Abstract
Organoid is an ideal in vitro model with cellular heterogeneity and genetic stability when passaging. Currently, organoids are exploited as new tools in a variety of preclinical researches and applications for disease modeling, drug screening, host-microbial interactions, and regenerative therapy. Advances have been made in the establishment of nasal and olfactory epithelium organoids that are used to investigate the pathogenesis of smell-related diseases and cellular/molecular mechanism underlying the regeneration of olfactory epithelium. A set of critical genes are identified to function in cell proliferation and neuronal differentiation in olfactory epithelium organoids. Besides, nasal epithelium organoids derived from chronic rhinosinusitis patients have been established to reveal the pathogenesis of this disease, potentially applied in drug responses in individual patient. The present article reviews recent research progresses of nasal and olfactory epithelium organoids in fundamental and preclinical researches, and proposes current advances and potential future direction in the field of organoid research and application.
Keywords: Organoid, Olfactory epithelium, Chronic rhinosinusitis, Nasal epithelium, Regeneration
Introduction
Although model organisms, such as mice and rats, are widely used in study on disease pathogenesis and drug screening, there still exists a tremendous gap between animal models and human body [1]. Besides, animal models have higher economic cost for feeding, transgenic line construction and maintenance, and more requirements for space and facility, compared to in vitro model [2]. Thus, establishment of a representative in vitro disease model is of great convenience and significance to study disease pathogenesis and investigate effective drugs [3]. With the development of biomedical technology, a variety of cell-based models mimicking real organs and tissues have been established [4]. Nonetheless, the classical 2D culture of cell lines and primary cells have obvious limitations to not fully recapitulate cell types, phenotypes, functions and microenvironment, thus are hard to faithfully mimic and reflect in vivo situation [5]. Recently, organoid, which is analogous to the architecture and functionality of original organs and tissues were established [6]. Organoids possess three-dimensional structures formed by progenitor/stem cells cultured in vitro [7]. The growth and differentiation in organoids are regulated by various growth factors and chemical cocktails, making organoids have similar cell composition, physiological functions and even structure as original tissues and organs [8]. Establishment of this highly bionic model in vitro facilitates to understanding the molecular mechanism of diseases and screening targeted drugs [9]. Massive production of organoids will have potential benefits in many fields, such as establishing new disease models, screening personalized drugs, and investigating pathogenesis [10].
Nasal mucosa, the transitional zone between squamous and columnar epithelium in the nasal vestibule, extends into the nasal cavity and is widely distributed in various walls and passages of the nasal cavity, as well as in the continuous mucosa of the nasopharynx, sinuses, and nasolacrimal ducts [11]. According to the histological structure and physiological function, nasal mucosa is divided into two parts: olfactory mucosa and respiratory mucosa. The olfactory mucosa is distributed in the middle of the nasal cavity, which is a pseudo-stratified columnar epithelium and is mainly composed of supporting cells, basal cells, and olfactory sensory neurons (OSNs), etc [12]. The olfactory mucosa is responsible for the regulation and control of olfaction. External odorants are first inhaled into nasal cavity and bind with olfactory receptors (ORs) expressed on the cilia of OSNs. These neurons then convert odor signals into electrical signals, which are subsequently transmitted to the olfactory bulb through olfactory nerve fibers. The olfactory bulb further processes signal and transmits to the olfactory cortex of the brain to complete the olfactory perception [13]. The respiratory mucosa in the anterior one-third of the nasal cavity is a pseudostratified and ciliated columnar epithelium, consisting of ciliated cells, columnar cells, goblet cells, and basal cells [14]. The respiratory mucosa mainly functions in defensing against airborne pollutants and inhaled pathogens as well as facilitating mucociliary clearance. The mucus secreted by respiratory mucosa construct a stable barrier to defense against various pathogens and particles, while the directional movement of cilia promotes the mucus secretion from the nasal cavity towards the esophagus, where it is transported to the digestive tract by swallowing [15]. These two mucosal epithelia provide a direct contact site to inhalable irritants, symbiotic organisms, and pathogens.
Olfactory and nasal epithelium organoids are mainly derived from olfactory and nasal epithelium, and are utilized to illuminate the mechanism underlying olfactory epithelium homeostasis and regeneration, as well as pathogenesis of nasal-related diseases. In this review, we summarized the recent advances in studies of olfactory and nasal epithelium organoids, especially in both fundamental research and preclinical applications. We further discussed current breakthroughs and challenges in the field of organoid research, and proposed the new progress in olfactory and nasal epithelium organoid technology.
Organoids derived from different sources
Organoid model is a technological breakthrough and has become an important tool for fundamental research and preclinical applications. The ‘organoid’ was firstly termed by Smith and Cochrae in 1946 to describe cystic teratoma [16]. The current organoid technology was firstly established by Hans Clevers and colleagues in 2009, when they succeeded to culture murine intestinal epithelial organoids in vitro [17]. These organ-like 3D cultures with stacked cell clusters were called organoids. Organoid growth simulates the formation of organs and differentiation into various types of functional cells [18]. The initiation of organoid culture requires the cultivation of stem/progenitor cells, either pluripotent stem cells (PSCs) or tissue-specific stem cells.
Organoids derived from adult tissue-specific stem cells
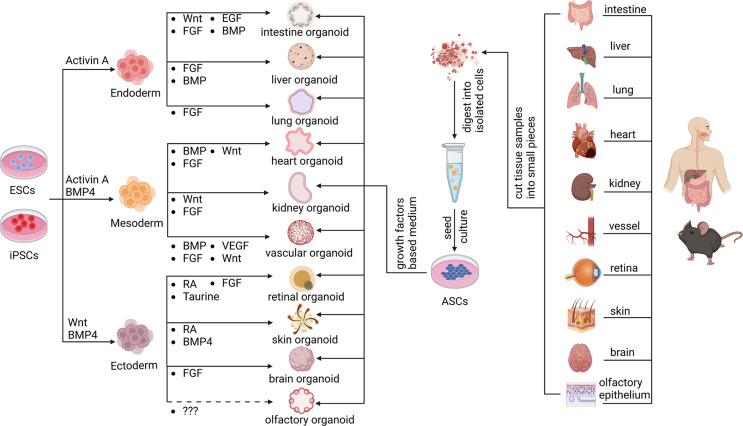
Adult stem cells (ASCs) are responsible for homeostasis and regeneration of epithelial tissues [19]. Epithelial organoid cultivation derived from adult tissue-resident stem cells provide ideal tools to advance epithelial tissue research [20]. In 2009, intestine organoid was firstly established by embedding ASCs from murine small intestinal epithelium into extracellular matrix (ECM)-rich hydrogel with specific chemical cocktail [17]. Subsequently, this technology has been widely applied to establish organoids from various tissues, while it mainly depends on the regenerative and renewal ability of epithelial cells [21]. Previous reports showed that versatile epithelial organoids derived from different organs have been constructed for multiple research purposes [22–24]. For instance, human lung organoids derived from single adult alveolar epithelial type II (AT2) cells significantly promoted the study for pathogenesis of lung diseases [25]. Besides, murine skin organoids generated from epidermal stem cells possessed the long-term expansion property (over 6 months), acting as a powerful model to investigate the epidermal homeostasis and disease progression in vitro [26]. This epithelial organoid type also includes mucosal organoid derived from murine oral epithelial stem cell [27], colon organoid derived from human colonic epithelium [28], kidney organoid from human tubular epithelium [29], and prostate organoids from murine luminal progenitor cells [30], and olfactory epithelium organoids from mouse Leucine-rich repeat-containing G-protein-coupled receptor 5(Lgr5) + basal cells [31] (Fig. 1).
Fig. 1.
Various organoids derived from either pluripotent stem cells (PSCs) or tissue-specific stem cells. Organoids derived from different tissues were established by using various adult tissue-specific progenitor cells, belonging to adult stem cells (ASCs). Meanwhile, organoids were also established via differentiation from induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) into cells of three germ layers (endoderm, mesoderm, ectoderm) through adding combination of growth factors. Currently, there is no report showing the generation of olfactory organoid from ESCs or iPSCs, and the specific growth factors necessary for this induction was unknown
One of the important determinants for organoid culture is the growth medium containing a tissue-specific growth factor cocktail. It was reported that Wnt signaling pathway plays a pivotal role in the maintenance of ASC stemness, which facilitates the generation of murine ASC-derived organoids [32]. Lgr5, an essential member of Wnt signaling, played crucial roles in regulating adult cell proliferation and stemness maintenance in versatile tissues, and thus was regarded as an important marker of adult stem cell [33]. A few types of ASC-derived organoids were constructed by using Lgr5 + stem cells. Murine intestinal, stomach and liver Lgr5(+) stem cells cultivated in 3D structures formed long-term passaging organoids, which simulated tissues of origin [34–36]. However, some normal adult tissues such as pancreas did not contain Lgr5 + stem cells, while injured pancreas by partial duct ligation showed increasing number of Lgr5 + cells. This also occurred in the olfactory epithelium, showing the recruitment of Lgr5 + cells in the injured tissue [37]. Thus, Lgr5 + cells were employed to guarantee long-term expansion of murine pancreatic or olfactory epithelium organoids in Rspondin1-based cultures [31, 38]. Rspondin1 is mainly used as an enhancer of Wnt signaling pathway to promote the proliferation of stem cells and maintain the stemness, which thus propels the proliferation and growth of organoids. Taken together, organoids were well established from tissue-specific stem cells. However, detailed comparison among various culture conditions for different organoid types are still lack, and it is hard to evaluate each condition for a specific type of organoid.
Organoids derived from pluripotent stem cells
Multiple types of organoids are derived from either induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) [39]. iPSC is a special subtype with self-renewal and capacity of pluripotent differentiation, forming various types of organoids under certain chemical induction [40]. Recent studies showed that three-dimensional culture technology combining with sequential addition of different growth factors facilitates the generation of brain organoids with typical cortical like structures from iPSCs [41]. Importantly, human brain organoids replicate the dynamic developmental process of human cerebral cortex and resemble the outer subventricular zone (oSVZ) region containing outer radial glia cells (oRGC). This provides an optimal model for studying cortex-related diseases such as autism spectrum disorder [42]. The hippocampus is important to memory formation and is severely damaged during the onset of Alzheimer’s disease [43]. Therefore, human hippocampal organoid derived from iPSCs have the potential to act as a promising tool to investigate pathogenesis of Alzheimer’s disease [44, 45]. An innovative strategy was described concerning differentiation of human iPSCs into hippocampal spheroids (HSs). This model was harnessed to reveal incipient pathogenic alteration in the hippocampi of AD patients, and offered a platform for screening treatment options against early stage of AD [46]. Apart from brain organoid, significant progress was achieved in other types of human iPSC-derived organoids, including heart organoid [47], retinal organoid [48], spinal cord organoid [49], kidney organoid [50], and skin organoid [51] (Fig. 1). In summary, simplicity and accessibility of iPSCs-derived organoids may replace the human tissues and avoid ethical issues, providing ideal and promising models for studying pathogenesis of some currently incurable diseases.
However, procedures for generating olfactory organoid from iPSCs was not reported yet. A few studies indicated potential growth factors regulating olfactory tissue development. To investigate the signal that initiated the formation of olfactory placode, Andrew et al. reported that activation of FGF pathway was required for olfactory placode formation and sufficient to induce it from cells within the preplacodal region in chicken lens. They also showed that FGF8 induced expression of genes specific for the presumptive olfactory region, subsequently functioning as olfactory markers [52]. In mouse, FGF8 altered stem cell marker expression and neurogenic patterns that directly reflected changes in bone morphogenetic protein (BMP) and Noggin expression in the nasal mesenchyme [53]. Besides, FGF8 has been implicated in patterning of both olfactory placode and subjacent frontonasal mesenchyme in mouse [54]. Recently, Rebecca and colleagues showed that BMP inhibition, wingless/integrated protein inhibition, retinoic acid inhibition, transforming growth factor alpha (TGFα) activation, and FGF8 activation were required in the induction of olfactory placode and differentiation of OSNs in human pluripotent stem cells [55]. Moreover, a recent study showed that functional OSNs were generated from human iPSCs via supplementation of FGF8 and dual SMAD inhibition, in which they analyzed the expression of OSN markers and validated their selective responsiveness to odorant compounds by measuring the membrane potentials [56]. In conclusion, although there are currently no reports on the cultivation of olfactory organoids derived from iPSCs, a few studies provide clues for potential growth factor candidates serving as critical elements to establish the induction procedures.
Advances in nasal organoids
Olfactory epithelium organoids
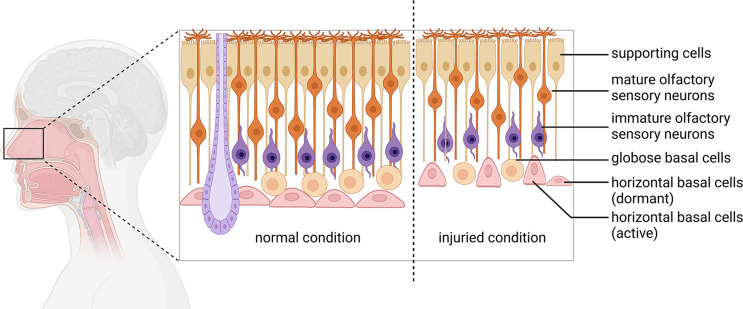
The sense of smell is of great significance to the life and health of mammals. Loss of olfactory function not only affects life quality, but also serves as an early indicator to many neurodegenerative diseases, such as Alzheimer’s Disease and Parkinson’s disease [57]. The olfactory epithelium undergoes continuous renewal throughout life, which mainly attributes to the presence of olfactory epithelium stem cells including mitotically active globose basal cells (GBC) and dormant horizontal basal cells (HBC) [58]. Once olfactory epithelium is severely damaged, HBCs are recruited from resting to active state, then generate GBCs to produce neurons and also differentiate into various types of olfactory epithelial cells, eventually regenerating olfactory epithelium [59]. Thus, the tissue-specific stem cells in olfactory epithelium provide an ideal source for organoid cultivation in vitro (Fig. 2).
Fig. 2.
Schematic diagram of cellular composition in the olfactory epithelium. The olfactory epithelium shows a pseudostratified epithelial structure composed of supporting cells (SCs), mature and immature olfactory sensory neurons (OSNs), globose basal cells (GBCs), and horizontal basal cells (HBCs). Under normal condition, the HBCs are flat and adherent to the basal lamina, whereas these cells are activated and switched into triangular shape after severe OE injury
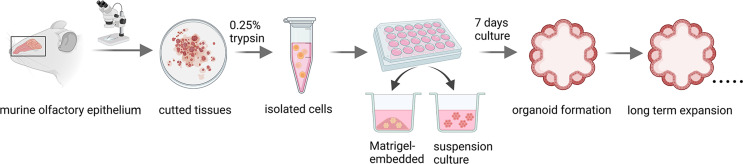
Multiple attempts have been made in culturing stem/progenitor cells and other cell types isolated from olfactory epithelium (Table 1). Previous study showed that P63 act as a ‘main controller’ of HBCs’ dormancy and activation [60]. Peterson and colleagues demonstrated that P63+ multipotent HBCs can be cultivated and passaged in vitro. This culture closely recapitulates the phenotype of HBCs in vivo, forming a 3D-culture spheroidal structure when embedded into growth factor-reduced Matrigel [61]. To investigate the alteration of HBCs with chronic inflammation, Chen and colleagues explained a crucial role of NF-kappaB signaling pathway using TNFα-treated primary-cultured HBCs [62]. Our team is committed to the establishment and development of olfactory epithelium organoid platform. We have successfully established clonal expansion of cultures from murine olfactory epithelium as well as from human olfactory mucosa using Matrigel-based three-dimensional system (Fig. 3). By using mouse olfactory epithelium organoid, we identified a few critical genes that regulates olfactory epithelial homeostasis and regeneration. Through activating or repressing Notch signaling by specific activator or inhibitor, we found that Notch signaling mainly affected aging-induced morphological alteration, cell proliferation and neuronal differentiation in olfactory epithelium organoids [63, 64]. Moreover, Lgr5 + stem cells in the olfactory epithelium formed 3D organoids in vitro. Importantly, proliferative capacity and neuronal generation in these Lgr5 + cells-derived organoids varied under different culture conditions, whereas VPA (a histone deacetylase inhibitor) and CHIR99021 (a Wnt agonist) induced the highest Lgr5 expression level, and LY411575 (a Notch inhibitor) resulted in the most abundant yield of OMP + mature sensory neurons [31]. Using this organoid platform, we revealed a new role of Chil4 (a chitinase-like protein expressed in supporting cells) in olfactory epithelium regeneration via communicating with inflammation, providing evidence that supporting cells modulate regeneration of sensory neurons [65]. Additionally, transmembrane protein 59 (Tmem59) was reported to be necessary in proliferation and neuronal generation in OE organoids [66]. Using OE organoids from aged mice, we found that Egr1 overexpression improves cell proliferation and sensory neuronal generation [67]. Study from Huang’s group showed that restraining matrix metalloproteases (MMPs) and prompting epidermal growth factor receptor (EGFR) expression not only restricted cell proliferation in mouse OE organoids, but also strongly repressed HBC proliferation post methimazole-induced OE injury [68]. The cultivation condition of murine olfactory organoids was applied in culture of human olfactory mucosa organoid, with a few modifications on addition of chemical cocktail. The olfactory cleft and superior turbinate tissue used for human olfactory organoid culture was dissected from patients undergoing surgery to access tumors on the skull base. These human olfactory organoids expressed marker genes such as Lgr5 (a marker for stem cell), NCAM1 (a marker for neuron cell), NGFR (a marker for neuronal cell), OMP (a marker of mature olfactory sensory neuron) and Krt5 (a marker for horizontal basal cell) [31]. In summary, the successful establishment of murine and human olfactory organoids provide reliable models for the investigation of molecular mechanisms underlying olfactory epithelium regeneration and other smell-related issues. This will be potentially applied in disease modeling, drug screening and establishing individualized precision medicine in the future.
Table 1.
Summary of reported methods for olfactory epithelium stem/progenitor cell culture
| Ref | Year | Source | Cultured Cell Type | Summary |
|---|---|---|---|---|
| [61] | 2019 | Mouse, rat, human | P63 + multipotent cells | P63 + multipotent olfactory epithelial cells were cultivated and passaged, and the cultured HBCs were modeled into a 3D-culture spheroidal structure embedded with growth factor-reduced Matrigel. |
| [62] | 2019 | Mouse | HBCs | Chronic inflammation by TNFα led to NF-κB activation in primary cultured HBCs. |
| [63] | 2020 | Mouse | Olfactory epithelium cells | Chemical cocktail regulated olfactory organoid growth and morphology via Notch signaling pathway. |
| [64] | 2018 | Mouse | Olfactory epithelium cells | Lesion induced generation of Notch1 + horizontal basal cells in olfactory epithelium organoids. |
| [31] | 2021 | Mouse, human | Lgr5 + olfactory epithelium progenitor cells | Expansion of olfactory epithelium/mucosa organoids was established in vitro, with generation of mature sensory neuron and functional response to odor stimulation. |
| [65] | 2021 | Mouse | Olfactory epithelium cells | Chil4 regulated cell proliferation and differentiation in olfactory epithelium organoids. |
| [66] | 2023 | Mouse | Olfactory epithelium cells | Tmem59 regulated cell proliferation, sustentacular and neuronal generation in olfactory epithelium organoids. |
| [68] | 2020 | Mouse | Olfactory epithelium cells | MMP and EGFR inhibition suppressed HBC proliferation in olfactory organoids. |
| [115] | 2024 | Mouse | Olfactory epithelium cells with impedance biosensors | The impedance device enabled real-time observation of morphological and physiological features in olfactory organoids from AD mice. |
| [67] | 2024 | Mouse | Aged olfactory epithelium cells | Egr1 overexpression recovered cell proliferation and neuronal generation in aged olfactory epithelium organoids. |
Fig. 3.
Method for olfactory organoid culture. Olfactory epithelial tissues were dissected under a stereomicroscope, and the tissues were cut into small pieces. Single cell suspension was made by adding 0.25% Trypsin, and then the cells were seeded into 24-well plate within Matrigel drops or in Matrigel-based medium. After cultivation for 7 days, organoids were formed and passaged for long-term expansion
Nasal epithelium organoids
The nasal mucosal epithelium, as an important physiological barrier, is the first defense line for the nose against external invasion. The formation of special tight junctions between nasal epithelial cells protect the mucosa from damage caused by pathogens such as bacteria, fungi, viruses, inhaled allergens, and other irritants [69]. Destruction of this epithelial barrier exposes nasal mucosa to the external substances, causing innate and adaptive immune responses. Human nasal epithelial stem and progenitor cells (hNESPCs) are important pluripotent stem cells for repairing nasal mucosal injury [70]. Tissue repairing maintains epithelial barrier function of nasal mucosa, while aberrant repair leads to the occurrence of nasal mucosal diseases [71]. Recently, there is an increasing number of studies on hNESPCs. However, due to complex experimental conditions and long culturing period, establishment of nasal epithelium model in vitro is challenging [72]. At present, the establishment of organoid model is an important carrier for studying the physiological functions of nasal mucosa. These in vitro cultured cells are close to the natural growth state of nasal mucosal epithelial cells, laying a good foundation for the study of function of nasal mucosal epithelium and related diseases (Table 2).
Table 2.
Summary of reported nasal epithelial organoids
| Ref | Year | Source | Technique | Summary |
|---|---|---|---|---|
| [78] | 2023 | Nasal epithelial cells from CRS patients | Air-liquid interface (ALI) culture | Vitamin D facilitated nasal epithelial recovery and host defense responses against influenza H1N1 and Staphylococcus Aureus infections. |
| [79] | 2020 | Primary human nasal epithelial cells from CRS patients | ALI culture | Different nasal irrigation solution had different effect on ALI-cultured human nasal epithelial cells. |
| [80] | 2018 | Nasal epithelial cells from CRS patients | ALI culture | IL-13Rα2 had a potential role in facilitating the genesis of CRS via interacting with ERK1/2 signal pathway in the nasal epithelium. |
| [81] | 2015 | Nasal epithelial cells from CRS patients | ALI culture | The positive interaction between TSLP, IL33, and Th2 contributed to the progress of nasal epithelial inflammation. |
| [82] | 2021 | Nasal epithelial cells from CRS patients | ALI culture | BMP-2 acted as a biomarker of CRS due to its capability to reflect the pathophysiology of nasal mucosa. |
| [83] | 2021 | Primary polyp and nasal epithelial cells of CRS patients | ALI culture | Staphylococcus aureus colonization and release of enterotoxin B destructed nasal epithelium structure via driving TLR, thus disrupting TLR triggering was a potential strategy to repress the CRS exacerbation. |
| [84] | 2017 | Primary human nasal epithelial cell cultures | ALI culture | Wnt/β-catenin signaling pathway prompted the inflammation and caused serious alterations coincident with those seen in the reconstruction process of nasal epithelium. |
| [85] | 2022 | Primary human nasal epithelial cells from CRS patients | 3D culture nasal organoids | Cultured nasal organoids were maintained for 20 days, with expression of markers for stem cells, goblet cells and ciliated cells. |
| [86] | 2018 | Sinonasal fibroblasts and sinonasal epithelial organoids from CRS patients and controls | Co-culture of organoids with fibroblasts | Malfunctional interaction between fibroblasts and epithelial cells prompted CRSwNP onset via dysregulated Wnt signaling pathway |
| [87] | 2022 | Nasal polyp tissue from CRS patients | 3D culture nasal organoids | Polyp-derived cells were cultivated in a 3D environment, with remaining differentiated state for a longer time and ciliary beating |
| [88] | 2023 | Nasal polyp tissues from CRS patients | Explant organoids culture | Crocin restrained nasal inflammation caused by ILC2 activation at low concentrations via blocking the activation of NF-κB signaling pathway. |
Chronic rhinosinusitis (CRS) is a common inflammatory disease affecting the mucosa and paranasal sinuses of the nasal cavity [73]. The pathological and physiological manifestations of CRS include inflammatory infiltration and tissue remodeling of the nasal mucosa, including eosinophil and neutrophil infiltration, polyp formation, goblet cell proliferation, and abnormal epithelial barrier function [74]. CRS is divided into two clinical subtypes based on the presence or absence of nasal polyps, including chronic rhinosinusitis with nasal polys (CRSwNP) and without nasal polys (CRSsNP) [75]. CRS is a multifactorial inflammatory disease. Development of effective disease model is essential for revealing the pathogenesis of CRS. The organoid technology facilitates establishing an optimal model to better understand CRS progression. Nasal mucosa epithelial cells were cultured on a porous support precoated with collagen by air-liquid interface (ALI) technology, with formation of pseudostratified ciliated columnar epithelium [76, 77]. ALI-based technology was harnessed to investigate the pathogenesis of CRS. For example, Fan et al. determined the role of Vitamin D in promoting epithelial repair and host defense against influenza H1N1 virus and Staphylococcus Aureus infections using an ALI-based nasal epithelial cell model [78]. Likewise, a similar ALI-cultured human nasal epithelial cell (hNEC) model was utilized to examine the effect of different nasal irrigation solution on epithelial mucociliary and barrier functionality [79]. Given to the essential function of human nasal epithelial cells (hNECs) inflammatory response in the onset of CRS, tremendous studies have illustrated various inflammatory factors using ALI-cultured hNECs, including IL-13Rα2 [80], Thymic stromal lymphopoietin (TSLP)-IL33-Th2 loop [81], BMP-2 [82], Toll-like receptor 2 (TLR2) [83], Wnt/β-catenin signaling [84], etc.
Comparatively, 3D-cultured organoid is superior in replicating the intricacy of originated organs and tissues than 2D culture. Ramezanpour and colleagues successfully cultured nasal epithelial organoids from primary hNECs, which were obtained by sterile nasal brushes from the inferior turbinate surface of CRS patients. These nasal epithelium organoids underwent multiple freeze-thaw cycles, thus facilitated constructing a biobank of nasal epithelial organoids from different patients and allow drug screening and potential preclinical applications in the future [85]. To verify the interactions between nasal epithelial cells and human sinonasal fibroblasts (hSNFs), Dobzanski et al. determined that nasal epithelial organoids co-cultured with hSNFs from CRSwNP patients altered epithelial cell morphology, and increased colony forming efficiency compared to epithelial cells co-cultured with healthy hSNFs [86]. Another study illustrated nasal organoids derived from CRSwNP patients, with observation of the ciliary beating for up to 20 days [87]. In addition, Xu et al. constructed an explant organoid model using nasal polyp tissues from CRSwNP patients. Using this model, they discovered that Crocin restrained nasal inflammation induced by ILC2 activation at low concentration via blocking the activation of NF-κB signaling pathway [88]. Collectively, nasal epithelial organoid, especially from CRS patients, will facilitate investigating pathogenesis of CRS, and potentially put forward personalized precision treatment for CRS.
Recent advances in organoid technology
Vascularized organoid
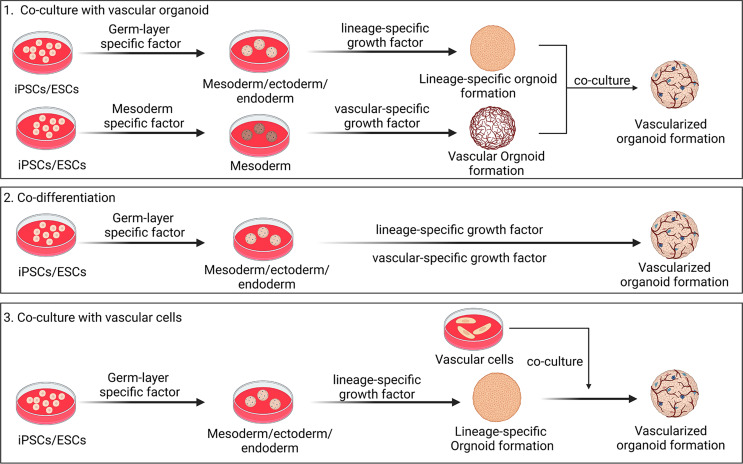
To better simulate the complexity in physiological structure in vivo of originated tissues, multiple bioengineering manipulation have been implemented to improve organoid technology. Based on the crucial role of blood supply in tissue genesis and function, it is preferable to generate vascular networks within organoids to realize the preclinical application [89]. A recent study reported vascularized human brain organoids equipped with blood-brain barrier like structures. In this study, vessel and brain organoids were cultured separately, and then co-cultured two types of organoids in single Matrigel droplet (one brain organoid was surrounded by two vessel organoids). After 40 days, the fused vascularized brain organoids were generated, and these organoids contained vascular network-like structures and displayed more neural progenitors, suggesting that increased blood supply promoted neural growth in brain organoid [90]. Different protocols of vascularized organoids have been developed (Fig. 4). From an anatomical perspective, vessel originates from mesoderm while cerebrum is from ectoderm [91]. Cakir et al. established a protocol for developing embryonic vasculature, beginning with the differentiation of mesoderm-originated angioblasts. In this strategy, appropriate combination of growth factors or genetic engineering induced the development of mesoderm together with ectoderm to generate vascularized brain organoids [92]. Human ETS variant 2 (hETV2) positive human embryonic stem cells (hESCs) boosted the formation of vascular-like network in human cortical organoids [92]. Vascularized brain organoids exhibited blood-brain barrier characteristic, such as enhanced tight junctions, accelerated nutrient transporters, and improved trans-endothelial electrical resistance [90]. Besides, Shi et al. developed a neoteric method for the production of vascularized human brain organoids through co-culturing hiPSCs with human umbilical vein endothelial cells (hUVECs) in vitro. These organoids formed a functional vascularization system as well as intercellular synaptic connections [93]. Above-mentioned methods were widely used to cultivate vascularized organoids from various human tissues and organs with specific modifications, including heart organoid [94, 95], retinal organoid [96], kidney organoid [97], and liver organoid [98]. Vascularized organoids promoted nutrient transportation, and facilitated to take away metabolites to prevent waste accumulation. In addition, vascularization in organoids was conducive to the growth and maturation, making them alike to the real organ, providing a certain physiological basis for in vivo transplantation, and facilitating in vivo revascularization and long-term survival after transplantation.
Fig. 4.
Overview of protocols for generation of vascularized organoids. Vascularized organoids are generated through co-culturing with vascular organoids, co-culturing with vascular cells, and organoid co-differentiation
Organoid-on-chip
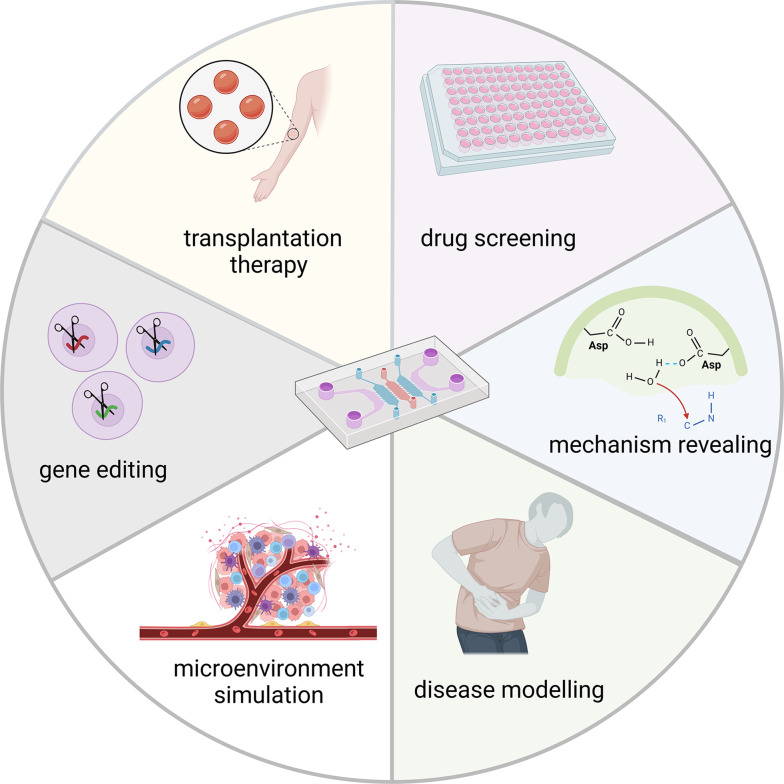
Organoid-on-chip, a cutting-edge technique, integrates the advantages of both organ chips and organoid technology, simulating different organs in vitro through a micro-device for cell culture [99]. Organ chip technology is developed based on microfluidic technology through implanting cells into a chip to mimic in vivo microenvironment, such as fluid and mechanical environment [100]. For instance, Rousset and colleagues established an experimental setup to control the residence time of single cells around 3D organ by combination of particle-flow control and organoid models in hanging drop networks [101]. Karina et al. established a personalized pancreatic ductal adenocarcinoma (PDAC) chip with functional vascular barrier. In this platform, they produced a vertically stacked-channel polydimethylsiloxane (PDMS)-free microfluidic chip that simulated blood vessel and pancreatic duct lumens, separated by a porous membrane and providing separate outflow collection. Besides, their device offered control of media flow rate. This system closely mimics the in vivo structure of PDAC and can be applied to assess drug sensitivity and predict therapy response [102]. By seeding organoid into chip, organoid-on-chip technology establishes culture conditions suitable for cells, such as flowing mechanical force and physiological hypoxia, thereby guaranteeing consistency between cell types, growth conditions and in vivo microenvironment mimicking [103]. This provides more efficient technological tool for disease modelling, drug screening, transplantation therapy exploitation, pathological mechanism uncovering, and gene editing (Fig. 5) [104]. Three aspects including more controllable microenvironment, modelling of multi-organ systems, increasing congruity between parallel experiments ensure that organoid-on-chip enables significant progresses in organoid research [99]. Multiple teams have devoted to recapitulating in vivo microenvironment using organoid chips. For instance, Zou et al. established a hepatocellular carcinoma organoid-on-chip simulating tumor microenvironment combining with mesenchymal stromal cells, peripheral blood mononuclear cells, and cancer-associated fibroblasts. Using this microengineered organoid-on-chip, they performed high-throughput drug screening to investigate potential immunological therapeutic strategy against hepatocellular carcinoma [105]. A mouse intestinal organoid-on-chip closely mimicked oxygen dynamics of damaged intestine and uncovered potential therapies [106]. Lee et al. developed a human kidney organoid-on-chip replicating shear stress of kidney using superior extracellular matrix, and this model was more sensitive to nephrotoxic drugs [107].
Fig. 5.
Multiple applications of organoid-on-chip. Organoid-on-chip provides ideal tools for drug screening and mechanism revealing, due to the faithful simulation of microenvironment and accurate disease modelling. As a promising platform, organoid-on-chip will be harnessed to exploit gene editing in vitro as well as transplantation therapy
Traditional organoid model showed limitation in mimicking organ-organ interactions, while organoid-on-chip may provide optimal solution for this dilemma. Promising works on organoid-on-chip technology put forward novel direction for organ-organ communication research during intricate physiological condition. Skardal et al. established a human heart-lung-liver model, consisted of 3D printed liver and heart organoids with microengineered lung tissues. Using this model, they investigated inter-organic response to bleomycin in term of cytokine-induced interactions between heart, lung, and liver [108]. Likewise, a similar device contained human liver, cardiac, and lung organoids was harnessed to evaluate the metabolic effect of liver drug on downstream toxicity in lung and cardiac organoids. More importantly, this device was expanded to construct a six-organoid model, including human liver, cardiac, lung, endothelium, brain, and testis organoids, which was utilized for precise presentation of drug interactions in vitro [109]. In contrary to high replication of biological process in vivo, in vitro-cultured organoids exhibit significant variability in size, structural organization, functional ability, and gene expression. Latest advances in organoid-on-chip technology proposes a tool to solve this problem by micro-engineering culture equipment. For example, Au et al. introduced a digital microfluidic system on human liver organoids, which allowed the organoids to move, merge, and split in a stylized category. Upon this platform, acetaminophen-mediated cell toxicity was analyzed without manual intervention [110]. To sum up, organoid-on-chip technology enhances the throughput and automation of organoid culture systems, which is of great significance for achieving large-scale, homogeneous, and standardized cultivation of organoids.
Conclusions and future directions
The sense of smell is an indispensable sensory function in human life, and its impact on the quality of human life exists in all aspects. Olfactory function affects the nutritional status of the human body via appetite and food preference, and helps people quickly identify dangers existing in the environment, such as leakage of natural gas, fire, dangerous chemical gases and rotten food [111]. Olfactory dysfunction not only impairs physical health, reduces the quality of life, and increases individual mortality, but also is the first symptom of many major neurodegenerative diseases [57]. Since the pathogenesis of olfactory dysfunction is unknown, there is still no targeted and effective therapy against this disease. In vitro disease model to study the pathogenesis of olfactory dysfunction is still lacking, while most of studies mainly depend on animal models [112, 113]. Establishment of a cell-based model in vitro will greatly reduce the number of animals to improve animal welfare, and will accelerate progress in understanding the pathogenesis of olfactory dysfunction due to lower cost, easier maintenance and simpler experimental operation of in vitro model. The current organoid models do not completely replace the animal model due to the limitation on faithfully simulating mucosa structure, including cell composition, alignment and microenvironment [114]. Recently, Liu and colleagues established a device combining impedance biosensor with olfactory organoids derived from Alzheimer’s disease (AD) mouse models, which were used to perform real-time detection for morphological and physiological alteration in olfactory organoids with progression of AD. This provided a novel model determining the pathogenesis and early diagnosis of olfactory dysfunction related neurodegenerative disease [115]. This is a breakthrough for the application of olfactory organoids, since they proposed a novel platform for uncovering mechanisms underlying the AD progression and association with olfactory disorders. However, it still does not fully reflect the state of nasal mucosa under pathological condition. Future efforts will be made to integrate pathological microenvironment into olfactory organoid.
Organoid culture model originated from nasal epithelial tissues is a representative system that favors the investigation of pathophysiology, host pathogen interaction, and preclinical development of CRS treatment. Many efforts to culture nasal airway epithelial cells as representative models have been made by using ALI technique [116]. However, the requirement for porous inserts impose restriction on the application of ALI cultures. Given to the application of organoids on high-throughput approach to analyze drug sensitivities in ovarian tumor patients [117], nasal organoids may propose the possibility of high-throughput screening for individual drug responses in CRS patients. In addition to CRS, other diseases such as allergic rhinitis and nasopharyngeal carcinoma seriously affect nasal mucosa function [118, 119]. However, organoid-based models for these diseases are still lack. Although currently there are many studies focused on CRS-derived organoids, the mechanism underlying CRS occurrence and progression are not fully captured.
Enormous breakthroughs have been made in organoid technology in the past decades. Although efforts were made to mimic the biological process of nasal and olfactory mucosa using organoids, they still did not faithfully recapitulate the real microenvironment of original tissue, and thus have not been broadly applied in disease modelling and preclinical applications for nasal diseases. Combining new organoid technologies such as vascularization and chip equipment with nasal mucosa organoid is an optimal choice to more realistically reflect the physiological microenvironment of nasal mucosa. To establish interaction among organoids from different tissues, and reduce the variability between organoids will be the main direction of future research on nasal mucosa organoids. These studies may open up new avenues for gaining a better understanding of human nasal epithelium development, function, and disorders.
Author contributions
Literature search and manuscript writing: Jinxia Liu and Yiqun Yu; Preparation of figures: Jinxia Liu; Conceptualization and supervision: Yiqun Yu and Yunfeng Zhang. All the authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China Grants (32070996 and 32271044 to Y.Y.); Science and Technology Commission of Shanghai Municipality (23ZR1409600 and 21140900600 to Y.Y.); Shanghai Municipal Education Commission, the Shanghai Oriental Scholar Program (GZ2022006 to Y.Y.); Shanghai Municipal Health Commission (GWVI-11.2-XD09 to Y.Y.); Fudan University, Shanghai Medical College, Young investigator for clinical and scientific research team (to Y.Y.).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors give consent for publication.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunfeng Zhang, Email: yfzhang@ioz.ac.cn.
Yiqun Yu, Email: yu_yiqun@fudan.edu.cn.
References
- 1.Rossi G, Manfrin A, Lutolf MP (2018) Progress and potential in organoid research. Nat Rev Genet 19(11):671–687. https://www.ncbi.nlm.nih.gov/pubmed/30228295 [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Zhang D, Wang D et al (2023) Cost-effective in vivo and in Vitro Mouse models for evaluating Anticryptosporidial Drug Efficacy: assessing Vorinostat, Docetaxel, and Baicalein. J Infect Dis 228(10):1430–1440. https://www.ncbi.nlm.nih.gov/pubmed/37418629 [DOI] [PubMed] [Google Scholar]
- 3.Sheridan MA, Fernando RC, Gardner L et al (2020) Establishment and differentiation of long-term trophoblast organoid cultures from the human placenta. Nat Protoc 15(10):3441–3463. https://www.ncbi.nlm.nih.gov/pubmed/32908314 [DOI] [PubMed] [Google Scholar]
- 4.Saitou M, Hayashi K (2021) Mammalian in vitro gametogenesis. Sci 374(6563):eaaz6830. https://www.ncbi.nlm.nih.gov/pubmed/34591639 [DOI] [PubMed]
- 5.Prior N, Inacio P, Huch M (2019) Liver organoids: from basic research to therapeutic applications. Gut 68(12):2228–2237. https://www.ncbi.nlm.nih.gov/pubmed/31300517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Owusu-Hammond C, Sievert D et al (2023) Stem cell-based Organoid models of Neurodevelopmental disorders. Biol Psychiatry 93(7):622–631. https://www.ncbi.nlm.nih.gov/pubmed/36759260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Jin H, Lou S et al (2024) Microfluidic droplet encapsulation-guided organoid growth promotes parental tumor phenotype recapitulation. Int J Cancer 154(1):145–154. https://www.ncbi.nlm.nih.gov/pubmed/37622267 [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Armendariz AI, Tata PR (2023) Recent advances in lung organoid development and applications in disease modeling. J Clin Invest 133(22):e170500. https://www.ncbi.nlm.nih.gov/pubmed/37966116 [DOI] [PMC free article] [PubMed]
- 9.Beydag-Tasoz BS, Yennek S, Grapin-Botton A (2023) Towards a better understanding of diabetes mellitus using organoid models. Nat Rev Endocrinol 19(4):232–248. https://www.ncbi.nlm.nih.gov/pubmed/36670309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Jiao D, Liu A et al (2022) Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol 15(1):58. https://www.ncbi.nlm.nih.gov/pubmed/35551634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geurkink N (1983) Nasal anatomy, physiology, and function. J Allergy Clin Immunol 72(2):123–128. https://www.ncbi.nlm.nih.gov/pubmed/6350406 [DOI] [PubMed] [Google Scholar]
- 12.Sokpor G, Abbas E, Rosenbusch J et al (2018) Transcriptional and epigenetic control of mammalian olfactory Epithelium Development. Mol Neurobiol 55(11):8306–8327. https://www.ncbi.nlm.nih.gov/pubmed/29532253 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi H (2024) Olfactory cilia, regulation and control of olfaction. Physiol Rep 12(19):e70057. https://www.ncbi.nlm.nih.gov/pubmed/39358841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo A, Won J, Gil CH et al (2022) Nasal symbiont Staphylococcus epidermidis restricts the cellular entry of influenza virus into the nasal epithelium. NPJ Biofilms Microbiomes 8(1):26. https://www.ncbi.nlm.nih.gov/pubmed/35418111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moratin H, Lang J, Picker MS et al (2024) The impact of NO(2) on Epithelial Barrier Integrity of a primary cell-based air-liquid interface model of the nasal respiratory epithelium. J Appl Toxicol 10.1002/jat.4717. https://www.ncbi.nlm.nih.gov/pubmed/39529574 [DOI] [PubMed]
- 16.Smith E, Cochrane WJ (1946) Cystic organoid teratoma; report of a case. Can Med Assoc J 55(2):151. https://www.ncbi.nlm.nih.gov/pubmed/20992760 [PubMed]
- 17.Sato T, Vries RG, Snippert HJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265. https://www.ncbi.nlm.nih.gov/pubmed/19329995 [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Zhang W, Wu X et al (2023) Organoid assessment technologies. Clin Transl Med 13(12):e1499. https://www.ncbi.nlm.nih.gov/pubmed/38115706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clevers H, Watt FM (2018) Defining adult stem cells by function, not by phenotype. Annu Rev Biochem 87:1015–1027. https://www.ncbi.nlm.nih.gov/pubmed/29494240 [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Chen X, Dowbaj AM et al (2022) Organoids. Nat Rev Methods Primers 2:94. https://www.ncbi.nlm.nih.gov/pubmed/37325195 [DOI] [PMC free article] [PubMed]
- 21.Yan HHN, Chan AS, Lai FP et al (2023) Organoid cultures for cancer modeling. Cell Stem Cell 30(7):917–937. https://www.ncbi.nlm.nih.gov/pubmed/37315564 [DOI] [PubMed] [Google Scholar]
- 22.Al-Ghadban S, Pursell IA, Diaz ZT et al (2020) 3D spheroids derived from human lipedema ASCs demonstrated similar adipogenic differentiation potential and ECM remodeling to Non-lipedema ASCs in Vitro. Int J Mol Sci 21(21):8350. https://www.ncbi.nlm.nih.gov/pubmed/33171717 [DOI] [PMC free article] [PubMed]
- 23.Luo L, Zhang W, Wang J et al (2021) A novel 3D culture model of human ASCs reduces cell death in spheroid cores and maintains inner cell proliferation compared with a nonadherent 3D culture. Front Cell Dev Biol 9:737275. https://www.ncbi.nlm.nih.gov/pubmed/34858974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann K, Obermayer B, Honzke K et al (2022) Human alveolar progenitors generate dual lineage bronchioalveolar organoids. Commun Biol 5(1):875. https://www.ncbi.nlm.nih.gov/pubmed/36008580 [DOI] [PMC free article] [PubMed]
- 25.Salahudeen AA, Choi SS, Rustagi A et al (2020) Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 588(7839):670–675. https://www.ncbi.nlm.nih.gov/pubmed/33238290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonekamp KE, Kretzschmar K, Wiener DJ et al (2019) Long-term expansion and differentiation of adult murine epidermal stem cells in 3D organoid cultures. Proc Natl Acad Sci U S A 116(29):14630–14638. https://www.ncbi.nlm.nih.gov/pubmed/31253707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seubert AC, Krafft M, Kretzschmar K (2021) Generation and characterization of murine oral mucosal organoid cultures. J Vis Exp (173). https://www.ncbi.nlm.nih.gov/pubmed/34398160 [DOI] [PubMed]
- 28.Jung P, Sato T, Merlos-Suarez A et al (2011) Isolation and in vitro expansion of human colonic stem cells. Nat Med 17(10):1225–1227. https://www.ncbi.nlm.nih.gov/pubmed/21892181 [DOI] [PubMed] [Google Scholar]
- 29.Schutgens F, Rookmaaker MB, Margaritis T et al (2019) Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 37(3):303–313. https://www.ncbi.nlm.nih.gov/pubmed/30833775 [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S, Hynes PG, Tillman HS et al (2015) Identification of different classes of Luminal Progenitor cells within prostate tumors. Cell Rep 13(10):2147–2158. https://www.ncbi.nlm.nih.gov/pubmed/26628377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren W, Wang L, Zhang X et al (2021) Expansion of murine and human olfactory epithelium/mucosa colonies and generation of mature olfactory sensory neurons under chemically defined conditions. Theranostics 11(2):684–699. https://www.ncbi.nlm.nih.gov/pubmed/33391499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maimets M, Rocchi C, Bron R et al (2016) Long-term in Vitro expansion of salivary gland stem cells driven by wnt signals. Stem Cell Rep 6(1):150–162. https://www.ncbi.nlm.nih.gov/pubmed/26724906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lau W, Peng WC, Gros P et al (2014) The R-spondin/Lgr5/Rnf43 module: regulator of wnt signal strength. Genes Dev 28(4):305–316. https://www.ncbi.nlm.nih.gov/pubmed/24532711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker N, Huch M, Kujala P et al (2010) Lgr5(+ ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6(1):25–36. https://www.ncbi.nlm.nih.gov/pubmed/20085740 [DOI] [PubMed] [Google Scholar]
- 35.Barker N, van Es JH, Kuipers J et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165):1003–1007. https://www.ncbi.nlm.nih.gov/pubmed/17934449 [DOI] [PubMed] [Google Scholar]
- 36.Huch M, Dorrell C, Boj SF et al (2013) In vitro expansion of single Lgr5 + liver stem cells induced by wnt-driven regeneration. Nature 494(7436):247–250. https://www.ncbi.nlm.nih.gov/pubmed/23354049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren W, Ma Z, Wang L et al (2022) Lgr5(+) cells are required and dynamically participate in olfactory epithelium regeneration: a revisiting shows Lgr5 expression in multiple cell lineages. Theranostics 12(13):5631–5644. https://www.ncbi.nlm.nih.gov/pubmed/35966594 [DOI] [PMC free article] [PubMed]
- 38.Huch M, Bonfanti P, Boj SF et al (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32(20):2708–2721. https://www.ncbi.nlm.nih.gov/pubmed/24045232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauth S, Karmakar S, Batra SK et al (2021) Recent advances in organoid development and applications in disease modeling. Biochim Biophys Acta Rev Cancer 1875(2):188527. https://www.ncbi.nlm.nih.gov/pubmed/33640383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhargava A, Sandoval Castellanos AM, Shah S et al (2022) An insight into the iPSCs-derived two-dimensional culture and three-dimensional organoid models for neurodegenerative disorders. Interface Focus 12(5):20220040. https://www.ncbi.nlm.nih.gov/pubmed/35992771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farcy S, Albert A, Gressens P et al (2022) Cortical Organoids to Model Microcephaly. Cells 11(14):2135. https://www.ncbi.nlm.nih.gov/pubmed/35883578 [DOI] [PMC free article] [PubMed]
- 42.Zang Z, Yin H, Du Z et al (2022) Valproic acid exposure decreases neurogenic potential of outer radial glia in human brain organoids. Front Mol Neurosci 15:1023765. https://www.ncbi.nlm.nih.gov/pubmed/36523605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheltens P, De Strooper B, Kivipelto M et al (2021) Alzheimer’s disease. Lancet 397(10284):1577–1590. https://www.ncbi.nlm.nih.gov/pubmed/33667416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Deng C, Meng Z et al (2024) Combined Catalpol and Tetramethylpyrazine Promote Axonal Plasticity in Alzheimer’s Disease by Inducing Astrocytes to Secrete Exosomes Carrying CDK5 mRNA and Regulating STAT3 Phosphorylation. Mol Neurobiol 61(12):10770–10791. https://www.ncbi.nlm.nih.gov/pubmed/38789892 [DOI] [PubMed]
- 45.Wu Y, Cheng J, Qi J et al (2024) Three-dimensional liquid metal-based neuro-interfaces for human hippocampal organoids. Nat Commun 15(1):4047. https://www.ncbi.nlm.nih.gov/pubmed/38744873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pomeshchik Y, Klementieva O, Gil J et al (2020) Human iPSC-Derived hippocampal spheroids: an innovative Tool for Stratifying Alzheimer Disease patient-specific Cellular phenotypes and developing therapies. Stem Cell Rep 15(1):256–273. https://www.ncbi.nlm.nih.gov/pubmed/32589876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song M, Choi DB, Im JS et al (2024) Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids. Cell Death Dis 15(5):308. https://www.ncbi.nlm.nih.gov/pubmed/38693114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otsuka Y, Imamura K, Oishi A et al (2024) Phototoxicity avoidance is a potential therapeutic approach for retinal dystrophy caused by EYS dysfunction. JCI Insight 9(8):e174179. https://www.ncbi.nlm.nih.gov/pubmed/38646933 [DOI] [PMC free article] [PubMed]
- 49.Wang Z, Liu R, Liu Y et al (2024) Human placenta decellularized Extracellular Matrix Hydrogel promotes the generation of human spinal cord organoids with Dorsoventral Organization from Human Induced Pluripotent Stem cells. ACS Biomater Sci Eng 10(5):3218–3231. https://www.ncbi.nlm.nih.gov/pubmed/38593429 [DOI] [PubMed] [Google Scholar]
- 50.Oishi H, Tabibzadeh N, Morizane R (2024) Advancing preclinical drug evaluation through automated 3D imaging for high-throughput screening with kidney organoids. Biofabrication 16(3). 10.1088/1758-5090/ad38df. https://www.ncbi.nlm.nih.gov/pubmed/38547531 [DOI] [PMC free article] [PubMed]
- 51.Kim MJ, Ahn HJ, Kong D et al (2024) Modeling of solar UV-induced photodamage on the hair follicles in human skin organoids. J Tissue Eng 15:20417314241248753. https://www.ncbi.nlm.nih.gov/pubmed/38725732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey AP, Bhattacharyya S, Bronner-Fraser M et al (2006) Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell 11(4):505–517. https://www.ncbi.nlm.nih.gov/pubmed/17011490 [DOI] [PubMed] [Google Scholar]
- 53.Forni PE, Bharti K, Flannery EM et al (2013) The indirect role of fibroblast growth factor-8 in defining neurogenic niches of the olfactory/GnRH systems. J Neurosci 33(50):19620–19634. https://www.ncbi.nlm.nih.gov/pubmed/24336726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaMantia AS, Bhasin N, Rhodes K et al (2000) Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron 28(2):411–425. https://www.ncbi.nlm.nih.gov/pubmed/11144352 [DOI] [PubMed]
- 55.Bricker RL, Bhaskar U, Titone R et al (2022) A molecular analysis of neural olfactory placode differentiation in human pluripotent stem cells. Stem Cells Dev 31(17–18):507–520. https://www.ncbi.nlm.nih.gov/pubmed/35592997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kikuta H, Tanaka H, Ozaki T et al (2024) Spontaneous differentiation of human induced pluripotent stem cells to odorant-responsive olfactory sensory neurons. Biochem Biophys Res Commun 719:150062. https://www.ncbi.nlm.nih.gov/pubmed/38740002 [DOI] [PubMed] [Google Scholar]
- 57.Fatuzzo I, Niccolini GF, Zoccali F et al (2023) Neurons, nose, and neurodegenerative diseases: olfactory function and cognitive impairment. Int J Mol Sci 24(3):2117. https://www.ncbi.nlm.nih.gov/pubmed/36768440 [DOI] [PMC free article] [PubMed]
- 58.Senf K, Karius J, Stumm R et al (2021) Chemokine signaling is required for homeostatic and injury-induced neurogenesis in the olfactory epithelium. Stem Cells 39(5):617–635. https://www.ncbi.nlm.nih.gov/pubmed/33470495 [DOI] [PubMed] [Google Scholar]
- 59.Schwob JE, Jang W, Holbrook EH et al (2017) Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J Comp Neurol 525(4):1034–1054. https://www.ncbi.nlm.nih.gov/pubmed/27560601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fletcher RB, Das D, Gadye L et al (2017) Deconstructing olfactory stem cell trajectories at single-cell resolution. Cell Stem Cell 20(6):817–830e8. https://www.ncbi.nlm.nih.gov/pubmed/28506465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peterson J, Lin B, Barrios-Camacho CM et al (2019) Activating a Reserve neural stem cell Population in Vitro enables Engraftment and Multipotency after transplantation. Stem Cell Rep 12(4):680–695. https://www.ncbi.nlm.nih.gov/pubmed/30930245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Reed RR, Lane AP (2019) Chronic inflammation directs an olfactory stem cell functional switch from Neuroregeneration to Immune Defense. Cell Stem Cell 25(4):501–513e. https://www.ncbi.nlm.nih.gov/pubmed/31523027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Tong M, Wang L et al (2020) Age-Dependent activation and neuronal differentiation of Lgr5 + basal cells in injured olfactory epithelium via Notch Signaling Pathway. Front Aging Neurosci 12:602688. https://www.ncbi.nlm.nih.gov/pubmed/33390928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai Q, Duan C, Ren W et al (2018) Notch Signaling regulates Lgr5(+) olfactory epithelium Progenitor/Stem cell turnover and mediates recovery of Lesioned olfactory epithelium in mouse model. Stem Cells 36(8):1259–1272. https://www.ncbi.nlm.nih.gov/pubmed/29664186 [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Ren W, Li X et al (2021) Chitinase-Like protein Ym2 (Chil4) regulates regeneration of the olfactory epithelium via Interaction with inflammation. J Neurosci 41(26):5620–5637. https://www.ncbi.nlm.nih.gov/pubmed/34016714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Z, Li W, Zhuang L et al (2023) TMEM59 ablation leads to loss of olfactory sensory neurons and impairs olfactory functions via interaction with inflammation. Brain Behav Immun 111:151–168. https://www.ncbi.nlm.nih.gov/pubmed/37061103 [DOI] [PubMed] [Google Scholar]
- 67.Li W, Wu T, Zhu K et al (2024) A single-cell transcriptomic census of mammalian olfactory epithelium aging. Dev Cell 59(22):3043–3058e8. https://www.ncbi.nlm.nih.gov/pubmed/39173624 [DOI] [PubMed] [Google Scholar]
- 68.Chen ZH, Luo XC, Yu CR et al (2020) Matrix metalloprotease-mediated cleavage of neural glial-related cell adhesion molecules activates quiescent olfactory stem cells via EGFR. Mol Cell Neurosci 108:103552. https://www.ncbi.nlm.nih.gov/pubmed/32918999 [DOI] [PubMed] [Google Scholar]
- 69.Na K, Lee M, Shin HW et al (2017) In vitro nasal mucosa gland-like structure formation on a chip. Lab Chip 17(9):1578–1584. https://www.ncbi.nlm.nih.gov/pubmed/28379223 [DOI] [PubMed] [Google Scholar]
- 70.Butler CR, Hynds RE, Gowers KH et al (2016) Rapid Expansion of Human Epithelial Stem Cells Suitable for Airway Tissue Engineering. Am J Respir Crit Care Med 194(2):156–168. https://www.ncbi.nlm.nih.gov/pubmed/26840431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu XM, Li CW, Chao SS et al (2014) Reduced growth and proliferation dynamics of nasal epithelial stem/progenitor cells in nasal polyps in vitro. Sci Rep 4:4619. https://www.ncbi.nlm.nih.gov/pubmed/24714674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C, Du H, Wang Y et al (2021) S100A11 regulates nasal epithelial cell remodeling and inflammation in CRSwNPs via the RAGE-mediated AMPK-STAT3 pathway. Mol Immunol 140:35–46. https://www.ncbi.nlm.nih.gov/pubmed/34653793 [DOI] [PubMed] [Google Scholar]
- 73.Desrosiers M, Diamant Z, Castelnuovo P et al (2024) Sustained efficacy of mepolizumab in patients with severe chronic rhinosinusitis with nasal polyps: SYNAPSE 24-week treatment-free follow-up. Int Forum Allergy Rhinol 14(1):18–31. https://www.ncbi.nlm.nih.gov/pubmed/37345861 [DOI] [PubMed] [Google Scholar]
- 74.Hamilos DL (2016) Chronic rhinosinusitis endotyping: sharpening the focus on inflammation. J Allergy Clin Immunol 137(5):1457–1459. https://www.ncbi.nlm.nih.gov/pubmed/27155037 [DOI] [PubMed] [Google Scholar]
- 75.Zhong B, Sun S, Tan KS et al (2023) Hypoxia-inducible factor 1alpha activates the NLRP3 inflammasome to regulate epithelial differentiation in chronic rhinosinusitis. J Allergy Clin Immunol 152(6):1444–1459e14. https://www.ncbi.nlm.nih.gov/pubmed/37777019 [DOI] [PubMed] [Google Scholar]
- 76.Capuana E, Fucarino A, Burgio S et al (2022) A dynamic air-liquid interface system for in vitro mimicking of the nasal mucosa. Biotechnol Bioeng 119(7):2004–2009. https://www.ncbi.nlm.nih.gov/pubmed/35320583 [DOI] [PubMed] [Google Scholar]
- 77.Luengen AE, Kniebs C, Buhl EM et al (2020) Choosing the right differentiation medium to develop Mucociliary phenotype of primary nasal epithelial cells in Vitro. Sci Rep 10(1):6963. https://www.ncbi.nlm.nih.gov/pubmed/32332878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao S, Huang Y, Zhang J et al (2023) Vitamin D promotes epithelial tissue repair and host defense responses against influenza H1N1 virus and Staphylococcus aureus infections. Respir Res 24(1):175. https://www.ncbi.nlm.nih.gov/pubmed/37407993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiao J, Yang J, Li J et al (2020) Hypertonic saline and seawater solutions damage sinonasal epithelial cell air-liquid interface cultures. Int Forum Allergy Rhinol 10(1):59–68. https://www.ncbi.nlm.nih.gov/pubmed/31610615 [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Li YY, Andiappan AK et al (2018) Role of IL-13Ralpha2 in modulating IL-13-induced MUC5AC and ciliary changes in healthy and CRSwNP mucosa. Allergy 73(8):1673–1685. https://www.ncbi.nlm.nih.gov/pubmed/29405354 [DOI] [PubMed] [Google Scholar]
- 81.Liao B, Cao PP, Zeng M et al (2015) Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy 70(9):1169–1180. https://www.ncbi.nlm.nih.gov/pubmed/26095319 [DOI] [PubMed]
- 82.Kim JY, Lim S, Lim HS et al (2021) Bone morphogenetic protein-2 as a novel biomarker for refractory chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 148(2):461–472e13. https://www.ncbi.nlm.nih.gov/pubmed/33667477 [DOI] [PubMed] [Google Scholar]
- 83.Martens K, Seys SF, Alpizar YA et al (2021) Staphylococcus aureus enterotoxin B disrupts nasal epithelial barrier integrity. Clin Exp Allergy 51(1):87–98. https://www.ncbi.nlm.nih.gov/pubmed/33090566 [DOI] [PubMed] [Google Scholar]
- 84.Boscke R, Vladar EK, Konnecke M et al (2017) Wnt signaling in Chronic Rhinosinusitis with nasal polyps. Am J Respir Cell Mol Biol 56(5):575–584. https://www.ncbi.nlm.nih.gov/pubmed/28059551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramezanpour M, Bolt H, Hon K et al (2022) Characterization of human nasal organoids from chronic rhinosinusitis patients. Biol Open 11(8):bio059267. https://www.ncbi.nlm.nih.gov/pubmed/35452072 [DOI] [PMC free article] [PubMed]
- 86.Dobzanski A, Khalil SM, Lane AP (2018) Nasal polyp fibroblasts modulate epithelial characteristics via wnt signaling. Int Forum Allergy Rhinol 8(12):1412–1420. https://www.ncbi.nlm.nih.gov/pubmed/30118173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franca CN, Bachi ALL, Kosugi EM et al (2022) Three-dimensional cell culture for the study of nasal polyps. Braz J Otorhinolaryngol 88(Suppl 5):S69–S74. https://www.ncbi.nlm.nih.gov/pubmed/34924329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiaodong X, Tao L, Jianmin L et al (2023) Crocin inhibits the type 2 inflammatory response produced by ILC2s in Eosinophilic nasal polyps. Am J Rhinol Allergy 37(6):656–669. https://www.ncbi.nlm.nih.gov/pubmed/37424236 [DOI] [PubMed] [Google Scholar]
- 89.Sato Y, Asahi T, Kataoka K (2023) Integrative single-cell RNA-seq analysis of vascularized cerebral organoids. BMC Biol 21(1):245. https://www.ncbi.nlm.nih.gov/pubmed/37940920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun XY, Ju XC, Li Y et al (2022) Generation of vascularized brain organoids to study neurovascular interactions. Elife 11:e76707. https://www.ncbi.nlm.nih.gov/pubmed/35506651 [DOI] [PMC free article] [PubMed]
- 91.He J, Zhang X, Xia X et al (2020) Organoid technology for tissue engineering. J Mol Cell Biol 12(8):569–579. https://www.ncbi.nlm.nih.gov/pubmed/32249317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cakir B, Xiang Y, Tanaka Y et al (2019) Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16(11):1169–1175. https://www.ncbi.nlm.nih.gov/pubmed/31591580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi Y, Sun L, Wang M et al (2020) Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol 18(5):e3000705. https://www.ncbi.nlm.nih.gov/pubmed/32401820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majid QA, Ghimire BR, Merkely B et al (2024) Generation and characterisation of scalable and stable human pluripotent stem cell-derived microvascular-like endothelial cells for cardiac applications. Angiogenesis 27(3):561–582. https://www.ncbi.nlm.nih.gov/pubmed/38775849 [DOI] [PMC free article] [PubMed]
- 95.Yang J, Lei W, Xiao Y et al (2024) Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation. Cell Prolif 57(8):e13631. https://www.ncbi.nlm.nih.gov/pubmed/38453465 [DOI] [PMC free article] [PubMed]
- 96.Kawai K, Ho MT, Ueno Y et al (2024) Hyaluronan improves photoreceptor differentiation and maturation in human retinal organoids. Acta Biomater 181:117–132. https://www.ncbi.nlm.nih.gov/pubmed/38705224 [DOI] [PubMed] [Google Scholar]
- 97.Peng K, Xie W, Wang T et al (2023) HIF-1alpha promotes kidney organoid vascularization and applications in disease modeling. Stem Cell Res Ther 14(1):336. https://www.ncbi.nlm.nih.gov/pubmed/37981699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harrison SP, Siller R, Tanaka Y et al (2023) Scalable production of tissue-like vascularized liver organoids from human PSCs. Exp Mol Med 55(9):2005–2024. https://www.ncbi.nlm.nih.gov/pubmed/37653039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Sci 364(6444):960–965. https://www.ncbi.nlm.nih.gov/pubmed/31171693 [DOI] [PMC free article] [PubMed]
- 100.Zhao Q, Cole T, Zhang Y et al (2021) Mechanical strain-enabled reconstitution of dynamic environment in Organ-on-a-Chip Platforms: a review. Micromachines (Basel) 12(7):765. https://www.ncbi.nlm.nih.gov/pubmed/34203533 [DOI] [PMC free article] [PubMed]
- 101.Rousset N, de Geus M, Chimisso V et al (2023) Controlling bead and cell mobility in a recirculating hanging-drop network. Lab Chip 23(22):4834–4847. https://www.ncbi.nlm.nih.gov/pubmed/37853793 [DOI] [PubMed] [Google Scholar]
- 102.Goluba K, Parfejevs V, Rostoka E et al (2024) Personalized PDAC chip with functional endothelial barrier for tumour biomarker detection: a platform for precision medicine applications. Mater Today Bio 29:101262. https://www.ncbi.nlm.nih.gov/pubmed/39381267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhi Y, Zhu Y, Wang J et al (2023) Cortical organoid-on-a-Chip with physiological hypoxia for investigating Tanshinone IIA-Induced neural differentiation. Research (Wash D C) 6:0273. https://www.ncbi.nlm.nih.gov/pubmed/38434243 [DOI] [PMC free article] [PubMed]
- 104.(2022) Organoids and organs on a chip. Nat Biotechnol 40(4):472. https://www.ncbi.nlm.nih.gov/pubmed/35418641 [DOI] [PubMed]
- 105.Zou Z, Lin Z, Wu C et al (2023) Micro-engineered Organoid-on-a-Chip based on mesenchymal stromal cells to predict immunotherapy responses of HCC patients. Adv Sci (Weinh) 10(27):e2302640. https://www.ncbi.nlm.nih.gov/pubmed/37485650 [DOI] [PMC free article] [PubMed]
- 106.Huang J, Xu Z, Jiao J et al (2023) Microfluidic intestinal organoid-on-a-chip uncovers therapeutic targets by recapitulating oxygen dynamics of intestinal IR injury. Bioact Mater 30:1–14. https://www.ncbi.nlm.nih.gov/pubmed/37534235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee HN, Choi YY, Kim JW et al (2021) Effect of biochemical and biomechanical factors on vascularization of kidney organoid-on-a-chip. Nano Converg 8(1):35. https://www.ncbi.nlm.nih.gov/pubmed/34748091 [DOI] [PMC free article] [PubMed]
- 108.Skardal A, Murphy SV, Devarasetty M et al (2017) Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7(1):8837. https://www.ncbi.nlm.nih.gov/pubmed/28821762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rajan SAP, Aleman J, Wan M et al (2020) Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater 106:124–135. https://www.ncbi.nlm.nih.gov/pubmed/32068138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Au SH, Chamberlain MD, Mahesh S et al (2014) Hepatic organoids for microfluidic drug screening. Lab Chip 14(17):3290–3299. https://www.ncbi.nlm.nih.gov/pubmed/24984750 [DOI] [PubMed] [Google Scholar]
- 111.Shin T, Kim J, Ahn M et al (2019) Olfactory dysfunction in CNS neuroimmunological disorders: a review. Mol Neurobiol 56(5):3714–3721. https://www.ncbi.nlm.nih.gov/pubmed/30191380 [DOI] [PubMed] [Google Scholar]
- 112.Ngwa HA, Kanthasamy A, Jin H et al (2014) Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology 43:73–81. https://www.ncbi.nlm.nih.gov/pubmed/24362016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen J, Li SS, Fang SM et al (2022) Olfactory dysfunction and potential mechanisms caused by volatile organophosphate dichlorvos in the silkworm as a model animal. J Hazard Mater 425:127940. https://www.ncbi.nlm.nih.gov/pubmed/34896704 [DOI] [PubMed] [Google Scholar]
- 114.Saorin G, Caligiuri I, Rizzolio F (2023) Microfluidic organoids-on-a-chip: the future of human models. Semin Cell Dev Biol 144:41–54. https://www.ncbi.nlm.nih.gov/pubmed/36241560 [DOI] [PubMed] [Google Scholar]
- 115.Liu M, Jiang N, Qin C et al (2024) Multimodal spatiotemporal monitoring of basal stem cell-derived organoids reveals progression of olfactory dysfunction in Alzheimer’s disease. Biosens Bioelectron 246:115832. https://www.ncbi.nlm.nih.gov/pubmed/38016198 [DOI] [PubMed]
- 116.Otter CJ, Fausto A, Tan LH et al (2023) Infection of primary nasal epithelial cells differentiates among lethal and seasonal human coronaviruses. Proc Natl Acad Sci U S A 120(15):e2218083120. https://www.ncbi.nlm.nih.gov/pubmed/37023127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phan N, Hong JJ, Tofig B et al (2019) A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun Biol 2:78. https://www.ncbi.nlm.nih.gov/pubmed/30820473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Watts AM, West NP, Smith PK et al (2022) Adult allergic rhinitis sufferers have unique nasal mucosal and peripheral blood immune gene expression profiles: a case-control study. Immun Inflamm Dis 10(1):78–92. https://www.ncbi.nlm.nih.gov/pubmed/34637606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou S, Huang H, Chen Q et al (2020) Long-term defects of nasal epithelium barrier functions in patients with nasopharyngeal carcinoma post chemo-radiotherapy. Radiother Oncol 148:116–125. https://www.ncbi.nlm.nih.gov/pubmed/32353641 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.