Summary
Introduction
Hospital-acquired infections (HAIs) present a global public health challenge, impacting patient safety and incurring substantial economic costs across healthcare settings. This study aims to accurately measure the financial burden of HAIs by analyzing real costs associated with various infections, providing insights for targeted prevention and management strategies.
Methods
This retrospective cohort study at a university hospital in Rome, Italy, analysed Hospital Discharge Records (HDR) from January to December 2018, focusing on patients with and without HAIs. The study employed ICD-9-CM codes, microbiology databases, and stratified analyses by infection site and microorganism. Cost increments were calculated using DRG reimbursement data. Propensity score matching compared infected patients with matched non-infected counterparts, simulating a randomized trial through two models: one adjusting for length of stay and mortality (less conservative), and one not using these factors as confounders (more conservative).
Results
In the study of 12,033 patients at Policlinico Universitario Tor Vergata, 10.07% developed an HAI, significantly raising mean DRG by 53.4% (€3,744 to €5,744). Propensity score analysis showed HAIs elevated costs by €4,695 (60.45%) in one model, and by €3,335 (31.15%) in another. Specific microbes and infection sites further influenced the cost impact, highlighting the need for targeted HAI prevention strategies.
Conclusion
Our study reveals the significant economic impact of hospital-acquired infections (HAIs), with a substantial increase in costs linked to specific microorganisms and infection sites. These findings highlight the need for effective HAI prevention strategies to enhance patient safety and reduce healthcare expenditures.
Keywords: Healthcare-associated infections (HAIs), Economic burden, Propensity score analysis, Hospitalization costs, Infection prevention
Introduction
Hospital-acquired infections (HAIs) represent a public health challenge globally, significantly impacting patient safety and healthcare systems. The World Health Organization (WHO) acknowledges their widespread occurrence, notably in high-risk areas such as intensive care units, with a more pronounced effect in low- and middle-income countries [1]. This situation calls for focused efforts to address the consequences for patient health and healthcare infrastructure.
The economic implications of HAIs are considerable, primarily due to extended hospital stays, the necessity for additional treatments, and the complexity of managing infections, especially those caused by antibiotic-resistant bacteria [2,3]. Studies within the United States highlight the significant economic burden HAIs impose, with estimates for 2016 ranging from $7.2 to $14.9 billion, primarily driven by Clostridioides difficile infections and surgical site infections, which together account for 79% of the total HAI-associated costs. [4,5].
Further research within the Central Texas Veterans Health Care System demonstrates the profound financial impact of HAIs, revealing substantial additional costs incurred before patient discharge. Notably, central line-associated bloodstream infections and catheter-associated urinary tract infections were identified as significant contributors to the financial strain. This study showcased that HAIs were associated with an extra pre-discharge expense of $29,412, and a 46.3% increase in the likelihood of post-discharge readmission, leading to additional post-discharge costs of $16,049 [6]. Roberts et al. identified that the costs attributable to HAIs ranged from $9,310 to $21,013, with variable costs between $1,581 and $6,824. Additionally, they noted that hospital stay length for patients with HAIs extended from 5.9 to 9.6 days. [7].
However, the literature presents limitations, such as small cohort sizes and a focus on specific HAIs, which may limit the generalisability of findings [8,9]. Moreover, many studies rely on cost estimates derived from national systems, which may not accurately capture the economic impact of each specific infection [4,5]. Additionally, certain cost evaluations do not account for the expenditures borne directly by healthcare facilities, potentially leading to inaccurate financial analyses. [7,10]. Research extending beyond hospital settings adds another layer of complexity, as the economic impact of HAIs in such environments can significantly differ from those within hospitals [11,12].
Acknowledging the critical nature of this issue [[13], [14], [15]], this study aims to bridge these gaps by providing a detailed assessment of the actual financial cost associated with HAIs, differentiating between various microorganisms and infection sites. Utilising real costs reported by the third-party payer and incorporating data from Hospital Discharge Records (HDR), this approach seeks to offer a more accurate depiction of the financial burden HAIs impose, thereby enriching the existing body of research.
By identifying and focusing on the most economically impactful infections, healthcare managers can allocate resources more efficiently and enhance overall patient care quality while simultaneously reducing unnecessary healthcare expenditures [16,17].
Methods
The study was conducted at the Tor Vergata University Hospital of Rome, Lazio, Italy. The data collection period spanned from January to December 2018 and forms part of a broader work focusing on the incidence of HAIs and associated factors published elsewhere [18,19]. Data pertaining to Hospital Discharge Records (HDR, in Italian Schede di Dimissione Ospedaliera, SDO) were retrieved from the Hospital Information System. The AREAS-ADT information system was utilised for data compilation, employing the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for diagnostic and procedural information. The ICD-9-CM is currently used in Italy for the Diagnosis Related Groups (DRG) system, which is the standard classification system to categorise hospital cases for billing and reimbursement purposes. Additionally, data concerning HAI were gathered from the database of the Complex Operational Unit of Microbiology.
The study design was a retrospective cohort study. HDRs of all patients admitted or discharged within the timeframe of January 1 to December 31, 2018, were included. To mitigate potential data distortion from prolonged hospital stay, which is often associated with an increased number of medical interventions and a correspondingly higher risk of acquiring HAIs, patients with hospital stays exceeding 60 days were excluded from the analysis.
Furthermore, patients whose HDR lacked information on the Diagnosis-Related Group (DRG) reimbursed by the third-party payer (in this case, the Lazio region, as per the Italian healthcare system) were also excluded.
The primary outcome of this study was the hospitalisation costs, measured using a top-down approach based on the reimbursement to the hospital by the third-party payer. This approach meant that, to evaluate the costs of a healthcare service such as hospitalisation, we did not calculate the value of each resource used by measuring its average quantity and price (bottom-up or ingredients method). Instead, we relied on the average cost of the service, which is based on a tariff that reflects the average cost. For hospitals, the tariff used is that of the DRG. Specifically, we utilised the DRGs value established by the Italian Ministry of Health determined by the hospital utilising version 24.0 of the Medicare DRGs, which in Italy is employed to specify the remuneration unit for acute hospital care provided by both public and accredited private hospitals within the National Health Service [20].
The study design incorporates a two-phase analytical approach. Initially, the presence of an HAI is considered as the dependent variable, exploring its relationship with a broad spectrum of variables, including patient demographics and other relevant factors. This initial phase serves to establish basic associations and patterns indicating the factors mostly associated with higher risk of contracting an HAI. Subsequently, the presence of HAI is considered as the principal independent variable in a propensity score matching analysis. This second phase is designed to evaluate the implications of HAIs on healthcare costs. Infections were considered based on laboratory reports for each patient, regardless of whether an infection diagnosis was present in the HDR. An infection was classified as an HAI only if it was detected through a test conducted at least 48 hours after hospital admission, adhering to standard criteria for distinguishing HAIs from community-acquired infections.
To comprehensively assess the economic impact of HAIs, the study employed a stratification of the collected data, considering the site where the sample tested has been collected (rectal, urinary, or respiratory tract, bloodstream and wound) and the type of microorganism detected. The microorganisms included in the analysis are those highlighted in Italy's National Plan to Combat Antibiotic Resistance including: Acinetobacter, Klebsiella, Clostridium, Enterococcus, Escherichia coli, Pseudomonas, Candida, and Staphylococcus infections [21].
Several other variables were included as potential confounders to ensure a robust examination of the primary outcome. These variables encompassed demographic factors, admission details, and clinical interventions, specifically: Sex, Age, Admission Modality (Categorised into scheduled, scheduled with pre-hospitalization, and urgent), Length of Stay (LOS), Intervention Category (Distinguished between medical and surgical interventions), Mortality Outcome, Education Level and Occupational Status. We also considered as possible confounders to be admitted in a Department at Risk of HAI and having received an Invasive Procedure at Risk of HAI. Departments at risk of HAI have been identified through univariate logistic regression analysis, comparing the infection risk across different hospital departments. Departments were considered at higher risk if they showed an Odds Ratio (OR) greater than 1 relative to the department with an infection rate closest to the overall sample's infection rate. Invasive Procedures at Risk of HAI were evaluated for their risk using logistic regression. Procedures were deemed high-risk if they had an OR greater than 1, were statistically significant when comparing infected patients against the non-infected, and after adjusting for age, education level, admission modality, type of intervention, and department at risk.
Statistical analysis
The statistical analysis included a comprehensive descriptive examination of the entire sample. For categorical variables, the number of observations and the proportion for each category level were calculated. Continuous variables were summarized using median values and interquartile ranges (IQR). Statistical significance of differences between infected and non-infected groups in terms of medians and proportions was assessed using Pearson's Chi-squared test for categorical variables, the Wilcoxon rank-sum test for continuous variables, and Fisher's exact test when appropriate.
Following the univariate analysis concerning infection risk, all variables identified as potential confounders were assessed for inclusion in the multivariable logistic regression to calculate propensity scores. Variables associated with an increased risk of infection, indicated by an Odds Ratio (OR) greater than 1 and a P-value less than 0.005, were included. Those presenting an indirect association were excluded. This association was attributed to clinical-related factors: the positive association between being disable/retired and the presence of HAI was interpreted as an indirect effect, primarily due to age, since being retired is inherently linked to older age.
Both mortality and LOS, which are associated with infection risk and increased costs, were subjected to clinical reasoning regarding their causal relationship with HAIs. a While HAIs can lead to increased mortality and prolonged LOS due to the complications and additional treatments required, reverse causality is also possible. To address this complexity, two models were proposed: Model 1) mortality and LOS were treated as mediators of the cost increase following an HAI, implying that their elevation is a consequence rather than a cause of an HAI. Therefore, they were not included as confounders in the propensity score calculation; Model 2) the underling patient frailty, which may manifest as increased mortality and LOS, was considered as a risk factor for HAIs, reflecting a predisposition both to get infected, and to risk of dying or to have a longer period of admission, and thus included as confounder for the calculation of propensity scores.
To balance covariates between the two study groups, a 1:1 propensity score matching analysis was conducted, with ties set to true and replacement to false. The impact of HAIs on cost increase was estimated using the Average Treatment Effect on the Treated (ATT). Estimates were reported in absolute terms (increase in Euros) and percentage terms by using as dependent variable the transformed logarithmic value of the cost in EUR. This analysis was further stratified by the site where the sample has been collected and by microorganism to delineate the cost implications associated with specific HAIs.
In the propensity score matching, especially in the stratified analysis by microorganism and by site where the number of infected patients was small and the number of non-infected patients was large, each execution of the model with statistical software yielded slightly different results due to the random selection of control matches. To ensure reproducibility, a fixed seed (set.seed(123)) was used in the statistical software. A sensitivity analysis on 100 iterations with different seed settings randomly assigned was subsequently performed to test the robustness of the model and the reliability of the conclusions drawn from the analysis.
The statistical analysis was conducted using RStudio software, version 4.3.1. For data cleaning and preparation, the tidyverse package was employed [22], for the regressions and to report the results we employed the gtsummary package [23], the Matching package was used to perform propensity score matching analysis [24].
Ethics
Our study adhered to the principles outlined in the Declaration of Helsinki. Approval for the research was granted by the Independent Ethics Committee of the University Hospital PTV in Rome, Italy, with the identifier 66.22 on April 1st, 2022. The research protocol included an exemption from requiring informed consent. To ensure confidentiality, all participant data were made anonymous before being analysed. This involved securely inputting the data into a password-protected Excel spreadsheet, which was only accessible to members of the research team involved in the study.
Results
The initial dataset of HDRs of patients admitted to the Tor Vergata University Hospital of Rome, comprised 12,218 individuals. Following the exclusion criteria, 185 patients (1.5% of total sample) with hospital stays exceeding 60 days and those with incomplete DRG information in their HDRs were removed from the analysis. This resulted in a final cohort of 12,033 subjects eligible for inclusion. Within this cohort, 1,212 patients, accounting for 10.1% of the total, were identified as having developed a healthcare-associated infection (HAI) during their hospital stay. This incidence underscores the significant challenge posed by HAIs within the hospital setting and highlights the necessity of targeted interventions to mitigate their occurrence and impact.
The descriptive analysis and univariate logistic regression, as depicted in Table I, elucidated the impact of healthcare-associated infections (HAIs) on hospitalization costs and identified several variables statistically associated with an increased risk of HAIs. The presence of HAIs was associated with a significant increment in hospital costs, from 3,744 to 5,744 Euros (+53.4%). Age emerged as a significant factor, with each additional year increasing the likelihood of acquiring an HAI (OR 1.02, 95% CI 1.02–1.03; P < 0.001). Among occupational statuses, retirees and individuals with disabilities were found to be at a higher risk (OR 1.69; 95% CI 1.49–1.93; P< 0.001). Urgent admission mode was strongly associated with HAIs (OR 2.17; 95% CI 1.84–2.58; P < 0.001), as was an extended length of stay (OR 1.16; 95% CI 1.15–1.17; P < 0.001).
Table I.
Descriptive statistics and univariate regression
| Characteristic | Descriptive statistics |
Univariate regression |
|||
|---|---|---|---|---|---|
| Non-infected, N = 10,821a | Infected, N = 1,212a | ORb | 95% CIb | P-value | |
| Real reimbursement (DRG) | 3,744 (2,142, 6,920) | 5,744 (3,800, 13,712) | 1.00 | 1.00, 1.00 | <0.001 |
| Log Real Reimbursement | 8.23 (7.67, 8.84) | 8.66 (8.24, 9.53) | 2.17 | 2.03, 2.33 | <0.001 |
| Sex | |||||
| Female | 5,982 (55%) | 671 (55%) | — | — | |
| Male | 4,839 (45%) | 541 (45%) | 1.00 | 0.88, 1.12 | >0.9 |
| Age | 65 (52, 76) | 71 (59, 81) | 1.02 | 1.02, 1.03 | <0.001 |
| Nationality | |||||
| Italian | 9,923 (92%) | 1,132 (93%) | — | — | |
| Non-Italian | 898 (8.3%) | 80 (6.6%) | 0.78 | 0.61, 0.98 | 0.041 |
| Education level | |||||
| Primary school | 1,389 (13%) | 175 (14%) | — | — | |
| Diploma | 6,778 (63%) | 855 (71%) | 1.00 | 0.84, 1.19 | >0.9 |
| University degree or higher | 2,654 (25%) | 182 (15%) | 0.54 | 0.44, 0.68 | <0.001 |
| Occupational status | |||||
| Employed/Student/Housewife | 4,513 (42%) | 365 (30%) | — | — | |
| Retired/Disable | 5,851 (54%) | 800 (66%) | 1.69 | 1.49, 1.93 | <0.001 |
| Unemployed | 457 (4.2%) | 47 (3.9%) | 1.27 | 0.91, 1.73 | 0.14 |
| Admission modality | |||||
| Scheduled | 2,389 (22%) | 166 (14%) | — | — | |
| Scheduled with prehospitalisation | 1,538 (14%) | 6 (0.5%) | 0.06 | 0.02, 0.12 | <0.001 |
| Urgent | 6,894 (64%) | 1,040 (86%) | 2.17 | 1.84, 2.58 | <0.001 |
| Days of stay | 4 (2, 8) | 22 (14, 33) | 1.16 | 1.15, 1.17 | <0.001 |
| Intervention category | |||||
| Medical | 4,974 (46%) | 731 (60%) | — | — | |
| Surgical | 5,847 (54%) | 481 (40%) | 0.56 | 0.50, 0.63 | <0.001 |
| Mortality outcome | |||||
| Survived | 10,473 (97%) | 966 (80%) | — | — | |
| Deceased | 348 (3.2%) | 246 (20%) | 7.66 | 6.42, 9.13 | <0.001 |
| Department | |||||
| Gastroenterology | 672 (6.2%) | 71 (5.9%) | — | — | |
| Cardiac Surgery | 285 (2.6%) | 53 (4.4%) | 1.76 | 1.20, 2.57 | 0.004 |
| Cardiology | 1,219 (11%) | 17 (1.4%) | 0.13 | 0.07, 0.22 | <0.001 |
| Coronary Care Unit | 671 (6.2%) | 17 (1.4%) | 0.24 | 0.14, 0.40 | <0.001 |
| Emergency Medicine | 342 (3.2%) | 164 (14%) | 4.54 | 3.35, 6.20 | <0.001 |
| Endocrinology and Diabetology | 112 (1.0%) | 81 (6.7%) | 6.85 | 4.71, 10.0 | <0.001 |
| Gynaecology | 246 (2.3%) | 1 (<0.1%) | 0.04 | 0.00, 0.18 | 0.001 |
| Infectious Diseases | 146 (1.3%) | 55 (4.5%) | 3.57 | 2.40, 5.29 | <0.001 |
| Intensive Care Unit | 63 (0.6%) | 49 (4.0%) | 7.36 | 4.71, 11.5 | <0.001 |
| Internal Medicine | 630 (5.8%) | 179 (15%) | 2.69 | 2.01, 3.63 | <0.001 |
| Lymphoproliferative Disorders | 105 (1.0%) | 106 (8.7%) | 9.55 | 6.66, 13.8 | <0.001 |
| Maxillofacial Surgery | 197 (1.8%) | 1 (<0.1%) | 0.05 | 0.00, 0.22 | 0.003 |
| Neurology | 1,455 (13%) | 108 (8.9%) | 0.70 | 0.51, 0.96 | 0.027 |
| Neurosurgery | 375 (3.5%) | 32 (2.6%) | 0.81 | 0.52, 1.24 | 0.3 |
| Oncology | 127 (1.2%) | 9 (0.7%) | 0.67 | 0.31, 1.31 | 0.3 |
| Ophthalmology | 88 (0.8%) | 0 (0%) | 0.00 | 0.00, 0.00 | >0.9 |
| Orthopaedics | 1,096 (10%) | 21 (1.7%) | 0.18 | 0.11, 0.29 | <0.001 |
| Otorhinolaryngology | 242 (2.2%) | 1 (<0.1%) | 0.04 | 0.00, 0.18 | 0.001 |
| Psychiatry and Clinical Psychology | 266 (2.5%) | 7 (0.6%) | 0.25 | 0.10, 0.51 | <0.001 |
| Respiratory Diseases | 250 (2.3%) | 74 (6.1%) | 2.80 | 1.96, 4.01 | <0.001 |
| Rheumatology | 42 (0.4%) | 4 (0.3%) | 0.90 | 0.27, 2.31 | 0.8 |
| Surgery | 1,374 (13%) | 120 (9.9%) | 0.83 | 0.61, 1.13 | 0.2 |
| Thoracic Surgery | 235 (2.2%) | 10 (0.8%) | 0.40 | 0.19, 0.76 | 0.009 |
| Urology | 339 (3.1%) | 23 (1.9%) | 0.64 | 0.39, 1.03 | 0.075 |
| Vascular Surgery | 244 (2.3%) | 9 (0.7%) | 0.35 | 0.16, 0.67 | 0.004 |
| Department at risk of HAI | |||||
| Low-risk-department | 8,888 (82%) | 451 (37%) | — | — | |
| High-risk-department | 1,933 (18%) | 761 (63%) | 7.76 | 6.84, 8.81 | <0.001 |
| Procedure at risk of HAI | 159 (1.5%) | 74 (6.1%) | |||
| FALSE | — | — | |||
| TRUE | 4.36 | 3.27, 5.76 | <0.001 | ||
Median (IQR); n (%).
OR = Odds Ratio, CI = Confidence Interval.
The departments of Cardiac Surgery, Emergency Medicine, Endocrinology and Diabetology, Infectious Diseases, Intensive Care Unit, Internal Medicine and Respiratory Diseases were found to have a markedly increased risk of HAIs compared with the department of Gastroenterology were the proportion of infected was similar to the average of the entire hospital. The overall OR associated with being admitted in one of these departments was 7.76 (95% CI 6.84–8.81; P < 0.001). Furthermore, the OR of in HAIs risk associated with undergoing high risk-invasive procedures was also correlated with an increased risk of infection was 4.36 (95% CI 3.27–5.76; P < 0.001).
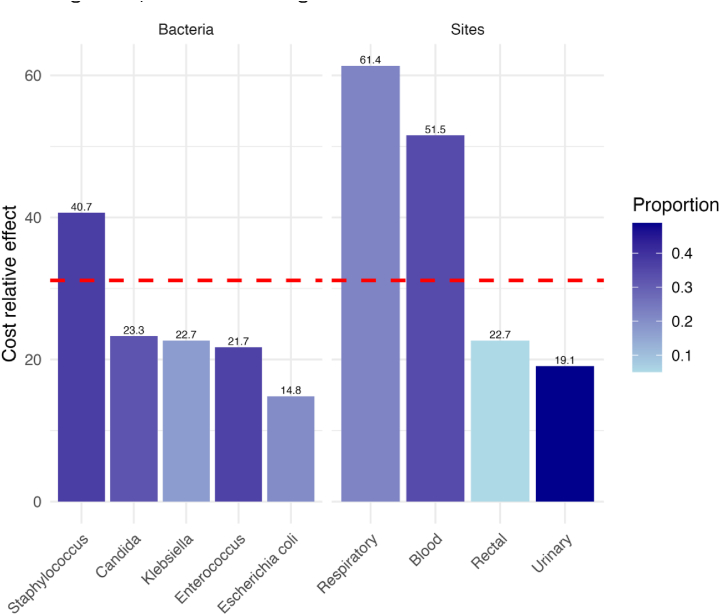
Having established the foundational relationship between HAIs and various risk factors through descriptive statistics and univariate logistic regression, we further explored these associations using a propensity score analysis to refine our understanding of the economic impact attributable to HAIs using the two distinct models, with results stratified by individual microorganisms and infection sites (Table II). The first model, which did not adjust for mortality and LOS as confounders, revealed a substantial cost increase associated with HAIs. The overall infection was linked to a significant rise in actual costs (estimated increase of €4,695, 95% CI 3,823-5,567, P <0.001) and an adjusted percentage increase of 60.45% (95% CI 49.76–71.91, P <0.001). Each bacterium and collection site demonstrated a significant upsurge in both actual and adjusted percentage costs, indicating the broad financial implications of HAIs. The second more conservative model incorporated mortality and LOS as confounders yielded a lower, yet significant, overall cost increase associated with HAIs (estimated increase of €3,335, 95% CI 2,386-4,284, P <0.001) and an adjusted percentage increase of 31.15% (95% CI 22.25–40.69, P <0.001). Notably, certain microorganisms were statistically linked to cost increases, including Klebsiella (real cost increase estimate of €4,504, 95% CI 1,497-7,510, P <0.01), Enterococcus (real cost increase estimate of €2,850, 95% CI 1,216-4,484, P <0.01), and Staphylococcus (real cost increase estimate of €4,660, 95% CI 2,906-6,414, P <0.001), among others. Specific collection sites such as bloodstream and respiratory tract were also associated with significant cost increases, illustrating the nuanced economic impact of HAIs depending on the type and location of infection. Figure 1 illustrates the relative difference in the cost increase associated with each site and microorganism, calculated using the more conservative Model 2.
Table II.
Results of propensity score by bacteria and collection site (Model 1 and 2)
| Microorganism and collection site | Number of infections | Proportion of infections | Cost effect (C.I – P-value) | Cost effect % (C.I – P-value) | |
|---|---|---|---|---|---|
| Model 1 - Not matched by Mortality and Days of stay | |||||
| Overall | 1212 | 4,695 (3,823- 5,567, P<0.001) | 60.45 (49.76–71.91, P<0.001) | ||
| Microorganism | Acinetobacter | 93 | 7.7% | 6,291 (2,887- 9,694, P=0.001) | 76.4 (38.95-123.95, P=0.001) |
| Klebsiella | 207 | 17.1% | 7,251 (4,939- 9,562, P<0.001) | 86.66 (56.72-122.33, P <0.001) | |
| Clostridium | 27 | 2.2% | 5,431 (-78-10,941, P=0.037) | 30.96 (-18.28-109.87, P=0.037) | |
| Enterococcus | 441 | 36.4% | 5,288 (3,784- 6,793, P<0.001) | 62.27 (44.76-81.9, P<0.001) | |
| Escherichia coli | 247 | 20.4% | 4,861 (2,851- 6,872, P<0.001) | 53.62 (28.57-83.56, P<0.001) | |
| Pseudomonas | 140 | 11.6% | 5,907 (3,448- 8,367, P<0.001) | 74.69 (50.7-102.5, P<0.001) | |
| Candida | 392 | 32.3% | 4,283 (3,018- 5,548, P<0.001) | 66.36 (50.14-84.32, P<0.001) | |
| Staphylococcus | 454 | 37.5% | 6,250 (4,950- 7,550, P<0.001) | 75.28 (60.21-91.77, P<0.001) | |
| Sites | Bloodstream | 417 | 34.4% | 9,507 (7,905-11,108, P<0.001) | 113.87 (93.14-136.82, P<0.001) |
| Urinary tract | 592 | 48.8% | 3,267 (2,351- 4,184, P <0.001) | 55.04 (42.11-69.14, P<0.001) | |
| Respiratory tract | 267 | 22.0% | 7,284 (5,332- 9,236, P<0.001) | 91.47 (69.35-116.47, P<0.001) | |
| Wound | 170 | 14.0% | 6,804 (4,782- 8,826, P <0.001) | 82.65 (56.58-113.06, P<0.001) | |
| Rectal tract | 62 | 5.1% | 7,258 (3,034-11,482, P <0.001) | 82.05 (0.31-143.52, P<0.001) | |
| Model 2 - Matched by Mortality and Days of stay | |||||
| Overall | 1212 | 3,335 (2,386-4,284, P<0.001) | 31.15 (22.25-40.69, P<0.001) | ||
| Microorganism | Acinetobacter | 93 | 7.7% | 2,909 (-824-6,641, P=0.07) | 6.35 (-15.84-34.4, P=0.39) |
| Klebsiella | 207 | 17.1% | 4,504 (1,497-7,510, P<0.01) | 22.67 (2.7-46.53, P<0.01) | |
| Clostridium | 27 | 2.2% | -468 (-8,688-7,752, P=0.86) | 4.48 (-33.4-63.9, P=0.73) | |
| Enterococcus | 441 | 36.4% | 2,850 (1,216-4,484, P<0.01) | 21.73 (8.24-36.9, P<0.01) | |
| Escherichia coli | 247 | 20.4% | 1,642 (-564-3,849, P =0.056) | 14.84 (-0.91-33.09, P<0.01) | |
| Pseudomonas | 140 | 11.6% | 2,832 (-18-5,682, P=0.06) | 23.96 (2.74-49.57, P <0.05) | |
| Candida | 392 | 32.3% | 2,513 (886-4,141, P <0.05) | 23.29 (9.36-39, P <0.01) | |
| Staphylococcus | 454 | 37.5% | 4,660 (2,906-6,414, P <0.001) | 40.66 (25.19-58.04, P <0.001) | |
| Sites | Bloodstream | 417 | 34.4% | 7,146 (5,040-9,253, P <0.001) | 51.55 (32.82-72.93, P <0.001) |
| Urinary tract | 592 | 48.8% | 1,400 (232-2,567, P <0.01) | 19.09 (8.62-30.57, P <0.01) | |
| Respiratory tract | 267 | 22.0% | 6,028 (3,603-8,454, P <0.001) | 61.35 (37.67-89.1, P <0.001) | |
| Wound | 170 | 14.0% | 2,552 (-180-5,284, P <0.05) | 7.87 (-9.38-28.39, P=0.067) | |
| Rectal tract | 62 | 5.1% | 4,044 (-527-8,615, P =0.10) | 22.67 (-0.06-60.55, P= 0.038) | |
Figure 1.
Relative difference in the cost increase associated with each site and microorganism, calculated using the more conservative Model 2.
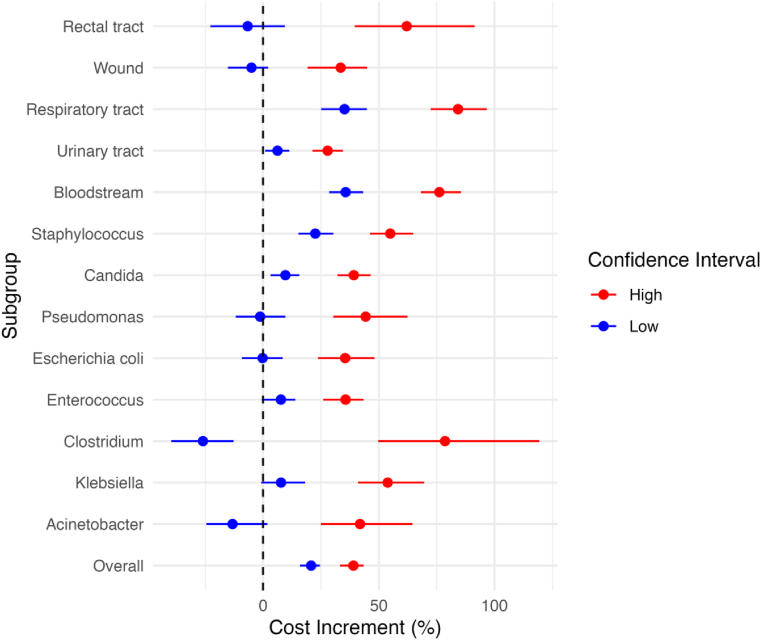
Figure 2 presents the outcomes of a univariate sensitivity analysis conducted to assess the robustness of the cost increase estimates from Model 2 against variations in the seed used for propensity score matching. This graph showcases the range of minimum and maximum values, along with the average of the upper and lower confidence interval margins derived from the propensity score analysis. Notably, rectal samples, despite being statistically associated with HAIs, are the only infection site where the minimum-maximum range of the lower confidence interval margin includes zero. This indicates that, depending on the seed variation, the association between infection in rectal tract and cost increases could be non-significant, underscoring the importance of considering seed variability in the propensity score matching process to ensure the reliability of the findings.
Figure 2.
Cost increment variations by microorganism and collection site.
Discussion
Our study has identified a significant increase in costs associated with HAIs, evident in both monetary terms and proportional values, evaluating the difference of cost in the propensity score using transformed in logarithmic values, providing a robust indicator applicable across various settings. The cost analysis was conducted using two models and both confirmed a substantial increase in costs linked to HAIs. Our findings are consistent with other studies that have demonstrated a rise in costs associated with HAIs [[5], [6], [7],16].
The study by Roberts et al. observes a broad variation in the total costs attributable to patients affected by Healthcare Associated Infections (HAIs), with a range between $11,299 and $21,013 and a significant attributable mortality rate of 6.1% [7]. Like in study, it adopts a design based on patients/admissions as the unit of analysis but considers the increase in costs as a proxy for the increase in hospital stay days, assuming a fixed daily cost of $2,015 USD, which is higher than the average cost in Italy. This difference could explain the extra cost of $19,344, higher than that estimated in our study. Nelson's research examines a smaller population than ours over a longer observation period and, unlike our study, further details the impact of HAIs, revealing a 46.3% increase in the likelihood of readmission along with significant additional costs, illustrating the influence of HAIs even after patient discharge. However, his analysis focuses on overall hospital expenses without providing per-admission costs [6]. Forrester's work estimates the overall costs of HAIs in the United States to be between $7.2 and $14.9 billion in 2016, identifying infections by Clostridioides difficile and surgical site infections as the most costly and prevalent, accounting for 79% of all infections [5]. Similar to our study, Forrester uses the ICD-9-CM system to select patients with HAIs from the admission registry, but, unlike our approach, does not use laboratory data confirmation to support an HAI diagnosis.
In general, our study distinguishes itself from existing literature by providing a cost per individual Healthcare Associated Infection (HAI) rather than an estimate of total costs. Moreover, by basing cost calculations on reimbursements determined through the DRG system, our study presents the actual expenditure per patient from the perspective of the third-party payer (the region). Finally, the identification of patients with HAIs was conducted by integrating data from the HDR, ensuring a comprehensive approach to recognizing and analyzing the incidence and economic impact of HAIs within the hospital setting.
Among the limitations of our study, it is crucial to acknowledge that not all HAIs were coded in the HDR dataset, which may have led to an underestimation of the calculated and reimbursed DRG and, consequently, might have resulted in an underestimated outcome of our analysis. As estimated in a previous paper on this study, approximately 30% of the infections identified through laboratory tests were not coded in the HDRs [18]. Furthermore, laboratory testing to confirm the suspicion of an HAI is performed only in the presence of symptoms, not as a screening measure. Consequently, some cases might not have been included in the study, leading to an underestimation of the prevalence of HAIs and consequent cost increase.
An additional aspect that partially limits our study is the use of the actual reimbursed DRGs as a measure of costs. While this method offers an indication of the actual increase in expenditure, it does not allow for the estimation of the real cost in terms of additional resources used, such as medical consultations, medications, and outpatient services, due to the absence of a bottom-up analysis, as was performed in other studies [4]. However, this method provides a realistic figure useful for healthcare planning purposes, namely the actual expenditure reimbursed by the third-party payer.
An inherent limitation of our study's design is the absence of randomization, which could result in the presence of confounders not included in the dataset, potentially influencing the outcomes. However, the use of propensity score analysis helps to mitigate this limitation, at least regarding the confounders available. The propensity score analysis is a strength of our work, as it allows for the creation of patient groups with the same level of susceptibility to HAIs, thereby facilitating a more accurate comparison between those who did and did not develop HAIs [25].
Finally, it should be noted that the data used in our study dates to 2018, which may limit their current relevance. However, this limitation might also reduce the bias associated with the effects of COVID-19, allowing us to analyse HAIs in a context less influenced by the pandemic. The analysis of the entire sample of one year's worth of hospital admissions has enabled us to eliminate a selection bias. Another strength of our study is that the HAIs included in the sample are all confirmed by laboratory analysis, in accordance with the guidelines of the European Centre for Disease Prevention and Control (ECDC).
This study's findings on the economic burden of HAIs at Policlinico Universitario Tor Vergata in Rome underscore critical public health implications. Firstly, the substantial costs associated with HAIs highlight the need for improved infection control measures, which can significantly enhance patient safety and reduce hospital expenditures. Secondly, the study emphasizes the importance of addressing antimicrobial resistance by reducing the incidence of HAIs, thereby preserving the effectiveness of existing antibiotics. Furthermore, the findings advocate for the implementation of robust HAI surveillance and reporting systems to inform targeted interventions and public health policies. Lastly, by demonstrating the potential for considerable cost savings through effective HAI prevention strategies, this study provides an economic rationale for healthcare managers and policy makers to invest in infection control practices.
Conclusions
Our research has demonstrated the substantial financial burden of HAIs. These infections have far-reaching consequences, impacting not only the healthcare system but also the lives of individuals and families. Therefore, it is imperative to implement economically advantageous prevention strategies, enhance infection prevention measures, develop and review policies, and strengthen surveillance systems to reduce expenses and improve the health and well-being of our patients. In this regard, further studies on cost-reduction interventions are essential to mitigate the impact of HAIs and ensure a healthier and more economically viable healthcare system for all. This study underscores the critical importance of comprehensive HAI prevention strategies, not only as a measure of improving patient care but also as a significant factor in controlling healthcare costs, highlighting the dual benefit of enhancing patient outcomes while achieving economic efficiency within the healthcare sector.
Conflict of interest statement
The authors declare no conflict of interest related to the study. This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
This study's dataset and the R code (version 4.3.2) utilised for data preparation, analysis, and the generation of publication-ready tables are available in the designated repository.
While the code is thoroughly commented to facilitate understanding, inquiries can be directed to stefano.orlando@uniroma2.it for further clarification.
For direct access to the dataset and code, refer to the following DOI: https://doi.org/10.17605/OSF.IO/MTQVX.
Credit author contributions
O.S. contributed to the conceptualization, methodology, formal analysis, and writing of the original draft. M.C. was responsible for formal analysis, data curation, and visualization. C.D.S. and C.M. participated in writing, review and editing, and data curation. F.C. provided support in formal analysis. L.G. and M.Ca. were involved in the investigation. G.L., D.D.G., E.B., and F.R. contributed to writing, review and editing. L.P. and L.E.G. supervised the project and were also involved in writing, review and editing. L.P. additionally participated in the investigation.
Funding statement
This research was co-funded by the Italian Complementary National Plan PNC-I.1 ‘Research initiatives for innovative technologies and pathways in the health and welfare sector,’ D.D. 931 of 06/06/2022, ‘DARE - DigitAl lifelong pRevEntion’ initiative, code PNC0000002, CUP N°: B53C22006470001.
References
- 1.World Health Organization . World Health Organization; Geneva: 2011. Report on the burden of endemic health care-associated infection worldwide [Internet]https://iris.who.int/handle/10665/80135 [cited 2024 Feb 8]. 40 p. Available from: [Google Scholar]
- 2.Mauldin P.D., Salgado C.D., Hansen I.S., Durup D.T., Bosso J.A. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2010;54:109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucidi C., Di Gregorio V., Ceccarelli G., Venditti M., Riggio O., Merli M. A cost analysis of a broad-spectrum antibiotic therapy in the empirical treatment of health care-associated infections in cirrhotic patients. Clinicoecon Outcomes Res. 2017;9:385–390. doi: 10.2147/CEOR.S130725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L. Cost of nosocomial infections in Wuhan No. 4 Hospital, China. Infect Control Hosp Epidemiol. 2000;21:4–5. doi: 10.1086/503209. [DOI] [PubMed] [Google Scholar]
- 5.Forrester J.D., Maggio P.M., Tennakoon L. Cost of Health Care–Associated Infections in the United States. J Patient Saf. 2022;18:e477. doi: 10.1097/PTS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 6.Nelson R., Ashby L., Bennett M., Bridges A., Chatterjee P., Choi H., et al. Excess Cost of Healthcare-Associated Infections in the Central Texas Veterans Health Care System. Open Forum Infect Dis. 2023;9(Supplement_2) doi: 10.1093/ofid/ofac492.1647. 2022 1. ofac 492.1647. [DOI] [Google Scholar]
- 7.Roberts R.R., Scott R.D.I., Hota B., Kampe L.M., Abbasi F., Schabowski S., et al. Costs Attributable to Healthcare-Acquired Infection in Hospitalized Adults and a Comparison of Economic Methods. Med Care. 2010;48:1026. doi: 10.1097/MLR.0b013e3181ef60a2. [DOI] [PubMed] [Google Scholar]
- 8.Lepelletier D., Ferréol S., Villers D., Richet H. [Methicillin-resistant Staphylococcus aureus nosocomial infections in ICU: risk factors, morbidity and cost] Pathol Biol. 2004;52:474–479. doi: 10.1016/j.patbio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Valaperta R., Tejada M.R., Frigerio M., Moroni A., Ciulla E., Cioffi S., et al. Staphylococcus aureus nosocomial infections: the role of a rapid and low-cost characterization for the establishment of a surveillance system. New Microbiol. 2010;33:223–232. [PubMed] [Google Scholar]
- 10.Heister T., Kaier K., Wolkewitz M. Estimating the burden of nosocomial infections: Time dependency and cost clustering should be taken into account. Am J Infect Control. 2017;45:94–95. doi: 10.1016/j.ajic.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Guest J.F., Keating T., Gould D., Wigglesworth N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-033367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyamogoba H., Obala A.A. Nosocomial infections in developing countries: cost effective control and prevention. East Afr Med J. 2002;79:435–441. doi: 10.4314/eamj.v79i8.8831. [DOI] [PubMed] [Google Scholar]
- 13.Kaier K., Wolkewitz M., Hehn P., Mutters N.T., Heister T. The impact of hospital-acquired infections on the patient-level reimbursement-cost relationship in a DRG-based hospital payment system. Int J Health Econ Manag. 2020;20:1–11. doi: 10.1007/s10754-019-09267-w. [DOI] [PubMed] [Google Scholar]
- 14.Scott RD 2nd, Culler S.D., Rask K.J. Understanding the Economic Impact of Health Care-Associated Infections: A Cost Perspective Analysis. J Infus Nurs Off Publ Infus Nurses Soc. 2019;42(2):61–69. doi: 10.1097/NAN.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 15.Tweddell S., Loomba R.S., Cooper D.S., Benscoter A.L. Health care-associated infections are associated with increased length of stay and cost but not mortality in children undergoing cardiac surgery. Congenit Heart Dis. 2019;14:785–790. doi: 10.1111/chd.12779. [DOI] [PubMed] [Google Scholar]
- 16.Plowman R., Graves N., Griffin M.A., Roberts J.A., Swan A.V., Cookson B., et al. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect. 2001;47:198–209. doi: 10.1053/jhin.2000.0881. [DOI] [PubMed] [Google Scholar]
- 17.López-Medrano F., San Juan R., Serrano O., Chaves F., Lumbreras C., Lizasoaín M., et al. [Impact of a non-compulsory antibiotic control program (PACTA): cost reductions and decreases in some nosocomial infections] Enferm Infecc Microbiol Clín. 2005;23:186–190. doi: 10.1157/13073141. [DOI] [PubMed] [Google Scholar]
- 18.Carestia M., Andreoni M., Buonomo E., Ciccacci F., Angelis L.D., Carolis G.D., et al. A novel, integrated approach for understanding and investigating Healthcare Associated Infections: A risk factors constellation analysis. PLoS One. 2023;18 doi: 10.1371/journal.pone.0282019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarente L., Mosconi C., Cicala M., De Santo C., Ciccacci F., Carestia M., et al. Device associated healthcare associated infection (DA-HAI): a detailed analysis of risk factors and outcomes in a university hospital in Rome, Italy. Infect Prev Pract. 2024;18 doi: 10.1016/j.infpip.2024.100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministero della Salute. Principali caratteristiche Diagnosis Related Groups (DRG) [Internet]. Available from: https://www.pnrr.salute.gov.it/portale/assistenzaOspedaliera/dettaglioContenutiAssistenzaOspedaliera.jsp?lingua=italiano&id=1349&area=ricoveriOspedalieri&menu=vuoto.
- 21.Iacchini S., Bellino S., D’Ancona F., Del Grosso M., Errico G., Pezzotti P., et al. Bollettino epidemiologico nazionale; 2021. Sorveglianza nazionale dell’antibiotico-resistenza AR-ISS: dati del primo semestre 2020. [Google Scholar]
- 22.Wickham H., Averick M., Bryan J., Chang W., McGowan L., François R., et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 23.Sjoberg D.D., Whiting K., Curry M., Lavery J.A., Larmarange J. Reproducible Summary Tables with the gtsummary Package. Rom Jahrb. 2021;13:570. doi: 10.32614/RJ-2021-053. [DOI] [Google Scholar]
- 24.Sekhon J.S. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching package for R. J Stat Software. 2011;42:1–52. doi: 10.18637/jss.v042.i07. [DOI] [Google Scholar]
- 25.Rosenbaum P.R., Rubin D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. doi: 10.2307/2335942. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study's dataset and the R code (version 4.3.2) utilised for data preparation, analysis, and the generation of publication-ready tables are available in the designated repository.
While the code is thoroughly commented to facilitate understanding, inquiries can be directed to stefano.orlando@uniroma2.it for further clarification.
For direct access to the dataset and code, refer to the following DOI: https://doi.org/10.17605/OSF.IO/MTQVX.