Abstract
Purpose
Up to 50% of high-risk non-muscle invasive bladder cancer (HR-NMIBC) patients fail Bacillus Calmette-Guérin (BCG) treatment, resulting in a high risk of progression and poor clinical outcomes. Biomarkers that predict outcomes after BCG are lacking. The antitumor effects of BCG are driven by a cytotoxic T cell response, which may be controlled by immune checkpoint proteins like Programmed Death Ligand 1 (PD-L1). Here, we hypothesized that PD-L1 protein expression could serve as a biomarker for BCG-failure.
Methods
HR-NMIBC patients who received ≥ 5 BCG instillations were included. Tissue microarrays were constructed from BCG-naïve tumors and recurrences and stained with the PD-L1 (SP142) antibody. PD-L1 status was defined as ≥ 5% tumor-infiltrating immune cells with membrane staining in the tumor area. Clinicopathological associations with PD-L1 positive tumors were investigated, and time-to-event analyses were performed comparing PD-L1 positive vs. negative tumors.
Results
432 BCG-naïve tumors and 160 recurrences were included, and 91% of patients received adequate BCG. In BCG-naïve tumors, PD-L1 was expressed in 7% of patients and PD-L1 expression was associated with stage T1 versus Ta disease (p = 0.015). PD-L1 expression was not associated with treatment failure after adequate BCG (p = 0.782) nor with progression-free survival (p = 0.732). Testing cut-offs of ≥ 1% and ≥ 10% PD-L1 positivity did not alter results. High PD-L1 expression was more frequent in tumor recurrences (14%) as compared to BCG-naïve tumors (p = 0.012).
Conclusion
PD-L1 expression in HR-NMIBC is not a biomarker of response to BCG. However, PD-L1 is higher in a subset of tumors that failed BCG treatment. More research is needed to determine the role of PD-L1 in tumors where BCG treatment failed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00345-024-05392-5.
Keywords: BCG, Bladder cancer, Immunotherapy, PD-L1, Prognosis, Progression, Recurrence
Introduction
Current bladder cancer (BC) risk stratification identifies patients at risk of recurring and progressive disease. The standard-of-care for patients with high-risk non-muscle invasive BC (HR-NMIBC) includes a transurethral resection of the bladder tumor (TURBT) followed by adjuvant intravesical instillations with Bacillus Calmette-Guérin (BCG) for up to three years [1]. Despite treatment, up to 50% of patients experience, recurrences, and 10–20% develop progression, which is associated with poor clinical outcomes [2]. No markers exist to predict response to treatment. Patients who do not respond to BCG suffer from BCG toxicity and a delay in surgery—a radical cystectomy (RC) with urinary diversion [1]. An early RC instead of BCG is associated with excellent long-term outcomes [3]. However, early RC in all patients results in overtreatment. Furthermore, most patients prefer bladder-sparing therapy since RC is associated with a reduced quality of life [4].
The cell surface protein Programmed Death Ligand 1 (PD-L1) binds to the Programmed Death 1 (PD-1) receptor on CD8 + T cells and suppresses a Th-1 immune response by inducing CD8 + T cell apoptosis, thereby suppressing antitumor immunity [5]. The antitumor effect of BCG is effectuated via a cytotoxic T cell response against residual tumor cells after TURBT [6]. Hence, it is hypothesized that the diminished effectivity of BCG therapy may be caused by upregulated PD-L1 expression, which could serve as a marker for lack of response to BCG. Evidence suggests that PD-L1 expression in tumor cells is associated with BC stage progression and poor clinical outcomes in advanced BC, but this is not the case for HR-NMIBC [7–10]. A recent review highlighted conflicting evidence and a lack of detailed information on BCG treatment [11]. As a result, the role of PD-L1 as a biomarker for predicting response to BCG in NMIBC is not fully established.
The role of PD-(L)1 immune checkpoint inhibitors (ICI) is also significant in BCG-unresponsive patients. Pembrolizumab was approved as monotherapy based on results from the phase 2 Keynote-057 study, but only 41% of patients had a complete response at 3 months [12]. Improving the success rate for ICIs by identifying patients who would benefit from ICI remains an unmet clinical need [13].
Here, we aimed to investigate if immunohistochemical (IHC) expression of PD-L1 can predict response to BCG in a large cohort of BCG-treated patients with pathological revision and long-term follow-up. In addition, we discuss if PD-L1 expression could provide a rationale for anti-PD-(L)1 ICI therapy in BCG-naïve tumors and recurrences.
Patients and methods
Patients
Patients with primary HR-NMIBC (Ta high-grade [HG]/T1/Tis) who received ≥ 5/6 BCG induction instillations were retrospectively included in the study between 2000 and 2018. Recruitment occured at four hospitals: Erasmus University MC, Franciscus Gasthuis & Vlietland and Amphia (all in the Netherlands), and Stavanger University Hospital (in Norway). All urologists were recommended to follow the then applicable European Association of Urology (EAU) guidelines on NMIBC during this study period; detailed follow-up protocols involving cystoscopy, cytology, and imaging (e.g. for upper tract and/or progressive disease) can be found in the supplemental methods. The BCG instillation schedule followed the Southwest Oncology Group (SWOG) protocol [1]. Follow-up concluded at the last visit or death, and was completed in September 2021.
Pathology
A large cohort (n = 509) of centrally reviewed HR-NMIBC tumors, including tumor recurrences, was used to construct tumor tissue microarrays (TMAs). Preferred regions of interest (ROI) were invasive tumor areas with tumor-infiltrating immune cells (ICs). The anti-PD-L1 antibody (VENTANA PD-L1 (SP142) Assay, Ventana Medical Systems, the companion diagnostic for atezolizumab) was used for IHC of TMAs. PD-L1/SP142 IHC was evaluated by two independent investigators, who were blinded to clinical outcomes during the assessment. Details on TMA construction, assessment, and IHC are provided in the supplements.
Definitions, endpoints & statistics
Adequate BCG was defined as receiving ≥ 5/6 BCG induction instillations plus ≥ 2/3 BCG maintenance instillations [14]. BCG failure was determined according to the 2021 EAU Guidelines on NMIBC [1]. The primary endpoint was defined as: a HG recurrence after the second round of BCG instillations (~ 6 months after induction), accounting for patients with a TaHG/Tis recurrence who may undergo a second round of BCG instillations. Patients who developed T1HG disease after BCG induction were also included in the primary endpoint. Secondary endpoints included recurrence at the first evaluation after BCG induction, 1-year high-grade recurrence-free survival (HG-RFS), 2-year progression-free survival (PFS), and 5-year cancer-specific survival (CSS). Progression was defined as the development of MIBC and/or (lymph) node metastatic disease. Chi-square testing was done to assess if PD-L1 expression was associated with clinicopathological parameters, and to determine if PD-L1 status differed between tumor recurrences vs BCG-naïve tumors or post-BCG random biopsies (in case of suspected recurrences) diagnosed as pT0. The Kaplan–Meier method with log-rank testing was used to estimate survival. The time variable was the duration between initial cancer diagnosis and HG recurrence, progression, or death from BC. Outcomes were deemed significant at p < 0.05. Analyses were done in R statistical software (v4.0.5) and SPSS Statistics (IBM® v26).
Results
Baseline study population and risk factors of BCG treatment failure
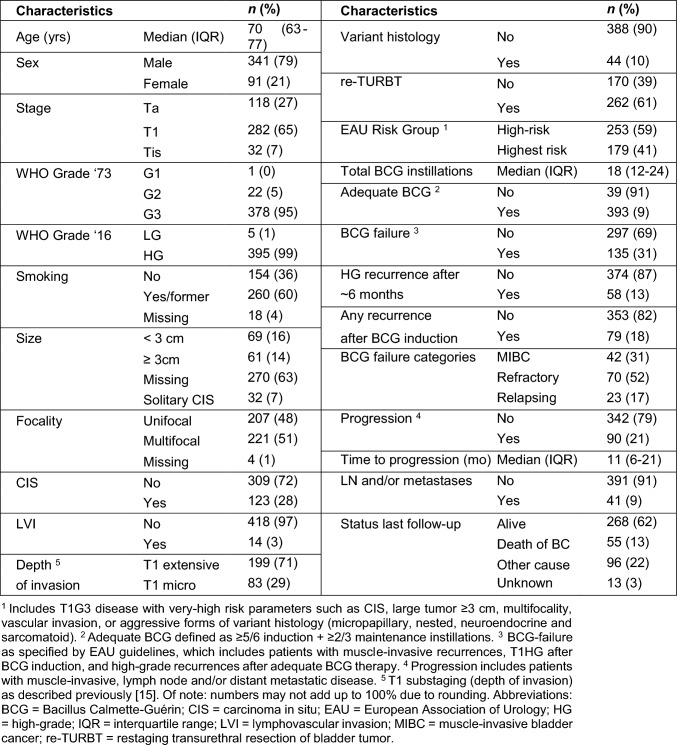
In BCG-naïve patients, N = 432 tumors with 1060 tumor cores were of sufficient quality for PD-L1 IHC, averaging approximately 2.5 cores per primary tumor. In 24/432 (%) tumors, PD-L1 information was used from the re-TURBT. Clinicopathological characteristics for included patients are summarized in Table 1. Ninety-one percent received adequate BCG, with a median clinical follow-up of 70 (IQR 45–98) months. According to the EAU guidelines on NMIBC, BCG treatment failed in 135/432 (31%) patients. Categories of BCG treatment failure are specified in Table 1. At baseline, tumor focality (p = 0.001), CIS (p = 0.03), LVI (p = 0.001), and the EAU very high-risk disease category (p = 0.003) were significantly correlated with BCG treatment failure.
Table 1.
Baseline patient and follow-up characteristics of N = 432 BCG-naïve high-risk non-muscle invasive bladder cancer patients
BCG Bacillus Calmette-Guérin, CIS carcinoma in situ, EAU European Association of Urology, HG high-grade, IQR interquartile range, LVI lymphovascular invasion, MIBC muscle-invasive bladder cancer, re-TURBT restaging transurethral resection of bladder tumor
aIncludes T1G3 disease with very-high risk parameters such as CIS, large tumor ≥ 3 cm, multifocality, vascular invasion, or aggressive forms of variant histology (micropapillary, nested, neuroendocrine and sarcomatoid)
bAdequate BCG defined as ≥ 5/6 induction + ≥ 2/3 maintenance instillations
cBCG-failure as specified by EAU guidelines, which includes patients with muscle-invasive recurrences, T1HG after BCG induction, and high-grade recurrences after adequate BCG therapy
dProgression includes patients with muscle-invasive, lymph node and/or distant metastatic disease
eT1 substaging (depth of invasion) as described previously [15]. Of note: numbers may not add up to 100% due to rounding
BCG-naïve HR-NMIBC patients have a low protein expression of PD-L1
We analyzed the number of cores with PD-L1 staining in any type of tumor-infiltrating IC. An example of a negative core and a positive core with PD-L1 staining in ≥ 5% ICs is illustrated in Figure S1. Using the recommended cut-off of ≥ 5% in ICs, we found that 29/432 (7%) of BCG-naïve tumors were considered PD-L1 positive based on the mean value of all cores. Due to known PD-L1 staining heterogeneity, we also investigated which fraction of cores scored positive if the core with the highest value was selected to determine PD-L1 status. Using the highest core, 69/432 (16%) tumors were positive for PD-L1 expression. The SP142 antibody is designed for ICs, but as suggested by the protocol and others, we also investigated staining on tumor cells (TCs). Using a ≥ 5% cut-off, 19/432 (4%, mean core expression) vs 40/432 (9%, highest expressed core) tumors were considered positive. All results on PD-L1 expression in ICs and TCs and other cut-offs used in literature are included in Table S1. To ensure that our findings were not influenced by PD-L1 spatial heterogeneity and the use of TMAs, we selected 30 samples (15 T1 responders vs. 15 non-responders to BCG) and checked if PD-L1 status based on TMAs differed from whole slide results, and this was not the case. In summary, whether the mean or highest PD-L1 core value was taken, and whether ICs or TCs were investigated, the number of positive tumors remained low.
PD-L1 expression in BCG-naïve tumors does not predict outcome after BCG treatment
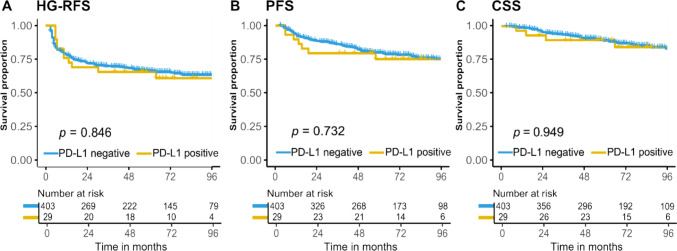
We aimed to determine if PD-L1 expression was associated with a HG recurrence after adequate BCG treatment, which occured in 58/432 (13%) patients. Additionally, 79/432 (18%) had a recurrence of any grade/stage after BCG induction. PD-L1 expression was not associated with BCG treatment failure after adequate BCG (p = 0.782) nor with recurrence after BCG induction (p = 0.626). Importantly, investigating TCs or using different cut-offs (≥ 1% or ≥ 10% PD-L1 staining determines if a tumor is PD-L1 positive), or selecting only patients with a re-TURBT and detrusor muscle in the specimen, did not alter these results. Survival analyses showed that PD-L1 expression was not associated with 1-year HG-RFS (76% in PD-L1 positive vs. 79% PD-L1 negative tumors, p = 0.846). In addition, no differences were found for 2-year PFS (79% vs. 89%, p = 0.732) or 5-year CSS (90% vs. 91%, p = 0.949) (Fig. 1). Selecting the highest expressed PD-L1 core (≥ 5% ICs) did not alter these results (Figure S2). We conclude that PD-L1 protein expression is not associated with outcomes after BCG treatment in HR-NMIBC patients.
Fig. 1.
Kaplan–Meier estimates of A high-grade recurrence-free survival (HG-RFS), B progression-free survival (PFS), and C cancer-specific survival (CSS) as assessed by mean PD-L1 status of ≥ 5% in N = 432 BCG-naïve high-risk non-muscle invasive bladder cancer patients
PD-L1 expression in BCG-naïve HR-NMIBC is associated with aggressive clinical features
Previously studies have shown that PD-L1 is associated with BC stage progression [8–10]. In our study, we confirmed that 25 out of 29 PD-L1 positive tumors had invasive T1 disease as compared to four with Ta disease (Table S2, p = 0.015). Contrary to other series, we did not find PD-L1 staining in patients with solitary CIS [8]. Recently, we published on the importance of T1 substaging in HR-NMIBC, in which we showed that patients with extensive disease into the lamina propria had a higher risk of BCG treatment failure than patients with micro-invasive disease [15]. Yet no difference in PD-L1 expression was found between tumors with T1 micro- vs extensive invasion (p = 0.361). Nonetheless, positive PD-L1 expression was correlated with patients having received a re-TURBT (p = 0.003), larger tumors (p = 0.045), and the EAU highest-risk disease criteria (p = 0.010) (Table S2). In brief, aggressive clinical features were associated with higher PD-L1 expression.
PD-L1 expression is more frequently observed in tumor recurrences
Next, we aimed to investigate PD-L1 expression in post-BCG tumor recurrences (N = 160). Overall results for ICs and TCs and different cut-offs, as well as for the mean expression of cores vs. the highest expressed core, are listed in Table S3. BCG treatment failures were highlighted (N = 128/160, 80%) as these tumors did not respond to BCG and might be candidates for anti-PD-(L)1 ICI therapy. PD-L1 was more frequently expressed post-BCG than in BCG-naive tumors (14% vs 7%, p = 0.012), regardless of whether a tumor was considered a BCG failure (14% vs 13%, p > 0.999). Finally, we investigated matching-pair tumors from patients with BCG-naïve tumors and tumor recurrences. In 19 out of 22 PD-L1 positive recurrences, matching BCG-naïve tissue was available, of which 18 were PD-L1 negative before BCG. In conclusion, high PD-L1 expression was more often observed in tumor recurrences as compared to BCG-naïve tumors.
Discussion
We investigated if PD-L1 protein expression was associated with response to BCG in a large and unbiased cohort of HR-NMIBC patients (N = 432) who received adequate BCG therapy with long-term clinical follow-up. We found that PD-L1 expression was positive in only 7% of tumors, and this expression was not associated with outcomes after BCG treatment in BCG-naïve tumors. Neither a change in the recommended 5% cut-off in tumor-infiltrating ICs nor an investigation of TCs altered these results. In addition, we found that aggressive tumor features were associated with increased PD-L1 expression, consistent with studies showing that PD-L1 overexpression correlates with BC stage progression [8, 9].
Previous studies showed that PD-L1 expression did not correspond with response to BCG, but the study populations consisted of small sample sizes (N = 22 to 186), and in 4/6 studies, varying antibodies were used [16–21]. Of particular interest are two studies that provide dissimilar evidence. Pierconti et al., investigated SP142, SP263, and 22C3 PD-L1 mAbs in N = 65 primary CIS patients, of which N = 37 developed HG recurring disease [22]. The percentage of samples that were PD-L1 positive based on tumor and immune cells was similar for SP142 and 22C3. SP263 showed inconsistent staining patterns. Only 22C3 was associated with recurring disease, while SP263 and SP142 did not differ between responders and non-responders, identical to the results presented in this study. A study by Kates et al. in N = 63 BCG-naïve tumors showed that PD-L1 (both 22C3 and SP142), using the combined positive score, was overexpressed in BCG non-responders (N = 32) [23]. Because of the patchy distribution of PD-L1, a whole slide analysis was performed. The percentage of SP142 staining is up to 28%, which could be because investigators used a selected cohort overrepresenting non-responders and a different scoring system. Based on contradictory findings and our study which indicates that PD-L1 expression has no prognostic value, we consider PD-L1 unfit as a biomarker for response to BCG.
We found that patients who recurred more often showed high PD-L1 expression. In 22 BCG-treated NMIBC patients that were sampled pre- and post-BCG, enhanced PD-L1 expression in tumor recurrences was seen, suggesting a PD-L1-mediated resistance mechanism [24]. In contrast, Boorjian et al. showed that patients who received BCG had lower PD-L1 expression at the time of RC, while others found that SP142 was lower in patients with a refractory recurrence [16, 25]. A study of N = 761 urothelial carcinoma patients investigating clinicopathological correlations for SP142 found that no intravesical BCG treatment before PD-L1 testing was associated with positive PD-L1 expression [26]. These findings directly contradict our results, yet multiple caveats must be addressed for the latter studies. The first study included HR-NMIBC patients who had a mixture of primary and recurring disease and not all patients received BCG [25]. In the second study, the mean expression of PD-L1 was generally very low (1.5%), differences were minimal (3% vs 0.6%) and measured in only 14 patients [16]. The third study included nephroureterectomies and stage T2-T4 tumors, which biases the primary analysis, and there was no BCG information given [26]. These caveats make it difficult to draw definitive conclusions.
Beyond its role as a prognostic biomarker, PD-L1 has long been recognized as a therapeutic target. In Keynote-057 (Cohort A, CIS only, N = 101), PD-L1 status was high (38%) in BCG unresponsive patients [12]. Neither the success rate by PD-L1 status nor the specific IHC antibody was mentioned. Considering the low durable response at 3 months from pembrolizumab, the high costs of ICI, other successful and less costly treatments like gemcitabine/docetaxel instillations becoming available and our data showing limited PD-L1 expression (14–25%) in recurring tumors, we argue that PD-L1 monotherapy is not an attractive approach in patients that failed BCG treatment [27]. Preclinical work in syngeneic mice and rats inoculated with BC cell lines and treated with anti-PD-L1 ICI in conjunction with BCG led to a tumor weight reduction and increased cytotoxic T cell immune responses as compared to BCG alone [28, 29]. Studies investigating a combination of PD-1/PD-L1 and BCG seem a more logical way forward. Currently, several of these studies are ongoing (e.g. POTOMAC or CREST). A combination strategy might have the potential to change treatment paradigms if side effects are tolerated.
This study had several limitations. First, we only used the SP142 antibody, while SP142 has shown higher interobserver variability, lower concordance, and lower overall expression as compared to other companion diagnostics [21, 30–32]. SP142 should be primarily scored in tumor-infiltrating immune cells and not in tumor cells, which is done in other antibodies and may explain the disparate outcomes. Nonetheless, all studies concluded that SP142 is useful to evaluate PD-L1 status in BC. Plus, the use of a single antibody for all samples prevents antibody-related bias. We found a low prevalence of BCG-naïve cores staining positive for PD-L1, and a potential explanation is that TMAs do not capture the intricate heterogeneity of the tumor microenvironment (TME). Nonetheless, we performed a whole slide analysis with different cut-offs and assessment techniques, but the results remained unaltered. A third limitation was that multiple TMA cores were lost during the production of the TMA, which could have affected endpoint analyses. Albeit we used triplicate cores, we advise using > 1 mm core sizes in the future. Finally, we did not investigate PD-L1 in the context of the TME. Analysis of immune cells such as CD4 + , CD8 + , T regs, and (innate) immune cells might help explain (adaptive) immune resistance mechanisms. Although interesting, this study aimed to assess whether PD-L1 alone could be used as a biomarker for response to BCG treatment.
Conclusions
IHC PD-L1 expression in BCG-naïve HR-NMIBC was not associated with BCG treatment failure. Based on low PD-L1 expression before BCG therapy, there seems to be no rationale for PD-L1 ICI monotherapy in BCG-naïve tumors. PD-L1 is higher in a subset of tumors that failed BCG treatment. More research is needed to determine the role of PD-L1 in tumors that failed BCG treatment, preferably within the context of the TME.
Material and/or code availability
Only trivial statistical code was used for the analysis of the data.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Roche Diagnostics Netherlands for the contribution of the PD-L1 (SP142) antibody.
Author contributions
“Conceptualization, FJ, EZ, and TZ; methodology, FJ, AM, TB, and TZ; validation, FJ and TZ; formal analysis, FJ; investigation, FJ, RH, AM and TZ, resources, VK, EB, KK, JB, and TZ; data curation, FJ; writing—original draft preparation, FJ; writing—review and editing, FJ, RH, VK, TB, KK, EZ, JB, TZ; visualization, FJ; supervision, JB, EZ and TZ; project administration, FJ and TZ; funding acquisition, TZ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by Roche Diagnostics, the Netherlands. A research agreement with Dr. Zuiverloon was used as material support to generate tumor tissue microarrays. The sponsor had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Erasmus Universitair Medisch Centrum Rotterdam,MRACE2017, Tahlita CM Zuiverloon.
Data availability
Clinical data cannot be uploaded due to privacy reasons.
Declarations
Conflict of interest
All other authors declare no conflicts of interest, including specific (non-)financial interests and relationships or employment relevant to the subject matter.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Erasmus University Medical Center (MEC-2018-1097) on April 9th, 2018.
Informed consent
Written informed consent was obtained from all subjects involved in the study to publish this paper if patients were alive. Patient consent was waived if patients were deceased. No patients can be identified in this publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babjuk M et al (2021) European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 2.Ge P et al (2018) Oncological outcome of primary and secondary muscle-invasive bladder cancer: a systematic review and meta-analysis. Sci Rep 8(1):7543–7543. 10.1038/s41598-018-26002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klaassen Z et al (2018) Treatment strategy for newly diagnosed T1 high-grade bladder urothelial carcinoma: new insights and updated recommendations. Eur Urol 74(5):597–608. 10.1016/j.eururo.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 4.Clements MB et al (2022) Health-related quality of life for patients undergoing radical cystectomy: results of a large prospective cohort. Eur Urol. 10.1016/j.eururo.2021.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12(4):252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redelman-Sidi G, Glickman MS, Bochner BH (2014) The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol 11(3):153–162. 10.1038/nrurol.2014.15 [DOI] [PubMed] [Google Scholar]
- 7.Ding X et al (2019) Clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Cancer Manag Res 11:4171–4184. 10.2147/cmar.S176937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inman BA et al (2007) PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 109(8):1499–1505. 10.1002/cncr.22588 [DOI] [PubMed] [Google Scholar]
- 9.Bellmunt J et al (2015) Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 26(4):812–817. 10.1093/annonc/mdv009 [DOI] [PubMed] [Google Scholar]
- 10.Boorjian SA et al (2008) T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 14(15):4800–4808. 10.1158/1078-0432.Ccr-08-0731 [DOI] [PubMed] [Google Scholar]
- 11.Nowak Ł, Krajewski W, Poterek A, Śliwa A, Zdrojowy R (2020) The prognostic value of programmed cell death protein ligand 1 in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin immunotherapy: current status. Arab J Urol. 19(1):67–70. 10.1080/2090598X.2020.1791562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balar AV et al (2021) Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol 22(7):919–930. 10.1016/S1470-2045(21)00147-9 [DOI] [PubMed] [Google Scholar]
- 13.de Jong FC, Rutten VC, Zuiverloon TCM, Theodorescu D (2021) Improving anti-PD-1/PD-L1 therapy for localized bladder cancer. Int J Mol Sci 22(6):2800. 10.3390/ijms22062800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamat AM et al (2016) Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the international bladder cancer group. J Clin Oncol 34(16):1935–1944. 10.1200/jco.2015.64.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong FC et al (2021) T1 substaging of nonmuscle invasive bladder cancer is associated with bacillus Calmette-Guérin failure and improves patient stratification at diagnosis. J Urol 205(3):701–708. 10.1097/ju.0000000000001422 [DOI] [PubMed] [Google Scholar]
- 16.Aydin AM, Baydar DE, Hazir B, Babaoglu B, Bilen CY (2020) Prognostic significance of pre-and post-treatment PD-L1 expression in patients with primary high-grade non-muscle-invasive bladder cancer treated with BCG immunotherapy. World J Urol 38(10):2537–2545. 10.1007/s00345-019-03065-2 [DOI] [PubMed] [Google Scholar]
- 17.Delcourt C et al (2020) Clinical interest of PD-L1 immuno-histochemistry expression as a predictive factor of Bacillus Calmette Guerin (BCG) efficacy in refractory high-risk non-muscle-invasive bladder cancer (NMIBC). World J Urol. 38(6):1517–1524. 10.1007/s00345-019-02896-3 [DOI] [PubMed] [Google Scholar]
- 18.Roumiguié M et al (2021) PD-L1 expression and pattern of immune cells in pre-treatment specimens are associated with disease-free survival for HR-NMIBC undergoing BCG treatment. World J Urol 39(11):4055–4065. 10.1007/s00345-020-03329-2 [DOI] [PubMed] [Google Scholar]
- 19.Martínez R et al (2019) Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. OncoImmunology. 8(8):1602460. 10.1080/2162402X.2019.1602460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wankowicz SAM et al (2017) Differential expression of PD-L1 in high-grade T1 vs muscle invasive bladder carcinoma and its prognostic implications. J Urol 198(4):817–823. 10.1016/j.juro.2017.04.102 [DOI] [PubMed] [Google Scholar]
- 21.Tretiakova M, Fulton R, Kocherginsky M, Long T, Ussakli C, Antic T, Gown A (2018) Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod Pathol 31(4):623–632. 10.1038/modpathol.2017.188 [DOI] [PubMed] [Google Scholar]
- 22.Pierconti F et al (2020) PD-L1 expression in bladder primary in situ urothelial carcinoma: evaluation in BCG-unresponsive patients and BCG responders. Virchows Arch 477(2):269–277. 10.1007/s00428-020-02755-2 [DOI] [PubMed] [Google Scholar]
- 23.Kates M et al (2020) Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clin Cancer Res 26(4):882–891. 10.1158/1078-0432.Ccr-19-1920 [DOI] [PubMed] [Google Scholar]
- 24.Hashizume A et al (2018) Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 9(75):34066–34078. 10.18632/oncotarget.26122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boorjian SA et al (2008) T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 14(15):4800–4808. 10.1158/1078-0432.Ccr-08-0731 [DOI] [PubMed] [Google Scholar]
- 26.Kim HS et al (2020) Programmed cell death-ligand 1 expression status in urothelial carcinoma according to clinical and pathological factors: a multi-institutional retrospective study. Front Oncol 10:568809. 10.3389/fonc.2020.568809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roumiguié M, Black PC (2022) Sequential gemcitabine plus docetaxel is the standard second-line intravesical therapy for BCG-unresponsive non-muscle-invasive bladder cancer: pro. Eur Urol Focus 8(4):1117–1120 [DOI] [PubMed] [Google Scholar]
- 28.Vandeveer AJ, Fallon JK, Tighe R, Sabzevari H, Schlom J, Greiner JW (2016) Systemic immunotherapy of non-muscle invasive mouse bladder cancer with avelumab, an anti–PD-L1 immune checkpoint inhibitor. Cancer Immunol Res 4(5):452–462. 10.1158/2326-6066.Cir-15-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y et al (2018) Bacillus Calmette-Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets Ther 11:2891–2899. 10.2147/OTT.S165840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rijnders M, van der Veldt AAM, Zuiverloon TCM, Grünberg K, Thunnissen E, de Wit R, van Leenders G (2019) PD-L1 antibody comparison in urothelial carcinoma. Eur Urol 75(3):538–540. 10.1016/j.eururo.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 31.Downes MR, Slodkowska E, Katabi N, Jungbluth AA, Xu B (2020) Inter- and intraobserver agreement of programmed death ligand 1 scoring in head and neck squamous cell carcinoma, urothelial carcinoma and breast carcinoma. Histopathology 76(2):191–200. 10.1111/his.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis H et al (2019) PD-L1 expression in urothelial carcinoma with predominant or pure variant histology: concordance among 3 commonly used and commercially available antibodies. Am J Surg Pathol 43(7):920–927. 10.1097/pas.0000000000001264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical data cannot be uploaded due to privacy reasons.