Abstract
Drying is a crucial unit operation within the functional foods and biopharmaceutical industries, acting as a fundamental preservation technique and a mechanism to maintain these products' bioactive components and nutritional values. The heat-sensitive bioactive components, which carry critical quality attributes, necessitate a meticulous selection of drying methods and conditions backed by robust research. In this review, we investigate challenges associated with drying these heat-sensitive materials and examine the impact of various drying methods. Our thorough research extensively covers ten notable drying methods: heat pump drying, freeze-drying, spray drying, vacuum drying, fluidized bed drying, superheated steam drying, infrared drying, microwave drying, osmotic drying, vacuum drying, and supercritical fluid drying. Each method is tailored to address the requirements of specific functional foods and biopharmaceuticals and provides a comprehensive account of each technique's inherent advantages and potential limitations. Further, the review ventures into the exploration of combined hybrid drying techniques and smart drying technologies with industry 4.0 tools such as automation, AI, machine learning, IoT, and cyber-physical systems. These innovative methods are designed to enhance product performance and elevate the quality of the final product in the drying of functional foods and biopharmaceuticals. Through a thorough survey of the drying landscape, this review illuminates the intricacies of these operations and underscores their pivotal role in functional foods and biopharmaceutical production.
Keywords: Functional foods, Biopharmaceuticals, Bioactive components, Drying methods
Introduction
Driven by evolving consumer preferences for convenience, health, and satisfaction, the food product market constantly pushes the envelope of innovation. One area of noteworthy progress has been in functional foods and biopharmaceuticals. These advancements cater to consumers’ inclination toward health-promoting foods that offer potential protection against diseases [1]. Functional foods are those that provide health benefits beyond their basic nutritional value. They can be naturally occurring, nutrient- or ingredient-enriched, and recognized for their diverse health-promoting properties. On the other hand, a nutraceutical is identified as a product extracted or purified from food sources and typically marketed in medicinal forms, which are not commonly associated with traditional food items. The term "nutraceutical" is often used synonymously with functional food, highlighting their shared health-benefiting characteristics. Despite their growing popularity, an acceptable, all-encompassing definition for these terms remains elusive, contributing to their continued interchangeability. The varieties and nuances of functional foods are numerous, ranging from foods naturally containing bioactive compounds to those synthesized to have an increased level of such compounds. The diversity of functional food categories and their examples is further elucidated in Table 1.
Table 1.
Different categories of functional foods
| Category | Definition | Examples |
|---|---|---|
| Basic foods |
Food/ food products that naturally contain the bioactive |
• Carrots with beta-carotene • Oat bran and barley cereals with beta-glucan • Green vegetables rich in lutein • Fruits, tea, and citrus containing neutralize free radical |
|
Processed foods with added bioactive |
The bioactive does not exist naturally in the food and is added during processing |
• Orange juice with added calcium • Milk with added omega-3 fatty acids • Salmon and other fish oils rich in omega-3 fatty acids • Cheese, meat products (a good source of Conjugated Linoleic Acid (CLA)) • Soy-based foods with Isoflavones: Daidzein Genistein • Yogurt and other dairy products (essential source of Lactobacillus) |
| Food ingredients synthesized to have more bioactive compounds | The level of the bioactive compounds is modified or concentrated beyond its natural level by traditional breeding, special livestock feeding, or genetic engineering |
• Yogurt with increased levels of probiotics • Tomatoes with increased levels of lycopene • Eggs with increased levels of omega-3 fatty acids • Foods fortified with indigestible carbohydrates |
Simultaneously, the field of biopharmaceuticals has seen tremendous strides. Biopharmaceuticals, comprising biomolecules such as proteins, nucleic acids, antibodies, enzymes, hormones, and vaccines, have been recognized and utilized for their immense therapeutic potential for decades [7, 8]. However, the efficient preservation and processing of these beneficial products pose a remarkable challenge due to their heat-sensitive nature. Both functional foods and biopharmaceuticals contain bioactive components that, while contributing to their health benefits, are highly susceptible to heat, and therefore, careful selection of drying methods and conditions is required [9, 10].
Drying, an integral part of the food and biopharmaceutical industry, serves as an effective preservation technique, ensuring a longer and safer shelf-life of the products. The drying process, however, must be conducted judiciously to minimize damage to the heat-sensitive bioactive components [9, 10]. The current review aims to amalgamate the research and advancements in drying methods, shedding light on their suitability for different biomaterials, their associated problems, and strategies to preserve the active ingredients in biopharmaceuticals, nutraceuticals, and functional foods.
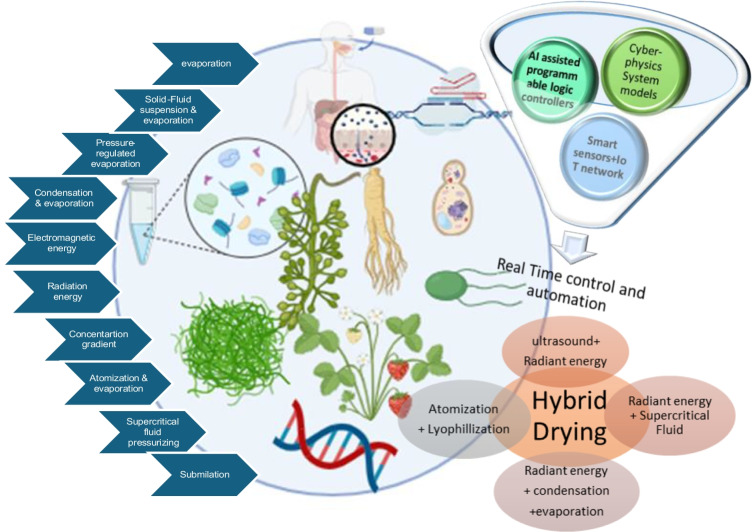
This review explores the nature of functional foods and biopharmaceuticals, addressing the challenges of drying these heat-sensitive materials. It will examine various drying techniques used to preserve diverse, valuable functional foods and biopharmaceuticals, elaborating on the advantages and disadvantages of each method. The discussion extends to hybrid drying technologies that enhance product performance and quality (Fig. 1). By providing a comprehensive overview of these drying techniques and their applications this review is poised to stimulate further research into developing even more effective preservation methods for these invaluable resources by providing a comprehensive overview of these drying techniques and their applications.
Fig.1.
Overview of the review methodology
History of Drying of Sensitive Bioproducts
The world has witnessed the emergence of new technologies in modern medicine and health care, followed by the Second World War. Freeze-dried plasma and antibiotics were the two remarkable medical advances made during wartime. After the discovery of penicillin by Alexander Fleming in 1928, a series of curious investigations were conducted to stabilize pure penicillin. Later, in 1939, Dr. Howard Florey and Ernst B. Chain, working at Oxford, used freeze-drying to stabilize penicillin, earning them the Nobel Prize in 1945 [11, 12]. Later, numerous research studies were done in the field of drying, and many were primarily focused on drying heat-sensitive products. At the end of the 19th century, spray-drying technology emerged, and a patent [13] was issued for spray-drying liquid eggs. The technique proved suitable for drying heat-sensitive biopharmaceutical products as well. With the advent of technology and research, different modifications and designs were studied in various drying methods to improve the quality and safety of dried products, retaining their functional and nutritional properties.
Major Concerns in Drying of Heat-Sensitive Materials
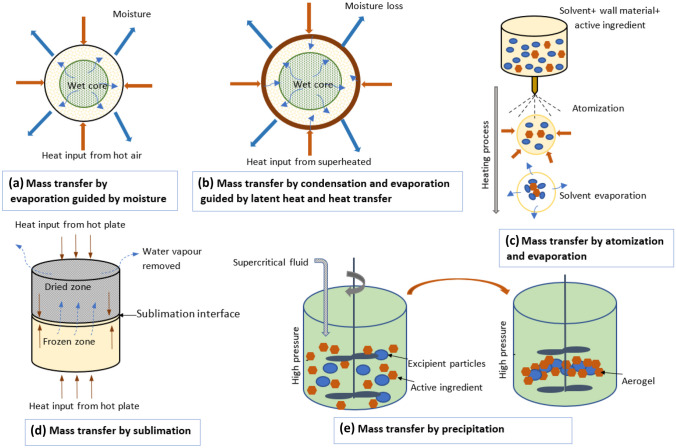
In the large-scale production of bioactive ingredients for nutraceuticals and functional foods, drying is a critical operation demanding significant energy. As demonstrated in Fig. 2, the removal of moisture in the drying process can occur in different ways: simple evaporation as in hot air or vacuum drying, condensation and evaporation as in superheated stem drying, atomization and evaporation as in spray drying, sublimation as in freeze drying, and precipitation as in supercritical fluid (SFC) drying [14, 15]. Several factors, such as temperature, humidity conditions, pressure, and exposure time, can influence the end product's quality and functionality. Although drying's primary objectives include microbial stability, reducing chemical degradation, facilitating storage, and minimizing transportation costs, researchers also aim to develop drying strategies to mitigate the loss and deformation of bioactive compounds in the dried product [16].
Fig. 2.
Different mechanisms of removing moisture in the drying process
Key indicators of product quality include cellular shrinkage, reduced rehydration capacity, absorbency, solid mobility, surface hardening, and the diminution of volatile aromatic compounds. The evolution of drying methods has led to the categorization of drying technologies into four generations. The first-generation drying technologies, being the most rudimentary, primarily relied on natural elements like sun and wind for drying. With the second generation, artificial heat sources such as ovens and stoves were introduced, greatly improving the reliability and control over the drying process. The third generation brought about mechanized drying techniques, employing hot air ovens, spray drying, and drum drying. This generation saw widespread use in industrial settings, demonstrating enhanced efficiency and control over drying conditions. Currently, we are in the era of fourth-generation drying systems, which use advanced technologies like microwave, infrared, radiofrequency, refractance window, heat pump fluid bed drying, and various hybrid systems. The central aim of these fourth-generation systems is to prioritize preserving food quality, ensuring the retention of essential nutritional components and taste attributes [17–20]. With each progressive generation, the field of drying technology becomes increasingly refined, balancing efficiency, energy consumption, and quality retention.
Drying processes are characterized by conductive and/or convective heat-transfer mechanisms. The primary aim of these processes is to diminish the concentration of residual volatile components in process streams rich in nonvolatile compounds. These procedures facilitate the transfer of energy from the outer surface to the interior of the wet material, resulting in the generation of internal heat within the solid substance. The different types of functional foods, including dairy, meat, grain, and plant-based functional food ingredients, are rich in bioactive elements such as vitamins, essential fatty acids, minerals, antioxidants, etc. These components, however, are highly susceptible to damage from high temperatures. The process of dehydrating these biological molecules may result in substantial chemical, physical, and nutritional degradation, including but not limited to browning reactions, lipid oxidation, colour and aroma depletion, and loss of vitamins and minerals [21].
Certain products are solvent-wet forms that are centrifuged before drying to minimize degradation. However, intense evaporation during drying can still cause a carry-over of solid product particles by the vapour flow. This carry-over can cause clogging of the filters and ducts, resulting in damage to the dryer system. Another common issue with biopharmaceutical and functional products is the agglomeration of particles and their hygroscopic nature, often leading to undesired “lumps” in the end product. Moreover, in the case of organic solids, in which the drying process is controlled by the diffusion of the liquid through the solid, larger lumps lead to longer processing times [8].
Moving forward, the discussion shifts to hybrid drying systems. These represent an advanced frontier in processing heat-sensitive materials. The forthcoming section will delve into these technologies, focusing on preserving the inherent qualities of functional foods and biopharmaceuticals.
Drying Systems for Heat-Sensitive Biomaterials
As most functional foods, nutraceutical foods, and biopharmaceutical ingredients are thermos-liable with a tendency for structural and functional deformation at extreme drying conditions, selecting the appropriate drying system is key. These drying methods and strategies are also chosen based on the nature of end products, such as food powders, flakes, leathers, or sheets from juices, purees, pastes, or suspensions [10]. Thermal degradation models for various biomolecules and nutrients are essential for understanding and predicting the behavior of heat-sensitive biomaterials under various temperature conditions [22]. Several numerical models aim to describe the kinetics of degradation reactions, assess the impact of temperature on biomaterial stability, and optimize processing parameters to minimize thermal degradation [23, 24]. Thermal degradation of the primary macro-nutrients such as carbohydrates, proteins, and lipids is sometimes essential for converting them to more digestible forms for enhancing nutrient intake. On the other hand, changes to micronutrients such as vitamins, minerals and other functional micronutrients may significantly affect on their functionality and bioavailability [24]. Table 2 summarizes the thermal degradation mechanism and models of macro-nutrients and micro-nutrients. The following section delves into an array of drying methods commonly used in the food and biopharmaceutical industries, specifically focusing on their application in the drying of functional foods and biopharmaceuticals. These methods are critical for preserving the products' quality, nutritional value, and bioactive properties while ensuring safety for consumption or use. The methods discussed include heat pump drying (HPD), freeze-drying, spray drying, vacuum drying, fluidized bed drying (FBD), superheated steam drying, infrared (IR) drying, microwave drying, osmotic drying, and supercritical fluid drying. Each method will be discussed in detail, highlighting its working principle, advantages, limitations, and particular applications in drying functional foods and biopharmaceuticals.
Table 2.
Thermal degradation mechanism and models of macro-nutrients and micro-nutrients
| Nutrient type | Mechanism and effects of thermal degradation | Models | Reference | |
|---|---|---|---|---|
| Macro-nutrients | Carbohydrate |
Mechanism • Temperature < 200 °C -loss of free water and hydroxyl groups in physical and polymeric changes • Temperature 220 °C -550 °C -formation of dehydrated anhydrides with structural and chemical changes • Temperatures > 550 °C further carbonization to degrade to smaller molecules CO2, H2, CH4, etc. Effects o Caramelization of carbohydrates leading to the formation of brown color, aroma and flavor compounds o Pyrolysis of carbohydrates, resulting in the breakdown of complex carbohydrates |
The Arrhenius equation and Reaction rate models: k = k0 · e−Ea/RT Ea is the activation energy (kJ mol −1), k is the rate constant, and k0 is the frequency/pre-exponential factor. R is the universal gas constant (8.314·10 −3 kJ mol −1 K −1), and T is the absolute temperature (°K) E.g.: Friedman model, Ozawa model, Kissinger model, Flynn-Wall-Ozawa model, Coast-Redfern model |
[24–30] |
| Proteins |
Mechanism • Temperature 100 °C -200 °C -spatial structure changes -thermal aggregation • Temperature > 200 °C – thermal degradation Effects o Denatured protein molecules undergo aggregation or covalent cross-linking, leading to the formation of insoluble protein aggregates o Pyrolysis of proteins, resulting in the breakdown of peptide bonds |
First and second-order Kinetic Models Arrhenius model and reaction model |
[31–33] | |
| Lipids |
Mechanism • Thermal oxidation 100 ~ 200 °C • Polymerization Effects o Free radicals formation leading to the formation of off-flavors, rancidity, and potentially harmful compounds such as lipid peroxides o Hydrolysis of lipids, resulting in the free fatty acids |
Second-order polynomial model Arrhenius model |
[24, 34–37] | |
| Micro-nutrients | Vitamins |
Mechanism • Thermal oxidation and degradation Effects o Lower bioactivity, irreversible binding to other molecules, or degradation to inactive compounds o 300–500 °C, some vitamins (Vitamin A) decomposes to form aromatic substances |
Vitamin C, D, & β-carotene- first-order reaction kinetic
Where, C0 -initial concentration of vitamin, Ct – measured concentration of vitamin at time t and k temperature- dependent rate constant |
[24, 38–42] |
| Minerals | Mineral stability and availability are reported to have minimal impact by drying processing than other macro- and micronutrients | - | [43, 44] | |
| Phenols, Flavonoids and Glycosides |
Mechanism • Maillard reaction Effects o Some polyphenols and flavonoids may increase during drying, but long-term exposure of heat reducing their concentration and bioavailability |
First-order reaction kinetic | [39, 45, 46] |
Heat Pump Drying
The Heat Pump Dryer System (HPDS) represents an innovative and energy-saving approach to drying and dehydration processes that harnesses low-grade energy to heat the drying medium. Heat pump drying technology is used in high-value foods and biomaterials where low-temperature drying generally ranges from 45 to 70 °C and well-controlled conditions are essential [47]. Its potential as a waste heat recovery system and high drying efficiency have boosted HPD’s popularity [48, 49]. Heat pumps can be classified into different designs, such as gas-engine-driven heat pumps [50–53], ground source heat pumps (GSHP), solar heat pumps [54–56], photovoltaic/thermal (PV/T) heat pumps [57], chemical heat pumps [58], and desiccant heat pumps [59, 60].
This technology is particularly suitable for high-value products, as it allows for controlled transient drying conditions in terms of temperature, humidity, and air velocity, thereby improving product quality attributes and reducing drying costs. HPD has proven to be a reliable method for biomaterials or food materials, including aquatic food products with a high content of phenolics, chlorophyll, ascorbic acid, phycocyanin, and antioxidant activity [48, 61–64]. Some of the advantages of HPD include [53, 65]:
Lower energy consumption (about 60%) for each unit of water removed, and therefore, higher energy efficiency with improved heat recovery
Well-controlled temperature profiles, making it highly suitable for heat-sensitive high-value products with better quality outcomes
Flexibility in drying conditions as it can generate temperatures typically ranging from -20 to 70 °C (with auxiliary heating) and a relative humidity of 15–80% (with a humidification system).
Despite these numerous benefits, HPD also has some limitations. The dryers require regular maintenance of components (compressor, refrigerant filters, etc.), and using CFCs in the refrigerant cycle presents environmental concerns. The technique is also not universally suitable for preserving all bioactive compounds. For instance, HPD can negatively affect ascorbic acid in functional food or biomaterials [62, 66].
Another notable hindrance to the widespread adoption of HPD is the constraints in achievable drying temperatures and the substantial capital required for setting up the system. However, these challenges do not overshadow the key benefits of this technology. Its ability to precisely control the operating temperature and relative humidity makes it ideal for drying functional foods, yielding minimal discoloration and ascorbic acid degradation. Despite the limits on temperature range, this precision positions HPD as a promising technology for enhancing the preservation of high-value foods and biomaterials.
Freeze Drying
Freeze drying, also known as lyophilization, is primarily utilized to remove water from sensitive biological molecules. This procedure prevents damage, enabling their preservation in a storable state that can be reconstituted simply by adding water. This method is optimal for preserving biopharmaceutical/nutraceutical products (Table 2) like antibiotics, bacteria, vaccines, diagnostic medications, protein-containing, biotechnological products, cells and tissues, and chemicals [67, 68]. Furthermore, freeze-drying is apt for preserving and drying various high-value functional foods like fruits, dairy products, meat proteins, eggs, etc. [69, 70].
Owing to the water being removed in its frozen state rather than its liquid state, the material's morphology, solubility, and chemical integrity are largely maintained after freeze-drying [71]. Freeze-drying is a three-phase process: initially, the product is frozen, decreasing the temperature to cause most of the water to crystallize, leaving only a small fraction unfrozen and incorporated within the product. Subsequently, the primary drying phase occurs, during which the chamber pressure is reduced to enable sublimation while heat is concurrently supplied to the product. The sublimation process is initiated from the material surface, which is driven by the vapor pressure gradient above the sublimation surface Pvi and the evaporated surface Pa and the rate of sublimation is computed by Eq. 1 [72].
| 1 |
where S is the sublimation rate, kg/(m2·s); Rd is the resistance inside the dry layer, m2/(Pa·s kg); Rs is the resistance to mass transfer from the dry surface to the resublimation surface, m2/(Pa·s kg); and Ri is the ice sublimation resistance, m2/(Pa·s kg).
And
| 1a |
Under the general assumption that the resistance of the convective mass transfer from the evaporation surface to the resublimation surface is negligible, the maximum sublimation rate possible can be computed as:
| 2 |
In primary stage drying, there is a moving interface of freeze-dried layer and frozen layer, and there is no distinctive boundary between the first and second phases of freeze-drying [72]. Equations 3a and 3b give the initial conduction heat transfer from the material surface to the sublimation interface and the frozen layer to sublimation interface, respectively [73].
| 3a |
| 3b |
where and are the heat flux through dried layer and frozen layer in (W), respectively; and are the thermal conductivity of dried layer and frozen layer in (W/mK), respectively; , , and are the surface area of the sublimation interface, frozen layer, and external surface in (m2), respectively; , , and are the temperature of the sublimation interface, external surface, and frozen layer in (K), respectively.
In the secondary drying phase, the product's temperature is increased to remove residual moisture, including bound and unfrozen water [74]. The heat transfer rate by conduction can be defined as the heat flux conducted through the frozen layer of the material (Eq. 3b). This technique has captivated researchers due to its capability to dry materials at lower temperatures, thereby maintaining their original colour, texture, and quality [70, 75]. The application of novel freeze-drying technologies such as Thin film freeze-drying (TFFD) enabled the production of uniform-sized aerosol particles for biopharmaceutical products such as Inhalation-based medication delivery. TFFD has several advantages over traditional freeze-drying processes in biopharmaceutical applications. TFFD uses an intermediate freezing rate, typically between 102 and 103 k/s, which is faster than standard freeze-drying [76]. This intermediate freezing rate improves the structural integrity and bioactivity of sensitive biopharmaceutical compounds enabling the production of engineered dry powders and facilitating precise dosing. Table 3 illustrates the freeze-drying conditions for different foods with bioactive components in FBD.
Table 3.
Freeze drying conditions for different foods with bioactive components
| Product | Drying condition | Drying Pressure (Pa) | References |
|---|---|---|---|
|
Green banana flours (Starch and crude fibre) |
Temperature: -47 to -50 °C |
700 | [77] |
| Brazilian ginseng root (beta-ecdysone & fructo-oligosaccharides) | Temperature: -40 °C | Atmospheric | [78] |
| Symbiotic drink with lactobacillus casei | Temperature: -49 °C | 1000 | [68] |
| Seabuckthorn berries (phenolic, carotenoids, fatty acids, and vitamin contents) | -20 to -50 °C shelf plate temperature | Atmospheric | [79, 80] |
| Blueberries (polyphenols, antioxidant activity, and ascorbic acid) | Temperature: -30 °C | Atmospheric | [81] |
| Submicron lactate dehydrogenase (LDH) protein particles | lyophilization (1 K/min) and spray freeze-drying (SFD) (106 K/s), temperature –50 to -140 oC | Atmospheric | [76] |
| Encapsulated Probiotic bacteria | chamber freeze-drier at -80 oC | 0.02 mbar | [82] |
| Encapsulated Spirulina Maxima in whey protein | Temperature: −50 °C | 0.04 mbar | [83] |
| Monoclonal antibodies formulated with lactose/leucine | Temperature - -100 °C | Atmospheric | [84] |
Among the key advantages of freeze-drying for food and biomaterial drying are:
Preservation of structural, biochemical, and immunological characteristics
Enhanced viability or activity rates, along with improved textural attributes, owing to drying at low temperatures
Effective recovery of volatile substances, maintaining structural integrity, surface area, and stoichiometric balances, leading to high product yield, prolonged shelf life, and decreased weight for easier storage, transportation, and handling [85]
Minimal oxidative reactions due to the absence of oxygen during drying, maintaining the quality of the final product.
However, the broad implementation of freeze-drying is constrained by the significant capital investment required. It is a high-energy, high-cost process for both operation and maintenance. Despite these limitations, freeze-drying remains an effective method for protein powder production. Nevertheless, issues such as ice formation, solute and protein concentration affecting protein stability, and potential cold denaturation during the freezing process are concerns. To address these issues, hybrid techniques such as combined spray- and freeze-drying, thin film freeze-drying, etc., have been developed, which involve spraying the product into a cryogenic medium, followed by the standard primary and secondary drying processes of freeze-drying [86–90].
Spray Drying
Spray drying, a popular particle formation and drying method, is particularly effective for continuously producing dry solids. These can be either powder or agglomerated particles derived from a liquid feedstock [91, 92]. This technology excels when the final product must meet specific quality standards, such as particle size distribution, residual moisture content, bulk density, and particle morphology.
The spray drying process involves rapid heat and mass transfer as the liquid feed is atomized into fine droplets and introduced into a hot airstream. The water evaporates from the droplet during this process, and the resulting dried powder is cooled and collected using cyclone separators. Spray drying modelling is one of the most commonly simulated models using computational fluid dynamics (CFD) [93]. The advanced computational power of CFD was reported to be effective in solving very sophisticated models such as the continuous phase flow model, droplet agglomeration models, particle droplet tracking, and wall depositions models [94]. The mechanism of increased surface area for evaporation of moisture from the atomized particles is attributed to the uniform and faster drying of spray droplets. Consequently, a single heat transfer equation can be utilized to model the heat flux to the droplet in the heating period and the following wet bulb temperature period (Eq. 4) [95].
| 4 |
where and are the drying medium and spray particle temperature in (K), respectively; Rp is the spray particle diameter in m; is the specific heat capacity of spray particle in (J/kg⋅K); represents spray particle mass; is mass flow rate and is the latent heat of vaporization in (J/kg).
The spray drying technique has widespread application in the biopharmaceutical industry [96] and in drying of encapsulated food ingredients [97] (Table 3). It is mainly used for microencapsulating the active ingredients of many biological materials, such as flavours, lipids, essential fatty acids, carotenoids, and more. The spray drying technique with microencapsulation was also reported to be a potential solution for manufacturing food additives for food fortification applications such as minerals [98]. The active ingredient is homogenized in an emulsion, which forms the microcapsules' coating. Subsequently, the active ingredient emulsion is spray-dried (Table 4).
Table 4.
Drying conditions for different encapsulated active ingredients in spray drying
| Encapsulated ingredient | Wall material | Air inlet temperature (°C) | Air outlet temperature (°C) | References |
|---|---|---|---|---|
| Anhydrous milk fat |
Whey proteins/lactose/ Maltodextrin |
160 | 80 | [99, 100] |
| Ethyl butyrate ethyl caprylate | Maltodextrin/gum arabic | 160 | 80 | [101, 102] |
| Caraway essential oil | Maltodextrin/Skim milk powder | 175–185 | 85–95 | [103] |
| Cardamom oleoresin | Gum arabic/modified starch/maltodextrin | 176–180 | 115–125 | [104, 105] |
| Bixin | Maltodextrin/gum arabic/modified starch | 180 | 130 | [106, 107] |
| d-Limonene | Maltodextrin/gum arabic/modified starch | 200 | 100–120 | [108, 109] |
| l-Menthol | Gum arabic | 180 | 95–105 | [110] |
| Black pepper oleoresin | Gum arabic/whey protein concentrate | 176–180 | 105–115 | [111, 112] |
| Cumin oleoresin | Maltodextrin/ gum arabic/modified starch | 158–162 | 115–125 | [113] |
| Arachidonyl l-ascorbate | Maltodextrin/gum arabic/soybean polysaccharides | 200 | 100–110 | [114] |
| Fish Oil | konjac glucomannan, Soybean protein isolate, potato starch | 200 | 80 | [115] |
| Fish oil | Sugar beet pectin/glucose syrup | 170 | 70 | [116, 117] |
| Short-chain fatty acid | Maltodextrin/gum arabic | 180 | 90 | [118, 119] |
| Hawthorn Berry polyphenols | β-cyclodextrin, whey protein isolate, gum arabic | 165 | [120] | |
| Lycopene | Gelatin/sucrose | 190 | 52 | [121, 122] |
| Turmeric oleoresin | Maltodextrin/gum arabic | 150–200 | 90 | [123] |
This prevalent method for drying liquid products has numerous advantages, including:
Drying time is comparatively less than other drying methods since the heat transfer rate is high
Good reconstitution capacity and product quality
Minimal chances of thermal denaturation as the droplet's surface temperature is maintained at the wet-bulb temperature, significantly lower than the drying gas temperature
Enhanced bioavailability of active ingredients and controlled release in encapsulated products
Improved control over particle size as the feed droplet size can be easily regulated.
However, it's essential to note that spray-dried products are thermoplastic and hygroscopic. As such, product recovery post-drying should be done swiftly and carefully to avoid the product sticking to the dryer walls, which could reduce overall efficiency. Moreover, these dried products are highly sensitive to moisture and temperature fluctuations during storage. Therefore, meticulous efforts must be made to maintain precise relative humidity and temperature levels during storage [124, 125].
Fluidized Bed Drying
Fluidized bed drying is widely applied in the drying of granular solids in various industries such as food, ceramics, biopharmaceuticals, and for drying phytochemicals like organic acids, carbohydrates, reducing sugars, lipids, and proteins [126–131]. This method is suitable for drying powders in the 50–2000 μm range, thanks to its high heat and mass transfer rates. FBD's effectiveness lies in its fluidization process, allowing for improved drying rates and reduced drying time. The fluidized bed drying process is another multiphase drying model, as the fluid and solid phases are in an interacting continuum. The drying process is governed by the continuum phase heat transfer from the drying medium into the solid phase. Therefore, the general continuum equation for heat, mass and momentum transfer for the fluid medium is set as the boundary conditions for the solid phase drying modelling. The solid phase dying is governed by diffusion equations (Eqs. 5a and 5b) for energy and mass transfer, respectively [132].
| 5a |
| 5b |
where, is the moisture content of the material in kg/kg; is the temperature of the material in K; is the specific heat capacity of the material; is the thermal conductivity of the material; is the density of the material kg/m3; is the effective radius of the material in m; Deff is the effective diffusivity coefficient m2/s.
In FBD, the product is subjected to a high flow velocity greater than its specific gravity. This flow lifts it above the periphery of the dryer mesh. It then decelerates and falls onto an annular zone between the central core and the equipment wall. This flow pattern establishes a unique solid–fluid suspension, ensuring uniform and faster drying [130]. This drying system has several advantages, such as:
Rapid drying speeds, facilitated by superior contact between gas and particles, results in high rates of heat and mass transfer
Enhanced thermal efficiency and a reduced flow area in comparison to traditional pneumatic dryers
Ease of control of the drying process by controlling the fluidization velocity and pressure drop.
However, the method does come with its limitations. It involves high power consumption, requiring suspending the entire bed in the gas phase, leading to a substantial pressure drop. There's an increased chance of attrition and, in some instances, granulation or agglomeration. FBD also has low flexibility for the type of product that can be dried (e.g., it is unsuitable for wet products). Furthermore, frequent issues during the drying of phytochemicals include instability within the drying bed, accumulation of products, coating on non-reactive substances, clumping of particles, and potential system failure. Moreover, there can be losses in bioactive components due to thermal degradation [130]. Therefore, precise control of the drying conditions is necessary for such products with bioactive components, as detailed in Table 5.
Table 5.
Drying conditions for different foods with bioactive components in fluidized bed drying and superheated steam drying
| Product and bioactive compound | Drying condition (air or superheated steam) | References |
|---|---|---|
| Phytochemicals |
Inlet temperature: 60 to 180 °C Feed flow rate: 3 to 12 g/min |
[130] |
| Green vegetables (broccoli) |
Inlet temperature: 60 to 80 °C Particle size: 1-3 cm Air flow rate: 1-3 m/s |
[133] |
| Pellet coated pharmaceuticals |
Inlet temperature: 90 °C Gas flow rate: 50 kg/h |
[134] |
| Soybeans |
Inlet temperature: 110–140 °C Air velocity: 2.4–4.1 m/s |
[135] |
| Probiotic bacteria | Inlet temperature: 40 °C | [136] |
| Bee pollen |
Inlet temperature: 40 °C Air velocity: 6.0 m/s |
[137] |
| Muskmelon seed |
Inlet temperature: 40–60 °C Air velocity: 7–11 m/s |
[138] |
| Wheat grains (dietary fibre and polyphenols) | Steam temperature: 110 to 180 °C | [139, 140] |
| Fish (omega-3 fatty acid) |
Steam temperature: 300 °C Flow rate: 150 kg/h |
[141] |
| Beef (Bioactive antihypertensive peptides) |
Steam temperature: 130 to 180 °C Flow rate: 35 -55 kg/h |
[142] |
| Shrimps (carotenoprotein, calcium) | Steam temperature: 120 to 180 °C | [143] |
| Soybeans (Lysine content) |
Steam temperature: < 135 °C Steam velocity: 3.2 m/s |
[144] |
| Oats (beta-glucan) |
Steam temperature: 110 to 160 °C Steam velocity: 0.35 to 1.0 m/s |
[145] |
| Waxy rice (Amylose content, Gamma-aminobutyric acid) |
Steam temperature: 130–150 °C Steam velocity: 3.5 m/s |
[146] |
Superheated Steam Drying
Superheated steam drying is a non-polluting, safe, and energy-efficient method [147–149]. Its capacity to dry materials at temperatures above 100 °C makes it widely applicable in the food industry, as illustrated in Table 5. This method offers various benefits, including reduced drying time and dryer size, ease of integration into production lines, and the ability to recover the energy supplied to the dryer in a usable form.
The drying medium in this method is superheated steam, which operates in a closed cycle, picking up moisture from the wet product in the drying chamber and then condensing the evaporated water in a heat exchanger [150, 151]. Since the drying occurs in a closed environment, the probability of oxidative reaction is minimal, preserving the quality and aroma of the dried material [15, 93]. Moreover, superheated steam drying resembles high-temperature short-time (HTST) treatment in which food gets decontaminated while drying [139, 140]. Low-pressure superheated steam is highly suitable for drying heat-sensitive products like fruits and vegetables, herbs, and other bioactive materials. Low-pressure superheated steam drying takes place in the pressure range of 5–10 kPa [147], at which the steam becomes saturated [152–154]. Compared to hot air-based drying, superheated steam drying has a faster drying rate, as part of the initial accelerated heat transfer is aided by latent heat contributed by the initial condensation and the subsequent free water evaporation (Eqs. 6a and 6b) followed by the diffusion model (Eqs. 5a and 5b) [15, 155].
| 6a |
| 6b |
where, Tm, Tsat, Tss are the material temperature, saturation temperature and superheated steam temperature in K, respectively; is the film condensation heat transfer coefficient; h is the convective heat transfer coefficient; and is the latent heat of condensation/evaporation.
Various scholars have utilized superheated steam drying to investigate its ability to preserve bioactive components, primarily antioxidant components, in multiple products. These include tea leaves, where studies have shown significant preservation of antioxidant properties compared to conventional oven drying methods [156], and in other products like onions, where low-pressure superheated steam drying has demonstrated better retention of bioactive components [157]. Another relevant work Suvarnakuta et al. [158] examined the effects of drying methods on the assay and antioxidant activity of xanthones in mangosteen rind. They concluded that hot air drying or low-pressure superheated steam drying at 75 °C is the most suitable drying method to maximize the quantity and quality of mangosteen.
Superheated steam drying has several advantages over hot air drying, including [159–162]:
Improved drying efficiency when compared to other drying techniques, especially with the closed-loop system.
Clean process without any emission of flue gases and odor emissions to the environment.
Absence of direct contact between the product and hot, oxygen-rich gas, reducing the likelihood of oxidation
Beyond drying, hot steam serves as a sterilizing agent
Improved control over the drying process by regulating the amount of steam introduced into the compressor, aiding in achieving precise product dryness.
The primary concern with superheated steam drying is the phenomenon known as initial condensation. This occurs when superheated steam comes into contact with a cold solid feed at ambient temperature, leading to vapor condensation on the material surface. This condensed moisture could increase the drying time unless the feed material is preheated by other means. A low-pressure superheated steam system is required to minimize prolonged drying of heat-sensitive bioactive compounds [144]. The full energy efficiency advantage of superheated steam drying can only be fully utilized in a closed-loop system, where the output steam is diverted elsewhere in the processing plant. Such design modifications could add to the system’s complexity and cost [157].
Infrared Drying
Infrared drying technology uses IR energy directly transferred from a heating element to the food, bypassing the need to heat the surrounding air. Thus, it helps to save energy and drying time. In IR drying, the radiant energy penetrates the product and converts it into heat, heating its surface and inner layers. This intense heating produces a higher heat and mass transfer rate than conventional drying methods. Recent research has highlighted this technique's capability to preserve bioactive components in foods post-drying, showing its effectiveness in maintaining the quality of various food products by preserving their phytochemical content and minimizing the loss of antioxidant activity [163–167].
The drying mechanism is also governed by the diffusion equation as explained in "Fluidized Bed Drying" section, and the energy balance is governed by the conduction, convection and radiation energy as given by Eq. 7 [168]. Lee et al. [169] studied the effects of far-IR drying on the antioxidant and anticoagulant activities of Ecklonia cava (brown seaweed) extracts. Their findings indicated that far-infrared radiation releases and activates low molecular weight bioactive compounds, such as polyphenols, due to its ability to heat materials without degrading their surface molecules [169, 170]. Senevirathne et al. [171] reported that far-infrared radiation drying at 80 °C is an effective and economical method for drying citrus press cakes with minimal loss of antioxidant activity.
| 7 |
where Tm, Tg, Tr are the material temperature, drying medium (hot air) temperature, and radiation temperature in K, respectively; is the material energy, is the material shape factor; is the Stefan-Boltzmann constant, W/cm2 ·K4; is the emissivity of the material; is Latent heat of vaporization, J/g; is the heat of desorption.
IR drying offers several advantages, including:
Reduced drying time due to higher dehydration rates and high heat transfer rates are achievable with compact heaters
High energy efficiency
Rapid process control while maintaining the final product's quality.
The IR drying systems suffer from disadvantages, especially for food and biomaterial drying, such as i) browning reactions resulting in darkening, ii) increased hardness, especially with increased IR power, and iii) deterioration of qualitative parameters [172–174].
Moreover, IR drying is an effective intermittent irradiation method when combined with convective air drying for heat-sensitive materials. An infrared-augmented convective dryer can rapidly remove surface moisture during the initial drying stages, followed by intermittent drying for the remainder of the process. This approach ensures a faster initial drying rate and offers better process control, as the IR power source can be easily cut off in the event of excessively high temperatures in the chamber, preventing overheating of the product. Ratseewo et al. [175] reported that far-infrared radiation drying of pigmented rice enhanced the content of total phenolic, flavonoid, tocopherols, anthocyanins, gallic and ferulic acids, and quercetin compared to traditional hot air drying. Overall, as demonstrated in Table 6, the IR drying method is reported to be appropriate for drying high-valued heat-sensitive food products.
Table 6.
Drying of different functional foods with bioactive components in Infrared Radiation drying
| Product and bioactive compound | Drying condition | References |
|---|---|---|
| Ecklonia cava (Brown seaweed) (antioxidants) |
Temperature: 40 to 80 °C Optimum temperature: 80 °C |
[169, 170] |
| Citrus press-cakes (antioxidants) |
Temperature: 40 to 80 °C Optimum temperature: 80 °C |
[171, 176] |
| Saffron (antioxidants and aroma compounds) |
Temperature: 50 to 80 °C Optimum condition: 80 °C for 30 s |
[177] |
| Gamguk flower (herb) (Chrysanthemum indicum L.) (phenolic and flavonoid) | Temperature: 50 °C | [178, 179] |
| Rice hulls (phenolic compounds) | Temperature: 100 °C | [180, 181] |
| Peanut hulls (antioxidants and radical scavenging activity) | Temperature: 150 °C for 60 min | [182] |
|
Ginkgo biloba seeds (Flavanoids and anti-oxidants) |
Temperature: 80 °C | [183] |
| Garlic (thiosulfinates, phenolic compounds and antioxidants) |
Temperature: 50 to 80 °C optimum temperature - < 70 °C |
[184] |
Microwave Drying
Microwave drying, or microwave-assisted drying, is a rapid drying technique extensively used in the food industry. This method involves transmitting microwave energy through the product, generating heat due to dipolar polarization and ionic conduction phenomena. This method is distinguished by its volumetric heating, propelled by electromagnetic radiation at 915 or 2,450 MHz frequencies. The heat is generated by the interaction between microwaves and the material, converting a portion of the electromagnetic energy into heat throughout the volume, primarily heating polar molecules like water in the product [185]. The heat transfer mechanism by internal heat generation results in a volumetric heating mechanism of electromagnetic energy supplied by microwaves. The volumetric heat flux is represented by the Eq. 8 [186].
| 8 |
where, V is the product volume in m3, and P is the Power in W, generated by the absorption of Microwave. This microwave power absorbed by water molecules (polar) is converted to heat.
Various studies have explored the potential of microwave drying in producing high-quality end products. The utilization of a two-stage microwave power system, which adjusts the power levels during the drying of functional food products, was proposed by [187, 188]. They proposed that adjusting the power levels of microwave energy (1- 2 kW/kg depending on the initial moisture content) could facilitate higher retention of β-carotene in dried carrots. Microwave-assisted vacuum drying has also been recognized as an appropriate drying method for thermolabile products, including certain foods (e.g., cranberries, carrots, garlic, mushrooms) and biopharmaceutical powders and granules [189]. Condurso et al. [190] found that microwave drying considerably increased the concentration of trisulfides and cyclic sulfur compounds, which contribute to the specific aroma of garlic and possess potent anticancer and chemoprotective properties, in Sicilian garlic compared to hot air drying. Moreover, Berteli et al. [191] studied the microwave vacuum drying process for biopharmaceutical granules and found that it is faster than other drying techniques. They highlighted several benefits:
Enhanced heat and mass diffusion through biomaterial due to its volumetric heating nature
Quicker formation of internal moisture gradients, leading to enhanced drying speeds
Accelerated drying rates achieved without raising the surface temperatures
Enhanced product quality, making it suitable for heat-sensitive products (such as carrots, garlic, mushrooms).
However, despite these advantages, further research is needed to address specific challenges associated with this method. These include problems such as non-uniform product heating and uneven distribution of the electromagnetic field in a microwave cavity [185] (Table 8).
Table 8.
A comprehensive summary of the various drying methods, highlighting their strengths and limitations and the suitability for biomolecules or bioproducts
| Drying Method | Suitable Biomolecules/Products | Benefits | Overall Limitation | References |
|---|---|---|---|---|
| Heat Pump Drying | High-value foods, aquatic products preserve essential amino acids | Lower energy consumption, well-controlled temperature profiles | Regular maintenance, environmental concerns, high capital cost | [63, 234, 235] |
| Freeze Drying | Biopharmaceuticals, high-value functional foods and ingredients such as Gelatin, isolated proteins, probiotics | Preserves structure, biochemical and immunological characteristics | High energy and cost, ice formation, protein stability issues | [79, 80, 82, 84, 236] |
| Spray Drying | Microencapsulation of biopharmaceuticals and biomaterials, food additives, gelatins, active biomolecules | Rapid drying, good reconstitution capacity, suitable liquid suspensions, higher foam expansion | Sensitive to moisture, potential for product sticking, non-uniform particle size, high temperatures driven denaturation | [111, 113, 122, 237, 238] |
| Fluidized Bed Drying | Granular solids, phytochemicals, coating/tableting for pharmaceutical and probiotics | Rapid drying speeds, enhanced thermal efficiency | High power consumption, attrition, unsuitable for highly wet products | [131, 133, 134, 136, 239] |
| Superheated Steam Drying | Fruits, vegetables, herbs, aquatic products | Non-polluting, preserves quality and aroma, faster drying rates | Complex setup, risk of overcooking, expensive equipment, | [143, 148, 154, 187] |
| Infrared Drying | Antioxidant-rich foods, herbs, nuts and seeds, green tea, fruit peels | Reduced drying time, high heat transfer rates, | Uneven heating, high initial setup cost, limited to certain products due to browning reactions and changes to functional properties | [164, 168, 173, 183, 240] |
| Microwave Drying | Heat-sensitive foods, biopharmaceutical powders. | Volumetric heating, enhanced heat and mass diffusion, improved energy efficiency. Suitable for hybrid drying | Non-uniform heating, high equipment cost, shielding needed | [188, 241, 242] |
| Osmotic Drying | Fruits, vegetables for bioactive compounds | Minimizes loss of functional components, preserves flavor, suitable as a pre-treatment | Texture changes, nutrient leaching, time-consuming | [243–246] |
| Vacuum Drying | Herbs, heat-sensitive foods, fruits, formulation of proteins | High-quality dried products, retains nutritional value, Suitable for hybrid drying for bioactive compounds | Expensive equipment, slower drying times, oxidation issues | [208, 247, 248] |
| Supercritical Fluid Drying | Proteins, peptides, sensitive biomolecules | Effective for heat-sensitive materials, minimal nutrient loss, suitable for bioactive compound extractions and hybrid drying | High pressure, residual solvents, complex setup, scaling challenges | [215, 219, 232, 233] |
Osmotic Drying
Osmotic dehydration is a critical process in drying functional foods such as grapes, berries, tomatoes, carrots, and mushrooms, as it minimizes the loss of functional components [192–196]. The technique operates on the principle of osmotic pressure difference caused by the salt and sugar concentration gradient between the cells of the food product and the surrounding medium. This method minimizes organoleptic and nutritional elements in the product, preserving its flavour and nutritional value [193, 196, 197].
Singh et al. [198] conducted studies on drying carrots by osmotic dehydration using sucrose (50° to 80°Brix) and salt solutions (5 to 15%). They reported that the drying occurs through a simultaneous process of water loss and solute diffusion, effectively drying the food product without excessive loss of nutrients following the Ficks diffusion equation. The osmotic pressure of the drying surface rises until it reach a critical level as the diffusion proceeds, resulting in cell membrane rupture. This facilitates increased cell permeabilization index which is measured by electro-physical measurements [199]. The chemical potential gradient, closely associated with the concentration gradient, represents the force exerted on each penetrant molecule during osmosis and diffusion. Under constant temperature and pressure conditions, the chemical potential (μ) can be described by the following equation [200, 201]:
| 9 |
where, is the partial derivative of the ratio of Gibbs free energy and number of moles of the penetrant. The chemical potential in a liquid phase as a function of temperature and water activity is determined by Eq. 10 [202].
| 10 |
where, is the standard chemical potential, R is the universal gas constant (J/Kmol), and T and are the absolute temperature (K) and water activity of the substance in the liquid phase, respectively.
García-Segovia et al. [203] investigated the effect of osmotic dehydration on Aloe Vera, focusing on retaining its immunomodulatory, anti-inflammatory, and antibacterial properties. Their research found that optimal results were achieved when osmotic drying was conducted at lower temperatures, specifically at 40 °C, demonstrating the potential for preserving bioactive compounds during the osmotic dehydration process.
Overall, the osmotic drying process is particularly effective for fruits and vegetables. The technique can dewater these items without compromising their nutritional and functional elements, preserving their inherent health benefits. Moreover, the capability to fine-tune the osmotic solution allows for optimization based on the specific properties of the food product, making it a versatile and efficient drying method. However, the technique has limitations, such as potential changes in texture and the need to remove the osmotic agents from the product after drying, which warrant further research and technological improvements. Also, the diffusion rate of water differs for different materials depending on their composition, geometry, and size, and this limits the drying rates, affecting their nutritional quality and organoleptic properties [199].
Pressure-Regulating Drying (Vacuum Drying)
Leveraging the universal gas laws, where temperature and pressure are directly proportional, pressure-regulating drying, commonly known as vacuum drying, has gained widespread attention among researchers. By reducing atmospheric pressure, vacuum drying enables water to evaporate at lower temperatures, making it an ideal choice for drying heat-sensitive food products like herbs, curry leaves, and carrots. This approach allows for achieving the desired dryness level without compromising the product's quality, as it operates in a pressure-regulated environment [204–206]. Typically, the operating pressure range varies from a vacuum to close to one atmosphere [151].
Orikasa et al. [207] investigated the effect of vacuum drying on the quality attributes of kiwi fruit. They reported that vacuum drying helps to improve the quality and nutritional value of the dried kiwifruit when compared to hot air drying by retaining l-ascorbic acid, a crucial vitamin. In another relevant study, Šumić et al. [208] noted a remarkable retention of functional elements such as phenols, anthocyanins, and total solids after vacuum-drying frozen sour cherries.
Moreover, the vacuum drying technique offers considerable flexibility and is less costly than freeze drying, making it a preferred choice for many custom or hybrid drying systems to preserve heat-sensitive biomolecules. Examples include vacuum-assisted microwave and vacuum foam drying [209, 210].
However, while vacuum drying offers several advantages, it's important to consider its potential limitations. Some challenges include the need for specialized equipment to maintain a constant vacuum, potential issues with oxidation, and slower drying times due to reduced pressure. Despite these challenges, the potential for high-quality dried products positions vacuum drying as an attractive method in the food industry. Continuous research and technological improvements can help address these challenges, increasing the efficiency and applicability of this method.
Supercritical Fluid Drying
Supercritical fluid drying is a relatively new drying method utilized in the food and biopharmaceutical field, especially in the drying of proteins [211–215]. This technique leverages the anti-solvent properties of SCFs to induce protein precipitation and remove water from formulations. SCFs, existing at temperatures and pressures beyond their critical points, exhibit distinctive characteristics of both liquid and gas states. Their density can exceed that of a liquid under increased pressure, yet they maintain the diffusivity and viscosity similar to a gas, facilitating effective mass transfer. When subjected to a supercritical jet of cosolvent, it dissolves the free water in the material and as penetrates deeper to dissolve entrapped water and bound water. The convective mass transfer is driven by the concentration gradient of cosolvent between the material surface and the fluid medium. This mechanism has it’s disadvantages as there is a high risk of removing water-soluble nutrients and bioactive compounds along with the water. Hence, solvent pressure, flow rate, temperatures, etc., impact the techno-functional properties of the dried material.
Supercritical carbon dioxide (CO2) is commonly employed in supercritical fluid drying due to its relatively low critical temperature of 31.5 °C, significantly lower than water's 374.4 °C. Additionally, the Food and Drug Administration recognizes it as a safe substance for food treatment applications. However, research in this drying area is somewhat limited, and potential issues such as residual CO2 in the product, which may alter the pH of the end product, need further investigation [213]. SCF drying is primarily utilized for drying foods and biopharmaceuticals where preserving the structures of the material pores is not critical [216]. Some notable patented applications of SFD in drying foods and biopharmaceuticals are detailed in Table 7.
Table 7.
Applications of SCD in Foods and biopharmaceuticals
| Compounds | SC-Solvents | Reference |
|---|---|---|
| Protein, peptides, nucleic acids, bacterial cells, antibodies, serums, liposomes, and viruses | Near supercritical CO2 | [217–222] |
| β-Carotene, α-tocoferol and rosmarinic acid | Supercritical CO2 | [223] |
| Strawberries (ascorbic acid, anthocyanins) | Supercritical CO2 | [224] |
| drug substance, liposome |
CO2 or other gases /co-solvent (ethanol) |
[225, 226] |
| Theophylline | ethanol/CO2 | [227] |
| Phenolic compounds (gallic acid resveratrol) | Supercritical CO2 | [228] |
| Salmon calcitonin | Supercritical CO2 | [229] |
| Insulin | Supercritical CO2 | [230] |
| Fenofibrate particles | Supercritical CO2 + ethanol | [231] |
| Green tea extract | Supercritical CO2 | [232, 233] |
While supercritical fluid drying presents a new approach for drying functional foods and biopharmaceuticals, several challenges persist. These include the need for high pressure, potential residual solvents in the product, and substantial investment for setup and operation.
In conclusion, "Drying System for Heat-Sensitive Biomaterials" section provided a comprehensive review of the several drying methods used in drying food and biopharmaceuticals. It is evident that the preservation and retention of the nutritional value and bioactive properties of functional foods and biopharmaceuticals during drying is an area that needs further research. Our thorough research of the published work also showcased that each reviewed method offers unique advantages and presents certain limitations, influencing its suitability for different applications. Despite the challenges associated with each method, ongoing research and development efforts are continually seeking to optimize these techniques and address their limitations. The following section will build on this foundation to explore hybrid drying methods that combine the strengths of multiple techniques, pointing toward the future of drying technology.
Hybrid Drying Methods: Innovation and Opportunities
The effectiveness and appropriateness of the aforementioned drying methods depend on the of biomaterial or bioactive compound type, the initial state of the material to be dried, and the desired final product form and functionality. Table 8 provides a comprehensive summary of the various drying methods, highlighting their strengths and limitations and the biomaterials for which each method holds the greatest application potential. As reported in Table 8, many of these drying techniques have limitations that could be minimized by combining the different techniques to improve the overall drying process, preserving the product integrity, efficacy, and quality of biopharmaceuticals and nutraceuticals while enhancing efficiency and cost-effectiveness.
The ongoing quest to retain the bioactive properties of foods and biopharmaceuticals during drying has sparked numerous innovations, including developing hybrid drying methods. By integrating two or more existing techniques, these hybrid methods are designed to leverage the strengths of each approach, thereby compensating for their limitations.
The industry and researchers increasingly recognize emerging hybrid techniques such as microwave-assisted vacuum drying, microwave sprouted bed drying, superheated steam fluidized bed drying, vacuum double-drum drying, spray freeze drying, and infrared-assisted drying in functional foods and biopharmaceuticals due to their superior efficiency and performance [8, 249, 250]. The advent of particle engineering, encapsulation, and the development of novel functional food ingredients in biopharmaceuticals have underscored the need for comprehensive research on tailored drying strategies and hybrid methods. Table 9 presents examples of hybrid drying methods and their applications in various functional foods.
Table 9.
Hybrid drying methods for various functional foods
| Functional Food Product | Drying method and condition | Reference |
|---|---|---|
| Viable Probiotics | Fluidized bed drying with encapsulation | [239] |
| Egg White Powder | Foam mat freeze-drying | [251] |
| Lactobacillus plantarum in aloe vera and agave fructans, whey protein | Spray Freeze-Drying | [238, 252, 253] |
| Apple pomace powder, blueberries | Microwave-assisted vacuum drying | [242, 254] |
| Biologics and Vaccines | Microwave Vacuum Drying | [209] |
| Passionflower (Passiflora alata) | Spray and spouted bed | [252] |
| Mexican plum fruit extract | Spray Drying and Spout-Fluid Bed Drying Microencapsulation | [255] |
| Wolfberry (Lycium barbarum L.) | Far-infrared radiation heating assisted pulsed vacuum drying (temperature of 65 °C, vacuum pressure for 15 min, and normal pressure for 2 min) | [256] |
| Pre-osmodehydrated watermelon | CO2 convective drying with Far-Infrared radiation heating assisted pulsed vacuum drying | [257] |
| Potato slices (Phenolic and Flavonoids) | Ultrasound-assisted far-infrared radiation drying (ultrasonic resonant frequency of 28 ± 0.5 kHz and temperature of 50 °C) | [258] |
| Pear slices (Phenolic and Flavonoids) | Contact ultrasound-assisted far-infrared radiation drying (ultrasonic resonant frequency of 28 ± 0.5 kHz and temperature of 30 °C) | [259] |
| Garlic Slices (allicin content) | Ultrasonic-assisted vacuum drying (ultrasonic resonant frequency of 40 kHz and temperature of 60 °C | [260] |
| Acai puree (anthocyanin, phenolic compounds, antioxidants) | Infrared-assisted freeze-drying | [261] |
| Polyphenol-enriched maple sugar | Vacuum double-drum drying (80 °C and 87.99 kPa) | [262] |
| Mulberry leaves extract | Supercritical fluid extraction and spray drying | [263] |
| Chrysanthemum cake (Phenols) | Infrared and Hot Air Drying | [264] |
Advancements and Future Directions in Drying Technologies
Novel Drying Techniques
The novel, fourth-generation dryers primarily focus on product quality, drying efficiency, time and temperature changes. This category's major types of dryers are high-vacuum, microwave, radio-frequency, and refractive window drying [265]. Among them, microwave and radio-frequency drying have gained comparatively faster commercial applications and attention from food processors and researchers over the others. Even though the technologies using electromagnetic heating, such as radio-frequency and microwave drying, have been researched for decades, the commercial application is still lagging behind the other types. The commercial-level scale-up of radio-frequency drying is limited by the large number of parameters that control the drying efficiency, such as dielectric, physical, and thermal characteristics of the biomaterial to be dried, voltage of electrode, electrode distance, etc. All these factors result in non-uniform heating and uneven distribution of temperature [266, 267]. Hence, there is ongoing research on novel drying technologies such as halogen drying [268] and refractive window drying [269–271]. Refractive window drying has recently been researched for its specific indirect heating of the material and its potential application for low-temperature and short-time processes to dry delicate, heat-sensitive products [272]. This novel drying technique is based on all three modes of heat transfer through conduction, convection, and radiation. It is ideally suitable for liquid materials where high moisture material is spread over a thin infra-red film; the refractive indices of the water and the material become similar, reducing reflection at the interface and enhancing the transmissivity of radiant energy to the product. The method is reported to maintain product temperature between 60–70 °C due to evaporative cooling and convective heat transfer to the ambient air above the material [271, 272]. Despite the greater research and development in novel drying techniques, the commercial application of these techniques in the biopharmaceutical and nutraceutical food industries is limited by various factors such as cost, scalability, infrastructure requirement, technical expertise, etc.
Integration of Automation and Control Technologies
The landscape of drying technologies for functional foods and biopharmaceuticals is transforming substantially by integrating intelligent automation and control technologies. As advancements in artificial intelligence (AI), machine learning, the Internet of Things (IoT), and cyber-physical systems continue, new opportunities for improving precision, efficiency, and sustainability in drying technologies are revealed.
Central to this transformation is the role of process automation, which involves using advanced software and hardware to manage and monitor drying processes. Control systems equipped with programmable logic controllers (PLCs) and supervisory control and data acquisition (SCADA) systems offer precise control over process variables such as temperature, humidity, and air velocity. These systems ensure consistent product quality while minimizing energy consumption and waste by maintaining optimal drying conditions. Furthermore, automation remarkably reduces the need for manual supervision, thereby cutting labour costs and human error [273, 274].
Implementing AI and machine learning has demonstrated its efficacy across various sectors, including biopharmaceuticals [275–277], drying technologies [278–280], and agri-food quality monitoring [281–283]. Alongside automation, incorporating AI and machine learning within drying technologies has unveiled new avenues for enhancing process efficiency. Machine learning algorithms can analyze historical and real-time data, enabling the prediction of optimal drying conditions and swift responses to changes in process variables. Likewise, predictive models serve to optimize drying schedules, reduce energy consumption, and improve product quality. Moreover, advanced control systems developed with the help of AI and profound learning neural networks can learn, adapt, and make autonomous decisions based on complex data inputs, thus managing the nonlinear and dynamic nature of drying processes [278, 280, 284–286].
The complexities and scale of modern drying processes necessitate managing and analyzing large volumes of data, a demand met by cloud computing and big data analytics. Cloud computing and big data analytics provide scalable computational resources and tools for extracting valuable insights from complex data sets. Such capabilities support advanced AI and machine learning applications, predictive modelling, and real-time process optimization [287, 288].
Smart sensors and IoT technologies complement these developments, facilitating real-time monitoring and control of drying processes. Smart sensors gather granular data on various process parameters and environmental conditions, while IoT ensures interconnectivity among these sensors, forming a comprehensive and synchronized data network [285, 287, 289, 290]. This real-time data fuels AI and machine learning systems, empowering predictive analytics, real-time adjustments, and proactive maintenance. Moreover, IoT integration can extend beyond individual drying systems to include entire production lines or even multiple manufacturing sites, fostering systemic efficiency and coherence.
Advancing this integration further, cyber-physical systems (CPS) represent the next fusion level between hardware and software in drying technologies. These systems tightly couple the computational (cyber) elements with the physical components of a drying process. Creating a digital twin – a real-time virtual replica of the physical process – is possible with CPS. Such digital twins can simulate and evaluate different process conditions and control strategies, yielding substantial improvements in system design and operation [291–294].
Ultimately, these integrated advancements in automation, AI, machine learning, IoT, and cyber-physical systems drive the evolution of drying technologies for functional foods and biopharmaceuticals. The continued exploration of these cutting-edge technological advancements will further shape and enhance the efficiency and sustainability of drying processes.
Trends and Research Directions
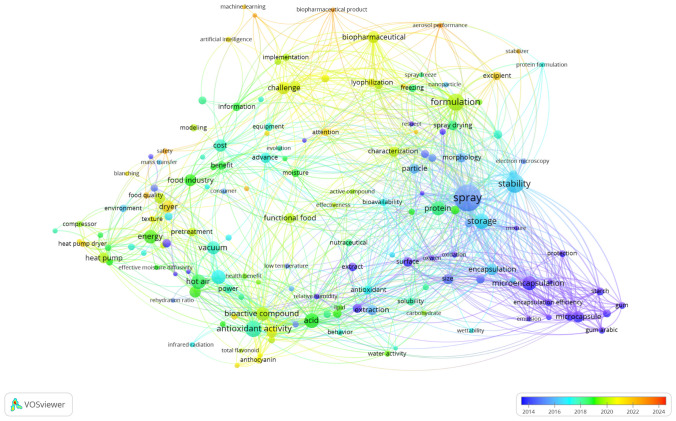
Drying technologies for functional foods and biopharmaceuticals have made remarkable strides, yet the future has potential for further innovation and refinement. A fundamental challenge lies in developing drying techniques that balance energy efficiency, cost-effectiveness, scalability, and preservation of nutritional and bioactive properties. To discern patterns and trends of advancement and innovations in the drying of biopharmaceuticals, nutraceuticals, and functional foods, a network visualization map was generated using VOSviewer as shown in Fig. 3. The figure visualizes the trends in drying of biopharmaceuticals, nutraceuticals, and functional foods over the years classified with keywords/terms with a technique of full counting generating 5 clusters of keywords. Based on the network visualization map, the advancement and innovations landscape in the drying of biopharmaceuticals, nutraceuticals, and functional foods showed that the earlier years of studies and focus were more skewed to the application of spray drying and micro-encapsulated spray-drying for biopharmaceuticals, nutraceuticals, and functional foods. This trend in technology and research has turned more towards hybrid drying systems and the application of machine learning, AI and IoT technology for improved hybrid drying systems for better drying efficiency and techno-functional properties of the final products in recent years.
Fig. 3.
Network visualization map on advancement and innovations landscape in drying of biopharmaceuticals, nutraceuticals, and functional foods
In the meantime, as discussed in "Advancements and Future Directions in Drying Technoligies" section, the digital age allows further integration of advanced technologies into these drying methods. Artificial intelligence, machine learning, and smart sensor technologies can profoundly transform drying processes. These technologies enable superior control, permit real-time modifications, and facilitate comprehensive optimization of drying processes, effectively ushering in a new era of intelligent and responsive drying techniques. These technologies use advanced sensors, data analytics, automation, and connection to improve drying efficiency, reduce energy consumption, and maintain product quality and safety. Preliminary studies on the IoT-based control system for smart drying technologies demonstrated the potential to preserve food's functional qualities and nutraceutical values, such as rehydration capacity, crude fibre, protein, and vitamin C levels, etc., compared to conventional drying method counterparts [295].
In parallel with technological innovations, the shift towards sustainable production systems necessitates a thorough understanding of the environmental impact and sustainability of drying techniques. This includes an evaluation of energy consumption, water usage, waste production, and how these techniques align with evolving regulatory requirements worldwide. The specific energy consumption (SEC) of drying technologies refers to the energy required to remove a unit of moisture from a product. Even though SEC is considered a good indicator of the energy performance of drying methods, it is often not proportional to the techno-functionality of this drying technology application in biopharmaceuticals and nutraceuticals. Figure 4 compares available data on SEC of different drying methods [296]. For instance, freeze-drying (lyophilization) is often preferred for biopharmaceuticals and certain nutraceuticals despite its relatively high energy consumption compared to other drying methods (Fig. 4). It has been reported to have a lower specific energy consumption (SEC) than freeze-drying, although it reduces total phenolic compounds [174]. Consequently, a hybrid infrared-freeze drying method has been reported effective for bioactive compounds to combine the benefits of both techniques, ensuring quality and energy efficiency [172, 174, 261]. Therefore, the choice of drying technology involves a trade-off between energy efficiency (as measured by SEC) and other factors such as product quality, safety, and regulatory compliance.
Fig. 4.
Comparison of specific energy consumption (SEC) for different drying techniques [161, 174, 245, 296–299]
From an economic perspective, detailed analyses are needed to evaluate the financial aspects of various methods as a function of techno-functionality. These would consider elements such as capital and operating costs and return on investment, providing insights into the economic feasibility of each method. This information would guide industries in making informed decisions on adopting these drying techniques.
Lastly, it is crucial to continue exploring the challenges and limitations inherent in different drying techniques, the potential integration of drying techniques for enhanced quality and sustainability, and the dedication of research efforts to finding potential pathways for digital transformation in the automation of these drying systems. This exploration will shape the evolution of drying technology, ensuring its practical viability and suitability for both functional foods and biopharmaceuticals.
Looking ahead, the emphasis should be on developing drying techniques that are not only efficient and sustainable but also economically viable. These methods must retain the quality of end products, ensuring that the therapeutic and nutritional benefits remain intact. Achieving harmony among these aspects will be pivotal in shaping the future landscape of drying techniques in the food, nutraceutical, and biopharmaceutical industries.
Conclusion
The process of drying functional foods and biopharmaceuticals poses unique challenges to industries due to the heat-sensitive nature of these products. Consequently, selecting the appropriate drying strategy and methods requires careful consideration, as different products require varying initial conditions to maintain their bioactive and functional components. Ongoing research focuses on enhancing existing systems and designing innovative hybrid solutions to improve drying outcomes. Functional foods and biopharmaceuticals are commonly dried under controlled conditions - either at lower temperatures or higher temperatures for brief periods - to safeguard the intrinsic functional properties. Critical to this process is a deep understanding of particle engineering for optimal rheology and microstructure and devising product-specific drying strategies. This understanding is fundamental given that several chemical instabilities, such as oxidation, aggregation, chemical bonding, and glycation, are commonplace in biomolecules. As such, optimizing various drying methods, including freeze-drying, vacuum drying, and superheated steam drying techniques, is essential for each category of these products, necessitating a comprehensive study of their nutritional and functional properties. The ongoing evolution of drying techniques is pivotal for the future of functional foods and biopharmaceuticals, seeking to balance quality retention, efficiency, and industrial feasibility in an ever-changing landscape. This comprehensive account of the advantages and limitations of each commonly used drying method provides researchers with a critical first building block to devise future innovative modifications to push the state-of-the-art into its future for drying products rich in bioactive volatiles.
Author Contribution
Dr. Rani P Ramachandran: Conceptualization; Investigation; Formal analysis, methodology and organization of the flow of information; Writing – original draft, Tables and Figures design, review and editing. Dr. Stefan Cenkowski & Dr. Jitendra Paliwal: Conceptualization; Investigation – review & editing. Dr. Mohammad Nadimi: Investigation;– review & editing, reformatting tables and figures; Funding acquisition: Dr. Jitendra Paliwal.
Funding
Open access funding provided by Agriculture & Agri-Food Canada library. This project was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (grant number RGPIN-2021-03350 received by Dr. J. Paliwal).
Availability of Data and Materials
Not applicable
Declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dable-Tupas G, Otero MCB, Bernolo L (2020) Functional foods and health benefits. Funct Foods Nutraceuticals 1:11 [Google Scholar]
- 2.Crowe KM et al (2013) Position of the academy of nutrition and dietetics: functional foods. J Acad Nutr Diet 113(8):1096–1103 [DOI] [PubMed] [Google Scholar]
- 3.Csapó J, Albert C, Szigeti TJ (2019) Functional foods. Élelmiszervizsgálati Közlemények 65(1):2340–2360 [Google Scholar]
- 4.John R, Singla A (2021) Functional foods: Components, health benefits, challenges, and major projects. J Environ Agric Energy 2:61–72 [Google Scholar]
- 5.Morais RMSC et al (2018) Functional dehydrated foods for health preservation. J Food Qual 2018:1739636 [Google Scholar]
- 6.Sajid AM et al (2021) Functional foods and human health: An overview. In: Muhammad Sajid A, Muhammad Haseeb A (eds) In Functional Foods. IntechOpen, Rijeka [Google Scholar]
- 7.Gervasi V et al (2018) Parenteral protein formulations: An overview of approved products within the European Union. Eur J Pharm Biopharm 131:8–24 [DOI] [PubMed] [Google Scholar]
- 8.Sharma A et al (2021) Innovative drying technologies for biopharmaceuticals. Int J Pharm 609:121115 [DOI] [PubMed] [Google Scholar]
- 9.ElGamal R et al (2023) Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 13:1580 [Google Scholar]
- 10.Hii CL et al (2021) Hybrid drying of food and bioproducts: a review. Drying Technol 39(11):1554–1576 [Google Scholar]
- 11.Bentley R (2009) Different roads to discovery; Prontosil (hence sulfa drugs) and penicillin (hence β-lactams). J Ind Microbiol Biotechnol 36(6):775–786 [DOI] [PubMed] [Google Scholar]
- 12.Ligon BL (2004) Penicillin: its discovery and early development. Seminars in Pediatric Infectious Diseases 15(1):52–57 [DOI] [PubMed] [Google Scholar]
- 13.Leo K, Meehan JJ, Sugihara TF (1963) Process of spray drying eggs. US3115413A
- 14.Emami F, Vatanara A, Park EJ, Na DH (2018) Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 10(3):131. 10.3390/pharmaceutics10030131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran RP, Paliwal J, Cenkowski S (2019) Computational modelling of superheated steam drying of compacted distillers’ spent grain coated with solubles. Food Bioprod Process 116:63–77 [Google Scholar]
- 16.Betoret E, Calabuig-Jiménez L, Barrera C, Rosa MD, Betoret E, Calabuig-Jiménez L, Barrera C, Rosa MD (2016) Sustainable drying technologies for the development of functional foods and preservation of bioactive compounds. In: Olvera JDR (ed) Sustainable Drying Technologies. 10.5772/64191 [Google Scholar]
- 17.Goyal MR, Veena N, Watharkar RB (eds) (2023) Advances in food proces engineering: novel processing, preservation, and decontamination of foods, 1st edn. Apple Academic Press. 10.1201/9781003303848
- 18.Kumar M, Madhumita M, Prabhakar PK, Basu S (2022) Refractance window drying of food and biological materials: Status on mechanisms, diffusion modelling and hybrid drying approach. Critical Reviews in Food Science and Nutrition 64(11):3458–3481. 10.1080/10408398.2022.2132210 [DOI] [PubMed] [Google Scholar]
- 19.Sruthy GN, Sandhya KR, Kumkum CR, Mythri R, Sharma M (2022) Chapter 10 - Thermal processing technologies for food . In: Tarafdar A, Pandey A, Sirohi R, Soccol C, Dussap C-G (eds) Current Developments in Biotechnology and Bioengineering. Elsevier, pp 263–300. 10.1016/B978-0-323-91158-0.00014-4 [Google Scholar]
- 20.Richter Reis F et al (2022) Trends in quality assessment and drying methods used for fruits and vegetables. Food Control 142:109254 [Google Scholar]
- 21.Guiné RPF (2018) The drying of foods and its effect on the physical-chemical, sensorial and nutritional properties. Int J Food Eng 2:93–100 [Google Scholar]
- 22.Djekić I et al (2023) Food quality 4.0: sustainable food manufacturing for the twenty-first century. Food Eng Rev 15(4):577–608 [Google Scholar]
- 23.Knoerzer K et al (2015) Multiphysics simulation of innovative food processing technologies. Food Eng Rev 7(2):64–81 [Google Scholar]
- 24.Xu E et al (2022) Heat-induced conversion of multiscale molecular structure of natural food nutrients: A review. Food Chem 369:130900 [DOI] [PubMed] [Google Scholar]
- 25.Nyoni B et al (2020) Modelling of thermal decomposition kinetics of proteins, carbohydrates and lipids using scenedesmus microalgae thermal data. Asian J Chem 32(11):2921–2926 [Google Scholar]
- 26.Osman AI et al (2022) Comprehensive thermokinetic modelling and predictions of cellulose decomposition in isothermal, non-isothermal, and stepwise heating modes. J Anal Appl Pyrol 161:105427 [Google Scholar]
- 27.Claude J, Ubbink J (2006) Thermal degradation of carbohydrate polymers in amorphous states: A physical study including colorimetry. Food Chem 96(3):402–410 [Google Scholar]
- 28.Barišić V, Kopjar M, Jozinović A, Flanjak I, Ačkar Đ, Miličević B, Šubarić D, Jokić S, Babić J (2019) The Chemistry behind Chocolate Production. Molecules 24(17):3163. 10.3390/molecules24173163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batiot B et al (2021) Origin and Justification of the use of the arrhenius relation to represent the reaction rate of the thermal decomposition of a solid. Appl Sci. 10.3390/app11094075
- 30.Akbar J et al (2012) Kinetics and mechanism of thermal degradation of pentose- and hexose-based carbohydrate polymers. Carbohyd Polym 90(3):1386–1393 [DOI] [PubMed] [Google Scholar]
- 31.Borzova VA et al (2016) Kinetics of thermal denaturation and aggregation of bovine serum albumin. PLoS ONE 11(4):e0153495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weijers M et al (2003) Heat-induced denaturation and aggregation of ovalbumin at neutral pH described by irreversible first-order kinetics. Protein Sci 12(12):2693–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quevedo M, Karbstein HP, Emin MA (2020) Denaturation behavior and kinetics of single- and multi-component protein systems at extrusion-like conditions. Polymers (Basel) 12(9):2145. 10.3390/polym12092145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang Y, Dong J, He X, Wang J, Li C, Dong L, Zhang Y, Zhou X, Wang H, Yi Y, Wang S (2022) Impact of Heating Temperature and Fatty Acid Type on the Formation of Lipid Oxidation Products During Thermal Processing. Front Nutr 9. 10.3389/fnut.2022.913297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D-Y et al (2020) Impact of different drying processes on the lipid deterioration and color characteristics of Penaeus vannamei. J Sci Food Agric 100(6):2544–2553 [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez A, Trigo M, Aubourg SP, Medina I (2021) Optimisation of healthy-lipid content and oxidative stability during oil extraction from squid (Illex argentinus) viscera by green processing. Mar Drugs 19(11):616. 10.3390/md19110616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cong S et al (2020) Characterization of the lipid oxidation process of robusta green coffee beans and shelf life prediction during accelerated storage. Molecules. 10.3390/molecules25051157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godoy HT, Amaya-Farfan J, Rodriguez-Amaya DB (2021) Chapter 8 - Degradation of vitamins. In: Rodriguez-Amaya DB, Amaya-Farfan J (eds) Chemical Changes During Processing and Storage of Foods. Academic Press, pp 329–383. 10.1016/B978-0-12-817380-0.00008-7 [Google Scholar]
- 39.ElGamal R et al (2023) Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: a comprehensive overview. Agronomy. 10.3390/agronomy13061580 [Google Scholar]
- 40.Ryley J, Kajda P (1994) Vitamins in thermal processing. Food Chem 49(2):119–129 [Google Scholar]
- 41.Demiray E, Tulek Y, Yilmaz Y (2013) Degradation kinetics of lycopene, β-carotene and ascorbic acid in tomatoes during hot air drying. LWT Food Sci Technol 50(1):172–176 [Google Scholar]
- 42.Dutta D et al (2006) Rheological characteristics and thermal degradation kinetics of beta-carotene in pumpkin puree. J Food Eng 76(4):538–546 [Google Scholar]
- 43.Shonte TT, Duodu KG, de Kock HL (2020) Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nettle leaves. Heliyon 6(5):e03938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korus A (2022) Effect of pre-treatment and drying methods on the content of minerals, B-group vitamins and tocopherols in kale (Brassica oleracea L. var. acephala) leaves. J Food Sci Technol 59(1):279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snoussi A et al (2021) Drying methodology effect on the phenolic content, antioxidant activity of Myrtus communis L. leaves ethanol extracts and soybean oil oxidative stability. BMC Chem 15(1):31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan S et al (2020) Effects of three drying methods on polyphenol composition and antioxidant activities of Litchi chinensis Sonn. Food Sci Biotechnol 29(3):351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loemba ABT, Kichonge B, Kivevele T (2023) Comprehensive assessment of heat pump dryers for drying agricultural products. Energy Sci Eng 11(8):2985–3014 [Google Scholar]
- 48.Shu B et al (2022) Newly generated and increased bound phenolic in lychee pulp during heat-pump drying detected by UPLC–ESI-triple-TOF-MS/MS. J Sci Food Agric 102(4):1381–1390 [DOI] [PubMed] [Google Scholar]
- 49.Singh A, Sarkar J, Sahoo RR (2020) Experiment on waste heat recovery-assisted heat pump drying of food chips: Performance, economic, and exergoeconomic analyses. J Food Process Preserv 44(9):e14699 [Google Scholar]
- 50.Hu Y, Feng Z, Song W (2023) Study on performance of a water-source gas engine-driven heat pump system for combined cooling and heating supply. Therm Sci Eng Prog 39:101726 [Google Scholar]
- 51.Jia L-L et al (2020) Experimental analysis of a novel gas-engine-driven heat pump (GEHP) system for combined cooling and hot-water supply. Int J Refrig 118:84–92 [Google Scholar]
- 52.Pawela B, Jaszczur M (2022) Review of gas engine heat pumps. Energies. 10.3390/en15134874 [Google Scholar]
- 53.Salehi F (2021) Recent applications of heat pump dryer for drying of fruit crops: a review. Int J Fruit Sci 21(1):546–555 [Google Scholar]
- 54.Badiei A, Golizadeh Akhlaghi Y, Zhao X, Shittu S, Xiao X, Li J, Fan Y, Li G (2020) A chronological review of advances in solar assisted heat pump technology in 21st century. Renew Sustain Energy Rev 132:110132 [Google Scholar]
- 55.Deef M et al (2023) Harnessing solar energy: a novel hybrid solar dryer for efficient fish waste processing. AgriEngineering 5:2439–2457 [Google Scholar]
- 56.Gu X et al (2022) Experimental and theoretical assessment of a solar assisted heat pump system for in-bin grain drying: A comprehensive case study. Renewable Energy 181:426–444 [Google Scholar]
- 57.Vaishak S, Bhale PV (2019) Photovoltaic/thermal-solar assisted heat pump system: Current status and future prospects. Sol Energy 189:268–284 [Google Scholar]
- 58.Dai S et al (2020) Thermodynamic analysis of a novel chemical heat pump cycle based on the physical-chemical thermal effects of reversible reaction. Energy Convers Manage 225:113419 [Google Scholar]
- 59.Çerçi KN, Hürdoğan E (2022) Performance assessment of a heat pump assisted rotary desiccant dryer for low temperature peanut drying. Biosys Eng 223:1–17 [Google Scholar]
- 60.Li W, Yin Y, Wang Y (2022) Performance evaluation of a heat pump-driven liquid desiccant dehumidification system integrated with fresh air supply. Energy Buildings 275:112473 [Google Scholar]
- 61.Sun D et al (2017) Study on combined heat pump drying with freeze-drying of Antarctic krill and its effects on the lipids. J Food Process Eng 40(6):e12577 [Google Scholar]
- 62.Garcìa LM et al (2021) Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. Leaves Horticulturae 7:10 [Google Scholar]
- 63.Zhu Z et al (2021) Effects of ultrasound pretreatment on the drying kinetics, water status and distribution in scallop adductors during heat pump drying. J Sci Food Agric 101(15):6239–6247 [DOI] [PubMed] [Google Scholar]
- 64.Thao BTT, Hung, PT, Lai ND, Tran TYN, Nguyen NQ, Pham TN, Tran TT, Bach LG, Dao TP (2024) Application of heat pump drying technology to produce dried mango products from Tu Quy mango (Mangifera india L.), Vietnam, on a pilot scale. Front Sustain Food Syst 8. 10.3389/fsufs.2024.1204303
- 65.Patel KK, Kar A (2012) Heat pump assisted drying of agricultural produce—an overview. J Food Sci Technol 49(2):142–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tajudin NHA et al (2019) Comparison of drying kinetics and product quality from convective heat pump and solar drying of Roselle calyx. Food Bioprod Process 118:40–49 [Google Scholar]
- 67.Authelin JR, Rodrigues MA, Tchessalov S, Singh SK, McCoy T, Wang S, Shalaev E (2020) Freezing of biologicals revisited: scale, stability, excipients, and degradation stresses. J Pharma Sci 109:44–61 [DOI] [PubMed] [Google Scholar]
- 68.Jouki M et al (2021) Encapsulation of Lactobacillus casei in quince seed gum-alginate beads to produce a functional synbiotic drink powder by agro-industrial by-products and freeze-drying. Food Hydrocolloids 120:106895 [Google Scholar]
- 69.Bhatta S, Stevanovic Janezic T, Ratti C (2020) Freeze-drying of plant-based foods. Foods 9(1):87. 10.3390/foods9010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Zhang Z, Hu L (2022) High efficient freeze-drying technology in food industry. Crit Rev Food Sci Nutr 62(12):3370–3388 [DOI] [PubMed] [Google Scholar]
- 71.Franks F, Auffret T (2007) Freeze-drying of pharmaceuticals and biopharmaceuticals. Royal Society of Chemistry. 10.1039/9781847557704
- 72.Nowak D, Jakubczyk E (2020) The freeze-drying of foods-the characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 9(10):1488. 10.3390/foods9101488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakagawa K, Ochiai T (2015) A mathematical model of multi-dimensional freeze-drying for food products. J Food Eng 161:55–67 [Google Scholar]
- 74.Mohammady M, Mohammadi Y, Yousefi G (2020) Freeze-drying of pharmaceutical and nutraceutical nanoparticles: the effects of formulation and technique parameters on nanoparticles characteristics. J Pharm Sci 109(11):3235–3247. 10.1016/j.xphs.2020.07.015 [DOI] [PubMed] [Google Scholar]
- 75.Colucci D, Prats-Montalbán JM, Ferrer A, Fissore D (2021) On-line product quality and process failure monitoring in freeze-drying of pharmaceutical products. Drying Technol 39:134–147 [Google Scholar]
- 76.Engstrom JD et al (2008) Formation of stable submicron protein particles by thin film freezing. Pharm Res 25(6):1334–1346 [DOI] [PubMed] [Google Scholar]
- 77.Ahmed J, Thomas L, Khashawi R (2020) Influence of hot-air drying and freeze-drying on functional, rheological, structural and dielectric properties of green banana flour and dispersions. Food Hydrocolloids 99:105331. 10.1016/j.foodhyd.2019.105331 [Google Scholar]
- 78.Vardanega R et al (2019) Obtaining functional powder tea from Brazilian ginseng roots: Effects of freeze and spray drying processes on chemical and nutritional quality, morphological and redispersion properties. Food Res Int 116:932–941 [DOI] [PubMed] [Google Scholar]
- 79.Gutiérrez L-F, Ratti C, Belkacemi K (2008) Effects of drying method on the extraction yields and quality of oils from quebec sea buckthorn (Hippophaë rhamnoides L.) seeds and pulp. Food Chem 106(3):896–904 [Google Scholar]
- 80.Araya-Farias M, Makhlouf J, Ratti C (2011) Drying of seabuckthorn (Hippophae rhamnoides L.) Berry: impact of dehydration methods on kinetics and quality. Drying Technol 29:51–359 [Google Scholar]
- 81.Reyes A et al (2011) Effect of operating conditions in freeze-drying on the nutritional properties of blueberries. Int J Food Sci Nutr 62(3):303–306 [DOI] [PubMed] [Google Scholar]
- 82.Moayyedi M et al (2018) Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J Funct Foods 40:391–399 [Google Scholar]
- 83.Mostafa Mohammed D et al (2024) Effect of Spirulina maxima microcapsules to mitigate testicular toxicity induced by cadmium in rats: Optimization of in vitro release behavior in the milk beverage. J Funct Foods 112:105938 [Google Scholar]
- 84.Hufnagel S et al (2022) Dry powders for inhalation containing monoclonal antibodies made by thin-film freeze-drying. Int J Pharm 618:121637 [DOI] [PubMed] [Google Scholar]
- 85.Oddone I, Barresi AA, Pisano R (2017) Influence of controlled ice nucleation on the freeze-drying of pharmaceutical products: the secondary drying step. Int J Pharm 524(1):134–140 [DOI] [PubMed] [Google Scholar]
- 86.Adali MB, Barresi AA, Boccardo G, Pisano R (2020) Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Processes 8:709–717 [Google Scholar]
- 87.Ishwarya SP, Anandharamakrishnan C, Stapley AGF (2015) Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci Technol 41(2):161–181 [Google Scholar]
- 88.Langford A et al (2018) Drying technologies for biopharmaceutical applications: Recent developments and future direction. Drying Technol 36(6):677–684 [Google Scholar]
- 89.Praphawatvet T, Cui Z, Williams RO (2022) Pharmaceutical dry powders of small molecules prepared by thin-film freezing and their applications – A focus on the physical and aerosol properties of the powders. Int J Pharm 629:122357 [DOI] [PubMed] [Google Scholar]
- 90.Rostamnezhad M et al (2022) Spray freeze-drying for inhalation application: process and formulation variables. Pharm Dev Technol 27(3):251–267 [DOI] [PubMed] [Google Scholar]
- 91.Miller DA, Ellenberger D, Porfirio T, Gil M (2022) Spray-drying technology. In: Williams Iii RO, Davis DA Jr, Miller DA (eds) In Formulating Poorly Water Soluble Drugs. Springer International Publishing, Cham, pp 377–452. 10.1007/978-3-030-88719-3_10 [Google Scholar]
- 92.Santos D, Maurício AC, Sencadas V, Santos, JD, Fernandes MH, Gomes, PS (2017) Spray drying: an overview. 10.5772/intechopen.72247
- 93.Ramachandran RP et al (2018) Computational fluid dynamics in drying process modelling - a technical review. Food Bioprocess Technol 11:271–292 [Google Scholar]
- 94.Samborska K et al (2022) Innovations in spray drying process for food and pharma industries. J Food Eng 321:110960 [Google Scholar]
- 95.O’Connell K, Olaleye AK, Van den Akker HEA (2023) A porous-crust drying model for a single dairy droplet. Chem Eng Res Des 200:741–752 [Google Scholar]
- 96.Ledet GA et al (2015) Spray-drying of biopharmaceuticals. In: Varshney D, Singh M (eds) In Lyophilized Biologics and Vaccines: Modality-Based Approaches. Springer, New York, NY, pp 273–297 [Google Scholar]
- 97.Furuta T, Neoh TL (2021) Microencapsulation of food bioactive components by spray drying: A review. Drying Technol 39(12):1800–1831 [Google Scholar]
- 98.Baldelli A, Ren M, Liang DY, Lai S, Hartono B, Sum K, Pratap-Singh A (2023) Sprayed microcapsules of minerals for fortified food. J Funct Foods 101:105401 [Google Scholar]
- 99.Hardas N et al (2000) Accelerated stability studies of microencapsulated anhydrous milk fat. LWT Food Sci Technol 33(7):506–513 [Google Scholar]
- 100.Young SL, Sarda X, Rosenberg M (1993) Microencapsulating properties of whey proteins. Microencapsulation of anhydrous milk fat. J Dairy Sci 76(10):2868–2877 [Google Scholar]
- 101.Liu X-D et al (2000) Retention of emulsified flavor in a single droplet during drying. Food Sci Technol Res 6(4):335–339 [Google Scholar]
- 102.Rosenberg M, Kopelman IJ, Talmon Y (1990) Factors affecting retention in spray-drying microencapsulation of volatile materials. J Agric Food Chem 38(5):1288–1294 [Google Scholar]
- 103.Bylaitë E, Rimantas Venskutonis P, Maþdþierienë R (2001) Properties of caraway ( Carum carvi L.) essential oil encapsulated into milk protein-based matrices. Eur Food Res Technol 212:661–670 [Google Scholar]
- 104.Al-Ismail KM et al (2015) Effect of microencapsulation of cardamom’s essential oil in gum Arabic and whey protein isolate using spray drying on its stability during storage. Qual Assur Saf Crops Foods 7:613–620 [Google Scholar]
- 105.Krishnan S, Bhosale R, Singhal RS (2005) Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohyd Polym 61(1):95–102 [Google Scholar]
- 106.Balakrishnan M et al (2021) Microencapsulation of bixin pigment by spray drying: Evaluation of characteristics. LWT Food Sci Technol 145:111343 [Google Scholar]
- 107.Barbosa MIMJ, Borsarelli CD, Mercadante AZ (2005) Light stability of spray-dried bixin encapsulated with different edible polysaccharide preparations. Food Res Int 38:989–994 [Google Scholar]
- 108.Jafari SM, He Y, Bhandari B (2007) Encapsulation of nanoparticles of d-limonene by spray drying: role of emulsifiers and emulsifying techniques. Drying Technol 25(6):1069–1079 [Google Scholar]
- 109.Soottitantawat A et al (2005) Influence of emulsion and powder size on the stability of encapsulated d-limonene by spray drying. Innov Food Sci Emerg Technol 6(1):107–114 [Google Scholar]
- 110.Soottitantawat A et al (2005) Microencapsulation of l-menthol by spray drying and its release characteristics. Innov Food Sci Emerg Technol 6(2):163–170 [Google Scholar]
- 111.Pascual Pineda LA et al (2021) Clustering function and minimum change in spreading pressure as key factor to predict storage conditions for black pepper oleoresin encapsulated by spray drying. Food Biosci 42:101215 [Google Scholar]
- 112.Shaikh J, Bhosale R, Singhal R (2006) Microencapsulation of black pepper oleoresin. Food Chem 94(1):105–110 [Google Scholar]
- 113.Kanakdande D, Bhosale R, Singhal RS (2007) Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohyd Polym 67(4):536–541 [Google Scholar]
- 114.Watanabe Y et al (2002) Suppressive effect of saturated acyl l-ascorbate on the oxidation of linoleic acid encapsulated with maltodextrin or gum arabic by spray-drying. J Agric Food Chem 50(14):3984–3987 [DOI] [PubMed] [Google Scholar]
- 115.Cui T et al (2021) Characterization and human microfold cell assay of fish oil microcapsules: Effect of spray drying and freeze-drying using konjac glucomannan (KGM)-soybean protein isolate (SPI) as wall materials. J Funct Foods 83:104542 [Google Scholar]
- 116.Drusch S (2007) Sugar beet pectin: A novel emulsifying wall component for microencapsulation of lipophilic food ingredients by spray-drying. Food Hydrocolloids 21(7):1223–1228 [Google Scholar]
- 117.Polavarapu S et al (2011) Physicochemical characterisation and oxidative stability of fish oil and fish oil–extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem 127(4):1694–1705 [Google Scholar]
- 118.Teixeira MI et al (2004) Characterization of short chain fatty acid microcapsules produced by spray drying. Mater Sci Eng C 24(5):653–658 [Google Scholar]
- 119.Yue H et al (2020) Development and optimization of spray-dried functional oil microcapsules: Oxidation stability and release kinetics. Food Sci Nutr 8(9):4730–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu J et al (2022) Microencapsulated hawthorn berry polyphenols alleviate exercise fatigue in mice by regulating AMPK signaling pathway and balancing intestinal microflora. J Funct Foods 97:105255 [Google Scholar]
- 121.Shu B et al (2006) Study on microencapsulation of lycopene by spray-drying. J Food Eng 76(4):664–669 [Google Scholar]
- 122.Souza ALR et al (2018) Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT 91:286–292 [Google Scholar]
- 123.Aniesrani Delfiya DS et al (2015) Microencapsulation of turmeric oleoresin by spray drying and in vitro release studies of microcapsules. J Food Process Eng 38:37–48 [Google Scholar]
- 124.Baumann JM, Adam MS, Wood JD (2021) Engineering advances in spray drying for pharmaceuticals. Annu Rev Chem Biomol Eng 12:217–240 [DOI] [PubMed] [Google Scholar]
- 125.Vega C, Roos YH (2006) Invited review: spray-dried dairy and dairy-like emulsions—compositional considerations. J Dairy Sci 89(2):383–401 [DOI] [PubMed] [Google Scholar]
- 126.Anand A, Gareipy Y, Raghavan V (2021) Fluidized bed and microwave-assisted fluidized bed drying of seed grade soybean. Drying Technol 39:507–527 [Google Scholar]
- 127.Di Renzo A, Scala F, Heinrich S (2021) First_page settings order article reprints open accesseditorial recent advances in fluidized bed hydrodynamics and transport phenomena—progress and understanding. Processes 9(4):639. 10.3390/pr9040639 [Google Scholar]
- 128.Luthra K, Sadaka SS (2020) Challenges and opportunities associated with drying rough rice in fluidized bed dryers: a review. Trans ASABE 63(3):583–595 [Google Scholar]
- 129.Mujaffar S, Ramsumair S (2019) Fluidized bed drying of pumpkin (Cucurbita sp.) Seeds. Foods. 10.3390/foods8050147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Souza CRF, Oliveira WP (2012) Drying of phytochemical preparations in a spouted bed: perspectives and challenges. Drying Technol 30(11–12):1209–1226 [Google Scholar]
- 131.Sozzi A et al (2021) Fluidized bed drying of blackberry wastes: Drying kinetics, particle characterization and nutritional value of the obtained granular solids. Powder Technol 385:37–49 [Google Scholar]
- 132.Kimwa MJ, Karuri N, Tanui J (2023) Computational modeling of spatial variation in moisture content and temperature distribution in corn at different superheated steam temperatures. Cogent Engineering 10(1):2216864 [Google Scholar]
- 133.Reyes A et al (2012) Analysis of the drying of broccoli florets in a fluidized pulsed bed. Drying Technol 30(11–12):1368–1376 [Google Scholar]
- 134.Hampel N et al (2013) Continuous pellet coating in a Wurster fluidized bed process. Chem Eng Sci 86:87–98 [Google Scholar]
- 135.Soponronnarit S et al (2001) Fluidised bed drying of soybeans. J Stored Prod Res 37(2):133–151 [DOI] [PubMed] [Google Scholar]
- 136.Poddar D et al (2022) Effect of fluidized bed drying, matrix constituents and structure on the viability of probiotic lactobacillus paracasei ATCC 55544 during storage at 4 °C, 25 °C and 37 °C. Microorganisms. 10.3390/microorganisms10010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kilic A (2020) Low temperature and high velocity assisted fluidized bed drying characteristics of bee pollen as bioactive food. J Food Process Eng 43(8):e13439 [Google Scholar]
- 138.Kaur S, Dhurve P, Arora VK (2022) Statistical approach to investigate the effect of vibro-fluidized bed drying on bioactive compounds of muskmelon (Cucumis melo) seeds. J Food Process Preserv 46(9):e16331 [Google Scholar]
- 139.Cenkowski S, Pronyk C, Zmidzinska D, Muir WE (2007) Decontamination of food products with superheated steam. J Food Eng 83:68–75 [Google Scholar]
- 140.Hu Y et al (2016) Microbial decontamination of wheat grain with superheated steam. Food Control 62:264–269 [Google Scholar]
- 141.Nygaard H, Hostmark O (2008) Microbial inactivation during superheated steam drying of fish meal. Drying Technol 26(2):222–230 [Google Scholar]
- 142.Speckhahn A, Srzednicki G, Desai DK (2010) Drying of beef in superheated steam. Drying Technol 28(9):1072–1082 [Google Scholar]
- 143.Prachayawarakorn S et al (2002) Desorption isotherms and drying characteristics of shrimp in superheated steam and hot air. Drying Technol 20(3):669–684 [Google Scholar]
- 144.Prachayawarakorn S, Prachayawasin P, Soponronnarit S (2006) Heating process of soybean using hot-air and superheated-steam fluidized-bed dryers. LWT Food Sci Technol 39(7):770–778 [Google Scholar]
- 145.Head DS et al (2010) Superheated steam processing of oat groats. LWT Food Sci Technol 43(4):690–694 [Google Scholar]
- 146.Chungcharoen T, Prachayawarakorn S, Tungtrakul P, Soponronnarit S (2014) Quality attributes of germinated high amylose and waxy rice in superheated steam and hot air drying. Drying Technol 33(7):876–885 [Google Scholar]
- 147.Alfy A, Kiran BV, Jeevitha GC, Hebbar HU (2016) Recent developments in superheated steam processing of foods-a review. Crit Rev Food Sci Nutr 56:2191–2208 [DOI] [PubMed] [Google Scholar]
- 148.Fang J et al (2023) Superheated steam processing: An emerging technology to improve food quality and safety. Crit Rev Food Sci Nutr 63(27):8720–8736 [DOI] [PubMed] [Google Scholar]
- 149.Patel SK, Bade MH (2020) Superheated steam drying and its applicability for various types of the dryer: The state of art. Drying Technol 39(3):284–305 [Google Scholar]
- 150.Bennamoun L, Ndukwu MC (2023) 11 - Superheated steam drying. In: Jafari SM, Malekjani N (eds) In Drying Technology in Food Processing. Woodhead Publishing, pp 341–377 [Google Scholar]
- 151.Chou SK, Chua KJ (2001) New hybrid drying technologies for heat sensitive foodstuffs. Trends Food Sci Technol 12:359–369 [Google Scholar]
- 152.Devahastin S, Mujumdar AS (2014) Superheated steam drying of foods and biomaterials. In: Tsotsas E, Mujumdar AS (eds) Modern Drying Technology. Wiley
- 153.Suvarnakuta P, Devahastin S, Soponronnarit S, Mujumdar AS (2005) Drying kinetics and inversion temperature in a low-pressure superheated steam-drying system. Ind Eng Chem Res 44(6):1934–1941. 10.1021/ie049675r [Google Scholar]
- 154.Suvarnakuta P, Devahastin S, Mujumdar AS (2007) A mathematical model for low-pressure superheated steam drying of a biomaterial. Chem Eng Process 46(7):675–683 [Google Scholar]
- 155.Ramachandran RP, Akbarzadeh M, Paliwal J, Cenkowski S (2017) Three-dimensional CFD modelling of superheated steam drying of a single distillers’ spent grain pellet. J Food Eng 212:121–135. 10.1016/j.jfoodeng.2017.05.025 [Google Scholar]
- 156.Roslan AS et al (2020) Effect of drying methods and parameters on the antioxidant properties of tea (Camellia sinensis) leaves. Food Prod Process Nutr 2(1):8 [Google Scholar]
- 157.Sehrawat R, Nema PK (2018) Low pressure superheated steam drying of onion slices: kinetics and quality comparison with vacuum and hot air drying in an advanced drying unit. J Food Sci Technol 55(10):4311–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suvarnakuta P, Chaweerungrat C, Devahastin S (2011) Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem 125(1):240–247 [Google Scholar]
- 159.Jittanit W, Angkaew K (2020) Effect of superheated-steam drying compared to conventional parboiling on chalkiness, head rice yield and quality of chalky rice kernels. J Stored Prod Res 87:101627 [Google Scholar]
- 160.Taechapairoj C et al (2003) Superheated steam fluidised bed paddy drying. J Food Eng 58(1):67–73 [Google Scholar]
- 161.Sobulska M, Wawrzyniak P, Woo MW (2022) Superheated steam spray drying as an energy-saving drying technique: a review. Energies. 10.3390/en15228546 [Google Scholar]
- 162.Romdhana H, Bonazzi C, Esteban-Decloux M (2015) Superheated steam drying: an overview of pilot and industrial dryers with a focus on energy efficiency. Drying Technol 33(10):1255–1274 [Google Scholar]
- 163.Delfiya DSA, Prashob K, Murali S, Alfiya PV, Samuel MP, Pandiselvam R (2021) Drying kinetics of food materials in infrared radiation drying A review. J Food Process Eng 45(6):e13810 [Google Scholar]
- 164.Doymaz I (2012) Drying of pomegranate seeds using infrared radiation. Food Sci Biotechnol 21:1269–1275 [Google Scholar]
- 165.Huang D et al (2021) Application of infrared radiation in the drying of food products. Trends Food Sci Technol 110:765–777 [Google Scholar]
- 166.Sakare P et al (2020) Infrared drying of food materials: recent advances. Food Eng Rev 12(3):381–398 [Google Scholar]
- 167.Pawar SB, Pratape VM (2017) Fundamentals of infrared heating and its application in drying of food materials: a review. J Food Process Eng 40(1) [Google Scholar]
- 168.Karaca Dolgun G, Aktaş M, Dolgun EC (2021) Infrared convective drying of walnut with energy-exergy perspective. J Food Eng 306:110638 [Google Scholar]
- 169.Lee S-H et al (2010) Antioxidative effect of Ecklonia cava dried by far infrared radiation drying. Food Sci Biotechnol 19(1):129–135 [Google Scholar]
- 170.Lee S-H, Jeon Y-J (2010) Effects of far infrared radiation drying on antioxidant and anticoagulant activities of Ecklonia cava extracts. J Korean Soc Appl Biol Chem 53(2):175–183 [Google Scholar]
- 171.Senevirathne M et al (2010) Effect of far-infrared radiation drying of citrus press-cakes on free radical scavenging and antioxidant activities. J Food Eng 97(2):168–176 [Google Scholar]
- 172.Antal T et al (2017) Comparative effects of three different drying methods on drying kinetics and quality of jerusalem artichoke (Helianthus tuberosus L.). J Food Process Preserv 41(3):e12971 [Google Scholar]
- 173.Lee S-C et al (2006) Effect of far-infrared irradiation on catechins and nitrite scavenging activity of green tea. J Agric Food Chem 54(2):399–403 [DOI] [PubMed] [Google Scholar]
- 174.Antal T et al (20117) Comparison of drying and quality characteristics of pear (Pyrus Communis L.) using mid-infrared-freeze drying and single stage of freeze drying. 13(4)
- 175.Ratseewo J, Meeso N, Siriamornpun S (2020) Changes in amino acids and bioactive compounds of pigmented rice as affected by far-infrared radiation and hot air drying. Food Chem 306:125644 [DOI] [PubMed] [Google Scholar]
- 176.Samarakoon K, Senevirathne M, Lee WW, Kim YT, Kim JI, Oh MC, Jeon YJ (2012) Antibacterial effect of citrus press-cakes dried by high speed and far-infrared radiation drying methods. Nurs Res Pract 6(3):187–194. 10.4162/nrp.2012.6.3.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pei Y et al (2021) Effects of ultrasound pretreatment followed by far-infrared drying on physicochemical properties, antioxidant activity and aroma compounds of saffron (Crocus sativus L.). Food Biosci 42:101186 [Google Scholar]
- 178.Kim W-W et al (2012) Effect of far infrared drying on antioxidant property, anti-inflammatory activity, and inhibitory activity in A549 cells of Gamguk (Chrysanthemum indicum L.) flower. Food Sci Biotechnol 21(1):261–265 [Google Scholar]
- 179.Prabawa SB et al (2023) The physicochemical quality of yellow chrysanthemum flower (Chrysanthemum indicum) brewed drink. Food Res
- 180.Ratseewo J et al (2022) Effects of far-infrared radiation drying on starch digestibility and the content of bioactive compounds in differently pigmented rice varieties. Foods. 10.3390/foods11244079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee S-C, Kim J-H, Jeng S-M, Kim D-R, Ha J-U, Na KC, Ahn DU (2003) Effect of far-infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem 51(15):4400–4403. 10.1021/jf0300285 [DOI] [PubMed] [Google Scholar]
- 182.Lee S-C et al (2006) Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem 94(4):489–493 [Google Scholar]
- 183.Boateng ID, Yang XM, Li YY (2021) Optimization of infrared-drying parameters for Ginkgo biloba L. seed and evaluation of product quality and bioactivity. Ind Crops Prod 160
- 184.Zhou L et al (2017) Drying of garlic slices (Allium Sativum L.) and its effect on thiosulfinates, total phenolic compounds and antioxidant activity during infrared drying. J Food Process Preserv 41(1):e12734 [Google Scholar]
- 185.Feng H, Yin Y, Tang J (2012) Microwave drying of food and agricultural materials: basics and heat and mass transfer modeling. Food Eng Rev 4:89–106. 10.1007/s12393-012-9048-x [Google Scholar]
- 186.Ravikumar M, Srinath MK, Prasad MSG (2023) Thermal modelling of microwave dehydration of fruit slice. Case Stud Therm Eng 51:103543 [Google Scholar]
- 187.Cárdenas-Bailón F, Pérez-Vázquez C, Osorio-Revilla G, Gallardo-Velázquez T (2019) Shrinkage modeling, drying kinetics and quality assessment of carrot cubes dried in a two stage spouted bed drying process. Emir J Food Agric 31:654–665 [Google Scholar]
- 188.Wang J, Xi YS (2005) Drying characteristics and drying quality of carrot using a two-stage microwave process. J Food Eng 68(4):505–511 [Google Scholar]
- 189.Kelen A et al (2006) Practical method for choosing diluent that ensures the best temperature uniformity in the case of pharmaceutical microwave vacuum drying of a heat sensitive product. Eur J Pharm Biopharm 62(1):101–109 [DOI] [PubMed] [Google Scholar]
- 190.Condurso C et al (2019) Influence of drying technologies on the aroma of Sicilian red garlic. LWT Food Sci Technol 104:180–185 [Google Scholar]
- 191.Berteli MN, Rodier E, Marsaioli A (2009) Study of the microwave vacuum drying process for a granulated product. Braz J Chem Eng 26(2):317–329 [Google Scholar]
- 192.Ahmed I, Qazi IM, Jamal S (2016) Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov Food Sci Emerg Technol 34:29–43 [Google Scholar]
- 193.Chandra S, Kumari D (2015) Recent development in osmotic dehydration of fruit and vegetables: a review. Crit Rev Food Sci Nutr 55(4):552–561 [DOI] [PubMed] [Google Scholar]
- 194.Lee WSL et al (2020) Assessing frost damage in barley using terahertz imaging. Opt Express 28(21):30644–30655 [DOI] [PubMed] [Google Scholar]
- 195.Ramya V, Jain NK (2017) A review on osmotic dehydration of fruits and vegetables: an integrated approach. J Food Process Eng 40(3):e12440 [Google Scholar]
- 196.Yadav AK, Singh SV (2014) Osmotic dehydration of fruits and vegetables: a review. J Food Sci Technol 51(9):1654–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ghosh PK et al (2004) Mass transfer kinetics model of osmotic dehydration of carrots. Trans ASAE 47(4):1179–1185 [Google Scholar]
- 198.Singh B, Kumar A, Gupta AK (2007) Study of mass transfer kinetics and effective diffusivity during osmotic dehydration of carrot cubes. J Food Eng 79(2):471–480 [Google Scholar]
- 199.Rastogi NK (2023) 9 - Developments in osmotic dehydration of foods. In: Jafari SM, Malekjani N (eds) In Drying Technology in Food Processing. Woodhead Publishing, pp 241–304
- 200.Struchtrup H (2014) The Chemical Potential. In: Struchtrup H (ed) In Thermodynamics and Energy Conversion. Springer, Berlin Heidelberg, Berlin Heidelberg, pp 455–465 [Google Scholar]
- 201.Asghari A, Zongo PA, Osse EF, Aghajanzadeh S, Raghavan V, Khalloufi S (2024) Review of osmotic dehydration: Promising technologies for enhancing products’ attributes, opportunities, and challenges for the food industries. Compr Rev Food Sci Food Saf 23(3):e13346. 10.1111/1541-4337.13346 [DOI] [PubMed] [Google Scholar]
- 202.Labuza TP, Altunakar B (2020) Water activity prediction and moisture sorption isotherms. In Water Activity in Foods 1:161–205 [Google Scholar]
- 203.García-Segovia P et al (2010) Osmotic dehydration of Aloe vera (Aloe barbadensis Miller). J Food Eng 97(2):154–160 [Google Scholar]
- 204.Orrego CE, Salgado N, Sarmiento LF (2023) 8 - Freeze drying and vacuum drying. In: Jafari SM, Malekjani N(eds) In Drying Technology in Food Processing Woodhead Publishing, pp 203–240
- 205.Parikh DM (2015) Vacuum drying: basics and application: part 1. Chem Eng 122(4):48 [Google Scholar]
- 206.Richter Reis F (2014) Studies on microwave-vacuum drying of foods. In: Reis R (ed) Vacuum Drying for Extending Food Shelf-Life F. Springer International Publishing, Cham, pp 29–38. 10.1007/978-3-319-08207-3_4 [Google Scholar]
- 207.Orikasa T et al (2014) Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. J Food Eng 125:51–58 [Google Scholar]
- 208.Šumić Z et al (2013) Optimization of frozen sour cherries vacuum drying process. Food Chem 136(1):55–63 [DOI] [PubMed] [Google Scholar]
- 209.Bhambhani A et al (2021) Evaluation of microwave vacuum drying as an alternative to freeze-drying of biologics and vaccines: the power of simple modeling to identify a mechanism for faster drying times achieved with microwave. AAPS PharmSciTech 22 [DOI] [PMC free article] [PubMed]
- 210.Jangle RD, Pisal SS. Vacuum foam drying: an alternative to lyophilization for biomolecule preservation. (0250–474X (Print)) [DOI] [PMC free article] [PubMed]
- 211.Gupta RB, Chattopadhyay P (2003) Method of forming nanoparticles and microparticles of controllable size using supercritical fluids with enhanced mass transfer. United States Patent- US 6620351 B2
- 212.Lebedev AE, Katalevich AM, Menshutina NV (2015) Modeling and scale-up of supercritical fluid processes. Part I: Supercritical drying. J Supercrit Fluids 106:122–132. 10.1016/j.supflu.2015.06.010 [Google Scholar]
- 213.Maltesen MJ, van de Weert M (2008) Drying methods for protein pharmaceuticals. Drug Discov Today Technol 5(2):e81–e88. 10.1016/j.ddtec.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 214.Parhi R, Suresh P (2013) Supercritical fluid technology: A review. J Adv Pharm Sci Technol 1(1):13–36 [Google Scholar]
- 215.Pravallika K, Chakraborty S, Singhal RS (2023) Supercritical drying of food products: An insightful review. J Food Eng 343:111375 [Google Scholar]
- 216.Zheng S et al (2010) Supercritical fluid drying: classification and applications. Recent Pat Chem Eng 3:230–244 [Google Scholar]
- 217.Truong-Le V, Pham B (2011) Preservation of bioactive materials by spray drying, In: W (PCT) (eds)
- 218.Nuchuchua O et al (2014) Scalable organic solvent free supercritical fluid spray drying process for producing dry protein formulations. Eur J Pharm Biopharm 88(3):919–930 [DOI] [PubMed] [Google Scholar]
- 219.Son W-S, Park HJ, Lee C-J, Kim S-N, Song SU, Park G, Lee Y-W (2020) Supercritical drying of vascular endothelial growth factor in mesenchymal stem cells culture fluids. J Supercrit Fluids 157:104710. 10.1016/j.supflu.2019.104710 [Google Scholar]
- 220.Lovalenti PM et al (2016) Stabilization of live attenuated influenza vaccines by freeze drying, spray drying, and foam drying. Pharm Res 33(5):1144–1160 [DOI] [PubMed] [Google Scholar]
- 221.Campardelli R et al (2016) Efficient encapsulation of proteins in submicro liposomes using a supercritical fluid assisted continuous process. J Supercrit Fluids 107:163–169 [Google Scholar]
- 222.Silva AS et al (2020) Rational design of multistage drug delivery vehicles for pulmonary RNA interference therapy. Int J Pharm 591:119989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gimenez-Rota C et al (2019) β-Carotene, α-tocoferol and rosmarinic acid encapsulated within PLA/PLGA microcarriers by supercritical emulsion extraction: Encapsulation efficiency, drugs shelf-life and antioxidant activity. J Supercrit Fluids 146:199–207 [Google Scholar]
- 224.Zambon A et al (2022) Microbial inactivation and drying of strawberry slices by supercritical CO2. J Supercrit Fluids 180:105430 [Google Scholar]
- 225.Winter G, Wiggenhorn M, Pellikaan H (2007) Preparation of powders containing colloidal particles. In: WIPO (eds)
- 226.Penoy N et al (2022) An innovative one step green supercritical CO2 process for the production of liposomes co-encapsulating both a hydrophobic and a hydrophilic compound for pulmonary administration. Int J Pharm 627:122212 [DOI] [PubMed] [Google Scholar]
- 227.Rodrigues MA, Padrela L, Geraldes V, Santos J, Matos HA, Azevedo EG (2011) Theophylline polymorphs by atomization of supercritical antisolvent induced suspensions. J Supercrit Fluids 58(2):303–312. 10.1016/j.supflu.2011.05.012 [Google Scholar]
- 228.Costa C et al (2022) Dry dosage forms of add-value bioactive phenolic compounds by supercritical CO(2)-assisted spray-drying. Molecules 27(6) [DOI] [PMC free article] [PubMed]
- 229.Cho W, Kim MS, Jung MS, Park J, Cha KH, Kim JS, Park HJ, Alhalaweh A, Velaga SP, Hwang SJ (2015) Design of salmon calcitonin particles for nasal delivery using spray-drying and novel supercritical fluid-assisted spray-drying processes. Int J Pharm 478(1):288–296 [DOI] [PubMed] [Google Scholar]
- 230.Du Z, Tang C, Guan Y-X, Yao S-J, Zhu Z-Q (2013) Bioactive insulin microparticles produced by supercritical fluid assisted atomization with an enhanced mixer. Int J Pharm 454(1):174–182. 10.1016/j.ijpharm.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 231.Kim J-S et al (2021) Preparation and characterization of fenofibrate microparticles with surface-active additives: application of a supercritical fluid-assisted spray-drying process. Pharmaceutics. 10.3390/pharmaceutics13122061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Meterc D, Petermann M, Weidner E (2008) Drying of aqueous green tea extracts using a supercritical fluid spray process. J Supercrit Fluids 45(2):253–259 [Google Scholar]
- 233.Atwi-Ghaddar S, Zerwette L, Destandau E, Lesellier E (2023) Supercritical Fluid Extraction (SFE) of Polar Compounds from Camellia sinensis Leaves: Use of Ethanol/Water as a Green Polarity Modifier. Molecules 28(14):5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deng Y et al (2015) Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem 171:168–176 [DOI] [PubMed] [Google Scholar]
- 235.Hamzeh A et al (2018) Effect of drying methods on gelatin from splendid squid (Loligo formosana) skins. Food Biosci 26:96–103 [Google Scholar]
- 236.Liu Y et al (2017) Effect of drying methods on physicochemical properties and in vitro hypoglycemic effects of orange peel dietary fiber. J Food Process Preserv 41(6):e13292 [Google Scholar]
- 237.Mad-Ali S et al (2016) Interfacial properties of gelatin from goat skin as influenced by drying methods. LWT 73:102–107 [Google Scholar]
- 238.Ceja-Medina LI, Ortiz-Basurto RI, Medina-Torres L, Calderas F, Bernad-Bernad MJ, González-Laredo RF, Ragazzo-Sanchez JA, Calderon-Santoyo M, González-Avila M, Andrade-González I, Manero O (2020) Microencapsulation of lactobacillus plantarum by spray drying with mixtures of Aloe vera mucilage and agave fructans as wall materials. J Food Process Eng 43(8)
- 239.Vorländer K et al (2023) Tableting of fluidized bed granules containing living microorganisms. Eur J Pharm Biopharm 187:57–67 [DOI] [PubMed] [Google Scholar]
- 240.Xu M, Tian G, Zhao C, Ahmad A, Zhang H, Bi J, Xiao H, Zheng J (2017) Infrared drying as a quick preparation method for dried tangerine peel. Int J Anal Chem 2017:6254793–6254811. 10.1155/2017/6254793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shi L et al (2021) Effect of a combined microwave-assisted drying and air drying on improving active nutraceutical compounds, flavor quality, and antioxidant properties of Camellia sinensis L. (cv. Longjing 43) flowers. Food Qual Saf 5:fyaa040 [Google Scholar]
- 242.Bhat IM, Wani SM, Mir SA, Naseem Z (2023) Effect of microwave-assisted vacuum and hot air oven drying methods on quality characteristics of apple pomace powder. Food Prod Process Nutr 5:26 [Google Scholar]
- 243.Özkan-Karabacak A et al (2022) Effect of osmotic dehydration pretreatment on the drying characteristics and quality properties of semi-dried (Intermediate) Kumquat (Citrus Japonica) slices by vacuum dryer. Foods. 10.3390/foods11142139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pandiselvam R et al (2022) Advanced osmotic dehydration techniques combined with emerging drying methods for sustainable food production: Impact on bioactive components, texture, color, and sensory properties of food. J Texture Stud 53(6):737–762 [DOI] [PubMed] [Google Scholar]
- 245.Masztalerz K, Łyczko J, Lech K (2021) Effect of filtrated osmotic solution based on concentrated chokeberry juice and mint extract on the drying kinetics. Molecules, energy consumption and physicochemical properties of dried apples. 10.3390/molecules26113274 [DOI] [PMC free article] [PubMed]
- 246.Pei Y-P et al (2023) Pulsed pressure enhances osmotic dehydration and subsequent hot air drying kinetics and quality attributes of red beetroot. Drying Technol 41(2):262–276 [Google Scholar]
- 247.Kumar V, Sharma VK, Kalonia DS (2009) In situ precipitation and vacuum drying of interferon alpha-2a: Development of a single-step process for obtaining dry, stable protein formulation. Int J Pharm 366(1):88–98. 10.1016/j.ijpharm.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 248.Krakowska-Sieprawska A et al (2022) Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 27(3) [DOI] [PMC free article] [PubMed]
- 249.Chhabra N, Arora M, Garg D, Samota MK (2024) Spray freeze drying - A synergistic drying technology and its applications in the food industry to preserve bioactive compounds. Food Control 155:110099 [Google Scholar]
- 250.Li G, Wang B, Li M, Wu R, Lv W, Li B (2024) Spouting technology in energy-carrying electromagnetic field drying of agricultural products. Food Eng Rev. 10.1007/s12393-023-09364-0 [Google Scholar]
- 251.Muthukumaran A, Ratti C, Raghavan VGS (2008) Foam-mat freeze drying of egg white and mathematical modeling part i optimization of egg white foam stability. Drying Technol 26(4):508–512 [Google Scholar]
- 252.Oliveira WP, Bott RF, Souza CRF (2006) Manufacture of standardized dried extracts from medicinal brazilian plants. Drying Technol 24(4):523–533. 10.1080/07373930600612073 [Google Scholar]
- 253.Anandharamakrishnan C, Rielly CD, Stapley AGF (2010) Spray-freeze-drying of whey proteins at sub-atmospheric pressures. Dairy Sci Technol 90(2):321–334 [Google Scholar]
- 254.Zielinska M, Michalska A (2016) Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem 212:671–680 [DOI] [PubMed] [Google Scholar]
- 255.Aguilera-Chávez SL, Gallardo-Velázquez T, Meza-Márquez OG, Osorio-Revilla G (2022) Spray drying and spout-fluid bed drying microencapsulation of mexican plum fruit (Spondias purpurea L) Extract and its effect on in vitro gastrointestinal bioaccessibility. Appl Sci 12(4):2213 [Google Scholar]
- 256.Xie L et al (2017) Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod Process 102:320–331 [Google Scholar]
- 257.Chakraborty R, Mondal P (2017) Effects of intermittent CO(2) convection under far-infrared radiation on vacuum drying of pre-osmodehydrated watermelon. J Sci Food Agric 97(11):3822–3830 [DOI] [PubMed] [Google Scholar]
- 258.Xi H et al (2020) Effect of ultrasonic power on drying process and quality properties of far-infrared radiation drying on potato slices. Food Sci Biotechnol 29(1):93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu Y et al (2019) Contact ultrasound strengthened far-infrared radiation drying on pear slices: Effects on drying characteristics, microstructure, and quality attributes. Drying Technol 37(6):745–758 [Google Scholar]
- 260.Chen Y et al (2020) Novel ultrasonic-assisted vacuum drying technique for dehydrating garlic slices and predicting the quality properties by low field nuclear magnetic resonance. Food Chem 306:125625 [DOI] [PubMed] [Google Scholar]
- 261.Oliveira NL et al (2021) Infrared-assisted freeze-drying (IRFD) of açai puree: Effects on the drying kinetics, microstructure and bioactive compounds. Innov Food Sci Emerg Technol 74:102843 [Google Scholar]
- 262.Bhatta S, Ratti C, Stevanovic T (2019) Impact of drying processes on properties of polyphenol-enriched maple sugar powders. J Food Process Eng 42
- 263.Sarkhel S et al (2022) Studies on supercritical fluid extraction and spray drying effect on the quality of instant tea of Mulberry leaves (Morus alba L.). Measure Food 7:100052 [Google Scholar]
- 264.Xu H, Wu M, Wang Y, Wei W, Sun D, Li D, Zheng Z, Gai F. (2022) Effect of combined infrared and hot air drying strategies on the quality of chrysanthemum (Chrysanthemum morifolium Ramat.) Cakes: drying behavior, aroma profiles and phenolic compounds. Foods. 10.3390/foods11152240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vega-Mercado H, Marcela Góngora-Nieto M, Barbosa-Cánovas GV (2001) Advances in dehydration of foods. J Food Eng 49(4):271–289 [Google Scholar]
- 266.Erdogdu F et al (2017) A computational study to design process conditions in industrial radio-frequency tempering/thawing process. J Food Eng 213:99–112 [Google Scholar]
- 267.Barua S et al (2023) 13 - Application of radio-frequency processing in the food industry. In: Jafari SM (eds) In Emerging Thermal Processes in the Food Industry. Woodhead Publishing, pp 343–374
- 268.Al-Hilphy AR et al (2021) Drying of sliced tomato (Lycopersicon esculentum L.) by a novel halogen dryer: Effects of drying temperature on physical properties, drying kinetics, and energy consumption. J Food Process Eng 44(3):e13624 [Google Scholar]
- 269.Mahanti NK, Chakraborty SK, Sudhakar S, Verma DK, Shankar S, Thakur M, Singh S, Tripathy S (2021) Refractance Window TM-Drying vs. other drying methods and effect of different process parameters on quality of foods: A comprehensive review of trends and technological developments. Future Foods 3:100024. 10.1016/j.fufo.2021.100024 [Google Scholar]
- 270.Topuz A, Feng H, Kushad M (2009) The effect of drying method and storage on color characteristics of paprika. LWT Food Sci Technol 42(10):1667–1673 [Google Scholar]
- 271.Silva NC et al (2024) Refractance window drying as an alternative method for brewer’s spent grain preservation. Appl Biosci 3:71–86. 10.3390/applbiosci3010005 [Google Scholar]
- 272.Zalpouri R et al (2022) Refractance window drying–a revisit on energy consumption and quality of dried bio-origin products. Food Eng Rev 14(2):257–270 [Google Scholar]
- 273.Holmes JF, Russell G, Allen JK (2013) 6 - Supervisory Control and Data Acquisition (SCADA) and related systems for automated process control in the food industry: an introduction. In: Caldwell DG (eds) In Robotics and Automation in the Food Industry. Woodhead Publishing, pp 130–142
- 274.Kondakci T, Zhou W (2017) Recent applications of advanced control techniques in food industry. Food Bioprocess Technol 10(3):522–542 [Google Scholar]
- 275.Kolluri S, Lin L, Liu R, Zhang Y, Zhang W (2022) Machine learning and artificial intelligence in pharmaceutical research and development: a review. AAPS J 24(1):19. 10.1208/s12248-021-00644-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Puranik A, Dandekar P, Jain R (2022) Exploring the potential of machine learning for more efficient development and production of biopharmaceuticals. Biotechnol Prog 38(6):e3291 [DOI] [PubMed] [Google Scholar]
- 277.Rathore AS et al (2023) Artificial intelligence and machine learning applications in biopharmaceutical manufacturing. Trends Biotechnol 41(4):497–510 [DOI] [PubMed] [Google Scholar]
- 278.Khan MIH et al (2022) Application of machine learning-based approach in food drying: opportunities and challenges. Drying Technol 40(6):1051–1067 [Google Scholar]
- 279.Martynenko A, Misra NN (2020) Machine learning in drying. Drying Technol 38(5–6):596–609 [Google Scholar]
- 280.Sun Q, Zhang M, Mujumdar AS (2019) Recent developments of artificial intelligence in drying of fresh food: A review. Crit Rev Food Sci Nutr 59(14):2258–2275 [DOI] [PubMed] [Google Scholar]
- 281.Divyanth LG et al (2022) Detection of coconut clusters based on occlusion condition using attention-guided faster R-CNN for robotic harvesting. Foods 11(23) [DOI] [PMC free article] [PubMed]
- 282.Hosainpour A et al (2022) Quality assessment of dried white mulberry (Morus alba L.) using machine vision. Horticulturae 8(11):1011 [Google Scholar]
- 283.Kheiralipour K, Nadimi M, Paliwal J (2022) Development of an Intelligent imaging system for ripeness determination of wild pistachios. Sensors 22(19):7134. 10.3390/s22197134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Aghbashlo M, Hosseinpour S, Mujumdar AS (2015) Application of artificial neural networks (ANNs) in drying technology: A comprehensive review. Drying Technol 33(12):1397–1462 [Google Scholar]
- 285.Martynenko A (2017) Computer vision for real-time control in drying. Food Eng Rev 9(2):91–111 [Google Scholar]
- 286.Zadhossein S et al (2023) Comparison of the energy and exergy parameters in cantaloupe (Cucurbita maxima) drying using hot air. Smart Agricul Technol 4:100198 [Google Scholar]
- 287.Mekala MS, Viswanathan P (2017) A novel technology for smart agriculture based on IoT with cloud computing. In 2017 International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC)
- 288.Rahul K et al (2022) Cloud computing: technological innovations in the food industry. In: Mor RS, Kamble SS, Sangwan KS (eds) Operations and Supply Chain Management in the Food Industry: Farm to Fork. Springer Nature Singapore, Singapore, pp 127–141 [Google Scholar]
- 289.Aghbashlo M, Hosseinpour S, Ghasemi-Varnamkhasti M (2014) Computer vision technology for real-time food quality assurance during drying process. Trends Food Sci Technol 39:76–84. 10.1016/J.TIFS.2014.06.003 [Google Scholar]
- 290.Rizalman MK et al (2022) Internet-of-Things for Smart Dryers: Enablers, State of the arts, Challenges, and Solutions. In 2022 IEEE International Conference on Artificial Intelligence in Engineering and Technology (IICAIET)
- 291.Dyck G et al (2023) Digital Twins: A novel traceability concept for post-harvest handling. Smart Agricul Technol 3:100079 [Google Scholar]
- 292.Nadimi M, Hawley E, Liu J, Hildebrand K, Sopiwnyk E, Paliwal J (2023) Enhancing traceability of wheat quality through the supply chain. Compr Rev Food Sci Food Saf 22(4):2495–2522. 10.1111/1541-4337.13150 [DOI] [PubMed] [Google Scholar]
- 293.Smetana S, Aganovic K, Heinz V (2021) Food supply chains as cyber-physical systems: a path for more sustainable personalized nutrition. Food Eng Rev 13(1):92–103 [Google Scholar]
- 294.Juckers A et al (2024) Digital twin enabled process development, optimization and control in lyophilization for enhanced biopharmaceutical production. Processes 12(1):211 [Google Scholar]
- 295.Mishra N et al (2023) Development of drying system by using internet of things for food quality monitoring and controlling. Energy Nexus 11:100219 [Google Scholar]
- 296.Martynenko AA, Alves Vieira GN (2023) Sustainability of drying technologies: system analysis. Sustain Food Technol 1(5):629–640 [Google Scholar]
- 297.Bimbenet J-J et al (2002) Heat balance of a multistage spray-dryer: principles and example of application. Lait 82(4):541–551 [Google Scholar]
- 298.Ye L et al (2021) Analysis of energy and specific energy requirements in various drying process of mint leaves. Case Stud Therm Eng 26:101113 [Google Scholar]
- 299.El-Mesery HS, Mwithiga G (2015) Performance of a convective, infrared and combined infrared- convective heated conveyor-belt dryer. J Food Sci Technol 52(5):2721–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable