Abstract
Background
Checkpoint inhibitor pneumonitis (CIP) that develops following immune checkpoint inhibitor (ICI) treatment can be difficult to distinguish from other common etiologies of lung inflammation in cancer patients. Here, we evaluate the bronchoalveolar lavage fluid (BAL) for potential biomarkers specific to CIP.
Methods
We conducted a retrospective study of patients who underwent standard of care bronchoscopy to compare the cytokines of interest between patients with and without CIP and with and without immune-mediated pulmonary diseases. Pulmonary diagnoses were determined by the treating clinician at the time of bronchoscopy and retroactively reviewed for agreement by the study team.
Results
Thirty-seven patients were included, and 24 (64.9%) had pulmonary infection, 2 (5.4%) had pulmonary edema, 6 (16.2%) had non-CIP drug-induced pneumonitis, 3 (8.1%) had CIP, 5 (13.5%) had immune-mediated ILD or autoimmune vasculitis, 4 (10.8%) had cancer progression, and 4 (10.8%) had nonimmune-mediated interstitial lung disease (ILD). IL-6 from the BAL was significantly higher in patients with CIP compared to those with cancer progression and nonimmune-mediated ILD, and IL-6 was significantly higher in patients with immune-mediated pulmonary diseases compared to cancer progression, nonimmune-mediated ILD, and infection.
Conclusions
BAL IL-6 distinguished CIP from other common, important causes of pulmonary infiltrates in patients with cancer, suggesting it may give insight into the pathophysiology of CIP and has potential as a biomarker.
Keywords: Immune checkpoint inhibitor pneumonitis, cancer, immunotherapy
Introduction
Immune checkpoint inhibitor (ICI) therapies have greatly advanced cancer treatment in the last decade. ICIs block physiologic regulators in the immune system, such as cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein-1 (PD-1), leading to upregulation of T cell function and revitalization and enhancement of antitumor activity [1]. ICIs have improved outcomes for patients with cancer; however, immune checkpoint inhibitor pneumonitis (CIP) remains a devastating and potentially fatal complication of ICI therapy.
Though most immune-related adverse events (iRAEs) are associated with improved overall survival [2, 3], we and others have shown that severe CIP may be associated with shorter survival [4, 5]. As ICIs are increasingly utilized in the neoadjuvant, adjuvant, and curative settings [6–8], identifying patients at highest risk of developing CIP prior to ICI treatment is of paramount importance. Furthermore, distinguishing CIP from alternate etiologies of lung inflammation (i.e., infection, cancer progression, and non-CIP-related interstitial lung disease [ILD]) in complex cancer patients with competing medical comorbidities is challenging and often impossible, and misattribution of CIP can lead to premature discontinuation or inappropriate delays in ICI treatment. We previously identified several objective pretreatment factors that may be predictive of CIP [9]. A corroborative predictive and diagnostic biomarker would help oncologists identify patients with the most favorable risk to benefit ratio from ICI treatment before treatment initiation and increase diagnostic certainty in patients that develop CIP after initiation of ICI therapy.
Materials and methods
Patient identification
Patients with available bronchoalveolar lavage (BAL) samples and diagnoses of cancer, infection, pulmonary edema, drug-related pneumonitis, autoimmune disease, and ILD between Sep 1, 2019, and Sep 1, 2023, were identified from the OSU BAL Repository (IRB 2019H0471), which includes clinical data and samples collected prospectively during clinically indicated bronchoscopies.
Clinical variables
The following were recorded for each included patient: age, sex, race, cancer type and stage, cancer status (active cancer was assigned if patients received systemic cancer treatment 12 months before BAL collection), clinical treatment course, BAL cell differential, and BAL cytokine concentrations. The final pulmonary diagnoses were determined by the treating clinical team and retrospectively reviewed by members of the study team for agreement utilizing serology, pathology, culture data, and imaging. If there were discrepancies between the treating clinician and study team members in determining a final pulmonary diagnosis, or if there was no leading diagnosis at the time of bronchoscopy, another member of the study team evaluated to make a final determination on the most likely pulmonary diagnosis. If multiple diagnoses were felt to be contributing, all were recorded. These determinations were made prior to cytokine measurements from the BAL. The study protocol was approved by the Ohio State University Institutional Board (2023C0071) and a waiver of consent was granted due to the retrospective nature of analysis.
BAL collection and specimen processing
The approach to obtaining the BAL (syringe pump ± wall suction into specimen trap) was left up to the discretion of the pulmonologist performing the bronchoscopy. As often as possible, a standardized approach was utilized with five syringes of 20 ml saline manually instilled and aspirated. From the BAL return, 15 mL were sent to the laboratory for clinical purposes, and the remainder was collected and retained for the repository. BAL specimens were passed through sterile surgical gauze and immediately centrifuged at 500 g for 15 min. Supernatant was aspirated and stored at − 80 °C. Cell pellets were resuspended with 10 mL of Hank’s balanced salt solution, for total cell counts. BAL cell differentials were obtained from the clinical laboratory as entered in the electronic medical record, and cytokines of interest (IFN-y, IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, and TNF-α) were measured in the supernatant on the first thaw cycle. Cytokines of interest were multiplexed using an electrochemiluminescence method read using the Meso QuickPlex SQ 120 (Meso Scale Discovery, 1601 Research Boulevard, Rockville, MD).
Statistical analysis
Patients' demographic and clinical characteristics were summarized using means (standard deviations) for continuous variables and frequencies (proportions) for categorical variables and further compared between patients with immune-mediated lung inflammation (CIP, drug-induced pneumonitis, autoimmune vasculitis, or ILD) and those with other diagnoses. To assess differences in cytokine levels and cell count differentials between patients with CIP, immune-mediated lung inflammation, and other diagnoses, we used the Kruskal–Wallis test to compare patients with CIP and immune-mediated lung inflammation with other diagnoses individually. For biomarkers that showed significant differences, we used box plots to visualize the results. Biomarkers were log-transformed in the box plots due to skewed distributions. For all comparisons, if a patient was diagnosed with both immune-mediated lung inflammation or CIP and another condition, immune-mediated lung inflammation and CIP were prioritized, and the patient was classified into the immune-mediated lung inflammation or CIP group. All analyses were conducted using SAS 9.4, with p-values less than 0.05 considered statistically significant.
Results
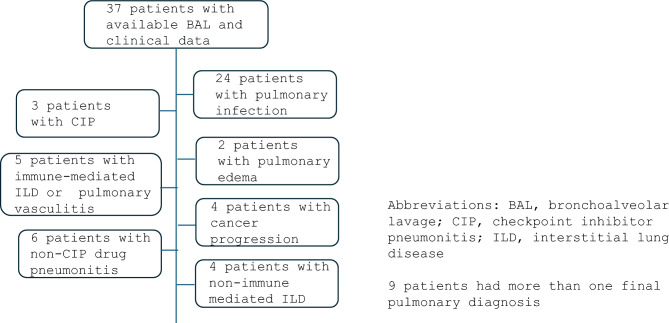
Thirty-seven patients that underwent clinically indicated bronchoscopies were included in this study. BAL was performed with syringe only in 26/37 (70.3%) patients and with a combination of syringe and wall suction in 11/37 (29.7%) patients. The mean instilled volume was 125.9 ± SD 60.6 mL, with mean return volume of 41.8 ± SD 18.3 mL. Of the 37 patients, 24 (64.9%) were determined to have pulmonary infection, 2 (5.4%) had pulmonary edema, 6 (16.2%) had non-CIP drug-induced pneumonitis, 3 (8.1%) had CIP, 5 (13.5%) had immune-mediated ILD or autoimmune vasculitis, 4 (10.8%) had cancer progression in the lungs, and 4 (10.8%) had nonimmune-mediated interstitial lung disease (ILD) (Fig. 1). Twenty-one of the 37 (56.8%) patients had active cancer and 16 did not. Eleven (29.7%) of the 37 patients received systemic corticosteroids within 7 days prior to BAL collection.
Fig. 1.
Patients by pulmonary diagnoses
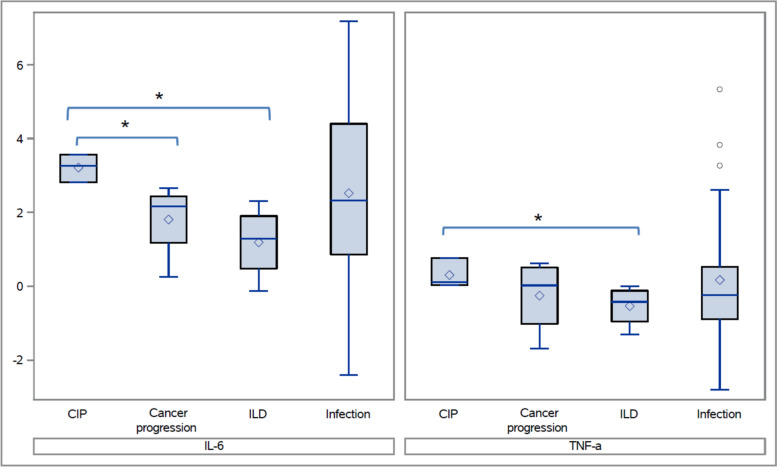
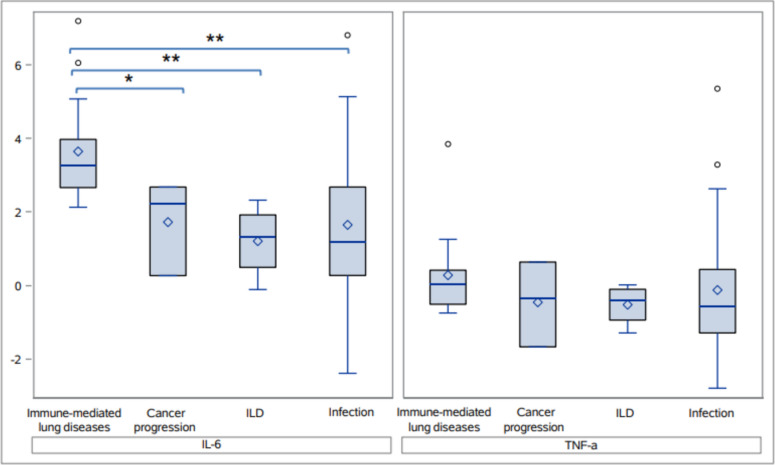
Patients with CIP had significantly higher levels of IL-6 from the BAL compared to those with cancer progression in the lungs (Median: 26.1 pg/mL vs. 8.7 pg/mL, which corresponds to 3.3 vs. 2.2 on the log scale as shown in the box plot, p = 0.033) and significantly higher IL-6 and TNF-α compared to those with nonimmune-mediated ILD (Median: 26.1 pg/mL vs. 3.7 pg/mL (3.3 vs. 1.3 on the log scale), p = 0.033 and 1.1 pg/mL versus 0.7 pg/mL (0.1 vs. − 0.4 on the log scale,), p = 0.033, respectively, Fig. 2). IL-6 was higher in CIP patients compared to those with pulmonary infection, but this difference was not statistically significant (Median: 26.1 pg/mL vs. 10.3 pg/mL, (3.3 vs. 1.3 on the log scale), p = 0.44, Fig. 2). There were no differences in the BAL cell differential and in the other cytokines of interest between CIP and pulmonary infection, cancer progression, and nonimmune-mediated ILD. When all immune-mediated pulmonary diagnoses were combined (CIP, non-CIP drug pneumonitis [10, 11], autoimmune pulmonary vasculitis, and immune-mediated ILD), IL-6 levels were significantly higher compared to those with cancer progression, nonimmune-mediated ILD, and infection (p = 0.044, p = 0.004, and p = 0.0078, respectively, Fig. 3). There were no significant differences noted in the BAL cell differentials and in other cytokines of interest. Patient demographic data between immune-mediated pulmonary diseases and alternate diagnoses were similar (Table 1).
Fig. 2.
Interleukin-6 and tumor necrosis factor alpha levels in bronchoalveolar lavage samples from patients with immune checkpoint inhibitor pneumonitis compared to patients with other common etiologies of lung inflammation. Box plot legend The central line in the box represents the median, while the diamond point within the box indicates the mean. The box edges correspond to the first (Q1) and third quartiles (Q3), and whiskers extend up to 1.5 times the interquartile range (IQR) above Q3 and below Q1. Dots beyond the whiskers represent outliers. CIP Checkpoint inhibitor pneumonitis; ILD Interstitial lung disease (specifically referring to nonimmune-mediated ILD), IL-6 Interleukin-6; TNF-α Tumor necrosis factor alpha. Note: * indicates p < 0.05 and ** indicates p < 0.01
Fig. 3.
Interleukin-6 and tumor necrosis factor alpha levels in bronchoalveolar lavage samples from patients with immune-mediated lung inflammation compared to patients with other inflammatory pulmonary processes. CIP Checkpoint inhibitor pneumonitis, ILD Nonimmune-mediated interstitial lung disease; IL-6 Interleukin-6; TNF-α Tumor necrosis factor alpha. Note: * indicates p < 0.05 and ** indicates p < 0.01
Table 1.
Demographics of cohort group of patients with immune-mediated lung inflammation compared to other diagnosis (pulmonary infection, cancer progression in the lungs, and interstitial lung disease)
| Immune-mediated lung inflammation (n = 14) | Other diagnosis (n = 23) | P value | ||
|---|---|---|---|---|
| Age | 63.6 ± 11 | 62.1 ± 13 | 0.712 | |
| Gender | Male | 8 (57.1%) | 12 (52.2%) | 1.00 |
| Female | 6 (42.9%) | 11 (47.8%) | ||
| Race | White | 13 (92.9%) | 20 (87.0%) | 1.00 |
| Other | 1 (7.1%) | 3 (13.0%) | ||
| BMI | 29.3 ± 7.2 | 37.3 ± 4.8 | 0.318 | |
| Smoking | Active smoker | 7 (50%) | 13 (56.5%) | 0.745 |
| Never smoker | 7 (50%) | 10 (43.5%) | ||
| Steroids given | Yes | 7 (50.0%) | 19 (82.6%) | 0.063 |
| No | 7 (50.0%) | 4 (17.4%) | ||
| Cancer history | No | 3 (21.4%) | 3 (13.0%) | 0.653 |
| Yes | 11 (78.6%) | 20 (87.0%) | ||
| Cancer status | Active cancer | 9 (64.3%) | 12 (52.2%) | 0.515 |
| Inactive/no cancer | 5 (35.7%) | 11 (47.8%) | ||
| Prior immunotherapy | No | 11 (78.6%) | 21 (91.3%) | 0.346 |
| Yes | 3 (21.4%) | 2 (8.7%) | ||
| Prior chemotherapy | No | 8 (57.1%) | 10 (43.5%) | 0.508 |
| Yes | 6 (42.9%) | 13 (56.5%) | ||
| Prior chest radiation | No | 10 (71.4%) | 17 (73.9%) | 1.00 |
| Yes | 4 (28.6%) | 6 (26.1%) | ||
Discussion
Major society guidelines have called for translational studies to isolate potential CIP biomarkers from the serum and BAL [12], but limited biomarker studies are available to date. To address this knowledge gap, we analyzed BAL from patients with CIP and other immune-mediated pulmonary diseases to identify differences in the cytokine profiles. In this study, we found that patients with CIP had higher levels of IL-6 in BAL specimens compared to patients with cancer progression and nonimmune-mediated ILD. We also found that patients with immune-mediated lung inflammation had higher levels of IL-6 in BAL compared to pulmonary infection, cancer progression, and nonimmune-mediated ILD. To our knowledge, our study is the first to show IL-6 as a biomarker from the BAL that can potentially differentiate CIP and immune-mediated lung inflammation from pulmonary infection, cancer progression in the lungs, and nonimmune-mediated ILD.
Several groups have examined IL-6 from the BAL in the setting of benign and malignant pulmonary processes. Hogea, et al., found that IL-6 from the BAL was higher in patients with malignant lung diseases [13], and Dowlati, et al., reported that IL-6 in the BAL from patients with lung cancer was higher than BAL from patients with chronic obstructive pulmonary disease (COPD) with acute pulmonary infections [14]. Kowalski, et al., showed that IL-6 was higher in the BAL of CIP patients compared to healthy control subjects, patients with lung cancer, and those with ILD [15]. We found that IL-6 from the BAL was higher in immune-mediated lung inflammation compared to the most common alternate etiologies for lung inflammation in cancer patients (infection, cancer progression, and non-CIP ILD), and our work corroborates prior studies showing BAL IL-6 as a promising biomarker for CIP. Utilization of this potential biomarker could lead to earlier detection of CIP, allowing for prompt immune suppression and prevention of more severe cases of CIP [16].
In addition to its potential as a diagnostic biomarker, IL-6 from the BAL may also have therapeutic implications for CIP. Tocilizumab, a monoclonal antibody that inhibits the IL-6 receptor to block the pro-inflammatory effects of IL-6, has been utilized for the treatment of iRAEs and CIP [17], though its role for CIP treatment is limited to steroid-refractory cases (pneumonitis not improving or worsening on systemic corticosteroids). Corticosteroids remain the mainstay of CIP treatment, but prolonged steroid treatment in cancer patients treated with ICIs may be associated with worsened progression-free and overall survival, in addition many other adverse effects [18]. Further research is needed to determine whether BAL IL-6 can be utilized to identify CIP patients most likely to benefit from early anti-IL-6 therapy.
There are several limitations to our study. Our CIP sample size was small, making it difficult to make definitive conclusions on cytokine differences among patients with CIP, cancer progression, nonimmune-mediated ILD, and pulmonary infection, though clear distinctions were observed even with the small sample size. Approximately, one third of patients received corticosteroids within 7 days prior to BAL collection, and the cytokine concentration at the time of BAL collection may not accurately reflect the cytokine concentration and composition of the pathogenic pulmonary process before corticosteroid administration. Several patients also had concurrent diagnoses, making it difficult to attribute identified cytokine compositions to one specific pulmonary disorder. However, as this is a real-world study of complex cancer patients, confident determination of a single pulmonary pathology is often difficult if not impossible. Additionally, BAL return was variable and differing volumes may affect cytokine measurements. Despite attempts to standardize BAL collection techniques [19], patient and clinician-specific factors impacted the approach and total instilled volume. Finally, given the retrospective review of clinical data, there is a possibility of unrecognized confounders that may misclassify the association between IL-6 from the BAL, CIP, and immune-mediated lung inflammation. However, given the paucity of studies surrounding analysis of potential biomarkers from the BAL in patients with and without CIP, we believe our study lends valuable insight into a previously understudied area of CIP.
Conclusion
BAL IL-6 distinguished CIP from other common, important causes of pulmonary infiltrates in patients with cancer, suggesting it may give insight into the pathophysiology of CIP and has potential as a biomarker. Further studies are needed to confirm the association between elevated IL-6 from the BAL and CIP.
Acknowledgements
This work was supported by the National Center for Advancing Translational Sciences [Award Number UL1TR002733], and the Ohio State University College of Medicine Office of Research and the Center for Clinical & Translational Science through the Richard P. Marie R. Bremer Medical Research Fund and William H. Davis Endowment for Basic Medical Research [Path to K award]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Davis/Bremer Research Fund, the Center for Clinical and Translational Science, the National Center for Advancing Translational Sciences, the National Institutes of Health, The Ohio State University Wexner Medical Center, or the university. Study data were collected and managed using Research electronic data capture (REDCap) tools hosted by the Clinical and Translational Science (CCTS) at the Ohio State University Center [UL1TR001070].
Author contributions
All authors contributed to the study conception and design. Data collection was done by MP, SC, LF, and KH. Material preparation was done by LF, DHO, and KH. Statistical analysis was done by SZ and LW. The first draft of the manuscript, including figures and tables, was written by MP, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Center for Advancing Translational Sciences [Award Number UL1TR002733], and the Ohio State University College of Medicine Office of Research and the Center for Clinical & Translational Science through the Richard P. Marie R. Bremer Medical Research Fund and William H. Davis Endowment for Basic Medical Research [Path to K award]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Davis/Bremer Research Fund, the Center for Clinical and Translational Science, the National Center for Advancing Translational Sciences, the National Institutes of Health, The Ohio State University Wexner Medical Center, or the university. Study data were collected and managed using Research electronic data capture (REDCap) tools hosted by the Clinical and Translational Science (CCTS) at the Ohio State University Center [UL1TR001070].
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study protocol was approved by the Ohio State University Institutional Board (2023C0071), and a waiver of consent was granted due to the retrospective nature of analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dwight H. Owen and Kevin Ho have contributed equally to this work.
References
- 1.Chuzi S, Tavora F, Cruz M et al (2017) Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 14(9):207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson AS, Goutam S, Stukalin I et al (2022) Association of immune-related adverse events, hospitalization, and therapy resumption with survival among patients with metastatic melanoma receiving single-agent or combination immunotherapy. JAMA Netw Open 5(12):e2245596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook S, Samuel V, Meyers DE et al (2024) Immune-related adverse events and survival among patients with metastatic NSCLC treated with immune checkpoint inhibitors. JAMA Netw Open 7(1):e2352302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Hao N, Yang S et al (2023) Predictive factors and prognosis of immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer patients. Front Oncol 26(13):1145143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong A, Riley M, Zhao S et al (2023) Association between pretreatment chest imaging and immune checkpoint inhibitor pneumonitis among patients with lung cancer. J Natl Compr Canc Netw 21(11):1164-1171.e5 [DOI] [PubMed] [Google Scholar]
- 6.Carlino MS, Larkin J, Long GV (2021) Immune checkpoint inhibitors in melanoma. Lancet 398(10304):1002–1014 [DOI] [PubMed] [Google Scholar]
- 7.Banna GL, Hassan MA, Signori A et al (2024) Neoadjuvant chemo-immunotherapy for early-stage non-small cell lung cancer: a systematic review and meta-analysis. JAMA Netw Open 7(4):e246837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821 [DOI] [PubMed] [Google Scholar]
- 9.Wong A, Riley M, Zhao S et al (2023) Association between pre-treatment chest imaging and pulmonary function abnormalities and immune checkpoint inhibitor pneumonitis. Cancer Immunol Immunother 72(6):1727–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolkove N, Baltzan M (2009) Amiodarone pulmonary toxicity. Can Respir J 16(2):43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuno O (2012) Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 13(1):39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears CR, Peikert T, Possick JD et al (2019) Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis. An official American thoracic society research statement. Am J Respir Crit Care Med 200(6):31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogea P, Tudorache E, Fira-Mladinescu O et al (2023) Serum and bronchoalveolar lavage fluid levels of cytokines in patients with lung cancer and chronic lung disease: a prospective comparative study. J Pers Med 13(6):998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowlati A, Levitan N, Remick SC (1999) Evaluation of interleukin-6 in bronchoalveolar lavage fluid and serum of patients with lung cancer. J Lab Clin Med 134(4):405–409 [DOI] [PubMed] [Google Scholar]
- 15.Kowalski B, Valaperti A, Bezel P et al (2022) Analysis of cytokines in serum and bronchoalveolar lavage fluid in patients with immune-checkpoint inhibitor-associated pneumonitis: a cross-sectional case-control study. J Cancer Res Clin Oncol 148(7):1711–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moodabagil M, Easterling R, Peng J, Abu-Sbeih H, Meara A, Donnelly E, Owen DH, Ho K (2024) Investigating risk factors and treatment options for severe, partially steroid responsive, and steroid-refractory checkpoint inhibitor pneumonitis. Oncologist 29(11):e1575–e1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroud CR, Hegde A, Cherry C et al (2019) Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 25(3):551–557 [DOI] [PubMed] [Google Scholar]
- 18.Petrelli F, Signorelli D, Ghidini M et al (2020) Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel) 12(3):546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikacenic C, Fussner LA, Bell J et al (2023) Research bronchoscopies in critically Ill research participants: an official American thoracic society workshop report. Ann Am Thorac Soc 20(5):621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.