Abstract
Background
Prior research indicates a potential link between dyslipidemia and endometriosis (EMs). However, the relationship between remnant cholesterol (RC) and EMs has not been thoroughly investigated. Consequently, looking into and clarifying the connection between RC and EMs was the primary goal of this study.
Methods
Following the screening of participants from the NHANES dataset spanning 2001 to 2006, a total of 1,840 individuals were incorporated into this research. A weighted multivariable logistic regression analysis was first performed to investigate the relation between RC and the likelihood of encountering EMs. To assess the degree of consistency in the link between RC and EMs across different populations, additional subgroup analyses were performed. In addition, the researchers used the extreme gradient boosting (XGBoost) technique and the area under the receiver operating characteristic curve (ROC) to evaluate how well RC recognized EMs. Lastly, both linear and nonlinear relationships were validated using generalized additive models (GAM), while dose-response connections were investigated through restricted cubic spline models.
Results
After accounting for all potential confounders, a strong correlation between RC and EMs was identified. In particular, an increase of one unit in RC was linked to a 135% rise in the likelihood of developing EMs. Analyses of subgroups revealed that these relationships remained stable across the majority of subgroups (interaction P-value > 0.05). Multivariable logistic regression demonstrated RC’s independent predictive value, maintaining statistical significance after adjusting for confounders. The AUC of 0.614 suggests RC’s moderate ability to discriminate EMs, outperforming traditional markers like LDL-C in sensitivity and specificity. Furthermore, XGBoost analysis identified RC as the most critical predictor among lipid-related and demographic variables. The relationship was further validated through GAM, which visually confirmed a linear trend, and RCS, which provided statistical evidence of linearity.
Conclusion
This study reveals a clear connection between RC and the likelihood of having EMs within the US population, suggesting RC as a potential marker for further investigation in understanding endometriosis risk.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02422-4.
Keywords: Cross-sectional study, Remnant cholesterol, Endometriosis, Generalized additive model, Extreme gradient boosting, Receiver operating characteristic curve, NHANES
Introduction
Endometriosis (EMs) is characterized by the aberrant growth of functioning endometrial tissue beyond the boundaries of the uterus, and it is dependent on estrogen [1, 2]. Manifestations of this condition include dysuria, irregular uterine bleeding, dyspareunia, dysmenorrhea, infertility, and chronic pelvic pain [3]. In more severe instances, it can result in widespread pelvic adhesions and considerable anatomical changes [4]. As one of the most prevalent benign gynecological disorders, EMs affect around 5–10% of women during their reproductive years worldwide [5, 6], substantially impairing both quality of life and reproductive potential [7]. Its prevalence is particularly high, with 30% of patients facing infertility and 45% of those suffering from chronic pelvic pain affected [8]. EMs undoubtedly represents a major global public health challenge. Despite its significant impact, there remains a lack of a single serum biomarker or non-invasive diagnostic tool with sufficient diagnostic accuracy [8]. Consequently, research into EMs risk factors and the development of reliable diagnostic biomarkers is of the utmost importance.
Previous research has indicated that dyslipidemia commonly occurs in individuals with EMs [9, 10]. For instance, Zhang H identified a link between elevated serum triglyceride and a heightened risk of EMs [11], whereas Nahar noted significantly lower levels of high-density lipoprotein cholesterol (HDL-C) and greater levels of low-density lipoprotein cholesterol (LDL-C) among patients with EMs [12]. Conversely, several researches have not been able to clearly establish a noteworthy link between LDL-C and EMs [11, 13]. These contradictory results emphasize the shortcomings of existing lipid biomarkers in the clinical assessment of EMs, highlighting the necessity for innovative lipid metrics to improve the forecasting and management of this disorder.
Remnant cholesterol (RC) has been identified as a new lipid marker, indicating the presence of cholesterol in triglyceride-rich lipoproteins, including chylomicron cholesterol, intermediate-density lipoprotein cholesterol, and very low-density lipoprotein cholesterol during non-fasting conditions [14]. Studies have established that RC is as reliable, or even more so, than LDL-C or TC in predicting cardiovascular disease [15]. Despite the substantial research into RC’s role in cardiovascular disease, its involvement in EMs remains largely unexplored. Notably, elevated RC levels have been associated with low-grade inflammation [16, 17], a condition implicated in the pathogenesis of EMs [18]. These findings imply that RC, as a significant marker of lipid metabolism, might be involved in the onset and advancement of EMs, potentially through its effects on chronic inflammatory processes.
As of now, there has been a lack of explicit research examining the connection between RC and EMs, especially among the American demographic. Clarifying the link and the predictive significance of RC in EMs could greatly improve the early detection of individuals who are at an elevated risk for this condition. Consequently, this study aims to evaluate the relation between RC levels and the likelihood of participants from the National Health and Nutrition Examination Survey (NHANES) experiencing EMs, which would offer essential insights for the prompt detection and prevention of EMs.
Materials and methods
Data source and ethics
The data and materials utilized in this research were sourced from NHANES, which is a cross-sectional program that represents the nation and gathers comprehensive information on demographics, dietary habits, health status, and laboratory results from the population of the United States [19]. Trained professionals collected these data through standardized questionnaires, interviews, and tests.
Every single person who took part in the NHANES study gave their agreement after being fully informed [19]. Approval for the data collection methods was granted by the Ethics Review Board of the National Center for Health Statistics (NCHS), which operates under the Centers for Disease Control and Prevention (CDC). The Human Research Protection Program at Michigan State University determined that this analysis does not involve human subjects, as it uses publicly available de-identified data [19].
Study population
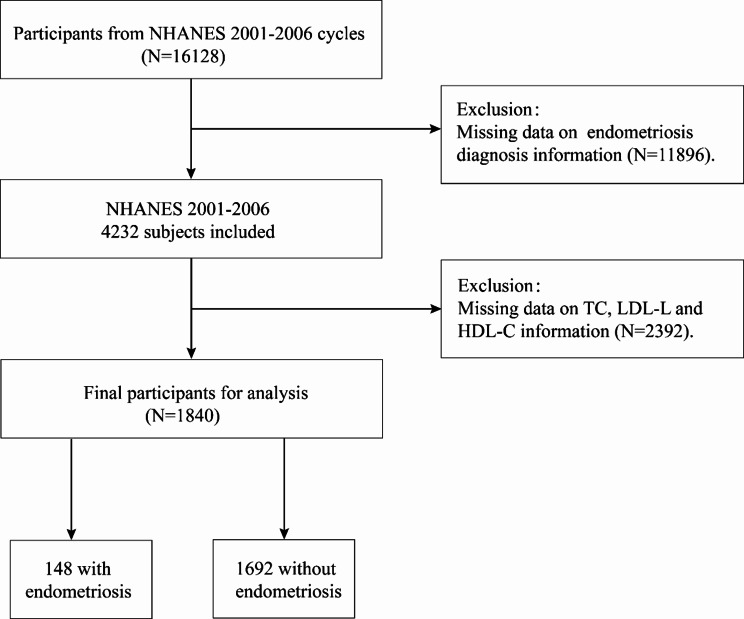
This research concentrated on EMs during the period from 2001 to 2006, which involved a total of 16,128 individuals. To ensure data integrity, individuals with missing EMs variables or unknown EMs status were initially excluded (n = 11,896). Subsequently, those lacking basic covariate data (TC, LDL-C, HDL-C) were systematically removed(n = 2,392). The final cohort included 1,840 participants for detailed analysis, as depicted in Fig. 1 and 2.
Fig. 1.
Flow chart of the screening process for the selection of endometriosis participants. NHANES, National Health and Nutrition Examination Survey; TC, Total Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; HDL-C, High-Density Lipoprotein Cholesterol
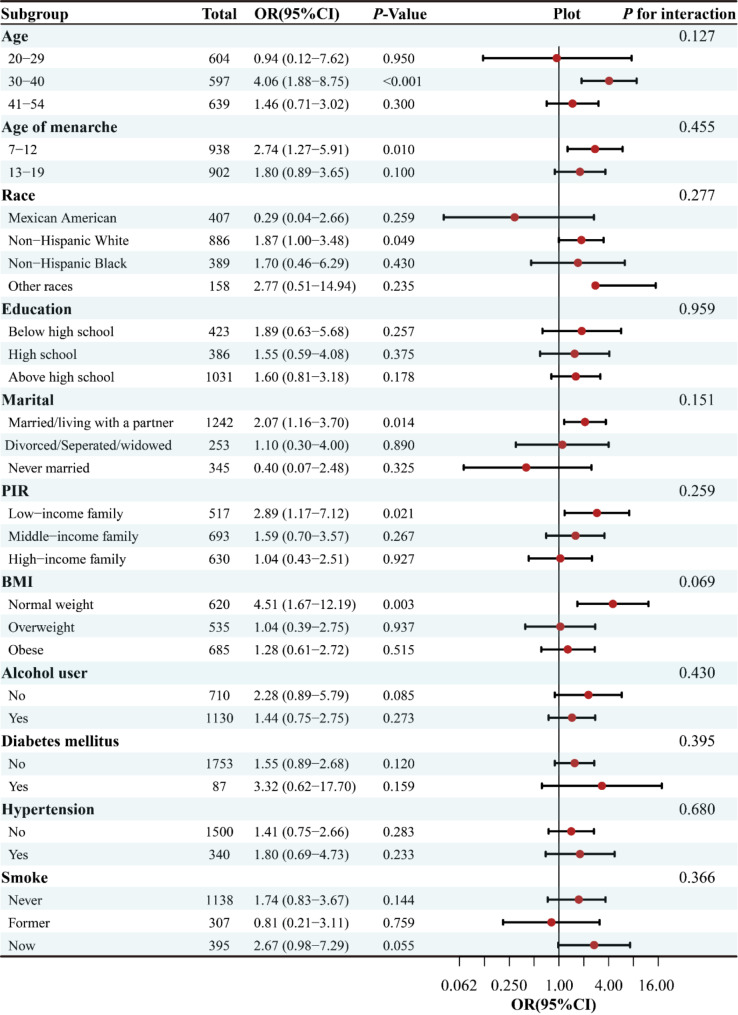
Fig. 2.
Stratified analyses of the association between RC with endometriosis according to baseline characteristics. The P value for interaction represents the likelihood of interaction between the variable and endometriosis. RC, Remnant Cholesterol; PIR, Poverty Income Ratio; BMI, Body Mass Index; OR, odds ratio; CI, confidence interval
Exposure and outcome variables
RC is determined by deducting the aggregate levels of LDL-C and HDL-C from TC [20]. TC and triglyceride (TG) levels are measured using enzymatic methods, while HDL-C levels are determined using either the heparin-manganese precipitation method or a direct immunoassay [21]. Subsequently, LDL-C levels are computed with the Friedewald equation, which uses the values of TC, TG, and HDL-C: [LDL-C] = [TC] – [HDL-C] – [TG/5] [22].
The outcome variable in this study was EMs. Data were obtained from NHANES through a questionnaire that asked participants if they had ever been informed by a healthcare professional, such as a doctor, that they had been diagnosed with EMs. A “yes” response indicated that the participant had EMs [23, 24].
Assessment of covariates
Covariates were chosen based on clinical insights and previous research [24–26], encompassing demographic factors, lifestyle behaviors, diet and health indicators. Demographic covariates included age, racial categories (classified into specific categories, including non-Hispanic White, Mexican American, non-Hispanic Black, and other racial identities) [27], educational attainment (ranging from not completing high school to high school completion and higher education) [28], poverty-to-income ratio (PIR, dispersed: <1.30, 1.30–3.50, and > 3.50) [26], and marital status (including divorced/separated/widowed, married/cohabitating, and never married) [29]. Lifestyle factors included age at menarche, alcohol use, smoking status, and BMI (categorized as < 25, 25.0-29.9, ≥ 30) [30]. Diet included energy intake, protein intake, dietary fiber intake and total sugars intake. Health indicators included hypertension and diabetes mellitus (DM), both recorded as binary variables (yes, no). Blood samples were utilized to assess lipid profiles, which comprised LDL-C and HDL-C [31, 32].
Statistical analysis
The research employed Multiple Imputation by Chained Equations (MICE) to address the issue of missing baseline variables. This method enabled the creation of five imputed datasets that incorporated crucial data points such as smoking and alcohol status, education, PIR, marital status, cardiovascular illnesses like hypertension and health indicator like diabetes. Given the high consistency of results across these datasets, the analyses were combined using Rubin’s rules to provide an accurate and comprehensive summary of the findings. To tackle the complex survey design that is typical of NHANES data gathering, a weighted methodology was used. Specifically, sample weights were calculated according to NHANES recommendations, using weight = WTSAF2YR/3 as the formula. This approach was systematically applied to datasets spanning three different cycles of NHANES, thereby augmenting the robustness and reliability of the results. In the weighted baseline table, participants were described based on their demographics and health characteristics. Categorical variables were displayed both as counts (n) and percentages (%). Meanwhile, continuous variables were conveyed as the mean along with the standard errors (SE), providing a numerical representation of data variation. Comparisons of continuous variables were conducted using the Mann-Whitney U test or the t test, contrasting groups with endometriosis against those without. In contrast, to examine any significant differences between groups, the Fisher’s exact tests and chi-squared tests were used to estimate categorical variables.
Using a multivariable logistic regression, this research looked at the relation between the RC and EMs. Odds ratios (OR) are presented for the estimates along with 95% confidence intervals (CI). Initially, RC was thought of as a continuous variable. Afterwards, to conduct a more comprehensive analysis, the data was subsequently separated into four equal halves called quartiles: Q1, which spanned from 0.150 to 0.380; Q2, which ranged from 0.380 to 0.545; Q3, from 0.545 to 0.813; and Q4, covering 0.813 to 2.073, with Q1 serving as the benchmark group. Model 1 does not incorporate any modifications or corrections. Model 2 took into consideration demographic considerations. Model 3 underwent further modifications to incorporate variables such as marital status, education level, BMI, PIR, smoke, alcohol, chronic issues like diabetes, and cardiovascular disorders like hypertension, lipid profiles like, age of menarche, dietary intake (engergy, protein, fiber and total sugars). In order to investigate the linear connections that exist between the quartiles of RC and EMs, trend tests were carried out. To evaluate the potential nonlinear association between RC and EMs, generalized additive models (GAM) and restricted cubic splines (RCS) were applied. GAM provided a flexible approach to explore the shape of the relationship by applying smoothing functions, enabling the visualization of potential deviations from linearity. RCS were used to further quantify and statistically test the relationship by fitting cubic polynomial functions to RC with smooth transitions at predefined knots (set at the 10th, 50th, and 90th percentiles). Two statistical tests were conducted: P for overall, to evaluate the overall association between RC and EMs, and P for non-linearity, to test for deviations from linearity. Visual inspection of the RCS-derived dose-response curve complemented the statistical tests and provided additional insights into the nature of the association. Subgroup analyses and interaction P values were computed to look at any interactions between stratified factors. Furthermore, the study utilized receiver operating characteristic (ROC) analysis to evaluate the diagnostic performance of RC along with three other lipid indicators in predicting endometriosis likelihood. The area under the curve (AUC) of the ROC was utilized to quantify this diagnostic value. Following this, the researchers applied the machine learning technique of the XGBoost algorithm to assess the relative importance of the chosen variables in relation to endometriosis. The data was analyzed statistically using the EmpowerRCH program and R version 4.4.1. Statistical significance was determined using p-values that were less than 0.05.
Results
Baseline characteristics of participants with and without endometriosis
The baseline characteristics of both the unweighted and weighted samples are presented in Table 1 and Supplementary Table 1. Among the 1,840 participants included in this study, 148 were diagnosed with endometriosis, resulting in a prevalence rate of 8.04%. The NHANES employs a complex multi-stage probability sampling method that incorporates weights to mitigate bias inherent in sample surveys. After adjusting for NHANES weights, the analysis encompassed a weighted total of 66,761,346 individuals. In the weighted sample, the mean age of individuals without endometriosis was 37.00 ± 0.34 years, compared to 40.63 ± 0.48 years for those with endometriosis. The study cohort was predominantly composed of non-Hispanic whites, who represented 68.70% of the total weighted sample population. Notably, women with endometriosis exhibited distinct characteristics compared to those without the conditionin particular, they were more prone to smoking and tended to have lower educational attainment. Furthermore, the mean levels of triglycerides, total cholesterol and RC were significantly elevated (P < 0.05).
Table 1.
Baseline characteristics of selected participants from the NHANES 2001–2006 characteristics of the women participants, Weighted
| Characteristics | Without endometriosis (N = 1692) |
With endometriosis (N = 148) |
P-value |
|---|---|---|---|
| Age (year), mean ± SE | 37.00 ± 0.34 | 40.63 ± 0.48 | < 0.001 |
| Race (n, %) | < 0.001 | ||
| Non-Hispanic White | 1138 (67.22) | 121 (81.63) | |
| Non-Hispanic Black | 220 (13.02) | 17 (11.26) | |
| Mexican American | 151 (8.95) | 2 (1.58) | |
| Other races | 183 (10.81) | 8 (5.53) | |
| Education (n, %) | 0.007 | ||
| Below high school | 271 (16.04) | 15 (9.80) | |
| High school | 357 (21.07) | 45 (30.67) | |
| Above high school | 1064 (62.89) | 88 (59.53) | |
| Marital (n, %) | 0.098 | ||
| Married/living with a partner | 1128 (66.65) | 108 (73.13) | |
| Divorced/Seperated/widowed | 240 (14.20) | 25 (16.77) | |
| Never married | 324 (19.15) | 15 (10.1) | |
| PIR (n, %) | 0.698 | ||
| Low-income family | 351 (20.72) | 31 (21.20) | |
| Middle-income family | 636 (37.61) | 49 (33.06) | |
| High-income family | 705 (41.67) | 68 (45.74) | |
| BMI (n, %) | 0.772 | ||
| Normal weight | 681 (40.23) | 57 (38.53) | |
| Overweight | 443 (26.19) | 42 (28.09) | |
| Obese | 568 (33.57) | 49 (33.38) | |
| Smoke (n, %) | < 0.001 | ||
| Never | 1021 (60.34) | 60 (40.40) | |
| Former | 271 (16.03) | 34 (22.95) | |
| Now | 400 (23.63) | 354 (6.65) | |
| Hypertension (n, %) | 0.114 | ||
| No | 1376 (81.35) | 113 (76.35) | |
| Yes | 316 (18.65) | 35 (23.65) | |
| Diabetes mellitus (n, %) | 0.473 | ||
| No | 1616 (95.53) | 143 (96.76) | |
| Yes | 76 (4.47) | 5 (3.24) | |
| Alcohol user (n, %) | 0.236 | ||
| No | 551 (32.55) | 39 (26.07) | |
| Yes | 1141 (67.45) | 109 (73.93) | |
| Age of menarche, mean ± SE | 12.64 ± 0.05 | 12.52 ± 0.21 | 0.570 |
| Blood lipid indices, mean ± SE | |||
| HDL-C | 58.34 ± 0.53 | 57.95 ± 1.84 | 0.842 |
| LDL-C | 112.49 ± 1.24 | 117.61 ± 2.93 | 0.105 |
| Triglyceride | 112.25 ± 60.48 | 132.53 ± 74.38 | 0.008 |
| Total cholesterol | 193.29 ± 1.29 | 202.06 ± 3.51 | 0.025 |
| Dietary, mean ± SE | |||
| Energy (Kcal) | 1945.42 ± 19.94 | 1881.56 ± 69.59 | 0.428 |
| Protein (gm) | 72.61 ± 0.95 | 68.14 ± 3.01 | 0.165 |
| Total sugar (gm) | 112.39 ± 2.27 | 112.88 ± 6.96 | 0.948 |
| Dietary fiber (gm) | 14.11 ± 0.28 | 12.46 ± 0.47 | 0.004 |
| RC, mean ± SE | 0.58 ± 0.01 | 0.68 ± 0.04 | 0.009 |
| RC group (n, %) | |||
| Q1 | 497 (29.40) | 28 (18.84) | |
| Q2 | 461 (27.23) | 41 (27.41) | |
| Q3 | 431 (25.46) | 38 (25.77) | |
| Q4 | 303 (17.91) | 41 (27.99) |
Data in the table: Weighted analyses for continuous variables: survey-weighted mean ± SE, P-value was by survey-weighted linear regression (svyglm); for categorical variables: survey-weighted percentage (95% CI), P-value was by survey-weighted chi-square test (svytable)
Abbreviation: RC, Remnant Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; HDL-C, High-Density Lipoprotein Cholesterol; PIR: Poverty Income Ratio; BMI: Body Mass Index
P - value less than 0.05 is expressed in bold
Higher RC is associated with endometriosis by weighted multivariate models and dose-response analysis
RC and the probability of experiencing EMs are positively correlated, as shown in Table 2, with this statistically significant relationship maintained across unadjusted, initially adjusted, and fully adjusted weighted logistic regression models. After controlling for all relevant covariates, there was a 135% rise in the prevalence of EMs for each unit increment in RC (95% CI of 1.17–4.74), indicating a robust positive link between RC and EMs. Further analysis categorized RC into quartiles, revealing that individuals in the upper quartile faced a considerably heightened risk in the fully adjusted model, with EMs occurring at rates 2.35 times greater than those in the lowest quartile (95% CI of 1.18 to 4.65). Comparable outcomes were noted in Model 1 and Model 2 as well. In addition, statistical significance was confirmed by a trend analysis that included all three models, which yielded a P value of under 0.05. The RCS model and GAM model were applied to explore the potential non-linear relationship between RC and EMs. The analysis revealed no significant evidence of non-linearity (P-non-linear > 0.05), indicating that the relationship between RC and EMs can be adequately explained by a linear model, with no substantial contribution from the non-linear terms (Supplementary Figs. 1 and 2).
Table 2.
Weighted multivariable logistic regression to assess the association of RC with endometriosis
| Model I | Model II | Model III | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| RC | 2.37 (1.38–4.06) | 0.002 | 2.32 (1.29–4.18) | 0.005 | 2.35(1.17–4.74) | 0.017 | ||
| Categories | ||||||||
| Q1 | 1.0 [Ref] | 1.0 [Ref] | 1.0 [Ref] | |||||
| Q2 | 1.57 (0.89–2.77) | 0.119 | 1.56 (0.88–2.77) | 0.128 | 1.49 (0.79–2.67) | 0.228 | ||
| Q3 | 1.58 (0.82–3.04) | 0.170 | 1.52 (0.80–2.89) | 0.201 | 1.46 (0.76–2.82) | 0.257 | ||
| Q4 | 2.44 (1.40–4.26) | 0.002 | 2.40 (1.33–4.34) | 0.004 | 2.35 (1.18–4.65) | 0.015 | ||
| P for trend | 1.30 (1.10–1.54) | 0.003 | 1.29 (1.08–1.55) | 0.006 | 1.28 (1.04–1.57) | 0.018 | ||
Model 1: no covariates were adjusted
Model 2: Adjusted for age, race
Model 3: Adjusted for age, race, education, marital status, PIR, BMI, smoke, alcohol, hypertension, diabetes, age of menarche, dietary energy, dietary protein, dietary fiber and total sugars
RC, Remnant Cholesterol; PIR: Poverty Income Ratio; BMI: Body Mass Index.CI, confidence interval; OR, odds ratios; Ref, reference
RC is in the quartile: Q1(0.150–0.380), Q2(0.380–0.545), Q3(0.545–0.813), Q4(0.813–2.073)
Correlation between RC and endometriosis in different subgroups
For the purpose of ensuring the robustness of the multivariable regression analysis results, this study conducted a more detailed analysis of the stratified association between RC and EMs across several subgroups, including race, age, educational background, PIR, marital status, BMI, chronic issues like diabetes, and cardiovascular disorders like hypertension, smoking status, and age at menarche. The outcomes from the stratified examination revealed a favorable link between RC and EMs in certain demographics, namely women aged 30–40 years, those who experienced menarche between 7 and 12 years old, non-Hispanic blacks, married women, low-income women, and those with a normal weight. Furthermore, tests for interactions did not reveal any statistically significant differences across all subgroups (all P values for interactions > 0.05).
RC may be a better indicator for identifying endometriosis
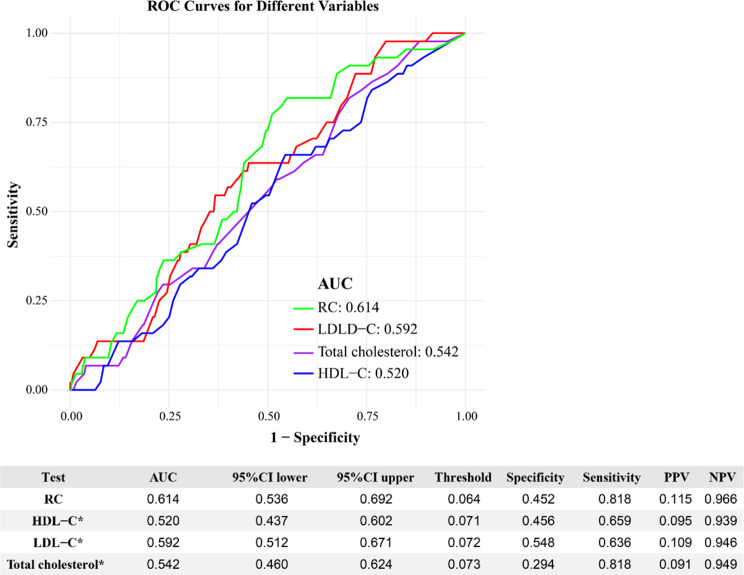
To evaluate the discriminatory ability of RC in predicting EMs, its performance was assessed alongside traditional lipid markers, including TC, HDL-C, and LDL-C. Figure 3 demonstrates the AUC values for these four markers. Among them, RC exhibited the highest AUC value of 0.614 (95% CI: 0.536–0.692), which meets the ‘possibly helpful’ threshold defined by JAMA, indicating its potential utility in clinical practice. In contrast, TC (AUC = 0.542), HDL-C (AUC = 0.520), and LDL-C (AUC = 0.592) all fell below this threshold, indicating poorer discriminatory ability. Additionally, RC achieved higher sensitivity (0.818) and specificity (0.614) compared to the other lipid markers, underscoring its potential as a more effective indicator for identifying individuals at risk of EMs.
Fig. 3.
Comparison of the predictive ability of four lipid related indicators for endometriosis. *When the AUC value of this indicator was compared to the AUC value of the RC the result obtained was P < 0.05. RC, Remnant Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; HDL-C, High-Density Lipoprotein Cholesterol
To further explore the predictive power of RC, we conducted multivariable logistic regression analyses (Table 3). After full adjustment for confounders, RC remained significantly associated with EMs (OR = 2.35, 95% CI: 1.17–4.74, P = 0.017). In contrast, TC, HDL-C, and LDL-C did not show significant associations with EMs after adjustment, further emphasizing RC’s distinct predictive value.
Table 3.
Weighted multivariable logistic regression to assess the association of other blood lipid indices with EMs
| Characteristic | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| RC | 2.37 (1.38–4.06) | 0.002 | 2.32 (1.29–4.19) | 0.006 | 2.35 (1.17–4.74) | 0.017 | ||
| HDL-C | 0.94 (0.52–1.72) | 0.843 | 0.84 (0.44–1.59) | 0.587 | 0.98 (0.53–1.84) | 0.960 | ||
| LDL-C | 1.18 (0.97–1.43) | 0.098 | 1.06 (0.86–1.31) | 0.591 | 1.01 (0.82–1.26) | 0.895 | ||
| Total cholesterol | 1.24 (1.04–1.48) | 0.020 | 1.13 (0.92–1.38) | 0.246 | 1.10 (0.89–1.35) | 0.351 | ||
Model 1: no covariates were adjusted
Model 2: Adjusted for age, race
Model 3: Adjusted for age, race, education, marital status, PIR, BMI, smoke, alcohol, hypertension, diabetes, age of menarche, dietary energy, dietary protein, dietary fiber and total sugars
RC, Remnant Cholesterol; PIR: Poverty Income Ratio; BMI: Body Mass Index.CI, confidence interval; OR, odds ratios; Ref, reference
RC is in the quartile: Q1(0.150–0.380), Q2(0.380–0.545), Q3(0.545–0.813), Q4(0.813–2.073)
Evaluating the relative importance of variables using XGBoost
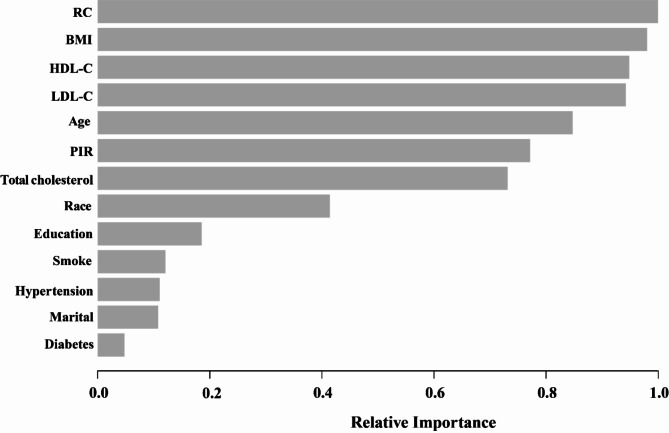
To support the robustness of the findings, the XGBoost algorithm was applied as a supplementary tool to validate the relative importance of variables in predicting EMs (Fig. 4). The selected variables encompassed age, PIR, BMI, marital status, race, education, smoking status, hypertension, diabetes, and blood lipid indicators, including HDL-C, LDL-C, and total cholesterol. The results from the XGBoost model indicated that RC, BMI, HDL-C, LDL-C, and age emerged as the five most important factors. Notably, RC was identified as the most significant indicator, exhibiting greater relative importance compared to blood lipid indicators such as HDL-C and LDL-C. These results align with the multivariable regression findings, further supporting RC’s central role as a predictive biomarker.
Fig. 4.
Using XGBoost modeling to assess the relative importance of specific variables for endometriosis. XGBoost, eXtremeGradient Boosting; RC, Remnant Cholesterol; BMI, Body Mass Index; HDL-C, High-Density Lipoprotein Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; PIR, Poverty Income Ratio
Discussion
This comprehensive research involving a sample of 1,840 adults showed a robust link between elevated levels of RC and a greater probability of experiencing EMs. The positive correlation persisted even after progressively adjusting for potential confounders across Models 1 to 3. Subgroup analyses indicated that the impact of RC on endometriosis was particularly pronounced among women aged 30–40 years, non-Hispanic Blacks, married individuals, those with normal weight, and participants with an age of menarche between 7 and 12 years. Multivariable logistic regression demonstrated RC’s independent predictive value, maintaining statistical significance after adjusting for confounders. AUC analysis (AUC = 0.614) further indicated moderate discriminatory ability, with RC outperforming LDL-C in sensitivity and specificity, underscoring its clinical potential. The XGBoost algorithm validated these findings by ranking RC as the most important predictor among lipid and demographic variables, reinforcing its relevance. To further evaluate the relationship, GAM visually established a consistent linear trend between RC and endometriosis, while RCS provided statistical evidence affirming this linearity. Together, these analyses present a coherent argument for RC’s potential as a candidate biomarker for endometriosis, indicating that it could partly serve as a risk element for this ailment.
Based on existing literature, this research represents the first exploration of the link between RC and EMs utilizing the NHANES database. Although previous studies on this subject have been somewhat restricted, current studies have clarified the significant link between irregularities in lipid metabolism and EMs [33–35]. Multiple investigations have demonstrated that patients with EMs are more likely to experience lipid metabolism disorders [9, 36], which are characterized by lower HDL-C levels and elevated TC and LDL-C levels [11–13, 37]. These traditional lipid markers have been instrumental in assessing the risk of EMs. As research has advanced, the scientific community has increasingly focused on additional potential blood lipid markers, among which RC has garnered significant attention from researchers. This study introduces RC into the realm of EMs research for the first time, elucidating its potential role as an emerging marker in this context. Unlike traditional blood lipid markers, RC primarily reflects the cholesterol content of residual particles in plasma [14]. Research has shown a strong link between increased RC levels and cardiovascular disease (CVD) [38, 39], and evidence suggests that EMs are also a risk factor for certain CVDs [40, 41]. Given that EMs and CVD may represent different manifestations of the same underlying pathological mechanism [42], RC might also be an essential factor in the development of EMs. This research highlights the promising role of RC in the early detection and intervention of EMs by exploring their relationship, thereby contributing to the understanding of lipid metabolism irregularities in the context of EMs studies.
Although RC may serve as a promising lipid marker for predicting endometriosis, the specific biological mechanisms underlying this association remain incompletely understood. Existing studies suggest several potential mechanisms that could account for the positive relation observed between RC levels and endometriosis. These mechanisms encompass inflammatory responses, immune regulation, and alterations in hormone levels. Initially, it is possible that RC contributes to the onset of endometriosis through the modulation of inflammatory responses. According to studies, people with endometriosis have significantly higher amounts of inflammatory factors in their peritoneal fluid, such as IL-8, TNF-α, and IL-1β [43]. These inflammatory elements are crucial in the advancement of the condition [44, 45]. By increasing the expression of these mediators, RC may trigger and perpetuate chronic inflammation [16], which in turn could hasten the growth and dissemination of ectopic endometrial tissue. Additionally, RC may influence the pathological mechanisms associated with endometriosis by altering the functions of immune cells, such as T cells and macrophages, that play a critical role in the invasion of ectopic endometrial tissues [46–49]. RC might also enhance the polarization of macrophages to a pro-inflammatory M1 phenotype, intensifying local inflammation and further facilitating lesion development [50–52]. Finally, RC may also impact the development of endometriosis by interfering with hormone metabolism, particularly the balance of estrogen and progesterone. Studies have demonstrated that blood lipid levels in endometriosis patients change significantly during hormone therapy, suggesting that RC may play a role in this process [53, 54]. In summary, the relationship between RC levels and endometriosis may stem from a variety of complex factors, including alterations in inflammatory responses, immune regulation, and hormone metabolism. To gain a deeper understanding of this association, future studies should integrate clinical and molecular biology methodologies to further elucidate these mechanisms and investigate the potential clinical utility of RC as a biomarker.
Meanwhile, the study’s subgroup analysis revealed that the relation between RC and EMs varied significantly across specific groups. Consequently, the study further investigated the potential reasons behind these subgroup differences. Previous research has indicated that African-American women typically enter puberty at an earlier age [55], resulting in prolonged exposure to elevated estrogen levels, which may increase the risk of endometriosis. Additionally, lower socioeconomic status may expose these women to a greater number of environmental pollutants during adolescence [56–58]. Collectively, these factors may amplify the relationship between RC and endometriosis. Moreover, those with poor PIR are more likely to experience malnourishment and limited access to medical care, which may exacerbate the impact of RC on the risk of endometriosis development. The research found that endometriosis risk was substantially increased in women whose menstrual cycles began before the age of twelve [59]. This association may be attributed to the longer duration of hormone exposure experienced by these women throughout their lives [60]. Extended exposure to estrogen is thought to provide a substantial role in the formation and maturation of ectopic endometrial tissue [61], which increases the likelihood of endometriosis. As a result, in specific groups, the significance of RC as a contributing factor to endometriosis may be more evident, whereas in other populations, this association might be diminished by the influence of additional factors.
RC, recognized as a non-invasive lipid marker, holds significant clinical relevance for the early screening and personalized treatment of endometriosis. A significant link was noted between levels of RC and the likelihood of developing endometriosis, particularly among high-risk groups characterized by early menarche, low socioeconomic status, and specific ethnic backgrounds. Routine monitoring of RC levels in these populations can aid healthcare providers in identifying individuals at high risk before the onset of symptoms, facilitating early intervention. This proactive approach not only reduces dependence on invasive diagnostic techniques but also enables more timely and effective treatment for patients. Furthermore, dynamic monitoring of RC levels can serve as a crucial reference for developing personalized treatment plans. Patients exhibiting elevated RC levels may present with a higher prevalence of estrogen-dependent lesions, indicating that these individuals may require more aggressive interventions, such as intensive hormonal therapy. Specifically, the effective reduction of estrogen levels in the body can be accomplished by using gonadotropin-releasing hormone (GnRH) agonists, antiprogestins, or aromatase inhibitors, which consequently diminishes the activity and proliferation of ectopic endometrial tissue. Conversely, for patients with lower RC levels, more conservative management strategies may be advisable to prevent overtreatment and mitigate potential side effects.
Strengths and limitations of the study
Multiple advantages boost the credibility of these results. This investigation marks the first instance of identifying a correlation between RC, a novel lipid indicator, and endometriosis. Utilizing a sample that is nationally representative from NHANES, along with the application of proper NHANES sampling weights, guarantees the representativeness of the American adults. Additionally, the integration of logistic regression, ROC analysis, and the supplementary use of machine learning (XGBoost) provides a comprehensive evaluation of RC’s predictive performance.
Nonetheless, several limitations must be acknowledged. The cross-sectional design of this study precludes establishing causal relationships between RC and endometriosis. Further prospective cohort studies and the underlying mechnism would be needed to explore temporal associations and the potential causal pathways. The use of self-reported endometriosis diagnoses introduces potential recall bias, which could affect the accuracy of the data. While the study controlled for several potential confounders, residual confounding remains a concern, as it is impossible to fully account for all variables that could influence the observed relationship.
Moreover, while RC demonstrated a moderate discriminatory ability (AUC = 0.614), its performance suggests that RC is more of a supplementary indicator rather than a definitive diagnostic tool. Further validation in prospective cohorts is needed to assess its reliability and clinical utility. The study also lack data on lifestyle factors, such as physical activity, which may have impacted the results and could be important confounders. Additionally, the absence of detailed clinical information regarding the severity or stage of endometriosis limits the ability to assess how RC might vary across different manifestations of the disease. Lastly, the unavailability of endometriosis variables in newer NHANES data cycles restricts the generalizability of findings to more contemporary populations, underscoring the importance of future studies to extend and validate these results.
Conclusion
This study found a significant association between elevated RC levels and the likelihood of developing endometriosis. These findings, supported by ROC analysis and feature importance ranking through XGBoost, suggest that RC may have potential as a biomarker for endometriosis. Additionally, the moderate diagnostic performance warrants caution in its clinical application, and further studies are needed to validate the role of RC in risk stratification and to explore its potential clinical uses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 2: Association between RC and endometriosis. Adjustments have been made for all covariates base on model 3. (A) Each red dot represents a separate sample, and the blue dots above and below the red dot represent 95% confidence intervals. (B) The red solid line is the smooth fitting curve between RC levels and EMs, while the blue dashed line reflects the 95% confidence intervals of the fitting. RC, Remnant Cholesterol; EMs, endometriosis.
Supplementary Material 3: Association between RC and endometriosis. Adjustments have been made for all covariates base on model 3. (A) Each red dot represents a separate sample, and the blue dots above and below the red dot represent 95% confidence intervals. (B) The red solid line is the smooth fitting curve between RC levels and EMs, while the blue dashed line reflects the 95% confidence intervals of the fitting. RC, Remnant Cholesterol; EMs, endometriosis.
Acknowledgements
To everyone who took part in the NHANES and helped out, thanks from the bottom of our hearts.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- Ems
Endometriosis
- RC
Remnant cholesterol
- ROC
Receiver operating characteristic curve
- AUC
Area under the curve
- MICE
Multiple Imputation via Chained Equations
- XGBoost
Extreme gradient boosting
- BMI
Body mass index
- GAM
Generalized additive models
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- CVD
Cardiovascular Disease
- PIR
Poverty income ratio
- OR
Odds ratio
- CI
Confidence interval
- SD
Standard deviation
Author contributions
Z.C., R.L., J.G., and X.Y. wrote the main manuscript text and Z.C. prepared all figures. All authors reviewed the manuscript.
Funding
This study was supported by Medical Research Fund Guangdong Province (A2024003), and Xinjiang Support Rural Science and Technology (SpecialCorrespondent) Program in Guangdong Province (KTPYJ 2023014).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was authorized by the NCHS Ethics Review Board, and individuals’ signed consent was required to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zeru Chen and Ruixuan Li contributed equally to this work.
Contributor Information
Yang Zhou, Email: zhouyang2009hi@126.com.
Mingzhu Cao, Email: 2019683081@gzhmu.edu.cn.
References
- 1.Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and Estrogen Signaling in the Endometrium: what goes wrong in endometriosis? Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed]
- 2.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–75. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Luo H, Zhu L, Yang W, Xiang H, Shi Q, Jin P. Dysmenorrhea pattern in adolescences informing adult endometriosis. BMC Public Health. 2024;24:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianconi L, Hummelshoj L, Coccia ME, Vigano P, Vittori G, Veit J, Music R, Tomassini A, D’Hooghe T. Recognizing endometriosis as a social disease: the European Union-encouraged Italian Senate approach. Fertil Steril. 2007;88:1285–7. [DOI] [PubMed] [Google Scholar]
- 6.Lamceva J, Uljanovs R, Strumfa I. The main theories on the pathogenesis of endometriosis. Int J Mol Sci 2023, 24. [DOI] [PMC free article] [PubMed]
- 7.Scheck S, Paterson ESJ, Henry CE. A promising future for endometriosis diagnosis and therapy: extracellular vesicles - a systematic review. Reprod Biol Endocrinol. 2022;20:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74:594–600. [PubMed] [Google Scholar]
- 9.Sahmani M, Ghaleh TD, Darabi M, Darabi M, Rashvand Z, Najafipour R. Lack of association between LIPC-514 C/T polymorphism of hepatic lipase and endometriosis in Iranian women. J Obstet Gynaecol Res. 2014;40:479–84. [DOI] [PubMed] [Google Scholar]
- 10.Maas A. Female-specific risk variables: from innocent bystanders to key players in cardiovascular risk prediction. Maturitas. 2024;186:107970. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Fan Y, Li H, Feng X, Yue D. Genetic association of serum lipids and lipid-modifying targets with endometriosis: trans-ethnic mendelian-randomization and mediation analysis. PLoS ONE. 2024;19:e0301752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahar K, Khanam NN, Chowdhury AA, Khan NJ, Mohamed Z. Association of Dyslipidemia with endometriosis: a Case Control Study. Mymensingh Med J. 2023;32:118–24. [PubMed] [Google Scholar]
- 13.Wang Z, Zhan C, Liao L, Luo Y, Lin S, Yan S. Bidirectional causality between the levels of blood lipids and endometriosis: a two-sample mendelian randomization study. BMC Womens Health. 2024;24:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varbo A, Nordestgaard BG. Remnant cholesterol and Triglyceride-Rich Lipoproteins in atherosclerosis progression and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2016;36:2133–5. [DOI] [PubMed] [Google Scholar]
- 15.Guo DC, Gao JW, Wang X, Chen ZT, Gao QY, Chen YX, Wang JF, Liu PM, Zhang HF. Remnant cholesterol and risk of incident hypertension: a population-based prospective cohort study. Hypertens Res. 2024;47:1157–66. [DOI] [PubMed] [Google Scholar]
- 16.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–309. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Weng X, Xu J, Wang W. Correlation between remnant cholesterol and hyperuricemia in American adults. Lipids Health Dis. 2024;23:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orisaka M, Mizutani T, Miyazaki Y, Shirafuji A, Tamamura C, Fujita M, Tsuyoshi H, Yoshida Y. Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging. Front Endocrinol (Lausanne). 2023;14:1324429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1 2013:1–37. [PubMed]
- 20.Qian S, You S, Sun Y, Wu Q, Wang X, Tang W, Dong X, Liu CF, Xu T, Cao Y, Zhong C. Remnant cholesterol and common carotid artery intima-media thickness in patients with ischemic stroke. Circ Cardiovasc Imaging. 2021;14:e010953. [DOI] [PubMed] [Google Scholar]
- 21.Xiao P, Wang Z, Lu Z, Liu S, Huang C, Xu Y, Tian Y. The association between remnant cholesterol and bone mineral density in US adults: the National Health and Nutrition Examination Survey (NHANES) 2013–2018. Lipids Health Dis. 2024;23:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie YY, Zhao L, Gao LJ, Xu RX, Gao Y, Dou KF, Guo YL, He YM. Association between remnant cholesterol and verbal learning and memory function in the elderly in the US. Lipids Health Dis. 2022;21:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Wang Y, Ji X, Kong W, Pan Z, Xu C, Geng Y, Miao J. Association between triglyceride-glucose index and risk of endometriosis in US population: results from the national health and nutrition examination survey (1999–2006). Front Endocrinol (Lausanne). 2024;15:1371393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu PW, Zhang XL, Yan XT, Qi C, Jiang GJ. Association between depression and endometriosis using data from NHANES 2005–2006. Sci Rep. 2023;13:18708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris HR, Eke AC, Chavarro JE, Missmer SA. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod. 2018;33:715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MS, Talge NM, Upson K. Urinary cadmium and endometriosis prevalence in a US nationally representative sample: results from NHANES 1999–2006. Hum Reprod. 2023;38:1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma XM, Li KX, Chen ZQ, Wu CM, Liao WZ, Guo XG. Impact of age, sex, and thyroid autoimmunity on the association between selenium intake and type 2 diabetes mellitus. BMC Public Health. 2024;24:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J, Chen T, Chen Y, Huang ZM, Li XJ, Chen HK, Huang YQ, Guo XG. The association between homocysteine and bacterial vaginosis: results from NHANES 2001–2004. Sci Rep. 2023;13:21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu EH, Liao WZ, Chen HK, Huang XY, Li RX, Liang HW, Guo XG. Association between serum vitamin E and bacterial vaginitis in women: a cross-sectional study. BMC Womens Health. 2024;24:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hastie CE, Padmanabhan S, Slack R, Pell AC, Oldroyd KG, Flapan AD, Jennings KP, Irving J, Eteiba H, Dominiczak AF, Pell JP. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:222–6. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Zhang K, Wang F, Wang M, Huang Q, Wei C, Gou Z. The LDL-C/ApoB ratio predicts cardiovascular and all-cause mortality in the general population. Lipids Health Dis. 2023;22:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Xu Y, Xu Y. The association of the platelet/high-density lipoprotein cholesterol ratio with self-reported stroke and cardiovascular mortality: a population-based observational study. Lipids Health Dis. 2024;23:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta M, Anitha M, Smith PB, Chiaro CR, Maan M, Chaudhury K, Patterson AD. Metabolomics reveals altered lipid metabolism in a mouse model of endometriosis. J Proteome Res. 2016;15:2626–33. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz CN, Torres-Reverón A, Appleyard CB. Metabolomics in endometriosis: challenges and perspectives for future studies. Reprod Fertil. 2021;2:R35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin X, Wang Q, Xu D, Sun Y, Xu W, Wang B, Yang Z, Hao L. Atorvastatin exerts dual effects of lesion regression and ovarian protection in the prevention and treatment of endometriosis. Eur J Pharmacol. 2024;964:176261. [DOI] [PubMed] [Google Scholar]
- 36.Yu M, Tang J, Huang Y, Guo C, Du P, Li N, Quan Q. HOXA10 regulates the synthesis of cholesterol in endometrial stromal cells. Front Endocrinol (Lausanne). 2022;13:852671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou G, Ren J, Huang Q, Nie X, Tong X, Cui YW, Hu R, Yao Q. Gene associations of lipid traits, lipid-lowering drug-target genes and endometriosis. Reprod Biomed Online. 2024;49:103856. [DOI] [PubMed] [Google Scholar]
- 38.Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, Lima J, Puri R, Nomura S, Tsai M, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. 2021;42:4324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang K, Yin S, Xiao Y, Wang J, Cui J, Wang J, Bai Y. Sexual dysfunction in patients with diabetes: association between remnant cholesterol and erectile dysfunction. Lipids Health Dis. 2024;23:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirillo M, Coccia ME, Petraglia F, Fatini C. Role of endometriosis in defining cardiovascular risk: a gender medicine approach for women’s health. Hum Fertil (Camb). 2022;25:745–53. [DOI] [PubMed] [Google Scholar]
- 41.Verit FF, Yildiz Zeyrek F, Zebitay AG, Akyol H. Cardiovascular risk may be increased in women with unexplained infertility. Clin Exp Reprod Med. 2017;44:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchandot B, Curtiaud A, Matsushita K, Trimaille A, Host A, Faller E, Garbin O, Akladios C, Jesel L, Morel O. Endometriosis and cardiovascular disease. Eur Heart J Open. 2022;2:oeac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voltolini Velho R, Halben N, Chekerov R, Keye J, Plendl J, Sehouli J, Mechsner S. Functional changes of immune cells: signal of immune tolerance of the ectopic lesions in endometriosis? Reprod Biomed Online. 2021;43:319–28. [DOI] [PubMed] [Google Scholar]
- 44.Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS. Systemic inflammation Induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b-5p and Let-7b-5p regulate macrophage cytokine production. J Clin Endocrinol Metab. 2018;103:64–74. [DOI] [PubMed] [Google Scholar]
- 45.Chopyak VV, Koval HD, Havrylyuk AM, Lishchuk-Yakymovych KA, Potomkina HA, Kurpisz MK. Immunopathogenesis of endometriosis - a novel look at an old problem. Cent Eur J Immunol. 2022;47:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Păvăleanu I, Balan RA, Grigoraş A, Balan TA, Amălinei C. The significance of immune microenvironment in patients with endometriosis. Rom J Morphol Embryol. 2023;64:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisovar A, Becker CM, Granne I, Southcombe JH. The role of CD8 + T cells in endometriosis: a systematic review. Front Immunol. 2023;14:1225639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z, Kong B, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: our understandings of their functions in uterus and ovary. Int Immunopharmacol. 2011;11:1442–50. [DOI] [PubMed] [Google Scholar]
- 49.Nie MF, Xie Q, Wu YH, He H, Zou LJ, She XL, Wu XQ. Serum and Ectopic Endometrium from Women with Endometriosis Modulate Macrophage M1/M2 Polarization via the Smad2/Smad3 Pathway. J Immunol Res 2018, 2018:6285813. [DOI] [PMC free article] [PubMed]
- 50.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo M, Zhao F, Cheng H, Su M, Wang Y. Macrophage polarization: an important role in inflammatory diseases. Front Immunol. 2024;15:1352946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan Y, La R, Jiang M, Xu W, Jiang D, Wang S, Huang L, Wu Q. The association between remnant cholesterol and rheumatoid arthritis: insights from a large population study. Lipids Health Dis. 2024;23:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skouby SO, Endrikat J, Düsterberg B, Schmidt W, Gerlinger C, Wessel J, Goldstein H, Jespersen J. A 1-year randomized study to evaluate the effects of a dose reduction in oral contraceptives on lipids and carbohydrate metabolism: 20 microg ethinyl estradiol combined with 100 microg levonorgestrel. Contraception. 2005;71:111–7. [DOI] [PubMed] [Google Scholar]
- 54.Teran AZ, Greenblatt RB, Chaddha JS. Changes in lipoproteins with various sex steroids. Obstet Gynecol Clin North Am. 1987;14:107–19. [PubMed] [Google Scholar]
- 55.Piekarski DJ, Johnson CM, Boivin JR, Thomas AW, Lin WC, Delevich K, E MG, Wilbrecht L. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res. 2017;1654:123–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiatt RA, Stewart SL, Hoeft KS, Kushi LH, Windham GC, Biro FM, Pinney SM, Wolff MS, Teitelbaum SL, Braithwaite D. Childhood socioeconomic position and Pubertal Onset in a cohort of multiethnic girls: implications for breast Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deardorff J, Abrams B, Ekwaru JP, Rehkopf DH. Socioeconomic status and age at menarche: an examination of multiple indicators in an ethnically diverse cohort. Ann Epidemiol. 2014;24:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iavazzo C, Vrachnis N, Gkegkes ID. Peritoneal bacteria contamination and endometriosis pathogenesis. Arch Gynecol Obstet. 2023;307:221–2. [DOI] [PubMed] [Google Scholar]
- 59.Lu MY, Niu JL, Liu B. The risk of endometriosis by early menarche is recently increased: a meta-analysis of literature published from 2000 to 2020. Arch Gynecol Obstet. 2023;307:59–69. [DOI] [PubMed] [Google Scholar]
- 60.Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emons G, Gründker C, Hanf V. [Are estrogens carcinogens?]. Zentralbl Gynakol. 2002;124:559–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 2: Association between RC and endometriosis. Adjustments have been made for all covariates base on model 3. (A) Each red dot represents a separate sample, and the blue dots above and below the red dot represent 95% confidence intervals. (B) The red solid line is the smooth fitting curve between RC levels and EMs, while the blue dashed line reflects the 95% confidence intervals of the fitting. RC, Remnant Cholesterol; EMs, endometriosis.
Supplementary Material 3: Association between RC and endometriosis. Adjustments have been made for all covariates base on model 3. (A) Each red dot represents a separate sample, and the blue dots above and below the red dot represent 95% confidence intervals. (B) The red solid line is the smooth fitting curve between RC levels and EMs, while the blue dashed line reflects the 95% confidence intervals of the fitting. RC, Remnant Cholesterol; EMs, endometriosis.
Data Availability Statement
No datasets were generated or analysed during the current study.