Abstract
The combined use of tocilizumab (TCZ) and immune checkpoint inhibitors (ICIs) in cancer treatment is gaining attention, but preclinical studies are lacking. Our study aims to investigate the synergistic anti-tumor effect of TCZ combined with ICIs and its role in treating immune-related adverse events (irAEs). The clinical significance of high interleukin-6 (IL-6) expression in tumor patients was analyzed from the Cancer Genome Atlas (TCGA) database. The expression levels of IL-6 were compared before and during the onset of ICIs-associated myocarditis patients. ICIs-related myocardial inflammatory injury and therapeutic lung cancer models were constructed in C57BL/6 J mice using murine-derived programmed death-1 (PD-1) inhibitors alone or in combination with TCZ. Possible inflammatory mechanisms were proposed and validated. The anti-tumor effects and mechanisms of both drugs in combination were assessed. Patients with high IL-6 expression had a poor prognosis, and those with ICIs-associated myocarditis exhibited elevated IL-6 from baseline. In the PD-1 inhibitors-associated myocardial inflammatory injury mouse model, the levels of IL-6 in the blood and cardiac tissues were significantly elevated. TCZ ameliorated immune myocardial inflammatory injury by inhibiting the IL-6/janus kinase 2 (JAK2)/signal transducer and activator of the transcription 3 (STAT3) pathway. The group treated with PD-1 inhibitors combined with TCZ showed significantly slower tumor growth than that treated with PD-1 inhibitors alone. TCZ resisted tumor growth by inhibiting the IL-6-JAK2-STAT3 pathway. By targeting the IL-6-JAK2-STAT3 pathway, TCZ can alleviate PD-1 inhibitors-associated myocardial inflammatory injury mediated by M1-polarized macrophages and plays a synergistic anti-tumor role by inhibiting lung cancer cell proliferation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03899-9.
Keywords: IL-6, Tocilizumab, Immune checkpoint inhibitors, Myocardial inflammatory injury
Introduction
Immune checkpoint blockade has changed the paradigm of tumor therapy, which significantly improves the response rate and durability of disease control [1]. Nevertheless, the immune checkpoint inhibitors (ICIs)-induced activation of the immune system may induce potentially serious or even fatal immune-related adverse events (irAEs) involving any organs [2, 3], with myocarditis reporting a mortality rate of up to 46%[4]. The treatment of irAEs, including myocarditis, is mostly empirical and based on the assumption that the underlying mechanism is an autoimmune response. Currently, the first-line treatment is usually high-dose corticosteroid shocks, and neither second-line treatment regimens nor hormonal combination regimens are recommended [5–7]. However, prolonged immunosuppression affects tumor prognosis, and steroid-associated toxicities such as osteoporosis, Cushing’s syndrome and infections also have a serious impact on patients’ quality of life [8]. Furthermore, some irAEs are corticosteroid-refractory and require additional and significant immunosuppression [9]. Therefore, a precise treatment paradigm reduces dependence on steroid therapy and potentially suppresses off-target autoimmunity while maintaining anti-tumor activation is urgently needed [10].
ICIs exhibit remarkable anti-tumor effectiveness, yet only a minority (ranging from 10 to 30%) yield long-lasting and robust responses. The use of single ICIs is hampered by their limited efficacy and susceptibility to drug resistance [11]. Combination therapy strategies based on ICIs are the future direction of exploration. Current research focuses on these innovative combinations of dual immunosuppressants, anti-angiogenesis agents, targeted therapies, chemotherapy, radiotherapy, and adoptive cell transfer therapy [12–15]. While these multimodal approaches enhance anti-tumor efficacy and durability, they also inevitably elevate the risk of adverse effects [16, 17]. The pursuit of ICIs combination therapy strategies that not only amplify anti-tumor efficacy but also maintain safety has consistently remained a pivotal concern.
Interleukin-6 (IL-6) plays a crucial role in the development and progression of various autoimmune diseases and tumors. Additionally, many case reports and retrospective clinical analyses have confirmed the significant elevation of serum IL-6 in patients with ICIs treatment-induced arthritis [18], pneumonia[19], enteritis [20] and hepatitis [21]. Tocilizumab (TCZ), a monoclonal antibody against the IL-6 receptor (IL-6R), has demonstrated significant anti-inflammatory effects in treating diseases, such as giant cell arteritis, rheumatoid arthritis and COVID-19 [22–24]. Recently recruiting NCT04691817, NCT04940299 and NCT03588936 clinical trials will reveal that the anti-IL-6R antibody TCZ combined with ICIs is safe and effective in anti-tumor therapy from the clinical point of view. And importantly, it is also reported that TCZ has significant effects in treating adverse reactions induced by oncology immunotherapy, including cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T cells [25], and PD-1 blockade-mediated lethal adverse events [9, 20, 26–28], especially myocarditis [9]. This suggests that the combination of TCZ and ICIs is an effective and promising anti-tumor combination strategy.
Based on the pro-inflammatory and tumor growth properties of IL-6, and the clinical safety and anti-inflammatory efficacy of TCZ, we intended to analyze the clinical significance of elevated IL-6 in cancer patients in the database and ICIs-associated myocarditis patients in our centers, and to investigate the ameliorative effect of TCZ on ICIs-related myocardial inflammatory injury and its synergistic anti-tumor effect in mice and cell models.
Materials and methods
Bioinformatics data analysis
Data on gene expression (ribonucleic acid-sequencing V2 (RNAseqV2)) and survival from The Cancer Genome Atlas (TCGA) were categorized, downloaded through the UCSC Cancer Browser and parsed in GraphPad Prism 7.0. Data on human immune myocarditis were obtained from Sequence Read Archive (SRA) databases (ERP120456 and SRP201972)[29] and sorted into the Trusted Platform Module (TPM) format. Myocarditis-related pathways were sorted and used in the Gene Set Enrichment Analysis (GSEA), and pathways with a Padj value of < 0.05 were taken as the ones that were significantly enriched for differentially expressed genes.
Patients
Non-small cell lung cancer (NSCLC) patients using ICIs in this center from February 2019 to August 2021 were recruited by this study. Inclusion criteria: (1) The patients with lung cancer confirmed by pathology in our center are not limited to the pathological types of lung cancer; (2) Treatment with immune checkpoint inhibitors, regardless of drug type; (3) patients with ICIs-related myocarditis diagnosed and treated according to National Comprehensive Cancer Network (NCCN) version 1.2020 and Chinese Society of Clinical Oncology (CSCO) guidelines, as well as Chinese Expert Consensus (2020 version) diagnostic criteria were collected; (4) Complete electrocardiogram, echocardiography, and laboratory examination before, during and after treatment, including cTNI, BNP, blood routine, etc.; (5) IL-6 levels increased compared to baseline when ICIs associated inflammatory injury occurred. The clinical part of our research has been approved by the Biomedical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University, and informed consent forms were obtained from the three patients.
Mice and cell lines
Male C57BL/6 mice (at the age of 5~6 weeks and with a weight of 19~21 g) were bought from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) and fed in the Experimental Animal Centre of Nanchang University. Lewis murine-derived lung adenocarcinoma cells (LLCs) and Raw264.7 murine-derived macrophages were provided by Procell Life Science & Technology Co., Ltd. (Wuhan, China). All experimental procedure involving animals has been approved by the Experimental Animal Ethics Review Committee of Nanchang University, with the ethical project approval number NCULAE-20221031141.
Chemicals and reagents
Programmed death-1 (PD-1) inhibitors (BE1046) were purchased from Bio X Cell. Lipopolysaccharide (LPS) (L2880) was bought from Sigma. TCZ was obtained from Roche. D-Luciferin potassium salt (D8390) was offered by Solarbio. Primary antibodies for Western blot (WB) detection were as follows: Anti-inducible nitric oxide synthases (iNOS) were obtained from Abmart (Shanghai, China); anti-phosphorylated STAT3 (STAT3), -pSTAT3 and -glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from ProteinTech (Wuhan, China); anti-janus kinase 2 (JAK2), -phosphorylated JAK2 (pJAK2) and -IL-6 were bought from Wanleibio (Shenyang, China). Primary antibodies used for immunohistochemistry (IHC) staining were as follows: Anti-F4/80, -lymphocyte antigen 6 complex, locus G (Ly6G), -CD3, -CD8, -CD4, -Ki67, -E-cadherin, -vimentin, -IL-6 and -iNOS were purchased from Servicebio (Wuhan, China). Antibodies used for immunofluorescence (IF) were the same as those for IHC staining.
Animal model construction
Mice were injected subcutaneously with 0.1 mL of 8 × 10^6/mL suspension stably transfected with luciferase-containing GV260 vector (GeneChem, Shanghai, China) of Lewis murine-derived LLCs at 2 cm below the right posterior axillary line. Then, tumor-bearing mice were randomly grouped 7 days after implantation (mean starting volume = 40 mm3). The group treated with PD-1 inhibitors was injected with 200 µg of InVivoPlus anti-mouse PD-1 inhibitors intraperitoneally on the 0th, 2nd and 4th days after tumor formation [30]. The same volume of vesicles was injected into control mice. A dose of 8 mg/kg TCZ was injected intraperitoneally once a week for four times in the administration group with a combination of TCZ and PD-1 inhibitors [31], and PD-1 inhibitors were administered as above. Tumor bioluminescence signals were measured by an in vivo imaging system (lumina3, PE) at the beginning of the administration and 3 weeks after the administration to assess tumor size. Mice were executed four weeks after the first dose administration, and heart tissues and blood were collected.
Cell model establishment
RAW264.7 macrophages at the stage of logarithmic growth were inoculated in a T25 culture bottle (approximately three million cells/bottle) and incubated in an incubator for 12 h. The corresponding culture bottle was added with a final concentration of 100 ng/mL LPS according to experimental design [32]. The medium was supplemented to 5 mL. Cellular proteins or RNA were extracted at 6 h after culture for subsequent experiments. A PD-1 inhibitors-inflammatory cell model was established by adding murine-derived PD-1 inhibitors at a final concentration of 20 µg/mL 1 h after the addition of LPS [33], and the rest was done as before. TCZ in the TCZ + PD-1 inhibitors cell model was added to incubation 24 h prior to PD-1 inhibitors at an ultimate concentration of 150 µg/mL [34], and the rest was done as before.
Enzyme-linked immunosorbent assay (ELISA)
Cardiac troponin I (cTNI), IL-6 and tumor necrosis factor-alpha (TNF-α) ELISAs were performed as per the instructions of the manufacturer (Neobioscience Technology Co. Ltd., Shenzhen, China). All tests were conducted in triplicate.
Echocardiography
The short-axis M ultrasound of mouse hearts was performed before tumor inoculation and PD-1 administration, and 4 weeks after PD-1 administration. Mice were treated with hair removal from the anterior thoracic region and subsequently anesthetized with isoflurane by inhalation. It was found that echocardiographic evaluation parameters evaluated cardiac function (Vevo TM 2100, Visual Sonics, Canada). Short axes were used for measuring left ventricular end-diastolic dimension (LVID;d), left ventricular end-systolic dimension (LVID;s), interventricular septal-diastolic thickness (IVS;d) and interventricular septal-systolic thickness (IVS;s). Ejection fraction (EF) and fraction shortening (FS) values were computed according to the equations as follows: EF% = [(LVEDV-LVESV)/LVEDV] × 100%, and FS% = [(LVID;d-LVID;s)/LVID;d].
Tissue and cell IF
Paraffin sections were first dewaxed with xylene, dehydrated with alcohol of different concentrations and later washed three times with phosphate buffer saline (PBS) after antigen repair. After being directly cleaned three times with PBS, cell slides in 24-well plates underwent 1-h incubation with 3% bovine serum albumin (BSA) at room temperature. Following that, samples were incubated with a specific primary antibody at the temperature of 4 ℃ overnight and a fluorescent secondary antibody at ambient temperature for 1 h. In the case of heterologous double-labeled staining, two different primary antibodies were mixed in a certain proportion with slices or slides at room temperature. In the event of heterologous double-labeling, two primary antibodies of different sources were blended in a certain ratio and incubated with the sample at 4 ℃ overnight. In addition, they were incubated with the secondary antibody of the corresponding species labeled with the primary antibody in a certain ratio at room temperature for 1 h. The rinsing of slices or slides with running water was followed by their sealing with anti-fluorescence quenching tablets and placement under a fluorescence microscope for observation.
Tumor cell model construction
Mouse-derived LLCs in the phase of logarithmic growth were taken and inoculated into 10 cm cell dishes. Four groups were set up: control group, TCZ group, RAW264.7 supernatant group, and TCZ group + RAW264.7 supernatant group. They were put in the incubator and incubated for 12 h. In accordance with the experimental design, the control group was added with medium; the TCZ group was added with a final concentration of 150 µg/mL TCZ; the RAW264.7 supernatant set was the RAW264.7 macrophage supernatant after 6 h of incubation with an ultimate concentration of 100 ng/mL LPS + 20 µg/mL PD-1 inhibitors, and was added with a twofold dilution of culture medium; the TCZ group + RAW264.7 supernatant group was supernatant at the above concentrations and TCZ at an ultimate concentration of 150 µg/mL. Groups received 24-h incubation for tumor cell function tests.
Wound healing
The migration ability of control, TCZ, RAW264.7 supernatant and TCZ + RAW264.7 supernatant groups was determined by transwell migration and wound healing assays. After digestion, centrifugation and resuspension, LLCs were inoculated in 6-well plates with 2 × 106 cells per well and then incubated in an incubator. After attachment, a wound healing test was carried out using a 200 μL sterile pipette, followed by the washing of cells with PBS three times and the addition and placement of the medium in the incubator at 37 °C. At last, images were photographed with a microscope at 0 and 48 h and analyzed by ImageJ.
Transwell experiments
Four groups of cell models were inoculated in Transwell chambers, with each group including 2 × 104 cells. Serum-free and 10% FBS-containing media were utilized in the upper and lower layers of the chamber, respectively. After 24-h placement in an incubator at 37 °C, they were washed, fixed and stained. Eventually, images were photographed with a microscope and parsed with ImageJ.
Education (EdU)
EdU experiments were performed to observe the proliferation ability of control group, TCZ group, RAW264.7 supernatant group, and TCZ group + RAW264.7 supernatant group. Four groups of cells were inoculated in plates with 96 wells, each of which contained 1.5 × 104 cells. EDU was diluted with whole medium to 50 μmol/L as per the instructions of YF594 Click-iT EDU (UE, Shanghai, China) Staining Kit. Next, 100 μL was added to every well and incubated for 2 h. The removal of the medium was followed by the fixing of cells with 4% paraformaldehyde, their neutralization with 2 mg/mL glycine solution and their washing twice with 3% bovine serum albumin. Afterward, 0.5% Triton X-100 was used as the osmotic enhancer to prepare the necessary Click-iT working solution and incubate it under dark conditions for 30 min. Then, 1 × Hoechst 33,342 solution was utilized for nuclear staining. At last, images were photographed by fluorescence microscopy and parsed by Image J.
Quantitative reverse transcription–polymerase chain reaction (PCR) (qRT-PCR)
Total RNA was separated from the heart by use of TRIzol reagent (TransGen Biotech, Beijing, China). Total RNA (2 µg) was employed for the synthesis of complementary deoxyribonucleic acids (cDNA) by utilizing a reverse transcription kit (Takara Biomedicals, Shiga, Japan). TAKARA SYBR Premix EX Tag II was applied in a CFX96 RT-PCR detection system (Bio-Rad, Hercules, California (CA), the United States of America (USA)) to perform amplification and qRT-PCR analysis. The relative messenger RNA (mRNA) expression levels of target genes were normalized to GAPDH. Then, three independent experiments were conducted. Specific primer pairs used were as follows: iNOS, 5′- GAGACAGGGAAGTCTGAAGCAC-3′ (forward) and 5′-CCAGCAGTAGTTGCTCCTCTTC-3′ (reverse); IL-1β, 5′-GAAATGCCACCTTTTGACAGTG-3′ (forward) and 5′- TGGATGCTCTCATCAGGACAG-3′(reverse); IL-6, 5′-TACCACTTCACAAGTCGGAGGC-3′ (forward) and 5′-CTGCAAGTGCATCATCGTTGTTC-3′ (reverse); TNF-α, 5′-CCTGTAGCCCACGTCGTAG- 3′ (forward) and 5′-GGGAGTAGACAAGGTACAACCC-3′ (reverse); IL-18, 5′-GACTCTTGCGTCAACTTCAAGG-3′ (forward) and 5′-CAGGCTGTCTTTTGTCAACGA-3′(reverse); GAPDH, 5′-ACGGATTTGGCCGTATTGG-3′ (forward) and 5′-CATTCTCGGCCTTGACTGTG-3′ (reverse).
Western blot analysis
Cells and heart tissues were lysed in a 1:1:100 configuration of phosphatase inhibitor: protease inhibitor: radio-immunoprecipitation assay (RIPA) lysis buffer. The supernatant was retained by centrifugation. Protein concentrations were detected and diluted to the same level with the bicinchoninic acid (BCA) method. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) protein gel electrophoresis and 5% skimmed milk were used to transfer proteins to polyvinylidene fluoride (PVDF) membranes at ambient temperature for 1 h. PVDF membranes clipped according to the target molecular weight were placed in a variety of particular primary antibodies at the temperature of 4 ℃ overnight. Tris Buffered Saline with Tween20 (TBST) was washed and placed in mouse or rabbit secondary antibodies corresponding to primary antibodies, and incubated at room temperature for 1 h. Bio-Rad gel imaging system exposure was used to take pictures. Image J software was used for analyzing images.
Flow cytometry
Flow cytometry was performed to detect macrophage M1 polarization in RAW264.7 macrophage model of control group, LPS group, PD-1 inhibitors group, and PD-1 inhibitors + TCZ group. The concentrations of the induction with LPS, PD-1 inhibitor, and TCZ are in line with the aforementioned descriptions. RAW264.7 cells were scraped off with a cell scraper and counted after resuspension in PBS. Besides, 1 × 106 cells were taken and resuspended with 100µL PBS. Then, 1µL CD16/32 blocker was added on ice for 10min and stained with 1.25µL fluorescently labeled CD86 (APC) antibody at 4 ℃ for 30min. Next, 100µL of membrane breaker A was added for fixation, and 100µL of membrane breaker B was added for membrane rapture. Finally, 2.5µL CD206 (PE) was added for the staining of cells at room temperature for 30min. Subsequently, 500µL flow staining buffer was used to resuspend cells on a CytoFLEX flow cytometer (Beckman, CA), and data analysis was performed.
Data statistics and analysis
Mean ± standard error was adopted to indicate all data. Unpaired sample T-tests were used to compare the two samples. Three groups or more were compared using one-way analysis of variance (ANOVA). Data were analyzed using Statistical Product and Service Solutions (SPSS) 25.0 software. It was considered that P < 0.05 showed a statistically significant difference. All experiments were conducted three times.
Results
IL-6 is elevated in ICIs associated myocarditis and is also a poor prognostic factor for tumors
Patient 1
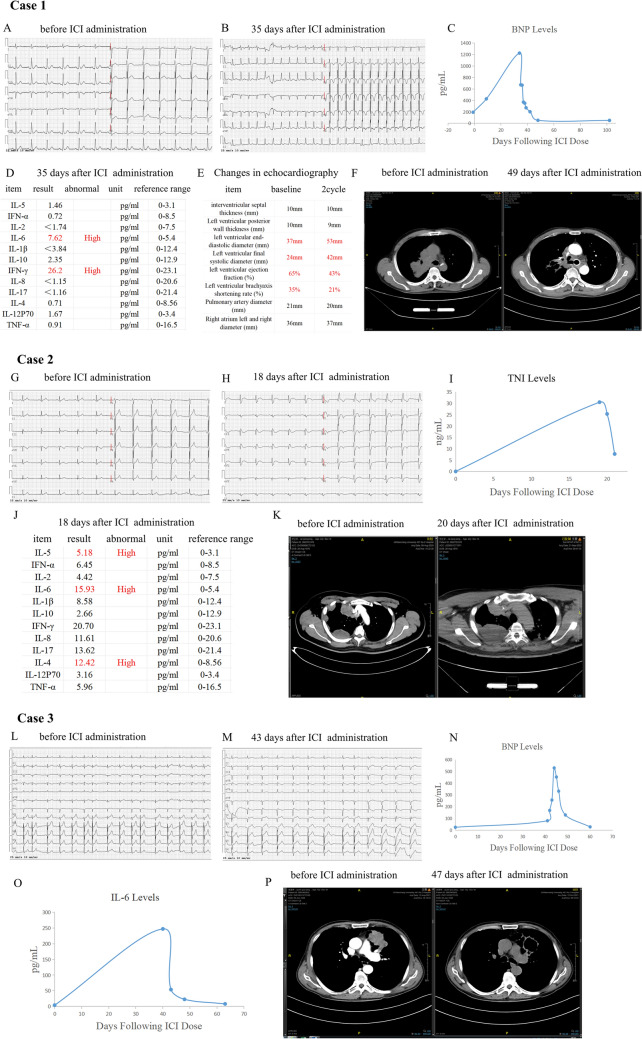
A 62-year-old male patient, diagnosed with squamous lung cancer, presented with chest tightness and shortness of breath 35 days following the administration of the initial 200 mg dose of Tislelizumab. Initially, his electrocardiogram (ECG) displayed a sinus rhythm, and his brain natriuretic peptide (BNP) levels were within the normal range. However, upon experiencing the aforementioned symptoms, his ECG revealed sinus tachycardia, with a heart rate of 149 beats per minute, accompanied by changes in the ST-segment and T-wave (ST-T). The BNP levels were significantly elevated, reaching 1224.59 pg/mL (normal range: 1–300 pg/mL). Furthermore, the results of cardiac ultrasound exhibited significant left atrial enlargement and decreased left ventricular function at the onset of myocarditis, while the baseline echocardiography suggested mild valvular regurgitation. Based on these findings, the patient was diagnosed with ICIs-related myocarditis. Noteworthily, the serum IL-6 level was higher than normal at 7.62 pg/mL (range of reference: 0–5.4 pg/mL). The reexamination images of computed tomography (CT) showed lung lesions were significantly reduced (Fig. 1A–F).
Fig. 1.
Clinical features before and after immunotherapy of three lung cancer patients. A-F Case 1. A, B Electrocardiogram (ECG) alterations before (A) and 35 days post (B) initiation immune checkpoint inhibitor (ICI) administration. C Temporal variation in brain natriuretic peptide (BNP) levels following ICI administration D Serum cytokine profiles after 35 days of ICI administration. E Changes in echocardiographic results before and after ICI treatment. F Chest CT images before (left) and 49 days after (right) starting ICI administration. G, K Case 2. G, H ECG alterations before (G) and 18 days post (H) initiation of ICI administration. I Temporal variation of cardiac troponin I (cTnI) levels following ICI Administration. J Serum cytokine profiles after 18 days of ICI administration. K Chest CT imaging before (left) and 49 days (right) following ICI administration. L–P Case 3. L, M ECG changes before (L) and 43 days after (M) starting ICI administration. N Temporal variation in BNP levels following ICI administration. O The changes of serum cytokine IL-6 levels following ICI administration. P Chest CT imaging before (left) and 47 days (right) following ICI administration
Patient 2
A 42-year-old male patient with squamous lung cancer presented with symptoms of low-grade fever, left-sided ptosis, dyspnea, and confusion 18 days after receiving the first dose of 200 mg Tislelizumab. Initial evaluation revealed a normal sinus rhythm on ECG and within-normal-range levels of troponin, creatine kinase (CK), CK-MB, and lactate dehydrogenase (LDH). However, upon developing the aforementioned clinical symptoms, the patient's ECG demonstrated notable changes, including an ST-segment up-regulation of 0.05–0.15 mV in leads I, avL and V2-V3, embryonic r-waves in leads avF and III, complete right bundle-branch block, and ST-T abnormalities. Further laboratory testing revealed a significant elevation in high-sensitivity troponin levels, reaching 30.50 ng/mL (normal range: 0–0.78 ng/mL). The echocardiographic results at the onset of myocarditis showed no significant abnormalities compared to those before ICIs treatment. To sum up, the patient was diagnosed with ICIs-related myocarditis combined with myositis. Notably, the IL-6 levels also were elevated at 15.93 pg/ml. Despite receiving methylprednisolone therapy and other symptomatic treatments, the patient's condition still progressed, leading to his automatic discharge from the hospital. During this period, the reexamination chest CT scan indicated an enlarged lesion (Fig. 1G–K).
Patient 3
A 73-year-old male patient, diagnosed with non-small cell carcinoma (containing the sarcomatoid carcinoma component), received two doses of Tislelizumab. Forty-three days after the initial dose, he began experiencing chest tightness and shortness of breath. Prior to the onset of these symptoms, his baseline ECG revealed sinus rhythm, and troponin levels were within the normal range. During the episode of chest tightness and dyspnea, the ECG displayed sinus arrhythmia, atrial premature contractions, complete right bundle branch block, and signs of right ventricular overload. Troponin levels were notably elevated, reaching 2.47 ng/ml, and with high level BNP. The echocardiographic results showed no significant abnormalities before and after ICIs treatment. These findings led to the diagnosis of ICIs-related myocarditis. And importantly, IL-6 levels were significantly increased, reaching 247.21 pg/mL. Following the administration of methylprednisolone, the patient's condition improved. A subsequent chest CT scan revealed a significant reduction in the lesion (Fig. 1L–P).
At the onset of three patients with ICIs-induced myocarditis, we observed that IL-6 was elevated. And myocardial inflammation is reduced and tumor regression occurs when anti-inflammatory therapy is effective. Conversely, tumor progression occurs when anti-inflammatory therapy is ineffective. Additionally, we have also collected information on the expression level of IL-6 and prognosis in lung cancer patients based on the TCGA database, and finds that patients with high IL-6 expression have a poor prognosis (Fig. S1).
Establishment of a mouse model of myocardial inflammatory injury induced by PD-1 inhibitors treatment for lung cancer
To further investigate the specific role of IL-6 in mediating ICIs-related myocardial inflammatory injury and its impact on anti-tumor therapy, we have established a mouse model of myocardial inflammatory injury induced by PD-1 inhibitors treatment for lung cancer. (see Fig. 2A for the schematic diagram). Our findings revealed a significant increase in the serum level of the myocardial injury marker cTNI in mice four weeks after the administration of PD-1 inhibitors (Fig. 2B). Additionally, an elevated serum TNF-α content was observed, which aligns with the inflammatory injury (Fig. 2C). Echocardiographic analysis further confirmed a marked deterioration in cardiac function in the PD-1 inhibitors group compared to the control group (Fig. 2D). The hematoxylin and eosin (HE) staining of the PD-1 inhibitors group exhibited cardiac structural disorders, cardiomyocyte edema hypertrophy, necrosis, nuclear consolidation and inflammatory cell infiltration compared with the control group (Fig. 2E). Consistent with myocardial histological results of ICIs-related myocarditis patients, we observed an increase in macrophages and T-lymphocytes in mouse model. The IHC of cardiac tissues from PD-1 inhibitors-treated mice demonstrated the positive expression of macrophages (F4/80+), neutrophils (Ly6G+) and T-lymphocytes (CD3+) compared with that from control mice, with T-lymphocytes being predominantly expressed at CD8+ (Fig. 2F–J).
Fig. 2.
Establishment of the PD-1 inhibitors-induced myocardial inflammatory injury model in tumor-bearing mice. A Mice model protocol and time points of drug administration. The analysis of cTNI (B) and TNF-α (C) in serum samples showed a significantly higher expression of inflammatory factors and myocardial injury markers in PD-1 inhibitors-treated mice. D Representative images of echocardiography exhibited that the cardiac function of control group and PD-1 inhibitors group changed. Indicators (LVEF, LVFS, IVS;d, IVS;s, LVID;d and LVID;s) at 4 weeks indicated impaired cardiac function. E The HE staining of control mice demonstrated no significant abnormalities, but PD-1 inhibitors-treated mice showed necrosis, myocardial structural disorders, edema hypertrophy, nuclear sequestration and inflammatory cell infiltration. F–J Representative IHC images of heart sections. PD-1 inhibitors-treated mice exhibited myocardial interstitial macrophages (F4/80 +) (F), Neutrophils (Ly6G +) (G), infiltration of T lymphocytes (H), and CD8 + T lymphocytes predominating among T lymphocytes (I–J). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, Original magnification, × 400
Elevated IL-6 expression in a mouse model of myocardial inflammatory injury induced by PD-1 inhibitors
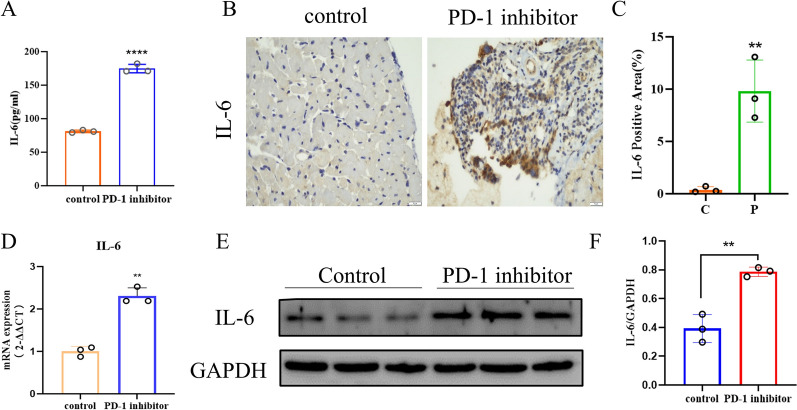
Utilizing the established mouse model, we assessed the expression of IL-6 in both mice cardiac tissue and serum. Our findings revealed that mice with PD-1 inhibitors group exhibited significantly higher serum levels of IL-6 compared to control mice (Fig. 3A). Additionally, tissue IHC staining revealed that IL-6 in the cardiac tissues of mice with PD-1 inhibitors group was positively expressed (Fig. 3B–C). The qRT-PCR analysis demonstrated a higher mRNA expression of IL-6 in the cardiac tissues of these mice (Fig. 3D). Similarly, WB experiments revealed increased IL-6 protein expression in the cardiac tissues of mice with PD-1 inhibitors group (Fig. 3E–F). In summary, IL-6 expression was elevated in the PD-1 inhibitors-induced myocardial inflammatory injury mouse model, consistent with clinical observations in patients.
Fig. 3.
Elevated IL-6 expression in the PD-1 inhibitors-induced myocardial inflammatory injury mouse model. A ELISA experiments revealed serum IL-6 levels in PD-1 inhibitors-treated mice compared with the control group. B, C IHC staining indicated that IL-6 was positively expressed in PD-1 inhibitors-treated mice. Original magnification, × 400. D qRT-PCR experiments indicated higher mRNA expression levels of IL-6 in PD-1 inhibitors-treated mice. E, F WB experiments revealed more protein expression of IL-6 in the cardiac tissues of PD-1 inhibitors-treated mice
PD-1 inhibitors promote IL-6 secretion by mediating macrophage M1 polarization
IL-6 is a multifunctional cytokine produced by various cell types, especially immune cells, and which possesses local paracrine function. To clarify which inflammatory cells types is associated with the secretion of the IL-6, we utilized IF double staining to co-localize IL-6 with different inflammatory cells. It was discovered that IL-6 was primarily localized to macrophages, while neutrophils and T-lymphocytes exhibited weak correlation with high IL-6 expression (Fig. 4A), which demonstrated that the synthesis and secretion of IL-6 are closely related to macrophages. It is well known that macrophages can turn into M1 pro-inflammatory and M2 anti-inflammatory types by means of polarization. To identify whether the secretion of IL-6 is mainly related to the M1 macrophage subtype, by using IF double staining, we observed F4/80 was co-localize with iNOS in PD-1 inhibitors group (Fig. 4B), indicating significant M1 polarization of macrophages. Similarly, WB experiments revealed the presence of M1 macrophage polarization in cardiac tissues following the addition of PD-1 inhibitors in mouse model (Fig. 4C, D). In addition, the RAW264.7 macrophage model showed that adding PD-1 inhibitors enhanced macrophage M1 polarization along with elevated IL-6 protein expression (Fig. 4E–G). In conclusion, macrophage M1 polarization and the secretion of IL-6 are closely related to myocardial inflammatory damage induced by PD-1 inhibitors.
Fig. 4.
Macrophage M1 polarization and the synthesis and secretion of IL-6 in PD-1 inhibitors-induced myocardial inflammatory injury mouse models. A The co-localization of macrophages (F4/80 +), T lymphocytes (CD3 +) and neutrophils (Ly6G +) with the inflammatory factor IL-6 suggested that IL-6 was primarily secreted by macrophages in PD-1 inhibitors-treated mice. B The co-localization of macrophages with iNOS indicated macrophage M1 polarization in PD-1 inhibitors-treated mice. C, D WB assay detected that the protein expression level of iNOS was higher in PD-1 inhibitors-treated mice than in control group. E–G WB assay demonstrated that adding PD-1 inhibitors enhanced the protein expression of IL-6 and iNOS in the cell model
Ameliorating effect of TCZ on myocardial inflammatory injury induced by PD-1 inhibitors
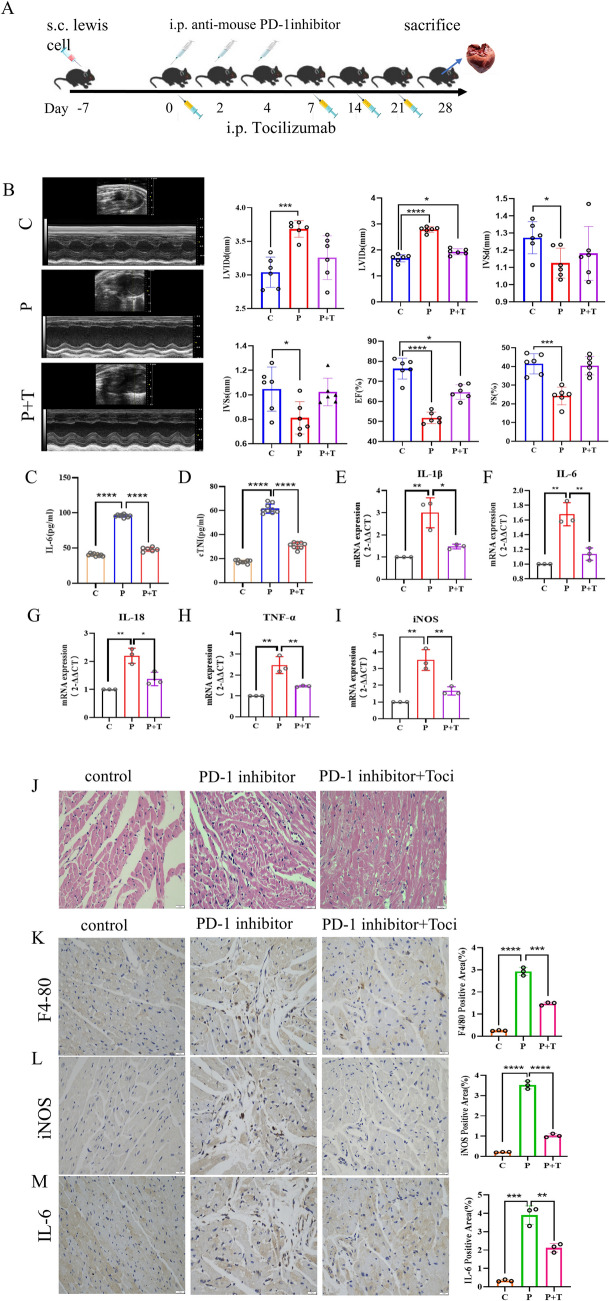
To explore whether the elevation of IL-6 level plays a promotional role in PD-1 inhibitors-induced myocardial inflammatory injury, we constructed the mouse model of PD-1 inhibitors combined with IL-6R receptor inhibitor tocilizumab, the schematic diagram is shown in Fig. 5A. It was noted that the cardiac function of mice in the TCZ treatment group was substantially restored compared with that in the PD-1 inhibitors group (Fig. 5B), and the content of serum IL-6 and cTNI in mice was decreased (Fig. 5C, D). Similarly, the cardiac tissues of mice in the TCZ treatment group, the IL-1β, IL-6, IL-18, TNF-α and iNOS were significantly decreased at the mRNA level (Fig. 5E–I). At the pathological level, HE staining (Fig. 5J) showed improvements in the structural disorders of cardiac tissues and a reduction in the inflammatory cell infiltration of mice in the TCZ-treated group. IHC staining (Fig. 5K–M) revealed that the heart tissue sections of mice with IL-6, F4/80 and iNOS exhibited a decrease in positive expression. In conclusion, TCZ attenuated PD-1 inhibitors-induced myocardial inflammation damage.
Fig. 5.
Attenuation of PD-1 inhibitors-induced myocardial inflammation by TCZ in mouse models. A Model protocol and time points of the drug administration of anti-IL-6R antibody TCZ in mice with myocarditis. B The ultrasound examination of the cardiac function of mice in control group, PD-1 inhibitors group, and PD-1 inhibitors + TCZ group at 28 days after drug administration revealed that the cardiac function of mice treated with PD-1 inhibitors + TCZ was basically restored. The heart function of mice in the PD-1 inhibitors group + TCZ group was basically recovered. C, D The analysis of inflammatory factors like (C) IL-6 and (D) cTNI in serum samples showed that myocardial injury and inflammation levels were decreased in the PD-1 inhibitors group + TCZ group compared to the PD-1 inhibitors group. E–I qRT-PCR experiments showed a significant decrease in the IL-1β, IL-6, IL-18, TNF-α and iNOS of the PD-1 inhibitors group + TCZ group compared to the PD-1 inhibitors group. J HE staining showed that PD-1 inhibitors + TCZ caused less structural damage to the heart and less inflammatory infiltration. K–M IHC staining revealed a decrease in the positive expression of IL-6, F4/80 and iNOS in the PD-1 inhibitors group + TCZ group. Original magnification, × 400
TCZ inhibits IL-6-JAK2-STAT3 inflammatory pathway, reducing macrophage M1 polarization and IL-6 production
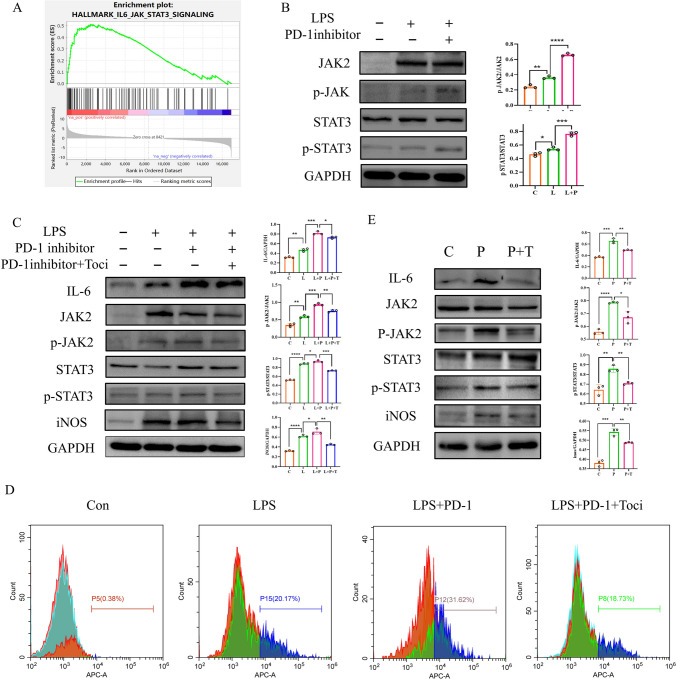
To further investigate the potential regulatory mechanism of IL-6 in PD-1 inhibitors-mediated myocardial inflammatory injury, by analyzing the sequencing data of human normal heart and ICIs-related myocarditis heart tissues, we discovered that the IL6-JAK2-STAT3 signaling pathway was enriched in the ICIs-related myocarditis group (Fig. 6A). In addition to its direct inflammatory injury to myocardium, whether IL-6 can further amplify the inflammatory effect by influencing the polarization and IL-6 secretion of macrophages remains to be investigated. Based on previously published literature, we hypothesized that PD-1 inhibition might promote inflammation through the IL-6-JAK2-STAT3 signaling pathway. To verify this, experiments were conducted in the RAW264.7 macrophage model, revealing an increase in the protein expression of p-JAK2/JAK2, p-STAT3/STAT3, and IL-6 following the addition of PD-1 inhibitors (Fig. 6B). However, the addition of TCZ, resulted in a decrease in the protein expression of p-JAK2/JAK2, p-STAT3/STAT3, iNOS and IL-6 (Fig. 6C). Additionally, we also have conducted flow cytometry analysis to assess the impact of tocilizumab on M1 polarization of macrophages. Using the RAW264.7 macrophage model, we divided the macrophage model into LPS, LPS + PD-1 inhibitor, LPS + PD-1 inhibitor + TCZ intervention, and control group. The results indicated that the level of M1 polarization macrophages significantly increased under the induction of LPS and PD-1 inhibitors. However, upon the intervention with tocilizumab, the number of M1 polarization macrophages decreased markedly (Fig. 6D). Similarly, in the mouse model, the TCZ group exhibited decreased protein expression of p-JAK2/JAK2, pSTAT3/STAT3, iNOS (Fig. 6E). Collectively, both mouse and cellular models showed that TCZ attenuated inflammation via the inhibition of the IL-6-JAK2-STAT3 pathway and weakened macrophage M1 polarization levels and IL-6 production.
Fig. 6.
Attenuation of inflammation by the inhibition of the IL6-JAK2-STAT3 pathway by TCZ. A GSEA analysis of IL6-JAK2-STAT3 signaling pathway enrichment in normal and immune myocarditis heart tissues. B WB experiments were conducted to detect the protein expression of IL-6, p-JAK2/JAK2 and p-STAT3/STAT3 in the RAW264.7 macrophage model. C WB experiments were performed to detect the protein expression of IL-6, p-JAK2/JAK2, p-STAT3/STAT3 and iNOS in RAW264.7 macrophage model of control group, LPS group, PD-1 inhibitors group, and PD-1 inhibitors + TCZ group. D Flow cytometry was performed to detect macrophage M1 polarization in RAW264.7 macrophage model of control group, LPS group, PD-1 inhibitors group, and PD-1 inhibitors + TCZ group. E WB assay for the protein expression of IL-6, p-JAK2/JAK2, p-STAT3/STAT3 and iNOS in heart tissues
Inhibition of tumor growth by TCZ in hormonal mice
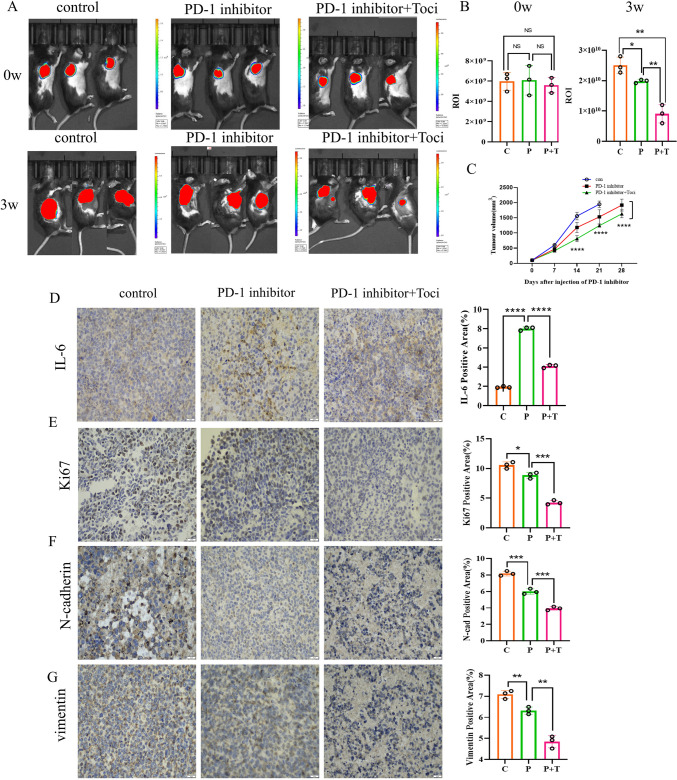
The use of TCZ may affect the defense against malignant tumors and the effect on the development and course of malignancy is not fully understood. To evaluate the anti-tumor therapeutic potential of TCZ in the context of ICIs therapy, tumor growth was evaluated in different groups of mouse models. It was found that the TCZ combined with PD-1 inhibitors group showed significantly slower tumor growth than the single-agent PD-1 inhibitors group (Fig. 7C). Similarly, the in vivo imaging system of mice at different time periods intuitively demonstrated that the combined group of mice showed even slower tumor growth (Fig. 7A, B). The IHC staining of mouse heart tissues revealed the positive expression of IL-6 in tumor tissues was decreased in the TCZ combined with PD-1 inhibitor group compared to the PD-1 inhibitor group (Fig. 7D). Additionally, tumor proliferation index Ki67, tumor invasion and metastasis-related molecules N-cadherin, and waveform protein vimentin (Fig. 7E–G) exhibited a decrease in PD-1 inhibitors-treated mice compared to the control group, and their expression was even lower in the TCZ-combined PD-1 inhibitors group.
Fig. 7.
Anti-tumor effect of PD-1 inhibitors enhanced by TCZ in mouse models. A, B In vivo imaging measurement of tumor ROI values in each group. C Tumor growth rates of control group, PD-1 inhibitors group, and PD-1 inhibitors group + TCZ group. D Positive expression of IL-6 in tumor tissues across three groups. E–G IHC staining detected tumor value-added invasive indexes, including Ki67 (E), N-cadherin (F) and vimentin (G), Original magnification, × 400
Inhibition of the JAK2-STAT3 pathway by TCZ against tumor growth
We have further verified the tumor-suppressing effect of TCZ through establishing in vitro culture models of tumor cells, including control group, TCZ group, RAW264.7 supernatant group, and TCZ + RAW264.7 supernatant group. Both Transwell (Fig. 8A) and wound healing assays (Fig. 8B) demonstrated that the TCZ group exhibited a significant decrease in migratory ability compared with the control one, and the decrease in migration ability was more obvious in the TCZ combined with RAW264.7 supernatant group. The effect of TCZ on LLC proliferation was further analyzed by EdU staining (Fig. 8C). It was found that the TCZ group showed a significant decrease in proliferation ability compared with the control one, and the TCZ combined with RAW264.7 supernatant group exhibited an even more significant decrease in this aspect. This indicates that TCZ is capable of inhibiting autocrine IL-6 secreted by tumor cells and paracrine IL-6 secreted by macrophages to achieve anti-tumor growth. In conclusion, both in vivo and ex vivo experiments showed that the TCZ combined with the PD-1 inhibitors group had a superior anti-tumor growth effect.
Fig. 8.
Enhanced anti-tumor effect of TCZ in cell models. A Transwell assay revealed the inhibited migration ability of LLC after the addition of TCZ, and the inhibition was more obvious after the addition of TCZ combined with RAW264.7 supernatant. B The wound healing array demonstrated a significant delay in the wound healing of cells after the addition of TCZ compared to the control group, and the delayed wound healing was more obvious after the addition of TCZ combined with RAW264.7 supernatant. C The rate of cell proliferation was reduced after the addition of EdU-stained TCZ compared to the control group, and the reduction was more obvious after the addition of TCZ combined with RAW264.7 supernatant. Scale bar: EdU, 50 μm; Transwell and wound healing arrays, 200 μm. D WB assay for the protein expression of IL-6, p-JAK2/JAK2, p-STAT3/STAT3 and iNOS in TCZ group, RAW264.7 supernatant group, and TCZ + RAW264.7 supernatant group. E TCZ counteracting tumor growth while reducing cardiac inflammation by inhibiting tumor cells and the macrophage IL-6 receptor signaling pathway
Is it true that TCZ inhibits IL-6R and thus the IL6-JAK2-STAT3 pathway against tumor growth? Western blot (WB) using cellular models has confirmed that in the TCZ group, the protein expression of IL-6, p-JAK2/JAK2 and p-STAT3/STAT3 was decreased compared with the control one (Fig. 8D). In addition, the protein expression of IL-6, p-JAK2/JAK2 and p-STAT3/STAT3 showed a more obvious decrease in the TCZ combined with RAW264.7 supernatant group. Therefore, TCZ may inhibit tumor growth through the IL-6-JAK2-STAT3 pathway.
Discussion
This study is the first basic one to explore TCZ combined with PD-1 inhibitors. Our results showed that patients with ICIs-related myocarditis have high serum IL-6 levels, correlating with anti-inflammatory and anti-tumor efficacy. TCZ modulates macrophage M1 polarization through the inhibition of the JAK2-STAT3 pathway, ameliorating PD-1 inhibitors-related myocardial inflammation in mice and inhibiting tumor growth. As a result, TCZ has the dual potential to synergize with PD-1 inhibitors against tumor growth while treating irAEs (Fig. 8E).
IL-6 is abundantly expressed in the tumor microenvironments (TMEs) of multiple solid tumors such as non-small cell lung cancer (NSCLC) [35], head and neck squamous cell carcinoma (HNSCC) [36], pancreatic [37], breast [38]and ovarian cancers [39], and melanoma [40]. Some research involving IL-6 signaling has found that aberrantly activating the downstream IL-6 signaling pathway promotes tumorigenesis, progression and metastasis [36, 41]. Additionally, it has been proved that STAT-3, the downstream effector of IL-6, directly stimulates the proliferation, activation and invasion of tumor cells [42]. These studies uncover the fundamental role of IL-6 in tumor pathogenesis and therapeutic resistance, theoretically bolstering the development of novel cancer therapeutics targeting this cytokine. Systemic and tumor-derived IL-6 can be an immune checkpoint hijacked by tumors to escape killing by tumor-reactive CD8+ T cells that are activated by ICIs treatment. Consequently, co-targeting drugs that inhibit the IL-6 pathway with ICIs are compelling.
The IL-6R blocking antibody TCZ is widely used in treating rheumatoid arthritis and has shown effectiveness in COVID-19 treatment [43]. Indeed, several clinical trials are already underway to investigate the potential of TCZ to enhance immune checkpoint blockade responses. These include NCT04691817, which explores the combination of TCZ and anti-PD-L1 Atezolizumab in NSCLC patients, and NCT04940299, which assesses the efficacy of combining anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) Ipilimumab, anti-PD-1 Nivolumab, and TCZ in treating patients with advanced melanoma, uroepithelial cancer, and EGFR-mutated NSCLC. At present, two preclinical studies have also demonstrated the anti-tumor potential of combining IL-6 blockade with ICIs, but neither employed TCZ. One study in melanoma mice showed that combining anti-PD-L1 with anti-IL-6 antibodies (MP5-20F3) led to improved tumor control, mediated by an increased production of IFN-γ from CD4+ and CD8+ T cells [44]. Another study in a colon cancer model demonstrated that IL-6 blocking augmented the anti-tumor activity of anti-CTLA-4 therapy. Higher concentrations of serum IL-6 after treatment with ICIs are correlated with the reduced survival of melanoma patients [45, 46]. This suggests that it is feasible to implement a co-targeting strategy of IL-6 and immune checkpoints in the setting of ICIs for tumor treatment. Our study demonstrated enhanced anti-tumor growth effect of TCZ in combination with anti-PD-1 therapy compared to ICIs alone, which can serve as a preclinical complement to these clinical trials.
Cytokine inhibitors have transformed the treatment landscape for a wide range of autoimmune diseases, which highlights their potential use in inhibiting irAEs [47]. Cytokines are both effector molecules of the efficacy of ICIs in cancer treatment and contributors to the development mechanism of irAEs. It has been demonstrated that the induction of IL-6 is linked with the development of irAEs, especially when the PD-1/PD-L1 axis is targeted [48, 49]. TCZ is mainly used as a secondary immunosuppressant in severe or complicated irAEs, including pneumonia [26], colitis [20], neurologic toxicity [27], steroid-refractory immune checkpoint inhibitor-related cholangiohepatitis (irCH) [21] and myocarditis [9]. Unlike glucocorticoids broadly suppressing inflammatory processes, cytokine inhibitors offer a more pertinent clinical approach to reduce ICIs-induced inflammation. Additionally, it reduces symptom duration and hospitalization [50], and has been shown to be effective in corticosteroid-resistant irAEs [51]. When used in conjunction with ICIs therapy, cytokine-targeted therapy reduces the incidence of irAEs or mitigates their severity, and prevents the development of preexisting autoimmune episodes [52]. Our studies have determined that IL-6R inhibition is unaffected by anti-tumor immune responses to PD-1 inhibitors and able to be combined with anti-tumor growth. Thus, TCZ can treat irAEs without affecting tumor therapy activity.
Excessive IL-6 release in response to inflammatory stimuli can potently activate the IL-6-JAK-STAT signaling pathway. The IL-6-JAK2-STAT3 signaling pathway is considered one of the three classical inflammatory pathways that have been most thoroughly studied among a variety of signaling pathways found to be associated with the development of inflammation [53]. The JAK2-STAT3 signaling pathway probably plays a critical part in malignant tumor development and has a close association with tumorigenesis, progression, metastasis, invasion and other biological behaviors. Its aberrant expression is of value in guiding tumor prognosis [54]. A great deal of research has shown the abnormally high activation of the IL-6-JAK2-STAT3 signaling pathway in various cancers, including gastric [55] and breast cancers [56], hepatocellular carcinoma [57], colorectal carcinoma [58], ovarian carcinoma [59] and lung cancer [60]. Hence, inhibiting and modulating the IL-6-JAK2-STAT3 signaling pathway facilitates the prevention, treatment and prognosis of ICIs-related myocardial inflammation and tumors, in line with the results of the current study. It is also an important tool for screening an important target for anti-inflammatory and anti-tumor drugs.
Certainly, our study has its limitations. Our research focused primarily on the anti-tumor effects of TCZ, lacking a detailed exploration of the combined anti-tumor mechanism. This aspect will be a crucial area for future elucidation. In addition, regarding the mouse model for ICIs-induced myocarditis, previous studies have demonstrated that the genetic knockout mouse model of CTLA4±PDCD1−/− can recapitulates the myocarditis status in mice [61, 62]. Nonetheless, given that our investigation delves into not just the influence of IL-6 and IL-6R inhibitor tocilizumab on PD-1 inhibitor-mediated myocardial inflammatory damage, but also its collaborative anti-tumor effects, the myocarditis animal model rooted in genetic knockout mice might not align perfectly with our research objectives. Moreover, our modeling approach can observe the PD-1 inhibitor-mediated impacts on cardiac function impairment and myocardial inflammatory injury [30].
Collectively, our study is the first to demonstrate the elevation of IL-6 levels in the serum of patients with ICIs-related myocarditis and mouse model with ICIs-induced myocardial inflammation damage, and highlights the potential of TCZ in targeting the IL-6/JAK2/STAT3 signaling pathway to alleviate ICIs-induced myocardial inflammation, while simultaneously enhancing anti-tumor immune responses. This also is the initial application of TCZ in combination with ICIs in an animal model, paving new avenues for immunotherapy combinations. However, future prospective studies are essential to further refine this immunotherapy combination and ensure the rational management of irAEs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The assistance of Yilin Xu, Peng Xu, Yali Yi and Zhiqin Lu were acknowledged.
Author contributions
YXC and YXL wrote the manuscript and mainly completed this experiment. YWL assisted in performing the experiment. DYL supervised this work. AWL contributed essential ideas and discussion. All authors gave their consent for publication.
Funding
This work gained support from the National Natural Science Foundation of China [grant numbers 82260628 and 82060577], the Natural Science Foundation of Jiangxi Province [grant number 20232ACB206042], the Wu Jieping Medical Foundation [grant number 320.6750.2024-16-10], and the Graduate Innovation Program of Nanchang University [grant number YC2023- B092].
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Animal experimentation care is in accordance with the institution's guidelines.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanxin Chen, Yuxi Luo and Yunwei Liu contributed equally to this work and share the first authorship.
References
- 1.Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33(17):1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F et al (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27(4):559–574 [DOI] [PubMed] [Google Scholar]
- 3.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F et al (2016) Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13(8):473–486 [DOI] [PubMed] [Google Scholar]
- 4.Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG et al (2019) Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 115(5):854–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Lacchetti C, Thompson JA (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J Oncol Pract 14(4):247–249 [DOI] [PubMed] [Google Scholar]
- 6.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv119–iv142 [DOI] [PubMed]
- 7.Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5(1):95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman AL (2001) Side effects of corticosteroid therapy. J Clin Gastroenterol 33(4):289–294 [DOI] [PubMed] [Google Scholar]
- 9.Doms J, Prior JO, Peters S, Obeid M (2020) Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann Oncol 31(9):1273–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, Miller WH Jr, Calabrese L (2020) Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol 17(8):504–515 [DOI] [PubMed] [Google Scholar]
- 11.Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17(12):e542–e551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England) 398(10294):27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546 [DOI] [PubMed] [Google Scholar]
- 14.Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S et al (2017) The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 12(2):194–207 [DOI] [PubMed] [Google Scholar]
- 15.Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R et al (2020) Three-year overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J thorac Oncol 15(2):288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y et al (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375(18):1749–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y, Zeng Z, Liu Y, Liu A (2023) Reflecting on the cardiac toxicity in non-small cell lung cancer in the era of immune checkpoint inhibitors therapy combined with thoracic radiotherapy. Biochim Biophys Acta 1878(6):189008 [DOI] [PubMed] [Google Scholar]
- 18.Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, Johnson DH, Uemura M, Diab A (2017) Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 76(12):2061–2064 [DOI] [PubMed] [Google Scholar]
- 19.Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR (2018) Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol (Stockholm, Sweden) 57(5):705–708 [DOI] [PubMed] [Google Scholar]
- 20.Holmstroem RB, Nielsen OH, Jacobsen S, Riis LB, Theile S, Bjerrum JT et al (2022) COLAR: open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J Immunother Cancer 10(9):e005111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moi L, Bouchaab H, Mederos N, Nguyen-Ngoc T, Perreau M, Fenwick C et al (2021) Personalized cytokine-directed therapy with tocilizumab for refractory immune checkpoint inhibitor-related cholangiohepatitis. J Thorac Oncol 16(2):318–326 [DOI] [PubMed] [Google Scholar]
- 22.Venkiteshwaran A (2009) Tocilizumab. MAbs 1(5):432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM et al (2021) Interleukin-6 receptor antagonists in critically Ill patients with covid-19. N Engl J Med 384(16):1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashraf FA, Anjum S, Hussaini A, Fraser A (2013) Refractory PMR with aortitis: life-saving treatment with anti-IL6 monoclonal antibody (tocilizumab) and surgical reconstruction of the ascending aorta. BMJ Case Reports 2013:bcr2013009523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, Pazdur R (2018) FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23(8):943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YH, Zhou Y, Liu YY, Zhang GJ, Xiao L, Li N, Qin HF, Wang JG, Zhang L (2021) Severe immune-related hyperthermia followed by immune-related pneumonitis with PD-1 inhibitor (sintilimab) in small cell lung cancer: a case report. Thoracic Cancer 12(11):1780–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picca A, Valyraki N, Birzu C, Kramkimel N, Hermine O, Zahr N, Berzero G, Psimaras D (2021) Anti-interleukin-6 and Janus kinase inhibitors for severe neurologic toxicity of checkpoint inhibitors. Neurol(R) Neuroimmunol Neuroinflamm 8(6):e1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S et al (2019) Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 25(3):551–557 [DOI] [PubMed] [Google Scholar]
- 29.Finke D, Heckmann MB, Salatzki J, Riffel J, Herpel E, Heinzerling LM et al (2021) Comparative transcriptomics of immune checkpoint inhibitor myocarditis identifies guanylate binding protein 5 and 6 dysregulation. Cancers 13(10):2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Liu Y, Xiong X, Zeng Z, Luo D, Liu A (2022) PD-1 inhibitor causes pathological injury to multiple organs in a Lewis lung cancer mouse model. Int Immunopharmacol 105:108551 [DOI] [PubMed] [Google Scholar]
- 31.Pope NH, Salmon M, Johnston WF, Lu G, Lau CL, Upchurch GR Jr, Ailawadi G (2015) Interleukin-6 receptor inhibition prevents descending thoracic aortic aneurysm formation. Ann Thorac Surg 100(5):1620–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He W, Frost MC (2016) CellNO trap: novel device for quantitative, real-time, direct measurement of nitric oxide from cultured RAW 267.4 macrophages. Redox Biol 8:383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Mi J, Liu W, Xiao S, Gao C (2019) Blocking of checkpoint receptor PD-L1 aggravates osteoarthritis in macrophage-dependent manner in the mice model. Int J Immunopathol Pharmacol 33:2058738418820760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM, Tung YC, Hsu HL (2019) MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer 18(1):42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abulaiti A, Shintani Y, Funaki S, Nakagiri T, Inoue M, Sawabata N, Minami M, Okumura M (2013) Interaction between non-small-cell lung cancer cells and fibroblasts via enhancement of TGF-β signaling by IL-6. Lung Cancer (Amsterdam, Netherlands) 82(2):204–213 [DOI] [PubMed] [Google Scholar]
- 36.Rašková M, Lacina L, Kejík Z, Venhauerová A, Skaličková M, Kolář M et al (2022) The role of IL-6 in cancer cell invasiveness and metastasis-overview and therapeutic opportunities. Cells 11(22):3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G et al (2011) Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19(4):456–469 [DOI] [PubMed] [Google Scholar]
- 38.Manore SG, Doheny DL, Wong GL, Lo HW (2022) IL-6/JAK/STAT3 signaling in breast cancer metastasis: biology and treatment. Front Oncol 12:866014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL (2018) IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res 10:6685–6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jobe NP, Rösel D, Dvořánková B, Kodet O, Lacina L, Mateu R, Smetana K, Brábek J (2016) Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem Cell Biol 146(2):205–217 [DOI] [PubMed] [Google Scholar]
- 41.Vilgelm AE (2023) Illuminating the mechanism of IL-6-mediated immunotherapy resistance. Cell Rep Med 4(1):100901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu H, Lee H, Herrmann A, Buettner R, Jove R (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14(11):736–746 [DOI] [PubMed] [Google Scholar]
- 43.Albuquerque AM, Eckert I, Tramujas L, Butler-Laporte G, McDonald EG, Brophy JM, Lee TC (2023) Effect of tocilizumab, sarilumab, and baricitinib on mortality among patients hospitalized for COVID-19 treated with corticosteroids: a systematic review and meta-analysis. Clin Microbiol Infect 29(1):13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y et al (2018) Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Can Res 78(17):5011–5022 [DOI] [PubMed] [Google Scholar]
- 45.Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M et al (2022) Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40(5):509-523.e506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, Weber J (2020) Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer 8(1):e000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang JH, Bluestone JA, Young A (2021) Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol 42(4):293–311 [DOI] [PubMed] [Google Scholar]
- 48.Yoshino K, Nakayama T, Ito A, Sato E, Kitano S (2019) Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: potential role of Th1-dominant immune response in immune-related adverse events: two case reports. BMC Cancer 19(1):1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka R, Okiyama N, Okune M, Ishitsuka Y, Watanabe R, Furuta J et al (2017) Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J Dermatol Sci 86(1):71–73 [DOI] [PubMed] [Google Scholar]
- 50.Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr, Abdel-Wahab N et al (2018) Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer 6(1):103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray-Brown W, Wilsdon TD, Weedon H, Proudman S, Sukumaran S, Klebe S, Walker JG, Smith MD, Wechalekar MD (2020) Nivolumab-induced synovitis is characterized by florid T cell infiltration and rapid resolution with synovial biopsy-guided therapy. J Immunother Cancer 8(1):e000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M (2019) Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 7(1):226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Li R (2023) Overview of the anti-inflammatory function of the innate immune sensor NLRC3. Mol Immunol 153:36–41 [DOI] [PubMed] [Google Scholar]
- 54.Liu X, Wang J, Wang H, Yin G, Liu Y, Lei X, Xiang M (2015) REG3A accelerates pancreatic cancer cell growth under IL-6-associated inflammatory condition: Involvement of a REG3A-JAK2/STAT3 positive feedback loop. Cancer Lett 362(1):45–60 [DOI] [PubMed] [Google Scholar]
- 55.Liu M, Li H, Zhang H, Zhou H, Jiao T, Feng M et al (2022) RBMS1 promotes gastric cancer metastasis through autocrine IL-6/JAK2/STAT3 signaling. Cell Death Dis 13(3):287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Yang P, Wang J, Zhang J, Ma Q, Jiang Y, Wu Y, Han T, Xiang D (2022) HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol 15(1):2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh AK, Bhadauria AS, Kumar U, Raj V, Maurya V, Kumar D et al (2018) Novel fused oxazepino-indoles (FOIs) attenuate liver carcinogenesis via IL-6/JAK2/STAT3 signaling blockade as evidenced through data-based mathematical modeling. Life Sci 201:161–172 [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Hu F, Li G, Li G, Yang X, Liu L, Zhang R, Zhang B, Feng Y (2018) Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis 9(2):25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim B, Kim HS, Kim S, Haegeman G, Tsang BK, Dhanasekaran DN, Song YS (2017) Adipose stromal cells from visceral and subcutaneous fat facilitate migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Res Treat 49(2):338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun C, Yang J, Cheng HB, Shen WX, Jiang ZQ, Wu MJ et al (2019) 2-Hydroxy-3-methylanthraquinone inhibits lung carcinoma cells through modulation of IL-6-induced JAK2/STAT3 pathway. Phytomed Int J Phytother Phytopharmacol 61:152848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma P, Liu J, Qin J, Lai L, Heo GS, Luehmann H et al (2024) Expansion of pathogenic cardiac macrophages in immune checkpoint inhibitor myocarditis. Circulation 149(1):48–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC et al (2021) A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov 11(3):614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.