Abstract
Blended care therapy (BCT), which augments live, video-based psychotherapy sessions with asynchronous digital tools, has the potential to increase access to evidence-based treatments for posttraumatic stress disorder (PTSD). However, its effectiveness in diverse, real-world settings is not well-understood. This evaluation aimed to assess clinical outcomes of a BCT program for PTSD symptoms. A retrospective cohort analysis was conducted of 199 adults who received an employer-offered BCT program for PTSD symptoms that delivered either cognitive processing therapy or prolonged exposure. PTSD symptom severity was regularly assessed using the PTSD Checklist for DSM-5 (PCL-5). Growth curve models were used to evaluate the trajectory of PTSD symptoms over the course of care, and an interaction term was added to assess outcomes by baseline PTSD symptom severity (i.e., PCL-5 ≥ 31 versus PCL-5 < 31). End-of-care reliable improvement and recovery were evaluated. On average, participants with baseline PCL-5 < 31 exhibited statistically significant declines in PTSD symptoms during care, while participants with baseline PCL-5 ≥ 31 showed statistically significantly steeper initial declines in PTSD symptoms that became less pronounced over time. Overall, 82.91% of participants demonstrated either reliable improvement or recovery in PTSD symptoms. This evaluation suggests BCT for PTSD symptoms can be beneficial in real-world settings. Future research should perform large-scale evaluations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83144-6.
Keywords: Posttraumatic stress disorder, Depression, Mental health, Psychotherapy, Telehealth, Digital health
Subject terms: Psychology, Health care, Outcomes research
Introduction
Worldwide, approximately 70% of adults experience a traumatic event across their lifetime and the estimated lifetime prevalence of posttraumatic stress disorder (PTSD) in trauma-exposed adults is 5.6%1. In the U.S alone, one study estimated that over 14 million civilian adults experience PTSD across their lifetime2. PTSD is associated with debilitating outcomes, including higher occurrences of disability and suicidal behaviors2–5. PTSD also results in substantial economic costs, with excess costs of $34.8 billion that are attributable to PTSD-related presenteeism and absenteeism in the U.S6. Despite its significant burden, the estimated past-year PTSD treatment prevalence was only 24% among U.S. civilians with lifetime PTSD7. When evaluating lifetime treatment prevalence, it is estimated PTSD goes untreated in 40.6% of U.S. civilian adults and treatment seeking is delayed by an average of 4.5 years among those who do eventually receive care2.
Research supports the effectiveness of evidence-based care, such as Cognitive Processing Therapy (CPT) or Prolonged Exposure (PE), for the treatment of PTSD symptoms8–11, as well as for symptoms of depression in adults with PTSD12,13. Treatment efficacy has been observed in those with clinically elevated levels of PTSD, as well as those with subclinical symptoms who are experiencing distress14. However, there exist significant barriers to receiving evidence-based care for PTSD15. For one, there is an insufficient number of providers who are able to provide specialized care such as CPT and PE15, and PTSD treatment is less readily available in community and private settings, particularly relative to the U.S. Department of Veterans Affairs (VA) Healthcare system15. Time constraints, affordability, transportation issues, and the stigma of seeking care for PTSD symptoms are also barriers to care16–19. PTSD symptoms, particularly avoidant behaviors (e.g., avoiding symptom triggers), are also factors that prevent treatment-seeking16,19. Given the significant and impairing consequences of PTSD, there is a strong need for treatment models that can better meet the care needs of impacted populations.
Evidence-based PTSD treatment delivered via telehealth show promise in mitigating common treatment barriers (e.g., travel-related issues)15,20, show comparable effectiveness to in-person care for PTSD15,21, and report similar levels of treatment satisfaction21,22. While internet-based interventions for PTSD symptoms (e.g., internet-based cognitive behavioral therapy [iCBT]) have also been found to be effective and could increase the accessibility of treatment23, as a standalone care option, dropout rates for internet-based interventions can be high24. Additionally, treatment effect sizes are larger in iCBT interventions for PTSD that include therapist involvement compared to those without therapist involvement23. Internet-based interventions that are delivered in between therapy sessions could increase the benefits of therapy; for example, they could facilitate the application of skills in day-to-day life25. Unsurprisingly, internet-based tools have been proposed as an approach to deliver between-session support for those receiving telehealth-based care for PTSD26. Altogether, this evidence provides support for a care model that blends therapy with digital tools in order to maximize treatment benefits.
In light of possible benefits as an effective and efficient care delivery model, blended care therapy that combines face-to-face therapy with digital tools has been evaluated for several mental disorders25. A growing body of literature has associated blended care therapy with positive clinical outcomes for common mental disorders25,27–29. There is also evidence that a blended care model can be effective and efficient for PTSD symptoms30,31. For instance, a recent study among veterans who were exposed to trauma found evidence-based blended care therapy for PTSD (i.e. weekly therapy sessions and digital modules) was associated with significantly lower PTSD and depression symptoms at the end of care and at 3-month follow-up30, providing preliminary support for this treatment approach.
Although research has reported positive outcomes for blended care therapy, the evidence is limited for blended care programs treating PTSD symptoms, particularly in populations outside of the VA. Therefore, the primary objective of this real-world evaluation was to evaluate changes in PTSD symptoms experienced by clients participating in an evidence-based blended care trauma treatment program delivered via telehealth. Among clients also presenting with clinically elevated depression symptoms, a secondary objective was to assess end-of-care changes in depression symptoms. This study preliminarily hypothesized participants receiving the evidence-based blended care trauma treatment program would experience significant improvements in clinical symptoms over the course of care and at the end of care.
Results
Table 1 describes participants’ baseline characteristics. On average, participants completed 7.97 (SD: 2.95) therapy sessions, and the average duration of care in the BCT Trauma program was 9.98 (SD: 4.46) weeks. Overall, 77.39% of participants attended all scheduled therapy sessions, 19.60% missed one session, 2.51% missed two sessions, and 0.50% missed three sessions. Upon stratification by treatment type, 78.18% of participants who received CPT completed all scheduled therapy sessions, and 76.40% of participants who received PE completed all scheduled therapy sessions.
Table 1.
Participant characteristics (N = 199).
| Participant characteristics | Distribution |
|---|---|
| Age, mean (SD) | 34.07 (9.26) |
| Gender, n (%) | |
| Female | 170 (85.43) |
| Male | 23 (11.56) |
| Other/missing | 6 (3.02) |
| Race and ethnicity, n (%) | |
| White | 108 (54.27) |
| Hispanic or Latino | 24 (12.06) |
| Asian or Pacific Islander | 15 (7.54) |
| Black or African American | 10 (5.03) |
| Multiple | 27 (13.57) |
| Other | 10 (5.03) |
| Prefer not to disclose/unknown | 5 (2.51) |
| Employee status, n (%) | |
| Employee | 155 (77.89) |
| Dependent | 32 (16.08) |
| Missing | 12 (6.03) |
| Baseline PCL-5 ≥ 31, n (%) | 112 (56.28) |
| Baseline PHQ-9 (n = 185) | |
| Baseline PHQ-9 ≥ 10, n (%) | 74 (40)a |
| BCT trauma program characteristics | |
| Received cognitive processing therapy, n (%) | 110 (55.28) |
| Received prolonged exposure, n (%) | 89 (44.72) |
| No. of therapy sessions completed, median [Q1,Q3] | 8.00 [6.00,10.00] |
| Duration of care (week), median [Q1,Q3] | 9.57 [7.00,13.57] |
PHQ-9 patient health questionnaire-9, PCL-5 PTSD checklist for DSM-5, BCT blended care therapy, SD standard deviation.
aPercent among those with PHQ-9 only.
PTSD symptoms
Paired samples t tests
Participants reported significant improvements in PTSD symptoms from pre-to post-treatment. In the entire sample, the mean PCL-5 scores changed from 34.39 to 18.29 (MD = 16.10, SDD = 14.92; p < .001; Hedge’s g = 1.07; Table 2). Among participants with baseline PCL-5 < 31, mean PCL-5 scores changed from 20.24 to 12.00 (MD = 8.24, SDD = 10.75; p < .001; Hedge’s g = 0.76). Among participants with baseline PCL-5 ≥ 31, mean PCL-5 scores changed from 45.38 to 23.18 (MD = 22.20, SDD = 14.90; p < .001; Hedge’s g = 1.48).
Table 2.
Changes in PTSD and depression symptoms from pre-to-post the BCT trauma program.
| Samples | Sample size | Baseline score, mean (SD) | Final score, mean (SD) | Paired differences, mean (SD) | 95% CI of the difference | T-value (df) | Hedges’s g |
|---|---|---|---|---|---|---|---|
| Outcome: PTSD symptoms (PCL-5) | |||||||
| Entire sample | 199 | 34.39 (15.32) | 18.29 (14.76) | 16.1 (14.92) | 14.01 to 18.18 | 15.21 (198)*** | 1.07 |
| Baseline PCL-5 ≥ 31 | 112 | 45.38 (10.35) | 23.18 (15.4) | 22.2 (14.9) | 19.41 to 24.99 | 15.77 (111)*** | 1.48 |
| Baseline PCL-5 < 31 | 87 | 20.24 (6.51) | 12.0 (11.14) | 8.24 (10.75) | 5.95 to 10.53 | 7.15 (86)*** | 0.76 |
| Outcome: depression symptoms (PHQ-9) | |||||||
| Baseline PHQ-9 ≥ 10 | 74 | 14.15 (3.69) | 8.86 (5.25) | 5.28 (5.08) | 4.11 to 6.46 | 8.95 (73)*** | 1.03 |
PHQ-9 patient health questionnaire-9, PCL-5 PTSD checklist for DSM-5, CI confidence interval, df degrees of freedom.
***p < 0.001.
Reliable improvement, recovery, clinically meaningful improvement
Overall, 165 participants (82.91%) experienced either reliable improvement or recovery in PTSD symptoms, and 129 (64.82%) experienced clinically meaningful improvement (Table 3). Among the 112 participants (56.28%) with PTSD symptoms above the clinical cutoff at baseline, 102 (91.07%) experienced reliable improvement or recovery, and 88 (78.57%) had clinically meaningful improvement in their PTSD symptoms by the end of care.
Table 3.
Reliable improvement, recovery, and clinically meaningful improvement.
| Samples | Sample size | Reliable improvementa | Recoveryb | Reliable improvement or recoveryc | Reliable improvement and recoveryd | Clinically meaningful improvemente |
|---|---|---|---|---|---|---|
| Outcome: PTSD symptoms (PCL-5) | ||||||
| Entire Sample | 199 | 164 (82.41) | – | 165 (82.91) | – | 129 (64.82) |
| Baseline PCL-5 ≥ 31 | 112 | 101 (90.18) | 80 (71.43) | 102 (91.07) | 79 (70.54) | 88 (78.57) |
| Outcome: depression symptoms (PHQ-9) | ||||||
| Baseline PHQ-9 ≥ 10 | 74 | 37 (50.00) | 45 (60.81) | 51 (68.92) | 31 (41.89) | – |
PHQ-9 patient health questionnaire-9, PCL-5 PTSD checklist for DSM-5.
aReliable improvement: ≥ 5-point decrease on the final PCL-5; or ≥ 6-point decrease on the final PHQ-9.
bRecovery: Final PCL-5 < 31 among those with baseline PCL-5 ≥ 31; or final PHQ-9 < 10.
cReliable improvement or recovery: ≥ 5-point decrease on the final PCL-5 or final PCL-5 < 31 among those with baseline PCL-5 ≥ 31; or ≥ 6-point decrease on the final PHQ-9 or final PHQ-9 < 10.
dReliable improvement and recovery: ≥ 5-point decrease on the final PCL-5 and final PCL-5 < 31 among those with baseline PCL-5 ≥ 31; or ≥ 6-point decrease on the final PHQ-9 and final PHQ-9 < 10.
eClinically meaningful improvement: ≥ 10-point decrease on the final PCL-5.
Growth curve models
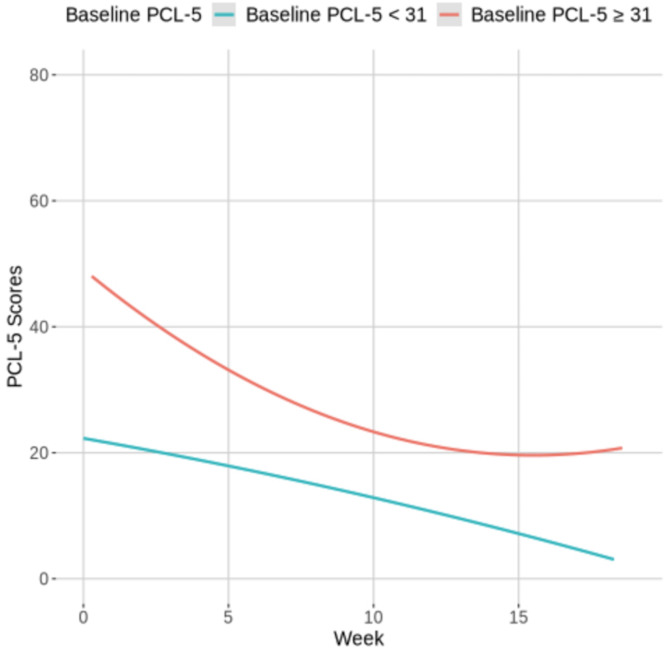
Coefficients for Model 1 are provided in the left column of Table 4. The intercept term (b = 25.20, p < .001) describes the average PCL-5 score at week 0 for participants reporting subclinical symptoms (i.e., baseline PCL-5 < 31), and the coefficient for the baseline symptom severity (b = 21.76, p < .001) describes the difference in average PCL-5 scores at week 0 for participants with baseline symptoms in the clinical range (i.e., baseline PCL-5 ≥ 31). The linear effect Week (b = − 2.53, p < .001) indicates that on average, participants exhibited a significant initial decrease in symptoms at a rate of more than 2 units of PCL-5 each week. In addition, the quadratic effect Week2 (b = 0.06, p < .001) suggests that the rate of decline diminished over time.
Table 4.
Growth curve modeling results of PTSD symptoms (PCL-5), b (95% confidence interval).
| Model 1 | Model 2 | |
|---|---|---|
| Intercept |
25.20 (22.99, 27.41) t = 22.34*** |
22.29 (19.90, 24.68) t = 18.31*** |
| Baseline PCL-5 ≥ 31 (Ref: PCL < 31) |
21.76 (19.09, 24.42) t = 16.00*** |
26.82 (23.67, 29.96) t = 16.73*** |
| Week |
− 2.53 (− 3.09, − 1.98) t = − 8.95*** |
− 0.81 (− 1.61, − 0.01) t = − 1.99* |
| Week2 |
0.06 (0.03, 0.10) t = 3.82*** |
− 0.01 (− 0.06, 0.04) t = − 0.51 |
| Week * Baseline PCL-5 ≥ 31 |
− 2.99 (− 4.04, − 1.95) t = − 5.64*** |
|
| Week2 * Baseline PCL-5 ≥ 31 |
0.14 (0.07, 0.20) t = 4.11*** |
|
| Deviance (− 2*Log likelihood) | 10,443.05 | 10,406.47 |
| Akaike inf. crit | 10,465.05 | 10,432.47 |
| Bayesian inf. crit | 10,523.04 | 10,501.01 |
PCL-5: PTSD Checklist for DSM-5. Baseline PCL-5 indicator is coded: 0 = PCL-5 < 31, 1 = PCL-5 ≥ 31. Model 1 residual degrees of freedom = 1428, and Model 2 residual degrees of freedom = 1426. Likelihood ratio test: 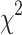 (2) = 36.57, p < 0.001.
(2) = 36.57, p < 0.001.
*p < 0.05; **p < 0.01; ***p < 0.001.
Coefficients for Model 2 are provided in the right column of Table 4, and Fig. 1 visually presents changes in PCL-5 scores by baseline PCL-5 severity. The presence of a Week*Baseline PCL-5 ≥ 31 interaction (bModel 2 = − 2.99, p < .001) indicates that participants with baseline symptoms in the clinical range exhibited a significantly steeper initial decline in PCL-5 scores. In addition, the interaction with the quadratic effect of time was significant (Week2*Baseline PCL-5 ≥ 31, b = 0.14, p < .001). The Week (bModel 2 = − 0.81, p < .05) and Week2 (b = − 0.01, p = .611) coefficients describe the predicted trajectory for participants with baseline PCL-5 < 31, demonstrating that PCL-5 scores significantly decreased during each week in care among participants with subclinical symptoms. Follow-up simple slopes analysis32 confirmed that the expected change in PTSD symptoms among those with baseline PCL-5 ≥ 31 was characterized by a much steeper initial decline (Week b = − 3.81, p < .001) and a more rapid recovery trajectory (Week2 b = 0.12, p < .001). The inclusion of age, gender, race and ethnicity, and treatment type (CPT or PE) as fixed effects in Model 2 did not improve model fit and were excluded from the final model (Supplementary Table S1). However, Supplementary Fig. S1 provides an exploratory visual presentation of growth curve modeling results of PCL-5 scores over the course of care by treatment type.
Fig. 1.
Changes in PCL-5 scores by baseline PCL-5 severity: visual presentation of growth curve modeling results. PCL-5 PTSD checklist for DSM-5. The growth curve model included baseline PCL-5 severity (PCL-5 ≥ 31 vs. < 31), and its interactions with week and week2 as fixed effects.
Depression symptoms
Among participants with PHQ-9 data (n = 185), 74 (40.0%) reported clinically elevated depression symptoms (PHQ-9 ≥ 10) at the beginning of the BCT Trauma program. The proportion of participants with clinically elevated depression symptoms was significantly higher among participants with baseline PCL-5 ≥ 31 (n = 65 out of 105, or 61.90%) compared to participants with baseline PCL-5 < 31 (n = 9 out of 80, or 11.25%; McNemar’s χ2(1) = 9.0, p < .001).
In the sub-sample with PHQ-9 ≥ 10 at the beginning of the BCT Trauma program, pre-post improvements were statistically significant (Table 2), with mean PHQ-9 scores changing from 14.15 to 8.86 (MD = 5.28, SDD = 5.08; p < .001; Hedge’s g = 1.03). In addition, 51 (68.92%) experienced reliable improvement or recovery in depression symptoms (Table 3).
PHQ-9 score changes over the entire course of care were also evaluated by including non-trauma focused therapy sessions before starting the BCT Trauma program. Overall, 100 (50.25%) participants reported elevated depression symptoms at the beginning of treatment (Supplementary Table S2). In this subset, 75 (75.00%) had reliable improvement or recovery by the end of care (inclusive of sessions prior to the BCT Trauma program). The changes in PHQ-9 over the entire treatment were statistically significant, and the mean PHQ-9 scores changed from 15.48 to 7.93 (MD = 7.55, SDD = 5.51; p < .001; Hedge’s g = 1.36).
Discussion
This evaluation assessed the clinical outcomes of a BCT program for PTSD symptoms that was offered as a mental health benefit by employers. Overall, 82.91% of participants experienced either reliable improvement or recovery in PTSD symptoms by the end of care, and this proportion was even higher (91.07%) in the subset with clinically elevated PTSD symptoms at the beginning of care. Similarly, participants with clinically elevated PTSD symptoms at baseline exhibited steep initial declines in symptoms during the early stages of treatment that became less steep over time, whereas participants with lower PTSD symptoms at baseline exhibited a more modest rate of decline in symptoms that remained consistent across treatment. These results provide evidence that a BCT Trauma Program can be beneficial among participants presenting with PTSD symptoms, including those whose baseline symptoms are subclinical yet may be experiencing distress related to PTSD symptoms.
Prior research on treatment for PTSD symptoms delivered using a blended care model has reported significant reductions in PTSD and depression symptoms among veterans30,31. A primary differentiator of the current study was positioning therapists in the lead of overall care with digital tools and activities supporting client practice, while other studies have primarily focused on digital module-delivered treatment with therapist support. The current study extends the previous literature by evaluating outcomes among participants receiving CPT or PE within a blended care model outside of a VA population. Importantly, in this study, the inclusion of treatment type as a variable in growth curve models did not improve model fit, suggesting either PE or CPT can be effective.
A high rate of participants completed all scheduled therapy sessions in this study (77.39%). In addition, completion rates (i.e., no missed scheduled therapy sessions) were high for both CPT (78.18%) and PE (76.40%). The completion rates observed in this study are in contrast to the literature that has generally reported lower retention for evidence-based PTSD treatments in real-world settings33,34. Because greater baseline PTSD symptom severity may contribute to dropout33,35, one potential factor explaining the high completion rates in this study could be the inclusion of participants with subclinical levels of PTSD symptoms. At the same time, although research suggests comparable attrition between in-person, face-to-face and video-based delivery of evidence-based PTSD treatment22, logistical barriers (e.g., travel) may still contribute to dropout15,33. As such, the high completion rates in this study could be at least partially attributable to the remote delivery of this program. It is also a strong possibility that the between-session support provided to clients through digital tools and provider feedback may have encouraged treatment completion. In order to inform real-world efforts to improve completion of evidence-based PTSD treatments, further research is needed to elucidate the factors contributing to the high completion rates observed in this evaluation.
This study suggests video-delivered BCT for PTSD can be an effective approach for delivering evidence-based care for PTSD symptoms. The effect size for pre-post change in PTSD symptoms was large (g = 1.07), and even larger in the subset with clinically elevated PTSD symptoms at baseline (g = 1.48). The latter effect size, in particular, was comparable to benchmarks estimated from randomized controlled trials of PE and CPT (intent-to-treat g’s = 1.38 and 1.69, respectively)36. Additionally, in this study the effect size for end-of-care depression outcomes (g = 1.03) was comparable to benchmarks for PE and CPT depression outcomes (intent-to-treat g’s = 0.97 and 0.91, respectively)36. As expected, when assessing depression outcomes over the entire course of care by including standard BCT sessions completed prior to starting the BCT Trauma program, the effect size for pre-post change in PHQ-9 scores over the entire treatment course was even larger (g = 1.36).
Strengths of the current study include the evaluation of clinical outcomes under real-world conditions and having an inclusive sample with regards to index trauma as well as to race and ethnicity. This study also assessed PTSD and depression symptoms using validated and reliable measures of symptom severity. Results may also be more generalizable to civilian populations compared with prior evaluations performed only among veterans. However, this study had several limitations. For one, as with any program that serves individuals seeking treatment, regression to the mean effects may also be contributing to some of the observed symptom reduction. In addition, although validated measures were used to assess PTSD and depression symptoms, structured clinical interviews for diagnoses were not conducted. Because the sample size was small and the program was delivered to employed populations and/or their dependents, further research is needed to examine whether results are generalizable. Finally, although consultation groups ensured model fidelity through in-depth session reviews, no formal fidelity measures were used in this evaluation.
In summary, in light of the substantial consequences associated with PTSD, such as the high economic burden (e.g., excess costs of $19,630 per person suffering from PTSD)6, increasing the accessibility of evidence-based treatment is essential. This real-world evaluation provides evidence for the clinical benefits of CPT and PE that are delivered completely virtually using a blended care model. These findings provide compelling data to encourage research on the potential role of BCT in improving access to evidence-based care for PTSD symptoms. More specifically, future research could assess whether BCT for PTSD symptoms could be applied to other clinical mental health programs to expand the reach of services to additional populations. Further research is warranted on how to optimize the quantity of care components (e.g., therapy sessions, digital content), particularly across severity levels, and a long-term evaluation to assess the durability of findings. Finally, because it is important to evaluate the scalability of such programs across different contexts, future research should assess outcomes of BCT for PTSD in large-scale, diverse populations.
Methods
Design and participants
Design
The present study used a retrospective cohort design. Participants were self-referred employees or their dependents who received blended care therapy (BCT) for PTSD symptoms (hereafter referred to as the BCT Trauma program) from Lyra Clinical Associates as an employer-offered mental health benefit. This analysis of de-identified data was determined to be exempt by the WCG Institutional Review Board (Puyallup, WA). All methods were performed in accordance with the relevant guidelines and regulations. Participants provided informed consent to take part in care and have their de-identified data used for research purposes as a part of that consent for care.
Participants
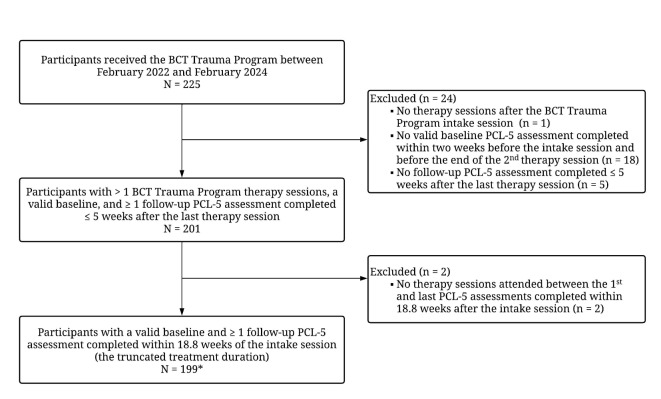
This study included participants who received the BCT Trauma program between February 2022 and February 2024. The initial sample included 225 participants. We excluded 1 participant who did not receive treatment after the intake session, 18 participants who did not complete the first PTSD symptom outcome assessment (i.e., PTSD Checklist for DSM-5 (PCL-5)37) within 14 days before the intake session and before the end of the second therapy session, and 5 participants who only submitted one PCL-5 assessment ≤ 5 weeks after the last treatment session (see Fig. 2 for details). To evaluate the impact of the BCT Trauma program over a typical course of treatment, we removed assessments collected > 18.8 weeks after the intake session (corresponding to mean + 1 standard deviation (SD) of the program treatment duration). Participants must also have received ≥ 1 therapy session between the first and last assessments, and as a result, 2 participants were removed. End-of-care assessment scores were the final valid assessment after baseline. The final sample included 199 participants, 105 (52.76%) of whom received non-trauma focused therapy sessions prior to starting the BCT Trauma Program.
Fig. 2.
Participant flowchart. BCT Blended care therapy. PCL-5 PTSD checklist for DSM-5. *Of the final sample, 105 (52.76%) received therapy sessions prior to starting the BCT trauma program.
Blended care therapy trauma program
The BCT Trauma Program was provided to clients who had experienced a criterion A traumatic stressor38 and were struggling with symptoms of PTSD. During the intake, information regarding the experience(s) of trauma were paired with assessments (e.g., PCL-5, Patient Health Questionnaire-9 (PHQ-9)39) to determine severity of PTSD symptoms and evaluate eligibility and fit with the program. The program did not have criteria to exclude participants due to PTSD symptom severity and included those presenting with mild to severe PTSD symptoms. Eligible clients began trauma-specific treatment (i.e., CPT or PE)40,41, which comprised live, video-based psychotherapy sessions with a therapist. Based upon the availability of providers in their state of residence, participants were offered either CPT or PE. Treatment was delivered by licensed therapists (e.g., licensed clinical psychologists, licensed clinical social workers, or licensed professional counselors) who were extensively vetted and underwent a BCT Trauma training program.
Modifications to CPT and PE based on existing research suggest the effectiveness of brief protocols42,43. Standard length of treatment was set at nine sessions (including the intake), with the flexibility to adjust treatment episode length based on treatment responsiveness in consultation with an expert trauma consultant. Clinicians used CPT, which excludes a trauma narrative. To fit the shortened protocol, the Challenging Beliefs Worksheet (CBW) and an abbreviated list of challenging questions were utilized similar to those supported in research in the CPT treatment manual40. Content related to the patterns of problematic thinking were removed, and therapists were encouraged to select three of the five CPT themes (Safety, Trust, Power/Control, Self-Esteem, Intimacy) for the final three sessions. Empirical support for modified CPT protocols can be found in the research literature42,44. For PE, modifications included shortening of the duration of the sessions (60 min vs. 90 min), which is supported as non-inferior in the literature45, removal of the breathing retraining in the first session, and shortened imaginal exposures (20–25 min vs. 30–45 min) during the actual session time45.
Therapy sessions were supported by between-session therapist-client messaging, the assignment of relevant digital tools that clients could access and use between sessions, and therapist-administered feedback on these assignments. Digital tools consisted of digital exercises and assessments that were based on CPT and PE protocols40,41. Examples of principles and skills that were reinforced through digital tools include explorations of trauma-related beliefs, understanding traumatic events and traumatic stress, and exposure to trauma-related reminders. Providers could also assign digital video lessons that were developed using transdiagnostic treatment approaches46–48, which have been described previously29.
Video-based therapy sessions with therapists, digital tools, and assessments were completed on a secure, HIPAA-compliant online platform developed by Lyra Health.
Blended care trauma training program
A training protocol in line with gold standard models for psychotherapy training programs was implemented as described below44. Providers from both groups (PE and CPT) attended a didactic training on trauma assessment (e.g., identifying Criterion A traumatic stressors, using the PTSD Checklist for DSM-5). Providers then attended either the PE or CPT didactic training. Instruction included in-depth explanations of how to implement each psychotherapy with fidelity as well as break-out sessions to practice elements of each therapy. Best practices for implementing PE or CPT using Lyra Health’s blended care digital tools were also discussed.
Providers were then assigned to consultation groups comprised of 1 PE or CPT subject matter expert and 6–7 providers. Consultation groups initially met weekly for 6–8 weeks and then bi-weekly for 2 months. Consultation groups ensured ongoing model fidelity by providing in-depth, ad hoc session reviews.
Measures
PTSD symptoms
The PCL-5 was assigned weekly (before sessions) to assess participants’ self-reported PTSD symptoms37. The PCL-5 is a 20-item measure based on the DSM-5 criteria that assesses the presence and severity of PTSD symptoms over the past-month. Responses were rated on a Likert-type scale, ranging from 0 (Not at all) to 4 (Extremely). Items were summed for a total severity score (range: 0 to 80). A PCL-5 score of 31 was previously found to be an optimal cutoff to identify those with a PTSD diagnosis49, and the current study used scores ≥ 31 to identify those with clinically elevated PTSD symptoms. Recovery was defined as falling below the clinical threshold at the end of care (final PCL < 31). The minimal threshold for reliable improvement (i.e., reliable change) was 5 points and the threshold for clinically meaningful change was 10 points50.
Depression symptoms
Based on providers’ assessment of participants’ initial depression symptoms levels, the Patient Health Questionnaire-9 (PHQ-9) was assigned before sessions on a weekly basis (n = 185 out of 199 participants)39. A score ≥ 10 was considered clinically elevated depression symptoms39, and recovery was defined as an end-of-care score below the clinical threshold (final PHQ-9 < 10). A score reduction of ≥ 6 points was considered reliable improvement51. End-of-care PHQ-9 scores were the final valid assessments that were completed along with the final valid PCL-5 assessments.
Demographics
Participants self-reported age, gender, and race and ethnicity. For analysis, participants who selected more than one category of race or ethnicity were re-categorized as “Multiple,” and those who selected “Native Hawaiian or Other Pacific Islander” or “American Indian or Alaska Native” were re-categorized as “Other” due to small sample sizes.
Statistical analysis
The clinical impact of the BCT Trauma program on the primary outcomes of PTSD symptoms was evaluated using the following methods: assessing the proportion of participants who exhibited reliable improvement and/or recovery, as well as clinically meaningful improvement; changes in PTSD symptoms from pre- to post-treatment using paired samples t-tests; and changes in PTSD symptoms over the course of treatment using growth curve analysis52,53. Participants with at least two valid outcome measures, regardless of program completion status, were included in these analyses.
Specifically, a pair of three-level (assessments nested under participants who were further nested under therapists) growth curve models were examined. Model 1 included linear (Week) and quadratic (Week2) fixed effects representing the amount of time between the intake session (Week = 0) and each PCL-5 assessment, as well as a random intercept and random effects of the linear and quadratic terms of time for participants. Because pre-treatment PTSD symptom severity may be linked to the strength of treatment outcomes54, baseline PTSD symptom severity represented by a dummy-coded indicator (i.e., 0 = baseline PCL-5 < 31; 1 = baseline PCL-5 ≥ 31) was also included as a fixed effect. Model 2 incorporated the interaction terms involving participants’ baseline severity and the two effects of time (linear, quadratic). An additional model was examined by adding participants’ age, gender, race and ethnicity, as well as the treatment type received (CPT or PE) as fixed effects to Model 2.
As a secondary outcome, among participants with clinically elevated depression symptoms at baseline (PHQ-9 ≥ 10), the proportion who achieved reliable improvement and/or recovery on the PHQ-9 was calculated and mean changes from pre-to post-treatment were evaluated using paired samples t-tests. As previously mentioned, prior to starting the BCT Trauma program, more than half of participants (n = 105; 52.76%) received non-trauma focused therapy sessions. Therefore, we also evaluated changes on the PHQ-9 across the entire treatment period, inclusive of prior BCT received (See Supplementary Table S2). Only baseline PHQ-9 scores submitted within 14 days of the first therapy session and before the second therapy session were included in the analysis of changes in PHQ-9 over the entire treatment period.
All analyses were conducted using R 4.2.3, and growth curve models were fitted using version 1.1.34 of the lmer library using full maximum likelihood estimation55.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author contributions
J.T.O., J.L.L and A.L. contributed to the study conceptualization, design, and manuscript preparation. L.W., S-Y.C., and R.E.W. contributed to the study design, data analysis, and manuscript preparation. S.T.M., N.F.B., and A.A.V. contributed to the manuscript preparation. C.C. contributed to the study conceptualization and manuscript preparation. All authors reviewed and approved the manuscript.
Data availability
All data supporting the findings are presented in the article and supplementary tables. As a business associate under HIPAA, Lyra Health is only permitted to use patient information as outlined in our Business Associate Agreements with our customers, which are covered entities. Many of Lyra Health’s customer agreements do not permit disclosure of de-identified patient data. Therefore, because of contractual restrictions, de-identified datasets cannot be shared.
Declarations
Competing interests
J.T.O., L.W., S-Y.C., S.T.M., A.V., J.L.L., C.C., and A.L. are salaried employees of, and have been granted equity in, Lyra Health, Inc. S.T.M, A.V., C.C. and A.L. are also salaried employees of Lyra Clinical Associates. N.F.B. is a former salaried employee of both Lyra Health Inc. and Lyra Clinical Associates, and has been granted equity in Lyra Health, Inc. R.E.W. serves as a paid consultant to Lyra Health. This study was funded by Lyra Health.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koenen, K. C. et al. Posttraumatic stress disorder in the world mental health surveys. Psychol. Med.47, 2260–2274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein, R. B. et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc. Psychiatry Psychiatr. Epidemiol.51, 1137–1148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson, P. PTSD as a public mental health priority. Curr. Psychiatry Rep.21, 1–12 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Akbar, R., Arya, V., Conroy, E., Wilcox, H. C. & Page, A. Posttraumatic stress disorder and risk of suicidal behavior: A systematic review and meta-analysis. Suicide Life Threat. Behav.53, 163–184 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Jellestad, L., Vital, N. A., Malamud, J., Taeymans, J. & Mueller-Pfeiffer, C. Functional impairment in posttraumatic stress disorder: A systematic review and meta-analysis. J. Psychiatr. Res.136, 14–22 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Davis, L. L. et al. The economic burden of posttraumatic stress disorder in the United States from a societal perspective. J. Clin. Psychiatry83, 40672 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Hale, A. C., Sripada, R. K. & Bohnert, K. M. Past-year treatment utilization among individuals meeting DSM-5 PTSD criteria: Results from a nationally representative sample. Psychiatr. Serv.69, 341–344 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Asmundson, G. J. et al. A meta-analytic review of cognitive processing therapy for adults with posttraumatic stress disorder. Cogn. Behav. Ther.48, 1–14 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Powers, M. B., Halpern, J. M., Ferenschak, M. P., Gillihan, S. J. & Foa, E. B. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin. Psychol. Rev.30, 635–641 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Lewis, C., Roberts, N. P., Andrew, M., Starling, E. & Bisson, J. I. Psychological therapies for post-traumatic stress disorder in adults: Systematic review and meta-analysis. Eur. J. Psychotraumatol.11, 1729633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnurr, P. P. et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: A randomized clinical trial. JAMA Netw. Open5, e2136921 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cusack, K. et al. Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clin. Psychol. Rev.43, 128–141 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Ronconi, J. M., Shiner, B. & Watts, B. V. A meta-analysis of depressive symptom outcomes in randomized, controlled trials for PTSD. J. Nerv. Ment. Dis.203, 522–529 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Korte, K. J., Allan, N. P., Gros, D. F. & Acierno, R. Differential treatment response trajectories in individuals with subclinical and clinical PTSD. J. Anxiety Disord.38, 95–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morland, L. A. et al. Advances in PTSD treatment delivery: Review of findings and clinical considerations for the use of telehealth interventions for PTSD. Curr. Treat. Options Psychiatry7, 221–241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouimette, P. et al. Perceived barriers to care among veterans health administration patients with posttraumatic stress disorder. Psychol. Serv.8, 212 (2011). [Google Scholar]
- 17.Kantor, V., Knefel, M. & Lueger-Schuster, B. Perceived barriers and facilitators of mental health service utilization in adult trauma survivors: A systematic review. Clin. Psychol. Rev.52, 52–68 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lehavot, K., Der-Martirosian, C., Simpson, T. L., Sadler, A. G. & Washington, D. L. Barriers to care for women veterans with posttraumatic stress disorder and depressive symptoms. Psychol. Serv.10, 203 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Smith, J. R., Workneh, A. & Yaya, S. Barriers and facilitators to help-seeking for individuals with posttraumatic stress disorder: A systematic review. J. Trauma. Stress33, 137–150 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kuhn, E. & Owen, J. E. Advances in PTSD treatment delivery: The role of digital technology in PTSD treatment. Curr. Treat. Options Psychiatry7, 88–102 (2020). [Google Scholar]
- 21.Scott, A. M. et al. Real-time telehealth versus face-to-face management for patients with ptsd in primary care: A systematic review and meta-analysis. J. Clin. Psychiatry83, 41146 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Shaker, A. A. et al. Psychiatric treatment conducted via telemedicine versus in-person modality in posttraumatic stress disorder, mood disorders, and anxiety disorders: Systematic review and meta-analysis. JMIR Ment. Health10, e44790 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sijbrandij, M., Kunovski, I. & Cuijpers, P. Effectiveness of internet-delivered cognitive behavioral therapy for posttraumatic stress disorder: A systematic review and meta-analysis. Depress. Anxiety33, 783–791 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Webb, C. A., Rosso, I. M. & Rauch, S. L. Internet-based cognitive behavioral therapy for depression: Current progress and future directions. Harv. Rev. Psychiatry25, 114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbe, D., Eichert, H.-C., Riper, H. & Ebert, D. D. Blending face-to-face and internet-based interventions for the treatment of mental disorders in adults: Systematic review. J. Med. Internet Res.19, e306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morland, L. A. et al. Telehealth and eHealth interventions for posttraumatic stress disorder. Curr. Opin. Psychol.14, 102–108 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Berger, T., Krieger, T., Sude, K., Meyer, B. & Maercker, A. Evaluating an e-mental health program (“deprexis”) as adjunctive treatment tool in psychotherapy for depression: Results of a pragmatic randomized controlled trial. J. Affect. Disord.227, 455–462 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Romijn, G. et al. Acceptability, effectiveness and cost-effectiveness of blended cognitive-behavioural therapy (bCBT) versus face-to-face CBT (ftfCBT) for anxiety disorders in specialised mental health care: A 15-week randomised controlled trial with 1-year follow-up. PLoS ONE16, e0259493 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lungu, A., Jun, J. J., Azarmanesh, O., Leykin, Y. & Chen, C.E.-J. Blended care-cognitive behavioral therapy for depression and anxiety in real-world settings: Pragmatic retrospective study. J. Med. Internet Res.22, e18723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cloitre, M. et al. Comparing the ratio of therapist support to internet sessions in a blended therapy delivered to trauma-exposed veterans: Quasi-experimental comparison study. JMIR Ment. Health9, e33080 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer, A. et al. A resource building virtual care programme: Improving symptoms and social functioning among female and male rural veterans. Eur. J. Psychotraumatol.12, 1860357 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiken, L. S. & West, S. G. Multiple Regression: Testing and Interpreting Interactions (Sage, 1991). [Google Scholar]
- 33.Najavits, L. M. The problem of dropout from “gold standard” PTSD therapies. Fprime Rep.7, (2015). [DOI] [PMC free article] [PubMed]
- 34.Kehle-Forbes, S. M., Meis, L. A., Spoont, M. R. & Polusny, M. A. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol. Trauma Theory Res. Pract. Policy8, 107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell, S., Mitchell, R., Shannon, C., Dorahy, M. & Hanna, D. Effects of baseline psychological symptom severity on dropout from trauma-focused cognitive behavior therapy for posttraumatic stress disorder: A meta-analysis. Traumatology29, 112 (2023). [Google Scholar]
- 36.Rubin, A., Parrish, D. E. & Washburn, M. Outcome benchmarks for adaptations of research-supported treatments for adult traumatic stress. Res. Soc. Work Pract.26, 243–259 (2016). [Google Scholar]
- 37.Blevins, C. A., Weathers, F. W., Davis, M. T., Witte, T. K. & Domino, J. L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. J. Trauma. Stress28, 489–498 (2015). [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association. Trauma- and stressor-related disorders. In Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 265–290 (American Psychiatric Association, 2013).
- 39.Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med.16, 606–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resick, P. A., Monson, C. M. & Chard, K. M. Cognitive Processing Therapy for PTSD: A Comprehensive Manual (Guilford Publications, 2017). [Google Scholar]
- 41.Foa, E. B., Hembree, E. A. & Rothbaum, B. O. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide (Oxford University Press, 2007). [Google Scholar]
- 42.Galovski, T. E., Blain, L. M., Mott, J. M., Elwood, L. & Houle, T. Manualized therapy for PTSD: Flexing the structure of cognitive processing therapy. J. Consult. Clin. Psychol.80, 968 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauch, S. A., Cigrang, J., Austern, D., Evans, A., for the STRONG STAR Consortium. Expanding the reach of effective PTSD treatment into primary care: Prolonged exposure for primary care. Focus15, 406–410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlin, B. E. & Cross, G. From the laboratory to the therapy room: National dissemination and implementation of evidence-based psychotherapies in the US Department of Veterans Affairs Health Care System. Am. Psychol.69, 19 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Nacasch, N. et al. Are 60-minute prolonged exposure sessions with 20-minute imaginal exposure to traumatic memories sufficient to successfully treat PTSD? A randomized noninferiority clinical trial. Behav. Ther.46, 328–341 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Barlow, D. H. et al. Unified Protocol for Transdiagnostic Treatment of Emotional Disorders: Therapist Guide (Oxford University Press, 2017). [Google Scholar]
- 47.Hayes, S. C., Strosahl, K. D. & Wilson, K. G. Acceptance and Commitment Therapy, Second Edition: The Process and Practice of Mindful Change (Guilford Press, 2011). [Google Scholar]
- 48.Linehan, M. M. DBT Skills Training Manual 2nd edn. (The Guilford Press, 2014). [Google Scholar]
- 49.Bovin, M. J. et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychol. Assess.28, 1379 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Weathers, F. W. et al. The PTSD checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov.
- 51.Gyani, A., Shafran, R., Layard, R. & Clark, D. M. Enhancing recovery rates: lessons from year one of IAPT. Behav. Res. Ther.51, 597–606 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singer, J. D. & Willett, J. B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence 644 (Oxford University Press, 2003). [Google Scholar]
- 53.Hoffman, L. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change (Routledge, 2015). [Google Scholar]
- 54.Mueser, K. T. et al. A randomized controlled trial of cognitive-behavioral treatment for posttraumatic stress disorder in severe mental illness. J. Consult. Clin. Psychol.76, 259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings are presented in the article and supplementary tables. As a business associate under HIPAA, Lyra Health is only permitted to use patient information as outlined in our Business Associate Agreements with our customers, which are covered entities. Many of Lyra Health’s customer agreements do not permit disclosure of de-identified patient data. Therefore, because of contractual restrictions, de-identified datasets cannot be shared.