Abstract
The global spread of the novel coronavirus disease 2019, caused by SARS-CoV-2 virus, impacts individuals of all age groups, including lactating women and children. Concerns have been raised regarding the potential transmission of SARS-CoV-2 from mother to child, following the discovery of SARS-CoV-2 RNA in human milk. Therefore, this study aims to investigate whether the Omicron novel coronavirus variants are transmitted through human milk. This study was conducted between March and May 2022 at Children’s Medical Center, the First Hospital of Jilin University, Lequn Branch. Fourteen lactating mothers and their breastfed children hospitalized with COVID-19 (Omicron variant) formed mother–child pairs, which constituted the test group. Additional 11 non-breastfed children of the same age hospitalized with COVID-19 (Omicron variant) participated in the study as the control group. Their clinical manifestations were observed, and the milk of lactating mothers with COVID-19 was collected for SARS-CoV-2 RNA detection. Milk samples from each lactating mother were collected consecutively for 2–18 days and subjected to polymerase chain reaction (PCR) testing forSARS-CoV-2 RNA detection. The time span for sample collection ranged from admission to discharged. The symptoms observed in mothers and children infected with the Omicron variant of COVID-19 were primary upper respiratory tract infection, with fever and cough being the main clinical manifestations. In total, 104 breast milk samples were collected from 14 lactating mothers with COVID-19, and all samples were negative for SARS-CoV-2 RNA. This study found no evidence of Omicron variants transmission through breast milk and accepts the safety of breastfeeding for novel coronavirus-positive mothers when contact precautions are taken. Our findings provide additional support for recommendations that lactating women with COVID-19 continue to breastfeed while taking precautions.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84838-7.
Keywords: Novel coronavirus pneumonia, COVID-19, Novel coronavirus Omicron variant, Breast milk, Breastfeeding, SARS-CoV-2
Subject terms: Diseases, Infectious diseases
Coronavirus disease 2019 (COVID-19), caused by the novel SARS-CoV-2 virus, is widespread worldwide and can occur in all age groups, including lactating women and children. The discovery of SARS CoV-2 RNA in human milk has raised concerns regarding whether breastfeeding can lead to mother-to-child transmission1. The scientific community, nursing experts, World Health Organization (WHO), and governments have not reached a consensus on the safety of breastfeeding for mothers who test positive for the novel coronavirus, and the recommendations regarding breastfeeding remain controversial. The WHO and Centers for Disease Control and Prevention encourage mothers with confirmed or suspected novel coronavirus infection to breastfeed directly while taking contact prevention measures2. China has implemented stringent measures to prohibit mothers with confirmed or suspected infection from breastfeeding3. The Omicron variant is spreading rapidly worldwide and has significant differences in clinical characteristics compared to previous of viruses4. In the spring of 2022, a pandemic emerged in Jilin Province, China. As reported by the Chinese Center for Disease Control and Prevention, the main circulating strain in China is the Omicron subtype strain BA.2, which is rapidly spreading. BA.2 caused major outbreaks in Jilin, Shanghai, and Hong Kong5. The epidemic spread through the family gathering, causing large-scale infection in women and children, including some lactating women. There is no answer to whether Omicron, a highly infectious COVID-19 variant, is transmitted through breast milk. Hence, this study investigated whether the novel Omicron coronavirus variants are transmitted through human milk.
Materials and methods
Subjects
This study was conducted at Children’s Medical Center, The First Hospital of Jilin University, Lequn Branch from March to May 2022. The hospital received patients diagnosed with COVID-19 in accordance with the Diagnosis and Treatment Plan for Novel Coronavirus Pneumonia (Trial Version 9)6 and established a pediatric area to manage children with COVID-19 and their families in a family ward. 14 lactating mothers and their 14 breastfed children hospitalized in the special area formed mother–child pairs as the test group. The age of the lactating mothers was 31.36 ± 4.63 years, while the age of their breastfed children (5 males and 9 females) was 6.5 (3.8,9.5) months. An additional 11 non-breastfed children (10 males and 1 female) of the same age group participated in the study as the control group, with the age of 9.0 (2.5,13.0) months. This study was approved by the ethics committee of the First Hospital of Jilin University (permit no. 2022–290). The nursing mothers and the guardians of all the children who participated in the trial signed an informed consent form.
Research methods
Clinical data and breast milk sample collection
Test participants received antiviral treatment with traditional Chinese medicine. Fourteen lactating mothers were administered Lianhua Qingwen capsules (YILING, China), and 14 breastfed children and 11 non-breastfed children were administered Jinhua Qinggan granules (JUXIECHANG, China). The appointed doctors collected the clinical data of the patients and the number of days required for the 14 breastfed children in the test group and the 11 non-breastfed children in the control group to yield a negative nucleic acid test result. Simultaneously, milk samples were collected from the 14 lactating mothers with COVID-19 for SARS CoV-2 detection. Samples were collected consecutively for 2–18 days from admission to discharged, and polymerase chain reaction (PCR) tests were conducted for SARS-CoV-2 RNA detection. Lactating mothers were admitted to the hospital after their nasopharyngeal swabs tested positive for nucleic acid and continued providing milk samples until their nucleic acid test showed negative results. They remained hospitalized to care for their breastfed children until the children were healthy to be discharged (Fig. 1). Milk samples were collected under sterile conditions, with mothers wearing masks, washing their hands with soapy water, and disinfecting their breast skin with 75% alcohol. After washing their hands again, they squeezed 5 ml of milk into a sterile collection container7, which were immediately sent for testing. Researchers tested for SARS-CoV-2 in human milk under sterile conditions and recorded the results.
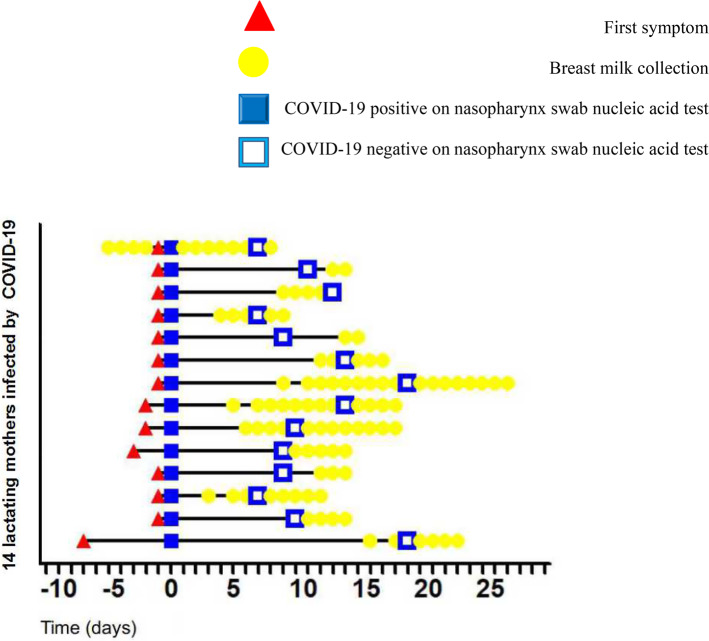
Fig. 1.
History, diagnosis, and collection of milk samples of lactating mothers infected by the Omicron variant (COVID-19: coronavirus disease 2019).
When the nucleic acid test performed using the nasopharyngeal swab of the lactating mother showed positive for the first time, the patient was diagnosed with COVID-19, and the day was recorded as day 0. (Triangle: The lactating mother first developed symptoms; Solid square: nucleic acid test performed using the nasopharyngeal swab of the lactating mother was positive for the first time, confirming the diagnosis of COVID-19; Hollow square: nucleic acid test performed using the nasopharyngeal swab from the lactating mother turned negative; Dot: breast milk sample collection time point.)
SARS CoV-2 detection
The breast milk samples were tested for SARS CoV-2 using a novel coronavirus 2019-nCoV nucleic acid detection kit, in accordance with the manufacturer’s instructions (Jiangsu Shuoshi Biotechnology Co., LTD, China). The results were quantitatively analyzed using fluorescence quantitative PCR. The lowest detection limit of the test reagent was 200 copies/mL, total number of amplification cycles was 45, and positive judgment threshold was 43.
Statistical analysis
Data were analyzed using GraphPad Prism version 9. Measurement data were presented as mean ± standard deviation or median (interquartile range). Normality tests were conducted on the measurement data, and independent sample t-tests were conducted to compare the two groups of measurement data that conformed to a normal distribution. For measurement data that did not conform to a normal distribution, the Mann–Whitney U test was used to compare the two groups. Chi-square or Fisher’s exact tests were used to compare categorical data. All tests were considered statistically significant at p < 0.05.
Results
Clinical characteristics of COVID-19 in 14 lactating mothers infected with the Omicron variant
The age of the 14 lactating mothers was assessed for normality using the Shapiro–Wilk test, which indicated a normal distribution (p = 0.622). The mean ± standard deviation method was represented as 31.36 ± 4.63 years old. All 14 lactating mothers had COVID-19-related clinical symptoms: fever (71.4%), chest tightness (7.1%), diarrhea (28.6%), nausea and vomiting (14.3%), cough (71.4%), expectoration (42.9%), gustatory deficit (7.1%), sore throat (50.0%), myalgia (28.6%), and fatigue (7.1%). One lactating mother’s lung CT scan showed viral pneumonia (7.1%). Twelve lactating mothers had not been vaccinated against COVID-19, accounting for 85.7%, whereas two had been vaccinated, accounting for 14.3% (Table 1).
Table 1.
Clinical characteristics of lactating mothers with COVID-19 of the Omicron variant.
| Value/number | |
|---|---|
| Basic characteristics | |
| Age (mean ± standard deviation) | 31.36 ± 4.63 |
| Clinical manifestations | |
| Asymptomatic (n, %) | 0 (0.0) |
| Fever (n, %) | 10 (71.4) |
| Headache (n, %) | 0 (0.0) |
| Chest tightness (n, %) | 1 (7.1) |
| Dyspnea (n, %) | 0 (0.0) |
| Diarrhea (n, %) | 4 (28.6) |
| Nausea and vomiting (n, %) | 2 (14.3) |
| Abdominal pain (n, %) | 0 (0.0) |
| Cough (n, %) | 10 (71.4) |
| Expectoration (n, %) | 6 (42.9) |
| Runny nose (n, %) | 0 (0.0) |
| Gustatory deficit (n, %) | 1 (7.1) |
| Sore throat (n, %) | 7 (50.0) |
| Myalgia (n, %) | 4 (28.6) |
| Fatigue (n, %) | 1 (7.1) |
| Anorexia (n, %) | 0 (0.0) |
| Hoarse voice (n, %) | 0 (0.0) |
| Viral pneumonia (n, %) | 1 (7.1) |
| Vaccinated against the novel coronavirus (n, %) | 2 (14.3) |
Note: instead of each patient showing only one symptom, a patient may have several symptoms simultaneously, resulting in the total of percentages exceeding 100%
Clinical characteristics of COVID-19 in children infected with the Omicron variant
COVID-19 pediatric patients infected with the Omicron variant were divided into two groups based on their breastfeeding status. The ages of 14 COVID-19 pediatric patients who received breastfeeding were tested for normality using the Shapiro–Wilk test. The results did not conform to a normal distribution (p = 0.001). The median (quartile) method was used to represent age at 6.5 (3.8, 9.5) months. The ages of COVID-19 pediatric patients who were not breastfed were tested for normality using the Shapiro–Wilk test. These results suggest that they conformed to a normal distribution (p = 0.070). The mean ± standard deviation method was used to represent age as 8.5 ± 5.0 months. To ensure comparability between the two groups (breastfed children and non-breastfed children), the median (quartile) method was still used to represent age as 9.0 (2.5, 13.0) months. No statistically significant difference was observed in age between the two groups of pediatric patients (p = 0.257). Among the COVID-19 pediatric patients who were breastfed and those who were not, there were five (35.7%) and ten (90.9%) male patients, respectively. The proportion of male COVID-19 pediatric patients who did not breastfeed was significantly higher than that of those who did (p = 0.012) (Table 2).
Table 2.
Clinical characteristics of children with COVID-19 of the Omicron variant.
| Breastfed children (n = 14) | Non-breastfed children (n = 11) | p-value | |
|---|---|---|---|
| Basic characteristics | |||
| Age (median, interquartile range) | 6.5 (3.8,9.5) | 9.0 (2.5,13.0) | 0.257 |
| Sex, male (n, %) | 5 (35.7) | 10(90.9) | 0.012 |
| Clinical manifestations | |||
| Asymptomatic (n, %) | 0 (0.0) | 0 (0.0) | - |
| Fever (n, %) | 11 (78.6) | 9 (81.8) | 0.622 |
| Headache (n, %) | 0 (0.0) | 0 (0.0) | - |
| Chest tightness (n, %) | 0 (0.0) | 0 (0.0) | - |
| Dyspnea (n, %) | 0 (0.0) | 0 (0.0) | - |
| Diarrhea (n, %) | 4 (28.6) | 0 (0.0) | 0.079 |
| Nausea and vomiting (n, %) | 0 (0.0) | 0 (0.0) | - |
| Abdominal pain (n, %) | 0 (0.0) | 0 (0.0) | - |
| Cough (n, %) | 11 (78.6) | 7(63.6) | 0.351 |
| Expectoration (n, %) | 0 (0.0) | 0 (0.0) | - |
| Runny nose (n, %) | 1 (7.1) | 1 (9.1) | 0.697 |
| Gustatory deficit (n, %) | 0 (0.0) | 0 (0.0) | - |
| Sore throat (n, %) | 0 (0.0) | 0 (0.0) | - |
| Myalgia (n, %) | 0 (0.0) | 0 (0.0) | - |
| Fatigue (n, %) | 0 (0.0) | 0 (0.0) | - |
| Anorexia (n, %) | 2 (14.3) | 0 (0.0) | 0.303 |
| Hoarse voice (n, %) | 1 (7.1) | 1 (9.1) | 0.697 |
| Viral pneumonia (n, %) | 2 (14.3) | 2 (18.2) | 0.604 |
| Vaccinated against the novel coronavirus (n, %) | 0 (0.0) | 0 (0.0) | - |
Note: instead of each patient showing only one symptom, one patient may have several symptoms simultaneously, resulting in the total of percentages exceeding 100%
The clinical manifestations of the two groups of pediatric patients are shown in Table 2. Among the 14 breastfed children in the experimental group, the clinical symptoms were as follows: fever (78.6%), diarrhea (28.6%), cough (78.6%), runny nose (7.1%), anorexia (14.3%), and hoarse voice (7.1%). Two patients showed viral pneumonia on lung CT, accounting for 14.3% (Table 2). Among the 11 non-breastfed patients in the control group, the clinical symptoms were as follows: fever (81.8%, 9/11), cough (63.6%), runny nose (9.1%), and hoarse voice (9.1%). Two patients showed viral pneumonia on lung CT, accounting for 18.2% (Table 2). None of the patients received the COVID-19 vaccination. No statistically significant difference in the composition ratio of the clinical manifestations was observed between the breastfed and non-breastfed groups (p > 0.05).
Collection of human milk and SARS-CoV-2 detection
In this study, 104 breast milk samples from 14 mothers with COVID-19 were collected over 2–18 consecutive days. All breast milk samples tested negative for SARS-CoV-2 (Fig. 1). Milk samples were collected from five lactating mothers within the first week of symptom onset. The earliest sample was collected four days prior to the onset of symptom. Additionally, a 27-years-old lactating mother was admitted to the pediatric special area to take care of her lactating child with COVID-19, who had no clinical symptoms at admission.
Comparison of disease outcome between breastfed and non-breastfed children
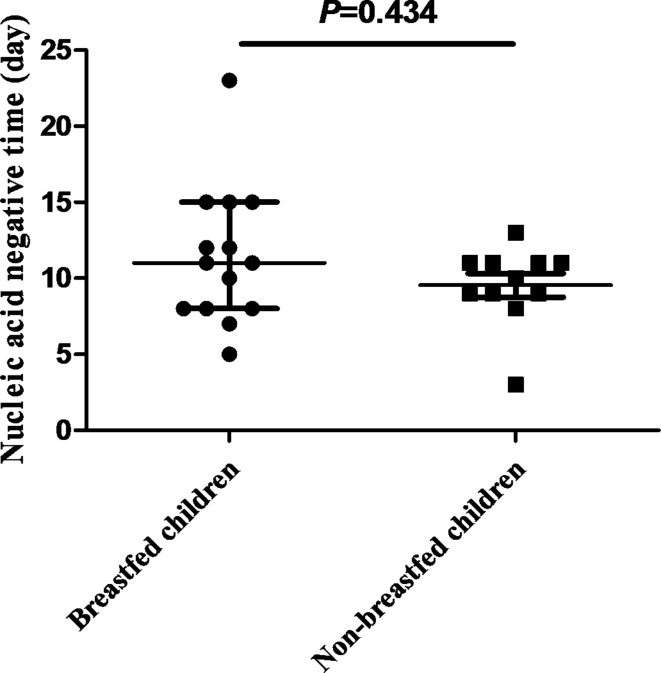
The nucleic acid-negative conversion days of 14 breastfed Omicron-infected children were tested for normality using the Shapiro–Wilk test, which followed a normal distribution (p = 0.166). The average conversion days were 11.4 ± 4.6 days, with a median and quartiles of 11.0 (8.0, 15.0) days. The nucleic acid negative conversion days of 11 non-breastfed children infected with Omicron were also tested for normality using the Shapiro–Wilk test; however, it did not conform to a normal distribution (p = 0.029). The average conversion time was 9.5 ± 2.6 days, with a median and interquartile range of 10.0 (9.0, 11.0) days. The two groups were compared using non-parametric methods, revealing no significant difference (p = 0.434) (Fig. 2).
Fig. 2.
Comparison of the number of days of negative conversion in the nucleic acid test between breastfed and non-breastfed children diagnosed with COVID-19 of the Omicron variant. p = 0.434, breastfed children group, n = 14; non-breastfed children group, n = 11.
Discussion
The Omicron variant of the SARS-CoV-2 virus (B.1.1.529) was first identified in Botswana in November 20218. On November 26, 2021, the WHO SARS-CoV-2 Evolution Technology Advisory Group defined this strain as having a variety of concerns. Compared with the other four variants (Alpha, Beta, Gamma and Delta), Omicron had the highest mutation rate. It has accumulated 50 mutations in the whole genome and at least 32 mutations in the spike protein, which enhances the infectivity and immune escape of Omicron variants4.
This study observed and recorded the clinical manifestations of lactating mothers and children with COVID-19 in both test and control groups. The findings indicated that the symptom in lactating mothers infected with the Omicron variant was upper respiratory tract infections, with primary clinical manifestations being fever, cough, expectoration, and sore throat. The symptoms of children infected with the Omicron variant primarily included upper respiratory tract infection with fever and cough. Most lung images of these lactating mothers and children showed no manifestations of viral pneumonia. Wolter et al.9 reported that the Omicron variant had milder clinical manifestation than the Delta variant; however, Duong et al.10 reported that the mutant variant leaded to an increased risk for severe cases. Currently, research on the Omicron variant is limited; therefore, its clinical manifestations have not been determined4,8–10. Previous studies have indicated that the mild clinical manifestations of Omicron infection may be related to vaccination9; however, most patients observed had no history of vaccination. Although the sample size of this study was small, the findings support the conclusion that the clinical manifestations of Omicron infection are mild.
Liu et al.2 reported that most studies did not find SARS-CoV-2 RNA in the breast milk of COVID-19 positive mothers, and only a few studies have observed the existence of SARS-CoV-2 RNA in the milk of infected lactating mothers. Chambers et al.11 reported that although SARS CoV-2 RNA was detected in human milk samples, no virus with replication ability was detected in the samples. This indicates that SARS CoV-2 RNA in human milk may not be infectious. Zhu et al.12 reported the presence of specific antibodies to the novel coronavirus in the milk of sick mothers, which may have a protective effect on their breastfed children. Blackshaw et al.13 reported that transmission routes other than milk, such as respiratory droplets, skin, contamination of breast pumps and milk containers, and close contact between the mother and baby, cannot be excluded during the breastfeeding process. Powell et al.14 demonstrated that most children are negative for SARS-CoV-2 when breastfeeding from their COVID-19 positive mothers while taking contact prevention measures. Thus, there is insufficient evidence to prove that the novel coronavirus increases the risk of infection in children through breast milk transmission and breastfeeding14. Although controversy exists among different organizations regarding the recommendations for breastfeeding, direct breastfeeding under the condition of taking contact prevention measures is often considered the first option for infected mothers2.
In this study, lactating mothers were taught cleaning techniques to avoid interference with the test results7. All breast milk samples tested negative for SARS-CoV-2. Previous studies on whether the milk of mothers with COVID-19 has virus transmissibility have primarily used milk collection at different time points. Milk samples were collected several times from each mother to detect SARS-CoV-2 RNA. The highlight of this study is that milk samples were collected for consecutive detection over 2–18 days. The time span of sample collection was from admission to negative nucleic acid conversion based on nasopharyngeal swabs and then to being discharged. Each lactating mother underwent a milk sample PCR test for SARS CoV-2 RNA detection every day to avoid missing transient changes in nucleic acids in the milk. One lactating mother, who was admitted to the pediatric special area to take care of her lactating child with COVID-19, had no clinical symptoms at admission, and the nasopharyngeal swab test result of SARS-CoV-2 RNA was negative. However, four days later, she developed a sore throat, and the nasopharyngeal swab test result for SARS-CoV-2 RNA was positive, and she was diagnosed with COVID-19. This lactating mother had her breast milk samples collected consecutively for 12 days—that is, from four days before the onset of COVID-19 symptoms, to her nasopharyngeal swab test result for SARS-CoV-2 RNA turning negative after treatment, and to her discharge. Although milk was collected continuously in this study, no SARS CoV-2 RNA was detected in any of the samples. Currently, there is a lack of understanding regarding COVID-19 caused by the Omicron variant. This study provides new data.
The proportion of male children with COVID-19 who were not breastfed in our study was higher than that of male children with COVID-19 who were breastfed, and the difference was statistically significant. Previous studies have shown that children do not appear to be at higher risk of severe illness based on sex15. We observed breastfed children in the test group and non-breastfed children in the control group received the same treatment. No difference was observed in the clinical severity between the two groups, and no statistical difference was observed in the number of days required for nucleic acids to turn negative. Previous studies have reported that breastfeeding is beneficial to the health of children7, and that there are specific antibodies to the novel coronavirus in the milk of sick mothers, which may have a protective effect on their breastfed children12. Therefore, it is speculated that breastfeeding may improve the clinical performance and prognosis of sick children. However, this study did not find this difference, which may be related to the small sample size and relatively mild illness in children.
Conclusions
Human milk is an important source of nutrition for infants; however, the discovery of SARS CoV-2 RNA in human milk has raised concerns about the safety of breastfeeding. This study investigated whether novel Omicron coronavirus variants are transmitted through human milk. We found no evidence of the transmission of Omicron variants through breast milk and accepted the safety of breastfeeding for novel coronavirus-positive mothers when contact precautions are taken. Although there are some limitations to our study, including the small sample size and lack of detections of SARS-CoV-2 specific antibodies, our findings provide additional support for recommendations that lactating women with COVID-19 continue to breastfeed while taking precautions, such as practicing hand and respiratory hygiene, wearing masks, washing their breasts with soap and warm water prior to breastfeeding, or expressing milk before feeding.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the families, pediatricians, nurses, and laboratory technicians who participated and contributed in this study during the COVID-19 pandemic. This study is the result of the joint efforts of various groups in the pediatric area.
Author contributions
Huiyi Jiang conducted the focus groups, led the analysis and interpretation of the qualitative data, and led the writing of the manuscript. Mengkun Wang and Yifei Li designed the quantitative survey, assisted with the quantitative analysis and data collection, and was a major contributor in writing the manuscript. Yifei Li and Jie Lei assisted with the quantitative analysis and data collection and assisted in writing the manuscript. All four of the authors have read and approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was approved by the ethics committee of the First Hospital of Jilin University (permit no. 2022-290). This research was performed in accordance with relevant guidelines/regulations. The nursing mothers and the guardians of all the children who participated in the trial signed an informed consent form. This study was conducted in accordance with both the ethical guidelines for life science and medical research involving human subjects and the rules established by the ethics committee.
Consent for publication
The authors gathered all original data and have consent to publish.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lou, F. et al. The benefits of breastfeeding still outweigh the risks of COVID-19 transmission. Front. Med.8, 703950. 10.3389/fmed.2021.703950 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu, X. et al. Recommendations for breastfeeding during coronavirus disease 2019 (COVID-19) pandemic. Int. Breastfeed. J.17, 28. 10.1186/s13006-022-00465-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho, W. B., Gibelli, M., Krebs, V. L. J., Calil, V. & Johnston, C. Expert recommendations for the care of newborns of mothers with COVID-19. Clinics (Sao Paulo, Brazil)75, e1932. 10.6061/clinics/2020/e1932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian, D., Sun, Y., Xu, H. & Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol.94, 2376–2383. 10.1002/jmv.27643 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obermeyer, F. et al. Analysis of 6.4 million SARS-CoV-2 genomes identifies mutations associated with fitness. Sci. (New York, N.Y.)376, 1327–1332. 10.1126/science.abm1208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahalingasivam, V. et al. COVID-19 and kidney disease: Insights from epidemiology to inform clinical practice. Nat. Rev. Nephrol.18, 485–498. 10.1038/s41581-022-00570-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire, M. K. et al. Best practices for human milk collection for COVID-19 research. Breastfeed. Med. Off. J. Acad. Breastfeed. Med.16, 29–38. 10.1089/bfm.2020.0296 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobrovitz, N. et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis.10.1016/s1473-3099(22)00801-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolter, N. et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet (London, England)399, 437–446. 10.1016/s0140-6736(22)00017-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duong, B. V. et al. Is the SARS CoV-2 omicron variant deadlier and more transmissible than delta variant? Int. J. Environ. Res. Public Health. 19, 10.3390/ijerph19084586 (2022). [DOI] [PMC free article] [PubMed]
- 11.Chambers, C. et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. Jama324, 1347–1348. 10.1001/jama.2020.15580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu, F., Zozaya, C., Zhou, Q., De Castro, C. & Shah, P. S. SARS-CoV-2 genome and antibodies in breastmilk: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Edition106, 514–521. 10.1136/archdischild-2020-321074 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Blackshaw, K. et al. The risk of infectious pathogens in breast-feeding, donated human milk and breast milk substitutes. Public Health Nutr.24, 1725–1740. 10.1017/s1368980020000555 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell, R. L. R. Safety of breast/chest-feeding by those infected by SARS-CoV-2. Curr. Opin. Clin. Nutr. Metab. care25, 129–132. 10.1097/mco.0000000000000816 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castagnoli, R. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr.174, 882–889. 10.1001/jamapediatrics.2020.1467 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.