Abstract
Heart rate variability (HRV) is a key indicator of cardiac autonomic function, making reliable assessment crucial. To examine the test-retest stability of resting HRV in healthy individuals, fifty participants attended two lab sessions within a week, at the same time of day. After a 5-minute acclimatization period, electrocardiogram and respiration were recorded at rest. For validation, average heart rate and RMSSD were assessed over 15 minutes using a validated open-source toolbox. Test-retest agreement was evaluated using intra-class correlation (ICC), and coefficients of variation (CV). Mean heart rate showed high stability (ICC = 0.81, CV = 6%), while RMSSD had lower concordance (ICC = 0.75) and greater variation (CV = 30%). These findings indicate good test-retest agreement for standard HRV features. However, a wide range of methodologies exists for assessing various properties of heart rate dynamics. This database is intended to support other researchers in testing additional HRV metrics to evaluate their reliability in healthy individuals.
Subject terms: Cardiovascular biology, Predictive markers
Background & Summary
Over the past few decades, heart rate variability (HRV) has become a reliable predictor of cardiovascular risk and overall mortality1. HRV measures fluctuations in heartbeat intervals influenced by both sympathetic and parasympathetic nervous systems. Low HRV has been linked to various medical conditions, including hypertension, chronic kidney disease, stroke, and dementia2–7. Given its clinical importance, accurately assessing HRV is crucial.
A review by Sandercock et al.8 found significant variability in HRV test-retest reliability, influenced by the study population, the type of HRV metric, and the test-retest interval8. HRV is commonly measured using time-domain (e.g., standard deviation of heartbeat intervals) and frequency-domain analyses (e.g., low- and high-frequency components)9. Nonlinear approaches have also been proposed for a more robust assessment of heart rate dynamics10–12. The reliability of HRV depends on the recording duration, with short-term (5–15 minutes) and long-term (24 hours) measurements providing different insights8.
At rest, there is a significant effect of respiration on HRV, a phenomenon known as respiratory sinus arrhythmia (RSA), where heart rate increases during inhalation and decreases during exhalation13–15. This cardiorespiratory coupling reflects the dynamic interaction between the autonomic nervous system and cardiac function, primarily mediated by vagal activity16. Different methods have been developed to assess this coupling. One common approach is time-domain analysis, which examines the variation in heartbeat intervals in relation to the respiratory cycle17,18. Frequency-domain analysis, on the other hand, measures the power of heart rate fluctuations within specific frequency bands associated with respiration, typically the high-frequency band19. A variety of other methods exist including nonlinear approaches such symbolic dynamics or phase-rectified signal averaging that offer a more complex assessment by evaluating the synchrony and complexity of the interactions between heart rate and respiration20–22.
In this study, we collected data to investigate the test-retest stability of resting HRV and cardiorespiratory coupling in healthy subjects.
Methods
Participants
Resting-state physiological recordings were obtained from 51 healthy volunteers (35 women, 16 men, 38 ± 16 years of age). All participants were recruited from the local community through word of mouth and advertisements on our website. One participant withdrew after the first session for personal reasons, leaving a total of 50 participants who completed both sessions in our laboratory. Demographic information is presented in Table 1.
Table 1.
Demographic information on sample of healthy volunteers who have completed two sessions.
| Feature | Mean ± SD | Range |
|---|---|---|
| Age [years] | 37.7 ± 16.1 | 19–73 |
| Gender [f/m] | n = 35 / n = 16 | — |
| BMI [kg/m²] | 22.9 ± 3.3 | 17–31 |
BMI: body mass index. SD: standard deviation.
Human subjects
The study concept, including consent for pseudonymized data sharing, was approved by the ethics committee of the medical faculty of the Friedrich-Schiller-Universität Jena (#5423-02/18). All research was performed in accordance with relevant guidelines and regulations. Informed written consent were obtained from all participants including consent to publish obtained data without identifying any individuals.
Procedures
All measurements were recorded at the Department of Psychosomatic Medicine and Psychotherapy at Jena University Hospital.
We performed two assessments one week apart at a similar time of day (mean difference 5 ± 43 min). The resting state recordings were made in the supine position. Participants were not allowed to exercise excessively, smoke or eat heavy meals in the two hours before the session.
ECG
An ECG (lead II) was recorded at 1000 Hz by an MP150 (ECG100C, BIOPAC systems inc., Golata, CA, USA). Pre-gelled Ag/AgCl electrodes (BlueSensor VL, Ambu GmbH, Bad Nauheim, GER) on the chest according to an adjusted Einthoven triangle23.
Respiration
Respiratory movements were recorded using a strain gauge transducer integrated into a belt, which was secured around the chest at the level of the lower end of the sternum (see Fig. 1). The acquired signal was low-pass filtered at 10 Hz and sampled at 1000 Hz (RESP100C, BIOPAC systems inc., Golata, CA, USA).
Fig. 1.
Cardiorespiratory recordings using the MP150 acquisition unit (BIOPAC Systems Inc.). Left: One ECG channel has been recorded between the electrodes red and white (lead II). A belt with a strain-gauge transducer acquired movement of the thorax during breathing. Right: The ECG and respiratory movement were sampled synchronously at 1000 Hz. Pictures from www.biopac.com adapted with permission.
Measurement protocol
Measurements were conducted in our temperature-controlled autonomic laboratory, maintained at 22 °C. The recording environment was quiet and fully shaded, with constant illumination provided by an indirect light source. Each session began with an interview, followed by a detailed explanation of the study’s purpose and design. Written consent was obtained from all participants.
Participants were then positioned comfortably on the examination tilt table, and electrodes and respiration belts were applied. For the resting state recording, participants were instructed to remain still and avoid movement, yawning, or coughing. A grey ellipse was displayed on a screen in front of them to allow free eye movement. An eye tracker monitored pupil size to ensure participants did not fall asleep.
Before data acquisition began, the instructor checked the signal quality and adjusted the electrodes and respiration belts if needed. Once the signal quality was deemed satisfactory, the recording proceeded for 20 minutes. The first five minutes were excluded from the analysis and removed from the data.
Parameters
To demonstrate plausibility of collected data, we investigated test-retest stability of standard time domain heart rate variability indices. Therefore, heart beats were extracted and analyzed using the open source PhysioNet Cardiovascular Signal Toolbox implemented in MATLAB (R2019a, The Mathworks, Natick, MA, USA). This toolbox has been validated previously24 and is freely available at PhysioNet.org25.
Data Records
Data are available at www.figshare.com (10.6084/m9.figshare.26359453.v1)26. Each recording is stored as a data file containing ECG and respiration signals for 15 minutes. The data are organized into columns, with the first column representing the time index in seconds, the second column containing the ECG signal in microvolts, and the third column representing the respiratory signal in volts. Additional information can be found in the table subjects.csv that is included in the repository.
Data anonymization
Files were pseudonymized to numbers 01-51 after random ordering. The table subjects.csv gives age in years, sex (man/woman), and body mass index in kg/m² for each participant.
Technical Validation
To assess the validity of the recorded data, we estimated the mean heart rate (HR) and RMSSD from the entire heart beat interval time series recorded during both the test and retest sessions (see Fig. 2). Stability and concordance were evaluated using coefficients of variation (CV), calculated as the ratio of the standard deviation to the mean of the two measurements, and intraclass correlation coefficients (ICCs) (two-way mixed model, single measure, absolute agreement; Shrout and Fleiss, 1979).
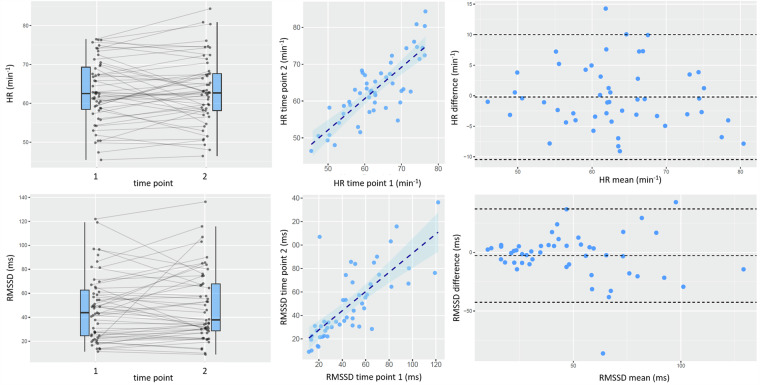
Fig. 2.
Test-retest stability of mean heart rate (HR, top row) and short-term heart rate variability (RMSSD, bottom row). Left: Changes from first to second session. Middle: Test-retest correlation. Right: Bland-Altman plots depicting differences (session 1 – session 2) over then average of both sessions. Dashed lines indicate mean difference and limits of agreement (1.96 standard deviations above and below).
As shown on the left side of Fig. 2, there were no significant differences between the test and retest estimates for either parameter. The coefficient of variation was CV = 5.8% for HR and CV = 29.6% for RMSSD. Both HR (ICC = 0.81, [0.71; 0.88]) and RMSSD (ICC = 0.75, [0.63; 0.84]) showed very high correlation between the test and retest sessions (Fig. 2, middle).
The Bland-Altman plot (Fig. 2, right side) depicts the difference between test and retest measurements over the average of the two measurements for the same subject. For HR, the mean bias was −0.2 min−1 with limits of agreement ranging from −10.5 to 10.0 min−1. The mean bias of RMSSD was −2.7 ms with limits of agreement from −42.6 to 37.2 ms. One outlier appeared to skew these statistics; specifically, in one subject, a high prevalence of premature heartbeats significantly elevated the RMSSD value during the second session (‘51_2.txt’).
However, even without sophisticated artifacts management or outlier correction these results indicate good reproducibility of standard HRV features that are comparable to previous reports.
Acknowledgements
A.S. is supported by the German Research Foundation (SCHU 3432/2−1; www.dfg.de) and the Interdisciplinary Center for Clinical Research Jena (AMSP17; www.uniklinikum-jena.de/izkf). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
A.S. performed data analysis and wrote the manuscript. F.L. collected and prepared the data and critically revised the manuscript. K.R. prepared and quality-controlled the data and critically revised the manuscript. Y.G. performed data analysis and critically revised the manuscript. K.B. conceived the study, prepared and critically revised the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Code availability
For the technical validation, we used code that is publicly available without restrictions (see methods section). In detail following functions/scripts were used: jqrs.m - QRS detector based on the Pan-Tompkins method (www.physionet.org/content/pcst) ada_f.m – adaptive filtering of heart beat time series (https://tocsy.pik-potsdam.de/ada.php).
Competing interests
All contributors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thayer, J. F., Yamamoto, S. S. & Brosschot, J. F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol.141, 122–131 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Brotman, D. J. et al. Heart rate variability predicts ESRD and CKD-related hospitalization. J. Am. Soc. Nephrol.21, 1560–1570 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dekker, J. M. et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC study. Circulation102, 1239–1244 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Fyfe-Johnson, A. L. et al. Heart Rate Variability and Incident Stroke: The Atherosclerosis Risk in Communities Study. Stroke47, 1452–1458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu, K. Y., Elliott, T., Knowles, M. & Howard, R. Heart rate variability in relation to cognition and behavior in neurodegenerative diseases: A systematic review and meta-analysis. Ageing Res. Rev.73, 101539 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reule, S. & Drawz, P. E. Heart rate and blood pressure: Any possible implications for management of hypertension. Curr. Hypertens. Rep.14, 478–484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaich, C. L. et al. Association of heart rate variability with cognitive performance: the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 9, (2020). [DOI] [PMC free article] [PubMed]
- 8.Sandercock, G. R. H., Bromley, P. D. & Brodie, D. A. The reliability of short-term measurements of heart rate variability. Int. J. Cardiol.103, 238–247 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Malik, M., Bigger, J., Camm, A. & Kleiger, R. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J.17, 354–381 (1996). [PubMed] [Google Scholar]
- 10.Baumert, M. et al. Multiscale entropy and detrended fluctuation analysis of QT interval and heart rate variability during normal pregnancy. Comput. Biol. Med.42, 347–52 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Miyabara, R. et al. Quantifying Effects of Pharmacological Blockers of Cardiac Autonomous Control Using Variability Parameters. Front. Physiol.8, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss, A., Schulz, S., Schroeder, R., Baumert, M. & Caminal, P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. A. Math. Phys. Eng. Sci.367, 277–296 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Laborde, S. et al. Effects of voluntary slow breathing on heart rate and heart rate variability: A systematic review and a meta-analysis. Neurosci. Biobehav. Rev.138, 104711 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Grossman, P. Respiratory and cardiac rhythms as windows to central and autonomic biobehavioral regulation: selection of window frames, keeping the panes clean and viewing the neural topography. Biol. Psychol.34, 131–61 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Eckberg, D. L. The human respiratory gate. J. Physiol.548, 339–352 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dick, T. E. et al. Cardiorespiratory Coupling: Common Rhythms in Cardiac, Sympathetic, and Respiratory Activities. in The Central Nervous System Control of Respiration (eds. Holstege, G., Beers, C. M. & Subramanian, H. H.) 209, 191–205 (Elsevier, 2014). [DOI] [PMC free article] [PubMed]
- 17.Grossman, P., van Beek, J. & Wientjes, C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology27, 702–714 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Grossman, P. & Taylor, E. W. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol.74, 263–285 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Garcia, A. J., Koschnitzky, J. E., Dashevskiy, T. & Ramirez, J. M. Cardiorespiratory coupling in health and disease. Auton. Neurosci. Basic Clin.175, 26–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz, S. et al. Cardiovascular and cardiorespiratory coupling analyses: a review. Philos. Trans. A. Math. Phys. Eng. Sci.371, 20120191 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Morales, J. et al. Model-Based Evaluation of Methods for Respiratory Sinus Arrhythmia Estimation. IEEE Trans. Biomed. Eng.68, 1882–1893 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Widjaja, D. et al. Cardiorespiratory information dynamics during mental arithmetic and sustained attention. PLoS One10, 1–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumann, A. & Bär, K.-J. Autonomic aging – A dataset to quantify changes of cardiovascular autonomic function during healthy aging. Sci. Data9, 1–5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vest, A. N. et al. An Open Source Benchmarked Toolbox for Cardiovascular Waveform and Interval Analysis. Physiol. Meas.39, 105004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberger, A. et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circ. [Online]101, e215–e220 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Schumann, A. et al. One-week test-retest stability of heart rate variability. figshare10.6084/m9.figshare.26359453.v1 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Schumann, A. et al. One-week test-retest stability of heart rate variability. figshare10.6084/m9.figshare.26359453.v1 (2024).
Data Availability Statement
For the technical validation, we used code that is publicly available without restrictions (see methods section). In detail following functions/scripts were used: jqrs.m - QRS detector based on the Pan-Tompkins method (www.physionet.org/content/pcst) ada_f.m – adaptive filtering of heart beat time series (https://tocsy.pik-potsdam.de/ada.php).