1. Introduction
Nuclear protein in testis carcinoma (NUT) is an exceptionally rare and invasive carcinoma that mainly arises in midline head, neck, and thoracic structures, and therefore this malignancy was originally referred to as NUT midline carcinoma. (Mengoli et al., 2018) It was renamed NUT carcinoma in 2015 due to its discovery outside the midline in several anatomical sites, including the salivary glands, lungs, pancreas, kidney, adrenal gland, bladder, ovary, and bone. (Mengoli et al., 2018, Jung et al., 2022) NUT carcinoma frequently possesses the BDR4-NUTM1 fusion oncoprotein, with NUT on chromosome 15 combining with bromodomain-containing protein 4 on chromosome 19. (Eagen and French, 2021) Alternative fusion variants have been detected, including combinations between NUTM1 and BRD3, NSD3, ZNF532, and ZNF592. (Eagen and French, 2021).
NUT carcinoma affects individuals with a median age varying between 16–24 years old. (Moreno et al., 2022) However, this lethal malignancy has affected patients of all ages over the last decade, ranging from 0-81.7 years. (Moreno et al., 2022) Its poor response to traditional chemotherapy and radiation leaves patients with a dismal prognosis and a median survival of 6.5 months. (Moreno et al., 2022) Immunohistochemical (IHC) staining with the anti-NUT C52 monoclonal antibody has demonstrated promise in diagnosing NUT carcinoma, with an 87 % sensitivity and 100 % specificity. (Li et al., 2021) Fluorescence in situ hybridization (FISH) to detect NUTM1 rearrangements in combination with IHC of the anti-NUT C52 antibody can reach a sensitivity of 100 % in diagnosing this malignancy. (Li et al., 2021) Recently, next generation sequencing (NGS) has emerged in the field, facilitating diagnosis of poorly differentiated tumors by detecting abnormal gene fusions. (Yang et al., 2021).
Recent case reports have highlighted its occurrence in ovarian tissues. Jiang et al. described a case of NUT carcinoma with MXI1-NUTM1 fusion presenting as extensive abdominopelvic lesions and bilateral ovarian masses, emphasizing the need for NUT testing in undifferentiated malignancies with such presentations. (Jiang et al., 2023 Jan) Similarly, Tamura et al. identified a novel MXD4-NUTM1 fusion in ovarian small round cell sarcoma, with immunohistochemical (IHC) staining showing high expression of the NUT protein. (Tamura et al., 2018 Nov) Another notable case of ovarian NUT carcinoma was reported in a 19-year-old female presenting with a pelvic mass and thoracic involvement. (Ball et al., 2012 Oct) The diagnosis was confirmed through nuclear immunohistochemistry and molecular analysis, highlighting the aggressive nature of this rare malignancy. (Ball et al., 2012 Oct) These findings highlight the diagnostic importance of IHC in ovarian NUT carcinoma, which can be misdiagnosed as other poorly differentiated malignancies without specific molecular testing.
Our case represents a unique gene fusion of one of the NUT family genes. NUTM2B fused to a MXI1- previously not described gene fusion in NUT carcinomas. It is considered the first detected MXI1-NUTM2B gene fusion in a poorly differentiated small blue cell malignancy of the ovary.
2. Case presentation
The patient is a 44-year-old female who initially presented to our gynecologic oncology clinic after identification of a pelvic mass on imaging. Her family history is only significant for her mother who has a history of lung cancer and a sister with cervical cancer.
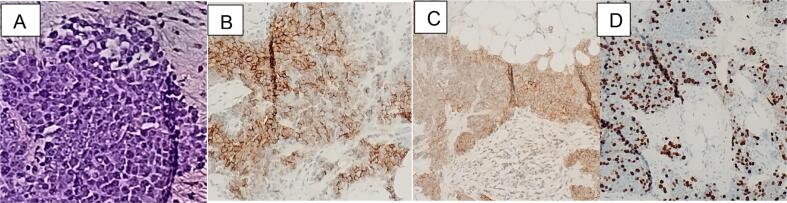
CT-Chest/Abdomen/Pelvis demonstrated bilateral solid adnexal masses, omental caking, and peritoneal carcinomatosis with little interval change (Fig. 1A). There was no gross evidence of metastatic disease involving the chest. There were a few nonenlarged borderline prominent cardio-phrenic lymph nodes bilaterally and trace perihepatic peri-splenic ascites. CT guided omental biopsy showed small round cell nests of malignant appearing infiltrating cells with scant amphophilic to eosinophilic vacuolated cytoplasm and large pleomorphic nuclei. The nuclei exhibited fine chromatin with variable numbers of inclusions and vacuolations. Immunostaining was positive only for CD56 and CD99 and a Ki-67 nuclear labeling of approximately 40 % in tumor cells (Fig. 2). The initial histologic findings were difficult to interpret, though suspicion of small cell carcinoma was high in the differential. However, following the discordant immune profile (negative synaptophysin, chromogranin, cytokeratins), the diagnosis of small cell was disfavored and less specific diagnosis of “small, round, blue cell” tumor was given while molecular profile and expert consultation was sought.
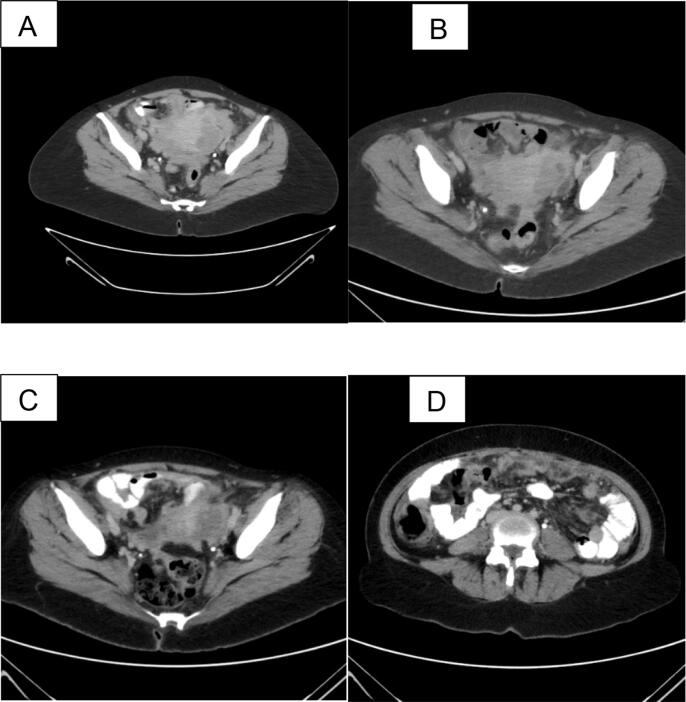
Fig. 1.
A. shows an image of pelvic disease prior to chemotherapy. B: shows disease burden in the pelvis after 3 cycles of chemotherapy with minimal change noted. C: notes after multiple additional cycles of chemotherapy with mild improvement in disease. D: shows worsening peritoneal disease after receiving greater than 6 cycles of chemotherapy.
Fig. 2.
A. Microscopic description shows an infiltrating malignant appearing neoplastic process. The tumor cells are arranged in infiltrating sheets with a desmoplastic fibrotic background stroma. They demonstrate a high nucleus to cytoplasm (N/C) ratio. The scant cytoplasm is eosinophilic where identifiable. The nuclei are pleomorphic with variable number of vacuolar inclusions, prominent and sometimes multiple nucleoli, with fine chromatin. There is scattered mitosis present. B, C, D. Immunohistochemical staining shows strong diffuse cytoplasmic and membranous reactivity to CD56 (B) and CD99 (C) and a Ki-67 (D) nuclear labeling of approximately 40% in tumor cells.
Given the extent of the disease, the decision was made to proceed with neoadjuvant chemotherapy. The treatment regimen of paclitaxel, carboplatin, and bevacizumab was discussed at an interdisciplinary tumor board and chosen based on its established efficacy as a first-line approach for advanced ovarian cancer. The patient received 3 cycles of carboplatin, paclitaxel, and bevacizumab with consideration of interval debulking surgery. Repeat CT scan after 3 cycles showed extensive peritoneal disease including omental caking, disease on liver capsule, serosal surface of bowel, and involving adnexal regions (Fig. 1B). There was a mild decrease in overall disease burden, with a decrease in measured thickness of omental caking and resolution of ascites. No new adenopathy, liver lesions, biliary dilation, or destructive bone lesions were present. CA-125 levels at this interval were 58.9. Given the decrease in CA125 level and improvement in CT scan, plan made to proceed with diagnostic laparoscopy with possible conversion based on intraoperative findings.
Intraoperative findings: Laparoscopic survey revealed evidence of partial treatment response. Minimal ascites was present. However, in the lower abdomen and pelvis the uterus was fixed to bilateral pelvic side walls with multiple loops of small bowel draped over uterine fundus obscuring direct visualization. Bilateral adnexa and posterior cul-de-sac were not visualized. Scattered peritoneal lesions were identified along with suspected mesenteric deposits causing folding of small bowel and limiting mobilization. In the upper abdomen we discovered scattered diaphragmatic lesions bilaterally consistent with at least partially treated disease. The peritoneal surface overlying the anterior abdominal wall was thickened and consistent with persistent disease. Plaque-like adhesions along the superior dome of the liver obscuring complete visualization.
The team aborted interval debulking surgery given burden of disease and unlikelihood of a complete (R0) cytoreduction. A biopsy of the anterior abdominal wall peritoneum was sent to pathology.
Pathological findings demonstrated a poorly differentiated malignant neoplasm composed of small round cells infiltrating fat and connective tissues with a morphology similar to that seen in the previous peritoneal biopsy (SKI24-60). The tumor cells were embedded in a collagenous stroma. The peritoneal biopsy was sent for a second opinion. Despite multiple additional immunohistochemical and molecular tests, a more definitive diagnosis could not be made. The tumor cells were negative for CDX2, CAM5.2, CK7, CK20, PAX8, WT1, GATA3, LCA, synaptophysin, chromogranin, desmin, EMA, SF1, OCT3/4, DUX4, AE1/AE3, and NUT. The tumor cells showed immunoreactivity (Fig. 1C-D) for CD56, CD99, and a high KI-67 labeling index. There was also retained nuclear expression of SMARCA4, SMARCA2 and INI1.
Tissue samples were sent for further genetic testing at CARIS Life Sciences, and a MXI1-NUTM2B gene fusion was detected by next generation sequencing with the Archer FusionPlex Solid Tumor panel (ArcherDX, Inc., Boulder, CO). Tumor cells were also positive for PLD-1, PR positive.
After completing a total of 6 cycles of her current chemotherapy regimen, the patient was subsequently referred for a second opinion and possible clinical trials. On recommendation of the consulting institution, the patient completed an additional two more cycles of Carboplatin, paclitaxel, and bevacizumab followed by repeat imaging that shows progressive disease with consideration being given to other treatment options including clinical trials (Fig. 1C, D). Per consulting institution, no BET inhibitor trials were available. Patient is now enrolled on a phase 1 clinical trial of “MDNA11, IL2 superkine with check-point inhibitor for advanced solid tumors.
At the time of this submission, the patient is 11 months from the time of her diagnosis and is alive with persistent disease.
3. Discussion
This case represents a novel finding of an MXI1-NUTM2B fusion in a poorly differentiated ovarian malignancy. This fusion has not been previously reported and is therefore the first documented case of such a fusion in ovarian cancer in our review. NUTM2B fusion with other genes has been reported in endometrial stromal sarcoma, clear cell sarcoma of the kidney, and other non-gynecologic malignancies.(Mariño-Enriquez et al., 2018, Kenny et al., 2016) In the case of a neonate with high-grade undifferentiated round-cell sarcoma of the gluteus, YWHAE-NUTM2B fusion gene was found in addition to BCOR positive immune-stained tumor cells. Overexpression of BCOR is thought to be the starting point of increased oncogenic potential, however the mechanisms are currently poorly understood. BCOR is thought to form a complex that affects gene transcription.(Guizard et al., 2019 Aug 8) These findings suggest that NUTM2B could be playing a significant role in tumorigenesis and varied tumor phenotypes. NUTM1 is present more often in midline cancers, which may highlight differences in NUTM1 versus NUTM2B genetic alterations.(Moreno et al., 2022) In our case, suspicion for ovarian primary cancer was high given the location of disease spread, and pattern of organ involvement.
The NUTM2B fusion partner, MXI1, is a member of the MAD gene family. It encodes a transcription factor that possesses a bHLH-Zip motif, similarly to that seen in members of the Myc protein family.(Zervos et al., 1993) This interaction moderates the activity of Myc through transcriptional inhibition, managing the growth of cells.(Zervos et al., 1993) Despite their typical role as MYC activity antagonists, MAD gene members like MXI1 may transform into MYC-like mimics by fusing with NUTM1.(McEvoy et al., 2021) This interaction may explain how NUTM1 fusions can progress into aggressive proliferative tumors. However, due to rarity of NUTM2B fusions with companion genes such as MXI1, the mechanism of this specific tumor development remains poorly understood. Further studies on how NUTM2B interacts with MXI1 are necessary to understand the evolution of this unusual tumor.
Clinical implications of the MXI1-NUTM2B fusion are yet to be elucidated. It is unclear whether this fusion follows the same clinical pattern as NUT carcinoma involving the NUTM1 gene. Patient is still alive 11 months after the initial diagnosis, with a continuously decreasing CA-125. This distinct fusion raises important questions about whether MXI1-NUTM2B represents a variant form of NUT carcinoma, or it is a completely unique type of poorly differentiated cancer.
The framework for therapies targeting NUT carcinomas is being further developed. Bromodomain Extra-Terminal (BET) inhibitors have been investigated for use in NUT carcinomas given the suspected epigenetic mechanisms in cancer gene overexpression. However, BET inhibitors are not yet FDA approved for use outside of clinical trials.(Piha-Paul et al., 2020).
This case emphasizes the importance of considering NUT carcinoma in the differential diagnosis of undifferentiated ovarian tumors by gynecologic oncologists, especially when conventional markers and histology do not yield definitive results. Utilizing advanced diagnostic tools such as NUT-specific immunohistochemistry and next-generation sequencing can facilitate accurate and timely diagnosis, enabling the exploration of targeted therapeutic strategies. Additionally, awareness of this entity underscores the need for multidisciplinary collaboration in managing such aggressive malignancies, including referral to clinical trials evaluating novel treatments like BET inhibitors.
Further research and additional case reports are essential to determine the prevalence and significance of the MXI1-NUTM2B fusion in ovarian and other malignancies. Comprehensive molecular characterization and functional studies will help clarify oncogenic mechanisms used. Continued documentation of such cases will be crucial in understanding whether this fusion follows the patterns observed in NUT carcinomas or if it delineates a new category of cancer with unique clinical and pathological features.
Financial Support.
No funding was provided to complete this work.
Relationship with Industry.
None of the authors have industry relationships related to this manuscript.
Declarations:
−Ethics approval and consent to participate: Not applicable.
−Consent for publication: Not applicable.
−Availability of data and material: Not applicable.
−Competing interests: Not applicable.
−Funding
No funding was received for this publication
−Authors' contributions: Mohammed Elshafey, MD: Writing introduction and case discussion, editing and reviewing; Malek Ghandour, MD: Writing case presentation, editing, and reviewing; Rebecca M. Adams, Medical Student: Contributing in writing introduction, editing, and reviewing; Daniel Neill, MD: Writing the pathological findings; Radhika Gogoi, MD: Review and editing.-Acknowledgements: Michael Petrich, MD: Providing radiology imaging.
CRediT authorship contribution statement
Mohammed Elshafey: Writing – review & editing, Writing – original draft. Malek Ghandour: Writing – review & editing. Rebecca M. Adams: Writing – review & editing. Daniel Neill: Writing – review & editing. Radhika Gogoi: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2024.101653.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ball A., Bromley A., Glaze S., French C.A., Ghatage P., Köbel M. A rare case of NUT midline carcinoma. Gynecol Oncol Case Rep. 2012 Oct;8(3):1–3. doi: 10.1016/j.gynor.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagen K.P., French C.A. Supercharging BRD4 with NUT in carcinoma. Oncogene. 2021;40(8):1396–1408. doi: 10.1038/s41388-020-01625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizard M., Karanian M., Dijoud F., Bouhamama A., Faure-Conter C., Hameury F., Tirode F., Corradini N. Neonatal Soft Tissue Sarcoma with YWHAE-NUTM2B Fusion. Case Rep Oncol. 2019 Aug 8;12(2):631–638. doi: 10.1159/000502227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang C, Hou Z, Wang Y, Qiao J, Li H. Case report: NUT carcinoma with MXI1::NUTM1 fusion characterized by abdominopelvic lesions and ovarian masses in a middle-aged female. Front Oncol. 2023 Jan 11;12:1091877. doi: 10.3389/fonc.2022.1091877. Erratum in: Front Oncol. 2023 Feb 17;13:1161821. doi: 10.3389/fonc.2023.1161821. PMID: 36741693; PMCID: PMC9890191. [DOI] [PMC free article] [PubMed]
- Jung M., Kim S.I., Kim J.W., Jeon Y.K., Lee C. NUT carcinoma in the pelvic cavity with unusual pathologic features. Int J Gynecol Pathol. 2022;41(3):292–297. doi: 10.1097/PGP.0000000000000801. [DOI] [PubMed] [Google Scholar]
- Kenny C., Bausenwein S., Lazaro A., et al. Mutually exclusive BCOR internal tandem duplications and YWHAE-NUTM2 fusions in clear cell sarcoma of kidney: not the full story. J Pathol. 2016;238(5):617–620. doi: 10.1002/path.4693. [PubMed: 27000436] [DOI] [PubMed] [Google Scholar]
- Li X., Shi H., Zhang W., et al. Immunotherapy and Targeting the Tumor Microenvironment: Current Place and New Insights in Primary Pulmonary NUT Carcinoma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.690115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño-Enriquez A., Lauria A., Przybyl J., et al. BCOR Internal Tandem Duplication in High-grade Uterine Sarcomas. Am J Surg Pathol. 2018;42(3):335–341. doi: 10.1097/PAS.0000000000000993. [PubMed: 29200103] [DOI] [PubMed] [Google Scholar]
- McEvoy C.R., Holliday H., Thio N., et al. A MXI1-NUTM1 fusion protein with MYC-like activity suggests a novel oncogenic mechanism in a subset of NUTM1-rearranged tumors. Lab Invest. 2021;101(1):26–37. doi: 10.1038/s41374-020-00484-3. [DOI] [PubMed] [Google Scholar]
- Mengoli M.C., Longo F.R., Fraggetta F., Cavazza A., Dubini A., Alì G., Guddo F., Gilioli E., Bogina G., Nannini N., Barbisan F., De Rosa N., Falconieri G., Rossi G., Graziano P. The 2015 World Health Organization Classification of lung tumors: new entities since the 2004 Classification. Pathologica. 2018;110(1):39–67. [PubMed] [Google Scholar]
- Moreno V., Saluja K., Pina-Oviedo S. NUT Carcinoma: Clinicopathologic Features, Molecular Genetics and Epigenetics. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.860830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piha-Paul S.A., Hann C.L., French C.A., et al. Phase 1 Study of Molibresib (GSK525762), a Bromodomain and Extra-Terminal Domain Protein Inhibitor, in NUT Carcinoma and Other Solid Tumors. JNCI Cancer Spectr. 2020;4 doi: 10.1093/jncics/pkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R., Nakaoka H., Yoshihara K., Mori Y., Yachida N., Nishikawa N., Motoyama T., Okuda S., Inoue I., Enomoto T. Novel MXD4-NUTM1 fusion transcript identified in primary ovarian undifferentiated small round cell sarcoma. Genes Chromosomes Cancer. 2018 Nov;57(11):557–563. doi: 10.1002/gcc.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Shen D., Shi J. Primary renal NUT carcinoma identified by next-generation sequencing: a case report and literature review. Int J Clin Exp Pathol. 2021;14(5):662. [PMC free article] [PubMed] [Google Scholar]
- Zervos A.S., Gyuris J., Brent R. Mxi1, a protein that specifically interacts with max to bind myc-max recognition sites. Cell. 1993;72(2):223–232. doi: 10.1016/0092-8674(93)90662-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.