Abstract
This study aimed to explore the effects of different brining times on the sensory, physicochemical properties, and volatile organic compounds (VOCs) of marinated grass carp (MGC). The results showed that different brining time changed the sensory quality, color and texture. The moisture content increased significantly with the extension of brining time, while the salt content, protein content, thiobarbituric acid reactive substances (TBARS), and total volatile basic‑nitrogen (TVB-N) decreased (p < 0.05). Free amino acids indicated that sweet amino acids significantly decreased, but bitter and umami amino acids increased. E-nose and E-tongue could clearly distinguish different MGC samples, and gas chromatography ion mobility spectrometry (GC-IMS) identified a total of 72 VOCs. Among them, 11 key VOCs were screened based on the variable importance of predicted component value (VIP) and relative odor activity value (ROAV), and they showed a high correlation with MGC quality. This study provides a theoretical foundation for enhancing the quality and improving the flavor of MGC.
Keywords: Grass carp, Marinade, GC-IMS, Flavor compounds, Correlation analysis
Graphical abstract
Highlights
-
•
The effect of brining time on the quality and flavor changes of MGC are unraveled.
-
•
Correlation of volatile compounds and quality was analyzed.
-
•
Brining time had significant effects on sensory and physicochemical properties.
-
•
E-nose and E-tongue could clearly distinguish different MGC samples.
-
•
11 VOCs were the key volatile compounds responsible for the flavor differences.
1. Introduction
Grass carp is one of the most widely distributed and abundant freshwater fishes in China, accounting for one fifth of China's freshwater aquaculture, and is an important economic fish (Zhou et al., 2024). Additionally, grass carp meat is rich in various unsaturated fatty acids and high quality protein and essential amino acids, which are popular among consumers (Li et al., 2024). Therefore, grass carp is widely used as one of the raw materials for daily cooking and aquatic product processing. However, due to its fishy flavor is one of the main factors affecting whether consumers buy it or not. In this study, the brining method was introduced in the processing of grass carp to increase consumers' desire to buy by masking the effect of spices Marinated products are traditional Chinese delicacies with a long history, which are favored by people for their excellent appearance, color, taste and flavor. However, among the many categories of marinated products, marinated grass carp products are relatively rare in the market.
Flavor is one of the most critical factors in assessing the quality of marinated products and is also an important basis for influencing consumers to purchase products (Han et al., 2020). The distinctive flavor of marinated products is mainly generated during the heating and cooking phase, which include heat-induced processes such as the Maillard reaction, lipid oxidation, protein degradation, and amino acids conversion (Wang et al., 2024). Marinading is a food processing technique in which the ingredients are first blanched or fried and then cooked by adding a pre-prepared marinade. This procedure is a crucial step in the processing of marinated products, contributing a strong fragrance and a excellent taste. During frying and brining, reactions such as protein degradation and lipid oxidation take place, resulting in the Maillard reaction, which leads to the formation of unique compounds of marinated products, including hexanal, octanal, and 2-pentyl furan (Qian et al., 2021). The flavor of meat products is also affected by the content of precursor substances such as free amino acids (sweet, umami, and bitter) and nucleotides, as well as their synergistic effects through reactions like the Maillard reaction and thermal degradation (Yu et al., 2021). Previous studies reported that aldehydes, ketones and esters play an important role in aroma formation during meat processing. In the study of Song et al. (2019) found that glutaraldehyde, phenylacetaldehyde and linalool reached the highest OAV values and contributed significantly to the aroma of braised meat. Another study indicated that different processing stages of Texas steak chicken produced specific key volatile flavor compounds, for example, the main flavors at the deep-frying stage were ethyl acetate, 1-hexanol, 4-methyl-2-pentanone (Yao et al., 2022).
Currently, studies on marinated products mainly focus on duck (Zhong et al., 2024), pork (Jiang et al., 2022) and beef (Pu et al., 2023), while fewer studies have been conducted on sensory characteristics, quality and flavor changes of marinated grass carp (MGC) during the brining stage. In order to deeply investigate the grass carp quality and flavor changes at different brining time periods. This study compared the commonalities and differences of flavor compositions at different brining time from the perspective of sensory, quality and flavor characteristics, screened the key VOCs, and further explored the relationship between the key VOCs and quality, so as to lay the foundation for the processing of marinated products.
2. Materials and methods
2.1. Sample preparation
15 fresh grass carps, each weighing about 2.0 ± 0.4 kg, was obtained from Changsheng Market (Nanchang, Jiangxi, China). They were packed in oxygenated plastic bags with water and transported alive to the laboratory within 1 h. These fish were sacrificed by a physical blow to the head, then scaled, gutted, decapitated, filleted into fillets (30 ± 2 g, 5 × 2 × 1.5 cm3), and rinsed in flowing tap water. All procedures were approved by the Commitment to Laboratory Animal Ethics and Welfare of Jiangxi Normal University (approval No. 20231018-A3). The fillets were marinated with 1.5 % salt, 3 % ginger and 5 % cooking wine for one hour at room temperature and then dried at 55 ± 0.5 °C for 21 h. Subsequently, the dried fish were randomly divided into seven portions and added to the pre-prepared marinated soup at a constant temperature of 65 °C. They were sauced and marinaded at a fish-to-marinade ratio of 1:2 for 0, 0.5, 1, 2, 4, 6, and 8 h respectively. A portion of each set of samples was retained for sensory evaluation, while the rest of the MGC samples were minced and randomly divided into 10 equal portions, vacuum-packed and stored at −80 °C.
2.2. Sensory evaluation
The sensory evaluation consists of five indicators (odor, appearance, chewiness, color and acceptability) and overall was calculated based on the weighted value of each indicator, and the methodology was adapted from Zhao et al. (2024), with minor adjustments. A sensory evaluation group consisting of 12 trained and experienced postgraduate students (6 males and 6 females) was formed to evaluate the odor, color, appearance, chewiness, and acceptability of MGC, with a total score of 100 points. To minimize the influence of subjective factors on the test results, the samples were password-numbered (using a 3-digit random number) and randomly assigned during the evaluation process. The sensory evaluation complied with laws and regulations, ethical and moral requirements and protected privacy of the participants, who gave their informed and explicit consent. The experiment was approved by the Commitment to Experimental Ethics and Welfare of Jiangxi Normal University (approval No. 20230820–005). There was no contact or communication between each member, and mouthwash was used before evaluating each sample. The sensory evaluation table is shown in Table S1, and the sensory evaluation was calculated based on the total score and was published as follows:
| (1) |
2.3. Determination of salt content
The salt content was measured using the assay method specified in the Chinese National Food Standard NFSS-2 (2016).
2.4. Determination of color
The colorimeter was calibrated with a whiteboard and zeroed with a blackboard. A WSC 2B portable precision colourimeter (Yidian Physical Optics Ltd., Shanghai, China) was used to determine the color coordinates of the fish meat, including luminance (L*), reddish-greenish tint (a*), and yellowish-blue tint (b*).
2.5. Determination of texture profile
Texture profiles was determined according to the method of Zhang et al. (2024). Measurements were done using a TA-XT Plus type texture analyzer (Stable Micro Systems Ltd., Godalming, UK), which was configured in TPA mode and equipped with a P/36R model probe. The trigger force was set to 5.0 g and the displacement distance was 15.0 mm. For the test parameter settings, the pre-test speed was 3.0 mm/s, the test speed was 1.0 mm/s and the post-test speed was 10.0 mm/s. The interval between the two compression man oeuvres was 5.0 s and 50 % of the sample was compressed during the test. During the data analysis phase, the key metrics of hardness, adhesiveness, springiness, cohesiveness and chewiness were focused on to fully assess the textural attributes of the samples.
2.6. Determination of moisture
The moisture content was determined by referring to the method of Xu et al. (2023), with slight modification. 0.5 g of sample was weighted in a rapid moisture analyzer (Guanya Technology & Science Co., Ltd., Shenzhen, China), and dried at 120 °C until a constant weight was achieved.
2.7. Determination of protein content
The process of extracting and quantifying protein was conducted in alignment with the National Standard for Food Safety delineated in GB 5009.228–2016, though slight alterations were implemented. The process involved weighting 0.2 g of fish meat in a digestion tube, adding 0.5 g CuSO4, 4.5 g K2SO4 and 10 mL H2SO4 for digestion. After the digestion solution was cooled to room temperature, 10 mL of distilled water was added. Protein content of samples was determined by a SKD-800 Kjeldahl Apparatus (Peiou Co., Ltd., Shanghai, China). H3BO3 solution, bromocresol green and methyl red were used as absorption indicators. After the sample was distilled for 4 min, it was titrated with 0.1 mol/L HCl standard titration solution. 20 mL distilled water was substituted for the digest of the sample as a blank control. The protein content was calculated as following:
| (2) |
where V1 and V2 represent the volume (mL) of HCl standard titration solution consumed by the sample and blank, respectively; C represents the concentration of standard HCl titration solution (mol/L); m represents the mass of fish (g); 0.014 represents the molar mass of nitrogen equivalent to titrate 1 mL of standard HCl titration solution (g/mol); F represents the coefficient for converting nitrogen into protein, and 100 is the unit conversion coefficient.
2.8. Determination of total volatile basic‑nitrogen (TVB-N)
The TVB-N content was extracted and measured as described by Zhang et al. (2024). Briefly, 10 g of ground fish meat was weighed in a digestion tube and 20 mL of distilled water was added and allowed to stand for 30 min. Subsequently, 1.0 g of MgO were added to the digestion tube and the TVB-N content in samples was measured using a SKD-800 Kjeldahl Apparatus (Peio Co., Ltd., Shanghai, China). The absorption indicator is H3BO3, bromocresol green and methyl red. After 4 min of distillation, titration was performed with 0.01 mol/L HCl standard titration solution. Distilled water (20 mL) was used as blank control. The formula for the calculation of TVB-N content was as follows:
| (3) |
where V1 and V2 represent the volume of HCl standard titration solution consumed by the sample and blank groups, respectively (mL); c indicates the concentration of HCl standard titration solution (mol/L); m represents the mass of sample (g); 14 represents the molar mass of nitrogen equivalent to titrate 1.0 mL of HCl standard titration solution (g/mol); and 100 is the unit conversion coefficient.
2.9. Determination of thiobarbituric acid reactive substances (TBARS)
The determination of TBARS values was based on the method described by Ali Ghoflgar Ghasemi et al. (2024) with minor adjustment. The procedure was as follows: first, add 25 mL of 20 % trichloroacetic acid (TCA) solution and 20 ml of distilled water to 5.0 g of sample. The mixture was homogenized using an T25 homogeniser (IKA Co., Ltd., Staufen, Germany) and then centrifuged at 7500 g for 10 min using a TGL-16 M high-speed refrigerated centrifuge (Lu Xiangyi Co., Ltd., Shanghai, China). After centrifugation, the supernatant was diluted to 50 mL, then, 5.0 mL of this mixed with 2.0 mL thiobarbituric acid (TBA) solution (0.02 mol/L) and heated for 40 min. Subsequently, the values were determined at 532 and 600 nm utilizing a U-2910 UV-V is spectrophotometer (Hitachi Co., Ltd., Tokyo, Japan).
2.10. Free amino acids (FAAs) analysis
The extraction and analysis of FAAs were carried out using a modified version of the method of Tian et al. (2020). Quantification was done with an L-8900 automated amino acid analyzer (Hitachi Co., Ltd., Tokyo, Japan). Briefly, 4 g minced fish meat was extracted with 3 % (m/m) sulfosalicylic acid and homogenized for 40 s (2 × 20 s, 7000 r/min). Then centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was mixed with 2 mL of hexane and the volume was adjusted to 50 mL with 0.02 mol/L HCl. The solution was filtered through a 0.22 μm filters and analyzed with an 835 to 50 amino acid auto-analyzer (Hitachi Co., Ltd., Tokyo, Japan).
Taste Active Value (TAV) indicates the degree of contribution of the taste substance to the overall taste of the samples. TAV >1 indicates that the substance has an important influence on the taste; TAV <1 means that it has no important effect on the taste. The formula is presented below:
| (4) |
where C represents taste substance content (mg/100 g); T represents taste substance content (mg/mL).
2.11. Electronic nose (E-nose) analysis
The E-nose analysis was conducted following the procedure described by Yu et al. (2018), with slight modification. 2.0 g of sample was weighed into a 20 mL headspace bottle and sealed with four layers of cling film, equilibrated in a water bath at 50 °C for 30 min, and inserted into the PEN3 E-nose (AirSense Analytics Co. Ltd., Schwerin, Germany) for measurement. The conditions were as follows: cleaning time 120 s, zero time 5 s, preparation time 5 s, measurement time 140 s, carrier gas flow rate 600 mL/min, injection flow rate 600 mL/min, and eigenvalue extraction time set at 137–139 s. Sensors and corresponding representative sensitive material types of PEN3 is showed in Table S2.
2.12. Electronic tongue (E-tongue) analysis
E-tongue analysis was performed following the method described by Shen et al. (2023). 10 g of fish meat was chopped into 200 mL beaker and homogenized by T25 homogeniser with 80 mL of distilled water at 4 °C for 10 min, and then centrifuged at 10000 g, 4 °C for 10 min. The supernatant was taken into a volumetric flask and volume-determined to 100 mL. The samples were tested with Insent MOS ASTREE E-tongue (Alpha M.O·S, Toulouse, France) equipped with single AHS, CTS, NMS, ANS, and SCS test sensors and two reference electrodes. Data was collected over 120 s with a 1.0 s period and 0 s delay. Values from the 120th second of each sensor were selected for graphing and analysis.
2.13. GC-IMS analysis
Sample preparation: 2.0 g of minced fish was weighed into a 20 mL headspace vial for incubation (60 °C, 15 min, 500 r/min) with a headspace injection volume of 100 μL and a needle temperature of 65 °C (Wu et al., 2023). Samples were analyzed using a GC-IMS instrument (Flavor Spec®, G.A.S. GmbH, Germany).
GC detection: An Agilent DB-wax column (30 m × 0.25 mm × 0.25 μm) at 60 °C was used with high-purity N2 carrier gas (purity ≥99.999 %). The carrier gas program initiates at 2 mL/min, holds for 2 min, increases to 10 mL/min from 2 to 10 min, then to 100 mL/min from 10 to 20 min, and finally to 150 mL/min from 20 to 30 min.
IMS detection: Use a 9.8 cm drift tube with 400 V/cm linear voltage and 65 °C IMS temperature. Apply high-purity N2 as drift gas (purity ≥99.999 %). Analyze substance spectra with Flavor Spec flavor analysis tool and qualify using GC × IMS Library Search software with NIST and IMS databases.1.3.10 Statistical analysis.
2.14. The relative odor activity value (ROAV) analysis
ROAV was employed to ascertain the influence of distinct odor constituents on the overall fragrance, utilizing the formula below:
| (5) |
Ti and Ci represent the sensory threshold (μg/kg) and relative content (%) of a specific VOC, respectively. Tstan and Cstan represent the sensory threshold (μg/kg) and relative content (%), respectively, of the VOC with the highest contribution to the overall volatile compounds.
2.15. Statistical analysis
All experiments were repeated three times (mean ± standard deviation). One-way ANOVA was performed on the data with SPSS version 16.0 statistical software. Significant differences were analyzed by Duncan's test (p < 0.05). OPLS-DA analysis was performed using SIMCA version 18. Flavor data were analyzed and collated using VOCal software with GC-IMS and GC × IMS Library Search (Flavor Spec®, GAS, Dortmund, Germany). Results were plotted by Origin 2021 and GraphPad Prism version 10.1.2.
3. Results and discussion
3.1. Changes in physicochemical properties
3.1.1. Sensory evaluation analysis
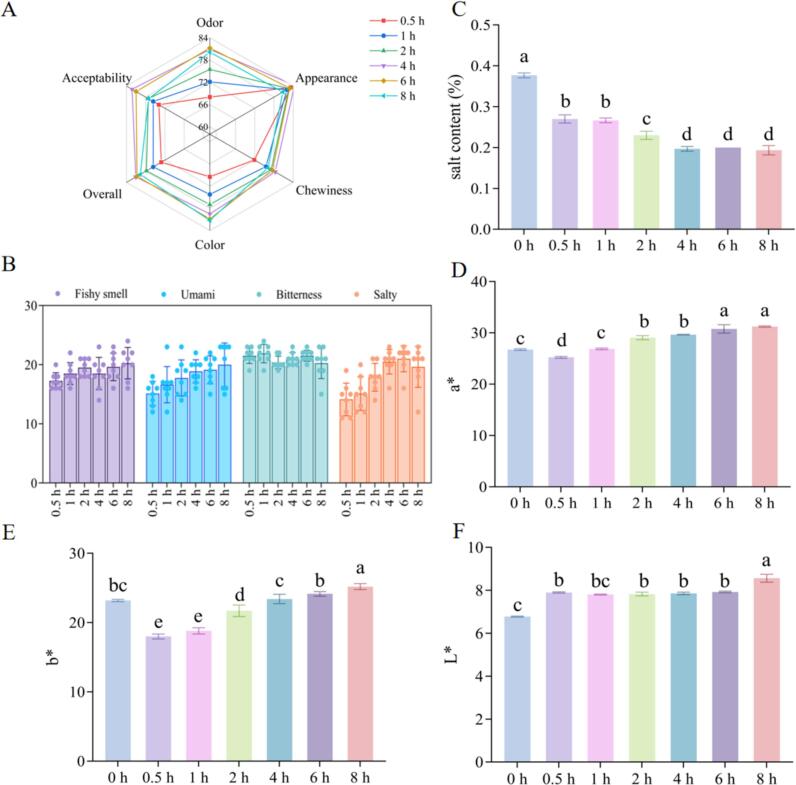
As shown in the radar chart in Fig. 1A, with the prolongation of brining time, the odor, chewiness, overall and acceptability scores of sensory all showed an increasing and then decreasing trend. The color score significantly increased (from 69.5 ± 5.21 to 82.88 ± 4.49), while the appearance score did not change significantly (p > 0.05). As mentioned, the overall score of MGC was 4 h > 6 h > 8 h > 4 h > 2 h > 1 h. This study further analyzed the change of sensory perception of odor (Fig. 1B), the scores of fishy smell and salinity significantly increased with the prolongation of brining time (p < 0.05). These findings indicated that the aromatic sauce-marinade derived from a blend of spices and seasonings, when heated, release scents that proficiently conceal the fishy odor, thereby substantially ameliorating the issue of fishiness (Dai et al., 2024).
Fig. 1.
Sensory evaluation (A, B), salt content (C), a* value (D), b* value (E) and L* value (F) of MGC with different brining time.
3.1.2. Salt content
The salt content of MGC was shown in Fig. 1C, its content decreased from 0.38 % to 0.13 % with the extension of brining time (p < 0.05). The downward trend went through two significant decreases, from 0 h to 0.5 h and 1 h to 4 h, respectively, and then the trend leveled off. Under the influence of osmotic pressure, the marinated soup continuously penetrated into the interior of the fish pieces, resulting in a gradual decrease in salt content. In addition, the first decreasing trend could also be related to the dissolution of precipitated salt from the surface of dried fish into the marinated soup. Once the salt on the surface of the MGC was completely dissolved into the marinated soup, the first leveling off period was emerged. After 4 h of treatment, a second plateauing period occurred and the diminution in salt content of MGC became less pronounced, which could be attributed to the internal and external osmotic pressures of the fish pieces reached an equilibrium state at this time.
3.1.3. Color
The color of MGC serves as a crucial indicator for quality control and consumer acceptance. The color attributes (a*, b* and L*) of MGC for different brining time were shown in Fig. 1D-F. Notably, the a* and b* values exhibited more pronounced alterations than the L* values, revealing a significant ascending trend with the exception of the 0 h (from 25.24 ± 0.14, 18.00 ± 0.29 to 31.22 ± 0.11, 25.19 ± 0.35, respectively). The L* value was significantly higher only at 8 h (8.56 ± 0.15), which may be attributed to the high salt content at 0–2 h and the high moisture content at 8 h (Xu et al., 2023). Furthermore, throughout the heating procedure, a Maillard reaction transpired between the marinated soup and grass carp, resulting in the formation of colored pigments (metmyoglobin). As the marinating process persisted, these pigmented substances gradually penetrated into the fish, causing the color of the fish to become darker and shift towards red and yellow, which was reflected by the increase in a* and b* values. Si et al. (2022) ‘s research findings revealed that an extension in heating duration induced Maillard and caramelization reactions in camel meat, consequently elevating its b* value.
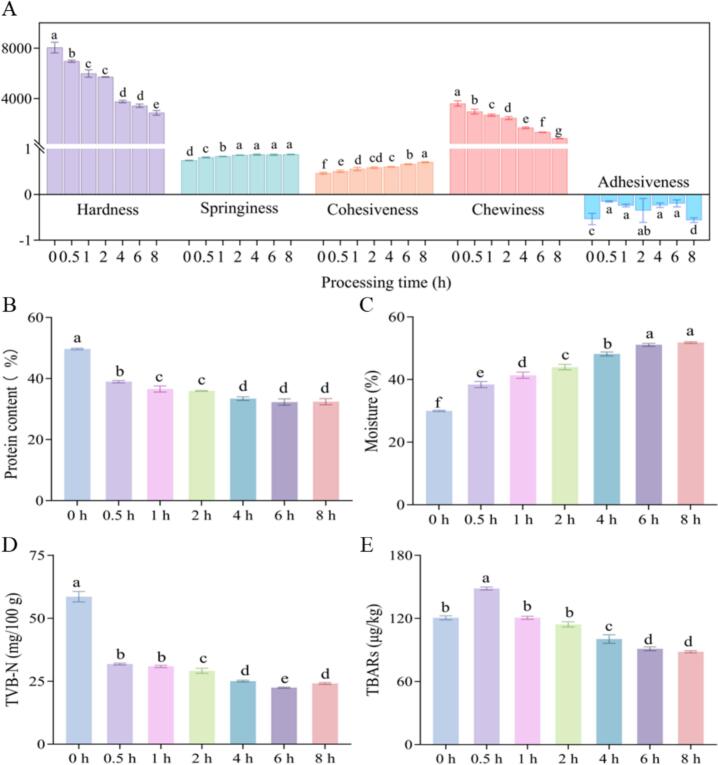
3.1.4. Texture profile
Texture attributes are a crucial metric in assessing the edible quality of MGC, including hardness, springiness, cohesiveness, chewiness and adhesiveness. TPA in the texture analyzer involves compressing the test probe twice to fully simulates the chewing process of food in the human mouth. The textural properties were presented in Fig. 2A, the chewiness and hardness of MGC significantly decreased from 8048.70 ± 347.42 g and 3608.53 ± 172.5 at 0 h to 2871.87 ± 149.77 g and 836.5 ± 15.24 at 8 h, respectively (p < 0.05). This aligned with our collective sensory perception of hardness and chewiness throughout the MGC's brining time. However, as the brining time increases, the springiness and cohesiveness of MGC significantly increased (p < 0.05). These findings indicated that varying brining time can notably modify its texture. A plausible explanation for this occurrence was that as the brining time is extended, the marinade soup further permeates the fish, elevating the water content of MGC, which can result in a decrease in hardness and chewiness. Additionally, denaturation and degradation of protein might cause the reticulation structure formed with water to break down, reducing the resistance of MGC to external forces and potentially leading to a slight increase in springiness.
Fig. 2.
Changes in the physicochemical properties of MGC with different brining time. Texture (A), protein content (B), moisture (C), TVB-N content (D), and TBARS content (E).
3.1.5. Protein and moisture content analysis
The crude protein content is a general term for various nitrogen-containing substances in fish meat, including true protein and nitrogen-containing compounds. As depicted in Fig. 2B, the protein content of MGC underwent a significant reduction from 49.67 % to 32.41 % (p < 0.05), which potentially attributed to the direct outflow of water-soluble and salt-soluble proteins into the marinated soup. Jiang et al. (2022) found that water-soluble proteins continued to leach into the brine as the curing time increased, which resulting in lower protein content of Thunnus obesus meat. It was noteworthy that the crude protein content exhibited a significant decline from 0 h to 4 h (p < 0.05), and the rate of decrease progressively diminished from 4 h to 8 h (p > 0.05). This might be due to the fact that aqueous-mediated substance migration profoundly influences the total quantity of nitrogenous compounds (crude protein content) in MGC. Once this migration reached equilibrium, the total amount of nitrogenous compounds undergone no further change. Dimakopoulou-Papazoglou and Katsanidis (2020) et al. stated that time affects the rate of mass transfer during osmotic processing, and equilibrium is reached between the water activity of the osmotic solution and the food after a certain period of time.
The moisture content, as illustrated in Fig. 2C, revealed that the moisture level of MGC at 0 h (29.97 %) was significantly lower than that of fresh grass carp, whose moisture level was approximately 77 % (Wen et al., 2015). This difference could be attributed to the drying process, which disrupted non-polar amino acids and led to the formation of sulfhydryl bonds, resulting in decreased water retention and increased water loss (Xu et al., 2022). Additionally, the moisture content of MGC increased significantly with the extension of brining time. This phenomenon might be due to the fact that the brining process can dilute and dissolve the salt concentration on the surface of the MGC, while the salt content inside the MGC remained higher than that on the surface, resulting in the continuous migration of water into the fish. Furthermore, there was no significant change in moisture content after 6 h of marinating. The possible reason was that the internal and external salt concentrations reached equilibrium and the moisture no longer migrated.
3.1.6. TVB-N and TBARS values
TVB-N value serves as a crucial indicator of fish freshness, which is mainly affected by endogenous enzymes and external microorganisms during fish processing and storage. The TVB-N content of the control group (0 h) reached 58.54 ± 1.70 mg/100 g, yet it was still fresh (Fig. 2D). According to Kim et al. (2020), the TVB-N value of hot-air dried fish reached 66.27 mg/100 g. Additionally, Rasul et al. (2018) found that the TVB-N value of dried fish prepared by different drying methods ranged from 37.58 mg/100 g to 45.03 mg/100 g. However, TVB-N of MGC decreased significantly with the increase of brining time (p < 0.05), especially it reached 22.44 ± 0.19 mg/100 g after 6 h of brining, which may be attributed to the fact that the alkaline nitrogenous compounds such as amines and ammonia reacted with certain compounds in the marinated soup, leading to the leaching of these compounds into the soup. In addition, the spices in the sauce-marinade had a variety of active components, such as polyphenols, flavonoids, terpenoids, and aldehydes, all of which had strong antioxidant activity, and these components inhibited the oxidation of proteins (Shah et al., 2014), thereby inhibiting the production of TVB-N.
TBARS is an important indicator for evaluating the degree of fat oxidation in meat and aquatic products. As shown in Fig. 2E, the TBARS values at different time decreased to varying degrees but remained below 2 mg/kg when compared to the MGC at 0 h. If the value is higher than the threshold, it was considered that the food had undergone rancidity (Senapati et al., 2017). This decline might be due to the fact that the brining temperature of 65 °C, which inhibited the growth and reproduction of most microorganisms in the fish meat, reduced the activity of endogenous enzymes, and slowed down the rate of lipid oxidation producing aldehydes, ketones, and other substances. Additionally, during the initial stage of marinading (0.5 h), the TBARS value peaked at 148.46 ± 1.16 μg/kg before decreasing significantly (p < 0.05). The possible reason was that the lipoxygenase, which promotes lipid oxidation within the fish during the 0.5 h marination period in MGC, did not completely lose its activity. As the brining time increased, the activity of this enzyme gradually weakened, which led to a decrease in TBARS value (Turhan et al., 2004).
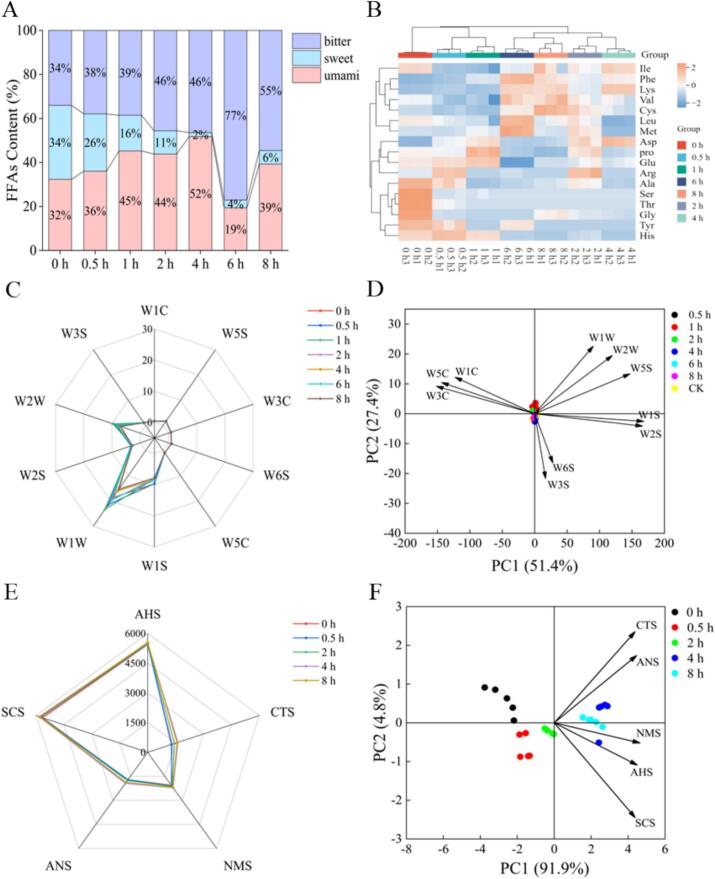
3.2. FAAs
The variations in the relative content of amino acids with different brining time were illustrated in Fig. 3A-B. Both bitter and umami amino acids exhibited a trend of initially increasing and then decreasing at 0 h to 8 h. Conversely, the relative contents of sweet amino acids showed a trend of decreasing and then increasing (p < 0.05). The profile of free amino acids (FAAs) significantly influenced the flavor of MGC (Hu et al., 2022). For instance, the relative content of sweet amino acids (e.g., Ser, Ala, Thr, Gly) was higher at 0 h but decreased significantly as brining time extended (p < 0.05), whereas the umami amino acids Glu and Asp were the highest at 1 h and 4 h, respectively. Furthermore, the total amount of FFAs in MGC decreased from 1206.33 ± 7.12 mg/100 g to 612 ± 42.61 mg/100 g with the extend of brining time (Table S3). The reason might have been the degradation of proteins and peptides leading to an increase in the FFAs, but since most of the amino acids were water-soluble, they dissolved out into the marinated soup. It was also possible that FAAs acted as intermediates in the Maillard reaction and were involved in lipid oxidation and thermal degradation processes, resulting in a complex series of biochemical reactions to produce VOCs (Chang et al., 2020). Studies have been reported that Glu undergone a series of complex reactions such as condensation, polymerization, and degradation to produce compounds like furfural, 2-butanone, and pyrrole (Gao et al., 2023). In addition, Li, Qu, et al. (2023) also demonstrated that oxidative degradation products of proteins and amino acids, as analyzed by metabolomics, can be served as intermediates in a Maillard reaction to generate the characteristic flavor of shrimp paste.
Fig. 3.
Stacked plots of relative content of savory, fresh, and bitter amino acids (A), heat map of free amino acid content (B), E-nose radar plot (C), E-nose load plot (D), E-tongue radar plot (E), and E-nose load plot (F) of MGC with different brining time.
The taste active value (TAV) was presented in Table 1, there were 6 FAAs in all samples with TAV >1, namely umami amino acid (Glu), sweet amino acid (Ala), and bitter amino acid (Val, Phe, Lys, His). These six FFAs significantly contribute to the distinctive flavor profile of MGC. Notably, the umami amino acid (Glu) exhibited the highest taste active value (TAV) among the analyzed samples, underscoring its pivotal role in shaping the taste of MGC. Furthermore, the prolongation of the brining process led to a substantial decrease in the TAV of Glu in grass carp, which aligned with the findings reported by Yang et al. (2020).
Table 1.
Free amino acid TAV of grass carp meat at different marinating temperatures.
| Aino acids | Flavoring properties | 0 h | 0.5 h | 1 h | 2 h | 4 h | 6 h | 8 h |
|---|---|---|---|---|---|---|---|---|
| Asp | umami | 0.24 | 0.43 | 0.31 | 0.31 | 0.36 | 0.13 | 0.21 |
| Thr | sweet | 0.12 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ser | sweet | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Glu | umami | 13.32 | 10.34 | 6.46 | 4.53 | 3.91 | 1.51 | 4.91 |
| Gly | sweet | 0.57 | 0.03 | 0.00 | 0.04 | 0.02 | 0.00 | 0.11 |
| Ala | sweet | 1.60 | 0.76 | 0.01 | 0.29 | 0.06 | 0.07 | 0.20 |
| Cys | odorless | – | – | – | – | – | – | – |
| Val | bitter | 1.10 | 0.60 | 0.30 | 0.45 | 0.39 | 0.48 | 0.73 |
| Met | bitter | 0.50 | 0.32 | 0.29 | 0.46 | 0.00 | 0.70 | 0.28 |
| Ile | bitter | 0.45 | 0.13 | 0.05 | 0.14 | 0.17 | 0.10 | 0.20 |
| Leu | bitter | 0.29 | 0.17 | 0.07 | 0.11 | 0.04 | 0.14 | 0.12 |
| Tyr | bitter | – | – | – | – | – | – | – |
| Phe | bitter | 0.72 | 0.81 | 0.82 | 0.90 | 1.10 | 1.63 | 1.73 |
| Lys | bitter | 0.64 | 0.95 | 0.33 | 1.24 | 2.62 | 2.19 | 3.09 |
| His | bitter | 10.80 | 10.10 | 3.98 | 0.90 | 0.00 | 0.32 | 0.00 |
| Arg | bitter | 0.12 | 0.35 | 0.00 | 0.21 | 0.00 | 0.01 | 0.00 |
| Pro | sweet | 0.22 | 0.13 | 0.17 | 0.11 | 0.04 | 0.02 | 0.01 |
3.3. E-nose analysis
The E-nose imitates the human olfactory system, is widely utilized for odor identification and aroma discrimination, and provides an overall spectrum of volatile compounds but does not provide specific information on the composition and quantity of volatile compounds (Chen et al., 2024). The results of the radar map of E-nose were presented in Fig. 3C. The highest response value was recorded for W1W, followed by W2W, W1S, and W2S, which suggested that sulfides, aromatic compounds, organic sulfides, alcohols, aldehydes and ketones, and methyl compounds played significant roles in the flavor profile of MGC. In addition, the response values of MGC on these differentiated sensors first increased and then decreased as the brining time increased.
The PCA analysis was shown in Fig. 3D. The contributions of PC1 and PC2 were 51.4 % and 27.4 %, respectively, with a cumulative contribution of 78.8 %, which indicateed that PC1 and PC2 could respond to the main information characteristics of volatiles with different brining times. Based on the E-nose findings, all the samples were significantly categorized into two segments: 0–2 h and 4–8 h. Notably, samples with 0.5 and 1 h brining time were positioned in the upper right quadrant and were positively correlated with W1W, W2W and W5S, and these sensors were associated with terpenes and organosulfur compounds, aromatic compounds, sulfur and chlorine compounds, and nitrogen oxides. Moreover, the 2 h sample was in the upper left quadrant and was positively correlated with the W1C, W3C, and W5C, indicating that hydrocarbons, ammonia, and aromatic compounds contributed more to the flavor of this sample. Additionally, the 4 h and 6 h samples were located in the lower right quadrant and were associated with sensitivity to Aromatic components and organic sulfides (W3S) and sensitivity to hydrogen (W6S). Therefore, based on the E-nose analysis of the overall aroma of grass carp meat, the types of volatile flavor components were further identified and analyzed by GC-IMS.
3.4. E-tongue analysis
E-tongue simulates the function of the human tongue, which rapidly reflects the overall taste information of the samples (Chen et al., 2023). E-tongue identified taste variations in MGC subjected to different brining durations (Fig. 3E). Notably, the response values for sourness (AHS) and bitterness (SCS) were greater than those of umami (NMS), saltiness (CTS), and sweetness (ANS) after brining. Furthermore, the perception of umami, saltiness, sweetness, and bitterness reached their peak at 4 h, thereafter gradually diminishing. These results suggested that MGC of 4 h obtained richest flavor perception.
The PCA was given in Fig. 3F, where PC1 and PC2 accounted for 91.9 % and 4.8 % of the variance contribution ratio, respectively. The total contribution of PC1 and PC2 was 96.7 %, which indicated that these two principal components reflect the information of the sample very effectively. The graph indicated that the distances of 4 h and 8 h from 0 h were the farthest, signifying a significant change in flavors. Moreover, the distribution of loading factors in the right quadrant aligned with the sample distribution at 4 h and 8 h, suggesting a positive correlation between the samples at these time points and the taste perception values.
3.5. GC-IMS analysis
3.5.1. Qualitative analysis of flavor compounds
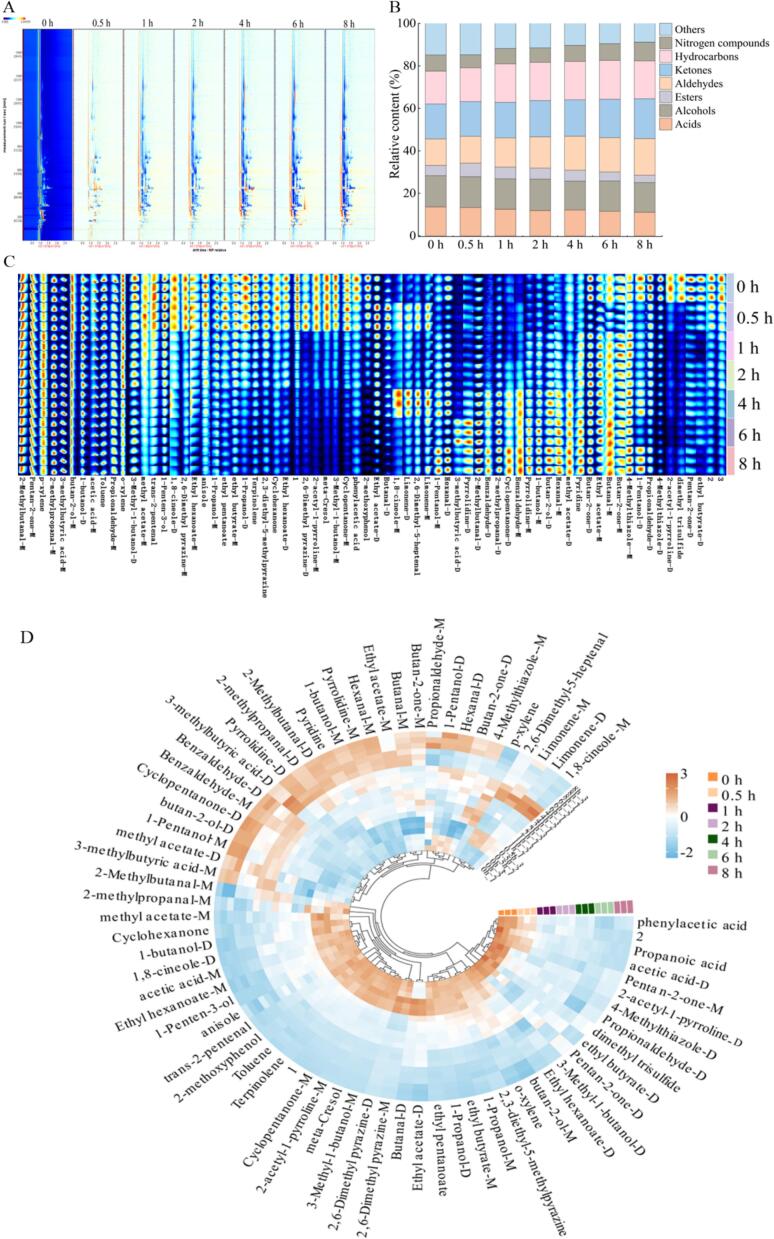
To further investigate the differences in volatile flavor components of grass carp at different stages of brining time, the MGC were determined by GC-IMS. In this research, 0 h were selected as a reference, and red indicated an increase in substance concentration, while blue indicated a decrease in the 2D spectra (Fig. 4A). The results showed that as the brining time lengthens, the discrepancies observed in the comparative graphs became progressively more substantial. This was particularly evident following a 4 h marinaded period, where there was a notable amplification in both the red and blue zones.
Fig. 4.
2D spectra of GC-IMS (A), flavor category stacked bar chart (B), characteristic fingerprint (C) and ring heat map of VOCs (D) of MGC with different brining time.
The relative contents of various volatile compounds were shown in Fig. 4B, where aldehydes, hydrocarbons, alcohols, ketones and other compounds were high in all MGC. Furthermore, a significant increase in the relative content of aldehydes, ketones and nitrogenous compounds with increasing brining time, while the relative content of acids and esters decreased. Similar findings were found in the study of Zhan et al. (2022) where brined chicken had the highest content of aldehydes, ketones and alcohols during brining, and the proportion of ketones tended to increase, and the variation in the content of these substances played an important role in the formation of the overall flavor of brined chicken. Therefore, the relative content of volatile flavor components was also one of the key factors affecting the formation of flavor in MGC, and the interaction of small molecular compounds with each other during the brining process produced different flavor profiles.
A total of 69 known volatile compounds and 3 unknown constituents were screened from the 7 sets of samples, of which the known constituents were mainly composed of 8 aldehydes, 4 ketones, 6 alcohols, 6 esters, 4 hydrocarbons, and 6 nitrogen-containing compounds (including dimers) (Fig. 4C-D). The flavor of MGC at 0.5 h and the flavor of the un-marinaded samples had a small difference, while the types and contents of flavor compounds changed significantly after 1 h. The results showed that the differences in the types and relative contents of flavor compounds such as phenylacetic acid, 3-methyl-1-butanol, ethyl hexanoate, propanol, anisole, methyl acetate, and 1-penten-3-ol, were not significant in the non-brined group and the sample brined for 0.5 h. However, with the increase of the brining time, glutaraldehyde, hexanal, 3-methylbutyric acid, cyclopentanone, 2-butanone, ethyl acetate, and butyraldehyde appeared gradually and their content increased gradually. Additionally, 2,6-Dimethyl-5-heptenal, Limonene-D, limonene-M and 1,8-cineole-M appeared higher only at 4 h.
3.5.2. Screening of key volatile flavor compounds
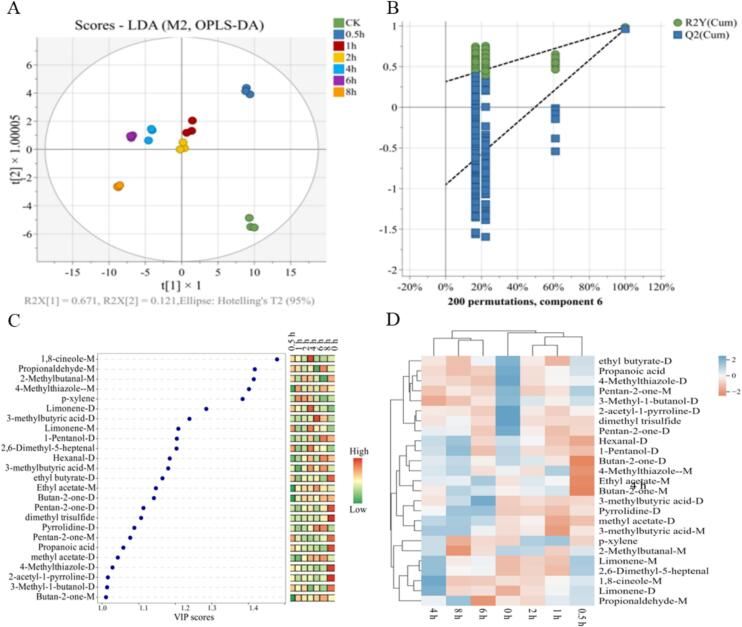
OPLS-DA was a supervised analysis method based on the partial least squares regression algorithm, which cloud effectively reduce the complexity of the data, visualize, discriminate analysis and prediction of the data, and made up for the deficiencies of the PCA analysis by predefined classifications in order to better determine the compositional variability of the volatile flavor components between samples (Kang et al., 2022). The relative content of the VOCs in Table S4 was used as the Y variable in the OPLS-DA model, whereas R2X and R2Y were used to denote the explanatory power of the X and Y matrices, and Q2 denoted the predictive power, where the closer R2 and Q2 were to 1.0, the better the predictive model was fitted. As shown in Fig. 5A-B, R2Y and Q2 were 0.98 and 0.96, and there was no overlap between the different MGC, indicating that the model was able to differentiate and predict the samples. The unmarinated group (0 h) was located in the lower right quadrant and far away from the other groups, indicating that the flavor profiles of the 0 h group were quite distinct from those of the marinated groups. In contrast, the 1 h and 2 h groups were positioned closely to each other on the flavor profile map, suggesting that they had similar flavor characteristics.
Fig. 5.
OPLS-DA score (A), cross-plot of 200 permutation tests (B), VIP score of MGC(C), and clustering heat map of MGC (D) for different brining time.
Variable important in the projection (VIP) was typically used in the analysis of key variables in the OPLS-DA model, VIP score values >1.0 were considered as key markers, and the larger values indicated that the key markers showed more significant flavor differences among samples at different brining time (Kang et al., 2022). As shown in Fig. 5C, a total of 21 key volatile flavor components (including dimers) were screened out, mainly including 2 alcohols, 4 esters, 4 aldehydes, 5 ketones, 3 acids, 2 alkanes, 4 nitrogen compounds and 1,8-cineole. The clustered heat map was shown in Fig. 5D, and the results indicated that the key volatile flavor substances could significantly differentiate between different brining time of MGC, mainly in the categories of 0 h, 0.5–2 h, and 4–6 h.
The ROAV was determined according to the relative content and threshold of compounds and was widely used in the analysis of key flavor (Ma et al., 2023). In this study, the lower threshold (0.1 μg/kg) and high relative content of 2-acetyl-1-pyrroline was set as ROAV value of 100.Within a certain range, the higher the ROAV value, the higher the contribution of the substance to the overall flavor of the MGC. Compounds with an ROAV ≥1 were considered as key flavor compounds, while those with 0.1 < ROAV <1 were thought to have a modifying effect on the flavor of the MGC (Guo et al., 2022). Due to its low threshold (0.1 μg/kg) and relatively high content, 2-acetyl-1-pyrroline was chosen as the key flavor substance for MGC, with an ROAVstan of 100. As shown in Table 2, a total of 18 flavor compounds with ROAV >1 was identified. Moreover, the ROAV value of key compounds increased with the extension of brining time, indicating that the key compounds were affected by marinating time and played an increasingly significant role in MGC.
Table 2.
ROAV of MGC at different marinating times.
| Compound | Threshold (μg/kg) | Odorant Description | ROAV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | 4 h | 6 h | 8 h | |||
| 3-Methyl-1-butanol | 4 | Rancid, pungent | 11.59 | 10.41 | 15.03 | 14.69 | 13.13 | 18.53 | 18.23 |
| Ethyl hexanoate | 2.3 | Fruity, strawberry | 1.42 | 1.32 | 2.15 | 2.14 | 2.13 | 2.49 | 2.38 |
| ethyl pentanoate | 5.9 | Orange, fruity | 1.01 | 1.09 | 1.83 | 1.61 | 1.73 | 2.01 | 2.29 |
| ethyl butyrate | 1 | Sweet,fruity | 9.90 | 9.86 | 18.17 | 16.33 | 18.17 | 20.34 | 21.85 |
| Ethyl acetate | 5 | Fruity, buttery | 4.58 | 7.43 | 11.17 | 9.22 | 13.01 | 11.24 | 8.34 |
| methyl acetate | 2 | Fruity, pineapple | 0.78 | 0.85 | 1.46 | 1.52 | 2.13 | 2.61 | 2.98 |
| Butanal | 8.2 | Penetrating,Spicy, grassy | 0.76 | 1.16 | 1.70 | 1.52 | 2.00 | 2.06 | 1.94 |
| 2-methylpropanal | 1.5 | Nutty, malty | 32.58 | 32.66 | 67.54 | 65.17 | 87.70 | 107.40 | 123.82 |
| Propionaldehyde | 15.1 | Floral, Pungent, Solvent | 1.15 | 1.00 | 2.01 | 1.86 | 2.53 | 2.60 | 3.62 |
| 2-Methylbutanal | 1 | Nutty,almond,Apple | 28.15 | 28.05 | 57.87 | 59.17 | 82.78 | 105.64 | 115.84 |
| Hexanal | 5 | Garlic, fresh, green, grassy, pungent, tallow, ishy | 1.97 | 1.31 | 3.51 | 3.94 | 6.24 | 6.79 | 10.61 |
| Limonene | 10 | Citrus, Mint | 0.53 | 0.94 | 1.58 | 1.38 | 3.05 | 2.57 | 2.75 |
| Toluene | 52.7 | Plastic,Sweet | 0.36 | 0.35 | 0.66 | 0.60 | 0.76 | 0.92 | 1.05 |
| 2,3-diethyl-5-methylpyrazine | 0.084 | Earth, Meat, Potato, Roast | 32.92 | 33.07 | 58.29 | 42.91 | 42.14 | 50.25 | 62.22 |
| 2-acetyl-1-pyrroline | 0.1 | Savory, Roast,Nutty | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 4-Methylthiazole | 55 | Green, Nut, Roasted Meat | 0.72 | 0.46 | 1.04 | 0.82 | 1.17 | 1.18 | 1.60 |
| 2-methoxyphenol | 1.6 | Nutty | 4.26 | 3.74 | 4.47 | 4.13 | 2.65 | 2.81 | 2.62 |
| 1,8-cineole | 1.1 | Camphor,Cool, Eucalyptol, Mint | 5.65 | 5.89 | 10.92 | 9.50 | 16.19 | 14.19 | 15.72 |
Note: The odor threshold values for these compounds were obtained using the Flavor-Base 10th Edition. (http://www.leffingwell.com/flavbase.htm). Odor descriptions were retrieved from Flavor Library | FEMA femaflavor.org).
3.5.3. Correlation analysis between key compounds and quality
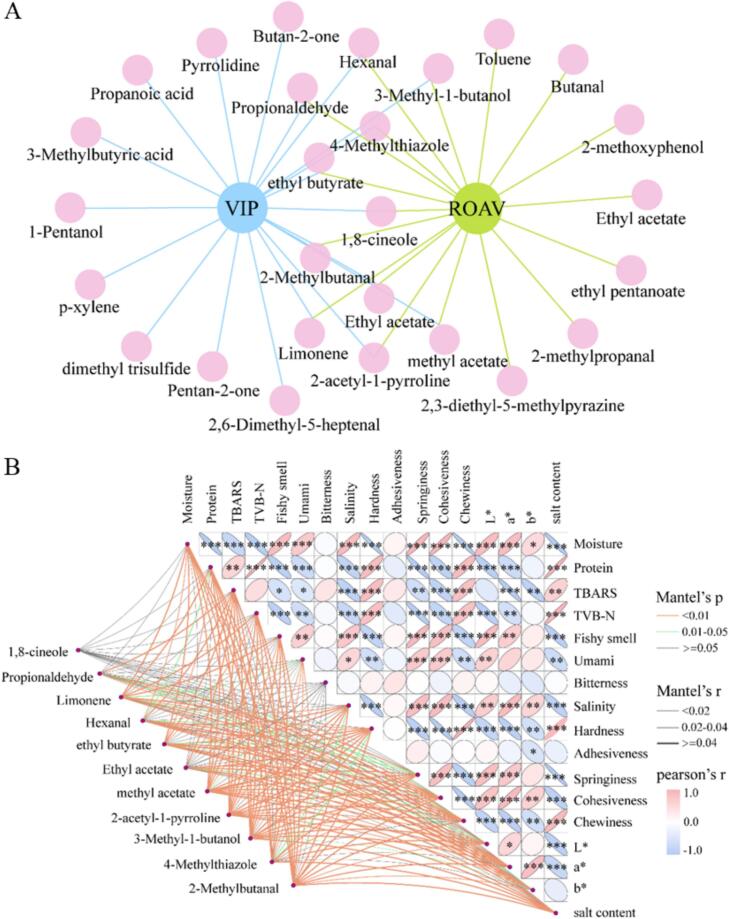
As shown in the venn diagram of Fig. 6A, a total of 11 characteristic flavor compounds were selected based on the conditions of VIP >1 and ROAV >1. The 11 potential flavors were 1,8-cineole, propionaldehyde, limonene, hexanal, ethyl butyrate, ethyl acetate, methyl acetate, 2-acetyl-1-pyrroline, 3-methyl-1-butanol, 4-methylthiazole and 2-methylbutanal, which possessed fruity, floral, minty, spicy, and roast meat aromas and played important roles in the flavor formation of the MGC. 1,8-cineole, as one of the characteristic aroma compounds in MGC, was originated from α-terpineol, a compound categorized as a terpenoid (Xi et al., 2024). Hexanal was produced by the oxidation of linoleic acid and linolenic acid (Chang et al., 2020),and played an important role in the flavor formation of MGC due to its low threshold value.
Fig. 6.
Wenn diagrams for key VOCs (A) and heat map of correlation combinations between key aroma compounds and quality factors (B) of MGC with different brining time.
The correlation between qualities and key flavor substances of MGC was illustrated in Fig. 6B, the results indicated that except for bitterness in the sensory, Adhesiveness in the texture and b* in the color, which had less effect on MGC, the rest of the qualities played an important role in determining the overall edible quality of MGC (p < 0.05). Notably, Moisture content significantly affected the physicochemical indices such as color, salt content, protein, TBARS and TVB-N of MGC. Chang et al. (2021) studied the effect of various frying conditions on quality properties of fried Spanish mackerel, which found that moisture significantly affected the color, texture, flavor and other properties of mackerel.
A mantel test analysis was conducted to analyze the relationship between quality and characteristic flavor compounds, which showed that all compounds, except for 1,8-cineole, have a certain influence on qualities (p < 0.05 & r > 0.04). In particular, there was a high correlation between 2-methylbutanol, 3-methyl-1-butanol, methyl acetate, 2-acetyl-1-pyrroline, 3-methyl-1-butanol, 4-methylthiazole and 2-methylbutanal and qualities. There has been a great deal of studies, which have demonstrated that during food processing, lipid oxidation, protein degradation, and Maillard reactions occur, which further promoted the production of flavor compounds (Yu et al., 2024). For example, leucine from protein degradation could further produce 3-methyl-1-butanol; linoleic acid from lipid oxidation could produce hexanal, and so on (Li et al., 2021). In conclusion, the qualities of MGC could directly affect its flavor formation, and the flavor substances could also change the edible quality of MGC.
4. Conclusion
This study explored the relationship between key VOCs and quality based on sensory assessment, physicochemical properties and VOCs changes. Key findings emphasized the role of brining time in modulating sensory characteristics, nutrient and oxidative stability, and improving product quality. Eleven key VOCs, including novel compounds such as 1,8-pinoresinol and 2-acetyl-1-pyrroline, were identified. These findings offer a fresh perspective on the biochemical pathways involved in fish product flavor formation and provide a scientific basis for targeted control of these compounds to optimize consumer acceptance and marketability. Strong correlations between specific VOCs and quality indicators like texture, color, and TBARS values provide the basis for precise quality control measures. This study provides a comprehensive framework for understanding MGC's unique flavor profile and highlights the need for future research on metabolic transformations in fish processing technologies. It has practical applications for improving quality of MGC and is of great importance to the food industry.
CRediT authorship contribution statement
Lu Zhang: Writing – review & editing, Supervision, Resources, Funding acquisition, Formal analysis. Yaqin Yu: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Qinhui Wen: Validation, Software. Shi Nie: Investigation, Data curation. Yang Hu: Methodology, Investigation. Chunming Tan: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation. Zongcai Tu: Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Key Research and Development Program of China, grant number 2022YFD2100902.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.102081.
Appendix A. Supplementary data
Table S1 Sensory evaluation criteria for MGC. Table S2 Sensors and corresponding representative sensitive material types of the PEN3 electronic nose. Table S3 Amino acid content of MGC. Table S4 The relative content of VOCs of GC-IMS analysis.
Data availability
Data will be made available on request.
References
- Ali Ghoflgar Ghasemi M., Hamishehkar H., Javadi A., Homayouni-Rad A., Jafarizadeh-Malmiri H. Natural-based edible nanocomposite coating for beef meat packaging. Food Chemistry. 2024;435 doi: 10.1016/j.foodchem.2023.137582. [DOI] [PubMed] [Google Scholar]
- Chang C., Wu G., Zhang H., Jin Q., Wang X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Critical Reviews in Food Science and Nutrition. 2020;60(9):1496–1514. doi: 10.1080/10408398.2019.1575792. [DOI] [PubMed] [Google Scholar]
- Chang L., Lin S., Zou B., Zheng X., Zhang S., Tang Y. Effect of frying conditions on self-heating fried Spanish mackerel quality attributes and flavor characteristics. Foods. 2021;10(1):98. doi: 10.3390/foods10010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Ning F., Zhao L., Ming H., Zhang J., Yu W., Yi S., Luo L. Quality assessment of royal jelly based on physicochemical properties and flavor profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue analyses. Food Chemistry. 2023;403 doi: 10.1016/j.foodchem.2022.134392. [DOI] [PubMed] [Google Scholar]
- Chen Q., Yang X., Hong P., Liu M., Li Z., Zhou C.…Liu S. GC-MS, GC-IMS, and E-nose analysis of volatile aroma compounds in wet-marinated fermented golden pomfret prepared using different cooking methods. Foods. 2024;13(3):390. doi: 10.3390/foods13030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., He S., Huang L., Lin S., Zhang M., Chi C., Chen H. Strategies to reduce fishy odor in aquatic products: Focusing on formation mechanism and mitigation means. Food Chemistry. 2024;444 doi: 10.1016/j.foodchem.2024.138625. [DOI] [PubMed] [Google Scholar]
- Dimakopoulou-Papazoglou D., Katsanidis E. Osmotic processing of meat: Mathematical modeling and quality parameters. Food Engineering Reviews. 2020;12(1):32–47. doi: 10.1007/s12393-019-09203-1. [DOI] [Google Scholar]
- Gao L., Zhang L., Liu J., Zhang X., Lu Y. Analysis of the volatile flavor compounds of pomegranate seeds at different processing temperatures by GC-IMS. Molecules. 2023;28(6):2717. doi: 10.3390/molecules28062717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Schwab W., Ho C.-T., Song C., Wan X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chemistry. 2022;376 doi: 10.1016/j.foodchem.2021.131933. [DOI] [PubMed] [Google Scholar]
- Han D., Zhang C.-H., Fauconnier M.-L., Mi S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and Duroc × (Landrac × Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Research International. 2020;130 doi: 10.1016/j.foodres.2019.108910. [DOI] [PubMed] [Google Scholar]
- Hu X., Li J., Zhang L., Wang H., Peng B., Hu Y., Liang Q., Tu Z. Effect of frying on the lipid oxidation and volatile substances in grass carp (Ctenopharyngodon idellus) fillet. Journal of Food Processing and Preservation. 2022;46(3) doi: 10.1111/jfpp.16342. [DOI] [Google Scholar]
- Jiang S., Xue D., Zhang Z., Shan K., Ke W., Zhang M.…Li C. Effect of sous-vide cooking on the quality and digestion characteristics of braised pork. Food Chemistry. 2022;375 doi: 10.1016/j.foodchem.2021.131683. [DOI] [PubMed] [Google Scholar]
- Kang C., Zhang Y., Zhang M., Qi J., Zhao W., Gu J., Guo W., Li Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chemistry. 2022;387 doi: 10.1016/j.foodchem.2022.132932. [DOI] [PubMed] [Google Scholar]
- Kim B.-S., Oh B.-J., Lee J.-H., Yoon Y.S., Lee H.-I. Effects of various drying methods on physicochemical characteristics and textural features of yellow croaker (Larimichthys Polyactis) Foods. 2020;9(2):196. doi: 10.3390/foods9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Deng N., Cai Y., Yang J., Ouyang F., Liu M., Wang J. Dynamic changes in postmortem quality of grass carp (Ctenopharyngodon idella) muscle: From the perspectives of muscle degradation and flavor evolution. Food Chemistry: X. 2024;23 doi: 10.1016/j.fochx.2024.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Qu S., Ma P., Zhang J., Zhao K., Chen L., Huang Q., Zou G., Tang H. Effects of chitosan coating combined with thermal treatment on physicochemical properties, bacterial diversity and volatile flavor of braised duck meat during refrigerated storage. Food Research International. 2023;167 doi: 10.1016/j.foodres.2023.112627. [DOI] [PubMed] [Google Scholar]
- Li P., Zhao W., Liu Y., Zhang A., Liu S., Song R., Zhang M., Liu J. Precursors of volatile organics in foxtail millet (Setaria italica) porridge: The relationship between volatile compounds and five fatty acids upon cooking. Journal of Cereal Science. 2021;100 doi: 10.1016/j.jcs.2021.103253. [DOI] [Google Scholar]
- Ma W., Zhu Y., Ma S., Shi J., Yan H., Lin Z., Lv H. Aroma characterisation of Liu-pao tea based on volatile fingerprint and aroma wheel using SBSE-GC–MS. Food Chemistry. 2023;414 doi: 10.1016/j.foodchem.2023.135739. [DOI] [PubMed] [Google Scholar]
- NFSS-2 . National food safety standard, determination of salt content in food. China Standards Press; 2016. National health and family planning commission of the People's Republic of China. GB 5009.42—2016. 2016. [Google Scholar]
- Pu X., Ruan J., Wu Z., Tang Y., Liu P., Zhang D., Li H. Changes in texture characteristics and special requirements of Sichuan-style braised beef for industrial production: Based on the changes in protein and lipid of beef. Foods. 2023;12(7):1386. doi: 10.3390/foods12071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M., Zheng M., Zhao W., Liu Q., Zeng X., Bai W. Effect of marinating and frying on the flavor of braised pigeon. Journal of Food Processing and Preservation. 2021;45(3) doi: 10.1111/jfpp.15219. [DOI] [Google Scholar]
- Rasul M., Majumdar B., Afrin F., Bapary M., Shah A.K.M. Biochemical, microbiological, and sensory properties of dried silver carp (Hypophthalmichthys molitrix) influenced by various drying methods. Fishes. 2018;3(3):25. doi: 10.3390/fishes3030025. [DOI] [Google Scholar]
- Senapati S.R., Kumar G.P., Singh C.B., Xavier K.A.M., Chouksey M.K., Nayak B.B., Balange A.K. Melanosis and quality attributes of chill stored farm raised whiteleg shrimp (Litopenaeus vannamei) Journal of Applied and Natural Science. 2017;9(1):626–631. doi: 10.31018/jans.v9i1.1242. [DOI] [Google Scholar]
- Shah M.A., Bosco S.J.D., Mir S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Science. 2014;98(1):21–33. doi: 10.1016/j.meatsci.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Shen C., Cai Y., Wu X., Gai S., Wang B., Liu D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC × GC-TOF-MS, GC-IMS, E-nose and E-tongue combined with chemometrics. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136257. [DOI] [PubMed] [Google Scholar]
- Si R., Wu D., Na Q., He J., Yi L., Ming L.…Ji R. Effects of various processing methods on the nutritional quality and carcinogenic substances of bactrian camel (Camelus bactrianus) meat. Foods. 2022;11(20):3276. doi: 10.3390/foods11203276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Fan L., Xu X., Xu R., Jia Q., Feng T. Aroma patterns characterization of braised pork obtained from a novel ingredient by sensory-guided analysis and gas-chromatography-olfactometry. Foods. 2019;8(3):87. doi: 10.3390/foods8030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li Z.J., Chao Y.Z., Wu Z.Q., Zhou M.X., Xiao S.T.…Zhe J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109456. [DOI] [PubMed] [Google Scholar]
- Turhan S., Ustun N.S., Altunkaynak T.B. Effect of cooking methods on total and heme iron contents of anchovy (Engraulis encrasicholus) Food Chemistry. 2004;88(2):169–172. doi: 10.1016/j.foodchem.2004.01.026. [DOI] [Google Scholar]
- Wang J., Yang P., Liu J., Yang W., Qiang Y., Jia W., Han D., Zhang C., Purcaro G., Fauconnier M.-L. Study of the flavor dissipation mechanism of soy-sauce-marinated beef using flavor matrices. Food Chemistry. 2024;437 doi: 10.1016/j.foodchem.2023.137890. [DOI] [PubMed] [Google Scholar]
- Wen J., Jiang W., Feng L., Kuang S., Jiang J., Tang L., Zhou X., Liu Y. The influence of graded levels of available phosphorus on growth performance, muscle antioxidant and flesh quality of young grass carp (Ctenopharyngodon idella) Animal Nutrition. 2015;1(2):77–84. doi: 10.1016/j.aninu.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Wang X., Hu P., Zhang Y., Li J., Jiang J., Zheng R., Zhang L. Research on flavor characteristics of beef cooked in tomato sour soup by gas chromatography-ion mobility spectrometry and electronic nose. LWT. 2023;179 doi: 10.1016/j.lwt.2023.114646. [DOI] [Google Scholar]
- Xi B.-N., Zhang J.-J., Xu X., Li C., Shu Y., Zhang Y., Shi X., Shen Y. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC–MS. Food Chemistry. 2024;435 doi: 10.1016/j.foodchem.2023.137547. [DOI] [PubMed] [Google Scholar]
- Xu N., Lai Y., Shao X., Zeng X., Wang P., Han M., Xu X. Different analysis of flavors among soft-boiled chicken: Based on GC-IMS and PLS-DA. Food Bioscience. 2023;56 doi: 10.1016/j.fbio.2023.103243. [DOI] [Google Scholar]
- Xu Y., Yan H., Xu W., Jia C., Peng Y., Zhuang X., Qi J., Xiong G., Mei L., Xu X. The effect of water-insoluble dietary fiber from star anise on water retention of minced meat gels. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111425. [DOI] [PubMed] [Google Scholar]
- Yang W., Shi W., Qu Y., Wang Z., Shen S., Tu L., Huang H., Wu H. Research on the quality changes of grass carp during brine salting. Food Science & Nutrition. 2020;8(6):2968–2983. doi: 10.1002/fsn3.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Cai Y., Liu D., Chen Y., Li J., Zhang M., Chen N., Zhang H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spec-trometry (HS-GC-IMS) Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.130989. [DOI] [PubMed] [Google Scholar]
- Yu D., Xu Y., Regenstein J.M., Xia W., Yang F., Jiang Q., Wang B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chemistry. 2018;242:412–420. doi: 10.1016/j.foodchem.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Yu X., Zhang W., Xin L., Xu S., Cheng J. Evaluation of flavor substances of rice bran kvass based on electronic nose and gas chromatography–mass spectrometry. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2024.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Wang G., Yin X., Ge C., Liao G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110696. [DOI] [PubMed] [Google Scholar]
- Zhan F., Sun L., Zhao G., Li M., Zhu C. Multiple technologies combined to analyze the changes of odor and taste in daokou braised chicken during processing. Foods. 2022;11(7):963. doi: 10.3390/foods11070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yu Y., Tan C., Nie S., Wen Q., Tu Z. Exploration of changes in sensory, physicochemical properties and microbial metabolic activities of grass carp meat with five thermal processing treatments during refrigerated storage. Food Chemistry: X. 2024;23 doi: 10.1016/j.fochx.2024.101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Zhang J., Wang S., Yu X., Zhang Q., Zhu C. Influence of heating temperatures and storage on the odor of duck meat and identification of characteristic odorous smell compounds. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2024.101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q., Xing Z., Teng F., Wu T., Pan S., Xu X. Evaluation of the aroma and taste contributions of star anise (I. Verum hook. F.) in braised duck leg via flavor omics combined with multivariate statistics. Food Research International. 2024;184 doi: 10.1016/j.foodres.2024.114209. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Xu Y., Xia W., Yu D., Wang B., Xu J. Insight into the role of lipids in odor changes of frozen grass carp (Ctenopharyngodon idella) based on lipidomics and GC–MS analysis: Impact of freeze-thaw cycles and heat treatment. Food Chemistry. 2024;459 doi: 10.1016/j.foodchem.2024.140436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sensory evaluation criteria for MGC. Table S2 Sensors and corresponding representative sensitive material types of the PEN3 electronic nose. Table S3 Amino acid content of MGC. Table S4 The relative content of VOCs of GC-IMS analysis.
Data Availability Statement
Data will be made available on request.