Abstract
Acute kidney injury (AKI) is associated with adverse hospitalization. Previous studies have reported that an elevated triglyceride glucose (TyG) index is significantly associated with the development of AKI in patients with cardiovascular disease, as well as in those undergoing surgery; however, the potential of the TyG index to predict AKI following neurotrauma remains unclear. Patients diagnosed with traumatic brain injury (TBI) in Chinese tertiary hospitals between January 2014 and December 2023 were included in this retrospective study. The outcome was the incidence of AKI. TyG was identified as an independent risk factor for AKI using logistic regression and propensity score matching (PSM). Finally, the association between TyG index and AKI was further assessed using multivariate logistic regression, restricted cubic spline (RCS) regression, and subgroup analysis. The present study enrolled 1,505 patients with TBI, of whom 66.45% were male, with an average age of 55.47 ± 17.32 years. The incidence of AKI was 9.4%. Multiple logistic regression analyses identified a relationship between the TyG levels and AKI risk. This relationship was retained after PSM. A significant positive correlation between TyG level and AKI was observed in all three models constructed using multivariate logistic regression. RCS regression analyses further indicated a linear increase in AKI risk with an increasing TyG index. In subgroup analyses, this correlation remained stable for the majority of the population but could be influenced by sex. TyG levels were positively correlated with the risk of AKI development in patients following TBI. As a predictive biomarker, the TyG index enables effective risk stratification and customization of management protocols to mitigate AKI in these patients, thus enhancing clinical outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84690-9.
Keywords: Insulin resistance, Triglyceride glucose index, Propensity score matching, Traumatic brain injury, Acute kidney injury, Gender differences
Subject terms: Medical research, Nephrology, Neurology
Introduction
Traumatic brain injury (TBI) is a severe condition with a high mortality rate of 30-40%, contributing to significant injury-related deaths globally1. In the United States, approximately 2.8 million individuals are affected by TBI annually, leading to around 56,000 fatalities, and these numbers are increasing2. Beyond neurological damage, TBI can lead to systemic complications, notably acute kidney injury (AKI), which exacerbates patient outcomes3.
Acute kidney injury (AKI) is a common systemic complication of TBI and a major clinical concern. Prior studies have reported that the incidence of AKI following TBI ranges from 9.2 to 24%, while its pathophysiological and therapeutic side effects may lead to patient deterioration4–6. Existing studies have confirmed that AKI is directly associated with prolonged hospital stay and increased ICU admission rates7,8, with the in-hospital mortality rate of critically ill patients with AKI reaching 62%9. Therefore, it is necessary for neurosurgeons and intensivists to identify high-risk patients with AKI at an early stage and administer nephrotoxic drugs appropriately or introduce clinical interventions early during treatment to improve the prognosis of patients with TBI.
Insulin resistance (IR), a multifaceted metabolic state, is identified as a critical risk factor contributing to both microvascular and macrovascular complications10. The Triglyceride Glucose Index (TyG), an emerging low-cost and easily accessible tool for assessing IR, is effective in predicting the risk of cardiovascular disease11, diabetes mellitus12, and associated complications13,14. Additionally, its straightforward nature and potential clinical applicability make it a promising candidate for evaluating IR and its consequences in a range of metabolic diseases. In a retrospective study involving 939 participants, TyG was identified as a risk factor for contrast-induced AKI following percutaneous coronary intervention in patients with type 2 diabetes. The model has a predicted area under the curve (AUC) of 0.785 and shows a strong performance in the prediction of re-cardiovascular diseases, which was confirmed in the validation set15. However, its predictive value as a potential biomarker for AKI following neurotrauma has not been fully explored.
This study aimed to elucidate the link between metabolic status and renal health, thereby addressing the current knowledge gap regarding the association between the TyG index and post-TBI-AKI. Fundamentally, this study explored a newly identified domain, the potential predictive utility of the TyG index for post-TBI renal health outcomes, aimed at assisting neurosurgeons in the early identification of patients at high risk for AKI, monitor renal function closely, and appropriately adjust the use of nephrotoxic medications.
Methods
Data selection
This observational study was conducted retrospectively at Fujian Provincial Hospital. This study included patients with TBI treated at the neurosurgery department between January 2014 and December 2023. All patients with TBI were admitted to the hospital within 48 h of injury. After examining the patient’s medical history and clinical presentation, the diagnosis of TBI was verified using CT and MRI, followed by neurosurgeons and intensivists offering logical treatment.
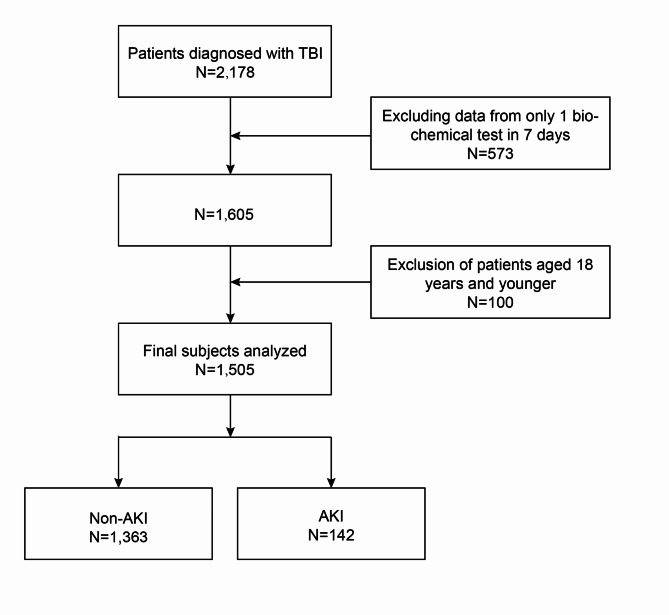
The inclusion criteria were as follows: (1) patients aged 18 years and above; (2) patients with a confirmed diagnosis of TBI based on CT or MRI findings. The exclusion criteria were as follows: (1) admission more than 24 h after the injury; (2) history of kidney-related disease, malignant hypertension, severe metabolic disease (including diabetic ketoacidosis, hypertonic hyperglycemia syndrome), severe liver disease, active cancer, or severe cardiovascular disease; (3) incomplete relevant clinical data; (4) history of surgery within 4 weeks before the TBI. (5) History of prior antiplatelet medications. This single-center retrospective analysis comprised 1505 patients (Fig. 1). This study was approved by the Ethics Committee of the Fujian Provincial Hospital (K2024-06-057), and adhered to the Declaration of Helsinki.
Fig. 1.
Participant selection flowchart.
Data collection
Through a literature review, This study collected clinical information and laboratory data of patients with TBI. These included demographic variables, such as sex, age, alcohol consumption, smoking, history of diabetes, and hypertension. General conditions on admission included temperature, heart rate, systolic blood pressure, diastolic blood pressure, occurrence of unequal pupils, and Glasgow Coma Scale (GCS) score. Laboratory tests included white blood cells, platelets, albumin, red blood cells, triglycerides, and fasting blood glucose measurements. Based on CT imaging, the type and location of anatomical injuries were recorded, including those in the frontal, temporal, parietal, and occipital lobes, as well as other positional, bilateral, epidural, subdural, subarachnoid, ventricular, skull base, and skull cap fractures. Treatment scenarios included antibiotics (vancomycin), dehydration drugs (mannitol and diuretics), and surgery. Adverse outcomes included ventilator use, ICU admission, length of hospital stay, and hospital death.
Definitions of exposed and ending variables
The primary outcome was AKI (stage I or higher) in patients with TBI. In this study, creatinine data from the first measurement on admission was used as a baseline, and a diagnosis of AKI was made if any one of the following three conditions were satisfied: (1) Urine output < 0.5 mL/kg/h within 6 h; (2) creatinine increase to more than 1.5 times the baseline value within 7 days; and (3) creatinine increase of ≥ 0.3 mg/dL within 48 h16. Because urine output in patients with brain injury is influenced by a variety of factors, it is difficult to document this in retrospective studies17. As such, only the serum creatinine level was used as a diagnostic indicator for AKI in this study. TyG was calculated using the following formula: TyG = Ln [fasting glucose × fasting triglycerides/2]18. The TyG was used as the exposure variable.
Statistical analysis
R version 3.6.4, SPSS version 25.0, and DecisionLink were used for the data analysis. This study initially performed one-way analysis of variance to assess the independent risk factors for AKI. Multivariate logistic regression was subsequently used to assess the correlation between these factors and AKI, and multiple covariate diagnoses were performed to determine whether TyG was an independent risk factor for AKI. To clarify the predictive efficacy of TyG, receiver operating characteristic (ROC) curves were generated to calculate the specificity, sensitivity, and area under the curve (AUC), based on which the optimal cut-off value was determined. Subsequently, TyG levels were transformed into categorical variables based on the optimal cutoff values. To minimize bias, propensity score matching (PSM) was used for variable adjustment. To further determine the correlation between TyG levels and AKI, three models were developed using multivariate logistic regression. Model 1 did not include any covariate adjustments; Model 2 involved adjustments for screening variables (age, temperature on admission, systolic blood pressure, leukocytes, TyG, platelets, hemoglobin, GCS, hypertension, history of diabetes mellitus, temporal lobe damage, subdural hemorrhage, bilateral brain injury, skull base, skull cap fracture, surgery, and use of antibiotics) that had a Statistically different on univariate analysis; and Model 3 was adjusted for all baseline characteristic variables except for adverse outcomes (ventilator use, ICU admission, length of stay, and in-hospital mortality). Restricted cubic spline (RCS) analysis was used to test the linear relationship between them. Finally, to clarify the heterogeneity of the relationship, subgroup analyses were performed based on age, sex, Glasgow Coma Scale (GCS) score, diabetes, and history of hypertension, and an interaction test was performed to test the stability of this relationship across subgroups.
Results
Characteristics of study population
This study included 1,505 patients diagnosed with TBI. Depending on whether AKI was present during the patients’ hospital stay, they were split into two groups. Of these, 1363 patients did not develop AKI, whereas 142 did (Table 1). The incidence of AKI was 9.4%, and the mean value of TyG was 7.02 ± 0.69. Furthermore, the average age of the patients was 55.47 ± 17.32 years, with males constituting 66.45% of the sample. Univariate analysis revealed that the AKI group was predominantly characterized by older age, higher body temperature on admission, higher systolic blood pressure, leukocytes, TyG, history of hypertension or diabetes mellitus, higher rate of damage to the temporal lobe, subdural hemorrhage, bilateral brain injury, skull base, skull cap fracture, increased need for surgery, and higher use of antibiotics than the non-AKI group. In addition, platelet and hemoglobin levels and GCS scores were lower in the AKI group.
Table 1.
Baseline characteristics of the non-AKI and AKI groups before and after propensity score matching.
| Variable names | Before PSM | P-value | After PSM | P-value | ||
|---|---|---|---|---|---|---|
| Non-AKI N = 1363 |
AKI N = 142 |
Non-AKI N = 258 |
AKI N = 139 |
|||
| Age | 54.84 ± 17.33 | 61.55 ± 16.04 | < 0.01 | 60.34 ± 16.61 | 61.40 ± 16.09 | 0.54 |
| Temperature | 36.7 ± 0.47 | 36.85 ± 0.71 | < 0.01 | 36.85 ± 0.65 | 36.85 ± 0.71 | 0.95 |
| Systolic blood pressure | 136.34 ± 20.86 | 141.45 ± 22.46 | 0.01 | 143.59 ± 23.81 | 141.08 ± 21.91 | 0.3 |
| Diastolic blood pressure | 78.75 ± 12.1 | 80.68 ± 13.9 | 0.07 | 81.58 ± 13.31 | 80.17 ± 12.30 | 0.3 |
| Blood platelet | 197.17 ± 60.79 | 179.92 ± 62.32 | < 0.01 | 178.95 ± 60.05 | 180.32 ± 62.73 | 0.83 |
| Haemoglobin | 127.14 ± 19.6 | 119.93 ± 23.77 | < 0.01 | 121.18 ± 22.75 | 120.11 ± 23.77 | 0.66 |
| Leucocyte | 11.42 ± 4.34 | 14.13 ± 6.45 | < 0.01 | 13.51 ± 5.46 | 13.99 ± 6.42 | 0.43 |
| Albumin | 40.02 ± 4.73 | 38.75 ± 6.82 | < 0.01 | 38.93 ± 6.02 | 38.73 ± 6.87 | 0.76 |
| TyG | 6.99 ± 0.68 | 7.29 ± 0.73 | < 0.01 | 7.12 ± 0.67 | 7.29 ± 0.74 | 0.02 |
| Gender (%) | 0.13 | 1 | ||||
| Female | 466 (34.19) | 39 (27.46) | 72 (27.91) | 39 (28.06) | ||
| Male | 897 (65.81) | 103 (72.54) | 186 (72.09) | 100 (71.94) | ||
| GCS (%) | < 0.01 | 0.98 | ||||
| Mild | 1086 (79.68) | 62 (43.66) | 118 (45.74) | 62 (44.60) | ||
| Moderate | 153 (11.23) | 33 (23.24) | 60 (23.26) | 33 (23.74) | ||
| Severe | 124 (9.10) | 47 (33.10) | 80 (31.01) | 44 (31.65) | ||
| Smoking (%) | 0.82 | 0.43 | ||||
| No | 1223 (89.73) | 126 (88.73) | 236 (91.47) | 123 (88.49) | ||
| Yes | 140 (10.27) | 16 (11.27) | 22 (8.53) | 16 (11.51) | ||
| Drinking (%) | 1 | 0.8 | ||||
| No | 1253 (91.93) | 130 (91.55) | 239 (92.64) | 127 (91.37) | ||
| Yes | 110 (8.07) | 12 (8.45) | 19 (7.36) | 12 (8.63) | ||
| Hypertension (%) | 0.04 | 1 | ||||
| No | 1018 (74.69) | 94 (66.20) | 170 (65.89) | 92 (66.19) | ||
| Yes | 345 (25.31) | 48 (33.80) | 88 (34.11) | 47 (33.81) | ||
| Diabetes (%) | 0.02 | 0.56 | ||||
| No | 1170 (85.84) | 111 (78.17) | 210 (81.40) | 109 (78.42) | ||
| Yes | 193 (14.16) | 31 (21.83) | 48 (18.60) | 30 (21.58) | ||
| Pupils are equal in size and round (%) | 0.78 | 0.43 | ||||
| No | 646 (47.40) | 65 (45.77) | 131 (50.78) | 64 (46.04) | ||
| Yes | 717 (52.60) | 77 (54.23) | 127 (49.22) | 75 (53.96) | ||
| Frontal lobe injury (%) | 0.09 | 0.82 | ||||
| No | 825 (60.53) | 75 (52.82) | 140 (54.26) | 73 (52.52) | ||
| Yes | 538 (39.47) | 67 (47.18) | 118 (45.74) | 66 (47.48) | ||
| Temporal lobe injury (%) | 0.02 | 0.45 | ||||
| No | 979 (71.83) | 88 (61.97) | 169 (65.50) | 85 (61.15) | ||
| Yes | 384 (28.17) | 54 (38.03) | 89 (34.50) | 54 (38.85) | ||
| Parietal lobe injury (%) | 0.08 | 1 | ||||
| No | 1270 (93.18) | 126 (88.73) | 231 (89.53) | 124 (89.21) | ||
| Yes | 93 (6.82) | 16 (11.27) | 27 (10.47) | 15 (10.79) | ||
| Occipital lobe injury (%) | 0.61 | 1 | ||||
| No | 1330 (97.58) | 137 (96.48) | 249 (96.51) | 134 (96.40) | ||
| Yes | 33 (2.42) | 5 (3.52) | 9 (3.49) | 5 (3.60) | ||
| Other positional injuries (%) | 1 | 1 | ||||
| No | 1316 (96.55) | 137 (96.48) | 251 (97.29) | 135 (97.12) | ||
| Yes | 47 (3.45) | 5 (3.52) | 7 (2.71) | 4 (2.88) | ||
| Ventricular hemorrhage (%) | 0.17 | 0.93 | ||||
| No | 1321 (96.92) | 134 (94.37) | 243 (94.19) | 132 (94.96) | ||
| Yes | 42 (3.08) | 8 (5.63) | 15 (5.81) | 7 (5.04) | ||
| Subarachnoid hemorrhage (%) | 0.19 | 0.62 | ||||
| No | 331 (24.28) | 27 (19.01) | 57 (22.09) | 27 (19.42) | ||
| Yes | 1032 (75.72) | 115 (80.99) | 201 (77.91) | 112 (80.58) | ||
| Epidural hemorrhage (%) | 0.49 | 1 | ||||
| No | 1094 (80.26) | 110 (77.46) | 200 (77.52) | 108 (77.70) | ||
| Yes | 269 (19.74) | 32 (22.54) | 58 (22.48) | 31 (22.30) | ||
| Subdural hemorrhage (%) | < 0.01 | 0.68 | ||||
| No | 708 (51.94) | 40 (28.17) | 79 (30.62) | 39 (28.06) | ||
| Yes | 655 (48.06) | 102 (71.83) | 179 (69.38) | 100 (71.94) | ||
| Bilateral brain damage (%) | < 0.01 | 0.91 | ||||
| No | 648 (47.54) | 41 (28.87) | 79 (30.62) | 41 (29.50) | ||
| Yes | 715 (52.46) | 101 (71.13) | 179 (69.38) | 98 (70.50) | ||
| Skull base fracture (%) | 0.046 | 0.46 | ||||
| No | 1268 (93.03) | 125 (88.03) | 234 (90.70) | 122 (87.77) | ||
| Yes | 95 (6.97) | 17 (11.97) | 24 (9.30) | 17 (12.23) | ||
| Skull cap fracture (%) | 0.035 | 0.83 | ||||
| No | 894 (65.59) | 80 (56.34) | 151 (58.53) | 79 (56.83) | ||
| Yes | 469 (34.41) | 62 (43.66) | 107 (41.47) | 60 (43.17) | ||
| Surgeries (%) | < 0.01 | 1 | ||||
| No | 1166 (85.55) | 77 (54.23) | 143 (55.43) | 77 (55.40) | ||
| Yes | 197 (14.45) | 65 (45.77) | 115 (44.57) | 62 (44.60) | ||
| Antibiotics (%) | < 0.01 | 0.78 | ||||
| No | 1344 (98.61) | 135 (95.07) | 248 (96.12) | 132 (94.96) | ||
| Yes | 19 (1.39) | 7 (4.93) | 10 (3.88) | 7 (5.04) | ||
| Dehydration drugs (%) | 0.11 | 0.47 | ||||
| No | 791 (58.03) | 72 (50.70) | 143 (55.43) | 71 (51.08) | ||
| Yes | 572 (41.97) | 70 (49.30) | 115 (44.57) | 68 (48.92) | ||
| Length of stay | 19.55 ± 17.5 | 35.23 ± 40.84 | < 0.01 | 27.19 ± 23.98 | 35.30 ± 41.17 | 0.01 |
| Deaths in hospital (%) | < 0.01 | 0.02 | ||||
| No | 1323 (97.07) | 117 (82.39) | 236 (91.47) | 115 (82.73) | ||
| Yes | 40 (2.93) | 25 (17.61) | 22 (8.53) | 24 (17.27) | ||
| ICU admission (%) | < 0.01 | 0.22 | ||||
| No | 1259 (92.37) | 103 (72.54) | 203 (78.68) | 101 (72.66) | ||
| Yes | 104 (7.63) | 39 (27.46) | 55 (21.32) | 38 (27.34) | ||
| Ventilator use (%) | < 0.01 | < 0.01 | ||||
| No | 1233 (90.46) | 78 (54.93) | 181 (70.16) | 76 (54.68) | ||
| Yes | 130 (9.54) | 64 (45.07) | 77 (29.84) | 63 (45.32) | ||
Unless specified otherwise, the mean ± standard deviation is used to indicate continuous values, while the percentage represents categorical variables.
TyG can predict the incidence of AKI
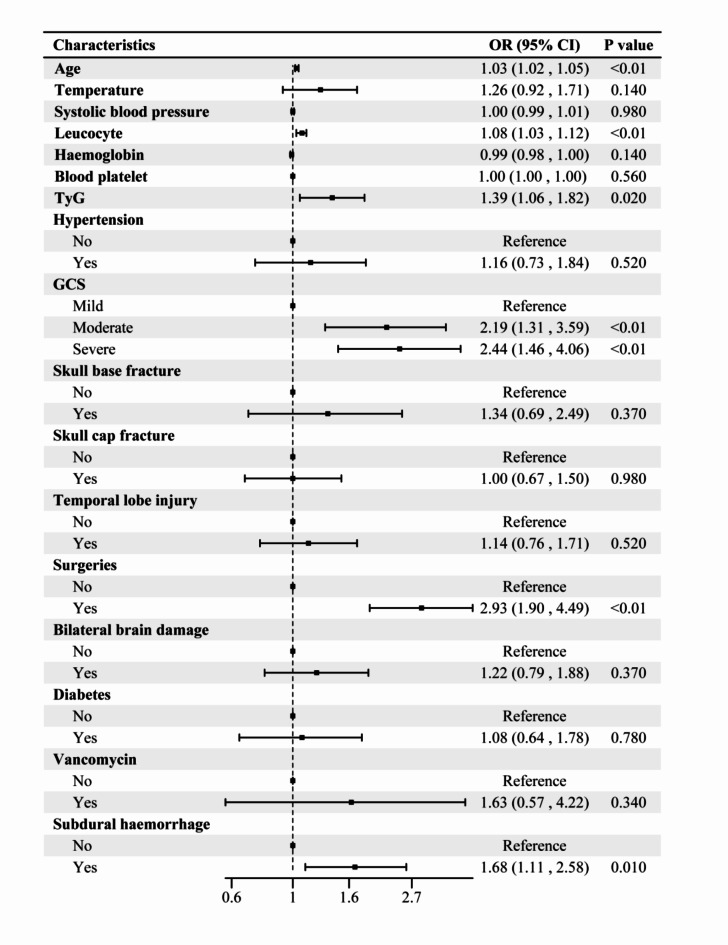
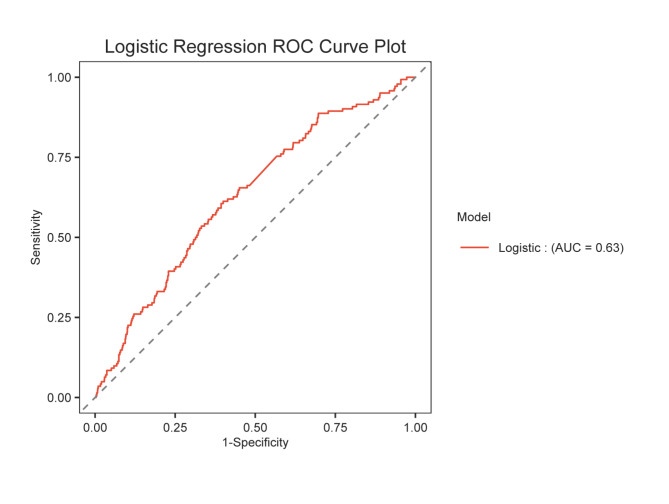
The independent variables included in the multifactorial analysis were age, admission temperature, systolic blood pressure, leukocyte count, TyG level, platelet count, hemoglobin level, GCS score, history of hypertension and diabetes mellitus, temporal lobe injury, subdural hemorrhage, bilateral brain injury, skull base and cap fractures, surgical intervention, and antibiotics (vancomycin) (Fig. 2). The results revealed that GCS score, age, leukocytes, TyG, subdural hemorrhage, and surgery were independent risk factors for AKI, and the variance inflation factor between the variables was less than 10 (Supplementary Table 1). Thus, TyG can indeed be used to predict the AKI. To evaluate TyG’s predictive power even more, ROC curves were created (Fig. 3). ROC curves were also calculated to predict TyG, with an AUC of 0.630 (95% CI 0.583–0.677), an optimal cutoff value of 7.0, sensitivity of 61.27%, and specificity of 60.01%.
Fig. 2.
Results of multivariate logistic regression analysis with independent predictors.
Fig. 3.
ROC curve of TyG index for prediction of AKI.
Propensity score matching
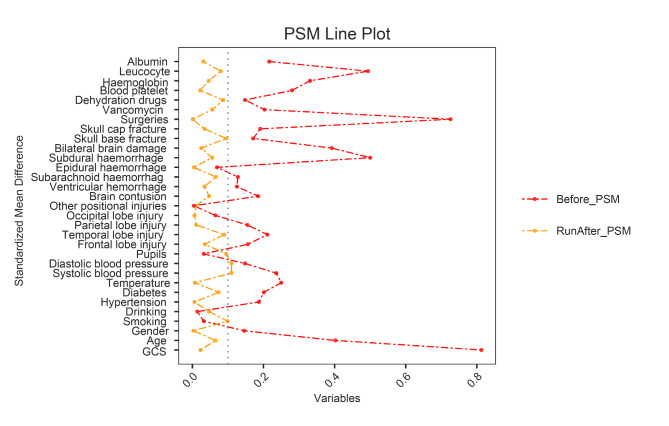
To eliminate the effects of confounders, matching was performed using the nearest neighbor propensity score matching (1:2) method. In this study, 139 patients with AKI completed matching, and the standardized mean difference following PSM was less than 0.1 (Fig. 4), indicating well-balanced and comparable baseline characteristics between the two groups. Table 2 displays the baseline clinical parameters of every subject following PSM. Consistent with the pre-matching period, TyG between the two groups after matching remained statistically significant (P = 0.02). Prior to PSM, the AKI group had longer lengths of stay (P<0.01), ventilator use (P<0.01), ICU admission (P<0.01), and in-hospital mortality (P<0.01) than the non-AKI group, and the differences were statistically significant. After controlling for confounding variables using PSM, the difference in ICU admission (P = 0.22) between the two groups was not significant. However, length of hospital stay (P = 0.01), ventilator use (P<0.01), and in-hospital mortality rates (P = 0.02) were statistically significant. These results suggest that the high prevalence of AKI during hospitalization and TyG levels are closely related and that adverse outcomes may occur during hospitalization.
Fig. 4.
Balance check for each variable after PSM. The horizontal axis indicates the standardized mean difference for each variable. The vertical axis indicates the type of variable.
Table 2.
Association between TyG and AKI.
| TyG | Model1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR(95% CI) | P-value | OR(95% CI) | P-value | OR(95% CI) | P-value | |
| Continuous | 1.70 (1.37, 2.11) | < 0.01 | 1.40 (1.07, 1.84) | 0.0149 | 1.39 (1.06, 1.83) | 0.0176 |
| Categories | ||||||
| < 7 | Reference | Reference | Reference | |||
| ≥ 7 | 2.37 (1.66, 3.37) | < 0.01 | 1.81 (1.22, 2.70) | < 0.01 | 1.77 (1.18, 2.64) | < 0.01 |
Model 1: no covariate adjustment.
Model 2: Adjusted for variables that were statistically different between the two groups, including temperature, age, systolic blood pressure, leukocytes, platelets, hemoglobin, GCS, hypertension, diabetes mellitus, temporal lobe damage, subdural hemorrhage, bilateral brain injury, skull base, skull cap fracture, surgeries, and antibiotics.
Model 3: adjusted for all.
Association between TyG and AKI
Three models were constructed to adjust for confounding factors and further assess the relationship between TyG and AKI (Table 2). The results showed a strong positive correlation between them. In the unadjusted model, the higher the TyG level, the higher the risk of AKI (model 1: OR = 1.70; 95% CI = 1.37, 2.11). In addition, this positive association was statistically significant in the partially and fully adjusted models (Model 2: OR = 1.40; 95% CI = 1.07, 1.84; Model 3: OR = 1.39; 95% CI = 1.06, 1.83). Subsequently, TyG was transformed into a categorical variable for further analysis based on its optimal cut-off value. The results revealed that the positive association of TyG with AKI remained stable in all models (model 1: OR = 2.37; 95% CI = 1.66, 3.37; model 2: OR = 1.81; 95% CI = 1.22, 2.70; model 3: OR = 1.77; 95% CI = 1.18, 2.64). Furthermore, the research observed a 77% increase in the risk of developing AKI for each unit increase in TyG in the higher TyG (TyG ≥ 7) group compared with participants in the lower TyG (TyG < 7) group after adjusting for all confounding variables (model 3; OR = 2.08; 95% CI = 1.25, 3.46).
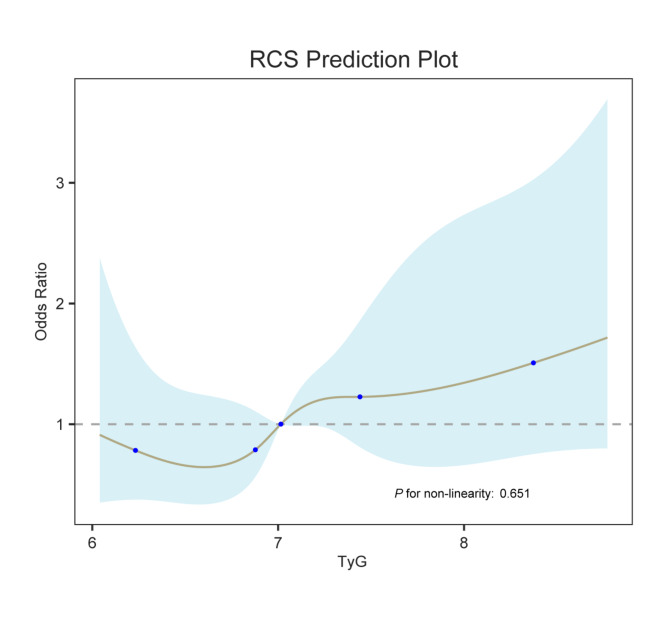
Dose-response relationship between AKI and TyG
Based on the model constructed above, the present study further explored the dose-response relationship between AKI and TyG using RCS analysis (Fig. 5). The results revealed a stable positive linear correlation between TyG level and AKI risk (P for nonlinearity = 0.651) in the fully adjusted model (Model 3).
Fig. 5.
Dose-response relationship between AKI and TyG.
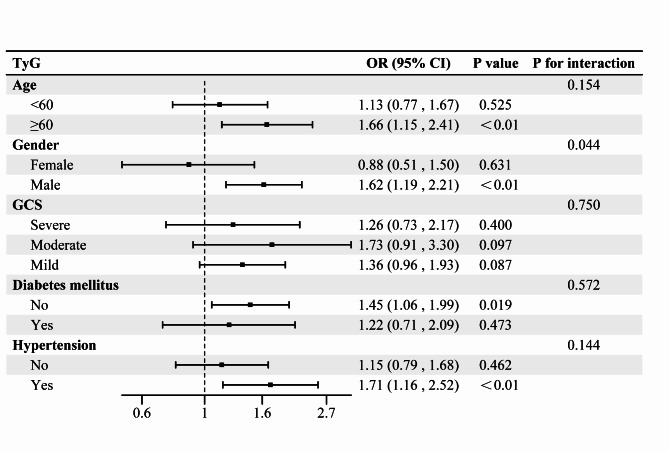
Subgroup analysis
In the present study, subgroup analyses according to age, sex, GCS score, diabetes mellitus, and history of hypertension were performed to explore whether this relationship was heterogeneous across subgroups (Fig. 6). The results indicated that the correlation between TyG and AKI was stronger in patients who were ≥ 60 years old, male, had a medium-sized craniocerebral injury, were non-diabetic, and had hypertension. Furthermore, the results of the interaction test revealed that the correlation between TyG and AKI remained stable in most of the population, but may have been affected by sex (P = 0.044).
Fig. 6.
Subgroup analysis.
Discussion
The present study established, for the first time, the predictive capability of TyG for AKI in hospitalized patients with TBI, thus providing insights into this correlation. The results indicated that an elevated index significantly and independently predicted the onset of AKI in patients with TBI. Even after PSM and multifactorial logistic regression were applied to adjust for potential confounders, a positive correlation remained. The dose-response curves further confirmed this positive linear correlation. The results of the subgroup analyses also revealed that the correlation between the TyG index and the incidence of AKI was stable in most subpopulations, but differed between the sex subgroups.
In previous studies, the homeostasis model assessment (HOMA) was found to be a relatively classical method for assessing IR. However, the HOMA is expensive, time-consuming, and invasive, which limits its use in routine clinical measurements19. Consequently, the TyG index has rapidly become a significant research focus as an effective and cost-efficient measure of IR20. Extensive research has further demonstrated that the TyG index outperforms the HOMA-IR in terms of effectiveness19,21,22. In an epidemiological survey of 14,948 women in the United States, different IR proxies were used to predict the incidence of female infertility, with results showing that the TyG index exhibited a higher area under the curve for predicting infertility than other IR alternatives23. TyG expression has also been correlated with the development and prognosis of cardiovascular diseases. A meta-analysis incorporating a large number of cohort studies explored the linear dose-response relationship between the TyG index and the incidence of cardiovascular disease using RCS curves. The results of this analysis revealed that the risks of coronary heart disease and cardiovascular disease increased by 35% and 23%, respectively, for each unit increase in the TyG index24. The association between TyG levels and AKI was also investigated. A retrospective study investigating the risk of AKI among patients with heart failure revealed that the incidence of AKI in these patients increased in conjunction with the TyG index. Notably, this association remained statistically significant, even after adjusting for potential confounders16. Fang et al. reported a similar positive linear correlation between TyG levels and AKI in patients with sepsis. Based on this, they suggested that early intervention in patients with sepsis and significantly elevated TyG index values might reduce the incidence or delay the progression of sepsis-associated AKI, thereby shortening the length of hospital stay8. Another study reported that TyG may be beneficial in identifying patients at high risk of AKI and poor renal function in those undergoing coronary artery revascularization25. These findings provide direct evidence for a link between AKI and TyG in patients with TBI. This study identifies for the first time the ability of the TyG to predict AKI in patients hospitalized with TBI and provides insights into this relationship. The findings show that a higher TyG index significantly and independently predicts the occurrence of AKI in TBI patients. The results of the subgroup analysis showed that the correlation between TyG index and AKI incidence may be affected by gender. Research by Golestaneh and his colleagues supports our conclusions. They conducted a prospective study that detailed the differences in AKI by sex, finding that men have a higher risk of AKI across all age groups. Furthermore, women over 55 who received estrogen therapy had a significantly lower risk of AKI compared to those who did not receive this treatment. This study suggests that this phenomenon may be attributed to the protective effect of estrogen on the kidneys26. In addition, we observed that the TyG index was more strongly correlated with the incidence of AKI in non-diabetic patients (OR = 1.45) than in diabetic patients (OR = 1.22). This observation could stem from the pronounced IR typically seen in diabetic patients, which results in elevated TyG levels. This continuous metabolic state may have reduced the sensitivity of TyG to AKI.
The underlying pathophysiological mechanisms that link the TyG index with AKI associated with TBI remain elusive. However, it has been suggested that a range of factors could influence this correlation. First, the TyG index primarily reflects the degree of IR in an individual and is assessed by measuring the fasting blood glucose and triglyceride levels. IR is associated with metabolic syndrome and elevated systemic inflammation27. After brain injury, IR may be exacerbated by enhanced stress responses and increased inflammatory activity28. This exacerbation of IR may further increase the risk of AKI through several mechanisms, including insulin-promoted sodium reabsorption in IR induced hyperinsulinemic states, which increases the glomerular filtration rate and ultimately leads to renal injury29 as well as the sympathetic nervous system and renin-angiotensin-aldosterone system, which may be improperly activated by IR, thereby increasing the risk of AKI30. Secondly, patients with higher TyG indices often exist in a more severe inflammatory state, which is a common systemic response to traumatic brain injury31. The cytokine interleukin-6 is released in the cerebrospinal fluid and serum following TBI32, ultimately influencing the development of AKI through the NF-κB/miR-26a-5p/interleukin-6 axis33. Oxidative stress is another key factor in cellular damage and organ dysfunction following traumatic brain injury34. IR may exacerbate the effects of oxidative stress34,35. Previous studies have indicated that IR associated with oxidative stress can trigger glomerular endothelial cell injury, tethered cell proliferation, and basement membrane thickening. Collectively, these processes lead to glomerulosclerosis and tubulointerstitial damage, which ultimately result in renal insufficiency36. In subgroup analyses, a significant effect of sex on the association between TyG and AKI was observed. This phenomenon may be primarily caused by differences in sex hormone levels, particularly estrogen. Through its protective effects on lipid metabolism, estrogen can reduce lipid deposition and exert anti-inflammatory effects, which may reduce the risk of AKI37,38. Furthermore, estrogen increases insulin sensitivity, further reducing the risk of kidney injury39.
Strengths and limitations
This study is the first to investigate the relationship between TyG levels and the incidence of AKI in patients with TBI, thus filling a gap in current research. In addition, this study collected and adjusted for potential confounders affecting AKI as much as possible based on the clinical electronic medical record information from hospitals to improve the accuracy of the results. Nevertheless, this study had some limitations. First, the study design was retrospective and observational, and preliminary evidence suggests that TyG can be used to stratify patients with TBI according to their risk of developing AKI. However, because of the characteristics of cross-sectional studies, causality remains uncertain at this time. Second, although this study adjusted for potential factors as much as possible by performing multivariate logistic regression and PSM, there may still be the limitation of not including all variables, which may have led to a persistent bias. Furthermore, as this was a single-center study, caution must be exercised when interpreting the results. Increasing the reliability of these findings would necessitate the inclusion of multicenter data. Moreover, this study only assessed the association of baseline TyG with AKI and its poor prognosis in patients with TBI, and did not consider dynamic changes in TyG. Finally, we recorded and analyzed the patient’s medication history, including antibiotics and antiplatelet agents, with a special focus on dehydrating medications commonly used in patients with traumatic brain injury (TBI), such as mannitol and diuretics. These drugs are necessary for some patients during treatment, despite their potential nephrotoxicity. However, due to the nature of retrospective studies, we were unable to accurately assess the exact extent of the effects of these drugs on renal function in patients. Future studies need to employ a prospective experimental design to more fully explore the effects of these drugs on renal function and to complement our findings.
Conclusion
This study confirms that the TyG index specifically predicts the occurrence of AKI in patients with TBI, demonstrating a significant correlation. By employing the TyG index as an early risk assessment tool, clinicians can proactively predict the AKI risk before the onset of significant renal impairment. Stratified patient management would enable precise adjustments in the use of nephrotoxic drugs and existing pharmacotherapies, thereby potentially reducing the incidence of adverse clinical events. This proactive approach to monitoring and intervention can significantly improve the quality of patient care and outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to express their gratitude to the following institutions: Fujian Provincial Key Laboratory of Medical Big Data Engineering and Fujian Provincial Key Laboratory of Emergency Medicine.
Abbreviations
- AKI
Acute kidney injury
- TyG
Triglyceride glucose
- TBI
Traumatic brain injury
- RCS
Restricted cubic spline
- ROC
Receiver operating characteristic
- IR
Insulin resistance
- AUC
Area under the curve
- GCS
Glasgow coma scale
- PSM
Propensity score matching
- HOMA
Homeostasis model assessment
Author contributions
S.L., L.C., J.W. and R.P. performed the experiments and collected the primary data. R.P., S.L., B.W., D.W. and H.G. wrote the main manuscript text and prepared the figures and tables. All authors reviewed the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study protocol was approved by the Ethics Committee of Fujian Provincial Hospital(K2024-06-057). The study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of Fujian Provincial Hospital waived the requirement of written informed consent because of the non-interventional design of this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rujun Pan, Shaojie Li and Baofang Wu contributed equally to this work.
Contributor Information
De Wei, Email: weidele@fjmu.edu.cn.
Hongzhi Gao, Email: gaohongzhi@fjmu.edu.cn.
References
- 1.Fonseca, J., Liu, X., Oliveira, H. P. & Pereira, T. Mortality prediction using medical time series on TBI patients. Comput. Methods Programs Biomed.242, 107806 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Taylor, C. A., Bell, J. M., Breiding, M. J. & Xu, L. Traumatic Brain Injury-Related Emergency Department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ.66, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, R. R., He, M., Gui, X. & Kang, Y. A nomogram based on serum cystatin C for predicting acute kidney injury in patients with traumatic brain injury. Ren. Fail.43, 206–215 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, R., Zhang, J., Xu, J., He, M. & Xu, J. Incidence and burden of acute kidney injury among traumatic brain-injury patients. Risk Manag. Healthc. Policy14, 4571–4580 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, N., Zhao, W-G., Xu, F-L., Zhang, W-F. & Gu, W-T. Neutrophil gelatinase-associated lipocalin as an early marker of acute kidney injury in patients with traumatic brain injury. J. Nephrol.26, 1083–1088 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Li, N., Zhao, W-G. & Zhang, W-F. Acute kidney injury in patients with severe traumatic brain injury: Implementation of the acute kidney injury network stage system. Neurocrit. Care14, 377–381 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Cheruku, S. R., Raphael, J., Neyra, J. A. & Fox, A. A. Acute kidney injury after cardiac surgery: Prediction, prevention, and management. Anesthesiology139, 880–898 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang, Y. et al. Triglyceride-glucose index predicts sepsis-associated acute kidney injury and length of stay in sepsis: A MIMIC-IV cohort study. Heliyon10, e29257 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, C-H. et al. Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PLoS One9, e109649 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes54, 1615–1625 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Huo, R-R., Liao, Q., Zhai, L., You, X-M. & Zuo, Y-L. Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: A nationwide prospective cohort study. Cardiovasc. Diabetol.23, 30 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, Q., Xiao, S., Jiao, X. & Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol.22, 279 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou, X-Z. et al. Association between different insulin resistance surrogates and all-cause mortality in patients with coronary heart disease and hypertension: NHANES longitudinal cohort study. Cardiovasc. Diabetol.23, 86 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan, L. et al. Association between insulin resistance and uncontrolled hypertension and arterial stiffness among US adults: a population-based study. Cardiovasc. Diabetol.22, 311 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu, Y. et al. Prognostic nutritional index combined with triglyceride-glucose index to contrast a nomogram for predicting contrast-induced kidney injury in type 2 diabetes mellitus patients with acute coronary syndrome after percutaneous coronary intervention. Clin. Interv Aging18, 1663–1673 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang, Z., Gong, H., Kan, F. & Ji, N. Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: Analysis of the MIMIC-IV database. Cardiovasc. Diabetol.22, 232 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggiore, U. et al. The relation between the incidence of hypernatremia and mortality in patients with severe traumatic brain injury. Crit. Care13, R110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alizargar, J., Bai, C-H., Hsieh, N-C. & Wu, S-F-V. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc. Diabetol.19, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son, D-H., Lee, H. S., Lee, Y-J., Lee, J-H. & Han, J-H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis.32, 596–604 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Guerrero-Romero, F. et al. Fasting triglycerides and glucose index as a diagnostic test for insulin resistance in young adults. Arch. Med. Res.47, 382–387 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Wu, T. D. et al. Association of Triglyceride-Glucose Index and Lung Health: a Population-based study. Chest160, 1026–1034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, Z. et al. The relationship between triglyceride-glucose index and albuminuria in United States adults. Front. Endocrinol. (Lausanne)14, 1215055 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia, W., Cai, Y., Zhang, S. & Wu, S. Association between different insulin resistance surrogates and infertility in reproductive-aged females. BMC Public Health23, 1985 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, X. et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc. Diabetol.21, 124 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi, Y. et al. Association of triglyceride glucose index with the risk of acute kidney injury in patients with coronary revascularization: a cohort study. Diabetol. Metab. Syndr.16, 117 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golestaneh, L. et al. Acute kidney Injury, and age: a prospective cohort study. Am. J. Kidney Dis.S0272-6386(24), 01037–01030 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Gluvic, Z. et al. Link between metabolic syndrome and insulin resistance. Curr. Vasc Pharmacol.15, 30–39 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Sekar, S. et al. Concussion/Mild traumatic brain Injury (TBI) induces brain insulin resistance: A positron emission tomography (PET) scanning study. Int. J. Mol. Sci.22, 9005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteghamati, A. et al. Insulin resistance is an independent correlate of increased urine albumin excretion: A cross-sectional study in Iranian type 2 diabetic patients. Diabet. Med.26, 177–181 (2009). [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo, R. A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia21, 165–171 (1981). [DOI] [PubMed] [Google Scholar]
- 31.Huang, D. et al. Positive association between different triglyceride glucose index-related indicators and psoriasis: Evidence from NHANES. Front. Immunol.14, 1325557 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue, T., Tanaka, S. & Okusa, M. D. Neuroimmune interactions in inflammation and acute kidney Injury. Front. Immunol.8, 945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, Y., Zhou, X. & Wu, Y. The miR-26a-5p/IL-6 axis alleviates sepsis-induced acute kidney injury by inhibiting renal inflammation. Ren. Fail.44, 551–561 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maciejczyk, M., Żebrowska, E. & Chabowski, A. Insulin resistance and oxidative stress in the brain: What’s new? Int. J. Mol. Sci.20, 874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong, S., Gan, S., Zhang, Y., Zhou, H. & Zhou, Q. Gamma-glutamyl transferase to high-density lipoprotein cholesterol ratio is a more powerful marker than TyG index for predicting metabolic syndrome in patients with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne). 14, 1248614 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin, Q. et al. Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Front. Immunol.14, 1185317 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao, X. et al. β2AR against myocarditis-lipid deposition depends on estrogenic environment in stress. J. Endocrinol.256, e220335 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Katzer, K., Hill, J. L., McIver, K. B. & Foster, M. T. Lipedema and the potential role of estrogen in excessive adipose tissue accumulation. Int. J. Mol. Sci.22, 11720 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Paoli, M., Zakharia, A. & Werstuck, G. H. The role of estrogen in insulin resistance: A review of clinical and preclinical data. Am. J. Pathol.191, 1490–1498 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.