Abstract
Infectious laryngotracheitis (ILT) is a highly contagious disease, usually controlled by vaccination with live attenuated vaccines. However, the latent infection and adverse reactions caused by the live attenuated vaccines against infectious laryngotracheitis virus (ILTV) have limited its use in poultry. Infectious bronchitis virus (IBV) is considered a potential vector for vaccine development, but the issue of poor stability in recombinant IBV expressing foreign genes has not yet been resolved. In this study, we designed a multi-epitope cassette (gD-T/B) containing multiple T and B cell epitopes of ILTV gD protein. The genetic stability of the full-length gD gene and the gD-T/B multi-epitope cassette replacing non-essential genes in IBV was systematically analyzed. We found that, at the same insertion site, the stability of inserting gD-T/B multi-epitope cassette was consistently higher compared to the full-length gD gene. This difference may be related to the presence of more signals affecting virus replication or transcription in larger heterologous genes. In addition, the stability of recombinant IBV varied depending on the genome region being replaced. When the gene 5 was replaced, rH120-Δ5ab-gD-T/B was maintained up to at least passage 20 (P20). Compared with the parental virus H120 strain, rH120-Δ5ab-gD-T/B showed similar growth kinetics. Clinical observations and scoring of clinical signs in the vaccination-challenge experiment showed that rH120-Δ5ab-gD-T/B provided 90% protection against virulent ILTV, effectively alleviating clinical signs caused by infection with a virulent strain of ILTV. Furthermore, rH120-Δ5ab-gD-T/B significantly reduced the replication and shedding of ILTV in the trachea. Overall, this study suggests that rH120-Δ5ab-gD-T/B is a promising candidate vaccine against ILTV.
Keywords: Infectious laryngotracheitis virus, Infectious bronchitis virus, Multiple epitopes, Stability, Vector vaccine
Introduction
Infectious laryngotracheitis (ILT) is an acute, highly contagious upper respiratory infectious disease caused by infectious laryngotracheitis virus (ILTV) (Ou and Giambrone, 2012). Strict biosafety management and vaccination are the main strategies for controlling ILT (Coppo et al., 2013a, 2013b). Although most of the commercial attenuated vaccines can provide clinical protection against ILTV virulent strains, latent infection and virulence reversion are important factors leading to ILT outbreaks (Hughes et al., 1991; Oldoni et al., 2009; Coppo et al., 2012; Garcia, 2017).
ILTV belongs to avian herpesvirus type I, with a genome size of approximately 150 kb and containing about 80 open reading frames (ORF) (Davison, 2010; Wu et al., 2022). Among them, glycoprotein D (gD) is located on the surface of virus particles, mediating virus adsorption to susceptible cells and the membrane fusion of virus-host cells (Di Giovine et al., 2011; Eisenberg et al., 2012). In addition, gD protein plays a crucial role in inducing a protective immune response in the body (Lazear et al., 2012; Pavlova et al., 2013; Zhao et al., 2014). In other herpesviruses, the above views have been confirmed. The gD protein of bovine herpesvirus type 1 (BHV-1) can stimulate a stronger cellular immune response than the gB and gC proteins, inducing more efficient production of neutralizing antibodies (Hutchings et al., 1990). For herpes simplex virus type 1 (HSV-1), gD protein induces the highest levels of antibodies, which can completely resist the challenge of HSV virulent strains (Lasky et al., 1984). What's more, the gD protein subunit vaccine of pseudorabies virus (PRV) can also protect against the lethal challenge of PRV virulent strains (Marchioli et al., 1987).
Infectious bronchitis virus (IBV), a global endemic virus, belongs to coronaviridae family (Quinteros et al., 2022). The total length of the IBV genome RNA is about 27.6 kb, which sequentially encodes 5′UTR-la/1b-S-3a-3b-E-M-5a-5b-N-3′UTR (Cavanagh, 2007; Peng et al., 2022). Globally, the effective prevention and control of IB often depends on vaccines (Zhao et al., 2023). IBV vector has unique advantages, including low cost, convenient immunization and induction of broad immune types, which is highly effective in large-scale application (Aston et al., 2019). Currently, many studies havd found that recombinant IBV was usually genetically unstable. However, the stability of recombinant virus was usually greatest when non-essential helper genes were replaced by heterologous genes (de Haan et al., 2003; Youn et al., 2005; Shen et al., 2009). When green fluorescent protein gene (GFP) and human renilla luciferase gene (hRluc) were replaced with ORF3, ORF5 and intergenic region (IR) of IBV genome respectively, it was found that hRluc gene replaced ORF5, resulting in the highest stability of the recombinant IBV. The hRluc gene could be stably inherited at the nucleic acid level up to 12 generations (Bentley et al., 2013). In this study, we designed and rescued a recombinant IBV vector vaccine carrying multiple epitopes of ILTV-gD protein, and evaluated its stability, growth characteristics and protective efficacy against challenge with ILTV virulent strain.
Materials and methods
Ethics statement
Institutional and national guidelines for the use and care of laboratory animals were closely followed. The use of animals in this study was approved by the South China Agricultural University Committee for Animal Experiments (approval ID: SYXK2019-0136).
Viruses and cells
Both the IBV H120 strain (GenBank No. ON350836.1) and the ILTV WG strain (GenBank No. JX458823.1) in this study were propagated in 9-day-old specific pathogen free (SPF) chicken embryos. BSR-T7/5 cells, a cell line stably expressing T7 RNA polymerase, were generously provided by Prof. Youming Zhang from Helmholtz Institute of Biotechnology, Shandong University. Chicken kidney cells (CK) were generated from 10-day-old SPF chicken embryos. The above cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), plus 1% Antibiotic-Antimycotic (10,000 I.U./mL of penicillin, 10,000 µg/mL of streptomycin) and incubated at 37°C with 5% CO2.
Plasmids construction and viral rescue
In order to construct a recombinant IBV vector with chimeric heterologous genes, according to our previous description, the Red/ET homologous recombination technology was used to complete the recombination in E. coli DH10B expressing Redα/Redβ recombinant protein (Shao et al., 2022). The gD gene and gD-T/B multiple epitope cassette of ILTV WG strain were synthesized by Sangon Biotech. The gD and gD-T/B containing the homologous sequence of the insertion site were amplified by overlapping PCR, and the length of the homologous sequence was about 40 bp. Then, a two-step recombination method was adopted. Briefly, in the first step, the E. Coli DH10B gyrA462, which expresses Redα/Redβ recombinant protein, was used to complete the “line-loop” recombination, and the screening gene ccdB-amp was replaced or inserted into the expression vector. Condly, the screening gene ccdB-amp was successfully replaced by gD or gD-T/B in the engineering bacterium E. Coli DH10B, and a correct recombinant was obtained through ccdB reverse screening.
According to the manufacturer's instructions, the recombinant IBV plasmid was co-transfected with pVAX1-H120-N into BSR-T7/5 cells using lipofectamine® 3000 transfection reagent (Thermo Fisher Scientific, USA). After incubation at 37°C for 4 h, the supernatant was discarded and the cells were washed twice with PBS. Fresh DMEM medium containing 2% FBS and 1% antibiotics was added and the culture was continued for 72 h. After three freeze-thaw cycles, the cell lysates were inoculated into 9-day-old SPF chicken embryos. 48 h after inoculation, the allantoic fluid of chicken embryos was harvested.
Stability detection of the recombinant IBVs
In order to detect the genetic stability, the recombinant IBVs were continuously passaged on chicken embryos for 20 generations. Refer to the manufacturer's instructions of Axyprep humoral Virus DNA/RNA Mini-Extraction Kit (Axygen, CA, United State) to extract the viral nucleic acids from P1 (1st passage) to P20 generations. The inserted full-length gene was amplified by HiScript II One Step RT-PCR Kit (Vazyme, Nanjing, China), and the primers were shown in Table 1.
Table 1.
Primers for detection of genetic stability by RT-PCR.
| Virus | Forward (5′-3′) | Reversed (5′-3′) | RT-qPCR Product (bp) |
|---|---|---|---|
| rH120-Δ3ab-gD | GGCATTATGCCTCTAATGAG | CCTACAAATATGTAAAGCGCTG | 1335 |
| rH120-Δ3ab-gD-T/B | 744 | ||
| rH120-ΔIR-gD | GTAGCAACAGGTGGAAGTAGC | AAGACGCGTTTTGGTCCGTG | 1348 |
| rH120-ΔIR-gD-T/B | 757 | ||
| rH120-Δ5ab-gD | TTGTTGTAGGTTGTGGTCCC | AAGGCTCTGCTTGTCCTGCT | 1345 |
| rH120-Δ5ab-gD-T/B | 754 |
To test the stable expression of heterologous proteins, rIBVs of P5, P10, and P20 generations were infected with single-layer CK cells at a multiplicity of infection (MOI) of 0.1. Meanwhile, rH120 infection group and no treatment group were set. 48 h after infection, CK cells were harvested. After protease lysis with 1% protease inhibitor, the supernatant was collected by centrifugation, and western blot analysis was performed using specific mouse monoclonal antibody (MAb) against gD and rabbit MAb against IBV-N (prepared in our laboratory).
Growth kinetics
9-day-old SPF chicken embryos were inoculated with the recombinant IBVs and parent virus H120 strain at a dose of 100 50% egg infection dose (EID50) via the allantoic route, respectively. The allantoic fluid of chicken embryos was harvested at 12, 24, 36, 48, 60 and 72 h after inoculation for EID50 measurement, and the growth kinetic curve of the recombinant virus was drawn using GraphPad Prism 8 software.
Immunization and challenge experiments
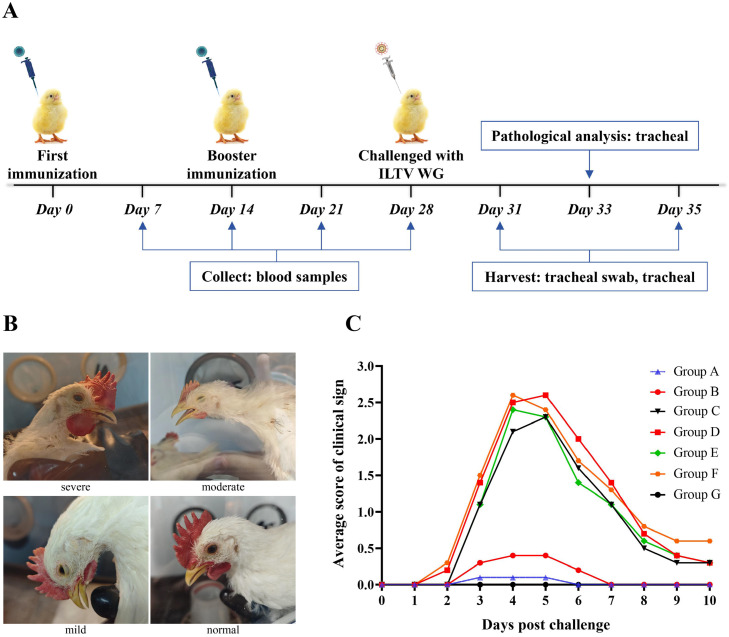
154 healthy one-day-old SPF chickens were randomly divided into 7 groups with 22 chickens in each group. Chickens in groups A and B were vaccinated with 100 uL of rH120-Δ5ab-gD-T/B via the oculonasal route at a dose of 104.0 EID50. Meanwhile, chickens in groups C and D were vaccinated with 100 uL of rH120 via the same route at a dose of 104.0 EID50 and served as vector controls. Moreover, chickens in groups E, F, and G were treated with equal amounts of PBS, and these served as unvaccinated controls. Based on the immunization schedule of the attenuated IBV vaccine used in clinical practice, a booster immunization was carried out 14 days post vaccination (dpv) following the same immunization protocol. At 28 dpv, chickens in groups A, C, and E were challenged with the virulent ILTV-WG strain via the intratracheal inoculation route at a dose of 103.5 EID50, respectively, while chickens in groups B, D, and F were challenged with the virulent ILTV at a dose of 104.0 EID50. Chickens in group G was inoculated with the same amount of PBS via the same route. For detailed information, please refer to Table 2. After challenge, mortality and clinical signs of ILT were recorded daily for 10 days. Briefly, chickens were rated on a scale of 0 to 3: normal (0), mild (1), moderate (2), and severe (3), according to clinically observed breathing patterns, conjunctivitis, and depression (Zhao et al., 2014; Hao et al., 2023).
Table 2.
Grouping for the immune challenge experiment and observation of clinical signs in chickens at different days post-challenge.
| Group | Vaccinationa | ILTV challenge doseb | Number of chickens showing clinical signs/number in groupc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| A | rH120-Δ5ab-gD-T/B | 103.5 EID50 | 0/10 | 0/10 | 1/10 | 1/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| B | rH120-Δ5ab-gD-T/B | 104.0 EID50 | 0/10 | 0/10 | 2/10 | 2/10 | 2/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| C | rH120 | 103.5 EID50 | 0/10 | 0/10 | 6/10 | 9/10 | 10/10 | 8/10 | 7/10 | 3/10 | 1/10 | 1/10 |
| D | rH120 | 104.0 EID50 | 0/10 | 1/10 | 7/10 | 10/10 | 10/10 | 9/10 | 8/10 | 4/10 | 2/10 | 1/10 |
| E | PBS | 103.5 EID50 | 0/10 | 0/10 | 5/10 | 9/10 | 10/10 | 7/10 | 6/10 | 3/10 | 2/10 | 1/10 |
| F | PBS | 104.0 EID50 | 0/10 | 1/10 | 7/10 | 10/10 | 10/10 | 8/10 | 7/10 | 4/10 | 2/10 | 2/10 |
| G | PBS | / | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
SPF chickens were immunized at one day of age by eye drop, and boosted according to the same immunization procedure at 14 days of age.
At 28 dpv, the chickens were challenged via intratracheal inoculation with the ILTV WG strain.
The marked chickens used for clinical observation.
Serum antibody detection
Blood samples were collected from 10 marked chickens in each group at 7, 14, 21, and 28 dpv. To determine the antibody titers against ILTV, all serum samples were detected using a commercial enzyme-linked immunosorbent assay (ELISA) test kit (BioChek, Netherlands).
Detecting viral load and shedding of ILTV in chickens
Tracheal swabs were collected from 10 marked chickens in each group at 3 and 7 days post challenge (dpc). Meanwhile, four unmarked chickens were randomly euthanized in each group, and 100 mg tracheal tissue samples were collected. The swab and tissue samples were treated according to the manufacturer's instructions, and viral DNA was extracted from the samples using Axyprop humoral Virus DNA/RNA Mini Extraction Kit (Axygen, CA, USA), with a final elution volume of 40 μL. The ILTV viral loads in the tracheal swab and tissue samples were quantified by real-time PCR, which amplified the UL27 gene (encoding glycoprotein B) of ILTV using the sense primer 5′-AGACACTGCATCTATGGACGTTGG-3′ and the antisense primer 5′-TGCTCCAGTTGGAGAAGAACATGC-3′. Real-time PCR assays were performed using C1000 TouchTM Thermal Cycler (Bio-rad, CA, USA) and Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The reaction system with a final volume of 20 μL included 10 μL of premix, 0.4 μL of each primer, 8.2 μL of nuclease free water, and 1 μL of template, with each sample repeating three reactions. The reaction was carried out at 95°C for 5 min, 40 cycles of 95°C for 10 s, and 60°C for 30 s.
Pathological analysis of trachea tissue
At 5 dpc, four unmarked chickens were randomly euthanized in each group, and the tracheas were collected and fixed with 10% neutral formalin solution. The tissue samples were stained with hematoxylin and eosin (H&E) after embedding in paraffin, and histopathological changes were observed under an upright microscope.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software Inc., La Jolla, CA, USA). The one-way analysis of variance (ANOVA) was adopted for multiple comparisons. Results were considered to be statistically significant if P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***).
Results
Construction of recombinant IBV expressing ILTV gD protein multiple epitope
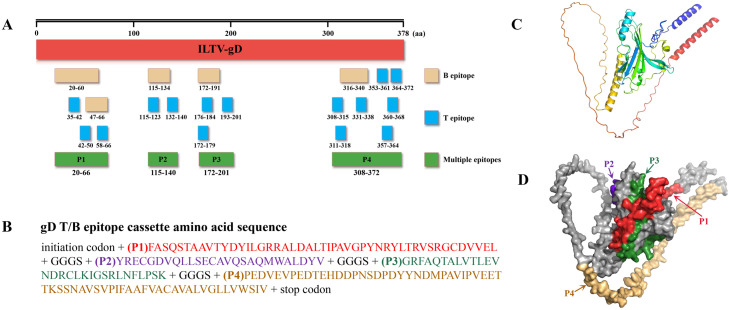
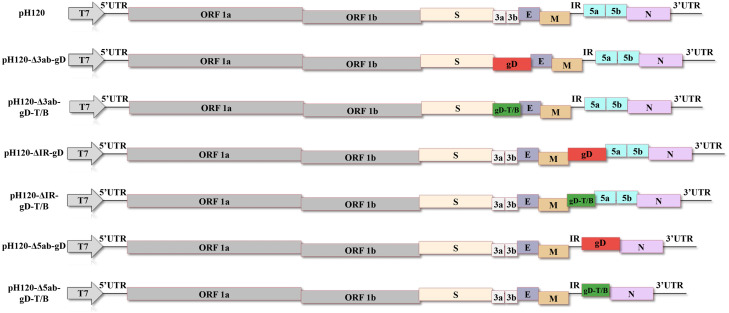
The positions of five B-cell epitopes and fifteen T-cell epitopes on the ILTV gD gene are shown in Fig. 1A, forming four multi-epitope regions which were assembled into one multi-epitope cassette by linking sequences (Fig. 1B). The three-dimensional (3D) structure of the gD protein was predicted using AlphaFold3 (Fig. 1C), and multi-epitope regions were labeled in the 3D structure of the gD protein using Pymol software (Fig. 1D). In order to develop a recombinant IBV expressing ILTV-specific immunogens, the H120 strain was used as a vector backbone. Red/ET homologous recombination technology was used to replace 3ab, 5ab and IR of H120 genome with gD gene and gD-T/B multi-epitope cassette, respectively (Fig. 2). The recombinant IBV plasmids were co-transfected with helper plasmid pVAX1-H120/N into BSR-T7/5 cells stably expressing T7 RNA polymerase by liposome method, and then incubated in 5% CO2 at 37°C for 72 h. Finally, the results of RT-PCR and genome sequencing confirmed that the recombinant IBVs were successfully rescued.
Fig. 1.
Design of the T/B multi-epitope cassette (gD-T/B) of ILTV gD protein. (A) The gD-T/B multi-epitope cassette consists of five B-cell epitopes and fifteen T-cell epitopes. Yellow boxes represent B-cell epitopes; blue boxes represent T-cell epitopes; green boxes represent multiple epitopes. (B) The amino acid sequence of the gD-T/B multi-epitope cassette contains four multiple epitopes (P1, P2, P3, P4) linked by flexible amino acids. (C) The reference glycoprotein D of ILTV. (D) The position of proposed multiple epitopes in the 3D structure of reference glycoprotein D of ILTV.
Fig. 2.
Schematic diagram of the construction of recombinant IBVs. Recombinant IBVs were constructed by inserting the full-length gD gene or gD-T/B multi-epitope cassette at different positions in the IBV genome using Red/ET homologous recombination technology. The genome schematic diagram of wild-type H120 strain and recombinant H120 strains, showing the position of foreign genes. The full-length gD gene is shown in a red box; the gD-T/B multi-epitope cassette is represented by a green box.
Genetic stability of recombinant IBVs
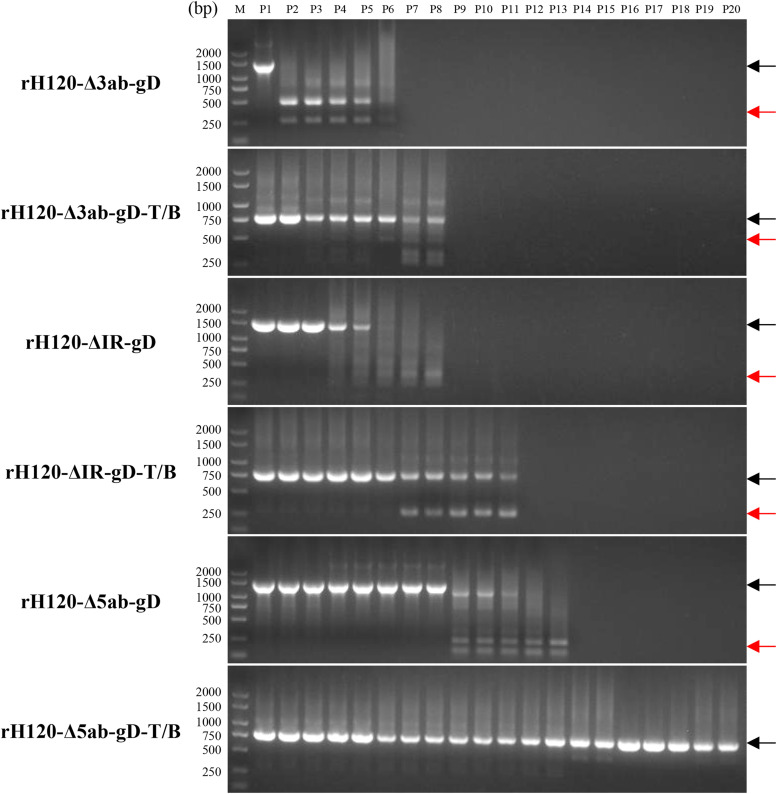
To further evaluate the potential of rIBV as a vaccine vector, the genetic stability of heterologous genes was first detected at the nucleotide level. Recombinant IBVs were continuously propagated in chicken embryos for 20 generations and the integrity of heterologous genes was detected by RT-PCR and genome sequencing (Fig. 3). The results had showed that the stability of recombinant IBVs varied depending on the inserted heterologous genes or insertion sites. Overall, recombinant IBVs with with the complete gD gene insertion generally exhibited lower genetic stability. rH120-Δ5ab-gD and rH120-ΔIR-gD experienced deletions of the heterologous gene at P9 and P6, respectively, while rH120-Δ3ab-gD encountered a deletion as early as at P2. In comparison, the stability of recombinant IBVs with gD-T/B multi-epitope cassette insertion was significantly improved. The heterologous genes in rH120-Δ3ab-gD-T/B and rH120-ΔIR-gD-T/B remained stable until P6, while the heterologous genes in rH120-Δ5ab-gD-T/B were stable for at least P20.
Fig. 3.
Genetic stability analysis of recombinant IBVs. The recombinant viruses rH120-Δ3ab-gD, rH120-Δ3ab-gD-T/B, rH120-ΔIR-gD, rH120-ΔIR-gD-T/B, rH120-Δ5ab-gD and rH120-Δ5ab-gD-T/B were continuously passaged in chicken embryos, and the allantoic fluid was harvested 48 h post-inoculation for identification. The foreign genes of the recombinant viruses were amplified by RT-PCR using primers from Table 1 and analyzed by agarose gel electrophoresis. Recombinant viruses that were determined to have deletions of foreign genes were not passaged further. The PCR products of the expected size were indicated by black arrows; the smaller PCR products that did not meet expectations were indicated by red arrows.
Antigenic analysis of rH120-Δ5ab-gD-T/B
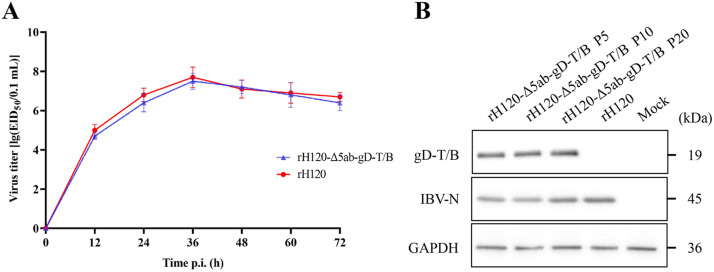
To further analyze the antigenicity of rH120-Δ5ab-gD-T/B and the stability of heterologous protein expression across different passages, rH120-Δ5ab-gD-T/B and rH120 were infected with CK cells at MOI=0.1. Cell lysates were harvested 48 hours post infection (hpi) and subjected to Western blot analysis. The results, as shown in Fig. 4A, demonstrated that protein bands approximately 19 kDa were observed in the rH120-Δ5ab-gD-T/B infection groups of P5, P10, and P15, indicating that the recombinant virus rH120-Δ5ab-gD-T/B could stably express the ILTV multi-epitope cassette gD-T/B protein at different generations. In contrast, only a protein band of approximately 45 kDa corresponding to IBV-N was observed in the rH120 infection group. No specific protein bands for gD-T/B and IBV-N were detected in the MOCK group.
Fig. 4.
Protein expression stability and growth characteristics of rH120-Δ5ab-gD-T/B. (A) Growth kinetics curves of the recombinant virus rH120-Δ5ab-gD-T/B and the parental virus rH120 in chicken embryos. (B) Expression of the gD-T/B multi-epitope cassette protein in generations P5, P10, and P20 of rH120-Δ5ab-gD-T/B was detected by Western blot.
Growth kinetics of rH120-Δ5ab-gD-T/B
To analyze the impact of replacing the 5ab gene of H120 with the gD-T/B multi-epitope cassette on viral replication, a comparative analysis of replication dynamics within chicken embryos was conducted between rH120-Δ5ab-gD-T/B and rH120. As shown in Fig. 4B, similar proliferation curves were observed for the recombinant virus rH120-Δ5ab-gD-T/B and the parental virus rH120. Similarly, rH120-Δ5ab-gD-T/B and rH120 reached peak titers at 36 hpi, after which the titers decreased slowly. Overall, rH120-Δ5ab-gD-T/B showed a slightly lower titer than rH120, with a difference from log10 0.2 to 0.4. The above results indicated that the replacement of the 5ab gene of H120 with the gD-T/B multi-epitope cassette had almost no impact on the virus's replication in chicken embryos.
Protective efficacy of rH120-Δ5ab-gD-T/B against ILTV challenge in chickens
To evaluate the protective efficacy of the recombinant virus rH120-Δ5ab-gD-T/B against ILTV challenge, immunization and challenge experiments were conducted, as illustrated in Fig. 5A. After challenged with the virulent ILTV WG strain, clinical signs of the chickens were recorded daily for 10 days (Table 2). As expected, no clinical symptoms were observed in group G, while the main clinical signs in groups C to F chickens were coughing up blood and severe respiratory distress (Fig. 5B). Chickens in groups C to F experienced a peak in clinical symptoms at 4 to 5 dpc, with mortality rates ranging from 10% to 20%, after which they began to gradually recover. Compared to groups C to F, the clinical scores of groups A and B were significantly lower. During the trial period, one chicken with severe symptoms and one with mild symptoms were observed in group B. Chickens in group A did not show clinical signs of moderate severity or above, and only one chicken showed mild symptoms of continuous sneezing at 3 dpc, but had fully recovered by 6 dpc (Fig. 5C). These results indicated that rH120-Δ5ab-gD-T/B could alleviate the clinical signs caused by challenge with a virulent ILTV strain.
Fig. 5.
Clinical observations and mean clinical sign scores after challenge with virulent ILTV. (A) Schematic diagram of the immune challenge experiment design. Blood samples were collected on days 7, 14, 21, and 28 for the detection of antibodies against ILTV. On days 31 and 35, tracheal swabs and tracheal tissues were collected for the detection of ILTV viral load. On day 33, tracheal tissues were collected for histopathological examination. (B) Clinical symptoms of chickens post challenge with virulent ILTV. (C) Mean clinical sign scores per group of chickens at 0 to 10 dpc. The data shown is the average value for each group of ten chickens.
Immune response induced by rH120-Δ5ab-gD-T/B in chickens
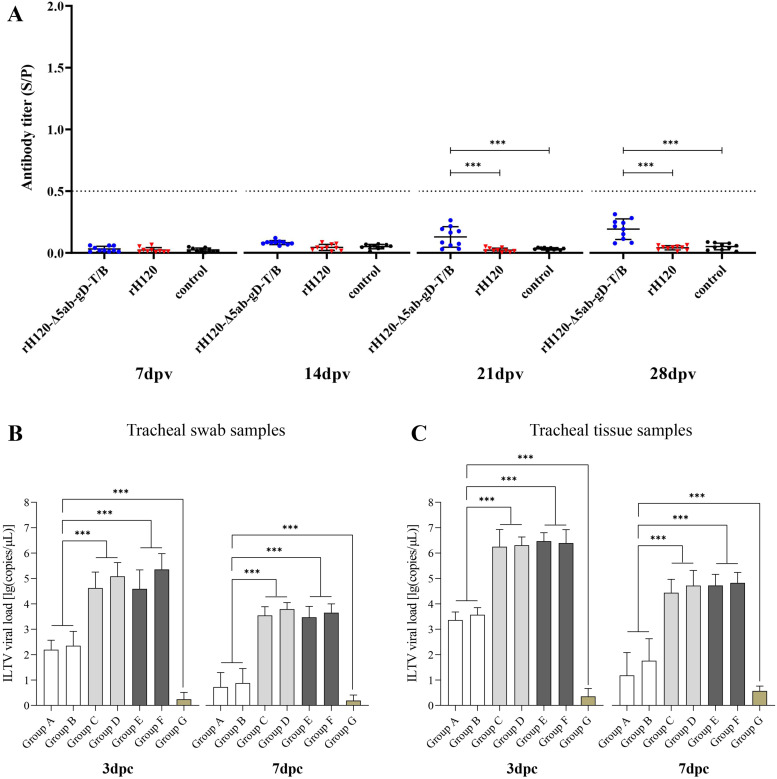
To assess the humoral immune response induced by rH120-Δ5ab-gD-T/B in chickens, serum samples were collected from each group at 7, 14, 21, and 28 dpv, and antibody titers against ILTV were determined by ELISA. The data showed that there was no statistically significant difference in ILTV antibody levels among the groups at 7 dpv and 14 dpv (P > 0.05). At 21 dpv and 28 dpv, compared to the control group and the rH120 immunized group, the antibody levels of the rH120-Δ5ab-gD-T/B immunized group showed a significant increase (P < 0.001), but did not reach the threshold for seropositivity (Fig. 6A).
Fig. 6.
Protective Efficacy of rH120-Δ5ab-gD-T/B against ILTV. (A) Humoral immune response induced by rH120-Δ5ab-gD-T/B. Serum samples were collected from the rH120-Δ5ab-gD-T/B immunized group, rH120 immunized group, and control group at 7, 14, 21, and 28 dpv. ILTV antibody titers were measured by ELISA. The data shown are measurements for each group of ten chickens. (B) Shedding of virulent ILTV in tracheal swabs was detected at 3 dpc and 7 dpc. Data presented are the means ± SD for ten chickens per group. (C) The viral genome load of virulent ILTV in tracheal tissues was detected at 3 dpc and 7 dpc. Data presented are the means ± SD for four chickens per group. The differences between groups are analyzed using ANOVA (***P < 0.001).
rH120-Δ5ab-gD-T/B reduced ILTV shedding and tissue viral load in trachea post challenge
The reduction of viral shedding and tissue viral load in the trachea post challenge is one of the important criteria for evaluating the efficacy of ILTV vaccines. Viral loads in tracheal swabs and tissues were quantified by qPCR at 3 and 7 dpc. As shown in Fig. 6B, at 3 and 7 dpc, the genome copies of ILTV in tracheal swabs of the rH120-Δ5ab-gD-T/B immunized groups (groups A and B) were significantly higher than thoes of the non-vaccinated/non-challenged group (group G) (P < 0.001), but significantly lower than thoes of the rH120 immunized groups (groups C and D) and the non-vaccinated/challenged groups (groups E and F) (P < 0.001). Similar results were also observed in the detection of viral load in tracheal tissue (Fig. 6C). These results indicated that rH120-Δ5ab-gD-T/B could reduce the shedding and replication of virulent ILTV strain in chickens.
Histopathological lesions in trachea post challenge
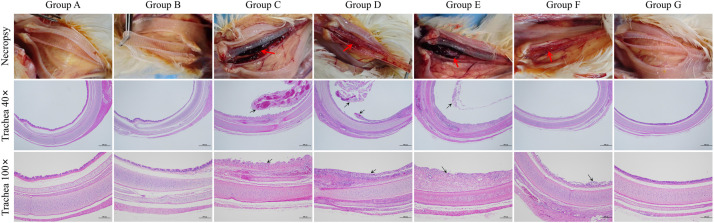
To assess the microscopic lesions in the trachea of chickens after ILTV challenge, necropsy was performed on chickens at 5 dpc to examine tracheal lesions, and histopathological studies were conducted on the trachea. Notably, extensive mucous secretion accompanied by red caseous material occluding the trachea was observed in groups C to F, while no significant lesions were observed in groups A, B, and G (Fig. 7). In addition, histopathological studies revealed significant pathological changes in the trachea of groups C to F, such as severe damage to the tracheal mucosa, sloughing of epithelium, increased thickness of tracheal mucosa, disappearance of cilia, and infiltration of inflammatory cells in the mucosa. The tracheas of groups A, B, and G were normal, with no pathological lesions.
Fig. 7.
Representative necropsy and histopathological analysis of the trachea post challenge with virulent ILTV. Four chickens from each group were euthanized at 5 dpc for tracheal lesion observation. Red arrows indicate gross lesions characterized by excessive mucous secretion or red caseous material obstructing the trachea. Additionally, tracheal tissues were collected for histopathological examination. Typical pathological changes are indicated by black arrows, such as severe damage to the tracheal mucosa, sloughing of epithelium, increased thickness of tracheal mucosa and disappearance of cilia.
Discussion
Currently, live attenuated vaccines were widely used to prevent ILT. In the current trend, live attenuated vaccines would remain the main method of ILT prevention and control for a long time. However, recent studies had reported that ILTV live attenuated vaccines could recombine with field strains, leading to immune failure and causing significant losses in the poultry industry (Fakhri et al., 2020; Perez et al., 2020; Sabir et al., 2020). Interestingly, a recent study had shown that ILTV field strains were gradually evolving towards chicken embryo origin (CEO) vaccine strains (Yi et al., 2024). In addition, the virulence of ILTV live attenuated vaccines was generally strong, causing significant reactions in animals after vaccination. Meanwhile, the latent infections caused by these vaccines were one of the important factors leading to ILT outbreaks (Bayoumi et al., 2020; Mossad et al., 2022). Continuous negative reports had caused scholars from various countries to hold a concerned attitude towards the use of ILTV live attenuated vaccines (Elshafiee et al., 2022; Ponnusamy et al., 2022; Santander-Parra et al., 2022). Therefore, the development of new ILTV vaccines was of great significance.
Coronaviruses were positive-strand RNA viruses with potential as immune vectors, yet the issue of poor stability of their recombinant viruses remained unresolved. The large RNA genome in coronaviruses allowed for genomic modification through mutation and recombination, providing additional plasticity that made these viruses inherently more prone to genetic instability (Su et al., 2016). In the past few years, IBV had been shown to accept and express foreign genes, but more research was focused on IBV vector vaccines expressing different genotypes of IBV S protein (Armesto et al., 2011; Keep et al., 2020). Therefore, we initiated a study on a bivalent IBV vector vaccine expressing ILT protective antigens, evaluating its stability and immunogenicity.
A key finding in our study was that the stability of inserting gD-T/B multi-epitope cassette was consistently higher compared to the full-length gD gene (Fig. 3). Previous study had shown that coronaviruses had helical nucleocapsid structures, which might be less restriction on genome size (de Haan et al., 2005). Moreover, an expansion of about 10% of its natural size (a total insertion size of 2.7 kb) in the coronavirus genome was indeed accepted without adversely affecting virus growth (de Haan et al., 2003). However, the larger gD gene is not as stable as the gD-T/B multi-epitope cassette, which may be related to the presence of more “heterologous signals” in larger heterologous genes. These “signals” could be secondary RNA structures or protein binding sites that affect viral replication or transcription, leading to larger foreign genes being more likely to be lost (de Haan et al., 2005).
The stability of foreign genes in recombinant coronaviruses is largely determined by the insertion site (de Haan et al., 2005; Bentley et al., 2013). The above view had been confirmed in this study. Regardless of whether the inserted heterologous gene was full-length gD gene or gD-T/B multi-epitope cassette, we observed that the stability of the recombinant virus was highest after replacing gene 5 (Fig. 3). When foreign gene (encoding green fluorescent protein) was inserted into the IBV genome in the form of fusion to the C-terminus or N-terminus of structural genes, unstable recombinant viruses were obtained (Shen et al., 2009). Similar results had also been reported in mouse hepatitis coronavirus (MHV) and transmissible gastroenteritis virus (TGEV) (Sola et al., 2003; Bosch et al., 2004). However, when heterologous genes replaced non-essential genes, foreign genes in the recombinant coronavirus genome had achieved higher stability (Sola et al., 2003; de Haan et al., 2005; Shen et al., 2009). Currently, there were no clear reports on the functions of IBV accessory proteins, but it had been confirmed that the absence of accessory proteins did not affect viral replication (Casais et al., 2005; Hodgson et al., 2006). Consistent with previous studies, in this study, replacing gene 5 with the gD-T/B multi-epitope cassette did not affect the replication of the recombinant virus (Fig. 4A).
As is well known, vaccine-induced immune responses play a crucial role in antiviral immunity and viral infection. Unexpectedly, in this study, the level of humoral immunity induced by rH120-Δ5ab-gD-T/B was low. Although a significant increase in antibodies against ILTV was detected after 21 dpv, it ultimately did not reach the threshold for serological positivity (Fig. 6A).
The possible reason was that the antigen coated in the ILTV ELISA antibody detection kit used in this study was the whole virus of ILTV, and the numerical value obtained by such kits might be low when detecting the level of antibodies against the gD-T/B multi-epitope cassette. In addition, multiple studies had shown that cell-mediated immunity was the main immune response in the case of ILTV infection (Fuchs et al., 2007; Coppo et al., 2013a, 2013b). Serological testing of specific antibodies against ILTV was a useful standard for assessing the immunogenicity of vector vaccines, but there was no positive correlation with clinical protective efficacy (Sabir et al., 2019). Therefore, in order to comprehensively evaluate the immunogenicity of the vector vaccines, it was necessary to assess both local and cell-mediated immunity.
Overall, rH120-Δ5ab-gD-T/B not only overcame the poor stability of recombinant IBV but also provided effective protection against virulent ILTV. Challenged with ILTV WG strain at a dose of 103.5EID50, rH120-Δ5ab-gD-T/B provided 90% protection effect under the premise that the morbidity in the non-vaccinated/challenged group was 100%. Meanwhile, rH120-Δ5ab-gD-T/B significantly reduced the replication and shedding of ILTV in the trachea, reducing the opportunity for ILTV to spread among chicken flocks and enter the environment.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by National Key R&D Program of China (2022YFD1801000), the Heyuan Branch, Guangdong Laboratory for Lingnan Modern Agriculture Project (DT20220003), the Construction project of modern agricultural science and technology innovation alliance in Guangdong province (2023KJ128), the China Agriculture Research System of MOF and MARA (CARS-42-13) and Provincial Science and Technology Special Fund Project for Zhongshan City (major special project + Task list management mode) (2021sdr003).
Footnotes
Scientific section: Immunology, Health and Disease
References
- Armesto M., Evans S., Cavanagh D., Abu-Median A.B., Keep S., Britton P. A recombinant avian infectious bronchitis virus expressing a heterologous spike gene belonging to the 4/91 serotype. PLoS One. 2011;6:e24352. doi: 10.1371/journal.pone.0024352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston E.J., Jordan B.J., Williams S.M., Garcia M., Jackwood M.W. Effect of pullet vaccination on development and longevity of immunity. Viruses. 2019;11 doi: 10.3390/v11020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi M., El-Saied M., Amer H., Bastami M., Sakr E.E., El-Mahdy M. Molecular characterization and genetic diversity of the infectious laryngotracheitis virus strains circulating in Egypt during the outbreaks of 2018 and 2019. Arch. Virol. 2020;165:661–670. doi: 10.1007/s00705-019-04522-4. [DOI] [PubMed] [Google Scholar]
- Bentley K., Armesto M., Britton P. Infectious bronchitis virus as a vector for the expression of heterologous genes. PLoS One. 2013;8:e67875. doi: 10.1371/journal.pone.0067875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., de Haan C.A., Rottier P.J. Coronavirus spike glycoprotein, extended at the carboxy terminus with green fluorescent protein, is assembly competent. J. Virol. 2004;78:7369–7378. doi: 10.1128/JVI.78.14.7369-7378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Davies M., Cavanagh D., Britton P. Gene 5 of the avian coronavirus infectious bronchitis virus is not essential for replication. J. Virol. 2005;79:8065–8078. doi: 10.1128/JVI.79.13.8065-8078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Coppo M.J., Noormohammadi A.H., Browning G.F., Devlin J.M. Challenges and recent advancements in infectious laryngotracheitis virus vaccines. Avian Pathol. 2013;42:195–205. doi: 10.1080/03079457.2013.800634. [DOI] [PubMed] [Google Scholar]
- Coppo M.J., Hartley C.A., Devlin J.M. Immune responses to infectious laryngotracheitis virus. Dev. Comp. Immunol. 2013;41:454–462. doi: 10.1016/j.dci.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Coppo M.J., Devlin J.M., Noormohammadi A.H. Comparison of the replication and transmissibility of two infectious laryngotracheitis virus chicken embryo origin vaccines delivered via drinking water. Avian Pathol. 2012;41:195–202. doi: 10.1080/03079457.2012.660132. [DOI] [PubMed] [Google Scholar]
- Davison A.J. Herpesvirus systematics. Vet. Microbiol. 2010;143:52–69. doi: 10.1016/j.vetmic.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., Haijema B.J., Boss D., Heuts F.W., Rottier P.J. Coronaviruses as vectors: stability of foreign gene expression. J. Virol. 2005;79:12742–12751. doi: 10.1128/JVI.79.20.12742-12751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., van Genne L., Stoop J.N., Volders H., Rottier P.J. Coronaviruses as vectors: position dependence of foreign gene expression. J. Virol. 2003;77:11312–11323. doi: 10.1128/JVI.77.21.11312-11323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovine P., Settembre E.C., Bhargava A.K., Luftig M.A., Lou H., Cohen G.H., Eisenberg R.J., Krummenacher C., Carfi A. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R.J., Atanasiu D., Cairns T.M., Gallagher J.R., Krummenacher C., Cohen G.H. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshafiee E.A., Hassan M., Provost C., Gagnon C.A., Ojkic D., Abdul-Careem M.F. Comparative full genome sequence analysis of wild-type and chicken embryo origin vaccine-like infectious laryngotracheitis virus field isolates from Canada. Infect. Genet. Evol. 2022;104 doi: 10.1016/j.meegid.2022.105350. [DOI] [PubMed] [Google Scholar]
- Fakhri O., Devlin J.M., Browning G.F., Coppo M., Quinteros J.A., Diaz-Mendez A., Lee S.W., Hartley C.A. Superinfection and recombination of infectious laryngotracheitis virus vaccines in the natural host. Vaccine. 2020;38:7508–7516. doi: 10.1016/j.vaccine.2020.09.064. [DOI] [PubMed] [Google Scholar]
- Fuchs W., Veits J., Helferich D., Granzow H., Teifke J.P., Mettenleiter T.C. Molecular biology of avian infectious laryngotracheitis virus. Vet. Res. 2007;38:261–279. doi: 10.1051/vetres:200657. [DOI] [PubMed] [Google Scholar]
- Garcia M. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet. Microbiol. 2017;206:157–162. doi: 10.1016/j.vetmic.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Hao X., Li J., Wang J., Zhou Z., Yuan X., Pan S., Zhu J., Zhang F., Yin S., Yang Y., Hu S., Shang S. Co-administration of chicken IL-2 alleviates clinical signs and replication of the ILTV chicken embryo origin vaccine by pre-activating natural killer cells and cytotoxic T lymphocytes. J. Virol. 2023;97 doi: 10.1128/jvi.01322-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson T., Britton P., Cavanagh D. Neither the RNA nor the proteins of open reading frames 3a and 3b of the coronavirus infectious bronchitis virus are essential for replication. J. Virol. 2006;80:296–305. doi: 10.1128/JVI.80.1.296-305.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C.S., Williams R.A., Gaskell R.M., Jordan F.T., Bradbury J.M., Bennett M., Jones R.C. Latency and reactivation of infectious laryngotracheitis vaccine virus. Arch. Virol. 1991;121:213–218. doi: 10.1007/BF01316755. [DOI] [PubMed] [Google Scholar]
- Hutchings D.L., van Drunen L.D.H.S., Babiuk L.A. Lymphocyte proliferative responses to separated bovine herpesvirus 1 proteins in immune cattle. J. Virol. 1990;64:5114–5122. doi: 10.1128/jvi.64.10.5114-5122.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep S., Sives S., Stevenson-Leggett P., Britton P., Vervelde L., Bickerton E. Limited cross-protection against infectious bronchitis provided by recombinant infectious bronchitis viruses expressing heterologous spike glycoproteins. Vaccines. 2020;8 doi: 10.3390/vaccines8020330. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L.A., Dowbenko D., Simonsen C.C., Berman P.W. Protection of mice from lethal Herpes Simplex virus infection by vaccination with a secreted form of cloned glycoprotein D. Nat. Biotechnol. 1984;2:527–532. [Google Scholar]
- Lazear E., Whitbeck J.C., Ponce-de-Leon M., Cairns T.M., Willis S.H., Zuo Y., Krummenacher C., Cohen G.H., Eisenberg R.J. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J. Virol. 2012;86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioli C.C., Yancey R.J., Petrovskis E.A., Timmins J.G., Post L.E. Evaluation of pseudorabies virus glycoprotein gp50 as a vaccine for Aujeszky's disease in mice and swine: expression by vaccinia virus and Chinese hamster ovary cells. J. Virol. 1987;61:3977–3982. doi: 10.1128/jvi.61.12.3977-3982.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossad Z., Moussa S.A., Saied M., Fathy M.M., Zanaty A.M. Molecular and genetic detection of infectious laryngeotrachitis disease virus in broiler farms after a disease outbreak in Egypt. Virusdisease. 2022;33:404–412. doi: 10.1007/s13337-022-00792-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldoni I., Rodriguez-Avila A., Riblet S.M., Zavala G., Garcia M. Pathogenicity and growth characteristics of selected infectious laryngotracheitis virus strains from the United States. Avian Pathol. 2009;38:47–53. doi: 10.1080/03079450802632031. [DOI] [PubMed] [Google Scholar]
- Ou S.C., Giambrone J.J. Infectious laryngotracheitis virus in chickens. World J. Virol. 2012;1:142–149. doi: 10.5501/wjv.v1.i5.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova S., Veits J., Mettenleiter T.C., Fuchs W. Identification and functional analysis of membrane proteins gD, gE, gI, and pUS9 of Infectious laryngotracheitis virus. Avian Dis. 2013;57(2 Suppl):416–426. doi: 10.1637/10332-082612-Reg.1. [DOI] [PubMed] [Google Scholar]
- Peng S., Wang Y., Zhang Y., Song X., Zou Y., Li L., Zhao X., Yin Z. Current knowledge on infectious bronchitis virus non-structural proteins: the bearer for achieving immune evasion function. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C.A., van der Meer F., Checkley S., Joseph T., King R., Ravi M., Peters D., Fonseca K., Gagnon C.A., Provost C., Ojkic D., Abdul-Careem M.F. Analysis of whole-genome sequences of infectious laryngotracheitis virus isolates from poultry flocks in Canada: evidence of recombination. Viruses. 2020;12 doi: 10.3390/v12111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy P., Sukumar K., Raja A., Saravanan S., Srinivasan P., Thangavelu A. Characterization of infectious laryngotracheitis virus isolates from laying hens during 2019-2020 outbreaks in Tamil Nadu, India. Arch. Virol. 2022;167:1819–1829. doi: 10.1007/s00705-022-05485-9. [DOI] [PubMed] [Google Scholar]
- Quinteros J.A., Noormohammadi A.H., Lee S.W., Browning G.F., Diaz-Mendez A. Genomics and pathogenesis of the avian coronavirus infectious bronchitis virus. Aust. Vet. J. 2022;100:496–512. doi: 10.1111/avj.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir A.J., Olaogun O.M., O'Rourke D., Fakhri O., Coppo M., Devlin J.M., Konsak-Ilievski B., Noormohammadi A.H. Full genomic characterisation of an emerging infectious laryngotracheitis virus class 7b from Australia linked to a vaccine strain revealed its identity. Infect. Genet. Evol. 2020;78 doi: 10.1016/j.meegid.2019.104067. [DOI] [PubMed] [Google Scholar]
- Sabir A.J., Adams T.E., O'Rourke D., Devlin J.M., Noormohammadi A.H. Investigation onto the correlation between systemic antibodies to surface glycoproteins of infectious laryngotracheitis virus (ILTV) and protective immunity. Vet. Microbiol. 2019;228:252–258. doi: 10.1016/j.vetmic.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Santander-Parra S.H., Nunez L., Buim M.R., Ferreira C., Loncoman C.A., Ferreira A. Detection and molecular characterization of infectious laryngotracheitis virus (ILTV) in chicken with respiratory signs in Brazil during 2015 and 2016. Braz. J. Microbiol. 2022;53:2223–2232. doi: 10.1007/s42770-022-00833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao G., Xie Z., Liang M., Liu Y., Song C., Feng K., Zhang X., Lin W., Fu J., Xie Q. Efficacy of recombinant Newcastle disease virus expressing HA protein of H9N2 Avian influenza virus in respiratory and intestinal tract. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Fang S.G., Chen B., Chen G., Tay F.P., Liu D.X. Towards construction of viral vectors based on avian coronavirus infectious bronchitis virus for gene delivery and vaccine development. J. Virol. Methods. 2009;160:48–56. doi: 10.1016/j.jviromet.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola I., Alonso S., Zuniga S., Balasch M., Plana-Duran J., Enjuanes L. Engineering the transmissible gastroenteritis virus genome as an expression vector inducing lactogenic immunity. J. Virol. 2003;77:4357–4369. doi: 10.1128/JVI.77.7.4357-4369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Zhang Z., Su X., Lu H., Li X., Yuan C., Liu Q., Teng Q., Geri L., Li Z. Biological characteristics of infectious laryngotracheitis viruses isolated in China. Viruses. 2022;14 doi: 10.3390/v14061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Li G., Mu Y., Cui S., Zhang D., Xu Q., Liang C., Wang M., Zhou S., Zhou H., Zhong M., Zhang A. Isolation, identification, molecular and pathogenicity characteristics of an infectious laryngotracheitis virus from Hubei province, China. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S., Leibowitz J.L., Collisson E.W. In vitro assembled, recombinant infectious bronchitis viruses demonstrate that the 5a open reading frame is not essential for replication. Virology. 2005;332:206–215. doi: 10.1016/j.virol.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhao Y., Zhang G. Key aspects of Coronavirus avian infectious bronchitis Virus. Pathogens. 2023;12 doi: 10.3390/pathogens12050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Spatz S., Zhang Z., Wen G., Garcia M., Zsak L., Yu Q. Newcastle disease virus (NDV) recombinants expressing infectious laryngotracheitis virus (ILTV) glycoproteins gB and gD protect chickens against ILTV and NDV challenges. J. Virol. 2014;88:8397–8406. doi: 10.1128/JVI.01321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]