Abstract
Sepsis is a major cause of morbidity and mortality worldwide. Among the various types of end-organ damage associated with sepsis, hepatic injury is linked to significantly higher mortality rates compared to dysfunction in other organ systems. This study aimed to investigate potential biomarkers of hepatic injury in sepsis patients through a multi-center, case–control approach. We enrolled three matched cohorts: 37 sepsis patients with hepatic dysfunction (S-HD), 37 sepsis patients without hepatic dysfunction (S-CON), and 18 healthy controls (HC). We measured five proposed biomarkers of hepatic dysfunction—ARG1, MDH1, GSTα, 5-NT, and SDH—using multiplex immunoassays. These biomarkers were compared to traditional markers of hepatic dysfunction, including albumin, bilirubin, ALT, AST, and GGT, across the cohorts using both conventional statistical methods and machine learning techniques. The median age of participants was comparable across cohorts: S-HD (65.0 years, IQR 49.5–82.5), S-CON (65.0 years, IQR 48.0–81.5), and HC (62.5 years, IQR 53.0–65.0; P = 0.794). Patients with hepatic dysfunction (S-HD) exhibited higher illness severity scores compared to those without hepatic dysfunction (S-CON): MODS scores were median 7.0 (IQR 4.0–10.0) in S-HD versus median 4.0 (IQR 2.0–7.0) in S-CON (P = 0.005), and SOFA scores were median 7.0 (IQR 4.0–11.0) in S-HD versus median 3.0 (IQR 2.0–6.0) in S-CON (P < 0.001). Hemoglobin and platelet counts were lower, while creatinine levels were higher in S-HD compared to S-CON (P < 0.05). On ICU Day 1, bilirubin, ALT, AST, GGT, and INR were significantly elevated in S-HD relative to S-CON (P ≤ 0.001), and albumin levels were lower (P < 0.05). Additionally, ARG1, GSTα, 5-NT, and SDH were significantly higher in S-HD patients on ICU Day 1 compared to S-CON (P < 0.05). ARG1, MDH1, and SDH showed positive correlations with AST, ALT, and MODS (P < 0.01). From ICU Day 1 to Day 7, ARG1, GSTα, SDH, and AST levels significantly decreased in S-HD patients (P < 0.05), whereas MDH1 and 5-NT levels did not. Among the proposed biomarkers, GSTα and 5-NT did not correlate with traditional hepatic dysfunction markers but were significant in identifying S-HD patients (feature importance 0.131 and 0.097, respectively) in a random forest classification model. This comprehensive model demonstrated excellent performance in distinguishing sepsis patients with hepatic injury, with sensitivity 0.93, specificity 0.94, NPV 0.94, PPV 0.94, and AUC 0.94. The biomarkers ARG1, MDH1, GSTα, 5-NT, and SDH show promise as novel indicators of hepatic dysfunction associated with sepsis. This study provides a foundational basis for subsequent research aimed at characterizing and clinically validating these markers. Future investigations should focus on integrating these potential biomarkers into routine laboratory assessments for sepsis and related hepatic injury.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-024-01545-3.
Keywords: Sepsis, Biomarkers, Hepatic dysfunction
Background
Sepsis is defined as life-threatening organ dysfunction occurring secondary to dysregulated host responses to infection [1]. Hepatic injury remains a clinically integral target of sepsis-induced organ injury and mediator of sepsis outcomes. Indeed, hepatic injury or failure occurs in 40% and 9% of septic patients, respectively [2]. Mortality rates are higher in those with sepsis-associated hepatic injury versus those without hepatic injury (54–68%) [3, 4]. Moreover, mortality rates double in critically ill sepsis patients with early hepatic injury, and hepatic injury confers a higher risk of death than other organ injury [5].
The impact of different sepsis phenotypes on the pathogenesis of hepatic injury remains poorly understood. Studies on sepsis phenotyping have identified specific patient subgroups, such as those with the δ phenotype, that exhibit disproportionately high rates of hepatic dysfunction and septic shock [6]. Similarly, hyperinflammatory sepsis endotypes are associated with more severe end-organ injury, including hepatic damage [7]. However, the molecular mechanisms through which these sepsis phenotypes may drive hepatic injury are not yet well characterized.
It is known that endotoxins, released during sepsis, reach the liver, triggering the accumulation of cytokines and danger-associated molecular patterns (DAMPs) within the hepatic sinusoids. This process activates hepatic stellate and Kupffer cells, which produce inflammatory mediators such as TNF, IL-6, IL-1β, and CCL2. These mediators recruit immune cells to the liver, contributing to a shift in hepatocytes from a homeostatic to an inflammatory gene expression profile. This is marked by the induction of acute-phase proteins, including serum amyloid A-1, IL-8, and CXCL1 [8, 9]. As a result, neutrophils and monocytes accumulate in the liver, where they play a dual role: aiding pathogen clearance while also driving immune-mediated hepatic injury, particularly through TNF-induced apoptosis [8, 10].
While inflammatory responses play a significant role in mediating hepatic injury in sepsis [2, 11, 12], other factors, such as hypoxia and cholestasis, also contribute to hepatic dysfunction. Hypoxic hepatic injury is the leading cause of marked transaminase elevation in critically ill patients [13, 14]. In septic shock, microthrombi, sinusoidal obstruction, and endothelial dysfunction impair hepatic perfusion, leading to hepatic hypoxia and subsequent liver injury [8]. Septic cholestasis, though less well understood, results from non-obstructive hepatic insults that disrupt bile formation and flow [8]. Cholestasis, typically indicated by elevated ALP or GGT levels, occurs in approximately 20% of ICU patients [5], and is primarily driven by inflammatory changes in bile acid metabolism[15, 16].
Hepatic dysfunction itself is both a risk factor for sepsis and a contributor to multiorgan failure [2]. Hepatic injury, particularly in the context of septic shock, significantly increases mortality in sepsis patients [14]. Although several biomarkers of hepatic injury have been proposed [17–20]. Most have been studied in the context of liver injuries unrelated to sepsis. Moreover, due to clinical heterogeneity and a lack of robust validation studies, none of these markers have achieved widespread clinical adoption.
Among biomarkers studied in sepsis, hyaluronic acid and bilirubin levels have been associated with an increased risk of mortality in patients with hepatic injury; however, their clinical utility remains limited due to insufficient validation [21]. Similarly, sTREM-1 and presepsin have shown promise as early diagnostic markers in sepsis patients with acute hepatic failure, outperforming traditional clinical markers such as leukocyte count, CRP, and procalcitonin. Despite this, their prognostic value for clinical outcomes is still unclear [18]. Indeed, limitations for prognostication are also present with traditional liver enzymes [22, 23], emphasizing the need for further molecular markers that are able to identify hepatic injury and impart prognostic knowledge. Yet, there are no studies that make comparisons of emerging hepatic biomarkers with the traditional hepatic enzymes (ALT, AST, ALP, GGT, bilirubin) used in clinical practice in attempts to augment diagnosis.
We hypothesized that a multiplex panel of novel hepatic injury biomarkers—arginase 1 (ARG1), α-glutathione S-transferase (GSTα), malate dehydrogenase 1 (MDH1), sorbitol dehydrogenase (SDH), and 5'-nucleotidase (5-NT)—would provide accurate, sensitive, and specific identification of hepatic dysfunction in sepsis. These markers have gained increasing use in monitoring hepatotoxicity during novel drug development [24], suggesting they may also enhance the ability of traditional hepatic markers to diagnose and prognosticate sepsis-associated liver injury. Our objectives were: 1) to determine whether these biomarkers can differentiate sepsis patients with hepatic injury from those without; 2) to apply machine learning techniques to assess the relative importance of each biomarker in identifying sepsis-associated hepatic injury; and 3) to explore correlations between these biomarkers and various clinical and laboratory parameters.
Methods
Cohort and demographics
Patients with sepsis, primarily due to community-acquired pneumonia, were retrospectively enrolled from two institutions: the University of British Columbia (Vancouver, British Columbia, Canada) and Western University (London, Ontario, Canada). Sepsis was defined according to the Sepsis-3 criteria [1]. Patients were matched by age, sex, source of sepsis, and causative organism, resulting in three cohorts: 37 sepsis patients with hepatic dysfunction (S-HD), 37 sepsis patients without hepatic dysfunction (S-CON), and 18 healthy controls (HC). Healthy controls were clinically well outpatients, without comorbidities, who were electively enrolled and phlebotomized for control plasma. The healthy control group had ages ranging from 32 to 78 years, with a median (IQR) of 62.5 (53.0–65.0).
Hepatic dysfunction in sepsis was defined by an International Normalized Ratio (INR) > 1.5, in addition to either elevated bilirubin (> 22 μmol/L), elevated AST (> 59 U/L), or elevated ALT (> 33 U/L), based on the upper limits of normal for each marker. Blood samples were collected in accordance with institutional protocols, with plasma isolated by centrifugation at 1600 × g for 15 min at 4 °C. Samples were obtained on the day of ICU admission (Day-1), and subsequently on Days 3, 5, and 7 post-admission. Plasma was aliquoted into 250 µL increments and stored at -80 °C until multiplex analysis.
Demographic data, baseline characteristics, and outcomes were systematically collected. Baseline characteristics included age, sex, and comorbidities such as cardiac disease, pulmonary disease, asthma, chronic kidney disease, hepatic disease, neurologic conditions, malignancy, hematologic disorders, HIV, diabetes, rheumatologic conditions, dementia, hypertension, and malnutrition. Admission organ function metrics (leukocyte count, hemoglobin, platelet count, creatinine, ALT, AST, GGT, INR, bilirubin) and treatments administered during hospitalization (antiviral agents, antibiotics, corticosteroids, antifungals, and vasopressors) were also recorded. Outcomes assessed included mortality, acute kidney injury, acute respiratory distress syndrome, and the need for renal replacement therapy or invasive mechanical ventilation. No patients were undergoing therapeutic anticoagulation.
Protein assays and measurement
Traditional hepatic function tests, including bilirubin, albumin, ALT, AST, and GGT, were measured in both sepsis cohorts on ICU Days 1, 3, 5, and 7. Additionally, five candidate hepatic biomarkers were analyzed using the MILLIPLEX MAP Human Hepatic Injury Magnetic Bead Panel—Toxicity Multiplex Assay (HLINJMAG-75 K, Millipore Sigma, Burlington, MA). This multiplex assay measures the following human analytes: hepatic-type arginase 1 (ARG1), α-glutathione S-transferase (GSTα), malate dehydrogenase 1 (MDH1), sorbitol dehydrogenase (SDH), and 5'-nucleotidase (5'-NT).
Statistics and machine learning
Continuous variables are reported as median with interquartile range (IQR), while categorical variables are presented as frequencies and percentages. Categorical variables were analyzed using chi-square tests or Fisher’s exact test as appropriate, and comparisons of continuous variables between groups (S-HD and S-CON) were made with Mann–Whitney U tests. Data analysis was performed using GraphPad Prism (Version 8.4.0; GraphPad Software, San Diego, CA, USA).
Temporal changes in protein expression were assessed using linear mixed models with maximum likelihood estimation. These models examined changes over time by including ICU Day and hepatic dysfunction as fixed effects and patient as a random effect, with a scaled identity covariance structure and random intercept. All statistical analyses were carried out using SPSS (Version 29; IBM Corporation, Armonk, NY, USA), with a significance level set at P < 0.05.
Machine learning techniques were employed to identify differences in circulating levels of traditional and putative hepatic markers between cohorts. A random forest classifier, comprising multiple decision trees, was utilized for group classification in each comparison [25]. To ensure conservative analysis, hyperparameter tuning was not performed; the classifier was constrained to 10 trees with a maximum depth of 3. A threefold cross-validation approach was applied, and metrics were averaged across the three folds. Classification metrics included balanced accuracy, receiver operating characteristic (ROC) area under the curve (AUC), F1 score, sensitivity, specificity, precision, negative predictive value (NPV), and positive predictive value (PPV) [26]. The F1 score, representing the harmonic mean of precision and recall (sensitivity), reflects the balance between these two metrics, with a higher F1 score indicating better performance in both areas. Feature importance was assessed as the mean across the three folds. For data visualization, nonlinear dimensionality reduction was performed using the t-distributed stochastic neighbor embedding (t-SNE) algorithm. All machine learning analyses were conducted using Python 3.13, Scikit-Learn (v.1.50), and BorutaPy (v.0.3) [27, 28].
Correlations between traditional and putative hepatic markers were assessed using Pearson correlation coefficients. Correlations were considered significant if P< 0.01. All statistical analyses were performed using SPSS (Version 29; IBM Corporation, Armonk, NY, USA). Heat maps illustrating Pearson correlation values between protein markers and clinical variables were generated in R (http://www.r-project.org) using the ggplot2 package (Version 3.5.1).
Results
Demographic and clinical characteristics
Baseline characteristics indicated that the three groups were comparable in terms of age, sex, co-morbidities, and interventions (Table 1). Among sepsis patients, both S-HD and S-CON groups primarily had viral infections (COVID-19 or influenza) or were culture-negative. Patients with hepatic dysfunction (S-HD) exhibited a greater degree of illness severity, as evidenced by significantly higher MODS (P = 0.005) and SOFA scores (P < 0.001). The S-HD cohort had lower levels of hemoglobin, platelets, and albumin (P < 0.05), along with elevated levels of creatinine, ALT, AST, GGT, INR, and bilirubin compared to the S-CON cohort (P ≤ 0.002). The most common infectious focus for both cohorts was a pulmonary source. Notably, S-HD patients had a three-fold higher mortality rate compared to S-CON patients (40.1% vs. 13.5%, P = 0.017). The incidence of acute kidney injury, the need for renal replacement therapy, acute respiratory distress syndrome, and mechanical ventilation requirements were similar between the sepsis cohorts (Table 1).
Table 1.
Demographic and Clinical Characteristics of Sepsis Patients
| Variable | S-HD (n = 37) | S-CON (n = 37) | HC (n = 18) | P value |
|---|---|---|---|---|
| Age, median (IQR) | 65.0 (49.5–82.5) | 65.0 (48.0–81.5) | 62.5 (53.0–65.0) | 0.794 |
| Male:Female, n | 23:14 | 23:14 | 11:7 | > 0.999 |
| MODS, median (IQR) | 7.0 (4.0–10.0) | 4.0 (2.0–7.0) | 0.005 | |
| SOFA, median (IQR) | 7.0 (4.0–11.0) | 3.0 (2.0–6.0) | < 0.001 | |
| Comorbidities, n (%) | ||||
| Cardiac disease | 15 (40.5) | 12 (32.4) | 0.629 | |
| Pulmonary disease | 5 (13.5) | 5 (13.5) | > 0.999 | |
| Asthma | 2 (5.4) | 2 (5.4) | > 0.999 | |
| Chronic kidney disease | 9 (24.3) | 9 (24.3) | > 0.999 | |
| Hepatic disease | 3 (8.1) | 2 (5.4) | > 0.999 | |
| Neurologic disease | 3 (8.1) | 10 (27.0) | 0.064 | |
| Malignancy | 2 (5.4) | 2 (5.4) | > 0.999 | |
| Hematologic disease | 1 (2.7) | 1 (2.7) | > 0.999 | |
| AIDS/HIV | 0 (0) | 3 (8.1) | 0.239 | |
| Diabetes | 9 (24.3 | 10 (27.1) | > 0.999 | |
| Rheumatologic disease | 3 (8.1) | 5 (13.5) | 0.479 | |
| Dementia | 3 (8.1) | 6 (16.2) | 0.309 | |
| Hypertension | 17 (45.9) | 16 (43.2) | > 0.999 | |
| Malnutrition | 0 (0) | 1 (2.7) | > 0.999 | |
| Admission laboratory values, median (IQR) | ||||
| White Blood Cells | 12.3 (6.1–20.7) | 11.0 (5.4–15.75) | 0.202 | |
| Hemoglobin | 115.5 (91.5–127.5) | 135.0 (111.5–141.0) | 0.014 | |
| Platelets | 169.5 (77.0–240.5) | 239.0 (163.0–296.0) | 0.002 | |
| Creatinine | 135.0 (106.0–280.5) | 91.0 (71.0–143.0) | 0.002 | |
| ALT | 60.0 (20.0–142.0) | 17.5 (12.3–33.3) | 16.0 (19.0–25.0) | < 0.001 |
| AST | 94.0 (47.0–198.0) | 30.5 (18.5–58.5) | 22.5 (18.0–27.0) | < 0.001 |
| GGT | 49.0 (25.0–74.0) | 20.5 (11.5–49.5) | 16.5 (11.0–23.0) | 0.001 |
| INR | 1.8 (1.6–2.1) | 1.1 (1.0–1.2) | < 0.001 | |
|
Bilirubin Albumin |
22.0 (13.0–31.0) | 4.0 (3.0–6.0) | 7.0 (4.0–8.0) | < 0.001 |
| 27.0 (21.7–36.0) | 32.5 (29.0–38.0) | 42.0 (40.0–45.0) | 0.033 | |
| Interventions, n (%) | ||||
| Antivirals | 5 (13.5) | 6 (16.2) | > 0.999 | |
| Antibiotics | 34 (91.8) | 34 (91.8) | > 0.999 | |
| Corticosteroid | 23 (62.1) | 24 (64.9) | > 0.999 | |
| Antifungals | 2 (5.4) | 2 (5.4) | > 0.999 | |
| Vasopressors | 18 (48.6) | 11 (29.7) | 0.152 | |
| Sepsis, n (%) organism identified | ||||
| Viral | 19 (51.4) | 17 (45.9) | 0.816 | |
| Gram positive | 4 (10.8) | 3 (8.1) | > 0.999 | |
| Gram negative | 2 (5.4) | 1 (2.7) | > 0.999 | |
| Fungal | 0 (0) | 1 (2.7) | > 0.999 | |
| Polymicrobial | 1 (2.7) | 2 (5.4) | > 0.999 | |
| None | 11 (29.7) | 13 (35.1) | 0.804 | |
| Infectious source, n (%) | ||||
| Pulmonary | 27 (72.9) | 34 (91.8) | 0.064 | |
| Urine | 3 (8.1) | 0 (0) | 0.239 | |
| Blood | 2 (5.3) | 0 (0) | 0.493 | |
| Skin | 0 (0) | 1 (2.7) | > 0.999 | |
| None | 5 (13.5) | 2 (5.4) | 0.429 | |
| Complications, n (%) | ||||
| Dead | 15 (40.1) | 5 (13.5) | 0.017 | |
| IMV | 11 (29.7) | 12 (32.4) | > 0.999 | |
| RRT | 5 (4.1) | 2 (5.4) | 0.492 | |
| ARDS | 4 (10.8) | 1 (2.7) | 0.375 | |
| AKI | 16 (43.2) | 9 (24.3) | 0.139 | |
Significant values at P < 0.05 values are bolded (Chi-Square, Fisher’s exact, or Mann–Whitney U test as appropriate)
Abbreviations: MODS (multiple organ dysfunction score), SOFA (sequential organ failure assessment), IMV (invasive mechanical ventilation), RRT (renal replacement therapy), ARDS (acute respiratory distress syndrome), AKI (acute kidney injury)
Comparison of circulating levels of putative and traditional hepatic biomarkers
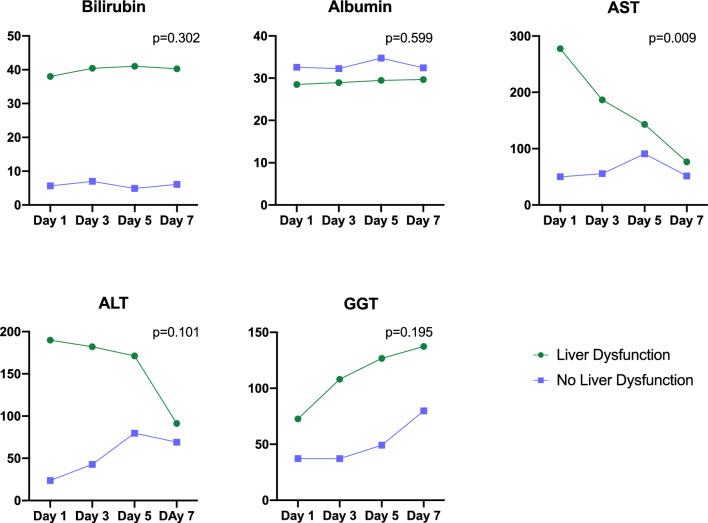
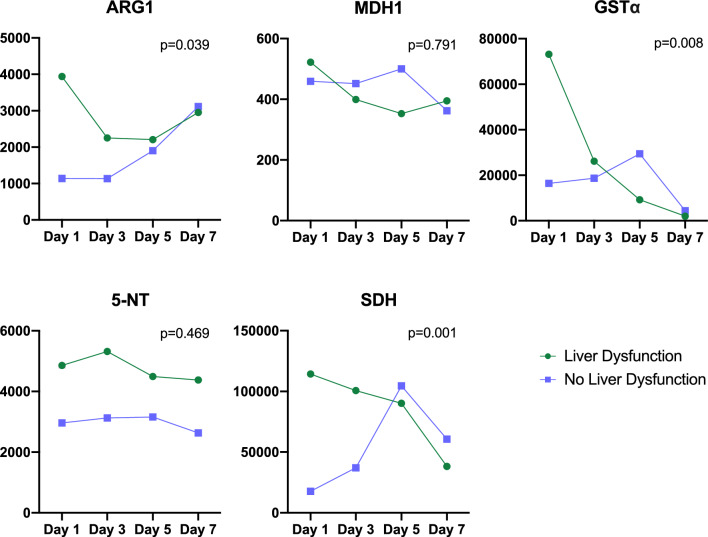
On ICU Day-1, bilirubin, ALT, AST, and GGT levels were significantly higher in S-HD patients compared to S-CON (P ≤ 0.001, Table 2). By ICU Day-7, only bilirubin remained significantly elevated in S-HD patients compared to S-CON. Additionally, ARG1, GSTα, and SDH levels were significantly higher in S-HD on ICU Day-1 (P < 0.05), and 5-NT was significantly elevated in S-HD compared to S-CON on both ICU Day-1 and Day-3 (P < 0.01, Table 3). Over the 7 ICU days, AST was the only marker to significantly decrease in S-HD patients compared to S-CON (P = 0.009, Fig. 1). Conversely, ARG1 (P < 0.039), GSTα (P = 0.008), and SDH (P = 0.001) all significantly decreased over the 7-day period in S-HD patients relative to S-CON (Fig. 2).
Table 2.
Comparison of Traditional Hepatic Dysfunction Biomarkers in S-HD and S-CON Patients Throughout ICU Stay
| Hepatic Test | S-HD | S-CON | P value |
|---|---|---|---|
| Bilirubin | |||
| Day-1 | 22.0 (13.0–31.0) | 4.0 (3.0–6.0) | < 0.001 |
| Day-3 | 15.0 (11.0–45.0) | 5.0 (3.0–7.0) | < 0.001 |
| Day-5 | 15.0 (8.8–54.5) | 5.0 (3.0–6.0) | < 0.001 |
| Day-7 | 15.0 (11.0–55.0) | 6.0 (4.0–7.0) | < 0.001 |
| Albumin | |||
| Day-1 | 27.0 (21.7–36.0) | 32.5 (29.0–38.0) | 0.033 |
| Day-3 | 30.0 (22.8–34.3) | 33.0 (27.0–37.0) | 0.695 |
| Day-5 | 31.0 (21.0–37.0) | 35.0 (31.3–38.8) | 0.042 |
| Day-7 | 31.0 (24.0–33.0) | 32.0 (27.0–37.0) | 0.274 |
| ALT | |||
| Day-1 | 60.0 (20.0–142.0) | 17.5 (12.3–33.3) | < 0.001 |
| Day-3 | 52.0 (21.0–156.0) | 22.0 (16.0–33.0) | 0.007 |
| Day-5 | 46.0 (30.3–146.3) | 27.5 (13.8–68.3) | 0.071 |
| Day-7 | 50.0 (24.0–100.0) | 51.0 (19.0–114.0) | 0.948 |
| AST | |||
| Day-1 | 94.0 (47.0–198.0) | 30.5 (18.5–58.5) | < 0.001 |
| Day-3 | 62.0 (37.0–222.0) | 34.0 (25.0–48.0) | < 0.001 |
| Day-5 | 81.0 (39.0–214.3) | 28.0 (23.3–60.5) | 0.004 |
| Day-7 | 78.0 (36.0–105.0) | 32.0 (22.0–80.0) | 0.089 |
| GGT | |||
| Day-1 | 49.0 (25.0–74.0) | 20.5 (11.5–49.5) | 0.001 |
| Day-3 | 74.0 (43.0–150.0) | 24.0 (12.0–52.0) | < 0.001 |
| Day-5 | 108.5 (69.5–172.8) | 30.5 (18.0–62.3) | < 0.001 |
| Day-7 | 94.0 (52.0–220.0) | 58.0 (17.0–108.0) | 0.071 |
Data is presented as median (IQR). P < 0.05 values bolded (Mann–Whitney U test)
Bilirubin is measured in μmol/L, albumin g/L, ALT, AST and GGT in U/L
Table 3.
Comparison of Putative Hepatic Dysfunction Biomarkers in S-HD and S-CON Patients Throughout ICU Stay
| Hepatic Test | S-HD | S-CON | P value |
|---|---|---|---|
| ARG1 | |||
| Day-1 | 1346.0 (387.7–5922.0) | 343.0 (309.5–1656.0) | 0.047 |
| Day-3 | 638.9 (304.0–1799.0) | 338.0 (184.0–768.2) | 0.191 |
| Day-5 | 1052.0 (328.4–3353.0) | 343.0 (304.0–1086.0) | 0.179 |
| Day-7 | 781.1 (328.8–1949.0) | 860.0 (349.5–1915.0) | 0.419 |
| MDH1 | |||
| Day-1 | 325.0 (315.0–355.0) | 325.0 (315.0–355.0) | 0.886 |
| Day-3 | 325.0 (315.0–350.5) | 325.0 (325.0–355.0) | 0.480 |
| Day-5 | 325.0 (315.0–355.0) | 325.0 (320.0–355.0) | 0.837 |
| Day-7 | 346.0 (322.5–355.0) | 325.0 (325.0–355.0) | 0.431 |
| GSTα | |||
| Day-1 | 3795.0 (913.4–35,195.0) | 1444.0 (926.3–2684.0) | 0.039 |
| Day-3 | 3494.0 (920.0–8711.0) | 1439.0 (634.1–1439.0) | 0.093 |
| Day-5 | 2329.0 (1320.0–9719.0) | 1729.0 (818.6–7023.0) | 0.527 |
| Day-7 | 1370.0 (553.8–2267.0) | 2843.0 (1572.0–6246.0) | 0.013 |
| 5-NT | |||
| Day-1 | 4161.0 (2642.0–6299.0) | 2769.0 (1596.0–4200.0) | 0.006 |
| Day-3 | 4055.0 (2074.0–7825.0) | 3023.0 (1403.0–4099.0) | 0.009 |
| Day-5 | 3843.0 (2260.0–4942.0) | 1692.0 (1017.0–4518.0) | 0.051 |
| Day-7 | 3733.0 (1456.0–7502.0) | 1447.0 (1110.0–4107.0) | 0.079 |
| SDH | |||
| Day-1 | 33,567.0 (15,480.0–169093.0) | 6645.0 (1625.0–24448.0) | 0.014 |
| Day-3 | 17,052.0 (3972.0–110293.0) | 9758.0 (1940.0–38457.0) | 0.317 |
| Day-5 | 52,233.0 (14,114.0–101592.0) | 18,321.0 (6131.0–61893.0) | 0.294 |
| Day-7 | 32,005.0 (5584.0–72385.0) | 43,917.0 (11,802.0–102244.0) | 0.316 |
Data is presented as median (IQR). P < 0.05 values bolded (Mann–Whitney U test)
Biomarkers are measured in pg/mL
Fig. 1.
Temporal trends of traditional hepatic dysfunction biomarkers throughout ICU stay. Linear mixed models with maximum likelihood estimation were employed to analyze changes over time in the circulating levels of traditional hepatic markers. Statistical significance was set at P < 0.05. The x-axis represents the day of ICU stay, and the y-axis shows the mean plasma concentration of each biomarker. Data for S-HD patients are depicted in green, while S-CON patients are shown in purple. Bilirubin levels did not exhibit a significant change throughout the ICU stay in S-HD patients compared to S-CON patients. Albumin levels remained stable throughout the ICU stay in S-HD patients relative to S-CON patients. AST levels significantly decreased over the ICU stay in S-HD patients compared to S-CON patients. ALT levels did not show significant changes throughout the ICU stay in S-HD patients compared to S-CON patients. GGT levels did not significantly change over time in S-HD patients compared to S-CON patients. Note: Bilirubin is measured in μmol/L, albumin in g/L, and ALT, AST, and GGT in U/L
Fig. 2.
Temporal trends of hepatic dysfunction biomarkers during ICU stay. Linear mixed models with maximum likelihood estimation were employed to assess changes over time in plasma levels of hepatic biomarkers. Statistical significance was set at P < 0.05 P < 0.05. The x-axis represents the day of ICU stay, and the y-axis depicts the mean plasma biomarker concentration (pg/mL). Biomarkers for the S-HD group are indicated in green, while those for the S-CON group are indicated in purple. ARG1 levels significantly decreased over the course of the ICU stay in the S-HD group compared to the S-CON group. MDH1 levels did not show significant changes over time in the S-HD group compared to the S-CON group. GSTα levels significantly decreased throughout the ICU stay in the S-HD group relative to the S-CON group. 5-NT levels did not change significantly over time in the S-HD group compared to the S-CON group, though they remained persistently elevated. SDH levels significantly decreased over the ICU stay in the S-HD group compared to the S-CON group
Random forest classification and feature reduction of hepatic biomarkers
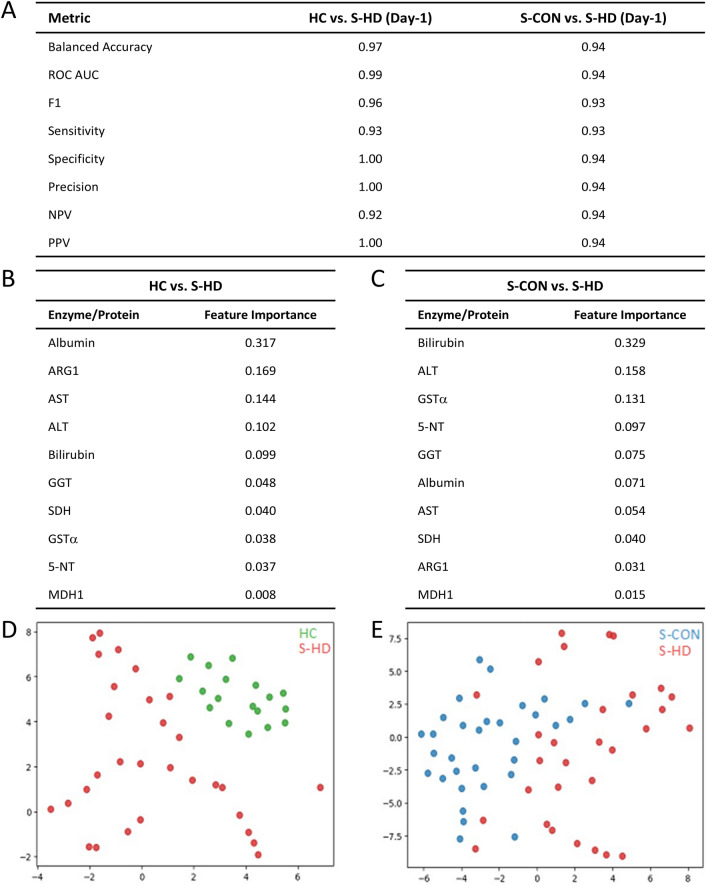
Random forest classification, feature reduction, and t-SNE analyses were employed to evaluate the accuracy of liver function tests (LFTs) and novel hepatic function biomarkers (Fig. 3). The combined use of LFTs and novel biomarkers demonstrated high accuracy, sensitivity, specificity, and precision in identifying S-HD patients (Fig. 3A). Feature ranking was performed to assess the relative importance of each biomarker in distinguishing hepatic dysfunction in sepsis. Among the ten biomarkers in the combined model, low albumin (feature importance 0.317) was the most effective in identifying hepatic dysfunction compared to healthy controls (HC) (Fig. 3B). Among the novel biomarkers, ARG1 (feature importance 0.169) was the most effective in differentiating S-HD patients from HC (Fig. 3B). When comparing S-HD to S-CON patients, bilirubin (feature importance 0.329) was the most indicative of hepatic dysfunction in the combined model (Fig. 3C). Of the five novel biomarkers, GSTα (feature importance 0.131) was most effective in distinguishing S-HD patients from S-CON (Fig. 3C). Both traditional and novel hepatic dysfunction biomarkers effectively separated HC from S-HD patients on ICU Day-1 (Fig. 3D). Although S-HD patients were distinguishable from S-CON patients, the separation was less pronounced compared to the HC-S-HD comparison (Fig. 3E).
Fig. 3.
Random forest classification, feature importance, and t-SNE analysis of biomarkers in sepsis patients with and without hepatic dysfunction. A) Random Forest classification was used to distinguish between healthy controls (HC), sepsis patients with hepatic dysfunction (S-HD), and sepsis patients without hepatic dysfunction (S-CON) based on traditional and putative hepatic markers. The model achieved high sensitivity, specificity, precision, and recall. B) Relative importance of biomarkers for differentiating HC from S-HD on ICU Day 1, as determined by Random Forest analysis. C) Relative importance of biomarkers for differentiating S-HD from S-CON on ICU Day 1, as determined by Random Forest analysis. D) t-SNE visualization of traditional and putative hepatic markers on ICU Day 1, plotting HC (green) versus S-HD (red) in 2D space. Axes are dimensionless and represent the reduced feature space. E) t-SNE visualization of traditional and putative hepatic markers on ICU Day 1, plotting S-CON (blue) versus S-HD (red) in 2D space. Axes are dimensionless and represent the reduced feature space.
Correlations of novel putative hepatic biomarkers and clinical parameters
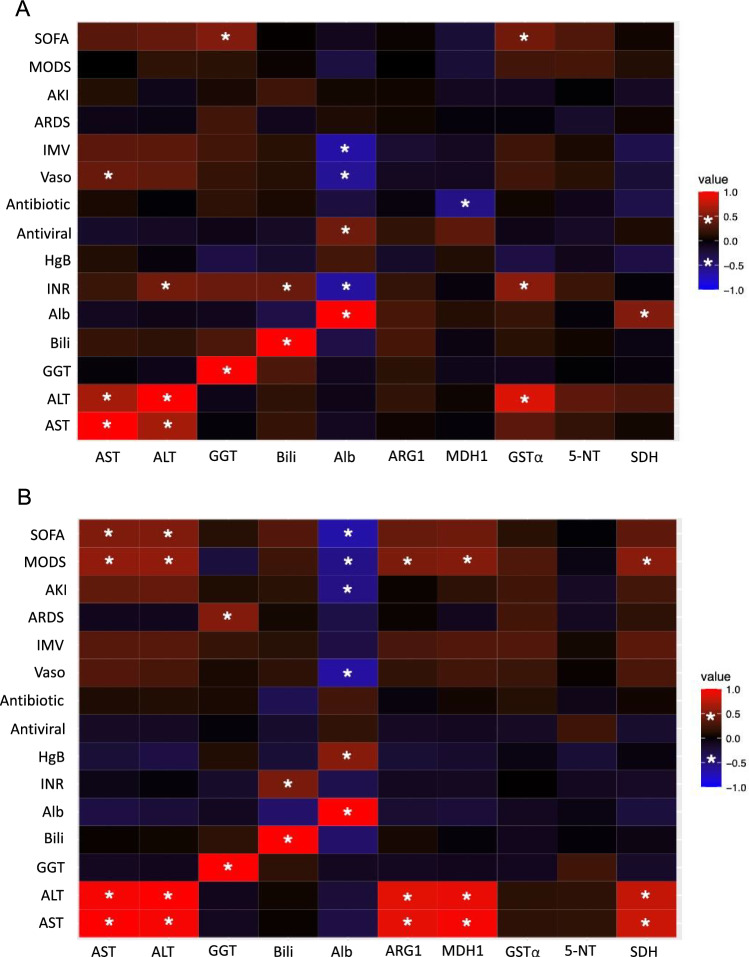
Correlations between our novel hepatic dysfunction biomarkers, clinical parameters, and liver function tests (LFTs) were examined in S-CON patients (Fig. 4A; Supplementary File 1). Notable associations were observed between these biomarkers and certain treatments. For instance, in S-CON patients, MDH1 showed a negative correlation with antibiotic use, while GSTα and SDH had positive correlations with the need for renal replacement therapy (RRT) on ICU Days 3 and 5. Additionally, GSTα was positively correlated with ALT, INR, and SOFA score, and SDH was positively correlated with albumin concentration (Fig. 4A).
Fig. 4.
Correlations between hepatic dysfunction biomarkers and clinical parameters on ICU day 1. Heat maps illustrate the correlations between traditional and putative hepatic dysfunction biomarkers and clinical variables for sepsis patients without hepatic dysfunction (S-CON) (A) and with hepatic dysfunction (S-HD) (B). Biomarkers are listed on the x-axis, and clinical parameters are listed on the y-axis. Significant correlations are defined by a Pearson R-value of ≥ 0.5 or ≤ − 0.5 with P < 0.01 P < 0.01, indicated by an asterisk (*). Positive correlations are shown in red, while negative correlations are shown in blue. SOFA (sequential organ failure assessment), MODS (multiple organ dysfunction score), AKI (acute kidney injury), ARDS (acute respiratory distress syndrome), IMV (invasive mechanical ventilation), Vaso (vasopressors), HgB (hemoglobin), INR (international normalized ratio), Alb (albumin), Bili (bilirubin), GGT (gamma glutamyl transferase), ALT (alanine aminotransferase), AST (aspartate aminotransferase)
In S-HD patients on ICU Day-1, ARG1, MDH1, and SDH were positively correlated with ALT, AST, and MODS (Fig. 4B). Correlations for ICU Days 3–7 are detailed in Supplementary File 1. Specifically, ARG1 and MDH1 were positively correlated with ALT, AST, and antifungal use on ICU Day-3. On ICU Day-5, MDH1 showed a positive correlation with ALT and AST, and by ICU Day-7, MDH1 was positively correlated with white blood cell count, ALT, and AST.
Discussion
We present a novel case–control study that identified five potential biomarkers of hepatic dysfunction in sepsis patients: ARG1, MDH1, GSTα, 5-NT, and SDH. This study combined traditional statistical methods with machine learning to assess the feature importance of these biomarkers in detecting hepatic dysfunction in sepsis. The integrated approach demonstrated that a combination of these novel and traditional hepatic injury biomarkers offered high sensitivity and specificity for identifying hepatic dysfunction. Notably, GSTα and 5-NT were found to be more crucial than GGT, albumin, or AST in this combined model for identifying sepsis patients with hepatic dysfunction. Despite the common occurrence of hepatic injury in sepsis, traditional liver markers have limited prognostic and diagnostic value [22, 23]. This study highlights previously uncharacterized biomarkers of hepatic injury in sepsis and establishes a basis for future clinical validation and research.
Our cohorts were well-matched by design for age, sex, and comorbidities and were similar to prior studies in sepsis [29–34]. S-HD patients had greater illness severity (MODS, SOFA) and higher mortality than S-CON patients [2]. Those with hepatic dysfunction received similar interventions as those without, but had higher ALT, AST, GGT, INR, bilirubin, and creatinine values, while hemoglobin, platelets, and albumin were lower. The source of sepsis in our cohorts was primarily of respiratory origin, with a significant proportion of cases infected with viruses or being culture-negative. Such a predominance of pulmonary infection is in keeping with pulmonary sepsis being a common precipitant of ICU admission and mortality [33, 35].
The S-HD cohort was identified based on elevated INR and increased levels of either bilirubin or transaminases. As anticipated, this cohort exhibited significantly higher levels of bilirubin, ALT, AST, and GGT on ICU Day 1 compared to the S-CON cohort. Additionally, our candidate biomarkers for hepatic dysfunction were effective in classifying patients with liver impairment, particularly early in the ICU admission. On ICU Day 1, S-HD patients had elevated levels of ARG1, GSTα, and SDH, while 5-NT levels were higher on both ICU Day 1 and Day 3 compared to the S-CON patients. Among traditional hepatic biomarkers, only AST showed a significant decrease throughout the ICU stay in patients with hepatic dysfunction compared to those without. In contrast, the levels of ARG1, GSTα, and SDH decreased significantly in patients with hepatic dysfunction, whereas 5-NT remained elevated throughout the ICU stay. These observations underscore the need for further investigation into the dynamic behavior of these biomarkers in relation to sepsis and hepatic injury.
Unlike conventional statistical methods, our machine learning approach elucidates the contribution of each biomarker to the identification of hepatic dysfunction. We present a biomarker panel that combines both traditional and novel markers, which effectively distinguishes hepatic dysfunction in sepsis patients from those without it and from healthy control subjects, demonstrating high specificity, sensitivity, and precision. Among the novel biomarkers, ARG1 was the most informative in differentiating S-HD patients from healthy controls (HC), while GSTα was the most informative in distinguishing S-HD from S-CON in a combined model of all ten biomarkers. Given our definition of hepatic dysfunction—characterized by elevated bilirubin or transaminases—it was anticipated that traditional biomarkers would perform well in distinguishing between sepsis patients with and without hepatic dysfunction. Indeed, bilirubin and ALT showed high feature importance for identifying S-HD compared to S-CON. However, GSTα and 5-NT exhibited higher feature importance than traditional biomarkers such as GGT and albumin, suggesting that these novel markers offer substantial potential for characterizing hepatic dysfunction in sepsis. These findings underscore the value of incorporating putative biomarkers, which provide additional and distinct information beyond the traditional markers that have long been used in clinical practice [36].
Clinically, the novel hepatic dysfunction biomarkers show correlations with traditional hepatic biomarkers and illness severity scores. On ICU Day 1, biomarkers such as ARG1, MDH1, and SDH correlated with admission levels of ALT, AST, and MODS in patients with hepatic dysfunction but showed no such correlations in patients without hepatic dysfunction. However, we did not observe any strong correlations with clinical interventions or patient outcomes, underscoring the complexity and variability of predicting outcomes in critical illness, sepsis, and hepatic failure [37–40]. Accurate disease prognostication remains a challenge in translational proteomics, irrespective of the disease state [41]. Traditional liver markers (AST, ALT, ALP, GGT, bilirubin, albumin) also face limitations in predicting disease severity, clinical outcomes, and treatment responses, despite their widespread clinical use [22]. Nevertheless, our exploratory study identifies putative markers of hepatic dysfunction in sepsis with high sensitivity and specificity, offering insights that extend beyond traditional liver markers. Further characterization of these putative biomarkers could enhance the range of biochemical tests available for evaluating hepatic injury in sepsis, potentially improving clinical assessment and management.
Arginase exists in two isoforms, with ARG1 being a cytoplasmic enzyme constitutively expressed throughout hepatic tissue, and ARG2 being found in the mitochondria of cells in the brain, intestines, and kidney [42]. ARG1 is a member of the urea cycle involved in converting L-arginine to urea and L-ornithine, which undergoes metabolism into proline and polyamides to drive collagen synthesis and cellular proliferation. Enzymatic deficiency leads to impaired ureagenesis [43]. ARG1 may also correlate with cirrhosis severity [44], and hepatic steatosis [45]. Hepatic-specific knockouts of ARG1 lead to profound arginase deficiency, which may result in neurologic symptoms [46]. In relation to hepatic disease, ARG1 may regulate nitric oxide levels and vascular function [47], modulate immune response [48], and facilitate tissue repair [49].
While MDH1 is expressed in the liver, it is also expressed in cardiac and skeletal muscle, kidney, spleen, intestine, and testes [50]. MDH1 catalyzes the reduction of aromatic alpha-keto acids in the presence of NADH. MDH1 plays an essential role in the malate-aspartate shuttle and the tricarboxylic acid cycle and is important in mitochondrial NADH supply for oxidative phosphorylation. MDH1 acetylation appears to regulate energy metabolism in acute hepatic failure [51, 52]. In relation to hepatic injury, MDH1 deacetylation appears to promote acute hepatic failure by regulating NETosis [52]. MDH1 has also been hypothesized to augment prognostication in sepsis when combined with lactate [53].
GSTα is predominantly expressed in the hepatic, kidney, and testes [54]. GSTα catalyzes the conjugation of the reduced form of glutathione to xenobiotic substrates for the purpose of detoxification. GSTα is a previously reported as a marker of hepatic damage [55], with increasing levels associated with hepatic damage in Hepatitis B or C infection [56, 57], and hemodialysis patients [58]. Moreover, previous reports corroborate our findings, suggesting GST is a marker of hepatic damage during polymicrobial sepsis [59].
5-NT is most heavily expressed in the liver, gastrointestinal tract, smooth muscle, and female reproductive tissue [60]. 5-NT is an enzyme that catalyzes the cleavage of 5’ nucleotides (dephosphorylation of nucleotides to nucleosides) with prominent expression in the plasma membrane of hepatocytes [61]. There is emerging evidence for 5-NT in regulating hepatic responses to injury. 5-NT activity has been reported to increase in obstructive jaundice, parenchymal hepatic disease, and hepatic metastases [62]. It is also found to be elevated in viral hepatitis, alcoholic hepatic disease, and cirrhosis [62].
SDH is highly expressed in liver, but is also expressed in most human tissues, being an enzyme that regulates carbohydrate metabolism, whereby sorbitol is converted to fructose. SDH is reported to be elevated in hepatic disease [63], while other studies report decreased SDH activity in hepatic injury [64]. Experimental inhibition of SDH appears to protect from ischemia–reperfusion induced injury via elevated glycolytic flux and enhanced sirtuin 1 activity [65]. SDH level also appears to facilitate the prognostication of recurrence-free survival in hepatocellular carcinoma patients [66].
Our study has several notable limitations. First, the sample size of sepsis patients was relatively small, though it aligns with the scope of other exploratory studies examining proteomic variations in sepsis [67]. Second, the level of transaminase elevation and hepatic dysfunction observed in our cohort might be considered mild. Nevertheless, these findings reflect the hepatic injury commonly seen in sepsis patients and do not address cases of severe hepatic decompensation or those who succumb to hepatic failure. Third, the majority of our sepsis patients had viral infections or culture-negative sepsis, suggesting that further investigation is needed to evaluate the utility of these putative hepatic dysfunction biomarkers in cohorts with predominantly bacterial sepsis. Fourth, since most cases of sepsis originated from pulmonary infections, additional research is required to explore sepsis from other sources. Investigating these biomarkers in relation to various causes of liver injury—such as toxic exposures, cirrhosis, and autoimmune liver disease—could reveal whether similar patterns occur outside the context of sepsis. Finally, the patients with hepatic dysfunction in our study had higher MODS/SOFA scores and mortality rates compared to those without hepatic dysfunction. This raises the possibility that the elevated levels of the putative biomarkers may be more reflective of overall illness severity rather than specific hepatic dysfunction.
Conclusions
Our study identifies five novel biomarkers—ARG1, MDH1, GSTα, 5-NT, and SDH—that exhibit high sensitivity and specificity in detecting hepatic dysfunction in sepsis. Utilizing machine learning, we present the first study to characterize a novel multiplex panel of circulating proteins, which enhances the ability to identify hepatic dysfunction compared to traditional liver function tests. However, these results require further validation before these biomarkers can be recommended for clinical use.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the front-line healthcare workers and the patients and their families for participating in this research study.
Abbreviations
- S-HD
Sepsis with hepatic dysfunction
- S-CON
Sepsis without hepatic dysfunction
- HC
Healthy control subjects
- MODS
Multiple organ dysfunction score
- SOFA
Sequential organ failure assessment score
- IMV
Invasive mechanical ventilation
- RRT
Renal replacement therapy
- ARDS
Acute respiratory distress syndrome
- AKI
Acute kidney injury
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
Gamma glutamyltransferase
- ARG1
Arginase 1
- MDH1
Malate dehydrogenase 1
- GSTα
Glutathione s-transferase
- 5-NT
5’-Nucleotidase
- SDH
Sorbitol dehydrogenase
- ICU
Intensive care unit
- IQR
Interquartile range
- INR
International normalized ratio
Author contributions
DDF conceived and designed the study. LVRN, MS, JAR and DDF collected human samples and clinical data. LRVN, MAP, MD, MRM and GC analyzed all data. LVRN and DDF wrote the manuscript with input from all other authors. All authors read and approved the final manuscript.
Funding
DDF received study funding from the London Health Sciences Foundation (https://lhsf.ca/), the London Community Foundation and the AMOSO Innovation Fund. The Captivate cohort research was funded by Dr. Russell’s CIHR grants # RN420682 – 439993, RN420682 – 439993 and RN474081 – 473647.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
Dr. Russell reports patents owned by the University of British Columbia (UBC) that are related to (1) the use of PCSK9 inhibitor(s) in sepsis, (2) the use of vasopressin in septic shock and (3) a patent owned by Ferring for use of selepressin in septic shock. Dr. Russell is an inventor on these patents. Dr. Russell is a shareholder in Molecular You Corp. Dr. Russell was a co-founder, Director and shareholder of Cyon Therapeutics Inc. (now closed) and Resolve Nanotherapeutics. Dr. Russell is the Senior Research Advisor of the British Columbia, Canada Post COVID – Interdisciplinary Clinical Care Network (PC-ICCN). Dr. Russell is no longer consulting for any industry. Dr. Russell reports receiving consulting fees in the last 3 years as a funded member of the Data and Safety Monitoring Board (DSMB) of an NIH-sponsored trial of plasma in COVID-19 (PASS-IT-ON) (2020-2021). Dr. Russell is the non-funded Chair of the Data and Safety Monitoring Board (DSMB) of a trial UC CISS II of stem cells in sepsis. Dr. Russell was a non-funded Science Advisor and member, Government of Canada COVID-19 Therapeutics Task Force (June 2020 – 2021). Dr. Russell has received grants for COVID-19 and for pneumonia research: 6 from the Canadian Institutes of Health Research (CIHR) and 3 from the St. Paul’s Foundation (SPF).
Ethics approval and consent to participate
This study was approved by the Western University, Human Research Ethics Board (REB #s 6970; #100036; # 6963). Our experimental methods are performed in accordance with ethical standards of the responsible committee on human experimentation with the Helsinki Declaration of 1975. Informed written consent was obtained from either participants or their legal guardians.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
James A. Russell, Email: Jim.Russell@hli.ubc.ca
Douglas D. Fraser, Email: douglas.fraser@lhsc.on.ca
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33(6):498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, Fang Q, Xu Q, Wang D, Jin Y. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35(11):2538–46. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. [DOI] [PubMed] [Google Scholar]
- 5.Kramer L, Jordan B, Druml W, Bauer P, Metnitz PG. Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Crit Care Med. 2007;35(4):1099-e1097. [DOI] [PubMed] [Google Scholar]
- 6.Seymour CW, Kennedy JN, Wang S. Chang C-CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H: Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha P, Kerchberger VE, Willmore A, Chambers J, Zhuo H, Abbott J, Jones C, Wickersham N, Wu N, Neyton L. Identifying molecular phenotypes in sepsis: an analysis of two prospective observational cohorts and secondary analysis of two randomised controlled trials. Lancet Respir Med. 2023;11(11):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strnad P, Tacke F, Koch A, Trautwein C. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14(1):55–66. [DOI] [PubMed] [Google Scholar]
- 9.Beyer D, Hoff J, Sommerfeld O, Zipprich A, Gaßler N, Press AT. The liver in sepsis: molecular mechanism of liver failure and their potential for clinical translation. Mol Med. 2022;28(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12(3):201–13. [DOI] [PubMed] [Google Scholar]
- 11.Szabo G, Romics L, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis. 2002;6(4):1045–66. [DOI] [PubMed] [Google Scholar]
- 12.Nesseler N, Launey Y, Aninat C, Morel F, Mallédant Y, Seguin P. Clinical review: the liver in sepsis. Crit Care. 2012;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Schöniger-Hekele M, Madl C. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37:1302–10. [DOI] [PubMed] [Google Scholar]
- 14.Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Kitzberger R, Warszawska J. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009;35:1397–405. [DOI] [PubMed] [Google Scholar]
- 15.Vanwijngaerden YM, Wauters J, Langouche L, Vander Perre S, Liddle C, Coulter S, Vanderborght S, Roskams T, Wilmer A, Van den Berghe G. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology. 2011;54(5):1741–52. [DOI] [PubMed] [Google Scholar]
- 16.Horvatits T, Drolz A, Rutter K, Roedl K, Langouche L, Van den Berghe G, Fauler G, Meyer B, Hülsmann M, Heinz G. Circulating bile acids predict outcome in critically ill patients. Ann Intensive Care. 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravindra KC, Vaidya VS, Wang Z, Federspiel JD, Virgen-Slane R, Everley RA, Grove JI, Stephens C, Ocana MF, Robles-Díaz M. Tandem mass tag-based quantitative proteomic profiling identifies candidate serum biomarkers of drug-induced liver injury in humans. Nat Commun. 2023;14(1):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Huang Z-B, Li H, Zheng X, Chen J-J, Wang X-B, Qian Z-P, Liu X-X, Fan X-G, Hu X-W. Early diagnostic biomarkers of sepsis for patients with acute-on-chronic liver failure: a multicenter study. Infectious diseases and therapy. 2021;10:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mookerjee RP. Prognosis and biomarkers in acute-on-chronic liver failure. InSeminars in Liver Disease 2016 (Vol. 36, No. 02, pp. 127-132). Thieme Medical Publishers. [DOI] [PubMed]
- 20.Fu S, Wu D, Jiang W, Li J, Long J, Jia C, Zhou T. Molecular biomarkers in drug-induced liver injury: challenges and future perspectives. Front Pharmacol. 2020;10:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen J-U, Peters L, Itenov TS, Bestle M, Thormar KM, Mohr TT, Lundgren B, Grarup J, Lundgren JD. Biomarker-assisted identification of sepsis-related acute liver impairment: a frequent and deadly condition in critically ill patients. Clin Chem Lab Med (CCLM). 2019;57(9):1422–31. 10.1515/cclm-2018-1350. [DOI] [PubMed] [Google Scholar]
- 22.Kluge M, Tacke F. Liver impairment in critical illness and sepsis: the dawn of new biomarkers? Ann Trans Med. 2019;7(S8):S258–S258. 10.21037/atm.2019.12.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Eldere A, Pirani T. Liver intensive care for the general intensivist. Anaesthesia. 2023;78(7):884–901. [DOI] [PubMed] [Google Scholar]
- 24.Goodsaid FM, Frueh FW, Mattes W. The predictive safety testing consortium: a synthesis of the goals, challenges and accomplishments of the critical path. Drug Discov Today Technol. 2007;4(2):47–50. [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Garreau D, von Luxburg U. When do random forests fail?. Advances in neural information processing systems. 2018;31
- 26.Bradley AP. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recogn. 1997;30(7):1145–59. [Google Scholar]
- 27.Kursa MB, Rudnicki WR. Feature selection with the Boruta package. J Stat Softw. 2010;36:1–13. [Google Scholar]
- 28.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V. Scikit-learn: machine learning in python. J Mach Learn Res. 2011;12:2825–30. [Google Scholar]
- 29.Van Nynatten LR, Slessarev M, Martin CM, Leligdowicz A, Miller MR, Patel MA, Daley M, Patterson EK, Cepinskas G, Fraser DD. Novel plasma protein biomarkers from critically ill sepsis patients. Clin Proteomics. 2022;19(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21. [DOI] [PubMed] [Google Scholar]
- 31.Sinapidis D, Kosmas V, Vittoros V, Koutelidakis IM, Pantazi A, Stefos A, Katsaros KE, Akinosoglou K, Bristianou M, Toutouzas K. Progression into sepsis: an individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect Dis. 2018;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Nynatten LR, Miller MR, Patel MA, Daley M, Filler G, Badrnya S, Miholits M, Webb B, McIntyre CW, Fraser DD. A novel multiplex biomarker panel for profiling human acute and chronic kidney disease. Sci Rep. 2023;13(1):21210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MA, Knauer MJ, Nicholson M, Daley M, Van Nynatten LR, Cepinskas G, Fraser DD. Organ and cell-specific biomarkers of Long-COVID identified with targeted proteomics and machine learning. Mol Med. 2023;29(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iosef C, Knauer MJ, Nicholson M, Van Nynatten LR, Cepinskas G, Draghici S, Han VK, Fraser DD. Plasma proteome of Long-COVID patients indicates HIF-mediated vasculo-proliferative disease with impact on brain and heart function. J Transl Med. 2023;21(1):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeganathan N, Yau S, Ahuja N, Otu D, Stein B, Fogg L, Balk R. The characteristics and impact of source of infection on sepsis-related ICU outcomes. J Crit Care. 2017;41:170–6. [DOI] [PubMed] [Google Scholar]
- 36.Mclntyre N, Rosalki S. Tests of the functions of the liver. In: Scientific foundations of biochemistry in clinical practice. Elsevier; 1994. p. 383–98. 10.1016/B978-0-7506-0167-2.50027-3. [Google Scholar]
- 37.Karvellas CJ, Bagshaw SM. Advances in management and prognostication in critically ill cirrhotic patients. Curr Opin Crit Care. 2014;20(2):210–7. [DOI] [PubMed] [Google Scholar]
- 38.Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, Baron RM, Bauer M, Buchman TG, Calfee CS. Redefining critical illness. Nat Med. 2022;28(6):1141–8. [DOI] [PubMed] [Google Scholar]
- 39.Herridge MS. Prognostication and intensive care unit outcome: the evolving role of scoring systems. Clin Chest Med. 2003;24(4):751–62. [DOI] [PubMed] [Google Scholar]
- 40.Sy E, Ronco JJ, Searle R, Karvellas CJ. Prognostication of critically ill patients with acute-on-chronic liver failure using the chronic liver failure–sequential organ failure assessment: a Canadian retrospective study. J Crit Care. 2016;36:234–9. [DOI] [PubMed] [Google Scholar]
- 41.Frangogiannis NG. Biomarkers: hopes and challenges in the path from discovery to clinical practice. Transl Res. 2012;159(4):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochemical Journal. 1998;336(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sin YY, Baron G, Schulze A, Funk CD. Arginase-1 deficiency. J Mol Med. 2015;93(12):1287–96. [DOI] [PubMed] [Google Scholar]
- 44.Purnamaningsih SM. The serum Arginase-1 correlation to child-pugh scores in predicting the severity of cirrhosis. Bali Medical Journal. 2017;6(3):606–10. [Google Scholar]
- 45.Alisi A, Comparcola D, De Stefanis C, Nobili V. Arginase 1: a potential marker of a common pattern of liver steatosis in HCV and NAFLD children. J Hepatol. 2015;62(5):1207–8. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne LL, Sin YY, Al-Dirbashi OY, Li X, Hurlbut DJ, Funk CD. Liver-specific knockout of arginase-1 leads to a profound phenotype similar to inducible whole body arginase-1 deficiency. Mol Genet Metab Rep. 2016;9:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163(7):3771–7. [PubMed] [Google Scholar]
- 49.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo ASY, Liew CT, Ngai SM, Tsui SKW, Fung KP, Lee CY, Waye MMY. Developmental regulation and cellular distribution of human cytosolic malate dehydrogenase (MDH1). J Cell Biochem. 2005;94(4):763–73. [DOI] [PubMed] [Google Scholar]
- 51.Shi C, Zhang Y, Chen Q, Wang Y, Zhang D, Guo J, Zhang Q, Zhang W, Gong Z. The acetylation of MDH1 and IDH1 is associated with energy metabolism in acute liver failure. Iscience. 2024;27(5):109678. 10.1016/j.isci.2024.109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Shi C, Guo J, Zhang D, Zhang Y, Zhang L, Gong Z. IDH1/MDH1 deacetylation promotes acute liver failure by regulating NETosis. Cell Mol Biol Lett. 2024;29(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, Du L, Zhang Z. Potential biomarkers in septic shock besides lactate. Exp Biol Med. 2020;245(12):1066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010;17(9):1373–80. [DOI] [PubMed] [Google Scholar]
- 55.Maina I, Rule JA, Wians FH Jr, Poirier M, Grant L, Lee WM. α-Glutathione S-transferase: a new biomarker for liver injury? J Appl Lab Med. 2016;1(2):119–28. [DOI] [PubMed] [Google Scholar]
- 56.Abdel-Moneim SM, Sliem H. Significance of serum alpha-glutathione S-transferase assessment in hepatitis C patients with different alanine aminotransferase patterns. Gastroenterol Res. 2011;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czuczejko J, Mila-Kierzenkowska C, Szewczyk-Golec K. Plasma α-Glutathione S-Transferase evaluation in patients with acute and chronic liver injury. Can J Gastroenterol Hepatol. 2019;2019:1–6. 10.1155/2019/5850787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boran M, Cetin S. Plasma alpha-glutathione S-transferase (alpha-GST) as a marker of liver damage in hemodialysis patients. Nephrol, Dial, Trans: Off Publ Eur Dial Trans Assoc-Eur Renal Assoc. 1998;13(5):1323–4. [DOI] [PubMed] [Google Scholar]
- 59.Koo DJ, Zhou M, Chaudry IH, Wang P. Plasma α-glutathione S-transferase: a sensitive indicator of hepatocellular damage during polymicrobial sepsis. Arch Surg. 2000;135(2):198–203. [DOI] [PubMed] [Google Scholar]
- 60.Alcedo KP, Bowser JL, Snider NT. The elegant complexity of mammalian ecto-5′-nucleotidase (CD73). Trends Cell Biol. 2021;31(10):829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schell MJ, Maurice M, Stieger B, Hubbard AL. 5’nucleotidase is sorted to the apical domain of hepatocytes via an indirect route. J Cell Biol. 1992;119(5):1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hyder MA, Hasan M, Mohieldein A. Comparative study of 5’-Nucleotidase test in various liver diseases. J Clin Diagn Res: JCDR. 2016;10(2):BC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiesner IS, Rawnsley HM, Brooks FP, Sentor JR. Sorbitol dehydrogenase in the diagnosis of liver disease. Am J Dig Dis. 1965;10:147–51. [DOI] [PubMed] [Google Scholar]
- 64.Asada M, Galambos JT. Sorbitol dehydrogenase and hepatocellular injury: an experimental and clinical study. Gastroenterology. 1963;44(5):578–87. [Google Scholar]
- 65.Zhang C, Li X, Liu Q. Sorbitol dehydrogenase inhibitor protects the liver from ischemia/reperfusion-induced injury via elevated glycolytic flux and enhanced sirtuin 1 activity. Mol Med Rep. 2015;11(1):283–8. [DOI] [PubMed] [Google Scholar]
- 66.Jeon D, Choi W-M, Kim J-S, Jung Y, Lee S-Y, Seo HR, Kim KM. Serum sorbitol dehydrogenase as a novel prognostic factor for hepatocellular carcinoma after surgical resection. Cancers. 2021;13(23):6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierrakos C, Vincent J-L. Sepsis biomarkers: a review. Crit Care. 2010;14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.