Abstract
Background
Radiation dermatitis (RD) or skin toxicity is one of the most common acute side effects of radiation in head and neck cancer patients. This study aims to correlate the pattern of volumetric-modulated arc therapy (VMAT) dose distribution to the skin with the grades of RD.
Materials and methods
80 plans of histopathologically proven squamous cell carcinoma head and neck patients already treated with definitive concurrent chemoradiation [66–70 Gy in 33–35# or 66 Gy in 30# in simultaneous integrated boost (SIB), with concurrent Cisplatin 100 mg/m2 3 weekly] at our institution between November 2022 and November 2023 were retrieved from our digital archives. For each plan, 1 ring structure was created 3mm below the external skin surface, and the parameters V40, V50, V60 and Dmax were collected from the same. These parameters were correlated with grades of RD as per Common Terminology Criteria for Adverse Events (CTCAE) v5.0. The statistical analysis was done using MedCalc software version 22.021.
Results
The incidence of G2/G3 RD was 52.5%, and its incidence was significantly correlated with all of the four parameters. Statistically significant (p < 0.001) dosimetric predictive accuracy was provided by 71.66 cc, 29.98 cc and 7.624 cc of the 3mm skin ring V40, V50 and V60, respectively.
Conclusion
The dose distribution pattern to a skin layer stationed 3mm below the surface may help predict the development of severe RD in head and neck cancer patients receiving concurrent chemoradiation.
Keywords: head and neck cancer, predictive accuracy, radiation dermatitis, squamous cell carcinoma
Introduction
Radiation dermatitis (RD) or skin toxicity is one of the most common acute side effects of radiation in head and neck cancer patients. If higher grade, it can cause unwanted delay or untimely stoppage of treatment, and considerable physical as well as psychological suffering of the patients. Radiobiologically, the development of RD has long been known to be dependent on dose-volume and overall-treatment-time [1]. Concurrent chemotherapy [2] increases the risk of RD significantly, as well as other acute side effects such as oral mucositis and dysphagia. Modern conformal techniques, like intensity-modulated radiotherapy (IMRT) [3] and volumetric-modulated arc therapy (VMAT), are now standards of care in head and neck squamous cell carcinoma. Though highly conformal as per target volume coverage and Organs-at-risk sparing [4, 5], these modern techniques have been observed to result in a relative overdosage of patient’s skin surface [6]. Apart from only a handful of previous studies, neither recommendations nor any guidelines are available [7] to use skin dose constraints to prevent the development of RD. Our study aims to correlate the pattern of VMAT dose distribution to the skin with the different grades of RD, and to assess whether specific skin dose-volume threshold values may be used as possible predictive factors for higher grades of toxicity in future studies. We hope that our study will help researchers in future to prospectively validate our data, and create a specific skin dose-constraint guideline so that treatment can be planned abiding those constraints to prevent acute skin toxicity of higher grade.
Materials and methods
Sample and treatment features
80 treatment plans of histopathologically proven inoperable non-metastatic head and neck squamous cell carcinoma patients of different subsites already treated with cisplatin-based definitive concurrent chemoradiation in VMAT technique at our institution between November 2022 and November 2023 were randomly selected, excluding the bolus-using plans (superficial gross tumour or skin infiltration). During treatment, concurrent cisplatin [8] was administered at a dose of 100 mg/m2 3 weekly for patients with an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–1 with dose modification, if needed, according to a kidney function test and general condition. For the different subsites, radiation dose was 66–70 Gy at conventional 2 Gy per fraction (33–35 fractions) in a phasic plan or 30 fractions in the simultaneous integrated boost (SIB) technique as per physician’s choice. Initial pre-treatment nutritional status was assessed using Simplified Nutritional Appetite Questionnaire (SNAQ) and noted down [9]. Standard supportive care measures, such as pain management, maintenance of oral hygiene and nutritional counselling, were implemented on a routine basis.
For each of the patients, a personalized thermoplastic head-neck-shoulder mask was created. An institutional Philips Brilliance 16-slice CT Scan machine was used to acquire simulation scans of 3mm slice width. Target volume contouring was done according to international consensus guidelines [10] at Varian Somavision workstation. 7mm margin was given for planning target volume (PTV) around the clinical target volume (CTV) as per institutional protocol. For PTV, a negative 3mm margin was delineated from patient’s body surface contour to avoid excessive accumulation of skin dose. Double arc-VMAT Treatment plannings were done using Eclipse v15.5 (VARIAN medical systems) software (RapidArc) and Anisotropic Analytical Algorithm (AAA) for dose calculation and optimization. The patients were treated in VARIAN TrueBeam linear accelerator (serial number-3279). Informed consent was obtained before starting treatment from all individual patients included in this study.
Analysis of skin dose distribution
The selected 80 VMAT plans were retrieved from our digital archive. For each plan, 1 new ring structure of 3 mm thickness was created below the external skin surface up to 3mm depth as all the PTV margins were cropped 3mm from the skin surface as per institution protocol, further supported by published data [11, 12], and thus this 3mm-thick ring structure was a perfect model to study the skin dose parameters. These volumes of interest extended between the upper and lower limits of PTV plus a fixed 1cm margin in both cranial and caudal directions. For this ‘3mm ring’ structure of each patient, the following parameters were collected — V40, V50, V60 and Dmax, where V40, V50 and V60 equal to the volume of the ring structure receiving a minimum of 40Gy, 50Gy and 60Gy dose respectively, and Dmax represents the maximum absolute point dose anywhere within the structure. These parameters were correlated with different grades of RD as per Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [13], as assessed by at least two of the authors at a time, once weekly during treatment and documented in the individual patient’s treatment record files.
Outcome measures and statistical analysis
Descriptive statistics were used to report patient ECOG Performance Status, age, sex, smoking history, disease [primary site, American Joint Committee on Cancer (AJCC) 8th edition tumor– node–metastasis (TNM) staging], treatment (RT compliance, RT schedule) and skin dose distribution (explorative dosimetric parameters) — related characteristics as median and range for continuous variables. These continuous variables were tested with Mann-Whitney test as it is a common nonparametric test for comparing two groups of independent samples [14], while categorical variables were tested by Fisher’s exact test due to its validity in analysing small sample of categorical variables and providing an exact p-value [15]. A p-value of < 0.05 was considered significant.
By plotting the receiver operating characteristic (ROC) curves, the optimal cutoff value was determined using Youden’s approach. The Youden Index is a commonly used summary measure of the ROC curve, and it measures the effectiveness of a diagnostic marker or a test, and enables the selection of an optimal threshold value (cutoff point) for the marker or test [16]. Sensitivity, specificity, and area under the curve (AUC) at the cutoff value were calculated in order to study the ability of the variables predicting the risk of developing higher grades of RD. Risk ratio (RR) for the association between each variable and the risk of developing higher grades of RD were obtained by univariate analysis.
All the statistical analysis was done using MedCalc software version 22.021.
Results
The selected 80 patients’ characteristic features are summarized in Table 1. In brief, median age was 65 years; 85% of the patients were male, and 67.5% of the sample population were heavy smokers (> 20 pack–years). 77.5% of the patients initially had moderate malnutrition as per SNAQ criteria, whereas 22.5% were well-nourished. The proportion of patients in subsets of oropharynx, hypopharynx and larynx were almost similar (28.75%, 25% and 27.5%, respectively) whereas 15% patients had disease of nasopharyngeal origin. Human papilloma virus (HPV) testing was not done in the oropharyngeal primary patients due to unavailability of the facility in our institution. A large proportion (63.75%) of the patients had stage IVA disease according to TNM staging 8th edition. TPF (docetaxel, carboplatin and fluorouracil)- based Induction chemotherapy was prescribed for only 5 of these 80 patients (6.25%). Overall, treatment compliance was good as per the patients’ attendance documented in the treatment room register, with only 6.25% having prolonged (> 4 days) RT treatment discontinuation due to toxicities. Treatment features and toxicity rates are shown in Table 2. 47.5% patients had grade 1 RD, 42.5% suffered from grade 2, and only 10% had grade 3 RD as per CTCAE criteria. No patients in our study suffered from grade 4 acute skin toxicity.
Table 1.
Patients’ characteristics
| Characteristics | No. of patients (%)(n = 80) |
|---|---|
|
| |
| Median age | |
| Years [range] | 65 (16–78) |
|
| |
| Sex | |
| Male | 68 (85%) |
| Female | 12 (15%) |
|
| |
| ECOG performance status | |
| 0 | 59 (73.75%) |
| 1 | 17 (21.25%) |
| 2 | 4 (5%) |
|
| |
| Initial nutritional status | |
|
| |
| Well nourished (BMI > 18.5, < 5% weight loss in last 6 months) | 18 (22.5%) |
|
| |
| Moderately malnourished (BMI > 18.5, 5–10% weight loss in last 6 months) | 62 (77.5%) |
|
| |
| Severely malnourished (BMI < 18.5, > 10% weight loss in last 6 months or > 5% in the last month) | 0 |
|
| |
| Smoking history (pack–years) | |
| 0 | 7 (8.75%) |
| 0–10 | 11 (13.75%) |
| 10–20 | 8 (10%) |
| > 20 | 54 (67.5%) |
|
| |
| Primary tumour subsite | |
| Nasopharynx | 12 (15%) |
| Oropharynx | 23 (28.75%) |
| Hypopharynx | 20 (25%) |
| Larynx | 22 (27.5%) |
| Others | 3 (3.75%) |
|
| |
| AJCC Stage (8 th edition) | |
| III | 24 (30%) |
| IVA | 51 (63.75%) |
| IVB | 5 (6.25%) |
ECOG — Eastern Cooperative Oncology Group; BMI — body mass index; AJCC — American Joint Committee on Cancer
Table 2.
Treatment characteristics and radiation dermatitis
| Characteristics | No. of patients |
|---|---|
|
| |
| VMAT schedule | |
| Sequential | 26 (32.5%) |
| SIB | 54 (67.5%) |
|
| |
| RT compliance | |
| No interruptions | 62 (77.5%) |
| Temporary interruptions | 18 (22.5%) |
| Median of interruptions (days, range) | 4 (1–16) |
| < 3 days | 13 (16.25%) |
| ≥ 4 days | 5 (6.25%) |
|
| |
| Radiation dermatitis (RD) | |
| Grade 1 | 38 (47.5%) |
| Grade 2 | 34 (42.5%) |
| Grade 3 | 8 (10%) |
| Grade 4 | 0 |
VMAT — volumetric-modulated arc therapy; SIB — simultaneous integrated boost; RT — radiation therapy
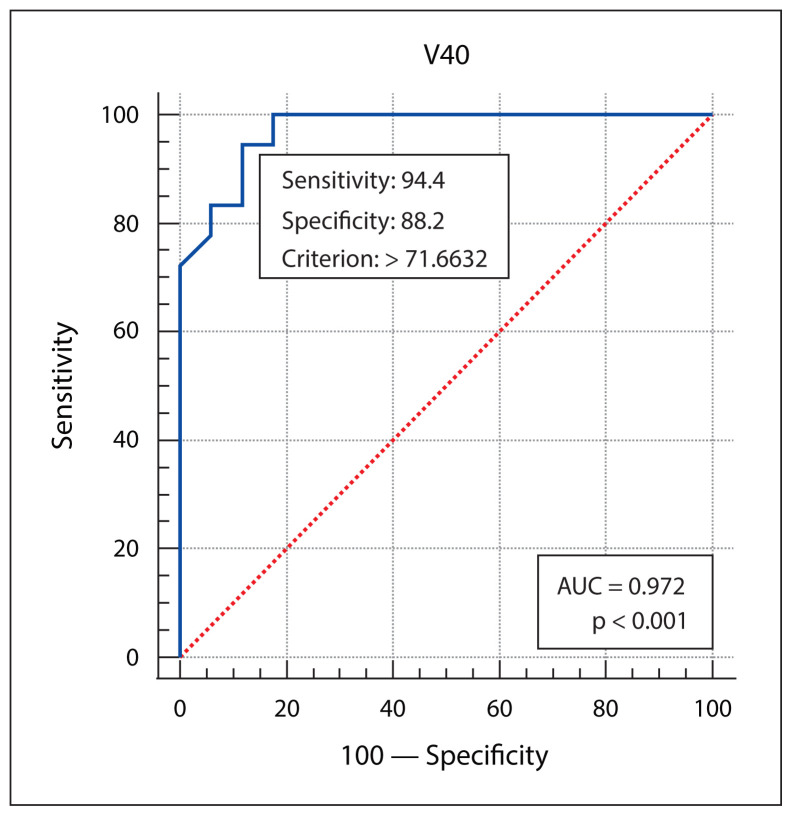
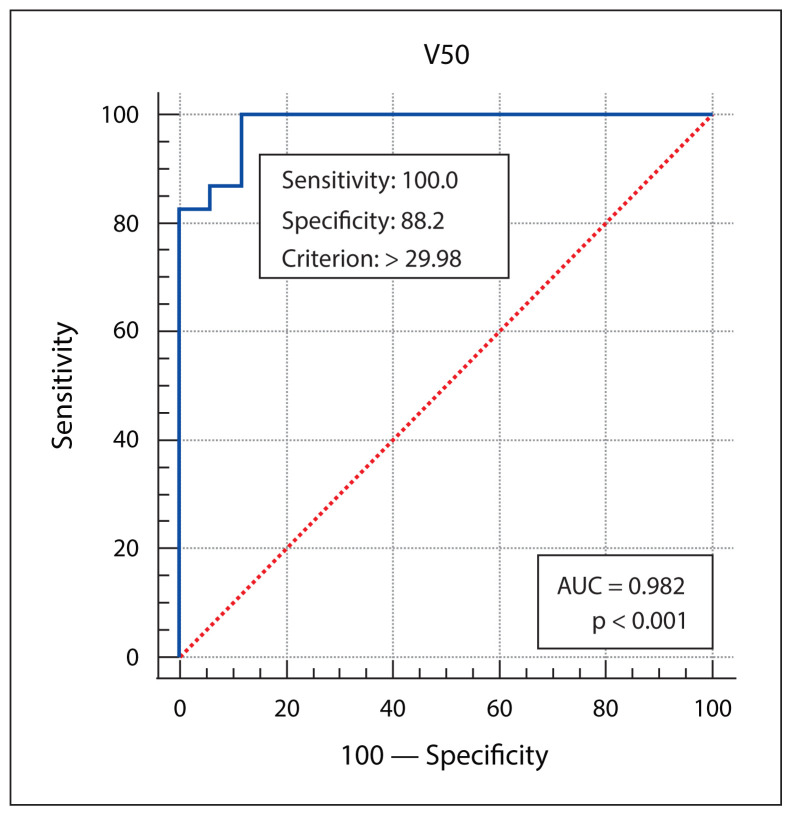
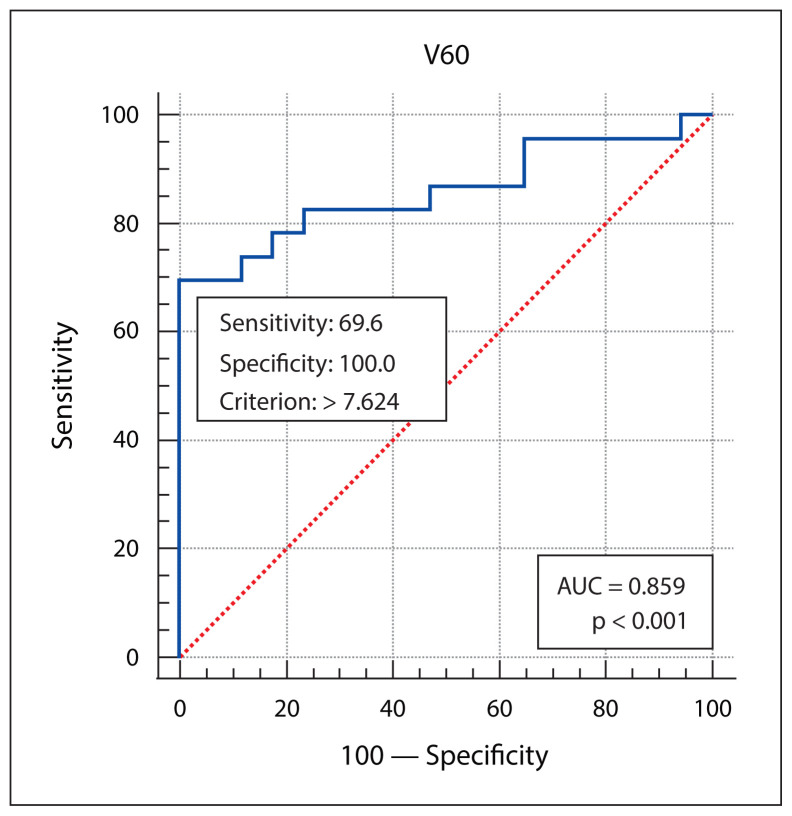
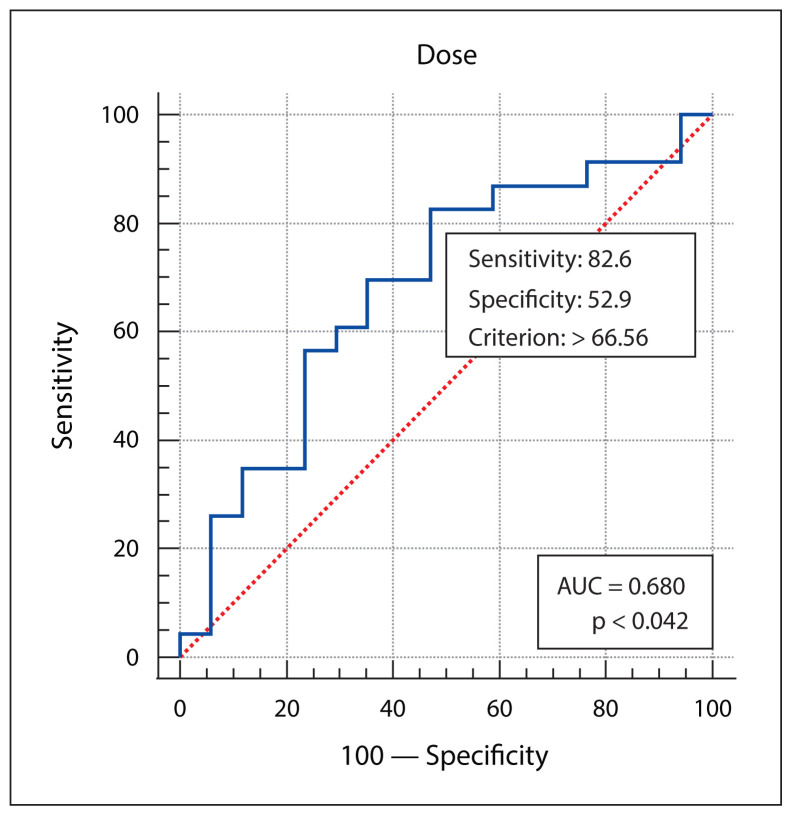
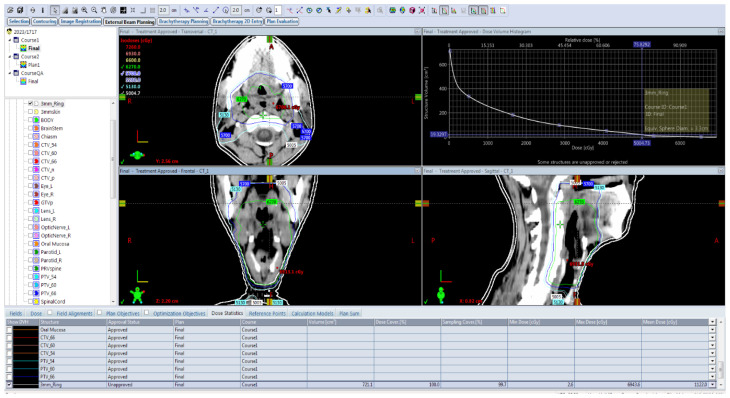
Patient, disease, treatment-related features and skin dose-volume parameters are summarized in Table 3 as per development of grade 1 and grade 2/3 RD. Statistically significant association of higher grade of RD was found with higher skin volumes receiving 40 Gy, 50 Gy or 60 Gy dose, and with the higher maximum point dose in the skin ring structure. From plotting ROC curves, statistically significant optimal cutoff values were found using Youden’s approach — 71.6632 cc for V40 (AUC = 0.972, p-value < 0.001), 29.98 cc for V50 (AUC = 0.982, p-value < 0.001), 7.624 cc for V60 (AUC = 0.859, p-value < 0.001), and 66.56Gy for Dmax (AUC = 0.680, p-value = 0.04). RR with 95% confidence interval (CI) were calculated by univariate analysis, and all of the 4 variables (V40, V50, V60 and Dmax) were significantly correlated with grade 2/3 skin toxicity as depicted in Table 4. V50 was found to have the best predictive accuracy among the four parameters (AUC 0.982) as the higher AUC values in ROC curve analysis indicate better test performance. Figures 1–4 show the ROC curves plotted in MedCalc software. Figure 5 shows the dose-volume histogram (DVH) of the 3mm ring structure in a sample VMAT treatment plan.
Table 3.
Distribution of variables according to the degrees of radiation dermatitis (RD)
| Characteristics | Grade 1 RD (n = 38) | Grade 2/3 RD (n = 42) | p-value |
|---|---|---|---|
|
| |||
| Age | |||
| < 65 years | 20 (51.28%) | 19 (48.71%) | 0.508822 |
| > 65 years | 18 (43.90%) | 23 (56.09%) | |
|
| |||
| Sex | |||
| Male | 33 (48.52%) | 35 (51.47%) | 0.66073 |
| Female | 5 (41.67%) | 7 (58.33%) | |
|
| |||
| ECOG PS | |||
| 0 | 28 (47.45%) | 31 (52.54%) | 0.994319 |
| 1 | 8 (47.06%) | 9 (52.94%) | |
| 2 | 2 (50%) | 2 (50%) | |
|
| |||
| Initial nutritional status | |||
| Well nourished | 11 (61.11%) | 7 (38.89%) | 0.188992 |
| Moderately malnourished | 27 (43.54%) | 35 (56.45%) | |
| Severely malnourished | 0 | ||
|
| |||
| Smoking history (pack–years) | |||
| 0 | 5 (71.43%) | 2 (28.57%) | |
| 0–10 | 6 (54.54%) | 5 (45.45%) | 0.49967 |
| 10–20 | 4 (50%) | 4 (50%) | |
| > 20 | 23 (42.59%) | 31 (57.41%) | |
|
| |||
| Primary subsite | |||
| Nasopharynx | 5 (41.67%) | 7 (58.33%) | |
| Oropharynx | 11 (47.83%) | 12 (52.17%) | 0.9254 |
| Hypopharynx | 9 (45%) | 11 (55%) | |
| Larynx | 12 (54.54%) | 10 (45.45%) | |
| Others | 1 (33.33%) | 2 (66.67%) | |
|
| |||
| Stage (AJCC 8 th edition TNM) | |||
| III | 10 (41.67%) | 14 (58.33%) | 0.7089 |
| IVA | 26 (50.98%) | 25 (49.02%) | |
| IVB | 2 (40%) | 3 (60%) | |
|
| |||
| VMAT schedule | |||
| Sequential | 13 (50%) | 13 (50%) | 0.7560 |
| SIB | 25 (46.296%) | 29 (53.7%) | |
|
| |||
| PTV (size, cc; median, range) | 161.4 (96.8–247) | 202.6 (128.9–274.6) | 0.1247 |
|
| |||
| Skin ring 3 mm V40 (size, cc; median, range) | 51.4182 (18.05–80.095) | 83.4892 (70.645–119.542) | < 0.00001 |
|
| |||
| Skin ring 3 mm V50 (size, cc; median, range) | 21.868 (4.37–37.812) | 47.93 (30.1146–85.06) | < 0.00001 |
|
| |||
| Skin ring 3 mm V60 (size, cc; median, range) | 2.647 (0.0012–7.264) | 10.4082 (0.000333–40.6) | < 0.00001 |
|
| |||
| Skin ring 3 mm D max | 66.56 (56.412–74.243) | 67.964 (60.697–74.26) | 0.030084 |
ECOG — Eastern Cooperative Oncology Group; AJCC — American Joint Committee on Cancer; PTV — planning target volume; VMAT — volumetric-modulated arc therapy; SIB — simultaneous integrated boost; PS — performance status
Table 4.
Univariate analyses
| Variable | Grade 1 RD (n = 38) | Grade 2/3 RD (n = 42) | Relative risk (95% confidence interval) | p-value |
|---|---|---|---|---|
|
| ||||
| V 40 | ||||
| ≥ 71.66 cc | 6 | 40 | 14.7826 | 0.0001 |
| < 71.66 cc | 32 | 2 | (3.8353–56.9772) | |
|
| ||||
| V 50 | ||||
| ≥ 29.98 cc | 6 | 42 | 57.2449 | 0.004 |
| < 29.98 cc | 32 | 0 | (3.6487–898.1137) | |
|
| ||||
| V 60 | ||||
| ≥ 7.624 cc | 2 | 18 | 2.2500 | < 0.0001 |
| < 7.624 cc | 36 | 24 | (1.5973–3.1694) | |
|
| ||||
| D max | ||||
| ≥ 66.56 Gy | 18 | 38 | 4.0714 | 0.0026 |
| < 66.56 Gy | 20 | 4 | (1.6346–10.1410) | |
Note: only statistically significant variables are shown; RD — radiation dermatitis; RR — relative risk; CI — confidence interval
Figure 1.
The receiver operating characteristic (ROC) curve analysis of skin ring 3 mm V40. AUC — area under the curve
Figure 2.
The receiver operating characteristic (ROC) curve analysis of skin ring 3 mm V50. AUC — area under the curve
Figure 3.
The receiver operating characteristic (ROC) curve analysis of skin ring 3 mm V60. AUC — area under the curve
Figure 4.
The receiver operating characteristic (ROC) curve analysis of skin ring 3 mm Dmax. AUC — area under the curve
Figure 5.
The dose-volume histogram (DVH) of the 3 mm ring structure in a sample volumetric-modulated arc therapy (VMAT) treatment plan
Discussion
IMRT is a highly precise conformal radiotherapy technique in which multiple small beamlets, each with a non-uniform intensity profile, create dose distributions that can accurately conform to convex and concave structures alike [17]. The introduction of IMRT into the treatment of head and neck cancer patients made the reduction of treatment-related morbidity possible along with exquisite dose distribution [18], although with a substantial risk of marginal geographic miss of target and increased integral dose. Lee et al. [6] in 2002 first considered the skin of the neck as a separate structure for IMRT optimization, and showed that the volume of the skin exposed to > 45 Gy could be brought down by 20% compared with regular IMRT planning, as well as decreasing its mean dose by 6%. Price et al. [19] applied a 4-mm negative margin to the PTV from the body surface contour, and found the occurrence of superficial hotspots above 110% to be minimal, along with better rotational IMRT plan conformity with this increase of PTV to skin distance. In another study by Penoncello et al. [20] published in 2016, VMAT, a special form of IMRT delivering single or double arcs of precise radiation beams while continuously rotating around the patient’s body [21] showed 5.6% reduction of mean dose to the skin compared with static IMRT. However, the more modern refinements of IMRT techniques introduced neither any specific constraints to use for the skin nor any indications how to contour it in the irradiated area. Different OAR guidelines [22] recommend 3–6 mm skin thickness to be contoured, the variation depending on different skin thicknesses in different regions of interest. Studer et al. [11] in 2011 found a positive association between the incidence of Grade 3/4 RD and the radiation dose delivered to the larger skin volume in patients receiving cetuximab as concurrent chemotherapy. It was assessed by measuring skin doses > 50 Gy and > 60 Gy in the subdermal area 3mm below the skin. Severe RD was found in V50 91cc and V60 50 cc where grade 0–2 reactions were seen in V50 61cc and V60 27cc. In 2019, Mori et al. [23] found that a 2-mm thick superficial body layer DVH was associated with the risk of developing acute RD in patients treated with tomotherapy. They found V56/V64 to be the most predictive parameters for grade 2/grade 3 RD. Optimal cutoff values were found to be 7.7 cc and 2.7 cc for V56 and V64, respectively. V64 < 3 cc constraints should keep the risk of Grade III toxicity lower than 10%. Also in 2019, Bonomo et al. [12] created 3 ring structures, 2 mm, 3 mm and 5 mm below the skin to assess the correlation between skin dose-volume and Grade III/IV RD in 90 head and neck cancer patients treated with tomotherapy. They compared a concurrent cisplatin cohort with a cetuximab cohort. In multivariate analysis, they found statistically significant correlation of > 10 kg weight loss and performance status > 1 with the development of severe RD. The best predictive accuracy they found was at 2mm: an AUC 0.61 with V50 of 19.9cc and V60 of 5.8 cc. Our retrospective work adds to the available literature providing hypothesis-generating results, though the limitations must be acknowledged. Firstly, the relatively smaller sample size reduces the generalizability of our results. Secondly, the data collected retrospectively from our institutional datasheets may have interobserver differences. Thirdly, all the patients underwent the VMAT treatment according to the original plan. No weight loss was noted down, and no adaptive RT was considered due to heavy workload and logistical reasons. For that reason, we could not correlate the occurrence of moderate to severe RD with significant anatomic change (weight loss). But we think that proper prospective validation with a larger sample size and multivariate analysis are warranted based on our results, and specific skin dose-constraints can be recommended in future international RT guidelines to decrease moderate to severe acute skin toxicity.
Conclusions
The dose distribution pattern to a 3 mm thick skin layer below the surface may help predict the development of moderate to severe radiation dermatitis in head and neck cancer patients receiving definitive concurrent chemoradiation. An optimum cut-off value of 29.98 cc for the volume of skin receiving at least 50 Gy radiation dose was found to be the strongest predictor of grade 2 and 3 radiation dermatitis in our study. This data may help future researchers to design large prospective studies incorporating these constraints. This will further validate the applicability of the present study in clinical setting to prevent radiation-induced skin toxicity.
Footnotes
Conflict of interest: The authors declare no potential conflict of interest.
Funding: None declared.
References
- 1.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25(26):4096–4103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 3.Nutting CM, Morden JP, Harrington KJ, et al. PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinde P, Jadhav A, Shankar V, et al. Assessment of dosimetric impact of interfractional 6D setup error in tongue cancer treated with IMRT and VMAT using daily kV-CBCT. Rep Pract Oncol Radiother. 2023;28(2):224–240. doi: 10.5603/rpor.a2023.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koiwai K, Hirasawa D, Sugimura M, et al. Impact of upgraded radiotherapy system on outcomes in postoperative head and neck squamous cell carcinoma patients. Rep Pract Oncol Radiother. 2022;27(6):954–962. doi: 10.5603/rpor.a2022.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee N, Chuang C, Quivey JM, et al. Skin toxicity due to intensity-modulated radiotherapy for head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2002;53(3):630–637. doi: 10.1016/s0360-3016(02)02756-6. [DOI] [PubMed] [Google Scholar]
- 7.Brodin NP, Tomé WA. Revisiting the dose constraints for head and neck OARs in the current era of IMRT. Oral Oncol. 2018;86:8–18. doi: 10.1016/j.oraloncology.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN guidelines. Head and Neck Cancers, version 2. 2023. http://www.nccn.org .
- 9.Serón-Arbeloa C, Labarta-Monzón L, Puzo-Foncillas J, et al. Malnutrition Screening and Assessment. Nutrients. 2022;14(12):2392. doi: 10.3390/nu14122392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grégoire V, Evans M, Le QT, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126(1):3–24. doi: 10.1016/j.radonc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Studer G, Brown M, Salgueiro EB, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81(1):110–117. doi: 10.1016/j.ijrobp.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Bonomo P, Talamonti C, Desideri I, et al. Analysis of skin dose distribution for the prediction of severe radiation dermatitis in head and neck squamous cell carcinoma patients treated with concurrent chemo-radiotherapy. Head Neck. 2020;42(2):244–253. doi: 10.1002/hed.25997. [DOI] [PubMed] [Google Scholar]
- 13.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf .
- 14.Nahm FS. Nonparametric statistical tests for the continuous data: the basic concept and the practical use. Korean J Anesthesiol. 2016;69(1):8–14. doi: 10.4097/kjae.2016.69.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HY. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod. 2017;42(2):152–155. doi: 10.5395/rde.2017.42.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 17.Gutiontov SI, Shin EJ, Lok B, et al. Intensity-modulated radiotherapy for head and neck surgeons. Head Neck. 2016;38(Suppl 1):E2368–E2373. doi: 10.1002/hed.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grégoire V, Langendijk JA, Nuyts S. Advances in Radiotherapy for Head and Neck Cancer. J Clin Oncol. 2015;33(29):3277–3284. doi: 10.1200/JCO.2015.61.2994. [DOI] [PubMed] [Google Scholar]
- 19.Price RA, Koren S, Veltchev I, et al. Planning target volumeto-skin proximity for head-and-neck intensity modulated radiation therapy treatment planning. Pract Radiat Oncol. 2014;4(1):e21–e29. doi: 10.1016/j.prro.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penoncello GP, Ding GX. Skin dose differences between intensity-modulated radiation therapy and volumetric-modulated arc therapy and between boost and integrated treatment regimens for treating head and neck and other cancer sites in patients. Med Dosim. 2016;41(1):80–86. doi: 10.1016/j.meddos.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Teoh M, Clark CH, Wood K, et al. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84(1007):967–996. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mir R, Kelly S, Xiao Y, et al. Organ at risk delineation for radiation therapy clinical trials: Global Harmonization Group consensus guidelines. Radiother Oncol. 2020;150:30–39. doi: 10.1016/j.radonc.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Mori M, Cattaneo GM, Dell’Oca I, et al. Skin DVHs predict cutaneous toxicity in Head and Neck Cancer patients treated with Tomotherapy. Phys Med. 2019;59:133–141. doi: 10.1016/j.ejmp.2019.02.015. [DOI] [PubMed] [Google Scholar]