Abstract
ErbB3 is markedly overexpressed in breast cancer cells and is associated with resistance and metastasis. Additionally, ErbB3 expression levels are positively correlated with low densities of tumor-infiltrating lymphocytes, a marker of poor prognosis. Consequently, ErbB3 is a promising therapeutic target for cancer immunotherapy. Here, we report the generation of ErbB3-targeted chimeric antigen receptor (CAR)-modified natural killer (NK) cells by transducing cord blood-derived primary NK cells using vsv-g envelope-pseudotyped lentiviral vectors. Transduced cells displayed stable CAR-expressing activity and increased cytotoxicity against ErbB3-positive breast cancer cell lines. Furthermore, anti-ErbB3 (aErbB3) CAR-NK cells strongly reduced the tumor burden in the SK-BR-3 xenograft mouse model without observable side effects. These findings underscore the potential of aErbB3 CAR-NK cells as targeted immunotherapy for ErbB3-positive breast cancer, suggesting a promising alternative to conventional treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03923-y.
Keywords: Chimeric antigen receptor (CAR), Natural killer (NK) cells, Breast cancer, ErbB3
Introduction
Chimeric antigen receptor (CAR)-T cell therapy has achieved remarkable success against relapsed or refractory hematologic malignancies, ultimately leading to the approval of six CAR-T products by the United States Food and Drug Administration [1–3]. Despite these advancements, CAR-T cell therapies encounter significant limitations in clinical settings, including immunotoxicity issues such as cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and hemophagocytic lymphohistiocytosis-like toxicity [4–6]. Additionally, the complex and time-consuming manufacturing process further complicates their application [7].
CAR natural killer (NK) cell therapy offers a promising alternative to CAR T cell therapy by addressing several limitations while potentially providing additional benefits [8]. CAR-NK cells exploit the distinct characteristics of NK cells and enable them to target various stress-related factors while minimizing the risk of immunotoxicity [9–11]. Unlike T cells that secrete interleukin (IL)-2 upon activation, NK cells secrete interferon (IFN)-γ, thus making it a relatively low-toxicity therapeutic option [12]. Furthermore, CAR-NK cells can be derived from both autologous and allogeneic sources, thereby addressing the manufacturing challenges associated with CAR-T therapies and facilitating patient-specific treatments and “off-the-shelf” solutions [13, 14]. Therefore, we aimed to develop CAR-NK cells capable of targeting tumor-associated antigens in solid cancers by focusing on NK cells, as this approach could adequately compensate for the limitations of T cells.
The ErbB family of receptors, including epidermal growth factor receptor (EGFR), ErbB2, ErbB3, and ErbB4, play a crucial role in the development and progression of various cancers [15, 16]. These plasma membrane-embedded receptors form active homo- and/or heterodimers upon ligand binding that then initiate MEK/MAPK and phosphatidylinositol 3-kinase (PI3K)/AKT signaling through tyrosine kinase activities [17]. Despite the remarkable clinical efficacy of antibody therapies such as ERBITUX and HERCEPTIN targeting EGFR and ErbB2, many patients do not respond to these treatments and eventually develop resistance [18–21]. To address these limitations, combination therapies targeting multiple ErbBs have been explored. However, continuous exposure to EGFR- or ErbB2-targeted treatments or to chemotherapeutics such as paclitaxel and tamoxifen often leads to the activation of ErbB3/PI3K/Akt bypass signaling through increased NRG1 expression, thus inducing resistance to these therapies [22–24]. Additionally, ErbB3 overexpression in various cancers, including head and neck small cell, breast, lung, gastric, ovarian, colon, prostate, and bladder cancers, is associated with poor survival rates and cancer recurrence [24, 25]. Thus, targeting ErbB3 is a promising therapeutic approach to overcome resistance mechanisms, particularly in combination with existing treatments.
In this study, we developed IL-15-secreting anti-ErbB3 (aErbB3) CAR-NK cells from umbilical cord blood mononuclear cells. The use of cytokine-releasing CAR constructs is a well-established method known to improve the persistence and functional activity of CAR immunotherapy [26, 27]. The developed aErbB3 CAR-NK cells exhibited robust, target-dependent cytotoxicity against ErbB3-expressing breast cancer cells both in vitro and in vivo. Notably, a single administration of aErbB3 CAR-NK cells resulted in sustained inhibition of tumor growth in animal models. Our findings showed the exceptional antitumor efficacy of ErbB3-targeted CAR-NK cells and suggest their potential for clinical immunotherapy in patients with ErbB3-positive breast cancer with limited treatment options.
Materials and methods
Production of CAR-NK cells using cord blood mononuclear cells
This study was approved by the Dong-A Institutional Review Board (approval number: 2–1,040,709AB-N-01–202302-BR-001-02), and all participants were provided written informed consent. Cord blood was centrifuged to obtain mononuclear cells using Lymphoprep™ (STEMCELL Technologies, British Columbia, Canada) and then cryopreserved according to the manufacturer’s instructions. NK cells were cultured in CTS™ AIM V™ SFM medium (Gibco, Billings, MT, USA) supplemented with 5% human platelet lysate (Helios, Sarasota, FL, USA) and 1X Antibiotic–Antimycotic (Gibco). NK cells were cultured with IL-2 (PeproTech, Cranbury, NJ, USA), IL-18 (PeproTech), and IL-21 (PeproTech) for 7 days and seeded at 1 × 106 cells/well into 24-well plates. The next day, the cells were pre-treated with 8 µM BX795 (MedChemExpress, Monmouth Junction, NJ, USA) for 30 min in a CO2 incubator. The lentivirus encoding CAR was then mixed with 8 µg/mL of polybrene (Santa Cruz Biotechnology, Dallas, TX, USA) and added to the cells at a multiplicity of infection of 10. Plates were centrifuged at 32 °C and 1000×g for 1 h to enhance viral integration.
Lentiviral vector construction and lentivirus production
We modified the pCDH-MSCV-MCS-EF1-copGFP lentiviral vector backbone (System Biosciences, Palo Alto, CA, USA) by removing the EF1 promoter and GFP. Subsequently, anti-CD19 (aCD19) and aErbB3 CAR constructs were cloned into multiple cloning-site regions. For the co-expression of IL-15 with CAR, the IL-15 sequence was linked to the aCD19 CAR and aErbB3 CAR constructs using a P2A sequence. The aErbB3 single-chain variable fragment (scFv) sequence was provided by ISU Abxis [28] (Seongnam, Republic of Korea). Lentiviral vectors were transfected into Lenti-X™ 293 T cells along with the helper plasmids pRRE (Addgene, Watertown, MA, USA), pRev (Addgene), and pMD2.G (Addgene) using D-fection reagent (Lugen Sci, Bucheon, Republic of Korea). Supernatants were collected 48 h after infection, filtered using a Minisart syringe filter (0.45 µm) (Sartorius, Göttingen, Germany), and concentrated using a Lenti-X™ concentrator (Takara Bio, Shiga, Japan) following the manufacturer’s protocol. The titers of the lentiviral particles were measured using a qPCR Lentivirus Titer Kit (Abcam, Cambridge, UK).
Cell lines
BT-474, MDA-MB-231, MDA-MB-453, OVCAR-3, Panc-1, and SK-BR-3 cells were purchased from the Korean Cell Line Bank (Seoul, Republic of Korea). Lenti-X™ 293 T cells were purchased from Takara Bio. All cell lines, except for Lenti-X™ 293 T and Panc-1, were cultured in RPMI-1640 medium (Welgene, Korea) supplemented with 10% fetal bovine serum (Gibco) and 1X antibiotic–antimycotic (Gibco). Lenti-X™ 293 T and Panc-1 cells were cultured in Dulbecco’s modified Eagle’s medium (Welgene) supplemented with 10% fetal bovine serum (Gibco) and 1X antibiotic–antimycotic (Gibco). All cells were incubated in a humidified 5% CO2 incubator at 37 °C and tested for mycoplasma contamination using the e-Myco™ Mycoplasma PCR Detection Kit (iNtRON Biotechnology, Seongnam, Republic of Korea) according to the manufacturer’s instructions.
Cytotoxicity assay
Each target cell was stained with CFSE (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions. Effector, aErbB3 CAR-NK, aCD19 CAR-NK and target cells were seeded at the indicated effector cell: target cell ratios for each cell line and co-cultured at 37 °C in a 5% CO2 incubator for 4 h. Thereafter, the cells were harvested, stained with fixable viability dyes (eBioscience™) to detect dead cells according to the manufacturer’s instructions and analyzed via flow cytometry. Dead target cells were identified in the CFSE+FVD+ region.
Degranulation assay
Target and effector cells (1 × 105 cells each) were co-cultured at a 1:1 ratio and at 37 °C in a 5% CO2 incubator for 4 h. GolgiStop™ Protein Transport Inhibitor (BD Biosciences, Franklin Lakes, NJ, USA) and FITC anti-human CD107a (BD Biosciences) were added according to the respective manufacturer’s instructions during co-culture to stain intracellular cytokines. After co-culture, the cells were stained for surface markers with the PE-Cy-7 anti-human CD56 antibody (BD Biosciences) and then fixed and permeabilized using a fixation/permeabilization solution (BD Biosciences) following the manufacturer’s protocol. FACS analysis was performed after the cells were washed to identify CD56+ cells expressing intracellular CD107a (CD56+CD107a+).
Quantification of cytokines using enzyme-linked immunosorbent assay (ELISA)
IFN-γ and IL-15 secreted by NK cells were quantified using ELISA. To assess IL-15 secretion, NK cells (1 × 106 cells/mL) were seeded into 24-well plates and incubated for 3 days at 37 °C in 5% CO2. The supernatants were collected for ELISA. To assess IFN-γ secretion, transduced NK cells (1 × 105) were co-cultured with target cells at 1:1 ratio in 96-well plates (200 µL/well) for 24 h at 37 °C in 5% CO2. ELISA assays for IFN-γ (Cloud-Clone Corporation, Katy, TX, USA) and IL-15 (Abcam) were performed according to the manufacturer’s instructions.
Bioinformatics analysis
Transcriptomic and proteomic data of breast cancer patients were obtained from The Cancer Genome Atlas Breast Cancer (TCGA-BRCA) database. This database was also used to determine the correlation between ErbB3 expression and T cell infiltration using Spearman’s and Pearson’s analyses. Breast cancer patient cohort datasets (GSE14999, GSE22384, GSE86374, GSE45436, GSE54236, and GSE76427) from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) were used as the BRCA validation sets. Statistical significance was set at P < 0.05.
Immunohistochemical staining
The Human Protein Atlas (HPA; http://www.proteinatlas.org/, accessed on May 2, 2024) was used to obtain immunohistochemical images and staining scores for ErbB3.
Animal study
Six-week-old female NOD-Prkdcem1Baek Il2rgem1Baek (NSGA) mice were obtained from GEM Biosciences Inc. (Cheongju, Republic of Korea). All animal experiments were conducted in accordance with the protocols approved by the Dong-A University Institutional Animal Care and Use Committee (DIACUC-23-05). NSGA mice (n = 5 per group) were used as animal models. The mice were subcutaneously injected with 1 × 107 SK-BR-3 breast cancer cells. Five days later, mice received intravenous injections of 5 × 106 aErbB3 CAR-NK cells and 5 × 106 aCD19 CAR-NK cells in 100 μL phosphate-buffered saline (PBS) via the tail vein. Both CAR-NK cell types were generated from a single cord blood donor. Tumor size was monitored twice weekly using a caliper. Three weeks after tumor establishment, the mice were euthanized to collect tumor tissue.
Flow cytometric analysis
Flow cytometry was performed to analyze the phenotype of the CAR-NK cells in the CD3-negative and CD56-positive populations. ErbB3 CAR expression in transduced NK cells was analyzed by flow cytometry using ErbB3-biotinylated protein (ACROBiosystems, Newark, DE, USA) for primary staining and anti-biotin-PE (Miltenyi Biotec, Gaithersburg, MD, USA) for secondary staining. aCD19 CAR expression in transduced NK cells was analyzed by flow cytometry using anti-FMC63 (ACROBiosystems) for primary staining and anti-mouse IgG1-PE-Cy-7 for secondary staining (BioLegend). ErbB3 expression in the cell lines was analyzed by flow cytometry using anti-ErbB3-PE antibody (BioLegend) to identify ErbB3-positive cells. The antibodies used in this study are listed in Table S1, and the specific staining methods are described in Supplementary Materials and Methods (Supplementary Information).
Statistical analysis
All experiments were replicated at least three times. Groups were statistically compared by performing the unpaired two-tailed Student’s t-test to calculate P-values using GraphPad Prism 9.0 (GraphPad Software, USA) unless otherwise stated. For in vivo experiments, a two-way ANOVA was performed to compare tumor volume between groups over time. Additionally, one-way ANOVA was used for the analysis of Figs. 5C, F, H, to compare multiple groups within the same dataset. Data are presented as the mean ± standard deviation unless otherwise stated. Statistical significance was set at P < 0.05.
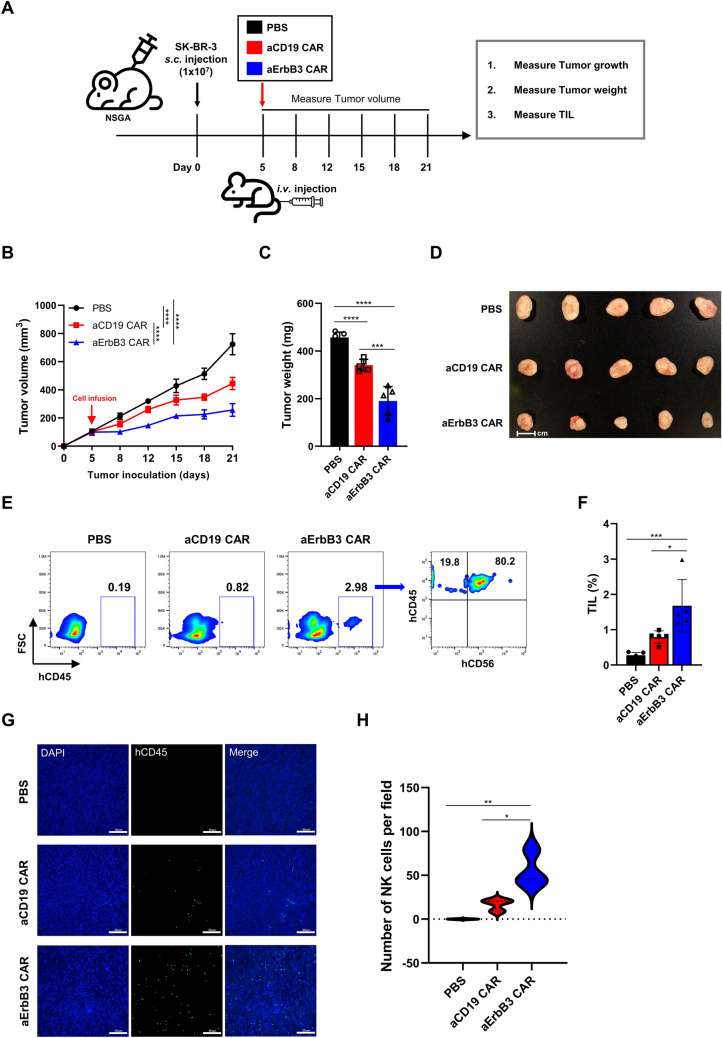
Fig. 5.
Antitumor effects of aErbB3 CAR-NK cells against SK-BR-3 in vivo. A Timetable of an in vivo experiment (n = 5 per group). Female NSGA mice (6 weeks old) were subcutaneously injected with 1 × 107 cells of the breast cancer cell line SK-BR-3. Five days later, they received 5 × 106 cells of aCD19 and aErbB3 CAR-NK cells. B SK-BR-3 tumor growth curve as calculated from caliper measurements of tumor growth twice weekly after subcutaneous injection. C Bar graph of tumor weight. D Image of excised tumors in mice. Scale bar, 1 cm. E Representative flow cytometric dot plots showing hCD45+ and hCD56+ CAR-NK cells infiltrating the tumor tissue in each treatment group. F Bar graph showing the percentage of tumor-infiltrating lymphocytes (TILs; hCD45+ cells) in each group. G Immunofluorescence staining of tumor sections to visualize CAR-NK cell infiltration. Tumor sections were stained with DAPI (blue) for nuclei and hCD45-FITC (green) for human CAR-NK cells. Images represent 400× magnification (scale bar, 50 μm). H Quantification of CAR-NK cell infiltration based on FITC-positive (hCD45.+) dots counted in 400× fields across three samples per group. Data are presented as the mean ± SD.*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 based on the results of a two-way ANOVA for the (B) and one-way ANOVA (C, F, H)
Results
ErbB3 is highly expressed in human breast cancer tissue and is associated with tumor-infiltrating lymphocytes (TILs)
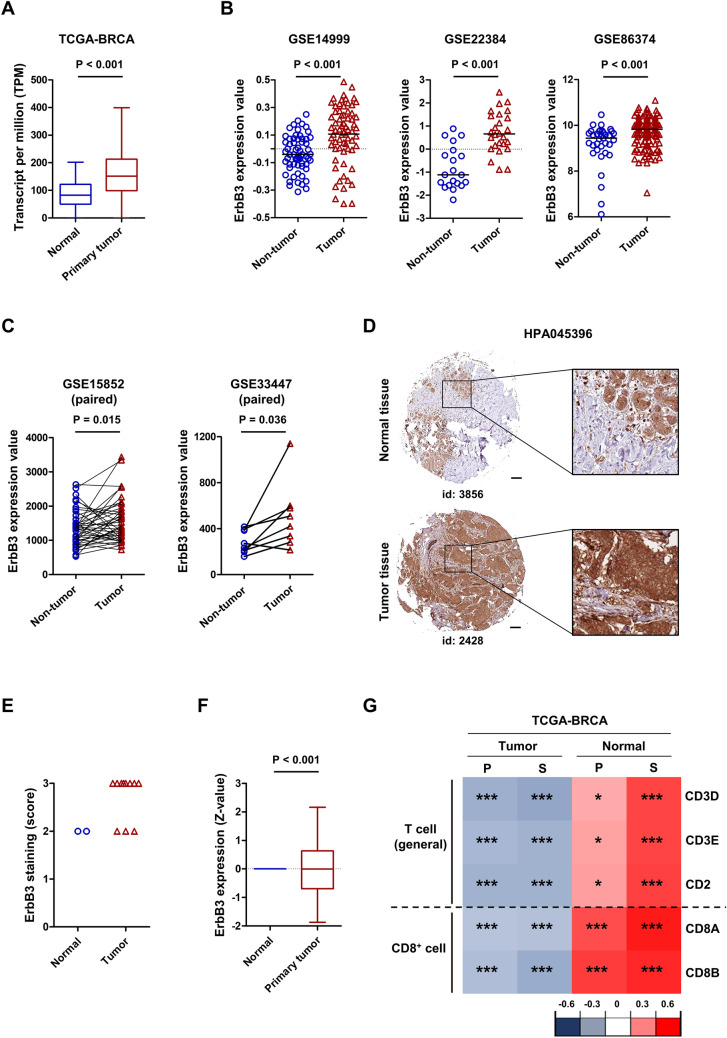
Given the established potential of ErbB3 as a therapeutic target for breast cancer, we analyzed its expression using the TCGA-BRCA database and observed that ErbB3 was upregulated in the tumor group compared to levels in the normal group (Fig. 1A). ErbB3 was significantly upregulated in breast tumors than in non-tumor tissues and BRCA-paired tumors (Fig. 1B, C). Using the HPA database, we further evaluated ErbB3 protein expression in normal and breast tumor tissues. As presented in Fig. 1D, E, breast tumor tissues exhibited higher ErbB3 protein expression than did their corresponding normal tissues, and this was consistent with the ErbB3 protein levels observed in the clinical proteomic tumor analysis consortium (CPTAC)-BRCA database (Fig. 1F). ErbB3 is also known for its role in mediating resistance to targeted therapies and is associated with poor prognosis in ErbB2-positive breast cancer patients [29, 30]. To determine whether ErbB3 was associated with “cold tumor” characteristics, we assessed the association between ErbB3 expression and T cell infiltration using the TCGA-BRCA database. ErbB3 expression was negatively correlated with T cells (CD3D, CD3E, and CD2) and CD8+ T cells (CD8A and CD8B) in breast tumors, while it was positively correlated in normal tissues (Fig. 1G). Collectively, these results imply that ErbB3 is a potential therapeutic target in tumors with immune escape properties affecting CD8+ T cell immunity in patients with breast cancer.
Fig. 1.
Impact of human ErbB3 expression in breast cancer. A Expression of ErbB3 in breast cancer according to the TCGA-BRCA database (normal n = 114, tumor n = 1097). B Validation of ErbB3 expression in cohorts from the GEO datasets, including GSE14999 (normal n = 61, tumor n = 68), GSE22384 (normal n = 20, tumor n = 26), GSE86374 (normal n = 35, tumor n = 124), C GSE15852 (normal n = 43, tumor n = 43), and GSE33447 (normal n = 8, tumor n = 8). D Representative immunohistochemical images and E staining scores of ErbB3 in breast tumor tissues and normal tissues according to the HPA. F Expression of ErbB3 protein according to the CPTAC-BRCA database. G Correlation between ErbB3 expression and T cell markers in the tumor and normal groups according to the TCGA-BRCA database. Association analysis was performed using Pearson’s (P) and Spearman’s (S) correlation. Statistical significance: *P < 0.05; **P < 0.01; ***P < 0.001 based on the results of Student’s t-test
Generation of ErbB3-specific CAR-NK cells expressing IL-15
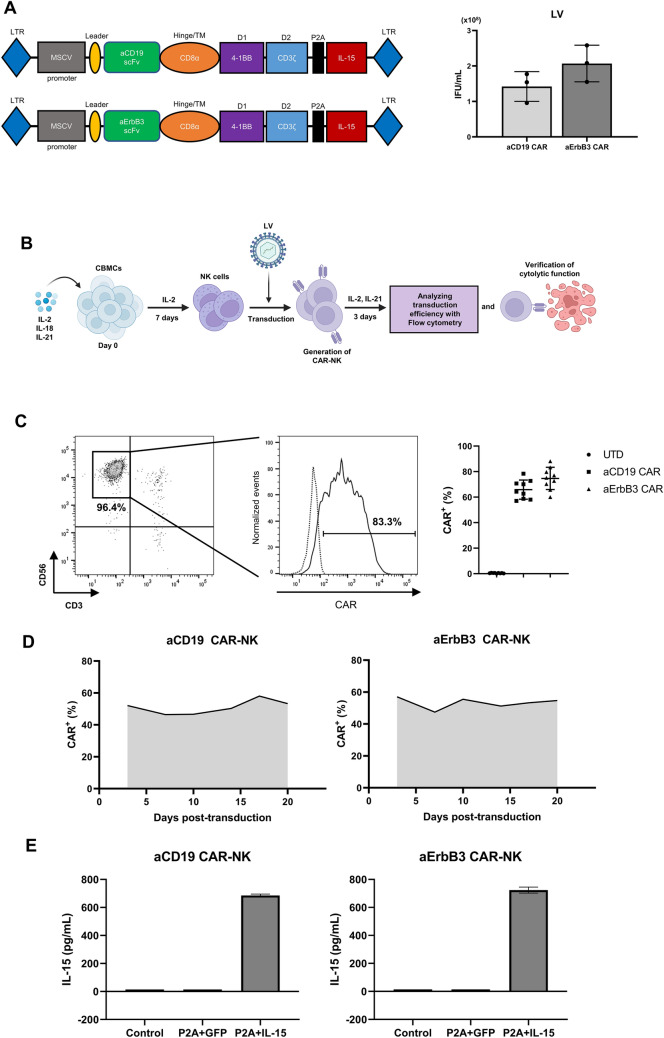
Considering the challenges of treating cold tumors characterized by high levels of immunosuppression and low T cell infiltration, we generated a construct comprising ErbB3-targeted CAR-NK cells with an immune modulator. The aErbB3 scFv sequence was inserted into the lentiviral vector backbone encoding a second-generation CAR designed to express IL-15, a cytokine that promotes antitumor effector cell proliferation, longevity, and cytotoxicity within the tumor microenvironment [31]. Additionally, we generated an IL-15-producing, aCD19-targeted CAR by integrating aCD19 scFv into the same construct (Fig. 2A, left panel). After constructing lentiviral vectors for aErbB3 and aCD19 CAR, we produced the corresponding lentiviruses and quantified their titers by performing a quantitative PCR. Both viruses were produced in sufficient quantities to transduce the NK cells (Fig. 2A, right panel). Transduced NK cells were expanded in cytokines for 3 days, and CAR-expressing NK cells were analyzed by flow cytometry (Fig. 2B). Transgene integration by the vsv-g envelope-pseudotyped lentivirus resulted in similar transduction rates between the aCD19 CAR-IL-15 and aErbB3 CAR-IL-15 vectors (Fig. 2C). CAR expression was stably maintained for 20 days post-transduction (Fig. 2D), and both groups exhibited similar levels of IL-15 secretion (Fig. 2E). We confirmed the high purity of the NK cells following transduction using flow cytometry (Supplementary Fig. S1). Taken together, our results demonstrate the successful development and validation of ErbB3-targeted CAR-NK cells characterized by stable CAR expression, efficient IL-15 co-expression, and a steady increase in NK cell purity over time.
Fig. 2.
Manufacture and validation of aErbB3 CAR-NK cells using cord blood-derived NK cells. A Schematic diagram depicting the construction of the second-generation aCD19 CAR and aErbB3 CAR encoded in a pCDH lentiviral vector with an MSCV promoter (left). Bar graph of the titer of the lentivirus produced (right). B Schematic representation of CAR-NK production from cord blood-derived NK cells and subsequent evaluation. C Representative flow cytometric analysis of CAR expression in CD3−CD56.+ subsets 3 days post-transduction (left). Summary data is presented as a scatter plot of the percentage of CAR expression in the non-transduced (UTD) and transduced cells (n = 9, mean ± SD) (right). D Determination of maintaining aCD19 CAR and aErbB3 CAR expression on transduced NK cells. E ELISA analysis of IL-15 secretion in aCD19 CAR-NK cells and aErbB3 CAR-NK cells. Control: Culture medium of non-transduced NK cells, P2A + GFP: culture medium of NK cells transduced with CAR lentivirus (P2A + GFP). P2A + IL-15: culture medium of NK cells transduced with CAR lentivirus (P2A + IL-15)
aErbB3 CAR-NK reacts specifically against ErbB3-positive cells
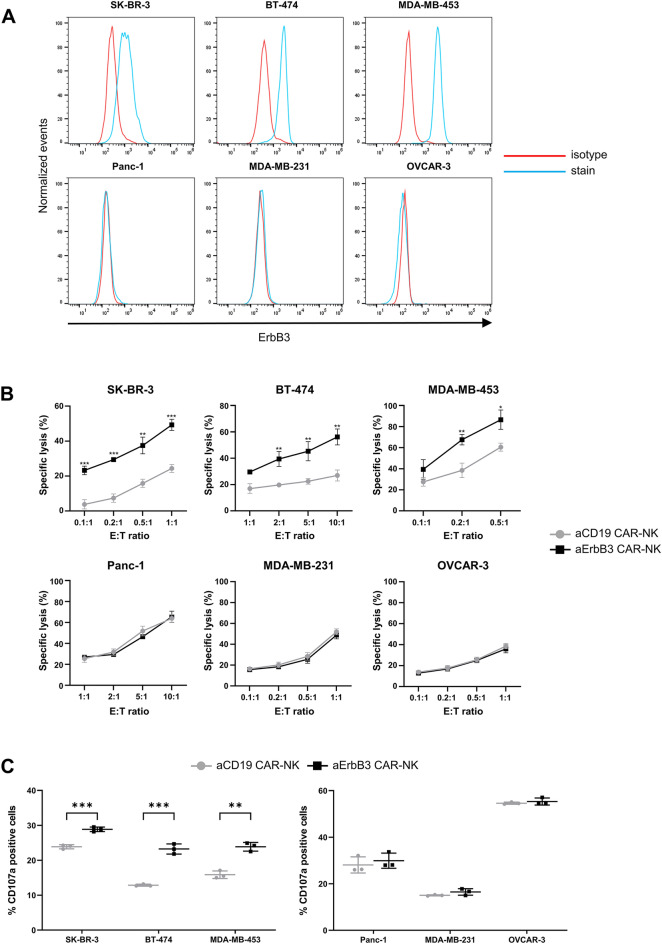
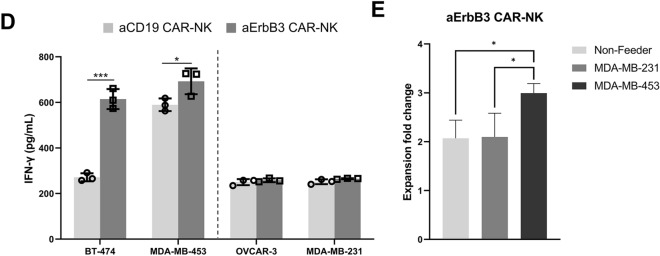
To analyze the target-specific cytotoxicity of aErbB3 CAR-NK cells, we screened ErbB3-positive target (SK-BR-3, BT-474, and MDA-MB-453) and ErbB3-negative (Panc-1, MDA-MB-231, and OVCAR-3) cell lines (Fig. 3A) and performed cytotoxicity assays on the selected cell lines to assess the antigen-specific antitumor activity of aErbB3 CAR-NK cells. Cytotoxicity was significantly increased in ErbB3-positive breast cancer cell lines, whereas no significant effect was observed in the ErbB3-negative cell lines (Fig. 3B). aCD19 CAR-NK cells efficiently lysed CD19-positive cells such as Raji, RS4;11, and Nalm-6 cells (Supplementary Fig. S2). For the NK cell degranulation assay, flow cytometry revealed significantly increased CD107a expression in aErbB3 CAR-NK cells co-cultured with ErbB3-positive cell lines but not with ErbB3-negative cell lines or in the control aCD19 CAR-NK cells (Fig. 3C). Additionally, IFN-γ production, as measured by ELISA was significantly increased upon co-culture with ErbB3-positive cell lines (Fig. 3D), and this was consistent with the observed increase in cytotoxicity and CD107a expression. aErbB3 CAR-NK cells were cultured under various conditions to assess their antigen-specific proliferation. No significant proliferation of CAR-NK cells was observed in the non-feeder group or the cells co-cultured with ErbB3-negative MDA-MB-231 cells. However, significant proliferation of CAR-NK cells was observed only when co-cultured with ErbB3-positive cells (Fig. 3E). Collectively, our results indicate that aErbB3 CAR-NK cells exert potent cytolytic activity against ErbB3-positive cells via ErbB3 antigen-specific effectors.
Fig. 3.
Antitumor activity of aErbB3 CAR-NK cells in vitro. A Flow cytometric analysis of ErbB3 expression in various cell lines. B Cytotoxicity of aErbB3 CAR-NK cells and aCD19 CAR-NK cells against each ErbB3-positive and -negative cell line. Flow cytometric analysis of CFSE+/FVD+-stained target cells after 4 h of co-culture at the indicated effector: target (E:T) ratio. C CD107a expression in aErbB3 CAR-NK cells and aCD19 CAR-NK cells when co-cultured with their respective cells. Target and effector cells were co-cultured at a 1:1 ratio for 4 h, and this was followed by flow cytometric analysis. D Quantification of IFN-γ present in supernatants collected after 24 h of co-culture with target cells through ELISA. E Antigen-specific proliferation of aErbB3 CAR-NK cells co-cultured with X-ray-irradiated MDA-MB-231 cells (ErbB3−) and X-ray-irradiated MDA-MB-453 cells (ErbB3+) for 5 days (E:T ratio = 1:2). Data are presented as the mean ± SD of three independent experiments (B, C, D, E) using CBMC CAR-NK cells from three donors. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 based on the results of Student’s t-test
Phenotypic comparison of aCD19 and aErbB3 CAR-NK cells
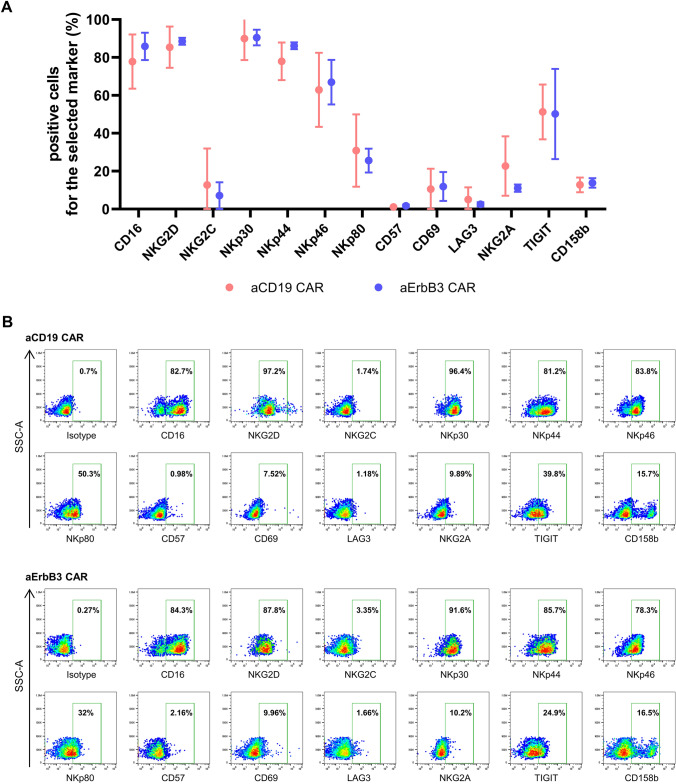
We conducted a comprehensive phenotypic analysis using flow cytometry to determine whether the enhanced functionality of aErbB3 CAR-NK cells resulted from the CAR-induced changes in cell phenotype. We evaluated the expression of key receptors (CD16, NKG2D, NKG2C, NKp30, NKp44, NKp46, NKp80, CD57, CD69, LAG3, NKG2A, TIGIT, and CD158b) in both aCD19 and aErbB3 CAR-NK cells and observed no statistically significant differences between the groups (Fig. 4A, B). Particularly, TIGIT showed relatively high expression, whereas NKG2A and CD158b exhibited low expression. Consequently, the lack of significant changes in receptor expression suggests that the increased cytotoxic activity of the CAR-NK cells is primarily attributable to the CAR construct.
Fig. 4.
Phenotyping of aCD19 and aErbB3 CAR-NK cells. A Comparative analysis of surface marker expression in aCD19 and aErbB3 CAR-NK cells. B Representative density plots illustrating the expression of these markers in aCD19 and aErbB3 CAR-NK cells. Data are presented as the mean ± SD of three independent experiments
aErbB3 CAR-NK cells inhibit the progression of SK-BR-3-derived tumors in vivo
Then, we investigated the in vivo efficacy of aErbB3 CAR-NK cells in controlling the growth of ErbB3-positive cells. SK-BR-3 cells were used to establish a mouse model. On day 5 post-tumor injection, the tumor-bearing mice were intravenously injected with PBS, aCD19 CAR-NK cells, or aErbB3 CAR-NK cells (5 × 106 cells/mouse). The tumor size was measured twice weekly using calipers until day 21, and the tumors were then excised (Fig. 5A). Both the aCD19 and aErbB3 CAR-NK cell groups exhibited significantly reduced tumor sizes and volumes compared to those of the PBS group (Fig. 5B–D). After tumor resection, we performed tumor dissociation and evaluated the infiltration level of CAR-NK cells in the tumor by flow cytometry. The results showed that the infiltration rate of aErbB3 CAR-NK cells was higher than that of the aCD19 CAR group (Fig. 5E, F). We also visually quantified the infiltrated CAR-NK cells in the tumor using immunofluorescence staining (Fig. 5G, H). The group treated with aCD19 CAR-NK cells exhibited a notable reduction in tumor size and volume compared to that of the PBS group, thus indicating that the innate immune activity of NK cells inhibited tumor growth. However, the aErbB3 CAR-NK cell group exhibited an even more pronounced tumor suppressive effect than the aCD19 CAR-NK cell group, thus suggesting the critical role of specific CAR-targeted tumor antigen recognition in enhancing antitumor efficacy. In summary, our study demonstrated the successful development and validation of aErbB3 CAR-NK cells that exhibited potent antitumor activity both in vitro and in vivo. These findings underscore the potential of aErbB3 CAR-NK cell therapy as a promising strategy that targets ErbB3-positive breast cancer cells, thus offering a novel approach for improving therapeutic outcomes in patients with ErbB3-positive breast cancer.
Discussion
Breast cancer is the most commonly diagnosed cancer in women worldwide and the leading cause of cancer deaths [32]. According to global cancer statistics, 2.3 million new cases were reported in 2020, making it the most common cancer and accounting for 11.7% of all cancers [33]. Breast cancer is a heterogeneous disease characterized by distinct molecular subtypes with different phenotypes, including ErbB2 activation, hormone receptor (estrogen and progesterone receptor) activation, and BRCA mutations [34]. To date, the most studied breast cancer-targeted therapies are for ErbB2-positive breast cancer, which accounts for approximately 20% of all breast cancer diagnoses and is characterized by a high recurrence rate and aggressive behavior [35]. Targeted therapies such as trastuzumab, pertuzumab, and lapatinib have significantly improved patient outcomes [36]. However, these therapies are often hindered by challenges such as drug resistance and limited efficacy in certain patient populations [29, 30, 36, 37].
ErbB3 mediates resistance to ErbB2-targeted therapeutics, as its activation can bypass ErbB2 inhibition and maintain pro-survival signaling pathways [30, 38, 39]. High ErbB3 expression levels are associated with poor response to ErbB2-targeted therapeutics and poor prognosis in patients with ErbB2-positive breast cancer [40]. The relationship between ErbB2 and ErbB3 in breast cancer is intertwined. ErbB2-overexpressing tumors often have increased ErbB3 expression [41], and the formation of ErbB2/ErbB3 heterodimers leads to enhanced signaling through the PI3K/Akt pathway that promotes cell survival and proliferation [38, 42]. The involvement of ErbB3 in drug resistance is not limited to ErbB2. ErbB3 also contributes to resistance to gefitinib, an EGFR-targeted therapy [43]. This interaction between ErbB2 and ErbB3 emphasizes the importance of targeting both receptors in therapeutic strategies for ErbB2-positive breast cancer [44]. In summary, although ErbB2 and ErbB3 are distinct members of the EGFR family, their interactions and signaling pathways play important roles in breast cancer development, progression, and response to therapy [40, 45]. Understanding the differences and interactions between ErbB2 and ErbB3 is essential for designing effective targeted therapies and improving treatment outcomes for patients with breast cancer.
In this study, we focused on obtaining cord blood-derived NK cells targeting human ErbB3. NK cells are highly cytotoxic effector cells that kill their targets in a non-antigen-specific manner without causing graft-versus-host disease [11, 46]. Cord blood is an easily accessible and ethically uncontroversial source of allogeneic and autologous NK cells [47, 48]. Our NK cell culture method produced high-purity NK cells and successfully transduced CARs. Expression rates were approximately 60–80%, and expression was maintained for up to 20 days post-transduction. CARs demonstrate their functionality by inducing specific lysis in cell lines expressing each target antigen. Further analysis to validate the specific cytotoxicity of CARs revealed no phenotypic differences between non-transfected cells and those transfected with CARs, thus suggesting that lentiviral transduction does not compromise genomic stability (data not shown). To determine the antitumor effects of aErbB3 CAR-NK cells in vivo, we established a xenograft model using NSGA mice. aErbB3 CAR-NK cells exhibited a higher antitumor effect than aCD19 CAR-NK cells without significant side effects such as spinal curvature or weight loss (Supplementary Fig. S3). As both groups were designed to produce IL-15, separate cytokine injections were unnecessary. Although they produce similar levels of IL-15, their antitumor effects differ noticeably. This highlights the critical role of targeting appropriate antigens in CAR-NK cell therapy for solid cancers. Nevertheless, even aErbB3 CAR-NK cells did not exhibit complete tumor remission, presumably due to the observation that the characteristics of solid tumors prevent the infiltration of large amounts of aErbB3 CAR-NK cells. Analysis of aErbB3 CAR-NK cells infiltrating the tumor showed that only a small percentage of cells infiltrated, with an average of 1.67% ± 0.74% (Fig. 5F). Additionally, the xenograft model of immunodeficient mice helps assess the direct cytotoxic effect of redirected effector cells on cancer cells but does not allow for the evaluation of adaptive immune responses [49].
Solid cancers are characterized by the development of a tumor microenvironment that exhibits multiple mechanisms of immune resistance [50]. To achieve the potential of CAR NK-cell therapy, strategies to increase migration and infiltration into solid tumors are required. For CAR NK-cell therapies targeting solid tumors to be successful, they must not be designed to simply target tumor antigens. They must also seek to convert “cold tumors” into “hot tumors” [51, 52]. Treating a “cold tumor” is more difficult than treating a “hot tumor”, as it possesses a significantly lower level of TILs and many various immune cells involved in immunosuppression [53]. The TIL level is essential due to the observation that it correlates with patient prognosis not only in breast cancer but also in various solid tumors [54, 55]. We observed that the level of ErbB3 expression correlates with the status of the tumor as a “cold” or “hot” tumor; therefore, we predict that it is important to regulate ErbB3 levels in breast cancer. However, the exact causal relationship between the levels of ErbB3 and TILs in breast cancer remains unknown, thus necessitating further research.
Collectively, our findings demonstrate that aErbB3 CAR-NK cells specifically target and inhibit cancer cells, suggesting that ErbB3-targeted immunotherapy may serve as a viable alternative for treating breast cancer. However, for clinical applications, it is essential to address concerns regarding safety and scalability. Phenotypic analysis (Fig. 4) revealed low expression of inhibitory receptors such as NKG2A and CD158b, raising the possibility of off-target effects on normal ErbB3-positive cells. Although TIGIT showed relatively high expression, suggesting potential tolerance mechanisms, further studies are needed to fully assess these interactions. Additionally, the lack of KIR genotyping in this study limits our ability to correlate specific KIR profiles with observed cytotoxicity, highlighting the need for future research to explore the balance between activating and inhibitory signals in CAR-NK cells. Advanced CAR designs, such as logic-gated CARs, may address these limitations by enhancing specificity and minimizing off-target effects [56]. Furthermore, our results demonstrated that CAR-NK cells could be expanded using K562-based feeder cells, achieving up to 806.6 ± 120.67-fold expansion (Supplementary Fig. S4). Nevertheless, further optimization and validation are required to ensure the reproducibility of these results for clinical-scale application.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dong-A University Hospital and its Cord Blood Bank for their support in this study.
Author contributions
SHK and JL designed the study. JL and JS conducted the experiments. JL, JS, WY, HC and S-HK performed data analysis. JL and JS prepared the drafts of the manuscript and figures. DJ, EC, SGJ, YSP, WCS, TDK, SL, HSL, HYL, JJK, TEK and JJP contributed to data extraction and provided feedback on the report. EYG and YHJ provided the input, edited, and approved the final version of the manuscript. All authors critically revised and approved the final manuscript.
Funding
This research received funding support from the ISU Abxis (ISU104-CAR-NK project). This work was also supported by a grant from the National Research Foundation (NRF-2023M3A9J4057877) in the Republic of Korea, and by the Dongnam Institute of Radiological & Medical Sciences funded by the Korean government (Grant no. 50593-2024).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juheon Lee and Jinhoo Song have contributed equally to this work.
Contributor Information
Jang-June Park, Email: jangjune.park@isu.co.kr.
Tae-Don Kim, Email: tdkim@kribb.re.kr.
Seok-Ho Kim, Email: cvaccine@dau.ac.kr.
References
- 1.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA et al (2010) Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116(20):4099–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H et al (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö et al (2017) Chimeric antigen receptor T cells in refractory B-Cell lymphomas. N Engl J Med 377(26):2545–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Teachey DT, Porter DL, Grupp SA (2015) CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125(26):4017–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanber K, Savani B, Jain T (2021) Graft-versus-host disease risk after chimeric antigen receptor T-cell therapy: the diametric opposition of T cells. Br J Haematol 195(5):660–668 [DOI] [PubMed] [Google Scholar]
- 6.Hines MR, Keenan C, Maron Alfaro G, Cheng C, Zhou Y, Sharma A et al (2021) Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy. Br J Haematol 194(4):701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine BL, Miskin J, Wonnacott K, Keir C (2017) Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev 4:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiossone L, Dumas PY, Vienne M, Vivier E (2018) Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18(11):671–688 [DOI] [PubMed] [Google Scholar]
- 9.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK et al (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105(8):3051–3057 [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Tian ZG, Zhang C (2018) Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin 39(2):167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskowski TJ, Biederstädt A, Rezvani K (2022) Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer 22(10):557–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Hu B, Stanley G, Harris ZM, Gautam S, Homer R et al (2023) IFN-γ is protective in cytokine release syndrome-associated extrapulmonary acute lung injury. Am J Respir Cell Mol Biol 68(1):75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heipertz EL, Zynda ER, Stav-Noraas TE, Hungler AD, Boucher SE, Kaur N, Vemuri MC (2021) Current perspectives on “off-the-shelf” allogeneic NK and CAR-NK cell therapies. Front Immunol 12:732135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegler EL, Zhu Y, Wang P, Yang L (2018) Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell 23(2):160–161 [DOI] [PubMed] [Google Scholar]
- 15.Hsu JL, Hung MC (2016) The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev 35(4):575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roskoski R Jr (2014) The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 79:34–74 [DOI] [PubMed] [Google Scholar]
- 17.Wang Z (2017) ErbB receptors and cancer. Methods Mol Biol 1652:3–35 [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345 [DOI] [PubMed] [Google Scholar]
- 19.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421(6924):756–760 [DOI] [PubMed] [Google Scholar]
- 20.Specenier P, Vermorken JB (2013) Cetuximab: its unique place in head and neck cancer treatment. Biologics 7:77–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ (1991) Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer 49(5):650–655 [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Gao L, Wang S, McManaman JL, Thor AD, Yang X et al (2010) Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-i receptor in breast cancer cells resistant to herceptin. Cancer Res 70(3):1204–1214 [DOI] [PubMed] [Google Scholar]
- 23.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J et al (2011) Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 3(99):99ra86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Lu A, Hu Z, Li J, Lu J (2024) ERBB3 targeting: a promising approach to overcoming cancer therapeutic resistance. Cancer Lett 599:217146 [DOI] [PubMed] [Google Scholar]
- 25.Bao Y, Cui J, Yue Y, Cao S, Li X, Liu L (2022) ERBB3 binding protein 1 promotes the progression of malignant melanoma through activation of the Wnt/ β-catenin signaling pathway. Cancer Cell Int 22(1):44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins ER, D’Souza RR, Klampatsa A (2021) Armored CAR T-cells: the next chapter in T-cell cancer immunotherapy. Biologics 15:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C et al (2023) CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer 22(1):20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong M, Yoo Y, Kim M, Kim JY, Cha JS, Choi MK et al (2021) A novel therapeutic Anti-Erb B3, ISU104 exhibits potent antitumorigenic activity by inhibiting ligand binding and ErbB3 heterodimerization. Mol Cancer Ther 20(6):1142–1152 [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Lyu H, Huang J, Liu B (2014) Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 13:105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445(7126):437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma S, Caligiuri MA, Yu J (2022) Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol 43(10):833–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M et al (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249 [DOI] [PubMed] [Google Scholar]
- 34.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P et al (2019) Breast cancer. Nat Rev Dis Primers 5(1):66 [DOI] [PubMed] [Google Scholar]
- 35.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182 [DOI] [PubMed] [Google Scholar]
- 36.Zhu K, Yang X, Tai H, Zhong X, Luo T, Zheng H (2024) HER2-targeted therapies in cancer: a systematic review. Biomark Res 12(1):16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Yang H, Yu X, Qin JJ (2022) Drug-resistant HER2-positive breast cancer: Molecular mechanisms and overcoming strategies. Front Pharmacol 13:1012552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiavue N, Cabel L, Melaabi S, Bataillon G, Callens C, Lerebours F et al (2020) ERBB3 mutations in cancer: biological aspects, prevalence and therapeutics. Oncogene 39(3):487–502 [DOI] [PubMed] [Google Scholar]
- 39.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP et al (2008) A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 68(14):5878–5887 [DOI] [PubMed] [Google Scholar]
- 40.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE (2003) The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 100(15):8933–8938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bièche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R (2003) Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer 106(5):758–765 [DOI] [PubMed] [Google Scholar]
- 42.Lyu H, Han A, Polsdofer E, Liu S, Liu B (2018) Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm Sin B 8(4):503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amin DN, Campbell MR, Moasser MM (2010) The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol 21(9):944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C et al (2012) HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res 72(10):2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spears M, Taylor KJ, Munro AF, Cunningham CA, Mallon EA, Twelves CJ et al (2012) In situ detection of HER2:HER2 and HER2:HER3 protein-protein interactions demonstrates prognostic significance in early breast cancer. Breast Cancer Res Treat 132(2):463–470 [DOI] [PubMed] [Google Scholar]
- 46.Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV (2021) Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol 14(1):73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M et al (2018) Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32(2):520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nham T, Poznanski SM, Fan IY, Vahedi F, Shenouda MM, Lee AJ et al (2018) Ex vivo-expanded natural killer cells derived from long-term cryopreserved cord blood are cytotoxic against primary breast cancer cells. J Immunother 41(2):64–72 [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Liao S, Xiao Z, Pan Q, Wang X, Shen K et al (2022) The development and improvement of immunodeficient mice and humanized immune system mouse models. Front Immunol 13:1007579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F et al (2019) The clinical role of the TME in solid cancer. Br J Cancer 120(1):45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S (2019) Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer 120(1):26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newick K, O’Brien S, Moon E, Albelda SM (2017) CAR T cell therapy for solid tumors. Annu Rev Med 68:139–152 [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Geng H, Liu Y, Liu L, Chen Y, Wu F et al (2023) Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm. 4(5):e343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brummel K, Eerkens AL, de Bruyn M, Nijman HW (2023) Tumour-infiltrating lymphocytes: from prognosis to treatment selection. Br J Cancer 128(3):451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savanur MA, Weinstein-Marom H, Gross G (2021) Implementing logic gates for safer immunotherapy of cancer. Front Immunol 12:780399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.