |
2,063,479 (1576334 to 2682673) |
2,319,803 (1688845 to 3158839) |
11.28 (8.63 to 14.7) |
6.14 (4.41 to 8.43) |
420.07 (321.25 to 545.38) |
239.19 (173.85 to 326.59) |
-1.82 (-2.04 to -1.59) |
-1.68 (-1.89 to -1.46) |
| Female |
23,873 (17662 to 32216) |
20,576 (14249 to 29551) |
904,995 (671721 to 1212350) |
803,700 (565144 to 1138842) |
10.36 (7.67 to 13.96) |
4.16 (2.88 to 5.99) |
386.97 (287.68 to 517.87) |
164.91 (115.79 to 233.95) |
-2.98 (-3.24 to -2.73) |
-2.80 (-3.03 to -2.57) |
||
| Male |
30,678 (22821 to 41314) |
39,879 (27541 to 57215) |
1,158,483 (862416 to 1555132) |
1,516,103 (1055688 to 2162210) |
12.12 (9.02 to 16.31) |
8.07 (5.57 to 11.6) |
450.36 (335.51 to 603.75) |
311.55 (216.67 to 445.48) |
-1.04 (-1.28 to -0.80) |
-0.92 (-1.14 to -0.69) |
||
| IHD | Both |
18,186 (12425 to 25798) |
26,133 (16061 to 39462) |
695,470 (474300 to 987319) |
968,125 (596430 to 1460928) |
3.70 (2.53 to 5.24) |
2.69 (1.66 to 4.07) |
138.53 (94.57 to 196.46) |
101.85 (62.79 to 153.83) |
-0.61 (-0.87 to -0.35) |
-0.61 (-0.86 to -0.37) |
|
| Female |
7755 (5167 to 11113) |
8224 (4824 to 12602) |
290,783 (194495 to 416797) |
297,271 (174337 to 456884) |
3.31 (2.21 to 4.74) |
1.69 (0.99 to 2.59) |
121.97 (81.65 to 174.34) |
62.04 (36.31 to 95.46) |
-1.94 (-2.20 to -1.68) |
-1.98 (-2.22 to -1.73) |
||
| Male |
10,431 (6919 to 15359) |
17,908 (10714 to 28158) |
404,687 (267838 to 593083) |
670,854 (402809 to 1047843) |
4.05 (2.69 to 5.96) |
3.67 (2.20 to 5.78) |
153.67 (101.79 to 225.35) |
140.49 (84.51 to 219.46) |
0.21 (-0.07 to 0.49) |
0.20 (-0.06 to 0.47) |
||
| Stroke | Both |
36,335 (26451 to 49299) |
34,268 (23988 to 47804) |
1,364,083 (994085 to 1844872) |
1,345,141 (946559 to 1854558) |
7.58 (5.52 to 10.27) |
3.44 (2.41 to 4.81) |
280.71 (204.97 to 379.06) |
136.7 (96.00 to 189.22) |
-2.56 (-2.79 to -2.32) |
-2.32 (-2.54 to -2.10) |
|
| Female |
16,105 (11227 to 22870) |
12,331 (8312 to 17817) |
611,661 (431020 to 859417) |
502,375 (344031 to 712207) |
7.04 (4.91 to 9.98) |
2.47 (1.66 to 3.57) |
263.88 (186.33 to 370.46) |
102.05 (69.72 to 144.99) |
-3.58 (-3.85 to -3.30) |
-3.25 (-3.48 to -3.01) |
||
| Male |
20,230 (14338 to 28296) |
21,937 (14725 to 31598) |
752,422 (533837 to 1050840) |
842,766 (570014 to 1201798) |
8.07 (5.73 to 11.28) |
4.39 (2.95 to 6.33) |
296.14 (210.47 to 413.26) |
170.56 (115.23 to 244.04) |
-1.84 (-2.08 to -1.60) |
-1.65 (-1.87 to -1.43) |
||
| LEPAD | Both |
30 (19 to 46) |
53 (32 to 83) |
3926 (2040 to 7374) |
6537 (3197 to 12046) |
0.01 (0 to 0.01) |
0.01 (0 to 0.01) |
0.83 (0.43 to 1.56) |
0.65 (0.32 to 1.20) |
-0.41 (-0.55 to -0.27) |
-0.67 (-0.83 to -0.51) |
|
| Female |
13 (8 to 21) |
20 (11 to 32) |
2552 (1246 to 5000) |
4054 (1780 to 7797) |
0.01 (0 to 0.01) |
0 (0 to 0.01) |
1.13 (0.55 to 2.21) |
0.81 (0.36 to 1.56) |
-1.54 (-1.71 to -1.36) |
-1.11 (-1.26 to -0.96) |
||
| Male |
17 (10 to 27) |
34 (19 to 56) |
1374 (756 to 2400) |
2483 (1307 to 4367) |
0.01 (0 to 0.01) |
0.01 (0 to 0.01) |
0.56 (0.31 to 0.97) |
0.49 (0.26 to 0.87) |
0.41 (0.17 to 0.65) |
-0.04 (-0.24 to 0.16) |
||
ASMR, age-standardized mortality rates; ASDR, age-standardized disability-adjusted life years rates; EAPC, estimated annual percentage change; CVD, cardiovascular diseases; IHD, ischemic heart disease; LEPAD, lower extremity peripheral arterial disease; CI, confidence intervals; UI, uncertainty intervals
Fig. 1.
EAPC in age-standardized mortality rates and age-standardized disability-adjusted life-year rates for CVD and subtypes induced by kidney dysfunction among 25–64 years globally and in China, 1992–2021. Error bars represent the 95% confidence intervals for the EAPC. EAPC, estimated annual percentage change; CVD, cardiovascular diseases; IHD, ischemic heart disease; LEPAD, lower extremity peripheral arterial disease
In China, from 1992 to 2021, the number of deaths and DALYs due to KD-induced IHD and LEPAD increased among individuals aged 25 to 64, while stroke-related deaths and DALYs decreased. Notably, the number of deaths and DALYs for stroke declined in females. The ASMR and ASDR showed downward trends (EAPCs < 0). The ASMR for KD-induced CVD decreased from 11.28 in 1992 to 6.14 in 2021 per 100,000 population (EAPC, -1.82 [95% CI, -2.04 to -1.59]), with IHD decreasing from 3.70 to 2.69 (EAPC, -0.61 [95% CI, -0.87 to -0.35]), stroke decreasing from 7.58 to 3.44 (EAPC, -2.56 [95% CI, -2.79 to -2.32]), while LEPAD remained at a low level of 0.01 (EAPC, -0.41 [95% CI, -0.55 to -0.27]) (Table 1; Fig. 1). However, between 2000 and 2004, there were brief increases in ASMR and ASDR (Figure S2). Notably, the ASMR and ASDR for male IHD, as well as the ASMR for male LEPAD showed upward trends (EAPCs > 0). Overall, the EAPCs for women in China were lower than those for men (Table 1; Fig. 1).
Globally and in China, the most significant decrease was observed in the burden of stroke. In 1992, the ASMR and ASDR for stroke in China were higher than the global level, but by 2021, these rates had fallen below the global level. Additionally, by 2021, the ASMR and ASDR for CVD and subtypes in China were lower than the global level. The decline in ASMR and ASDR for stroke in China outpaced the global level, whereas the decline for IHD and LEPAD was slower than the global trend (Table 1; Fig. 1).
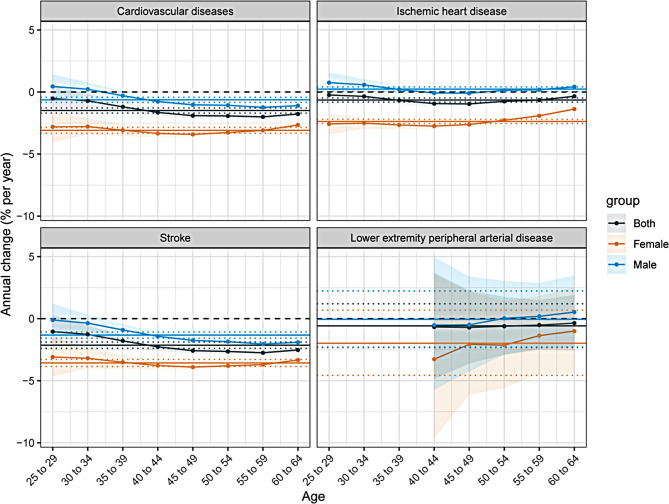
Time trends in different age groups among 25–64 years in China
Figure 2 presents the net and local drift calculated using the APC model. The horizontal solid line represents the net drift, while the curved solid line denotes the local drift, with dashed lines representing the 95% CI for each group. In China, the net drift for CVD is negative, indicating a decline in ASMR. Among men, the local drift for CVD in the 25–29 and 30–34 age groups shows a positive annual percentage change in mortality, indicating increased mortality rates in these groups. With increasing age, the annual percentage change gradually declines and stabilizes, suggesting that younger men are experiencing higher CVD mortality. Among women, two intersections between the net and local drift curves for CVD suggest improved survival in the 40–44 and 45–49 age groups. The net and local drift curves for stroke follow a similar pattern to those of CVD. For IHD, the net drift is positive in men and negative in women, indicating a rise in ASMR for men and a decline for women from 1992 to 2021. For LEPAD, the net drift is negative in both sexes, but the local drift curve rises with age, suggesting that LEPAD mortality is becoming more concentrated in older age groups.
Fig. 2.
The net drifts and local drifts for cardiovascular diseases and subtypes mortality induced by kidney dysfunction in China, 1992–2021. Lower extremity peripheral arterial disease is reported starting from individuals aged 40 years and older
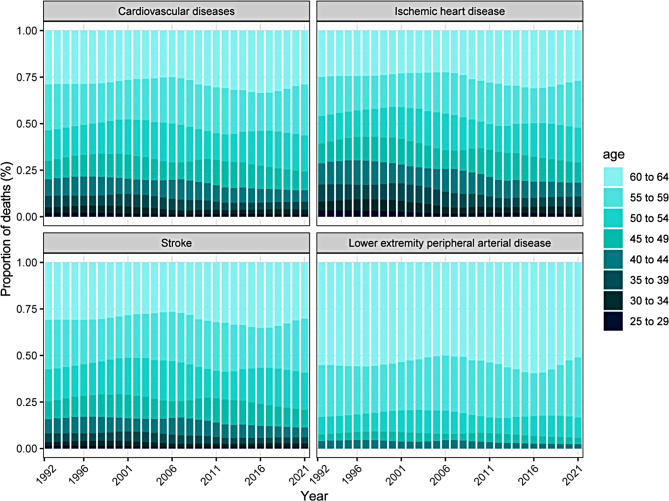
Figure 3 depicts temporal trends in the age distribution of CVD and subtypes among 25–64 years in China. Deaths from KD-induced CVD are increasingly shifting from individuals younger than 50 to those aged 50 and older, reflecting a growing burden of CVD mortality in older populations. The age distribution shift for IHD follows a similar pattern. Deaths from KD-induced stroke remain stable across age groups. The age distribution of LEPAD mortality indicates that individuals aged 55–59 and 60–64 account for a larger proportion. This reflects that LEPAD primarily affects older individuals.
Fig. 3.
The age distribution of deaths from cardiovascular diseases and subtypes induced by kidney dysfunction among 25–64 years in China, 1992–2021. The age groups include 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, and 60–64 years. Lower extremity peripheral arterial disease is reported starting from individuals aged 40 years and older
Age, period, and cohort effects on KD-induced CVD and subtypes among 25–64 years in China
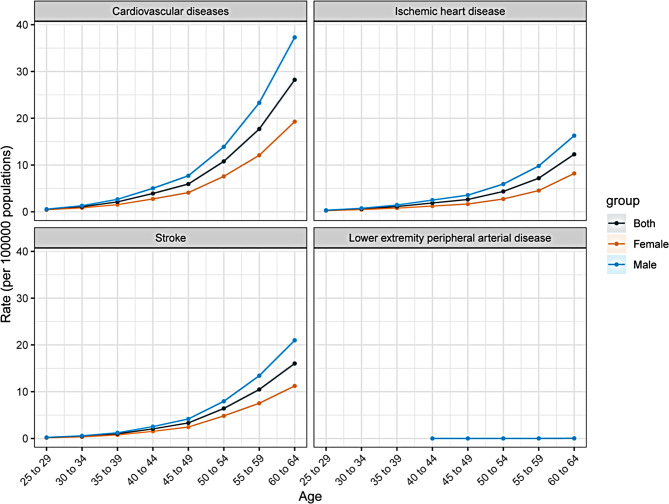
Figure 4 illustrates the longitudinal age curves for KD-induced CVD and subtypes among 25–64 years in China, representing age-specific mortality rates. A similar age-related risk pattern is observed for IHD and stroke, with the highest risk occurring in the 60–64 age group. Mortality rates increase steadily with age, and men exhibit consistently higher mortality rates than women. Due to limited data for LEPAD, a comprehensive picture of its age-related mortality remains unavailable.
Fig. 4.
Longitudinal age curve of mortality rates for cardiovascular diseases and subtypes induced by kidney dysfunction among 25–64 years in China. Lower extremity peripheral arterial disease is reported starting from individuals aged 40 years and older
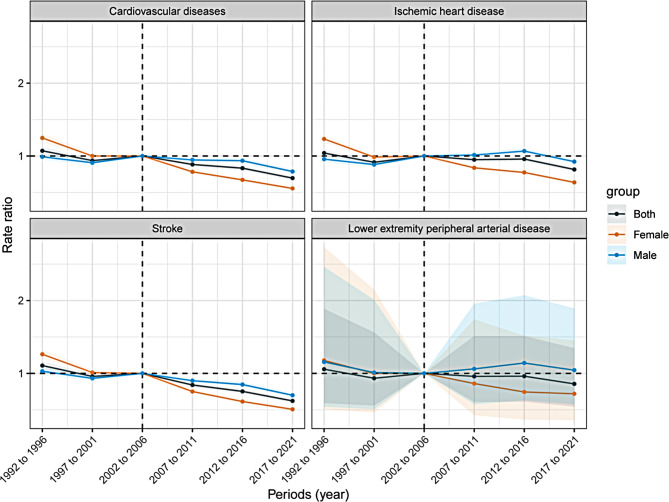
Figure 5 presents the period effects for KD-induced CVD and subtypes in China among 25–64 years, shown as the relative mortality risk across different time periods. From 1992 to 2021, the mortality risk for CVD and subtypes has declined in both sexes, though the decrease is modest, with the most pronounced reduction observed in stroke.
Fig. 5.
Period rate ratio of cardiovascular diseases and subtypes mortality induced by kidney dysfunction among 25–64 years in China
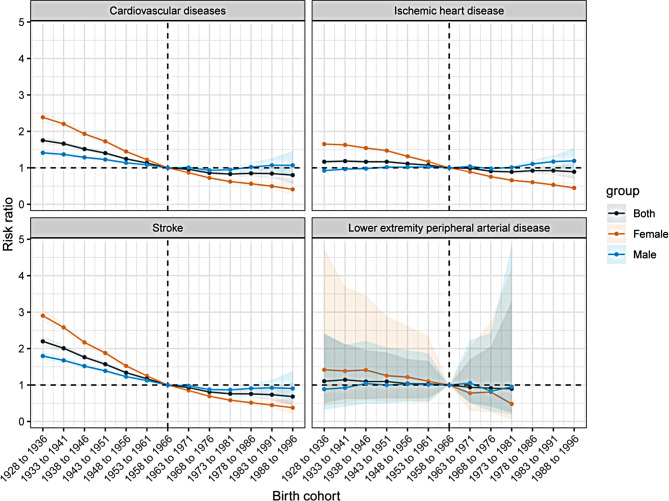
Figure 6 depicts the birth cohort effects for KD-induced CVD and subtypes in China, reflecting relative mortality risk across different birth cohorts. The trend of stroke has a steady decline in mortality risk from the 1900 to the 1990 birth cohorts. Notably, there is a slight upward trend in CVD mortality risk for men born between 1972 and 1981, indicating an increased risk in this cohort. For IHD, the mortality risk for men increases with each successive birth cohort. While the overall mortality risk for LEPAD shows a downward trend, a noticeable increase in risk is observed for the 1967–1976 birth cohort.
Fig. 6.
Cohort rate ratio of cardiovascular diseases and subtypes mortality induced by kidney dysfunction among 25–64 years in China
Additionally, women exhibit higher relative risks in the early period and birth cohort effects, but their rates of decline are more pronounced compared to men.
Discussion
To our knowledge, this is the first study to apply the APC model to analyze deaths and DALYs due to KD-induced CVD and subtypes among 25–64 years in China. Based on GBD 2021, from 1992 to 2021, the number of deaths and DALYs attributable to KD-induced CVD increases globally and in China among individuals aged 25–64. However, the ASMR and ASDR for CVD and subtypes show a declining trend. The burden of KD-induced CVD in China is lower than the global level, and the ASR of decline is significantly greater than the global trend, with the most notable reduction observed in stroke. In China and globally, males exhibit higher ASMR and ASDR than females, and the decline in IHD and stroke is less pronounced in males compared to females. Globally, the decline in LEPAD is more pronounced in males than in females. In contrast, the trends in CVD and subtypes in China diverge from the global pattern. While the ASMR and ASDR for CVD and subtypes have decreased in both sexes globally, the ASMR for IHD and LEPAD in males has shown an upward trend in China. As age increases, mortality rates for CVD and subtypes also rise, indicating an aging trend in overall mortality. However, it is notable that stroke mortality is increasingly affecting younger populations. Despite the overall decline in ASMR and ASDR over the past 30 years, the burden of IHD and LEPAD in Chinese males has been rising. To effectively mitigate this burden, China urgently needs to develop more comprehensive CVD prevention policies and improve access to healthcare services, particularly by increasing awareness and treatment of KD and its cardiovascular complications among the working-age population.
Our study reveals that the number of deaths and DALYs from KD-induced CVD continues to rise among the 25–64 age group in China. One contributing factor is the difficulty in detecting KD in its early stages, with many patients remaining unaware of their condition [27]. Additionally, KD diagnosis relies on biochemical tests, such as eGFR and urinary albumin excretion [28]. Compared to the simpler process of blood pressure measurement for hypertension, the complexity of diagnosing KD leads to diagnostic delays, accelerating kidney failure and increasing the incidence of CVD. Additionally, disparities in economic development, health education, and medical resources across regions in China contribute to poor treatment adherence and delayed interventions. Concerns about adverse drug reactions and the financial burden of treatment also contribute to insufficient management of KD, ultimately resulting in more death cases. To address this issue, regular kidney function screenings and routine treatments for the 25–64 age group could help mitigate the burden of KD-induced CVD, especially through targeted interventions for early-stage and high-risk populations. Among metabolic risk factors for CVD, high systolic blood pressure is the leading cause of CVD-related deaths and DALYs both globally and in China, while KD ranks fifth, accounting for approximately 7% of the CVD burden [29, 30]. Given the significant burden of CVD, the impact of KD on CVD cannot be overlooked. Previous studies have found that IHD and stroke are the leading causes of death in patients with KD [18]. Our findings show that the ASMR and ASDR for KD-induced CVD and subtypes in the 25–64 age group in China are lower than the global level and are on a declining trend. Notably, the reduction in CVD rates in China exceeds the global rate, likely due to advances in healthcare, particularly in kidney transplantation, peritoneal dialysis, and hemodialysis, which have yielded favorable outcomes in treating KD. Moreover, the Chinese government has introduced guidelines promoting healthy lifestyles for the prevention of metabolic CVD and included public health initiatives in the 14th Five-Year Plan, such as the Healthy China 2030 strategy and the Healthy China Action Plan (2019–2030) [31]. However, the burden of IHD and LEPAD in Chinese males has been increasing, consistent with previous research findings [7, 32]. This may be linked to the higher prevalence of metabolic syndrome in men, which includes conditions such as hypertension, hyperglycemia, and obesity, all significant risk factors for IHD and LEPAD. Additionally, the higher smoking rate among Chinese men further elevates the risk of CVD [33]. Therefore, early prevention and management of KD in men should be prioritized to reduce the burden of these related diseases.
Our findings suggest that the mortality burden from stroke is shifting toward younger populations, whereas LEPAD mortality remains concentrated in older individuals. This may indicate that current preventive measures for KD-induced stroke are more effective in older populations, while prevention efforts targeting younger age groups remain inadequate. The higher prevalence of LEPAD in older adults can be attributed to multiple factors, including the cumulative effects of atherosclerosis, vascular aging, increased oxidative stress and inflammation, declining metabolic function, and lifestyle factors. This also explains the reason for the low mortality rate of LEPAD among 25–64 years. Age is widely recognized as an independent risk factor for CVD mortality, and our results show a significant increase in KD-induced CVD mortality with advancing age [34, 35]. Aging contributes significantly to the burden of KD-induced CVD, partly because structural and functional changes in the kidneys increase susceptibility to acute kidney failure and chronic KD in older adults [36, 37]. Additionally, behavioral and metabolic changes associated with aging, such as reduced physical activity and inadequate nutrition, further exacerbate this risk [38]. These factors likely explain the observed increase in mortality rates for IHD and stroke with age in our study.
The APC analysis of sex differences reveals that males exhibit a higher mortality rate from CVD than females across all age groups. Cohort and period analyses show a general downward trend in both cohort and period relative risk, with females having higher relative risks in the early cohorts and periods. However, the decline is significantly greater in females than in males. Additionally, the risk of IHD has increased in males across birth cohorts. Overall, the burden of KD-induced CVD and subtypes is lower in females than in males within the 25–64 age group in China. This sex difference can be partially attributed to biological factors, such as the protective role of estrogen. Sex hormones and their receptors play a critical role in kidney function, with estrogen exerting protective effects and testosterone potentially impairing renal function [39]. Differences in vascular hemodynamics and other pathophysiological factors may also contribute to the observed sex differences [40–42]. Furthermore, behavioral risk factors are key contributors to this disparity; males generally have higher smoking rates and stress levels, whereas females tend to lead healthier lifestyles [43–45]. These sex differences may become more pronounced in the future. Despite recent advances in research on sex differences, we strongly recommend further exploration of the underlying mechanisms of KD-induced CVD sex differences. This will help identify sex-specific interventions in clinical practice, optimizing the reduction of health burdens. Developing CVD guidelines tailored to specific age and sex groups is critical for timely identification and management of CVD, ensuring the implementation of effective, individualized interventions that integrate both primary and secondary prevention strategies.
Hypertension, diabetes, obesity, dyslipidemia, and KD are not only metabolic risk factors for CVD, but also interact to collectively promote its development [46, 47]. Effective management of these metabolic diseases is therefore essential for reducing the global burden of CVD. To further alleviate the burden of KD-induced CVD, particularly IHD and LEPAD in the 25–64 age group, China should implement additional preventive measures. This includes promoting the use of diagnostic tests, such as serum creatinine and urinary protein, to ensure early screening and diagnosis in high-risk populations. Regular monitoring of blood pressure, blood glucose, and lipid levels is also critical to lowering the risk of CVD. Public health initiatives play a pivotal role in curbing the rising incidence of KD. Personal health education, routine kidney function screening, early kidney-protective treatments, and proper management of underlying diseases that impair kidney function can significantly reduce the burden of KD-induced CVD. In addition to dialysis and other renal replacement therapies, the accessibility of laboratory diagnostic services, healthcare providers’ awareness of KD treatment, patient counseling, and public education on the risks of KD remain insufficient. Policymakers should therefore place greater emphasis on KD and urgently introduce enhanced health education and support policies to mitigate its significant impact.
The strengths of this study include its use of the most recent GBD 2021 dataset and the application of an advanced APC model, marking the first analysis of mortality and DALYs related to KD-induced CVD and subtypes among the working-age population in China. However, there are several limitations. First, since GBD 2021 data are based on estimates rather than direct observations, this could introduce bias. Second, the data on LEPAD for 25–64 years in China are insufficient in this study, which may lead to less accurate results. Third, the analysis is limited to national-level data, lacking insights into regional and urban-rural disparities. Future research should incorporate more stratified data for a comprehensive analysis. Fourth, the APC model requires equal intervals between age groups and time periods, which may obscure subtle variations in age, period, and cohort effects. Lastly, due to limited data on LEPAD in GBD 2021, the results related to LEPAD may be less stable, and further studies with more robust data are needed to validate these findings.
Conclusion
This study provides valuable insights into the changing burden of KD-induced CVD and subtypes among the working-age population in China from 1992 to 2021. Significant progress has been made in reducing the burden of KD-induced CVD in China. Although the ASMR and ASDR for CVD, IHD, and LEPAD have declined, the absolute number of deaths and DALYs continues to rise, reflecting ongoing health challenges. Older populations remain the primary group affected by KD. Developing targeted strategies to address the unique risks faced by males, particularly in relation to IHD, is crucial to alleviating the burden of these diseases. Therefore, to mitigate the burden of KD-induced CVD and subtypes in the working-age population, public health authorities in China must advance guidelines targeting specific subtypes, genders, and age groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely thank the GBD team for allowing us to access their comprehensive database.
Author contributions
GZ: completed the initial manuscript and collected the data; HW: reviewed and revised the manuscript; PC and JZ: reviewed the manuscript and provided modification suggestions; LL: conceived and designed the study; HG: provided funding, conceived and designed the study. All authors worked together to produce the final manuscript.
Funding
The study was financially supported by the National Natural Science Foundation of China (Grant Nos. 52203166) and Talent Reserve Program (TRP), The First Hospital of Jilin University (No. JDYYCB-2023004).
Data availability
The datasets generated and/or analyzed during the current study are available in the GBD 2021. Publicly available datasets were analyzed in the current study. The data can be found here: http://ghdx.healthdata.org/gbd-results-tool. The analyzed data will be shared upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lingyun Liu, Email: tmxlly@jlu.edu.cn.
Hui Guo, Email: guohui315@jlu.edu.cn.
References
- 1.Mensah GA, Roth GA, Fuster V. The Global Burden of Cardiovascular diseases and Risk factors: 2020 and Beyond. J Am Coll Cardiol. 2019;74:2529–32. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Lond Engl. 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez AD, Adair T. Is the long-term decline in cardiovascular-disease mortality in high-income countries over? Evidence from national vital statistics. Int J Epidemiol. 2019;48:1815–23. [DOI] [PubMed] [Google Scholar]
- 4.Zhou M, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet Lond Engl. 2019;394:1145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, et al. Modifiable risk factors associated with cardiovascular disease and mortality in China: a PURE substudy. Eur Heart J. 2022;43:2852–63. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Colantonio LD. Cardiovascular Disease Risk Estimation in China. Ann Intern Med. 2019;170:340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, et al. [Direct economic burden of cerebrovascular disease, during 1993–2008 in China]. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. 2014;35:1263–6. [PubMed] [Google Scholar]
- 9.Trends. and disparities in China’s cardiovascular disease burden from 1990 to 2019 - PubMed. https://pubmed.ncbi.nlm.nih.gov/37596135/ [DOI] [PubMed]
- 10.Roth GA, et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet Lond Engl. 2020;395:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell-Wiley TM, et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;143:e984–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadi N, et al. The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2018;29:1609–16. [DOI] [PubMed] [Google Scholar]
- 14.Covic A, et al. Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol. 2018;6:319–31. [DOI] [PubMed] [Google Scholar]
- 15.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet Lond Engl. 2017;389:1238–52. [DOI] [PubMed] [Google Scholar]
- 16.Provenzano M, et al. Epidemiology of cardiovascular risk in chronic kidney disease patients: the real silent killer. Rev Cardiovasc Med. 2019;20:209–20. [DOI] [PubMed] [Google Scholar]
- 17.Sarnak MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108(17):2154–69. 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed]
- 18.Zhang S, et al. Global, regional, and national burden of kidney dysfunction from 1990 to 2019: a systematic analysis from the global burden of disease study 2019. BMC Public Health. 2023;23:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impaired Renal Function and Major Cardiovascular Events. in Young Adults - PubMed. https://pubmed.ncbi.nlm.nih.gov/37730288/ [DOI] [PubMed]
- 20.Arnett DK, ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. 2019: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 74, 1376–1414 (2019). [DOI] [PMC free article] [PubMed]
- 21.Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global burden and strength of evidence for. 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of Disease Study 2021 - PubMed. https://pubmed.ncbi.nlm.nih.gov/38762324/ [DOI] [PMC free article] [PubMed]
- 23.GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the global burden of Disease Study 2021. Lancet Lond Engl. 2024;403:2100–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin X, et al. The global, regional, and national disease burden of breast cancer attributable to low physical activity from 1990 to 2019: an analysis of the global burden of Disease Study 2019. Int J Behav Nutr Phys Act. 2022;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, Liu K, Fang S. Changing patterns of stroke and subtypes attributable to high systolic blood pressure in China from 1990 to 2019. Stroke. 2024;55:59–68. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Land KC. Age-period-cohort analysis: New models, methods, and empirical applications. New York: Chapman and Hall/CRC; 2016. 10.1201/b13902. [Google Scholar]
- 27.Chronic kidney disease. and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study - PubMed. https://pubmed.ncbi.nlm.nih.gov/27102194/ [DOI] [PubMed]
- 28.Executive summary of the KDIGO. 2021 clinical practice Guideline for the management of blood pressure in chronic kidney Disease - PubMed. https://pubmed.ncbi.nlm.nih.gov/33637203/ [DOI] [PubMed]
- 29.The burden of. cardiovascular diseases attributable to metabolic risk factors and its change from 1990 to 2019: a systematic analysis and prediction - PubMed. https://pubmed.ncbi.nlm.nih.gov/38455920/ [DOI] [PMC free article] [PubMed]
- 30.Hypertension and cardiovascular risk. General aspects - PubMed. https://pubmed.ncbi.nlm.nih.gov/29127059/ [DOI] [PubMed]
- 31.Tan X, Liu X, Shao H. Healthy China 2030: a vision for Health Care. Value Health Reg Issues. 2017;12:112–4. [DOI] [PubMed] [Google Scholar]
- 32.Mortality trend analysis of ischemic heart disease in. China between 2010 and 2019: a joinpoint analysis - PubMed. https://pubmed.ncbi.nlm.nih.gov/37016366/ [DOI] [PMC free article] [PubMed]
- 33.Liu Z, et al. Prevalence of tobacco dependence and associated factors in China: findings from nationwide China Health Literacy Survey during 2018-19. Lancet Reg Health West Pac. 2022;24:100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594:2061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Multimorbidity in Older Adults With Cardiovascular Disease. - PubMed. https://pubmed.ncbi.nlm.nih.gov/29747836/
- 36.Renal Aging. Causes and Consequences - PubMed. https://pubmed.ncbi.nlm.nih.gov/28143966/
- 37.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12:610–23. [DOI] [PubMed] [Google Scholar]
- 38.Merchant RA, Vathsala A. Healthy aging and chronic kidney disease. Kidney Res Clin Pract. 2022;41:644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valdivielso JM, Jacobs-Cachá C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28:1–9. [DOI] [PubMed] [Google Scholar]
- 40.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the Development of Cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2017;37:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart Br Card Soc. 2016;102:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasinger JH, Alexander BT. Gender differences in developmental programming of cardiovascular diseases. Clin Sci Lond Engl 1979. 2016;130:337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal NR, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. 2018;11:e004437. [DOI] [PubMed] [Google Scholar]
- 44.Davies RE, Rier JD. Gender disparities in CAD: women and ischemic heart disease. Curr Atheroscler Rep. 2018;20:51. [DOI] [PubMed] [Google Scholar]
- 45.Global regional. and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016 - PubMed. https://pubmed.ncbi.nlm.nih.gov/28919116/ [DOI] [PMC free article] [PubMed]
- 46.Matsushita K, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet Lond Engl. 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the GBD 2021. Publicly available datasets were analyzed in the current study. The data can be found here: http://ghdx.healthdata.org/gbd-results-tool. The analyzed data will be shared upon reasonable request to the corresponding author.