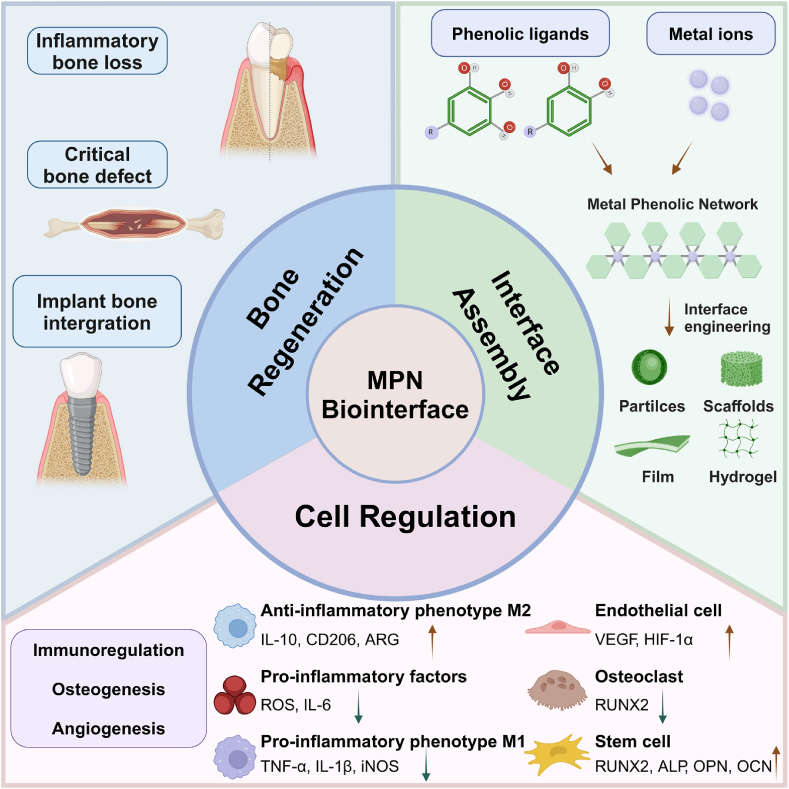
Abstract
Bone tissue regeneration presents a significant challenge in clinical treatment due to inadequate coordination between implant materials and reparative cells at the biomaterial-bone interfaces. This gap underscores the necessity of enhancing interaction modulation between cells and biomaterials, which is a crucial focus in bone tissue engineering. Metal-polyphenolic networks (MPN) are novel inorganic-organic hybrid complexes that are formed through coordination interactions between phenolic ligands and metal ions. These networks provide a multifunctional platform for biomedical applications, with the potential for tailored design and modifications. Despite advances in understanding MPN and their role in bone tissue regeneration, a comprehensive overview of the related mechanisms is lacking. Here, we address this gap by focusing on MPN biointerface-mediated cellular regulatory mechanisms during bone regeneration. We begin by reviewing the natural healing processes of bone defects, followed by a detailed examination of MPN, including their constituents and distinctive characteristics. We then explore the regulatory influence of MPN biointerfaces on key cellular activities during bone regeneration. Additionally, we illustrate their primary applications in addressing inflammatory bone loss, regenerating critical-size bone defects, and enhancing implant-bone integration. In conclusion, this review elucidates how MPN-based interfaces facilitate effective bone tissue regeneration, advancing our understanding of material interface-mediated cellular control and the broader field of tissue engineering.
Keywords: Metal-polyphenolic networks, Bone tissue regeneration, Biointerface, Stem cells, Immunoregulation
Graphical abstract
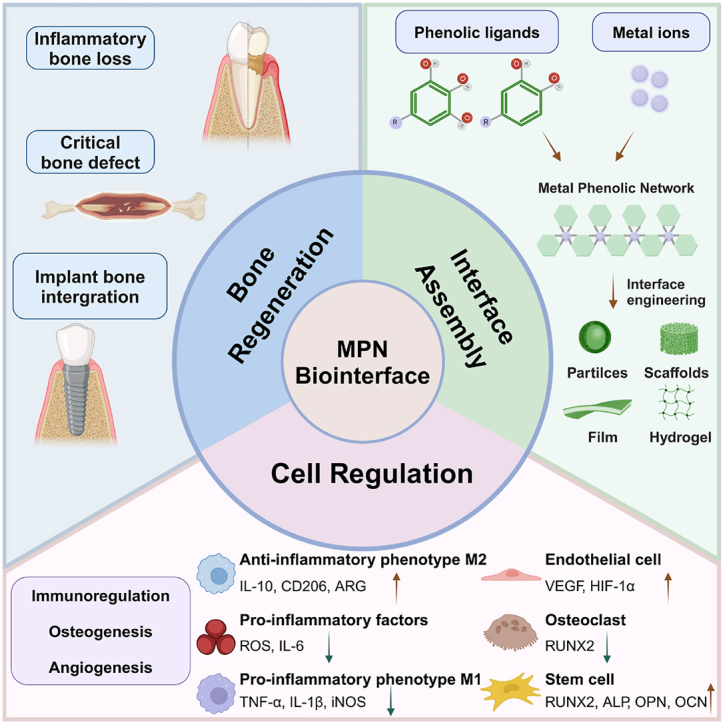
Schematic illustration of the interface assembly, cell regulation, and bone regeneration applications of MPNs.
1. Introduction
Bone defects caused by accidents, infectious diseases, or tumor resections contribute to patient deformity and mortality, imposing significant socioeconomic burdens [1]. The “gold standard” treatments for bone defects, including autologous and allogeneic bone grafts, have several limitations and disadvantages [2]. These include the risk of immune rejection, a scarcity of bone donors, the potential for infection, and the complexity of the morphology involved [3]. With the rapid advancement of biomedical technology and materials science, tissue engineering has emerged as an innovative approach for the repair of bone defects [[4], [5], [6]]. The specific approach in bone tissue engineering involves inoculating stem cells with multidirectional differentiation potential and bioactive growth factors onto functional biomaterial scaffold structures [7]. The biomaterial scaffolds hold significant promise as alternatives to traditional bone grafts.
The material interfaces play a crucial role in facilitating cell adhesion, proliferation, and differentiation, thereby establishing a conducive osteogenic microenvironment [8,9]. Over the years, research has demonstrated that the physicochemical properties (e.g., wettability, roughness, stiffness, and surface chemistry) of biomaterial scaffold interfaces play a crucial role in regulating various cell types (e.g., endothelial cells, osteoblasts, and stem cells) [[10], [11], [12]]. For instance, in comparison to a hydrophobic scaffold surface, a hydrophilic scaffold surface promotes greater cell adhesion, thereby establishing a foundation for osteogenic differentiation and matrix mineralization at the scaffold-bone interface [13]. Osteoblasts prefer rough surfaces [14]. The hardness of scaffold materials is expected to align with that of natural bone, as mesenchymal stem cells (MSCs) tend to assume an osteoblast-like morphology and efficiently elongate on harder surfaces, while they typically adopt elongated spindle shapes on softer surfaces [10]. The chemical functional groups (e.g., -CH3, -NH2, -COOH, and -OH) at the scaffold-bone interfaces are intricately linked to biocompatibility and immunogenicity, profoundly affecting cell adhesion, proliferation, and differentiation [15,16]. Accordingly, optimizing the physicochemical properties of material-biointerfaces is crucial for enhancing the effectiveness of bone healing.
To date, a range of surface modification technologies has been developed for the design of bone scaffolds [17]. These methods can be classified into three categories: chemical, physical, and biological approaches, each possessing distinct advantages and limitations [10,18,19]. Chemical modifications enable the precise tailoring and functionalization of surfaces; however, they may involve complex processes and pose potential toxicity risks [20,21]. Physical methods (e.g., simple physical absorption, plasma treatments, and laser ablation) offer simplicity and versatility without introducing chemical contaminants, though they may limit functionalization and stability [22,23]. Biological methods primarily employ natural extracellular matrix molecules, antibodies, aptamers, or growth factors as surface modifiers to develop functional scaffold materials [24,25]. However, these methods may encounter challenges related to stability and potential immune responses [26,27]. The choice of the above method depends on the specific requirements of application, taking into account factors such as bioactivity, stability, cost, and complexity. Thus, a simple yet effective surface modification approach is currently in high demand.
Metal-phenolic networks (MPN) are self-assembled supramolecular nanostructures formed by the coordination interactions between polyphenolic ligands and metal ions [28,29]. The use of MPNs as a surface coating strategy, initially proposed by Frank Caruso in 2013, provides a convenient method for combining phenolic ligands with metal ions on various substrate surfaces [30]. This results in materials that possess extensive adhesion, responsiveness to stimuli, antioxidant capacity, anti-inflammatory properties, and antibacterial activity [30,31]. MPNs have emerged as promising candidates for constructing bioactive material interfaces, interacting cell behaviors, and regulating biological processes [[32], [33], [34]]. While there are reviews on the fabrication and applications of MPNs in areas such as drug delivery and disease therapeutics, few have systematically explored the mechanisms by which MPNs regulate cellular behavior to promote bone tissue regeneration [31,[35], [36], [37]]. Therefore, there is a substantial need for a comprehensive summary of how MPNs, as bioactive interfaces, facilitate bone tissue regeneration.
In this review, we will elucidate the natural and material-mediated processes of bone healing, explore the impact of the MPN interface on critical cell fate, and provide an overview of the applications of MPNs in bone regeneration. This review aims to offer theoretical and technical support for bone tissue engineering utilizing MPNs and to encourage the application of material surface engineering in the field of biomedicine (Scheme 1).
Scheme 1.
Schematic illustration of the interface assembly, cell regulation, and bone regeneration applications of MPNs.
2. Natural and material interface-mediated bone healing process
2.1. Natural bone healing process
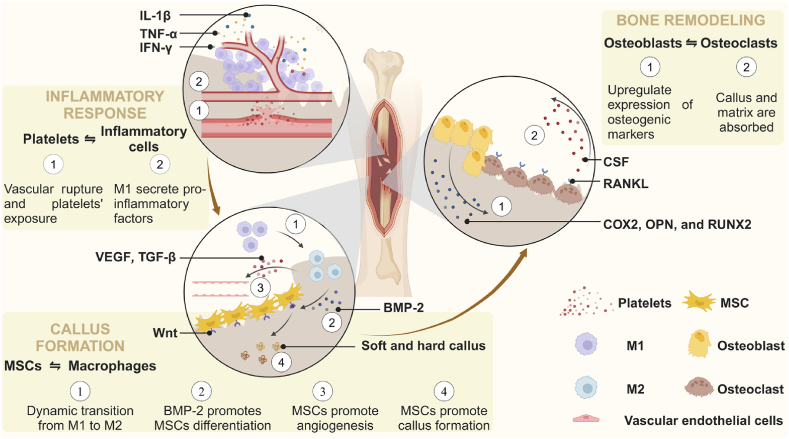
Bone defects are categorized as critical-size or non-critical-size based on their regenerative potential [38]. Non-critical-sized bone defects, characterized by smaller defect volumes, often demonstrate the natural healing capacity of bone [39]. In contrast, bone defects exceeding the critical size threshold will not heal without intervention [40,41]. In the development of animal models for experimental critical bone defects, rats, rabbits, dogs, pigs, and sheep are primarily utilized. The rat model for studying critical bone defects typically involves the creation of circular defects of ≥5 mm in diameter in the calvaria, femur, or mandible [42]. Rabbits models generally featured a 10 mm in diameter circular defect in the calvaria and an 11 mm diameter circular defect in the mandible [43]. The critical bone defect dog model commonly involves a 15 mm diameter and 4 mm deep defect in the mandible [44]. The critical bone defect porcine model mainly involves the preparation of a full-thickness cylindrical defect with a 25 mm diameter in the mandible [45]. The sheep model is primarily used for diaphyseal defects measuring ≥60 mm in size [46]. It is important to note that, at present, rats and rabbits are predominantly used for in vivo studies of biomaterials in laboratory settings, while the application of biomaterials in large animals remains relatively uncommon. This section focuses on the natural bone healing process of non-critical-size defects at the cellular and molecular levels, with the aim of providing inspiration and guidelines for the rational design of biomaterials that can replicate the natural healing process in critical-size defects. The natural healing process of bone tissue can be divided into three stages: (1) Inflammatory Response: The formation of a hematoma and various stimuli activate multiple inflammatory-related cascade signaling pathways within the stromal microenvironment of the bone defect. (2) Callus Formation: Activated and recruited cells coordinate an inflammatory response, promote osteogenesis, and secrete bone matrix components necessary for the formation of both soft and hard calluses. (3) Bone Remodeling: The bone matrix is shaped, calcified, and reconstructed under the precise regulation of osteoblasts and osteoclasts (Fig. 1).
Fig. 1.
Natural bone healing process at the cellular and molecular levels, including inflammatory response, callus formation, and bone remodeling.
Inflammatory Response: Immediately following a bone injury, platelets are exposed to the extravascular environment due to the rupture of blood vessels, which initiates a clotting cascade reaction. Simultaneously, inflammatory cells, including neutrophils and monocytes, are meticulously orchestrated by platelets and endothelial cells to migrate into the extracellular matrix of the hematoma [47]. Subsequently, macrophages undergo polarization into M1-phenotype macrophages (M1) and secrete pro-inflammatory factors, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). Monocyte-derived osteoclasts absorb necrotic bone fragments and temporary fibrin matrices from fractured bones [48]. The localized hypoxic and acidic microenvironment activates signaling pathways associated with osteogenesis and angiogenesis, thereby recruiting MSCs and fibroblasts. This process accelerates the formation of granulomas and replaces hematomas [40,41]. In summary, the initial inflammatory response and hematoma formation are essential prerequisites for subsequent bone healing.
Callus Formation: The recruitment of macrophages, MSCs, and fibroblasts aids in reducing inflammation and promoting the formation of bone matrix [49,50]. M2-phenotype macrophages (M2), which are smoothly reprogrammed from M1, produce anti-inflammatory cytokines such as interleukin-10 (IL-10), arginase-1 (ARG-1), and cluster of differentiation 206 (CD206) to mitigate inflammation. It is worth noting that the rapid phenotypic transition of macrophages from the M1 to M2 state plays a critical physiological role in successful endochondral ossification, maintaining cellular homeostasis, and osteogenesis [51]. M2 contribute to vascularization, immune response, and bone matrix deposition by secreting vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), and bone morphogenetic protein-2 (BMP-2) [52,53]. They subsequently facilitate the migration, homing, and osteogenic differentiation of MSCs [49,54]. MSCs are induced to undergo chondrogenic differentiation by oncostatin M and BMP-2, which are regulated by macrophages [55,56]. Synergistically, vascular endothelial cells (VECs) are stimulated by VEGF to promote angiogenesis, thereby establishing essential pathways for the transport of oxygen and nutrients necessary for bone regeneration. Chondrocytes produce cartilage to create a soft callus, which involves the participation of fibrous tissue. By upregulating the Wnt signaling pathway, MSCs enhance osteogenic differentiation by modulating runt-related transcription factor 2 (RUNX2) and Osterix (OSX) for endochondral ossification [57,58]. The woven bone forms a protective layer over the outer surface of the soft callus, enhancing its mechanical stability. Moreover, active fibroblasts secrete biological substances that promote the initial formation of the callus, while osteoblasts contribute to matrix mineralization and play a crucial role in the formation of the bony callus [40]. Additionally, the formation of neovascular networks is supported synergistically [48]. In summary, macrophages are influenced by the initial inflammatory response to mitigate inflammation and stimulate angiogenesis, while activated MSCs aggregate to promote the formation of bone matrix.
Bone Remodeling: Bone remodeling is a continuous physiological process characterized by a dynamic equilibrium between osteoblasts and multinucleated osteoclasts, which ensures the effective coexistence of callus remodeling and maintenance [59,60]. In particular, macrophages activated by the receptor activator of nuclear factor kappa-B ligand (RANKL) signaling pathway and colony-stimulating factor (CSF) differentiate into osteoclasts, which are responsible for bone callus resorption [61]. Both RANKL and CSF are essential factors in the formation and function of osteoclasts [62]. Simultaneously, osteoblasts upregulate the expression of osteogenic markers, including cyclooxygenase-2 (COX2), osteopontin (OPN), and RUNX2. They also enhance alkaline phosphatase (ALP) activity and promote calcium deposition, leading to the formation of a demineralized bone matrix [63]. It is beneficial for maintaining the homeostasis of callus reconstruction and preservation by balancing the activities of osteoblasts and osteoclasts [64]. Ultimately, the Haversian system is reconstructed, the standard bone shape is restored, and the bone healing process is finalized. In summary, the regulation of homeostasis and the biological functionality of osteoblasts and osteoclasts play a crucial role in the bone remodeling process.
In summary, the initial mild inflammatory response triggers the release of various cytokines, which activate macrophages and MSCs to participate in the formation of both soft and hard calluses. Immunoregulation aimed at reshaping a favorable osteogenic immune microenvironment is essential for mitigating the inflammatory response, promoting osteogenesis, and enhancing angiogenesis. The dynamic equilibrium between osteoblasts and osteoclasts is crucial for the maintenance phase of bone healing.
2.2. Material interface-mediated bone regeneration
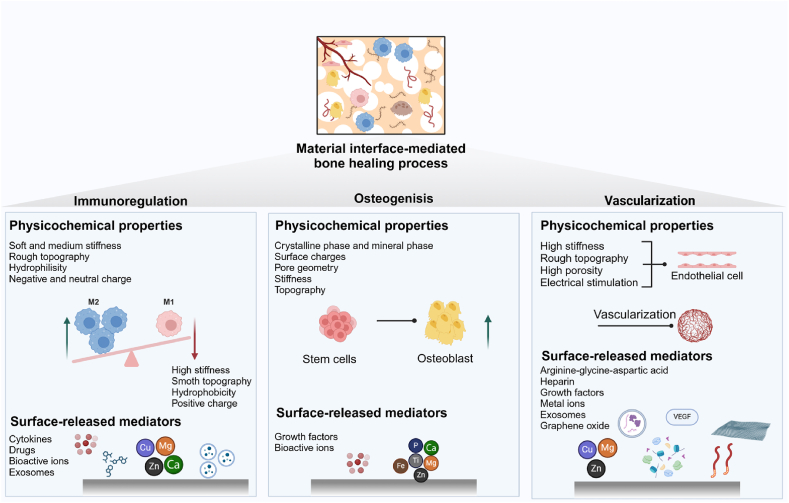
It is challenging for the body's innate repair capacity to address critical bone defects. With the integration of tissue engineering materials, complete bone regeneration is becoming a reality. In response to this challenge, researchers are actively developing biomaterials specifically tailored for the material-bone interface to enhance bone healing. These interfaces are engineered to regulate immunomodulation, induce osteogenesis, and promote vascularization, thereby facilitating bone regeneration mediated by the material interface (Fig. 2).
Fig. 2.
Material interface-mediated bone healing process, including immunoregulation, osteogenesis, and vascularization, influenced by physicochemical properties and surface modifications of these interfaces.
2.2.1. Immunomodulation
In recent years, research has revealed that immune cells, particularly macrophages, play a crucial regulatory role in the process of bone regeneration [65]. Current research indicates that biomaterials can foster a favorable osteoimmune microenvironment by recruiting and polarizing immune cells.
The recruitment and polarization of macrophages have been shown to be influenced by the physicochemical properties of material surfaces and the delivery of exogenous bioactive molecules [66]. The physicochemical properties of the material's surface (e.g., topography, stiffness, porosity, wettability, and surface charge) will directly influence macrophage behavior. For instance, compared to a planar surface, a grooved topographical surface can promote macrophage polarization toward the M2 by elongating cytoskeletal proteins and suppressing the expression of inflammatory factors [67,68]. Compared to the soft (11 kPa) and medium-stiffness (88 kPa) gels, stiff gels (323 kPa) generate mechanical stimuli that activate to the cell surface cation channels and promote differentiation towards M2 [69,70]. Hydrophilic or neutral biomaterials can promote M2 activation due to conformational changes in fibronectin and fibrinogen, whereas hydrophobic biomaterials tend to polarize towards M1 [71]. Scaffold surfaces with a positive surface charge are more likely to elicit inflammatory responses and activate M1 due to their interaction with negatively charged cell membranes, in contrast to surfaces with a negative or neutral charge [65]. The delivery of exogenous bioactive molecules to the scaffold-bone interfaces is a conventional method for enhancing the diverse functions of macrophages. Common delivery substances primarily include cytokines (e.g., IL-4 [72] and BMP-2 [73]), drugs (e.g., dexamethasone [74] and sitagliptin [75]), bioactive ions (e.g., Ca2+, Mg2+, Zn2+, and Cu2+) [[76], [77], [78], [79], [80]], and exosomes [81]. In summary, modifying physicochemical properties and delivering bioactive substances at the scaffold-bone interfaces are fundamental aspects of immunomodulation.
2.2.2. Osteogenesis
Proverbially, osteoconductivity and osteoinductivity are essential properties of implant biomaterials that enhance osteogenesis [82,83]. The key to effectively reinforcing osteogenesis lies in three crucial aspects: the physicochemical properties, metal ions, and bioactive molecules present on the material surfaces.
The surface physicochemical properties play a crucial role in regulating cell adhesion, proliferation, differentiation, and bone mineral formation [84]. Ceramics, which are non-metallic inorganic biomaterials, are widely recognized for their osteoconductive properties due to their crystalline and mineral phases that closely resemble those of natural bone tissue [83]. Surface charges generated by electrical stimulation influence cellular adhesion, proliferation, arrangement, migration, and differentiation through the calmodulin/calcineurin/nuclear factor of activated T-cells (CaM/Cn/NFAT) pathway, the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway, and Ca2+ influx [85,86]. The geometry of pores, including variations in channel size, shape, and curvature, can influence cellular functions [87]. The size and shape of the middle channel can enhance bone formation. High-curvature pores promote increased ectopic bone formation by activating mechanosensitive pathways. Additionally, the multicellular spatiotemporal organization of pre-osteoblasts suggests that these cells preferentially inhabit regions characterized by at least one negative principal curvature [78,88]. Biomimetic scaffolds are designed to replicate the properties of natural bone tissue, providing dynamic mechanical stimuli to promote the osteogenic differentiation of MSCs. And the MSCs will differentiate into osteoblasts on scaffolds that have been stiffened after 24 h, due to increased nuclear tension and histone acetylation [89]. The topographical cues, such as micro- and nano-structures in micro/nano-hybrid scaffolds, can facilitate osteogenic differentiation by activating various receptors on osteoblasts [90]. The bioactive ions (e.g., Ti4+, Mg2+, Fe3+, Zn2+, and Ca2+) released from scaffolds could stimulate the osteogenic differentiation of stem cells [91]. For example, the incorporation of Sr2+, Mg2+, and Li+ into bioceramics could reduce RANKL-induced osteoclasts formation and promote osteogenesis [[92], [93], [94]]. In addition, bioactive macromolecules (e.g., growth factors and stem cells) loaded onto the scaffolds can also participate in the osteogenic differentiation of stem cells at the interface of the scaffolds [[95], [96], [97]]. In summary, the physicochemical properties, metal ions, and bioactive molecules present on material surfaces play a significant role in osteogenesis during the bone healing process.
2.2.3. Vascularization
Given that bone is a highly vascularized tissue, angiogenesis is closely associated with the regeneration of bone tissue. Notably, in retrospect to the previous experiments on vascularization, modifications of the physicochemical properties and biochemical components of scaffold surfaces have been proposed. Compared to softer materials, stiffer materials promote endothelial cell migration and adhesion [98]. Increasing the roughness of material surfaces enhances endothelial cell adhesion, promotes cell growth, and stimulates angiogenesis [99]. High porosity in the implanted materials can create an optimal microenvironment for cell interactions and migration, thereby promoting the rapid vascularization of the defective tissue [100]. Electrical stimulation enhances the proliferation and tuberization of VECs and regulates the synthesis of crucial growth factors and cytokines (e.g., basic fibroblast growth factor, angiopoietin-2, thrombopoietin, and epidermal growth factor) during angiogenesis. This process occurs through the activation of the phosphoinositide 3-kinase (PI3K) and rho-associated protein kinase (ROCK) signaling pathways [101]. The components used for biochemical surface modification of scaffolds can influence the behavior of VECs. Scaffold surface modifications include arginine-glycine-aspartic acid [102,103], heparin [104], growth factors (e.g., VEGF [105]), metal ions (e.g., Cu2+, Mg2+, and Zn2+) [[106], [107], [108]], exosomes [109], and graphene oxide [110]. Herein, the vascular networks induced by the physicochemical properties and active components of scaffold materials could effectively promote angiogenesis and facilitate bone repair.
After implantation, the bioactive scaffold interfaces directly interact with the cells at the defect site, regulating the bone immune microenvironment, stimulating osteogenic activity, and enhancing vascularization. In order to efficiently facilitate the bone healing process at biointerfaces, several design principles must be considered.
-
-
Biocompatibility: Materials must not provoke a harmful immune response and should promote the activity of regenerative cells.
-
-
Bioactivity: Materials must actively engage with the biological environment to facilitate healing processes.
-
-
Degradability: Ideally, the material should degrade at a rate that aligns with new bone formation, gradually transferring the mechanical load to the healing tissue.
3. Metal-phenolic networks
MPNs represent an emerging class of organic and inorganic hybrid complexes formed by the combination of metal ions and polyphenols. These complexes create a composite network through various interactions, including coordination interactions, cation-π interactions, dynamic covalent interactions, and redox interactions [36,111]. MPNs can be effectively applied to a diverse range of material substrates, imparting various physicochemical functions that regulate cell fate [37,112]. In this section, we will provide a comprehensive introduction to composites and their physicochemical properties, establishing a foundation for the biological applications of MPNs as bioactive interfaces.
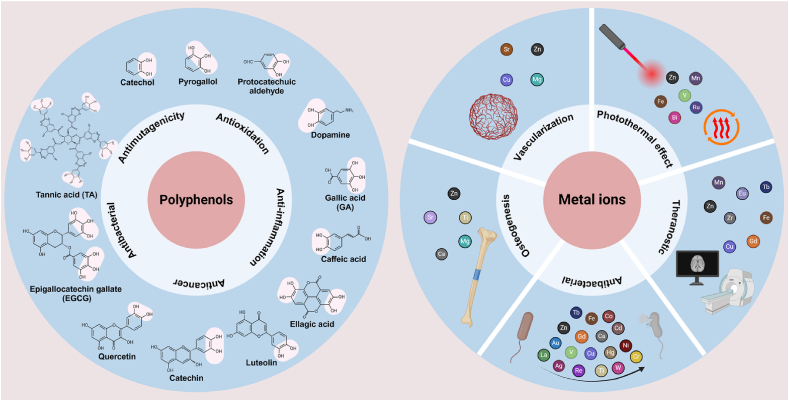
3.1. Flexible diversity of polyphenol ligands
Polyphenols, containing one or more phenol, catechol, or pyrogallol groups, are secondary metabolites primarily found in a diverse range of plant-based foods, including fruits, vegetables, tea leaves, and cereals [113]. In recent years, representative phenolic compounds, such as tannic acid (TA) [114], epigallocatechin gallate (EGCG) [115], pyrogallol (PG) [116], gallic acid (GA) [117], dopamine (DA) [118], and quercetin [119], have been extensively applied in the biomedical field [120,121]. Polyphenols exhibit distinctive characteristics, including antioxidant, anticancer, anti-inflammatory, antimutagenic, and antibacterial properties, which potentially provide protection against certain chronic diseases [122,123]. The natural bioactivities mentioned above are influenced by the number and position of phenol groups, which in turn affect cellular outcomes.
3.2. Diversity and functionality of metal ions
MPNs containing various metal ions, including transition metals and main group metals, exhibit a wide range of functionalities, such as antibacterial, osteogenic, and angiogenic properties [36]. According to their functionalities, the metal ions can be categorized into several types: those with photothermal properties [[124], [125], [126], [127]] (e.g., Zn2+, Mn2+, Fe3+, V3+, Bi3+, and Ru3+), theranostic functions [[128], [129], [130]] (e.g., Mn2+, Cu2+, Zn2+, Eu2+, Zr2+, Fe3+, Gd3+, and Tb3+), antimicrobial properties [[131], [132], [133], [134], [135], [136], [137], [138], [139], [140]] (e.g., Ag+, Au+, Cu2+, Ca2+, Zn2+, Co2+, Ni2+, Cd2+, Hg2+, La3+, Gd3+, Cr3+, Tb3+, V3+, Re3+, Fe3+, Ti4+, and W6+), osteogenic properties [[141], [142], [143], [144]] (e.g., Zn2+, Mg2+, Ca2+, Sr2+, and Ti4+), and vascularization [139,[145], [146], [147], [148], [149]] (e.g., Mg2+, Sr2+, Cu2+, and Zn2+). Versatile metallic elements offer a multitude of biochemical and biophysical signals that can modulate material properties, thereby enhancing our understanding of tissue regeneration engineering.
A summary of the various biological functions of polyphenols and metal ions is presented in Fig. 3. Although many natural polyphenols have been utilized for the assembly of MPNs, the exploration of synthetic polyphenols remains limited. Furthermore, we aim to expand the functional characteristics of polyphenols to enhance the applications of MPN assembly in the future. In addition, the toxicity of metal ions must be thoroughly considered when applying MPNs in biomedicine, as certain metal ions may pose biocompatibility concerns, even at very low doses.
Fig. 3.
Diversity and biologic activities of polyphenols and metal ions.
3.3. Physicochemical properties of MPNs
The physicochemical properties of MPNs play a crucial role in various biological functions. In this section, we will provide a brief overview of the assembly behavior, adhesion properties, controlled permeability, stimuli-responsiveness, and catalytic properties (Fig. 4).
Fig. 4.
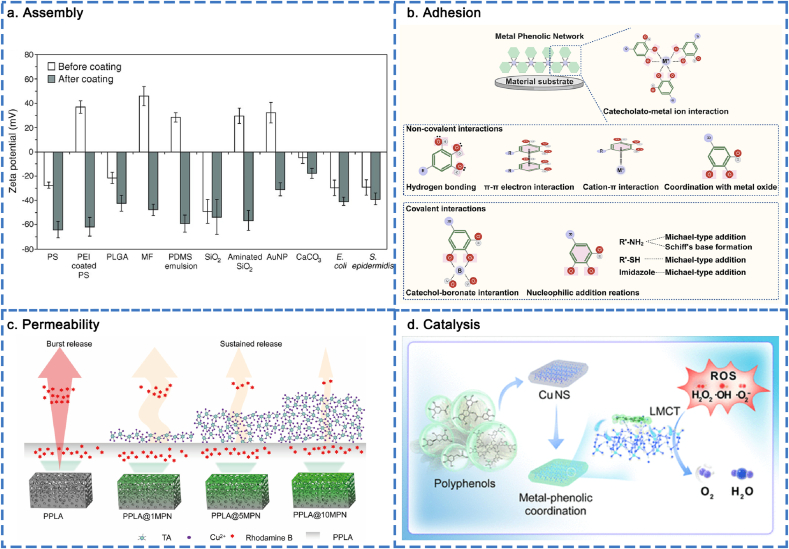
Physicochemical properties of MPNs. (a) MPNs assembled at different surface substrates. Reproduced with permission from Ref. [30]. Copyright 2013 Science. (b) Mechanism of extensive adhesiveness. (c) Permeability of MPNs. Reproduced with permission from Ref. [158]. Copyright 2021 ACS Appl. Mater. Interfaces. (d) Catalysis ability of MPNs. Reproduced with permission from Ref. [161]. Copyright 2024 ACS Nano.
3.3.1. Assembly
The “one-pot” assembly strategy, initially proposed by Frank Caruso in 2013, offers a straightforward method for combining phenolic ligands and metal ions on a variety of substrates, regardless of their composition, size, shape, or structure [30]. It has been reported that MPNs can be assembled on various surface substrates, including glass, gold (Au), polydimethylsiloxane (PDMS), poly (lactic-co-glycolic acid) (PLGA), silica (SiO2), aminated SiO2, cetyltrimethylammonium bromide-capped Au nanoparticles (Au NPs), calcium carbonate (CaCO3), Escherichia coli, and Staphylococcus epidermidis (Fig. 4a) [30]. The molar ratio of metal ions to polyphenols affects the thickness and roughness of MPN structures. It is worth mentioning that, compared to polyphenols, metal ions exert a more significant influence on the assembly process. The assembly process ends when the metal ions are exhausted, and MPNs gradually aggregate as the number of metal ions involved increases. The thickness of the MPN films depends on the selected metal and the metal feed concentration. Favorable results demonstrated that, compared to metals with different oxidation numbers (Cu2+/TA, Al3+/TA, and Zr4+/TA), the film thickness of Zr4+/TA was the thickest and Cu2+/TA capsules the thinnest. The film thickness of the capsules increased as the metal-to-ligand feed ratio changed from 1:3 to 3:1 [150]. The size of the MPN nanoparticles (NPs) can be controlled by adjusting the reaction time, precursor concentration, and the metal-to-ligand ratio [151]. MPN NPs gradually increased from 20 to 60 nm as the reaction time increased from 0 to 30 min and then plateaued. When the concentrations of the precursors were increased simultaneously, the size of the MPN NPs increased proportionally. The size of the NPs could also be increased by increasing the molar ratio of metal ions to polyphenols. To demonstrate the surface charges of MPNs, using TA as a model polyphenol, all selected metal ions (Cu2+, Fe3+, Al3+, Zr4+, and Ti4+) formed MPN NPs featuring ζ-potentials ranging from −30 to −50 mV. Using Fe3+ as a model metal ion, the use of smaller phenolic ligands (TA, EGCG, CAT, and GA) resulted in the formation of MPN NPs with more neutral ζ-potentials [152]. Increased concentrations of phosphate buffer (ranging from 0.1 to 10 mM) resulted in MPN NPs (Fe2+/quercetin) featuring ζ-potentials ranging from −30 to −40 mV. Changing the pH range from 4 to 8 led to the deprotonation of quercetin and insulted MPN NPs (Fe2+/quercetin) in a transition in ζ potential from −15 to −30 mV [151]. The deposition of MPNs layers contributed to the high stiffness of the resulting films, which was attributed to the clustered distribution of metal-catechol/pyrogallol complexes. Conversely, the removal of metal significantly leaded to a reduction in material stiffness [36,153]. Accordingly, MPNs can assemble on a variety of material substrates, and the assembly process can be precisely regulated through reaction time, precursor concentration, and metal-to-ligand ratio. This provides viable platforms for various material interfaces to implement MPN-based modification strategies.
3.3.2. Adhesion
Inspired by the remarkable underwater adhesion properties of mussel foot proteins that contain L-3,4-dihydroxyphenylalanine (DOPA), this adhesion mechanism can be utilized on a variety of substrate surfaces. The interactions between MPNs and the surfaces of substrates are classified as non-covalent and covalent interactions. The non-covalent interactions include hydrogen bonding, π-π electron interactions, cation-π interactions, coordination with metal oxide surfaces, and catecholato-metal ion interactions. The covalent interactions include catechol-boronate interactions and nucleophilic addition reactions (Fig. 4b) [154]. MPN-based interface modification serves as the foundation for waterproof adhesives, wherein the quinone-catechol reversible redox reaction involving silver and lignin enhances long-term adhesion to both hydrophilic surfaces (e.g., heart, lung, kidney, spleen, liver, bone, and muscle) and hydrophobic surfaces (e.g., polypropylene, Teflon, rubber, glass, nut shell, and steel) [155,156]. Accordingly, MPNs exhibit strong adhesion to a wide range of material surfaces through both non-covalent and covalent interactions.
3.3.3. Permeability
Cargo encapsulation and release can be achieved through the programmable permeability of MPNs. The intermolecular interactions (e.g., coordination, π-π stacking, hydrogen bonding, and electrostatic interaction) of MPNs can be modulated by intrinsic (i.e., building blocks) and extrinsic (i.e., environmental pH) parameters. This modulation allows for the dynamic gating of MPNs, including moderately permeable, highly permeable, or nearly impermeable states [157]. In this section, we primarily focus on the types of phenolic and metal building blocks, as well as the molar ratios of the precursors. The extrinsic parameters will be discussed in the next section. Specifically, increasing the Fe3+/TA molar ratio significantly altered the permeability of the Fe3+/TA capsules as the ratio increased from 0.5:1 to 3:1. And the continued increase beyond 3:1 resulted in additional nanoscale defects on the Fe3+/TA capsules, which translated to higher permeability. The impact of metal ions on capsule permeability was investigated using Fe3+/TA, Al3+/TA, and Cu2+/TA capsules. The strong binding affinity between Fe3+ and the catechol/galloyl groups of TA resulted in the formation of stable Fe3+/TA networks, which effectively reduced permeability. Al3+ and Cu2+ exhibited a significantly lower binding affinity to TA, leading to a decreased cross-linking density within the network. This reduction in cross-linking resulted in diminished steric hindrance between TA molecules, ultimately contributing to increased permeability. The effect of phenolic ligands on capsule permeability was examined using Fe3+/TA, Fe3+/GA, and Fe3+/PG capsules, all of which maintained the same molar ratio of phenolic groups. The carboxylic groups on GA could potentially provide additional driving forces (e.g., hydrogen bonding, and salt bridging of carboxylate) for capsule shrinkage, resulting in the lowest permeability, while the Fe3+/TA and Fe3+/PG capsules exhibited high permeability [157]. Accordingly, the adjustable permeability of MPNs offered valuable insights for designing smart hybrid materials intended for drug delivery applications. Our group reported a MPNs (Cu2+/TA) coating constructed on the surface of a porous poly (DL-lactide) (PPLA) scaffold. Based on the low permeability and molecular gating properties of the Cu2+/TA supramolecular structure, the PPLA@MPN scaffold achieved sustained release of the loaded growth factors (BMP-2). This endowed the scaffold with osteogenic capabilities and enhanced its performance in bone tissue engineering (Fig. 4c) [158].
3.3.4. Catalysis
MPNs are effective agents in the Fenton reaction. H2O2 can be activated by Cu+/Fe2+ to continuously produce highly reactive hydroxyl radicals (·OH), which have applications in various fields. In the MPN-participated Fenton reaction, polyphenols facilitated the valence transformation between Fe2+ and Fe3+, enabling the continuation of the Fenton reaction. According to Zhu and co-workers, a MPN (Fe3+/mitoxantrone) nanocatalyst was fabricated to catalytically convert endogenous H2O2 into highly reactive hydroxyl radical (·OH). This process enhances catalytic performance for irreversible ferroptosis by accumulating lethal lipid peroxides, ultimately leading to the death of tumor cells [159].
In addition, MPNs can function as a class of synthetic nanozymes that mimic the superoxide dismutase (SOD) and catalase (CAT) cascade, effectively eliminating various reactive oxygen species (ROS). Cu is essential in various natural enzymes (i.e., SOD) [160]. Our group reported an artificial biocatalyst, induced by phenolic ligand-metal charge transfer (LMCT), that utilized Cu2+ antioxidant nanozymes to mimic natural enzymes. This biocatalyst effectively eliminated ROS and facilitated the healing of chronic diabetic wounds (Fig. 4d) [161]. The MPN nanozyme demonstrated the ability to scavenge ROS through LMCT at a nanointerface. This finding offers an alternative strategy for developing metal-based nanozymes for the treatment of diabetic wounds and other diseases.
Accordingly, the catalytic ability of MPNs mainly serves to enhance ferroptosis through the Fenton reaction and mitigate oxidative stress.
3.3.5. Stimuli-responsiveness
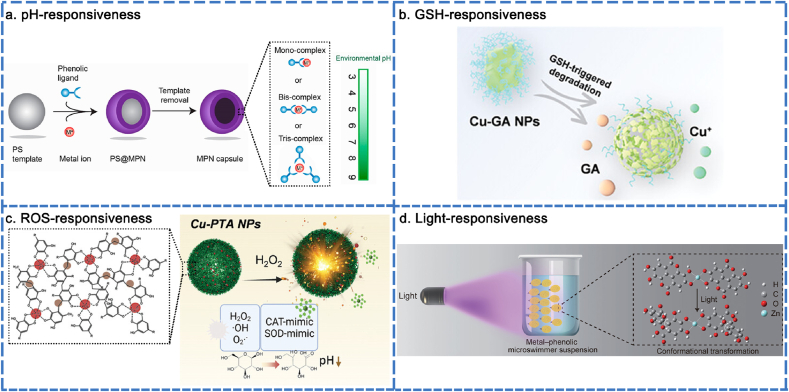
The stimuli-responsiveness of MPNs was harnessed to enhance the capabilities of smart nanodrug delivery systems, including improved loading efficiency, bioavailability, and on-demand delivery. This section focuses on the responsiveness of MPNs to various stimuli, including pH, glutathione (GSH), reactive oxygen species (ROS), ultraviolet (UV) light, and near-infrared (NIR) radiation.
The coordination interaction occurs when two or more phenolic ligands donate a non-bonding electron pair to the vacant orbitals of a metal ion. Mono-complexes form at low pH, while bis- and tris-complexes are produced at high pH. Mono-complexes are structurally unstable and prone to structural disintegration, whereas bis- and tris-complexes exhibit constitutional stability [36]. The conversion between bis- or tris-complexes and mono-complexes is achievable by adjusting the pH, which endows MPNs with controlled assembly and disassembly dynamics, enabling the on-demand loading and release of cargo. It is worth noting that raising the pH increases the permeability of MPN capsules. The elevated pH facilitates the deprotonation of phenolic ligands, leading to MPN capsules acquiring a more negative charge. The enhanced electrostatic repulsion between phenolic ligands, along with the increased tendency to form tris-state complexes, resulted in the loosening of internal molecular packing and the enlargement of pores within the film. MPN capsules can load cargo at a pH of 9. By reducing the pH to 4 after loading, the cargo can be encapsulated within the inner MPN capsules due to their increased hydrophobicity, closer packing, and near-impermeable properties. The cargo encapsulated in MPN capsules is released at varying pH levels. From pH 5 to 9, the capsules demonstrate increasing permeability. The programmable permeability of MPN capsules could be potentially useful for smart coatings (Fig. 5a) [157]. In another study, the pH-responsive drug release from MPN was also an intelligent nanodrug delivery platform. Cu2+/3,4,5-trihydroxybenzaldehyde (THBA) NPs were developed to load tobramycin (TOB) using a one-step self-assembly method based on a metal-phenolic network and Schiff base reaction to eradicate biofilms. The acidic environment disrupted the hydrogen bonds and metal coordination, resulting in the dissociation of NPs, which exert antibacterial effects [162]. Accordingly, pH can flexibly modulate the degree of MPN coordination to control drug loading and release.
Fig. 5.
Stimuli-responsiveness of MPNs. (a) pH-responsiveness of MPNs. Reproduced with permission from Ref. [157]. Copyright 2020 Chem. Mater. (b) GSH-responsiveness of MPNs. Reproduced with permission from Ref. [166]. Copyright 2023 Adv Healthcare Mater. (c) ROS-responsiveness of MPNs. Reproduced with permission from Ref. [167]. Copyright 2023 Adv Healthcare Mater. (d) Light-responsiveness of MPNs. Reproduced with permission from Ref. [168]. Copyright 2021 Adv. Mater.
Reports have revealed that elevated levels of ROS and significant depletion of GSH are advantageous strategies in cancer treatment [163]. MPNs have emerged as promising candidates for depleting intratumoral GSH. This can be achieved by conjugating GSH with specific molecules to form GS-X conjugates via Michael addition reaction, as well as by oxidizing GSH using metal ions, such as Fe3+ [164]. Favorable results indicated that Fe3+/Naphthazarin (Nap) NPs underwent GSH-responsive disintegration and reduction into Fe2+ and Nap. The released Nap disrupted intracellular redox homeostasis by depleting GSH, while Fe2+ launched the generation of ROS, resulting in remarkable tumor inhibition [165]. Similarly, Yu's group reported a Cu2+/GA NPs for tumor therapy through both GSH depletion and ROS generation. Through GSH-responsive dissociation, GA depleted excess GSH and disrupted the intracellular redox balance, leading to a reduction in unwanted ROS consumption (Fig. 5b). Cu2+ have also been reported to exhibit the ability to oxidize GSH, which could further deplete GSH and simultaneously generate Cu+, enhancing Fenton catalytic activity [166]. Taking into account the elaboration above, the GSH-responsive dissociation of MPNs holds significant potential for achieving optimal therapy by disrupting intracellular GSH levels and ROS generation.
In another study, the ROS-responsive degradation of MPNs was demonstrated in a microenvironment with elevated ROS levels, while the MPNs remained intact in a physiological environment. Cu2+/TA NPs exhibited a ROS-responsive release pattern. The abundant galloyl groups present in TA could modify the SOD to eliminate superoxide anions (·O2−). The dynamic changes in the oxidative state of Cu were sensitive to H2O2, which mimics CAT by decomposing H2O2 to produce O2 and ·OH. The varying concentrations of ROS resulted in dynamic changes in the morphology of Cu2+/TA NPs, transitioning from a blurred spherical shape to complete disappearance (Fig. 5c) [167]. In summary, the ROS-responsive degradation of MPNs maintained a balance between oxidation and antioxidation through the release of metal ions and polyphenols in response to stimuli.
Light (NIR/UV) driven directional motion is another aspect of the programmable nature of MPNs, which enhances their versatility in cargo loading. Frank Caruso and his colleagues reported on MPN (Zn2+/ellagic acid) microswimmers capable of swimming toward an external UV light source. The activated motion was generated through the conformational transformation of the phenolic ligands upon illumination, resulting in a significant twist in the planar molecule that propelled the particles forward. The UV-induced motion exhibited rapid and sustained positive phototaxis, with both the direction and speed of movement being highly regulated (Fig. 5d) [168]. In another report, the motion of NIR-driven microrobots was attributed to the temperature gradient and fluid distribution. The effective motion control increased the likelihood of particles encountering radicals in the solution, thereby enhancing antioxidative performance [169]. Accordingly, the light-driven microrobots offer a synthesis strategy for developing MPN-based, light-navigated microrobots for future medical treatments.
Summary, the various responses of MPNs induce structural and performance modifications that enable adaptation to complex environmental variations, thereby enhancing their practicality and feasibility in the biomedical domain.
4. MPN-mediated cell regulation
In this section, we describe the regulation of bone repair-related cells (macrophages, osteoblasts, and VECs) within the context of MPN interface-mediated bone regeneration.
4.1. Macrophage
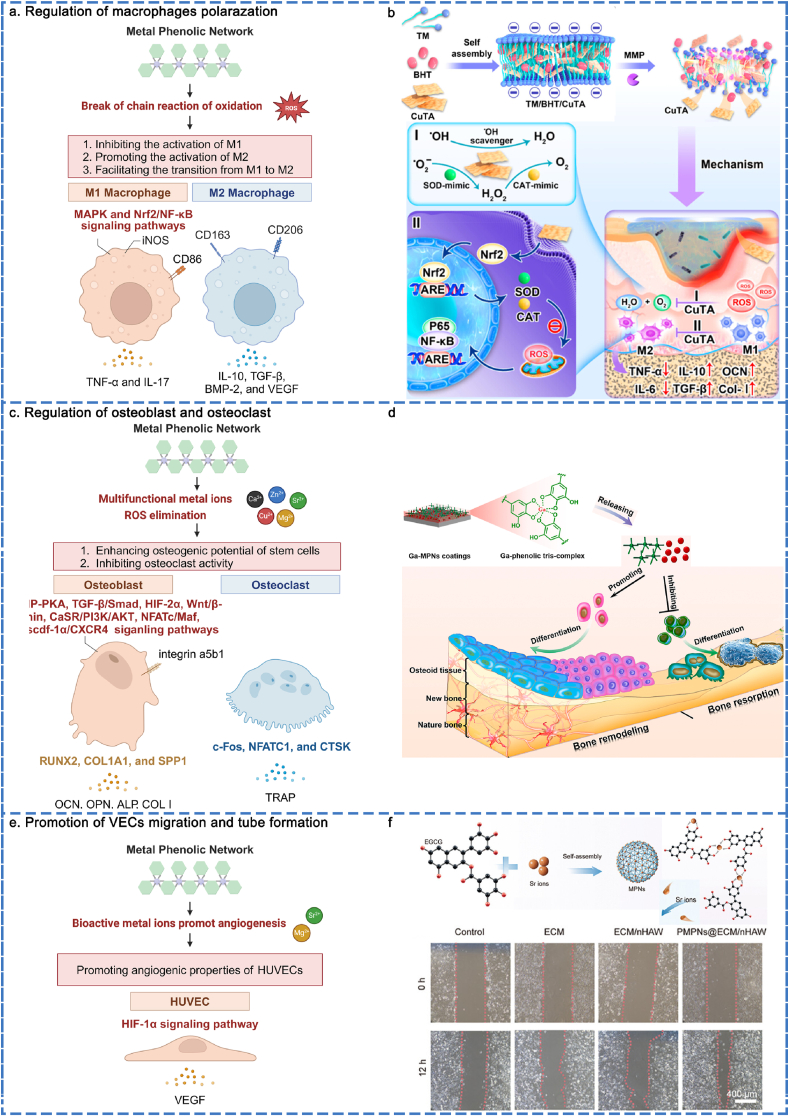
The immune response is a crucial factor in determining the fate of implanted materials, with macrophages playing a significant role in immunoregulation. It is a novel concept to design MPN interfaces with immunomodulatory functions aimed at regulating the immune inflammatory response and establishing a beneficial bone immune microenvironment through the modulation of macrophage polarization. MPNs possess the capability to inhibit the activation of the M1, promote the activation of M2, and facilitate the transition of phenotype from M1 to M2 (Fig. 6a).
Fig. 6.
MPN biointerace-mediated cell regulation of macrophages, osteoblasts, and VECs. (a) Regulation of macrophage polarization by MPNs. Reproduced with permission from Ref. [79]. Copyright 2023 ACS Nano. (b) Regulation mechanisms of macrophage polarization by MPNs. (c) Promoting osteoblasts differentiation and inhibiting osteoclasts differentiation. Reproduced with permission from Ref. [177]. Copyright 2023 Biomaterials. (d) Regulation mechanisms of osteoblasts promotion and osteoclasts inhibition. (e) Promotion of VECs migration. Reproduced with permission from Ref. [182]. Copyright 2024 Adv Healthcare Mater. (f) Regulation mechanisms of promoting VECs angiogenesis.
The most distinctive characteristic of polyphenols is the presence of catechol or pyrogallol groups attached to the benzene rings. The donation of hydrogen atoms and the transfer of electrons and protons from catechol or pyrogallol groups initiated the termination of the oxidation chain reaction [170]. Metal ions (e.g., Cu2+, Mg2+, Sr2+, and Zn2+) can modulate physiological and pathological immune responses, influencing macrophage polarization [171]. Accordingly, MPNs can be customized to modulate intracellular or microenvironmental levels of ROS, thereby demonstrating significant anti-inflammatory capabilities by inhibiting M1 polarization and promoting M2 polarization. According to Deng's group, a glucose-primed orthopedic implant was coated with Cu2+ and polydopamine (PDA). This coating dissociated in the low pH environment characteristic of diabetic infections and demonstrated significant efficacy in combating ROS. RNA sequencing analysis revealed that M1 activation-related pathways (e.g., MAPK, IL-17, TNF-α, and NF-ĸB signaling pathways) were downregulated by the suppression of the glycolysis pathway. In contrast, M2 polarization was significantly enhanced through the upregulation of fatty acid oxidation and oxidative phosphorylation [172]. Similarly, another report indicated that Mg2+/TA was incorporated into a filler through a blending electrospinning process, demonstrating sustained release behavior via pH-responsive mechanisms to scavenge ROS, thereby creating an anti-inflammatory environment. The results demonstrated that the proportion of CD206+ macrophages increased, while the proportion of iNOS+ macrophages decreased. It is worth noting that an anti-inflammatory microenvironment is positively correlated with a higher concentration of loaded Mg2+ within the physiological dose range [142]. Besides, our group developed a MPN (Sr2+/TA) coating for titanium implants. TA inhibited M1 polarization mainly due to its ability to scavenge ROS, while Sr2+ simultaneously promoted M2 polarization [173]. Similarly, Shin's group reported a gelatin cryogel loaded with MPNs, which were released in a sustained manner. The results revealed that the fraction of CD206+ cells was restored, while the percentage of CD206−/iNOS+ cells significantly decreased [174]. ROS was a crucial factor in inducing M1 activation and inhibiting M2. MPNs can compete with ROS to alleviate oxidative stress in the microenvironment, promoting M2 polarization and inhibiting M1 activity.
In another perspective, the dynamic transition from M1 to M2 macrophages plays a crucial role in regulating the inflammatory microenvironment. In an inflammatory state, macrophages release inflammatory mediators to eliminate pathogens and necrotic cells. Macrophage transition into a reparative state and release factors, such as VEGF, that promote tissue repair, thereby accelerating the healing process once inflammation subside. In recent years, MPNs have been integrated into biomaterials, effectively modulating the transition from M1 to M2 macrophages and enhancing the pro-healing properties of these materials. The Cu2+/TA nanozyme is an antioxidative cascade MPN nanozyme that possesses multiple ROS scavenging capabilities. Accordingly, Xu et al. constructed a Cu2+/TA nanozyme hydrogel system to facilitate the transformation of M1 to M2 by regulating the nuclear factor erythroid 2-related factor 2/nuclear factor kappa-light-chain-enhancer of activated B cells (Nrf2/NF-κB) pathway (Fig. 6b) [79]. Besides, Zhang's group reported a cellulose acetate membrane modified with MPNs (protocatechualdehyde (PCA)/Zn2+/Mg2+/Cu2+) to facilitate the transition from M1 to M2 macrophages, thereby creating a favorable osteoimmune microenvironment. The results demonstrated that the expression of pro-healing genes, including IL-10, TGF-β, BMP-2 and VEGF, has dramatically increased, while the expression of M2 phenotype markers CD163 and CD206 has also significantly increased [175]. In addition, a biomimetic cancellous bone scaffold was infused with Mg2+/tea polyphenol nanoclusters. As a controlled-release agent, Mg2+ and tea polyphenols could regulate the polarization of M1 toward M2, promote the expression of anti-inflammatory factors such as CD206, IL-10, and VEGF, and inhibit the expression levels of pro-inflammatory factors like IL-1β, iNOS, and TNF-α. This modulation supports the healing of infected bone defects in a severe inflammatory environment [176]. Accordingly, MPNs offer a promising platform for the smooth reprogramming of macrophages from the M1 to M2 phenotype, facilitating immunoregulation and fostering a favorable bone immune microenvironment.
In summary, the regulation of macrophage polarization mediated by MPNs primarily involves the inhibition of M1, the promotion of M2, and the dynamic transition from M1 to M2. The mechanisms underlying the aforementioned behaviors involve the MAPK and Nrf2/NF-ĸB signaling pathways.
4.2. Osteoblast
The regulation of the balance between osteogenesis and osteoclastogenesis is crucial for bone regeneration. MPNs have the capacity to enhance the osteogenesis of osteoblasts and secrete bone matrix, facilitating the repair of bone defects. At the same time, previous studies have reported that inhibiting osteoclast differentiation reduces bone matrix loss (Fig. 6c).
The biologically multifunctional metal ions (e.g., Ga3+, Zn2+, Mg2+, Sr2+, and Cu2+), which are composed of MPNs, are not only integral to bone tissue but also play a crucial role in osteogenic activities through various signaling pathways. The osteoinductive effects of polyphenols are considered crucial. Some studies suggest that polyphenols may contribute to osteogenesis by chelating mineral ions at the regeneration site and promoting cell adhesion, spreading, proliferation, and differentiation due to their high content of catechol or pyrogallol groups. Polyphenols can inhibit the receptor activator for nuclear factor-ĸ/receptor activator of nuclear factor kappa-B ligand/osteoporogeterin (RANK/RANKL/OPG) pathway by eliminating ROS and downregulating the maturation of osteoclasts. Accordingly, the regulation of osteogenic potential and osteoclast activity is aligned with the interaction of metal ions and polyphenols. The primary effect of Ga3+ is to inhibit bone resorption while promoting bone mineralization. Xu et al. reported the fabrication of an MPN (Ga3+/TA) coating on implants. The Ga3+/TA coating facilitated the sustained release of Ga3+ in situ, thereby partially mitigating the side effects associated with systemic administration. TA not only enhanced the fixation of biomacromolecules on substrate surfaces through noncovalent (e.g., electrostatic interactions, hydrogen bonds) and covalent (e.g., Schiff base reactions) reactions, but also reduced the loss of calcium ions through chelation. The results demonstrated that the Ga3+/TA coating upregulated the mRNA expression of osteogenic differentiation-related markers (e.g., RUNX2, ALP, COL I, and OCN) of MC3T3-E1 cells during the early stage and promoted the secretion of key proteins essential for osteogenesis (e.g., OCN and OPN) in the later stage. The mRNA expression of markers related to osteoclast activity (e.g., c-Fos, NFATC1, CTSK, and TRAP) was reduced [177]. Zn2+ activates the cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) pathway, leading to MAPK phosphorylation and promoting osteogenesis. Zn2+ also plays a crucial role in the transduction of transforming growth factor-β/small mother against decapentaplegic (TGF-β/Smad) signaling, contributing to various functions related to osteogenesis and tissue mineralization. Accordingly, Zhang's group designed a cellulose acetate membrane modified with Zn2+/PDA to preserve the osteogenic potential of the PDLSCs following H2O2 treatment [175]. Mg2+ is recognized for their role in osteogenesis by activating HIF-2α and Wnt/β-catenin signaling pathways in stem cells. This activation leads to an increased expression of osteogenic markers and promotes mineralization. Previous studies have revealed that the sustained and controlled release of Mg2+ from MPN NPs activated stem cells, increased mineralization, and promoted ALP activities, thereby exhibiting significant bone-enhancing effects [174,176]. Similarly, Gao and co-workers reported a nanofibrous composite made of Mg2+ and TA to enhance the adhesion, proliferation, and osteogenic differentiation of BMSCs by upregulating integrin α5β1 expression (Fig. 6d) [142]. Numerous studies have demonstrated the influence of Sr2+ in accelerating osteogenesis and enhancing osseointegration [178]. The mechanisms involved include the activation of wingless-related integration site/β-catenin (Wnt/β-catenin) [179], calcium-sensing receptor/phosphatidylinositide 3-kinase/protein kinase B (CaSR/PI3K/AKT) [180], and nuclear factor-activated T cells/musculoaponeurotic fibrosarcoma (NFATc/Maf) [181] pathways, of all which contribute to the bone anabolism in the initial stages. Sr2+ can reduce the formation of osteoclast and the expression of genes related to osteoclastogenesis. Accordingly, our group reported a surface modification strategy using MPN (Sr2+/TA). The Sr2+/TA modification significantly enhanced the recruitment and mobilization of endogenous stem cells through the activation of the stromal cell-derived factor-1α/Cys-X-Cys chemokine receptor 4 (SCDF-1α/CXCR4) signaling pathway, thereby promoting mineralization and late-stage osteogenesis [173]. Similarly, Liu et al. developed a Sr2+/EGCG modification on a biomimetic scaffold composed of extracellular matrix (ECM) and hydroxyapatite nanowires (nHAW). The Sr2+/EGCG modification induced osteogenic differentiation in stem cells and upregulated the expression of osteogenesis-related genes, such as RUNX2, COL1a1, and SPP1 [182]. As an osteogenesis-inducing factor, Cu2+-based MPNs (Cu2+/PDA) were modified on a glucose-primed orthopedic implant. The osteogenesis-promoting properties of PDA and Cu2+ facilitated the osteogenic differentiation of osteoblasts within a diabetic infectious microenvironment [172].
Overall, the diversity of metal ions offers greater potential for activating multiple osteogenesis-related signaling pathways while simultaneously inhibiting osteoclast-related signaling pathways. The regulation of MPNs for osteogenic enhancement primarily relied on the biological functions of metal ions, while polyphenols serve a supportive role.
4.3. Vascular endothelial cell
Angiogenesis is recognized as a prerequisite for successful osteogenesis. MPNs have a pronounced influence on enhancing vascularization. The corresponding mechanisms can be delineated as the direct upregulation of vascular-related gene expression, which leads to increased vascularization (Fig. 6e).
Bioactive metal ions (e.g., Mg2+, Sr2+, Cu2+, and Zn2+) play a crucial role in promoting angiogenesis. Mg2+ is known to upregulate the expression of VEGF by stimulating HIF-2α in BMSCs. Additionally, elevated levels of Mg2+ rendered HUVECs more sensitive toward motogenic factors. According to a recent study, researchers reported a gelatin cryogel incorporated with MPN (Mg2+/TA) NPs. The proliferation, migration, tube formation, expression of angiogenic genes, and angiogenic properties of HUVECs were significantly enhanced by Mg2+ [174]. Sr2+ has been reported to exhibit notable efficacy in promoting the formation of blood vessels. Xiao et al. reported on a 3D-printed scaffold coated with MPNs (Sr2+/EGCG). The controlled release of Si2+ enhanced the formation of tubular structured cells, as indicated by the nodes (primary stage of angiogenesis), meshes (middle stage of angiogenesis), total mesh area, and total tube length. This enhancement exhibited a concentration-dependent relationship with Si2+ through the HIF-1α signaling pathway (Fig. 6f) [149]. Similarly, Liu et al. developed a biomimetic scaffold modified with Sr2+ and EGCG. With the release of Sr2+, the recruitment and migratory capacity of HUVECs and vascularization were enhanced [182]. In another report, Xiao's group described a multifunctional nanoplatform that incorporated Cu2+ and EGCG to stimulate HUVECs to generate more junctions and meshes, thereby promoting angiogenesis and accelerating periodontal tissue repair [183]. Huang's group reported a Zn2+/EGCG coating applied to the titanium surface to enhance the migration of endothelial cells, increase the branch length, branch number, and node number of HUVECs, thereby promoting vascularized osseointegration [184].
Accordingly, the mechanisms by which MPNs promote vascularization mainly involve the enhancement of VEGF expression through the activation of HIF-1α signaling pathways via metal ions.
5. Application of MPNs in bone regeneration
Combining with metal ions and phenolic compounds, their dynamic interactions provide MPNs with synergistic advantages and properties that are specifically tailored to meet the requirements of the bone defect microenvironment. Polyphenols contain multiple phenolic hydroxyl groups, which confer antioxidant and anti-inflammatory properties. Specific metal ions have been reported to be beneficial for osteogenesis, angiogenesis, and immunomodulation. MPNs with various material morphologies, including coatings, NPs, and scaffolds, are extensively utilized in the field of bone regeneration. Compared to other material synthesis technologies, such as carbon dots [185], graphene [186], and metal-organic frameworks [187], MPNs exhibit superior adaptability due to their ability to in situ self-assemble on various substrates, demonstrate responsiveness to external stimuli, and offer a relatively simple, environmentally friendly fabrication process with tunable stability through the selection of different metal ions or polyphenol molecules. In contrast, other two-dimensional (2D) materials often involve intricate preparation processes, high energy consumption, limited biocompatibility, and minimal tunability in stability [188]. The assembly conditions of MPNs significantly influence their suitability for biomedical applications. For instance, metal ions and polyphenols can be introduced into an aqueous solution and assembled rapidly at room temperature, eliminating the need for specialized solvents and equipment typically required for hydrothermal reactions [151]. Therefore, it also establishes the necessary conditions for doped biologically active molecular substances to retain their biological activity during the assembly process. In this section, we discuss the primary applications of MPNs in three scenarios: the inhibition of inflammatory bone loss, the regeneration of critical-size bone defects, and the integration of implant-bone interfaces (see Table 1). The recent advancements in research regarding the applications of MPNs for bone regeneration in the aforementioned scenarios are detailed below.
Table 1.
MPNs applications for immunomodulation, osteogenesis, and vascularization in bone regeneration.
| Bone defect type | Applications | MPN modality | Substrate | Cell regulation mechanism | Reference |
|---|---|---|---|---|---|
| Inflammatory bone loss | Periodontitis | Cu2+/TA nanosheets | TM/BHT hydrogel | Macrophages were polarized from M1 to M2 through Nrf2/NF-κΒ pathway. The expression of COL-1 and OCN in MC3T3-E1 was up-regulated. | [79] |
| Zn2+/PDA nanoparticles | Silk fibroin/gelatin/Met patch | ROS was scavenged and M1 polarization was suppressed, leading to the upregulation of COL-1 and OCN expression in hPDLSCs. Additionally, the hPDLSCs osteogenic differentiation was promoted by Zn2+. | [193] | ||
| Sr2+/TA coating | Au/BMP-2 nanoparticles | The reduction of iNOS and oxidative stress in BMSCs promoted the osteogenic differentiation of BMSCs under inflammatory conditions. | [194] | ||
| Cu2+/TA coating | PLAM | Cu2+ mediated the migration and osteogenic differentiation of hPDLSCs through Erk1/2 and HIF-1α pathways and chemokine receptor Rnd3. The proinflammatory polarization of BMDMs was inhibited by regulating of macrophage TLR4 signaling pathway. | [195] | ||
| Fe3+/PC coating | Branched AuAg nanoparticles | The antioxidation and anti-inflammation of macrophages were inhibited by regulating the PI3K/Akt/Nrf2/NF-κB signaling pathway. | [196] | ||
| Fe3+/TA nanocomplexes | Treg cells | The percentage of CD25+Foxp3+ Tregs was increased. M2 polarization was promoted by Tregs- released IL-10 and TGF-β. BMSC osteogenesis was promoted via reversing the inhibited autophagy activity by Treg-derived TGF-β1. | [197] | ||
| Osteoarthritis | Mg2+/PDA coating | MoS2/PSB nanozyme | Mg/PDA coating could eliminate ROS/RNS, restrain the activation of NF-κB, and inhibit the block effect of Traf1 on Sox9 and expression of MMP-13, TNF-α, and IL-6 in macrophages. BMSCs could differentiate into chondrocytes through MAPK signaling pathway. | [201] | |
| Mg2+/DA microspheres | MDGM-Ps hydrogel microspheres | The directed cartilage differentiation of stem cells was induced by PI3K/AKT/HIF-1α pathway. The recruitment, migration, and chemotaxis of stem cells were enhanced. | [202] | ||
| Ce4+/EGCG nanoparticles | Membrane-EC nanoparticles | Reducing proinflammatory factors (iNOS and IL-1β) levels, promoting the anti-inflammatory phenotype conversion of macrophages, improving their inflammatory microenvironment, cartilage destruction, and bone erosion. | [203] | ||
| Mn2+/GA nanoparticles | Gelatin hydrogels | The expression of MMP13, IL-1β, and TNF-α were reduced in chondrocytes while COL2 level-associated with ECM synthesis and cartilage anabolism was significantly enhanced. | [204] | ||
| Sr2+/TA microspheres | Nanofibrous microspheres | Antiapoptotic and antioxidant pathways were stimulated by scavenging hydroxyl radicals. The expression of TNF-α and IL-1β in chondrocytes was inhibited. The apoptosis of chondrocytes was reduced. OPG/RANKL ratios were enhanced. | [205] | ||
| Critical bone defect | Crania | Fe3O4/TA nanoparticles | Silk fibroin hydrogel | mRNA and protein expression of OPN and OCN in BMSCs was increased via the cGMP/PKG/ERK pathway. | [206] |
| Zn2+/PCA coating | Cellulose acetate membrane | M2-to-M2 transition was efficiently promoted. Osteogenesis differentiation of PDLSCs was enhanced via cAMP-PKA and TGF-β/Smad signaling pathways. | [175] | ||
| Zn2+/TA nanoparticles | PCL nanofibers | Intracellular ROS scavenging capability was upregulated at the continuous release of Zn2+ and TA, reducing the probability of apoptosis. The osteogenesis differentiation of BMSCs was upregulated and osteoclastic related genes (TRAP, CTSK, RANKL, and MMP-9) was downregulated. | [207] | ||
| Sr2+/EGCG nanoparticles | ECM/nHAW/PDA scaffolds | Intracellular ROS in BMSCs was effectively eliminated, and reduced apoptosis. Osteogenic activity of BMSCs was enhanced by promoting the secretion of angiogenic cytokines of Sr2+. | [182] | ||
| Ag+/PDA nanoparticles | PVA hydrogel | Osteogenic differentiation of BMSCs was promoted by a favorable microenvironment induced by PDA. Ca2+ was enriched to provide nuclear sites for HAP formation by PDA. | [208] | ||
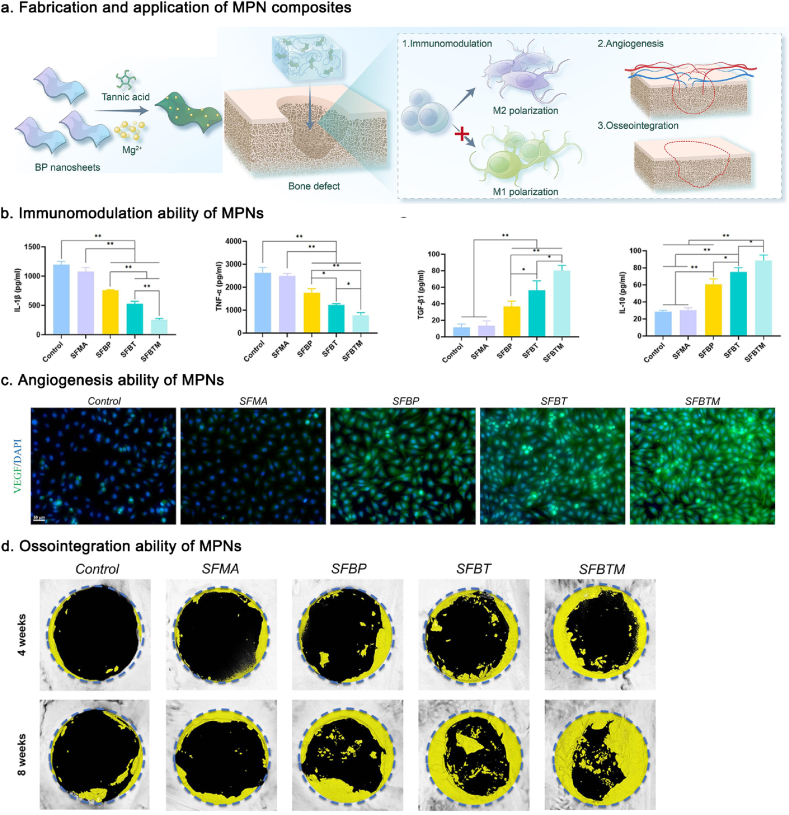
| Mg2+/TA coating | BP nanosheets | Macrophages were polarized towards M2. The release of TA and Mg2+ effectively improve the expression of VEGF and HIF-1α in HUVECs, and increase the expression of ALP, OPN, and COL-1 in BMSCs. | [209] | ||
| Mg2+/tea polyphenol nanoparticles | α/β TCP scaffold | Osteoblast differentiation and expression of OCN OPN, and RUNX2 were enhanced by Mg2+. Immune microenvironment was regulated by active phenolic hydroxyl groups and Mg2+. | [176] | ||
| Sr2+/TA nanoparticles | Biomimetic bone lamellae | The osteogenesis of osteoporotic BMSCs was promoted via PI3K/AKT signaling pathway. Angiogenesis ability was promoted by ERK. The differentiation of osteoclast precursors was inhibited by NF-κB signaling pathway. | [210] | ||
| Mg2+/TA nanoparticles | Gelatin cryogel | The Gelatin cryogel could inhibit M1 polarization and osteoclast differentiation via RANKL-induced pathway, enhance mineralization and ALP activities of stem cells by Wnt/β-catenin pathways, and upregulate expression of VEGF in HUVECs by HIF-2α pathway. | [174] | ||
| Femur | Fe3+/DA coating | HAp/GelMA hydrogel | The hydrogel regulated cellular affinities, proliferation, mineralization, osteogenic differentiation, and tissue ingrowth through interfacial nano-roughness properties and microstructural complexities of hydrogel surface. | [211] | |
| Mandible | Sr2+/TA particles | PCL nanofibrous membranes | The membrane reduced intracellular ROS via PI3K/Akt/NF-κB pathway, suppressed anti-inflammatory macrophage polarization, promoted BMSCs osteogenic differentiation, and decrease osteoclast activity, and RANKL/OPG ratio. | [212] | |
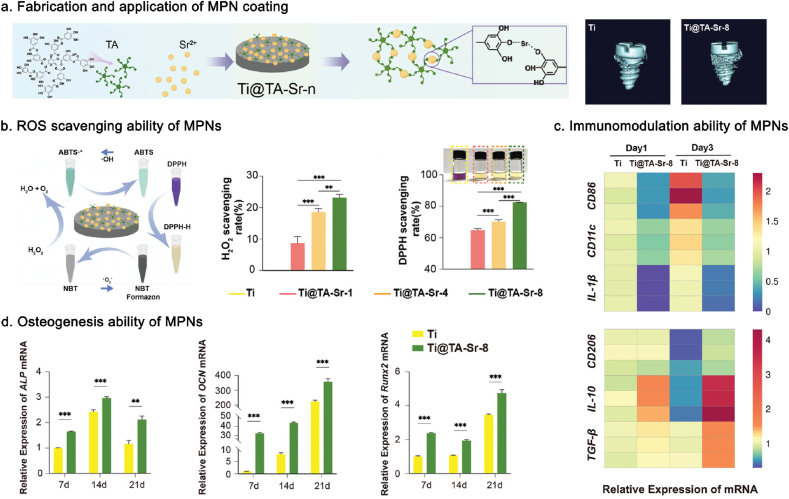
| Implant osseointegration | Femoral condyle | Ga3+/TA coatings | Ti | The MPN-coated Ti implant scavenged intracellular oxyradicals, promoted the proliferation of MC3T3-E1 cells, upregulated the expression of RUNX2, ALP, COL I, and OCN in MC3T3-E1 cells, and decreased the expression of c-Fos, NFATC1, CTSK, and TRAP in RAW264.7 cells. | [177] |
| Sr2+/TA coatings | Ti | The Sr2+/TA coated implants promoted recruitment and mobilization of endogenous stem cells via SCDF-1α/CXCR4 pathway, augmented osseointegration via Wnt/β-catenin, CaSR/PI3K/AKT, and NFATc/Maf pathways, and suppressed osteoclast differentiation via RANK/RNAKL/OPG pathway. | [173] | ||
| Sr2+/EGCG nanoparticles | Prussian blue nanomaterials/Ti | The MPN-modified Ti implants inhibited intracellular ROS, induced convert from M1 to M2, and enhanced the osteogenic differentiation of MSCs with the high expression of COL Ⅰ, ALP, and OCN. | [214] | ||
| Fe3+/PC coating | c (RGDfc) coating/Ti | The MPNs effectively enhanced the antioxidant and free radical scavenging capacity, and promoted the expression of COL Ⅰ, Cyclin D1, and Vinculin in MC3T3-E1 cells. | [215] | ||
| Zn2+/TA coating | ABL/Ti | Scavenge the cellular ROS accumulation, and induce M2 polarization. | [216] | ||
| Cu2+/TA nanopainting | Ti | The MAPK, cytokine-cytokine receptor interaction, NF-κB signaling pathways were activated in macrophages. | [218] | ||
| Sr2+/TA coating | PEEK | The implant could enhance the integrity and thickness of bone-like layers and new bone formation ability by dual reinforcement of a porous surface structure and the presence of Sr2+. | [219] | ||
| Cu2+/PDA nanoparticles | PEEK/Gox surface | The modified PEEK could enhance osteogenic differentiation of BMSCs, promote calcium nodule formation, down-regulate expression of MAPK, IL-17, TNF and NF-κB pathway in M1, and up-regulate oxidative stress-related peroxisome and glutathione metabolism to fight ROS. | [172] | ||
| ZnO/PDA nanoparticles | EDN1 coatings/PEEK | The coated PAEK implant could promote adhesion, proliferation, and osteo-differentiation of BMSCs through protein absorption by pyrogallol groups and elevate expression of HIF-1α and VEGF in HUVECs. | [220] | ||
| Fe3+/THPZB coating | Hydrogel coating/PAEK | The hydrogel coated PAEK could enhance MC3T3-E1 cells adhesion, proliferation, and osteogenic ability and HUVECs adhesion and migration, and activate the expression of VEGF and FGF. Inhibit the expression of CTSK, MMP-9, NFATC-2, and TRAP. | [221] | ||
| Proximal tibia | Fe3+/TA coating | HAp scaffold/TA | The scaffold could enhance the expression of OPN, OCN, COL1a2, and RUNX2 in MC3T3-E1, and elevate the expressions of ALP, RUNX2, OCN, and COL-I in BMSCs. | [217] |
5.1. Inflammatory bone loss inhibition
Periodontitis is an inflammatory disease that is initiated by oral microbiota plaque and exacerbated by the host's immune defenses. Periodontitis leads to the progressive and irreversible destruction of the supporting alveolar bone, ultimately resulting to tooth loss [189]. Supragingival and subgingival scaling are the primary clinical treatments for periodontitis, effectively controlling the progression of the tissue destruction, although they do not restore lost alveolar bone [190]. Currently, research into advanced NPs for immunomodulation, periodontal regeneration, and revascularization is an emerging trend [191,192]. Given their antioxidant, anti-inflammatory, and osteogenic properties, MPNs have been extensively explored for periodontal tissue regeneration. Accordingly, this section summarizes recent advances in the application of MPNs for periodontal tissue regeneration. In the treatment of periodontal tissue regeneration, MPN NPs and MPN coatings are commonly used assembly forms. MPN NPs can be adhered to hydrogels, fibrin, or cells for targeted delivery and can also be utilized for drug loading. Additionally, MPN coating can be applied to modify polylactic acid membranes for the regeneration of periodontal bone tissue.
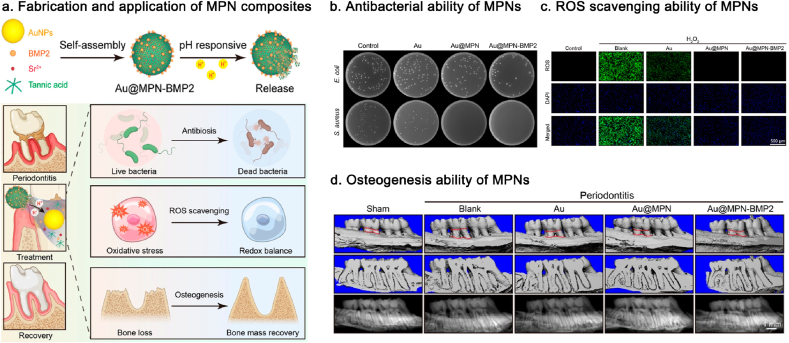
The excessive accumulation of ROS and matrix metalloproteinase (MMP) complicates the management of the intricate periodontal microenvironment. According to Xu et al., they developed a hydrogel system incorporating triglycerol monostearate/2,6-di-tert-butyl-4-methylphenol (TM/BHT) and MPN (Cu2+/TA) nanozyme. The Cu2+/TA nanozyme can be released in response to MMP activity in the inflammatory microenvironment. The released Cu2+/TA nanozyme contributed to the elimination of biofilms, the scavenging of multiple ROS, the modulation of macrophages from the M1 to M2 phenotype, and the promotion of osteogenic gene expression, thereby accelerating tissue regeneration in periodontitis [79]. Besides, MPNs can be loaded with bioactive molecules or drugs for localized delivery, enhancing drug utilization. Liu's group reported a silk fibroin/gelatin patch designed for use in diabetic periodontal regeneration. Due to its wide spread adhesion properties, Zn2+/PDA can be combined with silk fibroin, allowing for the loading of metformin for localized delivery. Accordingly, metformin endowed the patch with antiaging properties and the ability to activate M2 polarization. PDA enhances the reconstruction of the periodontal ligament through cell affinity. And Zn2+ promoted bone regeneration [193]. Similarly, Zhou et al. reported a multifunctional MPN (Sr2+/TA) composite loaded with BMP-2. The MPNs demonstrated remarkable antibacterial and antioxidant properties, while BMP-2 facilitated the osteogenic differentiation of BMSCs under inflammatory conditions and significantly prevented inflammatory bone loss (Fig. 7) [194]. In addition, MPNs could confer the inert interfaces with mimetic bioactivity. Current guided tissue regeneration barrier membranes lack the bioactivity necessary to regulate the bone repairing process. Accordingly, our group proposed a biomimetic strategy for bone tissue engineering that incorporates bioactive MPNs (TA/Cu2+) into porous polylactic acid membranes. The modification of the MPN nanointerface has the potential to regulate cellular physiology in a manner that promotes bone regeneration. This includes alleviating the pro-inflammatory polarization of BMDMs, inducing angiogenesis in HUVECs, and enhancing the attachment, migration and osteogenic differentiation of hPDLSCs. Collectively, these effects contribute to the regeneration of periodontal bone defects [195]. Besides porous polylactic acid membranes, MPNs can wrap around metal NPs due to their strong affinity, demonstrating potential as free-standing antioxidant films. For example, as reported by Zhou's group, the MPN (Fe3+/procyanidin) coating could confer anti-inflammatory activity to the surface of branched AuAg NPs, thereby alleviating the significant damage caused by the photothermal effects of these NPs. Accordingly, the collaboration between MPN's protective therapy and AuAg's photothermal therapy presents a novel and promising strategy for treating periodontitis [196]. In a recent study, Guo's group introduced MPN (Fe3+/TA) nanocomplexes loaded with the immunosuppressant rapamycin (RAPA). The RAPA-based MPN nanocomplexes could be assembled on Treg cells to facilitate the in situ release of RAPA, thereby maintaining the anti-inflammatory phenotype of regulatory T cells. This approach also guided a pro-resolving polarization of macrophages, enhanced osteogenic differentiation of BMSCs in the periodontal inflammatory milieu, and promoted the repair of hard tissue (alveolar bone) in vivo. This study cleverly linked T cell-based MPN therapy to the treatment of periodontal inflammation [197].
Fig. 7.
Fabrication of MPN composites loaded with BMP-2 and its application in treating periodontitis. Reproduced with permission from Ref. [194]. Copyright 2024 ACS Appl. Mater. Interfaces.
Besides inflammatory bone loss caused by periodontitis, arthritis is another significant manifestation of disease that lacks the ability for self-regeneration. Osteoarthritis (OA) is a chronic and degenerative disease that presents limited clinical options for effective management, contributing significantly to physical disabilities worldwide [198]. Conventional therapies are often insufficiently tailored to function effectively within the complex bone microenvironment [199,200]. Recent studies have explored the use of MPNs to promote cellular homeostasis and enhance bone regeneration. This section primarily describes the efforts of MPNs in the treatment of osteoarthritis. In the application of MPNs for the treatment of osteoarthritis, MPN nanoparticles and microspheres can be utilized for drug delivery and inflammation regulation.
The catechol groups of polyphenols can chelate metal ions through metal coordination bonds, allowing for the regulation and controlled release of these ions, which play a role in tissue repair. Accordingly, Li et al. fabricated a MoS2-based nanozyme through a stepwise modification of Mg2+-doped polydopamine. With the on-demand release of Mg2+, the MAPK signaling pathway was enhanced, allowing BMSCs to differentiate into chondrocytes. Mg2+ can also upregulate the expression of heat shock protein 70 (HSP70), which has the potential to promote the growth and proliferation of chondrocytes [201]. In addition, MPNs can serve as carriers for bioactive macromolecules. According to Peng's group, MPN (Mg2+/DA) loaded with platelet-derived growth factor-BB (PDGF-BB) was incorporated into hydrogel microspheres. Both PDGF-BB and Mg2+ were released at optimal concentrations. PDGF-BB recruited endogenous stem cells, while Mg2+ enhanced cartilage matrix synthesis by upregulating the expression of hypoxia-inducible factor 1-alpha (HIF-1α) and directing stem cell differentiation towards chondrogenesis [202]. In addition, given their robust antioxidant and anti-inflammatory activities, MPNs could self-assemble into NPs to diminish ROS and inflammatory cytokines. According to Zhao's group, they fabricated a MPN (Ce4+/EGCG) NPs that was camouflaged with a macrophage cell membrane to evade phagocytic clearance by macrophages and promote accumulation in activated inflammatory cells. The results demonstrated that the Ce4+/EGCG NPs could reduce inflammation and alter the phenotypes of macrophage cells [203]. Besides, due to the Schiff base reaction, MPNs can be utilized as a bioactive ingredient that is cross-linked with molecular chains (e.g., gelatin). According to Qian's group, they developed a nanocomposite hydrogel by incorporating with MPN (Mn2+/polygallate) through a cross-linking reaction. The synthesized hydrogel has the potential to protect cartilage by mitigating intra-articular ROS [204]. Furthermore, MPNs could serve as a platform to provide specific surface functional groups on materials, facilitating reversible interactions with various types of proteins and cells, thereby enhancing the bioactivity of these materials. Accordingly, Mo's group reported a surface modification barrier of MPNs (Sr2+/TA) on nanofiber microspheres. TA exhibited beneficial effects in protecting cartilage under OA conditions. Sr2+ had a positive intervention on cartilage matrix remodeling [205].
In conclusion, MPNs offer a promising platform for inhibiting inflammatory reaction and promoting the regeneration of bone loss associated with inflammation. With their antioxidant and anti-inflammatory abilities, MPNs can self-assemble into NPs to serve a therapeutic role locally. Due to their extensive adhesiveness, MPNs can bind to a variety of biological macromolecules or the surfaces of scaffold materials, thereby enhancing interfacial activity to modulate cellular behaviors. Metal ions can be released in a controlled and sustained manner to optimize their biological activity. MPNs can function as cargo carriers to deliver drugs or biomacromolecules locally.
5.2. Critical bone defect regeneration
Critical bone defects resulting from severe trauma, tumor resection, or congenital malformations continue to pose a significant clinical challenge in orthopedic surgery. At present, the “gold standard” for treating critical-size bone defects continues to rely on autologous/allogeneic bone grafts, as well as artificial bone grafts. Due to donor restrictions, immune rejection, infections, and other factors, outcomes such as uneven scar regeneration, delayed healing, and poor bone integration are inevitable. These issues can have a significant impact on clinical outcomes. It is essential to regulate the immune response, induce sustained osteogenic activity, and promote angiogenesis for the effective reconstruction of critical bone defects. Therefore, given their extensive modification capabilities and advanced biological functions, MPNs, or MPN-modified scaffold materials, have been thoroughly investigated for the treatment of critical bone defects. In the treatment of critical bone defects, the controlled-release MPN nanoparticles can be incorporated into fibrin hydrogels and hydroxyapatite scaffold materials. Additionally, MPN coating can be used to modify cellulose acetate membranes, PCL membranes, and hydroxyapatite scaffold materials.
The diverse adhesive properties of MPNs impart a range of adhesive characteristics to various biomaterials. For example, regenerated silk fibroin hydrogel exhibits excellent accessibility, biocompatibility, and biodegradability, which contribute to its extensive applications in the field of tissue engineering. However, inadequate adhesion at solid-state interfaces in wet and dynamic biological environments hinders the application of traditional silk protein hydrogels for biomedical purposes. Zou and colleagues introduced MPNs (Fe3O4/TA) into the silk fibroin hydrogel by utilizing the strong adhesive properties of the hydroxyl groups of TA to form cross-linked networks. The TA/Fe3O4-modified composite hydrogel exhibited significant osteogenic effects on BMSCs [206]. Besides, MPNs could modify biomaterials with inherent biological activities by creating a favorable immune microenvironment to meet the requirements of various clinical scenarios. Xie et al. reported on a cellulose acetate membrane modified with MPNs (Zn2+/PCA) to enhance bone regeneration by leveraging their immunomodulatory properties. The modification of MPNs could enhance the physicochemical properties, biocompatibility, and biological functions of cellulose acetate membranes, including antioxidation, immunomodulation, and pro-osteogenic potential. This enhancement aims to create a favorable osteoimmune microenvironment that accelerates the healing process [175]. Similarly, Tian's group reported a MPN (Zn2+/TA) coating strategy on poly (ε-caprolactone) (PCL) membrane. With a stable and constant release rate maintained for over 30 days, phenolic ligands and chelated metal ions significantly enhanced in vivo osteogenesis by manipulating a favorable osteoimmune microenvironment [207]. Besides, due to their strong antioxidant abilities, MPNs modification could serve as an innovative approach to managing ROS levels within the microenvironment of bone defects, thereby enhancing bone regeneration therapy. According to Zheng's group, they reported on MPNs (Sr2+/EGCG) coated scaffolds based on ECM and nHAW. The composite MPN-based scaffolds enhanced the differentiation potential of BMSCs in vitro and resulted in significant regenerative outcomes in rat cranial defects by improving the local microenvironment [182]. Furthermore, hydroxyapatite (HAP) is primarily a complex structure found in bone tissue. However, the limited hydroxyl groups on the surface of HAP restrict the application of HAP-based composites in bone tissue repair and regeneration. Accordingly, Lin et al. reported a chemical modification of Ag+/TA on the surface of HAP. TA could bind with growth factors on the surface of HAP, increasing the overall positive charge of the material and thereby enhancing its hydrophilicity. MPN-based HAP has the potential to promote cell proliferation and adhesion, thereby significantly enhancing the regeneration of bone tissue [208]. In addition to the aforementioned modifications of biopolymer materials, MPN can enhance the surface properties of black phosphorus (BP) to improve its stability in aqueous environment, particularly in cases where it is susceptible to oxidation. Wu et al. reported a MPNs (Mg2+/TA) coating chelated on BP to prevent the rapid oxidation of BP nanosheets in aqueous environments, while also enhancing the osteogenesis and osteo-immunomodulatory properties of BP (Fig. 8) [209]. β-Tricalcium phosphate (β-TCP) bioceramics are widely utilized in clinical treatments due to their mineralogical structure, which closely resembles that of natural bone, as well as their exceptional osteoinductive properties. However, ceramic-based scaffolds frequently fall short of meeting antimicrobial requirements. Accordingly, MPNs (Mg2+/tea polyphenols) NPs could be incorporated into β-TCP-based biomimetic scaffolds. The integrated biomimetic cancellous bone scaffold, designed with osteoinductive abilities, antimicrobial properties, and anti-inflammatory effects, was constructed to address infected bone defects [176]. In addition, the combined application of bioskiving and a carboxymethyl chitosan-stabilized amorphous calcium phosphate (ACP) mineralization solution has successfully simulated the nano- and micro-architecture of bone lamellae. However, the biomimetic bone lamella did not possess multiple bioactivities necessary for effectively repairing osteoporotic bone defects while maintaining hierarchical biomimicry. According to Yang et al., they doped Sr2+ into biomimetic bone lamellae to boost osteoporotic bone regeneration. The high phenolic hydroxyl content of TA contributes to its significant and sustained capacity for ROS scavenging. Accordingly, Sr2+/TA-modified biomimetic bone lamellae effectively treated osteoporotic bone defects through hierarchical biomimicry, ROS scavenging, and pro-osteogenic effects [210]. In addition, HAP nanorods may closely resemble bone apatite in terms of chemical composition and nanocrystalline structure. Ion-substituted HAP is biomimetic and more effectively promotes matrix maturation and osseointegration compared to pristine HAP. Incorporating inorganic nanomaterials into an organic matrix may enhance bioactivity compared to using singular components in bone tissue engineering. Accordingly, Peng's group synthesized a hydrogel that reacted with dopamine to form a catechol-modified hydrogel, which was subsequently coated onto a FeHAp nanorod through metal-catechol coordination. Accordingly, the interaction of the MPNs interface enhanced cell proliferation, matrix maturation, osteogenic potential, and vascularized bone reconstruction compared to unmodified scaffolds [211].
Fig. 8.
Fabrication of MPN-coated BP and its application in critical bone defect regeneration. Reproduced with permission from Ref. [209]. Copyright 2023 Chem. Eng. J.
In addition, the controlled release of metal ions is a crucial aspect of MPNs. For example, Sr2+, as a trace element in the human body, can bind to calcium-sensing receptor (CaSR) receptors on the surfaces of osteoblasts or osteoclasts. This binding activates downstream signaling pathways, such as the Wnt signaling pathway and the RANKL/RANK/OPG system, thereby inhibiting bone resorption and promoting osteogenesis. Accordingly, controlling the release of Sr2+, minimizing the initial burst release, and enhancing the release amount during the bone remodeling phase would further improve osteogenic efficacy by inhibiting osteoclastogenesis. Ren and colleagues developed MPNs (Sr2+/TA/EGCG/EGC) into polycaprolactone nanofibrous membranes using a blending electrospinning technique. The MPN-modified membranes exhibited a sustained release profile of phenolic ligands and Sr2+, which directly promotes the osteogenic differentiation of BMSCs [212]. In addition, Mg2+ not only exhibits osteogenic effects but also stimulates the expression of angiogenic growth factors and promotes immunomodulatory M2 polarization. However, several limitations must be addressed: the rapid corrosion of Mg2+ leads to early clearance, and an uncontrolled release of Mg2+ result in limited modulation of macrophage polarization, ultimately delaying bone healing. Shin et al. prepared multifunctional Mg2+/TA NPs within a gelatin cryogel to facilitate the sustained release of Mg2+ at the defect site, thereby enhancing bone regeneration [174].
In summary, the applications of MPNs in the regeneration of critical bone defects have been extensively studied. Benefiting from the extensive adhesive properties of polyphenols, MPNs impart unique adhesion to various materials, thereby expanding the range of applications for substrate materials. The modified substrate materials possess various bioactivities, such as antioxidant and anti-inflammatory properties, which help modulate cellular behavior. MPN coatings can enhance the surface hydrophilicity of materials, allowing for better adhesion of bioactive substances, such as growth factors. The metal ions released by MPNs can exert biological functions over an extended period.
5.3. Implant bone integration
Metal implants are widely utilized in orthopedic surgery, with titanium alloy being the most commonly used material in this field. Implants can ensure the stability of the fracture ends, promote joint formation, and contribute to bone healing. Implant site infections, immune rejection, and corrosion of the implant microenvironment can lead to implant mobility, looseness, or dislocation, ultimately resulting in implant failure. Therefore, designing bioactive modifications for implants to regulate the interface between the implant and bone tissue is crucial for addressing implant failure [213]. There has been significant exploration of MPNs for implant surface modification, owing to the synergistic bioactive effects of metal ions and polyphenols. MPN coatings on the surfaces of Ti/PEEK/PAEK implants represent a key strategy for enhancing implant osseointegration. Furthermore, MPN NPs can effectively load drug molecules onto these implants, enabling controlled release for inflammation regulation and promoting bone integration.
MPN-based coatings have been applied to the surfaces of titanium implants. Two or more galloyl groups from polyphenols can chelate with a metal ion to form a MPN network. Galloyl groups can interact with the surface of an implant substrate in various ways, including covalent bonds, hydrogen bonds, coordination bonds, π-π interactions, and cation-π interactions. This versatility enables the development of MPN-based coatings on implant substrate surfaces. Zhang et al. reported a Ga3+/TA coating on Ti-based implants. The catechol groups of TA are converted to quinone groups by ROS. MPNs-based coatings could be developed as a promising platform for the elimination of ROS and the controlled release of Ga3+. Long-term, low-dose administration of Ga3+ effectively exhibits antibacterial properties, reduces inflammation, promotes bone mineralization, and inhibits osteoclastogenesis. Accordingly, the Ga3+/TA coating enhanced the implants' adaptability to the complex microenvironments of bone defects [177]. Similarly, Sr2+ could enhance the osteogenic differentiation of osteoblasts while decreasing the expression of gene related to osteoclastogenesis. Accordingly, our group utilized TA and Sr2+ as building blocks to develop a multifunctional coating on Ti implants, aiming to enhance osseointegration through early immunomodulation. Sr2+/TA coatings enhanced osteo-immunomodulation and osseointegration at the bone-implant interface (Fig. 9) [173]. Considering the broad adhesiveness on various substrates, Cai et al. constructed a MPN network encapsulated in Prussian blue (PB) NPs which exhibited a high NIR absorption capacity and a high photothermal conversion efficiency. This design aimed to enhance the adhesion, proliferation, differentiation, and osteogenic gene expression of BMSCs. The Sr2+/EGCG/PB NPs were anchored to the surface of the titanium implant using a polydopamine adhesion layer. The early inflammatory microenvironment at the implant site resulted in the degradation of the MPNs. The responsive release of EGCG and Sr2+ was able to promote long-term osteogenic efficacy for low photothermal therapy [214]. In another context, grafting bioactive molecules, such as growth factors and extracellular matrix-like proteins, onto implant surfaces to provide essential bio-signals for osteointegration has been demonstrated as a potent strategy for improving implant osteointegration. However, the fragility of these bioactive molecules hinders the effectiveness of this approach. MPNs could provide versatile substrate modification due to their adhesive properties. Accordingly, Chen's group reported that a peptide ring structure increased stability and biocompatibility through MPN modification. Through the immobilization of peptides on the MPN interface, MPNs significantly enhanced cell viability, migration, and adhesion capacity, while also functioning as an intermediate layer with notable antioxidant activity [215]. In addition, MPNs could function as drug carriers to improve the loading efficiency of modified implant surfaces. According to Guo's group, they constructed a MPN (Zn2+/TA) network to enhance loading capacity of abaloparatide (ABL) through multiple molecular interactions and facilitate the long-term release of ABL over a period exceeding seven days [216]. Due to the bioinert nature of the implant surfaces, osseointegration surrounding the implant is compromised. Metal ion-modified hydroxyapatite nanorods can enhance the wetting behavior of composite scaffolds; however, issues such as aggregation and uncontrolled leaching remain limitations. According to Pei et al., they introduced the TA to specifically react with iron-phenolic interactions. Constructing a suitable interface between organic and inorganic materials to create cell-instructive scaffolds would boost osteointegration [217]. Infection-induced implant malfunction, or even failure, presents another significant challenge. Jiang's group deposited TA onto a titanium implant through interfacial molecular interactions, followed by interparticle locking facilitated by Cu2+ in MPNs. The MPNs on orthopedic implants rapidly disassembled upon the triggering of the acidic niche that induced by the bacterial metabolism. The bacteria were eradicated through the ·OH produced by the Cu2+-mediated Fenton-like reaction [218].
Fig. 9.
Fabrication of MPN-coated Ti implants and its application in implant bone integration. Reproduced with permission from Ref. [173]. Copyright 2024 Adv. Sci.
In addition to Ti implant surfaces, polyetheretherketone (PEEK) has an elastic modulus similar to that of human cortical bone, making it a promising candidate for replacing metallic implants. However, PEEK still has several limitations regarding its antimicrobial properties, such as the overuse of antibiotics contributing to the development of drug resistance and the biological toxicity associated with the use of Ag. Therefore, it is essential to develop a dual-functional coating for PEEK implants that exhibits both osseointegration and anti-infective properties. Li's group reported a MPN (Sr2+/TA) modification coating of bone implants via layer-by-layer assembly to enhance antibacterial activity in a pH-responsive manner and improve osseointegration [219]. Besides, diabetes is widely recognized for inducing disorders in bone metabolism, such as osteoporosis and alveolar bone resorption, which can adversely affect the osseointegration of implants in diabetic patients. In order to address the issues of hyperglycemia and excessive inflammation at the bone-implant interface in diabetic conditions, Deng and colleagues developed a modified orthopedic implant utilizing MPNs (Cu2+/PDA) to enhance osseointegration in diabetic patients. By leveraging the functions of PDA to improve the adhesion and proliferation of osteoblasts on the implant surface, along with the role of copper (Cu2+) in providing osteogenesis-inducing factors for osteogenic differentiation, the engineered implants significantly enhanced osseointegration and facilitated osteogenesis [172]. Besides, carbo-fiber-reinforced PEEK (CFRPEEK), achieved by incorporating short carbon fibers into PEEK, attains an elastic modulus comparable to that of human cortical bone, thereby mitigating complications associated with stress shielding. To enhance the bioinert surface and antimicrobial activity, zinc oxide NPs are emerging as one of the most promising inorganic antimicrobial materials. Jiang et al. reported the fabrication of active groups using PDA through PDA-assisted covalent immobilization to achieve a uniform distribution of ZnO. The hybrid PDA@ZnO NPs exhibit prolonged antibacterial activity while minimizing cytotoxic effects, in addition to demonstrating effective antimicrobial properties [220].
In addition to Ti and PEEK implants, poly (aryl ether ketone) (PAEK) also is a novel type of medical implant material that is increasingly being used in bone implants due to its exceptional properties. In order to expand the potential applications in biomedicine, Jian's group developed bioactive interfaces based on MPNs (Fe3+/THPZB). The processes of biomineralization, angiogenesis, osteogenesis, and osteoclastic activity were enhanced [221].
In conclusion, the modification of bone implants using MPNs has been extensively researched to enhance osseointegration at the implant-bone interface. MPN can be applied to the surface of the implant through various interactions to provide antibacterial, anti-inflammatory, antioxidant, and immunoregulatory functions, thereby transforming inert surfaces into biologically active interfaces. The metal ions can be released from MPNs in a controlled and sustained manner to promote osteogenesis and inhibit osteoclast activity. Polyphenols can promote cell adhesion, proliferation, and osteogenic differentiation. MPNs provide implant surfaces with a bioactive interface for the incorporation of growth factors or extracellular matrix proteins. MPNs can serve as cargo carriers to deliver drugs or biological macromolecules in situ.
6. Conclusions and perspectives
Timely and effective modulation of cell biology has always been crucial for bone tissue regeneration. MPNs can effectively integrate the biological and physicochemical properties of metal ions and polyphenols for bone tissue engineering. It employs an innovative, versatile, and tailored approach to the development of bone tissue engineering materials. Since the initial synthesis of MPNs in 2013, numerous reviews have addressed the synthesis methods, physicochemical properties, various application forms, and a broad spectrum of biomedical applications. To the best of our knowledge, this review is the first comprehensive examination of MPNs that encompasses recent research advancements in MPN-cell interaction behaviors and their applications in bone tissue engineering. This review offers a novel perspective on the clinical translation of MPNs in bone regeneration therapy for the future.
MPNs exert a significantly influence on bone regeneration activities through various interactions with multiple cell types, positioning them as versatile platforms for orchestrating the bone regeneration process. The unique structures of polyphenolic molecules, along with the versatile functions of metal ions, create material interfaces with distinct physicochemical properties—such as anti-inflammatory effects, antibacterial activity, pro-healing potential, and the promotion of osteogenesis—that can more effectively modulate cell behavior. This review offers a comprehensive understanding of the responses of bone repairing-associated cells to MPNs, aiming to inform rational material design and expanding the range of their potential clinical and biomedical applications. The cell types that need to be studied in the future can be expanded to T cells, neutrophils, and other cell types.
Although numerous successful applications of MPNs in bone tissue engineering have been reported, it is crucial to recognize the various challenges and limitations associated with current MPNs assembly and application systems that must be addressed and overcome. Natural polyphenols have been extensively studied, however, research on synthetic polyphenols remains limited. Further exploration of the physicochemical properties of polyphenolic substances is essential to expand the scope and enhance the functionality of MPNs. Additionally, carefully consideration must be given to the concentration and type of metal ions, as certain metal ions may exhibit toxicity even at low concentrations. Ensuring the biocompatibility of MPNs is a crucial prerequisite for facilitating the clinical translation. The potential long-term effects of MPNs, particularly concerning implantation materials, should be thoroughly investigated, as implants may remain in the body for extended periods ranging from months to years.
Considering that MPNs can be assembled within seconds in an aqueous solution, precisely regulating the rapid assembly kinetics to form a uniform and stable film presents a significant challenge. Currently, the assembly of MPNs primarily depends on laboratory exploration efforts, while the exploration of artificial intelligence and deep learning in this field remains underdeveloped. In the future, it is anticipated that the integration of MPNs synthesis with computer simulations will enable more efficient screening and prediction of MPNs assembly process. MPNs exhibit a diverse array of interactive forces, including both covalent and non-covalent interactions, which contribute to their distinctive properties. These interaction networks can be more clearly represented and analyzed through computer simulations, thereby enhancing our understanding of cellular responses to MPNs and enabling the real-time monitoring of the dynamic regenerative processes associated with cellular activities.
At present, the process of MPNs clinical conversion is obstacle-packed and long. Despite the progress made in the MPNs application of bone tissue regeneration, there are still some challenges and amelioration. Currently, the components of the MPNs system are straightforward, consisting of polyphenols and metal ions. As bioactive ingredients have been incorporated into MPNs, efforts to discover novel functions in clinical settings should be more diversified. We look forward to exploring additional biological entities that can be integrated into innovative intelligent MPN systems for diverse therapeutic applications in the future. MPNs present limitless possibilities for exploration, meriting the commitment and creativity of researchers. It is crucial that medical research involving MPNs draws from prior experiences to prevent overstated claims and unnecessary duplication of efforts. This approach will ensure meaningful progress and a thorough understanding of this novel material system.
CRediT authorship contribution statement
Ying Wang: Writing – review & editing, Writing – original draft, Formal analysis. Zhibang Li: Supervision, Formal analysis. Ruiqing Yu: Supervision, Formal analysis. Yi Chen: Supervision, Formal analysis. Danyang Wang: Supervision, Formal analysis. Weiwei Zhao: Writing – review & editing, Supervision, Funding acquisition, Formal analysis. Shaohua Ge: Supervision, Funding acquisition, Formal analysis. Hong Liu: Writing – review & editing, Supervision, Formal analysis. Jianhua Li: Writing – review & editing, Supervision, Funding acquisition, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Research Fund for the National Natural Science Foundation of China (82301081, 82470981, 82320108004), the Taishan Scholars Program of Shandong Province (tsqn201909180, tsqn202312344), National Key Research and Development Program of China (2023YFC250630), Shandong Province Key Research and Development Program (2024CXPT090, 2021ZDSYS18 and 2022CXGC020511), Shandong Province Major Scientific and Technical Innovation Project (No.2021SFGC0502), the Independent Training and Innovation Team in Jinan (No. 202228055), Natural Science Foundation of Shandong Province (CN) (ZR2024YQ055 and ZR2022QB103), Open Foundation of State Key Laboratory of Oral Diseases (SKLOD2024OF07), Young Scholars Program of Shandong University and Qilu Young Scholar Foundation of Shandong University.
Contributor Information
Weiwei Zhao, Email: zhaoweiwei@sdu.edu.cn.
Jianhua Li, Email: jianhua.li@sdu.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Feng B., Zhang M., Qin C., Zhai D., Wang Y., Zhou Y., et al. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact. Mater. 2023;22:127–140. doi: 10.1016/j.bioactmat.2022.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng P., Zhao R., Tang W., Yang F., Tian H., Peng S., et al. Structural and functional adaptive artificial bone: materials, fabrications, and properties. Adv. Funct. Mater. 2023;33(23) [Google Scholar]
- 3.Huang R.-L., Kobayashi E., Liu K., Li Q. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine. 2016;12:43–54. doi: 10.1016/j.ebiom.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 5.Hao J., Malek N.A.N.N., Kamaruddin W.H.A., Li J. Breaking piezoelectric limits of molecules for biodegradable implants. BME Mat. 2024;2(2) [Google Scholar]
- 6.Zhou J., Zhang Z., Joseph J., Zhang X., Ferdows B.E., Patel D.N., et al. Biomaterials and nanomedicine for bone regeneration: progress and future prospects. Explorations. 2021;1(2) doi: 10.1002/EXP.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins M.N., Ren G., Young K., Pina S., Reis R.L., Oliveira J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021;31(21) [Google Scholar]
- 8.Makvandi P., Josic U., Delfi M., Pinelli F., Jahed V., Kaya E., et al. Drug delivery (nano) platforms for oral and dental applications: tissue regeneration, infection control, and cancer management. Adv. Sci. 2021;8(8) doi: 10.1002/advs.202004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai M., Zhong W., Yan S., Ye T., Zheng R., Yang Z., et al. Diffusion-induced phase separation 3D printed scaffolds for dynamic tissue repair. BME Mat. 2024;2(3) [Google Scholar]
- 10.Amani H., Arzaghi H., Bayandori M., Dezfuli A.S., Pazoki-Toroudi H., Shafiee A., et al. Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv. Mater. Interfac. 2019;6(13) [Google Scholar]
- 11.Lopes D., Martins-Cruz C., Oliveira M.B., Mano J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240–275. doi: 10.1016/j.biomaterials.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilchrist A.E., Harley B.A.C. Engineered tissue models to replicate dynamic interactions within the hematopoietic stem cell niche. Adv. Healthcare Mater. 2022;11(7) doi: 10.1002/adhm.202102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda E., Levkin P.A. Emerging applications of superhydrophilic-superhydrophobic micropatterns. Adv. Mater. 2013;25(9):1234–1247. doi: 10.1002/adma.201204120. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.H., Jeon S., Qu X., Kang M.S., Lee J.H., Han D.-W., et al. Ternary MXene-loaded PLCL/collagen nanofibrous scaffolds that promote spontaneous osteogenic differentiation. Nano Converg. 2022;9(1):38. doi: 10.1186/s40580-022-00329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu T.-T., Cui F.-Z., Meng Q.-Y., Wang J., Wu D.-C., Zhang J., et al. Influence of surface chemistry on adhesion and osteo/odontogenic differentiation of dental pulp stem cells. ACS Biomater. Sci. Eng. 2017;3(6):1119–1128. doi: 10.1021/acsbiomaterials.7b00274. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Song Q., Yin W., Li C., An N., Le Y., et al. Bioactive scaffolds for tissue engineering: a review of decellularized extracellular matrix applications and innovations. Explorations. 2024 [Google Scholar]
- 17.Song J., Li L., Fang L., Zhang E., Zhang Y., Zhang Z., et al. Advanced strategies of scaffolds design for bone regeneration. BME Mat. 2023;1(4) [Google Scholar]
- 18.Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5(9):686–705. [Google Scholar]
- 19.Chaudhuri O., Cooper-White J., Janmey P.A., Mooney D.J., Shenoy V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584(7822):535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellanos M.I., Mas-Moruno C., Grau A., Serra-Picamal X., Trepat X., Albericio F., et al. Functionalization of CoCr surfaces with cell adhesive peptides to promote HUVECs adhesion and proliferation. Appl. Surf. Sci. 2017;393:82–92. [Google Scholar]
- 21.Li X., Wang B., Zhou S., Chen W., Chen H., Liang S., et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J. Nanobiotechnol. 2020;18(1):45. doi: 10.1186/s12951-020-00599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King W.J., Krebsbach P.H. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012;64(12):1239–1256. doi: 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Thian E.S., Wang M., Wang Z., Ren L. Surface design for antibacterial materials: from fundamentals to advanced strategies. Adv. Sci. 2021;8(19) doi: 10.1002/advs.202100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S., Dan X., Chen H., Li T., Liu B., Ju Y., et al. Developing fibrin-based biomaterials/scaffolds in tissue engineering. Bioact. Mater. 2024;40:597–623. doi: 10.1016/j.bioactmat.2024.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao X., Chen X., Sun Y., Yang P., Gu X., Dai X. Application of metal-organic frameworks-based functional composite scaffolds in tissue engineering, Regener. Biomaterials. 2024;11 doi: 10.1093/rb/rbae009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H.J., Casalini T., Hulsart-Billström G., Wang S., Oommen O.P., Salvalaglio M., et al. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials. 2018;161:190–202. doi: 10.1016/j.biomaterials.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Brennan M.Á., Layrolle P., Mooney D.J. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020;30(37) doi: 10.1002/adfm.201909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ejima H., Richardson J.J., Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–148. [Google Scholar]
- 29.Lin Z., Liu H., Richardson J.J., Xu W., Chen J., Zhou J., et al. Metal-phenolic network composites: from fundamentals to applications. Chem. Soc. Rev. 2024 doi: 10.1039/d3cs00273j. [DOI] [PubMed] [Google Scholar]
- 30.Ejima H., Richardson J.J., Liang K., Best J.P., van Koeverden M.P., Such G.K., et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341(6142):154–157. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J., Lin Z., Ju Y., Rahim M.A., Richardson J.J., Caruso F. Polyphenol-mediated assembly for particle engineering. Acc. Chem. Res. 2020;53(7):1269–1278. doi: 10.1021/acs.accounts.0c00150. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Shen L., Zhong Q.-Z., Li J. Metal-phenolic network coatings for engineering bioactive interfaces. Colloids Surf., B. 2021;205 doi: 10.1016/j.colsurfb.2021.111851. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G., Wang N., Ma Y., Zhai S., Ngai T., Ni S., et al. Metal coordination-driven assembly of stimulator of interferon genes-activating nanoparticles for tumor chemo-immunotherapy. BME Mat. 2024;2(2) [Google Scholar]
- 34.Shen L., Zhang Y., Feng J., Xu W., Chen Y., Li K., et al. Microencapsulation of ionic liquid by interfacial self-assembly of metal-phenolic network for efficient gastric absorption of oral drug delivery. ACS Appl. Mater. Interfaces. 2022;14(40):45229–45239. doi: 10.1021/acsami.2c15599. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., Xie L., Ju Y., Dai Y. Recent advances in metal-phenolic networks for cancer theranostics. Small. 2021;17(43) doi: 10.1002/smll.202100314. [DOI] [PubMed] [Google Scholar]
- 36.Geng H., Zhong Q.Z., Li J., Lin Z., Cui J., Caruso F., et al. Metal ion-directed functional metal-phenolic materials. Chem. Rev. 2022;122(13):11432–11473. doi: 10.1021/acs.chemrev.1c01042. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Miao Y., Yang L., Zhao Y., Wu K., Lu Z., et al. Recent advances in the development and antimicrobial applications of metal-phenolic networks. Adv. Sci. 2022;9(27) doi: 10.1002/advs.202202684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz J.P., Hollinger J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986;205:299–308. [PubMed] [Google Scholar]
- 39.Hoerth R.M., Seidt B.M., Shah M., Schwarz C., Willie B.M., Duda G.N., et al. Mechanical and structural properties of bone in non-critical and critical healing in rat, Acta. Biomaterials. 2014;10(9):4009–4019. doi: 10.1016/j.actbio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Holmes D. Closing the gap. Nature. 2017;550(7677):S194–S195. doi: 10.1038/550S194a. [DOI] [PubMed] [Google Scholar]
- 41.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5(8):584–603. [Google Scholar]
- 42.Ai Y., Dai F., Li W., Xu F., Yang H., Wu J., et al. Photo-crosslinked bioactive BG/BMSCs@GelMA hydrogels for bone-defect repairs. Mater. Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotagudda Ranganath S., Schlund M., Delattre J., Ferri J., Chai F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater. Today Bio. 2022;14 doi: 10.1016/j.mtbio.2022.100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J., Park S., Park J.-Y., Jung U.-W., Jung S., Oh Y., et al. Dual-phase blocks for regeneration of critical-sized bone defects. Nano Today. 2024;54 [Google Scholar]
- 45.Dewey M.J., Milner D.J., Weisgerber D., Flanagan C.L., Rubessa M., Lotti S., et al. Repair of critical-size porcine craniofacial bone defects using a collagen–polycaprolactone composite biomaterial. Biofabrication. 2022;14(1) doi: 10.1088/1758-5090/ac30d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparks D.S., Saifzadeh S., Savi F.M., Dlaska C.E., Berner A., Henkel J., et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020;15(3):877–924. doi: 10.1038/s41596-019-0271-2. [DOI] [PubMed] [Google Scholar]
- 47.Tuckermann J., Adams R.H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 2021;17(10):608–620. doi: 10.1038/s41584-021-00682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin S., Zhang W., Zhang Z., Jiang X. Recent advances in scaffold design and material for vascularized tissue-engineered bone regeneration. Adv. Healthcare Mater. 2019;8(10) doi: 10.1002/adhm.201801433. [DOI] [PubMed] [Google Scholar]
- 49.Salhotra A., Shah H.N., Levi B., Longaker M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aghazadeh Y., Khan S.T., Nkennor B., Nunes S.S. Cell-based therapies for vascular regeneration: past, present and future. Pharmacol. Ther. 2022;231 doi: 10.1016/j.pharmthera.2021.107976. [DOI] [PubMed] [Google Scholar]
- 51.Brown B.N., Ratner B.D., Goodman S.B., Amar S., Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin S.S., He D.Q., Luo D., Wang Y., Yu M., Guan B., et al. A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano. 2019;13(6):6581–6595. doi: 10.1021/acsnano.9b00489. [DOI] [PubMed] [Google Scholar]
- 53.Hankenson K.D., Gagne K., Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015;94(1):3–12. doi: 10.1016/j.addr.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Li B., Huang R., Ye J., Liu L., Qin L., Zhou J., et al. A self-healing coating containing curcumin for osteoimmunomodulation to ameliorate osseointegration. Chem. Eng. J. 2021;403(1) [Google Scholar]
- 55.Su N., Villicana C., Yang F. Immunomodulatory strategies for bone regeneration: a review from the perspective of disease types. Biomaterials. 2022;286 doi: 10.1016/j.biomaterials.2022.121604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang F., Yu Y., Liu S., Ming L., Zhang Y., Zhou Z., et al. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021;6(3):666–683. doi: 10.1016/j.bioactmat.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leucht P., Lee S., Yim N. Wnt signaling and bone regeneration: can't have one without the other. Biomaterials. 2019;196:46–50. doi: 10.1016/j.biomaterials.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 58.Hsu M.N., Huang K.L., Yu F.J., Lai P.L., Truong A.V., Lin M.W., et al. Coactivation of endogenous Wnt10b and Foxc2 by CRISPR activation enhances BMSC osteogenesis and promotes calvarial bone regeneration. Mol. Ther. 2020;28(2):441–451. doi: 10.1016/j.ymthe.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolamperti S., Villa I., Rubinacci A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 2022;10(1):48. doi: 10.1038/s41413-022-00219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim B.J., Koh J.M. Coupling factors involved in preserving bone balance. Cell. Mol. Life Sci. 2019;76(7):1243–1253. doi: 10.1007/s00018-018-2981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald M.M., Khoo W.H., Ng P.Y., Xiao Y., Zamerli J., Thatcher P., et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184(7):1940. doi: 10.1016/j.cell.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang W., Hou F., Gu Y., Saiding Q., Bao P., Tang J., et al. Local bone metabolism balance regulation via double-adhesive hydrogel for fixing orthopedic implants. Bioact. Mater. 2022;12:169–184. doi: 10.1016/j.bioactmat.2021.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L., You X., Zhang L., Zhang C., Zou W. Mechanical regulation of bone remodeling. Bone Res. 2022;10(1):16. doi: 10.1038/s41413-022-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collins M.T., Marcucci G., Anders H.-J., Beltrami G., Cauley J.A., Ebeling P.R., et al. Skeletal and extraskeletal disorders of biomineralization. Nat. Rev. Endocrinol. 2022;18(8):473–489. doi: 10.1038/s41574-022-00682-7. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Zhang H., Hu Y., Jing Y., Geng Z., Su J. Bone repair biomaterials: a perspective from immunomodulation. Adv. Funct. Mater. 2022;32(51) [Google Scholar]
- 66.Niu Y., Wang Z., Shi Y., Dong L., Wang C. Modulating macrophage activities to promote endogenous bone regeneration: biological mechanisms and engineering approaches. Bioact. Mater. 2021;6(1):244–261. doi: 10.1016/j.bioactmat.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutta S.D., Patil T.V., Ganguly K., Randhawa A., Lim K.-T. Unraveling the potential of 3D bioprinted immunomodulatory materials for regulating macrophage polarization: state-of-the-art in bone and associated tissue regeneration. Bioact. Mater. 2023;28:284–310. doi: 10.1016/j.bioactmat.2023.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bi Z., Cai Y., Shi X., Chen J., Li D., Zhang P., et al. Macrophage-mediated immunomodulation in biomaterial-assisted bone repair: molecular insights and therapeutic prospects. Chem. Eng. J. 2024;488 [Google Scholar]
- 69.Sridharan R., Cavanagh B., Cameron A.R., Kelly D.J., O'Brien F.J. Material stiffness influences the polarization state, function and migration mode of macrophages, Acta. Biomaterials. 2019;89(15):47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 70.Atcha H., Jairaman A., Holt J.R., Meli V.S., Nagalla R.R., Veerasubramanian P.K., et al. Mechanically activated ion channel piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021;12(1):3256. doi: 10.1038/s41467-021-23482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lv L., Xie Y., Li K., Hu T., Lu X., Cao Y., et al. Unveiling the mechanism of surface hydrophilicity-modulated macrophage polarization. Adv. Healthcare Mater. 2018;7(19) doi: 10.1002/adhm.201800675. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Feng Z., Liu X., Yang C., Gao R., Liu W., et al. Titanium alloy composited with dual-cytokine releasing polysaccharide hydrogel to enhance osseointegration via osteogenic and macrophage polarization signaling pathways, Regener. Biomaterials. 2022;9 doi: 10.1093/rb/rbac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan J., Zhang Q.-Y., Song Y.-T., Huang K., Jiang Y.-L., Chen J., et al. Accelerated bone defect regeneration through sequential activation of the M1 and M2 phenotypes of macrophages by a composite BMP-2@SIS hydrogel: an immunomodulatory perspective. Composites, Part B. 2022;243(15) [Google Scholar]
- 74.Majrashi M., Kotowska A., Scurr D., Hicks J.M., Ghaemmaghami A., Yang J. Sustained release of dexamethasone from 3D-printed scaffolds modulates macrophage activation and enhances osteogenic differentiation. ACS Appl. Mater. Interfaces. 2023;15(49):56623–56638. doi: 10.1021/acsami.3c09774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang G., Huang X., Wang T., Wang J., Zhao G., Wang H., et al. The impact of sitagliptin on macrophage polarity and angiogenesis in the osteointegration of titanium implants in type 2 diabetes. Biomed. Pharmacother. 2020;126 doi: 10.1016/j.biopha.2020.110078. [DOI] [PubMed] [Google Scholar]
- 76.Qiang H., Hou C., Zhang Y., Luo X., Li J., Meng C., et al. CaP-coated Zn-Mn-Li alloys regulate osseointegration via influencing macrophage polarization in the osteogenic environment, Regener. Biomaterials. 2023;10 doi: 10.1093/rb/rbad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C., Zhang W., Nie Y., Du X., Huang C., Li L., et al. Time-sequential and multi-functional 3D printed MgO2/PLGA scaffold developed as a novel biodegradable and bioactive bone substitute for challenging postsurgical osteosarcoma treatment. Adv. Mater. 2024;36(34) doi: 10.1002/adma.202308875. [DOI] [PubMed] [Google Scholar]
- 78.Li S., Yang H., Qu X., Qin Y., Liu A., Bao G., et al. Multiscale architecture design of 3D printed biodegradable Zn-based porous scaffolds for immunomodulatory osteogenesis. Nat. Commun. 2024;15(1):3131. doi: 10.1038/s41467-024-47189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Y., Luo Y., Weng Z., Xu H., Zhang W., Li Q., et al. Microenvironment-responsive metal-phenolic nanozyme release platform with antibacterial, ROS scavenging, and osteogenesis for periodontitis. ACS Nano. 2023;17(19):18732–18746. doi: 10.1021/acsnano.3c01940. [DOI] [PubMed] [Google Scholar]
- 80.Thoraval L., Thiébault E., Siboni R., Moniot A., Guillaume C., Jacobs A., et al. The acute inflammatory response to copper(II)-doped biphasic calcium phosphates. Mater. Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Lin Q., Zhang H., Wang S., Cui J., Hu Y., et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact. Mater. 2023;28:273–283. doi: 10.1016/j.bioactmat.2023.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armiento A.R., Hatt L.P., Sanchez Rosenberg G., Thompson K., Stoddart M.J. Functional biomaterials for bone regeneration: a lesson in complex biology. Adv. Funct. Mater. 2020;30(44) [Google Scholar]
- 83.Bai Y., Wang Z., He X., Zhu Y., Xu X., Yang H., et al. Application of bioactive materials for osteogenic function in bone tissue engineering. Small Methods. 2024;8(8) doi: 10.1002/smtd.202301283. [DOI] [PubMed] [Google Scholar]
- 84.Wu J., Feng C., Wang M., Wu H., Zhu X., Li X., et al. Whisker of biphasic calcium phosphate ceramics: osteo-immunomodulatory behaviors. Nano Res. 2022;15(10):9169–9182. [Google Scholar]
- 85.Li Z., He D., Guo B., Wang Z., Yu H., Wang Y., et al. Self-promoted electroactive biomimetic mineralized scaffolds for bacteria-infected bone regeneration. Nat. Commun. 2023;14(1):6963. doi: 10.1038/s41467-023-42598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X., Wang T., Zhang Z., Liu H., Li L., Wang A., et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Mater. Today. 2023;68:177–203. [Google Scholar]
- 87.Entezari A., Wu Q., Mirkhalaf M., Lu Z., Roohani I., Li Q., et al. Unraveling the influence of channel size and shape in 3D printed ceramic scaffolds on osteogenesis. Acta Biomater. 2024;180:115–127. doi: 10.1016/j.actbio.2024.04.020. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y., Wang Y., Lin M., Liu H., Pan Y., Wu J., et al. Cellular scale curvature in bioceramic scaffolds enhanced bone regeneration by regulating skeletal stem cells and vascularization. Adv. Healthcare Mater. 2024 doi: 10.1002/adhm.202401667. [DOI] [PubMed] [Google Scholar]
- 89.Li X., Liu S., Han S., Sun Q., Yang J., Zhang Y., et al. Dynamic stiffening hydrogel with instructive stiffening timing modulates stem cell fate in vitro and enhances bone remodeling in vivo. Adv. Healthcare Mater. 2023;12(29) doi: 10.1002/adhm.202300326. [DOI] [PubMed] [Google Scholar]
- 90.Lai X., Huang J., Huang S., Wang J., Zheng Y., Luo Y., et al. Antibacterial and osteogenic dual-functional micronano composite scaffold fabricated via melt electrowriting and solution electrospinning for bone tissue engineering. ACS Appl. Mater. Interfaces. 2024;16(29):37707–37721. doi: 10.1021/acsami.4c07400. [DOI] [PubMed] [Google Scholar]
- 91.Zhao R., Qian H., Zhu X., Zhang X., Chen Z., Yang X. Advancing osteoporotic bone regeneration through tailored tea polyphenols functionalized micro-/mano- hydroxyapatite bioceramics. Adv. Funct. Mater. 2024;34(32) [Google Scholar]
- 92.Shan Y., Bai Y., Yang S., Zhou Q., Wang G., Zhu B., et al. 3D-printed strontium-incorporated β-TCP bioceramic triply periodic minimal surface scaffolds with simultaneous high porosity, enhanced strength, and excellent bioactivity. J. Adv. Ceram. 2023;12(9):1671–1684. [Google Scholar]
- 93.Yang J., Shuai J., Siow L., Lu J., Sun M., An W., et al. MicroRNA-146a-loaded magnesium silicate nanospheres promote bone regeneration in an inflammatory microenvironment. Bone Res. 2024;12(1):2. doi: 10.1038/s41413-023-00299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Lei, Song Jing han, Li Song, na Y., Liu M.-y., Wan M.-c., et al. Advances in materials-based therapeutic strategies against osteoporosis. Biomaterials. 2023;296 doi: 10.1016/j.biomaterials.2023.122066. [DOI] [PubMed] [Google Scholar]
- 95.Zhou T., Zhou H., Wang F., Zhang P., Shang J., Shi L. An injectable carboxymethyl chitosan hydrogel scaffold formed via coordination bond for antibacterial and osteogenesis in osteomyelitis. Carbohydr. Polym. 2024;324(15) doi: 10.1016/j.carbpol.2023.121466. [DOI] [PubMed] [Google Scholar]
- 96.Hao J., Bai B., Ci Z., Tang J., Hu G., Dai C., et al. Large-sized bone defect repair by combining a decalcified bone matrix framework and bone regeneration units based on photo-crosslinkable osteogenic microgels. Bioact. Mater. 2022;14:97–109. doi: 10.1016/j.bioactmat.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu H., Jiao Y., Forouzanfar T., Wu G., Guo R., Lin H. High-strength double-network silk fibroin based hydrogel loaded with Icariin and BMSCs to inhibit osteoclasts and promote osteogenic differentiation to enhance bone repair. Biomater. Adv. 2024;160 doi: 10.1016/j.bioadv.2024.213856. [DOI] [PubMed] [Google Scholar]
- 98.Friend N.E., McCoy A.J., Stegemann J.P., Putnam A.J. A combination of matrix stiffness and degradability dictate microvascular network assembly and remodeling in cell-laden poly(ethylene glycol) hydrogels. Biomaterials. 2023;295 doi: 10.1016/j.biomaterials.2023.122050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guan Y., Racioppi L., Gerecht S. Engineering biomaterials to tailor the microenvironment for macrophage–endothelium interactions. Nat. Rev. Mater. 2023;8(10):688–699. [Google Scholar]
- 100.Li Y., Shi Y., Lu Y., Li X., Zhou J., Zadpoor A.A., et al. Additive manufacturing of vascular stents, Acta. Biomaterials. 2023;167:16–37. doi: 10.1016/j.actbio.2023.06.014. [DOI] [PubMed] [Google Scholar]
- 101.Sun J., Xie W., Wu Y., Li Z., Li Y. Accelerated bone healing via electrical stimulation. Adv. Sci. 2024 doi: 10.1002/advs.202404190. [DOI] [PubMed] [Google Scholar]
- 102.Gao J., Yu X., Wang X., He Y., Ding J. Biomaterial–rRelated cell microenvironment in tissue engineering and regenerative medicine. Engineering. 2022;13:31–45. [Google Scholar]
- 103.Ataie Z., Horchler S., Jaberi A., Koduru S.V., El-Mallah J.C., Sun M., et al. Accelerating patterned vascularization using granular hydrogel scaffolds and surgical micropuncture. Small. 2024;20(8) doi: 10.1002/smll.202307928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnbosco C., Zschoche S., Nitschke M., Hahn D., Werner C., Maitz M.F. Bioresponsive starPEG-heparin hydrogel coatings on vascular stents for enhanced hemocompatibility. Mater. Sci. Eng. C. 2021;128 doi: 10.1016/j.msec.2021.112268. [DOI] [PubMed] [Google Scholar]
- 105.Dhakane P., Tadimarri V.S., Sankaran S. Light-regulated pro-angiogenic engineered living materials. Adv. Funct. Mater. 2023;33(31) [Google Scholar]
- 106.Lu Y., Li L., Zhu Y., Wang X., Li M., Lin Z., et al. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS Appl. Mater. Interfaces. 2018;10(1):127–138. doi: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang W., Wang L., Zhang B., Shang S., Zhao C., Zhang W., et al. 3D printing of personalized magnesium composite bone tissue engineering scaffold for bone and angiogenesis regeneration. Chem. Eng. J. 2024;484(15) [Google Scholar]
- 108.Zhong C., Zhu H., Sheng Y., Wo J., You D., Sun G., et al. Biocompatibility and osteogenic potential of choline phosphate chitosan-coated biodegradable Zn1Mg, Acta. Biomaterials. 2024;175:395–410. doi: 10.1016/j.actbio.2023.12.014. [DOI] [PubMed] [Google Scholar]
- 109.Zhong Z., Li Y., Sun Z., Wu X., Li J., Jiang S., et al. Hypoxia-triggered exosome-mimetics accelerate vascularized osteogenesis. Mater. Today. 2024;73:16–29. [Google Scholar]
- 110.Xue H., Zhang Z., Lin Z., Su J., Panayi A.C., Xiong Y., et al. Enhanced tissue regeneration through immunomodulation of angiogenesis and osteogenesis with a multifaceted nanohybrid modified bioactive scaffold. Bioact. Mater. 2022;18:552–568. doi: 10.1016/j.bioactmat.2022.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mazaheri O., Lin Z., Xu W., Mohankumar M., Wang T., Zavabeti A., et al. Assembly of silicate–phenolic network coatings with tunable properties for controlled release of small molecules. Adv. Mater. 2024 doi: 10.1002/adma.202413349. [DOI] [PubMed] [Google Scholar]
- 112.Xie W., Guo Z., Zhao L., Wei Y. Metal-phenolic networks: facile assembled complexes for cancer theranostics. Theranostics. 2021;11(13):6407–6426. doi: 10.7150/thno.58711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang X., Li Z., Yang P., Duan G., Liu X., Gu Z., et al. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021;8(1):145–167. doi: 10.1039/d0mh01317j. [DOI] [PubMed] [Google Scholar]
- 114.Yang X., Shao J., Zhang Y., Wang T., Ge S., Li J. Microenvironment-driven fenton nanoreactor enabled by metal-phenolic encapsulation of calcium peroxide for effective control of dental caries. Adv. Healthcare Mater. 2024;13(10) doi: 10.1002/adhm.202303466. [DOI] [PubMed] [Google Scholar]
- 115.Lee S., Lee J., Byun H., Kim S.-J., Joo J., Park H.H., et al. Evaluation of the anti-oxidative and ROS scavenging properties of biomaterials coated with epigallocatechin gallate for tissue engineering, Acta. Biomaterials. 2021;124(1):166–178. doi: 10.1016/j.actbio.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Li L., Zhang C., Cao Z., Ma L., Liu C., Lan X., et al. Passivation protein-adhesion platform promoting stent reendothelialization using two-electron-assisted oxidation of polyphenols. Biomaterials. 2024;305 doi: 10.1016/j.biomaterials.2023.122423. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y., Zhou J., Yuan L., Wu F., Xie L., Yan X., et al. Neighboring carboxylic acid boosts peroxidase-like property of metal-phenolic nano-networks in eradicating streptococcus mutans biofilms. Small. 2022;19(3) doi: 10.1002/smll.202206657. [DOI] [PubMed] [Google Scholar]
- 118.Li X., Gao P., Tan J., Xiong K., Maitz M.F., Pan C., et al. Assembly of metal-phenolic/catecholamine networks for synergistically anti-inflammatory, antimicrobial, and anticoagulant coatings. ACS Appl. Mater. Interfaces. 2018;10(47):40844–40853. doi: 10.1021/acsami.8b14409. [DOI] [PubMed] [Google Scholar]
- 119.Osman M.E., Abo-Elnasr A.A., Mohamed E.T. Therapeutic potential activity of quercetin complexes against Streptococcus pneumoniae. Sci. Rep. 2024;14(1) doi: 10.1038/s41598-024-62782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reitzer F., Allais M., Ball V., Meyer F. Polyphenols at interfaces. Adv. Colloid Interface Sci. 2018;257:31–41. doi: 10.1016/j.cis.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 121.Xu L.Q., Neoh K.-G., Kang E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018;87:165–196. [Google Scholar]
- 122.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 123.Papuc C., Goran G.V., Predescu C.N., Nicorescu V., Stefan G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017;16(6):1243–1268. doi: 10.1111/1541-4337.12298. [DOI] [PubMed] [Google Scholar]
- 124.Liu T., Zhang M., Liu W., Zeng X., Song X., Yang X., et al. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. ACS Nano. 2018;12(4):3917–3927. doi: 10.1021/acsnano.8b01456. [DOI] [PubMed] [Google Scholar]
- 125.Zhao G., Wu H., Feng R., Wang D., Xu P., Jiang P., et al. Novel metal polyphenol framework for MR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces. 2018;10(4):3295–3304. doi: 10.1021/acsami.7b16222. [DOI] [PubMed] [Google Scholar]
- 126.Grape E.S., Flores J.G., Hidalgo T., Martínez-Ahumada E., Gutiérrez-Alejandre A., Hautier A., et al. A robust and biocompatible bismuth ellagate MOF synthesized under green ambient conditions. J. Am. Chem. Soc. 2020;142(39):16795–16804. doi: 10.1021/jacs.0c07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang S.J., Antonietti M., Fechler N. Self-assembly of metal phenolic mesocrystals and morphosynthetic transformation toward hierarchically porous carbons. J. Am. Chem. Soc. 2015;137(25):8269–8273. doi: 10.1021/jacs.5b04500. [DOI] [PubMed] [Google Scholar]
- 128.Dai Y., Cheng S., Wang Z., Zhang R., Yang Z., Wang J., et al. Hypochlorous acid promoted platinum drug chemotherapy by myeloperoxidase-encapsulated therapeutic metal phenolic nanoparticles. ACS Nano. 2018;12(1):455–463. doi: 10.1021/acsnano.7b06852. [DOI] [PubMed] [Google Scholar]
- 129.Yang Z., Dai Y., Shan L., Shen Z., Wang Z., Yung B.C., et al. Tumour microenvironment-responsive semiconducting polymer-based self-assembly nanotheranostics. Nanoscale Horiz. 2019;4(2):426–433. doi: 10.1039/C8NH00307F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai Y., Yang Z., Cheng S., Wang Z., Zhang R., Zhu G., et al. Toxic reactive oxygen species enhanced synergistic combination therapy by self-assembled metal-phenolic network nanoparticles. Adv. Mater. 2018;30(8) doi: 10.1002/adma.201704877. [DOI] [PubMed] [Google Scholar]
- 131.Liu G., Li K., Wang H., Ma L., Yu L., Nie Y. Stable fabrication of zwitterionic coating based on copper-phenolic networks on contact lens with improved surface wettability and broad-spectrum antimicrobial activity. ACS Appl. Mater. Interfaces. 2020;12(14):16125–16136. doi: 10.1021/acsami.0c02143. [DOI] [PubMed] [Google Scholar]
- 132.Zhang W., Besford Q.A., Christofferson A.J., Charchar P., Richardson J.J., Elbourne A., et al. Cobalt-directed assembly of antibodies onto metal-phenolic networks for enhanced particle targeting. Nano Lett. 2020;20(4):2660–2666. doi: 10.1021/acs.nanolett.0c00295. [DOI] [PubMed] [Google Scholar]
- 133.Yun G., Pan S., Wang T.-Y., Guo J., Richardson J.J., Caruso F. Synthesis of metal nanoparticles in metal-phenolic networks: catalytic and antimicrobial applications of coated textiles. Adv. Healthcare Mater. 2018;7(5) doi: 10.1002/adhm.201700934. [DOI] [PubMed] [Google Scholar]
- 134.Tu Q., Shen X., Liu Y., Zhang Q., Zhao X., Maitz M.F., et al. A facile metal–phenolic–amine strategy for dual-functionalization of blood-contacting devices with antibacterial and anticoagulant properties. Mater. Chem. Front. 2019;3(2):265–275. [Google Scholar]
- 135.Chen C., Zhang S., Zhang R., Sun P., Shi C., Abdalla M., et al. In situ tuning proangiogenic factor-mediated immunotolerance synergizes the tumoricidal immunity via a hypoxia-triggerable liposomal bio-nanoreactor. Theranostics. 2020;10(26):11998–12010. doi: 10.7150/thno.50806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang L., Li L., Wu H., Zhang B., Luo R., Wang Y. Catechol-mediated and copper-incorporated multilayer coating: an endothelium-mimetic approach for blood-contacting devices. J. Contr. Release. 2020;321(10):59–70. doi: 10.1016/j.jconrel.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Liu L., Xiao X., Li K., Li X., Yu K., Liao X., et al. Prevention of bacterial colonization based on self-assembled metal-phenolic nanocoating from rare-earth ions and catechin. ACS Appl. Mater. Interfaces. 2020;12(19):22237–22245. doi: 10.1021/acsami.0c06459. [DOI] [PubMed] [Google Scholar]
- 138.Xu Y., Sheng K., Li C., Shi G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano. 2010;4(7):4324–4330. doi: 10.1021/nn101187z. [DOI] [PubMed] [Google Scholar]
- 139.Xie Y., Chen S., Peng X., Wang X., Wei Z., Richardson J.J., et al. Alloyed nanostructures integrated metal-phenolic nanoplatform for synergistic wound disinfection and revascularization. Bioact. Mater. 2022;16:95–106. doi: 10.1016/j.bioactmat.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11(6):371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 141.Lee S., Chang Y.-Y., Lee J., Madhurakkat Perikamana S.K., Kim E.M., Jung Y.-H., et al. Surface engineering of titanium alloy using metal-polyphenol network coating with magnesium ions for improved osseointegration. Biomater. Sci. 2020;8(12):3404–3417. doi: 10.1039/d0bm00566e. [DOI] [PubMed] [Google Scholar]
- 142.Gao X., Wang Q., Ren L., Gong P., He M., Tian W., et al. Metal-phenolic networks as a novel filler to advance multi-functional immunomodulatory biocomposites. Chem. Eng. J. 2021;426 [Google Scholar]
- 143.Qiaoxia L., Yujie Z., Meng Y., Yizhu C., Yan W., Yinchun H., et al. Hydroxyapatite/tannic acid composite coating formation based on Ti modified by TiO2 nanotubes. Colloids Surf., B. 2020;196 doi: 10.1016/j.colsurfb.2020.111304. [DOI] [PubMed] [Google Scholar]
- 144.Chen X., Tan B., Wang S., Tang R., Bao Z., Chen G., et al. Rationally designed protein cross-linked hydrogel for bone regeneration via synergistic release of magnesium and zinc ions. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120895. [DOI] [PubMed] [Google Scholar]
- 145.Chen Z., Duan J., Diao Y., Chen Y., Liang X., Li H., et al. ROS-responsive capsules engineered from EGCG-Zinc networks improve therapeutic angiogenesis in mouse limb ischemia. Bioact. Mater. 2021;6(1):1–11. doi: 10.1016/j.bioactmat.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X., Wang Y., Liu J., Shi J., Mao D., Midgley A.C., et al. A metal-organic-framework incorporated vascular graft for sustained nitric oxide generation and long-term vascular patency. Chem. Eng. J. 2021;421(1) [Google Scholar]
- 147.Li Z., Huang X., Lin L., Jiao Y., Zhou C., Liu Z. Polyphenol and Cu2+ surface-modified chitin sponge synergizes with antibacterial, antioxidant and pro-vascularization activities for effective scarless regeneration of burned skin. Chem. Eng. J. 2021;419(1) [Google Scholar]
- 148.Ninan N., Forget A., Shastri V.P., Voelcker N.H., Blencowe A. Antibacterial and anti-Inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces. 2016;8(42):28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 149.Xiao S., Wei J., Jin S., Xia X., Yuan L., Zou Q., et al. A multifunctional coating strategy for promotion of immunomodulatory and osteo/angio-genic activity. Adv. Funct. Mater. 2023;33(4) [Google Scholar]
- 150.Guo J., Ping Y., Ejima H., Alt K., Meissner M., Richardson J.J., et al. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 2014;53(22):5546–5551. doi: 10.1002/anie.201311136. [DOI] [PubMed] [Google Scholar]
- 151.Xu W., Lin Z., Pan S., Chen J., Wang T., Cortez-Jugo C., et al. Direct assembly of metal-phenolic network nanoparticles for biomedical applications. Angew. Chem. Int. Ed. 2023;62(45) doi: 10.1002/anie.202312925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen J., Pan S., Zhou J., Lin Z., Qu Y., Glab A., et al. Assembly of bioactive nanoparticles via metal–phenolic complexation. Adv. Mater. 2022;34(10) doi: 10.1002/adma.202108624. [DOI] [PubMed] [Google Scholar]
- 153.Harrington M.J., Masic A., Holten-Andersen N., Waite J.H., Fratzl P. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science. 2010;328(5975):216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cui C., Liu W. Recent advances in wet adhesives: adhesion mechanism, design principle and applications. Prog. Polym. Sci. 2021;116 [Google Scholar]
- 155.Gan D., Xing W., Jiang L., Fang J., Zhao C., Ren F., et al. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019;10(1):1487. doi: 10.1038/s41467-019-09351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Geng H., Zhang P., Peng Q., Cui J., Hao J., Zeng H. Principles of cation−π interactions for engineering mussel-inspired functional materials. Acc. Chem. Res. 2022;55(8):1171–1182. doi: 10.1021/acs.accounts.2c00068. [DOI] [PubMed] [Google Scholar]
- 157.Chen J., Pan S., Zhou J., Zhong Q.-Z., Qu Y., Richardson J.J., et al. Programmable permeability of metal–phenolic network microcapsules. Chem. Mater. 2020;32(16):6975–6982. [Google Scholar]
- 158.Zhang Y., Li K., Shen L., Yu L., Ding T., Ma B., et al. Metal phenolic nanodressing of porous polymer scaffolds for enhanced bone regeneration via interfacial gating growth factor release and stem cell differentiation. ACS Appl. Mater. Interfaces. 2022;14(1):268–277. doi: 10.1021/acsami.1c19633. [DOI] [PubMed] [Google Scholar]
- 159.Zhu Y., Niu X., Wu T., Cheng J., Zou J., Pan Y., et al. Metal-phenolic nanocatalyst rewires metabolic vulnerability for catalytically amplified ferroptosis. Chem. Eng. J. 2024;485(1) [Google Scholar]
- 160.Liu Q., Zhang A., Wang R., Zhang Q., Cui D. A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano Lett. 2021;13(1):154. doi: 10.1007/s40820-021-00674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen Y., Yang X., Li K., Feng J., Liu X., Li Y., et al. Phenolic ligand–metal charge transfer induced copper nanozyme with reactive oxygen species-scavenging ability for chronic wound healing. ACS Nano. 2024;18(9):7024–7036. doi: 10.1021/acsnano.3c10376. [DOI] [PubMed] [Google Scholar]
- 162.Gao Q., Chu X., Yang J., Guo Y., Guo H., Qian S., et al. An antibiotic nanobomb constructed from pH-responsive chemical bonds in metal-phenolic network nanoparticles for biofilm eradication and corneal ulcer healing. Adv. Sci. 2024;11(22) doi: 10.1002/advs.202309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Niu B., Liao K., Zhou Y., Wen T., Quan G., Pan X., et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121110. [DOI] [PubMed] [Google Scholar]
- 164.Liu Z., Liu S., Liu B., Bian Y., Yuan M., Yang C., et al. Fe(III)-naphthazarin metal–phenolic networks for glutathione-depleting enhanced ferroptosis–apoptosis combined cancer therapy. Small. 2023;19(19) doi: 10.1002/smll.202207825. [DOI] [PubMed] [Google Scholar]
- 165.Liu Z., Liu S., Liu B., Meng Q., Yuan M., Ma X., et al. Tumor microenvironment-activatable metal-phenolic nanoformulations for ultrasound-boosted ferroptosis through triple regulatory pathways. Adv. Funct. Mater. 2024;34(44) [Google Scholar]
- 166.Zhao F., Yu H., Liang L., Wang C., Shi D., Zhang X., et al. Redox homeostasis disruptors based on metal-phenolic network nanoparticles for chemo/chemodynamic synergistic tumor therapy through activating apoptosis and cuproptosis. Adv. Healthcare Mater. 2023;12(29) doi: 10.1002/adhm.202301346. [DOI] [PubMed] [Google Scholar]
- 167.Li D., Li J., Wang S., Wang Q., Teng W. Dually crosslinked copper-poly(tannic acid) nanoparticles with microenvironment-responsiveness for infected wound treatment. Adv. Healthcare Mater. 2023;12(17) doi: 10.1002/adhm.202203063. [DOI] [PubMed] [Google Scholar]
- 168.Lin G., Richardson J.J., Ahmed H., Besford Q.A., Christofferson A.J., Beyer S., et al. Programmable phototaxis of metal–phenolic particle microswimmers. Adv. Mater. 2021;33(13) doi: 10.1002/adma.202006177. [DOI] [PubMed] [Google Scholar]
- 169.Guo Z., Liu T., Gao W., Iffelsberger C., Kong B., Pumera M. Multi-wavelength light-responsive metal–phenolic network-based microrobots for reactive species scavenging. Adv. Mater. 2023;35(10) doi: 10.1002/adma.202210994. [DOI] [PubMed] [Google Scholar]
- 170.Cao H., Yang L., Tian R., Wu H., Gu Z., Li Y. Versatile polyphenolic platforms in regulating cell biology. Chem. Soc. Rev. 2022;51(10):4175–4198. doi: 10.1039/d1cs01165k. [DOI] [PubMed] [Google Scholar]
- 171.Sun X., Zhou X., Shi X., Abed O.A., An X., Lei Y.L., et al. Strategies for the development of metalloimmunotherapies. Nat. Biomed. Eng. 2024;8(9):1073–1091. doi: 10.1038/s41551-024-01221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.He M., Wang H., Han Q., Shi X., He S., Sun J., et al. Glucose-primed PEEK orthopedic implants for antibacterial therapy and safeguarding diabetic osseointegration. Biomaterials. 2023;303 doi: 10.1016/j.biomaterials.2023.122355. [DOI] [PubMed] [Google Scholar]
- 173.Liu J., Shi Y., Zhao Y., Liu Y., Yang X., Li K., et al. A multifunctional metal–phenolic nanocoating on bone implants for enhanced osseointegration via early immunomodulation. Adv. Sci. 2024;11(18) doi: 10.1002/advs.202307269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jeong H., Byun H., Lee J., Han Y., Huh S.J., Shin H. Enhancement of bone tissue regeneration with multi-functional nanoparticles by coordination of immune, osteogenic, and angiogenic responses. Adv. Healthcare Mater. 2024 doi: 10.1002/adhm.202400232. [DOI] [PubMed] [Google Scholar]
- 175.Zhang Q.-Y., Tan J., Huang K., Nie R., Feng Z.-Y., Zou C.-Y., et al. Polyphenolic-modified cellulose acetate membrane for bone regeneration through immunomodulation. Carbohydr. Polym. 2023;305(1) doi: 10.1016/j.carbpol.2023.120546. [DOI] [PubMed] [Google Scholar]
- 176.Hu X., Chen J., Yang S., Zhang Z., Wu H., He J., et al. 3D printed multifunctional biomimetic bone scaffold combined with TP-Mg nanoparticles for the infectious bone defects repair. Small. 2024;20(40) doi: 10.1002/smll.202403681. [DOI] [PubMed] [Google Scholar]
- 177.Xu K., Mu C., Zhang C., Deng S., Lin S., Zheng L., et al. Antioxidative and antibacterial gallium (III)-phenolic coating for enhanced osseointegration of titanium implants via pro-osteogenesis and inhibiting osteoclastogenesis. Biomaterials. 2023;301 doi: 10.1016/j.biomaterials.2023.122268. [DOI] [PubMed] [Google Scholar]
- 178.Lee N.-H., Kang M.S., Kim T.-H., Yoon D.S., Mandakhbayar N., Jo S.B., et al. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121025. [DOI] [PubMed] [Google Scholar]
- 179.Cui X., Zhang Y., Wang J., Huang C., Wang Y., Yang H., et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact. Mater. 2020;5(2):334–347. doi: 10.1016/j.bioactmat.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ren W.-H., Xin S., Yang K., Yu Y.-B., Li S.-M., Zheng J.-J., et al. Strontium-doped hydroxyapatite promotes osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporotic rats through the CaSR-JAK2/STAT3 signaling pathway. Adv. NanoBiomed Res. 2022;2(9) [Google Scholar]
- 181.Saidak Z., Haÿ E., Marty C., Barbara A., Marie P.J. Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell. 2012;11(3):467–474. doi: 10.1111/j.1474-9726.2012.00804.x. [DOI] [PubMed] [Google Scholar]
- 182.Liu Z., Wang T., Zhang L., Luo Y., Zhao J., Chen Y., et al. Metal–phenolic networks-reinforced extracellular matrix scaffold for bone regeneration via combining radical-scavenging and photo-responsive regulation of microenvironment. Adv. Healthcare Mater. 2024;13(15) doi: 10.1002/adhm.202304158. [DOI] [PubMed] [Google Scholar]
- 183.P. Ming, B. Li, Q. Li, L. Yuan, X. Jiang, Y. Liu, et al., Multifunctional sericin-based biomineralized nanoplatforms with immunomodulatory and angio/osteo-genic activity for accelerated bone regeneration in periodontitis, Biomaterials 314 (2025) 122885. [DOI] [PubMed]
- 184.F. Jia, J. Guan, J. Wang, M. Li, Y. Zhang, L. Xie, et al., Zinc and melatonin mediated antimicrobial, anti-inflammatory, and antioxidant coatings accelerate bone defect repair, Colloids Surf., B 245 (2025) 114335. [DOI] [PubMed]
- 185.Wang B., Waterhouse G.I.N., Lu S. Carbon dots: mysterious past, vibrant present, and expansive future. Trends Chem. 2023;5(1):76–87. [Google Scholar]
- 186.Syama S., Mohanan P.V. Comprehensive application of graphene: emphasis on biomedical concerns. Nano Lett. 2019;11(1):6. doi: 10.1007/s40820-019-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun Y., Zheng L., Yang Y., Qian X., Fu T., Li X., et al. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano Lett. 2020;12(1):103. doi: 10.1007/s40820-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Huang H., Feng W., Chen Y. Two-dimensional biomaterials: material science, biological effect and biomedical engineering applications. Chem. Soc. Rev. 2021;50(20):11381–11485. doi: 10.1039/d0cs01138j. [DOI] [PubMed] [Google Scholar]
- 189.Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 190.Figuero E., Serrano J., Arweiler N.B., Auschill T.M., Gürkan A., Emingil G. Supra and subgingival application of antiseptics or antibiotics during periodontal therapy. Periodontol. 2023;2000 doi: 10.1111/prd.12511. [DOI] [PubMed] [Google Scholar]
- 191.Li J., Wang Y., Tang M., Zhang C., Fei Y., Li M., et al. New insights into nanotherapeutics for periodontitis: a triple concerto of antimicrobial activity, immunomodulation and periodontium regeneration. J. Nanobiotechnol. 2024;22(1):19. doi: 10.1186/s12951-023-02261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dang G.-p., Wei Y., Wan Q.-q., Gu J.-t., Wang K.-y., Wan M.-c., et al. Regulatory mechanisms and regeneration strategies of the soft–hard tissue interface in the human periodontium. BME Mat. 2024;2(3) [Google Scholar]
- 193.Gong J., Ye C., Ran J., Xiong X., Fang X., Zhou X., et al. Polydopamine-mediated immunomodulatory patch for diabetic periodontal tissue regeneration assisted by metformin-ZIF system. ACS Nano. 2023;17(17):16573–16586. doi: 10.1021/acsnano.3c02407. [DOI] [PubMed] [Google Scholar]
- 194.Mei H., Liu H., Sha C., Lv Q., Song Q., Jiang L., et al. Multifunctional metal–phenolic composites promote efficient periodontitis treatment via antibacterial and osteogenic properties. ACS Appl. Mater. Interfaces. 2024;16(11):13573–13584. doi: 10.1021/acsami.3c19621. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Y., Chen Y., Ding T., Zhang Y., Yang D., Zhao Y., et al. Janus porous polylactic acid membranes with versatile metal–phenolic interface for biomimetic periodontal bone regeneration, npj Regen. Méd. 2023;8(1):28. doi: 10.1038/s41536-023-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang H., Wang D., Huangfu H., Lv H., Qin Q., Ren S., et al. Branched AuAg nanoparticles coated by metal–phenolic networks for treating bacteria-induced periodontitis via photothermal antibacterial and immunotherapy. Mater. Des. 2022;224 [Google Scholar]
- 197.B. Zhang, G. Gong, Y. He, J. Liu, B. Wang, Y. Li, et al., Regulatory T cells engineered with polyphenol-functionalized immunosuppressant nanocomplexes for rebuilding periodontal hard tissue under inflammation-challenged microenvironment, Biomaterials 315 (2025) 122961. [DOI] [PubMed]
- 198.Jiang X., Sun Y., Lyu Y., Kang H., Zhao J., Zhang K., et al. Hydrogels for ameliorating osteoarthritis: mechanical modulation, anti-inflammation, and regeneration. BME Mat. 2024;2(2) [Google Scholar]
- 199.Meng X., Wang W.-D., Li S.-R., Sun Z.-J., Zhang L. Harnessing cerium-based biomaterials for the treatment of bone diseases, Acta. Biomaterials. 2024;183(15):30–49. doi: 10.1016/j.actbio.2024.05.046. [DOI] [PubMed] [Google Scholar]
- 200.Li L., Yin Y., Zhang S., Yang J., Li P., Zhou H., et al. Triggerable biomaterials-based osteomyelitis theranostics. BME Mat. 2024;4(29) [Google Scholar]
- 201.Yu P., Li Y., Sun H., Zhang H., Kang H., Wang P., et al. Mimicking antioxidases and hyaluronan synthase: a zwitterionic nanozyme for photothermal therapy of osteoarthritis. Adv. Mater. 2023;35(44) doi: 10.1002/adma.202303299. [DOI] [PubMed] [Google Scholar]
- 202.Yu H., Luo H., Chen J., Hu X., Chen Y., Zhong J., et al. Hydrogel microspheres for stem cell recruitment and induction of directed differentiation in osteoarthritis therapy. Chem. Eng. J. 2024;497(1) [Google Scholar]
- 203.Song X., Zheng Z., Ouyang S., Chen H., Sun M., Lin P., et al. Biomimetic epigallocatechin gallate–cerium assemblies for the treatment of rheumatoid arthritis. ACS Appl. Mater. Interfaces. 2023;15(28):33239–33249. doi: 10.1021/acsami.3c02768. [DOI] [PubMed] [Google Scholar]
- 204.Chen Q., Jin Y., Chen T., Zhou H., Wang X., Wu O., et al. Injectable nanocomposite hydrogels with enhanced lubrication and antioxidant properties for the treatment of osteoarthritis. Mater. Today Bio. 2024;25 doi: 10.1016/j.mtbio.2024.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen Y., Xu W., Shafiq M., Song D., Wang T., Yuan Z., et al. Injectable nanofiber microspheres modified with metal phenolic networks for effective osteoarthritis treatment, Acta. Biomaterials. 2023;157:593–608. doi: 10.1016/j.actbio.2022.11.040. [DOI] [PubMed] [Google Scholar]
- 206.Zou Y.-P., Liang H.-F., Wang B., Zhang Q.-C., Su D.-H., Lu S.-Y., et al. Precipitation-based silk fibroin fast gelling, highly adhesive, and magnetic nanocomposite hydrogel for repair of irregular bone defects. Adv. Funct. Mater. 2023;33(29) [Google Scholar]
- 207.Min H., Li K., Wang Q., Gao X., Xie L., Tian W. A novel filler of biocomposites for long-term self-regulated delivery of immunomodulatory and antibacterial components to accelerate bone regeneration. Composites, Part B. 2022;238(1) [Google Scholar]
- 208.Li X., Pang Y., Guan L., Li L., Zhu Y., Whittaker A.K., et al. Mussel-inspired antimicrobial hydrogel with cellulose nanocrystals/tannic acid modified silver nanoparticles for enhanced calvarial bone regeneration. Int. J. Biol. Macromol. 2024;270(2) doi: 10.1016/j.ijbiomac.2024.132419. [DOI] [PubMed] [Google Scholar]
- 209.Huang J., Tan Q.-C., Bai H., Wang J., Makvandi P., Ali Khan M., et al. Harnessing immunomodulation for efficient bone Regeneration: bioengineered black phosphorus-incorporated Self-Healing hydrogel. Chem. Eng. J. 2023;470 [Google Scholar]
- 210.Wu Z., Fan L., Chen C., Ma Y., Wu X., Li Y., et al. Promotion of osteoporotic bone healing by a tannic acid modified strontium-doped biomimetic bone lamella with ROS scavenging capacity and pro-osteogenic effect. Smart Mater. Med. 2023;4:590–602. [Google Scholar]
- 211.Hussain Z., Ullah I., Liu X., Mehmood S., Wang L., Ma F., et al. GelMA-catechol coated FeHAp nanorods functionalized nanofibrous reinforced bio-instructive and mechanically robust composite hydrogel scaffold for bone tissue engineering. Biomater. Adv. 2023;155 doi: 10.1016/j.bioadv.2023.213696. [DOI] [PubMed] [Google Scholar]
- 212.Ren L., Gong P., Gao X., Wang Q., Xie L., Tang W., et al. Metal–phenolic networks acted as a novel bio-filler of a barrier membrane to improve guided bone regeneration via manipulating osteoimmunomodulation. J. Mater. Chem. B. 2022;10(48):10128–10138. doi: 10.1039/d2tb01804g. [DOI] [PubMed] [Google Scholar]
- 213.Tang W., Fischer N.G., Kong X., Sang T., Ye Z. Hybrid coatings on dental and orthopedic titanium implants: current advances and challenges. BME Mat. 2024 [Google Scholar]
- 214.Gao P., Zuo Y., Yang Y., Yu J., Fan W., Bai R., et al. Multifunctional photothermal PB@EGCG-Sr nanocoating design on titanium surface: to achieve short-term rapid osseointegration and on-demand photothermal long-term osteogenesis. Chem. Eng. J. 2023;474 [Google Scholar]
- 215.Shou Z., Bai Z., Huo K., Zheng S., Shen Y., Zhou H., et al. Immobilizing c(RGDfc) on the surface of metal-phenolic networks by thiol-click reaction for accelerating osteointegration of implant. Mater. Today Bio. 2024;25 doi: 10.1016/j.mtbio.2024.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xu L., Fang J., Pan J., Qi H., Yin Y., He Y., et al. Zinc finger-inspired peptide-metal-phenolic nanointerface enhances bone-implant integration under bacterial infection microenvironment through immune modulation and osteogenesis promotion. Bioact. Mater. 2024;41:564–576. doi: 10.1016/j.bioactmat.2024.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hussain Z., Ullah I., Liu X., Shen W., Ding P., Zhang Y., et al. Tannin-reinforced iron substituted hydroxyapatite nanorods functionalized collagen-based composite nanofibrous coating as a cell-instructive bone-implant interface scaffold. Chem. Eng. J. 2022;438 [Google Scholar]
- 218.Zhang S., Chai Q., Man Z., Tang C., Li Z., Zhang J., et al. Bioinspired nano-painting on orthopedic implants orchestrates periprosthetic anti-infection and osseointegration in a rat model of arthroplasty. Chem. Eng. J. 2022;435(1) [Google Scholar]
- 219.Ding R., Yuan Z., Wang K., Peng P., Liang J., Wang K., et al. Dual-functional polyetheretherketone surface modification for enhanced osseointegration and antibacterial activity in pH-responsive manner. J. Mater. Sci. Technol. 2024;200:129–140. [Google Scholar]
- 220.Wang X., Pan L., Zheng A., Cao L., Wen J., Su T., et al. Multifunctionalized carbon-fiber-reinforced polyetheretherketone implant for rapid osseointegration under infected environment. Bioact. Mater. 2023;24:236–250. doi: 10.1016/j.bioactmat.2022.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li Y., Cheng X., Zhang X., Ma Z., Deng C., Liu C., et al. Biomimetic metal-phenolic network with cyclolactam hydrogel coating on PPENK implant facilitate bone repair. Chem. Eng. J. 2024;486 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.