Abstract
In this study, we identified cancer-associated fibroblast (CAF) molecular subtypes and developed a CAF-based prognostic model for breast cancer (BRCA). The heterogeneity of cancer-associated fibroblasts (CAFs) and their significant involvement in the advancement of BRCA were discovered employing single-cell RNA sequencing. Notably, we discovered that the RUNX1/SDC1 axis enhances BRCA cell invasion and metastasis. RUNX1 transcriptionally upregulates SDC1, which facilitates extracellular matrix remodeling and promotes tumor cell migration. This finding highlights the vital contribution of CAFs to the tumor microenvironment and provides new potential targets for therapeutic intervention. The predictive model showcased remarkable precision in anticipating patient outcomes and could guide personalized treatment strategies.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10565-024-09950-w.
Keywords: Cancer-associated fibroblasts, Breast cancer, Molecular subtypes, Predictive model, RUNX1/SDC1 axis, Targeted therapy
Introduction
Breast cancer (BRCA) stands out as the predominant cancer affecting women on a worldwide scale, displaying the highest incidence and mortality rates among gynecologic tumors (Goh et al. 2021). The emergence of BRCA heavily depends on the cell–cell interactions in the microenvironment, especially cancer-associated fibroblasts (CAF) (Sun et al. 2022). Cancer progression is significantly affected by the involvement of CAF as a major component of the tumor microenvironment (TME) (Barthel et al. 2022). Previous studies have indicated that the involvement of CAFs in tumor progression is evident in their secretion of a range of growth factors, chemokines, and matrix proteins, impacting processes such as proliferation, invasion, and metastasis (Shen et al. 2023). However, the specific mechanisms by which CAF function in BRCA and their impact on the prognosis of BRCA remain unclear. Therefore, in-depth research on the role of CAF in BRCA is of paramount importance for improving treatment strategies.
By independently analyzing each cell, single-cell technology is significantly superior to traditional methods in revealing heterogeneity within cell populations, facilitating the identification of unknown cell types (Suo et al. 2022). This technology allows for the precise differentiation of disease subtypes, optimizing treatment plans, supporting personalized medicine, and aiding in constructing cell lineages and developmental trajectories to deepen our understanding of cellular differentiation processes (Guo et al. 2023). Single-cell analysis also enhances the identification of biomarkers and therapeutic targets, providing a novel approach to studying the dynamic processes of cell responses to drugs (Van de Sande et al. 2023). These advantages make single-cell technology a formidable instrument for unraveling intricate biological processes (BPs) and disease mechanisms, thus opening new avenues for disease treatment and prevention. Previous studies have shown that CAF are not a homogeneous group of cells but comprise various subtypes with distinct differences in function, phenotype, and molecular characteristics (Bienkowska et al. 2021). However, the roles of these CAF subtypes in BRCA and whether there are commonalities among these subtypes remain unresolved questions (Chen et al. 2021). Through single-cell genomics technology, we may unravel the answers to these questions.
In tumor research, establishing prognostic models is valuable for assessing patient survival status and guiding treatment strategies (Tudor et al. 2020). Risk scoring based on cellular or genetic characteristics has shown promising applications in various tumors (Malecek and Mehta-Shah 2021; Calabrese 2021). Nevertheless, there are currently no reports on the role of CAF in the prognosis of BRCA or the establishment of a prognostic model based on CAF in BRCA (Piersma et al. 2020). Hence, constructing a new prognostic model for BRCA based on CAF might improve current treatment strategies.
Furthermore, CAF promotes the progression of tumors through direct or indirect interactions with cancer cells (Bhattacharjee et al. 2021). However, the molecular mechanisms underlying these interactions remain incompletely understood. Essential functions in tumor progression have been associated with certain genes within the CAF gene expression profile (Lin et al. 2023). SDC1, a glycoprotein, has been demonstrated to function in various cancers (Reszegi et al. 2022). Yet, the specific mechanisms of SDC1 in CAF and its influence on the invasion and metastasis of BRCA remain unclear. The substantial link between abnormal RUNX1 levels and tumor invasiveness, along with an adverse prognosis, has been established across diverse cancer types. It has been demonstrated through studies that heightened expression of RUNX1 in BRCA corresponds significantly with lymph node metastasis, heightened tumor proliferation, and adverse predictive indicators (Tuo et al. 2022). Moreover, RUNX1 is pivotal in overseeing multiple cancer-related signaling pathways, including Wnt/β-catenin and TGF-β/Smad, which are important for promoting tumor cell proliferation, migration, and invasion (Chen et al. 2024). Although there is currently no direct research reporting whether RUNX1 influences CAF function in BRCA by regulating SDC1 expression, given RUNX1's significant role in various tumor-related signaling pathways, we hypothesize that it may transcriptionally regulate SDC1 expression, thereby indirectly affecting CAF function and the invasive behavior of BRCA. This study aims to explore this hypothesis in depth to reveal whether RUNX1 is involved in the regulation of SDC1, thereby facilitating the function of CAF in propelling tumor advancement within the TME.
The aim of this research is to explore the participation of CAF in BRCA through single-cell genomics technology, establish a new CAF-related BRCA predictive model, and explore how CAF influences the invasion and metastasis of BRCA through the RUNX1/SDC1 axis. We hope this research will provide new theoretical and experimental foundations and positively impact the improvement of BRCA treatment strategies.
Materials and methods
Procurement of transcriptome sequencing data
The Cancer Genome Atlas (TCGA) project provided the RNA sequencing data and clinicopathological data concerning BRCA (TCGA-BRCA). Enclosed in the datasets were 113 samples from normal tissues adjacent to the tumors, as well as 1109 tumor samples. Details concerning 1095 subjects was obtainable, comprising futime, fustat, individual's age, sex, cancer grade, stage, T stage, M stage, and N stage. Additionally, the BRCA transcriptome sequencing dataset GSE39004 was retrieved from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/), which included 61 primary BRCA tissue samples. The GSE39004 dataset was integrated with the TCGA-THCA dataset, and the "combat" algorithm was employed to remove batch effects (Fig. S1) (Wang et al. 2022a, b).
Single-cell sequencing data download and preprocessing
The single-cell sequencing dataset GSE180286 specific to BRCA was sourced from the GEO database. Five primary BRCA tumors (GSM5457199, GSM5457202, GSM5457205, GSM5457208, GSM5457211) were selected for analysis, and processing of the data was achieved with the R package "Seurat". Criteria for quality assurance involved ensuring nFeature_RNA was limited between 200 and 5000, in addition to maintaining the mtRNA percentage below 20%, and a compilation of the top 2000 genes displaying significant variability was established through variance analysis (Meng et al. 2021).
Gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) analysis
Analysis of GO and KEGG pathway functions in the CAF cell lineage was accomplished through the utilization of the cluster Profiler R package (version 3.14.3), focusing on enriching biological processes (BPs), molecular functions (MFs), cellular components (CCs), and identified marker gene-enriched pathways. A significance threshold of less than 0.05 was deemed as statistically important (Yu et al. 2012).
Genetic mutation analysis
Information regarding genetic mutations, encompassing somatic changes and alterations in copy numbers (CNV) within the BRCA gene, was acquired from the GDC TCGA-BRCA database. The examination and visualization of somatic mutations in TC were executed by employing the maftools R package (version 2.6.05). Moreover, human chromosomal CNV profiles of CRGs were graphically represented through the RCircos R package (1.2.2 version). It is recommended to break down long sentences into shorter ones to enhance readability (Li et al. 2022).
Formation of protein–protein interaction (PPI) networks
The establishment of the PPI network employed the resources from the STRING database to investigate the potential interactions among the proteins encoded by the 30 identified CAF feature genes. Setting the interaction threshold at 0.4 corresponded to a medium level of confidence, and both known and predicted protein–protein associations were included. To visualize and analyze the PPI network, we employed Cytoscape software, which allowed for an in-depth exploration of the co-expression and interaction relationships between the proteins involved in extracellular matrix organization and tumor invasion pathways.
Correlation analysis
The application of the ggcorrplot software (v0.1.3) aided in the computation and depiction of the potential correlation between characteristic genes of CAF and gene expression present in BRCA for improvement (Li et al. 2022).
Consensual clustering and genetic clustering
Patients were assigned to specific molecular subtypes by utilizing the consensus clustering approach after the selection of characteristic genes related to CAF. The software "ConsensusClusterPlus" was applied for identifying the stability and quantity of clusters, with 1000 repetitions conducted for stability assurance. A catalogue of DEGs from consensus clustering was disclosed by applying the limma R software package and conditions of |log2(fold change)|> 1 and false discovery rate (FDR) < 0.05. Subsequently, patients were classified into diverse gene subgroups (Class A, Class B, and Class C) through unsupervised clustering, driven by the expression patterns of prognostic DEGs, to enable subsequent analyses. For a more thorough investigation into the clinical implications of consensus clustering and gene clustering techniques, the correlation between molecular subgroups, clinicopathological features, and prognostic outcomes was examined (Wilkerson and Hayes 2010). Clinical features included age, gender, TNM staging, and grading. Additionally, execution of the survival analysis using the Kaplan–Meier (KM) method was accomplished utilizing the survival toolkit available in the R software environment.
Gene set variation analysis (GSVA)
GSVA is a popular approach for estimating modifications in pathway and BP activity in a dataset of gene expression samples. This method employs the "GSVA" R package to interpret BP differences between the two CAF scoring subtypes. The gene set denoted as "c2.cp.kegg.v7.4.symbols.gmt" was sourced from the MSigDB database for the purpose of GSVA scrutiny (Hänzelmann et al. 2013).
Relationship between molecular subtypes and TME
Validation of TME attributes within diverse molecular categories involved the utilization of marker gene sets (C2.CP.KEGG (186 gene sets) and C5.GO. gene Ontology (10561 gene sets)) extracted from the MSigDB repository for GSVA analysis. An observed statistical significance corresponded to adjusted p-values under 0.05. The application of the deconvolution algorithm (CIBERSORT) aided in the computation of immune cell infiltration amounts (TICs) within individual BRCA specimens, utilizing the gene expression attribute matrix from the CIBERSORT platform. Comparing the gene expression level matrices of TCGA-THCA and GSE27155 datasets with the feature matrix of TICs led to the formulation of a TICs proportion matrix in BRCA tissues. CIBERSORT p-values < 0.05 were deemed appropriate for additional investigation. Additionally, the methodology used for determining the TICs in individual BRCA specimens involved the application of the single-sample gene set enrichment analysis (ssGSEA) algorithm (Wang et al. 2022a, b).
Construction of CAF risk scoring model
A univariate COX regression analysis was executed on the DEGs from the molecular subtypes of CAFs to pinpoint genes related to the survival of individuals diagnosed with BRCA. Subsequently, using consensus clustering analysis based on the expression of prognostic DEGs, individuals were categorized into different CAF gene subtypes, including the A subtype, the B subtype, and the C subtype. Next, employing the "caret" package in R, we arbitrarily divided every BRCA case from TCGA-BRCA and GSE39004 databases into training set (n = 539) and test set (n = 538) at a 1:1 ratio. The CAF Risk scoring system was developed in the training dataset by applying LASSO COX regression analysis through the "glmnet" package in R to reduce overfitting risks. The trajectory of every separate factor was examined and cross-validated for model establishment. A prognostic CAF Risk scoring system was formulated by applying Multivariate Cox analysis to pinpoint potential risk genes in the training set. Below is the formula for assessing CAF risk score: CRGs risk score = ∑ (Exp_i * coeff_i), where Exp_i represents the expression of key CAF-related risk genes, and coeff_i represents the risk coefficient. Examination of correlations was employed to assess the interrelation between risk scoring and diverse molecular or gene subtypes. Categorized by the median risk score, the group of 1077 BRCA patients was split into a high-risk group (n = 522) and a low-risk group (n = 555). Subsequently, utilization of KM survival analysis aimed to evaluate the variance in survival rates among the high-risk and low-risk cohorts. Finally, survival analysis and the creation of ROC curves were facilitated by classifying the test set and combined set into high-risk and low-risk divisions (Wang et al. 2022a, b).
Correlation of CAF risk assessment model with TME
In R language, boxplots were employed for evaluating the CAF expression in both high-risk and low-risk score categories, determining the TICs abundance within the TME of each C specimen through CIBERSORT, followed by correlation analysis to explore the link between TICs and prognostic risk genes (Sha et al. 2022).
Construction of column line plots and validation
Utilizing survival analysis methods in R packages, the independence of the CAF model from clinical features was evaluated through univariate and multivariate Cox regression analyses. Subsequently, relying on the multivariate Cox regression coefficients related to CAF attributes and clinical factors within the TCGA training group, we constructed column line plots and computed the concordance index (C-index) to verify the forecast capability of these graphs. For the validation process, calibration curves were generated by bootstrapping with 1000 iterations to evaluate the concordance between anticipated 1-year, 3-year, and 5-year overall survival (OS) probabilities and the actual observed values (Zheng et al. 2021).
Clinical sample collection
This study collected tissue samples from 55 BRCA patients, including tumor tissue and normal breast tissue located a minimum of 5 cm from the tumor. The clinical attributes of 55 individuals with BRCA are outlined in Table S1. All study participants agreed to the publication of the research results. Patients who had received cancer-related treatments such as radiation therapy, chemotherapy, and hormone therapy were excluded from the study. Patients with major chronic diseases, recent major surgeries or medical interventions in pregnancy or lactation, as well as those with immune disorders, were also excluded. These criteria ensured data consistency and accuracy of the research results. This study was granted permission by the the Clinical Ethics Committee of The First Hospital of China Medical University. Official authorization for animal experimentation protocols was granted by the Animal Ethics Committee of The First Hospital of China Medical University (No. CMUXN2022131) (Primac et al. 2019).
Isolation of NF (normal fibroblasts) and CAF
In simple terms, finely mincing and enzymatically digesting both tumor and non-tumor samples by employing type I collagenase (17100017, Gibco, USA) is essential prior to their cultivation in DMEM medium (11965092, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, 10100, GIBCO, USA). The ideal method for cell growth involves maintaining them in a humid atmosphere at 37°C, with 5% CO2 supplementation until NF/CAF attaches to the culture dish, and primary NF/CAF should be used before passage 6. Subsequently, immunofluorescence should be used to observe the expression of fibronectin as a fibroblast marker and cytokeratin as an epithelial cell marker to identify the isolated primary NF/CAF (Wen et al. 2019).
Cell culture
Wuhan Pronas Life Technologies Co., Ltd. (CL-0150A, China) supplied the human BRCA cell line MDA-MB-231. Cell cultivation took place in the RPMI 1640 medium (11875119, GIBCO, USA) encompassing 10% FBS (10100, GIBCO, USA) and 1% penicillin–streptomycin (10378016, GIBCO, USA). Incubation of cells occurred in a humidified environment with 5% CO2 at a temperature of 37 degrees Celsius. When undertaking lentiviral cell transfection, a 6-well plate was populated with 5 × 105 CAF cells. At the point of cell density hitting 70–90%, the cells were exposed to a medium comprising an optimal dosage of lentivirus (MOI = 10, working strength around 5 × 106 TU/mL) and 5 μg/mL polybrene (sourced from Merck, TR-1003, USA). Post 4 h of transfection, an identical medium amount was included to lessen the polybrene concentration, followed by a switch to fresh medium after 24 h. Fluorescent luciferase was used to assess gene transfection and cells with stable transfection were selected employing 60 μg/mL puromycin (Sangon Biotech, A100339, Shanghai, China) after 48 h. Sangon Biotech (Shanghai, China) offered the lentivirus packaging assistance. The following sequences are utilized in lentivirus-mediated gene silencing: sh-NC: 5'-CCTAAGGTTAAGTCGCCCTCG-3'; sh-SDC1: 5'-CCGACTGCTTTGGACCTAAAT-3'; sh-RUNX1: 5'-CCTACGATCAGTCCTACCAAT-3' (Sohn et al. 2018).
Dual-luciferase assay
The JASPAR database (https://jaspar.elixir.no/) was utilized to predict the presence of binding sites for RUNX1 and SDC1 at their respective promoters. The effect of RUNX1 on the transcriptional activity of the SDC1 promoter was investigated by co-transfecting CAF cells with Lipofectamine 2000 transfection reagent (Catalog number: 11668019, ThermoFisher, USA) and introducing oe-NC, oe-RUNX1, sh-NC, sh-RUNX1 plasmids, along with dual-luciferase reporter gene vectors containing the SDC1 promoter sequence (AATTGTTGTAA) and their corresponding mutant binding sites (TTAACAACATT). Renilla luciferase functioned as an internal control. Subsequent to transfection for 48 h, cellular collection and lysis were performed, and the assessment of luciferase activity was carried out using the Dual-Luciferase Reporter Gene Analysis System (Promega, Madison, WI, USA). Analyzing the ratio of firefly luciferase luminescence units (RLU) to Renilla luciferase luminescence units (RLU) allowed for the assessment of the activation level of the specific reporter gene (Taniue et al. 2016).
ChIP assay
The enrichment status of RUNX1 within the SDC1 gene promoter region was examined using a ChIP kit (Catalog number: KT101-02, Saicheng Biotechnology Co., Ltd., Guangzhou, China). The steps involved the fixation of cells at 70–80% confluence with 1% formaldehyde at room temperature (RT) for 10 min to create cross-links between DNA and proteins, followed by sonication to shear the cross-linked DNA–protein complexes into suitable-sized fragments. Centrifugation at 13000 rpm at 4 °C was performed to collect the supernatant, which was then divided into two tubes. In one test tube, overnight incubation occurred at 4 °C with Rabbit IgG (ab172730, 1:100 dilution, Abcam, UK) as the negative control antibody, while the other tube with a specific antibody against the target protein, Rabbit anti-RUNX1(1:100, ab272456, Abcam, UK). The separation of endogenous DNA–protein complexes was accomplished using the Protein Agarose/Sepharose precipitation method, followed by reversing the cross-links overnight at 65°C. Subsequently, DNA fragments were purified through phenol/chloroform extraction for the qPCR analysis of SDC1 gene promoter segments with the primers: Forward 5'-CCACAGAAAAACGCTGCGAA-3'; Reverse: 5'-CCAGATTCTCCCGTACGCTC-3' (Nelson et al. 2006).
Cell immunofluorescence staining
After counting the NF/CAF cells, they were dispersed and cultured in immunofluorescence chambers. Each well contained 2 × 105 cells. Upon reaching approximately 90% cell confluence, cells were rinsed three times with ice-cold PBS. 4% paraformaldehyde was used for cell fixation, adding 1 mL to individual wells, and then incubating at RT for 15 min. Upon three rounds of PBS rinses, cells were subjected to blocking utilizing 5% BSA, followed by a 30-min incubation period. Rabbit anti-α-SMA (#19245S, 1:200, Cell Signaling Technology, USA), Mouse anti-SDC1 (ab181789, 1:500, Abcam, UK), fibronectin (ab2413, 1:250, Abcam, UK), Cytokeratin (ab53280, 1:250, Abcam, UK) primary antibodies underwent an overnight incubation at 4°C, succeeded by three rounds of PBS rinsing. Subsequently, secondary antibodies, Goat anti-rabbit IgG H&L (Alexa Fluor 488) (1:200, ab150077, Abcam, UK) and Goat anti-mouse IgG H&L (Alexa Fluor 647) (1:200, ab150115, Abcam, UK) were applied and maintained for an hour at RT, then subjected to three PBS rinses. Cellular nuclei were labeled using PI (P1304MP, Invitrogen, USA) or DAPI (D9542, Sigma) dyes in low light conditions for a duration of 15 min. Ultimately, the slides were arranged with a fluorescence quencher and visually examined and imaged with a fluorescence microscope by Olympus, a Japanese manufacturer. The quantitative analysis was executed with Image-Pro Plus 6.0 software after three washes with PBS under dark conditions (Beneit et al. 2016).
Western blot
Utilization of RIPA lysis buffer (P0013B, Beyotime, Shanghai) encompassing 1% PMSF enabled the lysis of cells to obtain total proteins. Cell membrane proteins were extracted using the ProteoPrep® Membrane Extraction Kit (PROTMEM-1KT, MERCK). Each sample's protein concentration was gauged through the utilization of the BCA assay kit (P0011, Beyotime, Shanghai). The SDS-PAGE gels were developed within the 8% to 12% concentration range, adjusting the molecular weight of the identified protein bands. Matching quantities of protein samples were evenly inserted into all lanes using a micropipette to perform the process of electrophoresis separation. Transferring of proteins from the gel was done onto a PVDF membrane (1620177, BIO-RAD, USA), followed by blocking the membrane with 5% skim milk at RT for 1 h. Primary antibodies against α-SMA (#19245S, Cell Signaling Technology, USA), FAP-α (ab207178, Abcam), SNAI1 (PA5-23482, Invitrogen), MMP2 (ab86607, Abcam), SDC1 (ab128936, Abcam), RUNX1 (ab240639, Abcam), MMP9 (ab76003, Abcam), and GAPDH (ab8245, Abcam) were incorporated, followed by an overnight incubation of the membrane at a temperature of 4°C. Three 5-min washes were carried out on the membrane with 1 × TBST at RT. HRP-conjugated secondary antibodies, goat anti-rabbit IgG (ab6721, 1:2000) or goat anti-mouse IgG (ab6728, 1:2000), were included and kept at RT for 1 h. These antibodies were procured from Abcam in the UK, Cell Signaling Technology in the USA, and Invitrogen in the USA. Three wash cycles were performed on the membrane using 1 × TBST buffer at RT, lasting 5 min per cycle. Deployment of the ECL substrate (1705062, Bio-Rad, USA) resulted in the detection of protein bands, which were then imaged using the Image Quant LAS 4000C Gel Imaging System (GE, USA). The experiment incorporated GAPDH as an internal reference, and the relative expression quantity of the protein was determined by comparing the intensity of the target band with that of the reference band, enabling the evaluation of different protein expression levels (Wu and Yi 2018). The trial was reiterated three times for accuracy assessment.
ELISA
Supernatants from NF/CAF groups were collected and subjected to a human SDC1 ELISA assay kit (ab46506) for detection. This experiment aimed to detect the expression of secreted protein SDC1 following strict operational procedures (Fang et al. 2021).
Collection of NF/CAF conditioned medium
Seeded in 100 mm culture dishes, CAF and NF cells were cultured for 24 h at a cell density of 1 × 106 cells/mL, and the medium for culture was retrieved post a PBS rinse of the cells. Every well was supplied with 8 mL of serum-free medium, and post a 2-day incubation, the conditioned medium was amassed and strained using a 0.2 μm syringe filter to eradicate any remaining cells and detritus. To neutralize SDC1 in the conditioned medium of CAF, 25 μg/mL of human SDC1 antibody (ab128936) or its immunoglobulin G (IgG, ab109489) were introduced into the conditioned medium. Pursuing a 1-h incubation at RT, the medium was applied to the BRCA cells (Sun et al. 2021).
Cell scratch assay
The addition of 5 × 105 cells per well was followed by a 24-h culture period. In the next step, the medium for cultural growth was disposed of, and with the aid of a sterile pipette tip, a level scratch was meticulously crafted at the rear of the well, subsequently swapped with serum-exempt culture medium. Distances of the wounds were inspected utilizing an optical microscope (Leica, DM500) after 0 and 48 h of culture. Images were captured under an inverted microscope. The analysis of the scratch width in each well was performed using Image J software, and the cell migration capability was assessed through the comparison of scratch widths across the groups. The actual cell migration distance was determined by assessing the relative distance of cell migration to the scratch area measured from the original cell scratch area. Three rounds of experimentation were executed (Wei et al. 2020).
Transwell assay
Evaluation of cell invasion potential was conducted via the Transwell examination. First, ECM gel (EHS matrix E1270-1ML, Sigma) was refrigerated at 4°C overnight and subsequently thinned in medium devoid of serum at a ratio of 1:9 to achieve a concentration of 1mg/ml. Subsequently, each 24-well Transwell chamber (354480, Shanghai Yuhui Biotechnology Co., Ltd., China) was treated with 40 μl of ECM gel on the polycarbonate membrane and incubated at 37°C with 5% CO2 for 5 h to promote gel polymerization. After the gel had polymerized and formed, excess liquid was removed, and 70 μl of pure DMEM medium was introduced into individual chamber. The matrix gel was rehydrated by undergoing a 0.5-h incubation at 37°C in a humid incubation chamber as the subsequent step. The surplus culture medium was removed, and a 24-h serum-starvation period was applied to the cells. Upon completion of centrifugation, the cells were retrieved and reconstituted in DMEM medium minus FBS to achieve a concentration of 2.5 × 105/ml. Subsequently, the hydrated basement membrane of the upper chamber welcomed 0.2ml of cell suspension and pre-cooled DMEM medium with a 10% FBS concentration was introduced into the lower chamber, totaling 700 µl. Upon completion of a 24-h incubation interval under 37°C with 5% CO2 saturation, the compartment was eliminated, and cells present on the upper membrane and basal membrane were wiped away using a wet cotton swab. Subsequent to the fixation process utilizing methanol lasting 30 min, the cells underwent staining with 0.1% crystal violet dye for a period of 20 min. Following air-drying, observation using an inverted microscope and capturing of images occurred. The experiment was performed three times, and proportions of migrating cells through the membrane were calculated by selecting five arbitrary fields randomly (Li et al. 2020).
Nude mouse tumor model
Chosen were 42 female BALB/c nude rodents, 6 weeks of age (401, Beijing Vital River Laboratory Animal Technology Co., Ltd.), and housed under controlled conditions in an SPF-grade animal facility, maintained at 60–65% humidity and temperatures ranging between 22–25°C, following a 12-h cycle of light and darkness, with ad libitum access to sustenance and water. Post a week of acclimatization diet, the animal ethics commission sanctioned the experimental procedures and guidelines for animal utilization.
The animal models were separated into the following classifications: BC + NF group (injection of MDA-MB-231 and NF mixed solution); BC + CAF group (injection of MDA-MB-231 and CAF mixed solution); BC + CAF-sh-NC group (injection of MDA-MB-231 and CAF mixed solution infected with sh-NC lentivirus); BC + CAF-sh-SDC1 group (injection of MDA-MB-231 and CAF mixed solution infected with sh-SDC1 lentivirus); BC + CAF-oe-NC group (injection of MDA-MB-231 and CAF mixed solution infected with oe-NC lentivirus); BC + CAF-oe-SDC1 group (injection of MDA-MB-231 and CAF mixed solution infected with oe-SDC1 lentivirus). Each group consisted of 6 nude mice. On the 45th day after xenograft transplantation, the experimental animals were euthanized, and tumors and mouse lungs were collected for further study.
Subcutaneous Xenograft Tumor Model: MDA-MB-231 cells (1 × 106) were combined with an equivalent quantity of NF or CAF in 200μl PBS: Female nude mice, 4 weeks old, received subcutaneous injections of Matrigel prepared at a ratio of 1:1. Tumor volume (V = length × width2 × 0.5) was monitored weekly.
Lung Metastasis Model: MDA-MB-231-luciferase (1 × 106 cells) were blended with NF or CAF cells in 200μl of PBS at an equal ratio: Administered via the tail vein of nude mice, the 1:1 Matrigel mixture was coupled with a 0.2 mL cell suspension. After 4 weeks, the nude mice were placed in an IVIS Lumina XR imaging chamber (PerkinElmer, Waltham, MA, USA) for white light and bioluminescence imaging to observe lung metastasis. Subsequently, cervical dislocation was utilized for the euthanization of the mice, leading to the retrieval of their lungs. The calculation of the mean liver metastases count was executed by conducting H&E staining (Zhang et al. 2014; Vanden Borre et al. 2014).
H&E staining
Lung samples were obtained from nude mice carrying tumors and then preserved using 10% neutral formalin. Subsequently, the fixed lung tissues underwent embedding in paraffin prior to sectioning. These sections were deparaffinized with xylene and stained with hematoxylin, followed by eosin staining. After being rinsed with deionized water, a graded series of ethanol was used as a dehydrating agent and cleared with xylene. Neutral resin fixation was applied to the air-dried sections for subsequent examination under an optical microscope (Wei et al. 2019).
Immunohistochemical staining
Tumor tissues from nude mice, BRCA tissues, and corresponding normal adjacent tissues were procured. Formalin fixation was employed on the specimens, leading to the creation of paraffin sections with a thickness of 4 μm. Afterward, the samples were deparaffinized to water and subjected to standard immunohistochemical staining procedures. The antibodies used included α-SMA (#19245S, 1:250, Cell Signaling Technology, USA), SDC1 (Abcam, ab128936, 1:500), SNAI1 (PA5-23482, 1:200, Invitrogen), MMP2 (Abcam, ab86607, 1:200), and MMP9 (Abcam, ab76003, 1:500), all purchased from Abcam and Invitrogen. The assessment of staining outcomes involved the random selection of 5 regions through the microscope, with the quantification of cells displaying positive staining. Every assay was executed threefold (Wen et al. 2019).
Statistical applications and methodologies for data analysis
The analysis and manipulation of data were executed utilizing R 4.2.1, in conjunction with RStudio version 4.2.1 as the integrated development environment. File processing was executed employing Perl 5.30.0. The analysis of networks was executed with the assistance of Cytoscape version 3.7.2. Descriptive statistics were displayed as Mean ± Standard Deviation, with unpaired Student's t-test or Wilcoxon test utilized to compare normally distributed data across two groups, whereas one-way ANOVA followed by Tukey's post-hoc analysis was applied for comparisons among several groups. A P-value < 0.05 indicated statistical significance.
A logarithmic transformation was applied to meet the normality assumption for non-normally distributed data. Multiple imputation methods were primarily used to handle missing data to maintain data integrity and minimize bias. Additionally, to manage the surge of type I errors in multiple contrasts, the FDR control method was utilized to reduce the likelihood of chance findings, ensuring the rigor of statistical analysis and the reliability of results (Morris et al. 2017).
Results
Single-cell RNA sequencing identification of CAF in BRCA tissue
The research activity on BRCA, being a frequently encountered malignancy on a worldwide scale, is on the rise. Studies have found that the TME, especially CAF, significantly affects the prognosis of BRCA patients (Hu et al. 2022). CAF, as a major component of the TME, not only promotes tumor initiation and enhances cancer cell invasiveness but also triggers inflammation by generating pro-inflammatory cytokines that are accountable for immune tolerance and the metastasis of tumors (Gaggioli et al. 2007).
To further explore the role of CAF in BRCA and its impact on immunotherapy for BRCA patients, we analyzed the BRCA-related scRNA-seq dataset GSE180286 obtained from GEO. We selected 5 primary BRCA tumors (GSM5457199, GSM5457202, GSM5457205, GSM5457208, GSM5457211) for analysis. Data integration was performed employing the Seurat package. Our initial analysis focused on the gene counts (nFeature_RNA), mRNA molecule numbers (nCount_RNA), and percentage of mitochondrial genes (percent. mt) across all cells in the scRNA-seq dataset. The analysis revealed that most cells had nFeature_RNA < 5000, nCount_RNA < 20000, and percent.mt < 20% (Fig. S2A). Subsequently, by adhering to the thresholds of 200 < nFeature_RNA < 5000 and percent.mt < 10%, we sieved out inferior cells, culminating in an expression matrix comprising 21675 genes and 19565 cells. After filtering, the correlation analysis indicated good data quality, with a correlation coefficient of 0.12 between nCount_RNA and percent. mt and 0.92 between nCount_RNA and nFeature_RNA (Fig. S2B).
Additional scrutiny of the screened cell specimens entailed identifying genes with marked variability in gene expression levels and selecting the top 2000 variable genes for subsequent analysis (Fig. 1A). Cell cycle scoring was performed (Fig. S2C), and data underwent normalization. Then, the linear dimensionality reduction was conducted through the application of principal component analysis (PCA) on the chosen set of highly variant genes. The main gene expression heatmap for PC_1 – PC_6 was shown (Fig. S2D), and the arrangement of cells in PC_1 and PC_2 was showcased (Fig. S2E), showing evident discrepancies in batches between samples.
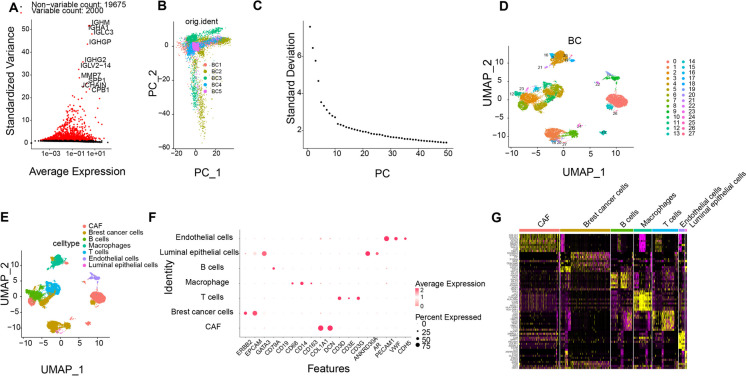
Fig. 1.
Cell Clustering and Annotation of scRNA-seq Data. Note: A Variance analysis for screening highly variable genes, where red represents the top 2000 highly variable genes, and black represents low variable genes, with the names of the top 10 highly variable genes labelled; B Distribution of cells in PC_1 and PC_2 after Harmony batch correction, with each point representing a cell; C Distribution of standard deviation (std) in PCs, with important PCs having larger standard deviation; D Visualization of UMAP clustering results illustrating the aggregation and distribution of cells in BRCA samples in two dimensions; E Visualization of UMAP clustering results showing the aggregation and distribution of cells from different sources, with each color representing a cluster; F Expression patterns of known cell lineage-specific marker genes in different clusters, where darker blue indicates higher average expression levels, and larger circles represent more cells expressing the gene; G Heatmap displaying the expression of the top 10 marker genes in 7 identified BRCA cell clusters (yellow denotes higher expression)
To mitigate batch discrepancies and enhance accurate cell clustering, the "harmony" R package was applied to batch correct the sample data to facilitate subsequent analysis (Fig. S2F), which successfully eliminated batch effects (Fig. 1B). Additionally, an ElbowPlot was produced to arrange PCs by the standard deviation (Fig. 1C), indicating that PC_1 to PC_20 efficiently incorporated the details from the chosen genes with substantial analytical importance.
Subsequently, UMAP visualization was utilized to categorize cells into 27 clusters based on the top 20 PCs (Fig. 1D). By pinpointing established marker genes specific to particular cell lineages in relevant literature and utilizing the resources of the CellMarker online database for cell categorization, a collective of 7 cell categories was successfully delineated. Clusters 1, 5, 8, 10, 14, 16, 18, and 23 were identified as BRCA cells marked by ERBB2, EPCAM, and GATA3 genes. Other cell types including B cells (clusters 2, 12, 15), Macrophages (clusters 3, 21, 25), T cells (clusters 4, 6, 11), Luminal epithelial cells (clusters 20, 27), and Endothelial cells (clusters 9, 19) were also annotated based on specific marker genes (Fig. 1E-F).
To validate the accuracy of annotation, the expression of 6 additional genes known as CAF markers (ACTA2, FAP, PDPN, PDGFRA, PDGFRB, and CAV1) was examined (Fig. S3A-B), confirming their expression in the annotated CAF cell clusters. Furthermore, differentially expressed marker genes were identified based on logFC > 1 threshold and adjPval < 0.05, showcasing the top 10 substantial distinctions within each cell grouping through a heatmap representation (Fig. 1G). Notably, upregulated genes in the CAF cell cluster were primarily known CAF markers, such as COL1A1, COL3A1, POSTN, and ACTA2.
In conclusion, we successfully analyzed the BRCA single-cell sequencing chip and accurately annotated the CAF cell cluster in BRCA.
Functional enrichment analysis of CAF marker genes in BRCA
In order to explore the functionalities of CAF within the context of BRCA, we pinpointed particular marker genes associated with cell clusters through the utilization of the "Find Markers" functionality offered in the "Seurat" toolkit. The CAF cell cluster's 577 DEGs underwent GO, KEGG, and GSEA enrichment analysis (Table S2). As shown in Fig. 2A-B, In the KEGG enrichment analysis, DEGs were primarily concentrated in pathways such as Focal adhesion, Regulation of actin cytoskeleton, Proteoglycans in cancer, Salmonella infection, and Pathways of neurodegeneration—multiple diseases. The circular plots displayed genes involved in Focal adhesion, Regulation of actin cytoskeleton, and Proteoglycans in cancer pathways, where darker colors beneath the genes indicated higher enrichment levels. Genes like COL1A1, COL1A2, LUM, TIMP3, DNC, FN1, COL6A3, COL6A1, and MYL9 were found to be enriched in the top three pathways.
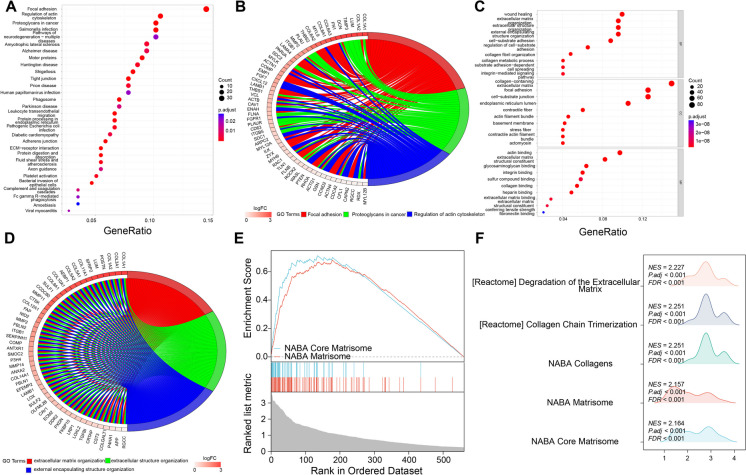
Fig. 2.
Functional Enrichment Analysis of Differential Genes in CAF Cell Populations. Note: A Bubble plot of KEGG enrichment analysis for differential genes in 577 CAF cell populations; B Circular plot showing the top three enriched pathways by KEGG; C Bubble plot of GO enrichment analysis for differential genes in 577 CAF cell populations; D Circular plot showing the top three enriched pathways by GO; E GSEA gene set enrichment analysis of 577 differential genes, illustrating enrichment scores and false discovery rates; F GSEA enrichment landscape plot reflecting the significance and direction of gene set enrichment in gene expression profile data
As depicted in Fig. 2C-D: In the GO enrichment analysis, we observed that the DEGs were primarily enriched in BPs such as wound healing (GO:0042060), extracellular matrix organization (GO:0030198), extracellular structure organization (GO:0030199), and external encapsulating structure organization (GO:0030200); CCs including collagen-containing extracellular matrix (GO:0062023), focal adhesion (GO:0005925), cell-substrate junction (GO:0030055), and endoplasmic reticulum lumen (GO:0005788); and MFs like actin binding (GO:0003779), extracellular matrix structural constituent (GO:0005201), glycosaminoglycan binding (GO:0005539), and integrin binding (GO:0005178). The circular plots illustrated the main genes enriched in the top three BPs.
Additionally, an analysis of GSEA enrichment was implemented on the 577 DEGs (Fig. 2E-F), unveiling substantial enrichment on NABA (Nidogen-1 and −2, Agrin and Basement membrane Associated proteins) Core Matrisome and NABA Matrisome. Through a mountain plot visualization, we displayed the top five gene sets enriched in the GSEA analysis. In summary, we presented the enriched signaling pathways, BPs, CCs, MFs, and gene set enrichments of DEGs in the CAF cell cluster.
Genetic and variant landscape of CAF feature genes in BRCA
Furthermore, we screened the 30 highest-ranking genes displaying the most notable variances in expression among the 577 DEGs in CAF, using a cutoff of Log2FC > 2, and named them CAF feature genes. Genetic mutations in these 30 genes within BRCA were investigated utilizing data sourced from the TCGA database. At the genetic level, we found that 26 genes had mutations in 148 out of 991 samples (14.93%). COL6A3, VCAN, FN1, and COL11A1 had high mutation frequencies, while CTGF, SFRP2, THY1, and CTHRC1 did not have any mutations in BRCA samples (Fig. 3A). Further analysis of the CNV of these 30 genes revealed significant deletions in POSTN, COL11A1, COL6A3, ACTA2, THY1, TAGLN, and VCAN; and significant amplifications in CTHRC1, COL1A1, COL3A1, COL5A2, ISLR, CCDC80, and AEBP1 (Fig. 3B). The distribution of CNV modifications related to these 30 genes on 23 chromosomes is illustrated in Fig. 3C. At the expression level, significant variation in the expression of these 30 genes was observed in TCGA-BRCA samples between normal and tumor tissues (Fig. 3D). At the protein level, we analyzed the interactions among the proteins encoded by these 30 genes through PPI analysis. As shown in Fig. 3E, there are strong interactions and co-expression relationships among these 30 genes.
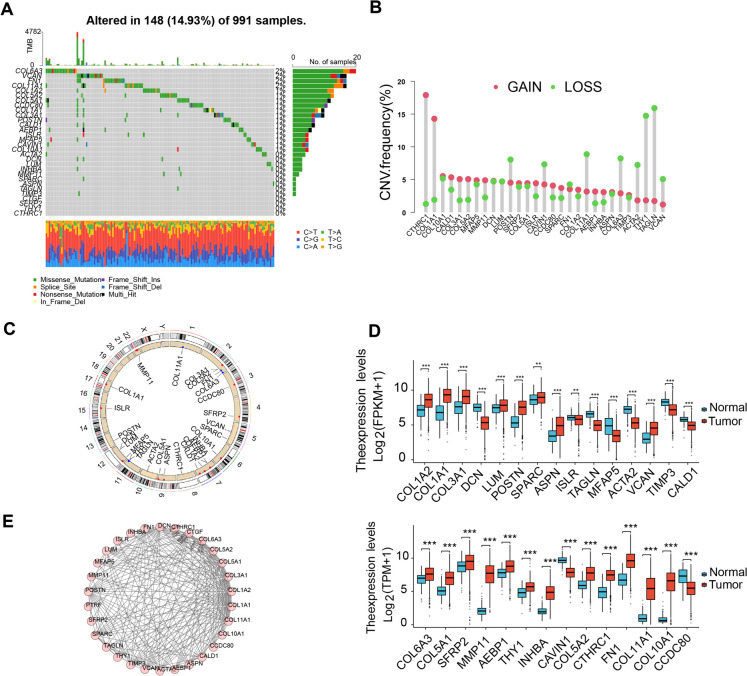
Fig. 3.
Genetic and Transcriptional Alterations of 30 CAF Feature Genes in BRCA. Note: A Mutation frequencies of 30 CAF feature genes in 991 BRCA patients from the TCGA cohort (the gray grid in the middle represents samples on the x-axis and genes on the y-axis, colored grids indicate gene mutations in the samples with mutation frequencies corresponding to the bar graph on the left where darker colors indicate higher mutation frequencies; the top bar graph reflects the total gene mutation status in each sample; different colors at the bottom reflect specific mutation types in the samples); B Frequencies of CNVs in the 30 CAF feature genes; C CNV alteration locations of 30 CAF feature genes on 23 chromosomes; D Expression distribution of the 30 CAF feature genes in normal and tumor tissues in TCGA-BRCA (***P < 0.001, normal n = 113, tumor n = 1109); E PPI analysis of the protein–protein interactions encoded by the 30 CAF feature genes
Identification of CAF-related molecular subtypes in BRCA based on 30 CAF feature genes
Firstly, we identified 8 CAF feature genes in TCGA-BRCA with prognostic value through Log-rank test and KM analysis. Fig. S4 indicate that high expression of LUM, INHBA, CTHRC1, COL5A2, COL11A1, and FN1 was linked to unfavorable outcomes among individuals with BRCA, whereas reduced levels of TAGLN and CALD1 were similarly linked to adverse prognostic implications. Furthermore, we combined the transcriptomic sequencing data of tumor tissues from 61 BRCA patients and clinical prognosis data from the GEO database GSE39004 dataset. Through consensus clustering analysis, we divided TCGA-BRCA and GSE39004 data into subtypes A (n = 250), B (n = 518), and C (n = 389) according to the expression variances observed in 30 characteristic genes of CAF (Fig. 4A). PCA analysis confirmed significant transcriptional differences in the expression profiles of these 30 CAF feature genes among subtypes A, B, and C (Fig. 4B). The heatmap showed that most genes were upregulated in subtype A and downregulated in subtype B and also displayed the association of these genes with the gender, age, T stage, and N stage of BRCA patients (Fig. 4C).
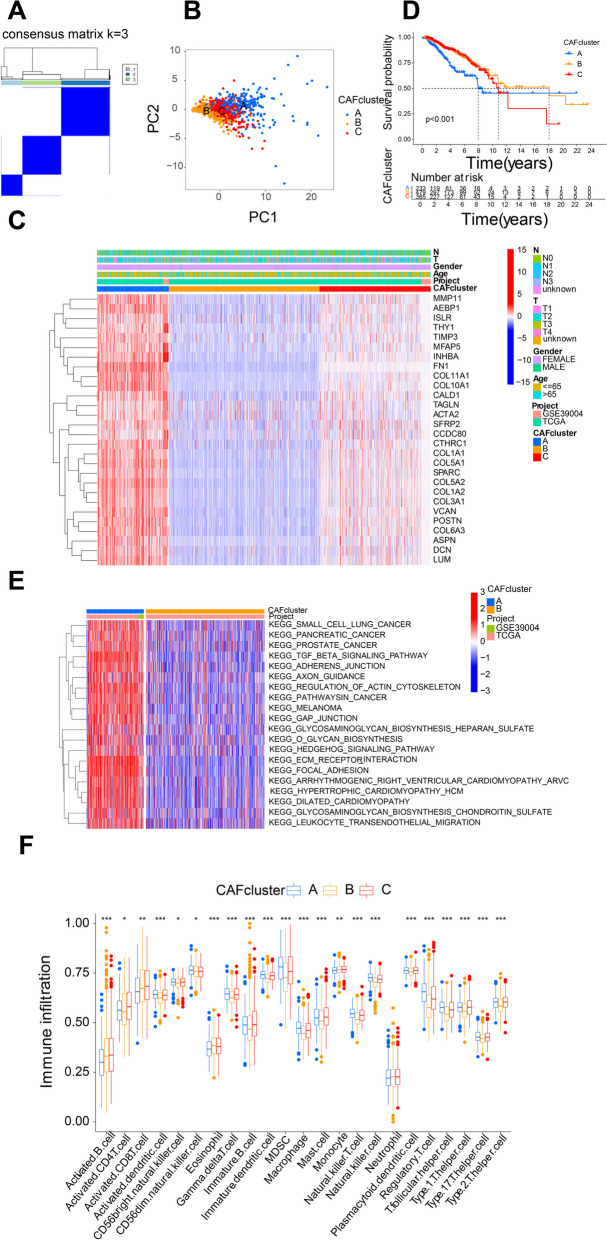
Fig. 4.
Identification of Molecular Subtypes of CAF Feature Genes in BRCA. Note: A Identification of three molecular subtypes (k = 3) of CAF feature genes through consensus clustering analysis of 1157 BRCA samples and their associated regions; B PCA depicting transcriptomic differences between different molecular subtypes; C Heatmap showing expression profiles of CAF feature genes in molecular subtypes A, B, and C, and the correlation between clinical-pathological features and different molecular subtypes; D Survival analysis of molecular subtypes A, B, and C using KM; E Pathway enrichment analysis of molecular subtypes A, B, and C through GSVA; F ssGSEA analysis of tumor immune cell infiltration among different molecular subtypes (* indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001)
Additional survival analysis revealed that subtype A displayed a less favorable survival outcome compared to subtypes B and C (Fig. 4D). To further observe the biological differences among these three subtypes, we performed pathway enrichment analysis using GSVA. We found that compared to subtype B, subtype A exhibited predominant enrichment in cancer-related pathways, and pathways related to ECM-receptor interaction and cell adhesion and migration (Fig. 4E). We also explored the role of CAF feature genes in the immune microenvironment of BRCA. Through ssGSEA, noticeable variances were noted in the infiltration of various immune cells within the three subcategories (Fig. 4F), with increased infiltration of most immune cells in subtype A, except for Activated B cell and Eosinophil, which showed decreased infiltration.
Through the above analysis, we identified 3 molecular subtypes in BRCA based on 30 CAF feature genes, and observed the pathways enriched in these subtypes and their relationship with immune cells.
Identification of differential genes from different molecular subtypes of CAF genes
In examining the potential biological tendencies of diverse CAF molecular subtypes, we defined conditions with |log2(fold change)| exceeding 1 and FDR less than 0.05, identifying 1432 DEGs among three subtypes (Fig. 5A). The investigation of relevant biological pathways involved the performance of GO and KEGG enrichment analyses. It was observed from the outcomes that the DEGs were notably enriched in extracellular matrix organization, extracellular vesicle organization, and extracellular structure organization processes, enriched in pathways such as the PI3K-Akt signaling pathway, Rap1 signaling pathway, proteoglycans in cancer, regulation of actin cytoskeleton, among others (Fig. 5B-C). Based on the enrichment analysis, we hypothesized that CAF characteristic gene molecular subtypes exert a crucial function in the extracellular matrix and tumor infiltration. Furthermore, univariate Cox regression analysis was undertaken, identifying 123 genes significantly associated with survival time (P < 0.05), which were later scrutinized (Table S3). Genes were classified into three subtypes based on differential gene expression levels, including gene subtypes A (n = 385), B (n = 592), and C (n = 180) (Fig. 5D). According to KM curves, subtype C showed a better prognosis than subtypes A and B (Fig. 5E). PCA substantiated remarkable variances in the gene expression profiles among gene subtypes A, B, and C (Fig. 5F).
Fig. 5.
Identification of CAF Gene Subtypes. Note: A DEGs between CAF molecular subtypes A, B, and C; (B-C) GO (B) and KEGG (C) enrichment analysis of these DEGs; D Identification of two gene subtypes (k = 3) and their associated regions through consensus clustering analysis based on the expression of prognostic-related DEGs; E Survival analysis of gene subtypes A, B, and C in BRCA patients; F PCA showing the transcriptomic variations among different gene subtypes
In summary, we identified differential genes from different molecular subtypes to characterize CAF gene subtypes, and observed the correlation between distinct gene subtypes and the prognostic implications for BRCA patients.
Constructing a BRCA risk assessment model based on CAF characteristic genes for accurate prognosis prediction in BRCA patients
A CAF risk assessment model was developed applying the prognosis DEGs' expression from various molecular subtypes. Firstly, three molecular subtypes, three gene subtypes, and two risk score groups were allocated to BRCA individuals (Fig. 6A). Risk scores for individual BRCA sample were computed within molecular and gene subtypes, showing higher risk values in subtype A compared to other subtypes in CAF molecular subtypes (Fig. 6B) and a significant increase in risk score for gene subtype A (Fig. 6C). Despite LASSO regression analysis showing 12 prognosis-related genes (Fig. S5A-B, Table S4) and displaying the distribution of these 12 genes in single-cell sequencing data (Fig. S6A-B). Subsequently, based on CAF risk scores, we identified the levels of the 12 prognosis-associated genes in the training set, demonstrating significant differences in these key risk score genes between high-risk and low-risk groups (Fig. 6D). The outcomes of CAF risk score in the training dataset indicated a shortened survival time as CAF risk score increased (Fig. 6E). KM survival curves illustrated a notable drop in survival period for high-risk group patients contrasted against the low-risk group (Fig. 6F). Moreover, the CAF risk scoring framework exhibited remarkable precision and sensitivity as shown by the survival rates at the end of 1 year, 3 years, and 5 years, manifesting AUC metrics of 0.714, 0.835, and 0.798, respectively (Fig. 6G). The results (Fig. 6H) show that the CAF risk score model performed the best among the various indicators, with an AUC value of 0.830, indicating that the model exhibits exceptional precision and strong discriminative ability in predicting patient prognosis. In contrast, diverse clinical aspects, encompassing age, gender, and distant metastasis, had weaker predictive power for prognosis, while tumor stage and lymph node status had a moderate effect on prognosis prediction (Fig. 6H).
Fig. 6.
Construction and Validation of a Prognostic Model for BRCA Based on DEGs. Note: A Sankey diagram illustrating the distribution of subtypes in groups based on different molecular subtypes, gene subtypes, risk scores, and survival outcomes; B Risk scores for the three CAF molecular subtypes; C Risk scores for the three gene subtypes; D Heatmap of 12 risk gene expression profiles in different risk groups in the training set (n = 539); E Distribution of CAF risk scores in the training set (n = 539); F Survival analysis of high and low CAF risk score groups in the training set (n = 539); G ROC curves representing the sensitivity and specificity of CAF risk score predictions for 1-year, 3-year, and 5-year survival in the training set; H The representation of the risk score prediction of the training group CAF for clinical pathological parameters and risk score by the ROC curve
Validating the precision of the CAF risk score system entailed the computation of CAF risk scores within the test set, combining expression data from TCGA-THCA and GSE27155. Patients harboring BRCA alterations were segregated into high-risk and low-risk classifications, showing the expression of the 12 key risk score genes in the combined and test sets (Fig. S7A and S8A). Fig. S7B and S8B display the correlation between patient survival rates and CAF risk scores in the combined and test datasets. Survival analysis in the combined and test sets revealed that BRCA patients with lower CAF risk scores had extended OS and better prognosis than high-risk patients, consistent with the results from the training set (Fig. S7C & S8C). Additionally, the CAF Risk scoring model upheld strong AUC metrics in both the combined and test sets (Fig. S7D & S8D), demonstrating the model's capability in precisely forecasting the survival likelihoods of BRCA individuals and its considerable prognostic significance.
Correlation analysis of CAF risk scoring model with BRCA TME
Next, we combined gene expression data from TCGA-BRCA and GSE39004 and uploaded it to the CIBERSORT network to calculate the assessments of 22 different types of immune cells. Subsequently, we investigated the relationship between CAF risk scoring and immune cells. The outcomes demonstrated an inverse relationship between the CAF risk scoring and the levels of B cells naïve, T cells CD8, T cells follicular helper, and Monocytes (p < 0.05) (Fig. 7A-D), while presenting a positive correlation with Macrophages M0, Neutrophils, and Macrophages M2 (p < 0.05) (Fig. 7E-G). Furthermore, no notable variances were observed in the StromalScore between CAF high and low-risk score groups, while ImmueScore and ESTIMATEScore exhibited decreased values in the CAF high-risk group (Fig. 7H). Further analysis revealed correlations between immune cell abundances and the expression of the 12 essential CAF-related risk genes. Research outcomes demonstrate an association between the activity of numerous immune cells and the presence of these 12 genes (Fig. 7I), which suggests that CAF risk scoring may suggest a negative outcome with varying immune infiltrates.
Fig. 7.
Correlation Analysis of TME and CAF Risk Scoring Model in BRCA. Note: A-G Correlation analysis between the CAF Risk Scoring model and tumor immune cells; H Differential analysis between CAF high/low risk score groups and immune/stroma/evaluation scores; I Correlation analysis between the abundance of tumor immune cells and 12 key risk scoring genes. * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001
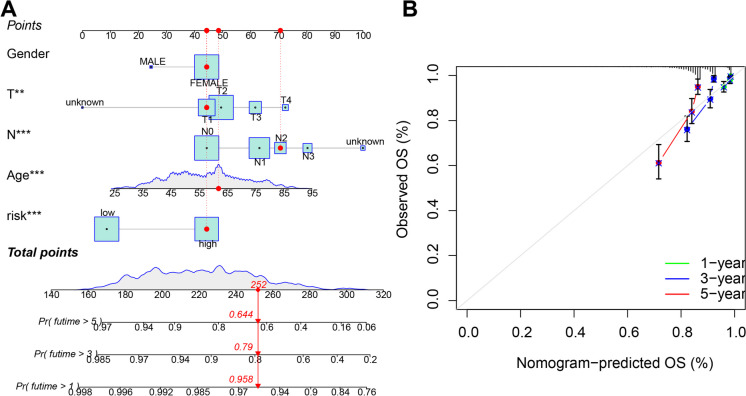
Construction of a nomogram for forecasting the outcome of BRCA patients
Given the significance of CAF risk scoring in the survival of BRCA subjects, a nomogram was formulated combining CAF risk scores with clinical-pathological features of BRCA patients for forecasting the 1-year, 3-year, and 5-year OS rates (Fig. 8A). The clinical-pathological features included gender, age, and TNM staging. Subsequent calibration plots demonstrated that the recommended chart showcased relatively precise performance among individuals with BRCA contrasted with the ideal model (Fig. 8B). Therefore, the model for forecasting the prognosis of BRCA patients, which relies on CAF gene features, is capable of accurately predicting patient survival rates and holds notable prognostic significance.
Fig. 8.
Construction and Validation of a Nomogram for Predicting Prognosis in BRCA Patients. Note: A Nomogram for predicting 1-year, 3-year, and 5-year OS in BRCA patients; B Calibration curves of the nomogram. The study includes 1077 BRCA samples with 522 high-risk scores and 555 low-risk scores
In vitro cell experiments demonstrate that CAF secrete SDC1 to stimulate migration and invasion of BRCA cells
After reviewing the information provided, we have identified that the CAF risk model constructed from these 12 risk genes holds significant prognostic value in BRCA. To further investigate the impact of CAF on BRCA function, we performed PPI analysis between these 12 risk genes and 30 CAF characteristic genes, which revealed a notable interaction between SDC1 and these 30 characteristic genes (Fig. 9A). In the TCGA-BRCA cohort, the level of SDC1 expression in tumor tissues surpassed that in healthy tissues extensively (Fig. 9B), and its high expression led to poorer prognosis for BRCA patients (Fig. 9C). Literature reports suggest that CAF-derived SDC1 participates in paracrine growth stimulation of BRCA cells and coordinates the alignment of extracellular matrix fibers from stromal cells, thus creating a microenvironment conducive to migration and invasion (Chute et al. 2018). Therefore, through conducting in vivo and in vitro experiments, we extensively confirmed the advancement stimulation of SDC1 secreted by CAFs on the progression of BRCA. Initially, we conducted immunohistochemical staining on collected adjacent normal tissue and BRCA tissue to observe the expression of CAF markers α-SMA and SDC1. The findings illustrated a noteworthy surge in the expression of α-SMA and SDC1 in BRCA tissue in relation to normal tissue (Fig. 9D).
Fig. 9.

Impact of CAF-Secreted SDC1 on Migration and Invasion of BRCA Cells. Note: A PPI analysis of 12 risk genes with 30 CAF feature genes (red represents CAF feature genes, blue represents risk genes); B Expression profile of SDC1 in TCGA-BRCA ( indicates ***P < 0.001, n = 1109); C KM curves of SDC1 in TCGA-BRCA; D Expression of SDC1 and α-SMA in BRCA tissue samples (n = 55) and adjacent normal tissue (n = 55) collected via immunohistochemical staining (scale bar = 50 μm); E Co-localization of α-SMA and SDC1 in immunofluorescence staining tissue (scale bar = 10 μm); F Expression of α-SMA in NF and CAF in immunofluorescence staining sections (scale bar = 25 μm); G Expression of α-SMA and FAP-α in NF and CAF detected by Western blot (** indicates P < 0.01); H Expression of secreted protein SDC1 in supernatant of NF and CAF detected by ELISA (** indicates P < 0.01); I Expression levels of SDC1 in BRCA cells in different groups detected by Western blot; J Cell scratch assay to assess cell migration in different groups; K Transwell assay to measure cell invasion ability in different groups (scale bar = 100 μm, *** indicates P < 0.001); L Expression levels of SNAI1, MMP2, and MMP9 in different cell groups detected by Western blot (** indicates P < 0.01). Cell experiments were repeated three times
Moreover, immunofluorescence experiments indicated a significantly higher co-localization of α-SMA and SDC1 in BRCA tissue than normal tissue (Fig. 9E). Subsequently, we isolated fibroblasts from normal and BRCA tissues, with fibroblasts from normal tissue labeled as NF and those from BRCA tissue labeled as CAF. Immunofluorescence observation of fibronectin and cytokeratin expression confirmed the accuracy of isolating NF and CAF cells (Fig. S9). Further identification of NF and CAF was carried out through immunofluorescence staining for α-SMA and Western blot analysis for α-SMA and FAP-α (Fig. 9F-G), and ELISA was employed to quantify the secretion of SDC1 in the culture supernatant of CAFs and NF, showing a considerable rise in SDC1 expression in CAF in contrast with NF cells (Fig. 9H). To assess whether CAF promotes the migration and infiltrative potential of BRCA cells through secreting SDC1, we co-cultured NF/CAF-conditioned medium with the BRCA cell line MDA-MB-231 and added SDC1 neutralizing antibodies to the CAF-conditioned medium. SDC1 expression was substantially higher in MDA-MB-231 cells under CAF conditions than in the NF group, as evidenced by Western blot analysis; upon addition of SDC1 neutralizing antibodies, the expression of SDC1 in MDA-MB-231 cells decreased significantly (Fig. 9I). Using wound healing (Fig. 9J) and Transwell assays (Fig. 9K), a notable enhancement was noted in the migratory and infiltrative capacities of MDA-MB-231 cells within the CAF group when contrasted with the NF group, which decreased upon the addition of SDC1-neutralizing antibodies. Furthermore, the protein levels associated with invasion and migration, SNAl1, MMP2, and MMP9, exhibited notably elevated expressions in the group of CAF relative to the NF group. Following the introduction of neutralizing antibodies targeting SDC1, these levels experienced a decline (Fig. 9L). It has been confirmed through cellular experiments conducted in vitro that CAF can discharge SDC1 to boost the motility and infiltration of BRCA cells.
RUNX1 in CAF promotes SDC1 transcription
To further investigate the upstream regulatory mechanism of SDC1, an assessment of the correlation existing between SDC1 and the other eleven risk genes was executed, revealing a significant positive correlation between SDC1 and the transcription factor RUNX1, with RUNX1 highly expressed in BRCA tissues (Fig. 10A-B). Additionally, RUNX1 expression was considerably escalated in CAF as opposed to NFs (Fig. 10C). Analysis using the JASPAR database (https://jaspar.elixir.no/) revealed a binding site, AATTGTTGTAA, in the promoter region of RUNX1 and SDC1 (Fig. 10D), suggesting that the transcription factor RUNX1 may regulate the transcription of SDC1, thereby influencing its expression. Subsequently, based on lentivirus-mediated silencing and overexpression of RUNX1 in CAF subgroups, RT-qPCR results showed (Fig. 10E) that overexpression of RUNX1 significantly increased its expression levels, while silencing RUNX1 led to a significant decrease in expression with better results observed for sh-RUNX1-1, for use in subsequent experiments. Moreover, the significant upregulation of SDC1 expression was observed upon overexpression of RUNX1, whereas a noteworthy downregulation of SDC1 expression was evident upon RUNX1 knockdown (Fig. 10F). Dual luciferase reporter assay data revealed a substantial escalation in SDC1 promoter activity with RUNX1 overexpression and a significant decrease with RUNX1 silencing, with no significant change observed in mutant groups (Fig. 10G). ChIP experiments revealed a significant enrichment of RUNX1 on the SDC1 promoter upon RUNX1 overexpression and a decrease upon RUNX1 silencing (Fig. 10H), indicating that RUNX1 in CAF may promote SDC1 transcription by binding to the SDC1 promoter region.
Fig. 10.
Transcriptional Regulation of SDC1 by RUNX1 in CAF. Note: A Correlation analysis between SDC1 and transcription factor RUNX1; B Expression levels of RUNX1 in BRCA tissues from TCGA dataset; C Western blot analysis of RUNX1 expression in NF and CAF; D Transcription binding sites of RUNX1; E RT-qPCR analysis of RUNX1 expression in cells after overexpression or silencing; F RT-qPCR analysis of SDC1 expression in cells after overexpression or silencing; G Dual-luciferase assay to assess the impact of RUNX1 on SDC1 promoter activity; H ChIP assay to detect enrichment of RUNX1 on the SDC1 promoter. All cell experiments were repeated 3 times, *P < 0.05, **P < 0.01
Facilitation of migration and invasion of BRCA cells in co-culture by RUNX1-induced upregulation of SDC1 in CAF
To elucidate the impact of RUNX1/SDC1 within CAF on the mobility and invasion of BRCA cells in a co-culture system, we manipulated CAF subgroups and assessed them using RT-qPCR. The results demonstrated (Fig. 11A) that silencing RUNX1 led to a marked upregulation of RUNX1 and SDC1 expression levels in CAF cells while overexpressing SDC1 increased SDC1 expression levels in CAF cells with no significant change in RUNX1. The abilities of BRCA cells to migrate and invade were determined through the implementation of Scratch and Transwell assays (Fig. 11B-C), showing a substantial drop in migration and invasion capabilities of MDA-MB-231 cells upon RUNX1 silencing, which were reversible by SDC1 overexpression. Besides, the protein levels associated with cell migration and invasion, comprising SNAl1, MMP2, and MMP9 significantly decreased upon RUNX1 silencing, and these results were reversed by SDC1 overexpression (Fig. 11D), indicating that the facilitation of cell migration and invasion in BRCA co-cultures is attributed to the elevation of SDC1 induced by RUNX1.
Fig. 11.
Impact of RUNX1/SDC1 in CAF on Migration and Invasion of BRCA Cells. Note: A RT-qPCR analysis of the expression levels of RUNX1 and SDC1 in different CAF groups; B Scratch assay to evaluate migration of BRCA cells in different groups; C Transwell assay to measure invasion ability of BRCA cells in different groups (scale bar = 50 μm); D Western blot analysis of SNAI1, MMP2, and MMP9 expression levels in different BRCA cell groups. All cell experiments were repeated 3 times, *P < 0.05, **P < 0.01
The in vivo metastasis of BRCA cells is promoted by CAF through the secretion of SDC1 in animal models
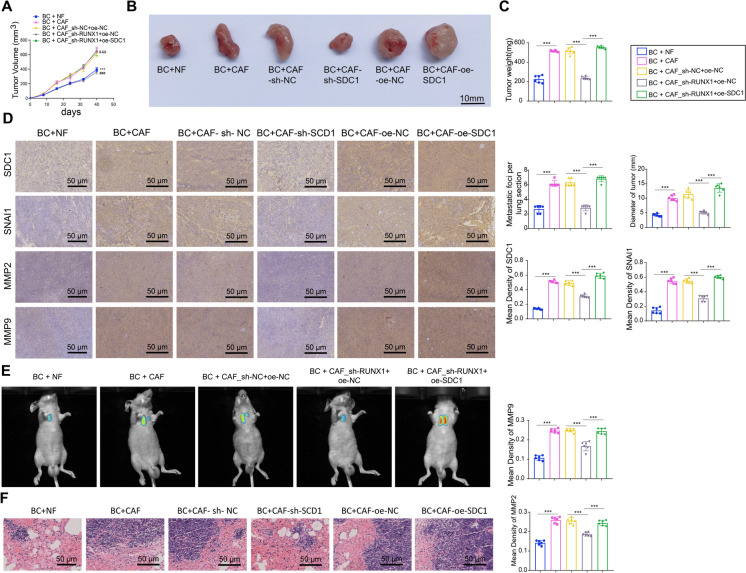
To further validate the role of the RUNX1/SDC1 axis within CAF in promoting tumor metastasis in vivo, nude mice were injected with MDA-MB-231 cells mixed with either NFs or CAF. The findings indicated that in contrast to the BC + NF group, the BC + CAF group exhibited increased tumor size and accelerated tumor growth rates. Silencing RUNX1 in CAF led to smaller tumors with slower growth rates, and these effects were reversed by SDC1 overexpression (Fig. 12A-C). Furthermore, immunohistochemistry examined the expression of SDC1, SNAl1, MMP9, and MMP2 in the tumors of the various groups, revealing that as opposed to the BC + NF group, the BC + CAF group illustrated a noteworthy surge in the expression of these proteins. Silencing RUNX1 in CAF reduced the expression of the mentioned proteins, and SDC1 overexpression in CAF reversed the effects of RUNX1 silencing (Fig. 12D). As expected, mice injected with BRCA cells mixed with BC + CAF_sh-RUNX1 + oe-NC showed improved lung metastasis with fewer metastatic nodules in the lungs compared to other nude mice, while overexpressing SDC1 in CAF increased lung metastasis (Fig. 12E-F).
Fig. 12.
Impact of CAF via the RUNX1/SDC1 Axis on In vivo Metastasis of BRCA Cells. Note: A Tumor growth curves of different groups of nude mice (compared to BC + NF group P < 0.05, *compared to BC + CAF-sh-NC group P < 0.05, &compared to BC + CAF-oe-NC group P < 0.05); (B-C) Representative images of tumors in different groups of nude mice (one from each group) and statistical analysis of tumor weight (*P < 0.05); D Immunohistochemistry to detect expression of SDC1, SNAI1, MMP2, and MMP9 in tumors of different mouse groups; E Lung metastasis images (left) and diameter analysis (right) in different groups of nude mice; F H&E staining to detect tumor metastatic foci in lung tissues of nude mice from different groups (*P < 0.05, scale bar = 50 μm). Six nude mice per group
These results from in vivo experiments further confirm that BRCA cell metastasis in vivo is facilitated by CAFs through the modulation of the RUNX1/SDC1 axis.
Discussion
Within this investigation, we applied single-cell multi-omics methodology for the first time to reveal the pivotal role of CAF in BRCA, especially in promoting invasion and metastasis of BRCA cells through secreting SDC1. This finding holds significant value for current BRCA research, as it uncovers the potential of CAF as a pioneering target for addressing BRCA. Cancer advancement is intricately connected with cellular communications in the microenvironment, and CAF, being a primary component of the TME, have long been recognized to significantly influence the progression of tumors (Wang et al. 2023; Ma et al. 2023). Therefore, this study presents a fresh perspective for further understanding and exploring the role of CAF in BRCA.
In bioinformatic analysis, we identified 30 highly expressed CAF characteristic genes and characterized molecular subtypes of CAF. These results reiterated the complexity and diversity of CAF. Previous studies have accumulated evidence suggesting that CAF are not a homogeneous cell population but rather comprised of various subtypes with distinct differences in functionality, phenotype, and molecular characteristics (Ge et al. 2021; Loke and Chisholm 2022; Sharma et al. 2021). However, how these CAF subtypes operate in tumors and whether commonalities exist among these subtypes remain unresolved issues.
In developing the risk-scoring model based on CAF characteristic genes, we employed machine learning techniques and validated the model's sensitivity and specificity through ROC curves. This Risk Scoring model may provide critical guidance for prognostic assessment in BRCA (Huntley et al. 2023; Parker et al. 2023). Particularly in clinical settings, early risk prediction based on this model could potentially offer more precise and personalized treatment strategies for patients (Luo et al. 2019). Furthermore, this highlights the applicability of machine-learning techniques in cancer research (Clift et al. 2023; Müller et al. 2023).
Through in-depth in vivo and in vitro experiments, it was uncovered that the participation of SDC1 is crucial in the function of CAFs, significantly promoting BRCA invasion and metastasis through SDC1 secretion. This discovery highlights a key mechanism by which CAFs influence BRCA progression. Although SDC1, a glycoprotein, has demonstrated significant involvement in multiple forms of cancer, its specific functional mechanism in CAF subtypes remains unclear (Lin et al. 2023; Reszegi et al. 2022). Our study not only fills this knowledge gap but also emphasizes the unique role of SDC1 in CAF subtypes, particularly in its interactions with the TME. Moreover, the establishment and confirmation of the CAF risk model indicate that the CAF subtype with high SDC1 levels is strongly linked to unfavorable outcomes among individuals with BRCA, providing a basis for the clinical application of the risk model.
Implicated in poor prognostic outcomes across multiple cancer types, the transcriptional regulator RUNX1 exerts its function by facilitating the migration and invasion of malignant cells (Fernández et al. 2023; Halperin et al. 2022). We focused specifically on the regulatory role of RUNX1 in CAFs. Although there are limited reports in the current literature regarding the regulatory relationship between RUNX1 and SDC1 (Lin et al. 2023; Reszegi et al. 2022), our in vivo and in vitro experiments confirmed that RUNX1 enhances the pro-tumor activity of CAFs by regulating SDC1 expression. These results underscore the vital function of the RUNX1/SDC1 axis in CAFs and further demonstrate the importance of this axis in BRCA invasion and metastasis. Based on these findings, the RUNX1/SDC1 axis presents a promising therapeutic target for BRCA, shedding light on the examination of the clinical relevance of the CAF risk model.
In summary, by employing single-cell multi-omics technology and extensive data analysis, this study reveals the significant role of CAF in BRCA growth and metastasis, highlighting how CAF promotes BRCA invasion and metastasis through secreting SDC1 (Fig. S10). Additionally, by delving into extensive datasets, a fresh prognostic algorithm was formulated centering CAF-related genes, exhibiting high sensitivity and specificity in prognostic prediction for BRCA patients, aiding in assessing patient survival risk more accurately and selecting more effective treatment strategies. Moreover, this research provides crucial clues for exploring the specific actions of CAF in BRCA and their potential molecular mechanisms, promising far-reaching implications for cancer treatment and prevention.
However, the principal restrictions of this investigation consist of confirming the promotion of BRCA invasion and metastasis by CAF through SDC1 secretion without unraveling the specific molecular mechanisms and the unclear role of SDC1 in other types of cancer. Furthermore, although the prognosis model related to CAF constructed in this study performed well in prognostic prediction for BRCA, its applicability to other cancer types requires further research validation. Lastly, the study primarily relied on publicly available database data, which may introduce data bias, necessitating verification in a larger and more diverse patient population.
Conclusions
For future research directions, we plan to delve deeper into the specific molecular mechanisms through which CAFs advance the invasion and metastasis of BRCA via the secretion of SDC1 and investigate the function of SDC1 in other types of cancer. Simultaneously, we aim to optimize and validate our prognostic model, evaluating its prognostic predictive capabilities in a broader range of cancer types and patient populations. We anticipate that these efforts will aid in identifying new targets for cancer therapy, improving cancer treatment efficacy, and ultimately realizing personalized cancer treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Batch correction using the "Combat" algorithm. Note: (A) Distribution of batch effects before ComBat correction; (B) Distribution of batch effects after ComBat correction. (JPG 797 KB)
Supplementary file2 Quality Control and PCA Dimensionality Reduction of scRNA-seq Data. Note: (A) Violin plots showing the number of genes per cell (nFeature_RNA), the number of mRNA molecules (nCount_RNA), and the percentage of mitochondrial genes (percent. mt) in scRNA-seq data; (B) Scatter plots depicting the correlation between filtered data nCount_RNA and percent.mt, nCount_RNA and nFeature_RNA; (C) Cell cycle status of each cell in scRNA-seq data, with S.Score representing the S phase and G2M.Score representing the G2M phase; (D) Heatmap of the top 20 significantly correlated genes with PC_1 – PC_6 in PCA, where yellow indicates upregulation and purple indicates downregulation; (E) Distribution of cells in PC_1 and PC_2 before batch correction, with each point representing a cell; (F) Batch correction process graph of Harmony, with the x-axis denoting the number of interactions. (JPG 1917 KB)
Supplementary file3 Expression Distribution of 9 Identified CAF Marker Genes in BRCA. Note: (A) Violin plots representing the distribution of 9 identified CAF marker genes in BRCA single-cell sequencing data; (B) UMAP clustering plots showing the distribution of the 9 identified CAF marker genes in BRCA single-cell sequencing data. (JPG 988 KB)
Supplementary file4 Prognostic Value of 8 CAF Feature Genes in BRCA. Note: KM curves of LUM, TAGLN, CALD1, INHBA, CTHRC1, COL5A2, COL11A1, and FN1 expression in TCGA-BRCA patients. (JPG 390 KB)
Supplementary file5 Identification of Representative Candidate Prognostic Genes. Note: (A-B) Impact of prognosis by LASSO regression analysis and partial likelihood deviation. (JPG 406 KB)
Supplementary file6 Expression Distribution of 12 CAF Risk Genes in BRCA. Note: (A) Violin plots showing the distribution of 12 risk genes in BRCA single-cell sequencing data; (B) UMAP clustering plots displaying the distribution of the 12 risk genes in BRCA single-cell sequencing data. (JPG 927 KB)
Supplementary file7 Validation of the CAF Risk Scoring Model in the Discovery Set. Note: (A) Heatmap of expression of 12 scoring genes in different risk groups of the Discovery Set (n = 1077); (B) Scatter plot of CAF risk score distribution and patient survival rates in the Discovery Set (n = 1077); (C) Survival analysis of high/low CAF risk score groups in the Discovery Set (n = 1077); (D) ROC curves predicting sensitivity and specificity of 1-year, 3-year, and 5-year survival rates based on the CAF risk score in the Discovery Set. (JPG 853 KB)
Supplementary file8 Validation of the CAF Risk Scoring Model in the Validation Set. Note: (A) Heatmap of expression of 12 scoring genes in different risk groups of the Validation Set (n = 538); (B) Scatter plot of CAF risk score distribution and patient survival rates in the Validation Set (n = 538); (C) Survival analysis of high/low CAF risk score groups in the Validation Set (n = 538); (D) ROC curves predicting sensitivity and specificity of 1-year, 3-year, and 5-year survival rates based on the CAF risk score in the Validation Set. (JPG 902 KB)
Supplementary file9 Immunofluorescence Identification of Separated NF/CAF. Note: Immunofluorescence observation of the expression of fibroblast marker gene fibronectin and endothelial cell marker gene Cytokeratin in NF/CAF. (JPG 217 KB)
Supplementary file10 Single-cell Multi-omics Technology-based Revelation of the Prognostic Value of Tumor-Associated Fibroblasts in BRCA and Identification of the Molecular Mechanism Through the RUNX1/SDC1 Axis Promoting BRCA Invasion and Metastasis. (JPG 2567 KB)
Acknowledgements
Not applicable.
Author contributions
Yunhao Wu and Jin Shang contributed equally to this work as co-first authors. Yunhao Wu conducted the primary experiments, including single-cell RNA sequencing analysis, and developed the CAF-based prognostic model. Jin Shang performed data interpretation and assisted in the development of the RUNX1/SDC1 axis analysis. Jiazi Jiang and Xiaoliang Liu supervised the research, provided clinical insights, and were responsible for manuscript preparation and critical revisions. All authors reviewed and approved the final manuscript.
Funding
Not applicable.
Data availability
The data that supports the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
Declarations
Ethical statement
This study was approved by the Clinical Ethics Committee of The First Hospital of China Medical University. All animal experiments were approved by the Animal Ethics Committee of The First Hospital of China Medical University (No. CMUXN2022131).
Consent to participate
Consent for publication was obtained from the participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Graphical Highlights
1. CAFs identified by single-cell RNA sequencing significantly promote BRCA progression via the RUNX1/SDC1 axis.
2. RUNX1 transcriptionally upregulates SDC1, enhancing the invasion and metastasis capabilities of BRCA cells.
3. The CAF-based prognostic model effectively predicts patient survival and highlights potential therapeutic targets.
Yunhao Wu and Jin Shang are regarded as co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiazi Jiang, Email: jzjiang@cmu.edu.cn.
Xiaoliang Liu, Email: shenhai.85@163.com.
References
- Barthel L, Hadamitzky M, Dammann P, et al. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022;41(1):53–75. 10.1007/s10555-021-09997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneit N, Fernández-García CE, Martín-Ventura JL, et al. Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis. Cardiovasc Diabetol. 2016;15(1):161. 10.1186/s12933-016-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S, Hamberger F, Ravichandra A, et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Invest. 2021;131(11):e146987. 10.1172/JCI146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowska KJ, Hanley CJ, Thomas GJ. Cancer-Associated Fibroblasts in Oral Cancer: A Current Perspective on Function and Potential for Therapeutic Targeting. Front Oral Health. 2021;2:686337. 10.3389/froh.2021.686337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. LNT and cancer risk assessment: Its flawed foundations part 1: Radiation and leukemia: Where LNT began. Environ Res. 2021;197:111025. 10.1016/j.envres.2021.111025. [DOI] [PubMed] [Google Scholar]
- Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18(12):792–804. 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang L, Yang M, et al. RUNX transcription factors: biological functions and implications in cancer. Clin Exp Med. 2024;24(1):50. 10.1007/s10238-023-01281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute C, Yang X, Meyer K, et al. Syndecan-1 induction in lung microenvironment supports the establishment of breast tumor metastases. Breast Cancer Res. 2018;20(1):66. 10.1186/s13058-018-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift AK, Dodwell D, Lord S, et al. Development and internal-external validation of statistical and machine learning models for breast cancer prognostication: cohort study. BMJ. 2023;381:e073800. 10.1136/bmj-2022-073800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Li HF, He MH, Yang M, Zhang JP. HDAC3 Downregulation Improves Cerebral Ischemic Injury via Regulation of the SDC1-Dependent JAK1/STAT3 Signaling Pathway Through miR-19a Upregulation. Mol Neurobiol. 2021;58(7):3158–74. 10.1007/s12035-021-02325-w. [DOI] [PubMed] [Google Scholar]
- Fernández NB, Sosa SM, Roberts JT, et al. RUNX1 Is Regulated by Androgen Receptor to Promote Cancer Stem Markers and Chemotherapy Resistance in Triple Negative Breast Cancer. Cells. 2023;12(3):444. 10.3390/cells12030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–400. 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Ge Y, Zhou L, Chen Z, et al. Identification of differentially expressed genes, signaling pathways and immune infiltration in rheumatoid arthritis by integrated bioinformatics analysis. Hereditas. 2021;158(1):5. 10.1186/s41065-020-00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CY, Patmore S, Smolenski A, et al. The role of von Willebrand factor in breast cancer metastasis. Transl Oncol. 2021;14(4):101033. 10.1016/j.tranon.2021.101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zhou B, Bie F, et al. Single-cell RNA sequencing analysis reveals transcriptional heterogeneity of multiple primary lung cancer. Clin Transl Med. 2023;13(10):e1453. 10.1002/ctm2.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin C, Hey J, Weichenhan D, et al. Global DNA Methylation Analysis of Cancer-Associated Fibroblasts Reveals Extensive Epigenetic Rewiring Linked with RUNX1 Upregulation in Breast Cancer Stroma. Cancer Res. 2022;82(22):4139–52. 10.1158/0008-5472.CAN-22-0209. [DOI] [PubMed] [Google Scholar]
- Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 2013;14:7. 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Li Z, Zheng B, et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun (Lond). 2022;42(5):401–34. 10.1002/cac2.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley C, Torr B, Sud A, et al. Utility of polygenic risk scores in UK cancer screening: a modelling analysis. Lancet Oncol. 2023;24(6):658–68. 10.1016/S1470-2045(23)00156-0. [DOI] [PubMed] [Google Scholar]
- Li S, Mai H, Zhu Y, et al. MicroRNA-4500 Inhibits Migration, Invasion, and Angiogenesis of Breast Cancer Cells via RRM2-Dependent MAPK Signaling Pathway. Mol Ther Nucleic Acids. 2020;21:278–89. 10.1016/j.omtn.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li X, Jiang P, Li R, et al. Analysis of cuproptosis in hepatocellular carcinoma using multi-omics reveals a comprehensive HCC landscape and the immune patterns of cuproptosis. Front Oncol. 2022;12:1009036. 10.3389/fonc.2022.1009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Chen Y, Zhang F, Zhu P, Yu L, Chen W. Single-cell RNA sequencing reveals the mediatory role of cancer-associated fibroblast PTN in hepatitis B virus cirrhosis-HCC progression. Gut Pathog. 2023;15(1):26. 10.1186/s13099-023-00554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke LHL, Chisholm RA. Measuring habitat complexity and spatial heterogeneity in ecology. Ecol Lett. 2022;25(10):2269–88. 10.1111/ele.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Stone BL, Koebnick C, et al. Using Temporal Features to Provide Data-Driven Clinical Early Warnings for Chronic Obstructive Pulmonary Disease and Asthma Care Management: Protocol for a Secondary Analysis. JMIR Res Protoc. 2019;8(6):e13783. 10.2196/13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Yang C, Peng A, et al. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol Cancer. 2023;22(1):170. 10.1186/s12943-023-01876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecek MK, Mehta-Shah N. Prognosis and risk stratification of peripheral T-cell lymphomas. Semin Hematol. 2021;58(2):70–7. 10.1053/j.seminhematol.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhao Q, An L, et al. A TNFR2-hnRNPK Axis Promotes Primary Liver Cancer Development via Activation of YAP Signaling in Hepatic Progenitor Cells. Cancer Res. 2021;81(11):3036–50. 10.1158/0008-5472.CAN-20-3175. [DOI] [PubMed] [Google Scholar]
- Morris R, Loftus A, Friedmann Y, Parker P, Pallister I. Intra-pelvic pressure changes after pelvic fracture: A cadaveric study quantifying the effect of a pelvic binder and limb bandaging over a bolster. Injury. 2017;48(4):833–40. 10.1016/j.injury.2017.01.046. [DOI] [PubMed] [Google Scholar]
- Müller M, Huber F, Arnaud M, et al. Machine learning methods and harmonized datasets improve immunogenic neoantigen prediction. Immunity. 2023;56(11):2650-2663.e6. 10.1016/j.immuni.2023.09.002. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Sova P, Bomsztyk K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34(1):e2. 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Mullins M, Cheang MCU, et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J Clin Oncol. 2023;41(26):4192–9. 10.1200/JCO.22.02511. [DOI] [PubMed] [Google Scholar]
- Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356. 10.1016/j.bbcan.2020.188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primac I, Maquoi E, Blacher S, et al. Stromal integrin α11 regulates PDGFR-β signaling and promotes breast cancer progression. J Clin Invest. 2019;129(11):4609–28. 10.1172/JCI125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszegi A, Tátrai P, Regős E, Kovalszky I, Baghy K. Syndecan-1 in liver pathophysiology. Am J Physiol Cell Physiol. 2022;323(2):C289–94. 10.1152/ajpcell.00039.2022. [DOI] [PubMed] [Google Scholar]
- Sha S, Si L, Wu X, et al. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front Immunol. 2022;13:922780. 10.3389/fimmu.2022.922780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, Medina-Saenz K, Miller PC, et al. Heterotypic clustering of circulating tumor cells and circulating cancer-associated fibroblasts facilitates breast cancer metastasis. Breast Cancer Res Treat. 2021;189(1):63–80. 10.1007/s10549-021-06299-0. [DOI] [PubMed] [Google Scholar]
- Shen W, Yao PA, Li W, et al. Cancer-associated fibroblast-targeted nanodrugs reshape colorectal tumor microenvironments to suppress tumor proliferation, metastasis and improve drug penetration. J Mater Chem B. 2023;11(9):1871–80. 10.1039/d2tb02253b. [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Jung DB, Lee H, et al. CNOT2 promotes proliferation and angiogenesis via VEGF signaling in MDA-MB-231 breast cancer cells [published correction appears in Cancer Lett. 2018 Sep 1;431:245–246. 10.1016/j.canlet.2018.05.002]. Cancer Lett. 2018;412:88–98. 10.1016/j.canlet.2017.09.052. [DOI] [PubMed]
- Sun L, Zhang X, Song Q, et al. IGFBP2 promotes tumor progression by inducing alternative polarization of macrophages in pancreatic ductal adenocarcinoma through the STAT3 pathway. Cancer Lett. 2021;500:132–46. 10.1016/j.canlet.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HF, Li LD, Lao IW, et al. Single-cell RNA sequencing reveals cellular and molecular reprograming landscape of gliomas and lung cancer brain metastases. Clin Transl Med. 2022;12(11):e1101. 10.1002/ctm2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C, Dann E, Goh I, et al. Mapping the developing human immune system across organs. Science. 2022;376(6597):eabo0510. 10.1126/science.abo0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniue K, Kurimoto A, Takeda Y, et al. ASBEL-TCF3 complex is required for the tumorigenicity of colorectal cancer cells. Proc Natl Acad Sci U S A. 2016;113(45):12739–44. 10.1073/pnas.1605938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor DV, Bâldea I, Lupu M, et al. COX-2 as a potential biomarker and therapeutic target in melanoma. Cancer Biol Med. 2020;17(1):20–31. 10.20892/j.issn.2095-3941.2019.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Z, Zhang Y, Wang X, et al. RUNX1 is a promising prognostic biomarker and related to immune infiltrates of cancer-associated fibroblasts in human cancers. BMC Cancer. 2022;22(1):523. 10.1186/s12885-022-09632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sande B, Lee JS, Mutasa-Gottgens E, et al. Applications of single-cell RNA sequencing in drug discovery and development. Nat Rev Drug Discov. 2023;22(6):496–520. 10.1038/s41573-023-00688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Borre P, McFadden DG, Gunda V, et al. The next generation of orthotopic thyroid cancer models: immunocompetent orthotopic mouse models of BRAF V600E-positive papillary and anaplastic thyroid carcinoma. Thyroid. 2014;24(4):705–14. 10.1089/thy.2013.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lin H, Su Q, Li C. Cuproptosis-related lncRNA predict prognosis and immune response of lung adenocarcinoma. World J Surg Oncol. 2022;20(1):275. 10.1186/s12957-022-02727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qin D, Tao Z, et al. Identification of cuproptosis-related subtypes, construction of a prognosis model, and tumor microenvironment landscape in gastric cancer. Front Immunol. 2022;13:1056932. 10.3389/fimmu.2022.1056932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li N, Liu Q, et al. Antiandrogen treatment induces stromal cell reprogramming to promote castration resistance in prostate cancer. Cancer Cell. 2023;41(7):1345-1362.e9. 10.1016/j.ccell.2023.05.016. [DOI] [PubMed] [Google Scholar]
- Wei L, Li J, Han Z, Chen Z, Zhang Q. Silencing of lncRNA MALAT1 Prevents Inflammatory Injury after Lung Transplant Ischemia-Reperfusion by Downregulation of IL-8 via p300. Mol Ther Nucleic Acids. 2019;18:285–97. 10.1016/j.omtn.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CY, Zhu MX, Lu NH, et al. Circular RNA circ_0020710 drives tumor progression and immune evasion by regulating the miR-370–3p/CXCL12 axis in melanoma. Mol Cancer. 2020;19(1):84. 10.1186/s12943-020-01191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Hou Y, Fu L, et al. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signalling. Cancer Lett. 2019;442:320–32. 10.1016/j.canlet.2018.10.015. [DOI] [PubMed] [Google Scholar]
- Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–3. 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Yi X. Down-regulation of Long Noncoding RNA MALAT1 Protects Hippocampal Neurons Against Excessive Autophagy and Apoptosis via the PI3K/Akt Signaling Pathway in Rats with Epilepsy. J Mol Neurosci. 2018;65(2):234–45. 10.1007/s12031-018-1093-3. [DOI] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–7. 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gaskins K, Yu Z, Xiong Y, Merino MJ, Kebebew E. An in vivo mouse model of metastatic human thyroid cancer. Thyroid. 2014;24(4):695–704. 10.1089/thy.2013.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Liu H, Ge Y, Wang X. Integrated single-cell and bulk RNA sequencing analysis identifies a cancer associated fibroblast-related signature for predicting prognosis and therapeutic responses in colorectal cancer. Cancer Cell Int. 2021;21(1):552. 10.1186/s12935-021-02252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Batch correction using the "Combat" algorithm. Note: (A) Distribution of batch effects before ComBat correction; (B) Distribution of batch effects after ComBat correction. (JPG 797 KB)
Supplementary file2 Quality Control and PCA Dimensionality Reduction of scRNA-seq Data. Note: (A) Violin plots showing the number of genes per cell (nFeature_RNA), the number of mRNA molecules (nCount_RNA), and the percentage of mitochondrial genes (percent. mt) in scRNA-seq data; (B) Scatter plots depicting the correlation between filtered data nCount_RNA and percent.mt, nCount_RNA and nFeature_RNA; (C) Cell cycle status of each cell in scRNA-seq data, with S.Score representing the S phase and G2M.Score representing the G2M phase; (D) Heatmap of the top 20 significantly correlated genes with PC_1 – PC_6 in PCA, where yellow indicates upregulation and purple indicates downregulation; (E) Distribution of cells in PC_1 and PC_2 before batch correction, with each point representing a cell; (F) Batch correction process graph of Harmony, with the x-axis denoting the number of interactions. (JPG 1917 KB)
Supplementary file3 Expression Distribution of 9 Identified CAF Marker Genes in BRCA. Note: (A) Violin plots representing the distribution of 9 identified CAF marker genes in BRCA single-cell sequencing data; (B) UMAP clustering plots showing the distribution of the 9 identified CAF marker genes in BRCA single-cell sequencing data. (JPG 988 KB)
Supplementary file4 Prognostic Value of 8 CAF Feature Genes in BRCA. Note: KM curves of LUM, TAGLN, CALD1, INHBA, CTHRC1, COL5A2, COL11A1, and FN1 expression in TCGA-BRCA patients. (JPG 390 KB)
Supplementary file5 Identification of Representative Candidate Prognostic Genes. Note: (A-B) Impact of prognosis by LASSO regression analysis and partial likelihood deviation. (JPG 406 KB)
Supplementary file6 Expression Distribution of 12 CAF Risk Genes in BRCA. Note: (A) Violin plots showing the distribution of 12 risk genes in BRCA single-cell sequencing data; (B) UMAP clustering plots displaying the distribution of the 12 risk genes in BRCA single-cell sequencing data. (JPG 927 KB)
Supplementary file7 Validation of the CAF Risk Scoring Model in the Discovery Set. Note: (A) Heatmap of expression of 12 scoring genes in different risk groups of the Discovery Set (n = 1077); (B) Scatter plot of CAF risk score distribution and patient survival rates in the Discovery Set (n = 1077); (C) Survival analysis of high/low CAF risk score groups in the Discovery Set (n = 1077); (D) ROC curves predicting sensitivity and specificity of 1-year, 3-year, and 5-year survival rates based on the CAF risk score in the Discovery Set. (JPG 853 KB)
Supplementary file8 Validation of the CAF Risk Scoring Model in the Validation Set. Note: (A) Heatmap of expression of 12 scoring genes in different risk groups of the Validation Set (n = 538); (B) Scatter plot of CAF risk score distribution and patient survival rates in the Validation Set (n = 538); (C) Survival analysis of high/low CAF risk score groups in the Validation Set (n = 538); (D) ROC curves predicting sensitivity and specificity of 1-year, 3-year, and 5-year survival rates based on the CAF risk score in the Validation Set. (JPG 902 KB)
Supplementary file9 Immunofluorescence Identification of Separated NF/CAF. Note: Immunofluorescence observation of the expression of fibroblast marker gene fibronectin and endothelial cell marker gene Cytokeratin in NF/CAF. (JPG 217 KB)
Supplementary file10 Single-cell Multi-omics Technology-based Revelation of the Prognostic Value of Tumor-Associated Fibroblasts in BRCA and Identification of the Molecular Mechanism Through the RUNX1/SDC1 Axis Promoting BRCA Invasion and Metastasis. (JPG 2567 KB)
Data Availability Statement
The data that supports the findings of this study are available on request from the corresponding author.
Not applicable.