Abstract
Myeloid cells accumulate extensively in most tumors and play a critical role in immunosuppression of the tumor microenvironment (TME). Like T cells, myeloid cells also express immune checkpoint molecules, which induce the immunosuppressive phenotype of these cells. In this review, we summarize the tumor-promoting function and immune checkpoint expression of four types of myeloid cells: macrophages, neutrophils, dendritic cells, and myeloid-derived suppressor cells, which are the main components of the TME. By summarizing the research status of myeloid checkpoints, we propose that blocking immune checkpoints on myeloid cells might be an effective strategy to reverse the immunosuppressive status of the TME. Moreover, combining nanotechnology, cellular therapy, and bispecific antibodies to achieve precise targeting of myeloid immune checkpoints can help to avoid the adverse effects of systemic administration, ultimately achieving a balance between efficacy and safety in cancer therapy.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03856-6.
Keywords: Immune checkpoint, Tumor microenvironment, Myeloid cells, Nanotherapy
Introduction
Immunotherapies including immune checkpoint blockade (ICB) and chimeric antigen receptor T cells (CAR-T) strategies have revolutionized the cancer treatment landscape. However, the efficacy of anti-PD-1 and anti-CTLA-4 therapies remains limited. In most cancers, only a small fraction of patients benefit from long-term ICB treatment. This limitation is primarily due to intrinsic drug resistance within the tumor and the immunosuppressive nature of the tumor microenvironment (TME) [1]. While CAR-T cell therapy has achieved remarkable success in hematological malignancies, it faces numerous obstacles in solid tumor treatments. Physical barriers including abnormal vascularization and the extracellular matrix restrict their ability to navigate and penetrate the tumor site. Furthermore, when CAR-T cells manage to infiltrate into the tumor, they are compromised by the suppressive immune cells, immune checkpoint expression, and the conditions of hypoxia and nutrient scarcity within the TME. These factors cumulatively diminish the potency and viability of CAR-T cells, thereby reducing their therapeutic efficacy against solid tumors [2]. Myeloid cells, in contrast to T cells, exhibit extensive infiltration in the majority of tumors [3]. Besides, tumor-educated myeloid cells, including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), tumor-infiltrating dendritic cells (TIDCs), and myeloid-derived suppressor cells (MDSCs), significantly contribute to immunosuppression of the TME [3]. Therefore, targeting myeloid cells presents a promising approach to overcome these challenges and enhance anti-tumoral immunity (supplementary Fig. 1).
Like T cells, myeloid cells also express inhibitory molecules, known as immune checkpoints. These checkpoints can extensively influence various functions of myeloid cells, including proliferation, migration, differentiation, and cytotoxicity [4, 5]. Blocking myeloid immune checkpoints is an effective strategy to reverse the immunosuppressive phenotype of tumor-infiltrating myeloid cells, offering a promising target for cancer therapy [4, 5]. A variety of myeloid immune checkpoint blockade therapies has entered clinical trials. Magrolimab (anti-CD47 IgG4) is a pioneering drug for myeloid checkpoint blockade. However, recent clinical trials associated with magrolimab showed frustrating outcomes, primarily due to the widespread expression of CD47 leading to severe side effects, making it difficult to strike a balance between safety and efficacy [6]. Consequently, there is an urgent need for more precise interventions to modulate immune checkpoints of myeloid cells within the TME. This review highlights the impact of immune checkpoint molecules on different types of myeloid cells. By summarizing recent clinical trials, we project potential future trajectories of myeloid checkpoint therapy and anticipate the novel therapeutic approaches that selectively target multiple immune checkpoint molecules on myeloid cells in the future.
Myeloid cell function in the TME
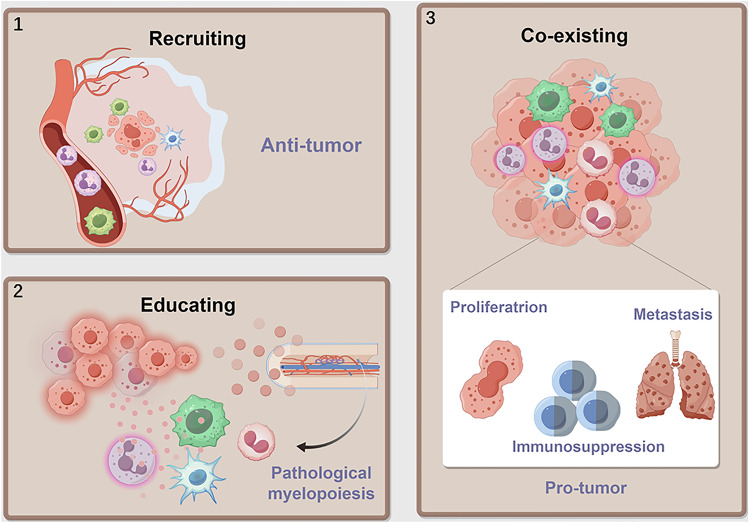
Myeloid cells are involved in all the stages of cancer progression. Tumor cells release cytokines to recruit myeloid cells. In the early stage of tumorigenesis, these myeloid cells induce an inflammatory response to trigger myelopoiesis and recruit other immune cells, which play a role in immune surveillance against tumors. However, under the education of TME, they are gradually reprogrammed to facilitate tumor progression. Persistent myelopoiesis also produces immunosuppressive MDSCs [5, 7]. In the following text, we will discuss the origins and functions of myeloid cells (Fig. 1).
Fig. 1.
Myeloid cells recruited to the TME are polarized into a pro-tumor phenotype. Part 1: Myeloid cells are recruited from the circulation to participate in anti-tumor immune responses. Part 2: However, within the tumor microenvironment (TME), they are polarized toward a pro-tumorigenic phenotype, which generates cancer-related inflammation driving pathological hematopoiesis, resulting in immunosuppressive myeloid-derived suppressor cells (MDSCs) production. Part 3: Ultimately, these myeloid cells within the TME coexist with the tumor cells, contributing to their proliferation, metastasis, and immune suppression. (By Figdraw)
Neutrophil
At the early stages of carcinogenesis, neutrophils with anti-tumor activity are recruited to the TME by cytokines produced by the tumor and surrounding cells [8, 9]. In contrast to neutrophils surrounding the tumor, intratumoral TANs demonstrate a higher propensity for promoting tumor growth and reduced mobility [10]. Similar to macrophages, neutrophils can be divided into N1 and N2 [10]. N2 is the predominant subtype of TANs in most malignancies and is correlated with an unfavorable prognosis in patients. Through the promotion of epithelial genetic instability, tumor cell proliferation, angiogenesis, and tissue remodeling, TANs contribute to cancer progression [11]. Furthermore, TANs play pivotal roles in tumor metastasis. Within premetastatic niches, neutrophils secrete BV8 and metalloproteinases 9 (MMP9) to induce angiogenesis [12], and release proteases to mediate the extracellular matrix (ECM) degradation, facilitating tumor extravasation and growth [13, 14]. Neutrophils can also entrap circulating tumor cells (CTCs) through direct ligation or release neutrophil extracellular traps (NET) to promote tumor metastasis [15, 16].
In contrast, intratumoral neutrophil infiltration is associated with improved overall survival (OS) in patients with colorectal cancer [17] and undifferentiated pleomorphic sarcoma [8]. Through the release of reactive oxygen species (ROS), NO, TNF-related apoptosis-inducing ligand (TRAIL), and TNF, N1 mediates anti-tumor response [18–20] and depletion of neutrophils leads to increased metastatic lesions of breast cancer [21]. A recent study highlights the pivotal role of neutrophils in determining immunotherapy efficacy. Successful immunotherapies elicit the expansion of neutrophils with an interferon gene signature, which is required for tumor control [22], and interferon-stimulated Ly6Ehi neutrophil is an accurate predictor of immunotherapy outcomes [23]. Besides, neutrophils can act as bystanders to eliminate tumor antigen escape variants during anti-tumor response mediated by T cells [24]. These results also underscore the immense potential of targeting neutrophils to impede tumor progression.
Macrophage
Macrophages are the most abundant immune cells in the TME. For a long time, it was thought that TAMs originated from bone marrow-derived monocytic precursors [25, 26]. However, tissue-resident macrophages (TRMs) also play an essential role in creating a supportive environment in the TME. While monocyte-derived macrophages are recruited later, TRMs participate in the formation of nurturing niches during carcinogenesis [25]. TAMs are sustained in the TME via TRM proliferation and monocyte differentiation [25].
Macrophages can induce antibody-dependent cellular cytotoxicity (ADCC), and phagocytosis (ADCP), and trigger adaptive immune responses against tumor cells [25]. Nevertheless, components of the TME, including hypoxia and cytokines, reprogram macrophages into the immunosuppressive M2 phenotype [25]. For example, tumor cells secrete interleukin-4 (IL-4) and Hedgehog ligand to direct M2 polarization [27]. Besides, hypoxia-induced factors (HIFs) in tumor cells boost the expression of checkpoint ligands, such as CD47 and HLA-G, which can bind with SIRPα and LILRB2 to hind the macrophage-mediated anti-tumor response [28, 29]. Most of the time, TAM infiltration correlates with poor prognosis for patients. TAMs facilitate tumor growth by promoting tumor vascularization, epithelial-to-mesenchymal transition (EMT), and ECM remodeling [30]. They also inhibit tumor cell clearance mediated by cytotoxic T lymphocytes (CTLs) via direct contact or secretion of soluble factors [30, 31] and recruit immunosuppressive T regulatory cells (Tregs) [30]. In addition, macrophages can facilitate lymphatic and hematogenous metastasis by interacting with cancer cells, the ECM, and other innate and adaptive immune cells [25].
DC
DCs can be divided into classical dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), and monocyte-derived dendritic cells (MoDCs) [32]. While cDCs excel in antigen presentation, pDCs are characterized by interferon secretion and MoDCs predominantly promote T cell differentiation in response to inflammation [32]. Specifically, this review focuses on cDCs, which possess the capacity to prime T cells and serve as a bridge between innate and adaptive immunity.
cDCs consist of two subtypes, namely, cDC1 and cDC2. In contrast to cDC2s, which lack cross-priming abilities and mainly elicit a CD4 + T cell response, cDC1s can present both endogenous and exogenous antigens to prime CD8 + T cells [32, 33]. DCs can also enhance the T cell response through cytokine secretion and direct conjugation via costimulatory molecules [32]. However, the TME hampers the recruitment of DCs and promotes the generation of tolerogenic DCs. Tumors expressing β-catenin reduce the presence of CC-chemokine ligand 4 (CCL4), leading to decreased infiltration of cDCs [34]. TME can also deactivate tumor-infiltrating NK cells, which normally secrete FMS-related tyrosine kinase 3 ligand (FLT3L) to support DC development and proliferation [35]. Moreover, vascular endothelial growth factor (VEGF) and IL-6 impede DC differentiation in TME [36, 37]. Versican, a type of toll-like receptor 2 (TLR2) ligand in the TME, can promote DCs to release IL-6 and IL-10 and upregulate their receptors on the cell surface through hyperphosphorylation of signal transducer and activator of transcription 3 (STAT3), facilitating the immunosuppression within the TME [37]. Tolerogenic DCs express fewer costimulatory molecules but higher levels of coinhibitory molecules to restrict anti-tumor activity [32]. Upon engagement of CTLA-4 and CD80/CD86, tolerogenic DCs secrete indoleamine 2,3-dioxygenase 1 (IDO1), which inhibits the response of CD8+ T cells, NK cells, and plasma cells, while inducing the differentiation of Tregs [38, 39]. However, this tolerogenic phenotype is reversible [40], indicating the potential for modulating DC function within the TME.
MDSC
MDSCs are immature myeloid cells that arise from pathological myelopoiesis and reprogramming of mature circulating monocytes in peripheral tissues [41]. MDSCs can be categorized into monocytic myeloid-derived suppressor cells (M-MDSCs), polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), and a small proportion of early stage myeloid-derived suppressor cells (e-MDSCs) [42]. Cytokines in the TME can recruit MDSCs and facilitate their expansion [43]. MDSCs play a pivotal role in establishing an immunosuppressive milieu within the TME, primarily through the secretion of various factors such as arginase 1, transforming growth factor beta (TGF-β), IL-10, inducible nitric oxide synthase (iNOS), and IDO [44]. Furthermore, the metabolic pathways of MDSCs can be influenced by the TME, favoring fatty acid oxidation, which amplifies their immunosuppressive capabilities [45]. MDSCs are crucial components of premetastatic niches, where they contribute to angiogenesis and tumor stemness and facilitate EMT to support tumor metastasis [46]. As immature myeloid cells, MDSCs can differentiate into other immunosuppressive or immunostimulating cells. For example, HIF-1α can induce MDSCs to differentiate into M2 TAMs [47]. Conversely, myeloid checkpoint blockade can redirect MDSCs toward an anti-tumor phenotype, which is discussed in detail in the following section.
Immune checkpoints in myeloid cells of the TME
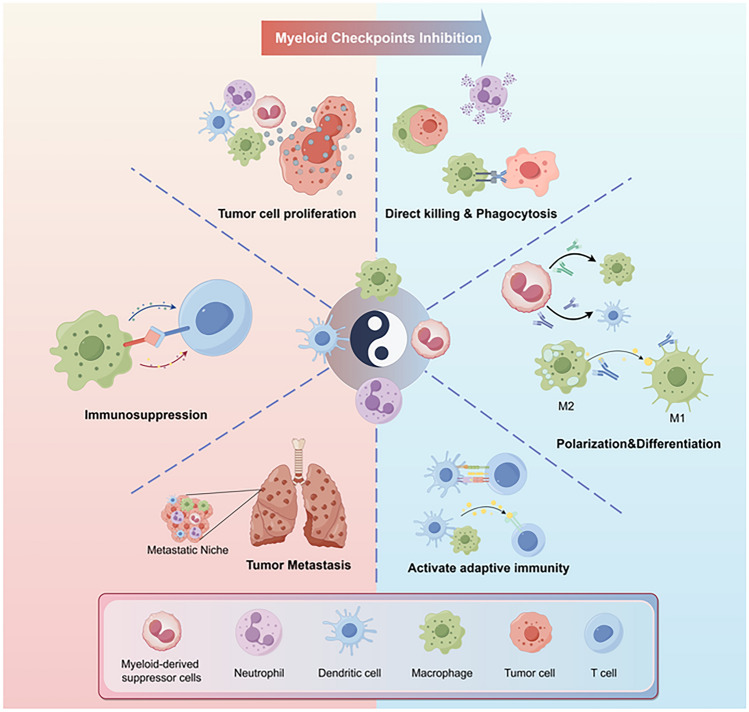
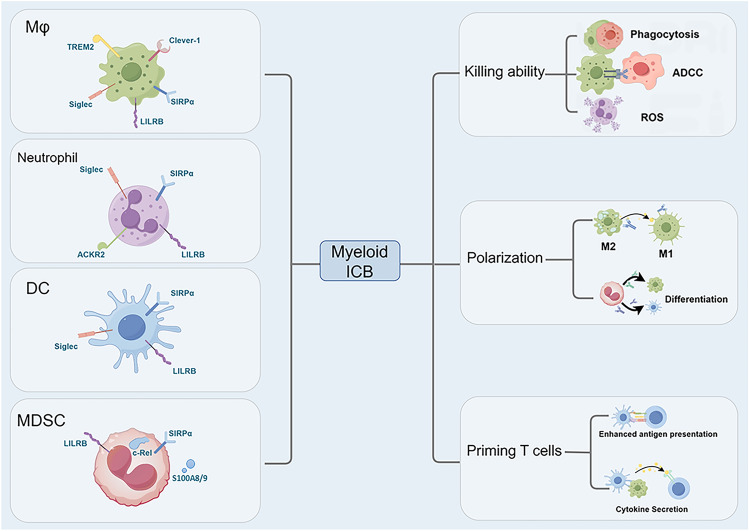
Myeloid cells express many immune checkpoints to prevent self-immunity. In the TME, myeloid checkpoints are upregulated to restrict their anti-tumor roles. Blockade of myeloid immune checkpoints can induce the immunostimulating phenotype of myeloid cells and alleviate immunosuppression in the TME [5], which provides novel therapeutic approaches for cancer treatment (Fig. 2).
Fig. 2.
The impact of immune checkpoint blockade on myeloid cells. Blocking immune checkpoints can enhance the tumor-killing ability of myeloid cells, including phagocytosis, ADCC (antibody-dependent cellular cytotoxicity), and the production of ROS (Reactive oxygen species), particularly in macrophages and neutrophils. Inhibition of myeloid immune checkpoints can also induce the anti-tumor phenotype of these cells. Myeloid checkpoint blockade can polarize M2 macrophages into M1 and promote the differentiation of immature MDSCs (myeloid-derived suppressor cells) into DCs (Dendritic cells) and macrophages. In addition, myeloid immune checkpoint blockade (ICB) can also enhance the antigen presentation and cytokine secretion of myeloid cells, thereby stimulating T cell activation. ACKR2, atypical chemokine receptor 2; Clever-1, common lymphatic endothelial and vascular endothelial receptor-1; LILRB, Leukocyte immunoglobulin-like receptor B; Mφ, macrophage; Siglec, sialic acid-binding immunoglobulin-like lectin; SIRPα, Signal regulatory protein α; TREM2, Triggering receptor expressed on myeloid cells 2. (By Figdraw)
Co-expressed immune checkpoints on myeloid cells
SIRPα: Signal regulatory protein α (SIRPα) is the first member identified in the signal regulatory protein family and is expressed across all kinds of myeloid cells [48]. SIRPα has three immunoglobulin superfamily (IgSF) domains in the extracellular region for ligand binding and a cytosolic domain equipped with both an immunoreceptor tyrosine-based inhibitory motif (ITIM), allowing signal transduction with SH2-containing phosphatase (SHP) [48]. CD47, the primary ligand of SIRPα, provides a "don’t eat me" signal to macrophages, preventing autologous phagocytosis in normal cells. For example, red blood cells (RBCs) from CD47−/− mice are rapidly cleared when transfused into wild-type (WT) recipients, and senescent erythrocytes with diminished CD47 levels are phagocytosed by splenic red pulp macrophages [49].
Tumor cells can upregulate CD47 to evade the macrophage attack [28, 50, 51]. Disrupting the CD47-SIRPα interaction promotes macrophage-mediated phagocytosis and limits tumor growth in vivo [52]. Anti-CD47 treatment also enhances the priming of T cell responses by macrophages [53]. The relationship between anti-CD47 and macrophage phenotypes is complex. Compared with M2 macrophages, anti-CD47 can induce higher phagocytosis rates in M1 macrophages [54]. However, M1 polarization reduces the phagocytosis of A549 and MCF-7 cells in response to anti-CD47 [55]. Although anti-CD47 cannot induce the transformation between M1 and M2 in vitro, it increases the presence of mouse M1 macrophages in vivo [54], suggesting that the effect on macrophage phenotypes is not direct. These paradox outcomes might contribute to the resistance of anti-CD47 therapy, necessitating further research to reveal the underlying mechanisms.
Ring et al. designed the anti-SIRPα antibody KWAR23 to enhance antibody-dependent cellular phagocytosis (ADCP) when combined with monoclonal antibodies (mAbs) targeting tumor-specific antigens, such as rituximab, trastuzumab, and cetuximab [56]. Intriguingly, KWAR23 does not augment the phagocytosis of non-opsonized tumor cells [56]. In fact, CD47-SIRPα only critically regulates tumor cells when tumor cells are decorated by “eat me” signals. These activating signals include calreticulin (CRT) and opsonins, such as the Fc domain of antibodies and complements [57, 58]. CRT, derived from macrophage secretions or endogenous pools, can interact with tumor cell-expressed epitopes and initiate phagocytosis via receptors like low-density lipoprotein receptor-related protein 1 (LRP-1) and C1Q [59]. In addition, signaling lymphocytic activation molecule family receptor 7 (SLAMF7) on tumor cells may activate phagocytosis by interacting with Mac1 on phagocytes. Some studies have suggested that SLAMF7 knockout impairs the phagocytic ability of macrophages targeting L1210 cells [60], whereas there is also a study reporting no correlation between SLAMF7 expression and phagocytosis induced by CD47 blockade [61]. A recent study revealed that SLAMF7, when interacting in cis with CD47 on the surface of tumor cells, effectively suppresses its potential to trigger phagocytosis [62]. The combination of the SLAMF7 antibody and the SIRPα antibody exhibited potent efficacy against cancer cells in both in vitro tissue cultures and within tumor-bearing mice. Given the low expression of SLAMF7 and SIRPα in RBCs, the combination might be a good strategy to avoid anemia induced by anti-CD47 [62] (supplementary Fig. 2). Besides, type I interferons (IFN) can reprogram tumor cell metabolism by activating oxidative phosphorylation, which is essential for CD47-SIRPα blockade efficacy [63].
As phagocytes, neutrophils also receive the “don’t eat me” signal mediated by CD47-SIRPα interactions [64]. Trogocytosis is a specialized form of phagocytosis mediated by neutrophils, which can mechanically disrupt the plasma membranes of tumor cells. CD11b/CD18 integrin-mediated neutrophil-tumor cell conjugation, essential for trogocytosis, is inhibited by CD47-SIRPα interactions. The inhibition is kindlin3-dependent and can be reversed by blocking CD47-SIRPα to activate integrins [65–67].
Anti-CD47 mAbs can improve T cell response to kill tumor cells, predominantly by promoting DC cross-priming [68]. ALX-148, a CD47 chimeric antibody composed of a modified SIRPα domain and an inactive human IgG1 Fc, modulates DC subsets, reducing CD8−DC while increasing CD8+ DCs, which are vital for cytotoxic T lymphocyte (CTL) cross-priming. Both DC subtypes upregulate the activation marker CD86 under the influence of ALX-148. The effects on CD8+ DCs were augmented in combination with anti-PD-1 therapy [69]. Furthermore, CD47 knockout tumor cells stimulate the proliferation of CD11c+ DCs, and vaccines using these DCs with CD47-defective tumor cells show superior efficacy compared to those using wild-type cells. Notably, SIRPα+ DCs display superior antigen-presenting ability compared to SIRPα−DCs, indicating potential targeting of the CD47-SIRPα axis to promote antigen presentation [70].
CD47 blockade increases phagocytosis of tumor DNA by CD103+ DCs, leading to increased secretion of CXCL9 and IL-12 via the cGAS-STING signaling pathway. This promotes the recruitment of NK cells and enhances their tumor-killing activity. Hypoxic TME conditions that impair ICB efficacy actually facilitate phagocytosis by CD103+ DCs, suggesting anti-CD47 therapy as a valuable alternative for ICB-resistant patients [71].
CD47 expression correlates with MDSC accumulation in TME. In a preclinical model of head-and-neck squamous cell carcinoma, anti-CD47 treatment decreased MDSC infiltration into the primary tumor and tumor-draining lymph nodes [72, 73]. Anti-CD47 inhibits the immunosuppressive function of MDSCs [74, 75]. Blockade of the CD47-SIRPα axis induces MDSC differentiation, leading to overexpression of major histocompatibility complex (MHC) II, CD86, and chemokines including macrophage chemoattractant protein 1 (MCP-1) and microtubule-associated protein 2 (MAP-2) [74]. Anti-SIRPα mAbs also reduce TGF-β and iNOS production by MDSCs [76]. Although chemotherapy can increase MDSC infiltration within the TME, dual anti-PD-L1 and anti-CD47 treatment can counteract this accumulation, especially in oxaliplatin (OXP) and FOLFOX regimens, thereby improving therapeutic outcomes [75].
LILRB: Leukocyte immunoglobulin-like receptor B (LILRB) is a member of the leukocyte immunoglobulin-like receptor family. The LILRB family consists of five members: LILRB1-5. Each member is equipped with intracellular ITIMs and external Ig-like domains for ligand binding. LILRB mainly interacts with MHC I, including classic MHC I(HLA-A, HLA-B, and HLA-C) and non-classic MHC I (HLA-E, HLA-F, and HLA-G) [77]. In addition, S100A8/A9, some myelin-associated proteins, and angiopoietin-like proteins (ANGPTL) are also identified as LILRB ligands [78].
LILRB1 is enriched in TAMs where its engagement with MHC I on tumor cells can lead to resistance to phagocytosis. Similar to SIRPα, LILRB1 also transmits a "do not eat me" signal to macrophages via ITIM/SHP signaling [5]. The recognition of MHC I by LILRB1 depends on the β2 microglobulin subunit of the MHC I complex, in contrast to LILRB2, which does not exhibit this dependency [79]. The interaction of LILRB2 with MHC I drives macrophages toward an immunosuppressive state besides inhibition of phagocytosis. Research by Chen et al. has demonstrated that blocking LILRB2 can enhance the phagocytic capacity of TAMs and promote a shift toward a more inflammatory M1 macrophage phenotype [80].
Upon stimulation, LILRB2 is upregulated on neutrophils to prevent excessive activation. Its interaction with HLA-G can inhibit neutrophil-mediated phagocytosis and the production of ROS [81]. LILRB2 also modulates neutrophil migration. Paired Ig-like receptor B (PIR-B) is a murine homolog of the human LILRB2. Pirb− neutrophils exhibit a stronger response to chemokines and increased affinity for integrins [82]. Therefore, the downregulation of LILRB2 expression augments the cytotoxic and migratory functions of neutrophils, potentially facilitating the TME infiltration of neutrophils to mediate anti-tumor response.
The upregulation of LILRB2 is also implicated in the induction of tolerogenic DCs [83]. LILRB2 promotes the secretion of IL-10 by DCs, which enhances HLA-G expression on T cells, amplifying the inhibitory effects mediated by LILRB2 [84]. Interestingly, PIR-B can compete with CD8+ T cells for MHCI to inhibit their proliferation and activation [85]. Moreover, the knockdown of PIR-B in mice increases immature DCs that are more likely to induce a tumor-promoting (helper) Th2 immune response [86]. However, binding ANGPTL2 to PIR-B can facilitate DC maturation and activation [87]. These seemingly contradictory findings underscore the complexity of PIR-B’s role and highlight the need for further investigation into its downstream signaling.
Despite its low abundance in peripheral blood, M-MDSCs demonstrate a significant correlation with survival, suggesting their potent immunosuppressive role [88]. PIR-A, the murine ortholog of human LILR, influences the differentiation of M-MDSCs into either M1 or M2 macrophages, depending on the balance of PIR-A and PIR-B signaling. The absence of PIR-B in MDSCs leads to increased PIR-A expression and a preference for M1 differentiation. Adoptive transfer of Pir-b− MDSCs reduces the activation of Tregs and angiogenesis, resulting in the inhibition of tumor growth and metastasis, and ultimately prolonging patient survival [89].
Galectin-8, identified as a novel ligand for LILRB4, has been found to induce M-MDSC-mediated promotion of tumor growth [90]. M-MDSCs constitutively express LILRB4. Blocking LILRB4 decreases inhibitory cytokine secretion and Treg activation induced by M-MDSCs [91]. Adoptive transfer of lilrb4−/− MDSCs suppresses tumor metastasis [92]. Furthermore, LILRB4 upregulates VEGF-A to promote tumor cell motility and angiogenesis in non-small cell lung cancer (NSCLC) [93]. The blockade of LILRB4 downregulates the VEGF-A and MMP9 production in MDSCs of tumor-bearing animals [92]. Additionally, sunitinib, an anti-angiogenic multikinase inhibitor, depletes MDSCs in the tumors and the circulation of preclinical models [94]. These findings suggest that anti-LILRB4 therapy, in combination with anti-angiogenic drugs, may offer a promising approach to cancer treatment. Given the shared expression of LILRB4 and similar derivation and functional characteristics between M-MDSCs and monocytic acute myeloid leukemia (AML) cells, targeting LILRB4 could emerge as a potential strategy for monocytic AML therapy [95].
Siglec: Sialic acid-binding immunoglobulin-like lectins (Siglecs) belong to IgSF characterized by 2 to 17 extracellular immunoglobulin domains. Most Siglecs possess ITIMs within their cytosolic domains, which indicates they might play similar roles with SIRPα and LILRB [96].
Siglec-9 recognizes the cancer-associated sialyl T glycoform of Mucin (MUC)1. This interaction induces a unique TAM phenotype with poor phagocytic ability, which correlates with poor prognosis in patients with breast cancer [97]. These TAMs can also suppress T cell responses and degrade the basement membrane, thereby facilitating tumor invasion [98]. Notably, the deletion of Siglec-9 in macrophages has been shown to enhance the recruitment and priming of T cells, which augments the efficacy of anti-PD-1 therapy [99]. Targeting Siglec-9 has shown promise in reducing the tumor burden in a humanized murine model [100]. Moreover, the combination between Siglec-9 and tumor-derived sialic acid is implicated in the differentiation of monocytes into immunosuppressive TAMs, a key factor contributing to poor prognosis in PDAC patients [101].
The interaction between Siglec-10 and CD24 has emerged as a potential therapeutic target. This ligation can activate SHP-1 and/or SHP-2 phosphatases associated with ITIMs, thereby mitigating TLR-mediated inflammation and reducing the phagocytic activity of macrophages [102]. Siglec-15 is also recognized as a target for cancer therapy. In murine models, Siglec-15 on TAMs interacts with Siglyl-Tn on tumor cells, leading to the release of TGF-β. Anti-Siglec-15 mAbs can promote T cell responses and M1 polarization, thereby limiting tumor growth in vivo and vitro [103]. IFN-γ upregulates PD-L1 and downregulates Siglec-15 in the TME, suggesting that Siglec-15 may serve as a potential target for cancer patients, especially those who are refractory to anti-PD-1/L1 therapy [104].
In neutrophils, Siglec-9 attenuates the production of ROS and NETs [105], [106]. The overexpression of sialic acid on tumor cells, via Siglec-9, inhibits ADCC of neutrophils [107, 108]. Siglec-E, a murine homolog of Siglec-9, modulates neutrophil activation in an epitope-specific manner. Studies have demonstrated that Siglec-E− neutrophils produce higher levels of ROS and express greater amounts of TRAIL and FasL when co-cultured with tumor cells. Furthermore, Siglec-E has been implicated in skewing macrophages toward an M1 phenotype in 3-methylcholanthrene-induced sarcomas. However, most studies have exhibited opposite outcomes using tumor cells from patients or specific cell lines [98, 99, 109, 110], indicating variability in Siglec-E-mediated immunosuppression. Considering that the immunosuppressive effects of Siglec-E are epitope-dependent, tumor cells may alter the sialic acid components on the cell surface to reverse the anti-tumor activity of immune cells.
Based on the above theory, tumor progression might be a matter of kinetics: neutrophils are initially recruited to eliminate the tumor, whereas tumor cells upregulate the ligands of Siglec-E to compromise immunity. Subsequently, macrophages migrate to the tumor site to substitute neutrophils. Macrophages exhibit an M1 phenotype via Siglec-E at an early stage. As the tumor progresses, changes in sialic acid on tumor cells alter the signal transmitted by Siglec-E, which skews macrophages into the M2 phenotype, resulting in immune escape [111].
Siglec-E also impedes the maturation and activation of DCs, impairing their antigen-presenting capabilities. The knockout of Siglec-E in tumor-bearing mice results in the upregulation of maturation markers in cDCs. When co-cultured with Siglec-E+ DCs, CD4+T cells showed reduced activation and proliferation. Siglec-10 can also reduce the immune response of DCs to damage-associated molecular patterns (DAMPs) [112]. Its homolog, Siglec-G, is found to be upregulated in tumors. The acidic environment within phagosomes can hinder the cross-presentation of exogenous antigens. NADPH oxidase 2 (NOX2), which promotes ROS production to alkalize phagosomes, is suppressed by Siglec-G through SHP-1 to compromise the cross-presenting function of DCs [113]. These insights highlight the complex role of Siglecs in modulating immune responses within the TME and underscore the need for a nuanced approach to targeting these receptors in cancer therapy.
Immune checkpoints on specific myeloid cells
Macrophage
Scavenger receptors: Scavenger receptors can recognize ligands widely, including pathogen-associated molecular patterns (PAMPs) and DAMPs, and mediate the clearance of dying cells and bacteria through endocytosis. According to their structure, cell type-specific expression, and recognition of host-derived ligands, they are categorized into the A-L subtype [114]. Within the TME, certain scavenger receptors enriched in TAMs have garnered attention as potential therapeutic targets [115]. One such receptor is the common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1), also known as stabilin-1 or FEEL1 [115]. Bexmarilimab, a drug that targets Clever-1, has progressed to clinical trials (supplementary Table 1), and the impact of Clever-1 on macrophages will be discussed in further detail below.
Clever-1 has been identified as a distinctive marker for immunosuppressive monocytes and macrophages [116]. A retrospective analysis identified Clever-1+ TAMs as a separate prognostic marker for poor OS in bladder urothelial carcinoma [117]. Notably, Clever-1hi monocytes can inhibit the Th1 response, whereas the blockade of Clever-1 enhances the production of pro-inflammatory cytokines and antigen presentation, thereby stimulating the activation of CD8+ T cells [118]. Clever-1 expression on TAMs also contributes to the clearance of certain tumor-inhibiting factors. For instance, secreted protein acidic and rich in cysteine (SPARC) inhibits tumor growth, angiogenesis, and invasion, and blockade of Clever-1-mediated SPARC clearance increases the sensitivity of the tumor to nab-paclitaxel [119]. Additionally, Stabilin-1-interacting chitinase-like protein (SI-CLP) is implicated in TAM recruitment and modulation of cell composition in the TME, thereby restricting tumor growth. However, its expression is downregulated and even absent in breast cancer [120], and the blockade of Clever-1 could serve as a strategy to reverse the situation [121]. Interestingly, Clever-1 is also known to induce an immune switch in TAMs, with macrophages in Clever-1-deficient mice demonstrating enhanced immunostimulatory activity. This is associated with a metabolic shift from oxidative phosphorylation to glycolysis, triggered by mTOR signaling [122]. These findings collectively suggest that Clever-1 plays an active role in fostering an immunosuppressive state within the TME, potentially contributing to resistance to immunotherapy. Supporting this notion, data from The Cancer Genome Atlas (TCGA) database have revealed significantly higher Clever-1 expression in non-responsive patients undergoing anti-CTLA-4 or anti-PD-1 therapy [116]. These insights highlight the potential of Clever-1 as a biomarker for predicting the efficacy of immunotherapy and underscore the need to explore combination therapies that include anti-Clever-1 agents alongside existing immune checkpoint blockers for cancer therapy.
TREM2: Triggering receptor expressed on myeloid cells 2 (TREM2) is a member of the IgSF family with a single extracellular V-type immunoglobulin domain and a short cytoplasmic domain lacking obvious signaling motifs. It connects with the DNAX activation protein (DAP)12 and DAP10 to conduct signals [123]. In both primary and metastatic tumors, TREM2 is enriched in monocyte-derived TAMs with an immunosuppressive phenotype [124, 125]. Compared to TREM2+ macrophages, TREM2− macrophages exhibited increased phagocytic activity and cytotoxic effects on human glioblastoma cells. TREM2 inhibition increases the presence of PD-1+ CD8+ T cells within the TME, suggesting a potential strategy for enhancing anti-tumor immunity [126, 127]. Recent research by Park et al. has illuminated that TREM2-expressing monocyte-derived macrophages may attenuate the recruitment and activation of NK cells within the tumor [128]. Furthermore, TREM2+ TAMs have been found to stimulate the proliferation of tumor cells in an immunosuppression-independent manner, underscoring a multifaceted role in tumor progression [129]. While elevated TREM2 expression is often correlated with a poor prognosis in a spectrum of cancers, it is crucial to note the heterogeneity in TREM2's role. For instance, one study has indicated that TREM2 may exert a protective influence in a diethylnitrosamine (DEN)-induced hepatocellular carcinoma model, and no significant difference in TREM2 expression was observed among patients with varying prognosis following targeted therapy [130]. These nuanced findings highlight the complexity of contextual background on TREM2's function in TAMs.
Neutrophil
ACKR2: Chemokines play an important role in sustaining inflammation and tumor development within the TME. Atypical chemokine receptor 2 (ACKR2) functions as a decoy and scavenger receptor for most chemokines. Studies have indicated that the depletion of ACKR2 is associated with primary tumor growth [131, 132]. However, neutrophils in Ackr−/− tumor-bearing mice upregulate the transcription of CCR1, CCR2, and CCR5, which increases the recruitment and tumor-killing activity of neutrophils in the metastatic niche, leading to a reduction of the metastatic lesions in preclinical models [133]. Additionally, CXCL14 promotes tumor metastasis by interacting with ACKR2 in tumor cells and cancer-associated fibroblasts (CAFs) [134, 135]. These findings collectively suggest that ACKR2 represents a promising therapeutic target for countering cancer metastasis.
MDSC
c-Rel: c-Rel, a member of the NF-κB family, is expressed in myeloid and lymphoid cells. Deletion of c-Rel in myeloid cells restricts tumor growth in murine models and decreases the proportion and immunosuppressive functions of MDSCs. c-Rel plays a critical role in regulating MDSC development and metabolism. MDSCs lacking c-Rel upregulate genes enriched in inflammatory response and cell cycle checkpoints, while downregulating genes enriched in glucose, amino acid, and lipid metabolism. Cytokines in the TME activate c-Rel, STAT3, and CCAAT/enhancer binding protein α (C/EBPα). These transcription factors enter the nucleus and interact with the enhancers or promoters of MDSC signature genes, which initiates the differentiation of MDSCs. Notably, the complex binds to the CCR2 and IL-1β genes, resulting in the production of CCR+Arg−IL-1βhi M-MDSCs (rMo), which exhibit a higher immunosuppressive activity. Interestingly, global deletion of c-Rel results in lower MDSC frequencies than myeloid-specific deletion in tumor-bearing mice, indicating that c-Rel in other cell types, besides myeloid cells, might affect MDSC migration. The blockade of c-Rel does not influence the frequency of myeloid and lymphoid cells in non-tumor-bearing naïve mice, suggesting that targeting c-Rel could be a promising approach to inhibit MDSCs specifically in the TME [136, 137].
S100A8/A9: S100A8 (MRP-8 or calgranulin-A) and S100A9 (MRP14 or calgranulin B) are members of the S100 protein family. In humans, S100A8 and S100A9 bind to each other to form polymers, particularly in the presence of Ca2+[138]. Within the TME, secretion of CCL2 by cancer cells and macrophages recruits MDSCs and initiates S100A8/A9 release via the CCL2-CCR2 axis [139–141]. The STAT3 signaling plays a pivotal role in the proliferation and activation of MDSCs, with phosphorylated STAT3 enhancing the expression of S100A9 [142, 143]. Elevated levels of S100A9 subsequently suppress the activation of BAX and caspase3, while upregulating the expression of Bcl-2, thereby attenuating the apoptosis of MDSCs [143]. S100A9 can disrupt the differentiation of myeloid cells, promoting their transformation into immunosuppressive MDSCs [144, 145]. S100A8/A9 can also act as a chemokine to recruit MDSCs [146–148]. Therefore, S100A8/A9 establishes an autocrine feedback loop that perpetuates the accumulation of MDSCs within the TME. The levels of S100A8/A9 in tumor tissues and patient serum can serve as biomarkers for MDSC-mediated immunosuppression [149, 150]. Additionally, S100A8 and S100A9 can bind to receptors on cancer cells, including advanced glycation end products (RAGE) and TLR4, which can promote tumor development and metastasis [151]. These results suggest their potential as therapeutic targets. However, their established roles in anti-infection response and maintaining immune homeostasis require careful consideration to minimize the potential side effects of targeting these proteins [151].
Targeting myeloid checkpoints for cancer therapy
Recently, many drugs that target myeloid checkpoints have been developed (Supplementary Table). CD47 has emerged as a particularly compelling target, given its pervasive expression across cancer cells and its role in emitting the "don't eat me" signal, which is pivotal in tumor immune evasion. However, the widespread expression of CD47 in healthy tissues, including erythrocytes and platelets, poses a hematotoxicity risk following anti-CD47 interventions [6]. Most anti-CD47 mAbs in clinical trials are IgG4 subtypes with low Fc activity. This attribute, while beneficial in mitigating side effects, concurrently presents a challenge in maximizing therapeutic efficacy. Magrolimab (GS-4721, Hu5F9-G4), an anti-CD47 IgG4 mAb, was a pioneer that advanced to clinical trials. However, its journey was not without setbacks. Magrolimab studies in hematologic tumors were discontinued due to futility and increased risk of death. Global enrollment for magrolimab's solid tumor studies has also been paused to reassess the risk–benefit profile across ongoing trials. Evorpacept (ALX-148), a fusion protein comprising an inert human IgG1 Fc fragment and a modified SIRPα D1 domain, stands out for its high-affinity binding to CD47. It inhibits the binding of wild-type SIRPα, thereby enhancing the phagocytosis of tumor cells while sparing normal RBCs, translating to improved patient tolerability [152]. However, history seems to repeat itself. The ASPEN-02 trial, which evaluated evorpacept in tandem with azacitidine for myelodysplastic neoplasm (MDS), was also terminated.
However, evorpacept's application in HER2-positive gastric cancer has shown promising results. An interim analysis from the phase 2 ASPEN-06 clinical trial indicated that the combination of evorpacept with trastuzumab and ramucirumab achieved an impressive objective response rate (ORR) of 52% in patients with advanced HER2-positive gastric cancer, eclipsing the standard therapy's 22% ORR. Furthermore, the 2024 AACR annual meeting highlighted the combination of evorpacept with R2 therapy (lenalidomide and rituximab), demonstrating robust anti-tumor efficacy and comparable toxicity to R2 therapy alone in a phase II single-arm trial for relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL) patients.
MK4830, an anti-LILRB2 IgG4, has shown a favorable safety profile in a clinical trial involving 84 patients, with a notable synergistic effect when combined with anti-PD-1 therapy. The combination of MK4830 and pembrolizumab resulted in a 45% response rate among patients previously unresponsive to anti-PD-1 or other combination therapies [153]. The CD24-Siglec-10 axis, which also transmits a "don't eat me" signal, offers a distinct advantage over anti-CD47 approaches, as the absence of CD24 on normal red blood cells and thrombocytes lessen the risk of anemia and thrombocytopenia. Despite this, drug development targeting CD24 faces significant challenges due to its limited immunogenicity, leaving most candidates in the preclinical stages. Siglec-15 has emerged as a promising target, given its mutually exclusive expression with PD-L1 and significant differential expression between tumor and normal cells. A phase I clinical trial demonstrated the safety of anti-Siglec-15 NC318, with the primary side effects being diarrhea and asymptomatic increases in amylase and lipase levels [154]. In a phase II clinical trial for NSCLC patients using a combination of NC318 and pembrolizumab, two patients achieved partial response (PR), and two patients achieved stable disease (SD) out of 18 patients, resulting in an ORR of 11%. Targeting immune checkpoint molecules that repolarize TAMs is also a promising strategy for cancer therapy. Bexmarilimab (FP-1305), a humanized IgG4 anti-Clever-1 antibody, can reprogram macrophages toward a pro-inflammatory state, thereby inducing CD8 + T cell-mediated anti-tumor responses [155, 156]. A phase I/II first-in-human clinical trial (MATINS; NCT03733990) has demonstrated the good safety and tolerability of Bexmarilimab [155]. In addition, PY314, a humanized mAb to deplete TREM2+TAMs, is also well tolerated in patients with renal cell carcinoma (RCC). However, the efficacy of bexmarilimab and PY314 still needs further research. Tasquinimod is an orally administered drug that disrupts the combination of S100A9 with RAGE and TLR4, demonstrating good tolerability in humans [157, 158]. In a phase III clinical trial, treatment with tasquinimod increased radiologic progression-free survival (PFS) in patients with metastatic castration-resistant prostate cancer (mCRPC). However, no impact on OS was observed [159]. Another phase II clinical trial also failed to detect the therapeutic benefits of tasquinimod in the treatment of advanced hepatocellular, gastric, ovarian, and renal cell carcinomas [160]. Recently, the role of Tasquinimod in myeloproliferative neoplasms (MPNs) has garnered attention, and corresponding clinical trials are currently recruiting participants (Supplementary Table 1).
We anticipate elucidating the future directions and efficacy of myeloid checkpoint blockade by analyzing preclinical and clinical data on CD47, a widely researched myeloid checkpoint with clinical potential. The clinical experiments of anti-CD47 demonstrated that achieving the balance between safety and therapeutic efficacy is challenging for myeloid checkpoint blockade with monotherapy. Therefore, combination therapy has attracted widespread attention. Despite suggestions of potential synergies between myeloid and T checkpoint blockades [25, 161, 162], the discontinuation of many clinical trials combining anti-CD47 and anti-PD-1 therapies indicates the complexity of this approach (Supplementary Table). Considering macrophage-mediated clearance of transferred T cells [163], the T cell toxicity of anti-CD47 might be the obstacle to the combination potential between myeloid and T checkpoint blockade. Chemotherapy and radiotherapy can induce the expression of pro-phagocytic signals such as CRT on the tumor cell membrane to enhance the efficacy of anti-CD47 therapy [164]. The combination of radiotherapy with CD47 blockade also induces the macrophage-mediated abscopal effect [165]. However, chemotherapy can inhibit the myelopoiesis of patients and the combination of magrolimab and azacitidine failed to obtain good clinical results. As for radiotherapy, more clinical trials are required to study its combinatory potential with myeloid checkpoint blockade. Recent studies have emphasized the importance of the Fc region of anti-CD47 antibodies for their anti-tumor activity [58]. Considering that tumor-specific mAbs can also provide Fc regions for the “eat me” signal, CD47/tumor-associated antigens (TAA) bispecific antibodies might be a more precise and effective strategy for cancer therapy.
Nanotherapy and cellular therapy have emerged as innovative approaches to realize in situ tumor delivery, thereby circumventing the adverse effects due to the wide expression of myeloid checkpoints or their ligands. Gao and colleagues developed a pH-responsive nanocarrier that releases CD47 inhibitor (RRX-001) in the acidic tumor microenvironment, minimizing the impact on normal cells. This nanocarrier also delivers a T-type calcium channel inhibitor (TTA-Q6), which upregulates CRT on the tumor cell surface, presenting an "eat me" signal for phagocytosis [166]. A pH-responsive nanoparticle also delivers an anti-CD47 drug and a senescence inducer to combat liver cancer. In this nanosystem, nanotechnology was used to coload lipid-soluble and water-soluble drugs to increase drug accumulation within the tumor and minimize systemic toxicity [167].
Bacterial outer membrane vesicles (OMVs) with high biocompatibility have emerged as a prevalent delivery platform in tumor immunotherapy. PEG/Se@OMV-CD47 nanobody (nb) is created by the fusion of CD47nb to ClyA on the surface of OMVs with the outer surface coated with a polyethylene glycol (PEG) layer containing diselenide bonds (PEG/Se). This coating shields the immunogenicity of PEG/Se@OMV-CD47nb in the systemic circulation, mitigating the risk of systemic immune activation from intravenous injection. Targeted radiation at the tumor site disrupts the PEG/Se layer, enabling controlled release of OMV-CD47nb within the TME, thereby enhancing local concentration and reducing systemic impact [168]. M1-derived extracellular vehicles (EVs) have an innate propensity to migrate toward the TME [169]. By decorating these EVs with an anti-tumor peptide RS17 that specifically binds to CD47 on cancer cells, the researchers achieved active targeting and enhanced local phagocytosis without affecting normal cells [169]. Peng’s team has designed a surface-engineered extracellular vesicles (SE-EVs) that display nanobodies against CDH17 on gastric cancer cells and load RRX-001, achieving a precise blockade of the CD47-SIRPα axis [170]. The combination of chimeric antigen receptor macrophages (CAR-M) and anti-CD47 has also shown promising efficacy. Researchers designed a cavity-injectable nanoporter-hydrogel superstructure to introduce glioma stem cell-targeted CAR genes into macrophages, increasing efficacy to prevent the relapse of glioblastoma after surgery [171].
Cancer vaccines stimulate tumor-specific immune responses, potentially synergizing with myeloid checkpoint inhibitors. This strategic combination may enhance anti-tumor immunity while minimizing the risk of autoimmune reactions. Yang and colleagues demonstrated that the removal of CD47 from tumor cells can significantly enhance the immunogenicity of tumor vaccines, thereby inducing a robust anti-tumor immune response in mouse models [70]. DCP-001 is a whole tumor cell vaccine derived from the myeloid leukemia cell line. After pre-incubation with DCP-001 and an anti-CD47 antibody, DCs increased the uptake of the tumor vaccine. DCP-001 can activate T cell response through intradermal injection, suggesting less hematotoxicity than systemic administration [172]. OVM can effectively activate DC vaccines and reactivate tumor-suppressed DCs by downregulating both SIRPa and CD47. Furthermore, the combination of PD-L1 blockade with the OVM further enhanced anti-tumor efficacy [173]. The innovative therapies, though not yet approved for clinical trials, represent a hopeful frontier in myeloid checkpoint blockade, particularly highlighting the potential of anti-CD47 interventions.
Perspective
In the complex dynamics between the immune system and cancer, myeloid cells have been recognized as the main components and pivotal modulators within the TME. Targeting the immune checkpoints on these cells holds promise to polarize them from pro-tumorigenic to anti-tumorigenic phenotype for reshaping the suppressive TME, thus potentiating the impact of cancer immunotherapy [25, 161, 162].
CD47 is the most extensively studied myeloid immune checkpoint, with its antagonist magrolimab advancing to phase III clinical trials. Despite this progress, the results have been frustrating. The expression of CD47 on RBCs constrains the potency of therapeutic interventions, complicating the balance between safety and efficacy. Therefore, the challenge of selectively targeting CD47 on tumor cells without impacting normal cells is a critical area of current investigation. Considering similar expression patterns and shared signaling pathways via ITIMs of SIRPα, LILRB2, and Siglecs, how to target tumor cells precisely but spare normal cells might be a common challenge of myeloid checkpoint blockade in the future. Nanotherapies, cell therapies, and bispecific antibodies provide a promising solution for this problem. Nanotherapies and cell therapies primarily act locally at the tumor site, while bispecific antibodies prevent on-target off-tumor binding. Some bispecific antibodies have already entered clinical trials (Supplementary Table), and the future of nanotherapies and cell therapies remains promising.
Previous reviews focus on the dissection biological roles of myeloid checkpoints. However, the expression of these molecules in various myeloid cells and their functions across different cell types is confusing. This is especially challenging for scientists with a strong focus on material development but limited biological expertise. Our article categorizes these molecules based on four types of myeloid cells, including macrophages, neutrophils, DCs, and MDSCs. We hope our review can help them to elucidate this problem and catalyze the development of precisely targeted therapies to combat cancer.
In addition to widely expressed checkpoints like SIRPα, LILRB2, and Siglecs, there is growing interest in immune checkpoints expressed on specific subsets of myeloid cells. For instance, ACKR2 has become a significant checkpoint in tumor metastasis. The unique roles of these checkpoints emphasize the need for a nuanced understanding of their functions and the development of targeted therapies.
It is also important to acknowledge that some immune checkpoints, such as PD-1 and VISTA, were not discussed in this review. Our review mainly focuses on the checkpoints with specific expression on myeloid cells. While these molecules also modulate myeloid cells, they primarily regulate anti-tumor response mediated by T cells. The multifaceted nature of these checkpoints and their impact on various immune cell types highlight the complexity of the immune system's regulatory mechanisms.
In summary, targeting myeloid immune checkpoints represents an important frontier in cancer immunotherapy. By elucidating the mechanisms of these checkpoints, we aim to catalyze the development of precisely targeted therapies to combat cancer. The future of this field promises an integration of innovative approaches, including nanotechnology, cell therapy, and bispecific antibodies, all aimed at enhancing the precision and effectiveness of immunotherapies while minimizing adverse effects. This advancement brings great hope to patients, offering the potential for more effective and safer cancer treatments.
Disclosure
Clinical data for recent clinical trials are not published but are available on the developers' official websites.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge Figdraw (figdraw.com) for providing assistance with graphical illustrations and clinicaltrials.gov for providing information for the Supplementary table.
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- ADCP
Antibody-dependent cellular phagocytosis
- AML
Acute myeloid leukemia
- ANGPTL
Angiopoietin-like protein
- CAR-M
Chimeric antigen receptor macrophage
- CAR-T
Chimeric antigen receptor T cell
- CCL
CC-chemokine ligand
- cDC
Classical dendritic cell
- C/EBPα
CCAAT/enhancer binding protein α
- Clever-1
Common lymphatic endothelial and vascular endothelial receptor-1
- CRT
Calreticulin
- CTC
Circulating tumor cell
- CTL
Cytotoxic T lymphocyte
- DAMP
Damage-associated molecular pattern
- DAP
DNAX activation protein
- ECM
Extracellular matrix
- e-MDSC
Early stage myeloid-derived suppressor cell
- EMT
Epithelial-to-mesenchymal transition
- EV
Extracellular vehicles
- FasL
Fas ligand
- HIF
Hypoxia-induced factor
- ICB
Immune checkpoint blockade
- IDO
Indoleamine 2,3-dioxygenase
- IFN
Interferon
- IgSF
Immunoglobulin superfamily
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- ITIM
Immunoreceptor tyrosine-based inhibitory motif
- LILRB
Leukocyte immunoglobulin-like receptor B
- mAb
Monoclonal antibody
- MDSC
Myeloid-derived suppressor cell
- MHC
Major histocompatibility complex
- M-MDSC
Monocytic myeloid-derived suppressor cell
- MMP
Metalloproteinases
- MoDC
Monocyte-derived dendritic cell
- NET
Neutrophil extracellular trap
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- OVM
Outer membrane vesicle
- PAMP
Pathogen-associated molecular pattern
- pDC
Plasmacytoid dendritic cell
- PIR-B
Paired Ig-like receptor B
- PMN-MDSC
Polymorphonuclear myeloid-derived suppressor cell
- RBC
Red blood cell
- ROS
Reactive oxygen species
- R/R NHL
Relapsed or refractory non-Hodgkin lymphoma
- SHP
SH2-containing phosphatase
- Siglec
Sialic acid-binding immunoglobulin-like lectin
- SLAMF7
Signaling lymphocytic activation molecule family receptor 7
- SIRP α
Signal regulatory protein α
- STAT
Signal transducer and activator of transcription
- TAM
Tumor-associated macrophage
- TAN
Tumor-associated neutrophil
- TGF-β
Transforming growth factor beta
- Th
T helper
- TIDC
Tumor-infiltrating dendritic cell
- TLR
Toll-like receptor
- TME
Tumor microenvironment
- TRAIL
TNF-related apoptosis-inducing ligand
- Treg
T regulatory cell
- TREM2
Triggering receptor expressed on myeloid cells 2
- VEGF
Vascular endothelial growth factor
- WT
Wild-type
Author’s contribution
CM wrote the main manuscript text, and CL and YT revised the manuscript accordingly. YL, ML, and YTs proofread and supervised the writing of the manuscript. All authors have reviewed the manuscript.
Funding
This review was supported by the Natural Science Foundation of China (81974377), 345 Talent Project of Shengjing Hospital (2022–2025), and the Outstanding Scientific Fund of Shengjing Hospital.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chao Lv and Yu Tian have contributed equally to this work.
Contributor Information
Chao Lv, Email: clu@cmu.edu.cn.
Yu Tian, Email: yu.tian@cmu.edu.cn.
References
- 1.O’Donnell JS, Teng MWL, Smyth MJ (2019) Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16:151–167. 10.1038/s41571-018-0142-8 [DOI] [PubMed] [Google Scholar]
- 2.Maalej KM et al. (2023) CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer 22:1723 10.1186/s12943-023-01723-z [DOI] [PMC free article] [PubMed]
- 3.Engblom C, Pfirschke C, Pittet MJ (2016) The role of myeloid cells in cancer therapies. Nat Rev Cancer 16:447–462. 10.1038/nrc.2016.54 [DOI] [PubMed] [Google Scholar]
- 4.Yi M et al (2023) Exploiting innate immunity for cancer immunotherapy. Mol Cancer 22:187. 10.1186/s12943-023-01885-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K, Smyth MJ (2020) Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol 17:1–12. 10.1038/s41423-019-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safety Concerns Prompt Pause of Magrolimab Trials. (2022) Cancer Discovery 12:877–878, 10.1158/2159-8290.Cd-nb2022-0012 [DOI] [PubMed]
- 7.Wang Y, Johnson KCC, Gatti-Mays ME, Li Z (2022) Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy. J Hematol Oncol 15. 10.1186/s13045-022-01335-y [DOI] [PMC free article] [PubMed]
- 8.Ponzetta A et al. (2019) Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell 178:346–360 e324, 10.1016/j.cell.2019.05.047 [DOI] [PMC free article] [PubMed]
- 9.Sody S et al. (2019) Distinct spatio-temporal dynamics of tumor-associated neutrophils in small tumor lesions. Front Immunol 10. 10.3389/fimmu.2019.01419 [DOI] [PMC free article] [PubMed]
- 10.Jaillon S et al (2020) Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 20:485–503. 10.1038/s41568-020-0281-y [DOI] [PubMed] [Google Scholar]
- 11.Shaul ME, Fridlender ZG (2019) Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 16:601–620. 10.1038/s41571-019-0222-4 [DOI] [PubMed] [Google Scholar]
- 12.Kowanetz M et al (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA 107:21248–21255. 10.1073/pnas.1015855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghajar CM et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15:807–817. 10.1038/ncb2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catena R et al (2013) Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov 3:578–589. 10.1158/2159-8290.CD-12-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczerba BM et al (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566:553–557. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- 16.Cools-Lartigue J et al (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 123:3446–3458. 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou G et al (2018) CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis 39:272–282. 10.1093/carcin/bgx142 [DOI] [PubMed] [Google Scholar]
- 18.Mahiddine K et al (2020) Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Invest 130:389–403. 10.1172/JCI130952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finisguerra V et al (2015) MET is required for the recruitment of anti-tumoural neutrophils. Nature 522:349–353. 10.1038/nature14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T (2004) Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res 64:1037–1043. 10.1158/0008-5472.can-03-1808 [DOI] [PubMed] [Google Scholar]
- 21.Granot Z et al (2011) Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20:300–314. 10.1016/j.ccr.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gungabeesoon J et al. (2023) A neutrophil response linked to tumor control in immunotherapy. Cell 186:1448–1464 e1420, 10.1016/j.cell.2023.02.032 [DOI] [PMC free article] [PubMed]
- 23.Benguigui M et al (2024) Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 42:253-265.e212. 10.1016/j.ccell.2023.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirschhorn D et al (2023) T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 186:1432-1447.e1417. 10.1016/j.cell.2023.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantovani A, Allavena P, Marchesi F, Garlanda C (2022) Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discovery 21:799–820. 10.1038/s41573-022-00520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discovery 17:887–904. 10.1038/nrd.2018.169 [DOI] [PubMed] [Google Scholar]
- 27.Hanna A et al (2019) Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. Oncoimmunology 8:1548241. 10.1080/2162402X.2018.1548241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H et al (2015) HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 112:E6215-6223. 10.1073/pnas.1520032112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garziera M, Scarabel L, Toffoli G (2017) Hypoxic modulation of HLA-G expression through the metabolic sensor HIF-1 in human cancer cells. J Immunol Res 2017:4587520. 10.1155/2017/4587520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bied M, Ho WW, Ginhoux F, Bleriot C (2023) Roles of macrophages in tumor development: a spatiotemporal perspective. Cell Mol Immunol 20:983–992. 10.1038/s41423-023-01061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christofides A et al (2022) The complex role of tumor-infiltrating macrophages. Nat Immunol 23:1148–1156. 10.1038/s41590-022-01267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wculek SK et al (2020) Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20:7–24. 10.1038/s41577-019-0210-z [DOI] [PubMed] [Google Scholar]
- 33.Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Cancer Res 79:4557–4566. 10.1158/0008-5472.CAN-18-3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spranger S, Bao R, Gajewski TF (2015) Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523:231–235. 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- 35.Barry KC et al (2018) A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med 24:1178–1191. 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohm JE et al (1999) Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. J Immunol 163:3260–3268 [PubMed] [Google Scholar]
- 37.Johnson DE, O’Keefe RA, Grandis JR (2018) Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 15:234–248. 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fallarino F et al (2003) Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 4:1206–1212. 10.1038/ni1003 [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, Mellor AL (2016) IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol 37:193–207. 10.1016/j.it.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu W et al. (2024) A programmable releasing versatile hydrogel platform boosts systemic immune responses via sculpting tumor immunogenicity and reversing tolerogenic dendritic cells. Biomaterials 305. 10.1016/j.biomaterials.2023.122444 [DOI] [PubMed]
- 41.Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, Umansky V (2024) Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol 21:147–164, 10.1038/s41571-023-00846-y [DOI] [PubMed]
- 42.Bronte V et al (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150. 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, Umansky V (2024) Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol 21:147–164. 10.1038/s41571-023-00846-y [DOI] [PubMed]
- 44.Toor SM, Elkord E (2018) Therapeutic prospects of targeting myeloid-derived suppressor cells and immune checkpoints in cancer. Immunol Cell Biol 96:888–897. 10.1111/imcb.12054 [DOI] [PubMed] [Google Scholar]
- 45.Hossain F et al (2015) Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res 3:1236–1247. 10.1158/2326-6066.CIR-15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66:97–110. 10.1146/annurev-med-051013-052304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corzo CA et al (2010) HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207:2439–2453. 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veillette A, Chen J (2018) SIRPα–CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol 39:173–184. 10.1016/j.it.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 49.Isenberg JS, Montero E (2024) Tolerating CD47. Clin Transl Med 14:e1584. 10.1002/ctm2.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Betancur PA et al (2017) A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun 8:14802. 10.1038/ncomms14802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo J et al (2015) Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 62:534–545. 10.1002/hep.27859 [DOI] [PubMed] [Google Scholar]
- 52.Weiskopf K et al (2016) CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 126:2610–2620. 10.1172/JCI81603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng D et al (2013) Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA 110:11103–11108. 10.1073/pnas.1305569110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M et al (2016) Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in vivo. PLoS ONE 11:e0153550. 10.1371/journal.pone.0153550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antonsen KW et al (2024) Proinflammatory polarization strongly reduces human macrophage in vitro phagocytosis of tumor cells in response to CD47 blockade. Eur J Immunol. 10.1002/eji.202350824 [DOI] [PubMed] [Google Scholar]
- 56.Ring NG et al (2017) Anti-SIRPalpha antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci USA 114:E10578–E10585. 10.1073/pnas.1710877114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Logtenberg MEW, Scheeren FA, Schumacher TN (2020) The CD47-SIRPalpha immune checkpoint. Immunity 52:742–752. 10.1016/j.immuni.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osorio JC, Smith P, Knorr DA, Ravetch JV (2023) The antitumor activities of anti-CD47 antibodies require Fc-FcγR interactions. Cancer Cell 41:2051-2065.e2056. 10.1016/j.ccell.2023.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng M et al (2019) Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 19:568–586. 10.1038/s41568-019-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J et al (2017) SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature 544:493–497. 10.1038/nature22076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He Y et al (2019) Cancer cell-expressed SLAMF7 is not required for CD47-mediated phagocytosis. Nat Commun 10:533. 10.1038/s41467-018-08013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Combining a first-in-class SLAMF7 antibody with SIRPα blockade for anti-tumor immunity. Nat Immunol 24:1978–1979, 10.1038/s41590-023-01673-0 (2023) [DOI] [PubMed]
- 63.Zhou H et al. Metabolic reprograming mediated by tumor cell-intrinsic type I IFN signaling is required for CD47-SIRPα blockade efficacy. Nat Commun 15. 10.1038/s41467-024-50136-z (2024) [DOI] [PMC free article] [PubMed]
- 64.Barrera L et al (2017) CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer 117:385–397. 10.1038/bjc.2017.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matlung HL et al. (2018) Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep 23:3946–3959. 10.1016/j.celrep.2018.05.082 [DOI] [PubMed]
- 66.van Rees DJ et al (2022) Sodium stibogluconate and CD47-SIRPalpha blockade overcome resistance of anti-CD20-opsonized B cells to neutrophil killing. Blood Adv 6:2156–2166. 10.1182/bloodadvances.2021005367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouti P et al (2021) Kindlin3-dependent CD11b/CD18-integrin activation is required for potentiation of neutrophil cytotoxicity by CD47-SIRPalpha checkpoint disruption. Cancer Immunol Res 9:147–155. 10.1158/2326-6066.CIR-20-0491 [DOI] [PubMed] [Google Scholar]
- 68.Liu X et al (2015) CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 21:1209–1215. 10.1038/nm.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kauder SE et al (2018) ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE 13:e0201832. 10.1371/journal.pone.0201832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y et al (2020) Vaccination with CD47 deficient tumor cells elicits an antitumor immune response in mice. Nat Commun 11:581. 10.1038/s41467-019-14102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S et al (2022) Blocking CD47 promotes antitumour immunity through CD103(+) dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J Hepatol 77:467–478. 10.1016/j.jhep.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 72.Wu L et al (2018) Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. Oncoimmunology 7:e1397248. 10.1080/2162402X.2017.1397248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan L et al (2023) A novel CD47-blocking peptide fused to pro-apoptotic KLA repeat inhibits lung cancer growth in mice. Cancer Immunol Immunother 72:4179–4194. 10.1007/s00262-023-03554-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pengam S et al (2019) SIRPalpha/CD47 axis controls the maintenance of transplant tolerance sustained by myeloid-derived suppressor cells. Am J Transplant 19:3263–3275. 10.1111/ajt.15497 [DOI] [PubMed] [Google Scholar]
- 75.Alimohammadi R et al (2023) Dual blockage of both PD-L1 and CD47 enhances the therapeutic effect of oxaliplatin and FOLFOX in CT-26 mice tumor model. Sci Rep 13:2472. 10.1038/s41598-023-29363-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pengam S et al (2019) SIRPα/CD47 axis controls the maintenance of transplant tolerance sustained by myeloid-derived suppressor cells. Am J Transplant 19:3263–3275. 10.1111/ajt.15497 [DOI] [PubMed] [Google Scholar]
- 77.Held W, Mariuzza RA (2008) Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat Rev Immunol 8:269–278. 10.1038/nri2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Touw W, Chen HM, Pan PY, Chen SH (2017) LILRB receptor-mediated regulation of myeloid cell maturation and function. Cancer Immunol Immunother 66:1079–1087. 10.1007/s00262-017-2023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ (2001) Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol 167:5543–5547. 10.4049/jimmunol.167.10.5543 [DOI] [PubMed] [Google Scholar]
- 80.Chen HM et al (2018) Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J Clin Invest 128:5647–5662. 10.1172/JCI97570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baudhuin J et al (2013) Exocytosis acts as a modulator of the ILT4-mediated inhibition of neutrophil functions. Proc Natl Acad Sci U S A 110:17957–17962. 10.1073/pnas.1221535110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H, Meng F, Chu CL, Takai T, Lowell CA (2005) The Src family kinases Hck and Fgr negatively regulate neutrophil and dendritic cell chemokine signaling via PIR-B. Immunity 22:235–246. 10.1016/j.immuni.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 83.Manavalan JS et al (2003) High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol 11:245–258. 10.1016/S0966-3274(03)00058-3 [DOI] [PubMed] [Google Scholar]
- 84.Gregori S et al (2010) Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116:935–944. 10.1182/blood-2009-07-234872 [DOI] [PubMed] [Google Scholar]
- 85.Endo S, Sakamoto Y, Kobayashi E, Nakamura A, Takai T (2008) Regulation of cytotoxic T lymphocyte triggering by PIR-B on dendritic cells. Proc Natl Acad Sci U S A 105:14515–14520. 10.1073/pnas.0804571105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ujike A et al (2002) Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(-/-) mice. Nat Immunol 3:542–548. 10.1038/ni801 [DOI] [PubMed] [Google Scholar]
- 87.Horiguchi H et al (2019) Dual functions of angiopoietin-like protein 2 signaling in tumor progression and anti-tumor immunity. Genes Dev 33:1641–1656. 10.1101/gad.329417.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Goeje PL et al (2015) Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. Oncoimmunology 4:e1014242. 10.1080/2162402X.2015.1014242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma G et al (2011) Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity 34:385–395. 10.1016/j.immuni.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y et al (2024) Discovery of galectin-8 as an LILRB4 ligand driving M-MDSCs defines a class of antibodies to fight solid tumors. Cell Rep Med 5:101374. 10.1016/j.xcrm.2023.101374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh L et al (2021) ILT3 (LILRB4) promotes the Immunosuppressive function of tumor-educated human monocytic myeloid-derived suppressor cells. Mol Cancer Res 19:702–716. 10.1158/1541-7786.MCR-20-0622 [DOI] [PubMed] [Google Scholar]
- 92.Su MT, Kumata S, Endo S, Okada Y, Takai T (2022) LILRB4 promotes tumor metastasis by regulating MDSCs and inhibiting miR-1 family miRNAs. Oncoimmunology 11:2060907. 10.1080/2162402X.2022.2060907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J et al (2021) ILT3 promotes tumor cell motility and angiogenesis in non-small cell lung cancer. Cancer Lett 501:263–276. 10.1016/j.canlet.2020.10.048 [DOI] [PubMed] [Google Scholar]
- 94.Liu J et al. (2023) Sunitinib attenuates reactive MDSCs enhancing anti-tumor immunity in HNSCC. Int Immunopharm 119 10.1016/j.intimp.2023.110243 [DOI] [PubMed]
- 95.Deng M et al (2021) Leukocyte immunoglobulin-like receptor subfamily B: therapeutic targets in cancer. Antib Ther 4:16–33. 10.1093/abt/tbab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Houtum EJH, Bull C, Cornelissen LAM, Adema GJ (2021) Siglec signaling in the tumor microenvironment. Front Immunol 12:790317. 10.3389/fimmu.2021.790317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao X et al (2017) Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget 8:30576–30586. 10.18632/oncotarget.15736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beatson R et al (2020) Cancer-associated hypersialylated MUC1 drives the differentiation of human monocytes into macrophages with a pathogenic phenotype. Commun Biol 3:644. 10.1038/s42003-020-01359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mei Y et al (2023) Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat Cancer. 10.1038/s43018-023-00598-9 [DOI] [PubMed] [Google Scholar]
- 100.Ibarlucea-Benitez I, Weitzenfeld P, Smith P, Ravetch JV (2021) Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc Natl Acad Sci USA 118. 10.1073/pnas.2107424118 [DOI] [PMC free article] [PubMed]
- 101.Rodriguez E et al (2021) Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat Commun 12:1270. 10.1038/s41467-021-21550-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li X et al (2024) Targeting CD24/Siglec-10 signal pathway for cancer immunotherapy: recent advances and future directions. Cancer Immunol Immunother 73:31. 10.1007/s00262-023-03606-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiao X et al. (2022) A novel immune checkpoint siglec-15 antibody inhibits LUAD by modulating mφ polarization in TME. Pharmacol Res 181, 10.1016/j.phrs.2022.106269 [DOI] [PubMed]
- 104.Wang J et al (2019) Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 25:656–666. 10.1038/s41591-019-0374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lizcano A et al (2017) Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood 129:3100–3110. 10.1182/blood-2016-11-751636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carlin AF et al (2009) Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336. 10.1182/blood-2008-11-187302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lustig M et al (2023) Disruption of the sialic acid/Siglec-9 axis improves antibody-mediated neutrophil cytotoxicity towards tumor cells. Front Immunol 14:1178817. 10.3389/fimmu.2023.1178817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan C et al. (2023) Sialic acids on tumor cells modulate IgA therapy by neutrophils via inhibitory receptors siglec-7 and siglec-9. Cancers (Basel) 15. 10.3390/cancers15133405 [DOI] [PMC free article] [PubMed]
- 109.Stanczak MA et al. (2022) Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci Transl Med 14. 10.1126/scitranslmed.abj1270 [DOI] [PMC free article] [PubMed]
- 110.Rodrigue, E et al. (2021) Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat Commun 12. 10.1038/s41467-021-21550-4 [DOI] [PMC free article] [PubMed]
- 111.Laubli H et al (2014) Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci USA 111:14211–14216. 10.1073/pnas.1409580111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen GY, Tang J, Zheng P, Liu Y (2009) CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323:1722–1725. 10.1126/science.1168988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ding Y et al (2016) The lectin Siglec-G inhibits dendritic cell cross-presentation by impairing MHC class I-peptide complex formation. Nat Immunol 17:1167–1175. 10.1038/ni.3535 [DOI] [PubMed] [Google Scholar]
- 114.Kazakova E, Iamshchikov P, Larionova I, Kzhyshkowska J (2023) Macrophage scavenger receptors: Tumor support and tumor inhibition. Front Oncol 12. 10.3389/fonc.2022.1096897 [DOI] [PMC free article] [PubMed]
- 115.Yu X, Guo C, Fisher PB, Subjeck JR, Wang XY (2015) Scavenger receptors: emerging roles in cancer biology and immunology. Adv Cancer Res 128:309–364. 10.1016/bs.acr.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hollmen M, Figueiredo CR, Jalkanen S (2020) New tools to prevent cancer growth and spread: a “Clever” approach. Br J Cancer 123:501–509. 10.1038/s41416-020-0953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang B et al (2020) Microlocalization and clinical significance of stabilin-1(+) macrophages in treatment-naive patients with urothelial carcinoma of the bladder. World J Urol 38:709–716. 10.1007/s00345-019-02853-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palani S, Elima K, Ekholm E, Jalkanen S, Salmi M (2016) monocyte stabilin-1 suppresses the activation of Th1 lymphocytes. J Immunol 196:115–123. 10.4049/jimmunol.1500257 [DOI] [PubMed] [Google Scholar]
- 119.Zhen Z et al (2017) Decorin gene upregulation mediated by an adeno-associated virus vector increases intratumoral uptake of nab-paclitaxel in neuroblastoma via inhibition of stabilin-1. Invest New Drugs 35:566–575. 10.1007/s10637-017-0477-5 [DOI] [PubMed] [Google Scholar]
- 120.Yin S et al (2020) SI-CLP inhibits the growth of mouse mammary adenocarcinoma by preventing recruitment of tumor-associated macrophages. Int J Cancer 146:1396–1408. 10.1002/ijc.32685 [DOI] [PubMed] [Google Scholar]
- 121.Kzhyshkowska J et al (2006) Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood 107:3221–3228. 10.1182/blood-2005-07-2843 [DOI] [PubMed] [Google Scholar]
- 122.Viitala M et al (2019) Immunotherapeutic blockade of macrophage clever-1 reactivates the CD8(+) T-cell response against immunosuppressive tumors. Clin Cancer Res 25:3289–3303. 10.1158/1078-0432.CCR-18-3016 [DOI] [PubMed] [Google Scholar]
- 123.Colonna M (2023) The biology of TREM receptors. Nat Rev Immunol. 10.1038/s41577-023-00837-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nalio Ramos R et al. (2022) Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell 185:1189–1207. 10.1016/j.cell.2022.02.021 [DOI] [PubMed]
- 125.Katzenelenbogen Y et al. (2020) Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell 182:872–885 e819, 10.1016/j.cell.2020.06.032 [DOI] [PubMed]
- 126.Sun R et al. (2023) TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma. Sci Adv 9:eade3559. 10.1126/sciadv.ade3559 [DOI] [PMC free article] [PubMed]
- 127.Molgora M et al. (2020) TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell 182:886–900. 10.1016/j.cell.2020.07.013 [DOI] [PMC free article] [PubMed]
- 128.Park MD et al (2023) TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat Immunol 24:792–801. 10.1038/s41590-023-01475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haensel D et al (2023) Skin basal cell carcinomas assemble a pro-tumorigenic spatially organized and self-propagating Trem2+ myeloid niche. Nat Commun 14:2685. 10.1038/s41467-023-37993-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Molgora M, Liu YA, Colonna M, Cella M (2023) TREM2: A new player in the tumor microenvironment. Semin Immunol 67:101739. 10.1016/j.smim.2023.101739 [DOI] [PubMed] [Google Scholar]
- 131.Vetrano S et al (2010) The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut 59:197–206. 10.1136/gut.2009.183772 [DOI] [PubMed] [Google Scholar]
- 132.Nibbs RJ et al (2007) The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest 117:1884–1892. 10.1172/JCI30068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Massara M et al (2018) ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat Commun 9:676. 10.1038/s41467-018-03080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang TM et al (2023) CXCL14 promotes metastasis of non-small cell lung cancer through ACKR2-depended signaling pathway. Int J Biol Sci 19:1455–1470. 10.7150/ijbs.79438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sjoberg E et al (2019) A novel ACKR2-dependent role of fibroblast-derived CXCL14 in epithelial-to-mesenchymal transition and metastasis of breast cancer. Clin Cancer Res 25:3702–3717. 10.1158/1078-0432.CCR-18-1294 [DOI] [PubMed] [Google Scholar]
- 136.Li T et al (2020) c-Rel Is a myeloid checkpoint for cancer immunotherapy. Nat Cancer 1:507–517. 10.1038/s43018-020-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li T et al (2022) c-Rel-dependent monocytes are potent immune suppressor cells in cancer. J Leukoc Biol 112:845–859. 10.1002/JLB.1MA0422-518RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pruenster M, Vogl T, Roth J, Sperandio M (2016) S100A8/A9: from basic science to clinical application. Pharmacol Ther 167:120–131. 10.1016/j.pharmthera.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 139.Eisenblaetter M et al (2017) Visualization of tumor-immune interaction - target-specific imaging of s100a8/a9 reveals pre-metastatic niche establishment. Theranostics 7:2392–2401. 10.7150/thno.17138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Y et al (2013) Premetastatic soil and prevention of breast cancer brain metastasis. Neuro Oncol 15:891–903. 10.1093/neuonc/not031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiu M et al (2019) Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol 12:945–957. 10.1038/s41385-019-0144-6 [DOI] [PubMed] [Google Scholar]
- 142.Liu YF et al (2017) Expansion and activation of granulocytic, myeloid-derived suppressor cells in childhood precursor B cell acute lymphoblastic leukemia. J Leukoc Biol 102:449–458. 10.1189/jlb.5MA1116-453RR [DOI] [PubMed] [Google Scholar]
- 143.Xu ZH et al (2021) Ze-Qi-Tang formula induces granulocytic myeloid-derived suppressor cell apoptosis via STAT3/S100A9/Bcl-2/Caspase-3 signaling to prolong the survival of mice with orthotopic lung cancer. Mediators Inflamm 2021:8856326. 10.1155/2021/8856326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ozbay Kurt FG et al. (2024) S100A9 and HMGB1 orchestrate MDSC-mediated immunosuppression in melanoma through TLR4 signaling. J Immunother Cancer 12. 10.1136/jitc-2024-009552 [DOI] [PMC free article] [PubMed]
- 145.Cheng P et al (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205:2235–2249. 10.1084/jem.20080132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang M et al (2019) S100A9 regulates MDSCs-mediated immune suppression via the RAGE and TLR4 signaling pathways in colorectal carcinoma. Front Immunol 10:2243. 10.3389/fimmu.2019.02243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Veirman K et al (2017) Extracellular S100A9 protein in bone marrow supports multiple myeloma survival by stimulating angiogenesis and cytokine secretion. Cancer Immunol Res 5:839–846. 10.1158/2326-6066.CIR-17-0192 [DOI] [PubMed] [Google Scholar]
- 148.Sinha P et al (2008) Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 181:4666–4675. 10.4049/jimmunol.181.7.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gielen PR et al (2016) Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro Oncol 18:1253–1264. 10.1093/neuonc/now034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Becker A et al (2015) Optical in vivo imaging of the alarmin S100A9 in tumor lesions allows for estimation of the individual malignant potential by evaluation of tumor-host cell interaction. J Nucl Med 56:450–456. 10.2967/jnumed.114.146688 [DOI] [PubMed] [Google Scholar]
- 151.Chen Y, Ouyang Y, Li Z, Wang X, Ma J (2023) S100A8 and S100A9 in Cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1878. 10.1016/j.bbcan.2023.188891 [DOI] [PubMed]
- 152.Lakhani NJ et al (2021) Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol 22:1740–1751. 10.1016/S1470-2045(21)00584-2 [DOI] [PubMed] [Google Scholar]
- 153.Siu LL et al (2022) First-in-class anti-immunoglobulin–like transcript 4 myeloid-specific antibody MK-4830 abrogates a PD-1 resistance mechanism in patients with advanced solid tumors. Clin Cancer Res 28:57–70. 10.1158/1078-0432.Ccr-21-2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Target AAI (2020) Siglec-15. Cancer Discov 10:7–8. 10.1158/2159-8290.Cd-nb2019-136 [Google Scholar]
- 155.Rannikko JH et al (2023) Bexmarilimab-induced macrophage activation leads to treatment benefit in solid tumors: the phase I/II first-in-human MATINS trial. Cell Rep Med 4:101307. 10.1016/j.xcrm.2023.101307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Virtakoivu R et al (2021) Systemic Blockade Of Clever-1 Elicits Lymphocyte Activation Alongside Checkpoint Molecule Downregulation In Patients With Solid Tumors: Results From A Phase I/II Clinical Trial. Clin Cancer Res 27:4205–4220. 10.1158/1078-0432.CCR-20-4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pili R et al (2011) Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol 29:4022–4028. 10.1200/jco.2011.35.6295 [DOI] [PubMed] [Google Scholar]
- 158.Armstrong AJ et al (2013) Long-term survival and biomarker correlates of tasquinimod efficacy in a multicenter randomized study of men with minimally symptomatic metastatic castration-resistant prostate cancer. Clin Cancer Res 19:6891–6901. 10.1158/1078-0432.Ccr-13-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sternberg C et al (2016) Randomized, double-blind, placebo-controlled phase III study of tasquinimod in men with metastatic castration-resistant prostate cancer. J Clin Oncol 34:2636–2643. 10.1200/jco.2016.66.9697 [DOI] [PubMed] [Google Scholar]
- 160.Escudier B et al (2017) A phase II multicentre, open-label, proof-of-concept study of tasquinimod in hepatocellular, ovarian, renal cell, and gastric cancers. Target Oncol 12:655–661. 10.1007/s11523-017-0525-2 [DOI] [PubMed] [Google Scholar]
- 161.DeNardo DG, Ruffell B (2019) Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol 19:369–382. 10.1038/s41577-019-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chamseddine AN, Assi T, Mir O, Chouaib S (2022) Modulating tumor-associated macrophages to enhance the efficacy of immune checkpoint inhibitors: A TAM-pting approach. Pharmacol Ther 231:107986. 10.1016/j.pharmthera.2021.107986 [DOI] [PubMed] [Google Scholar]
- 163.Yamada-Hunter SA et al (2024) Engineered CD47 protects T cells for enhanced antitumour immunity. Nature 630:457–465. 10.1038/s41586-024-07443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ye ZH, Yu WB, Huang MY, Chen J, Lu JJ (2023) Building on the backbone of CD47-based therapy in cancer: COMBINATION strategies, mechanisms, and future perspectives. Acta Pharm Sin B 13:1467–1487. 10.1016/j.apsb.2022.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nishiga Y et al (2022) Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect. Nat Cancer 3:1351–1366. 10.1038/s43018-022-00456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo Y, Bao Q, Hu P, Shi J (2023) Nanomedicine-based co-delivery of a calcium channel inhibitor and a small molecule targeting CD47 for lung cancer immunotherapy. Nat Commun 14:7306. 10.1038/s41467-023-42972-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gong K et al. (2024) Nanosystem delivers senescence activators and immunomodulators to combat liver cancer. Adv Sci (Weinh) 11, e2308310, 10.1002/advs.202308310 [DOI] [PMC free article] [PubMed]
- 168.Feng Q et al (2022) Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater 34:e2206200. 10.1002/adma.202206200 [DOI] [PubMed] [Google Scholar]
- 169.Tang L et al. (2023) Extracellular vesicles‐derived hybrid nanoplatforms for amplified CD47 blockade‐based cancer immunotherapy. Adv Mater 35. 10.1002/adma.202303835 [DOI] [PubMed]
- 170.Xia P et al. (2022) Surface‐engineered extracellular vesicles with CDH17 nanobodies to efficiently deliver imaging probes and chemo‐photothermal drugs for gastric cancer theragnostic. Adv Functional Mater 33. 10.1002/adfm.202209393
- 171.Chen C et al. (2022) Intracavity generation of glioma stem cell–specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med 14. 10.1126/scitranslmed.abn1128 [DOI] [PubMed]
- 172.Zuo H. et al. (2021) Transfer of cellular content from the allogeneic cell-based cancer vaccine dcp-001 to host dendritic cells hinges on phosphatidylserine and is enhanced by CD47 blockade. Cells 10. 10.3390/cells10113233 [DOI] [PMC free article] [PubMed]
- 173.Dan J et al (2023) Oncolytic virus M1 functions as a bifunctional checkpoint inhibitor to enhance the antitumor activity of DC vaccine. Cell Rep Med 4:101229. 10.1016/j.xcrm.2023.101229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.