ABSTRACT
Objective:
This study aims to determine the prevalence of Occult Hepatitis B and C Infections among Egyptian injection drug users (IDUs) and identify key risk factors contributing to their occurrence within this high-risk group.
Methods:
In this cross-sectional study, 200 Egyptian IDUs were assessed. Participants were negative for Hepatitis B surface antigen and hepatitis C virus (HCV) RNA, with anti-HCV positive patients who achieved sustained virologic response after treatment included. Quantitative polymerase chain reaction (PCR) was used to detect HCV RNA in plasma and peripheral blood mononuclear cells, while HBV DNA was identified via nested PCR. Comparisons were made between Occult Hepatitis B infection (OBI) positive and OBI negative subgroups, as well as between other comprehensive income (OCI) positive and OCI negative subgroups. A significance level of 0.05 was set, with P-values below this indicating statistical significance. Statistical comparisons between OBI and OCI-positive and negative groups were performed using the Mann–Whitney U test and Chi-square test.
Results:
OBI was found in 32% of IDUs, while OCI was detected in 42% of IDUs, and was present in 53.6% of seropositive individuals. All OBI patients showed a significant increase in all liver function tests, while OCI patients had significant elevations in alanine transaminase and aspartate transaminase values. HIV coinfection was identified in 39.1% and 26.1% of OBI and OCI cases respectively. OBI and OCI coinfection were detected in 31 patients.
Conclusion:
Hidden infections such as OBI and OCI remain an overlooked public health issue in Egypt’s IDU population. These findings highlight the need for targeted strategies to address these reservoirs of infection and could inform similar approaches in countries with comparable HBV/HCV epidemiology.
Keywords: Injection drug users, occult Hepatitis B virus, occult Hepatitis C virus
Introduction
Most global fatalities associated with viral hepatitis are attributed to the Hepatitis B and C viruses. Both viruses were found to be the most common causes of liver disease worldwide.[1] The World Health Organization established a target in 2015 to eliminate viral hepatitis by the year 2030, with a particular focus on Hepatitis B virus (HBV) and Hepatitis C virus (HCV). This objective aims to guarantee equitable availability of diagnostic and treatment resources on a global scale.[2] The yearly death rate for HBV-related disorders is close to 0.9 million people. Furthermore, HCV affects around 71 million individuals worldwide, is one of the leading causes of chronic hepatitis, and kills 0.4 million people each year. Around 300 million individuals worldwide are infected with the Hepatitis B and C viruses.[1]
Patients residing in areas with high endemicity are at a heightened risk of infection. Injection drug users (IDUs) are common hosts for blood-borne pathogens.[3] Over the last few decades, there have been dedicated international efforts to end epidemics caused by these blood-borne viruses through immunization, antiviral treatment, prevention of mother-to-infant transmission, and effective screening of blood and blood products, but the risk of transmission persists, particularly in association with injection drug use that increases the risk of disease transmission.[4]
Occult HBV infection presents as low levels of HBV DNA in the blood or liver and negative Hepatitis B surface antigen (HBsAg).[5] Occult Hepatitis B infection (OBI) can be classified as seropositive or seronegative based on HBV exposure indicators in the serum. Seropositive OBI is identified by the presence of anti-hepatitis B core (anti-HBc) antibodies and/or anti-hepatitis B surface (anti-HBs) antibodies, while seronegative OBI is defined by the absence of these antibodies.[6] The best way to diagnose OBI is to look for replication-competent HBV DNA in the liver. However, effective and standardized tests for detecting HBV DNA in the liver are still lacking. Real-time polymerase chain reaction (PCR) tests and nested PCR procedures are advised for achieving high specificity and sensitivity.[7]
OBI prevalence is influenced by HBV endemicity, population characteristics, and behavior, while detection is influenced by serological and molecular diagnostic sensitivity. The global frequency of OBI is still mostly unknown and often overestimated.[5]
Occult Hepatitis C infection is characterized by no detectable HCV RNA in serum with the presence of HCV RNA in hepatocytes and peripheral blood mononuclear cells (PBMCs).[8] There are two distinct types of Occult hepatitis C infection (OCI), namely, seronegative OCI (characterized by the absence of anti-HCV antibodies and serum HCV-RNA) and seropositive OCI (characterized by the presence of anti-HCV antibodies but the absence of serum HCV-RNA).[9] The preferred diagnostic approach for OCI is liver biopsy, which is invasive and has a risk of bleeding. Alternatively, a PCR test in PBMCs can be used for diagnosis.[9]
OBI and OCI have been reported in several high-risk populations, including IDUs. IDUs can transmit blood-borne infections and thus may contribute to HBV and HCV transmission.[9-11] In 2013, Matos et al., reported that around 12.7% of injectable drug users investigated had OBI. Furthermore, Hedayati-Moghaddam et al. reported in 2019 that 18.18% of 77 Iranian human immunodeficiency virus (HIV)-positive IDUs had OCI, whereas another Iranian research revealed a 9.57% OCI incidence among 115 HBV- and HIV-negative IDUs.[11,12] The present state of OBI and OCI, particularly in high-risk groups such as IDUs, is poorly understood.[6,13]
The issue of occult infections in this population has been largely overlooked in Egypt, a country that was known to have the highest prevalence of chronic HCV globally. What makes this study particularly significant, and novel is that it follows the highly successful nationwide HCV treatment campaign, which significantly reduced the infection rate. Despite these efforts, this specific high-risk group of IDUs remains under examined. As a high-risk group, IDUs could be a potential reservoir for both occult HCV and HBV, posing a risk for recurrence or undetected transmission. Hence, this work was essential to better understand the remaining epidemiological risks and their potential public health impact. In addition, the findings from this research could serve as a model for other countries with similar HCV or HBV epidemiology, providing valuable insights and guiding future public health strategies in addressing hidden reservoirs of infection.
As a result, the purpose of this study was to determine the prevalence of OBI and OCI among Egyptian IDUs as an initial attempt to address the issue within this population and, consequently, implement measures to restrict the spread of these viruses.
Methods
OBI and OCI have been documented in various high-risk cohorts, particularly among individuals who inject drugs. Intravenous drug users have the potential to transmit blood-borne infections, thereby playing a role in the transmission of HBV and HCV.[9]
A cross-sectional study of 200 Egyptian IDUs was conducted between April 2023 and January 2024. The current investigation followed the ethical guidelines outlined in the Declaration of Helsinki. The study has been authorized by the Ethics Committee of the Faculty of Pharmacy (registration number 202210PHH1). Participants received an explanation of the study’s purpose and signed written consent form. Participants had the liberty to withdraw from the study at any time without giving an explanation. Confidentiality of data and privacy was assured for all participants. Study participants were recruited from the addiction treatment department of El-Azazi Hospital for mental Health, Zagazig, Egypt.
A comprehensive historical assessment was conducted, including demographic factors such as level of education, age, presence of tattoos, past surgeries, blood transfusion, marital status, imprisonment history, and participation in syringe-sharing activities.
Inclusion criteria
Patients negative for HCV RNA and HBV surface antigen. Anti-HCV antibody-positive patients who had received direct-acting antiviral drugs, as well as HIV-positive patients (as indicated by patient file), were included in the study. Although the precise causes of OBI and OCI are not entirely understood, several factors are thought to be involved. Causes of OBI may include, chronic liver disease,[14] viral mutations,[15] and low viral load.[16] In addition, OCI causes may include past HCV infection,[17] weak or suppressed immune system, low virus load, genotype variation, and coinfections.[18]
Exclusion criteria
Patients positive for either HBsAg or HCV antibody were excluded from the study except for those who had received therapy and achieved sustained virologic response. The presence of possible causes of elevated liver enzymes, such as HCC or any indicators of liver cirrhosis reported in participants’ files, excluded the participant from the research.
Blood collection
Each participant provided 7 mL of peripheral blood, collected randomly at the time of admission; this sample was divided into two parts: 2 mL were collected in plain tubes (VacuLab® Plain Tubes, do not contain any additives) for separating serum and measuring aspartate aminotransferase, alanine aminotransferase, direct bilirubin, total bilirubin, albumin, and total protein. The remaining 5 mL were collected in ethylenediaminetetraacetic acid (EDTA) tubes (VacuLab® EDTA tubes, coated with K2EDTA) to separate plasma and peripheral blood monocytes for detection of HCV RNA and HBV DNA.
Serum separation
After clotting for 30 min at room temperature, whole blood was centrifuged for 30 min at 4°C and 3000 ×g. To eliminate the remaining cells, the supernatant was collected into sterile tubes and centrifuged again at 4000 ×g for 10 min at 4°C. Sera were stored at −80°C until use.
Isolation of PBMCs
In a 15- mL Falcon tube, 5 mL of pre-diluted blood (diluted at a ratio of 1:1 in phosphate buffer saline, pH 7.2) were cautiously layered on top of an equal volume of the density gradient solution (Histopaque®1077, Sigma Aldrich, Schnelldorf, Germany). Tubes were centrifuged for 30 min at 400 ×g. The mononuclear cells were retrieved from the interphase layer and subjected to two rounds of washing using a solution of phosphate-buffered saline containing 2% heat-inactivated fetal calf serum solution.[19]
Plasma separation
Two milliliters of EDTA blood were centrifuged at 2000 ×g for 15 min to remove cells and platelets. The resulting supernatant (plasma) was divided into 0.5 mL aliquots and stored at −20°C until use.
RNA Extraction from Plasma and PBMCs
Total RNA was isolated from plasma and PBMCs using the GeneJET Viral DNA/RNA purification kit (Catalog No. K0821, ThermoFisher Scientific, Waltham, MA, USA) following manufacturer’s guidelines.[20]
Detection of HCV RNA in Plasma and PBMCs
The Bosphore® HCV Quantification kit V3 (Anatolia Gene works, Istanbul, Turkey) was used to identify the presence of HCV RNA in both plasma and PBMCs. The kit detects all known HCV genotypes.
PCR reactions were 20 μL (final volume) and consisted of: 12 μL of PCR Master Mix, 8μL of RNA (5–50 ng) (sample, standard, positive, or negative control). Cycling conditions were 50°C for 30 min for reverse transcription, followed by inactivation of the reverse-transcriptase by incubation at 95°C for 15 min. This was followed by 50 cycles, each consisting of denaturation at 97°C for 30 s, annealing at 55°C for 80 s, and extension at 72°C for 15 s. The real-time PCR reactions were conducted using a StepOnePlus™ Real-Time PCR thermal cycler (Applied Biosystems, Bedford, MA, USA). Reactions were supplemented with the inclusion of four external quantitation standards, alongside the samples and the negative control (PCR-grade water).[21]
Detection of HBV DNA in Plasma by nested PCR
For the detection of HBV DNA, a nested PCR amplification of a 267 bp fragment of the partial S gene was performed. Viral DNA was extracted from plasma with the GeneJET Viral DNA/RNA Purification Kit. Nested PCR was performed using Phusion DNA polymerase (Thermo Scientific Company, Lithuania) and specific Partial S gene primers [Table 1].[22]
Table 1.
Primers used for detection of HBV DNA

In the initial reaction, 5 μL of extracted viral DNA (1–20 ng/mL), 10 μL of 2X Phusion Master Mix, and 10 picomoles each of “SS out” forward and reverse primers were combined. The reaction volume was then adjusted to 20 μL with water. Cycling conditions were as follows: Initial denaturation at 98°C for 30 s, followed by 35 cycles of 98°C for 10 s, 55°C for 20 s and 72°C 30 s. A final extension step at 72°C for 5 min was added. For the subsequent reaction, 1 μL of the PCR product from the first reaction was added to 10 μL of master mix, along with 10 picomoles each of “SS in” primers. Water was added to reach a final volume of 20 μL. Cycling conditions were as described above. PCR was performed in a Veriti 96 Thermal cycler (Applied Biosystems, USA).
PCR products were visualized by agarose gel electrophoresis (1.5% agarose) and ultraviolet (UV)-transillumination. Negative controls did not show any bands, positive samples had a single, sharp band 267 bp long.
Biochemical measurements
Serum aspartate transaminase (AST) and alanine transaminase (ALT) were quantified using enzymatic colorimetric methods as described by Reitman and Frankel in 1957[23] (Biodiagnostic kits Catalog No. AL 10 31 (45) and Catalog No. AS 10 61 (45), Biodiagnostic, Egypt). Serum direct and total bilirubin were measured colorimetrically utilizing Biodiagnostic kits Catalog No. BR 1111 and Catalog No. BR 1112 (Biodiagnostic, Egypt), respectively, following the method outlined by Walters and Gerarde.[24] Serum total protein and albumin were determined utilizing Biodiagnostic kits Catalog No. TP 20 20 and Catalog No. AB 10 10 (Biodiagnostic, Egypt), as previously described.[25] All these biochemical parameters were measured using a UV-visible spectrophotometer (UV-1601PC, Shimadzu, Japan).
Statistical analysis
Data analysis was conducted using the SPSS software (Statistical Package for the Social Sciences), version 26. To assess the normal distribution of quantitative variables, the Kolmogorov–Smirnov test was employed. Data were expressed as median (minimum-maximum) for numerical variables and frequencies percentages for the number of patients in each group for categorical variables. The Mann–Whitney U test was used to compare numerical variables between two groups. The Chi-square test was used to ascertain the statistical significance for categorical variables. The significance of the findings was assessed at a P < 0.05.
Results
The prevalence of OBI among IDUs
Sixty-four from the 200 recruited IDUs were positive for OBI, representing 32% of the study sample [Figure 1].
Figure 1.
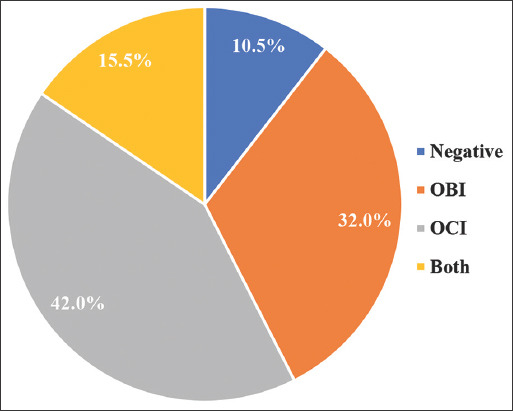
Viral infection distribution among injection drug users
Epidemiological and biochemical parameters in OBI-positive and OBI-negative IDUs
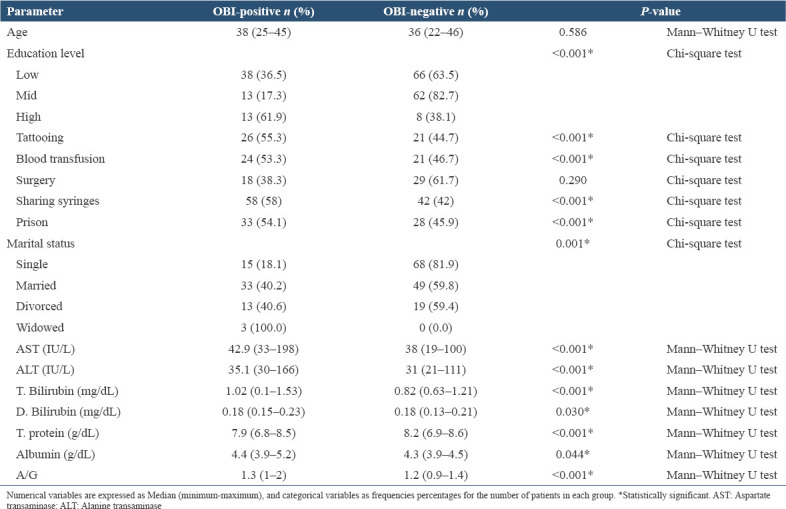
OBI prevalence significantly varied with education level (P < 0.001), tattooing (P < 0.001), blood transfusion (P < 0.001), sharing syringes (P < 0.001), previous history of prison (P < 0.001), and marital status (P = 0.001). No statistically significant association was found between OBI and age (P = 0.586) or surgical history (P = 0.290). Patients with OBI had higher levels of AST (P < 0.001), ALT (P < 0.001), and Total. Bilirubin (P < 0.001), Direct. Bilirubin (P = 0.030), Total. Protein (P < 0.001), Albumin (P = 0.044), and A/G ratio (P < 0.001) compared to OBI-negative patients [Table 2].
Table 2.
Epidemiological and biochemical parameters in OBI-positive and OBI-negative ID
The prevalence of OCI among IDUs
Eighty-four of the 200 recruited IDUs were positive for OCI, forming 42% of the study sample [Figure 1].
The prevalence of OCI among seropositive and seronegative
Of the 84 cases positive for OCI, 45 were seropositive, that is, positive for HCV Ab, representing 53.6% of OCI-positive cases. The remaining 39 OCI-positive cases were seronegative [Figure 2]. Statistical analysis confirmed a significant association between OCI and HCV antibody status (P < 0.001). Seropositive cases were 6.7 times more likely to have OCI, as indicated by an odds ratio of 6.719.
Figure 2.
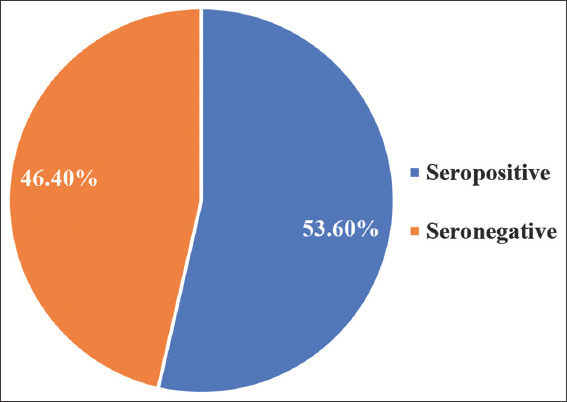
Distribution of seropositive and seronegative OCI among injection drug users
Epidemiological and biochemical parameters in OCI positive and OCI negative IDUs
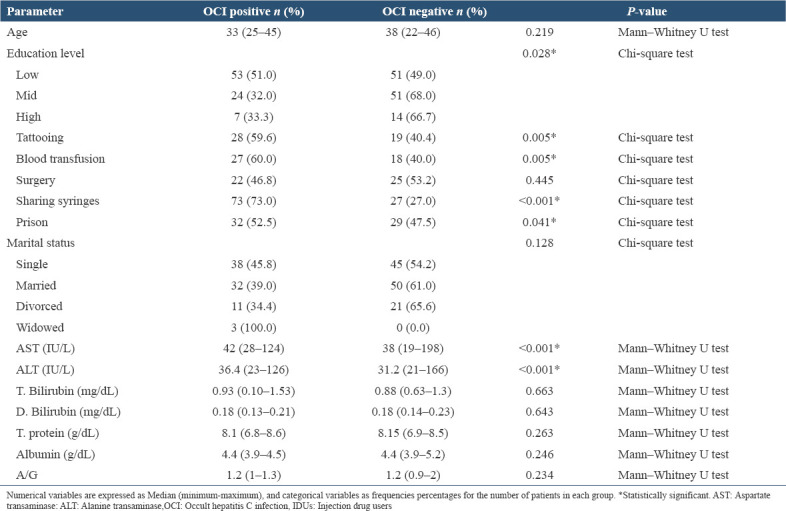
OCI prevalence significantly varied with education level (P = 0.028), sharing syringes (P < 0.001), history of incarceration (0.041), tattooing (P = 0.005), and blood transfusion (P = 0.005). No statistically significant association was found between OCI and age (P = 0.219), surgery (P = 0.445), and marital status (P = 0.128) [Table 2]. Patients with OCI exhibited higher levels of both AST (P < 0.001) and ALT (P < 0.001) compared to OCI-negative individuals. However, there were no significant differences in T. bilirubin, D. bilirubin, T. protein, albumin, or A/G ratio between the two groups [Table 3].
Table 3.
Epidemiological and biochemical parameters in OCI-positive and OCI-negative IDUs
Viral coinfection
Out of 23 HIV-positive individuals in the study cohort, nine were positive for OBI, six were positive for OCI and three were positive for both OBI and OCI. Over three-quarters (78.2%) of HIV-positive individuals in this study were coinfected with occult hepatitis viruses. This demonstrates a high prevalence in this demographic. In addition, 31 of the IDUs participating in this study were coinfected with OBI and OCI [Figure 3].
Figure 3.
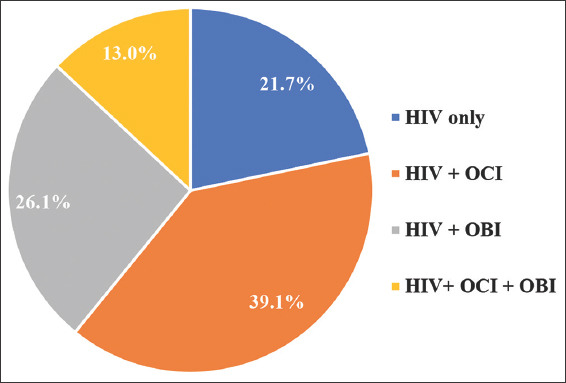
Distribution of HIV coinfection with occult hepatitis among injection drug users
Discussion
Occult Hepatitis B (OBI) and occult Hepatitis C (OCI) represent hidden reservoirs of infection that have been largely overlooked in high-risk populations, particularly in Egypt, where chronic HCV prevalence was historically the highest worldwide.[26] This study focused on a vulnerable group – IDUs – to assess the prevalence of these occult infections following Egypt’s highly successful large-scale HCV treatment campaign. Despite the campaign’s success in significantly reducing overt infections, this study identified that 32% of IDUs harbored occult HBV (OBI), while 42% had occult HCV (OCI). Notably, OBI and OCI were often codetected in individuals with HIV coinfection, suggesting that IDUs may still serve as a hidden reservoir for these viruses. These findings underscore the importance of continued surveillance and targeted interventions in this group to mitigate the risks of recurrence or undetected transmission.
Viral hepatitis stands as a pervasive and significant global health concern, particularly when transmitted through parenteral routes. In terms of both economic losses and public health impact, viral hepatitis is ranked as the third most prevalent infectious disease worldwide.[27]
Hepatitis B and C pose significant public health risks due to their transmission through blood contact, intravenous drug use, iatrogenic exposures, tattooing, and body piercings, resulting in a range of diseases and potentially triggering outbreaks and epidemics.[28,29] Notably, Egypt grapples with one of the highest prevalence rates of HCV globally and is classified as an intermediate site for HBV infection.[6]
Individuals who inject drugs suffer numerous hazards and adverse consequences.[30] Injection drug use leads to the transmission of blood-borne viruses such as HBV and HCV, causing significant morbidity and death.[31] According to a systematic review conducted in 2023, the prevalence of drug-related harms such as HCV and HIV infections was high among IDUs. This indicates that harm reduction measures are lacking and need to be developed and implemented.[31]
Occult hepatitis poses a more considerable challenge due to its inability to be diagnosed through routine tests, leading to a significant number of cases going undetected. This becomes especially problematic among high-risk groups, such as intravenous drug users, intensifying the risk of transmission within this particular demographic.[32]
Occult HBV infection is a complex clinical condition identified by low levels of HBV DNA in the blood or liver of individuals who test negative for HBsAg.[5] On the other hand, occult Hepatitis C is when the patient is positive for viral HCV RNA in their PBMCs, but negative for HCV RNA in their plasma. About 60% of OCI cases are diagnosed using the non-invasive method of OCI detection in PBMCs.[33] Both OBI and OCI have garnered increased attention due to their association with advanced liver fibrosis and cirrhosis.[34]
This research aimed at detection prevalence of OBI and OCI in Egyptian individuals who inject drugs, shedding light on their prevalence and correlation with specific epidemiological and clinical parameters.
Occult hepatitis B infection poses a significant public health concern, particularly among individuals at high risk of infection, such as those who inject drugs.[6]
In our current investigation, the presence of OBI accounted for approximately 32% of cases. Previous studies among Taiwanese intravenous drug users reported an OBI prevalence of approximately 41.1%.[35] while a study conducted in Iran found an overall frequency of OBI at 5.6%.[10] The variations in prevalence rates can be due to several factors such as sample size, technique of detection, and geographic viral location.[6]
Despite nested PCR being utilized in the Iranian study,[10] which typically offers higher sensitivity, the reported OBI prevalence among Iranian IDUs was considerably lower (5.6%) compared to the 32% reported in our study. The sensitivity cutoff used in that study was larger (700–1000 bp DNA products). While convenient for sequencing and genotyping, this cutoff may compromise sensitivity, potentially resulting in the under-representation of OBI cases. In contrast, our study employed a more sensitive PCR targeting a smaller 267 bp product, thereby enhancing the detection of OBI cases with low viral titers. This methodological difference might have contributed to the higher reported OBI prevalence in our study.
In this study, patients with OBI showed a statistically significant association between the presence of OBI and education level, tattooing, blood transfusion, sharing syringes, previous history of prison, and marital status. These results are consistent with different meta-analyses and studies that suggest that OBI prevalence is generally influenced by awareness of individual, blood donation, sharing objects among IDUs, and history of imprisonment.[6,34-36] Unfortunately, studies on the prevalence of blood-borne diseases in Egyptian IDUs are lacking.
The existence of OBI is considered a possible risk factor for chronic liver disease in various demographic groups. Furthermore, it contributes significantly to the development of serious liver conditions such as hepatocellular carcinoma and cirrhosis.[6,37,38] Our investigation revealed a significant increase in all liver function tests. Our results differ from previous reports, which report aberrations in ALT and AST only. This could be due to different geographical locations and different inclusion criteria. For example, a study conducted in Baltimore in 2004 found no association between AST and OBI, whereas ALT showed a statistically significant difference.[39] On the other hand, two studies conducted in Iran Viral coinfection with HIV were observed in this study; the rate of OBI- HIV and OCI-HIV coinfection was 39.1 and 26.1%, respectively.
This finding raises concerns about the adequacy of healthcare treatment for individuals living with HIV, particularly when considering the co-occurrence of hepatitis viruses. Co-infection with the occult forms, OBI and OCI, introduces complexities in the treatment and overall care of HIV patients, underscoring the need for integrated healthcare for this patient population. Our findings are consistent with Jamshidi et al., who reported that OBI and OCI occur frequently among HIV-positive IDUs representing 3.1% and 11.4% respectively, in an Iranian population. Another study on Iranian IDUs found that OCI-HIV coinfection was present in 18.1% of the study population.[34,40] on IDUs confected with viral hepatitis and HIV found flares of hepatic transaminases.[34,41]
Forty-two percent of the IDUs participating in this study tested positive for OCI. This high prevalence of OCI among IDUs in Egypt is a matter of worry and emphasizes the need to implement focused interventions that successfully address this problem. This discovery is consistent with the observation that IDUs are at a greater risk for HCV infection as OCI-positive individuals may contribute to HCV transmission. This increased risk is attributed to the practice of sharing infected needles and syringes.[9]
There is an apparent link between OCI and HCV antibody status. Those who tested positive for HCV antibody were 6.7 times more likely to develop OCI. This finding supports the research conducted by Kouroumalis and Voumvouraki, who reported that direct-acting antiviral drugs (DAAs) do not provide protection against reinfection. If follows that vulnerable populations, such as IDUs, may experience reinfection. In addition, studies have shown a significant incidence of OCI after treatment with DAAs. Therefore, it is recommended that one perform a dual testing for HCV RNA using both PBMCs and serum after the completion of therapy with DAAs, as well as during the confirmation of SVR.[42]
Our study revealed a statistically significant association between OCI and education level, suggesting that individuals with lower education levels may be at a higher risk of OCI. This association could be attributed to differences in awareness and knowledge about HCV transmission and prevention among individuals with different education levels. This finding agrees with the research conducted by Donyavi et al., which reported a significant correlation between OCI and educational achievement.[40]
The study found a strong association between sharing syringes and OCI among IDUs, indicating a significant transmission risk. In addition, a history of imprisonment was linked to OCI, emphasizing the need for interventions in correctional facilities. Our results are in agreement with the study performed by Sheikh et al.[43]
A comparative analysis was conducted to assess liver function markers in persons with OCI and those without OCI. Individuals diagnosed with OCI had higher serum levels of AST and ALT in comparison to OCI-negative individuals. Our findings are consistent with the studies conducted by Elkashef et al. and Saad et al., which proposed that OCI has the potential to cause mild liver damage. Therefore, it is advisable to do follow-up examinations for individuals with OCI to monitor the development of overt illness.[33,44]
The spread of HBV and HCV, along with HIV infection, poses a significant public health challenge. Diagnosing and treating these individuals has been challenging due to limited data. One of this study’s goals was to assess the prevalence of occult HCV and HBV infections in HIV-positive individuals.
Our study found that 31 IDUs were coinfected with OBI and OCI, and three of these also had HIV. Coinfection with the three viruses has been previously reported in an Iranian study.[34] The larger prevalence of hepatitis viruses among IDUs than HIV most likely reflects the widespread infection with hepatitis viruses, particularly HCV, in Egypt over the past 20 years.
Limitations
The study’s focus on male participants limits the generalizability of the findings to all IDUs. The sample size of 200 participants may not be large enough to detect subtler associations, and conducting the study in a single region limits its external validity.
Future directions
Future studies should include female participants to improve generalizability and expand the sample size across multiple regions for better representation. Longitudinal studies would help track the progression of hepatitis coinfections, and research on harm-reduction interventions, as well as additional risk factors such as socioeconomic status and mental health, would provide valuable insights. Molecular analysis of viral genotypes is also recommended for more targeted prevention and treatment strategies.
Conclusion
Over 70% of IDUs recruited for this study were infected with some form of occult hepatitis, 32% tested positive for OBI, 42% tested positive for OCI, and 15.5% were coinfected with both OBI and OCI. The high prevalence of OBI and OCI along with the significant coinfection results among Egyptian IDUs, a population previously understudied in this context considered as unique findings as this is the first research in Egypt to provide molecular evidence of these occult infections in IDUs and their clinical significance, which has not been reported earlier in Egyptian cohorts. As a result, this study is crucial for public health because it reveals the hidden burden of OBI and OCI among IDUs, who may unknowingly spread these infections. The findings advocate for changes in the screening protocols for Hepatitis B and C viruses to include molecular diagnostics, thus preventing further spread and enabling earlier intervention for those affected by these occult infections. This has significant implications for disease control and public health policy, particularly in Egypt, where IDUs represent a vulnerable and high-risk group for hepatitis transmission.
Ethics Approval and Consent to Participate
All participants completed an informed consent form, and all of them had the option to leave the study at any time without stating a reason, and the privacy and confidentiality of the obtained data were guaranteed.
Consent for Publication
All participants provided consent for publication, agreeing that their anonymized data may be used in this research and published globally, with privacy and confidentiality preserved.
Availability of Data and Materials
The data used in the study are available and will be provided by corresponding author on a reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
Kholoud A. Elkashef, Asmaa R. Abdel-Hamed, Noha M. Mesbah, Nelly R. Abdel Fattah, Fatma F. El-shaarawy and Ahmed Elsadek Fakhr: contributed to the study’s conception and design., Kholoud A. Elkashef, Nelly R. Abdel Fattah, Amal F. Gharib, and Mahmoud Amer: participated in sample collection. Kholoud A. Elkashef: analyzed the data and wrote the first draft of the manuscript. Asmaa R. Abdel-Hamed., Noha M. Mesbah, Amal F. Gharib, Ahmed Elsadek Fakhr, and Dina M. Abo-Elmatty: commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Gratitude is extended to all patients for their valuable participation and to the medical staff at El-Azazi Hospital for Mental Health for their support. Appreciation is also expressed to the General Secretariat of Mental Health for their efforts in contributing to the achievement of the WHO target for viral elimination by 2030.
References
- 1.Maqsood Q, Sumrin A, Iqbal M, Younas S, Hussain N, Mahnoor M, et al. Hepatitis C virus/Hepatitis B virus coinfection:Current prospectives. Antivir Ther. 2023;28:1–18. doi: 10.1177/13596535231189643. [DOI] [PubMed] [Google Scholar]
- 2.Ezzat S, Gamkrelidze I, Osman A, Gomaa A, Roushdy A, Esmat G, et al. Impacts of the Egyptian national screening and treatment programme for viral hepatitis C:A cost-effectiveness model. Liver Int. 2023;43:1417–26. doi: 10.1111/liv.15584. [DOI] [PubMed] [Google Scholar]
- 3.Osman HA, Ghweil AA, Sabry AM, Mahdy RE, Khodeary A. Management of patients with hepatitis B virus reactivation post-DAA treatment of chronic hepatitis c virus infection in HCV-HBV coinfected patients with pretreatment HBeAg seroconversion and early degree of hepatic fibrosis. Infect Drug Resist. 2019;12:3067–73. doi: 10.2147/IDR.S215974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awan A, Shakik S, Banack HR, Fisman DN, Simmons AE. Hepatitis B and C in individuals with a history of antipsychotic medication use:A population-based evaluation. PLoS One. 2023;12:3067–73. doi: 10.1371/journal.pone.0284323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubonja-Sonje M, Peru D, Abram M, Mohar-Vitezi B. Prevalence of occult hepatitis B virus infection and characterisation of hepatitis B surface antigen mutants among adults in western Croatia. Ann Hepatol. 2024;29:101156. doi: 10.1016/j.aohep.2023.101156. [DOI] [PubMed] [Google Scholar]
- 6.Azzam A, Khaled H, El-Kayal ES, Gad FA, Omar S. Prevalence of occult hepatitis B virus infection in Egypt:A systematic review with meta-analysis. J Egypt Public Health Assoc. 2023;98:1–16. doi: 10.1186/s42506-023-00138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397–408. doi: 10.1016/j.jhep.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Elbaz S, Mousa N, Elmetwalli A, Abdel-Razik A, Salah M, ElHammady A, et al. Unraveling IL-17 and IL-22 role in occult hepatitis C versus chronic hepatitis C virus infection. BMC Infect Dis. 2024;24:1–7. doi: 10.1186/s12879-024-09032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva E, Marques S, Leal B, Canhão B, Madaleno J, Simão A, et al. Occult hepatitis C infection identified in injection drug users with direct antiviral agents therapy and spontaneous resolution of hepatitis C virus infection. Virus Res. 2023;329:199104. doi: 10.1016/j.virusres.2023.199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asli M, Kandelouei T, Rahimyan K, Davoodbeglou F, Vaezjalali M. Characterization of occult hepatitis B infection among injecting drug users in Tehran, Iran. Hepat Mon. 2016;16:34763. doi: 10.5812/hepatmon.34763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matos MA, Ferreira RC, Rodrigues FP, Marinho TA, Lopes CL, Novais AC, et al. Occult hepatitis B virus infection among injecting drug users in the Central-West Region of Brazil. Mem Inst Oswaldo Cruz. 2013;108:386–9. doi: 10.1590/S0074-02762013000300019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedayati-Moghaddam MR, Soltanian H, Ahmadi-Ghezeldasht S. Occult hepatitis C virus infection in the Middle East and Eastern Mediterranean countries:A systematic review and meta-analysis. World J Hepatol. 2021;13:242–60. doi: 10.4254/wjh.v13.i2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbaga DS, Kenmoe S, Bikoï JN, Takuissu GR, Amougou-Atsama M, Okobalemba EA, et al. Global prevalence of occult hepatitis C virus:A systematic review and meta-analysis. World J Methodol. 2022;12:179–90. doi: 10.5662/wjm.v12.i3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitta C, Pollicino T, Raimondo G. Occult hepatitis B virus infection:An update. Viruses. 2022;14:1504. doi: 10.3390/v14071504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhang L, Dai Y, Zhang Y, Li J, Li X. Occult hepatitis B virus infection:Influence of S protein variants Hepatitis viruses. Virol J. 2016;13:1–11. doi: 10.1186/s12985-016-0464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocana S, Casas ML, Buhigas I, Lledo JL. Diagnostic strategy for occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1553. doi: 10.3748/wjg.v17.i12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naguib H, Abouelnaga SF, Elsayed MM. Occult hepatitis C virus infection in hemodialysis patients who achieved a sustained virological response to directly acting antiviral drugs:Is it a concern?Int Urol Nephrol. 2024;56:217–22. doi: 10.1007/s11255-023-03621-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim AY, Zur Wiesch JS, Kuntzen T, Timm J, Kaufmann DE, Duncan JE, et al. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 2006;3:2324–34. doi: 10.1371/journal.pmed.0030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Marco L, Manzini P, Trevisan M, Gillio-Tos A, Danielle F, Balloco C, et al. Prevalence and follow-up of occult HCV infection in an italian population free of clinically detectable infectious liver disease. PLoS One. 2012;7:e43541. doi: 10.1371/journal.pone.0043541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-Van Dillen PM, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:41–6. doi: 10.7150/ijms.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourkarim MR, Lemey P, Amini-Bavil-Olyaee S, Houspie L, Verbeeck J, Rahman M, et al. Molecular characterization of hepatitis B virus strains circulating in Belgian patients co-infected with HIV and HBV:Overt and occult infection. J Med Virol. 2011;83:1876–84. doi: 10.1002/jmv.22174. [DOI] [PubMed] [Google Scholar]
- 23.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Walters MI, Gerarde HW. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem J. 1970;15:231–43. [Google Scholar]
- 25.Doumas BT, Ard Watson W, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 26.Stroffolini T, Stroffolini G. Prevalence and modes of transmission of hepatitis C virus infection:A historical worldwide review. Viruses. 2024;16:1115. doi: 10.3390/v16071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisano MB, Giadans CG, Flichman DM, Ré VE, Preciado MV, Valva P. Viral hepatitis update:Progress and perspectives. World J Gastroenterol. 2021;27:4018–44. doi: 10.3748/wjg.v27.i26.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed MM, Hassan HE, Mohamed AA, Saleh AS. Knowledge and practice of nurses toward preventive measures of elderly patients with viral hepatitis B and C in the dialysis unit. NILES J Geriatr Gerontol. 2024;7:70–91. [Google Scholar]
- 29.Vilibic-Cavlek T, Zidovec-Lepej S, Ferenc T, Savic V, Nemeth-Blazic T, Vujica Ferenc M, et al. Seroprevalence trends and molecular epidemiology of viral hepatitis in croatia. Life (Basel) 2023;13:224. doi: 10.3390/life13010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins AB, Boyd J, Cooper HL, McNeil R. The intersectional risk environment of people who use drugs. Soc Sci Med. 2019;234:112384. doi: 10.1016/j.socscimed.2019.112384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degenhardt L, Webb P, Colledge-Frisby S, Ireland J, Wheeler A, Ottaviano S, et al. Epidemiology of injecting drug use, prevalence of injecting-related harm, and exposure to behavioural and environmental risks among people who inject drugs:A systematic review. Lancet Glob Health. 2023;11:e659–72. doi: 10.1016/S2214-109X(23)00057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ondigui JL, Kenmoe S, Kengne-Ndé C, Ebogo-Belobo JT, Takuissu GR, Kenfack-Momo R, et al. Epidemiology of occult hepatitis B and C in Africa:A systematic review and meta-analysis. J Infect Public Health. 2022;15:1436. doi: 10.1016/j.jiph.2022.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkashef KA, Emam WA, Mesbah NM, Abo-Elmatty DM, Abdel-Hamed AR. Prevalence of occult hepatitis C virus infection in Egyptian patients with lymphoma:A new vision. Diagnostics. 2022;12:1015. doi: 10.3390/diagnostics12041015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamshidi S, Bokharaei-Salim F, Esghaei M, Bastani MN, Garshasbi S, Chavoshpour S, et al. Occult HCV and occult HBV coinfection in Iranian human immunodeficiency virus-infected individuals. J Med Virol. 2020;92:3354–64. doi: 10.1002/jmv.25808. [DOI] [PubMed] [Google Scholar]
- 35.Lin C, Liu CJ, Chen PJ, Lai MY, Chen DS, Kao JH. High prevalence of occult hepatitis B virus infection in Taiwanese intravenous drug users. J Med Virol. 2007;79:1674–8. doi: 10.1002/jmv.20985. [DOI] [PubMed] [Google Scholar]
- 36.Eshraghi Mosa Abadi B, Kandelouei T, Eslami G, Asli M, Vaezjalali M. Prevalence and risk factors for occult hepatitis B and HIV infections among HCV infected intravenous drug users, Tehran, Iran. Arch Clin Infect Dis. 2018;13:67968. [Google Scholar]
- 37.Gissa SB, Minaye ME, Yeshitela B, Gemechu G, Tesfaye A, Alemayehu DH, et al. Occult hepatitis B virus infection among patients with chronic liver disease of unidentified cause, Addis Ababa Ethiopia. Sci Rep. 2022;12:13188. doi: 10.1038/s41598-022-17336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X, Yang L, Zhang P, Wang C, Luo S, Liu B, et al. Occult hepatitis B virus infection and liver fibrosis in Chinese patients. J Infect Dis. 2023;228:1375–84. doi: 10.1093/infdis/jiad140. [DOI] [PubMed] [Google Scholar]
- 39.Torbenson M, Kannangai R, Astemborski J, Strathdee SA, Vlahov D, Thomas DL. High prevalence of occult hepatitis B in Baltimore injection drug users? ?Hepatology. 2004;39:51–7. doi: 10.1002/hep.20025. [DOI] [PubMed] [Google Scholar]
- 40.Donyavi T, Bokharaei-Salim F, Khanaliha K, Sheikh M, Bastani MN, Moradi N, et al. High prevalence of occult hepatitis C virus infection in injection drug users with HIV infection. Arch Virol. 2019;164:2493–504. doi: 10.1007/s00705-019-04353-3. [DOI] [PubMed] [Google Scholar]
- 41.Azadmanesh K, Mohraz M, Aghakhani A, Edalat R, Jam S, Eslamifar A, et al. Occult hepatitis B virus infection in HIV-infected patients with isolated hepatitis B core antibody. Intervirology. 2008;51:270–4. doi: 10.1159/000160217. [DOI] [PubMed] [Google Scholar]
- 42.Kouroumalis E, Voumvouraki A. Hepatitis C virus:A critical approach to who really needs treatment. World J Hepatol. 2022;14:1–45. doi: 10.4254/wjh.v14.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheikh M, Bokharaei-Salim F, Monavari SH, Ataei-Pirkooh A, Esghaei M, Moradi N, et al. Molecular diagnosis of occult hepatitis C virus infection in Iranian injection drug users. Arch Virol. 2019;164:349–57. doi: 10.1007/s00705-018-4066-5. [DOI] [PubMed] [Google Scholar]
- 44.Saad Y, Zakaria S, Ramzy I, El Raziky M, Shaker O, Elakel W, et al. Prevalence of occult hepatitis C in egyptian patients with non alcoholic fatty liver disease. Open J Intern Med. 2011;1:33–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the study are available and will be provided by corresponding author on a reasonable request.


