Abstract
Cinnamic acid (CA) possesses important cardiovascular effects such as cardioprotective, antiatherogenic, antihyperlipidemic and antioxidant, which predicts its potential role in the treatment of hypertension. The study was executed to investigate the antihypertensive potential of CA in Sprague Dawley (SD) rats followed by evaluation in diverse vascular preparations. Invasive blood pressure monitoring technique was used in normotensive and hypertensive rats, under anesthesia. Isolated aortic rings from rat and rabbit, Langendorrf’s perfused isolated rabbit heart and guinea-pig right atria were used to probe the underlying mechanisms. The responses were recorded with pressure and force transducers connected to PowerLab Data Acquisition System. Intravenous administration of CA induced a respective 54% and 38% fall in mean arterial pressure (MAP) in the hypertensive and normotensive rats, respectively. In rat aortic rings, the CA exhibited muscarinic receptors-linked NO and indomethacin-sensitive endothelium-dependent (>50%) and calcium antagonistic and KATP-mediated endothelium-independent vasodilator effects. The CA showed negative inotropic and chronotropic effects in guinea-pig atrial strips. The CA suppressed force of ventricular contraction and heart rate while caused a 25% increase in coronary flow. This study supports the medicinal importance of CA as antihypertensive agent.
Keywords: Antihypertensive, Cinnamic acid, Endothelial nitric oxide, Hypertensive rats, Negative inotropic and chronotropic effects, Potassium and calcium channels
1. Introduction
Cinnamic acid (CA) is a naturally occurring bioactive aromatic carboxylic acid found in many medicinal important plants like Cinnamomum cassia (Chinese cinnamon), Panax ginseng, cocoa, fruits and vegetables [1]. Various investigations have found that CA and its derivatives have cardioprotective, antiatherogenic, antidiabetic, antioxidant, antiinflammatory and antihyperlipidemic potential [2–8]. CA notably helps in decreasing the body weight of obese rats and is able to reduce the activity of angiotensin converting enzyme in blood [9]. Hypertension is a major risk factor for cardiovascular diseases caused by oxidative stress, deterioration in nitric oxide (NO) dependent vasorelaxation, overexpression of L-type calcium (Ca++) channels, obesity (hyperlipidemia) and potassium (K+) channels inactivity [10,11]. In addition, reactive oxygen species (ROS) are believed to be responsible for the development of cardiovascular diseases, like hypertension, congestive heart failure and atherosclerosis [12]. The reported activities of CA predict its role in the treatment of hypertension, which has not been studied extensively in the past. We were interested to investigate the effects of CA on blood pressure in normotensive and hypertensive rat models, vascular tone (using rat and rabbit aorta) and cardiac performance (using isolated cardiac preparations) with extensive efforts made to explore the underlying mechanisms in vitro.
2. Materials and methods
2.1. Drugs and standards
The following reference chemicals were obtained from the sources specified: acetylcholine chloride ≥98%, ethylene glycol tetraacetic acid (EGTA) ≥98% (Alfa Aesar GmbH & Co, Karlsruhe, Germany), atropine sulfate ≥98% (Fluka-AG, Switzerland), Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME) ≥98%, indomethacin ≥99%, norepinephrine hydrochloride ≥98%, phenylephrine hydrochloride ≥98%, and verapamil hydrochloride ≥99% (Sigma Chemical Company, St. Louis, MO, USA), tetraethylammonium (TEA) (Sharlu-Spain), CA (BDH Laboratory Supplies, England), and Tween 80 (Chemie S.S., La Jota, Barcleona, Spain). Heparin injections (B. Braun, Germany), pentothal sodium powder ampoules (Abbott Laboratories, Pakistan) and water for injections (Otsuka, Ltd. Pakistan) were purchased from Shaheen Chemist, Abbottabad, KP, Pakistan. Stock solutions were made in saline, whereas CA was dissolved in Tween 80. The dilutions were made fresh on the day of experiment.
2.2. Experimental animals
All the experiments performed complied with the rulings of Institute of Laboratory Animal Resources, Commission on Life Sciences [13] and sanctioned by the Ethical Committee of Pharmacy Department, COMSATS University Islamabad, Abbottabad Campus, Pakistan (license no. PHM.Eth/CS-MO2-059-0721). Male adult Sprague–Dawley rats (180–250 g), local rabbits (1–1.5 kg) and guinea-pig (350–650 g) used in the study were bred and housed in the animal house of Department of Pharmacy, CUI, Abbottabad under a controlled environment (23–25 °C). Animals were provided with standard food and tap water ad libitum.
2.3. Invasive blood pressure measurement in rats under anesthesia
Rats were divided into groups having 6–7 rats each. Group 1 received a normal diet and water and acted as normal control. Group 2 was considered hypertensive control and received 8% sodium chloride in food and water for 6 weeks. After the end of the 6 weeks, the mean arterial pressure (MAP) of all the rats was measured before and after the intravenous administration of CA, and the % fall in MAP was calculated. These two groups were used to see the hypotensive and antihypertensive effect of CA.
2.3.1. Normotensive rats
As described [14,15], these experiments were performed on male Sprague–Dawley rats (180–250 g; n = 5–7 animals). Rats were anesthetized with an intraperitoneal injection of thiopental sodium (Pentothal, 50–90 mg/kg), minor mid-tracheal surgical incision (approximately 1 cm) was made to expose trachea, carotid artery and right jugular vein. The trachea was cannulated with a polyethylene tubing PE-20 to maintain spontaneous respiration. The right jugular vein was cannulated PE-50 to inject standard drugs and CA. The carotid artery was cannulated with similar tubing filled with heparinized saline (60 IU/mL) and connected to a pressure transducer (MLT 0699) coupled with bridge amplifier (N12128) and PowerLab (ML 846) Data Acquisition System (AD Instruments, Sydney, Australia). This connection was used for recording and measurement of systolic and diastolic blood pressures, calculated as mean arterial pressure (MAP). The body temperature of the rat was maintained by using an overhead lamp.
2.3.2. Hypertensive rats
The protocol of [16,17] was followed with some modifications. The rats (n = 5–7) were given a high-salt (8% NaCl) diet and water ad libitum for 6-weeks. One day prior to the experiment, the rats were given a normal diet and water. Subsequently the rats were used for in-vivo blood pressure measurement and the rats with 150–190 mmHg blood pressure were considered hypertensive and employed for experimentation.
2.3.3. Experimental protocol
The rats were equilibrated for 20–30 min (minutes); acetylcholine and norepinephrine were used to assure the stability of the rats towards hypotensive and hypertensive responses, respectively. Acetylcholine (1 μg/kg) 0.1 mL was gradually injected intravenously (i.v), which caused a fall in MAP. Approximately 5–10 min later, when the normal pattern of blood pressure was attained, norepinephrine (1 μg/kg) was slowly injected followed by a flush of 0.1 mL normal saline, which caused an increase in MAP. When the normal pattern of blood pressure resumed, rats were injected i.v with 0.1 mL normal saline or with the same volume of test substances. CA was administered intravenously at doses of 1, 3, 10, 30, 100 and 150 μg/kg. First the stock solution of CA was prepared. Then different doses of CA were prepared according to the average body weight and amount of volume injected. The blood pressure was allowed to return to the resting level between injections and normal blood pressure is achieved after 10–15 min. Different doses of CA and standards were then injected i.v, each dose was followed by a flush of 0.1 mL normal saline. Changes in blood pressures were calculated as the difference between the steady-state values prior and after each injection. The MAP was calculated as the diastolic blood pressure (BP) + 1/3 (systolic BP–diastolic BP).
2.4. In-vitro experimentation on isolated rat and rabbit vascular tissues
2.4.1. Rat thoracic aortic rings and assessment of vascular dysfunction
The procedure of [17] was followed with some modifications. Thoracic aorta was isolated from male Sprague–Dawley rats carefully to avoid any damage to the endothelium. The aorta was then transferred into the Kreb’s solution aerated with carbogen. The composition of Kreb’s solution was (mM): NaCl 118.2, NaHCO3 25.0, CaCl2 2.5, KCl 4.7, KH2PO4 1.3, MgSO4 1.2, and glucose 11.7 (pH 7.4). It was cautiously cleaned off fats and other connective tissues and then cut into rings 2–3 mm wide. In some rings, the endothelium was intentionally removed by gentle rubbing of the intimal surface with forceps. The rings with intact endothelium that produced less than 80% relaxation in response to acetylcholine (1 μM) were tossed away. Individual rings were suspended in 10 mL tissues baths at 37 °C aerated with carbogen. A preload of 2 g was applied to each preparation and incubated for 30 min. Changes in isometric tension were recorded and analyzed through a force transducer (MLT 0201) coupled with a bridge amplifier (N12128) and PowerLab (ML 846) Data Acquisition System (AD Instruments).
2.4.2. Involvement of endothelial mediators in vasodilation
A series of experiments were conducted to assess endothelium-dependent or independent effect of CA on isolated aortic rings. When the tension was at resting state or reached a plateau induced by PE (Phenylephrine) (1 μM), CA (μg/mL) were cumulatively added into the organ bath. Various concentrations of CA were administered carefully with 5–10 min interval. The maximum effect was recorded in 10 min after administration of single concentration of CA.
The rings with or without endothelium were always tested in parallel. To determine the underlying mechanisms, endothelium-intact rings were incubated with L-NAME (10 μM), indomethacin (1 μM) and atropine sulfate (1 μM) for 30 min before the addition of PE (1 μM). The test material was then added cumulatively, and the concentration response curves (CRCs) were constructed for the inhibitory responses.
2.4.3. Effect of Cinnamic acid on voltage-dependent Ca++-channels
High concentration of K+ (80 mM) was also used to depolarize the tissue, which produced sustained contractions, which allowed studying the effect on the voltage dependent Ca++ channels (VDCCs). The test material was then added cumulatively, and relaxation was expressed as the percentage of the contractions induced by high K+. This protocol was used to see if there is difference in vascular reactivity in two different vascular preparations from different species.
2.4.4. Effect of Cinnamic acid on K+-channels
A series of experiments were conducted to assess the involvement of Ca++-activated K+ channels in vasodilation through hyperpolarization as described previously [18–20]. The SD rat was sacrificed by cervical dislocation, thoracic aorta was immediately excised, placed in normal Kreb’s solution, cleaned from extra fatty tissues, cut into 2–3 mm rings and hanged in tissue organ bath aerated with carbogen. After equilibrium period, when the tension was at resting state or reached a plateau induced by PE (1 μM), test material was cumulatively added into the organ bath. To determine the underlying mechanisms, rat thoracic rings were incubated with TEA (Tetraethylammonium) (10 μM) for 30 min before the addition of PE (1 μM). The test material was then added cumulatively, and the CRCs were constructed for the inhibitory responses.
2.4.5. Effect on pre-contracted rabbit aortic rings
The protocol of Chan et al., 2006 [21] was followed with some modifications. PE (1 μM) or K+ (80 mM) was used to induce steady-state contractions. The CA was added in a cumulative manner to obtain concentration response curves and the relaxation was expressed as percent of agonist-induced contractions. These protocols allowed us to have an indirect approach to study the effect of CA on VDCCs or receptor-operated Ca++ Channels (ROCs) and Ca++ release from intracellular store(s).
2.4.6. Calcium channel blocking activity
In a set of experiments, an attempt was made to assure if the relaxation induced by the CA involved Ca++ influx through VDCCs. Aortic rings were washed four to five times with Ca++-free solution before the control CRCs of Ca++. When the control CRCs of Ca++ was found superimposable, then tissue was pretreated with CA for 30–45 min to test the possible calcium channel blocking effect. A parallel control was also run under similar experimental conditions.
2.4.7. Effect on intracellular Ca++ stores
In a set of experiments, the aim was to clarify whether the relaxation induced by CA is related to inhibition of intracellular Ca++. The rings were exposed to Ca++-free solution for 15 min before the application of PE (1 μM) to induce the first transient contraction. The composition of Ca++-free/EGTA Kreb’s solution was (mM): NaCl 118.2, NaHCO3 25.0, KCl 4.7, KH2PO4 1.3, MgSO4 1.2, EGTA (0.05 mM), and glucose 11.7 (pH 7.4). The rings were then washed three times with normal Kreb’s solution and incubated for at least 40 min for refilling of the intracellular stores. Subsequently, the medium was rapidly replaced with Ca++-free solution and the rings were incubated for another 15 min. The second contraction was then induced by PE (1 μM) in the presence of CA (μg/mL), which were added 30 min before the application of PE, both contractions were compared.
2.5. Langendorff’s perfused isolated rabbit heart
As described previously [22,23], healthy rabbits were sacrificed by cervical dislocation. The thorax was opened by an incision and the heart was removed immediately. The heart was transferred to a Petri dish containing Kreb’s-Henseleit solution, bubbled with carbogen at 37 °C. The pericardium and other adjacent fatty tissues were removed quickly. The aorta was cut at the point of branching, remaining 1 cm intact. The composition of Krebs–Henseleit buffer was (in mM): 118 NaCl, 4.7 KCl, 1.2MgSO47H2O, 1.25 CaCl2, 24 NaHCO3, 1.1 KH2PO4 and 10 glucose with pH 7.4. After gentle squeezing, the heart was attached to the cannula of Langendorrf’s apparatus through the aorta. The aerated and thermostatically maintained perfusion fluid is allowed to flow into the heart through the aorta. A thread was attached through a clip to the apex of the heart and passed through spontaneously rotating pulley attached to amplifier (N12128) for oscillography.
2.5.1. Protocol
After cannulation, retrograde perfusion via aorta was maintained. When the heart was attached to the experimental platform, perfusion pressure was maintained to reasonable heartbeat. Normally, 10–15 min is required to reach stability. Initially, control readings were taken and recorded for coronary flow (CF). All selected parameters were observed for 20 min prior to employing different concentrations of CA. A single isolated heart was used for complete dose–response curve, separately. An isolated heart that gave uneven pattern of the activity was tossed away.
2.5.2. Dosing cycle
After taking control data, various concentrations of CA (μg/mL) were administered gently into the rubber tubing with 5–10 min intervals. Measurements of the control and experimental data were taken. The maximum effect was recorded in 10 min after administration of single concentration of CA. Data was interpreted as heart rate (HR) was counted by number of beats per min. Force of Ventricular Contraction (FVC) was taken in cm from the baseline. The CF was measured in total number of drops per min (perfusate). Data of CA was compared with verapamil.
2.6. Effect of CA on cardiac contractility and force
As described previously [24,25], guinea-pigs (400–550 g) preferably male were sacrificed by cervical dislocation. The heart was immediately excised and then right atria were dissected out cautiously, in a Petri dish containing Kreb’s solution. The right atrium made into a strip with help of thread and mounted in 20 mL tissue bath filled with normal Kreb’s solution at 32 °C. The baths were bubbled with carbogen. The right atria maintained to produce stable beats at baseline tension 1 g of preload. Control data of acetylcholine (1 μM) and isoproterenol (1 μM) were taken twice at least. Then CA was employed into the tissue organ bath in additive manner to see effect on HR and force of contraction. The response was calculated as pretreated control. The magnitude variations in contractions of the tissue were recorded with a force-displacement transducer.
2.7. Statistics
All the data are expressed in mean ± standard error of the mean (SEM), and the median effective concentrations (EC50 values) are given with 95% confidence intervals. One-way analysis of variance (ANOVA) (followed by post hoc Tukey HSD test) used to calculated fall in MAP and two-way ANOVA (followed by post hoc Bonferroni test) used for percent vasorelaxation (SPSS 21 software, USA). The statistical parameters were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.001.
3. Results
3.1. Invasive blood pressure measurement in Normotensive and hypertensive rats
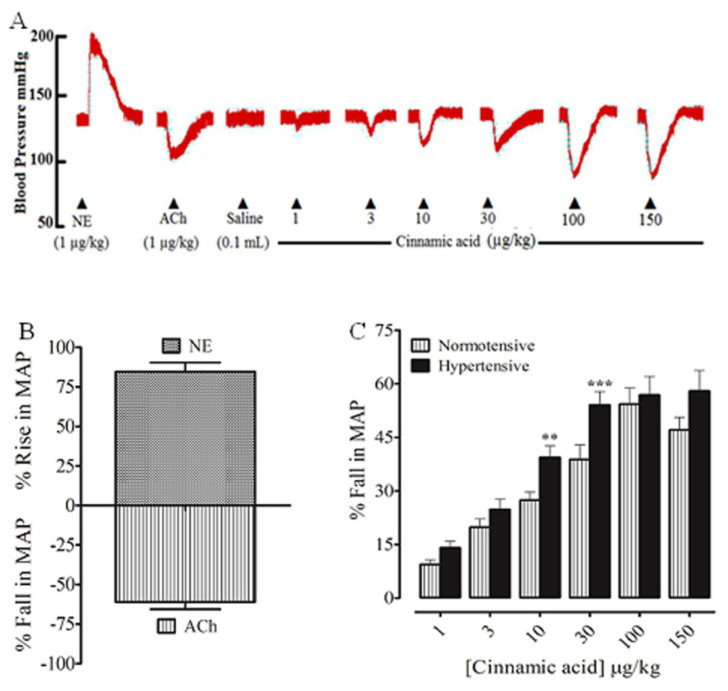
Prior to the injection of CA, the standards acetylcholine and norepinephrine were injected intravenously, which induced a fall and rise in MAP (Fig. 1A and B), respectively. Intravenous injection of CA to normotensive rats, under anesthesia, caused a dose-dependent decrease in MAP at dose of 1–100 μg/kg; next dose of 150 μg/kg did not produce further decline in MAP (Fig. 1A and C). The percent declines in MAP at the respective doses of 1, 3, 10, 30, 100 and 150 μg/kg were 9.43 ± 1.26, 19.84 ± 2.34, 27.40 ± 2.50, 38.79 ± 4.08, 54.29 ± 4.57 and 47.07 ± 3.97 mmHg (Fig. 1C), respectively.
Fig. 1.
A representative tracing (A) shows the effect of norepinephrine (NE), acetylcholine (ACh), and Cinnamic acid on MAP in rats under anesthesia. (B) The hypertensive and hypotensive effects of NE and ACh, respectively. (C) The blood pressure lowering response of the Cinnamic acid in normotensive and hypertensive rats under anesthesia. Values shown mean ± SEM (n = 6–7). One way ANOVA analysis followed by post hoc Tukey HSD test, **p < 0.01, ***p < 0.001 vs hypertensive.
In hypertensive rats under anesthesia, intravenous injection of CA caused a dose-dependent decrease in MAP at 1–30 μg/kg; next dose of 100 and 150 μg/kg did not exhibit further decline in MAP (Fig. 1C). The percent fall in MAP at the respective doses of 1, 3, 10, 30, 100 and 150 μg/kg were 14.06 ± 1.86, 24.81 ± 2.93, 39.38 ± 3.25, 54.07 ± 3.79, 56.85 ± 5.18 and 57.96 ± 6.74 mmHg (Fig. 1C), respectively.
3.2. Effect of Cinnamic acid on normotensive and hypertensive rat thoracic aortic rings
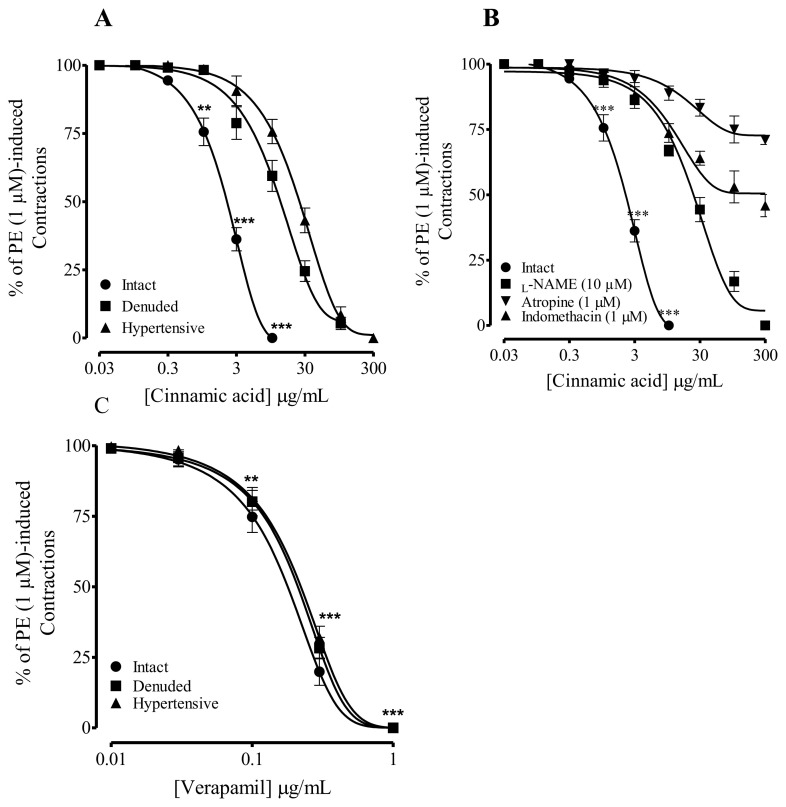
When tested in isolated rat aorta preparations from normotensive rats, pre-contracted with PE (1 μM), cumulative addition of CA caused endothelial-dependent (75%) vasorelaxation at 10 μg/mL (Fig. 2A). This relaxation to CA was reduced (50%) with denudation, the respective EC50 values of CA in the intact and denuded endothelium aortic rings isolated were 3.0 (1.0–5.0) and 17.9 μg/mL (11.3–24.7). In comparison, aortic rings from hypertensive rats, precontracted with PE (1 μM), cumulative effect of CA was partially (75%) endothelium-independent (Fig. 2A) with EC50 value of 30.0 μg/mL (19.8–40.2).
Fig. 2.
Response of Cinnamic acid on assorted parameters in isolated rat aorta preparations. (A) Endothelium-dependent vasorelaxant response of the Cinnamic acid precontracted with PE (1 μM), (B) the effect of Cinnamic acid in rat aortic rings pre-incubated with atropine, L-NAME and indomethacin, (C) the vasodilator effect of verapamil. Values shown mean ± SEM (n = 6–7). Two-way ANOVA followed by post hoc Bonferroni test, **p < 0.01 and ***p < 0.001 vs control.
To explore the underlying mechanisms of endothelium-dependent vasodilation induced by CA, isolated aorta from normotensive rats were pre-incubated with L-NAME (10 μM), atropine (1 μM) and indomethacin (1 μM).
In aortic rings preincubated with L-NAME (10 μM), blocked 50% the vasodilatory effect of CA at concentration of 30 μg/mL (Fig. 2B). Atropine and indomethacin preincubation blocked the effect of CA 75% and >85% (Fig. 2B), respectively. Preincubation with these blockers did not inhibit vasorelaxation to CA at higher concentrations (30–300 μg/mL), suggesting endothelium-independent mechanisms (Fig. 2B). Verapamil, a calcium channel blocker showed endothelium independent vasorelaxation in intact, denuded and hypertensive rats (Fig. 2C).
3.3. Effect of Cinnamic acid on K+-channels
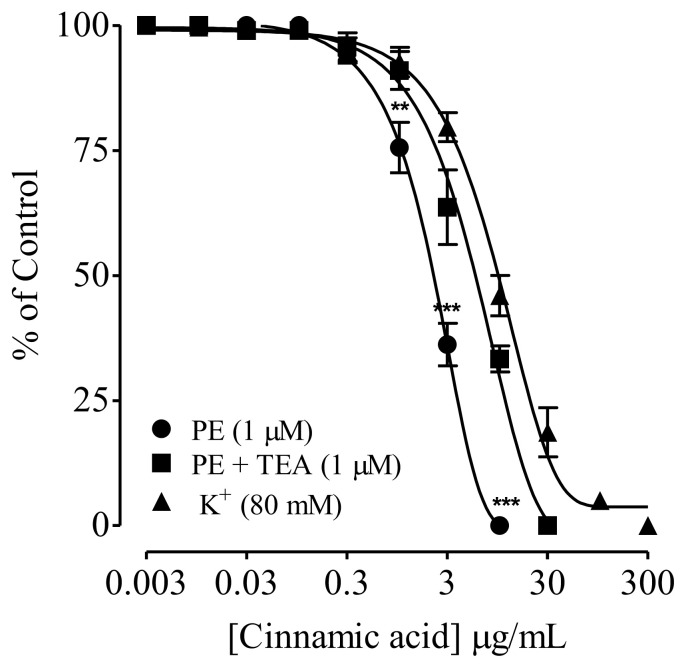
SD rat aortic rings preincubated with TEA (1 μM), blocked relaxation to CA against PE precontraction at higher 30 μg/mL, compared to 10 μg/mL (without TEA), with respective EC50 values of 3.0 (1.0–5.0) and 0.8 (0.3–1.3). CA also inhibited high K+(80 mM)-induced contractions in isolated rat aortic rings, with EC50 value of 16.4 μg/mL (10.6–22.2) (Fig. 3).
Fig. 3.
Effect of Cinnamic acid on phenylephrine sustained contractions with and without tetraethylammonium (TEA) and the endothelium-independent vasodilator effect of Cinnamic acid on K+ (80 mM)-induced contractions in isolated rat aorta rings. Values shown are mean ± SEM (n = 6–7). Two-way ANOVA followed by post hoc Bonferroni test, **p < 0.01 and ***p < 0.001 vs control.
3.4. Effects of cinnamic acid on voltage-dependent calcium channels
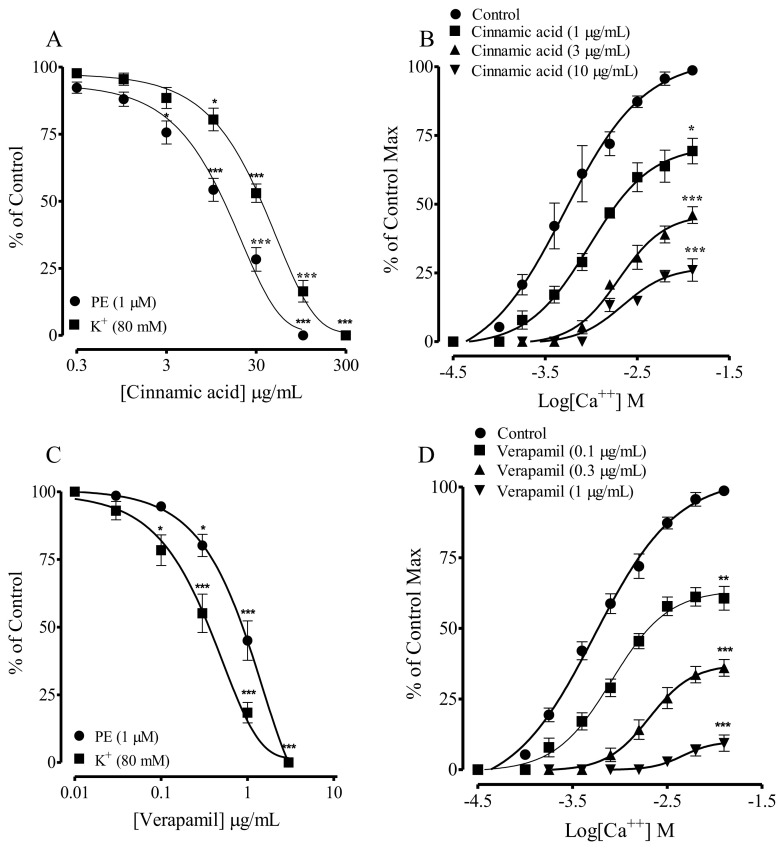
The calcium channel blocking effect of CA was further confirmed in rabbit aortic rings (due to pharmacological reasons). CA also inhibited high K+ (80 mM) induced contraction in isolated rabbit aortic rings with EC50 value of 42.1 (30.0–54.2), as shown in Fig. 4A. Preincubation of rabbit aortic rings with CA (1–30 μg/mL) induced concentration-dependent rightward non-parallel shift in the CaCl2 (Ca++) CRCs, in Ca++-free/EGTA medium (Fig. 4B), similar to the verapamil (Fig. 4D).
Fig. 4.
Concentration-dependent vasorelaxant response of (A) Cinnamic acid; (C) Verapamil on phenylephrine (PE 1 μM) and K+ (80 mM) precontractions and (B & D), respectively their effect on the Ca++ concentration–response curves, constructed in Ca++-free medium, in isolated rabbit aorta preparations. Values shown mean ± SEM (n = 6–7). Two-way ANOVA followed by post hoc Bonferroni test, **p < 0.01 and ***p < 0.001 vs control.
3.5. Effect on the performance of heart
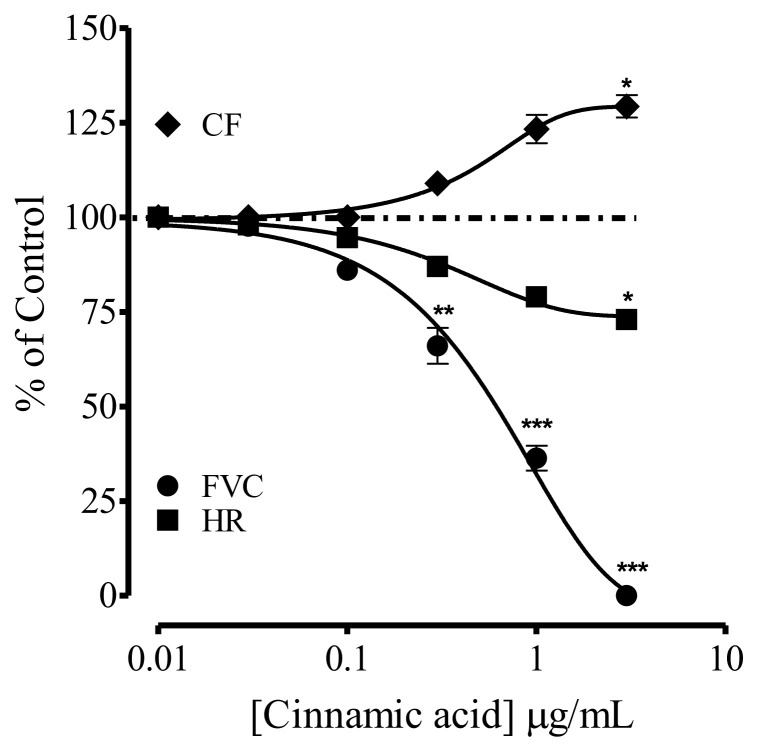
A series of experiments were performed to see the effect of CA on FVC, HR and CF. In isolated rabbit heart in the Langendorrf’s apparatus, injection of CA through the perfusing tubing, caused complete suppression of FVC, partially HR at the dose of 0.001–3.0 μg/mL, while it increased the CF up to 25% simultaneously (Fig. 5).
Fig. 5.
Effect of the Cinnamic acid on force of ventricular contraction (FVC), heart rate (HR) and coronary flow (CF) in isolated rabbit heart preparations in Langendorrf’s experiment. Values shown are mean ± SEM (n = 6–7). Two-way ANOVA followed by post hoc Bonferroni test, p* < 0.05 and p** < 0.01, ***p < 0.001 vs control.
3.6. Effect on cardiac contractility and force
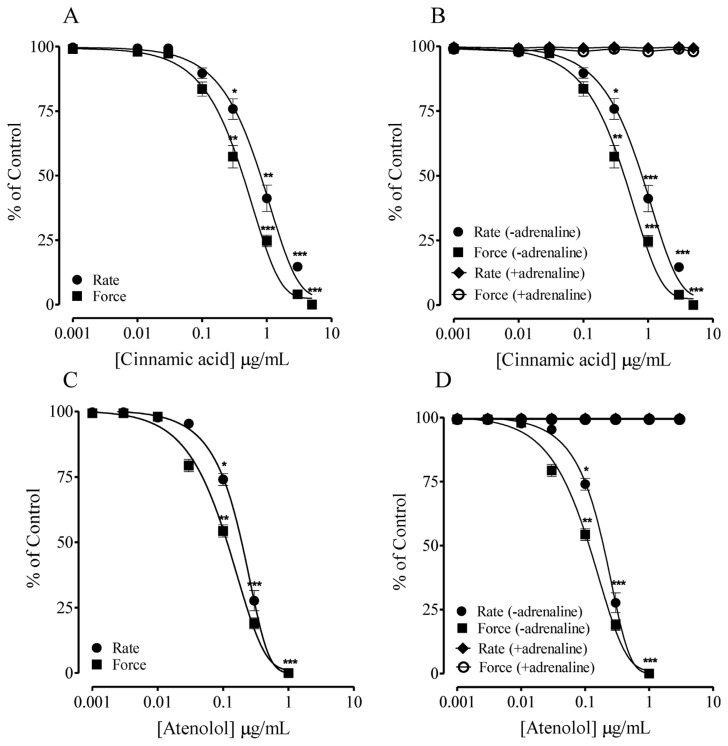
In isolated spontaneously beating guinea-pig right atrial (selected for pharmacological reasons) preparations, cumulative addition of the CA caused depressant effect on force and rate of contraction (Fig. 6A), in comparison with atenolol (Fig. 6C). Furthermore, pretreatment with adrenaline reverted the depressant response of CA on force and rate of contraction (Fig. 6B), suggesting the role of adrenergic receptors.
Fig. 6.
Effect of Cinnamic acid (A) on rate and force of contraction in spontaneously beating guinea-pig right atria. (B) Shows the effect of Cinnamic acid in the absence (-adrenaline) and presence (+adrenaline) of adrenaline (C) Shows the control response of Atenolol and (D) Atenolol in the absence (-adrenaline) and presence (+adrenaline) of adrenaline as control. Values shown are mean ± SEM (n = 6–7). Two-way ANOVA followed by post hoc Bonferroni test, p*< 0.05, **p < 0.01 and ***p < 0.001 vs control.
4. Discussion
CA is a naturally occurring constituent in many medicinal plants with important potential cardiovascular profile such as cardioprotective, antiatherogenic, antidiabetic, antioxidant, antiinflammatory and antihyperlipidemic [1,2,8]. In addition, CA reduces the activity of angiotensin converting enzyme, important enzyme in regulation of blood volume and blood pressure [9]. However, its role in the management of hypertension, with emphasis on the cardiovascular mechanisms, was not investigated in the past.
Intravenous injection of CA caused a dose-dependent (1–30 μg/kg) fall in systolic and diastolic blood pressures, expressed as MAP in the normotensive and high salt-induced hypertensive rats (representative of human hypertension). The dose response relationship of CA indicated that minimum effective dose is 1 μg/kg while the maximum effective dose is 100 μg/kg because further increase in dose did not produce any strong effect. CA was more effective antihypertensive in the hypertensive rats than the normotensives, suggesting its role in the management of hypertension. However, detail mechanistic studies were required to probe the underlying cardiovascular mechanisms. Increase in blood pressure results from imbalance in the peripheral vascular resistance (vascular tone) and or cardiac output. Therefore, the cardiovascular effects of CA were separately investigated on vascular and cardiac parameters.
In isolated rat aortic rings from the normotensive rats, CA induced a vasorelaxant effect, which was reduced (>60%) with removal of endothelium, indicating role of vascular endothelium. The vasorelaxant effect to CA also reduced (>75%) in rat aortic rings from the hypertensive rats, as high salt induces endothelium damage [16]. The vascular endothelium plays a pivotal role in modulating the vascular tone through the release of a variety of substances, including NO, prostaglandin I2 (PGI2) etc [14,26,27]. To probe the role of endothelial NO, rat aortic rings were preincubated with L-NAME, a NO synthase inhibitor, reversed (50%) the effect of CA, thus indicating partial role of NO. The vascular endothelium is coupled with muscarinic receptors, to investigate the effect on this signaling pathway, intact aortic rings were preincubated with atropine, a muscarinic receptor blocker. Interestingly, this preincubation also reversed the vasorelaxant effect of CA to a greater extent (>70%), indicating role of muscarinic receptors-linked NO pathway. In addition to NO, endothelial PGI2 also an important contributor to vascular relaxation, therefore aortic rings were preincubated with indomethacin, a prostaglandin inhibitor, which reversed (>80%). Thus these findings indicate that the endothelial-dependent vasorelaxation of CA is mediated through NO linked to muscarinic receptors and PGI2. These inhibitors and endothelium removal did not reversed effect of CA at higher concentrations (30–100 μM/mL), also suggesting its direct effect on vascular smooth muscle cells.
Important regulators for vascular smooth muscles (VSMs) include K+ and Ca++ channels. Cell membrane potassium channels are important vasomodulators of vascular tone [28], which are potential targets for antihypertensive drugs. The efflux of K+ causes hyperpolarization, closing of voltage-dependent Ca++ channels and restricting Ca++ entry, which promotes muscle relaxation [29]. To elucidate the mechanism of relaxation of CA as mediated by activation of K+-channels, sustained contractions were induced with phenylephrine in isolated rat aortic rings, where cumulative addition of CA induced relaxation. Preincubation of the aortic rings with TEA, a potassium channel blocker [28,29], abolished the effect of CA, at lower concentration, higher concentrations induced relaxation was independent of TEA, indicating role of K+ channels with additional mechanisms at higher concentrations.
In addition to K channels in VSMs, calcium channels contribute to vascular tone and play important role in the pathogenesis of hypertension. Therefore, Ca++ channel blockers are among the most effect antihypertensive medication [29]. To have insight into the effect of CA on vascular calcium channels, rat arotic rings were precontracted with high K+, where cumulative addition of CA induced a relaxant effect, suggesting a possible Ca++ channel blocker. High potassium (80 mM) is known to induce vascular smooth muscle cells contraction by increasing Ca++ influx through VDCCs and an agent which inhibits this influx is considered a Ca++ channel blocker [28,29]. The inhibitory effect of CA against the high K precontraction shows a possible Ca++ channel blocker that needed further confirmation. In a separate experiment, we used rabbit aortic rings (for some pharmacological reasons), due to stability, and contractile CRCs of CaCl2 were accomplished, in Ca++ free/EGTA medium, which purely shows Ca++ induced contractions. Interestingly, preincubation of the aortic rings with different concentrations of CA inhibited the development of contractile CRCs of CaCl2 with suppression of maximum effect, similar to verapamil, a conventional antihypertensive Ca++ channel blocker [29], confirming (indirectly) the Ca++ channel blocker potential of CA. Thus the vascular effects of CA are endothelium-dependent (mediated through Muscarinic receptors-linked NO and PGI2) and vascular smooth muscle-dependent (mediated through K+ activation and Ca++ channel blocking). These vascular effects of CA are responsible for the vascular component of decrease in blood pressure, observed in vivo. The effect of CA on heart performance was further evaluated to see how it decreased cardiac output in vivo.
To probe the dynamics of CA in heart, Langendorrf’s perfused isolated rabbit heart and right atria from guinea-pig (due to pharmacological reasons) were used. In the Langendorrf’s isolated perfused rabbit heart, bolus concentrations of CA completely suppressed FVC with partial inhibition of HR, while increased 25% coronary flow, suggesting negative inotropic and chronotropic effects. These effects on heart are responsible for the decrease in cardiac output that contributes to the antihypertensive effect of CA. The increase in CF might of therapeutic importance in cases of cardiac ischemia associated with hypertension. In isolated rabbit heart, it is hard to be convinced about the dynamics of CA as a synchronized physiological cardiac system that regulates FVC, HR and CF. Therefore, to further investigate the dynamics of CA, isolated guinea-pig right atrial strips (having inherent pace maker activity) were used in-vitro.
In isolated spontaneously beating guinea-pig right atrial strips, cumulative addition of CA inhibited the contractions, in comparison with atenolol, a selective cardiac β-adrenoceptor blocker [30]. To evaluate the role of cardiac β-adrenoceptor, atrial strips were preincubated with adrenaline, a β-adrenoceptor agonist, which completely reversed effect of CA, suggesting competitive type of interaction with cardiac β-adrenoceptor. This was further confirmed by preincubation of the atrial strips with adrenaline, which also abolished effects of atenolol. The findings indicate that CA decreases cardiac output through possible blocking of cardiac β-adrenoceptor and thus decrease in blood pressure.
The findings of this investigation show that CA induced antihypertensive effect, more potent in high salt-induced (8% NaCl) hypertensive rats. The antihypertensive effect is the outcome of vascular and cardiac effects of CA. The vasorelaxant effect is the result of synergistic effect of CA on endothelium and VSMs, mediated through muscarinic receptors-linked NO and PGI2 and activation of K+ channels and blockade of Ca++ channels while the decrease in cardiac output is due to blockade of cardiac βadrenoceptor. These mechanisms explain the antihypertensive effect of CA and suggest its role in the management of hypertension as an antihypertensive agent.
As CA has both calcium channel blocking and beta blocking activity. Previous literature has also shown that there are some compounds with dual action on both calcium channels and beta receptors. Carvedilol and its new analogs have been known for their nonselective beta blocking and calcium channel blocking effect [31]. Nabivolol produced β1 selective blocking activity coupled with endothelium/NO-mediated vasodilation [32]. The current investigation includes CA among the rare drugs with dual action.
Previous literature indicated that CA has the ability to reduce the activity of angiotensin converting enzyme (ACE) in blood, antihyperlipidemic and antiatherogenic potential [8,9]. It may provide the additional benefits of CA in the management of hypertension, hyperlipidemia and atherosclerosis. The antioxidant, antiinflammatory and cardioprotective effect [2–7] leads to its widespread potential in the management of various cardiovascular diseases. On the basis of current investigation and previous studies [1–9], CA has the potential to produce synergistic effects with other cardiovascular drugs such as diuretics, more potent calcium channel blocker and/or beta blocker, ACE inhibitors and statins to produce stronger effect. However, there is need to explore CA multiple pathways on molecular level and clinical trials for its use in the management of various cardiovascular diseases especially hypertension.
Supplementary Information
Funding Statement
This research is funded by NRPU No. 1554.
Footnotes
Author’s contributions: HMUDQ designed and carried out the experimental work and analyzed the statistical data and interpretation of results. US involved in acquisition of data, analysis and interpretation of the results. AJS and TK conceptualize the study, obtained finances, drafted and critically evaluated the manuscript. All authors read and approved the final manuscript.
Conflict of interest: The authors declare that they have no conflict of interest.
Funding: This research is funded by NRPU No. 1554.
References
- 1. Ruwizhi N, Aderibigbe BA. Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci. 2020;21:5712. doi: 10.3390/ijms21165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peperidou A, Pontiki E, Hadjipavlou-Litina D, Voulgari E, Avgoustakis K. Multifunctional cinnamic acid derivatives. Molecules. 2017;22:1247. doi: 10.3390/molecules22081247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adisakwattana S. Cinnamic acid and its derivatives: mechanisms for prevention and management of diabetes and its complications. Nutrients. 2017;9:163. doi: 10.3390/nu9020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song F, Li H, Sun J, Wang S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J Ethnopharmacol. 2013;150:125–30. doi: 10.1016/j.jep.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 5. Takeda Y, Tanigawa N, Sunghwa F, Ninomiya M, Hagiwara M, Matsushita K, et al. Morroniside cinnamic acid conjugate as an anti-inflammatory agent. Bioorg Med Chem Lett. 2010;20:4855–7. doi: 10.1016/j.bmcl.2010.06.095. [DOI] [PubMed] [Google Scholar]
- 6. De P, Bedos-Belval F, Vanucci-Bacque C, Baltas M. Cinnamic acid derivatives in tuberculosis, malaria and cardiovascular diseases-a review. Curr Org Chem. 2012;16:747–68. [Google Scholar]
- 7. Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem. 2012;12:749–67. doi: 10.2174/138955712801264792. [DOI] [PubMed] [Google Scholar]
- 8. Lapeyre C, Delomenède M, Bedos-Belval F, Duran H, Nègre-Salvayre A, Baltas M. Design, synthesis, and evaluation of pharmacological properties of cinnamic derivatives as anti-atherogenic agents. J Med Chem. 2005;48:8115–24. doi: 10.1021/jm050454c. [DOI] [PubMed] [Google Scholar]
- 9. Mnafgui K, Derbali A, Sayadi S, Gharsallah N, Elfeki A, Allouche N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J Food Sci Technol. 2015;52:4369–77. doi: 10.1007/s13197-014-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin. 2017;101:169–93. doi: 10.1016/j.mcna.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 12. Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–92. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National research council. guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 2011. [Google Scholar]
- 14. Furchgott RF, Zawadzki JV. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15. Gilani AH, Jabeen Q, Khan A, Shah AJ. A Gut modulatory, blood pressure lowering, diuretic and sedative activities of cardamom. J Ethnopharmacol. 2008;115:463–72. doi: 10.1016/j.jep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 16. Lawler JE. Hypertension produced by a high sodium diet in the borderline hypertensive rat (BHR) Clin Exp Hypertens. 1987;9:1713–31. doi: 10.3109/10641968709158968. [DOI] [PubMed] [Google Scholar]
- 17. Bibi R, Salma U, Bashir K, Khan T, Shah AJ. Antihypertensive activity of Sauromatum guttatum mediated by vasorelaxation and myocardial depressant effects. Arq Bras Cardiol. 2021;117:1093–103. doi: 10.36660/abc.20200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novakovic A, Bukarica LG, Kanjuh V, Heinle H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin Pharmacol Toxicol. 2006;99:360–4. doi: 10.1111/j.1742-7843.2006.pto_531.x. [DOI] [PubMed] [Google Scholar]
- 19. Shah AJ, Gilani AH. Blood pressure-lowering and vascular modulator effects of Acorus calamus extract are mediated through multiple pathways. J Cardiovasc Pharmacol. 2009;54:38–46. doi: 10.1097/FJC.0b013e3181aa5781. [DOI] [PubMed] [Google Scholar]
- 20. Janbaz KH, Akhtar T, Saqib F, Imran I, Zia-Ul-Haq M, Jansakul C, et al. Pharmacological justification of use of Solena heterophylla Lour in gastrointestinal, respiratory and vascular disorders. J Transl Med. 2015;13:134–45. doi: 10.1186/s12967-015-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan SS, Choi AO, Jones RL, Lin G. Mechanisms underlying the vasorelaxing effects of butyl idenephthalide, an active constituent of Ligusticum chuanxiong, in rat isolated aorta. Eur J Pharmacol. 2006;537:111–7. doi: 10.1016/j.ejphar.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 22. Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–50. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 23. Balderston SM, Johnson KE, Reiter MJ. Electrophysiological evaluation of cardiovascular agents in the isolated intact rabbit heart. J Pharmacol Methods. 1991;25:205–13. doi: 10.1016/0160-5402(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 24. Ayele Y, Urga K, Engidawork E. Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of leaf extract of Syzygium guineese (Wild) DC. Phytother Res. 2010;24:1457–62. doi: 10.1002/ptr.3141. [DOI] [PubMed] [Google Scholar]
- 25. Stephen F, Malcolm JL. A factor released from coronary vascular endothelium inhibits myocardial contractile performance. Am J Physiol. 1993;33:830–6. doi: 10.1152/ajpheart.1993.264.3.H830. [DOI] [PubMed] [Google Scholar]
- 26. Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–25. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 27. Hansen K, Nedergaard OA. Methodologic aspects of acetylcholine evoked relaxation of rabbit aorta. J Pharmacol Toxicol. 1999;41:153–9. doi: 10.1016/s1056-8719(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 28. Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010;584:2033–42. doi: 10.1016/j.febslet.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Godfraind T. Discovery and development of calcium channel blockers. Calcium antagonism and calcium entry blockade. Front Pharmacol. 2017;8:286. doi: 10.3389/fphar.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tesfamariam B, Allen GT. Beta 1-and beta 2-adrenoceptor antagonist activities of ICI-215001, a putative beta 3-adrenoceptor agonist. Br J Pharmacol. 1994;112:55. doi: 10.1111/j.1476-5381.1994.tb13028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload–induced Ca2+ release. Nat Med. 2011;17:1003–9. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta S, Wright HM. Nebivolol: a highly selective β1-adrenergic receptor blocker that causes vasodilation by increasing nitric oxide. Cardiovasc Ther. 2008;26:189–202. doi: 10.1111/j.1755-5922.2008.00054.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.