Abstract
Leishmaniasis, a neglected tropical disease caused by Leishmania parasites, continues to pose global health challenges. Current treatments face issues like resistance, safety, efficacy, and cost. This review covers the discovery, mechanisms of action, clinical applications, and limitations of key antileishmanial agents: pentavalent antimonials, amphotericin B, miltefosine, paromomycin, and pentamidine. Despite toxicity and resistance (antimonials), hospitalization needs and side effects (amphotericin B), regional efficacy variability (miltefosine), inconsistent outcomes (paromomycin), and severe side effects (pentamidine), these drugs are vital. Novel strategies to overcome the deficiencies of current therapies are highlighted, including combination regimens, advanced drug delivery systems, and immunomodulatory approaches. Comprehensive and cooperative efforts are crucial to fully realize the potential of advancements in antileishmanial pharmacotherapy and to reduce the unacceptable worldwide burden imposed by this neglected disease.
Introduction
Leishmaniasis, caused by the Leishmania species within the Trypanosomatidae family, is a significant global health challenge, with about 20 pathogenic species transmitted via sandfly bites [1,2]. The disease manifests in various forms, including visceral, mucosal, cutaneous, and mucocutaneous leishmaniasis (VL, ML, CL, and MCL), with severity influenced by the Leishmania strain and host immune response [3,4]. Outcomes range from self-healing cutaneous lesions to potentially fatal visceral diseases, illustrating the intricate parasite–host interactions [1]. Macrophages, pivotal in disease pathogenesis, are the primary host cells for Leishmania, highlighting complex parasite–host dynamics [5]. Despite significant research efforts, leishmaniasis continues to present challenges, including drug resistance and restricted access to treatments, which underscores the urgent need for novel therapeutic approaches.
Addressing these challenges requires improved drug therapy, as current treatments face issues such as toxicity, resistance, and limited availability, particularly in resource-poor regions. Initially, pentavalent antimonials (sodium stibogluconate and meglumine antimoniate) were primary treatments [1], but resistance, especially in regions like India, has reduced their use [6]. Amphotericin B, though effective, carries significant toxicities [7–9]. Liposomal amphotericin B, FDA-approved for leishmaniasis, is hampered by logistical issues in low-resource settings [10]. Miltefosine has been approved for the treatment of leishmaniasis [11], but other drugs like paromomycin and pentamidine are still in use. However, these treatments face significant challenges, such as resistance, high costs, and severe side effects [12], highlighting the pressing need for safer and more effective therapeutic options.
Our review discusses various currently available therapeutic strategies regarding leishmaniasis treatment, delving into the molecular mechanisms and evaluating the merits and drawbacks of mainstream drugs, including pentavalent antimonials, amphotericin B, miltefosine, paromomycin, and pentamidine. Being one of the most dangerous Neglected Tropical Diseases NTDs, leishmaniasis requires immediate attention and cutting-edge treatment approaches to overcome its multifaceted challenges. This requirement sets the setting for the following sections, where we will discuss new treatments and how to improve the ones that already exist.
Methods
A comprehensive literature search was conducted using databases such as PubMed, Scopus, Google Scholar, and Web of Science to identify relevant articles published from 2000 to March 2024 on antileishmanial drug discovery and development. Keywords used in the search included “leishmaniasis,” “antileishmanial drugs,” “drug resistance,” “treatment,” and “novel therapies.” The search was limited to articles published in English. Titles and abstracts were screened for relevance, and full-text articles were reviewed to extract key information on the mechanisms of action, clinical efficacy, and limitations of current treatments. Additional sources were identified through the reference lists of selected articles.
1. Pentavalent antimonial
Pentavalent antimonial compounds, including sodium stibogluconate and meglumine antimoniate, have been the cornerstone of leishmaniasis treatment for over 70 years. Discovered in the early 20th century, antimony potassium tartrate marked a significant advancement in antileishmanial therapy [13]. Despite its initial success against VL in various regions, the treatment faced setbacks due to high toxicity, lengthy treatment durations, and emerging parasite resistance [14]. A turning point came in 1947 with the introduction of the less toxic sodium stibogluconate, achieving up to 90% cure rates [15]. Even in recent years, pentavalent antimonials remain vital, albeit with growing resistance concerns [16,17].
1.1 Molecular mechanism of the inhibition of Leishmania by pentavalent antimony
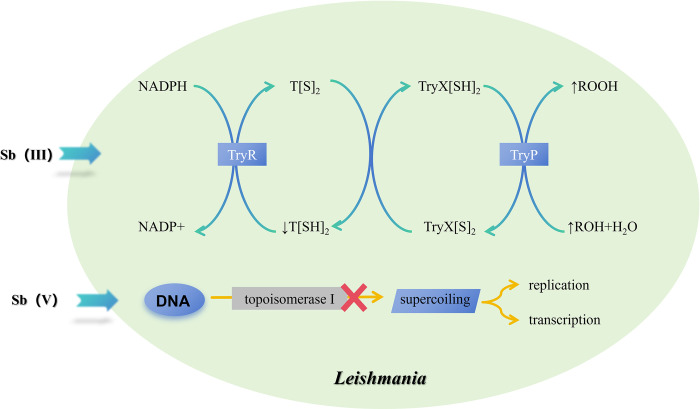
The antileishmanial activity of pentavalent antimonial (Sb(V)) compounds is understood through multiple interconnected mechanisms (Fig 1). Initially considered a prodrug, Sb(V) is bioreduced within Leishmania parasites to yield the trivalent form (Sb(III)), which directly imparts antiparasitic effects [18]. Sb(III) forms stable complexes with thiol groups, particularly glutathione in mammalian cells [19,20] and trypanothione in trypanosomatid parasites [21–23], disrupting critical cellular processes. Exposure to pentavalent antimonials prompts Leishmania to activate multidrug resistance transporters, expelling Sb(III)-thiol complexes and leading to thiol depletion, oxidative stress, and apoptosis [24–27]. Moreover, Sb(III) inhibits the trypanothione reductase system, pivotal for maintaining the intracellular redox balance within trypanosomatids, further exacerbating oxidative damage [28–30]. Crystallographic evidence elucidates the interaction of Sb(III) with the trypanothione reductase enzyme, underscoring a direct mechanism of action [31]. Moreover, pentavalent antimonials exhibit intrinsic antileishmanial effects, independent of bioreduction to Sb(III). Sodium stibogluconate and ureastibamine disrupt DNA topoisomerase I activity in Leishmania donovani, impeding DNA supercoiling, essential for replication and transcription [32,33]. Investigations also show Sb(V) forming stable complexes with adenine nucleosides, suggesting interference with nucleic acid metabolism [34]. These findings highlight the dual action of Sb(V), combining the generation of bioactive Sb(III) within cells and direct disruption of vital parasitic functions. Moreover, research on leishmaniasis and pentavalent antimonial compounds (such as SSG) also demonstrated that SSG affects the immune system [35]. This comprehensive approach, including both prodrug conversion and direct antileishmanial activity, illustrates the complexity of Sb(V)’s mechanism against Leishmania, offering insights for overcoming drug resistance and enhancing treatment efficacy.
Fig 1. Mechanism of action of pentavalent antimonials in Leishmania.
Upon administration, pentavalent antimony (Sb(V)) is bioreduced to trivalent antimony (Sb(III)) within Leishmania parasites, leading to 2 primary pathways of action. Sb(III) inhibits trypanothione reductase (TryR), disrupting the parasite’s redox balance and increasing oxidative stress. Concurrently, Sb(III) affects DNA topoisomerase I activity, impairing DNA supercoiling essential for replication and transcription. Additionally, Sb(V) shows intrinsic antileishmanial activity independent of its reduction to Sb(III), further complicating the parasite’s survival. Trypanothione peroxidase (TryP) participates in detoxifying reactive oxygen species (ROS), and the inhibition of TryR enhances ROS generation, leading to further damage to parasite macromolecules, including DNA and proteins, disrupting homeostasis and contributing to parasite death.
1.2 Current clinical applications of pentavalent antimonials
Pentavalent antimonial drugs, encompassing meglumine antimoniate (also known as Glucantime) and sodium stibogluconate (also called Pentostam), have historically been pivotal in treating all primary leishmaniasis forms, including CL, MCL, and VL [14,36,37]. Their use as the premier choice for CL and ML treatments stems from extensive clinical validation and their influence on the evolution of novel formulations and combination therapies [16]. According to the WHO 2010 guidelines, pentavalent antimonials are among the first-line treatment options for certain species of Leishmania, such as L. aethiopica in Old World CL. In the Indian subcontinent and East Africa, despite being administered intramuscularly or intravenously at a standard dosage of 20 mg Sb(V) per kg body weight daily over roughly a month, resistance in some regions has necessitated the search for optimized protocols and alternatives, underscoring the drug’s variable success and the pressing need for new strategies to address toxicity, resistance, and accessibility [1,6]. However, for other species like L. major, alternative therapies such as fluconazole are recommended as first-line treatments, particularly in North Africa, as endorsed by ASTMH/IDSA guidelines [38,39].
While pentavalent antimonial compounds initially showed promising therapeutic efficacy against leishmaniasis, varied clinical outcomes over time across different regions have become evident [40]. The side effects associated with pentavalent antimony are generally mild, including injection site pain, arthralgia, reversible peripheral neuropathy, and gastrointestinal discomfort. However, patients with HIV co-infection have a heightened risk of developing pancreatitis [41,42]. Prolonged use of higher doses has been linked to severe toxicities such as liver and renal failure, with some instances of significant cardiotoxicity, characterized by inverted T-waves, extended QTc interval on ECG, and potentially fatal arrhythmias [42–45]. The emergence of resistance to antimonial treatments significantly challenges their efficacy, with resistance leading to suboptimal outcomes and persistent infections, notably in India where failure rates surged dramatically between 1980 and 1997, reaching up to 65% in some areas [6,37,46]. The issue of drug resistance has prompted extensive research by scientists. Walker and colleagues found that S-adenosylmethionine synthetase (SAMS) and S-adenosylhomocysteine hydrolase (SAHH) were overexpressed in Sb(III)-resistant lines and isolates, which is the key molecule in Sb-resistance in Leishmania [47]. Analysis showed that Leishmania parasites overexpressing LABCG2 were resistant to antimony due to reduced Sb(III) accumulation via increased efflux. LABCG2 also transported thiols in the presence of Sb(III), as confirmed by biotinylation assays [48]. This underscores the need for new therapeutic options and optimized treatment protocols to counteract resistance and uphold the utility of pentavalent antimonials against leishmaniasis.
Several approaches have been explored to address the limitations of conventional pentavalent antimony therapy, including optimizing treatment protocols and combining therapies. For example, local antimony injections into CL lesions, endorsed by WHO, reduce side effects [49,50], and combination with cryotherapy shows efficacy against CL [16,51]. For VL, pentavalent antimony combined with other drugs like paromomycin has improved outcomes in Africa [52]. Sb(V) oxide and its complexes spontaneously form nanoaggregates or micelles in water, making it feasible to design new Sb(V) complexes with supramolecular assemblies for treating leishmaniasis effectively [53].
Beyond protocol optimization, advancing novel pentavalent antimony formulations is pivotal for enhancing antileishmanial efficacy. Liposome encapsulation improves solubility and targeted delivery [54,55], and designing amphiphilic Sb(V) molecules aims at better oral absorption for VL treatment [56]. Additionally, cyclodextrin complexes are developed to increase oral bioavailability [57]. These innovations aim to maintain pentavalent antimony’s clinical relevance through new combination regimens, administration methods, and addressing toxicity, resistance, and delivery challenges.
2. Amphotericin B
Amphotericin B (AmB), discovered in 1955 from Streptomyces nodosus, has been a cornerstone in treating serious fungal infections and shows broad activity against various pathogens including yeasts, dimorphic fungi, and molds [58,59]. Its antileishmanial potential was recognized early, with in vitro effectiveness established in 1960 and the first successful clinical use against VL reported in 1963 [8,9]. The primary challenge with AmB’s use is its insolubility in water, leading to the adoption of a nephrotoxic deoxycholate form as a second-line treatment for VL, CL, and MCL since the 1960s [60–62]. The development of liposomal delivery systems in the 1970s facilitated the creation of AmBisome, a liposomal formulation with improved bioavailability and reduced toxicity [62,63]. Although resistance to AmB in Leishmania species was historically considered a minor concern, emerging strains indicate potential patient hazards [64,65].
2.1 Molecular mechanism of the inhibition of Leishmania by amphotericin B
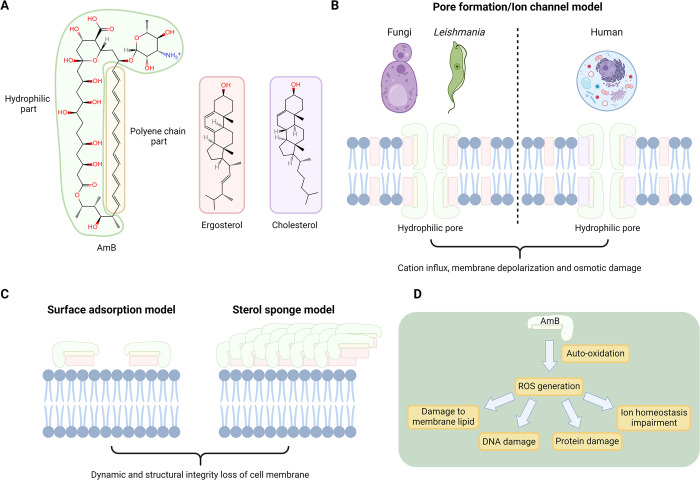
AmB exerts its potent antileishmanial effects predominantly through binding ergosterol in Leishmania and fungi cell membranes [66,67] significantly stronger than cholesterol in human cells [68,69], highlighting its preferential affinity that’s critical for its action. This preferential interaction is facilitated through hydrogen bonding and van der Waals forces [64,70], where AmB’s configurational compatibility with ergosterol, notably at C7, C22 double bonds, and C24 side-chain methylation [60,64], enhances its selective toxicity towards Leishmania by forming ion channels or lipid aggregates on membranes [61,70], leading to cell death through osmotic imbalance and ion homeostasis disruption. Recent studies further elucidate AmB’s mechanism, suggesting that beyond ion channel formation, AmB may aggregate on membrane surfaces to extract essential lipids, directly leading to Leishmania cell death [71], aligning with the sterol sponge model [72–74]. Additionally, structural studies using NMR and molecular dynamics simulations have revealed that AmB assembles into stable seven-molecule ion channels when interacting with ergosterol [75]. This formation, while established in fungal membranes, may also suggest a potential role in disrupting ergosterol-rich Leishmania membranes. Collectively, AmB has strong antileishmanial effects via intricate processes (Fig 2).
Fig 2. Chemical structure and proposed action mechanism of amphotericin B (AmB) against Leishmania parasites.
(A) The chemical structure of AmB, highlighting its polyene core that binds the membrane sterol ergosterol in fungi and Leishmania, as well as cholesterol in human. (B) The classical pore formation/ion channel model proposes AmB incorporated into the sterol-rich membrane, forming aqueous cytotoxic pores. (C) The ergosterol extraction mechanism is characterized by an alternative surface adsorption model and a sterol sponge model. (D) AmB-induced ROS generation, a result of auto-oxidation, further damages the parasite by targeting membrane lipids, DNA, proteins, and disrupting ion homeostasis, contributing to parasite cell death. Created with Biorender.com.
2.2 Current clinical applications of amphotericin B
Amphotericin B’s clinical application is substantially constrained by its poor water solubility and low oral bioavailability, driving the exploration of alternative formulations to circumvent these limitations [76,77]. Due to its molecular size, AmB tends to precipitate in acidic environments, resulting in an oral bioavailability of merely 0.3% [78,79]. Consequently, the deoxycholate form of AmB, which is more soluble, necessitates inconvenient hospitalization for intravenous administration due to its inherent toxicity [80,68]. To address these challenges, liposomal formulations, particularly AmBisome, have been developed. By encapsulating AmB within phospholipid bilayers, these formulations improve drug distribution to tissues and enhance plasma levels, which significantly reduces toxicity while retaining AmB’s efficacy against Leishmania [63,81–84]. Recent research emphasizes the importance of optimizing the physicochemical properties of liposomal AmB formulations for improved treatment outcomes in both cutaneous and visceral leishmaniasis, with ongoing advancements in topical and oral liposomal AmB formulations being explored [85]. Additionally, the development of pH-sensitive nanostructured lipid carriers (AmB-NLCs) has demonstrated promising results, with enhanced drug release under acidic conditions, potentially offering a targeted approach for localized leishmaniasis treatment [86]. This approach not only mitigates the side effects associated with traditional AmB but also enhances its therapeutic effectiveness, particularly in treating visceral leishmaniasis, underscoring the importance of advancements in drug delivery systems for leishmaniasis treatment [87,88]. Liposomal formulations have thus emerged as critical alternatives to traditional AmB, providing effective treatment options in regions with high leishmaniasis prevalence.
AmB became the primary therapy in Bihar, India, in the 1990s, addressing resistance to first-line drugs [70]. Its effectiveness is notable, but use is restricted due to the need for prolonged hospitalization and nephrotoxicity [62,68,80,89]. Treatment protocols vary, with dosages ranging from 7 to 20 mg/kg, potentially requiring up to 43 days to achieve near 100% cure rates against both antimony-sensitive and refractory cases [90]. Liposomal AmB (L-AmB) was introduced to reduce these drawbacks, leading to shorter hospital stays and improved outcomes [91–94]. A study in India reported a 95.7% cure rate with a single 10 mg/kg dose of L-AmB [95], prompting the WHO to recommend it as the first-line treatment in South Asia [96].
L-AmB enhances drug delivery to organs, allowing for high doses with less kidney damage [92] and nearly 100% cure rates [93]. This formulation has proven effective in both children and adults across various regions, including the Mediterranean, the Middle East, and Brazil, with doses of 20 mg/kg [97,98]. The Pan American Health Organization endorses L-AmB as the primary VL treatment, with 3 to 5 mg/kg doses showing up to 100% success in southern Europe [99]. While effective against VL, outcomes for ML and disseminated disease vary [87]. Topical L-AmB gel emerges as a new option for localized CL, offering an alternative to low-efficacy topicals and systemic treatments with toxicity risks [100]. A recent study showed mild local adverse reactions in less than 30% of CL patients [100].
Clinical applications have revealed variable AmB efficacy against different Leishmania strains and clinical manifestations. Uruguayan outbreak isolates associated with VL demonstrated higher infectivity and reduced drug sensitivity compared to South American reference strains [101]. In French Guiana, CL among military personnel showed significant treatment failures with pentamidine and L-AmB, necessitating alternative treatments [102]. A case of imported CL caused by Leishmania infantum in Korea was successfully treated with liposomal AmB [94]. The main concerns with AmB include its nephrotoxicity and severe infusion-related side effects, such as renal insufficiency and metabolic disorders [62,103], contrasting with L-AmB’s fewer adverse effects [94]. Advances have led to AmB derivatives with reduced renal toxicity and preserved antifungal efficacy [104], though their potential against diverse Leishmania strains remains under evaluation. Drug resistance is another challenge [105]; for example, in vitro studies indicate that L. infantum strains associated with VL in dogs showed resistance following miltefosine-allopurinol treatments, which also conferred cross-resistance to AmB [106]. Additionally, reports from Brazil have highlighted resistance to AmB in L. amazonensis strains, which are associated with CL, underscoring the need for vigilant resistance monitoring across different regions [107]. Similar AmB-miltefosine cross-resistance was observed in mutant and clinical relapse L. martiniquensis strains, affecting both VL and CL cases [108].
A significant drawback of the novel, commercial, low-toxicity amphotericin B lipid formulation is the economic burden. This product has a much higher price than the conventional amphotericin B deoxycholate, which limits its accessibility and affordability for many patients [109]. As the high expense of L-AmB hinders widespread use, costs are lowered in India with the 10 mg/kg single L-AmB dose scheme [95]. Further optimization continues on delivery matrices like chitosan and dendrimer nanoparticles to improve amphotericin B solubility, release, and toxicity against Leishmania major [110]. Combination strategies with short-course miltefosine may also enhance efficacy compared to miltefosine alone against VL spread in India [111].
3. Miltefosine
Miltefosine, an alkylphosphorylcholine compound with broad-spectrum antitumor, antiparasitic, and antifungal properties [112], is the only oral agent currently approved for leishmaniasis treatment, representing a significant advantage over injectable alternatives [113]. Initially developed as an anticancer drug, its efficacy against Leishmania parasites was serendipitously discovered in the late 1980s, marking it as a promising candidate for both VL and CL treatment [114]. Its unique oral administration convenience underscores miltefosine’s pivotal role in advancing leishmaniasis treatment modalities [11].
3.1 Molecular mechanism of the inhibition of Leishmania by miltefosine
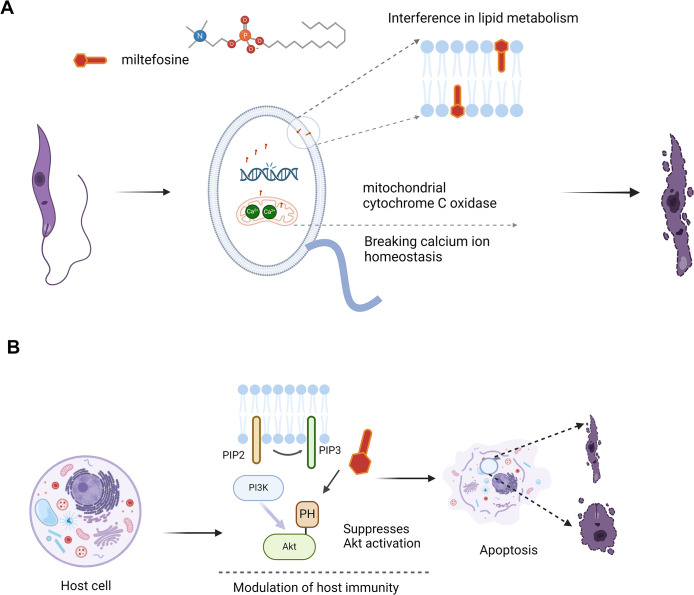
Miltefosine is a pleiotropic drug with multiple targets [115]. The research indicates that miltefosine may involve interfere with parasite lipid metabolism, induce programmed apoptosis-like death, modulate host immunity, and disrupt mitochondrial function [116]. Recent studies suggest that miltefosine may also exert antiparasitic effects by affecting calcium homeostasis in Leishmania [115,117]. However, miltefosine exerts its antileishmanial effects primarily through disrupting lipid metabolism within Leishmania parasites and interfering with their activation of host cell lipid signaling pathways, crucial for their survival. In the past, multiple hypotheses have been proposed for the mechanism of action about miltefosine in anti-Leishmania lipid metabolism [118]. For example, interfering with glycosylphosphatidylinositol (GPI) anchor biosynthesis and inhibiting glycosomal alkyl-specific acyl-CoA acyltransferase as the target of action to interfere with ether-phospholipid metabolism [119], but all have received challenges in recent years [120].
Specifically, it hampers the phosphatidylinositol 3-kinase (PI3K) signaling exploited by Leishmania to enter host cells and form an anti-apoptotic niche [121,122]. Miltefosine suppresses Akt activation, reversing PI3K-mediated survival signals, leading to apoptosis and infection control [123]. Though its exact target is unidentified, miltefosine likely competes with Akt pleckstrin homology (PH) domain for binding to PI(3,4)P2/PI(3,4,5)P3 phospholipids [124–126]. As a synthetic phosphatidylcholine analog, it is suggested to directly affect the parasite’s glycolipid, phospholipid, and sterol metabolism [118,127]. Omics profiling of miltefosine-treated L. donovani highlights its impact on biosynthesis pathways, underscoring the need for further research to detail its mechanisms and enhance treatment efficacy [128,129]. Thus, the multifaceted mechanism of action of miltefosine not only disrupts the basic survival pathway of Leishmania protozoa, but also has a modulatory effect on the immune system of the host (Fig 3). This emphasizes the imperative for in-depth study of miltefosine to further explore its therapeutic potential.
Fig 3. Mechanisms of anti-leishmanial action by miltefosine.
(A) Miltefosine disrupts Leishmania parasites through multiple mechanisms. It interferes with lipid metabolism by integrating into parasite membranes, disrupts calcium ion homeostasis, and inhibits mitochondrial cytochrome C oxidase, collectively leading to parasite death. (B) In host cells, miltefosine modulates the immune response by affecting the PI3K/Akt signaling pathway, inducing apoptosis in infected cells, which further enhances its antiparasitic efficacy. Created with Biorender.com.
3.2 Current clinical applications of miltefosine
Miltefosine, the only orally administered VL treatment to date [130], offers clear advantages over injectables. It is seen as safe, with mild gastrointestinal upset as the most common side effect [131]. Yet, its use in pregnant patients presents teratogenic risks [132], and there have been instances of ocular complications in post-kala-azar dermal leishmaniasis (PKDL) [133,134]. Additionally, reversible male reproductive toxicity has been reported [135], indicating the necessity for additional investigations. Miltefosine’s global utilization spans all major leishmaniasis forms, including VL, CL, MCL, and PKDL [136–140], notably approved in India for oral VL therapy as Impavido in 2002 [141]. Endorsed by WHO for PKDL treatment in East Africa, Bangladesh, India, and Nepal [142,143], it is recommended at 2.5 mg/day for 28 days for CL [140]. Its efficacy against New World CL rivals sodium stibogluconate [1], though bioavailability and efficacy vary by region and leishmaniasis type, partly due to pharmacogenomic differences [144]. Moreover, the FDA’s approval is based on studies that have shown that the susceptibility of Leishmania to miltefosine varies by Leishmania species, strains of a Leishmania species, and different geographic regions [145,146]. Despite these challenges, miltefosine’s oral administration, affordability, and safety profile maintain its significant role in global leishmaniasis treatment.
Despite emerging resistance issues [1], miltefosine remains a key player in combination therapies for leishmaniasis, favored for its cost-effectiveness and patient compliance. It is notably less expensive than L-AmB [147], offering economic advantages for VL treatment in the Indian subcontinent [140]. However, it is worth noting that due to the embryotoxicity of miltefosine, a contraceptive coverage period of 2 to 5 months is required after miltefosine use in the potential population, depending on the duration of treatment, which will limit its overall effectiveness [144]. Combining miltefosine with paromomycin or amphotericin B enhances its efficacy [144], which significantly reduce treatment duration and costs, thereby improving adherence [1,148]. In detail, the combination regimen of L-AmB 5 mg/kg single dose plus miltefosine 2.5 mg/kg per day in the treatment of visceral leishmaniasis was able to shorten the classical 28-day treatment by miltefosine to 7 days [148]. Moreover, synergies with antimony sodium gluconate have been noted [149]. Recent advancements, like thermotherapy and metal nanoparticle-encapsulated miltefosine, are tackling the challenge of prolonged treatment durations, showing promise in early studies for boosting efficacy and reducing toxicity [144,150]. These innovations underline miltefosine’s enduring significance in antileishmanial pharmacotherapy, supported by new therapies and combination treatments that extend its applicability against leishmaniasis.
4. Paromomycin
Paromomycin, an antibiotic first isolated from Streptomyces krestomuceticus in the 1950s, is unique for its clinically significant antileishmanial properties [151]. Recognized for its antileishmanial potential since 1961 through murine studies [152], its effectiveness against VL was confirmed in human trials by the Kenya Medical Research Institute (KEMRI) in Nairobi and the Hospital for Tropical Diseases in London later in the 1990s [153,154]. This led the Institute for OneWorld Health to develop an intramuscular paromomycin sulfate formulation, approved in India in 2006 for affordable VL treatment [1,151,155,156].
4.1 Molecular mechanism of the inhibition of Leishmania by paromomycin
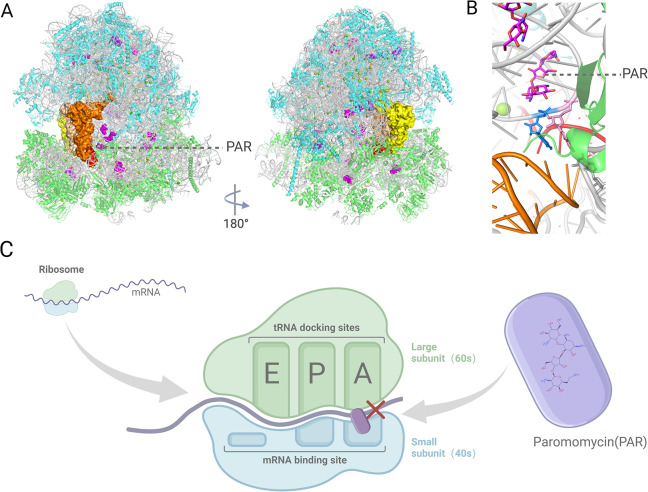
Paromomycin’s antileishmanial activity primarily involves disrupting ribosomal function and mitochondrial membrane potential, affecting protein synthesis and energy metabolism [157–159]. It likely targets Leishmania protein translation by binding to ribosomal RNA, similar to its antibacterial effects [160,161], specifically disrupting peptide chain translation by binding to the 30S ribosomal subunit and interacting with 16S rRNA [162]. Further, paromomycin impedes translation by enhancing ribosomal subunit association and preventing dissociation [161], with RNA sequencing and experiments identifying inhibitory interactions [160,163]. Cryoelectron microscopy has shown paromomycin binds the 91S subunit, disrupting tRNA recruitment [164], and also disrupts mitochondrial respiration and membrane potential [159], illustrating its broad antileishmanial mechanism (Fig 4).
Fig 4. Paromomycin targets the decoding center of the Leishmania cytosolic ribosome.
(A) A cryo-EM structure of the Leishmania cytosolic ribosome, showing 3 tRNAs positioned at the A-site (orange), P-site (beige), and E-site (yellow), with the mRNA in red. Paromomycin (PAR) is shown in purple, bound to the ribosomal RNA (rRNA), with ribosomal proteins in green and light blue representing the small (40S) and large (60S) subunits, respectively. (B) A close-up view of the PAR-binding pocket, highlighting its interaction with the decoding center of the ribosome, analogous to its binding site in bacterial ribosomes. (C) Paromomycin binds to the aminoacyl-tRNA recognition site on the small ribosomal subunit (40S), interfering with the translation process by causing mistranslation of the peptide chain, which compromises Leishmania growth. Created with Biorender.com.
4.2 Current clinical applications of paromomycin
Paromomycin, effective against VL and CL, is widely available and affordable, especially in endemic regions [156,165]. Its administration is mainly intramuscular for VL or topical for CL due to limited oral bioavailability [157,165]. The WHO recommended a paromomycin and sodium stibogluconate combination for VL in East Africa in 2010 [96], with subsequent studies validating up to 95% cure rates [166]. A 2015 Phase III trial in Bangladesh also highlighted a 94% efficacy of paromomycin monotherapy at 11 mg/kg for VL over 21 days, showing 94% efficacy and mild side effects [167]. In Israel since the 1990s, a topical ointment with 15% paromomycin and 12% reactive oxygen species has been utilized for CL, showing effectiveness against L. major, L. panamensis, L. mexicana, and L. braziliensis across various regions [168–171].
Nevertheless, the efficacy of paromomycin exhibits variability among different strains and populations [165]. Additionally, it is associated with adverse reactions such as localized pain at the injection site and transient auditory impairment, with a small percentage of patients experiencing nephrotoxic effects [156]. In light of these challenges, ongoing efforts focus on optimizing protocols and exploring novel delivery systems to enhance its risk-benefit ratio including the investigation of modified formulations such as 15% paromomycin with 10% urea [172] or 0.5% gentamicin [173,174] to mitigate adverse effects. Innovative paromomycin formulations like microspheres, liposomes, and hydrogels for both VL and CL are enhancing its efficacy and safety [175,165]. Khan and colleagues’ microsphere approach notably reduces nephrotoxicity [176], while liposomal formulations improve absorption and exhibit immunological benefits [177]. Solid lipid nanoparticles offer sustained release and reduced toxicity, increasing antileishmanial efficacy [178,179]. Biodistribution assays showed iontophoretic transport delivered higher PAR amounts to deeper skin layers than conventional ointment [180]. These advances are optimizing paromomycin’s therapeutic profile, promising to enhance its role in leishmaniasis treatment.
5. Pentamidine
Pentamidine, a synthetic amidine derivative synthesized in the late 1930s [181], initially treated VL before the 1950s [182] and later addressed drug-resistant CL in the 1970s [183]. Commercialized as an isethionate ester in 1984 [184,185], it now serves as a second-line option for leishmaniasis due to efficacy limits and toxicity [186]. As drug resistance escalates, elucidating pentamidine’s mechanisms of action and developing safer derivatives may unlock new possibilities for this old medication.
5.1 Molecular mechanism of the inhibition of Leishmania by pentamidine
Pentamidine’s effects on Leishmania parasites are notably complex and not yet fully understood. It interacts with various nucleic acids, disrupting nucleotide incorporation and oxidative phosphorylation, thereby affecting the biosynthesis of DNA, RNA, phospholipids, and proteins. It disrupts the MBNL1-CUG repeat complex in DM1, affecting alternative splicing of pre-mRNAs [187], and shows broad RNA-binding activity, including interactions with CUG RNA repeats and intron stem-loop RNA [188]. Furthermore, pentamidine’s nonspecific tRNA binding interferes with aminoacylation processes [189], adding another layer to its multifaceted inhibitory effects. Pentamidine may bind to the kinetoplast DNA, inhibiting mitochondrial respiratory chain complex II, inducing apoptosis through increased intracellular calcium [185]. Its competing with polyamines [190] significantly inhibits polyamine synthesis, critical for purine-lacking Leishmania [191]. These interactions highlight the need for thorough evaluation of pentamidine’s target engagement and its promiscuous binding behavior [192].
5.2 Current clinical applications of pentamidine
Previously a first-line leishmaniasis treatment, pentamidine’s use has declined due to adverse effects and new therapies, now serving mainly as a second-line option [186]. Its efficacy varies across Leishmania species, remaining a first-line recommendation for L. guyanensis-induced CL and MCL in several South American countries, backed by high cure rates and mild toxicity in trials [193,194]. Similarly, it is recommended for MCL from L. panamensis and diffuse CL from L. aethiopica, reflecting its species-specific effectiveness [195–197].
When primary treatments (pentavalent antimonials) fail, pentamidine serves as a secondary option for leishmaniasis [198]. Its efficacy, however, is inconsistent across studies: in Peru, only a 35% cure rate was reported for L. braziliensis infections, contrasting the 78% efficacy of meglumine antimoniate [199], whereas in Colombia, a study showed a 96% success rate [197]. This suggests that pentamidine’s effectiveness varies by region and Leishmania strain, highlighting the need for more research to define its precise therapeutic role.
Pentamidine’s clinical use is hampered by its safety profile and resistance development. Immediate reactions like hypotension, nausea, and vomiting, along with injection site pain, leukopenia, and hypoglycemia, underscore its toxicity [181]. Notably, glucose metabolism disorders affected 15.3% of patients, with a 3.6% incidence of acute kidney injury and widespread mild cardiovascular effects [200]. Resistance is also a significant issue, leading to over 30% failure rates in areas like India, necessitating dose increases that heighten toxicity risks [201]. These factors underline the urgent need for new treatments without these drawbacks to improve leishmaniasis care.
Conclusions and outlook
Leishmaniasis, a significant NTD caused by Leishmania spp., challenges global health. Historically treated with pentavalent antimonials, their use has declined due to toxicity and treatment failure. Other treatments like amphotericin B, miltefosine, and paromomycin face issues of cost, safety, and efficacy across species, stifling drug development due to insufficient investment and interest. Table 1 listed a further detailed comparison of the effectiveness, limitations of use, and side effects of the discussed antileishmanial drugs. However, recent efforts aim to overcome these barriers through advances in omics, combination regimens, immunomodulatory approaches, structure-based drug design, and other novel therapies. Emphasizing multidisciplinary approaches, global collaboration, and a balance of research aims is essential for advancing antileishmanial drug development and potentially eradicating the disease. To achieve this mission, the following issues central to antileishmanial pharmacotherapy warrant thorough discussion.
Table 1. Summary of key features of antileishmanial drugs of interest.
| Drug name | Route of administration | Effective against | Limitations and side effects | Cost of treating leishmaniasis |
Insight into drug improvements |
|---|---|---|---|---|---|
| Pentavalent antimonial | Intramuscular injection or intravenous infusion [1, 214] | VL: Leishmania donovani (East Africa): Highly effective (94% cure rate) Leishmania infantum (all regions): Highly effective (97% cure rate) PKDL: ● East Africa: Good efficacy in combination with paromomycin CL: Old World species (L. major, L. tropica, L. infantum): Highly effective New World species (L. braziliensis, L. panamensis, L. guyanensis): Highly effective ML: ● New World species: Moderate efficacy, may require combination therapy |
● Common side effects: Musculoskeletal pain, headache, nausea, and asthenia ● Cardiotoxicity, hepatotoxicity, nephrotoxicity, and pancreatitis as rare, and associated with cumulative doses [215–219] ● Abdominal colic, diarrhea, skin rashes, pancreatitis ● Painful to administer and prolonged treatment ● Drug resistance (in the Indian subcontinent) [1,6] |
Meglumine antimoniate (Glucantime): US$ 85 per patient cured [220] |
● Natural Cell-Penetrating Nanopeptide: Combined with Pentavalent Antimonial [221] ● Liposomal Encapsulation: Pentavalent Antimonials encapsulated in conventional liposomes [55–57] ● Polymer-Based Delivery Systems: Polyacryl starch microparticles containing covalently bound SSG [222] ● Cyclodextrin-Based Oral Formulation: Composition with MA and β-cyclodextrin enhances oral absorption in a murine model of CL [57] Topical Formulations for CL: Sb(V)-guanosine hydrogel highly effective against intracellular Leishmania amastigote [223–225] Amiodarone and Itraconazole: In hamsters, either alone or in combination, enhances glucantime activity in treating L. amazonensis lesions with no evident side effects [226] |
| Amphotericin B | Intravenous injection (for AmB deoxycholate and L-AmB) [80,68] or topical (for L-AmB gel) [100] | L-AmB: Showed efficacy against Leishmania infantum in a documented Korean case [94] Emerging resistance reported in some L. martiniquensis strains may confer cross-resistance [108] Topical L-AmB gel: Highly effective against patients with CL caused by L. major [100] |
AmB deoxycholate: ● Inherent nephrotoxicity [62,68,80] ● Longer hospitalization [89] ● Infusion reactions like fever, chills [103] L-AmB: ● Milder toxicity than amphotericin B deoxycholate [62,68,80–84,88] Topical L-AmB gel: ● Mild local adverse reactions like pain, itch, erythema, and discharge [100] |
US$ 659.79 (price adopted by WHO) or US$ 11,559.15 (price adopted by the Drug Regulation Board of Brazil) for treating an adult patient with VL in Brazil [227] | ● Improve liposomal formulations to reduce toxicity [228] ● Short-course combination with miltefosine [111] ● Nanoparticle incorporation to improve delivery [110] |
| Miltefosine (trade name: Impavido) |
Oral administration [116,145] | L. donovani was found to be the most sensitive, while L. major is not sensitive [116,229] | ● Gastrointestinal complaints ● Teratogenicity [116,144] ● Ocular complications [144,230] ● Reversible male reproductive toxicity [116,145] |
US$ 259.92 in the outpatient treatment regimen in Brazil [231] | ● Combination therapy [144] ● Formulation innovation [150] |
| Paromomycin | Intramuscular injection; intravenous injection; topical application [165] |
Strains: Efficacy against several Leishmania strains, including L. major, L. panamensis, L. mexicana, and L. braziliensis in varying regions [168–171] ● Advantageous regions: Africa and India [151] |
● Injection-site pain [157] ● Mild side effects [157] ● Laboratory strains developed resistance [165] |
Cost-effective: US$ ~10 per patient [232] | Develop some innovative formulations to enhance the efficacy and safety of paromomycin |
| Pentamidine | Intramuscular injection [165] | First-line recommendation for CL and MCL caused by L. guyanensis, which is endemic to Brazil, Colombia, French Guiana, and Suriname [193,194] | Efficacy varies between Leishmania species [165] ● Drug resistance [201] |
US$ 70 for relapsed patients [233] | Developing agents devoid of resistance and toxicity issues |
Given the outlined challenges, antileishmanial drug development faces critical issues, notably the suboptimal efficacy and drug resistance of current treatments, underscoring the need for safer and more effective alternatives. The adverse effects and safety concerns of drugs like pentavalent antimonials, along with the high costs and limited accessibility of treatments such as L-AmB and miltefosine, constrain their use, particularly in under-resourced areas. Furthermore, the administration routes of most existing agents, typically requiring parenteral injections, contribute to poor patient compliance and highlight the need for oral or topical options. Another key obstacle is the lack of treatment specificity against diverse Leishmania species and clinical manifestations, which hampers the development of tailored therapeutic regimens. To address these challenges, research advancements are crucial, particularly in the areas of combination therapies and novel drug delivery systems, such as nanoparticles, liposomes, and hydrogels, which offer promising avenues for enhancing treatment efficacy and reducing toxicity. Recent innovations in drug delivery have introduced advanced nanotechnology and liposomal carriers to boost bioavailability and mitigate toxicity. For example, pH-sensitive NLCs for AmB have been developed to enable targeted drug release under the acidic conditions typical of localized leishmaniasis lesions, thus meeting the need for localized treatments. Likewise, liposomal formulations, such as those created for AmB, enhance drug distribution and reduce side effects, proving particularly effective in the treatment of VL [62,63]. These technologies exemplify how innovations in drug delivery can expand treatment options [202] and improve outcomes for patients across various forms of the disease. Moving forward, the future of antileishmanial therapy hinges on continued innovation that addresses these diverse challenges, aiming for treatments that are not only efficacious but also safe, cost-effective, and widely accessible.
Additionally, rationale structural optimization guided by insights into drug–parasite interactions may yield derivatives with increased potency and selectivity. Developing novel formulations for diverse administration routes, including oral and topical options, could further expand therapeutic reach and access. New compounds play an important role in anti-parasitic treatment. Wyllie and colleagues have identified small molecule drugs that can inhibit the growth and reproduction of parasites by inhibiting the CRK12 enzyme [203]. As a prominent among drugs for treatment of infectious disease, macrocycles were more potent than miltefosine identified in a phenotypic screen of Leishmania infantum [204]. Melittin-containing fusion crystal [205], dioclea violacea lectin [206], 8-hydroxy-2-quinoline carbaldehyde derivatives [207], cyanotriazoles that rapidly cure trypanosome infections [208] also have been discovered to be used for treating leishmaniasis. Beyond traditional chemicals, newly developed gene editing methods also present opportunities to eliminate remaining parasites following therapy. To improve efficacy and reduce side effects, Lago and colleagues utilized topical rSm29 in conjunction with intravenous meglumine antimoniate for the treatment of cutaneous leishmaniasis [209]. As the conventional treatments often use drugs with high toxicity, A chitosan/collagen biomembrane, loaded with 2,3-dihydrobenzofuran can be employed for the treatment of CL [210]. Nahanji and colleagues enhanced the efficacy of fluconazole against Leishmania major for topical delivery using FLZ-nanoemulsions [211]. Besides, PA and AmpB together could form a promising new treatment strategy against Leishmania infections, offering enhanced efficacy without added toxicity [212]. Nanotechnology can enhance leishmaniasis treatment using drug-carrying nanosystems like metallic nanoparticles, liposomes, and polymeric/lipid nanoparticles, minimizing side effects, dose, and costs. Encapsulating antileishmanial drugs in nanosystems boosts bioavailability, sustained release, macrophage uptake, and target cell/tissue delivery, while enhancing efficacy and reducing toxicity [150]. Allahverdiyev and colleagues found Ag-NPs inhibited L. tropica promastigote proliferation and metabolic activity by 1.5–3× in the dark and 2–6.5× under UV light [213]. By combining strengths in parasitology, pharmacology, immunology, formulation science, and bioengineering, the next generation of antileishmanial regimens may be within reach.
The future of antileishmanial therapy looks promising, driven by innovations that broaden treatment possibilities. The development of broad-spectrum agents to combat various Leishmania species and manifestations is crucial. Enhancing current treatments through novel formulations and delivery systems, alongside multidisciplinary methods including immunopharmacology, gene editing, and bioengineering, could offer synergistic benefits, improving safety and efficacy. Additionally, uncovering unknown drug actions and resistance mechanisms is vital for creating targeted therapies. Achieving these advancements requires global collaboration and investment, emphasizing the need to address this neglected disease’s impact. This concerted effort could usher in a new era of improved outcomes for leishmaniasis patients.
Key Learning Points
Current antileishmanial drugs and their limitations
The review discusses the main antileishmanial drugs: pentavalent antimonials, amphotericin B, miltefosine, paromomycin, and pentamidine. Each of these drugs faces significant limitations, such as high toxicity, resistance issues, and variable efficacy across different regions and Leishmania species.
Drug resistance mechanisms
Leishmania parasites develop resistance through various mechanisms, including alterations in drug targets, increased efflux of drugs, and metabolic changes. Understanding these mechanisms is essential for developing new, more effective treatments.
Advancements in drug delivery systems
Innovative drug delivery methods, such as liposomal formulations and advanced drug delivery systems, have been developed to improve the efficacy and reduce the toxicity of antileishmanial drugs. Liposomal amphotericin B, for example, offers improved outcomes with reduced toxicity.
Combination therapies and new approaches
The review highlights the potential of combination therapies and new therapeutic strategies, including combination regimens, immunomodulatory approaches, and advanced drug delivery systems, to overcome the limitations of current treatments and improve patient outcomes.
Future directions and research needs
Continued research is needed to explore novel therapeutic targets, develop safer and more effective drugs, and implement comprehensive strategies to manage drug resistance. Collaborative efforts and multidisciplinary approaches are crucial for advancing antileishmanial pharmacotherapy and addressing the global burden of leishmaniasis.
Five Key Papers in the Field
Zulfiqar B, Avery VM. Assay development in leishmaniasis drug discovery: a comprehensive review. Expert Opin Drug Discov. 2022 Feb;17(2):151–166.
Altamura F, Rajesh R, Catta-Preta CMC, Moretti NS, Cestari I. The current drug discovery landscape for trypanosomiasis and leishmaniasis: Challenges and strategies to identify drug targets. Drug Dev Res. 2022 Apr;83(2):225–252.
Roquero I, Cantizani J, Cotillo I, Manzano MP, Kessler A, Martín JJ, McNamara CW. Novel chemical starting points for drug discovery in leishmaniasis and Chagas disease. Int J Parasitol Drugs Drug Resist. 2019 Aug;10:58–68.
Akhoundi M, Downing T, Votýpka J, Kuhls K, Lukeš J, Cannet A, Ravel C, Marty P, Delaunay P, Kasbari M, Granouillac B, Gradoni L, Sereno D. Leishmania infections: Molecular targets and diagnosis. Mol Aspects Med. 2017 Oct;57:1–29.
Andrade-Neto VV, Cunha-Junior EF, Faioes VDS, Pereira TM, Silva RL, Leon LL, Torres-Santos EC. Leishmaniasis treatment: update of possibilities for drug repurposing. Front Biosci (Landmark Ed). 2018 Jan 1;23(5):967–996.
Funding Statement
This work was supported by the National Key Research and Development Program of China [2021YFC2600200 and 2022YFC2305500 to Y.L.]; the General Program of National Natural Science Foundation of China [82172299 to Y.L.]; the Hubei Natural Science Fund for Distinguished Young Scholars [2022CFA068 to Y.L.], and the Hubei Public Health Youth Talents Program [to Y.L.].The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2 [DOI] [PubMed] [Google Scholar]
- 2.Courtenay O, Peters NC, Rogers ME, Bern C. Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform leishmaniasis transmission dynamics and control. PLoS Pathog. 2017;13:e1006571. doi: 10.1371/journal.ppat.1006571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Assche T, Deschacht M, da Luz RAI, Maes L, Cos P. Leishmania–macrophage interactions: Insights into the redox biology. Free Radic Biol Med. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 4.Hussain H, Al-Harrasi A, Al-Rawahi A, Green IR, Gibbons S. Fruitful Decade for Antileishmanial Compounds from 2002 to Late 2011. Chem Rev. 2014;114:10369–10428. doi: 10.1021/cr400552x [DOI] [PubMed] [Google Scholar]
- 5.Reithinger R, Dujardin J-C, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8 [DOI] [PubMed] [Google Scholar]
- 6.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, et al. Failure of Pentavalent Antimony in Visceral Leishmaniasis in India: Report from the Center of the Indian Epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121 [DOI] [PubMed] [Google Scholar]
- 7.Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen J-A, Sundar S. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980–2004. Lancet Infect Dis. 2005;5:763–774. doi: 10.1016/S1473-3099(05)70296-6 [DOI] [PubMed] [Google Scholar]
- 8.Furtado TA, Cisalpino EO, Santos UM. In vitro Studies of the Effect of Amphotericin B in Leishmania brasiliensis. Antibiot Amp Chemother. 1960;10:692–693. [PubMed] [Google Scholar]
- 9.Prata A. Treatment of kala-azar with amphotericin B. Trans R Soc Trop Med Hyg. 1963;57:266–268. doi: 10.1016/0035-9203(63)90183-4 [DOI] [PubMed] [Google Scholar]
- 10.Sundar S, Chakravarty J. An update on pharmacotherapy for leishmaniasis. Expert Opin Pharmacother. 2015;16:237–252. doi: 10.1517/14656566.2015.973850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smorenburg CH, Seynaeve C, Bontenbal M, Planting ASt, Sindermann H, Verweij J. Phase II study of miltefosine 6% solution as topical treatment of skin metastases in breast cancer patients. Anticancer Drugs. 2000;11:825. doi: 10.1097/00001813-200011000-00006 [DOI] [PubMed] [Google Scholar]
- 12.Santos SS, de Araújo RV, Giarolla J, Seoud OE, Ferreira EI. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: a review. Int J Antimicrob Agents. 2020;55:105906. doi: 10.1016/j.ijantimicag.2020.105906 [DOI] [PubMed] [Google Scholar]
- 13.Vianna G. Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. Arq Bras Med. 1912;4:426–428. [Google Scholar]
- 14.Frézard F, Demicheli C, Ribeiro RR. Pentavalent Antimonials: New Perspectives for Old Drugs. Molecules. 2009;14:2317–2336. doi: 10.3390/molecules14072317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman JD. Chemotherapy for Leishmaniasis: Biochemical Mechanisms, Clinical Efficacy, and Future Strategies. Rev Infect Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560 [DOI] [PubMed] [Google Scholar]
- 16.Berbert TRN, de Mello TFP, Wolf Nassif P, Mota CA, Silveira AV, Duarte GC, et al. Pentavalent Antimonials Combined with Other Therapeutic Alternatives for the Treatment of Cutaneous and Mucocutaneous Leishmaniasis: A Systematic Review. Dermatol Res Pract. 2018;2018:e9014726. doi: 10.1155/2018/9014726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman JD. Human Leishmaniasis: Clinical, Diagnostic, and Chemotherapeutic Developments in the Last 10 Years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684 [DOI] [PubMed] [Google Scholar]
- 18.Shaked-Mishan P, Ulrich N, Ephros M, Zilberstein D. Novel Intracellular SbV Reducing Activity Correlates with Antimony Susceptibility in Leishmania donovani *. J Biol Chem. 2001;276:3971–3976. doi: 10.1074/jbc.M005423200 [DOI] [PubMed] [Google Scholar]
- 19.Frézard F, Demicheli C, Ferreira CS, Costa MAP. Glutathione-Induced Conversion of Pentavalent Antimony to Trivalent Antimony in Meglumine Antimoniate. Antimicrob Agents Chemother. 2001;45:913–916. doi: 10.1128/AAC.45.3.913-916.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H, Yan SC, Cheng WS. Interaction of antimony tartrate with the tripeptide glutathione. Eur J Biochem. 2000;267:5450–5457. doi: 10.1046/j.1432-1327.2000.01605.x [DOI] [PubMed] [Google Scholar]
- 21.Fairlamb AH, Cerami A. Metabolism and Functions of Trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403 [DOI] [PubMed] [Google Scholar]
- 22.Krauth-Siegel RL, Meiering SK, Schmidt H. The Parasite-Specific Trypanothione Metabolism of Trypanosoma and Leishmania. 2003;384:539–549. doi: 10.1515/BC.2003.062 [DOI] [PubMed] [Google Scholar]
- 23.Yan S, Li F, Ding K, Sun H. Reduction of pentavalent antimony by trypanothione and formation of a binary and ternary complex of antimony(III) and trypanothione. J Biol Inorg Chem. 2003;8:689–697. doi: 10.1007/s00775-003-0468-1 [DOI] [PubMed] [Google Scholar]
- 24.Wyllie S, Cunningham ML, Fairlamb AH. Dual Action of Antimonial Drugs on Thiol Redox Metabolism in the Human Pathogen Leishmania donovani*. J Biol Chem. 2004;279:39925–39932. doi: 10.1074/jbc.M405635200 [DOI] [PubMed] [Google Scholar]
- 25.Sereno D, Holzmuller P, Mangot I, Cuny G, Ouaissi A, Lemesre J-L. Antimonial-Mediated DNA Fragmentation inLeishmania infantum Amastigotes. Antimicrob Agents Chemother. 2001;45:2064–2069. doi: 10.1128/AAC.45.7.2064-2069.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay R, Dey S, Xu N, Gage D, Lightbody J, Ouellette M, et al. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc Natl Acad Sci U S A. 1996;93:10383–10387. doi: 10.1073/pnas.93.19.10383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Fadili K, Messier N, Leprohon P, Roy G, Guimond C, Trudel N, et al. Role of the ABC Transporter MRPA (PGPA) in Antimony Resistance in Leishmania infantum Axenic and Intracellular Amastigotes. Antimicrob Agents Chemother. 2005;49:1988–1993. doi: 10.1128/AAC.49.5.1988-1993.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krauth-Siegel RL, Comini MA. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta BBA—Gen Subj. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A. Trypanothione: A Novel Bis(glutathionyl)spermidine Cofactor for Glutathione Reductase in Trypanosomatids. Science. 1985;227:1485–1487. doi: 10.1126/science.3883489 [DOI] [PubMed] [Google Scholar]
- 30.Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol Microbiol. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x [DOI] [PubMed] [Google Scholar]
- 31.Baiocco P, Colotti G, Franceschini S, Ilari A. Molecular Basis of Antimony Treatment in Leishmaniasis. J Med Chem. 2009;52:2603–2612. doi: 10.1021/jm900185q [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty AK, Majumder HK. Mode of action of pentavalent antimonials: Specific inhibition of type I DNA topoisomerase of Leishmaniadonovani. Biochem Biophys Res Commun. 1988;152:605–611. doi: 10.1016/s0006-291x(88)80081-0 [DOI] [PubMed] [Google Scholar]
- 33.Lucumi A, Robledo S, Gama V, Saravia NG. Sensitivity of Leishmania viannia panamensis to Pentavalent Antimony Is Correlated with the Formation of Cleavable DNA-Protein Complexes. Antimicrob Agents Chemother. 1998;42:1990–1995. doi: 10.1128/AAC.42.8.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demicheli C, Frézard F, Lecouvey M, Garnier-Suillerot A. Antimony(V) complex formation with adenine nucleosides in aqueous solution. Biochim Biophys Acta BBA—Gen Subj. 2002;1570:192–198. doi: 10.1016/s0304-4165(02)00198-8 [DOI] [PubMed] [Google Scholar]
- 35.Solomon M, Greenberger S, Milner M, Pavlotzky F, Barzilai A, Schwartz E, et al. Efficacy of systemic treatment for leishmania tropica cutaneous leishmaniasis. Acta Derm Venereol. 2022;102:adv00721–adv00721. doi: 10.2340/actadv.v102.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garza-Tovar TF, Sacriste-Hernández MI, Juárez-Durán ER, Arenas R. An overview of the treatment of cutaneous leishmaniasis. F1000Prime Rep. 2020;9. Available from: https://connect.h1.co/prime/reports/b/9/28/. doi: 10.12703/r/9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x [DOI] [PubMed] [Google Scholar]
- 38.Francesconi VA, Francesconi F, Ramasawmy R, Romero GAS, Alecrim MDGC. Failure of fluconazole in treating cutaneous leishmaniasis caused by leishmania guyanensis in the brazilian amazon: An open, nonrandomized phase 2 trial. PLoS Negl Trop Dis. 2018;12:e0006225. doi: 10.1371/journal.pntd.0006225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill N, Irwin A, Graham N, Leung C, Francis JR, Wall N, et al. Treatment of cutaneous leishmaniasis in a nonendemic country: A case series of children in australia. Pediatr Infect Dis J. 2022;41:e177. doi: 10.1097/INF.0000000000003445 [DOI] [PubMed] [Google Scholar]
- 40.Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Neto VA. Treatment of New World cutaneous leishmaniasis–a systematic review with a meta-analysis. Int J Dermatol. 2008;47:109–124. doi: 10.1111/j.1365-4632.2008.03417.x [DOI] [PubMed] [Google Scholar]
- 41.Pintado V, López-Vélez R. HIV-associated visceral leishmaniasis. Clin Microbiol Infect. 2001;7:291–300. doi: 10.1046/j.1198-743x.2001.00262.x [DOI] [PubMed] [Google Scholar]
- 42.Sasidharan S, Saudagar P. Leishmaniasis: where are we and where are we heading? Parasitol Res. 2021;120:1541–1554. doi: 10.1007/s00436-021-07139-2 [DOI] [PubMed] [Google Scholar]
- 43.Martínez FV, Guerrero ET, Arenas R, Cedillo MQ. Leishmaniasis en México. Med Cutánea Ibero-Lat-Am. 2011;39:163–183. [Google Scholar]
- 44.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. 2017;6:750. doi: 10.12688/f1000research.11120.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brummitt CF, Porter JAH, Herwaldt BL. Reversible Peripheral Neuropathy Associated with Sodium Stibogluconate Therapy for American Cutaneous Leishmaniasis. Clin Infect Dis. 1996;22:878–879. doi: 10.1093/clinids/22.5.878 [DOI] [PubMed] [Google Scholar]
- 46.Hirve S, Boelaert M, Matlashewski G, Mondal D, Arana B, Kroeger A, et al. Transmission Dynamics of Visceral Leishmaniasis in the Indian Subcontinent–A Systematic Literature Review. PLoS Negl Trop Dis. 2016;10:e0004896. doi: 10.1371/journal.pntd.0004896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker J, Gongora R, Vasquez J-J, Drummelsmith J, Burchmore R, Roy G, et al. Discovery of factors linked to antimony resistance in leishmania panamensis through differential proteome analysis. Mol Biochem Parasitol. 2012;183:166–176. doi: 10.1016/j.molbiopara.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 48.Perea A, Manzano JI, Castanys S, Gamarro F. The LABCG2 transporter from the protozoan parasite leishmania is involved in antimony resistance. Antimicrob Agents Chemother. 2016;60:3489–3496. doi: 10.1128/AAC.02813-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramalho DB, da Silva RE, de Senna MCR, Moreira HSA, Pedras MJ, de Avelar DM, et al. Meglumine antimoniate intralesional infiltration for localised cutaneous leishmaniasis: a single arm, open label, phase II clinical trial. Mem Inst Oswaldo Cruz. 2018;113:e180200. doi: 10.1590/0074-02760180200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brito NC, Rabello A, Cota GF. Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review. PLoS ONE. 2017;12:e0184777. doi: 10.1371/journal.pone.0184777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmanpour R, Razmavar MR, Abtahi N. Comparison of intralesional meglumine antimoniate, cryotherapy and their combination in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2006;45:1115–1116. doi: 10.1111/j.1365-4632.2006.02822.x [DOI] [PubMed] [Google Scholar]
- 52.Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019;20:1251–1265. doi: 10.1080/14656566.2019.1609940 [DOI] [PubMed] [Google Scholar]
- 53.Demicheli C, Vallejos VMR, Lanza JS, Ramos GS, Do Prado BR, Pomel S, et al. Supramolecular assemblies from antimony(V) complexes for the treatment of leishmaniasis. Biophys Rev. 2023;15:751–765. doi: 10.1007/s12551-023-01073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.New RRC, Chance ML, Thomas SC, Peters W. Antileishmanial activity of antimonials entrapped in liposomes. Nature. 1978;272:55–56. doi: 10.1038/272055a0 [DOI] [PubMed] [Google Scholar]
- 55.Collins M, Carter K, Baillie A, O’grady J. The Distribution of Free and Non-Ionic Vesicular Sodium Stibogluconate in the Dog. J Drug Target. 1993;1:133–142. doi: 10.3109/10611869308996069 [DOI] [PubMed] [Google Scholar]
- 56.Croft SL, Coombs GH. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 57.Demicheli C, Ochoa R, da Silva JBB, Falcão CAB, Rossi-Bergmann B, de Melo AL, et al. Oral Delivery of Meglumine Antimoniate-β-Cyclodextrin Complex for Treatment of Leishmaniasis. Antimicrob Agents Chemother. 2004;48:100–103. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 Years of Clinical Experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308 [DOI] [PubMed] [Google Scholar]
- 59.Donovick R, Gold W, Pagano JF, Stout HA. Amphotericins A and B, antifungal antibiotics produced by a streptomycete. I. In vitro studies. Antibiot Annu. 1955;3:579–586. [PubMed] [Google Scholar]
- 60.Morelle C, Mukherjee A, Zhang J, Fani F, Khandelwal A, Gingras H, et al. Well-Tolerated Amphotericin B Derivatives That Effectively Treat Visceral Leishmaniasis. ACS Infect Dis. 2021;7:2472–2482. doi: 10.1021/acsinfecdis.1c00245 [DOI] [PubMed] [Google Scholar]
- 61.Shirzadi MR. Lipsosomal amphotericin B: a review of its properties, function, and use for treatment of cutaneous leishmaniasis. Res Rep Trop Med. 2019;10:11–18. doi: 10.2147/RRTM.S200218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brüggemann RJ, Jensen GM, Lass-Flörl C. Liposomal amphotericin B—the past. J Antimicrob Chemother. 2022;77:ii3–ii10. doi: 10.1093/jac/dkac351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adler-moore JP, Proffitt RT. Development, Characterization, Efficacy and Mode of Action of Ambisome, A Unilamellar Liposomal Formulation of Amphotericin B. J Liposome Res. 1993;3:429–450. doi: 10.3109/08982109309150729 [DOI] [Google Scholar]
- 64.Alpizar-Sosa EA, Ithnin NRB, Wei W, Pountain AW, Weidt SK, Donachie AM, et al. Amphotericin B resistance in Leishmania mexicana: Alterations to sterol metabolism and oxidative stress response. PLoS Negl Trop Dis. 2022;16:e0010779. doi: 10.1371/journal.pntd.0010779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bansal R, Sen SS, Muthuswami R, Madhubala R. Stigmasterol as a potential biomarker for amphotericin B resistance in Leishmania donovani. J Antimicrob Chemother. 2020;75:942–950. doi: 10.1093/jac/dkz515 [DOI] [PubMed] [Google Scholar]
- 66.McCall L-I, Aroussi AE, Choi JY, Vieira DF, Muylder GD, Johnston JB, et al. Targeting Ergosterol Biosynthesis in Leishmania donovani: Essentiality of Sterol 14alpha-demethylase. PLoS Negl Trop Dis. 2015;9:e0003588. doi: 10.1371/journal.pntd.0003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodrigues ML. The Multifunctional Fungal Ergosterol. MBio. 2018;9:10.1128/mbio.01755-18. doi: 10.1128/mBio.01755-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect Dis Ther. 2021;10:115–147. doi: 10.1007/s40121-020-00382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Readio JD, Bittman R. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim Biophys Acta BBA–Biomembr. 1982;685:219–224. doi: 10.1016/0005-2736(82)90103-1 [DOI] [PubMed] [Google Scholar]
- 70.Kumari S, Kumar V, Tiwari RK, Ravidas V, Pandey K, Kumar A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Trop. 2022;235:106661. doi: 10.1016/j.actatropica.2022.106661 [DOI] [PubMed] [Google Scholar]
- 71.Alonso L, Mendanha SA, Dorta ML, Alonso A. Analysis of the Interactions of Amphotericin B with the Leishmania Plasma Membrane Using EPR Spectroscopy. J Phys Chem B. 2020;124:10157–10165. doi: 10.1021/acs.jpcb.0c07721 [DOI] [PubMed] [Google Scholar]
- 72.Lewandowska A, Soutar CP, Greenwood AI, Nimerovsky E, De Lio AM, Holler JT, et al. Fungicidal amphotericin B sponges are assemblies of staggered asymmetric homodimers encasing large void volumes. Nat Struct Mol Biol. 2021;28:972–981. doi: 10.1038/s41594-021-00685-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo X, Zhang J, Li X, Xiao E, Lange JD, Rienstra CM, et al. Sterol sponge mechanism is conserved for glycosylated polyene macrolides. ACS Cent Sci. 2021;7:781–791. doi: 10.1021/acscentsci.1c00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol. 2014;10:400–406. doi: 10.1038/nchembio.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umegawa Y, Yamamoto T, Dixit M, Funahashi K, Seo S, Nakagawa Y, et al. Amphotericin B assembles into seven-molecule ion channels: An NMR and molecular dynamics study. Sci Adv. 2022;8:eabo2658. doi: 10.1126/sciadv.abo2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thanki K, Date T, Jain S. Improved Oral Bioavailability and Gastrointestinal Stability of Amphotericin B through Fatty Acid Conjugation Approach. Mol Pharm. 2019;16:4519–4529. doi: 10.1021/acs.molpharmaceut.9b00662 [DOI] [PubMed] [Google Scholar]
- 77.Alencar ÉN, Sawangchan P, Kirsch LE, Egito EST. Unveiling the Amphotericin B Degradation Pathway and Its Kinetics in Lipid-Based Solutions. J Pharm Sci. 2021;110:1248–1258. doi: 10.1016/j.xphs.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 78.Italia JL, Yahya MM, Singh D, Ravi Kumar MNV. Biodegradable Nanoparticles Improve Oral Bioavailability of Amphotericin B and Show Reduced Nephrotoxicity Compared to Intravenous Fungizone. Pharm Res. 2009;26:1324–1331. doi: 10.1007/s11095-009-9841-2 [DOI] [PubMed] [Google Scholar]
- 79.Chaudhari MB, Desai PP, Patel PA, Patravale VB. Solid lipid nanoparticles of amphotericin B (AmbiOnp): in vitro and in vivo assessment towards safe and effective oral treatment module. Drug Deliv Transl Res. 2016;6:354–364. doi: 10.1007/s13346-015-0267-6 [DOI] [PubMed] [Google Scholar]
- 80.Nimtrakul P, Sermsappasuk P, Tiyaboonchai W. Strategies to enhance oral delivery of amphotericin B: a comparison of uncoated and enteric-coated nanostructured lipid carriers. Drug Deliv. 2020;27:1054–1062. doi: 10.1080/10717544.2020.1785050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujii G, Chang J-E, Coley T, Steere B. The Formation of Amphotericin B Ion Channels in Lipid Bilayers. Biochemistry. 1997;36:4959–4968. doi: 10.1021/bi962894z [DOI] [PubMed] [Google Scholar]
- 82.Adler-Moore JP, Gangneux J-P, Pappas PG. Comparison between liposomal formulations of amphotericin B. Med Mycol. 2016;54:223–231. doi: 10.1093/mmy/myv111 [DOI] [PubMed] [Google Scholar]
- 83.Adler-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother. 2002;49:21–30. doi: 10.1093/jac/49.suppl_1.21 [DOI] [PubMed] [Google Scholar]
- 84.Adler-Moore JP, Proffitt RT, Olson JA, Jensen GM. Tissue pharmacokinetics and pharmacodynamics of AmBisome (L-AmBis) in uninfected and infected animals and their effects on dosing regimens. J Liposome Res. 2017;27:195–209. doi: 10.1080/08982104.2017.1327543 [DOI] [PubMed] [Google Scholar]
- 85.Frézard F, Aguiar MMG, Ferreira LAM, Ramos GS, Santos TT, Borges GSM, et al. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics. 2023;15:99. doi: 10.3390/pharmaceutics15010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rebouças-Silva J, Tadini MC, Devequi-Nunes D, Mansur AL, Silveira-Mattos PS, de Oliveira CI, et al. Evaluation of in vitro and in vivo efficacy of a novel amphotericin B-loaded nanostructured lipid carrier in the treatment of leishmania braziliensis infection. Int J Nanomedicine. 2020;15:8659–8672. doi: 10.2147/IJN.S262642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chivinski J, Nathan K, Naeem F, Ekmekjian T, Libman MD, Barkati S. Intravenous Liposomal Amphotericin B Efficacy and Safety for Cutaneous and Mucosal Leishmaniasis: A Systematic Review and Meta-analysis. Open Forum. Infect Dis. 2023;10:ofad348. doi: 10.1093/ofid/ofad348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wasan E, Mandava T, Crespo-Moran P, Nagy A, Wasan KM. Review of Novel Oral Amphotericin B Formulations for the Treatment of Parasitic Infections. Pharmaceutics. 2022;14:2316. doi: 10.3390/pharmaceutics14112316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, Mathison CJN, et al. Recent Developments in Drug Discovery for Leishmaniasis and Human African Trypanosomiasis. Chem Rev. 2014;114:11305–11347. doi: 10.1021/cr500365f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giri OP, Singh AN. Experience with amphotericin B in sodium stibogluconate—unresponsive cases of visceral Leishmaniasis in north Bihar. J Assoc Physicians India. 1994;42:690–691. [PubMed] [Google Scholar]
- 91.Davidson RN, Di Martino L, Gradoni L, Giacchino R, Russo R, Gaeta GB, et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multi-centre trial. QJM Int J Med. 1994;87:75–81. doi: 10.1093/oxfordjournals.qjmed.a068903 [DOI] [PubMed] [Google Scholar]
- 92.Olson JA, Adler-Moore JP, Smith PJ, Proffitt RT. Treatment of Candida glabrata Infection in Immunosuppressed Mice by Using a Combination of Liposomal Amphotericin B with Caspofungin or Micafungin. Antimicrob Agents Chemother. 2005;49:4895–4902. doi: 10.1128/AAC.49.12.4895-4902.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohamed-Ahmed AHA, Brocchini S, Croft SL. Recent advances in development of amphotericin B formulations for the treatment of visceral leishmaniasis. Curr Opin Infect Dis. 2012;25:695. doi: 10.1097/QCO.0b013e328359eff2 [DOI] [PubMed] [Google Scholar]
- 94.Kim HJ, Kim EJ, Choi JW, Kim YC, Lee H-I, Shin H-I. A Rare Case of Imported Cutaneous Leishmaniasis Caused by Leishmania infantum in the Republic of Korea, 2021. Trop Med Infect Dis. 2023;8:223. doi: 10.3390/tropicalmed8040223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sundar S, Singh A, Agrawal N, Chakravarty J. Effectiveness of Single-Dose Liposomal Amphotericin B in Visceral Leishmaniasis in Bihar. Am J Trop Med Hyg. 2019;101:795–798. doi: 10.4269/ajtmh.19-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.World Health Organization. Control of the leishmaniases: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22–26 March 2010. World Health Organization; 2010. Available from: https://iris.who.int/handle/10665/44412. [Google Scholar]
- 97.Freire M, Badaró F, Avelar ME, Luz K, Nakatani MS, Teixeira R, et al. Efficacy and tolerability of liposomal amphotericin B (Ambisome) in the treatment of visceral leishmaniasis in Brazil. Braz J Infect Dis. 1997;1:230–240. [PubMed] [Google Scholar]
- 98.Sundar S, Chakravarty J. Liposomal Amphotericin B and Leishmaniasis: Dose and Response. J Glob Infect. 2010;2:159. doi: 10.4103/0974-777X.62886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saravolatz LD, Bern C, Adler-Moore J, Berenguer J, Boelaert M, den Boer M, et al. Liposomal Amphotericin B for the Treatment of Visceral Leishmaniasis. Clin Infect Dis. 2006;43:917–924. doi: 10.1086/507530 [DOI] [PubMed] [Google Scholar]
- 100.Horev A, Sagi O, Zur E, Ben-Shimol S. Topical liposomal amphotericin B gel treatment for cutaneous leishmaniasis caused by Leishmania major: a double-blind, randomized, placebo-controlled, pilot study. Int J Dermatol. 2023;62:40–47. doi: 10.1111/ijd.16407 [DOI] [PubMed] [Google Scholar]
- 101.Faral-Tello P, Greif G, Satragno D, Basmadjián Y, Robello C. Leishmania infantum isolates exhibit high infectivity and reduced susceptibility to amphotericin B. RSC Med Chem. 2020;11:913–918. doi: 10.1039/d0md00073f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henry K, Mayet A, Hernandez M, Frechard G, Blanc P-A, Schmitt M, et al. Outbreak of Cutaneous Leishmaniasis among military personnel in French Guiana, 2020: Clinical, phylogenetic, individual and environmental aspects. PLoS Negl Trop Dis. 2021;15:e0009938. doi: 10.1371/journal.pntd.0009938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26:223–227. doi: 10.1016/j.riam.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 104.Maji A, Soutar CP, Zhang J, Lewandowska A, Uno BE, Yan S, et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature. 2023;623:1079–1085. doi: 10.1038/s41586-023-06710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maertens J, Pagano L, Azoulay E, Warris A. Liposomal amphotericin B—the present. J Antimicrob Chemother. 2022;77:ii11–ii20. doi: 10.1093/jac/dkac352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonçalves G, Campos MP, Gonçalves AS, Medeiros LCS, Figueiredo FB. Increased Leishmania infantum resistance to miltefosine and amphotericin B after treatment of a dog with miltefosine and allopurinol. Parasit Vectors. 2021;14:599. doi: 10.1186/s13071-021-05100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferreira BA, Coser EM, de la Roca S, Aoki JI, Branco N, Soares GHC, et al. Amphotericin B resistance in leishmania amazonensis: In vitro and in vivo characterization of a brazilian clinical isolate. PLoS Negl Trop Dis. 2024;18:e0012175. doi: 10.1371/journal.pntd.0012175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mano C, Kongkaew A, Tippawangkosol P, Junkum A, Siriyasatien P, Jariyapan N. In vitro susceptibility to miltefosine of amphotericin B-resistant Leishmania (Mundinia) martiniquensis. Parasitol Res. 2023;122:3027–3035. doi: 10.1007/s00436-023-07992-3 [DOI] [PubMed] [Google Scholar]
- 109.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/CMR.9.4.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mehrizi TZ, Khamesipour A, Ardestani MS, Shahmabadi HE, Hoseini MHM, Mosaffa N, et al. Comparative analysis between four model nanoformulations of amphotericin B-chitosan, amphotericin B-dendrimer, betulinic acid-chitosan and betulinic acid-dendrimer for treatment of Leishmania major: real-time PCR assay plus. Int J Nanomedicine. 2019;14:7593–7607. doi: 10.2147/IJN.S220410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goswami RP, Rahman M, Das S, Tripathi SK, Goswami RP. Combination Therapy Against Indian Visceral Leishmaniasis with Liposomal Amphotericin B (FungisomeTM) and Short-Course Miltefosine in Comparison to Miltefosine Monotherapy. Am J Trop Med Hyg. 2020;103:308–314. doi: 10.4269/ajtmh.19-0931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Widmer F, Wright LC, Obando D, Handke R, Ganendren R, Ellis DH, et al. Hexadecylphosphocholine (Miltefosine) Has Broad-Spectrum Fungicidal Activity and Is Efficacious in a Mouse Model of Cryptococcosis. Antimicrob Agents Chemother. 2006;50:414–421. doi: 10.1128/AAC.50.2.414-421.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berman J (Josh). Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin Drug Metab Toxicol. 2008;4:1209–1216. doi: 10.1517/17425255.4.9.1209 [DOI] [PubMed] [Google Scholar]
- 114.Croft SL, Engel J. Miltefosine—discovery of the antileishmanial activity of phospholipid derivatives. Trans R Soc Trop Med Hyg. 2006;100:S4–S8. doi: 10.1016/j.trstmh.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 115.Benaim G, Paniz-Mondolfi A. Unmasking the mechanism behind miltefosine: Revealing the disruption of intracellular Ca2+ homeostasis as a rational therapeutic target in leishmaniasis and chagas disease. Biomolecules. 2024;14:406. doi: 10.3390/biom14040406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275 [DOI] [PubMed] [Google Scholar]
- 117.Pinto-Martinez AK, Rodriguez-Durán J, Serrano-Martin X, Hernandez-Rodriguez V, Benaim G. Mechanism of action of miltefosine on leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob Agents Chemother. 2017;62:10.1128/aac.01614-17. doi: 10.1128/aac.01614-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lux H, Heise N, Klenner T, Hart D, Opperdoes FR. Ether–lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether–lipid analogues in Leishmania. Mol Biochem Parasitol. 2000;111:1–14. doi: 10.1016/s0166-6851(00)00278-4 [DOI] [PubMed] [Google Scholar]
- 119.Lux H, Hart DT, Parker PJ, Klenner T. Ether lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for anti-leishmanial alkyl phospholipid analogues. In: Nigam S, Kunkel G, Prescott SM, editors. Platelet-Activating Factor and Related Lipid Mediators 2: Roles in Health and Disease. Boston, MA: Springer US; 1996. p. 201–211. doi: 10.1007/978-1-4899-0179-8_33 [DOI] [PubMed] [Google Scholar]
- 120.Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, et al. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by leishmania major *. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200 [DOI] [PubMed] [Google Scholar]
- 121.Kima PE. PI3K signaling in Leishmania infections. Cell Immunol. 2016;309:19–22. doi: 10.1016/j.cellimm.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruhland A, Leal N, Kima PE. Leishmania promastigotes activate PI3K/Akt signalling to confer host cell resistance to apoptosis. Cell Microbiol. 2007;9:84–96. doi: 10.1111/j.1462-5822.2006.00769.x [DOI] [PubMed] [Google Scholar]
- 123.Jangir S, Bala V, Lal N, Kumar L, Sarswat A, Kumar A, et al. Novel alkylphospholipid-DTC hybrids as promising agents against endocrine related cancers acting via modulation of Akt-pathway. Eur J Med Chem. 2014;85:638–647. doi: 10.1016/j.ejmech.2014.08.028 [DOI] [PubMed] [Google Scholar]
- 124.Chugh P, Bradel-Tretheway B, Monteiro-Filho CM, Planelles V, Maggirwar SB, Dewhurst S, et al. Akt inhibitors as an HIV-1 infected macrophage-specific anti-viral therapy. Retrovirology. 2008;5:11. doi: 10.1186/1742-4690-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd [DOI] [PubMed] [Google Scholar]
- 126.Hawkins PT, Stephens LR. Emerging evidence of signalling roles for PI(3,4)P2 in Class I and II PI3K-regulated pathways. Biochem Soc Trans. 2016;44:307–314. doi: 10.1042/BST20150248 [DOI] [PubMed] [Google Scholar]
- 127.Berman J. Miltefosine to treat leishmaniasis. Expert Opin Pharmacother. 2005;6:1381–1388. doi: 10.1517/14656566.6.8.1381 [DOI] [PubMed] [Google Scholar]
- 128.Armitage EG, Alqaisi AQI, Godzien J, Peña I, Mbekeani AJ, Alonso-Herranz V, et al. Complex Interplay between Sphingolipid and Sterol Metabolism Revealed by Perturbations to the Leishmania Metabolome Caused by Miltefosine. Antimicrob Agents Chemother. 2018;62:10.1128/aac.02095-17. doi: 10.1128/aac.02095-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Canuto GAB, Castilho-Martins EA, Tavares MFM, Rivas L, Barbas C, López-Gonzálvez Á. Multi-analytical platform metabolomic approach to study miltefosine mechanism of action and resistance in Leishmania. Anal Bioanal Chem. 2014;406:3459–3476. doi: 10.1007/s00216-014-7772-1 [DOI] [PubMed] [Google Scholar]
- 130.Musa AM, Mbui J, Mohammed R, Olobo J, Ritmeijer K, Alcoba G, et al. Paromomycin and Miltefosine Combination as an Alternative to Treat Patients With Visceral Leishmaniasis in Eastern Africa: A Randomized, Controlled. Multicountry Trial. Clin Infect Dis. 2023;76:e1177–e1185. doi: 10.1093/cid/ciac643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Planting AST, Stoter G, Verweij J. Phase II study of daily oral miltefosine (hexadecylphosphocholine) in advanced colorectal cancer. Eur J Cancer. 1993;29:518–519. doi: 10.1016/s0959-8049(05)80142-x [DOI] [PubMed] [Google Scholar]
- 132.Sindermann H, Engel J. Development of miltefosine as an oral treatment for leishmaniasis. Trans R Soc Trop Med Hyg. 2006;100:S17–S20. doi: 10.1016/j.trstmh.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 133.Saurabh S, Mahabir M. Adverse ocular events on miltefosine treatment for post-kala-azar dermal leishmaniasis in India. Trop Doct. 2020;50:37–42. doi: 10.1177/0049475519877317 [DOI] [PubMed] [Google Scholar]
- 134.Sundar S, Singh J, Dinkar A, Agrawal N. Safety and Effectiveness of Miltefosine in Post–Kala-Azar Dermal Leishmaniasis: An Observational Study. Open Forum. Infect Dis. 2023;10:ofad231. doi: 10.1093/ofid/ofad231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Thiel PPAM, Leenstra T, Kager PA, de Vries HJ, van Vugt M, van der Meide WF, et al. Miltefosine Treatment of Leishmania major Infection: An Observational Study Involving Dutch Military Personnel Returning from Northern Afghanistan. Clin Infect Dis. 2010;50:80–83. doi: 10.1086/648726 [DOI] [PubMed] [Google Scholar]
- 136.Bhattacharya SK, Sinha PK, Sundar S, Thakur CP, Jha TK, Pandey K, et al. Phase 4 Trial of Miltefosine for the Treatment of Indian Visceral Leishmaniasis. J Infect Dis. 2007;196:591–598. doi: 10.1086/519690 [DOI] [PubMed] [Google Scholar]
- 137.Ramesh V, Katara GK, Verma S, Salotra P. Miltefosine as an effective choice in the treatment of post-kala-azar dermal leishmaniasis. Br J Dermatol. 2011;165:411–414. doi: 10.1111/j.1365-2133.2011.10402.x [DOI] [PubMed] [Google Scholar]
- 138.Khandpur S, Chaturvedi P, Kumar U, Khaitan BK, Samantaray JC, Sharma VK. Oral miltefosine in post-kala-azar dermal leishmaniasis–experience in three cases. Int J Dermatol. 2010;49:565–569. doi: 10.1111/j.1365-4632.2010.04326.x [DOI] [PubMed] [Google Scholar]
- 139.Soto J, Toledo J, Valda L, Balderrama M, Rea I, Parra R, et al. Treatment of Bolivian Mucosal Leishmaniasis with Miltefosine. Clin Infect Dis. 2007;44:350–356. doi: 10.1086/510588 [DOI] [PubMed] [Google Scholar]
- 140.van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect Dis. 2010;10:184–194. doi: 10.1016/S1473-3099(10)70011-6 [DOI] [PubMed] [Google Scholar]
- 141.Reimão JQ, Pita Pedro DP, Coelho AC. The preclinical discovery and development of oral miltefosine for the treatment of visceral leishmaniasis: a case history. Expert Opin Drug Discovery. 2020;15:647–658. doi: 10.1080/17460441.2020.1743674 [DOI] [PubMed] [Google Scholar]
- 142.Pijpers J, Boer ML den, Essink DR, Ritmeijer K. The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia–A review and meta-analysis. PLoS Negl Trop Dis. 2019;13:e0007173. doi: 10.1371/journal.pntd.0007173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abongomera C, Battaglioli T, Adera C, Ritmeijer K. Severe post-kala-azar dermal leishmaniasis successfully treated with miltefosine in an Ethiopian HIV patient. Int J Infect Dis. 2019;81:221–224. doi: 10.1016/j.ijid.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 144.Palić S, Beijnen JH, Dorlo TPC. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int J Antimicrob Agents. 2022;59:106459. doi: 10.1016/j.ijantimicag.2021.106459 [DOI] [PubMed] [Google Scholar]
- 145.Soto JA, Berman JD. Miltefosine Treatment of Cutaneous Leishmaniasis. Clin Infect Dis. 2021;73:e2463–e2464. doi: 10.1093/cid/ciaa1461 [DOI] [PubMed] [Google Scholar]
- 146.US Food and Drug Administration. Label for IMPAVIDO (miltefosine) capsules, for oral use [prescribing information]. 2014 Mar. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf.
- 147.Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- 148.Griensven J van, Dorlo TP, Diro E, Costa C, Burza S. The status of combination therapy for visceral leishmaniasis: An updated review. Lancet Infect Dis. 2024;24:e36–e46. doi: 10.1016/S1473-3099(23)00353-5 [DOI] [PubMed] [Google Scholar]
- 149.Seifert K, Croft SL. In Vitro and In Vivo Interactions between Miltefosine and Other Antileishmanial Drugs. Antimicrob Agents Chemother. 2006;50:73–79. doi: 10.1128/AAC.50.1.73-79.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Registre C, Soares RDOA, Rubio KTS, Santos ODH, Carneiro SP. A Systematic Review of Drug-Carrying Nanosystems Used in the Treatment of Leishmaniasis. ACS Infect Dis. 2023;9:423–449. doi: 10.1021/acsinfecdis.2c00632 [DOI] [PubMed] [Google Scholar]
- 151.Davidson RN, den Boer M, Ritmeijer K. Paromomycin. Trans R Soc Trop Med Hyg. 2009;103:653–660. doi: 10.1016/j.trstmh.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 152.OI K. A study of experimental cutaneous leishmaniasis in white mice. Med Parazitol (Mosk). 1961;30:684–691. [PubMed] [Google Scholar]
- 153.Chunge CN, Owate J, Pamba HO, Donno L. Treatment of visceral leishmaniasis in Kenya by aminosidine alone or combined with sodium stibogluconate. Trans R Soc Trop Med Hyg. 1990;84:221–225. doi: 10.1016/0035-9203(90)90263-e [DOI] [PubMed] [Google Scholar]
- 154.Scott JAG, Davidson RN, Moody AH, Grant HR, Felmingham D, Scott GMS, et al. Aminosidine (paromomycin) in the treatment of leishmaniasis imported into the United Kingdom. Trans R Soc Trop Med Hyg. 1992;86:617–619. doi: 10.1016/0035-9203(92)90151-2 [DOI] [PubMed] [Google Scholar]
- 155.Neal RA. The effect of antibiotics of the neomycin group on experimental cutaneous leishmaniasis. Ann Trop Med Parasitol. 1968;62:54–62. doi: 10.1080/00034983.1968.11686529 [DOI] [PubMed] [Google Scholar]
- 156.Sundar S, Jha TK, Thakur CP, Sinha PK, Bhattacharya SK. Injectable Paromomycin for Visceral Leishmaniasis in India. N Engl J Med. 2007;356:2571–2581. doi: 10.1056/NEJMoa066536 [DOI] [PubMed] [Google Scholar]
- 157.Pokharel P, Ghimire R, Lamichhane P. Efficacy and Safety of Paromomycin for Visceral Leishmaniasis: A Systematic Review. J Trop Med. 2021;2021:e8629039. doi: 10.1155/2021/8629039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davis BD, Chen LL, Tai PC. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci U S A. 1986;83:6164–6168. doi: 10.1073/pnas.83.16.6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maarouf M, de Kouchkovsky Y, Brown S, Petit PX, Robert-Gero M. In VivoInterference of Paromomycin with Mitochondrial Activity ofLeishmania. Exp Cell Res. 1997;232:339–348. doi: 10.1006/excr.1997.3500 [DOI] [PubMed] [Google Scholar]
- 160.Fernández MM, Malchiodi EL, Algranati ID. Differential Effects of Paromomycin on Ribosomes of Leishmania mexicana and Mammalian Cells. Antimicrob Agents Chemother. 2011;55:86–93. doi: 10.1128/AAC.00506-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maarouf M, Lawrence F, Croft SL, Robert-Gero M. Ribosomes ofLeishmania are a target for the aminoglycosides. Parasitol Res. 1995;81:421–425. doi: 10.1007/BF00931504 [DOI] [PubMed] [Google Scholar]
- 162.Webster CM, Shepherd M. A mini-review: environmental and metabolic factors affecting aminoglycoside efficacy. World J Microbiol Biotechnol. 2022;39:7. doi: 10.1007/s11274-022-03445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fourmy D, Recht MI, Blanchard SC, Puglisi JD. Structure of the A Site of Escherichia coli 16S Ribosomal RNA Complexed with an Aminoglycoside Antibiotic. Science. 1996;274:1367–1371. doi: 10.1126/science.274.5291.1367 [DOI] [PubMed] [Google Scholar]
- 164.Shalev-Benami M, Zhang Y, Rozenberg H, Nobe Y, Taoka M, Matzov D, et al. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat Commun. 2017;8:1589. doi: 10.1038/s41467-017-01664-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matos APS, Viçosa AL, Ré MI, Ricci-Júnior E, Holandino C. A review of current treatments strategies based on paromomycin for leishmaniasis. J Drug Deliv Sci Technol. 2020;57:101664. doi: 10.1016/j.jddst.2020.101664 [DOI] [Google Scholar]
- 166.Kimutai R, Musa AM, Njoroge S, Omollo R, Alves F, Hailu A, et al. Safety and Effectiveness of Sodium Stibogluconate and Paromomycin Combination for the Treatment of Visceral Leishmaniasis in Eastern Africa: Results from a Pharmacovigilance Programme. Clin Drug Investig. 2017;37:259–272. doi: 10.1007/s40261-016-0481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jamil KM, Haque R, Rahman R, Faiz MA, Bhuiyan ATMRH, Kumar A, et al. Effectiveness Study of Paromomycin IM Injection (PMIM) for the Treatment of Visceral Leishmaniasis (VL) in Bangladesh. PLoS Negl Trop Dis. 2015;9:e0004118. doi: 10.1371/journal.pntd.0004118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.El-On J, Jacobs GP, Witztum E, Greenblatt CL. Development of topical treatment for cutaneous leishmaniasis caused by Leishmania major in experimental animals. Antimicrob Agents Chemother. 1984;26:745–751. doi: 10.1128/AAC.26.5.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Krause G, Kroeger A. Topical treatment of American cutaneous leishmaniasis with paramomycin and methylbenzethonium chloride: a clinical study under field conditions in Ecuador. Trans R Soc Trop Med Hyg. 1994;88:92–94. doi: 10.1016/0035-9203(94)90517-7 [DOI] [PubMed] [Google Scholar]
- 170.Arana BA, Mendoza CE, Rizzo NR, Kroeger A. Randomized, controlled, double-blind trial of topical treatment of cutaneous leishmaniasis with paromomycin plus methylbenzethonium chloride ointment in Guatemala. Am J Trop Med Hyg. 2001;65:466–470. doi: 10.4269/ajtmh.2001.65.466 [DOI] [PubMed] [Google Scholar]
- 171.Ozgoztasi O, Baydar I. A randomized clinical trial of topical paromomycin versus oral ketoconazole for treating cutaneous leishmaniasis in Turkey. Int J Dermatol. 1997;36:61–63. doi: 10.1046/j.1365-4362.1997.00022.x [DOI] [PubMed] [Google Scholar]
- 172.Asilian A, Jalayer T, Nilforooshzadeh M, Ghassemi RL, Peto R, Wayling S, et al. Treatment of cutaneous leishmaniasis with aminosidine (paromomycin) ointment: double-blind, randomized trial in the Islamic Republic of Iran. Bull World Health Organ. 2003;81:353–359. [PMC free article] [PubMed] [Google Scholar]
- 173.Salah AB, Buffet PA, Morizot G, Massoud NB, Zâatour A, Alaya NB, et al. WR279,396, a Third Generation Aminoglycoside Ointment for the Treatment of Leishmania major Cutaneous Leishmaniasis: A Phase 2, Randomized, Double Blind, Placebo Controlled Study. PLoS Negl Trop Dis. 2009;3:e432. doi: 10.1371/journal.pntd.0000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, et al. Topical Paromomycin with or without Gentamicin for Cutaneous Leishmaniasis. N Engl J Med. 2013;368:524–532. doi: 10.1056/NEJMoa1202657 [DOI] [PubMed] [Google Scholar]
- 175.Williams D, Mullen AB, Baillie AJ, Carter KC. Comparison of the Efficacy of Free and Non-ionic-surfactant Vesicular Formulations of Paromomycin in a Murine Model of Visceral Leishmaniasis. J Pharm Pharmacol. 1998;50:1351–1356. doi: 10.1111/j.2042-7158.1998.tb03358.x [DOI] [PubMed] [Google Scholar]
- 176.Khan W, Sharma SS, Kumar N. Bioanalytical method development, pharmacokinetics, and toxicity studies of paromomycin and paromomycin loaded in albumin microspheres. Drug Test Anal. 2013;5:453–460. doi: 10.1002/dta.339 [DOI] [PubMed] [Google Scholar]
- 177.Banerjee A, De M, Ali N. Combination Therapy with Paromomycin-Associated Stearylamine-Bearing Liposomes Cures Experimental Visceral Leishmaniasis through Th1-Biased Immunomodulation. Antimicrob Agents Chemother. 2011;55:1661–1670. doi: 10.1128/AAC.00524-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kharaji MH, Doroud D, Taheri T, Rafati S. Drug Targeting to Macrophages With Solid Lipid Nanoparticles Harboring Paromomycin: an In Vitro Evaluation Against L. major and L. tropica. AAPS PharmSciTech. 2016;17:1110–1119. doi: 10.1208/s12249-015-0439-1 [DOI] [PubMed] [Google Scholar]
- 179.Heidari-Kharaji M, Taheri T, Doroud D, Habibzadeh S, Badirzadeh A, Rafati S. Enhanced paromomycin efficacy by solid lipid nanoparticle formulation against Leishmania in mice model. Parasite Immunol. 2016;38:599–608. doi: 10.1111/pim.12340 [DOI] [PubMed] [Google Scholar]
- 180.de Sá FAP, Andrade JFM, Miranda TC, Cunha-Filho M, Gelfuso GM, Lapteva M, et al. Enhanced topical paromomycin delivery for cutaneous leishmaniasis treatment: Passive and iontophoretic approaches. Int J Pharm. 2023;648:123617. doi: 10.1016/j.ijpharm.2023.123617 [DOI] [PubMed] [Google Scholar]
- 181.Sands M, Kron MA, Brown RB. Pentamidine: A Review. Rev Infect Dis. 1985;7:625–6344. doi: 10.1093/clinids/7.5.625 [DOI] [PubMed] [Google Scholar]
- 182.Hazarika AN. Treatment of Kala-Azar with Pentamidine Isothionate. A Study of 55 Cases. Ind Med Gaz. 1949;84:140. [PMC free article] [PubMed] [Google Scholar]
- 183.Lai A Fat EJ, Vrede MA, Soetosenojo RM, Lai A Fat RF. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int J Dermatol. 2002;41:796–800. doi: 10.1046/j.1365-4362.2002.01633.x [DOI] [PubMed] [Google Scholar]
- 184.Basselin M, Denise H, Coombs GH, Barrett MP. Resistance to Pentamidine in Leishmania mexicana Involves Exclusion of the Drug from the Mitochondrion. Antimicrob Agents Chemother. 2002;46:3731–3738. doi: 10.1128/AAC.46.12.3731-3738.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mehta A, Shaha C. Apoptotic Death in Leishmania donovani Promastigotes in Response to Respiratory Chain Inhibition: COMPLEX II INHIBITION RESULTS IN INCREASED PENTAMIDINE CYTOTOXICITY *. J Biol Chem. 2004;279:11798–11813. doi: 10.1074/jbc.M309341200 [DOI] [PubMed] [Google Scholar]
- 186.Briones Nieva CA, Cid AG, Romero AI, García-Bustos MF, Villegas M, Bermúdez JM. An appraisal of the scientific current situation and new perspectives in the treatment of cutaneous leishmaniasis. Acta Trop. 2021;221:105988. doi: 10.1016/j.actatropica.2021.105988 [DOI] [PubMed] [Google Scholar]
- 187.Sztuba-Solinska J, Chavez-Calvillo G, Cline SE. Unveiling the druggable RNA targets and small molecule therapeutics. Bioorg Med Chem. 2019;27:2149–2165. doi: 10.1016/j.bmc.2019.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kelly ML, Chu C-C, Shi H, Ganser LR, Bogerd HP, Huynh K, et al. Understanding the characteristics of nonspecific binding of drug-like compounds to canonical stem–loop RNAs and their implications for functional cellular assays. RNA. 2021;27:12–26. doi: 10.1261/rna.076257.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ho JM, Bakkalbasi E, Söll D, Miller CA. Drugging tRNA aminoacylation. RNA Biol. 2018;15:667–677. doi: 10.1080/15476286.2018.1429879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Warren KS, Mahmoud AF. Tropical and Geographical Medicine. Trop Geogr Med. 1984. [cited 15 Jan 2024]. Available: https://www.cabdirect.org/cabdirect/abstract/19842016274. [Google Scholar]
- 191.Sheikh SY, Ansari WA, Hassan F, Faruqui T, Khan MF, Akhter Y, et al. Drug repositioning to discover novel ornithine decarboxylase inhibitors against visceral leishmaniasis. J Mol Recognit. 2023;36:e3021. doi: 10.1002/jmr.3021 [DOI] [PubMed] [Google Scholar]
- 192.Salazar-Villamizar ME, Escobar P. In vitro selection of ketoconazole-pentamidine-resistant Leishmania (Viannia) braziliensis strains. Exp Parasitol. 2022;233:108206. doi: 10.1016/j.exppara.2021.108206 [DOI] [PubMed] [Google Scholar]
- 193.Mitropoulos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: Updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309–322. doi: 10.1016/j.jaad.2009.06.088 [DOI] [PubMed] [Google Scholar]
- 194.Piccica M, Lagi F, Bartoloni A, Zammarchi L. Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: a systematic review. J Travel Med. 2021;28:taab065. doi: 10.1093/jtm/taab065 [DOI] [PubMed] [Google Scholar]
- 195.World Health Organization. Manual on case management and surveillance of the leishmaniases in the WHO European Region. 2017. Available from: https://www.who.int/publications-detail-redirect/9789289052511. [Google Scholar]
- 196.Soto J, Buffet P, Grogl M, Berman J. Successful Treatment of Colombian Cutaneous Leishmaniasis with Four Injections of Pentamidine. Am J Trop Med Hyg. 1994;50:107–111. doi: 10.4269/ajtmh.1994.50.107 [DOI] [PubMed] [Google Scholar]
- 197.Soto-Mancipe J, Grogl M, Berman JD. Evaluation of Pentamidine for the Treatment of Cutaneous Leishmaniasis in Colombia. Clin Infect Dis. 1993;16:417–425. doi: 10.1093/clind/16.3.417 [DOI] [PubMed] [Google Scholar]
- 198.Roussel M, Nacher M, Frémont G, Rotureau B, Clyti E, Sainte-Marie D, et al. Comparison between one and two injections of pentamidine isethionate, at 7 mg/kg in each injection, in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2006;100:307–314. doi: 10.1179/136485906X105561 [DOI] [PubMed] [Google Scholar]
- 199.Andersen EM, Cruz-Saldarriaga M, Llanos-Cuentas A, Luz-Cjuno M, Echevarria J, Miranda-Verastegui C, et al. COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS. Am J Trop Med Hyg. 2005;72:133–137. doi: 10.4269/ajtmh.2005.72.133 [DOI] [PubMed] [Google Scholar]
- 200.Blum J, Buffet P, Visser L, Harms G, Bailey MS, Caumes E, et al. LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014. J Travel Med. 2014;21:116–129. doi: 10.1111/jtm.12089 [DOI] [PubMed] [Google Scholar]
- 201.Wiwanitkit V. Interest in paromomycin for the treatment of visceral leishmaniasis (kala-azar). Ther Clin Risk Manag. 2012;8:323–328. doi: 10.2147/TCRM.S30139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang H, Lv P, Jiang J, Liu Y, Yan R, Shu S, et al. Advances in developing ACE2 derivatives against SARS-CoV-2. Lancet Microbe. 2023;4:e369–e378. doi: 10.1016/S2666-5247(23)00011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wyllie S, Thomas M, Patterson S, Crouch S, De Rycker M, Lowe R, et al. Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis. Nature. 2018;560:192–197. doi: 10.1038/s41586-018-0356-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Riu F, Ruppitsch LA, Duy Vo D, Hong RS, Tyagi M, Matheeussen A, et al. Discovery of a series of macrocycles as potent inhibitors of leishmania infantum. J Med Chem. 2024. [cited 2024 Oct 20]. doi: 10.1021/acs.jmedchem.4c01370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Habib M, Zheng J, Chan C-F, Yang Z, Wong ILK, Chow LMC, et al. A targeted and protease-activated genetically encoded melittin-containing particle for the treatment of cutaneous and visceral leishmaniasis. ACS Appl Mater Interfaces. 2024;16:49148–49163. doi: 10.1021/acsami.4c10426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barbosa FEV, de Lima DB, Dos Santos ALE, de Sousa VC, de Cássia Viana Carvalho R, de Moraes Alves MM, et al. Dioclea violacea lectin has potent in vitro leishmanicidal activity against leishmania infantum via carbohydrate recognition domain. Int J Biol Macromol. 2024;280:135665. doi: 10.1016/j.ijbiomac.2024.135665 [DOI] [PubMed] [Google Scholar]
- 207.Kumar J, Jyotisha QR, Jagruthi P, Arifuddin M, Qureshi IA. Discovery of 8-hydroxy-2-quinoline carbaldehyde derivatives as inhibitors for M1 aminopeptidase of leishmania donovani. Int J Biol Macromol. 2024;279:135105. doi: 10.1016/j.ijbiomac.2024.135105 [DOI] [PubMed] [Google Scholar]
- 208.Rao SPS, Gould MK, Noeske J, Saldivia M, Jumani RS, Ng PS, et al. Cyanotriazoles are selective topoisomerase II poisons that rapidly cure trypanosome infections. Science. 2023;380:1349–1356. doi: 10.1126/science.adh0614 [DOI] [PubMed] [Google Scholar]
- 209.Lago T, Peixoto F, Mambelli F, Carvalho LP, Guimarães LH, Carvalho AM, et al. Use of topical rSm29 in combination with intravenous meglumine antimoniate in the treatment of cutaneous leishmaniasis: A randomized controlled trial. Int J Infect Dis. 2024:147. doi: 10.1016/j.ijid.2024.107206 [DOI] [PubMed] [Google Scholar]
- 210.Braz EMA, Silva SCCC, Alves MMM, Carvalho FAA, Magalhães R, Osajima JA, et al. Chitosan/collagen biomembrane loaded with 2,3-dihydrobenzofuran for the treatment of cutaneous leishmaniasis. Int J Biol Macromol. 2024;280:135995. doi: 10.1016/j.ijbiomac.2024.135995 [DOI] [PubMed] [Google Scholar]
- 211.Nahanji MK, Mahboobian MM, Harchegani AL, Mohebali M, Fallah M, Nourian A, et al. Enhancing the efficacy of fluconazole against leishmania major: Formulation and evaluation of FLZ-nanoemulsions for topical delivery. Biomed Pharmacother. 2024;178:117109. doi: 10.1016/j.biopha.2024.117109 [DOI] [PubMed] [Google Scholar]
- 212.Candido ACBB, Pagotti MC, Santos DA dos, Paula LA de L, Veneziani RCS, Bastos JK, et al. Efficacy of diterpene polyalthic acid combined with amphotericin B against leishmania amazonensis in vitro. Pharmaceuticals. 2024;17:1243. doi: 10.3390/ph17091243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, et al. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int J Nanomedicine. 2011;6:2705–2714. doi: 10.2147/IJN.S23883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Frézard F, Martins PS, Barbosa MCM, Pimenta AMC, Ferreira WA, de Melo JE, et al. New insights into the chemical structure and composition of the pentavalent antimonial drugs, meglumine antimonate and sodium stibogluconate. J Inorg Biochem. 2008;102:656–665. doi: 10.1016/j.jinorgbio.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 215.Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, et al. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011;118:87–96. doi: 10.1016/j.actatropica.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 216.Clementi A, Battaglia G, Floris M, Castellino P, Ronco C, Cruz DN. Renal involvement in leishmaniasis: a review of the literature. NDT Plus. 2011;4:147–152. doi: 10.1093/ndtplus/sfr008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lyra MR, Passos SRL, Pimentel MIF, Bedoya-Pacheco SJ, Valete-Rosalino CM, Vasconcellos ECF, et al. PANCREATIC TOXICITY AS AN ADVERSE EFFECT INDUCED BY MEGLUMINE ANTIMONIATE THERAPY IN A CLINICAL TRIAL FOR CUTANEOUS LEISHMANIASIS. Rev Inst Med Trop São Paulo. 2016;58:68. doi: 10.1590/S1678-9946201658068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.El Jeri KH, Harzallah A, Barbouch S, Bacha MM, Kheder R, Turki S, et al. Visceral Leishmaniasis in Adults with Nephropathy. Saudi J Kidney Dis Transpl. 2017;28:95. doi: 10.4103/1319-2442.198159 [DOI] [PubMed] [Google Scholar]
- 219.Marques SA, Merlotto MR, Ramos PM, Marques MEA. American tegumentary leishmaniasis: severe side effects of pentavalent antimonial in a patient with chronic renal failure. An Bras Dermatol. 2019;94:355–357. doi: 10.1590/abd1806-4841.20198388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cardona-Arias JA, López-Carvajal L, Tamayo-Plata MP, Vélez ID. Comprehensive economic evaluation of thermotherapy for the treatment of cutaneous leishmaniasis in Colombia. BMC Public Health. 2018;18:185. doi: 10.1186/s12889-018-5060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Valentim Silva JR, de Barros NB, Aragão Macedo SR, Ferreira ADS, Moreira Dill LS, Zanchi FB, et al. A natural cell-penetrating nanopeptide combined with pentavalent antimonial as experimental therapy against cutaneous leishmaniasis. Exp Parasitol. 2020;217:107934. doi: 10.1016/j.exppara.2020.107934 [DOI] [PubMed] [Google Scholar]
- 222.Baillie AJ, Coombs GH, Dolan TF, Hunter CA, Laakso T, Sjöholm I, et al. Biodegradable microspheres‖: polyacryl starch microparticles as a delivery system for the antileishmanial drug, sodium stibogluconate. J Pharm Pharmacol. 1987;39:832–835. doi: 10.1111/j.2042-7158.1987.tb05126.x [DOI] [PubMed] [Google Scholar]
- 223.Frézard F, Demicheli C. New delivery strategies for the old pentavalent antimonial drugs. Expert Opin Drug Deliv. 2010;7:1343–1358. doi: 10.1517/17425247.2010.529897 [DOI] [PubMed] [Google Scholar]
- 224.Demicheli C, Santos LS, Ferreira CS, Bouchemal N, Hantz E, Eberlin MN, et al. Synthesis and characterization of Sb(V)–adenosine and Sb(V)–guanosine complexes in aqueous solution. Inorg Chim Acta. 2006;359:159–167. doi: 10.1016/j.ica.2005.09.003 [DOI] [Google Scholar]
- 225.Ferreira CS, da Rocha ICM, Neto RL, Melo MN, Frézard F, Demicheli C. Influence of the nucleobase on the physicochemical characteristics and biological activities of SbV-ribonucleoside complexes. J Braz Chem Soc. 2010;21:1258–1265. doi: 10.1590/S0103-50532010000700013 [DOI] [Google Scholar]
- 226.Anversa L, Salles Tiburcio MG, Batista LR, Cuba MB, Nogueira Nascentes GA, Martins TY, et al. Amiodarone and itraconazole improve the activity of pentavalent antimonial in the treatment of experimental cutaneous leishmaniasis. Int J Antimicrob Agents. 2017;50:159–165. doi: 10.1016/j.ijantimicag.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 227.de Assis TSM, Rosa DCP, Teixeira E de M, Cota G, Azeredo-da-Silva ALF, Werneck G, et al. The direct costs of treating human visceral leishmaniasis in Brazil. Rev Soc Bras Med Trop. 2017;50:478–482. doi: 10.1590/0037-8682-0133-2017 [DOI] [PubMed] [Google Scholar]
- 228.Seify R, Zahednezhad F, Zakeri-Milani P, Valizadeh H. Amphotericin B liposomal formulation: applicable preparation methods, challenges, and tips. Drug Dev Ind Pharm. 2023;49:367–376. doi: 10.1080/03639045.2023.2215006 [DOI] [PubMed] [Google Scholar]
- 229.Kumar A, Singh VK, Tiwari R, Madhukar P, Rajneesh Kumar S, et al. Post kala-azar dermal leishmaniasis in the Indian sub-continent: challenges and strategies for elimination. Front Immunol. 2023;14. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1236952. doi: 10.3389/fimmu.2023.1236952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chrusciak-Talhari A, Dietze R, Talhari CC, Silva RM da, Yamashita EPG, Penna G de O, et al. Randomized Controlled Clinical Trial to Access Efficacy and Safety of Miltefosine in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg. 2011;84:255–260. doi: 10.4269/ajtmh.2011.10-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Carvalho JP, de Assis TM, Simões TC, Cota G. Estimating direct costs of the treatment for mucosal leishmaniasis in Brazil. Rev Soc Bras Med Trop. 2021;54:e04542020. doi: 10.1590/0037-8682-0454-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Reguera RM, Pérez-Pertejo Y, Gutiérrez-Corbo C, Domínguez-Asenjo B, Ordóñez C, García-Estrada C, et al. Current and promising novel drug candidates against visceral leishmaniasis. Pure Appl Chem. 2019;91:1385–1404. doi: 10.1515/pac-2018-1102 [DOI] [Google Scholar]
- 233.WHA60.13 Control of leishmaniasis. [cited 2024 Jan 19]. Available from: https://www.who.int/publications-detail-redirect/wha60.13.