Abstract
Capsicum oleoresin (CO) is a concentrated extract derived from peppers (Capsicum annum L.) containing capsaicin (the active compound responsible for its pungency) and other bioactive components. The present study aimed to determine whether CO affects the energy expenditure and mitochondrial content of brown adipose tissue (BAT) in diet-induced obese mice. Four-week-old C57BL/6J mice were divided into three groups and fed with a normal chow diet, 45% high-fat diet (HF), or HF supplemented with 0.01% CO (HF+CO) for 16 weeks. The results showed that CO supplementation significantly suppressed weight gain and improved serum lipid profiles compared with HF feeding. The energy expenditure was significantly higher in the HF+CO group than in the HF group. Compared with the HF group, the HF+CO group had significantly upregulated the messenger RNA expression levels of uncoupling protein 1 (UCP1) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in BAT. The mitochondrial DNA content, which was reduced by HF intake, was significantly restored in the HF+CO group. Furthermore, the mitochondrial size and number were restored in the HF+CO group than in in the HF group. The activity of adenosine monophosphate-activated protein kinase (AMPK) in BAT was significantly increased in the HF+CO group than in the HF group. In conclusion, CO potentially inhibits weight gain by increasing energy expenditure in diet-induced obese mice. This beneficial effect is likely associated with the enhancement of mitochondrial content by upregulating key markers, including UCP1, PGC-1α, and AMPK, in BAT.
Keywords: brown adipose tissue, capsicum, energy metabolism, mitochondria, obesity
INTRODUCTION
Obesity results from an imbalance between energy intake and energy expenditure (Hill et al., 2012; Chouchani et al., 2019). When the caloric intake exceeds the number of calories burned, excess energy is stored as fat, leading to obesity (Hill et al., 2012). Owing to its ability to generate heat and consume energy, brown adipose tissue (BAT) is strongly associated with obesity (Harms and Seale, 2013).
Uncoupling protein 1 (UCP1), a protein located in the inner membrane of the mitochondria of brown adipose cells, is involved in the uncoupling process that dissipates the proton gradient generated by the electron transport chain to produce heat instead of adenosine triphosphate (ATP) (Chouchani et al., 2019). The activation of UCP1 increases energy expenditure, potentially eliciting effects such as fat loss owing to an elevated metabolic rate (Kim and Plutzky, 2016). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a transcription factor that promotes mitochondrial biogenesis and regulates mitochondrial oxidative metabolism. Moreover, it enhances UCP1 expression, thereby playing an important role in maintaining body temperature and regulating energy expenditure (Boström et al., 2012).
PGC-1α increases the mitochondrial number and promotes mitochondrial DNA (mtDNA) replication (Chen et al., 2022). The changes in the mtDNA content directly affect mitochondrial health and function (Scarpulla, 2008). Adequate mtDNA content, supported by effective mitochondrial replication, optimizes cellular energy production and plays an essential role in disease states or metabolic disorders (Muir et al., 2016).
When intracellular ATP levels are low, adenosine monophosphate-activated protein kinase (AMPK) is activated, promoting energy production and increasing energy expenditure (Hardie, 2011). AMPK can directly interact with and phosphorylate PGC-1α, whereas UCP1 is indirectly regulated through AMPK-mediated signaling pathways (Cantó and Auwerx, 2009; van der Vaart et al., 2021). AMPK stimulates mitochondrial biogenesis and enhances thermogenesis, thereby boosting energy consumption (Heidorn-Czarna et al., 2021; van der Vaart et al., 2021).
Capsicum oleoresin (CO) is extracted from the dried fruit of pepper (Capsicum annum L., which belongs to the Solanaceae family) using an organic solvent, and its main component is capsaicin (Haas et al., 1997). Dietary capsaicin has been reported to control obesity by reducing body weight and fat mass, possibly because of its metabolic effects that enhance energy metabolism and thermogenesis (Kawada et al., 1986a; Kawada et al., 1986b). CO exhibits various biological benefits, including antioxidant, antidiabetic, and anti-inflammatory properties (Sricharoen et al., 2017; Prathoshni et al., 2018). In particular, previous studies found that the nanoemulsion and microparticle forms of CO can prevent weight gain (Kim et al., 2014; da Silva Anthero et al., 2024). However, the effects of CO on the energy expenditure and mitochondrial function of BAT remain unknown. Therefore, the present study aimed to determine whether CO increases the energy expenditure and affects the mitochondrial content, including the UCP1, PGC-1α, and AMPK pathways, of BAT in mice fed with a high-fat diet (HF).
MATERIALS AND METHODS
Materials and reagents
CO (10,000 Scoville heat units) was provided by General Foods and Flavors. Serum triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) assay kits were purchased from Embiel. A hematoxylin and eosin (H&E) staining kit was procured from ScyTek Laboratories. RiboExTM reagent was obtained from GeneAll Biotechnology. Moloney murine leukemia virus (M-MLV) reverse transcriptase and 2X GreenStarTM qPCR Master Mix were purchased from Bioneer. A genomic DNA isolation kit was procured from Gentra Systems. An AMPK assay kit was obtained from MBL International. A bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Fisher Scientific.
Animals and diet
Three-week-old male C57BL/6J mice were acquired from DooYeol Biotech and used after 1-week acclimatization. The mice were individually housed in a room with a constant temperature (22°C±2°C) and humidity (55%±5%) and a 12-h light/dark cycle. They were divided into three groups (n=8 per group) and fed with experimental diets for 16 weeks. The experimental diets were categorized as follows: normal chow diet (NC; 2018S Rodent Diet, Harlan Teklad), HF, and HF supplemented with 0.01% CO (HF+CO). The mice were maintained on an HF diet (45% of total energy as fat) containing 23.0% (wt:wt) lard, 17.1% (wt:wt) casein, 12.2% (wt:wt) sucrose, 20.2% (wt:wt) starch, 15.5% (wt:wt) dextrose, 6.1% (wt:wt) cellulose, 4.3% (wt:wt) minerals, 1.2% (wt:wt) vitamins, 0.2% (wt:wt) L-cystine, and 0.3% (wt:wt) choline bitartrate to induce obesity. The diets were based on a modified AIN-93 diet (Reeves et al., 1993). During the 16-week intervention period, the body weight and food intake of mice were measured every week. Thereafter, all mice were euthanized, and serum, white adipose tissue (WAT), and BAT samples were collected for further experiments. The animal experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of Ewha Womans University (IACUC no. 20-051; approval date: September 18, 2020).
Serum lipids
Whole blood was collected from mice was allowed to coagulate at room temperature for 30 min, and subsequently centrifuged at 1,500 g for 20 min at 4°C to separate the serum. The serum TG, TC, and HDL-C levels were determined using a commercial assay kit via an enzyme colorimetric method. The low-density lipoprotein cholesterol (LDL-C) levels were calculated using Friedewald’s formula (Friedewald et al., 1972).
Histology
For BAT morphological analysis, H&E staining was performed as previously described (Jang et al., 2022). BAT was fixed in 10% neutral buffered formalin overnight at room temperature, embedded in paraffin, and sectioned at 6 μm thickness. Subsequently, the sections were stained with H&E in accordance with the manufacturer’s instructions. Digital images of the stained tissue sections were observed using the Olympus IX51 Inverted Microscope (400× magnification, Olympus).
Energy expenditure
The energy expenditure was assessed in accordance with the method of a previous study (Kim et al., 2023). The mice were housed in individual metabolic cages at 25°C and had ad libitum access to food and water. To accurately measure oxygen consumption (VO2) and carbon dioxide production (VCO2), O2 and CO2 analyzers were calibrated with highly purified gases, and VO2 and VCO2 were measured via indirect calorimetry (Oxylet, Panlab). Data were collected at 3-min intervals using a computer-assisted data acquisition software (Chart 5.2, ADInstruments) and recorded for 24 h. The energy expenditure was calculated using the following formula: energy expenditure (kcal/d/kg0.75)=[(3.815×VO2)+(1.232×VCO2)]×1.44 (Kim et al., 2023).
Real-time quantitative reverse-transcription polymerase chain reaction
Total RNA was isolated from BAT using the RiboExTM reagent, and the RNA concentration was quantified using a NanoDropTM spectrophotometer (Thermo Fisher Scientific). The mRNA levels were quantified using real-time quantitative reverse-transcription polymerase chain reaction (RT-qPCR), as described previously. Complementary DNA was synthesized using M-MLV reverse transcriptase. RT-qPCR analysis was performed on a QIAGEN Rotor-GeneⓇ Q Thermocycler using the 2X GreenStarTM qPCR Master Mix. The sense and antisense primers were as follows: β-actin, 5’-GGA CCT GAC AGA CTA CCT CA-3’ and 5’-GTT GCC AAT AGT GAT GAC CT-3’ (NM_007393); PGC-1α, 5’-GGG CCA AAC AGA GAG AGA GG-3’ and GTT TCG TTC GAC CTG CGT AA (NM_008904); and UCP-1, 5’-CAG GCT TCC AGT ACC ATT AG-3’ and 5’-CTT GGA CTG AGT CGT AGA GG-3’ (NM_009463). The relative mRNA quantification was normalized to β-actin and analyzed using the delta-delta Ct method (Livak and Schmittgen, 2001).
Transmission electron microscopy
To analyze the ultrastructure of the mitochondria in BAT, transmission electron microscopy (TEM) was performed in accordance with the method of a previous study (Jung et al., 2021). BAT was sliced into ≤2-mm-thick sections and post-fixed with 2% glutaraldehyde and 1% osmium tetroxide. The fixed tissue samples were then embedded in epoxy resin (Epon 812) and sectioned into 1-μm-thick slices using an ultramicrotome (Reichert-Jung). The sections were stained with 1% toluidine blue and observed under an H-7650 transmission electron microscope (20,000× magnification, Hitachi) at an accelerating voltage of 80 kV.
Mitochondrial DNA content
Total DNA was isolated from BAT using a genomic DNA isolation kit in accordance with the manufacturer’s instructions. DNA was extracted from 10 mg of BAT using a lysis buffer containing proteinase K. The relative mtDNA content was quantified by measuring the mitochondrial (cytochrome oxidase subunit 1, COX1) and nuclear (glyceraldehyde 3-phosphate dehydrogenase, GAPDH) genes using RT-qPCR (Jung et al., 2021). The sense and antisense primers were as follows: COX1, 5’-TAG CCG GAA ATC TAG CCC AT-3’ and 5’-GTT ATG GCT GGG GGT TTC AT-3’ (NC_005089); GAPDH, 5’-GGA GCC AAA AGG GTC ATC AT-3’ and 5’-TAC CAT GAG CCC TTC CAC AA-3’ (NC_000072).
AMPK activity
The AMPK activity in BAT was determined using an AMPK assay kit in accordance with the method of a previous study (Lee et al., 2011). The AMPK activity was measured via IRS-1 Ser 789 phosphorylation using the anti-mouse phospho-Ser 789 IRS-1 monoclonal antibody and peroxidase-coupled anti-mouse immunoglobulin G. The absorbance was then measured at 450 nm using a microplate reader (Varioskan Flash, Thermo Scientific). The AMPK activity was normalized to the protein content as analyzed using a BCA protein assay kit.
Statistical analysis
Data are expressed as the mean±standard error for eight animals in each group and were statistically analyzed using IBM SPSS version 25 (IBM Corp.). Significant differences between the HF and HF+CO groups were determined using the two-tailed Student’s t-test. Meanwhile, significant differences among three groups (NC, HF, and HF+CO) were verified using one-way analysis of variance and Tukey’s post hoc multiple-range test. Statistical significance was considered at P<0.05.
RESULTS
Body weight, food intake, and serum lipids
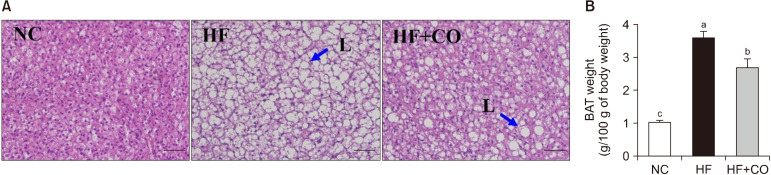
The body weight, food intake, and serum lipids of mice after 16-week supplementation with the experimental diets are shown in Table 1. During this period, the body weight of mice in the NC group increased from 17.40±0.35 to 26.21±0.29 g. By contrast, the body weight of mice in the HF group increased from 17.38±0.47 to 33.26±0.55 g, exhibiting a significantly greater increase than that of the NC group. Meanwhile, the body weight of mice in the HF+CO group increased from 17.37±0.52 to 30.84±0.64 g, demonstrating a significantly reduced body weight compared with that of the HF group. CO supplementation also significantly mitigated the body weight gain compared with HF feeding. The weight of WAT significantly increased by 5.99-fold in the HF group compared with that in the NC group. Nonetheless, it decreased by 45.4% in the HF+CO group compared with that in the HF group. Compared with that in the NC group, the food intake decreased in the HF and HF+CO groups. However, no significant differences in energy intake were observed among the three groups. The serum TG, TC, and LDL-C levels were significantly higher in the HF group than in the NC group. By contrast, the serum TG, TC, and LDL-C levels were significantly lower in the HF+CO group than in the HF group. No significant differences in serum HDL-C levels were observed among the three groups. As evidenced by H&E staining, HF intake resulted in an increase in lipid droplet size, whereas CO intake resulted in a decrease in lipid droplet size (Fig. 1A). The weight of BAT significantly increased by 3.56-fold in the HF group than in the NC group. Nonetheless, it decreased by 25.6% in the HF+CO group compared with that in the HF group (Fig. 1B).
Table 1.
Effects of CO on the body weight, food intake, and serum lipid profile of mice fed with an HF diet for 16 weeks
| NC | HF | HF+CO | |
|---|---|---|---|
| Initial body weight (g) | 17.40±0.35 | 17.38±0.47 | 17.37±0.52 |
| Final body weight (g) | 26.21±0.29c | 33.26±0.55a | 30.84±0.64b |
| Body weight gain (g/16 weeks) | 10.37±0.21c | 17.16±0.49a | 14.80±0.52b |
| WAT weight (g/100 g body weight) | 1.02±0.06c | 6.10±0.19a | 3.33±0.29b |
| Food intake (g/d) | 4.46±0.12a | 3.28±0.02b | 3.23±0.03b |
| Energy intake (kcal/d) | 15.53±1.17 | 15.22±0.09 | 15.00±0.12 |
| Serum lipids (mmol/L) | |||
| TG | 1.01±0.02c | 1.53±0.02a | 1.30±0.03b |
| TC | 3.39±0.15c | 4.73±0.12a | 4.10±0.15b |
| HDL-C | 2.11±0.09 | 1.90±0.04 | 2.31±0.14 |
| LDL-C | 0.81±0.17c | 2.14±0.14a | 1.35±0.12b |
The values are expressed as the mean±SEM (n=8).
Different superscript letters (a-c) within a row indicate significant differences at P<0.05.
NC, normal chow; HF, high-fat diet; HF+CO, HF+0.01% capsicum oleoresin; WAT, white adipose tissue; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Fig. 1.
Hematoxylin and eosin staining (A) and BAT weight (B) in mice fed with NC, HF, and HF+CO diets for 16 weeks. The values are expressed as the mean±SE (n=8). Different letters (a-c) indicate significant differences among the three groups (NC, HF, and HF+CO) at P<0.05. Scale bars=50 μm; magnification=400×. Blue arrows indicate lipid droplet (L). NC, normal chow; HF, high-fat diet; HF+CO, HF+0.01% capsicum oleoresin; BAT, brown adipose tissue.
Capsicum oleoresin increased the energy expenditure of mice
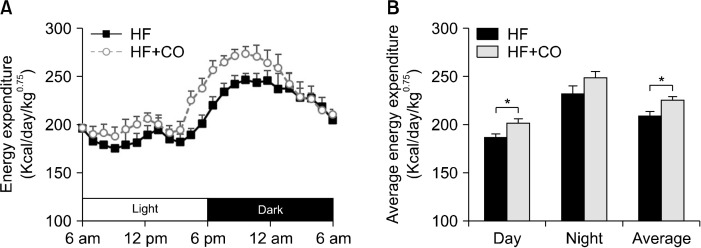
We measured the VO2- and VCO2-based energy expenditure values of mice to determine whether CO affects energy expenditure. The energy expenditure changes during the light and dark periods are presented in Fig. 2. The energy expenditure per unit body weight was significantly higher in the HF+CO group than in the HF group, with a particularly significant increase in energy expenditure during the light period.
Fig. 2.
Changes in energy expenditure (A) and average energy expenditure (B) in mice fed with HF and HF+CO diets for 16 weeks. The values are expressed as the mean± SE (n=8). *Significant differences between the HF and HF+CO groups at P<0.05. HF, high-fat diet; HF+CO, HF+0.01% capsicum oleoresin.
Capsicum oleoresin upregulated UCP1 and PGC-1α expression levels in BAT
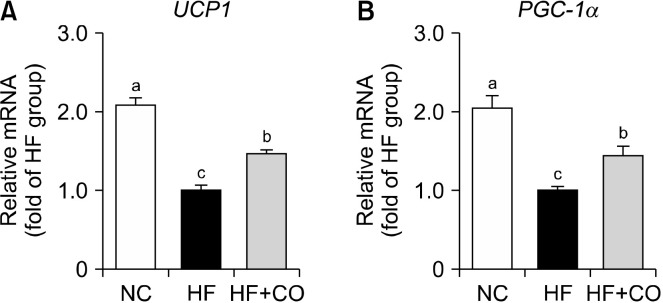
The mRNA expression levels of UCP1 and PGC-1α, which are genes related to energy expenditure in BAT, are shown in Fig. 3. In the HF group, the mRNA expression levels of UCP1 and PGC-1α were significantly downregulated by 51.9% and 51.0%, respectively, compared with those in the NC group. Conversely, the mRNA expression levels of UCP1 and PGC-1α in the HF+CO group were significantly upregulated by 1.47- and 1.44-fold, respectively, compared with those in the HF group.
Fig. 3.
UCP1 (A) and PGC-1α (B) gene expression levels in the brown adipose tissue of mice fed with NC, HF, and HF+CO diets for 16 weeks. The values are expressed as the mean±SE (n=8). Different letters (a-c) indicate significant differences among the three groups (NC, HF, and HF+CO) at P<0.05. NC, normal chow; HF, high-fat diet; HF+CO, HF+0.01% capsicum oleoresin; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; UCP1, uncoupling protein 1.
Capsicum oleoresin restored the mtDNA content of BAT
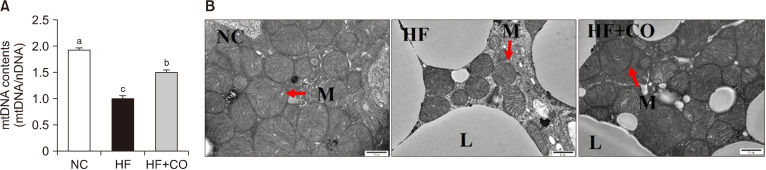
We investigated whether PGC-1α upregulation in BAT affects the mtDNA content through mitochondrial replication. The mtDNA content significantly decreased by 48.1% in the HF group compared with that in the NC group. Nevertheless, this reduction was significantly restored by 1.50-fold in the HF+CO group (Fig. 4A). Moreover, the mitochondrial size and number were also restored in the HF+CO group compared with those in the HF group, as observed via TEM (Fig. 4B).
Fig. 4.
Mitochondrial DNA (mtDNA) contents (A) and transmission electron microscopy images (B) of the brown adipose tissue of mice fed with NC, HF, and HF+CO diets for 16 weeks. The values are expressed as the mean±SE (n=8). Different letters (a-c) indicate significant differences among the three groups (NC, HF, and HF+CO) at P<0.05. Scale bar=2 μm; magnification=20,000×. Red arrows indicate mitochondria (M). NC, normal chow; HF, high-fat diet; HF+CO, HF+0.01% capsicum oleoresin; L, lipid droplet; nDNA, nuclear DNA.
Capsicum oleoresin activated AMPK in BAT
We examined the effects of AMPK activation, which promotes intracellular energy production and increases energy expenditure. The AMPK activity in BAT significantly decreased by 46.5% in the HF group compared with that in the NC group but significantly increased by 1.46-fold in the HF+CO group compared with that in the HF group (Fig. 5).
Fig. 5.
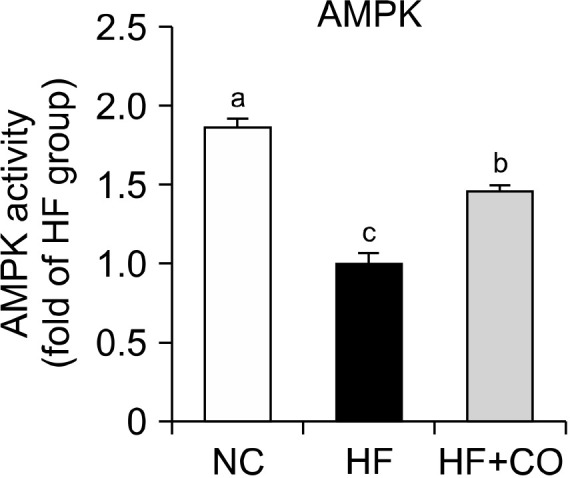
AMPK activity in the brown adipose tissue of mice fed with NC, HF, and HF+CO diets for 16 weeks. The values are expressed as the mean±SE (n=8). Different letters (a-c) indicate significant differences among the three groups (NC, HF, and HF+CO) at P<0.05. NC, normal chow; HF, high-fat diet; HF+CO, HF+0.01% capsicum oleoresin; AMPK, adenosine monophosphate-activated protein kinase.
DISCUSSION
Obesity is closely related to metabolic disorders such as diabetes, cardiovascular disease, hypertension, and fatty liver disease (Yang et al., 2022). Therefore, increasing energy expenditure and decreasing body fat can help alleviate the risk of these metabolic diseases (Hill et al., 2012). The role of brown fat in obesity and energy expenditure has attracted considerable attention. Unlike WAT, which primarily stores energy, BAT generates heat instead of storing energy (Harms and Seale, 2013). This thermogenic process positively impacts obesity and energy expenditure, making BAT a key focus in metabolic health studies.
Capsaicin, the active component of CO, and its analogs have been reported to increase energy expenditure and BAT thermogenesis (Yoneshiro et al., 2012; Leung, 2014; Saito, 2015; Saito et al., 2020). In particular, they have been found to enhance energy expenditure and fat burning in individuals with a high body mass index (Inoue et al., 2007). The effects of capsaicin and its analogs on energy expenditure and BAT activation have been reported in animal and human models. However, no studies have investigated these effects using CO. To the best of our knowledge, this study is the first to demonstrate that CO inhibits weight gain in HF-fed mice by increasing energy expenditure and regulating the molecular markers involved in regulating the mitochondrial content of BAT.
According to a previous study, capsaicin contributes to weight loss and improves blood lipid profiles while also reducing hepatic steatosis in an HF-induced obese mouse model (Kang et al., 2010). In a study on Japanese women, a high-fat, high-carbohydrate meal supplemented with red pepper (capsaicin) increased energy metabolism and promoted fat oxidation, potentially improving long-term weight loss and lipid profiles (Yoshioka et al., 1998). In our previous study, we showed that supplementation with 0.01% CO increased UCP2 expression and AMPK activation in the WAT of HF-fed rats (Kim et al., 2014). Therefore, in the present study, we set the CO dose to 0.01%. In this study, the weight gain in the HF group increased by 2-fold over a 16-week period compared with that in the NC group, whereas the weight gain in the HF+CO group was significantly suppressed by 29.5% compared with that in the HF group. The levels of serum lipids, including TG, TC, and LDL-C, were also significantly lower in the HF+CO group than in the HF group. The energy expenditure in the HF+CO group significantly exceeded that in the HF group. These findings suggest that CO improves the serum lipid profiles and inhibits weight gain by increasing energy expenditure in HF-fed mice.
UCP1 and PGC-1α interact with each other to regulate thermogenesis and energy metabolism (Boström et al., 2012; Chouchani et al., 2019). UCP1 directly generates heat in the mitochondria, thereby consuming energy (Chouchani et al., 2019). Alternatively, PGC-1α activates several thermogenic genes, including UCP1, to promote energy expenditure (Boström et al., 2012). PGC-1α stimulates mitochondrial biogenesis, whereas AMPK indirectly regulates UCP1 expression, which regulates thermogenesis in brown fat (van der Vaart et al., 2021; Chen et al., 2022). Capsicum chinense L. has been found to increase PGC-1α and UCP1 protein expression levels in the BAT of HF-induced obese mice (Han et al., 2024). In HF-induced obese mice, dietary capsaicin upregulates UCP1 protein and PGC-1α gene expression in WAT and subcutaneous fat, respectively, thereby stimulating heat production (Baskaran et al., 2016). Moreover, capsaicin intake increases the phosphorylation of skeletal muscle AMPK in HF-fed mice (Kim et al., 2010). In the present study, the mRNA expression levels of UCP1 and PGC-1α in BAT were significantly upregulated in the HF+CO group compared with those in the HF group. The mtDNA content, which was reduced by HF intake, was significantly restored in the HF+CO group. The mitochondrial size and number were also restored in the HF+CO group, as determined by TEM. Furthermore, the AMPK activity in BAT was significantly increased in the HF+CO group than in the HF group. Our results suggest that CO may enhance the mitochondrial function of BAT by upregulating UCP1, PGC-1α, and AMPK.
In conclusion, CO supplementation effectively suppressed weight gain and improved the lipid profiles of HF-fed mice. Furthermore, it significantly increased the energy expenditure, particularly during the light phase, and upregulated important markers, including UCP1, PGC-1α, and AMPK, in BAT. Moreover, CO restored the mitochondrial size, number, and mtDNA content, which were diminished by HF intake. These findings suggest that CO may help prevent obesity by enhancing energy expenditure and increasing the mitochondrial content in BAT. However, this study used a single concentration of CO without a positive control. Therefore, it has limitations in supporting the conclusion. Future studies should conduct additional experiments to evaluate the effects of CO using a positive control and various concentrations of CO.
Footnotes
FUNDING
This work was supported by the National Research Foundation of Korea (2019R1A2C1002861) and the Ewha Womans University Research Grant of 2024.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: YK. Analysis and interpretation: MSL. Data collection: MSL. Writing the article: MSL. Critical revision of the article: YK, IHK, MSL. Final approval of the article: YK, MD, IHK, MSL. Statistical analysis: MD, MSL. Obtained funding: YK. Overall responsibility: YK.
REFERENCES
- Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173:2369–2389. doi: 10.1111/bph.13514. https://doi.org/10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. https://doi.org/10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. https://doi.org/10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Qin Y, Liu B, Gao M, Li A, Li X, et al. PGC-1α-mediated mitochondrial quality control: Molecular mechanisms and implications for heart failure. Front Cell Dev Biol. 2022;10:871357. doi: 10.3389/fcell.2022.871357. https://doi.org/10.3389/fcell.2022.871357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019;29:27–37. doi: 10.1016/j.cmet.2018.11.002. https://doi.org/10.1016/j.cmet.2018.11.002. [DOI] [PubMed] [Google Scholar]
- da Silva Anthero AG, Bonetti CI, Bracht L, Cazarin CBB, Hubinger MD. The use of Capsicum oleoresin microparticles to mitigate hepatic damage and metabolic disorders induced by obesity. Food Res Int. 2024;195:114932. doi: 10.1016/j.foodres.2024.114932. https://doi.org/10.1016/j.foodres.2024.114932. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- Haas JS, Whipple RE, Grant PM, Andresen BD, Volpe AM, Pelkey GE. Chemical and elemental comparison of two formulations of oleoresin capsicum. Sci Justice. 1997;37:15–24. doi: 10.1016/S1355-0306(97)72136-1. https://doi.org/10.1016/s1355-0306(97)72136-1. [DOI] [PubMed] [Google Scholar]
- Han YY, Jo HN, Kim BM, Lee JS, Kim JM, Ryu DH, et al. Effects of NET-2201 (Capsicum chinense L. cv.) on brown adipose tissue activation and white adipose tissue browning in high-fat-diet-induced obese mice. Food Sci Biotechnol. 2024 doi: 10.1007/s10068-024-01692-z. https://doi.org/10.1007/s10068-024-01692-z. [DOI] [Google Scholar]
- Hardie DG. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. https://doi.org/10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. https://doi.org/10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Heidorn-Czarna M, Heidorn HM, Fernando S, Sanislav O, Jarmuszkiewicz W, Mutzel R, et al. Chronic activation of AMPK induces mitochondrial biogenesis through differential phosphorylation and abundance of mitochondrial proteins in Dictyostelium discoideum. Int J Mol Sci. 2021;22:11675. doi: 10.3390/ijms222111675. https://doi.org/10.3390/ijms222111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126:126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. https://doi.org/10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Matsunaga Y, Satoh H, Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids) Biosci Biotechnol Biochem. 2007;71:380–389. doi: 10.1271/bbb.60341. https://doi.org/10.1271/bbb.60341. [DOI] [PubMed] [Google Scholar]
- Jang S, Lee MS, Kang SA, Kim CT, Kim Y. Portulaca oleracea L. extract regulates hepatic cholesterol metabolism via the AMPK/microRNA-33/34a pathway in rats fed a high-cholesterol diet. Nutrients. 2022;14:3330. doi: 10.3390/nu14163330. https://doi.org/10.3390/nu14163330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Lee MS, Chang E, Kim CT, Kim Y. Mulberry (Morus alba L.) fruit extract ameliorates inflammation via regulating microRNA-21/132/143 expression and increases the skeletal muscle mitochondrial content and AMPK/SIRT activities. Antioxidants. 2021;10:1453. doi: 10.3390/antiox10091453. https://doi.org/10.3390/antiox10091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Goto T, Han IS, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18:780–787. doi: 10.1038/oby.2009.301. https://doi.org/10.1038/oby.2009.301. [DOI] [PubMed] [Google Scholar]
- Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr. 1986a;116:1272–1278. doi: 10.1093/jn/116.7.1272. https://doi.org/10.1093/jn/116.7.1272. [DOI] [PubMed] [Google Scholar]
- Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med. 1986b;183:250–256. doi: 10.3181/00379727-183-42414. https://doi.org/10.3181/00379727-183-42414. [DOI] [PubMed] [Google Scholar]
- Kim DH, Joo JI, Choi JW, Yun JW. Differential expression of skeletal muscle proteins in high-fat diet-fed rats in response to capsaicin feeding. Proteomics. 2010;10:2870–2881. doi: 10.1002/pmic.200900815. https://doi.org/10.1002/pmic.200900815. [DOI] [PubMed] [Google Scholar]
- Kim J, Han D, Lee MS, Lee J, Kim IH, Kim Y. Green tea and java pepper mixture prevents obesity by increasing energy expenditure and modulating hepatic AMPK/microRNA-34a/370 pathway in high-fat diet-fed rats. Antioxidants. 2023;12:1053. doi: 10.3390/antiox12051053. https://doi.org/10.3390/antiox12051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Lee MS, Jung S, Joo H, Kim CT, Kim IH, et al. Anti-obesity efficacy of nanoemulsion oleoresin capsicum in obese rats fed a high-fat diet. Int J Nanomedicine. 2014;9:301–310. doi: 10.2147/IJN.S52414. https://doi.org/10.2147/ijn.S52414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J. 2016;40:12–21. doi: 10.4093/dmj.2016.40.1.12. https://doi.org/10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kim IH, Kim CT, Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141:1947–1953. doi: 10.3945/jn.111.146050. https://doi.org/10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- Leung FW. In: Capsaicin as a Therapeutic Molecule. Abdel-Salam O, editor. Springer; 2014. Capsaicin as an anti-obesity drug; pp. 171–179. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. https://doi.org/10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Muir R, Diot A, Poulton J. Mitochondrial content is central to nuclear gene expression: Profound implications for human health. BioEssays. 2016;38:150–156. doi: 10.1002/bies.201500105. https://doi.org/10.1002/bies.201500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathoshni SM, Anitha R, Lakshmi T. The effect of capsicum oleoresin on nitric oxide production and nitric oxide synthase gene expression in macrophage cell line. Pharmacognosy Research. 2018;10:343–346. doi: 10.4103/pr.pr_46_18. [DOI] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. https://doi.org/10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Saito M, Matsushita M, Yoneshiro T, Okamatsu-Ogura Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: From mice to men. Front Endocrinol. 2020;11:222. doi: 10.3389/fendo.2020.00222. https://doi.org/10.3389/fendo.2020.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv Food Nutr Res. 2015;76:1–28. doi: 10.1016/bs.afnr.2015.07.002. https://doi.org/10.1016/bs.afnr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. https://doi.org/10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Sricharoen P, Lamaiphan N, Patthawaro P, Limchoowong N, Techawongstien S, Chanthai S. Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason Sonochem. 2017;38:629–639. doi: 10.1016/j.ultsonch.2016.08.018. https://doi.org/10.1016/j.ultsonch.2016.08.018. [DOI] [PubMed] [Google Scholar]
- van der Vaart JI, Boon MR, Houtkooper RH. The role of AMPK signaling in brown adipose tissue activation. Cells. 2021;10:1122. doi: 10.3390/cells10051122. https://doi.org/10.3390/cells10051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Liu S, Zhang C. The related metabolic diseases and treatments of obesity. Healthcare. 2022;10:1616. doi: 10.3390/healthcare10091616. https://doi.org/10.3390/healthcare10091616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012;95:845–850. doi: 10.3945/ajcn.111.018606. https://doi.org/10.3945/ajcn.111.018606. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, St-Pierre S, Suzuki M, Tremblay A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br J Nutr. 1998;80:503–510. doi: 10.1017/S0007114598001597. https://doi.org/10.1017/s0007114598001597. [DOI] [PubMed] [Google Scholar]