Abstract
Objective
This study aims to elucidate the therapeutic efficacy and safety of a taxane-based chemotherapy in combination with immune checkpoint inhibitors regimen in patients diagnosed with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC).
Methods
We retrospectively collected clinical data from 154 patients who received at least two cycles of PD-1 inhibitors in combination with a taxane-based chemotherapy as first-line treatment in seven hospitals in Hunan Province, between December 2018 and December 2023. These patients were subjected to long-term follow-up.
Results
The study included 154 eligible patients, with a median follow-up period of 21.5 months. The median PFS was 8.7 months, while the median OS was 16.7 months. The 12-month PFS rate was 43.6%, and the 12-month OS rate was 60.1%. At 24 months, the PFS rate was 34.4%, and the OS rate was 36.9%. With 26 complete responses (16.9%) and 52 partial responses (33.8%), the ORR was 50.6%. Stable disease was observed in 54 patients (35.1%), resulting in a disease control rate of 85.7%, while 22 patients showed progressive disease. In the univariate analysis, the distant organ metastasis had a statistically significant impact on both PFS and OS. Subsequent radiotherapy following this protocol also showed a statistically significant effect on PFS and OS. However, radiotherapy before recurrent metastasis did not significantly affect PFS, though it did have a significant impact on OS. Other factors analyzed did not show a statistically significant effect on PFS and OS. Multivariate analysis indicated that the distant organ metastasis and subsequent radiotherapy following this protocol were independent prognostic factors for PFS in patients with R/M HNSCC, and the latter was also an independent prognostic factor for OS in these patients. Regarding safety, during treatment anemia was observed in 97 patients, leukopenia in 64, neutropenia in 33, thrombocytopenia in 28, transaminase elevation in 46, hypothyroidism in 46 patients, and one patient stopped taking the medication due to a serious adverse reaction. No treatment-related deaths occurred.
Conclusion
The combination of PD-1 inhibitors with a a taxane-based chemotherapy regimen as a first-line treatment for R/M HNSCC patients demonstrates good therapeutic efficacy and acceptable safety profiles.
Keywords: Recurrent/Metastatic Head and Neck squamous cell carcinoma, Immunotherapy, Chemotherapy, Prognostic analysis, Real-world study
Introduction
The seven most common type of cancer worldwide is head and neck tumors [1], with squamous cell carcinoma being the most prevalent pathology, accounting for 90% of cases of head and neck tumors. Local recurrence or distant metastasis is frequent in head and neck squamous cell carcinoma. More than 60% of patients are diagnosed with locally advanced disease (stages III-IV, excluding M1). Despite comprehensive treatment, 40–60% of these patients eventually experience local recurrence or distant metastasis [2–4].
The prognosis for recurrent or metastatic HNSCC is dismay, with a median overall survival (mOS) of about one year [5].
Surgery is commonly used as a radical treatment for recurrent head and neck squamous carcinoma. At the same time, radiotherapy serves both as an adjuvant treatment post-surgery and as a radical treatment for patients unsuitable for surgery [6]. For patients who are not candidates for localized radical treatments, chemotherapy-based systemic treatments are primarily adopted [7]. In recent years, immune checkpoint inhibitors, such as PD-1 monoclonal antibodies, have rapidly advanced in treating advanced head and neck squamous carcinoma. The anti-tumor mechanism of PD-1 inhibitors involves blocking the interaction between PD-1 and its ligand PD-L1, thereby activating T cells and enhancing their ability to recognize and eliminate tumor cells, achieving anti-tumor effects [8, 9]. The KEYNOTE-048 study confirmed the efficacy of the pembrolizumab + platinum + 5-FU regimen [10]. However, the toxicity of 5-FU, its inconvenient administration, and the complications associated with its continuous multi-day infusion [11–15] highlight the urgent need to identify alternative chemotherapeutic agents. Immune checkpoint inhibitors (ICIs) have been widely adopted in recent years for cancer treatment. Despite their association with a range of potential adverse effects, including gastrointestinal, dermatological, hepatic, and endocrine toxicities, as well as possible risks of peripheral neuropathy, sensory neuropathy, and headache, their safety profile warrants further clinical investigation and evaluation [16]. Previous studies have shown that taxanes promotes the differentiation of human monocytes into pro-inflammatory M1 macrophages and enhances the capacity of these macrophages to present antigens to T cells [17]. Additionally, taxanes activates the TLR4 receptor and reprograms M2-polarized macrophages to an M1-like phenotype, thereby inducing a pro-inflammatory response that enhances anti-tumor effects [18]. Radiotherapy combined with taxanes increases PD-1 expression on CD4(+) T cells in patients with stage III-IV oropharyngeal cancer [19]. Additionally, taxanes combined with a recombinant vaccine enhances T-cell responses and antitumor activity [20]. These findings suggest a potential synergistic effect when combining taxanes with immune checkpoint inhibitors. An increasing number of studies have demonstrated that immune activation significantly influences the efficacy of cytotoxic drugs. Under certain conditions, these drugs not only exhibit direct tumoricidal effects but also stimulate immune responses, creating opportunities for immunotherapy to eliminate tumor dissemination and metastasis. This phenomenon is likely related to chemotherapy’s ability to reduce the number of cells that immune cells need to eliminate and to diminish the levels of immunosuppressive factors produced by cancer cells [21]. Several retrospective studies have indicated that HNSCC patients receiving immune checkpoint inhibitors show improved sensitivity to salvage chemotherapy, suggesting that immune checkpoint inhibitors may increase tumor sensitivity to chemotherapy [22, 23] Furthermore, taxanes and platinum-based drugs have distinct mechanisms of action and non-overlapping toxicities, making their combined use rational.
Clinical studies in China on the first-line use of PD-1 inhibitors combined with taxanes regimens for R/M HNSCC are limited. The only available data comes from a prospective phase II study conducted at the Cancer Hospital of the Chinese Academy of Medical Sciences, which utilized pembrolizumab and did not include any domestically produced PD-1 inhibitors [24]. Based on this gap, we designed the present research project.
Methods
Study design
This multicenter real-world study retrospectively collected clinical data from patients who received at least 2 cycles of first-line PD-1 inhibitor combined with taxanes chemotherapy at seven hospitals in Hunan Province, China, between December 2018 and December 2023. Patients involved were observed for an extended period. The study was approved by the Ethics Committee of The Second Xiangya Hospital (Approval number: 2023 Lun Audit No. K062).
Research subjects
Inclusion criteria
Patient ≥ 18 years old at the time of diagnosis of R/M HNSCC, with no gender limitations;
HNSCC patients with malignant tumors of the oral cavity, oropharynx, laryngopharynx, larynx, and nasal sinus confirmed by pathological histology or cytology;
The first-line treatment regimen after diagnosis of R/M HNSCC contains PD-1 inhibitors with taxanes chemotherapeutic agents, in combination with or without other drugs, and imaging is performed to assess the efficacy of treatment after at least 2 cycles of treatment;
Patients with at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [25];
Patients with metastatic or recurrent HNSCC for whom local radical therapy is not acceptable;
Exclusion criteria
Patients with nasopharyngeal carcinoma;
Patients who have had a second primary malignancy within the last 5 years or currently have one;
Patients with severe missing data that affects the analysis of results;
Patients who have previously received anti-PD-1 therapy;
Patients who received adjuvant therapy with a platinum/taxanes regimen, where the treatment interval with the regimen in this study is less than 6 months;
In this study, we collected data from HNSCC patients who received first-line treatment with PD-1 inhibitors combined with taxanes chemotherapeutic agents at seven hospitals in Hunan Province (Xiangya Second Hospital, Xiangya Hospital, Yueyang Central Hospital, Huaihua First People’s Hospital, Huaihua Fifth People’s Hospital, Hengyang Central Hospital, and Liling Traditional Chinese Medicine Hospital) between December 2018 and December 2023. Based on the inclusion and exclusion criteria, a total of 154 Chinese patients were included.
Treatment regimen
The antitumor regimen consisted of PD-1 inhibitors + taxanes chemotherapy drugs ± other drugs. Since this was a real-world study, the dosage was not strictly standardized but was determined by the treating clinician based on the patient’s physical condition and financial situation. The regimen was repeated every 3 weeks with efficacy assessments conducted after at least two cycles of treatment. For patients achieving stable disease, partial response, or complete response after multiple cycles of treatment with the above regimen, PD-1 inhibitors were continued until disease progression or for up to two years. Subsequent treatment regimens after progression were not restricted and were selected by the supervising clinician based on clinical judgment.
Data collection
Information collected
We collected the following patient information: hospitalization number, identity card number, patient and family phone numbers, name, gender, age, HPV infection status(P16 positivity was considered indicative of HPV infection in our study), PD-L1 CPS score, history of smoking, alcohol consumption, betel nut chewing, ECOG PS score, pathological findings, clinical diagnosis and staging, treatment regimen, therapeutic effects, blood counts, liver function, thyroid function, and treatment-related toxicities and side effects. Data were recorded using an electronic case system, ensuring privacy protection for sensitive information such as patient names and ID numbers.
Follow-up program
For all the patients, we collected information related to patients’ efficacy and adverse reactions every three months by telephone follow-up or outpatient review. The data includes overall survival, progression-free survival, and drug-related adverse reactions which will be recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE 5.0), without distinguishing which drugs caused them.
Study endpoints and efficacy assessment
The primary endpoints of this study were PFS and OS. PFS was defined as the interval from the beginning of treatment with a PD-1 inhibitor combined with a a taxane-based chemotherapeutic agent to the first occurrence of disease progression or death from any cause. OS was defined as the time from the beginning of the same treatment to the patient’s death. Median PFS refers to the time by which 50% of patients have achieved progression-free survival, and median OS is the time by which 50% of patients have achieved overall survival. The secondary endpoint was the ORR, which is the percentage of patients achieving complete response (CR) and partial response (PR) as assessed for efficacy. All measurable lesions had pre-treatment baseline measurements, and regular imaging and measurements were performed. Response was assessed per RECIST v1.1. Statistical Analysis.
Statistical analyses were conducted using R software (version 4.1.2, https://www.r-project.org/). Descriptive statistics were used to evaluate patient clinical characteristics, with constitutive ratios indicating their specific classifications. Survival curves were generated using the Kaplan-Meier method, and 95% confidence intervals (CIs) for median progression- PFS and OS were calculated. Univariate analyses were performed using the Log-rank test, while multivariate analyses were conducted with the Cox proportional hazards model. All p-values and confidence intervals were determined through two-sided tests, with a p-value of < 0.05 considered a statistically significant difference.
Results
Basic demographic and disease characteristics
The baseline characteristics of the 154 patients with R/M HNSCC are summarized in Table 1. A total of 116 patients (75.3%) were under the age of 60 years at the start of treatment, while 38 patients (24.7%) were 60 years of age or older, with a median age of 54 years (range:20–77 years). There were 140 male patients (90.0%) and 14 female patients (10.0%). These patients included 152 with an ECOG PS score of 1 and 2 with a score of 2. The sites of onset, in descending order of incidence, were tongue (34.4%), buccal (26.6%), larynx (11.7%), laryngopharynx (9.1%), gingiva (7.1%), oropharynx (5.2%), palate (1.9%), maxillary sinus (1.9%), floor of the mouth (1.3%), and nasal cavity (0.6%).In terms of PD-L1 CPS scores, 16 patients (10.4%) had a CPS < 1, 22 patients (14.3%) had a CPS between 1 and 20, 74 patients (48.1%) had a CPS ≥ 20, and the CPS score was unknown for 42 patients (27.3%). At the time of diagnosis, 104 patients (67.5%) had purely localized recurrence, 26 patients (16.9%) had purely distant metastasis, and 24 patients (15.6%) had both local recurrence and distant metastasis. Regarding HPV infection status, 68 patients (44.2%) were negative, 17 patients (11%) were positive, and 69 patients (44.8%) had an unknown status.
Table 1.
The baseline characteristics of 154 cases of recurrent/metastatic head and neck squamous cell carcinoma patients
| Characteristic | Cases (n) | |
|---|---|---|
| Age (years) | ||
| <60 | 116 | (75.3%) |
| ≥ 60 | 38 | (24.7%) |
| Gender | ||
| Male | 140 | (90.0%) |
| Female | 14 | (10.0%) |
| Primary Site | ||
| Tongue | 53 | (34.4%) |
| Buccal | 41 | (26.6%) |
| Larynx | 18 | (11.7%) |
| Laryngopharynx | 14 | (9.1%) |
| Oropharynx | 8 | (5.2%) |
| Palate | 3 | (1.9%) |
| Maxillary Sinus | 3 | (1.9%) |
| Floor of the Mouth | 2 | (1.3%) |
| Nasal Cavity | 1 | (0.6%) |
| ECOG PS Score | ||
| 1 | 152 | (98.7%) |
| 2 | 2 | (1.3%) |
| CPS Score | ||
| <1 | 16 | (10.4%) |
| ≥ 1, <20 | 22 | (14.3%) |
| ≥ 20 | 74 | (48.1%) |
| Unknown | 42 | (27.3%) |
| Recurrence/Metastasis | ||
| Local Recurrence Only | 104 | (67.5%) |
| Distant Metastasis Only | 26 | (16.9%) |
| Local and Distant | 24 | (15.6%) |
| HPV Infection Status | ||
| Negative | 68 | (44.2%) |
| Positive | 17 | (11.0%) |
| Unknown | 69 | (44.8%) |
Treatment characteristics
The treatment characteristics of the 154 patients are summarized in Table 2. For taxanes selection, 52 patients received docetaxel, 87 received albumin-bound taxanes, and 15 received taxanes liposomes. Regarding PD-1 inhibitors, 64 patients were treated with pembrolizumab, 27 with toripalimab, 21 with camrelizumab, 19 with sindilizumab, 16 with tislelizumab, and 7 with penpulimab. In addition, among the 154 patients who received a PD-1 inhibitor combined with a a taxane-based regimen, 131 were also treated with platinum agents (119 with cisplatin and 12 with nedaplatin), 7 were combined with cetuximab, 4 with nimotuzumab, 2 with anlotinib, and 1 with capecitabine. Seventeen patients did not receive any additional agents. Furthermore, 32 patients underwent radiotherapy before being diagnosed with recurrence/metastasis, and 48 received radiotherapy after treatment with this combination regimen.
Table 2.
The treatment characteristics of 154 cases of recurrent/metastatic head and neck squamous cell carcinoma patients
| Medicines | Cases (n) | |
|---|---|---|
| Taxanes | ||
| Docetaxel | 52 | (33.8%) |
| Albumin-bound taxanes | 87 | (56.5%) |
| Liposomal taxanes | 15 | (9.7%) |
| PD-1 Inhibitors | ||
| Pembrolizumab | 64 | (41.6%) |
| Toripalimab | 27 | (17.5%) |
| Camrelizumab | 21 | (13.6%) |
| Sindilizumab | 19 | (12.3%) |
| Tislelizumab | 16 | (10.4%) |
| Penpulimab | 7 | (4.5%) |
| Other medicines Combined | ||
| Platinum-based | 131 | (85.1%) |
| Cisplatin | 119 | (77.3%) |
| Nedaplatin | 12 | (7.8%) |
| Cetuximab | 7 | (4.5%) |
| Nimotuzumab | 4 | (2.6%) |
| Anlotinib | 2 | (1.3%) |
| Capecitabine | 1 | (0.6%) |
| No Other Medicines Combined | 17 | (11.0%) |
| Previous Radiotherapy | ||
| Yes | 32 | (20.8%) |
| No | 122 | (79.2%) |
| Subsequent Radiotherapy | ||
| Yes | 48 | (31.2%) |
| No | 106 | (68.8%) |
Treatment results
Overall efficacy
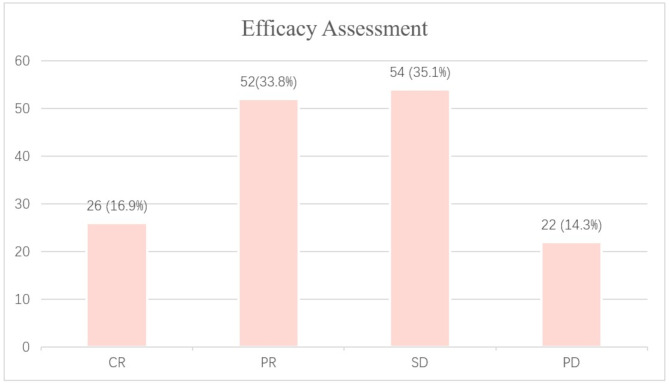
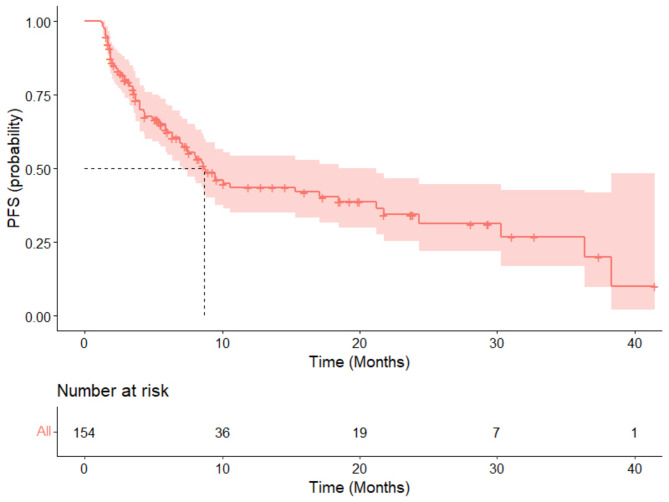
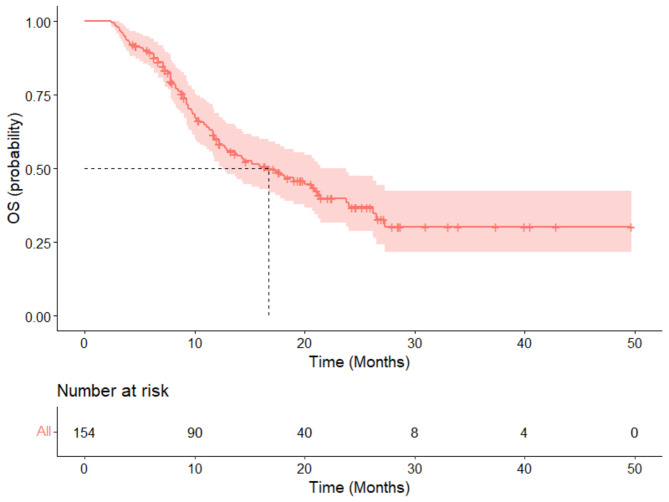
The median follow-up period for this study was 21.5 months (range: 2.4 months to 49.7 months). Efficacy data were available for all 154 patients. The results showed that 26 patients (16.9%) achieved a CR, and 52 patients (33.8%) had a PR, yielding an ORR of 50.6%. Additionally, 54 patients (35.1%) had SD, resulting in a DCR of 85.7%. Twenty-two patients experienced PD (Fig. 1). The median PFS for all patients was 8.7 months (95% CI: 7.1 to 21.2) (Fig. 2). The median overall survival was 16.7 months (95% CI: 12.5 to 23.8) (Fig. 3). The 12-month PFS rate was 43.6% (95% CI: 35–54.3%), and the 12-month OS rate was 60.1% (95% CI: 52.4–68.9%). At 24 months, the PFS rate was 34.4% (95% CI: 25.4–46.7%), and the OS rate was 36.9% (95% CI: 28.7–47.5%).
Fig. 1.
The efficacy assessment of 154 cases of recurrent/metastatic head and neck squamous cell carcinoma patients treated with first-line PD-1 inhibitors in combination with a taxane-based chemotherapy
Fig. 2.
The progression-free survival rate of 154 cases of recurrent/metastatic head and neck squamous cell carcinoma patients treated with first-line PD-1 inhibitors in combination with a taxane-based chemotherapy
Fig. 3.
The overall survival rate of 154 cases of recurrent/metastatic head and neck squamous cell carcinoma patients treated with first-line PD-1 inhibitors in combination with a taxane-based chemotherapy
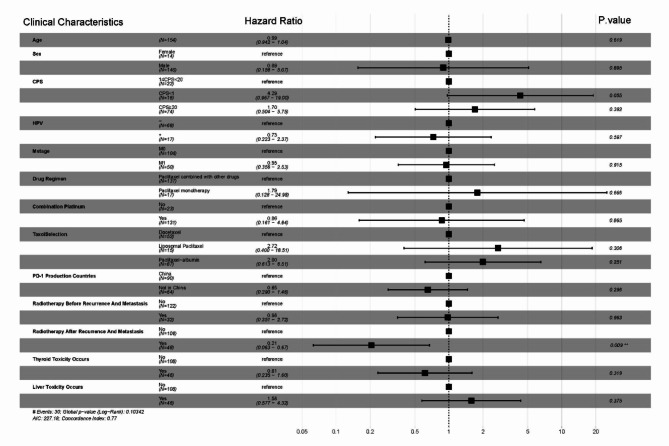
Univariate analysis of clinical characteristics and efficacy
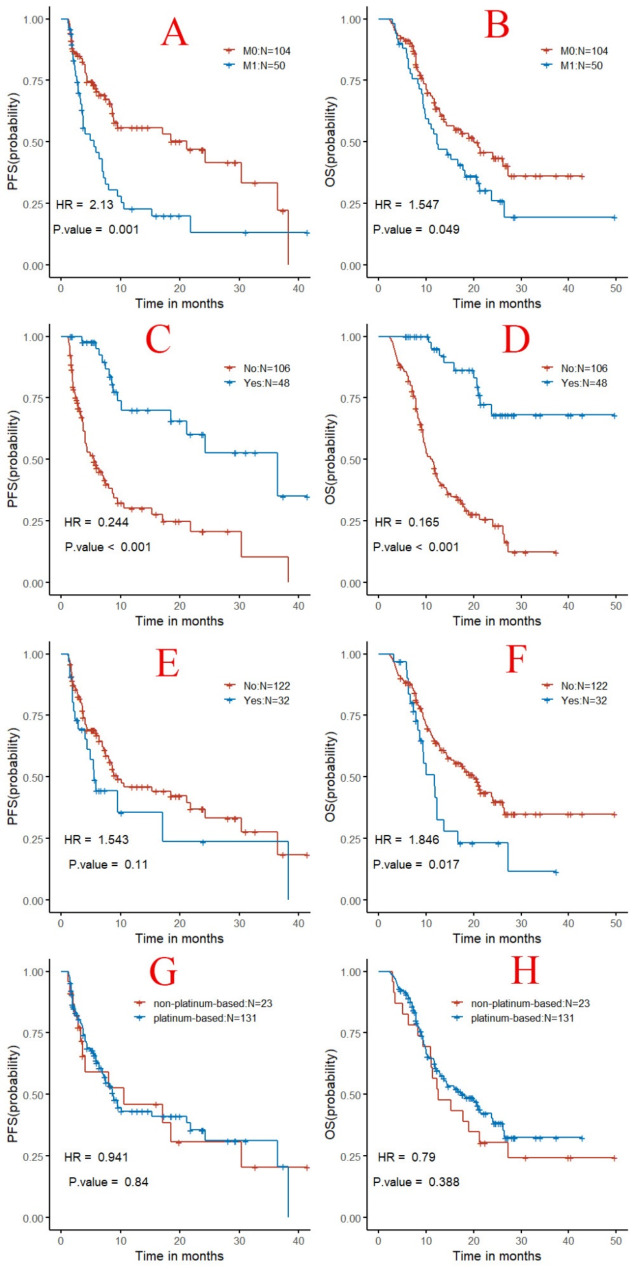
Univariate analysis of the data revealed that factors such as age, gender, CPS score, HPV infection status, primary tumor site, history of betel nut chewing, smoking, alcohol consumption, taxanes-only single-agent chemotherapy versus multi-agent chemotherapy with taxanes, platinum-based drug selection, a taxane-based drug selection, the origin of PD-1 inhibitors (imported vs. domestic), and the presence of thyroid or hepatic toxicity did not significantly impact PFS or OS (P ≥ 0.05). However, the presence of distant organ metastasis had a statistically significant impact on PFS (HR = 2.1, P = 0.001, Fig. 4A) and OS (HR = 1.5, P = 0.049, Fig. 4B). Subsequent radiotherapy in the study regimen significantly affected PFS (HR = 0.24, P < 0.001, Fig. 4C) and OS (HR = 0.16, P < 0.001, Fig. 4D). The radiotherapy before the diagnosis of recurrent/metastatic disease did not significantly influence PFS (Fig. 4E), but it did have a significant impact on OS (HR = 1.8, P = 0.019, Fig. 4F).
Fig. 4.
Kaplan-meier curves for univariate analysis of clinical characteristics in patients with recurrent/metastatic head and neck squamous cell carcinoma treated with pd-1 inhibitors in combination with a taxane-based chemotherapy. Note (A): Comparison of PFS survival curves based on the presence or absence of distant metastasis; (B): Comparison of OS survival curves based on the presence or absence of distant metastasis; (C): Comparison of PFS survival curves based on whether subsequent radiotherapy was administered; (D): Comparison of OS survival curves based on whether subsequent radiotherapy was administered; (E): Comparison of PFS survival curves based on whether radiotherapy was administered before recurrence/metastasis; (F): Comparison of OS survival curves based on whether radiotherapy was administered before recurrence/metastasis;
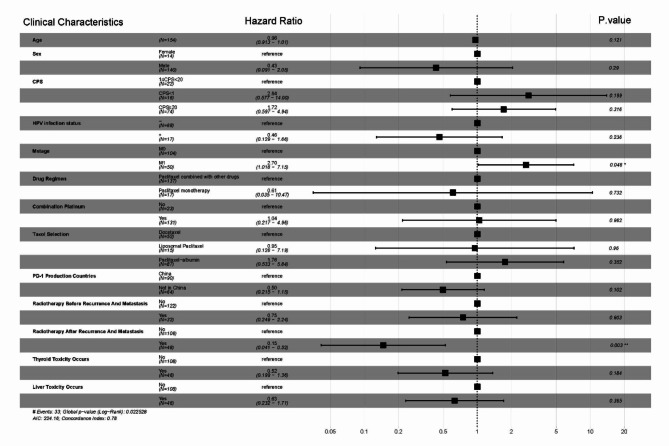
Multivariate analysis of clinical characteristics and efficacy
Multivariate analysis of patient clinical characteristics (including age, gender, CPS score, HPV infection status, presence of distant organ metastasis, use of single-agent or multi-agent chemotherapy, platinum-based drug selection, a taxane-based drug selection, the origin of PD-1 inhibitors, prior radiotherapy before recurrence/metastasis, subsequent radiotherapy after the therapy of this study, and presence of hepatic or thyroid toxicity) revealed that the presence of distant organ metastasis (HR = 2.7, P = 0.046) and subsequent radiotherapy after this regimen (HR = 0.15, P = 0.003) were independent prognostic factors for PFS in patients with R/M HNSCC (Fig. 5). Additionally, subsequent radiotherapy after this regimen (HR = 0.21, P = 0.009) was identified as an independent prognostic factor for OS in patients with R/M HNSCC (Fig. 6).
Fig. 5.
Multifactorial analysis of progression-free survival in 154 cases of recurrent/metastatic squamous cell carcinoma of the head and neck treated with first-line pd-1 inhibitors combined with a taxane-based chemotherapy
Fig. 6.
Multifactorial analysis of overall survival in 154 cases of recurrent/metastatic squamous cell carcinoma of the head and neck treated with first-line pd-1 inhibitors combined with a taxane-based chemotherapy
Safety
In terms of safety, the data are summarized in Table 3, which did not differentiate adverse reactions by the specific causative drug. Anemia was recorded in 97 patients (62.99%) during treatment, with grade 3–4 in 7.14% (11/154); Leukopenia was observed in 64 patients (43.51%), with 9.74% (15/154) being grade 3–4; Neutropenia occurred in 33 patients (21.43%), with 7.14% (11/154) being grade 3–4; Thrombocytopenia was noted in 28 patients (18.18%), with 1.95% (3/154) being grade 3–4; Elevated transaminase levels were reported in 46 patients (29.87%), with no cases of grade 3–4; Hypothyroidism was also reported in 46 patients (29.87%), with no cases of grade 3–4; One patient (0.65%) discontinued treatment due to severe adverse reactions (grade 4 bullous dermatitis, likely immune-related skin toxicity), which improved to grade 2 following hormone therapy. Most of the above adverse reactions were managed symptomatically and resolved without treatment-related mortality.
Table 3.
Adverse reactions
| Adverse Reaction Types | Severity Levels | All Grades, n (%) | Grade 3/4, n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | n | % | n | % | |
| Anemia | 53 | 33 | 9 | 2 | 97 | 62.99 | 11 | 7.14 |
| Leukopenia | 27 | 25 | 8 | 7 | 64 | 43.51 | 15 | 9.74 |
| Neutropenia | 9 | 13 | 6 | 5 | 33 | 21.43 | 11 | 7.14 |
| Thrombocytopenia | 19 | 6 | 1 | 2 | 28 | 18.18 | 3 | 1.95 |
| Elevated Transaminases | 44 | 2 | 0 | 0 | 46 | 29.87 | 0 | 0.00 |
| Hypothyroidism | 45 | 1 | 0 | 0 | 46 | 29.87 | 0 | 0.00 |
| Bullous Dermatitis | 0 | 0 | 0 | 1 | 1 | 0.65 | 1 | 0.65 |
Discussion
Currently, more clinical studies are needed to determine the optimal first-line treatment regimen for patients with R/M HNSCC. Table 4 summarizes the data on the efficacy of first-line treatment regimens in the studies of R/M HNSCC mentioned in this discussion. This study describes the clinical and treatment characteristics of patients with R/M HNSCC who received first-line treatment with PD-1 inhibitors combined with taxanes regimens in Hunan, China, and its surrounding areas in a real-world setting. It provided the median PFS and OS, as well as the 12-month and 24-month PFS and OS rates for this cohort of patients. The prognostic factors associated with receiving PD-1 inhibitors combined with taxanes regimens were also identified. After a literature search, it was found that this study is currently the first in China to describe the efficacy and safety of first-line PD-1 inhibitor combined with taxanes chemotherapy for the treatment of R/M HNSCC in a real-world setting.
Table 4.
Efficacy Data of First-Line treatment regimens in selected studies on R/M HNSCC
| Study Title | Study Population | Group | Patients(n) | ORR(%) | mPFS(Month) | mOS(Month) |
|---|---|---|---|---|---|---|
| Group 1: K | 301 | 16.9 | 2.3 | 11.5 | ||
| KEYNOTE-048 [10] | Global | Group 2: Cetuximab + PF | 300 | 36.0 | 5.1 | 10.7 |
| Group 3: K + PF | 281 | 36.3 | 4.9 | 13.0 | ||
| KEYNOTE-B10 [26] | US | Single-arm: K + Carboplatin + Paclitaxel | 101 | 49.0 | 5.6 | 13.1 |
| Black, C. M [27] | US | Single-arm: K + Platinum-based drug + Paclitaxel | 83 | NA | 8.7 | 14.9 |
| Fuereder, T [28] | Austria | Single-arm: K + Docetaxel | 22 | 22.7 | 5.8 | 21.3 |
| Gui, L [24] | China | Single-arm: K + TP | 20 | 80 | 6.9 | NA |
| Our study | China | Single-arm: PD-1 Ab + Taxanes | 154 | 50.6 | 8.7 | 16.7 |
Note: Some clinical study protocols are unnamed and are referred to by the first author’s name in this table; Abbreviations for drugs in the groups: K: Pembrolizumab, PF: Platinum combined with fluorouracil, TP: Albumin-bound taxanes combined with cisplatin
Regarding clinical characteristics, the median age, gender distribution, ECOG PS score, CPS score, and HPV positivity rate in our study population are similar to those observed in the KEYNOTE-048 trial’s immunotherapy combined with the chemotherapy group. However, there were notable differences in the primary tumor sites. The KEYNOTE-048 study reported the highest proportion of oropharyngeal cancers, whereas this study found a greater prevalence of oral cancers, which may be related to the higher incidence of oral cancers in the Hunan region due to betel quid chewing. Additionally, this study observed a lower proportion of patients with distant organ metastases compared to the KEYNOTE-048 study (32% vs. 73%).
In terms of efficacy, the ORR for the pembrolizumab combination chemotherapy group in the KEYNOTE-048 study was 36.3% [10]. Interim data from the KEYNOTE-B10 study reported an ORR of 49% [26]. A prospective phase II study presented by Chinese researchers at the same conference, which included 20 patients with recurrent/metastatic HNSCC treated with pembrolizumab combined with albumin-bound taxanes and cisplatin, achieved an ORR of up to 80% [24]. Conversely, a prospective phase I/II study in Austria involving 22 patients with recurrent/metastatic HNSCC treated with pembrolizumab and docetaxel reported an ORR of only 22.7% [28]. The present study observed an ORR of 51.9%. A prospective phase II study conducted by Japanese researchers, which included 35 patients with R/M HNSCC who had previously received PD-1 inhibitor and platinum-based therapy, reported an ORR of 69.6% for second-line treatment with paclitaxel and biweekly cetuximab [29]. These results indicate that similar regimens can yield significantly different ORRs across various regions and populations, highlighting the need for further clinical research to clarify efficacy variations between different regions and populations. A multicenter real-world study conducted in the United States, which involved first-line treatment of recurrent/metastatic HNSCC with pembrolizumab, platinum-based agents, and taxanes, reported a median real-world time to next treatment (rwTTNT) of 8.7 months (n = 83) [27], which is similar to the findings of this study. An Austrian study involving 22 patients with recurrent/metastatic HNSCC who received first-line treatment with pembrolizumab and docetaxel reported a median PFS of 5.8 months [28]. In a prospective phase II study conducted by Japanese researchers involving 35 patients with R/M HNSCC, the mPFS for second-line treatment with paclitaxel and biweekly cetuximab, following prior treatment with PD-1 inhibitor and platinum-based therapy, was reported to be 5.5 months [29]. Additionally, a prospective phase II study presented by Chinese researchers at the 2022 ESMO Annual Meeting, which included 20 patients treated first-line with pembrolizumab, albumin-bound taxanes, and cisplatin, showed a median PFS of 6.9 months [24]. In this study, the median PFS was 8.7 months, with a 12-month PFS rate of 43.6%, both of which are higher than the results reported in the KEYNOTE-048 study for the immunotherapy combination chemotherapy group (median PFS: 4.9 months, 12-month PFS rate: 17%). However, the median PFS of the above regimens did not exceed 10 months, indicating that R/M HNSCC remains highly susceptible to progression even after systemic therapy. This high propensity for progression is a major factor contributing to the poor prognosis of patients with R/M HNSCC. Therefore, there is a need for further exploration of medication use for patients entering the maintenance phase of treatment. The median OS in this study was 16.7 months, which was slightly higher than that of the KEYNOTE-048 study immune combination chemotherapy group (13.0 months), while the median OS of the KEYNOTE-B10 study published interim data was 13.1 months [26] A real-world study in the United States that included 83 patients reported a median rwOS of 14.9 months with first-line use of pembrolizumab combined with platinum and taxanes for patients with R/M HNSCC [27]. In an Austrian study that included 22 patients with R/M HNSCC, a median OS of up to 21.3 months was achieved with the first-line use of pembrolizumab and docetaxel [28]. In a prospective phase II study conducted by Japanese researchers involving 35 patients with R/M HNSCC, the mOS for those receiving second-line treatment with paclitaxel and biweekly cetuximab, after prior treatment with PD-1 inhibitor and platinum-based therapy, was reported to be 13.3 months [29]. In this study, the 12-month OS rate was 60.1% and the 24-month OS rate was 36.9%. In comparison, the KEYNOTE-048 study’s immune combination chemotherapy group had 12-month and 24-month OS rates of 53% and 29%, respectively [10], suggesting that substituting taxanes for 5-Fu might be a more effective treatment option.
Regarding factors affecting efficacy, this study did not find a correlation between HPV infection status and treatment outcomes. In contrast, most previous real-world studies on R/M HNSCC have shown a better prognosis for HPV-positive patients. This discrepancy may be related to the low rate of HPV detection in this study. In this study, patients in the population with a CPS < 1 had poorer OS, but the small sample size prevented finding statistically significant differences. There were also no significant differences in efficacy between patients with CPS scores of 1–19 and those with CPS scores ≥ 20. This may be due to the low CPS detection rate in the study population and the variability in the platforms of the reagents used for CPS testing across different hospitals, which could affect the results analysis. In addition, we found that patients who had the opportunity to undergo radiotherapy after treatment with this regimen had better PFS and OS than those who failed to gain access to radiotherapy, further demonstrating the importance of radiotherapy in HNSCC. This study also revealed that hypopharyngeal carcinoma had a poorer OS compared to other types of HNSCC (HR = 2.13, P = 0.088). However, due to the small sample size for hypopharyngeal carcinoma (N = 14), the result was not statistically significant. Previous research has similarly indicated that hypopharyngeal carcinoma generally has a worse prognosis than other head and neck squamous cell carcinomas [30]. Additionally, this study compared the efficacy based on the selection of platinum-based drugs, the type of taxanes used, and the origin of the PD-1 inhibitors (imported vs. domestic). No significant differences in efficacy were observed among these factors. Meanwhile, a recent head-to-head comparison between sindilizumab and pembrolizumab in patients with advanced non-small cell lung cancer conducted by Professor Wu Yilong’s team did not reveal any statistically significant differences in efficacy between the two treatments [31]. This indicates that imported and domestic PD-1 inhibitors have comparable efficacy. These clinical characteristics mentioned have not been addressed in previous studies on R/M HNSCC, providing valuable reference points for drug selection tailored to patients with different economic conditions.
In terms of safety, the TPExtreme and KEYNOTE-B10 studies have confirmed both the efficacy and safety of taxanes. Other studies have also demonstrated the good tolerability of docetaxel [32, 33]. In our study, no fatal adverse reactions were recorded, and only one patient discontinued treatment due to adverse reactions.
This study represents the first real-world investigation in China to describe the efficacy and safety of a PD-1 inhibitor combined with a taxane-based chemotherapy regimen as a first-line treatment for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). Currently, first-line treatment decisions for R/M HNSCC continue to rely on findings from the KEYNOTE-048 study. The publication of our findings provides clinicians with valuable real-world evidence to support the use of a PD-1 inhibitor in combination with taxane-based chemotherapy for this patient population. When contextualized alongside recently released data from other countries on similar treatment regimens, our results suggest that PD-1 inhibitors combined with taxane-based chemotherapy are poised to replace the treatment paradigm established by the KEYNOTE-048 study. This regimen has the potential to become the most prevalent first-line treatment approach for R/M HNSCC in the near future. In addition to analyzing conventional outcomes such as objective response rate (ORR), progression-free survival (PFS), and overall survival (OS), our study also compared the efficacy across different platinum agents, taxane agents, and sources of PD-1 inhibitors (imported versus domestically manufactured). Notably, no significant differences in efficacy were observed based on these clinical characteristics. These findings address a gap in prior R/M HNSCC clinical studies and provide valuable guidance for drug selection, especially for patients with varying economic circumstances.
This study shares several limitations commonly observed in real-world studies. For instance, the inability to control for confounding biases through study design, missing data, and potential issues with data accuracy. A notable example involves performance status (PS) scores: among the recorded data, 152 patients were categorized as having a PS score of 1, while only 2 patients were categorized as having a PS score of 2. Such a distribution is unlikely in clinical practice. After consulting clinical staff from participating institutions, it was determined that this discrepancy arose because, in some cases, healthcare providers did not conduct detailed PS assessments for patients with better physical conditions to save time, and inaccurately recorded patients with a true PS score of 0 as having a PS score of 1. Although this does not affect the prognostic outcome data of this study, it may impact the reliability of the multivariate analysis of prognostic factors.Additionally, the patient population in this study was primarily from Hunan Province and surrounding regions, limiting its generalizability to a national level. The relatively small sample size and short follow-up period further constrain the study’s findings. Post-progression treatment strategies, which are critical to patient outcomes, were not explored in this study. Furthermore, comorbidities, which could influence both treatment efficacy and prognosis, were also not analyzed. Another limitation is the exclusion of patients who discontinued treatment after the first cycle, which may restrict the reliability of the conclusions when compared with other clinical studies. There were 42 patients whose PD-L1 combined positive score (CPS) was unknown, and variations in CPS detection platforms across hospitals might have introduced bias into the results. CPS has been established as a prognostic factor for recurrent and metastatic HNSCC patients, and its absence for a subset of patients could limit the study’s findings. Similarly, HPV infection status was unknown for 69 patients in the study. The lack of this data may have impacted the conclusions regarding the correlation between HPV infection status and prognosis. We look forward to further clinical research to address these gaps.
Conclusions
This study is the first in China to describe the efficacy and safety of first-line use of PD-1 inhibitors combined with a paclitaxel-based chemotherapeutic regimen for the treatment of recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) in a real-world setting. The results demonstrate that the PD-1 inhibitor combined with a paclitaxel-based regimen provides good therapeutic efficacy as a first-line treatment for patients with R/M HNSCC. Additionally, no treatment-related deaths were observed, suggesting an acceptable safety profile.
Acknowledgements
Not applicable.
Abbreviations
- CPS
Combined Positive Score
- CR
Complete response
- ECOG PS
Eastern cooperative oncology group performance status
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human papillomavirus
- mOS
Median overall survival
- mPFS
Median progression-free survival
- NCI-CTCAE
National cancer institute-common terminology criteria for adverse events
- ORR
Objective response rate
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response evaluation criteria in solid tumors
- R/M HNSCC
Recurrent/metastatic head and neck squamous cell carcinoma
- rwTTNT
real-world time to next treatment
- rwOS
real-world overall survival
- SD
Stable disease
Author contributions
MO: Writing – original draft, Writing – review & editing, Conceptualization. HS: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. XL: Writing – review & editing, Writing – original draft, Validation, Methodology, Conceptualization. HW: Writing – review & editing, Writing – original draft, Data curation, Investigation. FD: Writing – review & editing, Writing – original draft, Data curation. ES: Writing – review & editing, Writing – original draft, Visualization, Resources, Investigation. GP: Writing – review & editing, Writing – original draft, Methodology, Resources, Data curation. HW: Writing – review & editing, Writing – original draft, Validation, Data curation. YZ: Writing – review & editing, Writing – original draft, Visualization, Data curation. HX: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Data curation. BL: Writing – review & editing, Writing – original draft, Visualization, Validation, Data curation. SH: Writing – review & editing, Writing – original draft, Validation, Supervision. YH: Writing – review & editing, Writing – original draft, Data curation. PL: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Funding
China International Medical Exchange Foundation, Simcere Anti-Tumor Special Fund Clinical Project, Intratumoral Injection of Recombinant Human Endostatin Combined with Chemotherapy for the Treatment of Exophytic Recurrent and Metastatic Malignant Tumors of the Head and Neck, Registration Number: ChiCTR1900022364; Project Number: Z-2014-06-18384.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Second Xiangya Hospital (Approval number: 2023 Lun Audit No. K062).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Ouyang, Hanquan Sun and Xiaoyu Liu contributed equally to this work and share the first authorship.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based Chemother plus Cetuximab head neck cancer. 2008;359(11):1116–27. [DOI] [PubMed] [Google Scholar]
- 3.Gupta B, Johnson NW, Kumar NJO. Global epidemiology of head and neck cancers: a continuing challenge. 2016;91(1):13–23. [DOI] [PubMed]
- 4.Viale PHJJotapio. The American Cancer Society’s facts & figures: 2020 edition. 2020;11(2):135. [DOI] [PMC free article] [PubMed]
- 5.Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13(1):35–46. [DOI] [PubMed] [Google Scholar]
- 6.Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(Suppl 7):vii252–61. [DOI] [PubMed] [Google Scholar]
- 7.Rajendra A, Noronha V, Joshi A, Patil VM, Menon N, Prabhash K. Palliative chemotherapy in head and neck cancer: balancing between beneficial and adverse effects. Expert Rev Anticancer Ther. 2020;20(1):17–29. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–41. [DOI] [PubMed] [Google Scholar]
- 9.Bagchi S, Yuan R, Engleman EG. Immune Checkpoint inhibitors for the treatment of Cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. [DOI] [PubMed] [Google Scholar]
- 10.Harrington KJ, Burtness B, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic Head and Neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. 2023;41(4):790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein T. Skin reactions to 5-fluorouracil. N Engl J Med. 1977;297(6):337–8. [DOI] [PubMed] [Google Scholar]
- 12.Saif MW. Capecitabine versus continuous-infusion 5-fluorouracil for colorectal cancer: a retrospective efficacy and safety comparison. Clin Colorectal Cancer. 2005;5(2):89–100. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, Das AK, William M. 5-Fluorouracil-induced acute coronary syndrome. Med J Aust. 2019;211(6):255–e71. [DOI] [PubMed] [Google Scholar]
- 14.Ozer M, Dumas B, Horta L, Sadrzadeh H. 5-Fluorouracil associated neurovascular toxicities. Curr Probl Cancer. 2021;45(6):100746. [DOI] [PubMed] [Google Scholar]
- 15.Hara T, Nishikawa K, Sakatoku M, Oba K, Sakamoto J, Omura K. Phase II study of weekly paclitaxel, cisplatin, and 5-fluorouracil for advanced gastric cancer. Gastric Cancer. 2011;14(4):332–8. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo A, Santoni M, Mollica V, Logullo F, Rosellini M, Marchetti A, et al. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert Opin Drug Metab Toxicol. 2021;17(12):1455–66. [DOI] [PubMed] [Google Scholar]
- 17.Millrud CR, Mehmeti M, Leandersson K. Docetaxel promotes the generation of anti-tumorigenic human macrophages. Exp Cell Res. 2018;362(2):525–31. [DOI] [PubMed] [Google Scholar]
- 18.Wanderley CW, Colon DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, et al. Paclitaxel reduces Tumor Growth by Reprogramming Tumor-Associated macrophages to an M1 Profile in a TLR4-Dependent manner. Cancer Res. 2018;78(20):5891–900. [DOI] [PubMed] [Google Scholar]
- 19.Parikh F, Duluc D, Imai N, Clark A, Misiukiewicz K, Bonomi M, et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res. 2014;74(24):7205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(4):e1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh K, Daste A, Martin N, Pons-Tostivint E, Auperin A, Herrera-Gomez RG, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019;121:123–9. [DOI] [PubMed] [Google Scholar]
- 23.Sakai A, Ebisumoto K, Iijima H, Yamauchi M, Teramura T, Yamazaki A, et al. Chemotherapy following immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: clinical effectiveness and influence of inflammatory and nutritional factors. Discov Oncol. 2023;14(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gui L, He X, Yang J, Liu P, Yan Q, Shi YJAO. 683P pembrolizumab plus nabpaclitaxe and platinum as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma: a prospective phase II study. 2022;33:S855–6. [DOI] [PubMed]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer (Oxford, England: 1990). 2009;45(2):228 – 47. [DOI] [PubMed]
- 26.Dzienis M, Cundom J, Fuentes C, Hansen A, Nordlinger M, Pastor A et al. 651O Pembrolizumab (pembro) + carboplatin (carbo) + paclitaxel (pacli) as first-line (1L) therapy in recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): phase VI KEYNOTE-B10 study. 2022;33:S839–40.
- 27.Black CM, Zheng D, Hair GM, Ai L, Wang L, Goto D, et al. Real-world use of first-line pembrolizumab + platinum + taxane combination regimens in recurrent / metastatic head and neck squamous cell carcinoma. Front Oncol. 2024;14:1348045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuereder T, Minichsdorfer C, Mittlboeck M, Wagner C, Heller G, Putz EM, et al. Pembrolizumab plus Docetaxel for the treatment of recurrent/metastatic head and neck cancer: a prospective phase I/II study. Oral Oncol. 2022;124:105634. [DOI] [PubMed] [Google Scholar]
- 29.Koyama T, Kiyota N, Boku S, Imamura Y, Shibata N, Satake H, et al. A phase II trial of paclitaxel plus biweekly cetuximab for patients with recurrent or metastatic head and neck cancer previously treated with both platinum-based chemotherapy and anti-PD-1 antibody. ESMO Open. 2024;9(6):103476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du E, Mazul AL, Farquhar D, Brennan P, Anantharaman D, Abedi-Ardekani B, et al. Long-term survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and human papillomavirus status. Laryngoscope. 2019;129(11):2506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggie Liu SY, Huang J, Deng JY, Xu CR, Yan HH, Yang MY, et al. PD-L1 expression guidance on sintilimab versus pembrolizumab with or without platinum-doublet chemotherapy in untreated patients with advanced non-small cell lung cancer (CTONG1901): a phase 2, randomized, controlled trial. Sci Bull (Beijing). 2024;69(4):535–43. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs H, Pammer J, Minichsdorfer C, Posch D, Kornek G, Aretin MB, et al. Modified biweekly cisplatin, docetaxel plus cetuximab (TPEx) as first-line treatment for patients with recurrent/metastatic head and neck cancer. Med Oncol. 2018;35(3):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falco A, Leiva M, Blanco A, Cefarelli G, Rodriguez A, Melo J, et al. First-line cisplatin, docetaxel, and cetuximab for patients with recurrent or metastatic head and neck cancer: a multicenter cohort study. World J Clin Oncol. 2022;13(2):147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.