Abstract
The detection of skeletal remains using human remain detection dogs (HRD) is often reported anecdotally by handlers to be a challenge. Limited studies have been conducted to determine the volatile organic compounds (VOCs) emitted from bones, particularly when there is limited organic matter remaining. This study aimed to determine the VOCs emitted from dry, weathered bones and examine the detection performance of HRD dogs on these bones when used as training aids. The VOCs of four different bones (clavicle, rib, humerus, and vertebrae) from three cadavers were collected using sorbent tubes and analyzed using comprehensive two-dimensional gas chromatography‒time-of-flight mass spectrometry (GC × GC‒TOFMS). Subsequently, the responses of the HRD dogs to the bone samples were recorded over two separate two-day trials. A total of 296 VOCs were detected and classified into chemical classes, with aromatics and linear aliphatics being the most abundant classes. Several differences in the chemical class distribution were observed between the bone types, but the number and intensity of the VOCs were similar between the bone samples. During the HRD dog training, a higher false detection rate was observed on the first day of each trial; however, the detection rate improved to 100 % on the second day of each trial. Although the dogs are capable of detecting bones, they require exposure to and training with a diverse range of skeletal remains to enhance their efficiency. This is necessary due to the variations in the types and intensity of VOCs compared to earlier decomposition stages involving soft tissue.
Keywords: Forensic taphonomy, Decomposition chemistry, Skeletal remains, Canine detection, Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GCxGC-TOFMS)
Highlights
-
•
GC × GC‒TOFMS is valuable in detecting reduced odour profiles in human bone.
-
•
Characterization of VOC profiles across various bone types revealed differences in their chemical signatures.
-
•
Effective training of Human Remains Detection dogs requires repeat exposure to human bones.
1. Introduction
The process of human decomposition has been studied extensively in the literature [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]] and has been commonly classified into five observable phases: fresh, bloat, active decay, advanced decay, and dry remains/skeletonization [5,13]. The decomposition continuum is extremely complex and depends on several internal (body composition, health issues, cause of death, etc.) and external (temperature, weather, fauna, flora, etc.) factors [4,11,12,14,15]. During these stages, the degradation of macromolecules such as proteins, lipids, and carbohydrates, release a variety of volatile organic compounds (VOCs) which contribute to the decomposition odour [7,9,[16], [17], [18], [19]]. Human remains detection (HRD) dogs rely on these VOCs to locate human remains during search and recovery operations following suspicious disappearances or mass disasters [[20], [21], [22], [23], [24]]. HRD dogs are trained using a variety of decomposition odour sources known as training aids [23,25]. Regular training with these aids (human tissues, blood, bones, etc.), which closely resemble the odour signature of human remains, results in enhanced recovery rates during search operations [[26], [27], [28], [29]].
The success rate of HRD dogs has been shown to decrease when searching for older or skeletonized remains [25]. Compared to the other decomposition stages, the skeletal remains stage is composed mostly of bone and other hard tissue with very little soft tissue remaining [1,2]. Bones contain less organic matter compared to soft tissue, which often reduces the level of emitted VOCs that are detectable to HRD dogs making their detection more challenging. The characterization and variability of bone VOCs have been minimally examined as the majority of studies have focused on soft tissue decomposition [17,18,30,31]. Consequently, the lack of sufficient data on VOCs emitted from human bones represents a critical gap in the literature, which hinders the development of effective HRD dog training for locating skeletal remains.
The complexity of the decomposition odour matrix requires advanced collection, separation, and identification methods for its analysis [[32], [33], [34]]. Sorbent tubes, which contain materials designed to capture VOCs within the tubes, are used to collect volatile compounds that accumulate in the headspace above the samples. The sorbent tubes are then heated through a process known as thermal desorption (TD) to release the trapped VOCs for analysis [33,35]. In previous studies, gas chromatography (GC) has been used to separate odour mixtures into identifiable VOCs, however, in more recent years, there has been a transition to comprehensive two-dimensional gas chromatography (GC × GC) [[36], [37], [38], [39]]. The coupling of two different columns with different stationary phases in succession enables a higher peak capacity and selectivity compared to conventional GC-MS [[40], [41], [42], [43], [44], [45], [46]]. These columns are serially connected via a modulator, which allows efficient trapping and reinjection of volatile analytes onto the second-dimension column. The GC × GC is typically coupled with a time-of-flight mass spectrometer (TOFMS) for compound identification. TOFMS is preferably used because of the fast acquisition rate and minimal signal distortion [32,37,38,41]. Since fewer VOCs are emitted from bones, using GC × GC-TOFMS allows for the determination of a more complete VOC profile.
This study aims to determine the VOCs emitted from different types of human bones using TD-GC × GC-TOFMS. Additionally, the detection performance of various HRD dogs in both indoor and outdoor training scenarios was examined. This allowed for additional insights into the dogs' proficiency in locating human bones as well as validating the use of bones as training aids for the search of skeletal remains. The results will help direct recommendations to enhance HRD dogs’ success rates in both training and operational scenarios.
2. Materials and methods
2.1. Bone collection
The human bones utilized for this research were collected from the Research in Experimental and Social Thanatology (REST) facility affiliated with the University of Quebec at Trois-Rivieres (UQTR). The REST facility consists of a high-security outdoor area that permits donation of cadavers for the physical, chemical, and biological study of human decomposition in a Canadian environment [47]. This site was chosen to replicate the environment in which law enforcement agencies could search for human remains in real-case scenarios [48].
Bones from three different cadavers (donors) were used for this research (Table 1). Donor selection was based on the stage of decomposition and the availability of bones to avoid disturbing any remaining tissues. For each donor, four types of bones, with little to no remaining soft tissues, were collected for analysis: the right clavicle, the first right rib, the right humerus, and three lumbar vertebrae (L2, L3, L4). The collection and sampling of the bones was authorized by the ethics subcommittee of the teaching and research laboratory in anatomy at UQTR (CER-09-148-06.05).
Table 1.
Personal and demographic information regarding the REST Donors (n = 3), including the date of arrival and days since deposition at the time of the last VOC collection.
| Donor ID | Date of death (dd/mm/yyyy) | Sex | Age | Height (cm) | Weight (kg) | Date of arrival at REST (dd/mm/yyyy) | Days since deposition |
|---|---|---|---|---|---|---|---|
| Donor 1 | August 07, 2020 | M | 71 | 173 | 70.0 | August 10, 2020 | 854 |
| Donor 2 | September 27, 2020 | F | 69 | 165 | 54.0 | September 28, 2020 | 805 |
| Donor 3 | November 01, 2020 | M | 72 | 180 | 85.3 | November 02, 2020 | 769 |
2.2. VOC collection
The bones were collected and transported to the UQTR laboratory to reduce the background VOCs being collected during the sample collection process. After each sampling period, the bones were returned to their respective donors at the REST facility to allow the decomposition process to continue. Bones were sampled individually five times between June 2022 and November 2022. VOCs were collected by placing an aluminum hood (38 cm × 38 cm x 38 cm) over each bone for 15 min to allow headspace accumulation. The headspace was drawn onto a Tenax TA/Carbograph 5TD stainless steel sorbent tube (Markes International Ltd, Bridgend, UK) using an ACTI-VOC pump (Markes International Ltd, Bridgend, UK). The samples were collected in triplicates at a constant flow rate of 100 mL/min for 5 min. Control samples were also collected to determine the background VOCs in the laboratory environment where the headspace was collected. Sorbent tubes were capped with brass long-term storage caps fitted with PTFE ferrules, placed in a sealed glass mason jar and stored at 4 °C in a refrigerator until analysis.
2.3. VOC analysis
Prior to analysis, 0.2 μL of 10 mg/L bromobenzene (GC grade, Sigma-Aldrich) in methanol (HPLC grade, Sigma-Aldrich) was added as an internal standard to each sorbent tube using eVOL® XR handheld automated analytical syringe (SGE Analytical Science, Wetherill Park, NSW, Australia). Samples were analyzed using a Markes TD 100-xr multitube autosampler thermal desorption (TD) (Markes International Ltd., Bridgend, UK) coupled to the LECO Pegasus® BT 4D GC × GC-TOFMS system (LECO Corporation, St-Joseph, MI, USA). This system is equipped with a secondary oven, a quad-jet dual-stage modulator, and a unit mass TOFMS.
Each sorbent tube was heated at 300 °C for 5 min to allow thermal desorption of the compounds before being collected onto a general-purpose cold trap at −10 °C. This trap was desorbed with a split ratio of 50.5:1 at 280 °C for 5 min at a desorption flow rate of 20 mL/min. The desorbed analytes were injected onto the first-dimension column through an uncoated deactivated silica fused transfer line (1.2 m) maintained at 150 °C. The first-dimension column was a mid-polar Rxi®-624Sil MS (Restek Corporation, Bellefonte, PA, USA) (30 m × 0.25 mm ID × 1.40 μm df), and the second-dimension column was a polar Stabilwax (Restek Corporation, Bellafonte, PA, USA) (2 m × 0.250 mm ID × 0.25 μm df). The first-dimension oven was set to 35 °C and held for 7 min before increasing at 4 °C/min to 230 °C where it was held for 1 min. The helium (high purity, Linde Inc., Trois-Rivieres, Quebec, Canada) used as the carrier gas was maintained at a constant pressure of 122.7 kpa. The modulation period was 6 s with a 1.20 s hot pulse. The offset temperature for the second oven was +5 °C relative to the first-dimension GC oven temperature and the offset temperature for the modulator was +15 °C relative to the second-dimension oven. The transfer line was held at 250 °C and the TOFMS was operated in electron ionization (EI) mode at 70 eV and the ion source was kept at 250 °C, with a mass range of 29–450, an acquisition delay of 430 s, and an acquisition rate of 250 spectra/s.
2.4. Data analysis
ChromaTOF® (version 5.51.6.0; LECO) was used for initial data processing, including peak finding (minimum signal-to-noise ratio of 100), baseline integration, non-targeted deconvolution (NTD®), and spectral library matching. The National Institute of Standards and Technology (NIST 2017) mass spectral library was used to identify compounds with a spectral match required to combine GC × GC sub-peaks set to a minimum of 500. The minimum similarity for matches was set to 600 and the minimum similarity before hit was set to 700. Data processing tasks such as peak table alignment, peak area normalization to internal standard, sample triplicate and control comparison, sample triplicate merging, and filtering of miscellaneous peaks such as acetone, oxygen, solvent and column bleed, were performed using custom R scripts in R Studio ® (version 2023.03.0 + 386). A list of compounds with their respective normalized peak areas was generated. Further details on the custom R scripts can be found in previous studies [49].
2.5. HRD dog trials
Two separate HRD dog trials, spanning two days each, were conducted in October 2022 and May 2023 at the Ontario Provincial Police (OPP) Canine Unit involving certified HRD dog/handler teams from police agencies across Ontario. These trials involved exposing HRD dogs to the human bones from Donor 1 obtained from REST, with training authorized by the animal ethics committee at UQTR (N°2022-S.F.3.). The bones from Donor 1 were used for the dog trial because they were the oldest remains sampled in this study. A total of 22 HRD dogs participated in the study, with the number of dogs varying for each trial based on their availability.
During the trials, human bones were concealed in various indoor and outdoor locations. All the scenarios were single-blind trials where the HRD dogs' handler was unaware of the location of the target odour. Indoor trials took place in furnished rooms resembling an apartment and an office (Trial 1: Day 1, and Trial 2: Day 2). The search areas for the outdoor training were conducted in an acre of woodland and grassland (Trial 1: Day 2, and Trial 2: Day 1). As all trials were designed to simulate real-case scenarios, the research environment served as the control (i.e. background VOC profile) for the HRD dog trials. A detailed description of the trials and location of the location of the concealed bones is provided in Table 2. Handlers had the option to allow their dogs to search either off-leash or on-leash, with the dogs working only a few minutes at a time to prevent fatigue. When a dog indicated a final response, the handler would request the location confirmation before rewarding the dog. The HRD dogs’ responses were recorded as shown in Table 3. No true negative (TN) responses were recorded, as no distractors or blank odours were used in these trials due to the type of search scenario being used for training.
Table 2.
Description of the HRD dog trials conducted in October 2022 and May 2023 and the location of all concealed bones.
| Trial 1 |
Trial 2 |
|||
|---|---|---|---|---|
| Day 1 |
Day 2 |
Day 1 |
Day 2 |
|
| Apartment | Forest | Building backyard | Office | |
| Clavicle (C) | in a drawer | in a trunk | – | – |
| Rib (R) | in a drawer | at the foot of a tree | – | – |
| Humerus (H) | in a cabinet | under a fallen tree | on the grass, close to a fence | within a brick wall structure |
| Vertebrae (V) | in a locker | in a trunk | – | – |
Table 3.
Responses recorded for the HRD dog in the two-days trials conducted in October 2022 and May 2023.
| Response | Description |
|---|---|
| True positive (TP) | The dog correctly alerted to the target odour (human bones) |
| Partial positive (PP) | The dog showed a behaviour change, and narrowed down the target odour, but did not alert |
| False positive (FP) | The dog incorrectly gave a positive alert to a location without the target odour |
| False Negative (FN) | The dog did not give an alert at a location where there was a target odour |
These results were used to calculate each scenario's detection and false response rates using the following statistical measures:
| (1) |
| (2) |
| (3) |
3. Results and discussion
3.1. VOC profiles of human bone samples
From the sampling of the bones, 296 VOCs were identified. All VOCs were either only found in the human bones or found with a higher normalized peak area when compared to the controls. The detected VOCs included all classes of compounds previously identified in decomposition odour studies: acids, alcohols, aldehydes, aromatics, cyclic aliphatics, ester and analogues, ethers, halogen-containing, ketones, linear aliphatics, nitrogen-containing and sulphur-containing. The list of all the classified VOCs detected in the human bones can be found in the Supplementary Material section as an excel file sheet labelled “Supplementary Material – VOCs list”.
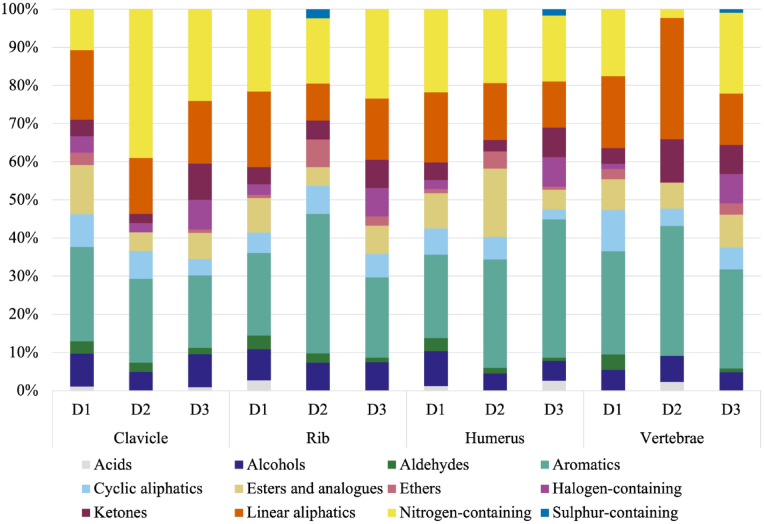
Fig. 1 represents the abundance of chemical classes in each donor's bone (clavicle, rib, humerus and vertebrae). The overall trend of each chemical class is very similar between the clavicle, the rib, the humerus and the vertebrae of each donor however, there are some differences in the abundance of VOCs between class types. The most abundant classes for all four of the bones are the aromatics (18.97–36.59 %; n = 55), linear aliphatics (9.75–31.81 %; n = 65), and nitrogen-containing compounds (2.27–39.04 %; n = 43), followed by esters and analogues (4.88–17.91 %; n = 33), alcohols (4.47–9.20 %; n = 30), cyclic aliphatics (2.58–10.81 %; n = 22), and ketones (2.43–11.36 %; n = 22). The halogen-containing compounds (0–7.69 %; n = 7), acids (0 %–2.70 %; n = 7), aldehydes (0–4.05 %; n = 6), ethers (0–7.31 %; n = 4) and sulphur-containing compounds (0–2.43 %; n = 2) were the classes with the least number of compounds where n represents the total number of different VOCs.
Fig. 1.
VOC class abundance (%) for the clavicle, rib, humerus and vertebrae (obtained from three different donors) analyzed five times between June 2022 to November 2022.
Some compound classes were not detected in some bone samples. Sulphur-containing compounds were detected only in three bone samples (D2 rib; D3 humerus; D3 vertebrae) and acids were only identified in 50 % of the bone samples (D2 clavicle; D2-D3 rib; D2 humerus; D1-D3 vertebrae). Ethers were not detected in two bone samples (D2 clavicle & D2 vertebrae), while halogen-containing compounds were not detected in three bone samples (D2 rib, D2 humerus and D2 vertebrae).
Of the 296 VOCs detected, the most abundant chemical classes were the aromatics, followed by the linear aliphatics and the nitrogen-containing compounds. These classes have been reported as the most abundant in other decomposition VOC studies as well [17,31,32,50]. Both aromatic compounds and nitrogen-containing compounds may result from the degradation of amino acids from proteins. During the process of bone decomposition, bacterial collagenase will degrade the protein from collagenases present in the bones into amino acids, then further degradation can result in the emission of aromatics and nitrogen-containing VOCs [32,33]. The nitrogen-containing compounds can also result from the degradation of the nitrogen-containing nucleic acids from residual DNA. Linear aliphatic compounds may be the result of lipid degradation [32,33]. Some residual lipid tissues, such as bone marrow, can still be present in dry bone, resulting in the emission of these compounds [51].
Ethers were among the least abundant VOCs, which is consistent with previous studies [31,52]. However, sulphur-containing compounds were one of the least abundant classes of VOCs identified in this study, despite their prevalence in soft tissue decomposition VOC studies [17,53]. This may be due to the lack of soft tissues on the bone samples analyzed in this study. The sulphur-containing compounds result from the breakdown of sulphur-containing amino acids such as cysteine and methionine [32,37,53]. Sulphur-containing compounds are known to be particularly scent heavy and their reduced presence in dry bone may influence the detection efficiency of HRD dogs.
Halogen-containing compounds were detected in 83 % of bones in this study. Halogen-containing compounds usually are not present in high abundance in decomposition VOCs studies [31,54], and thus there is minimal information about their origin. Hypothetically, halogen-containing compounds could result from residual soil on the bones or an accumulation of halogen-containing compounds in the bones through nutrition. Some halogens can be found in food and water [55,56] and accumulate in bones throughout a donor's life [[57], [58], [59]], subsequently being released during the degradation process.
3.2. Comparison of VOC profiles across bone types
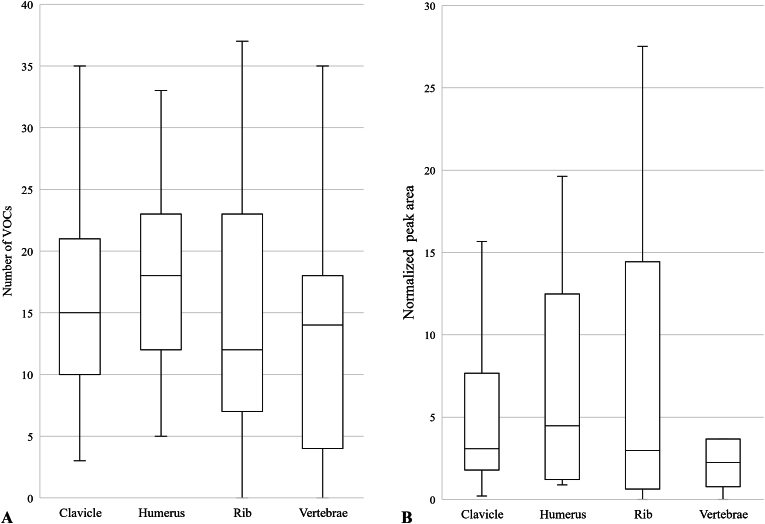
Fig. 2 highlights the variation in the number of VOCs (A) and the normalized peak areas (B) emitted from each bone. Fig. 2A shows the median and the variability of the total number of VOCs detected for each sampling of the bones (total of 15 sampling points per bone). Fig. 2B represents the distribution's central tendency and variability of the sum of the VOCs normalized peak area for each sampling of the bones (total of 15 sampling points per bone). The peaks have been normalized to the internal standard and the peak area is proportional to the concentration of the VOCs.
Fig. 2.
Variation of the number of VOCs (A) and the normalized peak area (B) for the clavicle, rib, humerus, and vertebrae analyzed between June 2022 and November 2022.
In Fig. 2A, the humerus has the highest median (n = 18) of the overall number of VOCs, followed by the clavicle (n = 15), the vertebrae (n = 14) and the rib (n = 12). However, no significant difference is observed between the number of VOCs produced by each bone. There is minor variation observed between the variability of the four bones. The rib bone has the most variation of the number of VOCS between samples/sampling periods (n = 16), followed by the vertebrae (n = 14), the humerus (n = 11) and the clavicle (n = 11).
In Fig. 2B, the humerus has the highest median of the normalized peak area (n = 4.46), followed by clavicle (n = 3.08), rib (n = 2.97) and vertebrae (n = 2.25). The median line is overlapping for every bone, except the humerus median line which lies outside the vertebrae box plots. However, the difference between the humerus and the vertebrae is not statistically different.
Between the number of VOCs and the normalized peak area of the four types of bones, no significant difference was observed. However, some inter-donor variability was observed and may be attributed to the bone composition of each individual [60]. Some bones contain more bone marrow and are more vascularized than others. Bone marrow is composed mostly of fat (lipid), water, and protein and can be found in different proportions in the bones. Thus, during bone degradation, a bone with more bone marrow will produce more decomposition products of lipids and protein, which include oxygenated, nitrogen-containing, and aromatic volatile organic compounds [32]. The humerus has more bone marrow compared to the rib or the clavicle, which explains why there are more VOCs detected in it [61,62]. Furthermore, vascularized bones, such as the humerus or vertebrae, have more pores, and a high porosity in bones allows for a greater circulation of water, and microorganisms leading to their degradation. The microorganism activities will be more important, leading to the degradation of collagen and bone marrow [[62], [63], [64], [65], [66], [67]]. Collagen is a widespread protein in the human body and its degradation during the decomposition process could lead to nitrogen-containing and aromatic VOCs [32]. Given that the clavicle, rib, humerus and vertebrae have different porosity, they can be impacted differently during the decomposition process, resulting in a variation of the number and intensity of the VOCs detected.
In a study conducted using the same sample collection and analysis as the current study, Dargan et al. (2022) [25] identified between 23 and 430 significant VOCs per sample from decomposed lower limbs, with a total of 1495 different compounds detected across their study. In comparison, the present study identified 296 unique VOCs, approximately five times fewer than those reported by Dargan et al. (2022), with a range of 0–46 VOCs per sample. This disparity can be attributed to differences in the decomposition state of the samples between the studies. The lower limbs analyzed by Dargan et al. (2022) retained decomposed soft tissue, whereas the samples in the present study were completely skeletonized. Decomposing soft tissues emit a broader variety of VOCs compared to skeletonized remains, leading to significant differences in the VOC profiles [25].
3.3. HRD dog detection rates
The responses of HRD dogs exposed to the different human bones during trials conducted in October 2022 (Trial 1) and May 2023 (Trial 2) are presented in Table 4. The first day of Trial 1 and the second day of Trial 2 were indoor trials, and the second day of Trial 1 and the first day of Trial 2 were outdoor trials. On day 1 of Trial 1, false responses from the HRD dogs were recorded for the clavicle (detection rate = 91 %), the rib (detection rate = 55 %), and the vertebrae (detection rate = 72 %) of the same donor (Donor 1). The humerus had a detection rate of 100 %. During day 2 of Trial 1, a 100 % detection rate for all bone samples was recorded. On day 1 of trial 2 which occurred 7 months later, a detection rate of less than 10 % was recorded, while on day 2 of trial 2, the detection rate was 100 %. These responses indicate that the HRD dogs had a lower detection rate on the first day of each trial, and on the second day, the detection rate increased to 100 %. This could be attributed to the fact that by the second day of each trial, the dogs had already been exposed to the bone samples and were more familiar with the scent.
Table 4.
Responses of HRD dogs to human bones (C=Clavicle, R=Rib, H=Humerus, V=Vertebrae) during trials conducted in October 2022 (Trial 1) and May 2023 (Trial 2). (Here TP = True positive, FN=False negative, FP=False positive, PP= Partial positive, Dash (−) = HRD absent from the trial).
| Trial 1 |
Trial 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 2 |
Day 1 |
Day 2 |
|||||||
| HRDD ID # | C | R | H | V | C | R | H | V | H | H |
| HRDD 1 | TP | TP | TP | TP | TP | TP | TP | TP | FP | TP |
| HRDD 2 | TP | FN | TP | FN | TP | TP | TP | TP | FP | – |
| HRDD 3 | TP | FP | TP | TP | TP | TP | TP | TP | FN | – |
| HRDD 4 | TP | FP | TP | FN | TP | TP | TP | TP | FP | – |
| HRDD 5 | TP | TP | TP | TP | TP | TP | TP | TP | - | – |
| HRDD 6 | – | – | – | – | TP | TP | TP | TP | FP | TP |
| HRDD 7 | – | – | – | – | TP | TP | TP | TP | FP | TP |
| HRDD 8 | TP | FP | TP | TP | – | – | – | – | TP | – |
| HRDD 9 | FN | PP | TP | TP | – | – | – | – | – | – |
| HRDD 10 | TP | TP | TP | TP | – | – | – | – | – | – |
| HRDD 11 | TP | TP | TP | TP | – | – | – | – | – | – |
| HRDD 12 | TP | TP | TP | FN | – | – | – | – | – | – |
| HRDD 13 | TP | TP | TP | TP | – | – | – | – | – | – |
| HRDD 14 | – | – | – | – | – | – | – | – | FN | TP |
| HRDD 15 | – | – | – | – | – | – | – | – | PP | TP |
| HRDD 16 | – | – | – | – | – | – | – | – | PP | TP |
| HRDD 17 | – | – | – | – | – | – | – | – | FN | TP |
| HRDD 18 | – | – | – | – | – | – | – | – | FP | TP |
| HRDD 19 | – | – | – | – | – | – | – | – | FN | – |
| HRDD 20 | – | – | – | – | – | – | – | – | FP | – |
| HRDD 21 | – | – | – | – | – | – | – | – | - | TP |
| HRDD 22 | – | – | – | – | – | – | – | – | FP | – |
| Positive response | 10 | 6 | 11 | 8 | 7 | 7 | 7 | 7 | 1 | 9 |
| Partial Positive response | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Negative response | 1 | 4 | 0 | 3 | 0 | 0 | 0 | 0 | 12 | 0 |
Additionally, the handlers confirmed that the dogs exhibited a quicker and more confident response on the second day of training compared to the first day of each trial. For a dog to be able to detect their target efficiently, they must be frequently exposed to the target odour. When training HRD dogs, the handlers do not regularly use skeletonized remains due to the challenges of ethically acquiring material as training aids. As noted previously, the VOC profile of hard tissue like bone differs from the VOC profile of soft tissues which could influence the detection efficacy of the dogs. Dargan et al. (2022) [25] reported a high HRD dog detection rate of 98.4 % for decomposed lower limbs containing soft and hard tissue, while the present study observed an overall detection rate of 84 %. This comparatively lower detection rate can be attributed to the absence of soft tissue in the current study, which reduces VOC diversity and intensity, as well as the limited training of HRD dogs on skeletonized tissue. These findings underscore the need for future studies to focus on training HRD dogs with skeletonized remains to enhance detection performance and ensure comprehensive capabilities across various decomposition states.
Additional factors affecting the detection rate of the dogs can include the dog's energy level or year of training, the air movement/wind and the environment [24,26,[68], [69], [70], [71]]. A dog with a lower energy level or less experience may find it more challenging to recognize a target odour like bone that produces fewer VOCs than soft tissue training aids. The temperature, wind, humidity, and searching environment are also factors that affect the detection of HRD dogs. Further, these factors also influence the concentration and spread of odour. Thus, the false responses and challenges faced by certain HRD dogs during the first day of the Trial 2 can be attributed to these environmental factors. Additionally, obstacles in the search environment, such as buildings, trees, furniture, etc., can block or deviate the odour's path which can explain the difficulties faced by the HRD dogs in locating ribs, clavicle, and vertebrae during the first day of Trial 1, and the humerus of the first day of Trial 2.
During the first day of Trial 2 (outdoor search), more than 50 % of the dogs mistakenly reacted to the building instead of the bone. This observation can be attributed to the placement of the humerus. The bone was placed on the grass beside a building, with the wind blowing toward the building. The building could have potentially blocked the trajectory of the odour leading to the accumulation of the VOCs in this area [28] which could have caused some HRD dogs to alert and give positive responses to the building rather than the bone. These environmental factors could have a more significant impact when searching for bones and skeletal remains, as the already limited VOCs emitted from the bones may be further dispersed, reducing the concentration of VOCs at the odour source. Therefore, it is essential to consider such factors when conducting skeletal remains operational search.
Although no statistically significant differences were observed in the VOC profiles of the bones in this study, it is still recommended to train HRD dogs with a robust set of training aids. Variations in the VOC profiles may occur with other bone compositions, particularly from different donors. Furthermore, since bones have limited organic matter resulting in the reduction of the odour intensity compared to soft tissues [51], HRD dogs may not be familiar with less odorous remains. Thus, frequent exposure and training with bones and/or skeletal remains is necessary to increase their effectiveness in operational searches.
This study has several limitations related to the number of samples and the number of dogs participating in the trial. Given that the VOC profile can vary due to the intrinsic factors of the donors, using a sample size of only three donors may not be representative of a comprehensive VOC profile. Equally, while not significant, there was visible variation noted between the VOC profile of each bone type. Thus, further studies should focus on analyzing different bones and/or a complete skeleton, as well as using a larger number of donors. For the HRD dogs in this study, the participating dogs varied between each trial, with some dogs participating in one day of one trial or both days of only one trial and not the other. Consistently using the same dogs across all trials and extending dog trials for a longer period would provide a more reliable conclusion on their detection capabilities.
4. Conclusion
This study aimed to establish the VOCs emitted from human bones and examine the detection performance of HRD dogs to these samples. Aromatics and linear aliphatic compounds were the most abundant classes detected, likely originating from collagen protein degradation. In contrast, sulphur-containing compounds were the least abundant, differing from the VOC decomposition studies of soft tissues, which may affect HRD dogs' detection capabilities since sulphur-containing compounds are highly odorous. While variations were observed in the general VOC profile of the different bone types, there was no significant difference in the quantity or intensity of the compounds.
For the detection capabilities of the HRD dogs in this study, it was established that they were able to locate human bones with little to no soft tissue. However, they required frequent exposure to the bone samples. Thus, it is recommended that HRD dogs receive regular exposure to skeletal remains during training to maintain and improve their detection accuracy.
CRediT authorship contribution statement
Frédérique Ouimet: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Darshil Patel: Writing – review & editing, Validation, Supervision, Software, Methodology, Data curation. Marissa Tsontakis: Investigation, Formal analysis. Clifford Samson: Supervision, Resources, Project administration, Conceptualization. Shari L. Forbes: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support of Canada 150 Research Chairs [C150-2017-12], Natural Sciences and Engineering Research Council of Canada [RGPIN/6098/2019], and Université du Québec à Trois-Rivières. The authors wish to acknowledge Dr. Rushali Dargan, Dr. Agathe Ribéreau-Gayon, Jerika Ho and all the other members of the Forbes Research Team at Université du Québec à Trois-Rivières for helping with sampling, data collection, and revision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fsisyn.2024.100566.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Vass A.A. Beyond grave - understanding human decomposition. Microbiol. Today. 2001;28:190–192. [Google Scholar]
- 2.Hau T.C., Hamzah N.H., Lian H.H., Amir Hamzah S.P.A. Decomposition process and post mortem changes: review. Sains Malays. 2014;43:1873–1882. [Google Scholar]
- 3.Janaway R.C., Percival S.L., Wilson A.S. Microbiology and Aging: Clinical Manifestations. Humana Press; 2009. Decomposition of human remains; pp. 313–334. [DOI] [Google Scholar]
- 4.Carter D., Tibbett M. Soil Analysis in Forensic Taphonomy. CRC Press; 2008. Cadaver decomposition and soil; pp. 29–51. [DOI] [Google Scholar]
- 5.Carter D.O., Yellowlees D., Tibbett M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften. 2007;94:12–24. doi: 10.1007/s00114-006-0159-1. [DOI] [PubMed] [Google Scholar]
- 6.Forbes S.L. Soil Analysis in Forensic Taphonomy. CRC Press; 2008. Decomposition chemistry in a burial environment; pp. 203–223. [DOI] [Google Scholar]
- 7.Dent B.B., Forbes S.L., Stuart B.H. Review of human decomposition processes in soil. Environ. Geol. 2004;45:576–585. doi: 10.1007/s00254-003-0913-z. [DOI] [Google Scholar]
- 8.Wescott D.J. Recent advances in forensic anthropology: decomposition research. Forensic Sci Res. 2018;3:327–342. doi: 10.1080/20961790.2018.1488571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbes S.L., Perrault K.A., Comstock J.L. Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. first ed. 2017. Microscopic post-mortem changes: the chemistry of decomposition; pp. 26–38. [DOI] [Google Scholar]
- 10.Goff M.L. Early post-mortem changes and stages of decomposition in exposed cadavers. Exp. Appl. Acarol. 2009;49:21–36. doi: 10.1007/s10493-009-9284-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C., Byard R.W. Factors and processes causing accelerated decomposition in human cadavers - an overview. J Forensic Leg Med. 2011;18:6–9. doi: 10.1016/j.jflm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Cockle D.L., Bell L.S. The environmental variables that impact human decomposition in terrestrially exposed contexts within Canada. Sci. Justice. 2017;57:107–117. doi: 10.1016/j.scijus.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Clark M.A., Worrell M.B., Pless J.E. CRC Press; 1997. Forensic Taphonomy: the Postmortem Fate of Human Remains. [DOI] [Google Scholar]
- 14.Boyero L., Cardinale B.J., Bastian M., Pearson R.G. Biotic vs. abiotic control of decomposition: a comparison of the effects of simulated extinctions and changes in temperature. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietro Campobasso C., Di Vella G., Introna F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001;120:18–27. doi: 10.1016/S0379-0738(01)00411-X. [DOI] [PubMed] [Google Scholar]
- 16.Vass A.A. Odor mortis. Forensic Sci. Int. 2012;222:234–241. doi: 10.1016/j.forsciint.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Perrault K., Stuart B., Forbes S. A longitudinal study of decomposition odour in soil using sorbent tubes and solid phase microextraction. Chromatography. 2014;1:120–140. doi: 10.3390/chromatography1030120. [DOI] [Google Scholar]
- 18.Stadler S., Desaulniers J.P., Forbes S.L. Inter-year repeatability study of volatile organic compounds from surface decomposition of human analogues. Int. J. Leg. Med. 2015;129:641–650. doi: 10.1007/s00414-014-1024-y. [DOI] [PubMed] [Google Scholar]
- 19.Deo A., Forbes S.L., Stuart B.H., Ueland M. Profiling the seasonal variability of decomposition odour from human remains in a temperate Australian environment. Aust. J. Forensic Sci. 2020;52:654–664. doi: 10.1080/00450618.2019.1637938. [DOI] [Google Scholar]
- 20.Johnston J.M. Auburn University; 1999. Canine Detection Capabilities: Operational Implications of Recent R & D Findings. [Google Scholar]
- 21.Nawn K. California State Polytechnic University; 2018. Sniffing Out Decomposition: Investigating the Reliability of Human Remains Detection Dogs, Master of Arts in Applied Anthropology. [Google Scholar]
- 22.Glavaš V., Pintar A. Human remains detection dogs as a new prospecting method in archaeology. J. Archaeol. Method Theor. 2019;26:1106–1124. doi: 10.1007/s10816-018-9406-y. [DOI] [Google Scholar]
- 23.Dargan R., Forbes S.L. Cadaver‐detection dogs: a review of their capabilities and the volatile organic compound profile of their associated training aids. WIREs Forensic Science. 2021;3 doi: 10.1002/wfs2.1409. [DOI] [Google Scholar]
- 24.Browne C., Stafford K.J. The use of scent-detection dogs. Ir. Vet. J. 2006;59:97–104. https://www.researchgate.net/publication/261663456 [Google Scholar]
- 25.Dargan R., Samson C., Burr W.S., Daoust B., Forbes S.L. Validating the use of amputated limbs used as cadaver detection dog training aids. Frontiers in Analytical Science. 2022;2 doi: 10.3389/frans.2022.934639. [DOI] [Google Scholar]
- 26.Sorg M.H., David E., Rebmann A.J., Sorg M.H., David Edward. In: Forensic Osteology Advances in the Identification of Human Remains. Second. Reichs K.J., editor. Charles C Thomas; Springfield: 1998. Cadaver dogs, taphonomy, and postmortem interval in the northeast; pp. 120–144. [Google Scholar]
- 27.Osterkamp T. Detector Dogs and Scent Movement - How Weather, Terrain, and Vegetation Influence Search Strategies. CRC Press; 2020. The dog's nose and scent; pp. 9–49. [DOI] [Google Scholar]
- 28.Stejskal S.M. second ed. CRC Press; 2022. Death, Decomposition, and Detector Dogs: from Science to Scene. [DOI] [Google Scholar]
- 29.Coli A., Stornelli M.R., Giannessi E. The dog vomeronasal organ: a review. Dog Behavior. 2016;2:24–31. doi: 10.4454/db.v2i1.27. [DOI] [Google Scholar]
- 30.Rosier E., Loix S., Develter W., Van de Voorde W., Tytgat J., Cuypers E. Time-dependent VOC-profile of decomposed human and animal remains in laboratory environment. Forensic Sci. Int. 2016;266:164–169. doi: 10.1016/j.forsciint.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Knobel Z., Ueland M., Nizio K.D., Patel D., Forbes S.L. A comparison of human and pig decomposition rates and odour profiles in an Australian environment. Aust. J. Forensic Sci. 2019;51:557–572. doi: 10.1080/00450618.2018.1439100. [DOI] [Google Scholar]
- 32.Stefanuto P.-H., Rosier E., Tytgat J., Focant J.-F., Cuypers E. In: Taphonomy of Human Remains. Schotsmans E.M.J., Marquez-Grand N., Forbes S.L.F., editors. John Wiley & Sons Ltd.; 2017. Profiling volatile organic compounds of decomposition; pp. 39–52. [DOI] [Google Scholar]
- 33.Martin C., Verheggen F. Odour profile of human corpses: a review. Forensic Chemistry. 2018;10:27–36. doi: 10.1016/j.forc.2018.07.002. [DOI] [Google Scholar]
- 34.Stefanuto P.H., Perrault K., Stadler S., Pesesse R., Brokl M., Forbes S., Focant J.F. Reading cadaveric decomposition chemistry with a new pair of glasses. Chempluschem. 2014;79:786–789. doi: 10.1002/cplu.201402003. [DOI] [Google Scholar]
- 35.Verheggen F., Perrault K.A., Megido R.C., Dubois L.M., Francis F., Haubruge E., Forbes S.L., Focant J.F., Stefanuto P.H. The odor of death: an overview of current knowledge on characterization and applications. Bioscience. 2017;67:600–613. doi: 10.1093/biosci/bix046. [DOI] [Google Scholar]
- 36.Brasseur C., Dekeirsschieter J., Schotsmans E.M.J., de Koning S., Wilson A.S., Haubruge E., Focant J.F. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J. Chromatogr. A. 2012;1255:163–170. doi: 10.1016/j.chroma.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 37.Dekeirsschieter J., Stefanuto P.H., Brasseur C., Haubruge E., Focant J.F. Enhanced characterization of the smell of death by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOFMS) PLoS One. 2012;7 doi: 10.1371/journal.pone.0039005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadler S., Stefanuto P.H., Brokl M., Forbes S.L., Focant J.F. Characterization of volatile organic compounds from human analogue decomposition using thermal desorption coupled to comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Anal. Chem. 2013;85:998–1005. doi: 10.1021/ac302614y. [DOI] [PubMed] [Google Scholar]
- 39.Rosier E., Cuypers E., Dekens M., Verplaetse R., Develter W., Van De Voorde W., Maes D., Tytgat J. Development and validation of a new TD-GC/MS method and its applicability in the search for human and animal decomposition products Forensic Toxicology. Anal. Bioanal. Chem. 2014;406:3611–3619. doi: 10.1007/s00216-014-7741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dallüge J., Beens J., Brinkman U.A.T. Comprehensive two-dimensional gas chromatography: a powerful and versatile analytical tool. J. Chromatogr. A. 2003;1000:69–108. doi: 10.1016/S0021-9673(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 41.Stefanuto P.H., Perrault K.A., Stadler S., Pesesse R., Leblanc H.N., Forbes S.L., Focant J.F. GC × GC-TOFMS and supervised multivariate approaches to study human cadaveric decomposition olfactive signatures. Anal. Bioanal. Chem. 2015;407:4767–4778. doi: 10.1007/s00216-015-8683-5. [DOI] [PubMed] [Google Scholar]
- 42.Bertsch W. Two-dimensional gas chromatography. Concepts, instrumentation, and applications-Part 1: fundamentals, conventional two-dimensional gas chromatography, selected applications. J. High Resolut. Chromatogr. 1999;22:647–665. doi: 10.1002/(SICI)1521-4168(19991201)22:12<647::AID-JHRC647>3.0.CO;2-V. [DOI] [Google Scholar]
- 43.Bertsch W. Two-dimensional gas chromatography. Concepts, instrumentation, and applications-Part 2 : comprehensive two-dimensional gas chromatography. J. High Resolut. Chromatogr. 1999;23:167–181. doi: 10.1002/(SICI)1521-4168(20000301)23:3<167::AID-JHRC167>3.0.CO. 2-2. [DOI] [Google Scholar]
- 44.Mondello L., Tranchida P.Q., Dugo P., Dugo G. Comprehensive two-dimensional gas chromatography-mass spectrometry: a review. Mass Spectrom. Rev. 2008;27:101–124. doi: 10.1002/mas.20158. [DOI] [PubMed] [Google Scholar]
- 45.Marriott P., Shellie R. Principles and applications of comprehensive two-dimensional gas chromatography. Trends Anal. Chem. 2002;21:573–583. doi: 10.1016/S0165-9936(02)00814-2. [DOI] [PubMed] [Google Scholar]
- 46.Venkatramani C.J., Phillips J.B. Comprehensive two-dimensional gas chromatography applied to the analysis of complex mixtures. J. Microcolumn Sep. 1993;5:511–516. doi: 10.1002/mcs.1220050604. [DOI] [Google Scholar]
- 47.Ribéreau-Gayon A., Carter D.O., Forbes S. Developing a new scoring method to evaluate human decomposition in a humid, continental (Dfb) climate in Quebec. J. Forensic Sci. 2023;68:536–548. doi: 10.1111/1556-4029.15201. [DOI] [PubMed] [Google Scholar]
- 48.Pecsi E.L., Bronchti G., Crispino F., Forbes S.L. Perspectives on the establishment of a canadian human taphonomic facility: the experience of REST[ES] Forensic Sci. Int. 2020;2:287–292. doi: 10.1016/j.fsisyn.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel D. Université du Québec à Trois-Rivières; Thesis: 2022. Identifying the Transition from Ante-mortem Odour to Post-Mortem Odour. [Google Scholar]
- 50.Vass A.A., Smith R.R., Thompson C.V., Burnett M.N., Dulgerian N., Eckenrode B.A. Odor analysis of decomposing buried human remains. J. Forensic Sci. 2008;53:384–391. doi: 10.1111/j.1556-4029.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 51.Collins M.J., Nielsen-Marsh C.M., Hiller J., Smith C.I., Roberts J.P., V Prigodich R., Wess T.J., Csapò J., Millard A.R., Turner-Walker G. 2002. The Survival of Organic Matter in Bone: a Review. [Google Scholar]
- 52.Forbes S.L., Perrault K.A., Stefanuto P.H., Nizio K.D., Focant J.F. Comparison of the decomposition VOC profile during winter and summer in a moist, mid-latitude (Cfb) climate. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Statheropoulos M., Agapiou A., Zorba E., Mikedi K., Karma S., Pallis G.C., Eliopoulos C., Spiliopoulou C. Combined chemical and optical methods for monitoring the early decay stages of surrogate human models. Forensic Sci. Int. 2011;210:154–163. doi: 10.1016/j.forsciint.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Dargan R. Université du Québec à Trois-Rivières; Thesis: 2022. Comparing the Decomposition Odour between Cadavers and Human Remains Used as Cadaver Detection Dog Training Aids. [Google Scholar]
- 55.Fuge R. Sources of halogens in the environment, influences on human and animal health. Environ. Geochem. Health. 1988;10:51–56. doi: 10.1007/BF01758592. [DOI] [PubMed] [Google Scholar]
- 56.Mello P.A., Barin J.S., Duarte F.A., Bizzi C.A., Diehl L.O., Muller E.I., Flores E.M.M. Analytical methods for the determination of halogens in bioanalytical sciences: a review. Anal. Bioanal. Chem. 2013;405:7615–7642. doi: 10.1007/s00216-013-7077-9. [DOI] [PubMed] [Google Scholar]
- 57.Ashley D.L., Bonin M.A., Cardinali F.L., Mccraw J.M., V Wooten J. Measurement of volatile organic compounds in human blood. Environ. Health Perspect. 1996;10:871–877. doi: 10.1289/ehp.96104s5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Currey J.D. Bones: Structure and Mechanics. first ed. Princeton University Press; 2002. The structure of bone tissue; pp. 3–26. [Google Scholar]
- 59.Glock G.E., Lowater F., Murray M.M. The retention and elimination of fluorine in bones. Biochem. J. 1941;35:1235–1239. doi: 10.1042/bj0351235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hedges R.E.M. Bone diagenesis: an overview of processes. Archaeometry. 2002;44:319–328. doi: 10.1111/1475-4754.00064. [DOI] [Google Scholar]
- 61.Coard R. One bone, two bones, wet bones, dry bones: transport potentials under experimental conditions. J. Archaeol. Sci. 1999;26:1369–1375. doi: 10.1006/jasc.1999.0438. [DOI] [Google Scholar]
- 62.Florencio-Silva R., Sasso G.R.D.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson E.C., Adams R.H. Biology of bone: the vasculature of the skeletal system. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kendall C., Eriksen A.M.H., Kontopoulos I., Collins M.J., Turner-Walker G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018;491:21–37. doi: 10.1016/j.palaeo.2017.11.041. [DOI] [Google Scholar]
- 65.Emmons A.L., Mundorff A.Z., Keenan S.W., Davoren J., Andronowski J., Carter D., Debruyn J.M. Characterizing the postmortem human bone microbiome from surface-decomposed remains. PLoS One. 2020;15 doi: 10.1371/journal.pone.0218636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emmons A.L., Mundorff A.Z., Hoeland K.M., Davoren J., Keenan S.W., Carter D.O., Campagna S.R., DeBruyn J.M. Postmortem skeletal microbial community composition and function in buried human remains. mSystems. 2022;7 doi: 10.1128/msystems.00041-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karr L.P., Outram A.K. Bone degradation and environment: understanding, assessing and conducting archaeological experiments using modern animal bones. Int. J. Osteoarchaeol. 2015;25:201–212. doi: 10.1002/oa.2275. [DOI] [Google Scholar]
- 68.Jones K.E., Dashfield K., Downend A., Otto C.M. Search-and-rescue dogs: an overview for veterinarians. J. Am. Vet. Med. Assoc. : Disaster Medicine. 2004;225:854–860. doi: 10.2460/javma.2004.225.854. [DOI] [PubMed] [Google Scholar]
- 69.Mesloh C., Wolf R.A. Scent as forensic evidence and its relationship to the law enforcement canine, orlando. 2000. https://www.researchgate.net/publication/238774972
- 70.Polgár Z., Kinnunen M., Újváry D., Miklósi Á., Gácsi M. A test of canine olfactory capacity: comparing various dog breeds and wolves in a natural detection task. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rebmann A.J., David Edward, Sorg M.H., Koenig Marcia. first ed. CRC Press; 2000. Cadaver Dog Handbook - Forensic Training and Tactics for the Recovery of Human Remains. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.