Abstract
Background
Due to its strong immunogenicity and tumor specificity, neoplastic antigen has emerged as an immunotherapy target with wide therapeutic prospect and clinical application value. Anti-programmed death-1 (PD-1) antibodies reinvigorate T cell-mediated antitumor immunity. So, we conducted single-arm trial to assess the safety and efficacy of PD-1 blockade(Camrelizumab)-activated neoantigen specific cellular therapy (aNASCT) on advanced relapsed non-small lung cancer(NSCLC)(ClinicalTrials.gov NCT03205930).
Methods
Neoantigenic peptides were designed and manufactured according to the whole exome sequencing and RNA sequencing of fresh biopsy tissues and peripheral blood as well as bioinformatics analysis. All participants received subcutaneous injection of mature dendritic cells(mDCs) loaded with neoantigens on day 8 and an intravenous infusion of PD-1 blockade-activated autologous cytotoxic T lymphocytes (CTLs) induced by mDCs on day 27 for a period defined as 28 days (4 weeks). Enrolled patients received at least three cycles of therapy. The safety and efficacy of the treatment were evaluated by evaluating adverse reactions, progression-free survival (PFS), overall survival (OS).
Results
A total of 13 patients with advanced relapsed NSCLC were enrolled in this study. All 13 patients received at least three cycles of aNASCT treatment, of which two patients received at most 12 cycles of treatment. Treatment-related adverse events (AEs) occurred in 4/13 (30.8%)patients with transient fever below 38℃.The objective response rate (ORR) across the 13 enrolled patients was 7 of 13 (53.85%,95% CI 0.29–0.77).The disease control rate (DCR) was 8 of 13 (61.54%,95% CI 0.36–0.82). The median PFS was 11 months (95% CI 6.1–15.9), and the median OS was 15 months(95% CI 11.5–18.5).
Conclusions
Our findings indicate that aMASCT therapy was safety and immunogenicity of patients with advanced relapsed NSCLC, suggesting its promising potential in cancer immunotherapy.
Keywords: Neoantigen, Cell therapy, Immunotherapy, Advanced non-small cell lung cancer
Introduction
Cancer immunotherapy, encompassing cell-based cancer immunotherapies and immune checkpoint inhibitors(ICIs),has revolutionized the treatment paradigm [1, 2]. Cell-based cancer immunotherapies, including activation and amplification of the patient’s autologous T lymphocytes in vitro and then returning them to the body to kill tumor cells [3], have demonstrated remarkable efficacy in advanced cancers [4, 5]. Multiple antigen-stimulating cell therapy (MASCT) is the first therapeutic intervention combining DCs vaccines and ACT in a single treatment modality to elicit both active and passive immune response [6].Our previous study has demonstrated that due to the weak immunogenicity of antigen, MASCT treatment for patients with non-small cell lung cancer (NSCLC) who were intolerant to standard therapies was still unsatisfactory [7]. Consequently, effective and low toxicity therapies for advanced NSCLC are urgently needed.
Tumor neoantigens, which are mutated peptides that bind with the major histocompatibility complex (MHC) on the surface of malignant cells, have the potential to be recognized by T cells and elicit robust and specific antitumor immune responses. A growing body of evidence indicates that immunotherapy targeting neoantigens has significant antitumor effects on various cancers [8, 9]. In support of these findings, specific cell therapy targeting neoantigens may strengthen patients´ endogenous immune response against cancer [10]. Meanwhile, NSCLC is recognized as one of the most ICI-sensitive cancers. ICI can restore suppressed immune responses to tumors by inhibiting ligand-receptor interactions involving lymphocyte regulatory molecules [11]. Mutation-derived neoantigens may be critical tumor-specific targets for ICI-reactivated CTLs [12]. We reasoned that this strategy could augment the response rates and durability of responses over the results of PD-1 inhibition alone by inducing, expanding, and broadening the tumor-directed T cell repertoire. Therefore, we analyzed the data from our phase I/IIa trial to assess the safety and efficacy of PD-1(Camrelizumab) blocked neoantigen specific cellular therapy(aNASCT) in advanced NSCLC after standard treatment.
Materials and methods
Study design and patients
Between June 2017 to June 2020, Patients aged between 18 and 75 years were provided informed consent and enrolled to a prospective, single center, single-arm, pilot clinical trial (NCT03205930). The clinical trial was approved by the Ethics Committee of Lianyungang Clinical College of Nanjing Medical University (2,017,008) on February 23,2017. The study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Eligible patients were clinically or radiographically evident, pathologically confirmed stage IV or recurrent metastatic NSCLC, including squamous cell carcinoma and adenocarcinoma, who failed multiple lines of therapy(at least two systemic therapies). Other inclusion criteria included a life expectancy of over three months, an eastern cooperative oncology group (ECOG) score of 0–2,adequate hematologic and organ functions, tumor lesions could be evaluated by response evaluation criteria in solid tumors version 1.1 (RECIST 1.1), cessation of any cancer therapy for at least one month before enrollment. Patients were excluded if they had received prior immunotherapy, including anti-CTLA-4 or anti-PD-1/PD-L1 therapies, because these were not regarded as the standard treatment for NSCLC in China in 2017. Patients were also excluded if they had an active autoimmune disease. The patients enrolled were undergoing tumor biopsies and blood withdrawing, including formalin-fixed paraffin-embedded (FFPE) samples, biopsy specimen, serum samples and serous effusions.
Study treatment
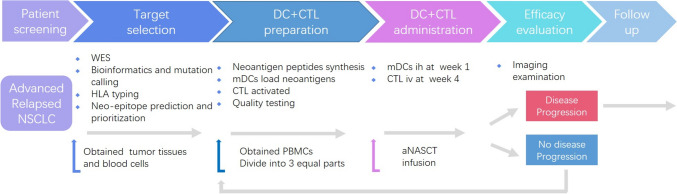
All participants were treated with a subcutaneous injection of 1–10 × 107 mature dendritic cells (mDCs) loaded with neoantigens on day 8 and an intravenous infusion of PD-1 blockade-activated autologous cytotoxic T lymphocytes (CTLs) induced by mDCs on day 27 (1–10 × 109 per infusion cycle) (Fig. 1). A treatment cycle was defined as 28 days (4 weeks). Eligible participants were exposed to repeat cycles of therapeutic regimen until one of the following events occurred: progressive disease (PD); death; unmanageable toxic effects; consent withdrawn from the study; received up to 12 cycles or discontinuation owing to the physician’s decision.
Fig. 1.
The treatment protocol of aNASCT for patients with advanced replased NSCLC. WES: whole exome sequencing; DC: dendritic cell; CTL: autologous cytotoxic T lymphocytes
Study endpoints and assessments
The primary endpoints of this study included safety and feasibility. Safety endpoints will be assessed based on the occurrence of adverse events, with a threshold of at least 80% of patients treated needing to be monitored for safety outcomes. The secondary endpoints included progression-free survival (PFS, the duration from time of enrollment to PD)overall survival (OS, the duration from the time of enrollment to death), objective response rate (ORR, including rate of complete response (CR) plus partial response (PR)), and disease control rate (DCR, including CR, PR, and stable disease (SD)).
The tumor assessment of each patient, including contrast-enhanced computed tomography and magnetic resonance imaging (MRI) of the brain, was conducted before the first treatment. Tumor responses were assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria after three cycles and subsequently every 12 weeks based on the investigator's assessment. Toxicity assessments, adverse events, laboratory values, ECG, and vital signs were assessed regularly and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v.4.03) for 36 months after the final cycle or until the patient was lost to follow-up.
Whole exome sequencing
FFPE tumor samples and matched autologous peripheral blood mononuclear cells (PBMCs) samples of each patient were sent to the facility of Geneseeq Technology Inc (Nanjing, China) for subsequently whole exome sequencing( WES) analysis [13, 14].
Bioinformatics and mutation calling
All raw data output from sequencing were filtered out adapter and trimming low quality reads with Illumina illustration. Clean reads were aligned to the reference genome hg19 using BWA. Duplicate site were marked using Picard. The paired tumor and normal data were used to detected single nucleotide variations (SNV) and small insertions/deletions (Indel) using Mutect2. All detected variation were annotated by Annovar and filtered with frequency more than 5% and over than 20 supported reads for further analysis.
Human leukocyte antigen (HLA) typing
HLA class I major loci A, B, C were typed at four-digit resolution by Polysolver [15]using normal sample’s WES sequence data.
Neoantigenic peptides(neopeptides) prediction and prioritization
All mutation were annotated by Annovar. NeoORF were annotated manually and translated into amino acid sequence. The 31 long peptides which containing mutation in middle site and extend 15 amino acids of both sides were selected for epitopes prediction. All possible 8-11mers peptides sequences were computed affinity and stability cooperated with the patient’s HLA-I subtypes using netMHC4.0/netMHCpan3.0 and netMHCstab/netMHCpan 2.0.
Epitopes with stability more than 2 h were defined as positive presented epitopes. Neo-peptide which contained 31 amino acids were classified as four levels: Neopeptides with more than two positive epitopes were defined as level 4.Otherwise, neopeptides containing only single epitope with affinity value lower than 150 nM were defined as level 3, and neopeptides containing single epitope affinity value which ranged from 150 to 500 nM were defined as level 2, and affinity value higher than 500 nM were defined as level1. All neopeptides were ranked by high levels and within same level ranked by the mutation frequency. For each patient, the top 1–15 neopeptides were selected for further peptides synthesis and using in further aNASCT cell culture.
Generation and assessment of aNASCT
MASCT is the first therapeutic intervention combining DCs vaccines and ACT in a single treatment modality to elicit both active and passive immune response, MASCT was prepared as our published study [7]. Peripheral blood mononuclear cells (PBMCs) from patients were obtained by density gradient centrifugation using Lymphoprep (Nycome-dPharma, Oslo, Norway) and incubated 1.5 h at 37 °C in a saturated 5% CO2 incubator. After that, adherent monocytes were cultured with GM-CSF (1000 U/mL) and IL-4 (500 U/mL) in AIM-V (Gibco, Carlsbad, CA) to differentiate into immature DCs (imDCs). The imDCs were pulsed by a peptide pool of neoantigens (1 μg/mL/peptide) and then cultured with a maturing cocktail (IL-6, 1000 U/mL; TNF-α, 1000 U/mL; IL-1β, 10,000 U/mL; PEG2, 1 μg/mL; Poly I:C, 10 μg/mL) to differentiate into antigen-presenting mDCs. In order to prepare for the activated CTL infusion, frozen non-adherent PBMCs were co-cultured with antigen-loaded mDCs in the presence of IL-2 (1000 U/mL; R&D Systems Inc, Minneapolis, MN) about four weeks. The anti-CD3 antibody (50 ng/mL; eBioscience, Inc., San Diego, CA) was added 3 days after co-culturing. The autologous T cells were then incubated with 1.5 mg Camrelizumab (a fully human IgG4 monoclonal antibody against PD-1) [16]ex vivo for 40 min in a 37 °C thermostat, referred to as aNASCT, and finally transferred to patients.
Flow cytometry analysis
The circulating regulatory T cells(Tregs)from patients were assessed using flow cytometry as previously described [7]. Antibodies for surface markers and intracellular protein staining were obtained from BD Biosciences including anti-CD80, -CD83, -CD86, -CD3, -CD4, -CD8, -CD25, -CD127, -PD-1, -IL-2,IL-4,IL-6,IL-10,TNF- α,-IFNγ. All the flow cytometry assays were determined using a FACSCalibur Flow Cytometer (BD Pharmingen), and the data were analyzed by the FlowJo software (Tree Star Inc.).
IFN-γ enzyme-linked immunospot assay (ELISPOT)
IFN-γ ELISPOT kit (U-CyTech Biosciences, Netherlands) was used to detect the levels of peptide reactivity in the CTL. The ELISPOT assay was conducted and analyzed according to standard protocol. About 2 × 105 cells were incubated with the neoantigen peptides pool, positive peptides or irrelevant peptides using a 48-well ELISPOT plate overnight at 37 °C in a saturated 5% CO2. The resulting spots were scanned by Elispot CTL Reader (Cell Technology Inc, Columbia, MD) and analyzed with Elispot software (AID, Strassberg, Germany).
Statistical analysis
Patient characteristics, clinical outcomes were presented using simple descriptive statistics. GraphPad Prism 9.0 software was used to plot survival curves and perform data analyses. Data samples were compared using a 2-tailed t-test. The Kaplan–Meier curves was used to estimated PFS and OS with corresponding 95% CIs. P < 0.05 was considered significant in all the analyses.
Results
Patient characteristics and neoantigen identification
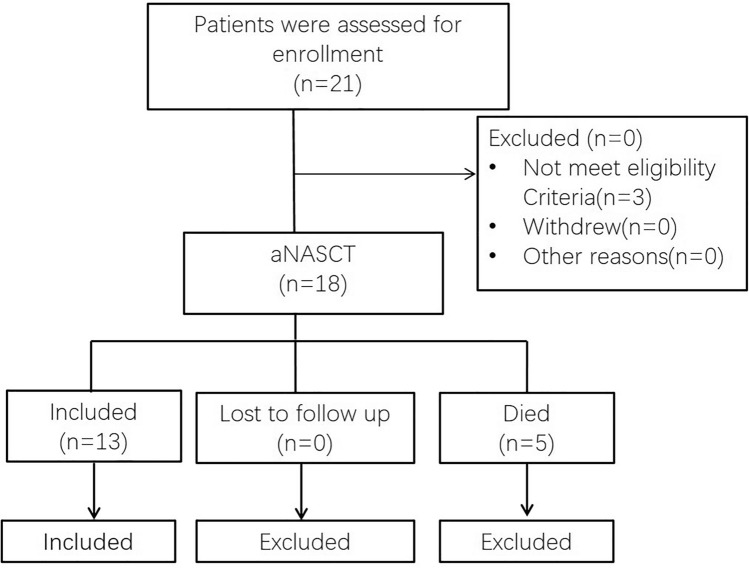
We conducted a single-arm, pilot study to test the efficacy of aNASCT in patients with relapsed advanced lung cancer(ClinicalTrials.gov NCT03205930). The aNASCT formulation and the overall schedule of administration are shown in Fig. 1. From June 2017 to June 2020, Lianyungang Clinical College of Nanjing Medical University recruited 21 patients with advanced non-small cell lung cancer who had relapsed under standard multiline treatment. The 21 patients must not have previously undergone immunotherapy, such as anti-CTLA-4 or anti-PD-1/PD-L1 treatments, and they should not have any active autoimmune diseases. Except for three patients who had failed biopsies, tumor and blood samples were collected for high-throughput sequencing from 18 of 21 patients. After performing WES on the DNA from both tumor and blood samples, five patients were excluded due to death from rapid tumor progression. The participant flow through this study is outlined in Fig. 2. The demographics and baseline clinical characteristics of the included 13 patients are listed in Table 1. All patients enrolled in the study completed follow-up, the median follow-up was 15 months.
Fig. 2.
Flow chart of patients with advanced replased NSCLC receiving aNASCT treatment
Table 1.
Baseline clinical characteristics of patients
| Patient ID | Sex | Age | Pathology | ECOG PS | Driver genes | Prior antitumor treatment | First-line treatment | Second-line treatment | Third-line treatment | PD-L1 TPS | Treatment cycle |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P01 | M | 67 | A | 0 | WT | Sur + C | Sur |
bone metastasis C:AP |
C: docetaxel | 1 | 10 |
| P02 | F | 64 | S | 0 | EGFR exon19Del | T + C | T: gefitinib |
brain metastasis T: Axitinib |
C:AP | 0 | 4 |
| P03 | M | 73 | S | 1 | WT | Sur + C | Sur |
lung metastasis C:TP |
bone metastasis C: docetaxel |
5 | 6 |
| P04 | F | 41 | S | 1 | WT | C + R + An | C:TP |
bone metastasis:R C: docetaxel |
An | 0 | 9 |
| P06 | F | 56 | A | 0 | WT | C | C:AP | C: GP | C: docetaxel | 20 | 12 |
| P08 | M | 63 | A | 1 | EGFR exon19Del、TP53 | T + C | T: gefitinib |
brain metastasis T: Axitinib |
C:AP | 0 | 3 |
| P09 | M | 44 | A | 0 | WT | C + B | C:AP | C + B: B + AP | C + B: B + docetaxel | 15 | 12 |
| P11 | M | 61 | A | 1 | WT | C + B + An | C:AP | C + B: B + AP | An | 5 | 4 |
| P13 | M | 39 | A | 0 | WT | C + B | C:AP | C + B: B + AP | C + B: B + docetaxel | 0 | 9 |
| P14 | M | 58 | S | 1 | WT | C + An | C:TP | C: docetaxel | An | 40 | 9 |
| P16 | F | 63 | S | 1 | WT | C + R + An | C:TP |
brain metastasis:R C: docetaxel |
An | 55 | 3 |
| P17 | M | 60 | A | 1 | EGFR L858R | T + C | T: gefitinib | T: Axitinib | C:AP regimen | 2 | 6 |
| P21 | M | 67 | A | 2 | WT | C + B + An | C:AP | B + C:B + AP | An | 0 | 3 |
M: male, F: female, A: adenocarcinoma, S: squamous cell carcinoma, ECOG PS: Eastern cooperative oncology Group performance status, WT: wild-type, EGFR: epidermal growth factor receptor, Sur: surgery, C: Chemotherapy, R: Radiotherapy, B: Bevacizumab, An: Anlotinib, T: targeted therapy, AP: Pemetrexed plus Cisplatin, TP: Paclitaxel plus Cisplatin, GP: Gemcitabine plus Cisplatin
To produce the personalized neoantigen peptide, patients firstly underwent 416 gene panel sequencing and human leukocyte antigen (HLA)-I typing as outlined in Table 2. After stepwise filtering criteria, the top 3–15 neopeptides according to the prediction algorithm were selected for each patient (Table 2). The turn-around time of the whole process was mostly between four months.
Table 2.
Patient HLA, number of neoepitopes
| Patient ID | HLA I binders | Nonsynonymous mutations HLA I binders | Number of expressed neoepitopes | Neopeptides in aNASCT |
|---|---|---|---|---|
| P01 | HLA-A*11:01:01/HLA-A*01:06:01 | 25 | 8 | 8 |
| HLA-B*14:01:01/HLA-B*15:02:01 | ||||
| HLA-C*08:02:01/HLA-C*08:01:01 | ||||
| P02 | HLA-A*11:02:01/HLA-A*24:02:01 | 27 | 11 | 11 |
| HLA-B*39:01:01/HLA-B*51:01:01 | ||||
| HLA-C*14:02:01/HLA-C*07:02:01 | ||||
| P03 | HLA-A*11:01:01/HLA-A*02:06:01 | 53 | 35 | 10 |
| HLA-B*13:01:01/HLA-B*46:01:01 | ||||
| HLA-C*03:04:01/HLA-C*01:03:01 | ||||
| P04 | HLA-A*11:02:01/HLA-A*11:02:01 | 17 | 9 | 9 |
| HLA-B*52:01:01/HLA-B*13:01:01 | ||||
| HLA-C*12:02:01/HLA-C*03:04:01 | ||||
| P06 | HLA-A*24:02:01/HLA-A*02:03:01 | 14 | 6 | 6 |
| HLA-B*39:01:01/HLA-B*48:01:01 | ||||
| HLA-C*07:02:01/HLA-C*08:01:01 | ||||
| P08 | HLA-A*03:01:01/HLA-A*03:01:01 | 77 | 26 | 10 |
| HLA-B*57:01:01/HLA-B*15:17:01 | ||||
| HLA-C*06:02:03/ HLA-C*07:01:02 | ||||
| P09 | HLA-A*24:02:01/HLA-A*11:01:01 | 26 | 8 | 8 |
| HLA-B*48:01:01/HLA-B*48:01:01 | ||||
| HLA-C*08:01:01/HLA-C*07:02:01 | ||||
| P11 | HLA-A*30:01:01/HLA-A*33:03:01 | 22 | 14 | 14 |
| HLA-B*44:03:01/HLA-B*13:02:01 | ||||
| HLA-C*06:02:01/HLA-C*14:03:01 | ||||
| P13 | HLA-A*30:01:01/HLA-A*30:01:01 | 18 | 8 | 8 |
| HLA-B*40:01:01/HLA-B*15:01:20 | ||||
| HLA-C*04:03:01/HLA-C*03:03:01 | ||||
| P14 | HLA-A*24:02:01/HLA-A*02:01:01 | 53 | 15 | 15 |
| HLA-B*35:01:01/HLA-B*15:01:01 | ||||
| HLA-C*03:01:01/HLA-C*07:02:01 | ||||
| P16 | HLA-A*24:02:01/HLA-A*24:02:01 | 3 | 3 | 3 |
| HLA-B*40:01:02/HLA-B*54:01:01 | ||||
| HLA-C*01:02:01/HLA-C*03:04:01 | ||||
| P17 | HLA-A*11:02:01/HLA-A*11:01:01 | 18 | 10 | 10 |
| HLA-B*13:01:01/HLA-B*35:01:01 | ||||
| HLA-C*03:03:01/HLA-C*03:04:01 | ||||
| P21 | HLA-A*03:01:01/HLA-A*01:01:01 | 301 | 158 | 10 |
| HLA-B*57:01:01/HLA-B*15:17:01 | ||||
| HLA-C*06:02:03/HLA-C*07:01:02 |
Feasibility of aNASCT manufacturing
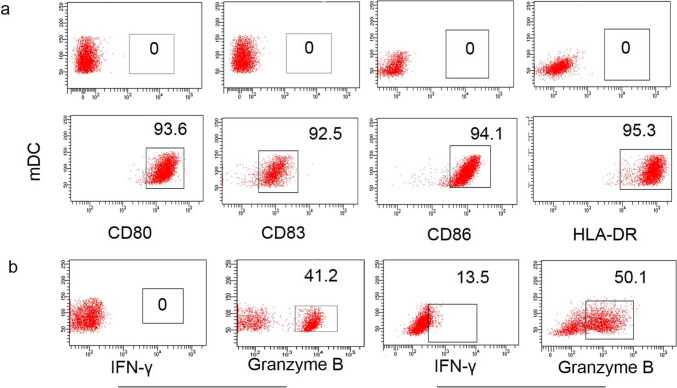
In total, 13 patients underwent aNASCT treatment. PBMCs were collected by leukapheresis for aNASCT following peptide synthesis. Each patient received no less than three cycles of treatment. The immunophenotypes and characteristics of mDCs or CTLs should be evaluated before treatment. Almost all mDCs expressed mature molecular markers (CD80+, CD83+, CD86+, HLA-DR+), while imDCs did not expressed these markers (Fig. 3a). In addition, a considerable percentage of CTLs expressed granzyme B and IFN-γ compared with PBMC (Fig. 3b), which indicated that mDCs and CTLs were activated before infusion. These data establish the feasibility of this advanced manufacturing platform and support further study.
Fig. 3.
Characteristics of mDCs or CTL. a Flow cytometry was used to detect the expression of markers of maturation in imDCs and mDCs; b The expression of IFN-γ and granzyme B of PBMC and PD-1 blockade-activated CTL
Safety of aNASCT
aNASCT treatments were well tolerated. The most common observed immune-related adverse events (AEs) was transient fever (4/13, 30.8%), but almost all fevers were mild and below 38 °C,and resolved within 12 h spontaneously. Other immune-related AEs were not observed. (Table 3).
Table 3.
Treatment-related AEs in all treated patients
| AEs | All treated patients(n = 13) | |
|---|---|---|
| Grade 1–2 | Grade 3–4 | |
| Fever | 4 | 0 |
| Flu-like symptoms | 0 | 0 |
| Chills | 0 | 0 |
| Dizziness | 0 | 0 |
| Fatigue | 0 | 0 |
Efficiency of aNASCT
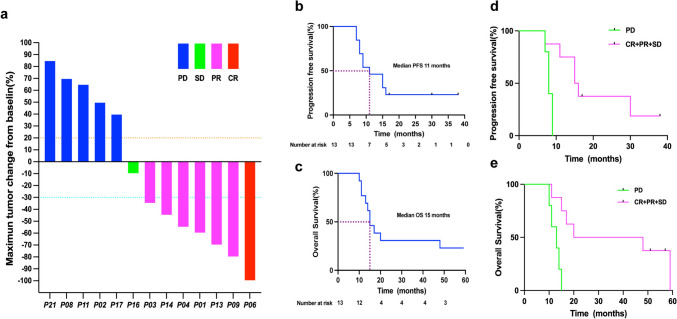
All 13 patients received at least three cycles of aNASCT treatment, of which two patients received at most 12 cycles of treatment, and two patients received only nine cycles of treatment due to the COVID-19 (Table 1). The tumor response of the remaining 13 patients is shown in Fig. 4. Patients with CR, PR, SD, and PD were 1, 6, 1, 5.The objective response rate (ORR) was 7 of 13 (53.85%,95% CI 0.29–0.77), while the disease control rate(DCR) was 8 of 13 (61.54%,95% CI 0.36–0.82). As of June 30, 2024, the median PFS was 11 months (95% CI 6.1–15.9), and the median OS was 15 months(95% CI 11.5–18.5) (Fig. 4b–c). The median PFS was 15.5 months in the effective treatment group (CR + PR + SD), and eight months in the ineffective treatment group (PD) (P = 0.0044). The median OS was 34 months in the effective treatment group, and 13 months in the ineffective treatment group(P = 0.0039) (Fig. 4d–e). Figure 5 is the swimmer's plot of the 13 patients treatment with aNASCT.
Fig. 4.
Presupposition analysis of the clinical activity of patients given aNASCT. a Waterfall plot of the best observed response for 13 patients treated with aNASCT; b–c PFS and OS of patients given aNASCT; d–e PFS and OS of patients in the effective treatment group(CR + PR + SD) and ineffective treatment group(PD)
Fig. 5.
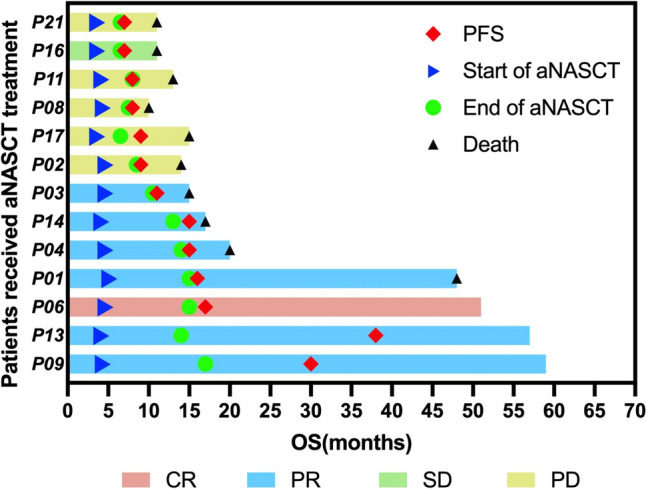
The swimmer's plot of the 13 patients treatment with aNASCT
IFN-γ ELISPOT of aNASCT
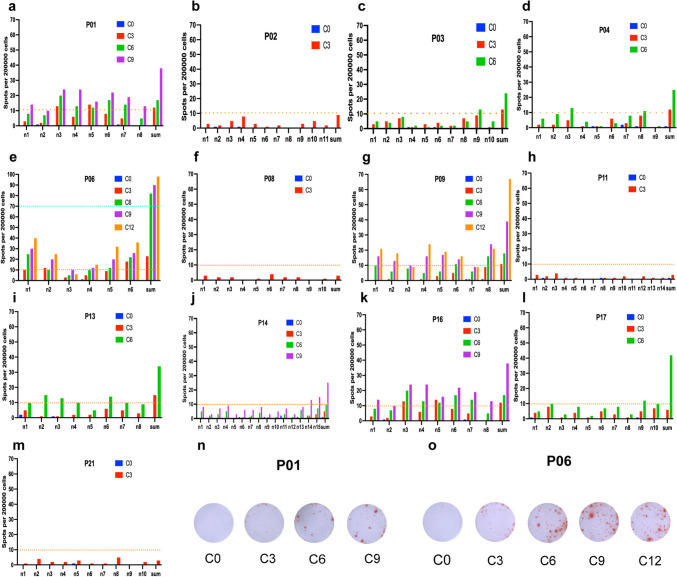
To detect neoantigen peptide specific immune responses, IFN-γ ELISPOT was detected in vitro by autologous PBMC before aNASCT treatment and after each three cycles of treatment, respectively (Fig. 6). As shown in Fig. 6, after a period of treatment with aNASCT, most of the predicted synthesized new antigen peptides had obvious response, and the response rate increased with the increase of treatment period. However, there was no significant difference in the number of positive spots in patients who did not respond to treatment compared with those before treatment. The results indicated that aNASCT therapy did not induce a significant specific immune response in patients who did not respond to treatment.
Fig. 6.
IFN-γ ELISPOT Assays were detected in vitro by autologous PBMC before and after each three cycles of aNASCT treatment, respectively. a–m IFN-γ ELISPOT of 13 patients before and after each three cycles of aNASCT treatment. n–o IFN-γ ELISPOT of the summed neopeptides in patient P01 and P06. n1-15: neo-peptide predicted for each patient sum: all neopeptides synthesized by each patient. C0/3/6/9/12: before and after each 3/6/9/12 cycles of aNASCT treatment. P0-21: patient 0–21
Analysis of the circulating T cells
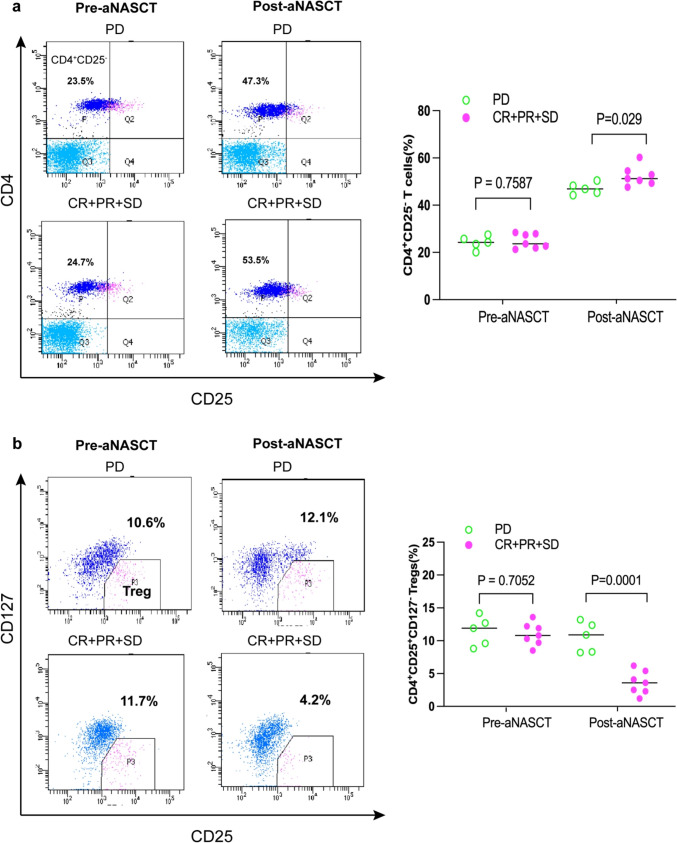
The infiltration of T cells forms the foundation of antitumor immunity. Therefore, monitoring lymphocyte subpopulations is crucial for assessing the effectiveness of antitumor therapy. In order to understand the activation of T cells in the tumor microenvironment during treatment, we used flow cytometry to detect the cells in PBMC of patients before and after treatment. There was no significant difference in CD3+ T cells, CD3+ CD4+ T cells, and CD3+ CD8+ T cells among PBMCs before and after aNASCT treatment. Before treatment with aNASCT, there was no significant difference in CD4+ CD25−T cells and Tregs in PBMC between the effective and ineffective groups (P > 0.05). After three cycles of aNASCT treatment, the percentage of CD4+ CD25−T cells increased (P < 0.05) in all groups( Fig. 7a). Tregs in the effective treatment group was significantly decreased than pretreatment(P = 0.029), while Tregs in the ineffective treatment group was not significantly decreased(P = 0.7587) ( Fig. 7b).These results revealed the important role of Tregs in tumor microenvironment in the treatment of advanced NSCLC by aNASCT.
Fig. 7.
Trends in circulating T cells in the effective treatment group(CR + PR + SD) and the ineffective treatment group(PD) before and after aNASCT treatment. The frequency of CD4 + CD25 − T cells a and CD4 + CD25 + CD127 − tregs b in patients’ PBMCs was measured at the beginning of the first treatment and measured after three cycles of aNASCT treatment
Discussion
This study included patients with stage IV NSCLC who had failed standard treatment and were diagnosed with metastases or local recurrences by imaging. Such patients have higher tumor mutational burden and poor prognosis. Our previous single-center prospective study showed that the PFS of pretreated advanced NSCLC patients, who were treated with aMASCT, was 4.5 months, and the OS was only 8.2 months [7]. The limited efficacy of MASCT may be related to the weak immunogenicity of tumor cells: most tumor cells only express low levels of tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs), and their immunogenicity is so weak that the tumor cells are not sufficient to stimulate the body to produce a strong enough immune response. On the other hand, it may be related to the antigenic modulation of tumor cells: the immune response of the host to tumor antigens may lead to the reduction or loss of antigens on the surface of tumor cells, thus evading immune attack. Therefore, the selection of appropriate antigenic targets is a critical barrier for the success of cancer immunotherapy. Neoantigens are tumor-specific and expressed in tumor cells only, thus neoantigens can bypass the host central or peripheral immune tolerance to elicit or augment neoantigen‐specific antitumor immunity with strong neoantigen binding. NSCLC bears a high mutational load [17]. Direct targeting of neoantigens with personalized NSCLC, which bears a high mutational load, provides an alternative approach to enhance tumor immunotherapy.
In this study, we designed a prospective clinical trial to evaluate the safety and efficacy of aNASCT for patients with recurrent metastatic NSCLC. The study is novel for combining mDCs loaded with neoantigens and PD-1 blockade-activated CTLs. This approach addresses a critical gap in cancer immunotherapy by integrating two potent immune-modulating strategies to enhance antitumor responses. Furthermore, the use of WES and bioinformatics for neoantigen prediction and prioritization, allowing for personalized immunotherapy tailored to the unique mutational landscape of each patient's tumor. However, the disadvantage was that the design and production of neoantigenic peptides took about four months. The personalized neoantigen typically require extracting samples from patients and analyzing mutations through techniques such as WES to identify the neoantigens with the highest potential for immune activation. This process is not only time-consuming but also involves complex bioinformatics analyses and laboratory operations, which may lead to the deterioration of the patient's condition, while waiting for treatment. In this study, five patients were excluded due to disease progression, at last only 13 patients were treated with aNASCT. This clinical issue can be analyzed from multiple perspectives. Firstly, the long production time may affect the opportunity for patients to receive timely treatment, especially in cases of rapid tumor progression. For example, in a study on advanced esophageal squamous cell carcinoma (ESCC), the combination of personalized mRNA vaccines with PD-1 inhibitors showed good efficacy, but the lengthy production cycle could delay the optimal treatment timing [18]. Secondly, methods to improve the efficiency of neoantigen screening and vaccine production, such as using high-throughput screening technologies or automated platforms, are expected to shorten production time. To address this issue, our research center continuously improved its measures by collaborating and communicating with other centers. Initially, the time from the screening and synthesis of neoantigen peptides to the start of aNASCT treatment was shortened from 5 to 3.5 months.
In this study, we show that aNASCT is safe and tolerable and can be conveniently administered for patients. All of the adverse events were low-grade and transient. Among the 13 patients, four patients experienced transient fever below 38 °C (30.77%, 4/13) for less than 12 h, while other patients did not show immune-related treatment responses. Previously, our research team reported the incidence of aMASCT treatment-related adverse events was 56.3%. The results showed that aNASCT demonstrated good safety in the treatment of advanced NSCLC.
Although only one of the enrolled patients achieve CR, this study demonstrated a 53.85% ORR and 61.54% DCR in patients with recurrent advanced NSCLC who were treated with aNASCT. As of June 30, 2024, the median PFS was 11 months (95% CI 6.1–15.9), and the median OS was 15 months (95% CI 11.5–18.5).
Our previous study showed that the median PFS was 4.5 months and OS was 8.2 months in relapsed NSCLC patients receiving aMASCT [7]. The results indicated that patients benefited from the activation of the immune system by aNASCT, resulting in improved PFS and OS. Compared with similar research results, the aNASCT treatment for advanced NSCLC in this study has shown good efficacy, but due to the small sample size, more reliable experimental data require further research with an expanded sample size.
Furthermore, we have measured the percentage of neoantigens that can stimulate the response of CD4+CD25− T cells and CD4+CD25+CD127− Tregs. As shown in, after three cycles of aNASCT treatment, there was no significant change in the ineffective group, which may be the reason of poor immune response to aNASCT treatment. Tregs are generally considered to be cells with immunosuppressive functions [19, 20]. Based on the above results, ineffective antitumor immune responses in part of patients with advanced NSCLC contributed to tumor progression and poor outcomes, which may be related to the tumor-suppressive microenvironment caused by immune imbalance.
This study was a pooled analysis of a single-center, single-arm trial. A major limitation is the small sample size, as only 13 patients were included in the analysis. This limited cohort may reduce the generalizability of the findings and the statistical power to detect significant differences or rare adverse events. Additionally, the complex nature of the treatment protocol, involving multiple cycles of mDCs and PD-1 blockade-activated CTLs, also poses challenges in standardizing the treatment and monitoring processes. Another limitation is that the reasons for effective and ineffective treatments are unclear, and it remains uncertain whether corresponding markers can be easily detected for pre-evaluating therapeutic effects.
Conclusions
In conclusion, our study demonstrates that aNASCT is feasible, safe, and capable of inducing specific T cell immunity and therapeutic benefits. Next, we are planning to conduct basic and clinical trials in order to improve the therapeutic effect of tumor neoantigen therapy. Finally, we believe that tumor neoantigens are currently considered a promising field in cancer immunotherapy.
Acknowledgements
We thank the patients enrolled in our clinical trials.
Abbreviations
- aNASCT
PD-1 blockade(camrelizumab)-activated neoantigen specific cellular therapy
- ACT
Adoptive cellular therapy
- AEs
Adverse events
- CI
Confidence intervals
- CR
Complete response
- CTL
Cytotoxic T lymphocytes
- DCR
Disease control rate
- DCs
Dendritic cells
- ECOG
Eastern cooperative oncology group
- ELISPOT
Enzyme-linked immunospot assay
- HLA
Human leukocyte antigen
- HR
Hazard ratios
- ICIs
Immune checkpoint inhibitors
- MASCT
Multiple antigen specific cellular therapy
- MDSC
Myeloid-derived suppression cells
- MHC
Major histocompatibility complex
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
- PD
Progressive disease
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response evaluation criteria in solid tumors version 1.1
- SD
Stable disease
- SNV
Single nucleotide variations
- Tregs
Regulatory T cells
- WES
Whole exome sequencing
Authors contributions
Xiaodong Jiang, Yun Qiao, Kaiyuan Hui and Chenxi Hu participated in the conception and design of this study. Yun Qiao, Liang Liu, Changhong Dong and Xiaodong Jiang contributed to the recruitment of patients and acquisition and analysis and review of the data. Yun Qiao, Kaiyuan Hui, Chenxi Hu, Mei Wang, Wen Sun conducted the in vitro experiments, analyzed the data, and wrote the manuscript. Xiaodong Jiang supervised the drafting of the manuscript. All authors critically reviewed each draft and approved the version to be published.
Funding
This work was supported by the Jiangsu Province Key Research and Development Plan (Social Development) Project (BE2017684), the Social Developmental Project of Jiangsu Province (BE2022769), Jiangsu Provincial Key Medical Disciplines Development Program (JSDW202234), and Young doctor science and technology project of Lianyungang commission of health (QN202406).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was conducted using a protocol approved by the medical ethics committee of Lianyungang Clinical College of Nanjing Medical University (2017008). All methods and procedures associated with this study were conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, and Chinese law. These trials were registered with the Clinical Trials Registry (NCT03205930).
Informed consent
Informed consent for protocol therapy, the analysis of blood samples for research purposes and the use of generated data for publication was obtained from all patients enrolled in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yun Qiao, Kaiyuan Hui and Chenxi Hu were contributed equally to this article.
References
- 1.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD (2016) The future of cancer treatment: immunomodulation, CARs and combi- nation immunotherapy. Nat Rev Clin Oncol 13(5):273–290. 10.1038/nrclinonc.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng MWL, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell infiltration and PD-L1. Can Res 75(11):2139–2145. 10.1158/0008-5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita H, Vesely MD, Koboldt DC, Charles G, Rickert, Robert, Schreiber D (2012) Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482(7385):400–404. 10.1038/nature10755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan QZ et al (2015) Adjuvant cellular immunotherapy in patients with resected primary non-small cell lung cancer. Oncoimmunology 4(9):e1038017. 10.1080/2162402X.2015.1038017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanen J (2017) Converting cold into hot tumors by combining immunotherapies. Cell 170(6):1055–1056. 10.1016/j.cell.2017.08.031 [DOI] [PubMed] [Google Scholar]
- 6.Han Y et al (2017) Dynamic and specific immune responses against multiple tumor antigens were elicited in patients with hepatocellular carcinoma after cell-based immunotherapy. J Transl Med 15(1):64. 10.1186/s12967-017-1165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang LJ, Wen YX, Hu R, Lei W, Xia YY, Hu CX, Qiao Y, Geng XW, Chen T, Fei JY, Hui KY, Jiang XD (2019) Safety and efficacy of PD-1 blockade-activated multiple antigen-specific cellular therapy alone or in combination with apatinib in patients with advanced solid tumors: a pooled analysis of two prospective trials. Cancer Immunol Immunother 68(9):1467–1477. 10.1007/s00262-019-02375-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal R, Cadieux EL, Salgado R (2019) Neoantigen-directed immune escape in lung cancer evolution. Nature 567(7749):479–485. 10.1038/s41586-019-1032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarchoan M et al (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17(9):569. 10.1038/nrc.2017.74 [DOI] [PubMed] [Google Scholar]
- 10.Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L (2023) Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther 8(1):9. 10.1038/s41392-022-01270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 12.Ding Z et al (2021) Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther 6(1):26. 10.1038/s41392-020-00448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y, Shao Y, Shi X, Lou G, Zhang Y, Wu X, Tong X, Yu X (2016) Mutational profiling of non-small-cell lung cancer patients resistant to first-generation EGFR tyrosine kinase inhibitors using next generation sequencing. Oncotarget 7(38):61755–61763. 10.18632/oncotarget.11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen F et al (2019) Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Invest 129(5):2056–2070. 10.1172/JCI99538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla SA et al (2015) Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat Biotechnol 33(11):1152–1158. 10.1038/nbt.3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MoH et al (2018) Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR- 1210, an anti-PD-1 antibody in advanced solid tumours: a dose- escalation, phase 1 study. Br J Cancer 119(5):538–545. 10.1038/s41416-018-0100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuben A et al (2020) Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun 11(1):603. 10.1038/s41467-019-14273-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Peng X, Li J et al (2024) Personalized mRNA vaccine combined with PD-1 inhibitor therapy in a patient with advanced esophageal squamous cell carcinoma. Am J Cancer Res 14(8):3896–3904. 10.62347/NVFB3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CL et al (2018) Safety and activity of PD-1 blockade-activated DC-CIK cells in patients with advanced solid tumors. Oncoimmunology 7(4):e1417721. 10.1080/2162402X.2017.1417721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176(6):1248–1264. 10.1016/j.cell.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.