Highlights
-
•
Our team described our manual clinical trial screening process and subsequent success in improving trial enrollment.
-
•
Data showed that, over time, “screen no trial available” (SNTA) rates stayed stable, but enrollment rates increased.
-
•
An understanding of our catchment and a portfolio matched to the population led to enrollment well above national average.
Abstract
Objective
There is no standard clinical trial screening process in gynecologic oncology. In our low resource, highly diverse gynecologic oncology patient population, we sought to create an equitable, adaptable, manual screening process.
Methods
Our objective is to describe our clinical trial screening process and success in improving trial enrollment. An Institutional Review Board (IRB) approved quality improvement (QI) project was implemented in July 2022 to evaluate trial access. Screenable events were defined. Potential patients were those with a screenable event: new patients or diagnoses, regimen changes, progressions, and recurrences. Events were categorized into screen positive or screened no trial available. Screen positives were further categorized as screen positive, enrollment failure events or enrollments. Data about patients were collected via weekly research team meetings. Monthly meetings occurred to review progress. The data were compared to trials available, number of patients with trail available, and those that enrolled. Reasons for enrollment fails were tracked.
Results
Over time, “screen no trial available” (SNTA) rates stayed stable, but enrollment rates increased. Patient preference accounted for 32.8 % of enrollment failures (n = 42), pre-existing symptoms 23.4 % (n = 30), and location 21.1 % (n = 27). During increased employee turnover, there was a rise in enrollment fails due to staffing (n = 6, 4.7 %). We describe an effective process of clearly defining and tracking our patient population and ‘screenable events’ for which all patients are screened and offered trial participation if eligible.
Conclusions
We show that we improved understanding of the patient population, built a clinical trial portfolio better matched to population served, exceeded national averages for enrolling patients on trials, and are improving number eligible.
1. Introduction
In modern cancer care, clinical trials are a crucial tool for furthering research and treatment development, and are used to drive innovation to deliver cutting edge care. Clinical trials have the promise to improve cancer survival, making diagnoses that once seemed bleak more optimistic. Treatment at a hospital that participates in clinical trials results in improved patient outcomes. (Bouzalmate-Hajjaj et al., 2022) Evaluations of hospital programs, specifically analyzing cardiology and obstetric units, that enroll patients on trials show that a pro-trials culture is associated with improved guideline adherence and patient outcomes. (Majumdar et al., 2008, West et al., 2005) Additionally, a 2021 study by Jones et al. showed that when racial barriers to trial enrollment were overcome, there were equal estimates of progression-free survival across races. (Jones et al., 2021) Similarly, a survey conducted by Du Bois et al. showed that for their cohort of participants with ovarian cancer, clinical trials provided patients with opportunities for better quality of care, with their ability to participate, not their outcomes, being limited by social determinants and individual level factors. (Bois et al., 2005) These data are incredibly encouraging, as they show what a powerful tool clinical trials can be. However, the most recent estimates from the 2021 American Society of Clinical Oncology Quality Care Symposium show that clinical trial enrollment, nationally, is 6.3 %. (Unger and Fleury, 2021) Previous estimates suggested that trial enrollment was less than 5 %. (Unger and Fleury, 2021) Given these modest numbers, one can see that there is considerable room for improvement.
Beyond opening clinical trials, it is paramount to ensure that the trials available meet the needs of patients and communities within the catchments of hospital systems. Furthermore, trial participation is fraught with design, implementation, and access inequities and barriers, resulting in unacceptably low representation of minority patient populations on cancer clinical trials. (Brangman, 2022) Gynecologic oncology (gyn onc) is equally affected by these issues, thus the need to implement and describe an equitable screening process is critical. In the 2015 paper investigating minority participation in published Gynecologic Oncology Group (GOG) gynecologic oncology trials, Scalici et al. showed that observed enrollment of Black patients on trial was significantly lower than expected (83 % enrollment of White patients vs 8 % enrollment of Black patients). (Scalici et al., 2015) Furthermore, this disparity appears to be widening. Enrollment of Black patients was found to have decreased with time, with there being 2.8-times less Black participant enrollment seen between 2009–2013 compared to 1994–2002. (Scalici et al., 2015) There appears to be a higher impact seen in Pharma funded trials, with the finding that National Institutes of Health (NIH) funded trials have a 4-times higher level of minority patient enrollment than non-NIH funded trials. (Ma et al., 2021).
At this time, there is no standardized screening process that has been universally adapted for the field of gyn onc and beyond. Moreover, there is a paucity of published material about structuring a clinical trial enrollment strategy. This includes both the process of equitably screening patients and tailoring an institution’s clinical trial portfolio to match patient population. Previous studies have shown a positive impact of pre-screening patients on trial enrollment – at Bellevue Hospital, implementing pre-screening led to a 4.6-times increase in patient enrollment in clinical trials. (Wu et al., 2022) Additional studies have looked at the use of artificial intelligence (AI) technologies for screening for trial participants. (Use, 2023) However, limitations exist for both methods. Mainly, neither the pre-screening or AI strategies have been recommended or implemented in a standardized fashion. It is also important to note that many patients become eligible for trials after a recurrence or progression, and they are not being identified from a “new diagnosis” or “new patient” group. This highlights the need to clearly define best practices on when patients presenting for cancer care should be screened for clinical trial enrollment.
Furthermore, the time required to assess a patient’s eligibility is increasingly complex due to many trial specific inclusion and exclusion criteria, molecular requirements, pre-screening periods, and the need for a clear understanding of prior treatment history and treatment response. These are all very complicated clinical factors that are inconsistently documented in the medical record. Therefore, with such heterogeneous events triggering trial eligibility, if these patient’s oncologists and care teams are not actively engaged in the trial enrollment process, this could compromise the ability for patients to have access to a potentially life-saving clinical trial. Treating physician recommendation has been shown to be an incredibly powerful force for improving clinical trial enrollment. (Gregg et al., 2014, Millar et al., 2022).
With all the above barriers in mind, we aimed to define, streamline, and improve clinical trial enrollment to better serve the patients seen in our medium-sized, mixed academic and community gyn onc practice. This need was compounded in the setting of Coronavirus (COVID-19) resource shunting and, subsequent instability with research personnel. Thus, within our low resource, highly diverse patient population, we have created an efficient manual screening process that incorporates patients of all disease statuses. Our objective is to describe our experience with this process and our success, specifically surrounding changes in clinical trial availability for cancer patients and trials enrollment over time.
2. Methods
This study is a quality improvement project that was implemented in July of 2022 through Louisiana State University in New Orleans to evaluate trial enrollment among patients seen in all gyn onc clinics included in our healthcare network. This program is a collaboration between University Medical Center (UMC) and the Louisiana Cancer Research Center (LCRC), who also serve as the Gulf-South National Cancer Institute Community Oncology Research Program (NCORP) lead, a minority and underserved NCORP. The project was defined as an internal quality improvement effort to, first, capture objective clinical trial enrollment based on a defined screening process and, next, use that data to identify unmet need in the population. We identified the disease processes most seen for which there was no trial available. This allowed us to identify which trials could be opened to meet that unmet need and enhance enrollment.
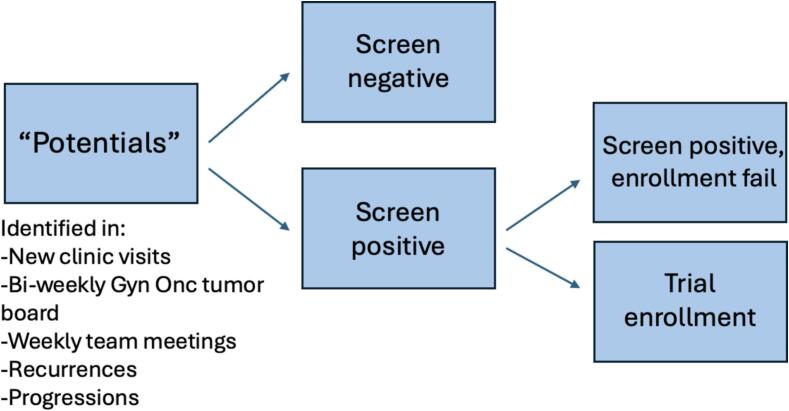
The initial step was to define our “trial denominator”; i.e., event at which a patient would be eligible for clinical trial screening. Trial enrollment screening points were defined as: the start of all gyn onc clinic visits, patients presented at bi-monthly gynecology tumor boards, weekly gynecology pre-operative meetings, and by keeping track of patients marked as “potentials” on a shared electronic medical record (EMR) (Epic Hyperspace Production (PRD) Host version 100.2312.4.0) list. For the intents of our process, we defined potential patients to include 1. new patients such as pre-operative, new cancer, or referrals for second opinions, 2. established patients with a new cancer diagnosis, 3. established cancer patients who have had a change in their regimen, or 4. those with a progression and/or recurrence. Further description of screenable events is detailed in Table 1.
Table 1.
Description of Screenable Events.
| Event Name | Description |
|---|---|
| Initial Diagnosis | When a new patient presents to clinic, the doctors will review their medical history and case presentation to determine if they may be eligible for one of the open trials. Based on the referral note and physical exam, the doctors will be able to enter the initial meeting with an idea of potential trials for that specific patient, whether they be surgical trials, treatment trials, or patient quality of life (QOL) trials. |
| Maintenance | After a patient has received their initial treatment intervention, they may be eligible for treatment trials or other trials focused on social support. |
| Progression | If a patient was never found to have “no evidence of malignancy (NEM)”, and their cancer persists or advances, their treatment plan may change, which could make them eligible for a new trial. |
| Recurrence | If a patient is found to have malignancy after being categorized as “NEM”, based on their cancer type and characteristics, they may be eligible for a myriad of clinical trials. |
Throughout a given week, any patient meeting the above criteria was added to a shared “potential trial patient list” in the EMR by their treating clinician. In order to constantly update and review this list, a standing, 15 min, weekly virtual meeting was held in which the three gynecologic oncologists, four clinical trials coordinators, two research nurses, and one research fellow collaborated to evaluate if all potential patients were being considered, and if potential trial patients were eligible and “screened positive” or if there was no trial available for these patients. The definitions for screening positive versus screening negative (also known as “screened no trial available”) are presented in Table 2.
Table 2.
Description of Trial Screening Outcomes.
| Screening Outcome | Description |
|---|---|
| Screen Positive | The patient met all inclusion criteria for the trial for which they are eligible. This includes their specific cancer stage, histology, age, performance status, other demographic details, and previous treatment regimens. |
| Screen Negative (“Screened No Trial Available”) | The patient does not meet inclusion criteria for the trial for which they were considered. |
| Screen Positive, Enrollment Failure | The patient meets all inclusion criteria for the trial for which they are eligible. However, for a different reason, the patient is not enrolled on trial. These reasons include, but are not limited to, patient preference, patient location, and staffing. |
| Successful Enrollment | The patient meets all inclusion criteria for the trial for which they are eligible, and are successfully enrolled on trial. |
For all patients that screened positive, the team then discussed the next steps to get these patients on trial – whether that be additional medical workup or simply confirming each patient meets their specific trial’s eligibility criteria – to ensure the patient was optimized to move forward in the enrollment process. This pool of patients was followed up on and patients were categorized as the following: “successful enrollments” or “screen positive enrollment failure” events. A flow chart depicting this procedure is displayed in Fig. 1. Definitions for these terms are presented in Table 2. Data were followed regularly and held in a shared database.
Fig. 1.
Flow Chart of Potential Trial Patients in Clinic.
At least once monthly, one gynecologic oncologist (AMJ) compiled data regarding the number of patients screened, disease presentations for which there was no trial available, how many patients who screened positive were ultimately enrolled, how many patients who screened positive were enrollment failures, and the reasons thereof. Outcome measures were tracked, including: the percent of cancer patients for whom no trial was available, the percent of patients who screened positive that were enrolled on trial, and the number of screenable events. Subsequently, these data were presented monthly to review the diagnosis landscape for patients and identify unmet need. Additionally, open trials were reviewed for each type of cancer (endometrial, ovarian, and cervical) and future trials were presented before they were opened to ensure there was a population in need and confirm no competing trials were opened. Dynamic factors including the hiring of new clinical trial staff, changes to the clinical trial portfolio, and healthcare system priorities, were followed to account for their potential impact on trial enrollments and to optimize resource utilization.
For data evaluation, categorical variables were summarized in groups (yes/no screening led to enrollment) using counts and percentages. Continuous variables were summarized using means and standard deviations. Categorical comparisons across groups were made using Fisher exact tests, while continuous comparisons were made using Wilcoxon rank sum tests. Multivariable logistic regression was performed to determine if certain trial factors were associated with enrollment while adjusting for certain patient factors like distance, insurance status, race, and marital status.
3. Results
This program was implemented over a 19-month period (June 2022 to December 2023). The average age of participants was 60.67 years (SD = 13.45). While there were 44 patients with more than one screening event (n = 35 with 2 events, n = 7 with 3 events, and n = 2 with 4 + events), we chose not to adjust for the repeated measures on each patient because most of the patients (n = 118) were only screened for one trial, for n = 162 screen events over this time-period. The most common cancer was endometrial and the most common stage was stage I. There was an equal number of Black and White patients included in the patient population, with n = 72 and n = 63 patients included, respectively. Complete demographics are presented in Table 3.
Table 3.
Patient Demographic Information.
| Counts (N = 151) | Percent Enrolled (%) | p-value | |
|---|---|---|---|
| Race | 0.125 | ||
| Asian | 4 | 75 | |
| Black or African American | 72 | 31.9 | |
| White | 63 | 34.9 | |
| Unknown/Not reported | 12 | 58.3 | |
| Ethnicity | 0.111 | ||
| Hispanic/LatinX | 10 | 60 | |
| Not Hispanic | 136 | 33.8 | |
| Insurance | 0.142 | ||
| Private | 40 | 32.5 | |
| Medicare | 72 | 30.6 | |
| Medicaid | 29 | 55.2 | |
| Free Care (Hospital Covers Costs) | 4 | 50 | |
| Unspecified | 7 | 42.9 | |
| Cancer Type | 0.032 | ||
| Cervical | 23 | 56.5 | |
| Endometrial | 93 | 35.5 | |
| Ovarian | 28 | 25 | |
| Vaginal | 2 | 100 | |
| Vulvar | 2 | 0 | |
| Genetic predisposition | 3 | 0 | |
| Cancer Stage | 0.087 | ||
| I | 57 | 42.1 | |
| II | 8 | 75 | |
| III | 34 | 35.3 | |
| IV | 38 | 28.9 | |
| No cancer | 3 | 0 | |
| Unknown | 11 | 18.2 | |
| Number of screenable events | 0.148 | ||
| 1 | 109 | 42.2 | |
| >1 | 42 | 40.2 |
In our population, there was a significantly higher percentage of patients with cervical cancer (56.5 %) who enrolled on trial compared to patients with endometrial (35.5 %) or ovarian (25 %) cancer (p = 0.032). Also, there was a higher number of patients with early-stage cancers (stages I and II) who enrolled on trial compared to patients with later stage/advanced cancer (stages III and IV), but findings were not significant (p = 0.087).
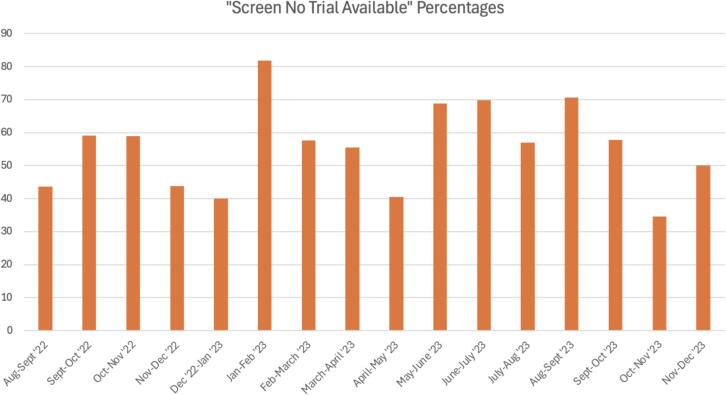
Regarding data collected to assess how well the open, active clinical trials were meeting patient need, each month our team recorded: the number of patients seen with no cancer, the number of patients screened who were confirmed to have cancer, the number of patients who “successfully enrolled” on trial, and the number of patients who were “enrollment failures”. Over time, “screen no trial available” rates stayed stable, but enrollment rates increased. These results are displayed in Fig. 2 and Fig. 3.
Fig. 2.
Monthly Trials Data for “Screen No Trial Available” Percentages. In Fig. 2, it is seen that the percentages recorded for “Screen No Trial Available” events had no significant trend. Fluctuations in rates were most likely due to physician trends in referral patterns or changes in patient load, rather than true effects due to the impacts of opening trials. The denominator used was total patients who were “screen negative” events for each month, including cancer patients for whom there was no trial available and patients who were found to have benign disease presentations.
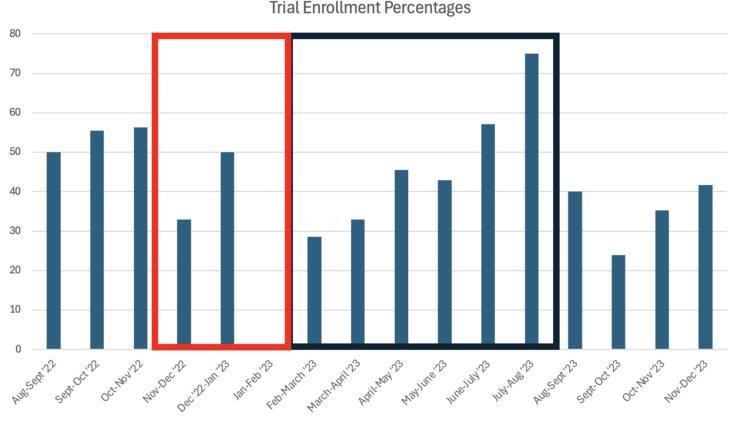
Fig. 3.
Monthly Trials Data for Enrollment Percentages. Fig. 3 depicts the percentage of analytical cancer cases able to enroll on trial compared to the total number of cancer patients screened in clinic. The denominator used was total patients who were “screen positive” events for each month. There was a consistent increase in trial enrollment when the team was operating at full strength, and not during known, predictable, lulls. A black box was placed around the time-period when the trials team was fully operational. A red box was placed around the “holiday lull”: a known drop in trial enrollment during the months around the holidays where patients are less likely to enroll, due to trial follow-up requirements. After the first expected “holiday lull” captured in the data, enrollment rates steadily increased from February 2023 to August 2023. During August 2023, there was a complete turnover of clinical trial coordinators, and a subsequent drop in enrollment rates was noted. After only two months, though, enrollment numbers began to rise back to pre-turnover levels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Data were also collected on the documented reasons for enrollment failures in that patient population. Patient preference was the most cited barrier and accounted for 32.8 % of enrollment failures (n = 42). Pre-existing symptoms or conditions accounted for 23.4 % of enrollment failures (n = 30), and patient location/distance from treatment center accounted for 21.1 % (n = 27). During the time of employee turnover, there was a rise in enrollment failures due to staffing issues (n = 6, 4.7 %). For patients who declined trial participation, regardless of the reason, they were still able to be treated by the team at Louisiana State University-New Orleans and UMC; declining trial participation did not impact their therapeutic relationship with our providers.
4. Discussion
After detailing the manual screening process and the results of its implementation, it is evident that team involvement, proper staffing, and patient engagement are vital components to the success of a mixed-setting clinical trials program. This screening process harnesses the capabilities of the EMR system to facilitate the pre-screening process and discussions around patients who may or may not be trial eligible. The high level of collaboration between investigators, physician team, clinical staff, and clinical trials coordinators has fostered an environment that emphasizes thoroughness, leading to higher accrual rates than the national average.
Provider involvement has been shown to be a positive force toward improving trial enrollment among patient populations. (Gregg et al., 2014, Program, 2010) Specifically, in a study evaluating trial recruitment in the primary care setting, the importance of a doctor’s influence on patient decision-making was emphasized. (Millar et al., 2022) Lack of physician familiarity with the clinical trial procedures is a cited barrier to trial success due to lack of patient referral. The benefits of a manual screening process evaluated in this paper are first, driven by clinician evaluation, then regular occurrence of brief meetings to discuss potential patients, which allows all team members to be familiar with available protocol criteria and emphasizes the importance of provider-patient interactions for informing patients about clinical trial enrollment opportunities.
Additionally, by regular review of clinical trial screening and ongoing enrollments, our team was able to identify areas of unmet need and pursue opening trials that fill those gaps. Evaluation of the data showed that a significant majority of patients that enrolled on trial enrolled on cervical cancer trials as compared to trials for other disease sites (p = 0.032). In the years’ worth of data that were collected for this specific study, the team opened five new trials, three of which were appropriate for cervical cancer patients. Our ability to measure and react to the “screen no trial available” events was critical to making our portfolio meet our catchment. This physician led, highly engaging manual screening process showed how efficiently the trials menu could be catered to the exact needs of our patient population.
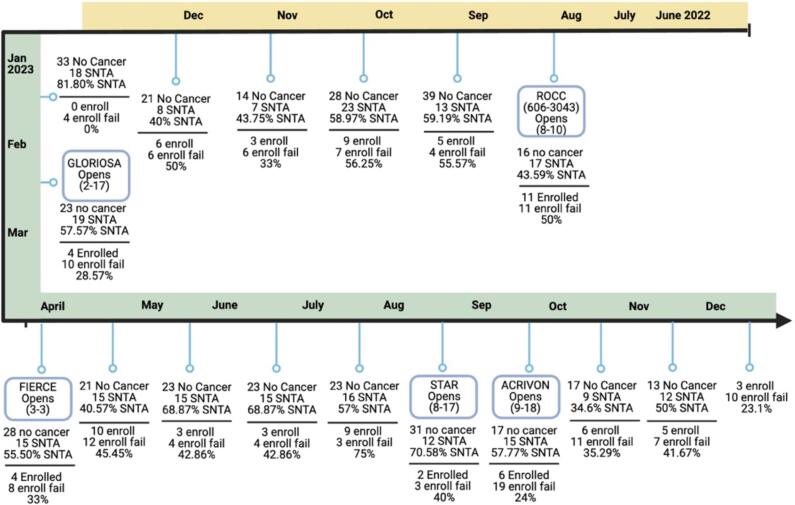
Another strength of our approach was the way that causes for enrollment failures was accounted for, which allowed us to better understand the issues our patients prioritized when considering clinical trial participation. This provided our team with a more robust understanding of the types of trials that are attractive to the patients we care for. A timeline of trial openings compared to findings from the data is presented in Fig. 4. Though there is no way to counteract a “patient preference” based decision to decline enrolling on trial, patient location was found to be a notable barrier to participation, which is modifiable. With this information, the team had evidence to provide continued support of our Virtual Nursing Program, and our partnership with a community clinic in Lafayette Louisiana, approximately 135 miles away. This ultimately led to the successful expansion of this site to our NCORP roster to support our gyn onc enrollments remotely. The data not only influenced trials being opened, but also informed the team of ways to better reach our patient population by expanding our follow-up availability.
Fig. 4.
Timeline of Patient Data and Trial Openings. In Fig. 4, data about patient enrollment were compared to the timeline of trial openings and closures to better characterize how the trials landscape evolved. It is shown that, after the only month where recorded enrollment was 0 patients, the Gloriosa (Platinum sensitive recurrent ovarian) and FIERCE (early stage endometrial) trials were opened. Trial enrollment steadily increased with these openings, up until the point of total employee turnover.
Beyond patient, cancer, and trial factors, our initiative also tracked real-world impacts to trial enrollment, such as seasonal lulls and workforce instability. There were two distinct drops in the rates of trial enrollment seen in clinical trials in this patient population: one in the winter of 2022 and one in the early fall of 2023. As previously mentioned, when considering wintertime, it is paramount to note that there has historically been decreased trial enrollment around the holidays.(Chaudhari et al., 2020) This is assumed to be due to a patient’s desire to spend time with their families and avoid extensive clinical visits during a time with so much natural social programming. However, enrollment rates rose to their original levels following this period in 2022. In this instance, enrollment failures were increased due to patient preference, not lack of availability of trials or staffing.
Regarding the 2023 decrease in enrollment rates, it is important to note “staffing issues” as a new and prominent cause of enrollment failure. During the transitional period this cancer center endured, the number of staff was incompatible with the demand of the open clinical trials. The data, which showed that staffing was a cause of unmet patient need, helped promote the hiring of additional clinical research coordinators. Not only did this evaluation impact trials opening, but it has helped shape a more effective team structure, propagating continued success.
There are many strengths of this study. Firstly, this is a novel approach for exploring a trials screening process. The current data on this subject is sparse, thus following this dynamic data detailing our process, and providing the results showing that it is effective adds to current understanding of a complex and variable process. In addition, the manual screening process presented can be tailored to many institutions and cancer trials.
While we argue that this is an easily replicated model for improving enrollment across a variety of small to medium gyn onc groups, we also acknowledge that the weaknesses of this study include that this is just one of many approaches used in current practice, is certainly not exhaustive, and does not compare other approaches. Other limitations include our unexpected high staff turnover seen during the data collection period. This real-world problem was addressed and rectified as soon as possible, but is still included as it gives additional context to the reader.
To have a flourishing clinical trials program, adequate and appropriate enrollment is vital. By remaining patient-focused and creating a collaborative, well-delegated team with a high level of communication, the gyn onc team at Louisiana State University-New Orleans has found replicable success with our clinical trials program. Further, this success was accomplished in a high-diversity, low resource health care setting, often left behind in clinical trial participation. In detailing our methods, we hope to act as a blueprint for developing programs to foster strong, data driven trials menus at other major cancer treatment centers.
CRediT authorship contribution statement
M. Klein: Writing – review & editing, Writing – original draft, Investigation, Data curation, Conceptualization. H. Pirzadah: Writing – review & editing, Writing – original draft, Conceptualization. Y. Magharehabed: Writing – review & editing, Writing – original draft. A. Chapple: Formal analysis, Data curation. N. Nair: Writing – review & editing, Supervision, Conceptualization. A. Jernigan: Writing – review & editing, Writing – original draft, Supervision, Project administration, Data curation, Conceptualization. T. Castellano: Writing – review & editing, Writing – original draft, Supervision, Project administration, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bois A.D., Rochon J., Lamparter C., Pfisterer J. Pattern of care and impact of participation in clinical studies on the outcome in ovarian cancer. Int. J. Gynecol. Cancer. 2005;15(2) doi: 10.1136/ijgc-00009577-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Bouzalmate-Hajjaj A., Massó Guijarro P., Khan K.S., Bueno-Cavanillas A., Cano-Ibáñez N. Benefits of participation in clinical trials: an umbrella review. Int. J. Environ. Res. Public Health. 2022;19(22):15368. doi: 10.3390/ijerph192215368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangman S.A. Achieving diversity in study populations: the importance of community engagement. J. Am. Geriatr. Soc. 2022;70(11):3080–3086. doi: 10.1111/jgs.18043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N., Ravi R., Gogtay N.J., Thatte U.M. Recruitment and retention of the participants in clinical trials: challenges and solutions. Perspect. Clin. Res. 2020;11(2):64–69. doi: 10.4103/picr.PICR_206_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg J.R., Horn L., Davidson M.A., Gilbert J. Patient enrollment onto clinical trials: the role of physician knowledge. J Cancer Educ off J Am Assoc Cancer Educ. 2014;29(1):74–79. doi: 10.1007/s13187-013-0548-z. [DOI] [PubMed] [Google Scholar]
- Jones N., Wilhite A., Paladugu R., et al. Eliminating racial disparities in endometrial cancer clinical trial enrollment in the Deep South: a pathway to equity. Gynecol. Oncol. 2021;162:S6. doi: 10.1016/S0090-8258(21)00660-0. [DOI] [Google Scholar]
- Ma M.A., Gutiérrez D.E., Frausto J.M., Al-Delaimy W.K. Minority representation in clinical trials in the united states: trends over the past 25 Years. Mayo Clin. Proc. 2021;96(1):264–266. doi: 10.1016/j.mayocp.2020.10.027. [DOI] [PubMed] [Google Scholar]
- Majumdar S.R., Roe M.T., Peterson E.D., Chen A.Y., Gibler W.B., Armstrong P.W. Better outcomes for patients treated at hospitals that participate in clinical trials. Arch. Intern. Med. 2008;168(6):657–662. doi: 10.1001/archinternmed.2007.124. [DOI] [PubMed] [Google Scholar]
- Millar M.M., Taft T., Weir C.R. Clinical trial recruitment in primary care: exploratory factor analysis of a questionnaire to measure barriers and facilitators to primary care providers’ involvement. BMC Prim Care. 2022;23(1):311. doi: 10.1186/s12875-022-01898-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Program I of M (US) C on CCT and the NCG, Nass SJ, Moses HL, Mendelsohn J. Physician and Patient Participation in Cancer Clinical Trials. In: A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. National Academies Press (US); 2010. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK220370/. [PubMed]
- Scalici J., Finan M.A., Black J., et al. Minority participation in gynecologic oncology group (GOG) studies. Gynecol. Oncol. 2015;138(2):441–444. doi: 10.1016/j.ygyno.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J.M., Fleury M. Nationally representative estimates of the participation of cancer patients in clinical research studies according to the commission on cancer. J. Clin. Oncol. 2021;39(28_suppl):74. doi: 10.1200/JCO.2020.39.28_suppl.74. [DOI] [Google Scholar]
- Use of artificial intelligence for cancer clinical trial enrollment: a systematic review and meta-analysis - PubMed. Accessed November 29, 2023. https://pubmed.ncbi.nlm.nih.gov/36688707/. [DOI] [PMC free article] [PubMed]
- West J., Wright J., Tuffnell D., Jankowicz D., West R. Do clinical trials improve quality of care? A comparison of clinical processes and outcomes in patients in a clinical trial and similar patients outside a trial where both groups are managed according to a strict protocol. Qual. Saf. Health Care. 2005;14(3):175–178. doi: 10.1136/qshc.2004.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Yakubov A., Abdul-Hay M., et al. Prescreening to increase therapeutic oncology trial enrollment at the largest public hospital in the united states. JCO Oncol Pract. 2022;18(4):e620–e625. doi: 10.1200/OP.21.00629. [DOI] [PubMed] [Google Scholar]