Abstract
Background
Female pelvic peritoneal adhesions (FPPA) represent a significant global health burden. Dietary habits play a crucial role in health outcomes, yet their influence on FPPA remains unclear. This study aims to explore the bidirectional causal relationships between 72 eating habits and FPPA using sex-stratified Mendelian randomization (MR).
Methods
We employed a bidirectional MR approach, utilizing single nucleotide polymorphisms (SNPs) significantly associated with 72 different eating habits as instrumental variables. The causal relationships were assessed using five MR methods, including inverse variance weighting (IVW). After Bonferroni correction, eating habits with a p-value < 0.05 were considered to have a significant causal relationship with FPPA. For those habits with significant associations, reverse MR was conducted to assess potential reverse causality. Sensitivity analyses, including IVW, MR-Egger, and leave-one-out tests, were performed to ensure the robustness of the results.
Results
Before Bonferroni correction, five eating habits showed potential associations with FPPA, including non-oily fish intake (OR: 0.989, 95% CI: 0.982–0.995, p=0.000521), side salad intake: OR 1.003 (95% CI: 1.001–1.006), p=0.007779, poultry intake: OR 1.005 (95% CI: 1.001–1.009), p=0.018016, spirits intake: OR 1.010 (95% CI: 1.001–1.019), p=0.036152, hard cheese intake: OR 0.995 (95% CI: 0.991–1.000), p=0.043784. After correction, only non-oily fish intake remained significantly associated with a lower risk of FPPA. No reverse causal relationship was observed between non-oily fish intake and FPPA, and sensitivity analyses revealed no abnormalities, further confirming the robustness of the findings.
Discussion
Our study identifies non-oily fish intake as a protective dietary factor against FPPA, with no evidence of reverse causality. These findings highlight the importance of dietary interventions in managing FPPA risk and suggest potential avenues for future research and public health strategies.
Keywords: female pelvic peritoneal adhesions, Mendelian randomization, eating habits, non-oily fish intake, causal relationship, sensitivity analysis
Introduction
Female pelvic peritoneal adhesions (FPPA) are fibrous bands that form between tissues and organs within the pelvic cavity, often as a result of inflammation, surgical procedures, or infection.1 These adhesions can lead to significant clinical complications, including chronic pelvic pain, infertility, bowel obstruction, and dyspareunia.2 As a common sequelae of pelvic surgery, particularly in gynecological operations such as hysterectomy, myomectomy, and cesarean section, FPPA represents a substantial burden on women’s health worldwide.3 The incidence of adhesions following pelvic surgery is alarmingly high, with studies indicating that up to 90% of women undergoing major gynecological procedures develop some degree of adhesion formation. Despite their prevalence, the pathophysiology of FPPA remains complex and multi-factorial, involving a cascade of inflammatory responses, tissue injury, and abnormal wound healing.4
Globally, the burden of FPPA is immense, with significant implications for healthcare systems.5 The economic costs associated with the diagnosis, treatment, and management of adhesion-related complications are substantial, leading to increased healthcare expenditures.6 For instance, in the United States alone, it is estimated that adhesion-related complications contribute to approximately $1.3 billion in additional healthcare costs annually. These costs are driven by the need for repeated surgical interventions, prolonged hospital stays, and long-term medical management. Beyond the financial implications, FPPA also exerts a profound impact on the quality of life of affected women.7 Chronic pelvic pain, which is one of the most debilitating symptoms of FPPA, can severely limit a woman’s ability to perform daily activities, work, and engage in social and intimate relationships. The psychological burden of living with chronic pain, coupled with the anxiety and stress associated with infertility and sexual dysfunction, further exacerbates the overall impact of FPPA on women’s well-being.
Moreover, FPPA is a leading cause of secondary infertility, which remains a major public health concern globally.8 Adhesions can distort the normal anatomy of the pelvic organs, impairing the function of the fallopian tubes and leading to ectopic pregnancies, miscarriage, or the inability to conceive.9 In regions with limited access to advanced reproductive healthcare, the impact of FPPA on fertility can be particularly devastating, contributing to a cycle of poverty and reduced socioeconomic opportunities for women. The burden of FPPA is not evenly distributed across the globe, with women in low- and middle-income countries (LMICs) facing a disproportionate share of the impact.10 In these settings, limited access to safe surgical care, a higher prevalence of pelvic infections, and inadequate postoperative follow-up contribute to a higher incidence and severity of adhesions. Additionally, cultural stigmas associated with infertility and chronic pelvic pain may prevent women from seeking timely medical care, further exacerbating the burden of FPPA in these regions.
Dietary habits are a critical factor influencing the health and disease outcomes in women.11–13 The relationship between eating habits and the development of various diseases in women has been extensively studied, with evidence suggesting that diet plays a pivotal role in both the prevention and progression of numerous conditions.14 For instance, dietary patterns rich in fruits, vegetables, whole grains, and lean proteins have been associated with a reduced risk of cardiovascular diseases, certain cancers, and metabolic disorders.15 Conversely, diets high in processed foods, saturated fats, and sugars have been linked to an increased risk of obesity, type 2 diabetes, and inflammatory conditions.16 These associations highlight the complex interplay between nutrition and women’s health, where diet can act as both a protective and risk factor depending on its composition.
In the context of gynecological health, eating habits have been implicated in the development and progression of conditions such as poly-cystic ovary syndrome (PCOS), endometriosis, and pelvic inflammatory disease (PID). For example, dietary intake of omega-3 fatty acids, found in foods like fish and flax-seeds, has been shown to reduce inflammation and may alleviate symptoms in conditions like endometriosis.17 Similarly, diets high in antioxidants and fiber are thought to support hormonal balance and reduce the risk of developing conditions like PCOS.18 The influence of diet on hormonal regulation, inflammation, and metabolic function underscores its importance in managing and potentially preventing gynecological conditions. However, despite these associations, establishing a clear causal relationship between specific dietary habits and the risk of developing FPPA has been challenging. This is where Mendelian randomization (MR) emerges as a powerful tool.
Mendelian randomization is an analytical method that leverages genetic variants as instrumental variables to estimate the causal effect of an exposure, such as a dietary habit, on an outcome, like disease development. The approach is grounded in the principle that genetic variants are randomly assorted at conception and thus are less likely to be confounded by environmental or behavioral factors that typically complicate observational studies. This makes MR a particularly robust method for studying the causal relationships between eating habits and disease outcomes, providing insights that are less prone to biases such as reverse causation and confounding. By using genetic proxies for dietary habits, MR can help disentangle the complex web of associations and identify whether specific dietary patterns directly contribute to the risk of developing conditions like FPPA.
In this study, we employ MR to investigate the causal relationship between 73 distinct eating habits and the development of FPPA in women. Given the established links between diet and inflammatory processes, hormonal regulation, and metabolic health, it is plausible that certain dietary habits could influence the risk of FPPA. By applying MR, we aim to determine whether these dietary habits are merely associated with FPPA or if they play a direct causal role in its development. This approach not only strengthens the validity of our findings but also provides a clearer understanding of how diet may contribute to the pathogenesis of FPPA, potentially guiding future dietary recommendations and interventions aimed at reducing the burden of this condition. Moreover, the sex-stratified nature of our study allows us to focus specifically on women, recognizing that dietary impacts can differ significantly between sexes due to biological, hormonal, and lifestyle factors. Women often experience unique health challenges related to their reproductive system, and these can be influenced by dietary habits in ways that differ from men. By concentrating on a female cohort, our research acknowledges and addresses these differences, aiming to provide more relevant and targeted insights into the role of diet in women’s health.
Data Source
All GWAS summary data utilized in this study were exclusively derived from female participants to enhance the precision of our analysis. The GWAS data for 72 dietary habits, encompassing drinking water intake, low-calorie drink intake, fizzy drink intake, orange juice intake, instant coffee intake, filtered coffee intake, sugar added to coffee intake, standard tea intake, milk intake, and red wine intake, were sourced from the results of GWAS round 2 conducted by the Neale Lab. Detailed information, including the population recruitment criteria and the genetic data quality control measures, can be accessed at http://www.nealelab.is/uk-biobank. The imputed genotypes were based on the HRC, UK10K, and 1000 Genomes reference panels, as released by UK Biobank in March 2018. The UK Biobank is an extensive biomedical database and research resource that provides comprehensive genetic and health data from approximately half a million UK participants. It is designed to advance the prevention, diagnosis, and treatment of a wide range of severe and life-threatening diseases. Detailed information regarding the dietary habits dataset is available in Table S1.
The GWAS summary data for FPPA were fetched from IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/, id = ukb-b-6413).
These GWAS sample populations needed to be predominantly of European descent. The GWAS populations for exposure and outcome have some overlap; therefore, we optimized the selection of MR methods to minimize the impact of this overlap.19 The list of covariates varies between original GWASs, but always included sex and age. Details can be found in the original studies. The data analyzed in this secondary study is publicly available from existing, published GWASs and therefore the ethical approval and informed consent have been obtained by all original studies. This is a secondary analysis based on summary statistics from existing, published studies. The ethical approval and informed consent have been obtained by all original studies.
Genetic Instrumental Variables (IVs) Selection
The selection of instrumental variables is critical for ensuring the validity and robustness of causal inferences in Mendelian randomization analysis. To be valid, IVs must meet three essential assumptions: (1) the genetic variants must be associated with the exposures of interest; (2) the genetic variants must be independent of con-founders; and (3) the genetic variants should affect the outcomes exclusively through the exposures, without influencing other pathways. In this study, specific criteria were established for the selection of IVs. Firstly, each SNP was required to show a significant association with the 72 dietary habits, meeting the genome-wide significance threshold of P < 5E-6. Secondly, linkage disequilibrium (LD) was evaluated using European sample data from the 1000 Genomes Project reference panel, retaining only the most significant SNPs within an R2 < 0.001 and a clumping window of 10,000 kb. Lastly, SNPs with a minor allele frequency (MAF) of ≤ 0.01 were excluded to minimize potential biases due to genetic pleiotropy. The final set of SNPs that satisfied these criteria was utilized as instrumental variables in the TSMR analysis.
Two-Sample Mendelian Randomization Analysis
In this study, Mendelian randomization was utilized to explore the causal relationships between dietary habits and various types of abortions in women. The analysis required comprehensive datasets for both exposures and outcomes, which included SNP rsIDs, effect sizes (beta coefficients), alleles (effect and other), standard errors (SEs), effect allele frequencies (EAFs), and P-values. To avoid potential issues caused by palindromic SNPs (SNPs with identical alleles on both DNA strands), such SNPs were excluded from the analysis. The MR analysis employed five methods: Inverse Variance Weighted (multiplicative random effects), Weighted Median, Inverse Variance Weighted, Inverse Variance Weighted (fixed effects), and Weighted Mode. Inverse Variance Weighted was used as our primary method to determine whether a significant relationship exists.
These methods were chosen because, even with significant correlations due to confounding, the two-sample MR approaches—whether fixed-effect, (multiplicative) random-effects meta-analysis, weighted median estimator, or weighted mode estimator—perform comparably to their application in one-sample MR, with the exception of MR-Egger, which can exhibit bias that reflects the direction and magnitude of the confounding.19 After applying Bonferroni correction, dietary habits with a P-value < 0.05 were considered to have a significant causal relationship with abortions.
For these significant dietary habits, reverse Mendelian randomization was conducted to investigate potential reverse causal relationships between dietary habits and abortion risk, utilizing SNPs associated with abortions (P < 5E-6) as IVs and employing the same methods as in the primary analysis. The list of covariates varied between the original GWASs but always included sex and age. Further details can be found in the original studies. The MR analyses were performed using the “TwoSampleMR” package (version 0.5.10) in R (version 4.3.3).
Sensitivity Analysis
In this study, heterogeneity refers to the variability in causal estimates derived from different genetic variants used as instrumental variables, which may signal inconsistencies in the estimated causal effects. We assessed heterogeneity using the MR Egger and Inverse Variance Weighted (IVW) methods, with a Q_pval < 0.05 considered indicative of significant heterogeneity. To evaluate pleiotropy, where a genetic variant may influence multiple phenotypic traits and potentially confound the causal estimates, we employed the MR Egger method. A p-value < 0.05 was taken as evidence of pleiotropy. The robustness of the causal estimates and the influence of individual genetic variants were further examined using the leave-one-out method, which involves systematically excluding each genetic variant in turn to assess its impact on the overall causal estimate. The results of the MR analysis were visually represented using scatter plots, forest plots, and leave-one-out plots to enhance the clarity and interpretation of the findings.
Results
Following the instrumental variable (IV) selection strategy outlined earlier, this study identified a total of 1,824 SNPs as IVs for the 72 dietary habits, with the number of IVs per dietary habit ranging from 9 to 116. These selected SNPs were subsequently extracted from the GWAS summary data for female pelvic peritoneal adhesions. Detailed information on all IVs, including SNP rsIDs, effect sizes (beta coefficients), alleles (effect and other), standard errors (SEs), effect allele frequencies (EAFs), and P-values, is provided in Table S2.
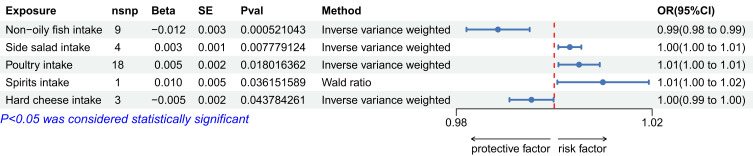
Before Bonferroni correction, five dietary habits showed potential associations with female pelvic peritoneal adhesions:
-
1)
Non-oily fish intake: OR 0.989 (95% CI: 0.982–0.995), p=0.000521
-
2)
Side salad intake: OR 1.003 (95% CI: 1.001–1.006), p=0.007779
-
3)
Poultry intake: OR 1.005 (95% CI: 1.001–1.009), p=0.018016
-
4)
Spirits intake: OR 1.010 (95% CI: 1.001–1.019), p=0.036152
-
5)
Hard cheese intake: OR 0.995 (95% CI: 0.991–1.000), p=0.043784
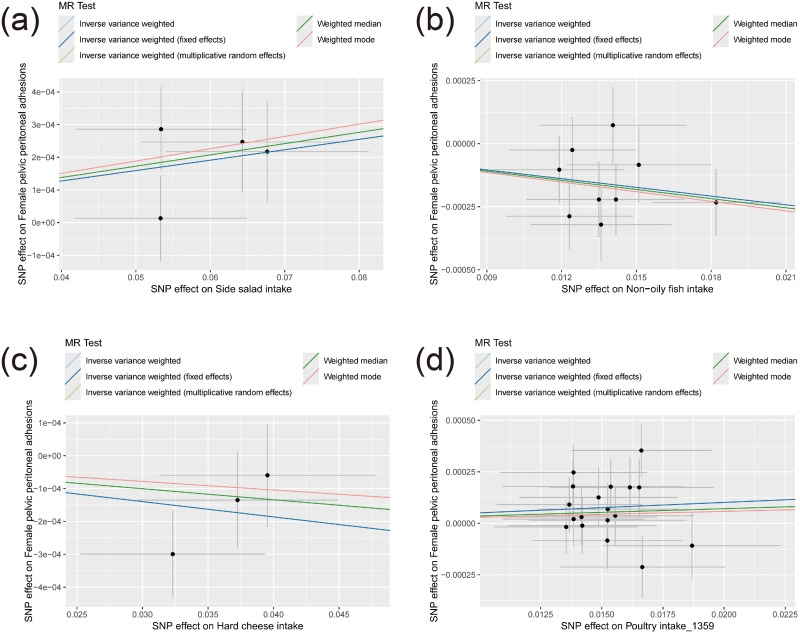
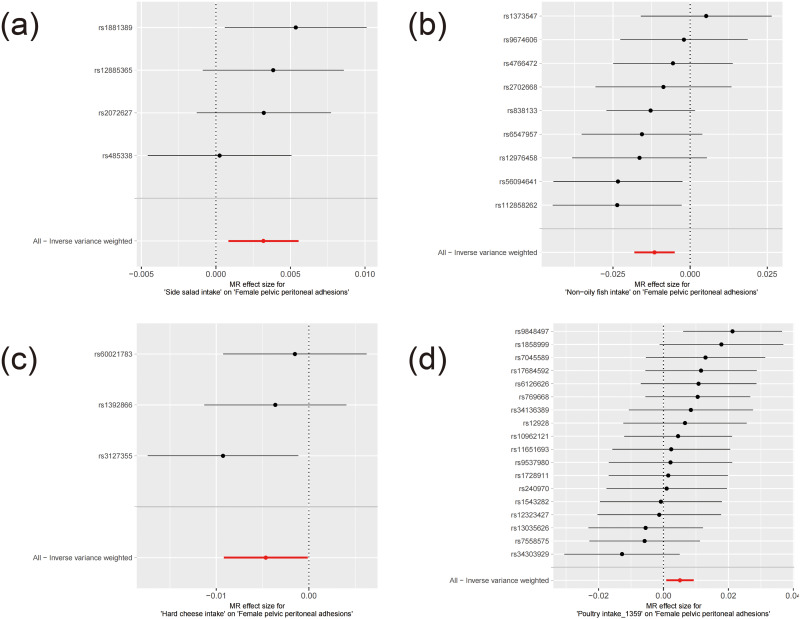
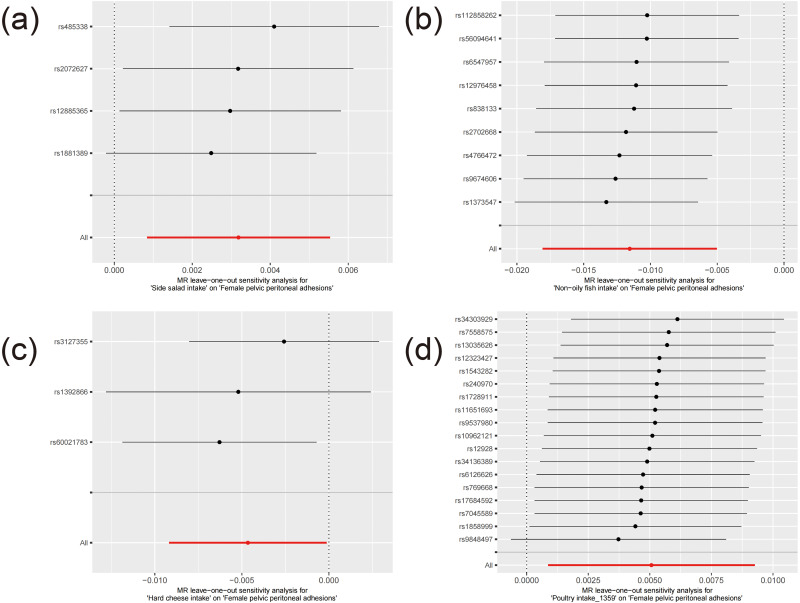
These associations are illustrated in Figures 1–3. After applying Bonferroni correction, non-oily fish intake was found to have a significant causal relationship with female pelvic peritoneal adhesions: OR 0.989 (95% CI: 0.982–0.995), p=0.000521. The complete results of the MR analyses, including those from all five MR methods, are available in Table S3. Sensitivity analysis showed no evidence of heterogeneity or pleiotropy for this finding, as detailed in Tables S4 and S5. As depicted in Figure 4, the leave-one-out analysis indicated that no single SNP significantly influenced the result.
Figure 1.
The forest plot of potential associations with female pelvic peritoneal adhesions.
Figure 2.
The scatter plots for MR results of potential associations with female pelvic peritoneal adhesions. (a–d) represents respectively Side salad intake, Non−oily fish intake, Hard cheese intake, SNP effect on Poultry intake_1359.
Figure 3.
The forest plot for MR results of potential associations with female pelvic peritoneal adhesions. (a–d) represents respectively Side salad intake, Non−oily fish intake, Hard cheese intake, SNP effect on Poultry intake_1359.
Figure 4.
The leave-one-out plots of potential associations with female pelvic peritoneal adhesions. (a–d) represents respectively Side salad intake, Non−oily fish intake, Hard cheese intake, SNP effect on Poultry intake_1359.
For the reverse MR analysis, three IVs were identified for female pelvic peritoneal adhesions. The reverse MR results did not reveal any causal relationship. Detailed results from all MR methods, as well as the heterogeneity and pleiotropy tests, can be found in Tables S6–S9.
Discussions
In this study, we employed a comprehensive Mendelian randomization approach to explore the causal relationships between 72 dietary habits and female pelvic peritoneal adhesions. Utilizing genetic instrumental variables derived exclusively from female participants, our analysis revealed potential associations between five dietary habits and FPPA before Bonferroni correction, with non-oily fish intake demonstrating a significant causal relationship after correction. We further strengthened the validity of our findings through sensitivity analyses, which indicated no evidence of heterogeneity or pleiotropy, thereby enhancing the robustness of our results. Additionally, a reverse MR analysis was conducted to investigate the possibility of reverse causality between FPPA and dietary habits, which did not reveal any significant causal relationships.
The finding that non-oily fish intake has a significant causal relationship with FPPA after Bonferroni correction, with an odds ratio (OR) of 0.989 (95% CI: 0.982–0.995), p=0.000521, suggests that this dietary habit exerts a protective effect against the development of FPPA. The OR being less than 1 indicates that higher consumption of non-oily fish is associated with a reduced risk of FPPA, pointing to a beneficial role of this dietary component in mitigating the formation of pelvic adhesions.
Non-oily fish, which typically includes varieties such as cod, haddock, and tilapia, is rich in essential nutrients like high-quality protein, vitamins (such as vitamin D and B12), and minerals (such as selenium).20 However, the protective effect observed in our study is likely attributable to the presence of omega-3 fatty acids, albeit in lower concentrations than in oily fish.21 Omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been extensively studied for their anti-inflammatory properties.22 These fatty acids are known to modulate inflammatory responses by altering the production of eicosanoids, cytokines, and reactive oxygen species, all of which play critical roles in the pathophysiology of adhesions.23 FPPA is primarily driven by inflammatory processes following surgical trauma, infection, or endometriosis. The peritoneal healing process involves a complex interplay of pro-inflammatory and anti-inflammatory signals, with imbalances often leading to excessive fibrosis and adhesion formation. The anti-inflammatory effects of omega-3 fatty acids could potentially disrupt this pathological process by reducing the expression of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), which are known to promote fibroblast proliferation and extracellular matrix deposition.24 Additionally, omega-3 fatty acids may enhance the resolution of inflammation by promoting the synthesis of specialized pro-resolving mediators (SPMs) such as resolvins and protectins, which facilitate tissue repair and prevent chronic inflammation. Moreover, the consumption of non-oily fish might contribute to a balanced dietary intake that supports overall metabolic and hormonal health, factors that are intricately linked to the development of gynecological conditions.25 For instance, a diet that includes fish could help in maintaining optimal levels of adiponectin, an anti-inflammatory adipokine that has been shown to have protective effects against adhesion formation. Lower levels of adiponectin have been associated with increased inflammation and fibrosis, suggesting that dietary factors influencing adiponectin levels could indirectly impact the risk of FPPA. The protective role of non-oily fish intake might also be related to its influence on systemic oxidative stress.26 The antioxidant properties of selenium, commonly found in fish, may reduce oxidative damage to peritoneal tissues, thereby preventing the excessive fibroblast activity that leads to adhesion formation. Oxidative stress is a known contributor to the pathogenesis of FPPA, as it can exacerbate the inflammatory response and promote fibrotic changes within the peritoneal cavity. By counteracting oxidative stress, nutrients found in non-oily fish could contribute to a more favorable healing environment, thereby reducing the likelihood of adhesion formation. Additionally, it is important to consider the broader dietary context in which non-oily fish intake occurs. Individuals who consume higher amounts of non-oily fish may also engage in other health-promoting behaviors, such as increased consumption of fruits, vegetables, and whole grains, which are rich in anti-inflammatory and antioxidant compounds. These synergistic effects of a balanced diet could further enhance the protective role of non-oily fish against FPPA. However, the protective effect observed in this study may also be influenced by genetic factors that modulate individual responses to dietary intake. Genetic polymorphisms related to fatty acid metabolism, inflammatory pathways, and antioxidant defenses could determine how effectively a person benefits from the nutrients found in non-oily fish. Future research should aim to explore these genetic interactions to better understand the variability in dietary impacts on FPPA risk.
In addition to our findings, recent studies have provided further evidence supporting the use of fractional CO2 laser in improving quality of life and symptoms in postmenopausal women with vulvo-vaginal atrophy (VVA). For instance, a study demonstrated that fractional CO2 laser treatment significantly improved sexual function and reduced symptoms of VVA, as measured by the Female Sexual Function Index (FSFI) and the Vaginal Health Index (VHI).27 Similarly, another study highlighted the safety and efficacy of fractional CO2 laser in gynecological cancer survivors, with notable improvements in both sexual function and overall quality of life.28 These findings align with our results, reinforcing the notion that fractional CO2 laser is a valuable therapeutic option for women suffering from VVA, particularly those with a history of gynecological cancer.
Several limitations should be acknowledged, which may affect the interpretation and generalizability of our findings. Firstly, the study relies on summary-level data from genome-wide association studies, which inherently limits the granularity of the analysis. Although GWAS provide robust associations between genetic variants and traits, they do not account for potential interactions between genetic variants and environmental factors, including other dietary habits or lifestyle factors. This means that the causal estimates derived from Mendelian randomization might not fully capture the complexity of dietary influences on FPPA, particularly in real-world settings where multiple factors interact dynamically. Secondly, our study focused exclusively on female participants of European descent, which, while enhancing the internal validity of our findings, limits their external applicability. The genetic architecture and dietary patterns of other populations may differ significantly, potentially leading to different associations between diet and FPPA in non-European populations. This population-specific focus also means that the findings may not be generalizable to women from other ethnic backgrounds, who might have different genetic susceptibilities or dietary exposures. Future research should aim to replicate these findings in more diverse populations to determine the universality of the observed associations. Another limitation lies in the selection and definition of dietary habits. The 72 dietary habits examined in this study were defined based on self-reported data, which is subject to recall bias and measurement error. Such inaccuracies could weaken the associations between the dietary habits and FPPA, leading to either underestimation or overestimation of the true causal effects. Moreover, the dietary habits were assessed in isolation, without considering their potential interactions or cumulative effects. In reality, dietary habits often co-occur and may have synergistic or antagonistic effects on health outcomes. The exclusion of these interactions may result in a simplified view of the relationship between diet and FPPA. Furthermore, the MR analysis itself, while powerful, is not without its own limitations. One major assumption of MR is the absence of pleiotropy, where genetic variants used as instrumental variables influence the outcome through pathways other than the exposure of interest. Although we employed sensitivity analyses to detect and account for pleiotropy, the possibility of unmeasured or unknown pleiotropic effects cannot be entirely ruled out. This is particularly relevant given the complex nature of dietary patterns, which may influence multiple biological pathways beyond those directly related to FPPA. Additionally, the MR approach assumes that the relationship between the genetic variants and the exposure (in this case, dietary habits) is linear and that the effect of the exposure on the outcome is also linear. However, dietary effects on health outcomes are often non-linear, with potential threshold effects or diminishing returns at higher levels of intake. Our analysis may not fully capture these non-linearities, which could lead to oversimplified conclusions regarding the impact of specific dietary habits on FPPA risk. Lastly, while our study explored reverse causality, the limited number of instrumental variables available for FPPA constrained the power of the reverse MR analysis. This limitation might have led to an underestimation of potential reverse causal relationships, where FPPA could influence dietary habits, rather than the other way around. Further studies with larger and more comprehensive datasets are needed to fully explore these bidirectional relationships.
Conclusion
This study provides novel evidence that non-oily fish intake may have a protective effect against the development of FPPA, as demonstrated through Mendelian randomization analysis. By leveraging genetic instrumental variables derived from large-scale GWAS data, we were able to establish a potential causal relationship that suggests the beneficial role of specific dietary habits in reducing FPPA risk. Despite the limitations inherent in this study, our findings underscore the importance of considering dietary interventions as part of the broader strategy for managing and preventing gynecological conditions such as FPPA. These results also highlight the need for further research to explore the underlying mechanisms and to validate these associations in more diverse populations and through longitudinal studies.
Funding Statement
There is no funding to report.
Data Sharing Statement
The genotype and phenotype data are available on application from the GWAS Catalog or the published article and its supplementary files, the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/, id = ukb-b-6413), UK Biobank (http://www.nealelab.is/uk-biobank).
Ethics Approval and Consent to Participate
This study complies with the Declaration of Helsinki and was approved by the ethics committee of Changning Maternity and Infant Health Hospital, East China Normal University (CNFBLLAR-2024–007).
Author Contributions
Tiantian Dai was the first author. Yi Zhang was the corresponding author. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
- 1.Tabibian N, Swehli E, Boyd A, Umbreen A, Tabibian JH. Abdominal adhesions: a practical review of an often overlooked entity. Ann Med Surg. 2017;15:9–13. doi: 10.1016/j.amsu.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007;9(Suppl 2):25–34. doi: 10.1111/j.1463-1318.2007.01358.x [DOI] [PubMed] [Google Scholar]
- 3.Imudia AN, Kumar S, Saed GM, Diamond MP. Pathogenesis of intra-abdominal and pelvic adhesion development. Semin Reprod Med. 2008;26(4):289–297. doi: 10.1055/s-0028-1082387 [DOI] [PubMed] [Google Scholar]
- 4.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18(4):260–273. doi: 10.1159/000050149 [DOI] [PubMed] [Google Scholar]
- 5.Al-Jabri S, Tulandi T. Management and prevention of pelvic adhesions. Semin Reprod Med. 2011;29(2):130–137. doi: 10.1055/s-0031-1272475 [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A, Spedicato M, Mutinati M, et al. Peritoneal adhesions in human and veterinary medicine: from pathogenesis to therapy. A review. Immunopharmacol Immunotoxicol. 2010;32(3):481–494. doi: 10.3109/08923970903524367 [DOI] [PubMed] [Google Scholar]
- 7.ten Broek RP, Issa Y, van Santbrink EJ, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ. 2013;347:f5588. doi: 10.1136/bmj.f5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canis M, Botchorishvili R, Bourdel N, Gremeau AS, Curinier S, Rabischong B. Pelvic adhesions and fertility: where are we in 2018? J Visc Surg. 2018;155(Suppl 1):S11–S15. doi: 10.1016/j.jviscsurg.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Dawood AS, Elgergawy AE. Incidence and sites of pelvic adhesions in women with post-caesarean infertility. J Obstet Gynaecol. 2018;38(8):1158–1163. doi: 10.1080/01443615.2018.1460583 [DOI] [PubMed] [Google Scholar]
- 10.Vrijland WW, Jeekel J, van Geldorp HJ, Swank DJ, Bonjer HJ. Abdominal adhesions: intestinal obstruction, pain, and infertility. Surg Endosc. 2003;17(7):1017–1022. doi: 10.1007/s00464-002-9208-9 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Gomez E, Luque-Vara T, Moya-Fernandez PJ, Lopez-Olivares M, Gallardo-Vigil MA, Enrique-Miron C. Factors influencing dietary patterns during pregnancy in a culturally diverse society. Nutrients. 2020;12(11):3242. doi: 10.3390/nu12113242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James DC. Factors influencing food choices, dietary intake, and nutrition-related attitudes among African Americans: application of a culturally sensitive model. Ethn Health. 2004;9(4):349–367. doi: 10.1080/1355785042000285375 [DOI] [PubMed] [Google Scholar]
- 13.Kant AK. Dietary patterns: biomarkers and chronic disease risk. Appl Physiol Nutr Metab. 2010;35(2):199–206. doi: 10.1139/H10-005 [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Gao ZJ, Yu X, Wang P. Dietary regulation in health and disease. Signal Transduct Target Ther. 2022;7(1):252. doi: 10.1038/s41392-022-01104-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestel PJ, Mori TA. Dietary patterns, dietary nutrients and cardiovascular disease. Rev Cardiovasc Med. 2022;23(1):17. doi: 10.31083/j.rcm2301017 [DOI] [PubMed] [Google Scholar]
- 16.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcinkowska A, Gornicka M. The role of dietary fats in the development and treatment of endometriosis. Life. 2023;13(3):654. doi: 10.3390/life13030654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahid R, Iahtisham Ul H, Mahnoor, et al. Diet and lifestyle modifications for effective management of polycystic ovarian syndrome (PCOS). J Food Biochem. 2022;46(7):e14117. doi: 10.1111/jfbc.14117 [DOI] [PubMed] [Google Scholar]
- 19.Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50(5):1651–1659. doi: 10.1093/ije/dyab084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sargent JR. Fish oils and human diet. Br J Nutr. 1997;78(Suppl 1):S5–13. doi: 10.1079/bjn19970131 [DOI] [PubMed] [Google Scholar]
- 21.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x [DOI] [PubMed] [Google Scholar]
- 22.Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51(3):232–237. doi: 10.1016/j.plipres.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2(3):355–374. doi: 10.3390/nu2030355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson BZ, Stevenson AW, Prele CM, Fear MW, Wood FM. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines. 2020;8(5):101. doi: 10.3390/biomedicines8050101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamiol-Milc D, Biernawska J, Liput M, Stachowska L, Domiszewski Z. Seafood intake as a method of non-communicable diseases (NCD) prevention in adults. Nutrients. 2021;13(5):1422. doi: 10.3390/nu13051422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Li Z, Gao Y, Xu B, Zhang W, Wu IXY. Fish oil supplementation and risk of incident systemic lupus erythematosus: a large population-based prospective study. Nutr J. 2024;23(1):63. doi: 10.1186/s12937-024-00965-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Donato V, D’Oria O, Scudo M, et al. Safety evaluation of fractional CO(2) laser treatment in post-menopausal women with vaginal atrophy: a prospective observational study. Maturitas. 2020;135:34–39. doi: 10.1016/j.maturitas.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 28.D’Oria O, Giannini A, Buzzaccarini G, et al. Fractional Co2 laser for vulvo-vaginal atrophy in gynecologic cancer patients: a valid therapeutic choice? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2022;277:84–89. doi: 10.1016/j.ejogrb.2022.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genotype and phenotype data are available on application from the GWAS Catalog or the published article and its supplementary files, the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/, id = ukb-b-6413), UK Biobank (http://www.nealelab.is/uk-biobank).