Abstract
Mixed-mode sorbents exhibit two or more primary retention mechanisms, which can enhance the selectivity and capacity of the extraction process in a single step. In this study, a facile approach was proposed to prepare functionalized metal-organic frameworks (MOFs) by post-synthetic oxidation. The composites could be varied independently for each processing step, resulting in four frameworks to meet different sample pretreatment requirements. Then, the fabricated MOFs were used as sorbents for the extraction and enrichment acidic and neutral compounds on an online solid phase extraction. The sorbents exhibited a dual retention mechanism combining hydrophilic-lipophilic balance and cation exchange interactions. Excellent linearity was observed over a range of 0.5–5000 μg kg-1 for the parabens and 10–50,000 μg kg-1 for the sulfonamides in pre-cooked foods. The detection limits were 0.02 and 1.27 μg kg-1, respectively. This method provided a novel mixed-mode framework for simultaneous determination of acidic and neutral compounds in complex samples.
Keywords
Sulfonic acid functionalized metal-organic frameworks
Parabens
Sulfonamides
Online solid phase extraction
Pre-cooked foods
Graphical abstract
Highlights
-
•
Functional MOFs were prepared to meet the requirements of different sample pretreatment.
-
•
The sorbents exhibited the performances of hydrophilic-lipophilic-balance and cation-exchange interaction.
-
•
Mixed-mode sorbents can simultaneously extract acidic and neutral compounds.
-
•
Online SPE-HPLC-DAD method was developed to simultaneously determine paraben and sulfonamide in pre-cooked foods.
1. Introduction
Nowadays, the pre-cooked food market is flourishing in China and the size of the industry will exceed 630 billion yuan in 2025. A majority of people are wary of pre-cooked food and not without reason: Without a transparent national standard in place, no one can be sure they tick all the boxes for safety (Rangel-Huerta et al., 2022). The State Administration for Market Supervision and six other departments jointly issued a notice stressing that preservatives must not be added to pre-cooked food. Parabens are one of the most widely used preservatives in food products (Huang et al., 2021). Liao et al. found that almost all (99 %) of food samples collected from nine cities in China contained at least one of the parabens (C. Liao, Chen, & Kannan, 2013). Parabens are potentially toxic due to their endocrine disrupting potential and possible links with breast cancer (S. Huang et al., 2024; Pulcastro & Ziv-Gal, 2024). In addition, sulfonamides are widely used as growth promoters added to feed in agricultural and aquaculture systems (Dong et al., 2024; Huang et al., 2024; Shirani et al., 2022). The results of food monitoring and sampling in many cities of China have repeatedly reported excessive levels of sulfonamides in pre-cooked food. In general, vegetables easily absorb antibiotics and the detected frequency is 58.1 % (Jin et al., 2020a; Jin et al., 2020b). Therefore, the simultaneous determination of sulfonamides and parabens in pre-cooked food is of great importance for food safety supervision.
Chromatographic methods coupled with mass spectroscopy are widely used for the simultaneous determination of sulfonamides and parabens (Jian et al., 2024; Nasrollahi et al., 2023). However, the matrix of pre-cooked foods is complex. Sample pretreatment is a critical and often complex phase of the analytical process. The effectiveness of pretreatment not only directly affects the final analytical results but can also adversely affect the performance of mass spectrometers (Cao & Zhou, 2024). Solid-phase extraction (SPE) is an effective approach to concentrate target analytes while eliminating matrix interferences. In particular, the on-line SPE system can achieve high sensitivity because all components are introduced into the analytical system (Folorunsho et al., 2023; Liu et al., 2024). The essence of these SPE approaches is the use of appropriate sorbents. In general, the Oasis HLB column is proposed to extract sulfonamides under acidic conditions (pH = 3) (Zheng et al., 2022). However, using a single on-line column, e.g., HLB or C18, lipids that potentially caused the matrix effect cannot be removed form complex food sample (T. Li et al., 2020). The mixed-cation-exchange (MCX) sorbent was then applied to construct SPE systems for extraction of basic/neutral chemicals and removal of sample matrix (Yan et al., 2024). However, the low retention efficiency of the neutral compounds on the phase backbone is still a shortboard. Thus, the MCX column connected with another HLB column in series in on-line SPE to determine sulfonamides.
Nowadays, several new materials can also be used in the preparation of mixed-mode sorbents to improve their retention ability. Chen et al., prepared nanofibrous membrane modified polystyrene sulfonic acid for extraction of 13 sulfonamides in food samples (R. Chen et al., 2016). However, preparation of functional materials remains a major challenge. Metal-organic frameworks are used in a variety of applications due to their versatile structure, large surface area, tunable porosity, and tailorable chemistry. The MOFs have shown great potential for sample pretreatment due to the wide range of possible chemical interactions with the target chemicals including H-bonding, π-π, and hydrophobic/hydrophilic interactions(Yang et al., 2020; Zhang et al., 2019). MOFs can be used as mixed-mode sorbents after sulfonic acid group modification for the simultaneous extraction of multiple chemicals in complex samples. However, the preparation of a sulfonic acid-functionalized sorbent is a major technical challenge. Phang et al., prepared UIO-66 (Zr) frameworks with sulfonic acid groups using 2,5-dimercaptoterephthalic acid, which isn't a commercially available reagent (Phang et al., 2015). Alternatively, these sorbents can be prepared by grafting cysteamine followed by the subsequent oxidation of the sulfides (Mortazavi et al., 2019). However, dehydration solvents, high costs, and elevated reaction temperatures are required.
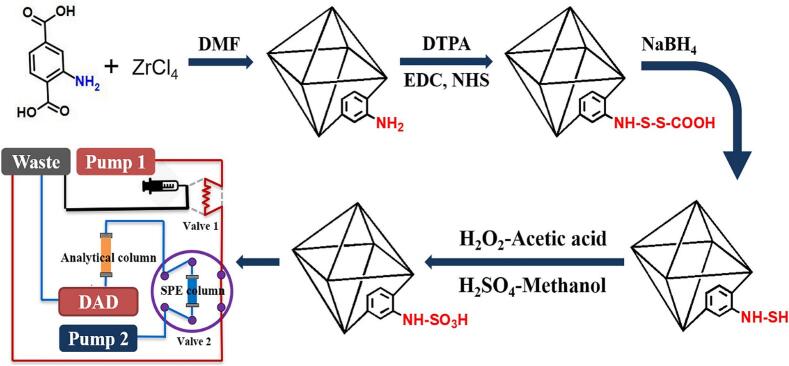
In this study, an amine-functionalized UiO-66 (NH2-UiO-66) was prepared, and amino groups were converted to thiols by post-synthetic oxidation at room temperature using a commercial reagent. Then, the performance of SO3-UiO-66 was then characterized. This sorbent was packaged in a steel cartridge and coupled to the online SPE-HPLC system for the simultaneous determination of sulfonamides and parabens in pre-cooked food samples.
2. Experimental
2.1. Chemicals and regents
Sulfadiazine (SDZ), sulfamethazine (SMZ), sulfamethoxazole (SMX), methyl paraben (MeP), ethyl paraben (EtP), propyl paraben (PrP), butyl paraben (BuP), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), zirconium chloride (ZrCl4), 2-aminoterephthalic acid (NH2-H2BDC), sodium borohydride (NaBH4), 3, 3′-dithiodipropionic acid standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, anhydrous ethanol, ammonium hydroxide, methanol, concentrated HCl, acetonitrile, N, N-dimethylformamide (DMF) and hydrogen peroxide (H2O2) were purchased from Sinopharm Chemical Reagent (Shanghai, China). C18 sorbents were obtained from Sigma-Aldrich (Bellefonte, PA, USA). Ultrapure water was obtained from a Milli-R04 purification system (Millipore, Darmstadt, Germany). Standard stock solutions (1 mg mL−1) were prepared in acetonitrile and stored at 4 °C.
2.2. Preparation of UiO-66-SO3H composites
The preparation of UiO-66-NH2 was carried out according to a previously reported method with minor modifications (Y. Li et al., 2018). Briefly, ZrCl4 (1.25 g) was dissolved in 10 mL of conc. HCl and 50 mL DMF via ultrasonication. 2-aminoterephthalic acid (1.34 g) was dissolved in a separate round-bottomed flask containing 100 mL DMF. After mixing, the two mixtures were stirred under ultrasonication for 10 min and then kept at 80 °C overnight. The resulting solids were separated by centrifugation at 10000 rpm for 10 min and washed thoroughly with DMF and ethanol, respectively. The resulting materials were dried under vacuum and activated by heating at 150 °C for 5 h to obtain a pale-yellow powder.
The resulting UiO-66-NH2 (50 mg) was dispersed in 15 mL of water and activated by adding 1 mmol EDC and 1 mmol NHS for 30 min. 3, 3′-dithiodipropionic acid (1 mmol) was dissolved in 5 mL DMF and added to the mixture to introduce disulfides. After stirring at 40 °C for 24 h, the products were collected by centrifugation, washed with ethanol, and dried overnight at room temperature under vacuum.
The dried products were incubated in 80 mL of methanol containing 1 g NaBH4 at room temperature for 12 h to yield UiO-66-SH. After washing with methanol/water (9/1, v/v), oxidation of the thiol groups was performed to prepare UiO-66-SO3H. Briefly, UiO-66-SH (0.3 g) was oxidized in 20 mL 30 % H2O2 at room temperature for 2 h. After collecting by centrifugation, the products were suspended in 0.02 M H2SO4 (20 mL) and allowed to react for a further 30 min. The resulting materials (UiO-66-SO3H) were washed several times with water until the pH value of the supernatant reached 7 and then dried overnight at room temperature under a vacuum.
2.3. Characterization
SEM analysis was used to study the morphologies of the functional MOFs using a Sirion 200 instrument (FEI, Eindhoven, The Netherlands). FTIR spectra of the materials were recorded using a Bruker Vertex 70 spectrometer (Ettlingen, Germany) based on the KBr pellet technique. XRD patterns were obtained using Philips X Pert diffractometer with CuKα radiation (λ = 1.5417 Å) in the refraction mode. Surface areas and pore size distributions were measured using a Micromeritics ASAP 2020 volumetric adsorption analyzer. XPS assays were carried out using a VG multi-lab 2000 spectrometer (Thermo Fisher, Pittsburgh, PA, USA).
2.4. Online analysis procedure
An on-line SPE-HPLC system was performed using an HP-1200 HPLC system from Agilent Technologies (Palo Alto, CA, USA) equipped with two quaternary pumps (1 and 2), a column thermostat, a DAD detector, a 7725i injection valve (value 1), a sample loop (10 mL) and a 6-port switching valve (value 2). The UiO-66-SO3H was packed into a stainless-steel tube (4.6 mm × 10 mm) as an SPE column for the extraction of parabens and sulfonamides. A commercial C18 column (150 mm × 2.1 mm i.d., particle size 5 μm, Supelco, Bellefonte, PA, USA) was used as the analytical column. Pump 1 was used for SPE column conditioning and sample injection. Pump 2 was used for the transfer of the target compounds from the SPE column to the analytical column and subsequent chromatographic separation (Fig. 1).
Fig. 1.
Schematic representation of preparation of UiO-66-SO3H and its application in the online-SPE-HPLC system.
The gradient elution procedures and valve switching procedures for Pumps 1 and 2 are shown in Table 1. Once the six-port valve (valve 2) was set to the loading position, the sample extracts in the loop were passed through the SPE column at a flow rate of 1 mL min−1. A solution of acetonitrile/water at pH 4.0 (9/1, v/v) was used as a washing solution to remove the sample matrices. The value 2 was switched from the “load” to the “injection” position and the analytes retained on the SPE column were eluted into the analytical column in a back-flush mode using methanol containing 0.1 % ammonium hydroxide. Based on the back-flush mode and control of the valve switching time, very little eluting solution was transferred to the analytical column, reducing the effect of eluting on the chromatographic separation.
Table 1.
Concurrent on-line SPE procedure and HPLC solvent gradient programs.
| Time (min) |
Pump 1 |
Valve 1 position |
Time (min) |
Pump 2 |
Valve 2 position |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aa (%) |
Bb (%) |
Event | Aa (%) |
Dd (%) |
Cc (%) |
Event | ||||
| 0 | 100 | 0 | Loading | Load | 0 | 0 | 0 | 100 | Eluting | Inject |
| 20 | 100 | 0 | Load | 0.5 | 0 | 0 | 100 | Inject | ||
| 21 | 100 | 0 | Inject | 1 | 15 | 85 | 0 | Separation | Load | |
| 31 | 100 | 0 | Inject | 2 | 15 | 85 | 0 | Load | ||
| 32 | 90 | 10 | Washing | Load | 22 | 60 | 40 | 0 | Load | |
| 35 | 90 | 10 | Load | 32 | 60 | 40 | 0 | Load | ||
| 35 | 15 | 85 | 0 | Equilibration | Load | |||||
Acetonitrile
Aqueous solution at pH 4.0
Methanol solutions containing 0.1 % ammonium hydroxide
1 % Acetic acid solution.
The chromatographic separation was performed in a gradient elution mode using acetonitrile and 1 % acetic acid solution as the mobile phases. The gradient elution conditions were as follows: 15 % acetonitrile for 1 min, changed to 60 % for 22 min, held for 10 min and then returned to the initial composition (15 % acetonitrile) for 3 min to equilibrate the analytical column. The online extraction and chromatographic separation were completed in less than 35 min. The flow rate was 1 mL min−1, the column temperature was 35 °C and the DAD was set to 270 nm.
2.5. Determination of sulfonamides and parabens in pre-cooked food samples
The pre-cooked yu-shiang shredded pork, Chinese sauerkraut fish, stewed beef with potatoes and kung pao chicken were collected from local restaurants. One gram of sample was weighed, diluted in 100 mL of hot water and ultrasonicated for 10 min. After centrifugation for 15 min at 5000 rpm, the supernatant was filtered through paper and 50 mL of the solution was shaken with 10 mL of acetonitrile for 30 min. The resulting solution was stored overnight in a refrigerator at -20 °C and then rapidly centrifuged to remove the proteins and lipids. Subsequently, 0.1 g C18 was added in to the supernatant and vortexed for 30 s. The mixture was centrifuged at 5000 rpm for 15 min to obtain the supernatant. This solution was introduced into the online SPE HPLC-DAD system for the simultaneous determination of sulfonamides and parabens in food samples.
3. Results and discussion
3.1. Preparation and characterization of functional MOFs
In this study, functionalized MOFs were achieved by post-synthetic oxidation of thiol-decorated frameworks to generate sulfonic acid groups. There are several current methods to prepare MOFs-SO3H but the ligands are not commercially available and the reaction conditions are harsh (T. F. Chen, Han, et al., 2019). Therefore, we used 3,3′-dithiodipropionic acid to modify the UiO-66-NH2 to provide disulfides at room temperature. This group was reduced with NaBH4 to form the thiol-decorated frameworks and further oxidized to prepare UIO-66-SO3H. The presence of acidic (-SO3H) and basic (-NH-) groups along with the framework provided this material with hydrophilic-lipophilic balance, cation exchange and π-π interaction capabilities, and therefore can be used as a mixed-mode sorbent. The functionalization of material could be varied independently for each processing step and we can obtain NH2-UiO-66, COOH-UiO-66, SO3-UiO-66, and so on. Even for the SO3-UiO-66, the retention window between neutral and basic chemicals can also be altered by varying the number of sulfonic acid groups. Thus, the MOF material can be used as a versatile tool to meet the requirements of different sample pretreatment. In this study, SO3-UiO-66 is just used as a novel mixed-mode sorbent for simultaneous determination of sulfonamides and parabens.
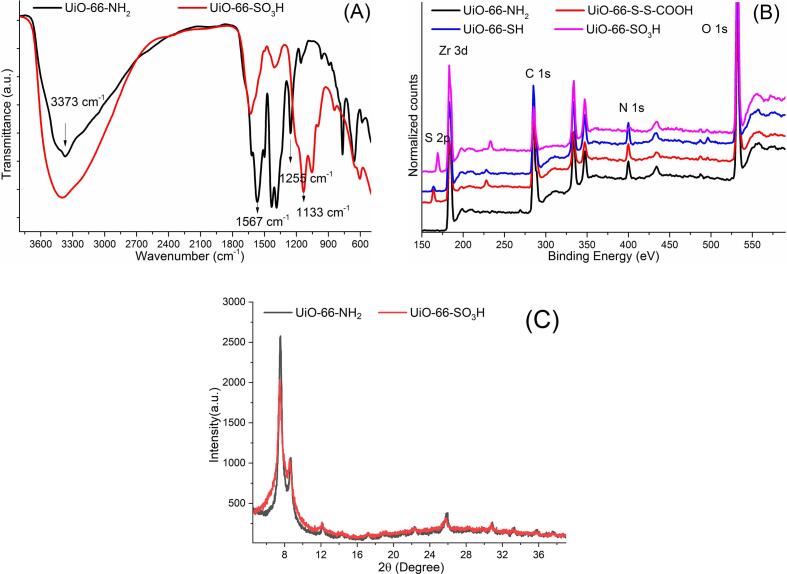
FT-IR and XPS were used to characterize the binding properties of the resulting materials (Fig. 2). The UiO-66-NH2 generated a peak at 3371 cm−1, which could be attributed to symmetric and anti-symmetric vibrations of the -NH2 groups on the organic linker. The peak at 1567 cm−1 was attributed to the occurrence of the reaction of Zr4+ with -COOH (Ding et al., 2020). The peaks corresponding to S2p, Zr3d, C1s, Zr3p, and O1s were clearly identified in the survey scan spectrum. The Zr3d spectra and the S2p (163.5 eV) confirmed the presence of UiO-66 and 3, 3′-dithiodipropionic acid, respectively. After oxidation of the thiol groups, the characteristic peak at 1133 cm−1 in the FT-IR spectrum was attributed to the -SO3H groups. The peak at 166.8 eV was assigned to the C-SOx-C linkage (Fig. 2). The appearance of three distinctive peaks at 2θ = 7.53, 8.66, and 25.82° indicates that UiO-66-NH3 was successfully synthesized. The XRD pattern of UiO-66-SO3H matched well with that of UiO-66-NH3. These results indicated that the sulfonic acid groups were successfully formed and this process did not destroy the original MOF structure. This material has two retention mechanisms (cation exchange and reversed phase) as it is formed from the MOF with hydrophilic and lipophilic regions and the sulfonic acid groups on its surface.
Fig. 2.
(A) The Fourier transform infrared (FT-IR) spectra of UiO-66-NH2 and UiO-66-SO3H; (B) the X-ray photoelectron spectra of UiO-66-NH2, UiO-66-S-S-COOH, UiO-66-SH and UiO-66-SO3H; (C) the XRD patterns of spectra of UiO-66-NH2 and UiO-66-SO3H.
Nitrogen absorption isotherm curves and the pore size distributions indicated that UiO-66-SO3H had a surface area of about 1844 m2 g−1 with domain pore sizes of 2.1 nm. These were close to the original values for UiO-66-NH2 (1963 m2 g−1 and 2.4 nm, respectively). These results also indicated that the sulfonic acid modifications did not damage the MOF framework. It was consistent with the morphology of the functional materials. However, SEM analysis indicated that UiO-66-NH2 displayed rounded crystals with an average particle size of 150 nm while the surfaces of UiO-66-SO3H became rough although the average sizes were unchanged (Fig. 3). The above results also indicated that surface adsorption is the predominant mechanism for the MOF framework.
Fig. 3.
SEM micrographs of (A) UiO-66-NH2 and (B) UiO-66-SO3H samples.
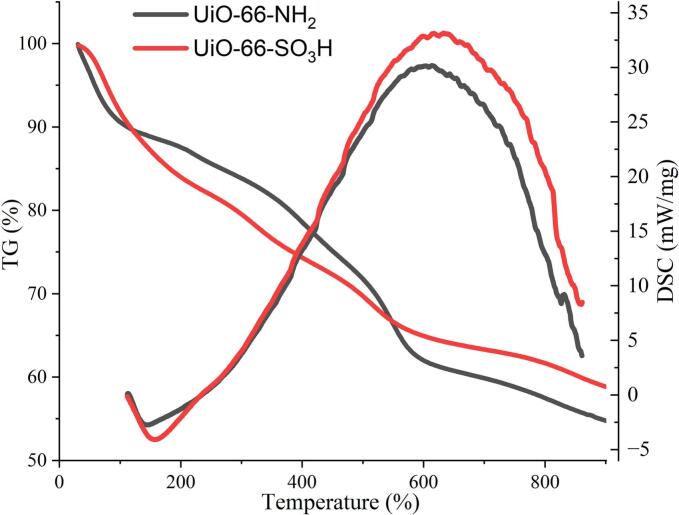
In general, with the same coordination environments, high-valent metals with high charge density (hard acids), including Zr4+, Cr3+, Al3+, Fe3+, etc., tend to coordinate with O donor ligands (hard bases) to form MOFs with strong coordination bonding, thus presenting good chemical stability. Leo et al., reported that sulfonic acid functionalized MOF material was stable in the acid-catalyzed reaction and the sulfur content remained constant after the reaction (Leo et al., 2022). In our study, the thermal stability of UiO-66-SO3H was confirmed by TG analysis (Fig. 4). The weight loss by about 10 % was found in the temperature range of 30–110 °C. The big weight loss observed around 110 and 600 °C for UiO-66-NH2 and UiO-66-SO3H, respectively, which are attributed to collapse the structure of MOFs and buried the organic linkers. Formation of sulfonic acid groups did not destroy the stability of original MOF structure. We found that the adsorption capacity of UiO-66-SO3H was stable at pH ranging from 2 to 10 and the RSD value was not more than 5.6 %, indicating that it was stable in the on-line extraction.
Fig. 4.
TG-DSC curves of (A) UiO-66-NH2 and (B) UiO-66-SO3H samples.
3.2. Optimization of On-line SPE
The UiO-66-SO3H was able to interact with the target molecules through ion exchange and hydrophilic-lipophilic interactions. Therefore, it can be used as a mixed-mode sorbent for online SPE-HPLC to simultaneously determine sulfonamides and parabens. The extraction parameters of the online SPE were optimized based on the spiked food samples. Based on the retention mechanism, a water miscible solvent, such as methanol or acetonitrile, was used to extract the target compounds. We found that the extraction efficiencies of sulfonamides and parabens were maximal when acetonitrile solution was used as the loading solvent. Loading volumes were then tested to achieve the optimum concentration factor and thereby lower detection limits. We found that recoveries were greatly reduced when the loading volume was >20 mL. The effect of loading flow rate (0.5–5 mL min−1) on the extraction efficiency and backpressure of the SPE column was also studied. Increasing the flow rate can save analytical time but also decrease extraction efficiency due to a short contact time. When the flow rates were > 1 mL min−1, the recoveries of parabens and sulfonamides were < 75 %. Therefore, 1.0 mL min−1 was selected for the following experiments.
Effective cleaning was an essential step for the on-line SPE system. This polymeric mixed-mode sorbent features reversed-phase and strong cation exchange retention mechanisms. In reversed-phase separations, the washing tends to be more polar than the surface of mixed-mode sorbent and is typically a mixture of water and an organic solvent. Thus, the mixture of acetonitrile/water and methanol/water were used as washing solutions to reduce matrix interference. When the ratio of acetonitrile/water was 9/1 and the washing time was 3 min, the matrix of the blank samples could be removed from the SPE column but the recoveries of sulfonamides were < 80 %. Therefore, the pH value of the washing solution was further optimized as the pKa values of paraben range from 8.1 to 8.8 and sulfonamides have two dissociation constants (pKa1 = 1.6–2.65; pKa2 = 5.7–8.43)(Bogdanova et al., 2020). We found that the retention of parabens on the sorbents was independent of pH (Mokhtari et al., 2020). However, the extraction efficiencies of sulfonamides were optimal at pH 4 (Fig. 5). This was most likely due to the electrostatic interactions between the negatively charged surfaces of UiO-66-SO3H and the positively charged sulfonamide species(Ahsan et al., 2018). Finally, acetonitrile-water (9/1, v/v, pH = 4) was used as a washing solution.
Fig. 5.
Influence of pH on the extraction efficiencies. The pH values were changed in the range of 2, 4, 5, 6, 8 and 9, respectively.
Finally, the mobile phase was optimized to elute and separate all test compounds and the back-flushing mode was used to prevent peak broadening. We found that parabens and sulfonamides could be rapidly eluted using methanol containing 0.1 % ammonium hydroxide. The results indicated that 1.0 min was sufficient to rapidly elute parabens and sulfonamides from the SPE column. Then, the gradient elution program was optimized for baseline separation of the analytes. We found that no significant interfering signal was obtained after a full working day. The proposed method can be applied to determine parabens and sulfonamides in complex samples.
3.3. Method validation
The On-line SPE-HPLC system using UiO-66-SO3H as sorbent was proposed for the simultaneous determination of parabens and sulfonamides in pre-cooked food samples. In this study, we evaluated its performance and determined the linear range, precision, limit of detection (LOD, S/N = 3), and limit of quantification (LOQ, S/N = 10). Under optimal conditions, the linearity ranges were 10–50,000 μg kg−1 and 0.5–5000 μg kg−1 for sulfonamides and parabens, respectively, with correlation coefficients >0.998 (Table 2). The European Union Commission Regulation No 37/2010 sets the maximum residue limits (MRLs) for all sulfonamides to 100 μg kg−1 for muscle, fat, liver, and kidney from all food-producing species and for bovine, ovine, and caprine milk. In China, no parabens are allowed to be added to food. Liao et al., determined the concentrations of six parabens in 13 categories of food samples collected from nine cities in China, and the mean value was 39.3 μg kg−1. It is shown that the proposed method can be used to simultaneously determine sulfonamides and parabens in pre-cooked food samples.
Table 2.
Performance of On-line SPE-HPLC method for determination of parabens and sulfonamides using UiO-66-SO3H as the sorbents.
| Linearity (μg kg−1) |
Correlation coefficient | LOD (μg kg−1) |
LOQ (μg kg−1) |
Intra-day precision | Inter-day precision | |
|---|---|---|---|---|---|---|
| SDZ | 10–50,000 | 0.9983 | 1.27 ± 0.35 | 3.96 ± 2.15 | 6.6 % | 5.3 % |
| SMZ | 10–50,000 | 0.9983 | 0.17 ± 0.04 | 0.58 ± 0.31 | 5.5 % | 4.2 % |
| SMX | 10–50,000 | 0.9986 | 1.16 ± 0.40 | 4.28 ± 2.33 | 6.9 % | 6.6 % |
| MeP | 0.5–5000 | 0.9990 | 0.12 ± 0.06 | 0.42 ± 0.19 | 6.2 % | 7.9 % |
| EtP | 0.5–5000 | 0.9991 | 0.11 ± 0.08 | 0.46 ± 0.18 | 5.1 % | 8.5 % |
| PrP | 0.5–5000 | 0.9989 | 0.06 ± 0.04 | 0.29 ± 0.09 | 3.6 % | 6.1 % |
| BuP | 0.5–5000 | 0.9995 | 0.02 ± 0.01 | 0.13 ± 0.06 | 4.7 % | 5.5 % |
In these assays, the intra-day RSD (n = 5) ranged from 3.6 to 6.9 % and the inter-day RSD (n = 5) ranged from 4.2 to 8.5 %, indicating good reproducibility. Recommended acceptable precision as provided by CODEX Guidelines is <15 %, when the analyte concentration > 100 μg kg−1. It can be seen that use of automated pretreatment ensures the stability of the entire method. Due to the high yield of the MOF material, large quantities of SPE columns can be prepared from the same batch to ensure method reproducibility. The sorbents prepared in different batches may have performance errors, which can be resolved by mixing several batches of materials and fine-tuning the SPE column loading.
After concentration on the SPE column, the target compounds were completely transferred to the analytical column. Therefore, the enhancement factors ranged from 120 to 257. The LODs of the sulfonamides were changed from 0.17 to 1.27 μg kg−1 and these values of the parabens were changed from 0.02 to 0.12 μg kg−1, respectively. The recoveries of sulfonamides and parabens ranged from 84.3 to 89.5 %. After sample analysis, the SPE column was retained using methanol. Even after 100 cycles, the peak areas of the target compounds decreased by only 4.6–8.7 %, indicating high structural stability of the MOFs. We did not observe any decrease in extraction efficiency due to swelling of the adsorbents. As can be seen from the comparative chromatograms, the extraction efficiency of the proposed method was better than that using UiO-66-NH2 as the sorbent, suggesting that the extraction performance of MOFs can be changed via different functional modifications (Fig. 6). Various sorbents, such as magnetic polymers, carbon materials, organic framework materials and so on, have been reported to determine sulfonamides or parabens, respectively (X. L. Chen, Ai, et al., 2019; Z. P. Chen, Yu, et al., 2019; Jiang et al., 2020; Makkliang et al., 2018; Moga et al., 2020; Nasir et al., 2019; Razavi & Es'haghi, 2019; Sadeghi & Olieaei, 2019; Yazdi et al., 2018, Hu, Luan, Cui, & Yang, 2024, Barzallo, Palacio, March, & Ferrer, 2023, Pasupuleti, Hsieh, Pasupuleti, & Huang, 2022, Tekin, Karlidag, Ozdogan, Kocoglu, & Bakirdere, 2022). A comparison with other sorbents showed that our materials have high extraction efficiencies and a dynamic linear range to simultaneously determine these compounds in complex samples (Table 3).
Fig. 6.
Comparison of online-SPE-HPLC chromatograms for determination of parabens and sulfonamides (2 mg kg−1). Target compounds: 1. Sulfadiazine (SDZ); 2. Sulfamethazine (SMZ); 3. Sulfamethoxazole (SMX); 4. Methylparaben (MeP); 5. Ethylparaben (EtP); 6. Propylparaben (PrP); 7. Butylparaben (BuP). Blank line: this chromatogram is obtained using UiO-66-NH3 as a sorbent. Red line: this chromatogram is obtained using UiO-66-SO3H as a sorbent. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Comparison of the determination of parabens and sulfonamides using different sorbents.
| Analyte | Sorbents | Detection | Leaner range (mg Kg−1) | LODs (μg Kg−1) | Ref. |
|---|---|---|---|---|---|
| Sulfonamides | 2-(hexyloxy) naphthalene-sulfate doped polyaniline polyme | Off-line SPE HPLC-UV | 0.03–50 | 9.2–13.5 | Chen et al., 2019 |
| Poly(ethylene glycol) diacrylate-based polymers | Off-line SPE HPLC-DAD | 0.1–100 | 7.5–16.2 | Moga, Vergara-Barberan, Lerma-Garcia, Herrero-Martinez and Simo-Alfonso, 2020 | |
| Thiol-functionalized magnetic carbon nanotubes | Off-line SPE HPLC-DAD | 0.5–500 | 0.11–0.17 | Nasir et al., 2019 | |
| Oasis MCX resin | 3D SPE HPLC-DAD | 0.02–1 | 0.6–6.0 | Barzallo, Palacio, March, & Ferrer, 2023 | |
| Ttryptophan based hypercrosslinked porous polymer | pipette tip SPE HPLC-DAD | 0.01–1 | 0.94–7.47 | Hu, Luan, Cui, & Yang, 2024 | |
| UiO-66-SO3H | On-line SPE HPLC-DAD | 0.01–50 | 0.17–1.27 | This work | |
| Parabens | Curcumin loaded magnetic graphene oxide | Off-line SPE HPLC-DAD | 0.001–5 | 0.4–1 | Razavi and Es'haghi, 2019 |
| Metal organic framework materials | Off-line SPE HPLC-DAD | 0.005–0.5 | 0.59–1.37 | Jiang et al., 2020 | |
| Reduced graphene oxide modified iron nanoparticles | Off-line SPE HPLC-DAD | 0.0001–0.1 | 0.02–1.27 | Tekin, Karlidag, Ozdogan, Kocoglu, & Bakirdere, 2022 | |
| Magnetic-activated carbon | Off-line SPE HPLC-DAD | 0.0003–1 | 0.1–0.3 | Pasupuleti, Hsieh, Pasupuleti, & Huang, 2022 | |
| UiO-66-SO3H | On-line SPE HPLC-DAD | 0.0005–5 | 0.02–0.12 | This work |
3.4. Simultaneous determination of parabens and sulfonamides in real samples
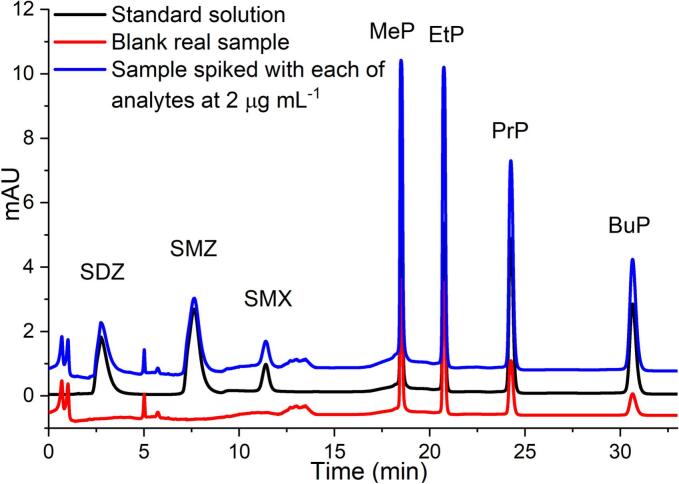
We applied the proposed method to the determination of parabens and sulfonamides in pre-cooked food samples collected from local restaurants. Sulfonamides are the most commonly consumed antibiotics in China and worldwide. Jin et al. showed that the detected frequency of sulfonamides in vegetable samples was 58.1 % and the residual levels varied between different types of vegetables(Caixia Jin, Shan Wei, Ruilian Sun, Wei Zou, Xingli Zhang, Qixing Zhou, et al., 2020). However, we did not detect sulfonamides in pre-cooked samples, because fresh vegetables are rarely used for pre-cooked food production, due to the difficulty of preservation. We found that parabens were present in most of the pre-cooked samples, with concentrations ranging from 0 and 2.72 μg kg−1. A previous study reported that the majority (>90 %) of food samples from the United States and China contained measurable levels of parabens(C. Y. Liao, Liu, & Kannan, 2013). Parabens are often found in the eggs, canned foods, baked goods and so on, which are the main ingredients of pre-cooked foods (Monteagudo et al., 2021). The chromatograms obtained for spiked Chinese sauerkraut fish samples showed little sample matrix interference and high extraction recoveries with more than 120-fold enrichment (Fig. 7).
Fig. 7.
Chromatograms of blank Chinese sauerkraut fish samples (red line), standard solution (2 mg kg−1, blank line), and sample spiked with each of analytes at 2 mg kg−1 (blue line), respectively. Target compounds: 1. Sulfadiazine (SDZ); 2. Sulfamethazine (SMZ); 3. Sulfamethoxazole (SMX); 4. Methylparaben (MeP); 5. Ethylparaben (EtP); 6. Propylparaben (PrP); 7. Butylparaben (BuP). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The spiked recoveries of sulfonamides ranged from 96.1 to 104.8 % with RSDs <8.5 %. The spiked recoveries of parabens ranged from 95.1 to 105.5 % and the RSDs <7.3 % (Table 4). These results indicate that this method can be applied to simultaneously determine parabens and sulfonamides in pre-cooked food samples.
Table 4.
Analytical results for the determination of parabens and sulfonamides in pre-cooked foods using the On-line SPE-HPLC method.
| Analytes | Spiked concentration (μg kg−1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Yu-shiang shredded pork |
Chinese sauerkraut fish |
Stewed beef with potatoes |
kung pao chicken |
||||||
| 10 | 100 | 10 | 100 | 10 | 100 | 10 | 100 | ||
| SDZ | Natural samples | -a | – | – | – | ||||
| Recovery (%)b | 98.4 | 101.8 | 96.1 | 100.7 | 102.1 | 100.8 | 97.5 | 97 | |
| RSD (%) | 3.3 | 5.1 | 5.6 | 4.8 | 4.3 | 3.9 | 5.1 | 7.4 | |
| SMZ | Natural samples | – | – | – | – | ||||
| Recovery (%) | 97.2 | 99.3 | 104.8 | 97 | 102.7 | 98.8 | 98.5 | 103.3 | |
| RSD (%) | 3.9 | 7.4 | 5.1 | 4.8 | 7.9 | 6.5 | 8.5 | 4.3 | |
| SMX | Natural samples | – | – | – | – | ||||
| Recovery (%) | 100.5 | 97.2 | 100.2 | 99.4 | 101.6 | 97.5 | 103.3 | 101.6 | |
| RSD (%) | 3.6 | 5.6 | 4.7 | 5.4 | 4.7 | 3.2 | 3.7 | 2 | |
| MeP | Natural samples | 1.57 | 1.24 | 0.58 | – | ||||
| Recovery (%) | 101.2 | 99.2 | 100.7 | 100.5 | 98.5 | 102.1 | 96.2 | 99.7 | |
| RSD (%) | 6.8 | 3.2 | 1.5 | 2.5 | 4.9 | 6.3 | 3.9 | 5.5 | |
| EtP | Natural samples | 1.58 | – | 0.92 | – | ||||
| Recovery (%) | 102.1 | 101.9 | 98.1 | 102.3 | 103.7 | 102.6 | 99.3 | 97.4 | |
| RSD (%) | 4.8 | 6.2 | 7.7 | 3.1 | 2.7 | 4.4 | 6.3 | 6.7 | |
| PrP | Natural samples | 0.71 | – | 2.72 | – | ||||
| Recovery (%) | 105.8 | 97.2 | 101.5 | 97.4 | 106.2 | 102.3 | 97.8 | 99.6 | |
| RSD (%) | 4.7 | 5.3 | 4.6 | 3.8 | 5 | 3.6 | 8.2 | 7.3 | |
| BuP | Natural samples | 0.46 | – | – | – | ||||
| Recovery (%) | 103.8 | 94.3 | 95.1 | 99.8 | 101.1 | 100.2 | 99.3 | 103.4 | |
| RSD (%) | 5.9 | 6.2 | 5.8 | 4.3 | 6.6 | 4.8 | 5.5 | 6.9 | |
-: No detected
Spiked recovery is obtained by adding a known amount of analyte into the natural test sample matrix.
4. Conclusions
In this study, we proposed a simple and efficient approach for the preparation of sulfonic acid functional MOFs based on a post-synthetic oxidation strategy. This material could be used for extraction and enrichment acidic and neutral compounds, based on the dual retention mechanism combining hydrophilic-lipophilic balance and cation exchange interactions. Thus, it could be used as a sorbent to simultaneously determine paraben and sulfonamides in pre-cooked food samples based on an on-line SPE-HPLC system. The enhancement factors ranged from 120 to 257 and the LODs reached the μg kg−1 level. Due to the high stability, no significant reduction in peak area was observed after 100 cycles. The spiked recoveries of sulfonamides ranged from 96.1 to 104.8 % and 95.1–105.5 %, respectively, with RSDs <8.5 %. The proposed method is reliable and sensitive for simultaneous determination of parabens and sulfonamides. This study provides an approach for the preparation of multifunctional MOFs and their application in sample pretreatment. This study provides an approach for the preparation of mixed-mode MOF sorbents, which combine an effective reversed-phase chemistry with strong ion-exchange sites on a single particle. They have superior applications in the extraction of compounds that are not retained or not well resolved by typical methods or in the removal of highly complex sample matrices, not limited to pre-cooked foods.
CRediT authorship contribution statement
Min Fang: Writing – original draft, Software, Resources, Project administration. Jianyang Ke: Methodology, Formal analysis. Zhaojie Wang: Methodology, Formal analysis. Qing Fu: Software, Investigation. Qing Yang: Data curation. Lin Xu: Software, Investigation. Yuepeng Lu: Supervision, Data curation. Yong Yang: Validation, Project administration. Xiaoming Jiang: Supervision, Resources. Yongning Wu: Supervision, Resources. Zhiyong Gong: Supervision, Resources. Xin Liu: Writing – review & editing, Visualization, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the National Natural Science Foundation of China (Grant No. 32001772), the Open Research Fund of the Key Laboratory of Edible Oil Quality and Safety,State Administration for Market Regulation (Grant No. SYYKF202307), and the Science and Technology Program of State Administration for Market Regulation of China (NO. 2023MK151).
Contributor Information
Min Fang, Email: fangmin0227@126.com.
Xin Liu, Email: liuxinhook@whpu.edu.cn.
Data availability
Data will be made available on request.
References
- Ahsan M.A., Islam M.T., Hernandez C., Kim H., Lin Y.R., Curry M.L.…Noveron J.C. Adsorptive removal of sulfamethoxazole and bisphenol a from contaminated water using functionalized carbonaceous material derived from tea leaves. Journal of Environmental Chemical Engineering. 2018;6(4):4215–4225. [Google Scholar]
- Barzallo D., Palacio E., March J., Ferrer L. 3D printed device coated with solid-phase extraction resin for the on-site extraction of seven sulfonamides from environmental water samples preceding HPLC-DAD analysis. Microchemical Journal. 2023:190. [Google Scholar]
- Bogdanova P., Pochivalov A., Vakh C., Bulatov A. Supramolecular solvents formation in aqueous solutions containing primary amine and monoterpenoid compound: Liquid phase microextraction of sulfonamides. Talanta. 2020;216 doi: 10.1016/j.talanta.2020.120992. [DOI] [PubMed] [Google Scholar]
- Cao Z., Zhou J. Research progress on pretreatment technology for the analysis of amphetamine biological samples. Journal of Separation Science. 2024;47(16) doi: 10.1002/jssc.202400337. [DOI] [PubMed] [Google Scholar]
- Chen R., Yang Y., Qu B., Li Y., Lu Y., Tian L.…Ramakrishna S. Rapid determination of sulfonamide residues in pork by surface-modified hydrophilic electrospun nanofibrous membrane solid-phase extraction combined with ultra-performance liquid chromatography. Analytical and Bioanalytical Chemistry. 2016;408(20):5499–5511. doi: 10.1007/s00216-016-9648-z. [DOI] [PubMed] [Google Scholar]
- Chen T.F., Han S.Y., Wang Z.P., Gao H., Wang L.Y., Deng Y.H.…Li G.S. Modified UiO-66 frameworks with methylthio, thiol and sulfonic acid function groups: The structure and visible-light-driven photocatalytic property study. Applied Catalysis B: Environmental. 2019:259. [Google Scholar]
- Chen X.L., Ai L.F., Cao Y.Q., Nian Q.X., Jia Y.Q., Hao Y.L.…Wang X.S. Rapid determination of sulfonamides in chicken muscle and Milk using efficient graphene oxide-based monolith on-line solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Food Analytical Methods. 2019;12(1):271–281. [Google Scholar]
- Chen Z.P., Yu C., Xi J.B., Tang S., Bao T., Zhang J. A hybrid material prepared by controlled growth of a covalent organic framework on amino-modified MIL-68 for pipette tip solid-phase extraction of sulfonamides prior to their determination by HPLC. Microchimica Acta. 2019;186(6) doi: 10.1007/s00604-019-3513-7. [DOI] [PubMed] [Google Scholar]
- Ding L., Shao P.H., Luo Y., Yin X.C., Yu S.P., Fang L.L.…Luo X.B. Functionalization of UiO-66-NH2 with rhodanine via amidation: Towarding a robust adsorbent with dual coordination sites for selective capture of ag(I) from wastewater. Chemical Engineering Journal. 2020;382 [Google Scholar]
- Dong Y., Chen Y., Wang R., Hong Z., Fan W., Huang Z., Wang G. Exploration of porous imine-based covalent organic framework for solid-phase extraction of five trace sulfonamides in food samples. Journal of Separation Science. 2024;47(1) doi: 10.1002/jssc.202300535. [DOI] [PubMed] [Google Scholar]
- Folorunsho O., Bogush A., Kourtchev I. A new on-line SPE LC-HRMS method for simultaneous analysis of selected emerging contaminants in surface waters. Analytical Methods. 2023;15(3):284–296. doi: 10.1039/d2ay01574a. [DOI] [PubMed] [Google Scholar]
- Hu Z.J., Luan X.L., Cui Y.Y., Yang C.X. Novel phenazine-based microporous organic network for selective and sensitive determination of trace sulfonamides in milk samples. Anal Chim Acta. 2024;1326:343138. doi: 10.1016/j.aca.2024.343138. [DOI] [PubMed] [Google Scholar]
- Huang K., Zhang X., Wang B., Wang X., You Y., Tang H.…Jing T. Accurate assessment of parabens exposure in healthy Chinese female adults: Findings from a multi-pathway exposure assessment coupled with intervention study. Environmental Research. 2021;193 doi: 10.1016/j.envres.2020.110540. [DOI] [PubMed] [Google Scholar]
- Huang S., Qi Z., Liu H., Long C., Fang L., Tan L., Yu Y. A large-scale survey of urinary parabens and triclocarban in the Chinese population as well as the influencing factors and health risks. The Science of the Total Environment. 2024;926 doi: 10.1016/j.scitotenv.2024.171799. [DOI] [PubMed] [Google Scholar]
- Jian N., Dai Y., Liu H., Wu N., Liu L.E., Wu D., Wu Y. Simple, fast and eco-friendly micro-solid phase extraction based on thiol and ionic liquid bi-functional nanofibers membrane for the determination of sulfonamides in environmental water. Analytica Chimica Acta. 2024;1288 doi: 10.1016/j.aca.2023.342163. [DOI] [PubMed] [Google Scholar]
- Jiang Y.X., Qin Z.C., Song X.N., Piao H.L., Li J.K., Wang X.H.…Sun Y. Facile preparation of metal organic framework-based laboratory semi-automatic micro-extraction syringe packed column for analysis of parabens in vegetable oil samples. Microchemical Journal. 2020;158 [Google Scholar]
- Jin C., Wei S., Sun R., Zou W., Zhang X., Zhou Q.…Huang L. The forms, distribution, and risk assessment of sulfonamide antibiotics in the manure-soil-vegetable system of feedlot livestock. Bulletin of Environmental Contamination and Toxicology. 2020;105(5):790–797. doi: 10.1007/s00128-020-03010-9. [DOI] [PubMed] [Google Scholar]
- Jin C., Wei S., Sun R., Zou W., Zhang X., Zhou Q.…Huang L. The forms, distribution, and risk assessment of sulfonamide antibiotics in the manure–soil–vegetable system of feedlot livestock. Bulletin of Environmental Contamination and Toxicology. 2020;105(5):790–797. doi: 10.1007/s00128-020-03010-9. [DOI] [PubMed] [Google Scholar]
- Leo P., Crespí N., Palomino C., Martín A., Orcajo G., Calleja G., Martinez F. Catalytic activity and stability of sulfonic-functionalized UiO-66 and MIL-101 materials in friedel-crafts acylation reaction. Catalysis Today. 2022;390-391:258–264. [Google Scholar]
- Li T., Wang C., Xu Z., Chakraborty A. A coupled method of on-line solid phase extraction with the UHPLC–MS/MS for detection of sulfonamides antibiotics residues in aquaculture. Chemosphere. 2020;254 doi: 10.1016/j.chemosphere.2020.126765. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu J.T., Zhang K.H., Lei L., Lei Z.L. UiO-66-NH2@PMAA: A hybrid polymer-MOFs architecture for pectinase immobilization. Industrial & Engineering Chemistry Research. 2018;57(2):559–567. [Google Scholar]
- Liao C., Chen L., Kannan K. Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environment International. 2013;57-58:68–74. doi: 10.1016/j.envint.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Liao C.Y., Liu F., Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environmental Science & Technology. 2013;47(8):3918–3925. doi: 10.1021/es400724s. [DOI] [PubMed] [Google Scholar]
- Liu Y.M., Wang S., Dickenson A., Mao J., Bai X., Liao X. An on-line SPE-LC-MS/MS method for quantification of nucleobases and nucleosides present in biological fluids. Analytical Methods. 2024;16(16):2505–2512. doi: 10.1039/d4ay00100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkliang F., Kanatharana P., Thavarungkul P., Thammakhet-Buranachai C. A miniaturized monolith-MWCNTs-COOH multi-stir-rod microextractor device for trace parabens determination in cosmetic and personal care products. Talanta. 2018;184:429–436. doi: 10.1016/j.talanta.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Moga A., Vergara-Barberan M., Lerma-Garcia M.J., Herrero-Martinez J.M., Simo-Alfonso E.F. Poly(ethylene glycol) diacrylate-based solid-phase extraction for determination of sulfonamides in meat samples. Microchemical Journal. 2020;157 [Google Scholar]
- Mokhtari M., Hamaizi H., Garcia M.D.G., Galera M.M. Synthesis and characterization of a sulfonic species-based mesoporous sorbent for the pre-concentration of nine personal care products in wastewater and swimming pool water. Microchemical Journal. 2020;153 [Google Scholar]
- Monteagudo C., Robles-Aguilera V., Salcedo-Bellido I., Galvez-Ontiveros Y., Samaniego-Sanchez C., Aguilera M.…Rivas A. Dietary exposure to parabens and body mass index in an adolescent Spanish population. Environmental Research. 2021;201 doi: 10.1016/j.envres.2021.111548. [DOI] [PubMed] [Google Scholar]
- Mortazavi S.S., Abbasi A., Masteri-Farahani M., Farzaneh F. Sulfonic acid functionalized MIL-101(Cr) metal-organic framework for catalytic production of Acetals. Chemistryselect. 2019;4(25):7495–7501. [Google Scholar]
- Nasir A.N.M., Yahaya N., Zain N.N.M., Lim V., Kamaruzaman S., Saad B.…Hirota Y. Thiol-functionalized magnetic carbon nanotubes for magnetic micro-solid phase extraction of sulfonamide antibiotics from milks and commercial chicken meat products. Food Chemistry. 2019;276:458–466. doi: 10.1016/j.foodchem.2018.10.044. [DOI] [PubMed] [Google Scholar]
- Nasrollahi S.S., Moosavi N.S., Yamini Y. Determination of parabens in different samples using green analytical chemistry approaches since 2015. TrAC Trends in Analytical Chemistry. 2023;166 [Google Scholar]
- Pasupuleti R.R., Hsieh J.-R., Pasupuleti V.R., Huang Y.-L. Eco-friendly magnetic Solid-Phase extraction and deep eutectic solvent for the separation and detection of parabens from the environmental water and urine samples. Microchemical Journal. 2022;178 [Google Scholar]
- Phang W.J., Jo H., Lee W.R., Song J.H., Yoo K., Kim B., Hong C.S. Superprotonic conductivity of a UiO-66 framework functionalized with sulfonic acid groups by facile Postsynthetic oxidation. Angewandte Chemie, International Edition. 2015;54(17):5142–5146. doi: 10.1002/anie.201411703. [DOI] [PubMed] [Google Scholar]
- Pulcastro H., Ziv-Gal A. Parabens effects on female reproductive health - review of evidence from epidemiological and rodent-based studies. Reproductive Toxicology. 2024;128 doi: 10.1016/j.reprotox.2024.108636. [DOI] [PubMed] [Google Scholar]
- Rangel-Huerta O.D., Uhlig S., Ivanova L., Dang T.T., Rode T.M., Noriega Fernandez E., Faeste C.K. Metabolomics workflow for quality control of differently-processed pre-cooked chicken fillets. Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.131006. [DOI] [PubMed] [Google Scholar]
- Razavi N., Es'haghi Z. Curcumin loaded magnetic graphene oxide solid-phase extraction for the determination of parabens in toothpaste and mouthwash coupled with high performance liquid chromatography. Microchemical Journal. 2019;148:616–625. [Google Scholar]
- Sadeghi S., Olieaei S. Nanostructured polyaniline based pipette tip solid phase extraction coupled with high-performance liquid chromatography for the selective determination of trace levels of three sulfonamides in honey and milk samples with the aid of experimental design methodology. Microchemical Journal. 2019;146:974–985. [Google Scholar]
- Shirani M., Parandi E., Nodeh H.R., Akbari-Adergani B., Shahdadi F. Development of a rapid efficient solid-phase microextraction: An overhead rotating flat surface sorbent based 3-D graphene oxide/ lanthanum nanoparticles @ Ni foam for separation and determination of sulfonamides in animal-based food products. Food Chemistry. 2022;373(Pt A) doi: 10.1016/j.foodchem.2021.131421. [DOI] [PubMed] [Google Scholar]
- Tekin Z., Karlidag N.E., Ozdogan N., Kocoglu E.S., Bakirdere S. Dispersive solid phase extraction based on reduced graphene oxide modified Fe(3)O(4) nanocomposite for trace determination of parabens in rock, soil, moss, seaweed, feces, and water samples from Horseshoe and Faure Islands. J Hazard Mater. 2022;426:127819. doi: 10.1016/j.jhazmat.2021.127819. [DOI] [PubMed] [Google Scholar]
- Yan Y., Yang R., Wang Y., Wu Y., Gu X., Qiao X. Esterified styrene-maleic acid copolymer modified silica as mixed-mode polymer-brush stationary phases for chromatographic separation. Journal of Chromatography. A. 2024;1732 doi: 10.1016/j.chroma.2024.465227. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Wang Y.B., Pan M.F., Xie X.Q., Liu K.X., Hong L.P., Wang S. Synthesis of magnetic metal-organic frame material and its application in food sample preparation. Foods. 2020;9(11) doi: 10.3390/foods9111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi M.N., Yamini Y., Asiabi H. Fabrication of polypyrrole-silver nanocomposite for hollow fiber solid phase microextraction followed by HPLC/UV analysis for determination of parabens in water and beverages samples. Journal of Food Composition and Analysis. 2018;74:18–26. [Google Scholar]
- Zhang Y.Y., Li G.L., Wu D., Li X.T., Yu Y.X., Luo P.J.…Wu Y.N. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. Trac-Trends in Analytical Chemistry. 2019;121 [Google Scholar]
- Zheng M., Tang S., Bao Y., Daniels K.D., How Z.T., El-Din M.G.…Tang L. Fully-automated SPE coupled to UHPLC-MS/MS method for multiresidue analysis of 26 trace antibiotics in environmental waters: SPE optimization and method validation. Environmental Science and Pollution Research International. 2022;29(12):16973–16987. doi: 10.1007/s11356-021-15947-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.