Highlights
-
•
CircPTPN11 is time- and dose-dependent upon CVB5 infection.
-
•
CircPTPN11 is specific to intestinal tissue.
-
•
CircPTPN11 inhibits CVB5 replication by activating IRF3 in the IFN-I pathway.
-
•
CircPTPN11 can target SIRPA through sponge adsorption of miR-152-3p to exert its role.
Keywords: Coxsackieviruses group B5 (CVB5); Circular RNAs (circRNAs); IFN-I pathway; circRNA-miRNA-mRNA network; Hand, foot and mouth disease (HFMD)
Abstract
Coxsackievirus B5 (CVB5) is a major pathogen responsible for hand-foot-mouth disease, herpangina, and even severe death. The mechanisms underlying CVB5-induced diseases are not fully elucidated, and no specific antiviral treatments are currently available. Circular RNAs (circRNAs), a closed-loop molecular structure, have been reported to be involved in virus infectious diseases. However, their roles and mechanisms in CVB5 infection remain largely unknown. In this study, we identify that CircPTPN11 is significantly upregulated following CVB5 infection in RD cells. Characteristic analysis reveals that the expression of CircPTPN11 is both time- and dose-dependent upon CVB5 infection and is specific to intestinal tissue. Moreover, CircPTPN11 inhibits CVB5 replication by activating IRF3 in the type-I interferon (IFN-I) pathway. Further underneath mechanism shows that CircPTPN11 indirectly regulates CVB5 replication by sponging miR-152-3p, and miR-152-3p influences CVB5 replication by interacting with the gene coding for signal regulatory protein alpha (SIRPA). In conclusion, this study suggests that CircPTPN11 targets SIRPA by sponging miR-152-3p, thereby inhibiting the replication and proliferation of CVB5. These findings provide a molecular target for the diagnosis and treatment of CVB5 infection.
1. Introduction
Coxsackievirus B5 (CVB5) was first isolated in 1952 from the Faulkner strain and is mainly transmitted via the fecal-oral route, particularly among children under 5 years of age (He et al., 2022). Symptoms of CVB5 infection include hand, foot, and mouth disease (HFMD), aseptic meningitis, viral encephalitis, and acute flaccid paralysis, with severe cases to death (Liu et al., 2023). There have been global epidemics of CVB5 infection, with particularly severe impacts in Asian, posing a significant threat to the health of infants (Alhazmi et al., 2023; Huang et al., 2023; Mone et al., 2023). Currently, no effective treatments or vaccines are available. CVB5 belongs to the Enterovirus genus of the Picornaviridae family, with a genome approximately 7400 bp, consisting of single-stranded RNA of positive sense. The viral RNA encodes a polyprotein precursor of 2200 amino acids, which is cleaved into three segments: P1, P2, and P3. The P1 precursor encodes four structural proteins (VP1, VP2, VP3, and VP4) that form the viral nucleocapsid, while the P2 and P3 gene regions encode seven nonstructural proteins (NSPs) that possess various enzymatic activities (Shen, 2018; Tan et al., 2023).
Circular RNAs (circRNAs) are a novel and highly conserved class of non-coding RNAs (ncRNAs) in mammals. They are derived from pre-mRNAs with a length varied from fewer than 100 to 1000 nucleotides (nt). Unlike linear RNAs, circRNAs lack both a 5′cap structure and a 3′-poly(A) tail, forming a closed-loop structure (Chen, 2020; Lun et al., 2023). Most circRNAs are composed of exons and are located in the cytoplasm which is involved in a variety of biological processes, including cancer, inflammation, fibrosis, and viral infection (He and Zhu, 2023; Ju et al., 2023). CircRNAs as “sponge” for microRNA (miRNA), binding to them and thereby relieving the suppression of its target mRNAs, ultimately influencing gene expression. Additionally, circRNAs can enhance or disrupt protein interactions, acting as either facilitator or competitor. Furthermore, circRNAs have the capability for cap-independent translation (Chen et al., 2022b; Dong et al., 2023; Misir et al., 2022).
A growing body of research suggests that circRNAs play important regulators in viral replication and may serve as candidate biomarkers and targets for antiviral treatments. For example, Zhang et al. found that the increased levels of the circ_0004812 facilitated the replication of Hepatitis B virus (HBV) by sponging miR-1287-5p, which promoted the expression of follistatin-related protein (FSTL), ultimately inhibiting the production of IFN-α and IFN-β (Zhang and Wang, 2020). Similarly, Yang et al. observed that circSIAE was significantly decreased in Hela cells after Coxsackievirus B3 (CVB3) infection. CircSIAE targets amino-acid kinase 2 (TAOK2) by sponging miR-331-3p, activating the nuclear factor κB (NF-κB) and inhibiting the replication and proliferation of CVB3 (Yang et al., 2021). These findings highlight the complex biological functions of circRNAs in regulating viral mechanisms. However, the role of circRNAs in CVB5 replication remains unknown.
In our previous work, we analyzed circRNAs expression profiles of RD cells infected with CVB5 (Li et al., 2022) and selected several circRNAs to further illustrate their roles and mechanisms following CVB5 infection. In this research, we identified a novel human exon circRNA, named CircPTPN11, which is derived from the protein tyrosine phosphatase non-receptor type 11 (PTPN11) gene. The levels of CircPTPN11 significantly increase after CVB5 infection in RD cells and are highly inducible in intestinal tissue. We also found CircPTPN11 inhibits CVB5 replication by activating IRF3 in the type-I interferon (IFN-I) pathway. Additionally, CircPTPN11 indirectly regulates CVB5 replication by sponging miR-152-3p, which in turn influences CVB5 replication through interaction with the gene coding for signal regulatory protein alpha (SIRPA). In all, CircPTPN11 acts as a positive regulator of host antiviral immunity by targeting SIRPA through miR-152-3p adsorption, thereby inhibiting the replication and proliferation of CVB5. These findings provide a potential molecular target for the diagnosis and treatment of CVB5 infection.
2. Materials and methods
2.1. Cell culture and viral infection
Human Rhabdomyosarcoma cells (RD), human embryonic kidney 293T (HEK 293T), monkey African green kidney cells (Vero), human neuroblastoma cells (SH-SY5Y), normal human colon tissue cells (CCD 841), normal human epithelial cells (HIEC-6), Hep G2 cells and human peripheral blood mononuclear cells (THP-1) were cultured in Dulbecco's modified Eagle medium (DMEM) (Servicebio, China) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (NEWZERUM, New Zealand). All the cells were incubated at 37 °C with 5 % CO2 incubator.
The CVB5 strain (GenBank: MH201081.1), isolated from HFMD patients in Kunming, Yunnan Province, was cultured in RD cells. The virus titer was measured by the 50 % Tissue Culture Infectious Dose (TCID50) according to the Reed-Muench formula (Svensson et al., 1999).
2.2. Plasmid construction and transfection
The CircPTPN11 and SIRPA gene were cloned into the pcDNA3.1(+) vector, and siCircPTPN11 (siCircPTPN11-1: GAATTAAGAAAAGCAAGAG; siCircPTPN11-2: TAAGAAAAGCAAGAGGAAA), miR-152-3p mimic (UCAGUGCAUGACAGAACUUGG), and inhibitor (AGUCACGUACUGUCUUGAACC) were synthesized (Ribobio, China). All the plasmids were transfected into RD cells using Lipofectamine 3000 Reagent (Invitrogen, USA) according to the instructions.
2.3. Real-time quantitative PCR (qPCR)
Total RNA was extracted from the cells using RNAiso plus reagent (TaKaRa, Japan). The RNA was quantified following the instructions of the One-Step TB Green Prime Script RT-PCR Kit (TaKaRa, Japan) on a 7500 real-time PCR system. The cycling conditions: 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, extension and annealing at 60 °C for 34s. The relative RNA levels were calculated using the 2−ΔΔCt method. All the primers are listed in Table S1.
2.4. Cytoplasmic nucleus separation
The PARIS Kit (Invitrogen, USA) was used to separate cytoplasmic and nuclear components. Briefly, RD Cells were harvested, washed, and lysed. The cells were then suspended in ice-cold Cell Disruption Buffer for 10min. After centrifugation, the supernatant was collected as the cytoplasmic fraction, and the precipitation was retained as the nuclear fraction. RNA was extracted using a 2 × lysis/binding solution and analyzed by reverse transcription (RT)-qPCR. GAPDH or U6 were used as the control genes.
2.5. Western blotting
Cells were collected and lysed in radioimmunoprecipitation assay (RIPA) buffer (Solarbio, China) containing 1 % protease inhibitor and 1 % phosphatase inhibitor (Absin, China). Protein concentration was calculated using a bicinchoninic acid (BCA) protein assay kit. Proteins were separated on 8–12 % SDS-PAGE gel and transferred onto methanol-activated polyvinylidene difluoride filter (PVDF) membranes. The membranes were blocked with 5 % skimmed milk and incubated with primary and secondary antibodies. The bands were visualized using an enhanced chemiluminescence (ECL) detection system and scanned with Image J software, the ratios of the target protein to the internal reference protein were calculated. The antibodies included IFN Signaling Pathway Antibody Sampler Kit (CST, USA), SIRPA Antibody (CUSABIO, China) GAPDH Antibody (ABclonal, USA) and α-Tubulin Antibody (ABclonal, USA).
2.6. Dual-luciferase reporter assay
CircPTPN11 wild-type, CircPTPN11 with a 160–167 bp mutation, SIRPA and SIRPA with 657–663 bp mutation were cloned into the multicloning region of the pmirGLO vector using Cloning kits (TaKaRa, Japan) respectively, the constructs named CircPTPN11-WT, CircPTPN11-Mut, SIRPA-WT, and SIRPA-Mut. These wild-type or mutated plasmids were transfected with miR-152-3p-mimic or miR-NC into HEK 293T cells. Luciferase activity was measured using a dual luciferase reporter assay system (Vazyme, China) after 24 h. The effect of gene transcriptional regulation was analyzed by measuring the ratio of Firefly to Revilla fluorescence.
2.7. RNase R treatment
Total RNA was extracted from cells and treated with RNase R (Beyotime, China) by incubating at 37 °C for 20 min, then deactivated at 70 °C for 10min. GAPDH or U6 served as the endogenous control and the relative circRNA levels were calculated using the 2-ΔΔCt method.
2.8. Immunofluorescence assay
The cells were washed and fixed with 4 % paraformaldehyde at room temperature. The cells were permeabilized with 0.2 % Triton X-100 and then blocked with 5 % BSA at room temperature. The cells were incubated with primary antibodies overnight at 4 °C, followed by incubation with the secondary antibodies A23410 and A23220 (Scientific, China) for 1 h. The nuclei were stained with Diisopropanolamine (DAPI) for 2min. Finally, the staining was observed with the Nikon confocal microscope.
2.9. Enzyme-linked immunosorbent assay (ELISA)
Cells were transfected with plasmids and infected with CVB5. After 24 h post-infection, the supernatant was collected. IFN-I production was measured by the ELISA kit (MEIMIAN, China) according to the manufacturer's instructions.
2.10. Statistical analysis
All experiments were carried out three times independently, and the data were expressed as mean ± SD. The student's t-test was carried out for statistical analysis using Prism software (GraphPad Prism9). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. CircPTPN11 is highly expressed in CVB5-infected RD cells
To investigate the role of CircPTPN11 after CVB5 infected RD cells, we performed RNA sequencing (RNA-seq) to analyze circRNA transcriptomes and identified 10 circRNAs that were highly differentially expressed according to log2-fold changes in IFN pathway. The results confirmed that the level of CircPTPN11 was consistent with the RNA-seq results, identifying it as a candidate circRNA by RT-qPCR (Fig. 1A-B). According to the Circbank database, CircPTPN11 is formed by reverse splicing of the linear transcript from exons 14–16 of the protein tyrosine phosphatase non-receptor type 11 (PTPN11) gene with a length of 231 nucleotides (Fig. 1C). Sanger sequencing confirmed head-to-tail splicing, revealing that the junction site of the CircPTPN11 is located between 1777 nt (exon 13) and 2589 nt (exon 15) of the PTPN11 gene (Fig. 1D). To further characterize CircPTPN11, the stabilities of CircPTPN11 was confirmed using RNase R treatment and divergent primer PCR, validating the ring formation of CircPTPN11 (Fig. 1E-F). Finally, we examined the subcellular localization of CircPTPN11. It is mainly distributed in the cytoplasm, regardless of CVB5 infection, accounting 75 % for of the total CircPTPN11 (Fig. 1G).
Fig. 1.
Characterization of CircPTPN11 in RD cells. (A) The levels of 10 candidate circRNAs identified from the RNA-seq analysis. (B) RT-qPCR was performed to confirm the levels of 10 candidate circRNAs according to RNA-seq results. (C) The genomic location and junction of CircPTPN11. (D) Sanger sequencing confirmation of the head-to-tail splicing of CircPTPN11. (E) RT-PCR confirmation of the head-to-tail splicing of CircPTPN11. (F) The level of CircPTPN11 with and without RNase R treatment. (G) The sub-cellular distribution of CircPTPN11 in the RD cells with or without CVB5 infection. Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, ***P < 0.001 vs. the control group.
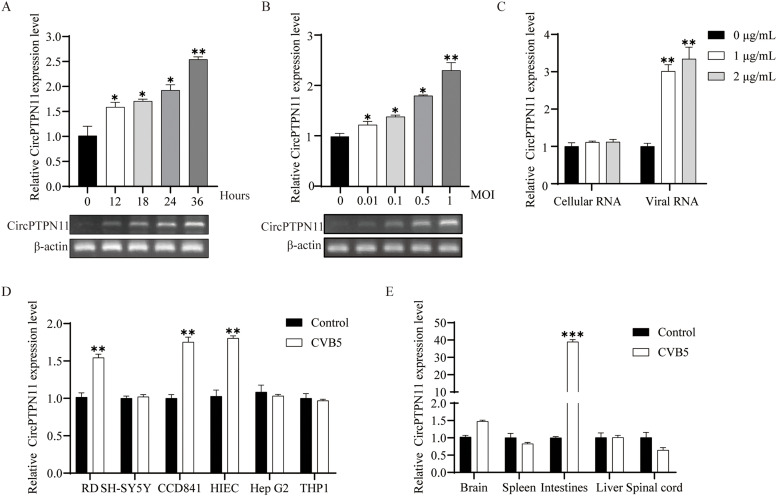
Next, we found CircPTPN11 exhibits highly inducible expression in RD cells in a dose- and time-dependent during viral infection (Fig. 2A-B). RNA was extracted from CVB5 virions (Viral RNA) and transfected into RD cells. Meanwhile, untransfected RD cells (Cellular RNA) were used as control to detect the level of circRNA. The level of CircPTPN11 was significantly induced by viral RNA, consistent with the effects of poly (I: C) treatment (Fig. 2C and Figure S1). All the results suggest that an increment of CircPTPN11 is associated with viral RNA accumulation during CVB5 replication. To further verify the specificity of CircPTPN11 expression, various cell lines suitable for CVB5 infection infected with it. The results showed that CircPTPN11 was highly inducible in CCD 841, HIEC-6 cells, and RD cells (Fig. 2D). Also we detected the levels of CircPTPN11 in different tissues in vivo. The results showed that CVB5 effectively infected the tissues (Figure S2) and a high level of CircPTPN11 was detected in the intestinal tissues (Fig. 2E), indicating that CircPTPN11 maybe specific to intestinal tissue. Additional results revealed that CircPTPN11 level is not affected by IFN-β, IFN-γ, LPS, and TNF-α (Figure S3). All results suggest that CircPTPN11 is involved in CVB5 infection and warrants further paid attention.
Fig. 2.
The levels of CircPTPN11 are induced by viral infection. (A) RD cells were infected with CVB5 (MOI=1) for the indicated times. RT-qPCR and gel-electrophoresis were performed to determine the levels of CircPTPN11. (B) RD cells were infected with CVB5 at varying MOIs for 24 h. RT-qPCR and gel-electrophoresis were performed to determine the levels of CircPTPN11. (C) RD cells were transfected with different amounts of cellular or viral RNAand harvested at 24HPI. The levels of CircPTPN11 were analyzed by qRT-PCR. (D) Various cell lines were infected with CVB5 (MOI=1) for 24 h. Cells were harvested and the levels of CircPTPN11 were analyzed by qRT-PCR. (E) Three-day-old BALB/c mice were intraperitoneally injected with CVB5, and tissue samples were collected 10 days later. RT-qPCR was performed to determine the levels of CircPTPN11 in different tissues. Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group.
3.2. CircPTPN11 inhibits CVB5 replication
To evaluate the role of CircPTPN11 in CVB5 replication, we successfully transfected RD cells with constructs designed to either overexpress or knockdown of CircPTPN11 (Figure S4-S5). RD cells were transfected with pcDNA3.1-CircPTPN11 plasmid and subsequently infected with CVB5. Cells and supernatants were harvested at 24 h post-infection (HPI). The results showed that the levels of CVB5 VP1 mRNA and protein (Fig. 3A-B), as well as CVB5 loads, were both decreased (Fig. 3C). Subsequently, RD cells were transfected with siCircPTPN11-1 to knockdown its expression and further confirm the function of CircPTPN11. The results demonstrate that knockdown of CircPTPN11 led to an increase in the levels of CVB5 VP1 mRNA and protein (Fig. 3D-E), as well as CVB5 loads (Fig. 3F). Together, all results suggest that CircPTPN11 inhibits the replication and proliferation of CVB5.
Fig. 3.
CircPTPN11 inhibits CVB5 replication. (A-C) RD cells were transfected with either pcDNA3.1-CircPTPN11 or pcDNA3.1 (an empty vector), and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells and supernatants were harvested at 24HPI, and the levels of CVB5 VP1 were analyzed by qRT-PCR (A), and Western blotting (B). CVB5 titers were analyzed by TCID50 assay (C). (D-F) RD cells were transfected with either siCircPTPN11 or siNC (an empty vector), and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells and supernatants were harvested at 24HPI, and the levels of CVB5 VP1 were analyzed by qRT-PCR (D), and Western blotting (E). The CVB5 titers were analyzed by TCID50 assay (F). Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group.
IFN-Ⅰ is critical as the first line of host defense against viruses, therefore we examined the production of IFN-I and the expression of effector genes. The results confirmed that overexpression of CircPTPN11 significantly increased the production of IFN-β and IFN-α2 (Fig. 4A), as well as the mRNA expression levels of IFN-β, IFN-α2 and interferon-stimulated genes (ISGs) (Fig. 4B), indicating that CircPTPN11 acts as a positive regulator of IFN-I during CVB5 infection. Next, we explored the effects of CircPTPN11 on key proteins involved in regulating the IFN-I pathway. Western blotting analysis showed that overexpression of CircPTPN11 activated IRF3 and promoted phosphorylation of IRF3 (p-IRF3) (Fig. 4C), subsequently leading to p-IRF3 dimerization (Fig. 4D) and nuclear translocation (Fig. 4E-F). Together, these results suggest that CircPTPN11 enhances host antiviral immunity by triggering the IFN-I signaling pathway via IRF3 activation.
Fig. 4.
CircPTPN11 activates promotes IFN-I via IRF3 to promote the expression of ISGs. RD cells were transfected with either pcDNA3.1-CircPTPN11 or pcDNA3.1 (an empty vector), and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells and supernatants were harvested at 24 HPI. (A) The secretion of IFN-α2 and IFN-β were determined by ELISA. (B) The expression of vital ISGs and IFN-I mRNA were determined by RT-qPCR. (C) The expression of MDA5, RIG-I, IKKε/p-IKKε, TBK1/p-TBK1 and IRF3/p-IRF3 were determined by Western blotting. (D) The expression of (p-IRF3)2 was determined by Western blotting. (E) The expression of IRF3 and p-IRF3 in nuclear and cytoplasmic fractions were determined by Western blotting. (F) The expression of p-IRF3 in nuclear and cytoplasmic fractions were visualized by Immunofluorescence assay. Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. the control group.
3.3. miR-152-3p acts as a sponge for CircPTPN11 and promotes CVB5 replication
CircRNAs have been shown to act as miRNA sponges to regulate gene expression, therefore, the potential miRNAs associated with CircPTPN11 were investigated. Using the CircBank, CSCD and Circinteractome databases, seven putative miRNAs (miR-3974, miR-152-3p, miR-6881, miR-4288, miR-3124, miR-3666, and miR-4295-3p) were found to overlap across these databases as possible targets for CircPTPN11. To further verify the downstream miRNAs targets of CircPTPN11, total RNA was extracted following overexpression or knockdown of CircPTPN11 in RD cells. RT-qPCR analysis revealed that overexpression of CircPTPN11 changed the levels of miR-152-3p, miR-6881, miR-4288 and miR-4295-3p (Fig. 5A), while knockdown of CircPTPN11 only increased miR-152-3p level (Fig. 5B). Therefore, we identified miR-152-3p as a potential downstream target of CircPTPN11. Also, the level of miR-152-3p decreased following CVB5 infection in RD cells (Figure S6). The highly conserved binding site between miR-152-3p and CircPTPN11 is shown in Fig. 5C. To validate our prediction, HEK 293T cells were co-transfected with miR-152-3p, pmirGLO-CircPTPN11-WT or pmirGLO-CircPTPN11-Mut, which resulted in significantly lower luciferase activity in the CircPTPN11-WT group compared to the CircPTPN11-Mut group (Fig. 5D), confirming the physical binding of miR-152-3p to CircPTPN11. Then we successfully induced either overexpression or knockdown of miR-152-3p (Figure S7-S8). subsequently, RD cells were transfected with either a miR-152-3p mimic or inhibitor, RT-qPCR analysis showed that knockdown of miR-152-3p increased the level of CircPTPN11, while overexpression of miR-152-3p reduced the level of CircPTPN11 (Fig. 5E). Further investigation into the effect of miR-152-3p on CVB5 replication revealed that miR-152-3p inhibitors decreased the expression of CVB5 VP1, while increasing the expression of IRF3/p-IRF3, ISGs, and IFN-β (Fig. 5F-G). Conversely, overexpression of miR-152-3p promoted the expression of CVB5 VP1, while reducing the expression of IRF3/p-IRF3, ISGs, and IFN-β (Fig. 5H-I). All results indicate that miR-152-3p is a downstream target of CircPTPN11 that can promote the replication of CVB5 via modulating the IRF3-mediated IFN-I pathway.
Fig. 5.
miR-152-3p is targeted by CircPTPN11 and promotes CVB5 replication. (A) RD cells were transfected with either pcDNA3.1-CircPTPN11 or pcDNA3.1 (an empty vector), and then the cells were harvested at 24 h post-transfection. The levels of miRNA were determined by RT-qPCR. (B) RD cells were transfected with either siCircPTPN11 or siNC (an empty vector), and then the cells were harvested at 24 h post-transfection. The levels of miRNA were determined by RT-qPCR. (C) The prediction of the binding site of miR-152-3p on CircPTPN11. The binding site sequences in CircPTPN11 were mutated, and the construct was named CircPTPN11-Mut. (D) HEK 293T cells were transfected with miR-152-3p, pmirGLO-CircPTPN11-WT or pmirGLO-CircPTPN11-Mut, and then the cells were harvested at 24 h post-transfection. The luciferase reporter assay was performed to measure luciferase activity. (E) RD cells were transfected with either miR-152-3p-inhibitor or miR-152-3p-mimic, and then the cells were harvested at 24 h post-transfection. The levels of CircPTPN11 were determined by RT-qPCR. (F-G) RD cells were transfected with miR-152-3p-inhibitor, and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells were harvested at 24 HPI. The levels of IRF3/p-IRF3 and VP1 were determined by Western blotting (F), the levels of vital ISGs, IFN-β and VP1 mRNA were determined by RT-qPCR (G). (H-I) RD cells were transfected with miR-152-3p-mimic, and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells were harvested at 24 HPI. The levels of IRF3/p-IRF3 and VP1 were determined by Western blotting (H), the levels of vital ISGs, IFN-β and VP1 mRNA were determined by RT-qPCR (I). Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, vs. the control group.
3.4. SIRPA is a downstream target gene of miR-152-3p and suppresses CVB5 replication
Three databases, including CSCD, ENCORI, and TargetScan, were used to predict downstream target genes of miR-152-3p, identifying the gene encoding SIRPA as the target (Fig. 6A). The relationship between SIRPA and miR-152-3p was assessed by Western blotting. The results showed that the expression of SIRPA was decreased upon transfection with miR-152-3p-mimic (Fig. 6B), while transfection with the miR-152-3p inhibitor increased SIRPA expression (Fig. 6C). Additionally, the expression of SIRPA was significantly decreased in RD cells following CVB5 infection (Figure S9). The predicted binding site of miR-152-3p on SIRPA is shown in Fig. 6D. To verify the prediction, HEK 293T cells were transfected with miR-152-3p, SIRPA-WT or SIRPA-Mut. The results showed significantly lower luciferase activity in the SIRPA-WT group compared to SIRPA-Mut group (Fig. 6E), confirming the physical binding of miR-152-3p to SIRPA. Furthermore, overexpression of the SIRPA in RD cells suppressed the expression of CVB5 VP1, while activating the expression of IRF3/p-IRF3, IFN-β, and ISGs (Fig. 6F-G). All results indicate that SIRPA is a downstream target of miR-152-3p and inhibits CVB5 replication via the IRF3-mediated IFN-I pathway.
Fig. 6.
SIRPA is a downstream target gene of miR-152-3p and suppresses CVB5 replication. (A) Downstream targets genes of miR-152-3p were identified by database screening. (B) RD cells were transfected with miR-152-3p-mimic, and then the cells were harvested at 24 h post-transfection. The expression of SIRPA was determined by Western blotting. (C) RD cells were transfected with miR-152-3p- inhibitor, and then the cells were harvested at 24 h post-transfection. The expression level of SIRPA was determined by Western blotting. (D) The prediction binding site of miR-152-3p on SIRPA. Their binding site sequences in SIRPA were mutated and the construct was named CircPTPN11-Mut. (E) HEK 293T cells were transfected with miR-152-3p, SIRPA-WT or SIRPA-Mut, and then the cells were harvested at 24 h post-transfection. The luciferase reporter gene assay was performed to measure luciferase activity. (F-G) RD cells were transfected with either pcDNA3.1-SIRPA or pcDNA3.1 (an empty vector), and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells were harvested at 24 HPI. The expression of IRF3/p-IRF3 and VP1 were determined by Western blotting (F), the levels of vital ISGs, IFN-β and VP1 mRNA are determined by RT-qPCR (G). Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, vs. the control group.
3.5. Inhibition of CVB5-activate IFN by the CircPTPN11/miR-152-3p/SIRPA axis
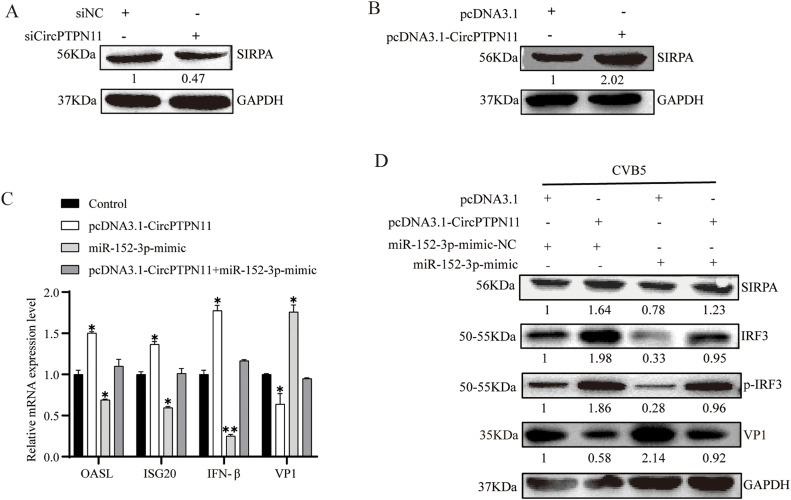
First, we examined whether CircPTPN11 affects SIRPA expression. The expression of SIRPA decreased upon transfection with siCircPTPN11 (Fig. 7A), while transfection with pcDNA3.1-CircPTPN11 increased SIRPA expression (Fig. 7B). These results confirm that circPTPN11 regulates the expression of SIRPA. Next, to assess the impact of these molecules on IFN-I regulation and CVB5 replication, RD cells were co-transfected with CircPTPN11 or/and miR-152-3p-mimic, followed by CVB5 infection. The results showed that co-transfection with CircPTPN11 and miR-152-3p-mimic restored the expression of OASL, ISG20, and IFN-β mRNA, as well as the expression of SIRPA, IRF3/p-IRF3 and VP1 protein compared to controls (Fig. 7C-D). These results indicate that overexpression of CircPTPN11 inhibits the expression of SIRPA by targeting miR-152-3p, thereby inhibiting CVB5 replication via IRF3-mediated IFN-I pathway. Conversely, knockdown of CircPTPN11 promoted the expression of SIRPA by targeting miR-152-3p, activating CVB5 replication via IRF3-mediated IFN-I pathway. Together, CircPTPN11 inhibits the replication of CVB5 and modulates the IFN-Ⅰ pathway by targeting miR-152-3p/SIRPA axis.
Fig. 7.
Inhibition of CVB5-Induced ISGs by the CircPTPN11/miR-152-3p/SIRPA Axis. (A) RD cells were transfected with either siCirPTPN11 or siNC (an empty vector), and then the cells were harvested at 24 h post-transfection. The expression of SIRPA were determined by Western blotting. (B) RD cells were transfected with either pcDNA3.1-CirPTPN11 or pcDNA3.1 (an empty vector), and then the cells were harvested at 24 h post-transfection. the expression of SIRPA were determined by Western blotting. (C-D) RD cells were transfected with pcDNA3.1-CircPTPN11 and/or miR-152-3p-mimic, and then the cells infected with CVB5 (MOI=1) at 24 h post-transfection. Cells were harvested at 24 HPI. The levels of OASL, ISG20, IFN-β and VP1 mRNA were determined by RT-qPCR (C), the expression of SIRPA, IRF3/p-IRF3 and VP1 were determined by Western blotting (D). Data are representative of three independent experiments with similar results. Data are representative of three independent experiments and are plotted as the mean ± S.D. *P < 0.05, **P < 0.01 vs. the control group.
4. Discussion
HFMD typically starts with a feeling of unwellness and a low-grade fever in children under 5 years old, followed by the appearance of flat, red spots on the hands and the feet, as well as painful lesions in the throat. In most cases, HFMD is self-limiting and children recover within 7 to 10 days. However, growing evidence suggests a potential risk for more serious complications, including permanent paralysis, and central neurological complications, resulting in death. Epidemiological investigations have demonstrated that enterovirus 71 (EV71), CVA (CVA16 and CVA6), and CVB (CVB2 and CVB5) account for the majority of HFMD cases. In recent years, CVB5 has emerged as a common pathogen for HFMD (Saguil et al., 2019; Zhang et al., 2022). Unfortunately, no specific antiviral drugs are available and current vaccines do not provide cross-protection.
Research into the regulation of viruses has traditionally focused on the involvement of protein-coding genes, however, in recent years, scientists have discovered that an entire class of molecules, termed non-coding RNAs (ncRNAs), plays crucial regulatory roles in both virus and host. An explosion of studies on ncRNA biology has since shown that they are a diverse and prevalent group of RNAs, and they could potentially serve as new biomarkers or therapies for HFMD (Chen et al., 2022a). Accumulating evidence demonstrates that miRNA, long non-coding RNA (lncRNA) and circRNA are three important types of ncRNAs that play vital regulatory roles during viral infection. To date, multiple studies have shown that miRNAs are closely linked to enterovirus infection as they participate in immune response, signaling pathways and cell apoptosis (Guo et al., 2023; Wang et al., 2024). In our previous study, we revealed the expression profile of lncRNAs in CVB5-infected cells and demonstrated that LINC1392 could regulate the melanoma differentiation-associated gene 5 (MDA5) by interacting with ELAV Like RNA Binding Protein 1 (ELAVL1) to inhibit CVB5 infection (Li et al., 2023). However, fewer studies have explored how circRNA regulates enterovirus replication. In our research, we focused on advances in understanding the modulatory role of circRNAs during CVB5 infection. We chose 10 circRNAs that are involved in the IFN pathway, due to limitations of the types and quantities of circRNAs, the RNA-sequencing were not highly consistent with RT-PCR results. We identified CircPTPN11 based on consistent results from both RNA sequencing and RT-PCR analyses. The level of CircPTPN11 was increased in RD cells infected with CVB5. Also, we found CircPTPN11 inhibited CVB5 replication and proliferation by activating IRF3 in the IFN-I pathway.
The mechanism of circRNAs in human cancers have been elucidated first. Certain circRNAs are involved in either promoting or suppressing cancer by acting as post-transcriptional regulators through binding with miRNAs or RNA-binding proteins (RBPs) (Kohansal et al., 2024; Zhou et al., 2020). One of the most prominent roles of circRNAs is their ability to act as miRNA sponges, directly binding to corresponding miRNAs to inhibit their activity and regulate the expression of target genes (Dong et al., 2023; Misir et al., 2022). With these functions have become more established, circRNAs have also contributed to the evolution of our knowledge of virus-host interactions. We hypothesized that CircPTPN11 might inhibit the replication of CVB5 by acting as a miRNA sponge. According to CircBank, CSCD and Circinteractome databases, miR-152-3p was identified as a target miRNA of CircPTPN11, and a dual luciferase assay supported the binding sites between them. Although the function of miR-152-3p has been well studied in cancer and recognized as an onco-suppressor, its role in viruses remains relatively unexplored (Liu et al., 2022; Moya et al., 2019; Ramalho-Carvalho et al., 2018). MiRNAs are highly stable in both fresh serum and plasma, and miR-152-3p has shown potential as biomarkers for Dengue and human papilloma virus (HPV) 16 infection, but lack of research on its role in viral diseases (Nilsen et al., 2019; Ouyang et al., 2016). In our study, we found miR-152-3p plays a positive role in CVB5 replication via the IRF3-mediated IFN-I pathway. Further functional studies identified that SIRPA as a downstream target gene of miR-152-3p. SIRPA is a well-known inhibitor of phagocytosis by interacting with CD47, blocking the activation of macrophages and dendritic cells (Cham et al., 2020). Notably, SIRPA has also been shown to inhibit viral entry and reduce infection by various viruses through endocytic pathways (Sarute et al., 2021). Here we demonstrate that SIRPA activates the IRF3-mediated IFN-Ⅰ pathway to inhibit CVB5 replication.
In summary, our research shows that CircPTPN11 acts as a sponge for miR-152-3p, inhibiting the replication of CVB5 by up-regulating SIRPA via the IRF3-mediated IFN-I pathway (Fig. 8). These findings enrich our understanding of the functional roles of circRNA in enterovirus replication and provide novel insights for developing therapeutic strategies against CVB5 infection.
Fig. 8.
A proposed model for CircPTPN11 inhibition CVB5 replication. CircPTPN11 acts as a sponge for miR-152-3p, inhibiting the replication of CVB5 by up-regulating SIRPA via IRF3-mediated IFN-I pathway.
CRediT authorship contribution statement
Jingru Gao: Writing – original draft, Software, Methodology, Data curation. Fan Yang: Software, Funding acquisition, Data curation. Jihong Zhang: Investigation. Heng Yang: Writing – review & editing, Supervision. Wei Chen: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgments
Acknowledgements
We thank Timothy Mahony for his expert advice and linguistic assistance on the manuscript.
Funding
National Natural Science Foundation of China (No. 82360388), Young Talents Support Program of Yunnan Province (Ten Thousand People Plan, YNWR-QNBJ-2019-178), Kunming University of Science and Technology & the First People's Hospital of Yunnan Province Joint Special Project on Medical Research (No. KUST-KH2022002Y), Project of Yunnan Provincial Key Laboratory of Clinical Virology (No. 202205AG070053-02).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2024.199508.
Contributor Information
Heng Yang, Email: yangheng2008.cool@163.com.
Wei Chen, Email: wchen@kust.edu.cn.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Alhazmi A., Nekoua M.P., Mercier A., Vergez I., Sane F., Alidjinou E.K., Hober D. Combating coxsackievirus B infections. Rev. Med. Virol. 2023;33(1):e2406. doi: 10.1002/rmv.2406. [DOI] [PubMed] [Google Scholar]
- Cham L.B., Adomati T., Li F., Ali M., Lang K.S. CD47 as a potential target to therapy for infectious diseases. Antibodies. 2020;9(3) doi: 10.3390/antib9030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020;21(8):475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- Chen W., Li J., Li J., Zhang J., Zhang J. Roles of non-coding RNAs in virus-host interaction about pathogenesis of hand-foot-mouth disease. Curr. Microbiol. 2022;79(9):247. doi: 10.1007/s00284-022-02928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang J., Wang C., Liu M., Zou Q. Deep learning models for disease-associated circRNA prediction: a review. Brief. Bioinform. 2022;23(6) doi: 10.1093/bib/bbac364. [DOI] [PubMed] [Google Scholar]
- Dong J., Zeng Z., Huang Y., Chen C., Cheng Z., Zhu Q. Challenges and opportunities for circRNA identification and delivery. Crit. Rev. Biochem. Mol. Biol. 2023;58(1):19–35. doi: 10.1080/10409238.2023.2185764. [DOI] [PubMed] [Google Scholar]
- Guo H., Zhu Y., Zou Y., Li C., Wang Y., De G., Lu L. Enterovirus 71 induces pyroptosis of human neuroblastoma SH-SY5Y cells through miR-146a/CXCR4 axis. Heliyon. 2023;9(4):e15014. doi: 10.1016/j.heliyon.2023.e15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wei H., Wei L., Fan H., Yan D., Zhao H., Zhu S., Ji T., Xiao J., Lu H., Wang W., Guo Q., Yang Q., Xing W., Zhang Y. Molecular epidemiology reveals the co-circulation of two genotypes of Coxsackievirus B5 in China. Viruses. 2022;14(12) doi: 10.3390/v14122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Zhu Q. Circular RNAs: emerging roles and new insights in human cancers. Biomed. Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115217. [DOI] [PubMed] [Google Scholar]
- Huang S., Zhang Y., Zhang W., Chen M., Li C., Guo X., Zhu S., Zeng H., Fang L., Ke B., Li H., Yoshida H., Xu W., Deng X., Zheng H. Prevalence of non-polio enteroviruses in the sewage of Guangzhou City, China, from 2013 to 2021. Microbiol. Spectr. 2023;11(3) doi: 10.1128/spectrum.03632-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M., Kim D., Son G., Han J. Circular RNAs in and out of cells: therapeutic usages of circular RNAs. Mol. Cells. 2023;46(1):33–40. doi: 10.14348/molcells.2023.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohansal M., Alghanimi Y.K., Banoon S.R., Ghasemian A., Afkhami H., Daraei A., Wang Z., Nekouian N., Xie J., Deng X., Tang H. CircRNA-associated ceRNA regulatory networks as emerging mechanisms governing the development and biophysiopathology of epilepsy. CNS Neurosci. Ther. 2024;30(4):e14735. doi: 10.1111/cns.14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li J., Teng P., Yang F., Zhang J., Sun B., Chen W. Long noncoding RNA 1392 regulates MDA5 by interaction with ELAVL1 to inhibit coxsackievirus B5 infection. Virol. Sin. 2023;38(5):699–708. doi: 10.1016/j.virs.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Teng P., Yang F., Ou X., Zhang J., Chen W. Bioinformatics and screening of a circular RNA-microRNA-mRNA regulatory network induced by Coxsackievirus group B5 in human rhabdomyosarcoma cells. Int. J. Mol. Sci. 2022;23(9) doi: 10.3390/ijms23094628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li L., Bai J., Li L., Fan J., Fu Z., Liu J. Long noncoding RNA plasmacytoma variant translocation 1 promotes progression of colorectal cancer by sponging microRNA-152-3p and regulating E2F3/MAPK8 signaling. Cancer Sci. 2022;113(1):109–119. doi: 10.1111/cas.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen J., Zhang M., Guo W., Feng C., Liu J., Xu L., Gao N., Ma S. Coxsackievirus B: the important agent of hand, foot, and mouth disease. J. Med. Virol. 2023;95(3):e28669. doi: 10.1002/jmv.28669. [DOI] [PubMed] [Google Scholar]
- Lun J., Guo J., Yu M., Zhang H., Fang J. Circular RNAs in inflammatory bowel disease. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1307985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misir S., Wu N., Yang B.B. Specific expression and functions of circular RNAs. Cell Death. Differ. 2022;29(3):481–491. doi: 10.1038/s41418-022-00948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mone K., Lasrado N., Sur M., Reddy J. Vaccines against group B Coxsackieviruses and their importance. Vaccines. 2023;11(2) doi: 10.3390/vaccines11020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya L., Meijer J., Schubert S., Matin F., Batra J. Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289 expression as biomarker for prostate cancer diagnosis. Int. J. Mol. Sci. 2019;20(5) doi: 10.3390/ijms20051154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen A., Jonsson M., Aarnes E.K., Kristensen G.B., Lyng H. Reference microRNAs for RT-qPCR assays in cervical cancer patients and their application to studies of HPV16 and hypoxia biomarkers. Transl. Oncol. 2019;12(3):576–584. doi: 10.1016/j.tranon.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X., Jiang X., Gu D., Zhang Y., Kong S.K., Jiang C., Xie W. Dysregulated serum MiRNA profile and promising biomarkers in dengue-infected patients. Int. J. Med. Sci. 2016;13(3):195–205. doi: 10.7150/ijms.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Carvalho J., Gonçalves C.S., Graça I., Bidarra D., Pereira-Silva E., Salta S., Godinho M.I., Gomez A., Esteller M., Costa B.M., Henrique R., Jerónimo C. A multiplatform approach identifies miR-152-3p as a common epigenetically regulated onco-suppressor in prostate cancer targeting TMEM97. Clin. Epigenet. 2018;10:40. doi: 10.1186/s13148-018-0475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguil A., Kane S.F., Lauters R., Mercado M.G. Hand-foot-and-mouth disease: rapid evidence review. Am. Fam. Physician. 2019;100(7):408–414. [PubMed] [Google Scholar]
- Sarute N., Cheng H., Yan Z., Salas-Briceno K., Richner J., Rong L., Ross S.R. Signal-regulatory protein alpha is an anti-viral entry factor targeting viruses using endocytic pathways. PLoS Pathog. 2021;17(6) doi: 10.1371/journal.ppat.1009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. Recombination analysis of coxsackievirus B5 genogroup C. Arch. Virol. 2018;163(2):539–544. doi: 10.1007/s00705-017-3608-6. [DOI] [PubMed] [Google Scholar]
- Svensson L., Hjalmarsson A., Everitt E. TCID50 determination by an immuno dot blot assay as exemplified in a study of storage conditions of infectious pancreatic necrosis virus. J. Virol. Methods. 1999;80(1):17–24. doi: 10.1016/s0166-0934(99)00018-x. [DOI] [PubMed] [Google Scholar]
- Tan M., Suo J., Zhang Z., He W., Tan L., Jiang H., Li M., He J., Pan Y., Xu B., Yan L., Bin S., Gan Z., Sun Y., Jiang H., Sun Q., Zhang Z. Molecular characterization of coxsackievirus B5 from the sputum of pneumonia children patients of Kunming, Southwest China. Virol. J. 2023;20(1):74. doi: 10.1186/s12985-023-02019-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Z., Li H., Wang M., Qiu Y., Lu L. miR-29b-3p regulates cardiomyocytes pyroptosis in CVB3-induced myocarditis through targeting DNMT3A. Cell Mol. Biol. Lett. 2024;29(1):55. doi: 10.1186/s11658-024-00576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Li Y., Wang Y., Qiao X., Liu T., Wang H., Shen H. The circRNA circSIAE inhibits replication of coxsackie virus B3 by targeting miR-331-3p and thousand and one amino-acid kinase 2. Front. Cell Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.779919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang Z. Circular RNA hsa_circ_0004812 impairs IFN-induced immune response by sponging miR-1287-5p to regulate FSTL1 in chronic hepatitis B. Virol. J. 2020;17(1):40. doi: 10.1186/s12985-020-01314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang Y., Li H., Liu L. Hand-foot-and-mouth disease-associated enterovirus and the development of multivalent HFMD vaccines. Int. J. Mol. Sci. 2022;24(1) doi: 10.3390/ijms24010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.Y., Cai Z.R., Liu J., Wang D.S., Ju H.Q., Xu R.H. Circular RNA: metabolism, functions and interactions with proteins. Mol. Cancer. 2020;19(1):172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.