Abstract
In this study, the endophytic fungus Coniothyrium chaingmaiense-KUMBMDBT-25 was isolated from the healthy stem of Euphorbia tirucalli, mass cultivated by submerged fermentation, and extracted using ethyl acetate as a solvent. The extract was subjected to GC-MS analysis. The synthesized Con-AgNPs were characterized through various bioanalytical methods. The synthesis was confirmed by Bio- spectrophotometry, which showed an absorption peak at 404 nm. FTIR analysis verified the reduction and capping of Con-AgNPs, displaying peaks corresponding to various functional groups. SEM-EDAX and HR-TEM examinations revealed that the Con-AgNPs were spherical, and EDAX analysis confirmed the presence of silver atoms at 3 keV. XRD studies revealed the crystalline structure of Con-AgNPs. DLS and Zeta potential tests determined the size and stability of the synthesized Con-AgNPs, which were 65.81 nm. The Con-AgNPs demonstrated strong antibacterial activity against P. aeruginosa (14.06 ± 0.11 mm, 10 mg/mL) and effective antifungal activity against A. flavus (13.03 ± 0.05 mm, 10 mg/mL). Con-AgNPs exhibited notable biological attributes, including a cytotoxic effect of up to 38.82% and 19.15% at 200 µg/mL in an MTT assay measuring cell viability. Additionally, the nanoparticles demonstrated significant anti-inflammatory effects in both in vitro and in vivo studies, validating the biological and pharmacological potential of Con-AgNPs.
Keywords: Endophytic fungus, Coniothyrium chaingmaiense, GC-MS, Con-AgNPs, Pharmacological properties
Subject terms: Biochemistry, Biological techniques, Biotechnology, Cancer, Microbiology, Molecular biology
Introduction
Nanotechnology is one of the fastest growing fields of study due to its widespread applicability in science and industry1. Now a days it has become a rapidly growing field of science that involves the synthesis of various NPs. NPs can be created naturally or artificially2. NPs can be synthesized using a variety of chemical and physical methods; however, these methods are inefficient, unfriendly to the environment, capital intensive, and toxic3. Both bottom-up and top-down methods are utilized for synthesis of NPs. Solvothermal, co-precipitation, sonochemistry, pyrolysis, and green methods are bottom-up processes, while pulsed laser ablation, spray pyrolysis, and ball milling are top-down. Chemical and top-down methods are inefficient, polluting, and time-consuming. To overcome these constraints, scientists intend to synthesis NPs from bacteria, fungi, and plants. Environmentally friendly plant-mediated synthetic technologies are being developed. Nanomaterial scientists have prioritized eco-friendly nanoscale material manufacturing technologies in recent years4–6.
Nanoparticles are defined as materials that contain at least one external element and range in size from around 1 to 100 nm. The promising potential of metal- and metal-oxide-based nanoparticles for developing new strategies for developing antibacterial drugs and overcoming antibacterial resistance have been shown. Shape, size, and surface area are the primary morphological characteristics that determine the antibacterial potential of NPs7. Green nanoparticles offer a novel vehicle for targeted and safer delivery of medicines, a possible cancer therapy alternative. Green synthesis produces Ag, Cu, Au, ZnO, Se, CuO, and other NPs with distinct biological activity. Metal oxide NPs have been studied for a decade due to their many technical applications. NPs are an interesting inorganic material used in semi-conductors, energy conservation, textiles, cosmetics, electronics, health care, catalysis, and chemical sensors. NPs have diverse biological functions, including targeted drug delivery, anti-inflammatory, wound healing, antibacterial, anti-cancer, and bioimaging8–10.
The plant-based synthesis of NPs has transformed drug research. Biogenic NPs possess the capacity to function as biocatalysts while concurrently exhibiting cytotoxicity and causing membrane damage. One study indicates that membrane rupture and free radical generation are two of the most common ways of NPs-induced damage to cells. The interaction of NPs with fungal hyphae and spores has been shown to inhibit fungal proliferation11. AgNPs, like other metal NPs, have numerous critical applications, including fuel cells, fluorescent labelling, single electron transistors, and DNA/RNA detection via specific probes. They may also be beneficial in biomedical diagnostic devices, nano computers, biosensors, agriculture, and medicine12. AgNPs are used in medicines to treat a variety of conditions, including burns and open wounds13. Synthesized AgNPs are used in biological methods to develop the demand for beneficial and expensive habitat expansion. Additionally, advantageous machinery for material synthesis was conducted to the exploration of biomimetic processes for synthesis14. The living organisms, and in addition to plants, bacteria and fungi are the most advantageous microorganisms for the biologically active synthesis of AgNPs, particularly those with high resistance to metal were easy to handle and also cover diverse extracellular proteins that imply on the AgNPs determined15.
Endophytic fungus lives inside its host plant without causing disease symptoms. Endophytic fungi are also ideal for synthesizing AgNPs13. Endophytes can synthesize a variety of materials that are useful in modern medicine and agriculture. Endophytes have been most broadly considered for their ability to synthesize antioxidants, antidiabetic, antibacterial, antiviral, and anticancer compounds, and their use in the synthesis of AgNPs has also been specified to some extent13. Multiple investigations have recorded the utilization of fungi in the internal or external synthesis of metal NPs through the action of reducing enzymes. There have been numerous reports documenting the production of metal NPs coated with fungus. T. purpureogenus, obtained from T. baccata Linn, produced AgNPs measuring 49.3 nm in size and exhibiting a face-centered cubic crystalline structure. The AgNPs shown significant antibacterial and free radical scavenging properties16. According to a paper by Dinesh et al.17, AgNPs derived from the endophytic fungus P. cinnamopurpureum (C. orchioides) have demonstrated antibacterial properties. The study conducted by Vardhana et al.18 found that AgNPs produced by the endophytic fungus Phyllosticta sp., which was isolated from A. retroflexus, showed strong antimicrobial action against bacterial infections.
The present study aimed to isolate endophytic fungus C. chaingmaiense -KUMBMDBT-25 which belongs to the phylum Ascomycota and class Dothideomycetes, isolated from a E. tirucalli. The fungus was utilized for the synthesis of AgNPs and the synthesized Con-AgNPs which was characterized by using various bioanalytical techniques such as Bio-spectrophotometer, Fourier transform infrared (FTIR) spectroscopy, Scanning electron microscopy – energy dispersive analysis of X-ray (SEM-EDX), High-resolution Transmission electron microscopy (HR-TEM), X-ray diffraction (XRD), Dynamic Light Scattering (DLS) and Zeta potential. Further also determined its antibiotic enhancing, antibacterial, minimum inhibitory concentration, antifungal, antioxidant, anticancer and anti-inflammatory properties.
The result is the first report on the synthesis of Con-AgNPs by the endophytic fungus C. chaingmaiense-KUMBMDBT-25, utilising a biological technique, as per the literature survey. This work demonstrates that the endophytic fungus C. chaingmaiense secretes a greater quantity of proteins, resulting in enhanced nanoparticle production. This fungus exhibits several benefits over many biological agents; most filamentous C. chaingmaiense fungi demonstrate significant resistance to metals, characterised by strong wall-binding capacity and internal metal uptake capability. Moreover, they are readily cultivable on a wide scale, facilitating the acquisition of sufficient biomass for processing; however, silver nanoparticles synthesized from plant sources exhibit less stability compared to those synthesized by fungal mediation. The utilization of plants for the manufacture of silver nanoparticles results in a reduction of plant diversity. Considering all factors, we identified the endophytic fungus C. chaingmaiense as the best option for the synthesis of Con-AgNPs.
Results and discussion
Isolation, identification and molecular characterization of endophytic fungus
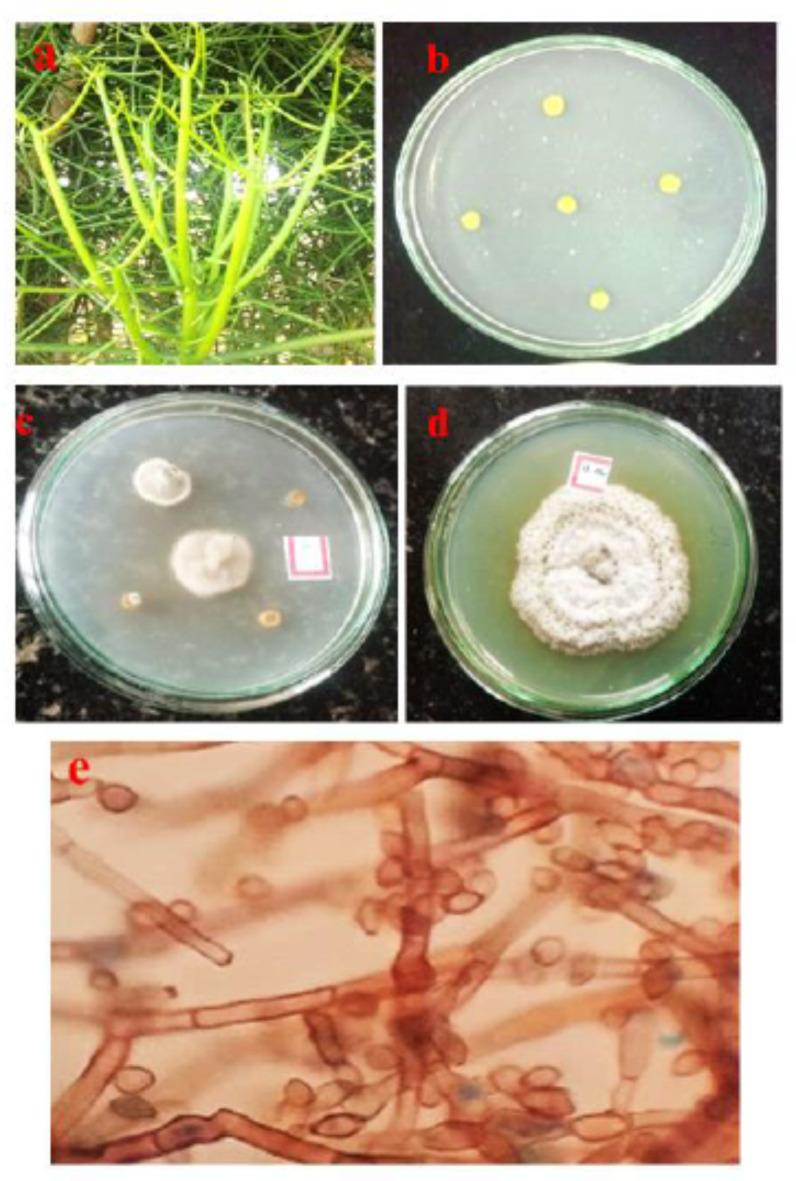
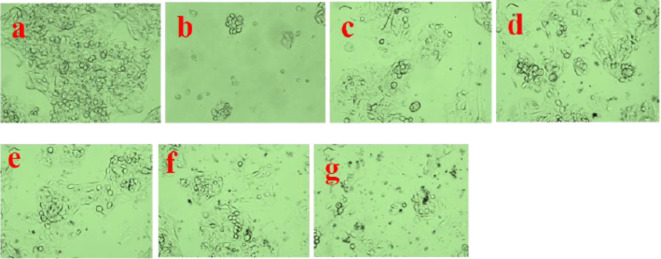
Medicinal plant E. tirucalli stem was used to isolate the endophytic fungus. The isolated endophytic fungus was whitish green colour, the cell morphology was recorded with respect to spore chain (Fig. 1, a, b, c, d and e). Fungus characterized through PCR amplification of the 18s rRNA gene using the ITS1 primer and ITS4 primer, the 18s rRNA gene was identified using Sanger’s dideoxy Nucleotide sequence of the amplified ITS region. Blastn analysis showed significant multiple sequence alignment and pairwise results that revealed to identity with the sequence of Coniothyrium chaingmaiense-KUMBMDBT-25 and was deposited in NCBI GenBank with accession number (MW029905) of isolate. The sequencing files are in ABI format, which can be viewed using software like MEGA- Version 7.0.14 to build a phylogenetic tree using the neighbour-joining (NJ) (Fig. 2) method with nucleotide pairwise genetic distance corrections. In a study conducted by Tayung and Jha19, a total of 77 isolates from 18 distinct genera were found in the healthy inner bark of T. baccata, which is similar to our results. The endophytes were obtained by isolating them using different mycological media, which included media that were modified with bark extract from the host plant. The highest number of endophytes was obtained by isolating them on malt extract agar medium supplemented with bark extract, whereas the lowest number was obtained on water agar medium.
Fig. 1.
(a) Euphorbia tirucalli medicinal plant, (b) plant sample placed on a PDA media, (c) endophytic fungus grown from surface sterilized stem segment of E. tirucalli on PDA media after 48 h, (d) colony morphology of endophytic fungus C. chaingmaiense, (e) microscopic view of C. chaingmaiense.
Fig. 2.
Phylogenetic analysis of C. chaingmaiense strains with ITS sequences of closely related fungal strains.
Gas-chromatography and mass spectroscopy
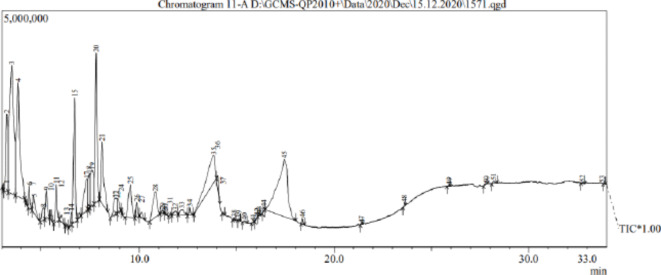
Bioactive compounds present in the extract was analyzed and identified by GC-MS analysis. The compounds were identified by comparing their retention time with the National institute of standard and technology (NIST) data base. The obtained results indicate the existence of 51 compounds in the chromatogram based on their retention time (Fig. 3; Table 1). The major bioactive compounds of peak 45 (RT 17.444, Area percent 16.20) was Glucitol d-Glucitol, peak 3 (RT 3.506, Area percent 16.05) was Propanoic acid, 2-hydroxy-, ethyl ester, peak 4 (RT 3.829, Area percent 11.03) was Butanal, 3-hydroxy-, peak 20 (RT 7.831, Area percent 7.51) was 2-Furancarboxaldehyde, 5-(hydroxymethyl). The majority of the bioactive compound found had a wide range of biological applications and also it helps to synthesis of Con-AgNPs. Similarly, to the results we found, GC-MS analysis of the crude ethyl acetate extract from endophytic C. gloeosporioides from L. corammendalica revealed antimicrobial compounds like 2-methyl-3-methyl-3-hexene, 3-ethyl-2,4-dimethyi-pentane, diethyl pythalate, and hexadecanamide. Another study investigated P. thyrsiflorus endophytic fungi that produced volatile chemicals. According to Devi and Singh20, the main ingredients were phenol, 2,4-bis(1,1-dimethylethyl), 1-Hexadecene, 1-Hexadecanol, hexadecanoic acid, octadecanoic acid methyl ester, and 1-Nonadecene.
Fig. 3.
GC-MS Chromatogram of ethyl acetate extract of endophytic fungus C. chaingmaiense.
Table 1.
List of identified bioactive compounds of an ethyl acetate extract of endophytic fungus C. Chaingmaiense.
| Sl. No. | Compound | Molecular formula | Molecular weight (g/mol) | Retention time | Peak | Area % |
|---|---|---|---|---|---|---|
| 01 | Glucitol d-Glucitol | C6H14O6 | 182 | 17.444 | 45 | 16.20 |
| 02 | Propanoic acid, 2-hydroxy-, ethyl ester | C5H10O3 | 118 | 3.506 | 3 | 16.05 |
| 03 | Butanal, 3-hydroxy- | C4H8O2 | 88 | 3.829 | 4 | 11.03 |
| 04 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | C6H6O3 | 126 | 7.831 | 20 | 7.51 |
| 05 | 3-Deoxy-d-mannoic lactone | C6H10O5 | 162 | 13.822 | 35 | 7.22 |
| 06 | 1,2,3-Propanetriol, monoacetate | C5H10O4 | 134 | 8.140 | 21 | 4.65 |
| 07 | Butanoic acid, 2-ethyl-3-oxo-, methyl ester | C7H12O3 | 144 | 3.245 | 2 | 4.09 |
| 08 | Propanal, 2,3-dihydroxy- | C3H6O3 | 90 | 7.635 | 19 | 3.21 |
| 09 | 2-Formyl-9-[.beta.-d-ribofuranosyl]hypoxanthine | C11H12N4O6 | 296 | 10.823 | 28 | 2.94 |
| 10 | Phenol, 2-methoxy-3-(2-propenyl) | C10H12O2 | 164 | 9.549 | 25 | 2.77 |
| 11 | 3-Furanmethanol 3-Furylmethanol | C5H6O2 | 98.1 | 3.033 | 1 | 0.06 |
| 12 | 2-Furancarboxaldehyde, 5-methyl- | C7H10N2O | 138.17 | 4.204 | 5 | 0.07 |
| 13 | 2,4-Dihydroxy-2,5-dimethyl-3(2 H)-furan-3-one | C6H8O4 | 144.12 | 4.396 | 6 | 0.74 |
| 14 | 2 H-Pyran-2,6(3 H)-dione | C5H4O3 | 112.08 | 4.613 | 7 | 1.04 |
| 15 | Octane, 1-propoxy- Ether, octyl propyl n-Octyl propyl ether | C11H24O | 172.31 | 5.131 | 8 | 0.88 |
| 16 | Pentanoic acid, 4-oxo- | C5H8O3 | 116.11 | 5.279 | 9 | 1.52 |
| 17 | 2,5-Dimethyl-4-hydroxy-3(2 H)-furanone | C6H8O3 | 128.13 | 5.524 | 10 | 0.31 |
| 18 | 3-Hepten-2-one, 3-methyl- | C8H14O | 126.2 | 5.791 | 11 | 1.61 |
| 19 | Butanedioic acid, monomethyl ester | C5H8O4 | 132.11 | 6.051 | 12 | 0.40 |
| 20 | 2 H-Pyran-3(4 H)-one, dihydro-6-methyl- | C6H10O2 | 114.14 | 6.315 | 13 | 0.09 |
| 21 | Butanoic acid, 2-methyl-3-oxo-, ethyl ester | C7H12O3 | 144.17 | 6.561 | 14 | 0.54 |
| 22 | 1,3-Dioxolane-4-methanol | C4H8O3 | 104.1 | 6.956 | 16 | 0.24 |
| 23 | dl-Glyceraldehyde dimer | C6H12O6 | 180.16 | 7.483 | 18 | 1.92 |
| 24 | 1,2-Dioxetane, 3,4,4-trimethyl-3-[[(trimethylsilyl)oxy]methyl]- | C9H20O3Si | 204.34 | 8.809 | 22 | 1.06 |
| 25 | Methyl 4-acetyl-5-oxohexanoate | C9H14O4 | 186.2 | 8.891 | 23 | 0.85 |
| 26 | (3-Ethoxy-4,5-dihydro-isoxazol-5-ylmethyl)-amine | C6H12N2O2 | 144.17 | 9.075 | 24 | 0.03 |
| 27 | DL-Proline, 5-oxo-, methyl ester | C22H25NO6 | 399.4 | 9.864 | 26 | 0.61 |
| 28 | 1,2-Cyclopropanedicarboxylic acid, 3-methylene-, diethyl ester | C10H14O4 | 198.22 | 10.120 | 27 | 0.36 |
| 29 | 2,4-Methylene-D-epirhamnitol | C7H14O5 | 178.18 | 11.199 | 29 | 0.10 |
| 30 | Cyclohexyl methyl ethylphosphonate | C9H19O3P | 206.22 | 11.323 | 30 | 0.04 |
| 31 | Piracetam | C6H10N2O2 | 142.16 | 11.569 | 31 | 0.06 |
| 32 | D-Glucitol, 1,4-anhydro- | C18H34O6 | 346.5 | 11.844 | 32 | 0.26 |
| 33 | d-Mannitol, 1,4-anhydro- 1,4-Anhydro-d-mannitol | C13H18Br2O5 | 414.09 | 12.184 | 33 | 0.55 |
| 34 | Ethyl N-(o-anisyl)formimidate | C10H13NO2 | 179.22 | 12.580 | 34 | 0.28 |
| 35 | 3-Deoxy-d-mannonic acid | C6H12O6 | 180.16 | 14.019 | 36 | 0.44 |
| 36 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | 268.4 | 14.300 | 37 | 0.04 |
| 37 | Tricyclo[3.3.3.0]undecan-3-one | C11H16O | 164.24 | 14.856 | 38 | 0.06 |
| 38 | Adamantane, 1-isocyanato- | C11H15NO | 177.24 | 15.094 | 39 | 0.05 |
| 39 | 1-Cyclohepten-3,6-dione, 2,5,5-trimethyl-, dioxime | C10H16N2O2 | 196.25 | 15.386 | 40 | 0.09 |
| 40 | 3-Methoxy-4-tetrazol-1-yl-phenylamine | C8H9N5O | 191.19 | 15.853 | 41 | 0.02 |
| 41 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.5 | 16.057 | 42 | 0.07 |
| 42 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C11H18N2O2 | 210.27 | 16.155 | 43 | 0.04 |
| 43 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 16.383 | 44 | 0.10 |
| 44 | Acetamide, 2-(4-benzyloxyphenoxy]-N-(3-tolyl)- | C22H21NO3 | 347.4 | 18.374 | 46 | 0.16 |
| 45 | Phenol, 4-pentyl- | C11H16O | 164.24 | 21.410 | 47 | 0.03 |
| 46 | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)- | C30H50 | 410.7 | 23.612 | 48 | 0.09 |
| 47 | .beta.-Sitosterol acetate | C31H52O2 | 456.7 | 25.893 | 49 | 0.03 |
| 48 | 3-Ethoxy-1,1,1,5,5,5-hexamethyl-3-(trimethylsiloxy)trisiloxane | C11H32O4Si4 | 340.71 | 27.824 | 50 | 0.06 |
| 49 | gamma.-Sitosterol | C29H50O | 414.7 | 28.196 | 51 | 0.14 |
| 50 | Silicic acid, diethyl bis(trimethylsilyl) ester | C10H28O4Si3 | 296.58 | 32.746 | 52 | 0.02 |
| 51 | Pentasiloxane, dodecamethyl- | C12H36O4Si5 | 384.84 | 33.872 | 53 | 0.02 |
Fungal mediated synthesis and characterization of Con-AgNPs
Recent scientific interest in the NPs synthesis approach arises from its ease of usage, cost efficiency, and potential for large-scale manufacture. Biosynthesis was used to synthesize Ag-NPs from Coniothyrium chaingmaiense strain. The synthesis of AgNPs from fungal filtrate of C. chaingmaiense was carried out. After 24 h of incubation, the reaction mixture’s light brown colour was changed to a dark brown colour (Fig. 4, a and b) indicates that the synthesis process converted silver ions to Con-AgNPs. Bio-spectrophotometer was used to determine the absorbance of sample supernatant. Similar to our results21, the fungus may release reductase, which decreases Ag + ions in the solution. Previous research has demonstrated the role of decreasing nicotinamide adenine dinucleotide (NADH) and the NADH-dependent nitrate reductase enzyme in metal nanoparticle formation. Proteins also cover metal nanoparticles, such as AgNPs, to prevent aggregation and keep them stable in the media.
Fig. 4.
(a) Cell filtrate of endophytic fungus C. chaingmaiense, (b) Color change to brown after treating with 1 mM AgNO3.
Bio-spectrophotometer
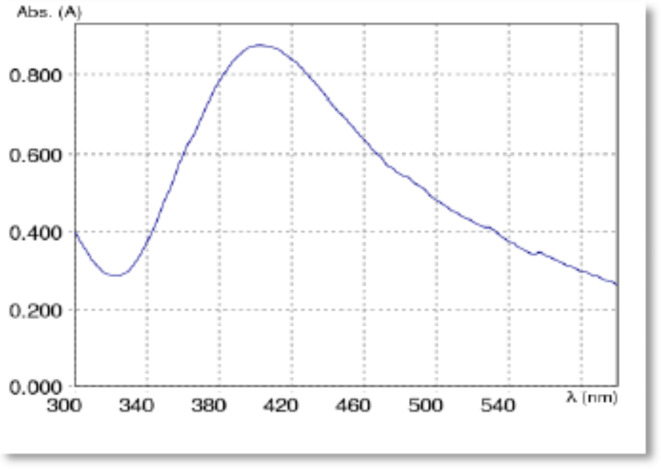
Bio-spectrophotometer was conducted within the standard wavelength range of 200 to 700 nm to observe the biosynthetic and environmentally friendly interaction between plant extract and metallic salt22. The Bio-spectrophotometer is an extremely useful and reliable technique for the primary characterization of synthesized Con-AgNPs, and it is also used to determine the synthesis and stability of Con-AgNPs. A sharp peak in the spectrum Plasmon resonance (SPR) at 404 nm was observed (Fig. 5). The SPR peak at 404 nm confirmed the synthesis of Con-AgNPs. Hu et al.23 isolated AgNPs from the endophytic fungus T. purpureogenus, with an absorbance peak at 418 nm and a wavelength range of 390 to 420 nm.
Fig. 5.
Bio-spectrophotometer analysis graph of synthesized Con-AgNPs.
Fourier transform infrared spectroscopy
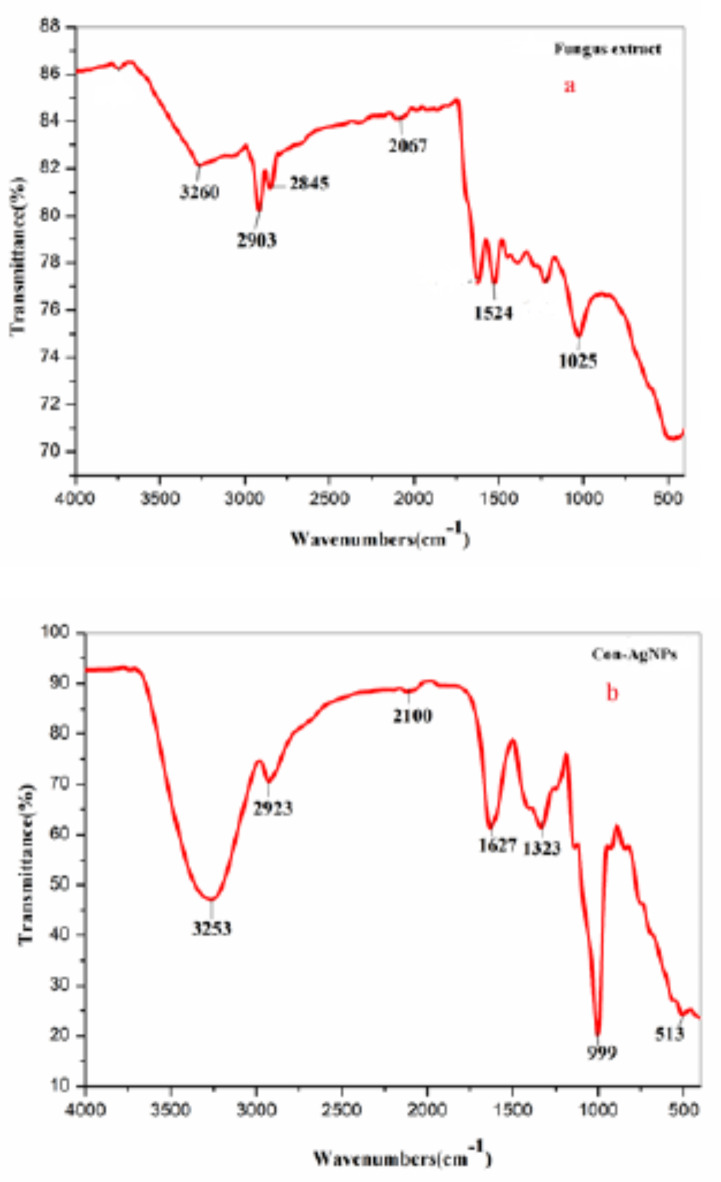
FTIR analysis of Con-AgNPs revealed the connection of several functional groups and their corresponding vibrational modes, including alkanes, phenols, alcohols, aromatics, alkenes, alkyl halides, and aliphatic amines, which showed as stretching vibration peaks of AgNPs. FTIR revealed the presence of protein-like structures on the surface, which are believed to play a significant role in stabilizing Con-AgNPs. During the synthesis process, a bio-based molecule (amino acids) functions as a capping/stabilizing agent, hence reducing the necessity for hazardous substances24. This study utilized FTIR to investigate the interaction between silver ions and the bioactive compounds in C. chaingmaiense fungal extracts responsible for stabilizing Con-AgNPs. The FTIR spectra of the C. chaingmaiense extract (Fig. 6.a) exhibited a peak at 3260 cm− 1, indicative of the stretching vibration of hydroxyl groups (-OH)25, bands at 2903 and 2845 cm− 1 associated with the aliphatic CH stretch26, a peak at 2067 cm− 1 corresponding to the stretching of (C-N) groups27, a band at 1524 cm− 1 attributed to the bending vibration of the (NH) C = O group17, bands at 1025 cm− 1 linked to the stretching of alcohol (-OH) and (C = O) groups28. The FTIR analysis of Con-AgNPs (Fig. 6.b), revealed an intense peak at 3253 cm− 1, which corresponds to the stretching vibrations of the hydroxy group, H-bonded OH stretch. The peak observed at 2923 cm− 1 can be attributed to the H-bonded OH stretch. Assigned to Methyl C-H asym. sym. stretch, the band at 2100 cm− 1 corresponds to the stretching vibrations of the cyanide ion, the peak at 1627 cm− 1 corresponds to the stretching vibrations of the primary amine, NH bend, the band at 1323, 999, and 513 cm− 1 corresponds to the stretching vibrations of the primary or secondary, OH in plane bend, Silicate ion, and aliphatic respectively, the obtained data was compared to Nandiyanto et al.29, these findings are consistent with previous reports Ramalingmam et al.30, worked on synthesis of AgNPs using an endophytic fungus, C. lunata and their antimicrobial potential. Netala et al., Netala et al.31–33, have also worked on synthesis of AgNPs using an endophytic fungus and its antimicrobial studies. These findings confirm the presence of protein in the samples, which may have acted as a capping and stabilizing agent during the synthesis of AgNPs.
Fig. 6.
(a) FTIR spectra of C. chaingmaiense extract. (b) FTIR spectra of synthesized Con-AgNPs.
Scanning electron microscopy
Scanning electron microscopy was used to analyse the synthesized Con-AgNPs. The predominant particles are round and can be categorized as NPs, measuring between 29.72 and 40 nm, with differing degrees of aggregation34. The surface morphology and shape of the synthesized Con-AgNPs were determined using SEM analysis at various magnifications. The SEM images revealed that the synthesized Con-AgNPs was spherical in nature (Fig. 7, a, b, c and d). Similar to our study, Ramalingmam et al.30 worked on AgNPs synthesized endophytic fungus C. lunata scanning electron microscopy images revealed that synthesized AgNPs have spherical shape in nature.
Fig. 7.
(a–d) Scanning Electron Micrographs of synthesized Con-AgNPs.
Energy dispersive analysis of X-ray
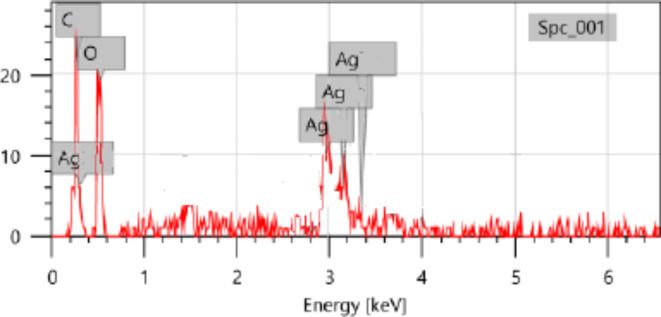
The EDAX technique can be employed for the elemental analysis of biosynthesized nanoparticles35. The EDAX spectrum provides both a qualitative and quantitative assessment of the elements involved in the formation of AgNPs. The EDAX spectrum profiles of the NPs revealed strong silver atom signals at approximately 3Kev, confirming the presence of elemental silver reduction of silver ions to Con-AgNPs (Fig. 8), as well as weaker carbon and oxygen atom signals. The results align with previous study conducted by Popli et al.36, Cladosporium sp., an endophytic fungus responsible for the synthesis of AgNPs. The researchers performed an EDAX analysis, which indicated that the synthesized AgNPs exhibited significant energy absorption in the range of 2.5 to 4 keV.
Fig. 8.
EADX spectra of synthesized Con-AgNPs.
HR-TEM analysis
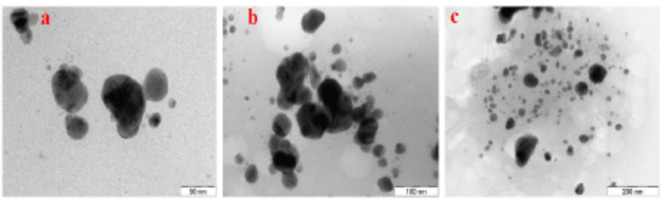
TEM is an effective method for analyzing NPs dimensions and structure, however it poses difficulties in sample preparation and image interpretation37. High-resolution transmission electron microscopy (Fig. 9) has disclosed the precise dimensions and morphology of the synthesized Con-AgNPs. The synthesized Con-AgNPs were nearly spherical in shape when observed at various magnifications. In a manner similar to our investigation, Singh et al.13, examined biologically synthesized AgNPs that were extracted from the healthy leaves of R. sativus. The findings of the TEM analysis indicate that AgNPs are virtually spherical and have a size distribution of 10 to 30 nm.
Fig. 9.
(a–c) HR-TEM images of synthesized Con-AgNPs.
X-ray diffraction
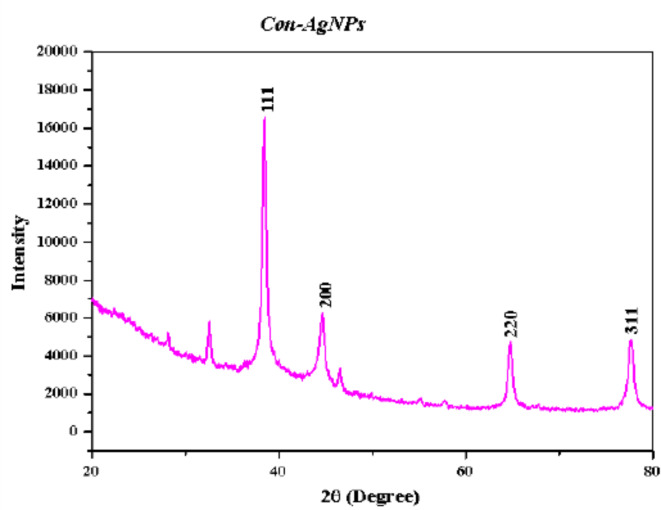
The phase and crystallographic structures of synthesized Con-AgNPs, an X-ray diffraction pattern was obtained. The Bragg reflection peaks were observed at 2Ө values of 38.50°, 44.67°, 64.71°, and 77.61°, corresponding to the (111), (200), (220) and (311) (Fig. 10), silver planes were observed, with all peaks corresponding to silver’s face-centered cubic (FCC) lattice phase. The obtained data was compared to the joint committee on powder diffraction standards (JCPDS) file no. 03-0921, which indicated that these sharp Bragg peaks indicated the presence of a capping agent that stabilized the Con-AgNPs. The result indicate that the AgNPs formed are crystalline in nature. The XRD pattern also displayed some extra peaks which were unassigned. Bio-organic phase on particle surfaces generated extra peaks. The major phase is silver, according to the results. However, inorganic moieties may have other crystalline phases which correspond to the XRD pattern as impurities. These peaks were generated by crystalline impurities of leftover plant extracts on nanoparticle surfaces38. Previous reports are verified by these results. The characterization of AgNPs was assessed by Azmath et al.39, using cell-free filtrates of the fungi A. niger and F. semitectum, which is comparable to our investigation. When the synthesized NPs were subjected to XRD analysis, Braggs peak values of 38.2°, 44.42°, 64.5°, and 77.4° indicated that they were crystallized.
Fig. 10.
XRD pattern of synthesized Con-AgNPs.
Zeta potential
The zeta potential of the synthesized Con-AgNPs found as -18.0 mV (Fig. 11) with single peak which indicates the stability of synthesized Con-AgNPs. The report obtained revealed that the synthesized Con-AgNPs were very stable. Similar to our study, with earlier report13, worked on synthesized AgNPs from endophytic fungus Alternaria sp are characterized by using zeta potential. Sharma et al.16, also synthesized AgNPs and tested their antibacterial efficacy against T. baccata-isolated T. purpureogenus. The zeta potential of the synthesized nanoparticles is -19.5 mV.
Fig. 11.
Zeta potential analysis of synthesized Con-AgNPs.
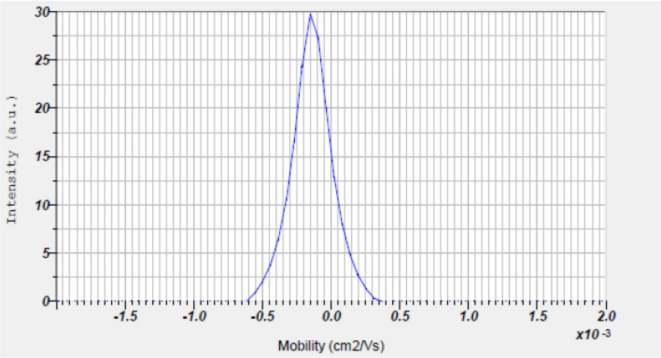
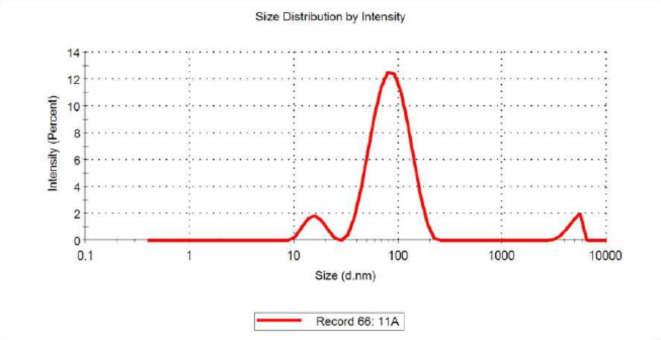
Dynamic light scattering
Particle size analysis was employed to calculate the hydrodynamic radii or size of the synthesized Con-AgNPs. Dynamic light scattering evidenced the average size of the synthesized Con-AgNPs 73.21 nm. The obtained peak indicated that the quality of synthesized Con-AgNPs was good (Fig. 12). In a similar manner to our investigation40, investigated the endophytic fungus Nemania sp. that was extracted from T. baccata L.S, a type of Iranian Yew, for its ability to synthesize antibacterial AgNPs. The focus of their study was the measurement of particle size for the synthesized NPs. The results showed that the average particle size, represented by the D mean number, was 33.54 nm. Additionally, the size distribution of the NPs indicated that the largest frequency of particles with a size of 26.9 nm was observed. Furthermore, the average volume was 69.43.
Fig. 12.
Dynamic light scattering of synthesized Con-AgNPs.
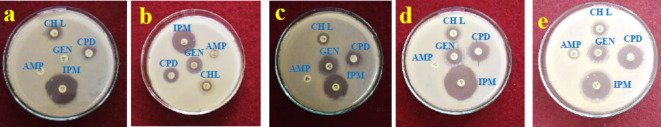
Antibiotic enhancing activity of Con-AgNPs
AgNPs can interact with bacterial cell membranes, resulting in damage and the release of intracellular components into the external environment. The antibacterial activity of AgNPs is attributed to their interaction with and inhibition of bacterial cell membranes. The antibacterial, antifungal, and other biological activities are significantly influenced by the size and concentration of NPs; smaller sizes can easily traverse bacterial protective barriers and cause damage41. Antibiotic enhancing activity of the synthesized Con-AgNPs was determined at 1 mg/mL concentrations using the disc diffusion method. After 24 h, a zone of inhibition was observed around the antibiotic disc impregnated with Con-AgNPs, as shown (Figs. 13 and 14), Con-AgNPs enhanced the activity of strong antibiotics (Gentamicin 9 diameter) and (Ampicillin 3.5 diameter) combined with Con-AgNPs against pathogenic bacteria E. coli formed maximum zone of inhibition was observed (Table 2). Rahi and Parmer2 reported similar results while investigating the antibiotic-enhancing properties of AgNPs produced by the endophytic fungus Penicillium sp. It was found that the control region, which consisted of antibiotics alone and silver nitrate, did not exhibit any zone of inhibition in the cases of E. coli, S. aureus, and P. aeruginosa. When AgNPs and amoxicillin were combined, the greatest level of inhibition (13.69-fold increase) was observed against E. coli.
Fig. 13.
Standard antibiotic enhancing activity against test bacterial pathogens, (a) E. coli, (b) S. aureus, (c) P. aeruginosa, (d) S. typhi, (e) K. pneumoniae.
Fig. 14.
Antibiotic enhancing activity of Con-AgNPs against pathogenic bacteria (a) E. coli, (b) S. aureus, (c) P. aeruginosa, (d) S. typhi, (e) K. pneumoniae.
Table 2.
Zone of inhibition values of antibiotic enhancing activity Con-AgNPs.
| Test pathogenic bacteria | Measurement of zone of inhibition in diameter (mm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Antibiotic disc (10 µg) and Con-AgNPs (1 mg/mL) | |||||||||||||||
| Chloramphenicol (CHL) | Cefpodoxime (CPD) | Gentamicin (GEN) | Ampicillin (AMP) | Imipenem (IPM) | |||||||||||
| Ab (A) | Ab + Ag NP(B) | (B2 − A2)/A2 | Ab (A) | Ab + Ag NP(B) | (B2 − A2)/A2 | Ab (A) | Ab + Ag NP(B) | (B2 − A2)/A2 | Ab (A) | Ab + AgNP(B) | (B2 − A2 )/A2 | Ab (A) | Ab + AgNP (B) | (B2 − A2 )/A2 | |
| E. coli | 16.03 ± 0.05 | 21 ± 00 | 0.72 | 13.03 ± 0.05 | 19 ± 00 | 1.31 | 6 ± 00 | 19 ± 00 | 9 | 6 ± 00 | 13.06 ± 0.11 | 3.5 | 25.03 ± 0.05 | 27.06 ± 0.17 | 0.16 |
| S.aureus | 13.06 ± 0.11 | 21 ± 00 | 1.82 | 16.06 ± 0.11 | 17.0 ± 0.05 | 0.11 | 15.06 ± 0.11 | 19.03 ± 0.05 | 0.59 | 6 ± 00 | 10 ± 00 | 1.77 | 22.03 ± 0.05 | 26.1 ± 0.17 | 0.39 |
| P. aeruginosa | 11.06 ± 0.11 | 17 ± 00 | 1.32 | 14.06 ± 0.11 | 23.1 ± 0.17 | 1.69 | 17.1 ± 0.17 | 21.1 ± 0.11 | 0.51 | 6 ± 00 | 10 ± 00 | 1.77 | 21.06 ± 0.11 | 32.03 ± 0.05 | 1.31 |
| S. typhi | 17.13 ± 0.23 | 18 ± 00 | 0.12 | 21.06 ± 0.11 | 20.03 ± 0.05 | 0.08 | 17.03 ± 0.05 | 20.06 ± 0.11 | 0.38 | 6 ± 00 | 10 ± 00 | 1.77 | 26.06 ± 0.11 | 30.1 ± 0.17 | 0.33 |
| K. pneumoniae | 15.06 ± 0.11 | 19 ± 00 | 0.6 | 14.06 ± 0.11 | 21.1 ± 0.17 | 1.25 | 15.06 ± 0.11 | 18.03 ± 0.05 | 0.43 | 6 ± 00 | 10 ± 00 | 1.77 | 25.03 ± 0.05 | 30.03 ± 0.05 | 0.43 |
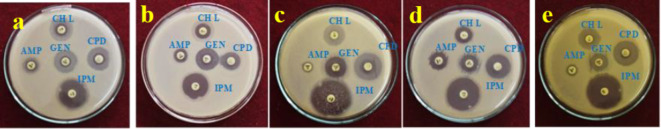
Antibacterial activity of Con-AgNPs
The presence of a thicker peptidoglycan coating in gram-positive bacteria, gram-negative strains are more susceptible and require higher dosages of NPs. NPs may impede bacterial growth by changing the membrane potential for ATP generation or decreasing the ribosome’s tRNA binding capability, disrupting its protein synthesis pathway. NPs have high antibacterial activity because their small size and broad surface area enable quick dispersion and increase microbial interaction. As initial choices, NPs bind to bacterial DNA and membrane proteins’ phosphoric and thiol groups, restricting bacterial growth42. The antibacterial activity of the synthesized Con-AgNPs was determined by measuring the diameter of the zone of inhibition in mm using the agar well diffusion method. The antibacterial effect of different concentrations of Con-AgNPs (10 mg/mL, 5 mg/mL, and 2.5 mg/mL) after 24 h of incubation. Was evaluated the zone of inhibition was observed. The highest antibacterial activity of Con-AgNPs was observed against E. coli (10 mg/mL, 14.1 ± 0.17 mm) other test bacteria as shown in (Figs. 15, 16 and 17; Table 3), these findings indicated that synthesized Con-AgNPs exhibited a significant zone of inhibition against gram negative bacteria when compared to gram positive bacteria. Netala et al.32, conducted a study on the antibacterial characteristics of AgNPs that were synthesized utilizing an endophytic fungus. AgNPs shown effective antibacterial activity against S. aureus, P. aeruginosa, and K. pneumonia. Among the tested bacteria, P. aeruginosa exhibited the largest zone of inhibition, measuring 18.5 mm, followed by K. pneumonia with 13.6 mm, and S. aureus with 11.6 mm.
Fig. 15.
Standard antibiotics against test bacteria pathogens (a) E. coli, (b) S. aureus, (c) P. aeruginosa, (d) S. typhi, (e) K. pneumoniae.
Fig. 16.
Antibacterial activity of Con-AgNPs against bacterial pathogens (a) E. coli, (b) S. aureus, (c) P. aeruginosa, (d) S. typhi, (e) K. pneumoniae.
Fig. 17.
The zone of inhibition values indicated antibacterial activity of Con-AgNPs against bacterial pathogens. Each value is given as a mean ± SD. ***Significance at P < 0.05.
Table 3.
Zone of inhibition values of antibacterial activity of Con-AgNPs against bacterial pathogens.
| Test pathogenic bacteria | Measurement of zone of inhibition in diameter (mm) | |||||
|---|---|---|---|---|---|---|
| Standard Antibiotics (mg/mL) | Con-AgNPs (mg/mL) | |||||
| streptomycin (STM) | Ciprofloxacin (CPFX) | Chloramphenicol (CHL) | 10 | 5 | 2.5 | |
| E. coli | 20.1 ± 0.17 | 29.13 ± 0.23 | 24.06 ± 0.11 | 14.1 ± 0.17 | 13.06 ± 0.11 | 12.1 ± 0.17 |
| S. aureus | 10.16 ± 0.23 | 35.93 ± 0.11 | 28.03 ± 0.05 | 13.03 ± 0.05 | 11.06 ± 0.11 | 10.06 ± 0.11 |
| P. aeruginosa | 23.06 ± 0.09 | 25.96 ± 0.11 | 25.1 ± 0.17 | 14.06 ± 0.11 | 12.13 ± 0.23 | 11.06 ± 0.11 |
| S. typhi | 21.16 ± 0.28 | 27.93 ± 0.11 | 25.13 ± 0.23 | 13.1 ± 0.17 | 12.03 ± 0.05 | 11.13 ± 0.23 |
| K. pneumoniae | 23.16 ± 0.28 | 35.03 ± 0.05 | 29.1 ± 0.17 | 12.06 ± 0.11 | 11.06 ± 0.11 | 10.16 ± 0.28 |
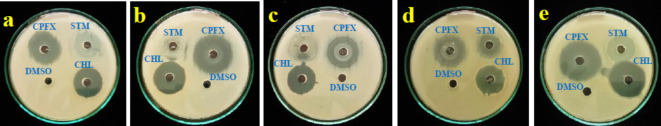
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) method is the most common way for pharmaceutical companies to test how well their drugs kill bacteria. The MIC test was quick, easy to repeat, and showed different levels of sensitivity for each material that was studied. It is possible to identify the smallest amount of a test solution that inhibits all bacterial growth using the MIC assay43. Con-AgNPs synthesized from the endophytic fungus C. chaingmaiense have a minimum inhibitory concentration against some human pathogenic bacteria. The study results revealed that the minimum inhibitory concentration was effective against E. coli (Fig. 18.a, b, c, and d Table 4). The results show that synthesized Con-AgNPs have a lowest minimum inhibitory concentration. In a manner akin to our research, Rani et al.44, investigated the MIC activity of AgNPs that were produced by the endophytic fungus A. terreus. The MIC values of A. terreus against a panel of reference bacterial isolates ranged from 11.43 to 308 µg/mL. The ATCC isolates of S. typhi, E. coli, S. aureus, S. flexneri, and S. marcescens had a minimum inhibitory concentration of 11.43 µg/mL. P. mirabilis and E. faecalis had a MIC of 102 µg/mL, whereas P. aeruginosa had a MIC of 32 µg/mL.
Fig. 18.
(a–c) Minimum inhibitory concentration of Streptomycin, Ciprofloxacin and Chloramphenicol against bacterial pathogens. (d) Minimum inhibitory concentration of Con-AgNPs against bacterial pathogens.
Table 4.
MIC values of Con-AgNPs against bacterial pathogens.
| Test pathogenic bacteria | STM (mg/mL) | CPFX (mg/mL) | CHL (mg/mL) | Con-AgNPs (mg/mL) |
|---|---|---|---|---|
| E. coli | 1.25 | 1.25 | 1.25 | 0.0625 |
| S. aureus | 1.25 | 0.03125 | 0.25 | 0.125 |
| P. aeruginosa | 1.25 | 0.03125 | 0.25 | 0.25 |
| S. typhi | 1.25 | 0.0625 | 1.25 | 0.125 |
| K. pneumoniae | 1.25 | 1.25 | 1.25 | 0.125 |
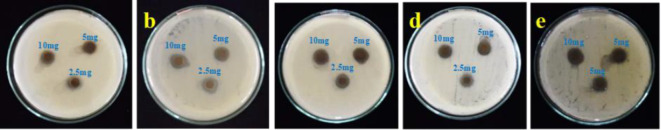
Antifungal activity of Con-AgNPs
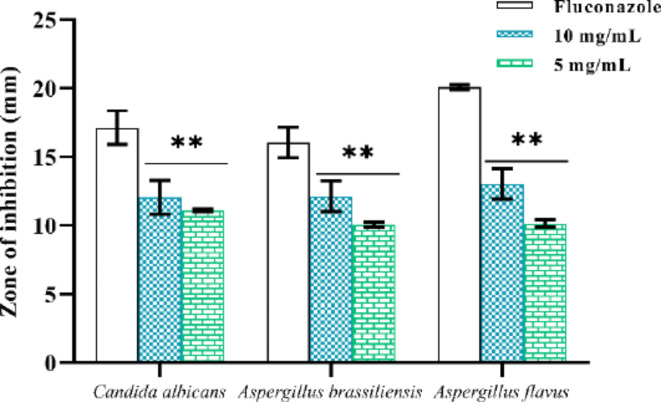
NPs showed significant antifungal activity through several methods. These encompass the development of reactive oxygen species, the damage of cell walls, and the impact on hyphae and spores. Nano-particles, specifically, can impede fungal proliferation through producing oxidative stress and disrupting intracellular physiological equilibrium. The antifungal efficacy of NPs is dependent upon their capacity to adhere to fungal spores, with diminutive particles exhibiting superior binding affinity. Biomolecule coronas formed under pathophysiological conditions can facilitate fungal resistance to NPs by diminishing their physical adsorption to spores45. The antifungal activity of the synthesized Con-AgNPs was determined using the agar diffusion method. Antifungal activity against standard fungal pathogens was determined by loading wells with different concentrations of Con-AgNPs (10 mg/mL and 5 mg/mL) respectively. After incubation for 2–3 days at 27 ± 2 ℃, the diameter of the clear zones of inhibition (ZOI) was measured and recorded, as shown in (Table 5; Figs. 19 and 20). The strong antifungal activity of Con-AgNPs against A. flavus (10 mg/mL 13.03 ± 0.05 mm) formed the maximum zone of inhibition. The results revealed that synthesized Con-AgNPs showed very good antifungal activity. In line with our previous research Yugandhar et al.46, we assessed the antimicrobial properties of AgNPs synthesized from the stem bark extract of S. alternifolium. The study focused on five fungi, namely A. flavus, P. chrysogenum, T. harzianum, A. solani, and A. niger, grown on a medium of potato dextrose agar. The results showed that these fungi exhibited the largest inhibition zones.
Table 5.
Zone of inhibition values of antifungal activity of Con-AgNPs against fungal pathogens.
| Test pathogenic fungi | Measurement of zone of inhibition in diameter (mm) | ||
|---|---|---|---|
| Standard antibiotics (mg/mL) | Con-AgNPs (mg/mL) | ||
| Fluconazole (FCZ) | 10 | 5 | |
| Candida albicans | 17.13 ± 0.23 | 12.06 ± 0.11 | 11.1 ± 0.17 |
| Aspergillus brassiliensis | 16.06 ± 0.11 | 12.13 ± 0.03 | 10.06 ± 0.11 |
| Aspergillus flavus | 20.1 ± 0.17 | 13.03 ± 0.05 | 10.16 ± 0.28 |
Fig. 19.
Fluconazole showed antifungal activity against test fungal pathogens (a) C. albicans, (b) A. brassiliensis, (c) A. flavus. Con-AgNPs against test fungal pathogens, (d) C. albicans, (e) A. brassiliensis, (f) A. flavus.
Fig. 20.
The zone of inhibition values indicated antifungal activity of Con-AgNPs against fungal pathogens. Each value is given as a mean ± SD. ***Significance at P < 0.05.
In vitro antioxidant activity of Con-AgNPs
DPPH radical scavenging activity
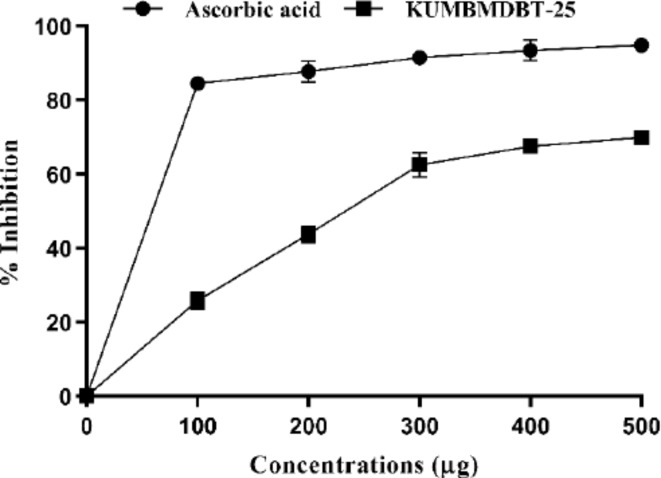
DPPH is a stable molecule and a typical free radical that can be reduced by absorption of hydrogen or an electron from donating ions. The antioxidant-reducing capability of the biosynthesized AgNPs has been evaluated by analyzing changes in color formation. The antioxidant activity of biosynthesized AgNPs is related to the functional groups attached to them47. Radical scavenging characteristics of synthesized Con-AgNPs by measuring changes in the absorbance of the 1, 1-diphenyl-2-picrylhydrazyl radical at 517 nm to understand more about its antioxidative mechanisms. synthesized Con-AgNPs scavenged the DPPH radicals in a dose-dependent manner (Fig. 21). In terms of radical scavenging activity, the Con-AgNPs at a concentration of 100–500 µg/mL, showed radical scavenging action with an IC50 of 135. µg/mL, whereas Ascorbic acid had an IC50 of 18.55 µg/mL. Netala et al.31, conducted a study on the antioxidant activity of AgNPs produced by P. microspora, an endophytic fungus. They found that the ability to remove free radicals increased when the concentration of the nanoparticles reached 100 µg/mL. The concentration of 200 µg/mL resulted in a viability of 33.63%, with IC50 values of 120.44 µg/mL, which were notably low.
Fig. 21.
DPPH free radical scavenging activity of Con-AgNPs.
ABTS radical cation decolorization assay
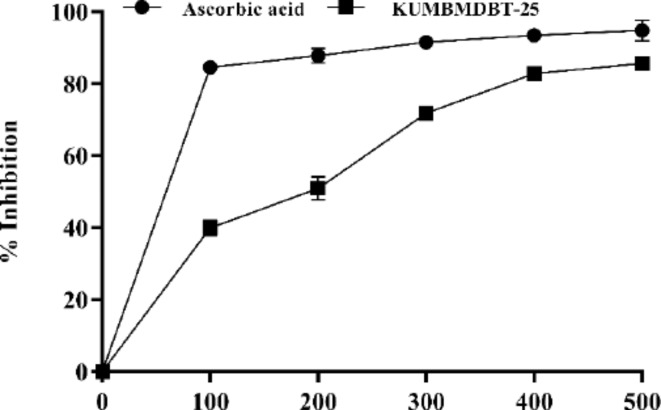
Reactive oxygen species are responsible for the breakdown of membrane lipids in plant cell membranes, as well as the degradation of nucleic acids and amino acids, resulting to a shift in plant metabolic pathways. The oxidative stress responses and plant biomolecules contribute to the capping and reduction of NPs48. The antioxidant property of the Con-AgNPs has been shown by the inhibition of ABTS free radicals. The ABTS radical was scavenged by synthesized Con-AgNPs in a concentration-dependent manner (100–500 µg/mL). Con-AgNPs exhibited increased activity as a radical scavenger (Fig. 22). The results show the scavenging activity of (ABTS) in terms of percent inhibition. The results indicated that the IC50 for synthesized Con-AgNPs was 121.2 µg/mL at a concentration of 100 µg/mL, whereas Ascorbic acid had an IC50 of 11.37 µg/mL. At P < 0.05, values with different superscripts are statistically distinct. Each value is represented as mean ± SD of three independent experiments. Similar to this, Nallappan et al.49, used L. acutangular leaf extract to study the green biosynthesis of AgNPs. They found that at different concentrations ranging from 50 to 300 µg/mL, the biologically synthesized AgNPs showed activity ranging from 47.9 to 85.2% in the ABTS radical cation decolorization assay.
Fig. 22.
ABTS free radical scavenging activity of Con-AgNPs.
Measurement of cell viability by MTT assay
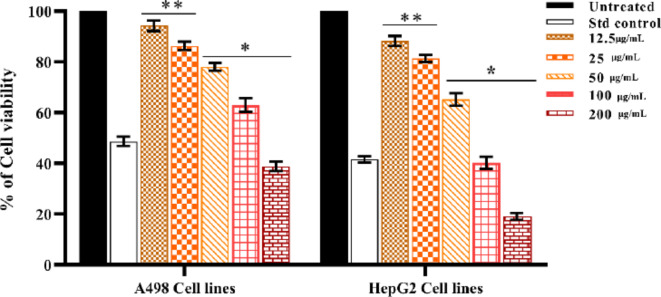
The hazardous properties of NPs present a significant chance for cancer therapy because of their beneficial anti-cancer potential. The three main mechanisms behind the cytotoxic effects of NPs are the generation of reactive oxygen species (ROS) and the development of DNA damage10. HepG2 and A498 cell lines were treated with the Synthesized by Con-AgNPs (Figs. 23 and 24). The cytotoxicity of cancer cells treated with Con-AgNPs was observed to be dose-dependent; as the concentration of Con-AgNPs increases, so does the cytotoxicity of the cells. The A498 and HepG2 cell lines were observed for 24 h to determine the percentage of cell viability based on the IC50 value. The concentrations ranged from 12.5 to 200 µg/mL. Figure 25 illustrates the percentage of A498 cell lines at 200 µg/mL (38.82%) and HepG2 cell lines at 200 µg/mL (19.15%). The A498 cell lines exhibited moderate toxicity with higher IC50 values at 154.92 µg/mL, while the HepG2 cell lines exhibited significantly cytotoxic nature with low IC50 values at 70.35 µg/mL after a 24-hour incubation period. Several studies have emphasised the significance of the surface properties of AgNPs and their ability to effectively infiltrate and target different types of cancer cells, such as human lung fibroblast (WI38), mammary gland breast cancer (MCF-7), human amnion (WISH), hepatocellular carcinoma (HepG-2), breast carcinoma (MCF-7), colon carcinoma (HCT-116), and lung cancer cells50.
Fig. 23.
Percentage cell viability of Con-AgNPs against A498 cell lines at different concentration, (a) Untreated, (b) Std control, Cisplatin, (c) 12.5 µg/mL, (d) 25 µg/mL, (e) 50 µg/mL, (f) 100 µg/mL, (g) 200 µg/mL.
Fig. 24.
Percentage cell viability of Con-AgNPs against HepG2 cell lines at different concentration, (a) Untreated, (b) Std control, Cisplatin, (c) 12.5 µg/mL, (d) 25 µg/mL, (e) 50 µg/mL, (f) 100 µg/mL, (g) 200 µg/mL.
Fig. 25.
Percentage cell viability of synthesized Con-AgNPs against HepG2 cell lines. Each value is given as a mean ± SD. ***Significance at P < 0.05.
In vitro anti-inflammatory activity of synthesized Con-AgNPs
Egg Albumin Denaturation test
Inflammation causes protein denaturation inside inflamed cells, which in turn impairs the functionality of the corresponding cells or tissues. Enzymes lose their ability to function when their secondary and tertiary structures are denatured by exposure to extremely acidic or basic environments; this process is known as denaturation. An agent that successfully suppresses protein denaturation would be an ideal substitute for an anti-inflammatory drug51. The potential anti-inflammatory effects of Con-AgNPs against heat-induced denaturation of egg albumin were investigated in this study. At doses ranging from 10 to 250 µg/mL, synthesized Con-AgNPs had values for mean inhibition of protein denaturation (Fig. 26). In our study, the anti-inflammatory activity of egg albumin denaturation was determined by using in-vitro anti-inflammatory activity of X. granatum extracts synthesized with AgNPs52.
Fig. 26.
Percentage inhibition of Con-AgNPs and Diclofenac against egg albumin denaturation. Each value is given as a mean ± SD. ***Significance at P < 0.05.
Membrane stabilization test by hypotonicity-induced hemolysis
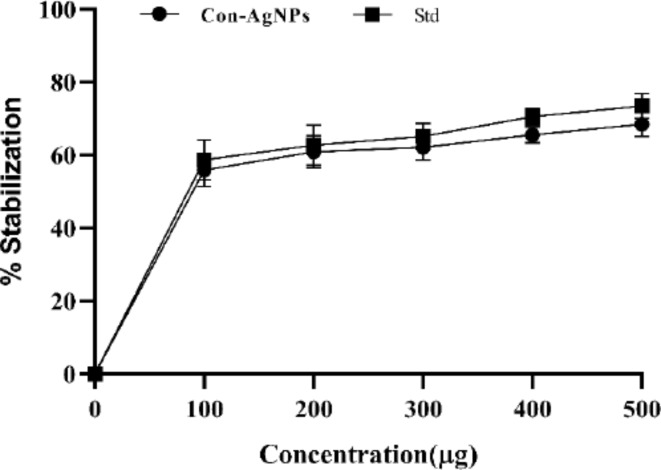
The membrane of human red blood cells (HRBCs) is similar to the lysosomal membrane. Thus, a drug that promotes the stability of the erythrocyte membrane may likewise boost the stability of the lysosomal membrane. This can subsequently inhibit the release of lysosomal enzymes and proteases, hence contributing to cellular inflammation and damage51. HRBC membrane stabilization is a popular method for assessing anti-inflammatory activity in vitro. Because the membrane of an erythrocyte or RBC is analogous to the lysosomal membrane, the test sample may also stabilize lysosomal membranes. As shown in the results, Con-AgNPs have significant anti-inflammatory activity at various concentrations, percentage stabilization of synthesized Con-AgNPs, and standard drug Diclofenac sodium (Fig. 27). Bagur et al.53, who worked on synthesized AgNPs of the endophytic fungus E. rostrata, performed a membrane stabilization test using hypotonicity-induced hemolysis.
Fig. 27.
Percentage inhibition of Con-AgNPs and Diclofenac against membrane stabilization test by hypotonicity-induced hemolysis.
In vivo anti-inflammatory activity of synthesized Con-AgNPs
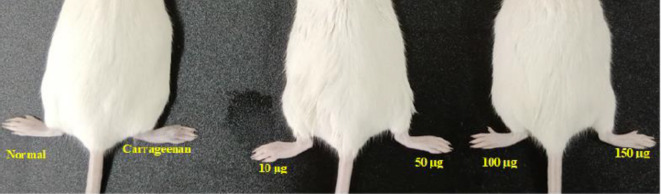
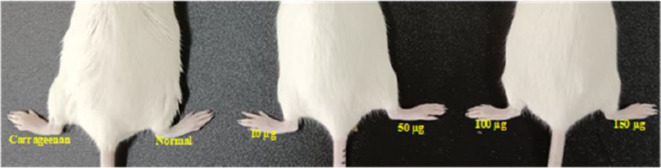
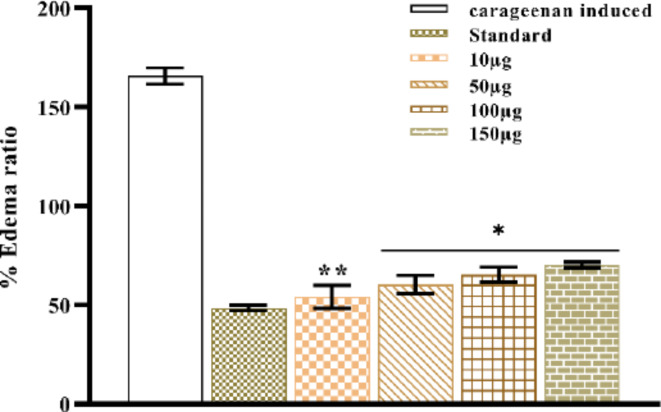
Carrageenan causes paw edema in mice. The paw inflammation induced by the chemical carrageenan is extensively utilized in research to facilitate the development of novel anti-inflammatory drugs. Carrageenan injection stimulates an increase in histamine, serotonin, and prostaglandin-like substances in the paw, leading to the formation of edema. The anti-inflammatory characteristics of NPs are expected to be significant as they inhibit histamine, serotonin, and prostaglandin35. Subcutaneous carrageenan injection increased the size of the mouse paws due to edema, indicating acute paw inflammation in our studies. Figures 28 and 29 show the thickness of the mice’s paws after treatment with Con-AgNPs and Diclofenac sodium. Fungal nanoparticles and Diclofenac sodium showed %inhibition when compared to mice given Diclofenac at 1 h (Fig. 30; Table 6). The paws of different groups of mice were photographed, including normal control and carrageenan-induced paw edema animals treated with Diclofenac or synthesized Con-AgNPs. These pictures showed the significant reduction in paw thickness caused by Diclofenac sodium. Comparable to our findings Rafie et al.54, assessed the anti-inflammatory properties of aqueous leaf extracts, Terminalia species-derived AgNPs, and a reference medication in a rat model of carrageenan-induced paw edema (Indomethacin). T. catappa, T. mellueri, and T. bellerica/AgNPs showed maximal edema inhibition percentages of 95.7%, 93%, and 92.13%, respectively, at a concentration of 50 mg kg-1 body weight. These effects are comparable to the regularly used medicine indomethacin, which has a 92.1% effect.
Fig. 28.
Carrageenan induced paw edema at different concentrations of standard drug Diclofenac.
Fig. 29.
Carrageenan induced paw edema at different concentrations of Con-AgNPs.
Fig. 30.
Percentage inhibition of carrageenan induced paw edema at different concentrations of Con-AgNPs. Each value is given as a mean ± SD. ***Significance at P < 0.05.
Table 6.
Percentage inhibition values of carrageenan induced paw edema at different concentrations of Con-AgNPs.
| Percentage of edema ration | |||||
|---|---|---|---|---|---|
| Control | Con-AgNPs concentration (µg/mL) | Standard drug | |||
| carrageenan induced | 10 µg/ml | 50 µg/ml | 100 µg/ml | 150 µg/ml | Diclofenac sodium |
| 155.69 ± 4.02 | 50.06 ± 1.81 | 60.38 ± 0.81 | 65.32 ± 0.83 | 70.28 ± 0.40 | 58.50 ± 1.21 |
Histopathological analysis
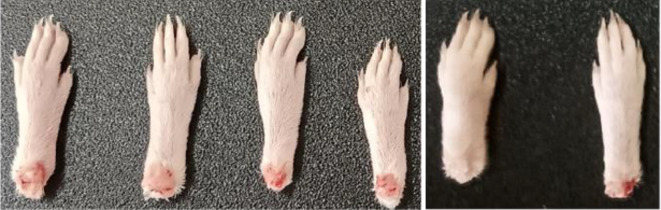
Carrageenan-induced paw inflammation in Swiss Albino female mice was examined histopathologically (Fig. 31). Following euthanasia, the legs were preserved in a 10% buffered Formalin solution before histological sections were prepared using the paraffin method. All ethanol sections were dehydrated, cleared in xylene, and then embedded in paraffin wax. Hematoxylin stains were used to stain transverse Sects. (4–5 m in thickness) mounted on glass slides. All sections were examined to assess inflammatory responses (Fig. 32).
Fig. 31.
Histopathological studies of inflammations in legs of Swiss albino female mice.
Fig. 32.
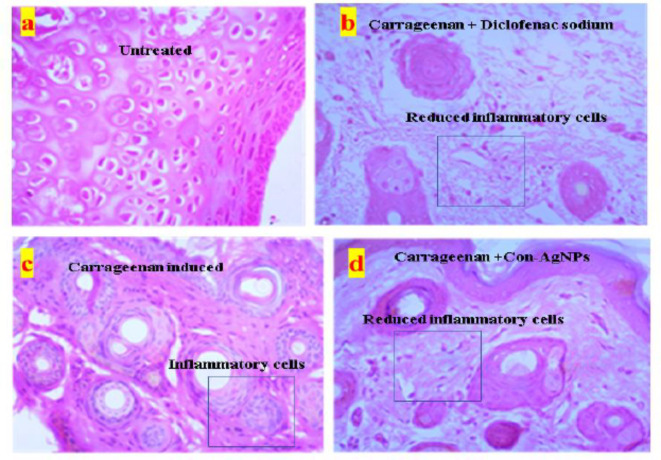
Histopathological studies of Con-AgNPs, (a) Untreated control, (b) Standard drug, Diclofenac shows the faster decrease in the inflammation, (c) Carrageenan paw edema shows the faster increase in the inflammation, (d) Synthesized silver nanoparticles show the gradual decrease in the inflammation with respect to time interval.
Conclusion
The present research explored the endophytic fungus C. chaingmaiense, which is the first reported instance of implementing a biological approach to synthesize Con-AgNPs. This process provides a simple, economical, and eco-friendly alternative to chemical and physical methods for synthesizing NPs. It is also non-toxic. The Con-AgNPs that were synthesized exhibited significant expansion, as shown by Dynamic light scattering. The average size of the synthesized Con-AgNPs was found to be 73.21 nm, with a spherical form and high crystallinity. Furthermore, these NPs were highly stable, as indicated by a zeta potential of -18.0 mv. The Con-AgNPs produced in this investigation shown exceptional antibiotic-enhancing, antibacterial, minimum inhibitory concentration, antifungal and antioxidant properties. Additionally, the study measured cell viability using the MTT assay and assessed anti-inflammatory activity. The synthesized Con-AgNPs have significant pharmacological and biological value.
Materials and methods
Isolation and identification of the endophytic fungus
E. tirucalli medicinal plant was collected from Chitradurga district of Karnataka, INDIA. Collected sample were surface sterilized as described by13. Plant sample was properly washed in running water for five min to eliminate adhered debris. First, the surface was sterilized using 70% ethanol for 60 s, then sodium hypochlorite for 3 min, and finally, 75% ethanol for 30 s, autoclaved distilled water was then used to rinse the surface sterilized sample three times and allowed to dry in laminar airflow. The plant stems were cut into tiny pieces and placed in petri dishes containing potato dextrose agar (PDA, Himedia Pvt. Ltd. INDIA). In order to achieve pure culture, isolated endophytic fungus was sub cultured on potato dextrose agar plates (PDA) and incubated at 28 °C for 5 to 7 days. After isolation, fungus culture was identified to genus level based on microscopic and cultural characters. This culture stock was then kept in refrigerator 4℃ for further use.
Molecular characterization of the endophytic fungus
The Chromous Biotech gDNA minispin kit and the CTAB (Cetyltrimethylammonium Bromide) technique were used to isolate the genomic DNA1,55. The fungal genome’s internal transcribed spacer (ITS) sections were sequenced. The ITS region of the endophytic fungal genome was amplified using the primers ITS1-5′-CCGTAGGTGAACCTGCGG-3′ (forward primer) and ITS4-5′-TCCTCCGCTTATTGATATGC-3′ (reverse primer). Amplification was carried out in a CG palm cycler using 50 ng of DNA template, 1.5 mM MgCl2, 0.4 U Taq DNA polymerase, 200 M dNTP, and 20 Picomoles per litre (pmol/L) of each primer. The amplification cycles were altered which included five min at 94 °C for initial denaturation, thirty cycles at 94 °C for synthesis, one min at 55 °C, one min at 72 °C, and seven min at 72 °C for final extension. To validate the band, the amplified product was tested on 1.2% w/v Agarose gel electrophoresis and ethidium bromide was employed for straining. The band was recorded using a gel documentation machine, and the PCR product was purified using a gel extraction kit. The ITS sequences obtained was analysed using the Basic Local Alignment Search Tool (BLAST), NCBI (http://www.ncbi.nlm.nih.gov).
Biomass preparation of endophytic fungus
The endophytic fungus C. chaingmaiense was cultivated by submerged fermentation. To prepare media, employed a modified approach3. The loop contains a whole colony of endophytic fungus C. chaingmaiense was inoculated to 100 mL of potato dextrose broth for 7–14 days, the flask was incubated at 28℃. After harvesting filtrate was collected by passing it through a Whatman No.1 filter paper. The metabolites in culture filtrate were extracted with equal amount of ethyl acetate. The resultant residue was dried in a vacuum evaporator to obtain the extract56. The extract was subjected to GC-MS analysis and also the collected culture filtrate was further used for Con-AgNPs synthesis.
Gas-chromatography and mass spectroscopy
In order to ascertain the presence of secondary metabolites in the extract, a GC-MS analysis was implemented (Shimadzu QP 2010 Plus). The column utilized was RtxR-5, which had an inner diameter of 0.25 mm, a film coating of 0.25 m, and a length of 30 m. The sample was filtered using a Whatman filter (0.2 m). The carrier gas was 99.999% pure helium at a flow rate of 1 mL/min in split mode. A column with an inlet temperature of 280 °C was injected with 1µL of an endophytic fungus extract, C. chaingmaiense. The oven temperature was initially set at 70 °C for two min, and it was successively raised to 320 °C at a rate of 7 °C per min. The ion sources were maintained at a temperature of 250 °C. The detector operates in scan mode from 30 to 500 Da atomic units, and the mass spectra of compounds present in endophytic fungus extract were obtained using electron ionisation at 70 eV. The procedure lasted a total of 22.5 min, which included a three- min solvent delay. The NIST05s library database was utilized to compare the extracted spectrum of the endophytic fungus C. chaingmaiense56.
Fungal mediated synthesis and characterization of Con-AgNPs
Silver nitrate was purchased from Himeda Laboratories Pvt.Ltd. INDIA., for the synthesis of Con-AgNPs. 100 mL of 1mM silver nitrate was mixed with 100 mL of cell filtrate in a flask and incubated at 28 ± 2℃ in dark condition for 24 h., filtrate (without AgNO3 solution) was taken as control, after 24-h incubation, cell filtrate solution with silver nitrate solution turned into light brown to dark brown that indicated the synthesis of Con-AgNPs. At first, characterization of Con-AgNPs was done by using Bio-spectrophotometer as described by31,32, with little modification. The synthesis of Con-AgNPs was measured at 200 nm to 800 nm. In order to separate synthesized Con-AgNPs from cell filtrate solution, centrifugation was done at 15,000 rpm for 15 min, this was done for 3 times with continuous washing of the pellet with sterile distilled water and ethanol, the supernatant was discorded and pellet was collected. Pellet was transferred to petri dish, placed in hot air oven at 50 ℃ for 4 h for drying. Moisture free Con-AgNPs were obtained and crushed using pestle and mortar. The obtained powder of Con-AgNPs was subjected to further characterization such as FTIR, SEM-EDAX, HR-TEM, XRD, DLS and Zeta potential. The functional groups involved in the production and stability of Con-AgNPs was investigated using FTIR analysis (Model Perkin- Elmer), and was recorded in range of 500–4000 cm‾1 with the resolution of 2 cm− 1. Further, to know the shape, surface morphology and elemental composition of Con-AgNPs, SEM-EDX was performed (Model, SEM HITACHI SU1510 and Ametek EDAX, Mahwah, NJ). Size, phase and crystallographic structures of Con-AgNPs were determined by subjecting AgNPs to XRD analysis (utima IV X-ray powder diffractometer Model). Stability of the Con-AgNPs was detected by measuring zeta sizer (Malvern instrument)13,57–62.
Antibiotic enhancing activity of Con-AgNPs
The evaluation of antibiotic enhancing activity of synthesized Con-AgNPs with some important commercial antibiotics was done by the disc diffusion method described by Rahi et al.2. At the beginning of this activity, the test bacterial pathogens such as Escherichia coli [MTCC-1599], Staphylococcus aureus [MTCC-4734], Pseudomonas aeruginosa [MTCC-1934], Salmonella typhi [MTCC-734], Klebesilla pneumoniae [MTCC-7028] were inoculated on Mueller -Hinton Agar (MHA) medium by swab inoculation method. Then 1 mg/mL of synthesized Con-AgNPs were dissolved in Dimethyl Sulfoxide (DMSO), 10 µg concentration of standard antibiotics discs [(Chloramphenical (CHL), Cefpodoxime (CPD), Gentamicine (GEN), Ampicillin (AMP) and Imipenem (IPM) were collected from Himedia Laboratories Pvt. Ltd. INDIA)] impregnated with 1 mg/mL concentration of synthesized Con-AgNPs were placed on Mueller -Hinton Agar (MHA, Himedia Laboratories Pvt. Ltd. INDIA), aseptic condition was maintained and incubated at 37℃ for 24 h. Standard antibiotic disc was employed as positive control, whereas Dimethyl Sulfoxide (DMSO) was employed as negative control. Formation of zone of inhibition was observed (Fold increase area was calculated by the equation (B2-A2)/ A2), where zone of inhibition of each antibiotic (A) and antibiotic + AgNPs (B). In case of absence of zone of inhibition (A) were considered as 6 mm, the measurement was estimated in triplicates the average ± standard deviation (SD) were calculated.
Antibacterial activity of Con-AgNPs
The synthesized Con-AgNPs was subjected to antibacterial activity by agar well diffusion method as outlined by Bauer et al. with little modification63,64. The antibacterial activity was tested against standard bacterial human pathogens gram positive (Staphylococcus aureus [MTCC-4734]), gram negative (Escherichia coli [MTCC-1599], Pseudomonas aeruginosa [MTCC-1934], Salmonella typhi [MTCC-734], Klebesilla pneumoniae [MTCC-7028]), the test bacterial pathogens were first inoculated on Mueller-Hinton Agar (MHA, Himedia Laboratories Pvt. Ltd. INDIA) medium by swab inoculation method, then the wells were made by sterile borer. A positive control (Streptomycin, Ciprofloxacin and Chloramphenicol) was employed while a negative control DMSO (Dimethyl Sulfoxide) was used. Incubated for 24 h at 37 °C, the standard antibiotics (1 mg/mL concentration) and Con-AgNPs (10 mg/mL, 05 mg/mL and 2.5 mg/mL) were loaded under aseptic conditions and incubated for 24 h. The experiment was performed in triplicates and results were expressed in mean ± standard deviation.
Minimum inhibitory concentration
MIC assay was performed in accordance with, Rani et al. Each well of the microtitre plate received 50 µL of each sterile Mueller Hinton broth44. To the first row of the microtitre plate, 4 mg/mL of Con-AgNPs solution dissolved in DMSO (Dimethyl Sulfoxide) and standard antibiotics Streptomycin, Ciprofloxacin, and Chloramphenicol were added, followed by serial dilution across. 100 µL of tested bacterial pathogens such as Escherichia coli [MTCC-1599], Staphylococcus aureus [MTCC-4734], Pseudomonas aeruginosa [MTCC-1934], Salmonella typhi [MTCC-734], Klebesilla pneumoniae [MTCC-7028] inoculums (approximately 1.5 × 108 CFU/mL) were added to each well. A positive control (tested bacterial pathogens) was employed while a negative control Mueller Hinton broth (MHB) was used. A positive control (tested bacterial pathogens) was used, while Mueller Hinton broth (MHB) was used as a negative control. Plates were wrapped in cling wrap to prevent bacteria dehydration and incubated at 37 °C for 24 h. To avoid errors, experiments were carried out in triplicate. Following incubation, 10 µL of Alamar Blue dye (resazurin) was added to each well and the plate was incubated in the dark for 3 h. Bacterial growth was indicated by a change in colour from purple to pink or colourless. The MIC value for AgNPs was determined as the lowest concentration at which no colour change was observed.
Antifungal activity of Con-AgNPs
The antifungal activity of synthesized Con-AgNPs was determined as described by Soni et al., with minor modifications65. Agar well diffusion method was used to evaluate the antifungal activity of synthesized Con-AgNPs against standard fungal pathogens such as Candida albicans [MTCC-1637], Aspergillus brassiliensis [MTCC-1344] and Aspergillus flavus [MTCC-9606]. The spore suspension of test fungi were made in sterile water with tween 20 and it was swab inoculated on sterile Sabouraud Dextrose Agar (SDA, Himedia Laboratories Pvt. Ltd. INDIA) medium using the spread plate method. The wells were made with a sterile borer on inoculated Sabouraud Dextrose Agar (SDA) medium, and standard antifungal FCZ (Fluconazole,1 mg/mL) was used as positive control, different concentrations of Con-AgNPs (10 mg/mL and 5 mg/mL) and a negative control DMSO (Dimethyl Sulfoxide) were loaded under aseptic conditions. Plates were incubated in upright position for 2–3 days at 27 °C, the formation of a zone of inhibition was spotted, and the average standard deviation (SD) was computed after measuring in triplicates.
In vitro antioxidant activity of Con-AgNPs
DPPH radical scavenging activity
The free radical scavenging activity was positive, according to Rhaman et al.66. To 140 µL of an ethanolic solution containing 1mM DPPH, varied concentrations of sample solution (100–500 µg/mL) were added. The tubes were incubated in the dark for 5 min at 37 °C. The absorbance was measured at 517 nm against a blank. As a standard, L- ascorbic acid is employed. The radical scavenging activity of the synthesized Con-AgNPs, expressed as a percentage of inhibition, was calculated using the equation below.
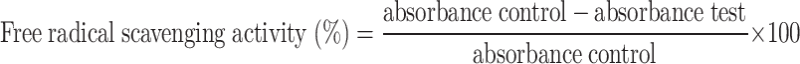
ABTS radical cation decolorization assay
The ABTS radical cation decolorization experiment, according to Sreeram et al., was a success67. To generate an ABTS + solution, 7 mM ABTS was combined with 2.45 mM potassium persulfate in water (1:1). This reaction mixture was maintained at room temperature in a container for 16 to 20 h. We generated an ABTS + solution with methanol as a diluting agent that had an absorbance of 0.7 at 734 nm. After 30 min, different concentrations of synthesized Con-AgNPs (100 to 500 µg/mL) were added to make up to 4mL of ABTS + solution, and the absorbance at 734 nm was measured. The results, which were provided as percentages, were measured using percent scavenging of Con-AgNPs. The radical cation decolorization assay of the synthesized AgNPs, expressed as a percentage of inhibition, was calculated using the equation below.

Measurement of cell viability by MTT assay
The MTT assay was used to determine the effect of synthesized Con-AgNPs on the viability of Human Heptaocellular adenocarcinoma cell line (HepG2) and Human renal (Kidney) adenocarcinoma cell line (A498). HepG2 and A498 cell lines were procured from the National Centre for Cell Science, Pune, INDIA. The cell lines were grown ideological and maintained at 37 °C in 5% CO2 in DMEM containing 10% foetal calf serum and 1% antibiotic solution containing penicillin and streptomycin until the monolayer was sub-confluent. Spectrophotometric analysis of MTT reduction assay was performed as per article Giridasappa et al.; Akther et al.,68,69. When cell lines attained confluency, they were trypsinized, passaged, and transferred at a density of 30 × 103 cells in 100 µL volume per well in a 96 multiwell plate using the mitochondrial succinate dehydrogenase. The reaction plates were incubated in a CO2 incubator at 37 °C for 24 h. A partial monolayer is formed; cancer and fibrocystic cell lines were washed twice with 1×PBS pH 7.4 and replaced with a fresh medium containing various dilutions of prepared Con-AgNPs (12.5–200 µg/mL) and incubated for 24 h at 37 °C. The supernatant was then discarded, and 10 µL of MTT (5 mg/mL) was combined and cultured for 4 h to allow the cells to absorb the dye. The media was replaced with 100 µL DMSO, and the activity was measured at 570 nm. When compared to the control (without test sample).
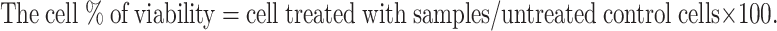
Evaluation of in vitro anti-inflammatory activity of synthesized Con-AgNPs
Egg albumin denaturation test
The egg albumin denaturation test, as described previously Gupta et al.70, was used to assess the in vitro anti-inflammatory activity of the synthesized Con-AgNPs. The reaction mixture (5mL) contained 5 different concentrations of produced Con-AgNPs (10, 50, 100, 150, and 200 µg/mL), 0.2 mL of egg albumin, 2.8 mL of phosphate buffered saline (PBS, pH 6.4), and 2 mL of each. An equivalent volume of distilled water was used as a control. After being incubated at (37 °C) for 15 min, the mixtures were heated at 70 °C for 5 min. After cooling, absorbance was measured at 660 nm using a vehicle as a blank. Diclofenac sodium was used as a control drug and was treated similarly to measure absorbance at final concentrations of 10, 50, 100, 150, and 200 µg/mL. The experiment was carried out three times.
The percentage inhibition of protein denaturation was calculated using the following formula.

Where Acontrol is the absorbance of solution without silver nanoparticles, Asample is the absorbance of solution with sample silver nanoparticles/standard.
Membrane stabilization test
Suspension of Human Red Blood Cells (HRBC) preparation Human blood was collected in a centrifuge tube with 2 mg disodium EDTA added as an anticoagulant, then centrifuged at 1000 g for 10 min. After centrifugation, the erythrocyte pellet was gently resuspended and washed three times with ten volumes of pyrogen-free saline (0.9% NaCl). The suspension was centrifuged again at 1000 g for 10 min, and the pellet was gently resuspended in normal saline to 10.0% (v/v)70.
Hypotonicity-induced hemolysis
Synthesized Con-AgNPs were dissolved in isotonic phosphate buffer solution as well as distilled water (hypotonic solution) at the required concentration (100–500 µg/mL) in centrifuge tubes. The control tubes held 5 mL of distilled water. As a positive control, Diclofenac sodium was dissolved in isotonic phosphate buffer solution to the required concentration (100–500 µg/mL). Each tube received 0.1 mL of erythrocyte suspension, which was gently mixed. The tubes were then incubated in a water bath for 30 min at 37 °C. The tubes were then centrifuged for 5 min at 3000 rpm. The absorbance at 540 nm of the supernatant is measured to determine the haemoglobin content due to hemolysis. The percentage of hemolysis was calculated by assuming the hemolysis produced in the presence of distilled water as 100%. The percent inhibition of hemolysis by the extract was calculated according to the Equation53.
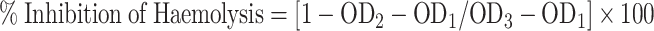
Where, OD1, OD2, and OD3 are the absorbance of the test sample in an isotonic solution, the absorbance of the test sample in a hypotonic solution, and the absorbance of the control sample in a hypotonic solution, respectively.
In-vivo anti-inflammatory activity of synthesized Con-AgNPs
Experimental mice
Female Swiss albino mice weighing 25 to 35 g were obtained from the Animal Facility of Liveon Biolabs Pvt. Ltd., Tumkur. All animal experiments were carried out in accordance with the guidelines of the Committee for the Purpose of Supervision of Experiments on Animals (CPCSEA), India’s national regulatory body for animal experiments with approval number were consented by the Office of Institutional Animal Ethical Committee (IAEC) for the Purpose of CPCSEA under the rules 5(a) of the “Breeding of experiments on animal. The experimental procedure received approval from the Institutional Ethical Committee established under the CPCSEA, Government of India. The registration number is 123/PO/C/99/CPCSEA and the sanctioned letter number is SSPT/IAEC.Clear/152/2016-17, dated 01/10/2016. The mice were provided with water and food ad libitum. The animals were anesthetized with sodium pentobarbital anesthesia and euthanized via cervical dislocation, with utmost care taken to minimize any suffering or pain. The approaches were executed in compliance with the applicable rules and regulation. The study was conducted in accordance with the ARRIVE criteria (https://submission.springernature.com/submission/f36b1992-9396-42df-93cc-eff077a11641/file/d2a32db4-e884-4cab-9041-dbc16f29ea6c).
Carrageenan-induced paw edema in mice
Carrageenan-induced mouse paw edema is a well-known model of acute inflammation for evaluating anti-inflammatory drugs. The anti-inflammatory effect of synthesized Con-AgNPs was evaluated in mice using carrageenan-induced paw edema, as previously described with minor modifications70,71. To induce inflammation, 0.1 mL of 1% carrageenan was subcutaneously injected into the right hind paw of mice. Mice were given intra-peritoneal injections of Diclofenac sodium (10 mg/kg) and synthesized Con-AgNPs (10 µg to 150 µg/mouse) one hour before receiving carrageenan. The carrageenan-induced edema feet were dissected two hours after the animals were sacrificed, and the paw edema was assessed for histopathology. The dissected paws were weighed, compared to the controls, and stored in 10% formalin.
The inhibitory effect was determined by using following formula:
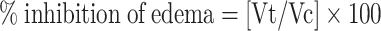
Where Vc is the edema volume in the control group and Vt is the edema volume in tested group.
Statistical analysis
The data collected from the experiment were analysed statistically and presented as mean values with standard deviations (SD). The Prism Pad software (Version 5.0) developed by GraphPad Software, Inc., San Diego, CA, USA, was employed to conduct a one-way ANOVA with Dunnett’s test. Statistically significant P-values were defined as those that were less than 0.05 (p < 0.05).
Acknowledgements
Thanks to the Department of Microbiology, Kuvempu University, Karnataka, for providing laboratory facilities and financial support to carry out this research work. Department of Studies and Research in Biochemistry, Tumkur University, Tumakuru, Karnataka, INDIA., for providing facilities to perform anti-inflammatory analysis. SN thank Vision Group on Science and Technology (VGST), Government of Karnataka, Karnataka and Department of Science and Technology - Empowerment and Equity Opportunities for Excellence in Science (DST-EMEQ) for financial assistance. This work is also funded by Researchers Supporting Project number (RSP2024R31), King Saud University, Riyadh, Saudi Arabia.
Abbreviations
- PCR
Polymer chain reaction
- CTAB
Cetyl trimethyl ammonium bromide
- BLAST
Basic local alignment search tool
- NCBI
National Center for Biotechnology information
- ITS
Internal transcribed spacer
- ANOVA
Analysis of variance
- MEGA
Molecular evolutionary genetics analysis
- KUMBMDBT
Kuvempu University Microbiology Manjunatha D B Thippeswamy
- FTIR
Fourier transform infrared spectroscopy
- SEM
Scanning electron microscopy
- HR-TEM
High resolution-transmission electron microscopy
- EDAX
Energy dispersive analysis of X-ray
- XRD
X-ray diffraction
- DLS
Dynamic light scattering
- AgNPs
Silver nanoparticles
- NPs
Nanoparticles
- Con-AgNPs
Coniothyrium chaingmaiensev derived-silver nanoparticles
Author contributions
MD-Analysis of research work in laboratory, methodology, software, data analysis and manuscript writing, MGT, NS, TRS guide for the experimentation of anti-inflammatory activity, reviewing. PJ, SHV-manuscript correction and editing, TB- designee and correction of research work. SM, HR, SN, AK, SV- manuscript correction and editing.
Funding
Researchers Supporting Project number (RSP2024R31), King Saud University, Riyadh, Saudi Arabia.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thippeswamy Basaiah, Email: thippeswamyb272@yahoo.in.
Sachin Naik, Email: snaik@ksu.edu.sa.
References
- 1.Govindappa, M., Lavanya, M. & Aishwarya, P. Synthesis and characterization of Endophytic Fungi, Cladosporium perangustum mediated silver nanoparticles and their antioxidant, Anticancer and Nano-toxicological study. Bionanosci. 10, 928–941. 10.1007/s12668-020-00719-z (2020). [Google Scholar]
- 2.Rahi, D. K. & Parmar, A. S. Mycosynthesis of silver nanoparticles by an endophytic Penicillium species of Aloe vera root, evaluation of their antibacterial and antibiotic enhancing activity. (2014).
- 3.Barkhade, T. Extracellular biosynthesis of silver nanoparticles using Fungus Penicillium species. (2018). 10.5281/zenodo.1164148
- 4.Al-Radadi, N. S. et al. Zingiber officinale driven bioproduction of ZnO nanoparticles and their anti-inflammatory, anti-diabetic, anti-Alzheimer, anti-oxidant, and anti-microbial applications. Inorg. Chem. Commun. 140, 109274 (2022). [Google Scholar]
- 5.Khan, M. I. et al. Monotheca Buxifolia Driven synthesis of Zinc Oxide Nano Material its characterization and Biomedical Applications. Micromachines. 13, 668 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafar, S. et al. Development of Iron nanoparticles (FeNPs) using biomass of Enterobacter: its characterization, Antimicrobial, Anti-alzheimer’s, and enzyme inhibition potential. Micromachines. 13, 1259 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan, H. et al. The Aquilegia pubiflora (himalayan columbine) mediated synthesis of nanoceria for diverse biomedical applications. RSC Adv. 10, 19219–19231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jan, H. et al. Plant-based synthesis of Zinc Oxide nanoparticles (ZnO‐NPs) using Aqueous Leaf Extract of Aquilegia pubiflora: their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in Vitro Properties. Oxidative Med. Cell. Longev. 4786227 2021a (2021). [DOI] [PMC free article] [PubMed]
- 9.Faisal, S. et al. Paraclostridium benzoelyticum Bacterium-Mediated Zinc Oxide Nanoparticles and Their In Vivo Multiple Biological Applications. Oxidative Medicine and Cellular Longevity. 1–15 (2022a). (2022). [DOI] [PMC free article] [PubMed]
- 10.Jan, H. et al. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO‐NPs) Using Aqueous Leaf Extract of Aquilegia pubiflora : Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other In Vitro Properties. Oxidative Medicine and Cellular Longevity. 4786227 (2021b). (2021). [DOI] [PMC free article] [PubMed]
- 11.Faisal, S. et al. Fagonia cretica-Mediated synthesis of Manganese Oxide (Mno2) nanomaterialse their characterization and evaluation of their bio-catalytic and enzyme inhibition potential for maintaining flavor and texture in Apples.Catalysts. 12, 558 (2022b). [Google Scholar]
- 12.Rodrigues, A. G., Ping, L. Y. & Marcato, P. D. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl. Microb. Biotechnol. 97, 775–782. 10.1007/s00253-012-4209-7 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Singhal, G., Bhavesh, R. & Kasariya, K. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart. Res. 13, 2981–2988. 10.1007/s11051-010-0193-y (2011). [Google Scholar]
- 14.Bala, M. & arya, V. Biological synthesis of silver nanoparticles from aqueous extract of endophytic fungus aspergillus fumigatus and its antibacterial action. Int. J. Nanomaterials Biostructures. 3 (2), 37–41 (2013). [Google Scholar]
- 15.Mistry, H., Thakor, R. & Patil, C. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnol. Lett. 43, 307–316. 10.1007/s10529-020-03008-7 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Sharma, A., Sagar, A., Rana, J. & Rani, R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces Purpureogenus isolated from Taxus baccata Linn. Micro Nano Syst. Lett. 10, 2. 10.1186/s40486-022-00144-9 (2022). [Google Scholar]
- 17.Dinesh, B. et al. Antibacterial activity of AgNPs synthesized using endophytic fungus Penicillium Cinnamopurpureum, Spectroscopy latters, 20–34, (2022). 10.1080/00387010.2021.2010764
- 18.Vardhana, J., Aparna, A. & Ramarajan, S. Synthesis and antibacterial activity of silver nanoparticles from Endophytic Fungi Phyllosticta Sp isolated from Amaranthus retroflexus - A plant weed. Int. J. Pharm. Sci. Rev. Res. 51, 48–52 (2018). [Google Scholar]
- 19.Tayung, K. & Jha, D. K. Antimicrobial endophytic fungal assemblages inhabiting bark of Taxus baccata L. of Indo-Burma mega biodiversity hotspot. Indian J. Microbiol., S74–S81, 10.1007/s12088-010-0056-3(2010). [DOI] [PMC free article] [PubMed]
- 20.Devi, N. N. & Singh, M. S. GC-MS analysis of metabolites from endophytic fungus Colletotrichum gloeosporioides isolated from Phlogacanthus Thyrsiflorus Nees. Int. J. Pharm. Sci. Rev. Res. 23, 392–395 (2013). [Google Scholar]
- 21.El-Baky, N. A. & Amara, A. F. Recent approaches towards control of fungal diseases in plants: an updated review. J. Fungi, 7–900. (2021). [DOI] [PMC free article] [PubMed]
- 22.Abdullah et al. Green synthesis and characterization of copper and nickel hybrid nanomaterials: investigation of their biological and photocatalytic potential for the removal of organic crystal violet dye. J. Saudi Chem. Soc. 26, 101486 (2022). [Google Scholar]
- 23.Hu, X., Kandasamy, S., Tieyan, J. & Myeong-Hyeon, W. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces Purpureogenus. Int. J. Nanomed. 14, 3427–3438. 10.2147/IJN.S200817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizwan, M. et al. Enterobacter hormaechei -Driven Novel Biosynthesis of Tin Oxide nanoparticles and evaluation of their anti-aging, cytotoxic, and enzyme inhibition potential. ACS Omega. 8, 27439–27449 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nischitha, R. & Shivanna, M. B. Screening of secondary metabolites and antioxidant potential of endophytic fungus Penicillium Citrinum and host Digitaria bicornis by spectrophotometric and electrochemical methods. Arch. Microbiol. 204, 206. 10.1007/s00203-022-02795-z (2022). [DOI] [PubMed] [Google Scholar]
- 26.Prasher, I. B. & Dhanda, R. K. GC-MS analysis of secondary metabolites of Endophytic Nigrospora Sphaerica isolated from Parthenium hysterophorus. Int. J. Pharm. Sci. Rev. Res. 44, 217–223 (2017). [Google Scholar]
- 27.Nischitha, R. & Shivanna, M. B. Metabolite fingerprinting, in vitro antimicrobial and antioxidant activities and in-silico docking in Alloteropsis cimicina and its endophytic fungus Penicillium Pinophilum. Mol. Biol. Rep. 48, 4021–4037. 10.1007/s11033-021-06410-0 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Gill, H. & Vasundhara, M. Isolation of taxol producing endophytic fungus Alternaria brassicicola from non-taxus medicinal plant Terminalia Arjuna. World J. Microbiol. Biotechnol. 35, 74. 10.1007/s11274-019-2651-8 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Nandiyanto, A. B. D., Oktiani, R. & Ragadhita, R. How to read and interpret FTIR Spectroscope of Organic Material. Indonesian J. Sci. Technol. 4, 97. 10.17509/ijost.v4i1.15806 (2019). [Google Scholar]
- 30.Ramalingmam, P., Sathiyabama, M. & Prabha, T. Biosynthesis of silver nanoparticles using an endophytic fungus, Curvularia lunata and its antimicrobial potential. J. Nanosci. Nanoengineering. 1, 241–247 (2015). [Google Scholar]
- 31.Netala, V. R. et al. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 5683–5696. 10.2147/IJN.S112857 (2016). [DOI] [PMC free article] [PubMed]
- 32.Netala, V. R., Kotakadi, V. S., Bobbu, P., Gaddam, S. A. & Tartt, V. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and antimicrobial studies. 3 Biotech. 6, 132. 10.1007/s13205-016-0433-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netala, V. R., Ghosh, S. B., Bobbu, P. & Tartte, V. Endophytic fungal assisted synthesis of silver nanoparticles, characterization and antimicrobial activity. Asian J. Pharm. Clin. Res. 113–116. (2015).
- 34.Faisal, S. et al. Edible mushroom (Flammulina Velutipes) as biosource for silver nanoparticles: from synthesis to diverse biomedical and environmental applications. Nanotechnology. 32, 065101 (2021a). [DOI] [PubMed] [Google Scholar]
- 35.Faisal, S. et al. Vivo Analgesic, anti-inflammatory, and anti-diabetic screening of Bacopa monnieri -synthesized copper oxide nanoparticles. ACS Omega. 7, 4071–4082 (2022c). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popli, D. et al. Endophyte fungi, Cladosporium species-mediated synthesis of AgNPs possessing in vitro antioxidant, anti-diabetic and anti-alzheimer activity, artificial cells, Nano medicine, and biotechnology, S676–S683, (2018). 10.1080/21691401.2018.1434188 [DOI] [PubMed]
- 37.Faisal, S. et al. Curcuma longa MedsynthesisthescopperCoxide Oxide, Nickel Oxide and Cu-Ni Bimetallic Hnanoparticlesticharacterizationzatioevaluationuation for Antimicroanti-parasiticasiticytotoxicopotentialsntials. Coatings. 11, 849 (2021e). [Google Scholar]
- 38.Nagaraja, S. K. et al. Biomimetic synthesis of silver nanoparticles using Cucumis sativus var. Hardwickii fruit extract and their characterizations, anticancer potential and apoptosis studies against Pa-1 (human ovarian teratocarcinoma) cell line via flow cytometry. Appl. Nanosci. 13, 3073–3084 (2023). [Google Scholar]
- 39.Azmath, P., Baker, S., Rakshith, D. & Satish, S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 24, 140–146. 10.1016/j.jsps.2015.01.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farsi, M. & Farokhi, S. Biosynthesis of Antibacterial Silver nanoparticles by Endophytic Fungus Nemania sp. isolated from Taxus baccata L. (Iranian Yew). Zahedan J. Res. Med. Sci. 2010.5812/zjrms.57916 (2018).
- 41.Abdullah, Al-Radadi, N. S., Hussain, T., Faisal, S. & Shah, A. R. Novel biosynthesis, characterization and bio-catalytic potential of green algae (Spirogyra hyalina) mediated silver nanomaterials. Saudi J. Biol. Sci. 29, 411–419 (2022b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ullah, R. et al. In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres. Green. Process. Synthesis. 10, 101–111 (2021). [Google Scholar]
- 43.Vassallo, J., Besinis, A., Boden, R. & Handy, R. D. The minimum inhibitory concentration (MIC) assay with Escherichia coli: an early tier in the environmental hazard assessment of nanomaterials? Ecotoxicol. Environ. Saf. 162, 633–646 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Rani, R., Sharma, D., Chaturvedi, M., Jp, Y. & Green Synthesis, characterization and antibacterial activity of silver nanoparticles of Endophytic Fungi Aspergillus terreus. J. Nanomed. Nanotechnol. 0810.4172/2157-7439.1000457 (2017).
- 45.Niazi, S. K. et al. GC-MS based characterization, Antibacterial, Antifungal and anti-oncogenic activity of Ethyl acetate extract of aspergillus Niger strain AK-6 isolated from Rhizospheric Soil. CIMB. 45, 3733–3756 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yugandhar, Haribabu, R., Savithramma, N. & Synthesis (eds), characterization and antimicrobial properties of green-synthesised silver nanoparticles from stem bark extract of Syzygium alternifolium (Wt.) Walp, 3 Biotech, 5:1031–1039, DOI (2015). 10.1007/s13205-015-0307-4 [DOI] [PMC free article] [PubMed]
- 47.Nagaraja, S. K., Niazi, S. K., Bepari, A., Assiri, R. A. & Nayaka, S. Leonotis nepetifolia Flower Bud Exmediateddiated Green Synthesis of Snanoparticlestitheir characterizationzationinand In evaluationuation of Biological Applications. Materials. 15, 8990 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faisal, S. et al. Bio-catalytic activity of Novel Mentha arvensis intervened Biocompatible Magnesium Oxide nanomaterials. Catalysts. 11, 780 (2021c). [Google Scholar]
- 49.Nallappan, D. et al. Green biosynthesis, antioxidant, antibacterial, and anticancer activities of silver nanoparticles of Luffa acutangula leaf extract. Biomed. Res. Int. 1–28. 10.1155/2021/5125681 (2021). [DOI] [PMC free article] [PubMed]
- 50.El-Naggar, N. E., Hussein, M. H. & El-Sawah, A. A. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in -vitro and in -vivo assessment of anticancer activities, Sci. Rep, 8 (1), 8925. (2018). 10.1038/s41598-018-27276-6 [DOI] [PMC free article] [PubMed]
- 51.Sangeeta, M. K. et al. In-vitro evaluation of Talaromyces Islandicus mediated Zinc Oxide nanoparticles for Antibacterial, Anti-inflammatory, bio-pesticidal and seed growth promoting activities. Waste Biomass Valor. 15, 1901–1915 (2024). [Google Scholar]
- 52.Das, S. K., Behera, S., Patra, J. K. & Thatoi, H. Green synthesis of sliver nanoparticles using Avicennia officinalis and Xylocarpus granatum extracts and in vitro evaluation of antioxidant, antidiabetic and anti-inflammatory activities. J. Cluster Sci. 30, 1103–1113. 10.1007/s10876-019-01571-2 (2019). [Google Scholar]
- 53.Bagur, H. et al. Biogenically synthesized silver nanoparticles using endophyte rungal extract of Ocimum tenuiflorum and evaluation of biomedical properties. J. Cluster Sci. 31, 1241–1255. 10.1007/s10876-019-01731-4 (2020). [Google Scholar]
- 54.Rafie, H. M. E. & Hamed, M. A. A. Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv. Nat. Sci. NanoSci. NanoTechnol. 1–11. 10.1088/2043-6262/5/3/035008 (2014).
- 55.Bhat, M. P. et al. Aspergillus Niger CJ6 extract with antimicrobial potential promotes in-vitro cytotoxicity and induced apoptosis against MIA PaCa-2 cell line. Environ. Res. 229, 116008 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Kanjana, M., Kanimozhi, G., Udayakumar, R. & Panneerselvam, A. GC-MS analysis of Bioactive compounds of Endophytic Fungi Chaetomium Globosum, Cladosporium tenuissimum and Penicillium Janthinellum. J. Biomedical Pharm. Sci. Volume. 2, 123 (2019). [Google Scholar]
- 57.Devi, L. & Joshi, S. Ultrastructures of silver nanoparticles biosynthesized using endophytic fungi. J. Microsc Ultrastruct. 3, 29. 10.1016/j.jmau.2014.10.004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Govindappa, M., Farheen, H., Chandrappa, C. P., Channabasava, Rai, R. V. & Raghavendra, V. B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. NanoSci. NanoTechnol. 1–9. 10.1088/2043-6262/7/3/035014 (2016).
- 59.Magdi, H. M., Mohamed, H. E. M. & Marwa, M. Ae-a. biosynthesis of silver nanoparticles using fungi and biological evaluation of mycosynthesized silver nanoparticles. Egypt. Soc. Experimental Biology. 10 (1), 1–12 (2014). [Google Scholar]
- 60.Rudrappa, M. et al. Myco-Nanofabrication of Silver nanoparticles by Penicillium Brasilianum NP5 and their Antimicrobial, Photoprotective and Anticancer Effect on MDA-MB-231 breast Cancer cell line. Antibiotics. 12, 567 (2023a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudrappa, M. et al. PeniciCitrinumtrinum NP4 mediated production, extraction, physicochemical characterization of the melanin, and its anticancer, apoptotic, photoprotection properties. Int. J. Biol. Macromol. 245, 125547 (2023b). [DOI] [PubMed] [Google Scholar]
- 62.Nayaka, S. et al. Biosynthesis, characterization, and in vitro assessment on cytotoxicity of actinomycete-synthesized silver nanoparticles on Allium cepa root tip cells. Beni-Suef Univ. J. Basic. Appl. Sci. 9, 51 (2020). [Google Scholar]
- 63.Bauer, A. W., Kirby, W. M. M., Sherris, J. C. & Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496. 10.1093/ajcp/45.4_ts.493 (1966). [PubMed] [Google Scholar]
- 64.Shashiraj, K. N. et al. Exploring the Antimicrobial, Anticancer, and apoptosis inducing ability of Biofabricated Silver nanoparticles using Lagerstroemia speciosa Flower buds against the human osteosarcoma (MG-63) cell line via Flow Cytometry. Bioengineering. 10, 821 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soni, N. & Prakash, S. Antimicrobial and mosquitocidal activity of microbial synthesized silver nanoparticles. Parasitol. Res. 114, 1023–1030. 10.1007/s00436-014-4268-z (2015). [DOI] [PubMed] [Google Scholar]
- 66.Rahman, M. M., Islam, M. B., Biswas, M., Khurshid & Alam, A. H. M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 810.1186/s13104-015-1618-6 (2015). [DOI] [PMC free article] [PubMed]
- 67.Sreeram, N. P., Henning, S. M. & Niu, Y. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 54, 1599–1603. 10.1021/jf052857r (2006). [DOI] [PubMed] [Google Scholar]
- 68.Giridasappa, A., Ismail, S. M., Rangappa, D. & Antioxidant Antiproliferative and antihemolytic properties of phytofabricated silver nanoparticles using Simarouba glauca and Celastrus Paniculatus extracts. Appl. Nanosci. 11, 2561–2576. 10.1007/s13204-021-02084-z (2021). [Google Scholar]
- 69.Akther, T., Mathipi, V., Kumar, N. S., Davoodbasha, M. & Srinivasan, H. Fungal-mediated synthesis of pharmaceutically active silver nanoparticles and anticancer property against A549 cells through apoptosis. Environ. Sci. Pollut. Res. 13649–13657. 10.1007/s11356-019-04718-w (2019). [DOI] [PubMed]
- 70.Gupta, A. K. et al. Analgesic and anti-inflammatory properties of Gelsolin in Acetic Acid Induced Writhing, tail immersion and Carrageenan Induced Paw Edema in mice. PLoS ONE. 10, e0135558. 10.1371/journal.pone.0135558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghorbanzadeh, B. et al. A study of the mechanisms underlying the ant-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 47, 292. 10.4103/0253-7613.157127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.