Abstract
Introduction
Compared to platinum-based therapies, a combination of ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) has demonstrated improved outcomes in advanced non-small cell lung cancer (NSCLC), albeit with higher rates of immune-related adverse events (irAEs). This multicenter retrospective study evaluated the efficacy and safety of nivolumab and ipilimumab with or without chemotherapy (NI and NICT) in real-world clinical settings.
Methods
We enrolled 215 treatment-naïve NSCLC patients who received NI or NICT between December 2020 and May 2023 at 14 institutions in Japan. Severe irAEs (Grade ≥ 3) were assessed using the Common Terminology Criteria for Adverse Events. Progression-free survival (PFS) and overall survival (OS) were evaluated using Kaplan–Meier methods and propensity score matching.
Results
Of 215 patients, 104 and 111 received NI and NICT, respectively. The median PFS was 5.3 and 5.9 months for NI and NICT, respectively. The median OS was 22.1 and 19.2 months for NI and NICT, respectively. High fever within 3 weeks of treatment initiation and high tumor burden were indicators of severe irAEs. Grade 3 or higher irAEs occurred in 36.5% patients in the NI group and 50.5% patients in the NICT group, with higher treatment-related mortality in the NICT group (5.4% vs. 1.9% in NI).
Conclusions
NI and NICT showed comparable efficacies in PFS and OS. However, NICT had a higher incidence of severe irAEs and treatment-related mortality. High tumor burden and early high fever were predictors of severe irAEs. Further research is warranted to optimize the efficacy and safety of NICT for NSCLC treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03890-4.
Keywords: Non-small cell lung cancer, Ipilimumab, Nivolumab, Immune-related adverse events, Chemotherapy
Introduction
Recently, the combination of ipilimumab, an anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), and nivolumab, an anti-programmed cell death 1 (anti-PD-1), has demonstrated improved outcomes in untreated advanced non-small cell lung cancer (NSCLC) compared to platinum-based therapies, as evidenced by the CheckMate227 and CheckMate9LA trials [1, 2]. However, this combination therapy is linked to a higher incidence of immune-related adverse events (irAEs) compared to monotherapy with anti-PD-1 or anti-programmed cell death ligand 1 (PD-L1) antibodies [1]. Despite the relatively high rate of irAE-related discontinuation observed in these prospective studies, patients who experienced discontinuation showed a better prognosis than the overall population, with treatment-related mortality rates ranging from 1 to 2% [1, 2]. Contrarily, a phase III trial in Japan comparing nivolumab and ipilimumab with chemotherapy (NICT) and pembrolizumab with chemotherapy was halted owing to numerous severe irAEs, including fatalities, in the NICT arm [3]. Consequently, NICT treatment has raised significant safety concerns related to uncontrolled and potentially fatal AEs in Japan [3].
NICT prevents early progression by incorporating two cycles of platinum combination therapy with nivolumab and ipilimumab (NI) [2]. In clinical practice, the combination of immune checkpoint inhibitors (ICIs) and chemotherapy is frequently selected for patients with high tumor burdens, tumor-related clinical symptoms, and rapid disease progression. In contrast, ICIs alone are often preferred for older patients, those with poor performance status (PS), and those with medical comorbidities, which may introduce a clinician bias in treatment selection [4]. In our earlier single-center retrospective study, we identified total tumor burden before treatment initiation as a predictor of Grade ≥ 3 severe irAEs in NICT or NI for NSCLC [5]. Interestingly, concomitant chemotherapy with NI did not predict severe irAEs in our investigation. Furthermore, while NI is widely utilized for tumors in various organs, NICT is specifically available for NSCLC alone, and comprehensive safety data are insufficient to ascertain whether NICT is poorly tolerated in real-world clinical practice.
Multi-death cases have been observed in the NICT arm of a phase III trial discontinued in Japan [3]. One of the most severe or potentially fatal irAEs is cytokine release syndrome (CRS), characterized by high fever as its most common initial symptom, followed by hypotension and respiratory failure [6]. Fever also manifests in other life-threatening irAEs such as pneumonitis, myocarditis, hepatitis, and endocrine disorders such as adrenal insufficiency. Combination therapy involving ipilimumab and an anti-PD-1 antibody is associated with earlier onset and increased severity of irAEs compared to treatment with anti-PD-1 antibody alone [7]. Therefore, fever should be managed cautiously and promptly upon treatment initiation.
Here, we conducted a multicenter, retrospective, observational study to compare the efficacy and safety of NI and NICT in real-world clinical practice. We aimed to investigate whether concomitant chemotherapy or total tumor volume influences the severity and fatality of irAEs. Additionally, we explored whether the early onset of high fever after treatment initiation correlates with the development and prognosis of severe or fatal irAEs.
Patients and methods
Patient selection and data collection
This multicenter, retrospective observational study was conducted in collaboration with the Sapporo Medical University Thoracic Oncology Team (START) at 14 institutions in Hokkaido, Japan. Consecutive treatment-naïve NSCLC patients who received NI or NICT between December 2020 and May 2023 were enrolled in the study. The NICT arm included chemotherapy combinations of either carboplatin and paclitaxel or carboplatin/cisplatin and pemetrexed. Inclusion criteria included unresectable advanced or recurrent NSCLC. Exclusion criteria included mutations of epidermal growth factor receptor, rearrangements of anaplastic lymphoma kinase, prior chemoradiation or immune checkpoint inhibitor (ICI) therapy, other malignancies, and missing or incomplete data. Based on sample size calculations, 225 patients were included to ensure sufficient statistical power for comparing Grade 3 or higher irAEs and median survival between treatment groups.
Patient characteristics
Data collected included age, sex, performance status, smoking history, histopathology, disease stage, PD-L1 expression, chest imaging, and treatment response. Baseline tumor burden was measured using RECIST v1.1, and PD-L1 expression was assessed using the Dako PD-L1 IHC 22C3 PharmDx test.
Endpoint definitions
Treatment response was evaluated per RECIST v1.1, and severe pneumonitis was defined as Grade ≥ 3 ICI-related toxicities. Primary endpoints included progression-free survival (PFS), overall survival (OS), and the incidence of Grade 3 or higher irAEs. Secondary endpoints included the relationship between chemotherapy, tumor volume, early onset of fever, and the severity of irAEs.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. The Mann–Whitney U test and Fisher's exact test were used to analyze differences between groups. PFS and OS were compared using Kaplan–Meier curves and log-rank tests. Cox proportional hazards models were used to identify the risk factors for survival. Logistic regression was applied to assess irAE predictors, and propensity score matching (PSM) was performed to adjust for potential confounders. Sensitivity analyses were conducted to validate results across subgroups.
Ethical considerations
The study was approved by the Institutional Review Board of Sapporo Medical University Hospital (352–103) and adhered to the Declaration of Helsinki. An opt-out consent process was implemented.
The detailed methodology is available as supplementary material (Supplementary data).
Results
Patient inclusion and characteristics
In total, 225 patients who received nivolumab plus ipilimumab with or without chemotherapy for advanced NSCLC were identified. Of these, three patients were excluded owing to a lack of PD-L1 data, and seven patients were excluded because their lesions could not be measured. Finally, 215 patients were included in the study (Supplementary Fig. 1), and their characteristics are summarized in Table 1. Among these, 104 patients (48%) received NI and 111 patients (52%) received NICT. Age was higher in the NI group than in the NICT group (p < 0.001), with more patients older than 70 years. The tumor stage was significantly different between the two groups, with more recurrent cases in the NI group (p = 0.03). No significant differences were observed between the two groups in terms of sex, smoking history, PS, histology, PD-L1 expression, or metastatic sites.
Table 1.
Baseline characteristics of patients included in the study
| Characteristics | No. (%) | NICT (n = 111) | p | |
|---|---|---|---|---|
| NI (n = 104) | ||||
| Age | < 70 | 28 (26.9) | 67 (60.4) | < 0.001 |
| ≥ 70 | 76 (73.1) | 44 (39.6) | ||
| Sex (%) | Female | 33 (31.7) | 28 (25.2) | 0.36 |
| Male | 71 (68.3) | 83 (74.8) | ||
| Smoking status (%) | Ex/Current | 89 (85.6) | 104 (93.7) | 0.07 |
| Never | 15 (14.4) | 7 (6.3) | ||
| ECOG PS (%) | 0–1 | 95 (91.3) | 108 (97.3) | 0.08 |
| > 1 | 9 (8.7) | 3 (2.7) | ||
| Histology (%) | NSQ | 63 (60.6) | 69 (62.2) | 0.89 |
| SQ | 41 (39.4) | 42 (37.8) | ||
| PD-L1 expression (%) | ≥ 50% | 25 (24.0) | 33 (29.7) | 0.37 |
| 1–49% | 38 (36.5) | 31 (27.9) | ||
| < 1% | 41 (39.4) | 47 (42.3) | ||
| Stage (%) | III | 17 (16.3) | 22 (19.8) | 0.03 |
| IV | 64 (61.5) | 79 (71.2) | ||
| Rec | 23 (22.1) | 10 (9.0) | ||
| Brain metastasis (%) | 14 (13.5) | 23 (20.7) | 0.21 | |
| Liver metastasis (%) | 8 (7.7) | 12 (10.8) | 0.49 | |
| Bone metastasis (%) | 24 (23.1) | 37 (33.3) | 0.10 | |
| Pleural metastasis (%) | 23 (22.1) | 29 (26.1) | 0.53 |
NI nivolumab and ipilimumab, NICT nivolumab, ipilimumab, and chemotherapy, NSQ non-squamous cell carcinoma, SQ squamous cell carcinoma, ECOG PS Eastern Cooperative Oncology Group Performance Status, PD-L1 Programmed cell death ligand 1, Rec recurrence
Efficacy
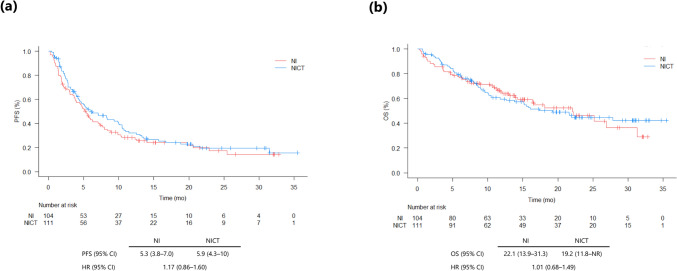
The data cut-off date was November 30, 2023, and the median follow-up for all patients included in the study was 11.8 (0.3–35.5) months. The median PFS duration was 5.3 months (95% CI: 3.8–7.0) in the NI group and 5.9 months (95% CI: 4.3–10.0) in the NICT group (HR 1.17; 95% CI: 0.86–1.60) (Fig. 1a). In total, 64 patients were alive in the NI group, and 59 patients were alive in the NICT group at the time of data cut-off. The median OS was 22.1 months (95% CI: 13.9–31.3) in the NI group and 19.2 (95% CI: 11.8–NR [not reached]) in the NICT group (HR 1.01; 95% CI: 0.68–1.49), respectively (Fig. 1b).
Fig. 1.
Kaplan–Meier curves of progression-free survival (PFS) (a) and overall survival (OS) (b) in patients with non-small cell lung cancer treated with nivolumab and ipilimumab (NI) or nivolumab, ipilimumab, and chemotherapy (NICT). Abbreviations: HR hazard ratio, CI confidence interval, mo months
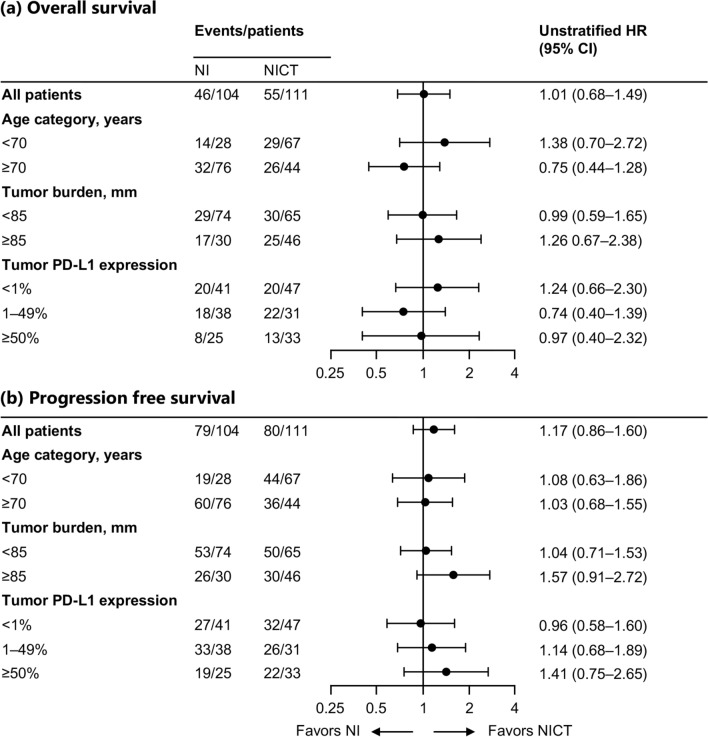
We evaluated the PFS and OS in the selected subgroups of patients according to age (< 70 years and ≥ 70 years), overall tumor burden (< 85 mm and ≥ 85 mm), PD-L1 TPS (< 1%, 1–49% and ≥ 50%) (Fig. 2), and high fever after treatment initiation (< 22 days and ≥ 22 days). In the patients grouped by age, there were no significant differences in PFS and OS between NI and NICT in both younger (< 70 years) and older (≥ 70 years) patients. A trend was observed for longer OS in younger patients who received NICT and older patients treated with NI (Supplementary Fig. 2a, 2b). However, the interaction test performed using COX proportional hazards model for age and chemotherapy combination revealed no significant results. In the context of high tumor burden (≥ 85 mm), the NICT group exhibited a better PFS during the observation period than the NI group, although there was no statistically significant difference (2.65 vs. 4.17 months [HR 1.57; 95% CI: 0.91–2.72]) (Supplementary Fig. 3a). Contrarily, in patients with low tumor burden (< 85 mm), there was no significant difference in PFS between the NI and NICT groups, and the Kaplan–Meier curves were almost overlapping. (Supplementary Fig. 3b). Regardless of the tumor burden, OS was not significantly different between the NI and NICT groups (Supplementary Fig. 3a, 3b). Furthermore, there were no significant differences in PFS and OS between the NI and NICT groups, regardless of the PD-L1 TPS expression levels (Supplementary Fig. 4a–c).
Fig. 2.
Subgroup analysis of overall survival (a) and progression-free survival (b). The HR and its 95% CI were calculated with an unstratified Cox regression model. All subgroup analyses were exploratory. Abbreviations: NI nivolumab and ipilimumab, NICT nivolumab, ipilimumab, and chemotherapy, HR hazard ratio, CI confidence interval, PD-L1, Programmed cell death ligand 1
The ORRs were 44.2% (46/104) for NI and 51.4% (57/111) for NICT, while the progressive disease rates were 26.0% (27/104) for the NI group and 21.6% (24/111) for the NICT group (Supplementary Table 1).
Safety and toxicity
At the date cut-off, the treatment discontinuation rates were 85.7% (90/105) and 89% (99/111) for the NI and NICT groups, respectively. Disease progression was the most common reason for treatment discontinuation. Furthermore, 34 of 104 (32.7%) patients in the NI group and 40 of 111 (36.0%) patients in the NICT group discontinued treatment due to AEs. Table 2 shows a list of Grade 3 treatment-related AEs and fatal events observed when treated with steroids (i.e., irAEs). Grade 3 or higher irAEs were reported in 38 of 104 patients (36.5%) in the NI group and 56 of 111 patients (50.5%) in the NICT group. The most frequently reported Grade 3 or higher irAE in patients treated with NI was pneumonia, reported in 18 of 104 patients (17.3%). The most frequently reported Grade 3 or higher irAE in patients treated with NICT was rash, reported in 23 of 104 (20.7%) patients.
Table 2.
Treatment-related Grade ≥ 3 and fatal adverse events in patients treated with steroids
| Total | No. (%) | |||||
|---|---|---|---|---|---|---|
| NI (n = 104) | NICT (n = 111) | |||||
| ≥ Grade 3 | Grade 4 | Grade 5 | ≥ Grade 3 | Grade 4 | Grade 5 | |
| 38 (36.5) | 8 (7.7) | 2 (1.9) | 56 (50.5) | 14 (12.6) | 6 (5.4) | |
| Pneumonitis | 16 (15.4) | 2 (1.9) | 13 (11.7) | 2 (1.8) | 5 (4.5) | |
| Rash | 4 (3.8) | 20 (18.0) | 3 (2.7) | |||
| Liver dysfunction | 10 (9.6) | 4 (3.8) | 11 (9.9) | 2 (1.8) | ||
| Colitis | 1 (1.0) | 10 (9.0) | 1 (0.9) | |||
| Endocrine disorder | 4 (3.8) | 5 (4.5) | ||||
| Cytokine release syndrome | 1 (1.0) | 1 (1.0) | 7 (6.3) | 1 (0.9) | 1 (0.9) | |
| Nephritis | 5 (4.5) | 3 (2.7) | ||||
| Infusion-related reaction | 3 (2.7) | 1 (0.9) | ||||
| CPK elevation | 2 (1.9) | 1 (1.0) | ||||
| Pancreatitis | 1 (1.0) | 1 (1.0) | 1 (0.9) | |||
| Arthritis | 1 (1.0) | 1 (0.9) | ||||
| CNS disorder | 2 (1.8) | 1 (0.9) | ||||
| Myositis | 1 (1.0) | 1 (1.0) | ||||
| Pulmonary edema | 1 (1.0) | |||||
| Sclerosing cholangitis | 1 (1.0) | |||||
| Uveitis | 1 (1.0) | |||||
| Gastrorrhagia | 1 (0.9) | 1 (0.9) | ||||
| Neutropenia | 1 (0.9) | 1 (0.9) | ||||
| Gastritis | 1 (0.9) | |||||
| Sepsis | 1 (0.9) | |||||
| Fatigue | 1 (0.9) | |||||
| Peripheral nerve disorder | 1 (0.9) | |||||
| Anemia | 1 (0.9) | |||||
| Bronchial hemorrhage | 1 (0.9) | |||||
NI nivolumab and ipilimumab therapy, NICT nivolumab, ipilimumab, and chemotherapy, CPK creatine phosphokinase, CNS central nervous system
The number of patients with multiple Grade 3 irAEs or ≥ Grade 4 irAEs reported was 12 of 104 (11.5%) in the NI group and 31 of 111 (27.9%) in the NICT group. Treatment-related deaths occurred in 2 of 104 patients (1.9%) in the NI group and in 6 of 111 patients (5.4%) in the NICT group. Treatment-related deaths were caused by CRS and pancreatitis in one patient each in the NI group and CRS in one patient and pneumonitis in five patients in the NICT group.
PSM analysis
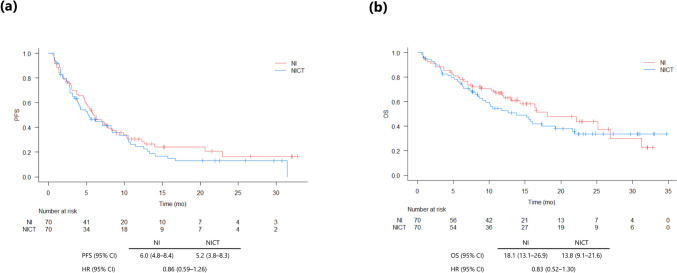
To control for unbalanced conditions at baseline between the groups, we performed PSM with age, sex, smoking status, PS, histology, PD-L1 TPS, and stage as adjustment factors, and the 1:1 matching yielded match pairs of 70 patients in the two groups, with no differences in any of the characteristics (Supplementary Table 2). The median PFS duration was 6.0 months (95% CI: 4.8–8.4) in the NI group and 5.2 months (95% CI: 3.8–8.3) in NICT group (HR 0.86; 95% CI: 0.59–1.26) (Fig. 3a). The median survival duration was 18.1 months (95% CI: 13.1–26.9) in the NI group and 13.8 months (95% CI: 9.1–21.6) in the NICT group (HR 0.83; 95% CI: 0.52–1.30) (Fig. 3b).
Fig. 3.
Kaplan–Meier curves of progression-free survival (PFS) (a) and overall survival (OS) (b) in patients with non-small cell lung cancer treated with nivolumab and ipilimumab (NI) or nivolumab, ipilimumab, and chemotherapy (NICT) after propensity score matching. Abbreviations: HR hazard ratio, CI confidence interval, mo months
Univariate and multivariate analyses
Univariate and multivariate analyses were used to examine the relationship between pretreatment clinical parameters and an onset high fever of about 38 ℃ or higher within 21 days of treatment initiation, and Grade 3 or higher irAE. Tumor burden was included in multivariate analysis because it is associated with the risk of irAEs [4]. Tumor burden and early high fever, but not age, smoking status, histology, and treatment, correlated with ≥ Grade 3 irAE (Table 3). In the multivariate analysis, including all variables of univariate analyses, the ORs of high tumor burden and early high fever were 2.13 (95% CI = 1.13–4.01, p = 0.02) and 2.52 (95% CI = 1.25–5.09, p = 0.01), respectively.
Table 3.
Risk factors for ≥ Grade 3 immune-related adverse events
| Variable | All patient (n = 215) | ||||||
|---|---|---|---|---|---|---|---|
| Univariate analysis | p | Multivariate analysis | p | ||||
| OR | 95%CI | OR | 95%CI | ||||
| Age (y.o.) | < 70 vs. ≥ 70 | 0.56 | 0.31–1.01 | 0.052 | 0.609 | 0.32–1.14 | 0.123 |
| Tumor burden (mm) | < 85 vs. ≥ 85 | 2.89 | 1.57–5.40 | < 0.001 | 2.13 | 1.13–4.01 | 0.020 |
| Histology | NSQ vs SQ | 1.13 | 0.62–2.10 | 0.067 | 1.5 | 0.81–2.78 | 0.202 |
| Smoking status | Never vs. Former/Current | 3.91 | 1.23–16.47 | 0.012 | 3.02 | 0.91–10.0 | 0.071 |
| Treatment | NI vs. NICT | 1.76 | 0.99–3.17 | 0.054 | 1.07 | 0.57–2.03 | 0.824 |
| Early high fever | No vs. Yes | 4.05 | 2.08–8.09 | < 0.001 | 2.52 | 1.25–5.09 | 0.010 |
≥ G3 irAEs (n = 94)
y.o. years old, OR odds ratio, CI confidence interval, NSQ non-squamous cell carcinoma, SQ squamous cell carcinoma, NI nivolumab and ipilimumab therapy, NICT nivolumab, ipilimumab, and chemotherapy
The relationship between two or more Grade 3 irAEs or Grade 4 or more irAEs (more severe or fatal irAEs), pretreatment clinical parameters, and early high fever was investigated using univariate and multivariate analyses. Stage IV disease and early high fever, but not tumor burden or treatment, were correlated with more severe or fatal irAEs (Supplementary Table 3). In the multivariate analysis, including all variables of univariate analyses, the ORs of Stage IV and early high fever were 2.84 (95% CI = 1.14–7.04, p = 0.024) and 3.51 (95% CI = 1.61–7.70, p = 0.0017), respectively. The Kaplan–Meier curves of PFS and OS based on the presence/absence of early high fever are presented in Supplementary Fig. 5a and b.
In addition, univariate and multivariate analyses were performed for pneumonitis and CRS, which caused treatment-related deaths (Supplementary Tables 4 and 5). In multivariate analysis incorporating all factors from univariate analysis, pneumonitis and CRS were significantly associated with patients 70 years or older and with early high fever, with ORs of 2.67 (95% CI = 1.03–6.95, p = 0.044) and 8.35 (95% CI = 1.43–48.9, p = 0.018) respectively.
Discussion
The findings of our retrospective observational study demonstrated that PFS and OS did not differ between treatment-naïve NSCLC patients who received NI or NICT therapy. However, the safety profile showed a higher incidence of Grade 3 or higher treatment-related AEs, including treatment-related deaths, in the NICT group. In addition, a high tumor burden and early high fever were identified as significant predictors of severe irAEs. Hence, the efficacies of NI and NICT were comparable, and the benefits of concomitant chemotherapy were unclear in our study.
NICT is a combination of nivolumab and ipilimumab with chemotherapy (two cycles) as the first-line treatment for patients with NSCLC. The clinical rationale for this regimen is that the addition of chemotherapy to the combination of nivolumab and ipilimumab may provide early disease control during treatment, while minimizing the side effects associated with an entire course of chemotherapy [1, 2]. However, no clinical trials have compared NI and NICT, and the significance of combining chemotherapy with NI remains unclear.
In the phase 3 study, the ORR for NICT (Checkmate9LA) was 38.2% and progressive disease (PD) rate was 9% [8], while the ORR for NI (Checkmate227) was 35.9% for PD-L1 ≥ 1%, 27.3% for PD-L1 < 1% and PD rate was 22.7% and 24.1%, respectively [1]. The 5-year survival rate for NICT was 18% (PD-L1 ≥ 1% in 18% and PD-L1 < 1% in 22%), comparable to the 5-year survival rates for NI, PD-L1 ≥ 1% in 24% and PD-L1 < 1% in 19% [1, 9].
In our study, the ORRs for NI and NICT were 44.2% and 51.4%, while the PD rates were 26.0% and 21.6%, respectively, with slightly higher ORRs and slightly lower PD rates for NICT, similar to those in a phase 3 study [1, 2]. PFS and OS were not significantly different in the survival curves, although they were slightly better in the NICT than in the NI group during the early treatment phase. In the PSM adjusted for age, sex, smoking history, PS, histology, PD-L1 expression, and disease stage, the early treatment advantage of NICT disappeared, and the PFS and OS of the two groups were similar. In practice, the ORR increases and the PD rate decreases when NI is combined with chemotherapy; however, NICT is not superior to NI in terms of long-term prognosis [1, 9], suggesting that chemotherapy combinations should not be used readily and, in the case of symptomatic tumors, rapid tumor progression or large tumor volumes requiring a short-term response, cancer therapists should fully consider the risks and benefits on a case-by-case basis.
In our NICT group, 50.5% AEs were Grade 3 or higher irAE, while 5.4% were Grade 5 irAE; in contrast, in the NI group 36.5% AEs were Grade 3 or higher irAE and 1.9% AEs were Grade 5 irAE. Thus, a higher rate of serious AEs was noted in NICT. Although NICT showed a manageable safety profile in the Checkmate 9LA trial [2], a Japanese phase III trial comparing NICT with pembrolizumab in combination with platinum (JCOG2007) showed that treatment-related deaths were more frequent in the NICT arm (7.5%, 11/147 patients), owing to which the trial was discontinued [3]. Contrastingly, in a prospective observational study in Japan (LIGHT-NING study), the incidence of ≥ Grade 3 irAE was 32.1% and 27.0% in the NICT and NI groups, respectively, while the incidence of treatment-related deaths in the NICT and NI groups was 2.8% and 3.5%, respectively; notably, these findings are similar to those of the Checkmate 9LA study, which reported 2% treatment-related deaths [2, 10]. In this retrospective study and the LIGHT-NING studies, ≥ Grade 3 irAEs were more common in the NICT group, consistent with the trend [10]. Possible reasons for the differences in reported treatment-related deaths between studies may include various treatment interventions used for AEs in different study formats (prospective interventional studies, prospective observational studies, and retrospective observational studies). Moreover, confounding factors other than the clinical backgrounds of the patients may cause variations in treatment-related mortality between trials. One such factor is the COVID-19 pandemic in the historical context.
The TERAVOLT trial, which investigated the impact of SARS-CoV-2 infection on patients with thoracic malignancies, reported that ICI monotherapy or combination therapy with ICI and chemotherapy does not increase the risk of mortality due to COVID-19 [11]. Notably, COVID-19 in patients undergoing ICI treatment for solid tumors is associated with an increased frequency of Grade 3 or higher irAEs, and the combination of anti-CTLA-4 antibodies has been identified as a risk factor [12]. Although ipilimumab promotes an antitumor effect by suppressing regulatory T cells via CTLA-4, it may not be able to suppress anti-viral immune responses appropriately during SARS-CoV-2 infection; it may also enhance cytokine storms, which has been implicated in severe COVID-19 [13, 14]. The fact that COVID-19 during ICI treatment does not increase the frequency of irAEs, but increases their severity, and that COVID-19 promotes the development of autoimmune diseases indicates that COVID-19 can considerably impact the human immune system even during ICI treatment [12, 15]. A patient in the NICT arm of the JCOG 2007 trial who developed treatment-resistant CRS after COVID-19 died without any treatment benefits [16]. In addition to COVID-19 that can induce CRS from inflammatory pathology during NI or NICT treatment [16, 17]. CRS development can also be triggered by radiotherapy, bacterial infection, pleurodesis, and COVID-19 vaccines [16, 18–20]; therefore, clinicians should be aware of inflammatory triggers during ICI treatment. In Hokkaido, where the participating centers of our study were located, there was an explosion of COVID-19 cases after January 2022 owing to the prevalence of the omicron strain [21]. Six of seven deaths from interstitial lung disease or CRS occurred during the COVID-19 epidemic in Hokkaido. Although this study did not examine the details of COVID-19 vaccination status or COVID-19 infection, an association between the COVID-19 epidemic and the development of fatal irAEs was suggested.
Fever during ICI treatment should always be considered as a potential trigger of severe irAEs. Infection was reported as the most common cause (67%) of fever in patients with cancer, whereas in current cancer treatments with ICIs, fever due to various irAEs is a differential diagnosis [22]. For instance, in pneumonitis, which is relatively common and can be fatal if severe, the incidence of fever ranges from 12 to 33% [23]. Conversely, in CRS, which is less common but potentially fatal, high fever is the most frequent initial symptom [24]. The median time from the start of ICI therapy to the onset of CRS is 11 days, and early high fever after treatment initiation requires careful attention [24]. Our study revealed that high tumor burden and early high fever were clinical factors associated with Grade 3 or higher irAEs, which was consistent with our previous report [5]. In our study, early high fever was associated with more severe, multiple Grade 3 or Grade 4 or higher irAEs, and CRS. Moreover, older age is a risk factor for severe pneumonitis. Although the combined use of chemotherapy was not significantly associated with any risk, all five deaths from pneumonitis and seven of eight cases of CRS occurred in the NICT group; therefore, the effect of combined chemotherapy on the severity of irAEs needs to be investigated further.
In our study, the incidence of Grade 3 or higher pneumonitis was 15.4% in the NI group and 11.7% in the NICT group, which was significantly higher than the Grade 3 or higher pulmonary toxicity rate of 1.7%–3.3% reported in previous phase III trials [1, 2]. One of the contributing factors to this higher incidence is the proportion of elderly patients in our cohort. In the CM227 and CM9LA trials, 47.5% and 41% of the patients were 65 years or older, respectively; moreover, 9.9% and 10% of the patients were 75 years or older, respectively [1, 2]. In contrast, in our study, 84.6% of patients in the NI group and 59.5% in the NICT group were 65 years or older, with particularly high proportions of elderly patients in the NI group. In the NI group, 53.8% were 75 years or older and 14.4% in the NICT group. Generally, the incidence of ICI-related pulmonary toxicity, such as irAE, significantly increases with age [25]. Therefore, the high incidence of pneumonitis in this study can be attributed to high proportion of patients with underlying diseases such as chronic obstructive pulmonary disease and interstitial pneumonia due to the ageing population. It was assumed that the fragility of the lung tissue increases in older patients.
Our study has several limitations. First, it was a retrospective study, which may overestimate PFS and underestimate AEs. Second, management of AEs may have differed among centers. Third, we only investigated Grade 3 or higher AEs treated with steroids as irAEs; therefore, we may have overestimated AEs that were not inherently irAEs and did not investigate low-grade AEs. In addition, the relationship between low-grade irAE and early-stage high fever could not be examined. Moreover, this study was unable to verify the effects of early intervention with steroids for early high fever. In the future, prospective studies must be conducted to verify whether the administration of steroids for non-infectious high fever in the early stages improves patient outcomes and prognosis. Finally, there may have been differences in treatment strategies adopted at various participating centers once the AEs resolved.
In conclusion, our multicenter retrospective study showed no significant differences in PFS or OS between the NI and NICT groups. AEs were more frequent and severe in the NICT group than in the NI group. Patients with high fever within three weeks of treatment initiation were more likely to develop severe or fatal irAEs and had a poorer prognosis. Therefore, patient observation during early treatment and intervention for AEs is important. Future follow-up of the patients enrolled in this study will continue to investigate the association of NI and NICT treatment strategies with long-term prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Editage (www.editage.com) for their assistance with English language editing.
Abbreviations
- CRS
Cytokine release syndrome
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IHC
Immunohistochemistry
- irAE
Immune-related adverse event
- NI
Nivolumab and ipilimumab
- NICT
Nivolumab and ipilimumab with chemotherapy
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
- ORR
Objective response rate
- OS
Overall survival
- PFS
Progression-free survival
- PS
Performance status
- PSM
Propensity score matching
- TPS
Tumor proportion score
Author’s contribution
All authors had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization; TS, Data curation; All authors, Formal analysis; TS, Funding acquisition; TS, Investigation; TS, Methodology; TS, Project administration; TS, YN and TI, Resources; TS, Software; TS, Supervision; HN, MT and HC, Validation; TS, Visualization; TS, Writing—original draft; TS, Writing—review and editing; All authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request. No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
Financial Interests: Dr Toshiyuki Sumi reported personal fee from Ono Pharmaceutical and AstraZeneca outside the submitted work. Other authors declare they have no financial interests.
Ethics approval
This study was conducted with the approval of the Institutional Review Board of Sapporo Medical University Hospital (352–103) and was performed in accordance with the provisions of the Declaration of Helsinki.
Consent to participate
Informed consent was not obtained from all patients; instead, the opt-out method was used. Comprehensive information about the research was made available at the hospital facility and website.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brahmer JR, Lee J-S, Ciuleanu TE, Bernabe Caro R, Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R, Pluzanski A, Burgers J (2023) Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J Clin Oncol 41(6):1200–1212. 10.1200/JCO.22.01503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone DP, Ciuleanu TE, Schenker M, Schenker M, Cobo M, Bordenave S, Juan-Vidal O, Menezes J, Reinmuth N, Richardet E, Cheng Y, Mizutani H (2024) Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the CheckMate 9LA randomized trial. J Immunother Cancer 12(2):e008189. 10.1136/jitc-2023-008189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiraishi Y, Hakozaki T, Nomura S, Kataoka T, Tanaka K, Miura S, Sekino Y, Ando M, Horinouchi H, Ohe Y, Okamoto I (2022) A multicenter, randomized Phase III study comparing platinum combination chemotherapy plus pembrolizumab with platinum combination chemotherapy plus nivolumab and ipilimumab for treatment-naive advanced non-small cell lung cancer without driver gene alterations: JCOG2007 (NIPPON study). Clin Lung Cancer 23(4):e285–e288. 10.1016/j.cllc.2021.10.012 [DOI] [PubMed] [Google Scholar]
- 4.Uehara Y, Hakozaki T, Kitadai R, Narita K, Watanabe K, Hashimoto K, Kawai S, Yomota M, Hosomi Y (2022) Association between the baseline tumor size and outcomes of patients with non-small cell lung cancer treated with first-line immune checkpoint inhibitor monotherapy or in combination with chemotherapy. Transl Lung Cancer Res 11(2):135–149. 10.21037/tlcr-21-815 [DOI] [PMC free article] [PubMed]
- 5.Sumi T, Koshshino Y, Sekikawa M, Nagahisa Y, Matsuura K, Shijubou N, Kamada K, Watanabe H, Michimata H, Nagayama D, Tanaka Y (2022) Risk factors for severe immune-related adverse events after first-line pembrolizumab monotherapy or combination chemotherapy for non-small-cell lung cancer. Investig New Drugs 40(6):1298–1305. 10.1007/s10637-022-01310-x [DOI] [PubMed] [Google Scholar]
- 6.Liu LL, Skribek M, Harmenberg U, Gerling M (2023) Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: case report and systematic review of the literature. J Immunother Cancer 11(3):e005841. 10.1136/jitc-2022-005841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16(9):563–580. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A (2021) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22(2):198–211. 10.1016/S1470-2045(20)30641-0 [published correction appears in Lancet Oncol. e92. 10.1016/S1470-2045(21)00082-6. 2021 March;22(3)]. [DOI] [PubMed]
- 9.Reck M, Ciuleanu TE, Schenker M, Bordenave S, Cobo M, Juan-Vidal O, Reinmuth N, Richardet E, Felip E, Menezes J, Cheng Y (2024) Five-year outcomes with first-line (1L) nivolumab + ipilimumab + chemotherapy (N + I + C) vs C in patients (pts) with metastatic NSCLC (mNSCLC) in CheckMate 9LA. J Clin Oncol 42(16_suppl):8560. 10.1200/JCO.2024.42.16_suppl.8560
- 10.Imai H, Kijima T, Azuma K, Kishi K, Saito H, Yamaguchi T, Tanizaki J, Yoneshima Y, Fujita K, Watanabe S, Kitazono S (2024) First-line nivolumab plus ipilimumab with or without chemotherapy for Japanese patients with non-small cell lung cancer: LIGHT-NING study. Jpn J Clin Oncol 54(4):452–462. 10.1093/jjco/hyad195 [published correction appears in Jpn J Clin Oncol. hyae069. 10.1093/jjco/hyae069. 2024 May 22]. [DOI] [PMC free article] [PubMed]
- 11.Garassino MC, Whisenant JG, Huang LC et al (2020) COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 21(7):914–922. 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo M, Liu J, Miao R, Ahmed Z, Yu J, Guan J, Ahmad S, Zhou S, Grove A, Manoucheri M, Socinski MA (2022) A Single Center retrospective study of the impact of COVID-19 infection on immune-related adverse events in cancer patients receiving immune checkpoint inhibitors. J Immunother 45(9):389–395. 10.1097/CJI.0000000000000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber J (2009) Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 58(5):823–830. 10.1007/s00262-008-0653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z, Jiang X, Dai X, Li B (2022) The dynamic role of FOXP3+ Tregs and their potential therapeutic applications during SARS-CoV-2 infection. Front Immunol 13:916411. 10.3389/fimmu.2022.916411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng K, Li X, Yang D, Chan SC, Zhou J, Wan EY, Chui CS, Lai FT, Wong CK, Chan EW, Leung WK (2023) Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine 63:102154. 10.1016/j.eclinm.2023.102154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiraishi Y, Tokito T, Toyozawa R, Inagaki C, Nokihara H, Kawashima Y, Ohe Y, Okamoto I (2024) Five cases of cytokine release syndrome in patients receiving cytotoxic chemotherapy together with nivolumab plus ipilimumab: A case report. J Thorac Oncol 19(2):337–343. 10.1016/j.jtho.2023.10.010 [DOI] [PubMed] [Google Scholar]
- 17.Dinoto A, Rossato F, Corradetti T, Gioulis M, Marsala SZ, Ferracci F (2022) Multifocal nivolumab immune-related adverse effects during asymptomatic SARS-CoV-2 infection: causality or casuality? Neurol Sci 43(5):2967–2968. 10.1007/s10072-022-05916-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker CA, Kim SK, Budhu S, Matsoukas K, Daniyan AF, D’Angelo SP (2018) Cytokine release syndrome after radiation therapy: Case report and review of the literature. J Immunother Cancer 6(1):1. 10.1186/s40425-017-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumi T, Suzuki K, Koshino Y, Ikeda T, Watanabe H, Yamada Y, Chiba H (2023) Cytokine releasing syndrome following pleurodesis during combination chemo-immunotherapy for lung adenocarcinoma. JJLC 63(7):971–976. 10.2482/haigan.63.971 [Google Scholar]
- 20.Sumi T, Koshino Y, Michimata H, Nagayama D, Watanabe H, Yamada Y, Chiba H (2022) Cytokine release syndrome in a patient with non-small cell lung cancer on ipilimumab and nivolumab maintenance therapy after vaccination with the mRNA-1273 vaccine: a case report. Transl Lung Cancer Res 11(9):1973–1976. 10.21037/tlcr-22-388 [DOI] [PMC free article] [PubMed]
- 21.Data on COVID-19 [Hokkaido]. Accessed 16 July 2024. https://www.harp.lg.jp/opendata/dataset/1369.html.
- 22.Toussaint E, Bahel-Ball E, Vekemans M, Georgala A, Al-Hakak L, Paesmans M, Aoun M (2006) Causes of fever in cancer patients (prospective study over 477 episodes). Support Care Cancer 14(7):763–769. 10.1007/s00520-005-0898-0 [DOI] [PubMed] [Google Scholar]
- 23.Cadranel J, Canellas A, Matton L, Darrason M, Parrot A, Naccache JM, Lavolé A, Ruppert AM, Fallet V (2019) Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev 28(153):190058. 10.1183/16000617.0058-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tay SH, Toh MMX, Thian YL, Vellayappan BA, Fairhurst AM, Chan YH, Aminkeng F, Bharwani LD, Huang Y, Mak A, Wong AS (2022) Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: a case series of 25 patients and review of the literature. Front Immunol 13:807050. 10.3389/fimmu.2022.807050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Tian T, Zhang Y, Zhou S, Hu P, Zhang J (2021) Age-Associated Changes in Adverse Events Arising From Anti-PD-(L)1 Therapy. Front Oncol 11:619385. Published 2021 May 13. 10.3389/fonc.2021.619385 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request. No datasets were generated or analysed during the current study.