Abstract
Objective
Depression is a common comorbidity in cardiovascular disease (CVD), and both conditions are associated with chronic inflammation. The systemic immune-inflammation index (SII) has emerged as a promising marker of systemic inflammation, but its role in association with depressive symptoms, particularly in the context of CVD, remains unclear. This study aims to investigate the association of SII with depressive symptoms in individuals with and without CVD using cross-sectional data from NHANES (2005–2016).
Methods
A total of 29,479 participants from the National Health and Nutrition Examination Survey (NHANES) 2005–2016 waves were included. Depressive symptoms were assessed through Patient’s Health Questionnaire (PHQ-9). SII was calculated as the platelet count × neutrophil count/lymphocyte count. In order to determine the relationships between SII and depressive symptoms in participants with and without CVD, binary logistic regression model and smooth curve fitting were used. We also performed sensitivity analyses and subgroup analysis.
Results
The total prevalence of depressive symptoms was 8.73% among the 29,479 participants analyzed. After adjusting for confounding factors, a higher SII was significantly associated with increased depressive symptoms in the total population (OR per SD increase: 1.101, 95% CI: 1.060–1.144, P < 0.0001). This association was stronger in participants without CVD (OR: 1.121, 95% CI: 1.073–1.172, P < 0.0001) compared to those with CVD (OR: 1.055, 95% CI: 0.973–1.144, P = 0.19571). Participants in the highest SII tertile had a significantly higher risk of depressive symptoms compared to those in the lowest tertile, particularly in the non-CVD group (OR: 1.161, 95% CI: 1.026–1.313, P = 0.01765).
Conclusion
The SII is independently associated with an increased risk of depressive symptoms, particularly in individuals without CVD. These findings suggest that the SII may serve as a valuable predictor of depressive symptoms in the general population, with potential implications for early screening and intervention strategies. Further research is needed to elucidate the mechanisms underlying this association and to explore the clinical utility of SII in depressive symptoms assessment, especially in the context of cardiovascular health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-024-03314-5.
Keywords: Systemic immune-inflammation index, Depression, Cardiovascular disease, Inflammation, NHANES
Introduction
Depression is a common mental health disorder that affects millions of people worldwide and is a leading cause of disability [1]. It is particularly prevalent among individuals with cardiovascular disease (CVD), with studies estimating that up to 20–40% of CVD patients experience depressive symptoms [2]. The relationship between depression and CVD appears to be bidirectional, with each condition increasing the risk of developing or worsening the other [3]. This comorbidity is associated with poorer health outcomes, increased mortality, and higher healthcare costs, highlighting the need for effective screening and early intervention strategies [4].
Chronic inflammation has emerged as a potential common pathway linking depression and CVD [5]. Several inflammatory markers, such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have been associated with both conditions [3]. Recently, the systemic immune-inflammation index (SII), calculated as platelet count × neutrophil count/lymphocyte count, has gained attention as a novel marker of systemic inflammation [6]. The SII has shown promise in predicting outcomes in various diseases, including cancer, nonalcoholic fatty liver disease, and cardiovascular disorders [7–9]. However, its potential role in predicting depression risk, particularly in the context of CVD, remains largely unexplored.
Given the complex interplay between depression, CVD, and inflammation, investigating the predictive value of the SII for depression could provide valuable insights into the underlying mechanisms and potential screening tools. This study aims to examine the association between SII and depressive symptoms in a large, nationally representative sample of US adults, with a particular focus on comparing this relationship in individuals with and without CVD. By clarifying the potential of SII as a predictor of depression, especially in the context of cardiovascular health, this research may contribute to the development of more targeted screening and intervention strategies for these interrelated conditions.
Methods
Study design and participants
This study utilized data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2005 and 2016. The analyses are cross-sectional, assessing the association between systemic immune-inflammation index (SII) and depressive symptoms for each participant at a single point in time. NHANES is a cross-sectional, nationally representative survey of the non-institutionalized civilian population in the United States, designed to assess the health and nutritional status of adults and children. The survey combines interviews, physical examinations, and laboratory tests, providing comprehensive data on a wide range of health topics. NHANES uses a complex, multistage probability sampling design to select participants, ensuring that the sample is representative of the US population across all ages, races, and ethnicities. The survey protocols were approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent.
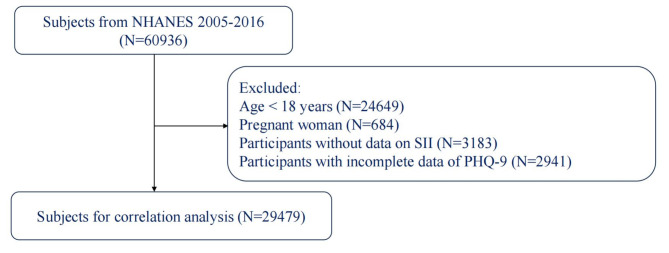
From the original dataset of 60,936 subjects in the NHANES 2005–2016 cycles, we applied several exclusion criteria to define our study population (Fig. 1). We excluded participants younger than 18 years of age (n = 24,649) and pregnant women (n = 684) to focus on the adult, non-pregnant population. Additionally, we removed participants lacking data necessary for calculating the systemic immune-inflammation index (SII) (n = 3,183) and those with incomplete responses to the Patient Health Questionnaire-9 (PHQ-9) for depression assessment (n = 2,941). After applying these exclusion criteria, our final analytic sample consisted of 29,479 participants.
Fig. 1.
Flow chart of participant selection from the National Health and Nutrition Examination Survey (NHANES) 2005–2016
Assessment of depressive symptoms
Depressive symptoms were evaluated using the Patient Health Questionnaire-9 (PHQ-9), a widely validated screening tool for depression [10]. The PHQ-9 consists of nine items that assess the frequency of depressive symptoms over the past two weeks, corresponding to the diagnostic criteria for major depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [11]. Participants rated each item on a four-point Likert scale ranging from 0 (not at all) to 3 (nearly every day), resulting in a total score between 0 and 27. The internal consistency of the PHQ-9 in our sample was good (Cronbach’s α = 0.84). In line with previous research and clinical guidelines, we defined clinically significant depressive symptoms as a PHQ-9 score ≥ 10 [12, 13]. This cut-off has demonstrated high sensitivity and specificity for identifying major depression in various populations [14].
Calculation of the SII Index
The SII is calculated using complete blood count parameters as: SII = platelet count × neutrophil count / lymphocyte count [6, 15, 16]. The platelet, neutrophil and lymphocyte counts are typically measured using automated hematology analyzers and reported as 1000 cells/µl. SII integrates three types of inflammatory cells - platelets, neutrophils and lymphocytes - to provide a comprehensive assessment of the balance between host inflammatory and immune status [8, 17]. This novel index was first proposed by Hu et al. in 2014 and has since been widely investigated as a marker of systemic inflammation in various conditions [6]. The SII is considered advantageous over traditional inflammatory markers as it reflects both inflammatory and immune system components [18]. Furthermore, SII index was categorized into quartiles: Q1 (< 332.12), Q2 (332.12–465.75), Q3 (465.75–656.5), and Q4 (> 656.5).
Assessment of potential covariates
Potential covariates were selected based on their established associations with CVD and depression in previous literature and their availability in the NHANES database. Sociodemographic factors included age, sex, race/ethnicity (Mexican American, Non-Hispanic Black, Non-Hispanic White, Other), education level (below high school, high school graduate, above high school), marital status (unmarried, married), and family poverty income ratio. Health-related factors encompassed body mass index (BMI), smoking status (never, former, current), alcohol consumption (no, yes), history of malignancy, diabetes, and hypertension. Medication use was also considered, including statins, antidepressants, antidiabetic agents, and antihypertensive medications. These variables were assessed through standardized questionnaires, physical examinations, and laboratory tests conducted as part of the NHANES protocol. BMI defined as weight in kilograms divided by the square of height in meters (kg/m2). Hypertension was defined as having an average systolic blood pressure exceeding 140 mmHg, a diastolic blood pressure greater than 90 mmHg, or self-reported use of antihypertensive medication. Diabetes was identified by any of the following criteria: being informed by a doctor or health professional that one has diabetes, currently taking insulin, a fasting glucose level of ≥ 126 mg/dL, or a glycohemoglobin level of ≥ 6.5%. A former smoker is defined as an adult who has smoked at least 100 cigarettes in their lifetime but has quit smoking at the time of the interview. A never smoker is an adult who has never smoked or has smoked fewer than 100 cigarettes in their lifetime. Participants who have ever been diagnosed with any of the following conditions-congestive heart failure, coronary heart disease, angina/angina pectoris, heart attack, or stroke-are classified as having CVD. For more detailed information on the measurement procedures, additional details can be found on the NHANES website (www.cdc.gov/nchs/nhanes/).
Statistical analysis
All statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) and EmpowerStats (X&Y Solutions, Inc, Boston, MA), and accounted for the complex survey design of NHANES, including sampling weights, stratification, and clustering. Baseline characteristics were presented as weighted means (95% confidence intervals) for continuous variables and weighted percentages (95% confidence intervals) for categorical variables. Differences between participants with and without CVD were assessed using survey-weighted linear regression for continuous variables and survey-weighted chi-square tests for categorical variables. The association between SII and depression was examined using survey-weighted logistic regression models. SII was analyzed both as a continuous variable (per standard deviation increase) and as a categorical variable (tertiles). Three models were constructed with progressive adjustment for potential confounders: Model 1 was unadjusted; Model 2 adjusted for sex, age, BMI, race/ethnicity, education level, marital status, and family poverty income ratio; Model 3 further adjusted for history of malignancy, diabetes, hypertension, and use of statins, antidepressants, antidiabetic agents, and antihypertensive medications. Subgroup analyses were performed to assess the consistency of the association across various demographic and clinical characteristics. A smooth curve fitting was conducted to examine potential non-linear relationships between SII and depression. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
A total of 29,479 participants were included in this analysis. Table 1 presents the baseline characteristics of participants stratified by CVD status. Compared to those without CVD, participants with CVD were older (64.61 vs. 46.10 years, P < 0.0001), had a higher BMI (30.58 vs. 28.79 kg/m², P < 0.0001), and lower family poverty income ratio (2.54 vs. 3.07, P < 0.0001). The CVD group had a higher proportion of males (54.36% vs. 48.80%, P < 0.0001) and Non-Hispanic Whites (75.30% vs. 68.85%, P < 0.0001). They also had higher rates of diabetes (33.66% vs. 10.03%, P < 0.0001), hypertension (75.35% vs. 34.55%, P < 0.0001), and depression (14.03% vs. 6.97%, P < 0.0001). Medication use, including statins, antidepressants, and antidiabetic agents, was significantly higher in the CVD group (all P < 0.0001). Notably, the mean PHQ-9 score was significantly higher in the CVD group (4.29 vs. 2.88, P < 0.0001), indicating a higher prevalence of depressive symptoms.
Table 1.
Baseline characteristics of the participants in CVD and non-CVD groups
| Variables | Non-CVD | CVD | P |
|---|---|---|---|
| PHQ-9 score | 2.882 (2.791 ,2.972) | 4.293 (4.034 ,4.553) | < 0.0001 |
| Age, years | 46.098 (45.622 ,46.573) | 64.608 (63.932 ,65.284) | < 0.0001 |
| Family Poverty Income Ratio | 3.070 (2.995 ,3.145) | 2.538 (2.442 ,2.633) | < 0.0001 |
| BMI, kg/m2 | 28.790 (28.618 ,28.961) | 30.576 (30.227 ,30.926) | < 0.0001 |
| Sex | < 0.0001 | ||
| Female | 51.197 (50.574 ,51.819) | 45.645 (43.262 ,48.047) | |
| Male | 48.803 (48.181 ,49.426) | 54.355 (51.953 ,56.738) | |
| Race/Ethnicity | < 0.0001 | ||
| Mexican American | 8.626 (7.237 ,10.253) | 4.654 (3.558 ,6.066) | |
| Non-Hispanic Black | 10.444 (9.084 ,11.980) | 11.297 (9.676 ,13.150) | |
| Non-Hispanic White | 68.845 (65.940 ,71.609) | 75.295 (72.348 ,78.024) | |
| Other | 12.085 (10.929 ,13.344) | 8.754 (7.435 ,10.281) | |
| Education level | < 0.0001 | ||
| Below high school | 15.600 (14.351 ,16.937) | 24.248 (22.051 ,26.590) | |
| High school graduate | 22.276 (21.269 ,23.317) | 26.801 (24.517 ,29.215) | |
| Above high school | 62.123 (60.206 ,64.004) | 48.951 (45.910 ,52.000) | |
| Marital status | 0.0004 | ||
| Unmarried | 35.682 (34.357 ,37.030) | 40.029 (37.364 ,42.754) | |
| Married | 64.318 (62.970 ,65.643) | 59.971 (57.246 ,62.636) | |
| Malignancy | < 0.0001 | ||
| No | 91.015 (90.453 ,91.547) | 78.280 (76.036 ,80.368) | |
| Yes | 8.985 (8.453 ,9.547) | 21.720 (19.632 ,23.964) | |
| Smoking status | < 0.0001 | ||
| never | 55.699 (54.445 ,56.947) | 37.950 (35.757 ,40.193) | |
| former | 23.711 (22.711 ,24.740) | 39.328 (37.308 ,41.385) | |
| now | 20.590 (19.593 ,21.625) | 22.722 (20.733 ,24.842) | |
| Diabetes | < 0.0001 | ||
| No | 89.970 (89.353 ,90.555) | 66.338 (64.200 ,68.412) | |
| Yes | 10.030 (9.445 ,10.647) | 33.662 (31.588 ,35.800) | |
| Hypertension | < 0.0001 | ||
| No | 65.450 (64.415 ,66.470) | 24.652 (22.489 ,26.952) | |
| Yes | 34.550 (33.530 ,35.585) | 75.348 (73.048 ,77.511) | |
| Alcohol drinker | 0.0089 | ||
| No | 49.159 (46.727 ,51.596) | 43.123 (39.048 ,47.294) | |
| Yes | 50.841 (48.404 ,53.273) | 56.877 (52.706 ,60.952) | |
| Statins use | < 0.0001 | ||
| No | 86.728 (86.070 ,87.360) | 42.397 (39.987 ,44.844) | |
| Yes | 13.272 (12.640 ,13.930) | 57.603 (55.156 ,60.013) | |
| Antidepressants | < 0.0001 | ||
| No | 87.765 (87.006 ,88.485) | 77.802 (75.693 ,79.776) | |
| Yes | 12.235 (11.515 ,12.994) | 22.198 (20.224 ,24.307) | |
| Antidiabetic agents | < 0.0001 | ||
| No | 93.061 (92.561 ,93.529) | 74.494 (72.508 ,76.383) | |
| Yes | 6.939 (6.471 ,7.439) | 25.506 (23.617 ,27.492) | |
| Antihypertensive Medications | < 0.0001 | ||
| No | 94.522 (94.071 ,94.941) | 89.105 (87.388 ,90.614) | |
| Yes | 5.478 (5.059 ,5.929) | 10.895 (9.386 ,12.612) | |
| Depression | < 0.0001 | ||
| No | 93.027 (92.533 ,93.490) | 85.971 (84.123 ,87.635) | |
| Yes | 6.973 (6.510 ,7.467) | 14.029 (12.365 ,15.877) |
For continuous variables: survey-weighted mean (95% CI), P-value was by survey-weighted linear regression (svyglm); For categorical variables: survey-weighted percentage (95% CI), P-value was by survey-weighted Chi-square test (svytable)
Association between SII and depression in participants with and without CVD
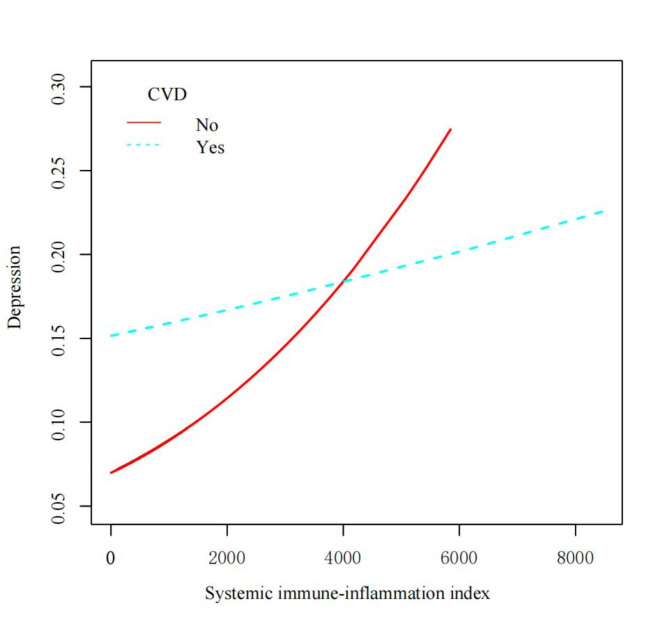
Figure 2 illustrates the relationship between the SII and the probability of depression, stratified by CVD status. The smoothed curves reveal a generally positive association between SII and depressive symptoms for both groups. However, the relationship appears to be stronger and more linear in participants without CVD compared to those with CVD. For participants without CVD, the probability of depression increases steadily with increasing SII values, showing a nearly linear trend. The curve starts at a lower probability of depression for low SII values and rises consistently as SII increases. In contrast, the curve for participants with CVD shows a more complex pattern. It begins at a higher baseline probability of depression compared to the non-CVD group, even at low SII values. The curve then demonstrates a slight increase in depression probability as SII increases, but the slope is less steep compared to the non-CVD group.
Fig. 2.
Smooth curve fitting of the relationship between the systemic immune-inflammation index (SII) and the probability of depression, stratified by cardiovascular disease (CVD) status
Table 2 presents the association between SII and depressive symptoms among participants with and without CVD. In the fully adjusted model (Model 3), a significant association between SII and depressive symptoms was observed in the total population (OR per SD increase: 1.101, 95% CI: 1.060–1.144, P < 0.00001). When stratified by CVD status, the association remained significant in participants without CVD (OR: 1.121, 95% CI: 1.073–1.172, P < 0.00001), but not in those with CVD (OR: 1.055, 95% CI: 0.973–1.144, P = 0.19571). When SII was categorized into tertiles, participants in the highest tertile had a significantly higher risk of depression compared to those in the lowest tertile in the total population (OR: 1.166, 95% CI: 1.043–1.303, P = 0.00689) and in participants without CVD (OR: 1.161, 95% CI: 1.026–1.313, P = 0.01765). However, this association was not statistically significant in participants with CVD (OR: 1.228, 95% CI: 0.940–1.603, P = 0.13154).
Table 2.
Association between systemic immune-inflammation index (SII) and risk of depression among participants with and without CVD
| Exposure | Non-CVD | CVD | Total |
|---|---|---|---|
| Model 1 | |||
| SII (per SD change) | 1.168 (1.123, 1.215) < 0.00001 | 1.033 (0.965, 1.106) 0.35074 | 1.129 (1.091, 1.168) < 0.00001 |
| SII tertile | |||
| Low | 1(Reference) | 1(Reference) | 1(Reference) |
| Middle | 0.992 (0.883, 1.115) 0.89629 | 0.983 (0.758, 1.274) 0.89589 | 0.990 (0.890, 1.102) 0.85997 |
| High | 1.331 (1.190, 1.487) < 0.00001 | 1.244 (0.985, 1.570) 0.06646 | 1.314 (1.188, 1.453) < 0.00001 |
| Model 2 | |||
| SII (per SD change) | 1.128 (1.080, 1.179) < 0.00001 | 1.060 (0.979, 1.147) 0.14965 | 1.107 (1.066, 1.150) < 0.00001 |
| SII tertile | |||
| Low | 1(Reference) | 1(Reference) | 1(Reference) |
| Middle | 0.969 (0.854, 1.100) 0.62800 | 0.917 (0.687, 1.224) 0.55720 | 0.965 (0.860, 1.083) 0.54325 |
| High | 1.181 (1.045, 1.335) 0.00785 | 1.233 (0.946, 1.608) 0.12127 | 1.183 (1.059, 1.322) 0.00297 |
| Model 3 | |||
| SII (per SD change) | 1.121 (1.073, 1.172) < 0.00001 | 1.055 (0.973, 1.144) 0.19571 | 1.101 (1.060, 1.144) < 0.00001 |
| SII tertile | |||
| Low | 1(Reference) | 1(Reference) | 1(Reference) |
| Middle | 0.969 (0.853, 1.100) 0.62296 | 0.926 (0.692, 1.238) 0.60270 | 0.966 (0.860, 1.084) 0.55289 |
| High | 1.161 (1.026, 1.313) 0.01765 | 1.228 (0.940, 1.603) 0.13154 | 1.166 (1.043, 1.303) 0.00689 |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
Model 1 adjust for: None
Model 2 adjust for: Sex, age, BMI, race/ethnicity, education level, marital status, family poverty income ratio;
Model 3 adjust for: Sex, age, BMI, race/ethnicity, education level, marital status, family poverty income ratio, malignancy, diabetes, hypertension, statins use, antidepressants, antidiabetic agents, antihypertensive medications.
The trend analysis showed a significant dose-response relationship between SII tertiles and depressive symptoms in both CVD and non-CVD groups (P for trend < 0.001), suggesting a gradual increase in depressive symptoms with increasing SII levels. These results indicate that the association between SII and depressive symptoms is more pronounced in individuals without CVD, suggesting potential differences in the inflammatory mechanisms underlying depression in the presence or absence of CVD.
Subgroup analysis and sensitivity analysis
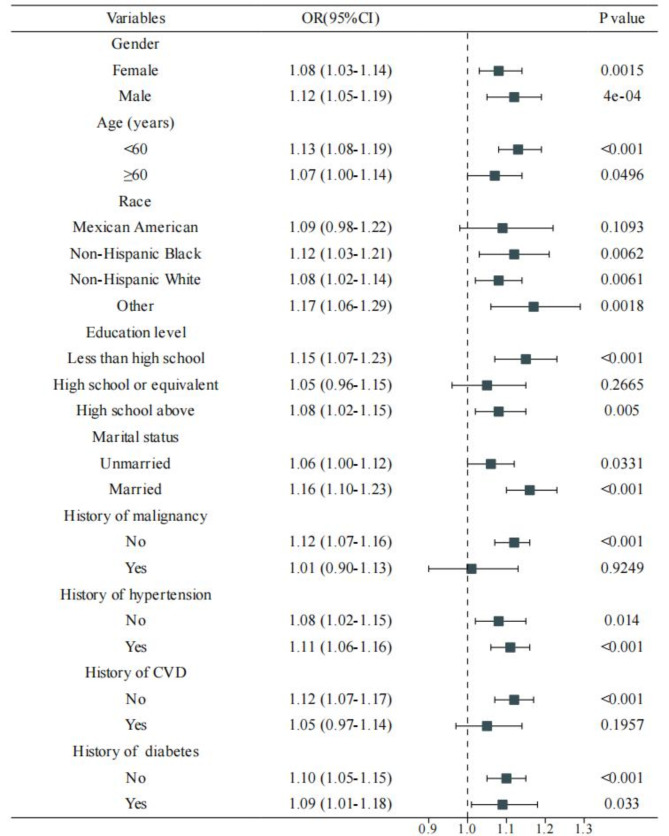
Subgroup analyses were conducted to assess the consistency of the association between SII and depression across various demographic and clinical characteristics (Fig. 3). The positive association between SII and depressive symptoms was generally consistent across most subgroups, with some notable variations. The association was stronger in males, younger participants (< 60 years), and those with less than high school education. Interestingly, the association was significant in participants without a history of malignancy but not in those with a history of malignancy. Consistent with our main findings, the subgroup analysis confirmed a stronger association in participants without CVD (OR: 1.12, 95% CI: 1.07–1.17, P < 0.001) compared to those with CVD (OR: 1.05, 95% CI: 0.97–1.14, P = 0.1957).
Fig. 3.
Forest plot showing the association between systemic immune-inflammation index (SII) and depression across various subgroups
To assess the robustness of our findings, we conducted a sensitivity analysis by excluding participants with a history of malignancy (Table S1). The results remained largely consistent with our main analysis. In the fully adjusted model, the association between SII and depression remained significant in the total population and in participants without CVD, while it was not statistically significant in participants with CVD. However, when SII was categorized into tertiles, the association became statistically significant in participants with CVD after excluding those with malignancy.
These subgroup and sensitivity analyses strengthen the overall association between SII and depression, while also highlighting potential variations across different demographic and clinical subgroups. The consistency of results after excluding participants with malignancy strengthens the validity of our findings.
Discussion
In this large-scale study utilizing data from the NHANES, we investigated the association between the SII and depression in individuals with and without CVD. Our findings reveal a significant positive association between SII and depressive symptoms in the overall population, with a notably stronger relationship observed in individuals without CVD. It is important to note that the cross-sectional design of our study precludes the ability to infer causality or longitudinal predictions between SII and depressive symptoms. Specifically, we found that a higher SII was associated with an increased risk of depression in the total population, with this association being more pronounced in participants without CVD compared to those with CVD. Furthermore, our analysis revealed a dose-response relationship, with participants in the highest SII tertile demonstrating a significantly higher risk of depression compared to those in the lowest tertile, particularly in the non-CVD group (OR: 1.161, 95% CI: 1.026–1.313). These findings highlight the potential value of SII as a predictor of depression, especially in individuals without CVD.
The observed association between higher SII and increased depression corresponds with the growing body of evidence supporting the role of inflammation in the pathophysiology of depression [19, 20]. The SII, which integrates information on platelets, neutrophils, and lymphocytes, provides a comprehensive measure of systemic inflammation that may reflect the complex interplay between immune activation and depression. Several mechanisms may underlie this association. Elevated inflammatory markers can affect neurotransmitter metabolism, neuroendocrine function, and neural plasticity, all of which are implicated in the development of depressive symptoms [19, 21]. Furthermore, pro-inflammatory cytokines can activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to increased cortisol levels and subsequent alterations in mood and behavior [22]. The stronger association observed in participants without CVD is particularly interesting. This finding suggests that in the absence of established cardiovascular disease, systemic inflammation as measured by SII may play a more prominent role in depression. In individuals with CVD, the relationship between inflammation and depression may be confounded by other factors related to cardiovascular health, medication use, or lifestyle changes associated with CVD management. The dose-response relationship observed in our study further strengthens the evidence for a potential causal link between systemic inflammation and depression risk, highlighting the importance of considering inflammatory markers in depression risk assessment and management strategies.
Our findings extend previous research on inflammatory markers and depression by focusing on the SII, a composite index that may offer advantages over single inflammatory markers. While previous studies have demonstrated associations between individual inflammatory markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) with depression risk, results have been inconsistent [23, 24]. The SII, by incorporating multiple components of the immune system, may provide a more comprehensive and stable measure of systemic inflammation. Our results are consistent with studies that have shown positive associations between other inflammatory indices, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), and depression risk [25, 26]. However, our study is novel in its exploration of SII in relation to depression risk, particularly in the context of CVD status. By demonstrating a stronger association in non-CVD participants, our findings contribute to the growing understanding of the differential impact of inflammation on mental health in various clinical contexts.
The subgroup analyses in our study revealed important insights into the relationship between SII and depression risk across different demographic and clinical characteristics. Notably, we observed a stronger association in males, younger participants (< 60 years), and those with less than high school education. These findings suggest that the impact of systemic inflammation on depression risk may vary across different population subgroups. The stronger association in males is particularly interesting, as it contrasts with the generally higher prevalence of depression in females [27]. This finding may indicate that inflammatory processes play a more significant role in the pathophysiology of depression in males, or that males are more susceptible to the depressogenic effects of inflammation. The stronger association in younger participants suggests that early-life inflammation may have a more profound impact on mental health, highlighting the importance of early intervention and prevention strategies. The increased vulnerability observed in individuals with lower education levels may reflect the complex interplay between socioeconomic factors, inflammation, and mental health, underscoring the need for targeted interventions in disadvantaged populations [28].
The potential clinical implications of our findings are substantial. The SII, being a readily available and cost-effective biomarker derivable from routine blood tests, could serve as a valuable tool for identifying individuals at higher risk of depression, particularly in non-CVD populations [6, 29]. This could facilitate early screening and intervention strategies, potentially improving outcomes and reducing the burden of depression. In clinical practice, elevated SII levels could prompt more thorough mental health assessments and closer monitoring of depressive symptoms. Furthermore, our findings suggest that anti-inflammatory interventions may have potential as preventive or therapeutic strategies for depression, especially in individuals without CVD [30, 31]. However, it is crucial to note that while our study demonstrates an association, it does not establish causality, and further research is needed to determine the clinical utility of SII in depression risk assessment and management.
Our study has several strengths that enhance the reliability and generalizability of our findings. First, the large sample size derived from the NHANES database provides robust statistical power and enhances the representativeness of our results. Second, we conducted comprehensive adjustments for a wide range of potential confounding factors, including demographic characteristics, lifestyle factors, and comorbidities, thereby reducing the likelihood of false associations. Third, the use of smooth curve fitting allowed us to visualize the non-linear relationships between SII and depression risk, providing a more nuanced understanding of this association. Despite these strengths, several limitations should be considered when interpreting our results. First, the cross-sectional nature of our study precludes the establishment of causal relationships between SII and depression symptoms. Longitudinal studies are needed to elucidate the temporal relationship between systemic inflammation and the development of depressive symptoms. Second, while we adjusted for a comprehensive set of confounding factors, the possibility of residual confounding due to unmeasured variables cannot be ruled out. Third, our assessment of depressive symptoms relied on PHQ-9 rather than clinical diagnoses, which may have introduced some misclassification bias. Future studies incorporating structured clinical interviews for depression diagnosis would be valuable. A limitation of this study is the reliance on a single SII measurement due to the cross-sectional design of NHANES. While SII is a robust indicator of systemic inflammation, its variability over time-potentially influenced by transient or chronic factors-remains unaccounted for in our analysis. Future longitudinal studies with serial SII measurements are needed to better understand its stability and role in predicting depressive symptoms. Another limitation of our study is the uncertain clinical significance of the observed associations. Although we found statistically significant associations between SII and depressive symptoms, the lack of data on other established risk factors for clinical depression, such as previous depressive episodes, family history, or psychosocial stressors, limits our ability to assess the clinical relevance of these findings fully. Future research incorporating these factors may provide more clarity on the practical importance of inflammation in the development of depression. Furthermore, the mean PHQ-9 scores in both the CVD and non-CVD groups were within the normal range, which means that our study primarily captures mild depressive symptoms. This limits our ability to assess the strength of the association between SII and depression in individuals with moderate or severe depression. Further research focusing on more severe cases of depression is needed to better understand the role of systemic inflammation in these populations.
Conclusion
Our study provides strong evidence for an independent association between the SII and depression risk, particularly in individuals without cardiovascular disease. These findings highlight the potential of SII as a valuable predictor of depression symptoms in the general population, with implications for early screening and intervention strategies. The observed dose-response relationship and the consistency of results across various subgroups strengthen the validity of our findings. While further research is needed to establish causality and explore the clinical utility of SII in depression assessment, our study contributes significantly to the growing body of evidence linking inflammation and mental health. By elucidating the differential impact of systemic inflammation on depression risk in CVD and non-CVD populations, our findings open new avenues for understanding the complex interplay between cardiovascular health, inflammation, and depression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
LD conceived the study and wrote the first draft of manuscript, HCJ were responsible for data collection and analysis, and LXG was responsible for figure visualization and revising manuscript. All authors reviewed and approved the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 82300363), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ23H020001), and Zhejiang Province Clinical Key Specialty Construction Project (Grant No. 2024-ZJZK-0001).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The need to obtain written informed consent from each patient was waived due to the retrospective, record-based nature of this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dan Liu and Chaojie He contributed equally to this work.
References
- 1.Global regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis, for the Global Burden of Disease Study 2019. The lancet Psychiatry, Pubmed Central PMCID, PMC8776563 Health, the Royal Academy of Engineering, the US Academy of Medical Sciences, the US National Institutes of Health., Conselho Nacional de Desenvolvimento Científico e Tecnológico, the UK Medical Research Council, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul; and consulting fees from the United Nations Children’s Fund, outside the submitted work. P B Mitchell reports grants from the Australian National Health and Medical Research Council; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen Australia, outside the submitted work. G C Patton reports support for the present manuscript from the Australia National Health and Medical Research Council. J B Soriano reports participation in the Institute for Health Metrics and Evaluation’s Tobacco Advisory board, outside the submitted work. D J Stein reports royalties or licenses from Elsevier and the American Psychiatric Press; consulting fees from Johnson & Johnson, Lundbeck, Sanofi, and Vistagen; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier and Takeda, outside the submitted work. M B Stein reports grants or contracts from the US National Institute of Mental Health, US Department of Defense, and US Department of Veterans Affairs; consulting fees from Aptinyx, Acadia Pharmaceuticals, Bionomics, Boehringer-Ingelheim, Clexio, EmpowerPharm, Engrail, GW Pharmaceuticals, Janssen, Kazz Pharmaceuticals, and Roche/Genentech; stocks from Pfizer; holds stock options in Epivario and Oxeia Biopharmaceuticals, and owns mutual funds that might contain pharmaceutical stocks; and is the Editor-in-Chief of Depression and Anxiety, Deputy Editor of Biological Psychiatry, and Co-Editor-in-Chief of UptoDate (Psychiatry), outside the submitted work. C E I Szoeke acknowledges support for the present manuscript from National Health and Medical Research Council Australia funding (1032350 and 1062133) paid to the University of Melbourne; and acknowledges payment for expert testimony from the Victorian Department of Health, and for leadership or fiduciary role in board, society, committee or advocacy group, paid or unpaid with the American Medical Association, outside the submitted work. All other authors declare no competing interests. Epub 2022/01/14. eng.
- 2.Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–72. PubMed PMID: 24282187. Epub 2013/11/28. eng. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur Heart J. 2020;41(17):1687–96. PubMed PMID: 30698764. Pubmed Central PMCID: PMC10941327. Epub 2019/01/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha MK, Qamar A, Vaduganathan M, Charney DS, Murrough JW. Screening and management of Depression in patients with Cardiovascular Disease: JACC State-of-the-art review. J Am Coll Cardiol. 2019;73(14):1827–45. PubMed PMID: 30975301. Pubmed Central PMCID: PMC7871437. Epub 2019/04/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–56. PubMed PMID: 32553197. Pubmed Central PMCID: PMC7381373. Epub 2020/06/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin cancer Research: Official J Am Association Cancer Res. 2014;20(23):6212–22. PubMed PMID: 25271081. Epub 2014/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Qu Y, Wen F, Yu R, He X, Jia H, et al. Prognostic nutritional index and systemic immune-inflammation index are prognostic biomarkers for non-small-cell lung cancer brain metastases. Biomark Med. 2021;15(13):1071–84. PubMed PMID: 34397267. Epub 2021/08/17. eng. [DOI] [PubMed] [Google Scholar]
- 8.Xiao S, Wang Z, Zuo R, Zhou Y, Yang Y, Chen T, et al. Association of systemic Immune inflammation index with All-Cause, Cardiovascular Disease, and Cancer-related mortality in patients with Cardiovascular Disease: a cross-sectional study. J Inflamm Res. 2023;16:941–61. PubMed PMID: 36908696. Pubmed Central PMCID: PMC9999722. Epub 2023/03/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao E, Cheng Y, Yu C, Li H, Fan X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med. 2023;55(1):2197652. PubMed PMID: 37052341. Pubmed Central PMCID: PMC10115001. Epub 2023/04/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. PubMed PMID: 11556941. Pubmed Central PMCID: PMC1495268. Epub 2001/09/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strong DR, Kahler CW, Colby SM, Griesler PC, Kandel D. Linking measures of adolescent nicotine dependence to a common latent continuum. Drug Alcohol Depend. 2009;99(1–3):296–308. PubMed PMID: 18938047. Pubmed Central PMCID: PMC2655729. Epub 2008/10/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levis B, Benedetti A, Thombs BD. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ (Clinical research ed). 2019;365:l1476. PubMed PMID: 30967483. Pubmed Central PMCID: PMC6454318 at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work other than that described above; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years with the following exceptions: NJ and SP received a grant, outside the submitted work, from the University of Calgary Hotchkiss Brain Institute, which was jointly funded by the Institute and Pfizer; Pfizer was the original sponsor of the development of the PHQ-9, which is now in the public domain; JCNC is a steering committee member or consultant of Astra Zeneca, Bayer, Lilly, MSD, and Pfizer and has received sponsorships and honorariums for giving lectures and providing consultancy, and her affiliated institution has received research grants from these companies; UH was an advisory board member for Lundbeck and Servier, a consultant for Bayer Pharma, and a speaker for Roche Pharma and Servier and has received personal fees from Janssen, all outside the submitted work; MI has received a grant from Novartis Pharma and personal fees from Meiji, Mochida, Takeda, Novartis, Yoshitomi, Pfizer, Eisai, Otsuka, MSD, Technomics, and Sumitomo Dainippon, all outside of the submitted work; no other relationships or activities that could appear to have influenced the submitted work. Epub 2019/04/11. eng. [DOI] [PMC free article] [PubMed]
- 13.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ: Can Med Association J = J de l’Association medicale canadienne. 2012;184(3):E191–6. PubMed PMID: 22184363. Pubmed Central PMCID: PMC3281183. Epub 2011/12/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel JS, Oh Y, Rand KL, Wu W, Cyders MA, Kroenke K, et al. Measurement invariance of the patient health questionnaire-9 (PHQ-9) depression screener in U.S. adults across sex, race/ethnicity, and education level: NHANES 2005–2016. Depress Anxiety. 2019;36(9):813–23. PubMed PMID: 31356710. Pubmed Central PMCID: PMC6736700. Epub 2019/07/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X, et al. Association of depressive symptoms with Incident Cardiovascular diseases in Middle-aged and older Chinese adults. JAMA Netw open. 2019;2(12):e1916591. PubMed PMID: 31800066. Pubmed Central PMCID: PMC6902756. Epub 2019/12/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Shao W, Zhu Q, Zhang R, Sun T, Wang B, et al. Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011–2016. J Translational Med. 2023;21(1):691. PubMed PMID: 37794370. Pubmed Central PMCID: PMC10548719. Epub 2023/10/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahemuti N, Jing X, Zhang N, Liu C, Li C, Cui Z et al. Association between systemic immunity-inflammation index and hyperlipidemia: a Population-based study from the NHANES (2015–2020). Nutrients. 2023;15(5). PubMed PMID: 36904176. Pubmed Central PMCID: PMC10004774. Epub 2023/03/12. eng. [DOI] [PMC free article] [PubMed]
- 18.Qin Z, Li H, Wang L, Geng J, Yang Q, Su B, et al. Systemic Immune-inflammation index is Associated with increased urinary albumin excretion: a Population-based study. Front Immunol. 2022;13:863640. PubMed PMID: 35386695. Pubmed Central PMCID: PMC8977553. Epub 2022/04/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. PubMed PMID: 26711676. Pubmed Central PMCID: PMC5542678. Epub 2015/12/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatrica Scandinavica. 2017;135(5):373–87. PubMed PMID: 28122130. Epub 2017/01/26. eng. [DOI] [PubMed] [Google Scholar]
- 21.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. PubMed PMID: 23644052. Pubmed Central PMCID: PMC3741070. Epub 2013/05/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. PubMed PMID: 16316783. Pubmed Central PMCID: PMC3392963. Epub 2005/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkanova V, Ebmeier KP, Allan CL, CRP. IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–44. PubMed PMID: 23870425. Epub 2013/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 24.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–15. PubMed PMID: 26065825. Pubmed Central PMCID: PMC4566946. Epub 2015/06/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain, behavior, and immunity. 2020;89:594–600. PubMed PMID: 32738287. Pubmed Central PMCID: PMC7390748. Epub 2020/08/02. eng. [DOI] [PMC free article] [PubMed]
- 26.Mocking RJT, Nap TS, Westerink AM, Assies J, Vaz FM, Koeter MWJ, et al. Biological profiling of prospective antidepressant response in major depressive disorder: associations with (neuro)inflammation, fatty acid metabolism, and amygdala-reactivity. Psychoneuroendocrinology. 2017;79:84–92. PubMed PMID: 28262603. Epub 2017/03/07. eng. [DOI] [PubMed] [Google Scholar]
- 27.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–8. PubMed PMID: 25133871. Pubmed Central PMCID: PMC4561502. Epub 2014/08/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. PubMed PMID: 24228900. Pubmed Central PMCID: PMC3846682. Epub 2013/11/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566. PubMed PMID: 30002404. Pubmed Central PMCID: PMC6043609. Epub 2018/07/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23(4):2324–32. PubMed PMID: 30734486. Pubmed Central PMCID: PMC6433686. Epub 2019/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, et al. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disord. 2015;17(3):269–77. PubMed PMID: 25257835. Epub 2014/09/27. eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.