Abstract
Background
Immune checkpoint inhibitors (ICIs) show optimal treatment effects on recurrent or metastatic nasopharyngeal carcinoma(R/M NPC). Nonetheless, whether metastatic sites impact ICIs efficacy remains unclear.
Methods
We performed a secondary analysis of R/M NPC patients treated with KL-A167, a programmed cell death-ligand 1(PD-L1) inhibitor, based on a multicenter, single-arm, phase II study from China between 2019 and 2021 years, which represents the first and most comprehensive analysis of the effectiveness of a PD-L1 inhibitor in patients who have been previously treated. The Cox proportional hazard model was utilized to evaluate the association between sites and PFS and OS. Sensitivity analysis and subgroup analysis were carried out to confirm the reliability of our findings.
Results
A total of 153 R/M NPC patients were included. The mean age was 47 years and 81% of patients were males. All patients in our study had distant metastasis, with a majority (n = 69) presenting with more than 2 sites of distant metastasis upon admission. The collected sites of metastasis included liver, lung, lymph and bone. Among the 153 patients, 37.9% (58 patients) received anti-PD-L1 treatment for a minimum of 6 months, and 17.6% (27 patients) were treated for at least 12 months. By conducting multivariate analysis, R/M NPC patients with non-liver metastases presented significantly longer progress-free survival (PFS, HR:1.67, CI:1.09–0.2.55, p = 0.018) and overall survival (OS, HR:2.52, CI:1.49–4.28, p < 0.001) compared with those with liver metastasis. The median PFS (72 vs. 144 days, p < 0.0001) and OS (730 vs. 305 days, p < 0.0001) were significantly longer for patients with non-liver metastases. However, lung, bone and lymph node metastasis had no statistical significance on PFS and OS (p > 0.005). Our sensitive analysis showed liver metastases patients with less other site metastases (0 or 1) had shorter OS compared to non-liver metastases patients with more other metastases(≥ 2). Furthermore, subgroup analysis indicated the robustness evidence liver metastasis indeed a valuable prognostic factor for survival.
Conclusions
Compared to patients with other metastatic sites, R/M NPC patients with liver metastasis have poor survival patterns when receiving anti-PD-L1 therapy. Our study provides rational evidence for the urgent need to explore more efficacy treatment modalities for NPC patients with liver metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03905-0.
Keywords: Metastatic sites, Nasopharyngeal carcinoma, Anti-PD-L1, Survival
Introduction
Nasopharyngeal carcinoma (NPC) exhibits a distinctive geographic pattern, with a notably high incidence rate observed in Southern China [1, 2]. Meanwhile, the prevalence of NPC is more pronounced in male than female populations [3, 4].
Epidemiological reviews indicated that the survival of NPC patients increased modestly during the past years [1, 5]. Despite the satisfied local control rate with advanced radiotherapy, dealing with the distant metastatic sites in NPC remains a pivotal challenge. Distant metastases were observed in more than 15% of NPC patients who received intensity-modulated radiotherapy before [6]. Patients with metastatic NPC who have failed first-line treatment do not have a universally agreed-upon standard of care [7].
The emergence of immune checkpoint blockers (ICB) has transformed the clinical landscape for advanced cancer patients, especially for those who have a recurrent or metastatic (R/M) disease. Emerging evidence indicated that immune microenvironment played a vital important role in the metastatic and recurrence of NPC, which suggested the potential treatment benefits of immunotherapy in these patients [8–10].
Additionally, how to make tailored clinical decision for selecting the appropriate NPC patients to elect to ICB therapy represented a critical topic. Burdens of tumor metastasis have been explored as the potential biomarkers for responsiveness to ICB. The preliminary clinical evaluation suggested that pembrolizumab displayed a lower level of effectiveness in non-small cell lung cancer (NSCLC) and melanoma patients who exhibited liver metastasis [11]. Meanwhile, some studies also determined the consistent findings in some solid tumors include gastrointestinal tumors, melanoma and breast cancer treated with immunotherapy [12–15]. A retrospective study by Bilen MA et al. found that among gastrointestinal tumors and melanoma patients who had received more than two lines of systemic therapy prior to immunotherapy, liver metastasis was associated with significantly shorter OS(HR: 2.63). Nonetheless, limited evidence was available for predicting the survival probabilities in NPC patients underwent the ICB treatment. Notably, one retrospective study revealed that R/M NPC patients without liver metastasis could get more survival benefits from the anti-PD-1 therapy [16]. However, whether the same survival patterns were shown in NPC patients undergoing anti-PD-L1 therapy remains unknown.
To fill this research gap, we aim to explore the survival of R/M patients with different distant metastatic sites when they received the anti-PD-L1 therapy, based on one multicenter, single-arm, phase II study from China between 2019 and 2021 years. In addition, we also investigate the prognostic role of number of metastatic sites in R/M NPC patients.
Methods
Study design and data sources
A secondary analysis from one multicenter, prospective, clinical trial for comparing the prognosis of metastatic NPC patients who had varied metastatic sites was conducted. One hundred fifty three patients who were administered KL-A167 were included in the intention-to-treat (ITT) analysis at 42 accredited hospitals in China from February 26, 2019, to January 13, 2021. The eligibility and exclusion criteria were detailed in a prior publication [5].
Variables of interest
Patients’ information was collected during the entire study period including. Computed tomography (CT) and magnetic resonance imaging (MRI) of the nasopharynx, head, neck, chest, abdomen, and pelvis identified distant metastases in the lung, liver, lymph nodes, and bones.
Study outcome
We measured two clinical outcomes: PFS and OS. PFS was determined from the first dose of anti-PD-L1 until death and OS from the first dose to progression of clinical or radiographic findings. RECIST v 1.1 was used to assess response to treatment.
Ethic approval
All data used were from the prior study that adhered to the guidelines and principles outlined in the Helsinki Declaration and the Good Clinical Practice guidelines outlined by the International Council for Harmonization. Before enrolling in the study, all patients provided written informed consent to participate.
Statistical analysis
Statistical analysis was conducted using R version 4.3.2. Survival analysis was conducted using the Kaplan–Meier method, and comparison between groups with diverse sites of metastasis was performed using the log-rank test. The univariate analysis, multivariate analysis and subgroup analysis of PFS and OS were tested by the Cox proportional hazard model. The multivariate analysis of sites of metastases and subgroup analysis included factors were: gender, age, T stage, N stage, liver metastases, lung metastases, bone metastases, lymph node metastases and the total number of metastatic sites. All the tests conducted were two-tailed, and a p-value of less than 0.05 was considered statistically significant.
Result
Patient characteristics
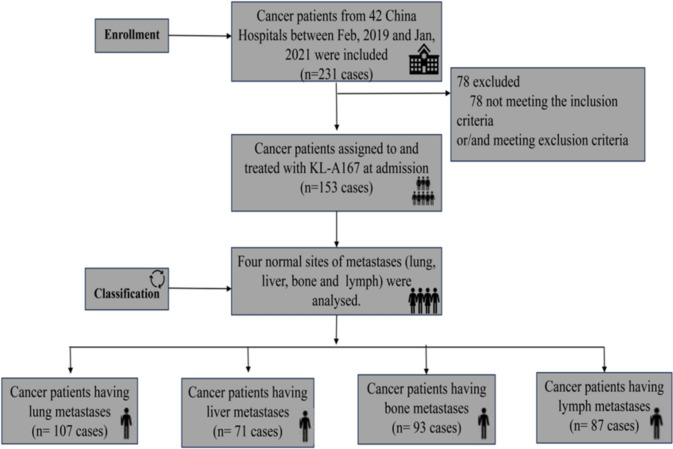
A total of 231 patients were screened at 42 qualified Chinese hospitals from February 26, 2019 to January 13, 2021, of whom 153 received anti-PD-L1. Patient enrollment and participating sites were presented in Supplementary Table 1.
Approximately 21.7 months were followed up by the median follow-up as of July 13, 2021. All of the study population exhibited metastatic sites and 69 patients suffered from numerous metastatic sites (≥ 3), with liver (71 cases), lung (107 cases), bone (93 cases), and lymph node (87 cases) observed in various sites (Fig. 1). In addition, there were a few other metastatic sites in the study cohort, such as the brain and spleen. Due to the small number, they have been excluded from our study.
Fig. 1.
Trial profiles
Analyses of survival in patients with different metastatic sites.
The association between the different metastatic sites (liver, lung, bone, lymph node) with the PFS and OS were analyzed. The univariate analyses of the metastatic sites concerning clinical outcomes were presented in Table 1. In univariate analysis, R/M NPC patients with non-liver metastases presented longer PFS (HR = 1.97, 95%CI: 1.40–2.76, p < 0.001) and OS (HR = 2.57, 95%CI: 1.73–3.81, p < 0.001).
Table 1.
Univariate survival analysis of baseline metastatic sites
| Outcome | Liver | Lung | Bone | Lymph | ||||
|---|---|---|---|---|---|---|---|---|
| Yes(n = 71) | No(n = 82) | Yes(n = 107) | No(n = 46) | Yes(n = 90) | No(n = 63) | Yes(n = 87) | No(n = 66) | |
| Median PFS(Q1,Q3), day | 43(40–162) | 127(46–305) | 85(42–236) | 45(41–243) | 49(41–208) | 128(43–271) | 85(42–249) | 82(41–210) |
| HR(95%CI) | 1.97(1.40–2.76) | 0.90(0.63–1.30) | 1.47(1.04- 2.08) | 0.90(0.65 −1.26) | ||||
| P-value | < 0.001 | 0.585 | 0.030 | 0.549 | ||||
| Median OS(Q1,Q3), day | 246(127–499) | 592(317–816) | 434(218–786) | 373(169–616) | ||||
| HR (95%CI) | 2.57 (1.73 – 3.81) | 0.67 (0.45 – 1.01) | 1.32 (0.88 – 1.97) | 1.36 (0.91 – 2.04) | ||||
| P-value | < 0.001 | 0.056 | 0.175 | 0.131 | ||||
Q1—First quartile Q3—Third quartile
Baseline characteristics of patients with or without liver metastasis are presented in Table 2. The NPC patients without liver metastasis presented fewer total number of metastatic sites, lower ECOG score, fewer lung metastases, and higher sum of tumor maximum diameters (P < 0.05). The mean age of patients with liver metastasis was 47.38, and 47.72 for those without. The majority of patients (81.7%) in both groups were male. All 153 patients had non-keratinizing NPC, with 51 cases (62.2%) of undifferentiated subtype in non-liver metastasis, and 46 cases (64.8%) in liver metastasis. Among the patients with liver metastasis, 52 cases (73.2%) received ≥ 5 cycles of chemotherapy, and 61 cases (77.4%) were observed in non-liver metastasis cohort. As shown in supplementary Table S2, chemotherapy drugs previously used to treat advanced diseases have been studied.
Table 2.
Patient characteristics in Non-Liver metastases and Liver metastases sample
| Characteristic | Non-Liver metastases(n = 82) | Liver metastases (n = 71) | P |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 47.72 (10.31) | 47.38 (9.26) | 0.832 |
| < 45 | 29 (35.4) | 23 (32.4) | 0.699 |
| ≥ 45 | 53 (64.6) | 48 (67.6) | |
| Gender | |||
| Male | 67 (81.7) | 58 (81.7) | 0.998 |
| Female | 15 (18.3) | 13 (18.3) | |
| ECOG | |||
| 0 | 40 (48.8) | 19 (26.8) | 0.005 |
| 1 | 42 (51.2) | 52 (73.2) | |
| Tumor stage | |||
| T0 ~ T2 | 24 (29.3) | 28 (39.4) | 0.232 |
| T3 ~ T4 | 26 (31.4) | 24 (33.8) | |
| Tx | 32 (59.3) | 19 (26.8) | |
| Node stage | |||
| N0 ~ N2 | 44 (53.6) | 40 (56.3) | 0.763 |
| N3 ~ N4 | 13 (15.8) | 13 (18.3) | |
| Nx | 25 (30.6) | 18 (25.4) | |
| BMI | |||
| Mean (SD) | 21.45 (2.90) | 21.79 (3.47) | 0.506 |
| Smoking history | |||
| Yes | 33 (40.2) | 22 (31.0) | 0.234 |
| No | 49 (59.8) | 49 (69.0) | |
| Histology (WHO) | |||
| Undifferentiated nonkeratinising | 51 (62.2) | 46 (64.8) | 0.484 |
| Differentiated nonkeratinising | 22 (26.8) | 14 (19.7) | |
| Nonkeratinising (differentiation unknown) | 9 (11.0) | 11 (15.5) | |
| Prior radiotherapy | |||
| Yes | 77 (93.9) | 69 (97.2) | 0.561 |
| No | 5 (6.1) | 2 (2.8) | |
| Number of radiotherapies | |||
| 1 | 35 (42.7) | 40 (56.3) | 0.561 |
| 2 | 18 (22) | 11 (15.5) | |
| 3 or more | 24 (29.3) | 18 (25.4) | |
| Unknown | 5 (6.0) | 2 (2.8) | |
| Number of chemotherapies | |||
| < 5 | 21 (25.6) | 19 (26.8) | 0.872 |
| ≥ 5 | 61 (74.4) | 52 (73.2) | |
| Sum of longest diameter of target lesion, mm | |||
| Mean (SD) | 59.80 (39.6–99) | 38.75 (24.75–66) | 0.001 |
| The number of involved sites | |||
| < 3 | 61 (74.4) | 23 (32.4) | 0.001 |
| ≥ 3 | 21 (25.6) | 48 (67.6) | |
| The time from initial diagnosis to first dose, days | |||
| Median (range) | 425 (228.75–842.50) | 539 (321–1008) | 0.198 |
| Bone metastases | |||
| Yes | 36 (43.9) | 24 (33.8) | 0.202 |
| No | 46 (56.1) | 47 (66.2) | |
| Lung metastases | |||
| Yes | 16 (19.5) | 30 (42.3) | 0.002 |
| No | 66 (80.5) | 41 (57.7) | |
| Lymph node metastases | |||
| Yes | 34 (41.5) | 32 (45.1) | 0.653 |
| No | 48 (58.5) | 39 (54.9) | |
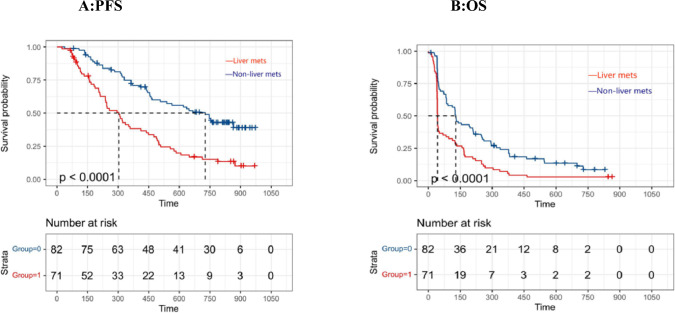
The KM plot depicting the relationship between liver metastases and PFS and OS was illustrated in Fig. 2A and Fig. 2B, respectively. Patients with non-liver metastases showed a significantly longer PFS and OS in the analysis. (PFS:305 vs. 730 days, p < 0.0001; OS: 72 vs 144 days, p < 0.0001).
Fig. 2.
Kaplan–Meier plot of progression-free survival (PFS) and overall survival (OS) stratified by presence of liver metastases. Time = Days
Analyses of survival in patients with different metastatic sites.
In stepwise multivariate analysis, the presence of non-liver metastases remained significantly associated with improved PFS (HR = 1.67, 95%CI: 1.09–2.55, p = 0.018), and OS (HR = 2.52, CI: 1.49–4.28, p < 0.001). Meanwhile, compared to other factors only ECOG PS had statistical significance on survival in the multivariate analysis. The multivariate analyses concerning clinical outcomes were presented in Table S3(PFS) and Table S4(OS).
Sensitivity analysis
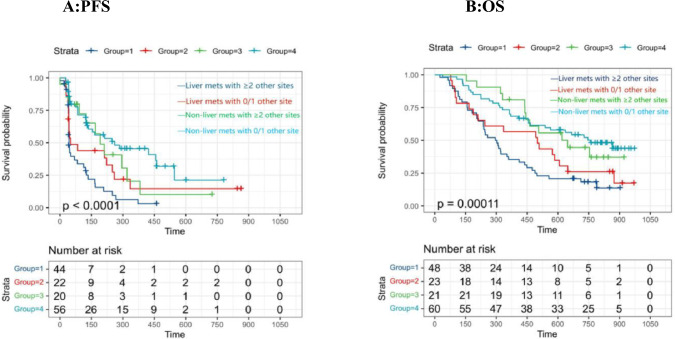
A sensitivity analysis was performed to evaluate the robustness and reliability of the correlation between non-liver metastasis and clinical outcomes. First, the patients were re-divided into four groups. The KM were displayed in Fig. 3 to show the survival of all populations. Figure 3A shows the PFS of four group patients who were enrolled into the study according to metastatic sites. Among the patients with liver metastasis, patients with a smaller number of other metastatic sites showed longer PFS than patients with a greater number of other metastatic sites (Group1 vs Group2,p < 0.0001).
Fig.3.
Kaplan–Meier plot of progression-free survival (PFS) and overall survival (OS) stratified by patients. Patients are classified into four groups based on liver metastases and total number of metastases. Group 1: Liver metastases with > 2 other metastases sites. Group 2: Liver metastasis with 0 or 1 other metastasis site. Group 3:Non-liver metastases with > 2 other metastases sites. Group 4: Non-liver metastasis with 0 or 1 other metastasis site
Figure 3B shows the OS of four group patients. Meanwhile, patients without liver involvement still had longer OS compared to patients with liver metastases (Group4 vs Group2, Group3 vs Group1). Moreover, liver metastases patients with 0 or 1 other site metastases had shorter OS compared to non-liver metastases patients with even more than 2 other metastases (Group2 vs Group3, p = 0.00011). Compared to more total number of metastatic sites, liver metastasis posed greater risk for OS.
Multivariate subgroup analysis:
Besides, the Cox proportional hazard model was utilized to perform multivariate subgroup analysis on PFS and OS (shown in Fig S1 and Fig S2, respectively). In subgroup analysis, heterogeneity was existed in two groups of liver metastasis and non-liver metastasis including the bone metastasis (P for interaction < 0.05). In the liver metastasis cohort, patients without bone metastasis had significantly longer OS and most patients had past radiotherapy history. No significant interaction was observed in other subgroups (P for interaction > 0.05).
Discussion
With the advancement of immunotherapy, the approval of PD-1 inhibitors has been granted for the treatment of NPC patients with R/M. Clinical outcomes for this patient population are poor, despite PD-1 inhibitors demonstrated antitumor activity. So far, there are limited therapeutic options for this patient cohort [17, 18].
In this study, we conducted a secondary analysis of R/M NPC patients treated with PD-L1 inhibitors based on a multicenter, single-arm, phase II study. In our previous study, the safety profile of PD-L1 had been demonstrated in patients to be acceptable, as well as promising efficacy. In this study, metastasis to the liver was identified as an independent prognostic factor for OS (HR = 2.52, CI: 1.49–4.28, p < 0.001). Regardless of the total number of metastasis sites, patients included in this cohort who had liver metastasis experienced shorter PFS and OS compared to those without liver metastasis. The findings of this study further contribute to the existing body of research that has investigated the prognostic significance of liver metastasis in cancer patients undergoing immunotherapy, particularly in non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma (RCC), and skin cancer [18–21]. This study examined various sites of metastasis and clinical outcomes in NPC patients with who were treated with anti-PD-L1.
The similar study findings that in R/M NPC patients who received anti-PD-1, various metastatic sites had varying effects on survival, and significant OS benefit from anti-PD-1 was observed in patients without liver metastasis, which were revealed by Ma et al [16]. Our patient cohort receiving anti-PD-L1 therapy in this study represents a distinct population. All patients in the cohort are individuals with NPC who exhibited at least one metastatic site rather than just local recurrence. Additionally, participants in phase 2 clinical trials were administered novel immune checkpoint blockade (ICB) agents.
Research indicates that the overall prognosis for metastatic patients undergoing immune checkpoint blockade (ICB) therapy is influenced by the total number of metastatic sites. Qiao et al. found that in lung cancer patients, more metastatic organs involved were associated with poorer response to immune checkpoint inhibitors (ICIs) [22]. Nevertheless, our study findings revealed that in R/M NPC patients treated with PD-L1 therapy, liver metastasis served as a negative prognostic factor, irrespective of the total number of metastatic organs involved. This may be explained by the liver being anatomically, histologically, and functionally unique [23, 24]. The liver possesses exceptional immunological characteristics due to its abundant and diverse array of antigen-processing cells which allow for the induction of T cell tolerance toward harmless nutrients and commensal bacteria-derived antigens from the gastrointestinal tract. Simultaneously, the liver exhibits potent immune responses against pathogenic microbes. It is anatomically unique in the liver receives blood through the portal vein and releases it via the central vein, organized within a hexagonal lobular pattern where capillaries with fenestrations facilitate direct interaction between circulating T cells and hepatocytes [25]. In preclinical models, Li and colleagues revealed that liver metastases have been observed to divert activated CD8 + T cells from the systemic circulation. Within the liver, activated CD8 + T cells undergo apoptosis following an interaction with monocyte-derived macrophages [26]. However, CD8 + T cells are an important component in ICB [27, 28]. Hence, liver metastasis could potentially disrupt the immune-regulatory mechanisms within the body, subsequently impacting the treatment response of NPC patients who received PD-L1 therapy. The mechanism that NPC patients with liver metastasis may experience diminished clinical benefit from PD-L1 inhibitor therapy should be explored further.
Including our findings of NPC patients, the presence of liver metastasis has been found as a poor predictive factor for patients receiving ICB treatment across multiple cancer types [29–31]. Thus, NPC patients with metastatic disease in the liver should be considered for different or more aggressive treatment. Previous studies have demonstrated that NPC patients with liver metastases may experience clinical benefits from transcatheter hepatic artery chemoembolization (TACE) or partial hepatectomy [32, 33]. Given these previous findings in surgery, patients with NPC who have metastases confined to the liver may derive benefits from undergoing liver resection prior to initiating anti-PD-L1 treatment. Considering that all patients had advanced-stage, surgical treatment was not clinically feasible. However, the combination treatment of transcatheter hepatic artery chemoembolization (TACE) and PD-L1 therapy may hold clinical promise for these individuals [32, 33]. Liu. et al. (2023) found that NPC patients with liver metastases receiving chemotherapy plus PD-1 inhibitor obtained longer PFS than those received chemotherapy alone [34]. Clinical outcomes for our cohort patients may improve with combination chemotherapy in addition to PD-L1 therapy. Additionally, a new treatment that LDH inhibition synergizes with IL-21 could promote CD8 + T cell stemness and antitumor immunity was presented by Hermans and colleagues [35]. Furthermore, liver-directed radiotherapy reshapes the liver's immune microenvironment [26, 36]. Using these results, radiation therapy to the liver or Hermans’ new method before initiating PD-L1 may improve survival in our cohort of patients with liver metastasis.
This study has some noteworthy highlights. First, this is a second analysis from a well-designed phase 2 study focusing on the immunecheckpoint inhibitor outcomes of previously treated R/M NPC patients. Second, this is the first study to assess the prognostic effects of pre-treatment and longitudinal variation of metastatic sites of baseline on NPC patients. Moreover, the sensitive analyses with interaction tests support the robust correlation between liver metastasis of baseline and prognosis of R/M NPC patients.
Admittedly, our study also has some limitations that should be addressed in the following works. Firstly, this is a single-arm design without control groups for comparison. We accounted for this by controlling for baseline disease characteristics and conducting the large sample size of our study. Meanwhile, well-designed randomized controlled trial (RCT) are underway for the future. Furthermore, it is crucial to acknowledge that all patients in the study had non-keratinizing NPC histology. Whether our findings might be applied to other histological types of NPC patients should be further explored. Additionally, this study only focused on the four most frequent metastatic sites, with independent analyses performed on each site. However, given the limited number of patients with brain metastases and isolated liver metastases, the predictive value of these sites could not be fully assessed. This conclusion requires clinical studies with larger sample sizes to be further confirmed.
Conclusion
This study identifies liver metastasis as an independent negative prognostic factor in previously treated R/M NPC patients undergoing immunotherapy, regardless of the number of metastatic sites. These findings suggest that liver metastasis should be carefully considered when selecting immunotherapeutic strategies for NPC patients. Given the diminished benefit of immunotherapy in this subgroup, alternative or combined treatment modalities such as transcatheter hepatic artery chemoembolization(TACE), liver-directed radiotherapy, or combination chemotherapy with PD-L1 inhibitors may be warranted to enhance clinical outcomes. Furthermore, our study highlights the need for large-scale, prospective research to refine the treatment approach for NPC patients with liver metastasis.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
LYT: design, definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. MY: design, definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. WZG: design, definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. LZR: design, definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis. PYY:design, definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis. YYJ: manuscript preparation, manuscript editing, and manuscript review. GK: design, definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis. SG: design, definition.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuantai Li, Yu Min and Zhigong Wei contributed equally to this work.
Contributor Information
Ping Ai, Email: aiping00222@163.com.
Ye Chen, Email: huaxichenye@163.com.
Xingchen Peng, Email: pxx2014@163.com.
References
- 1.Chen Y-P et al (2019) Nasopharyngeal carcinoma. The Lancet 394:64–80 [DOI] [PubMed] [Google Scholar]
- 2.Wong KCW et al (2021) Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol 18:679–695 [DOI] [PubMed] [Google Scholar]
- 3.Chen W et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132 [DOI] [PubMed] [Google Scholar]
- 4.Wang H-Y et al (2016) A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin J Cancer 35:41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y et al (2023) Efficacy and safety of KL-A167 in previously treated recurrent or metastatic nasopharyngeal carcinoma: a multicenter, single-arm, phase 2 study. Lancet Reg Health West Pac 31:100617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AWM, Ma BBY, Ng WT, Chan ATC (2015) Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 33:3356–3364 [DOI] [PubMed] [Google Scholar]
- 7.Hong S et al (2021) Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as first-line therapy for recurrent or metastatic nasopharyngeal carcinoma: final overall survival Analysis of GEM20110714 phase III study. J Clin Oncol 39:3273–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J-Y, Wei X-L, Wang Y-Q, Wang F-H (2022) Current status and advances of immunotherapy in nasopharyngeal carcinoma. Ther Adv Med Oncol 14:175883592210962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masterson L et al (2020) Immune checkpoint inhibitors in advanced nasopharyngeal carcinoma: beyond an era of chemoradiation? Int J Cancer 146:2305–2314 [DOI] [PubMed] [Google Scholar]
- 10.Feng C et al (2021) The prognostic significance of APOBEC3B and PD-L1/PD-1 in nasopharyngeal carcinoma. Appl Immunohistochem Mol Morphol AIMM 29:239–244 [DOI] [PubMed] [Google Scholar]
- 11.Lee JC et al (2022) The liver-immunity nexus and cancer immunotherapy. Clin Cancer Res 28:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumodhee S et al (2022) Impact of radiotherapy schedule on survival of patients treated with immune-checkpoint inhibitors for advanced melanoma and non-small cell lung cancer. cancer radiother. J Soc Franc Radio Oncol. 26:1045–1053 [DOI] [PubMed] [Google Scholar]
- 13.Zou Y et al (2023) The single-cell landscape of intratumoral heterogeneity and the immunosuppressive microenvironment in liver and brain metastases of breast cancer. Adv Sci 10:2203699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pires Da Silva I et al (2020) Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti–PD-1 therapy. Cancer 126:86–97 [DOI] [PubMed] [Google Scholar]
- 15.Bilen MA et al (2019) Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer 19:857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y et al (2021) Copy number loss in granzyme genes confers resistance to immune checkpoint inhibitor in nasopharyngeal carcinoma. J Immunother Cancer 9:e002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu C et al (2017) Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1–positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol 35:4050–4056 [DOI] [PubMed] [Google Scholar]
- 18.Topalian SL et al (2019) Five-year survival and correlates among patients with advanced melanoma, renal cell Carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol 5:1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumeh PC et al (2017) Liver metastasis and treatment outcome with Anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 5:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumeh PC et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pires Da Silva I et al (2020) Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti –PD-1 therapy. Cancer. 126:86–97 [DOI] [PubMed] [Google Scholar]
- 22.Qiao M et al (2021) Efficacy of immune-checkpoint inhibitors in advanced non-small cell lung cancer patients with different metastases. Ann Transl Med 9:34–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzolini G, Ochoa MC, Morales-Kastresana A, Sanmamed MF, Melero I (2012) The liver, liver metastasis and liver cancer: a special case for immunotherapy with cytokines and immunostimulatory monoclonal antibodies. Immunotherapy 4:1081–1085 [DOI] [PubMed] [Google Scholar]
- 24.Juza RM, Pauli EM (2014) Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat 27:764–769 [DOI] [PubMed] [Google Scholar]
- 25.Andersson ER (2021) In the zone for liver proliferation. Science 371:887–888 [DOI] [PubMed] [Google Scholar]
- 26.Yu J et al (2021) Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 27:152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang P et al (2018) Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med 24:1550–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins L et al (2022) Cancer-associated fibroblasts suppress cd8+ t-cell infiltration and confer resistance to immune-checkpoint blockade. Cancer Res 82:2904–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sridhar S et al (2019) Prognostic significance of liver metastasis in durvalumab-treated lung cancer patients. Clin Lung Cancer 20:e601–e608 [DOI] [PubMed] [Google Scholar]
- 30.Sonpavde G et al (2020) Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 Inhibitors. J Urol 204:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y et al (2021) Metastatic sites as predictors in advanced NSCLC treated with PD-1 inhibitors: a systematic review and meta-analysis. Hum Vaccines Immunother 17:1278–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim SJM et al (2016) Metastasectomy for metachronous pulmonary and hepatic metastases from nasopharyngeal carcinoma: report of 6 cases and review of the literature: resection of pulmonary and hepatic metastases from NPC. Head Neck 38:E37–E40 [DOI] [PubMed] [Google Scholar]
- 33.Huang J et al (2014) Partial hepatectomy for liver metastases from nasopharyngeal carcinoma: a comparative study and review of the literature. BMC Cancer 14:818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu G-Y et al (2023) Development of a prognostic model to identify the metastatic nasopharyngeal carcinoma patients who may benefit from chemotherapy combination PD-1 inhibitor. Front Immunol 14:1069010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermans D et al (2020) Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8 + T cell stemness and antitumor immunity. Proc Natl Acad Sci 117:6047–6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong J, Le TQ, Massarelli E, Hendifar AE, Tuli R (2018) Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer 6:46 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.