Abstract
With national and global health policymakers facing numerous complex decisions related to achieving and maintaining polio eradication, we expanded our previously developed dynamic poliovirus transmission model using information from an expert literature review process and including additional immunity states and the evolution of oral poliovirus vaccine (OPV). The model explicitly considers serotype differences and distinguishes fecal-oral and oropharyngeal transmission. We evaluated the model by simulating diverse historical experiences with polioviruses, including one country that eliminated wild poliovirus using both OPV and inactivated poliovirus vaccine (IPV) (USA), three importation outbreaks of wild poliovirus (Albania, the Netherlands, Tajikistan), one situation in which no circulating vaccine-derived polioviruses (cVDPVs) emerge despite annual OPV use and cessation (Cuba), three cVDPV outbreaks (Haiti, Madura Island in Indonesia, northern Nigeria), one area of current endemic circulation of all three serotypes (northern Nigeria), and one area with recent endemic circulation and subsequent elimination of multiple serotypes (northern India). We find that when sufficient information about the conditions exists, the model can reproduce the general behavior of poliovirus transmission and outbreaks while maintaining consistency in the generic model inputs. The assumption of spatially homogeneous mixing remains a significant limitation that affects the performance of the differential equation-based model when significant heterogeneities in immunity and mixing may exist. Further studies on OPV virus evolution and improved understanding of the mechanisms of mixing and transmission may help to better characterize poliovirus transmission in populations. Broad application of the model promises to offer insights in the context of global and national policy and economic models.
Keywords: Polio eradication, dynamic modeling, disease outbreaks
1. INTRODUCTION
The World Health Assembly resolved in 1988 to eradicate polio globally.(1) Since then, the Global Polio Eradication Initiative (GPEI) worked with countries to successfully eradicate one of the three wild poliovirus serotypes (i.e., type 2 in 1999),(2) certify three of the six World Health Organization (WHO) regions as polio-free, and interrupt apparent transmission of indigenous wild polioviruses types 1 and 3 (WPV1 and WPV3, respectively) in all but three countries (i.e., Afghanistan, Nigeria, and Pakistan). Currently, all countries remain at risk of outbreaks due to importations of wild poliovirus from the remaining reservoirs of indigenous or reestablished poliovirus transmission(3) and at risk of outbreaks of circulating vaccine-derived poliovirus (cVDPV) as long as oral poliovirus vaccine (OPV) use continues.(4) Managing these risks requires focusing on managing population immunity,(5) for which countries face numerous different vaccine choices and delivery strategies.(6) Completing the eradication of WPV2 requires that countries coordinate and agree on a minimum global policy that they implement nationally to achieve eradication.(6,7) Ending all cases of poliomyelitis will require that countries coordinate and agree to the synchronized cessation of the different serotypes of OPV, including the imminent decision about cessation of all type 2-containing OPV.(7,8)
Mathematical models of poliovirus transmission can help us understand population immunity and its dynamic interaction with outbreaks and vaccination policies. Economic evaluation of policy alternatives requires dynamic poliovirus transmission models to correctly estimate the risks and benefits of the alternatives.(9–12) We previously developed a dynamic poliovirus transmission model(13) to support economic analyses of post-eradication policies,(11,14) which also yielded important dynamic insights related to achieving eradication.(10,15) To address policies at a highly aggregate level (i.e., ultimately at a global level, but while considering differences by income group), the model sought to minimize complexity while maintaining the ability to characterize the impact of major policy choices on the expected cases from outbreaks triggered by exogenously generated random events.(14) Specifically, the prospective outbreak model(13) used model inputs reflecting “average serotypes” and assumed secondary OPV infection rates independent of population immunity levels, although later adaptations of the model include OPV transmission, but not evolution, as part of the dynamic model.(16) Recent changes in poliovirus vaccine options and global policies motivate the development of an expanded poliovirus transmission and evolution model. Specifically, given the GPEI’s strategic shift after 2005 to focus increasingly on individual serotypes using monovalent OPV types 1 and 3 (mOPV1 and mOPV3) and since 2010 on bivalent types 1 and 3 OPV (bOPV) rather than trivalent OPV (tOPV) for supplementary immunization activities (SIAs) and the possibility of cessation of all type-2 containing OPV (OPV2), explicit consideration of population immunity and risks for each serotype becomes much more important.(5) With significant uncertainty remaining about cVDPV risks after OPV cessation and the appropriate response strategies, the evolution of OPV and its dynamic interaction with population immunity requires better assessment to support the management of cVDPV risks.(17) In addition, recent pursuit of an aggressive research agenda to stimulate the development of more affordable inactivated poliovirus vaccine (IPV) options may substantially increase the attractiveness of policies involving IPV. Discussion of various IPV immunization options (e.g., using a single dose of IPV) combined with the potential impact of waning on population immunity to poliovirus transmission,(18) motivate the consideration of an expanded set of immunity states, including states for various IPV doses with or without infection with live poliovirus (LPV, including WPV, OPV, or any OPV-related live virus) and for multiple stages of waning.(5,19) In addition, given that IPV protects much better against oropharyngeal than fecal excretion,(18) fully capturing the differences between the vaccines requires explicitly distinguishing fecal-oral and oropharyngeal transmission.
This article describes our expanded poliovirus transmission model for use in risk, decision, and economic analyses to help inform current and future polio policy questions. We present the results of an iterative process of modeling past experiences with polioviruses in different contexts. We base the selection of generic model inputs largely on an extensive expert literature review process(18,19) and setting-specific inputs on the best available data for each situation. The iterative process ensures internally consistent assumptions about the many highly uncertain model inputs(18,19) and serves to demonstrate the ability of the model to replicate different features of poliovirus transmission and evolution.
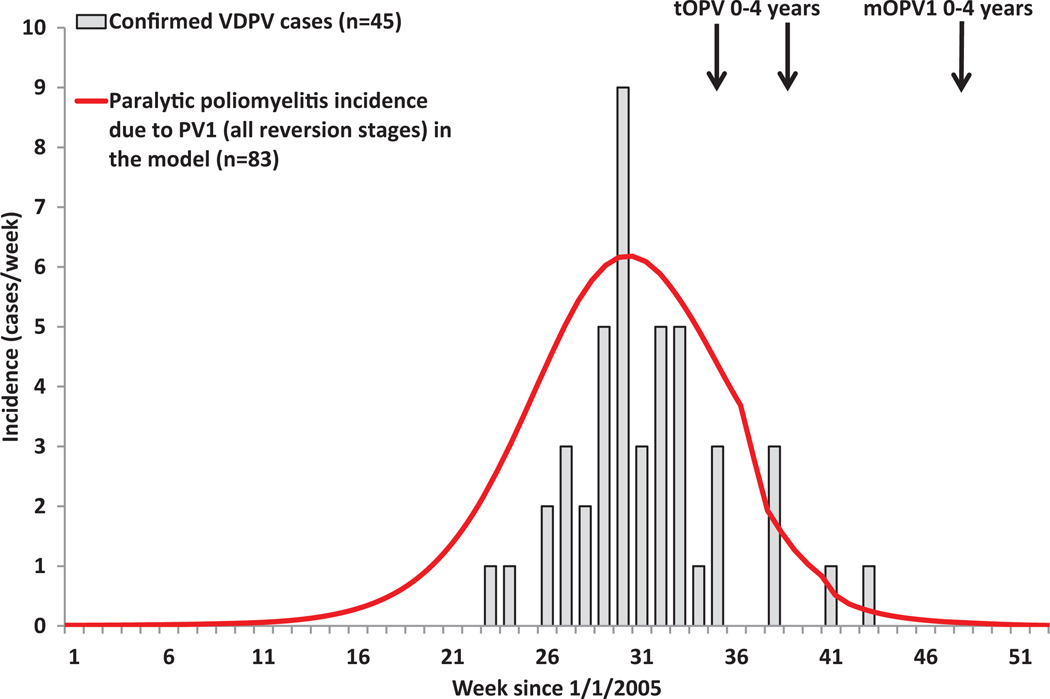
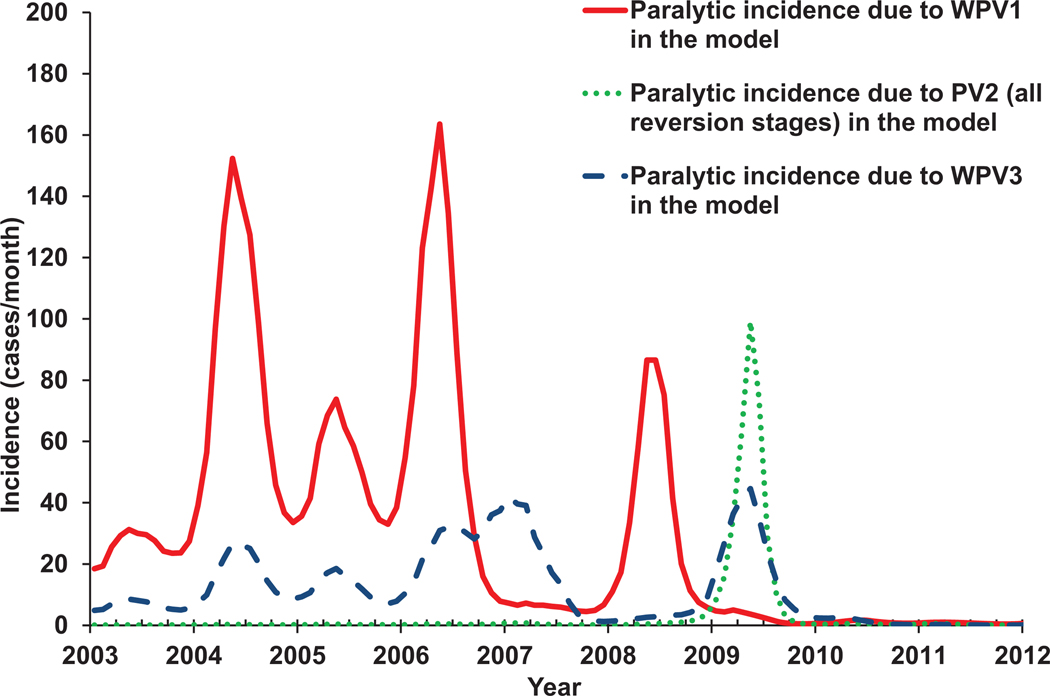
We assume familiarity with polioviruses(17,18,20–25) and prior poliovirus transmission models.(13,26–29) The next section provides an overview of the model structure and inputs and describes the methods for our application of the model to multiple situations selected to test the model on different types of poliovirus dynamics. We present the results of application of the model to one country that eliminated WPV using both OPV and IPV (the USA), three polio-free countries that experienced WPV importation outbreaks (Albania, the Netherlands, Tajikistan), one country in which no cVDPVs emerge despite annual OPV use in campaigns and no routine OPV immunization (Cuba), three places that experienced cVDPV outbreaks (Haiti, Madura Island in Indonesia, northern Nigeria), one area with ongoing endemic transmission of WPV1, WPV3, and cVDPV2 (northern Nigeria), and one area with recent endemic circulation and elimination of WPV1 and WPV3 (northern India). In each situation, we used the best publicly available data to characterize the setting-specific population dynamics and vaccination history. We discuss the performance of the model and important issues and limitations with the hope that our transparent and comprehensive analysis will facilitate assessments of further use of this and other models to support policy and economic analyses. Specifically, the model may help assess the tradeoffs in costs, risks, and benefits of current and future global vaccination options, including cessation of type 2-containing OPV and the use of IPV,(7) determine optimal SIA vaccine(s), scope, and frequency in specific countries,(6) and explore outbreak response options after OPV cessation.
2. METHODS
2.1. Model Structure
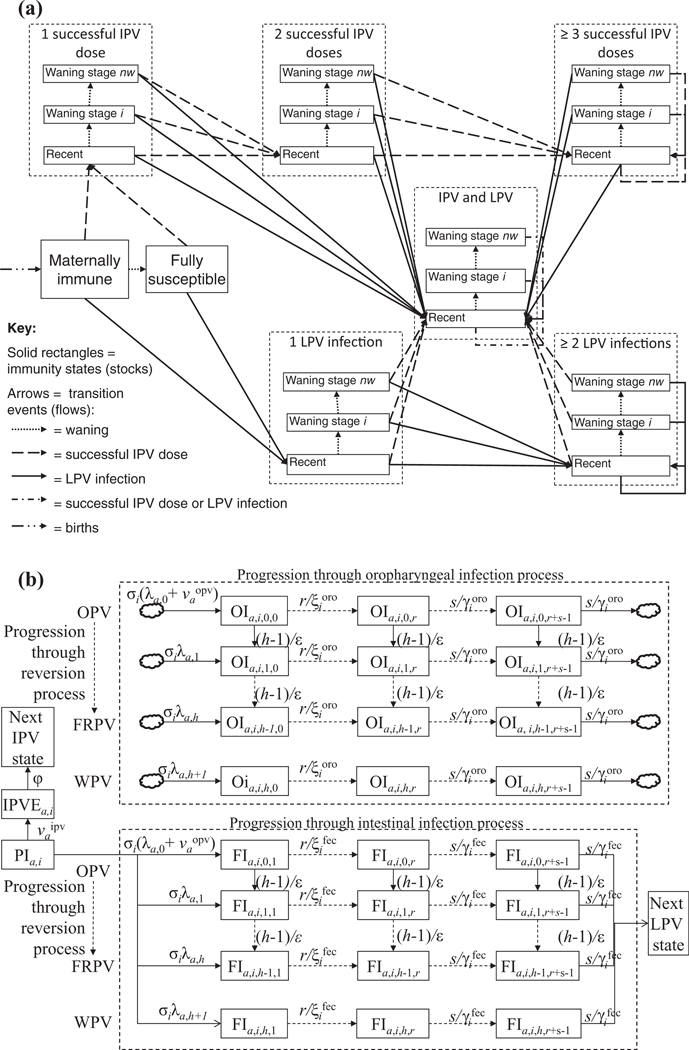
Fig. 1 provides the model structure in the form of two diagrams, which expand on the high-level conceptual diagram presented elsewhere (see Fig. 1 in Thompson et al.(5)). Fig. 1(a) shows the flows between eight immunity states as a result of various epidemiological events (see Appendix A1 for all of the model equations). An expert review process identified the eight states in Fig. 1 as the minimum set needed to characterize significantly different states with respect to poliovirus transmission, although each state reflects a distribution because individuals and viruses vary.(18,19) We included immunity states for a single successful dose of vaccine to accommodate the possibility of exploring real policy options under discussion that might rely on delivery of a single dose.(7) We characterize each immunity state by the: (1) average relative susceptibility to infection compared to fully susceptible individuals , (2) average latent period and average duration of infectiousness (both different for fecal and oropharyngeal infections), and (3) average relative infectiousness compared to fully susceptible individuals (also different with respect to fecal-oral and oropharyngeal transmission), defined as the daily probability of infecting others by an infected individual in a given immunity state divided by the daily probability of infecting others by a previously fully susceptible infected individual in an identical situation. Individuals in the fully susceptible state never experienced (1) infection with a LPV or (2) effective immunization with IPV, and they lack any residual maternal immunity. We assume that fully susceptible individuals may contract paralytic poliomyelitis upon infection at a serotype-specific paralysis-to-infection rate (PIR). We assume that children born to mothers with any active recent or historical immunity, not including immunity from a single IPV dose, receive maternal antibodies at birth, which we assume protect them to some extent from infection and infectiousness until they age into the fully susceptible state after a short time, and also reduce their PIR by a fixed fraction during this time . We assume that maternally immunes not infected with a LPV or successfully vaccinated become fully susceptible as they age into the second age group, which in the model always starts at age 3 months. While the model structure accommodates different PIRs by age (in the form of a relative PIR compared to the first age group, or ), we used serotype-specific but age-independent values for PIR given the large uncertainties about actual PIRs due to our inability to observe predominantly asymptomatic infections. For all immunity states other than maternally immunes, we assume that in the absence of further successful IPV vaccinations or LPV infections, waning occurs over stages as characterized by increasing relative susceptibility, duration of infectiousness, and relative infectiousness. However, we assume that any active immunity from IPV or LPV provides lifelong protection from paralytic poliomyelitis.
Fig. 1.
Overview of the model structure.
(a) Flows between immunity states as a result of epidemiological events.
(b) Infection and reversion processes.
Acronyms: FRPV = fully-reverted poliovirus; IPV = inactivated poliovirus vaccine; OPV = oral poliovirus vaccine; WPV = wild poliovirus
Symbols: = partially infectible in age group a and immunity state
= IPV-exposed individual from immunity state and age group a.
= individual in age group a from immunity state , infected with virus strain j and in fecal (oropharyngeal) infection stage
= force-of–infection to age group a for virus strain
= force-of-IPV(OPV)-vaccination to age group a as a result of routine and supplementary immunization
= relative susceptibility for immunity state
= average duration of the fecal (oropharyngeal) latent period for immunity state
= average duration of the fecal (oropharyngeal) infectious period for immunity state
= IPV immunity delay
= number of reversion stages
= number of latent stages
= number of infectious stages
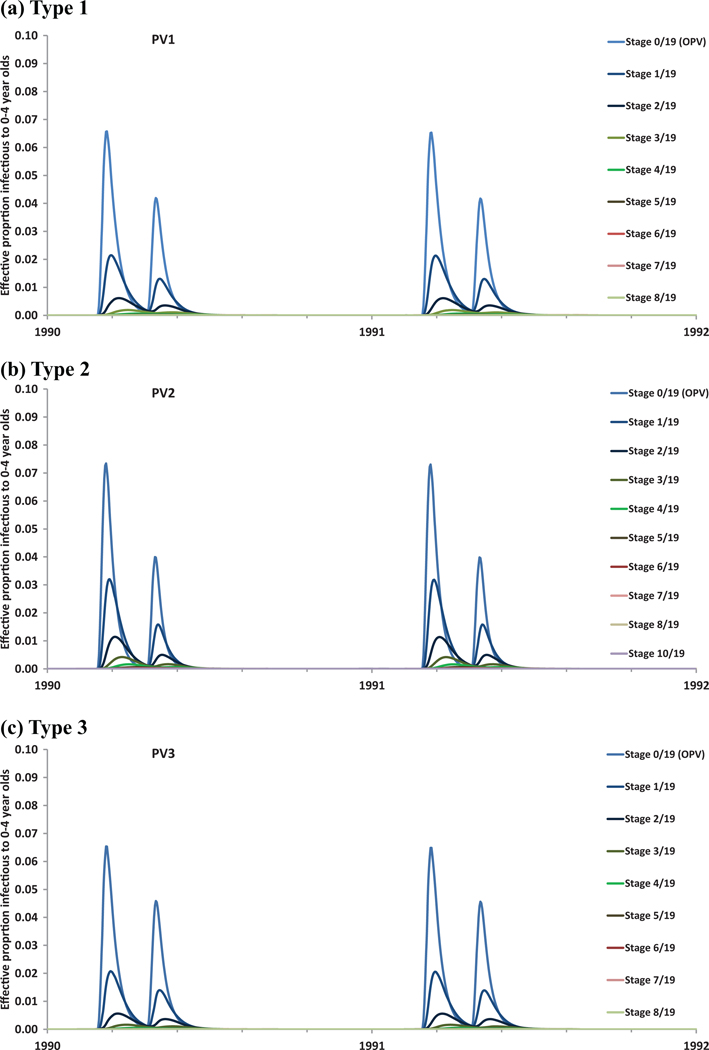
Fig. 1(b) shows model characterization of the infection and reversion process for partially infectibles in a given immunity state . We use a discrete number of reversion stages to model the reversion process by which OPV viruses eliminate attenuating mutations over time as they adapt to the human gut and revert toward WPV-like properties.(22–24,30) We assume the same PIR and basic reproductive number (R0)(31) as typical homotypic WPVs for the last reversion stage.(17) This differential equation-based (DEB) model does not attempt to simulate the exact random process by which individual viruses eliminate mutations and change properties, but instead defines each discrete reversion stage as a hypothetical virus strain characterized by a distinct average PIR and average relative R0 compared to WPV.(17) However, like any multi-stage expiry process in a DEB model, the reversion process implies that any inflow into the first stage results in a gamma distribution over the time to reach the last reversion stage, with the mean equal to the total duration of the reversion process and other parameters determined by the number of stages.(32,33) The model does not account for the possibility that the virus mutates towards a lower reversion stage. Specifically, the model distinguishes reversion stages and different virus strains ranging from the OPV viruses with all attenuating mutations intact for (i.e., the OPV virus as given to vaccinees) to the fully-reverted poliovirus (FRPV) for to WPV for . We assume equidistant reversion stages, with characterizing the average time for the OPV virus to reach the last reversion stage (i.e., to acquire the properties of a typical homotypic WPV). Observations for from VDPV or vaccine-associated paralytic poliomyelitis (VAPP, i.e., very rare cases of paralytic poliomyelitis associated with the vaccine in OPV recipients or their close contacts(34)) cases are conditional on the occurrence of substantial reversion and therefore may represent underestimates of the actual average reversion time for all healthy OPV recipients in any given population.(17)
In Fig. 1(b), relative susceptibility determines the relative rate at which individuals in this immunity state become infected compared to fully susceptible individuals, and the absolute rate depends on the force of infection of age group a and virus strain . The force of infection for virus strain and age group a depends on the assumed R0 for the given virus strain and the product of the setting-specific age-mixing matrix (35,36) and the number of people in each age group residing in infectious states with strain ,(31) weighted according to their relative infectiousness and the relative importance of their transmission mode (i.e., fecal-oral or oropharyngeal) (see equations in Appendix A1). We further include seasonality by oscillating R0 according to a sine function characterized by a peak day (i.e., day of each year when the sine function becomes maximum) and an amplitude (i.e., , defined as the difference between the peak or trough and the average R0, relative to the average ). For each age group, the mixing matrix governs the relative weight that all age groups carry onto the force of infection. We assume that does not depend on the virus strain and in the absence of empirical data on mixing by age for fecal-orally spread infections we assume highly simplified mixing matrices based on the preferential mixing model described below.(37–39) OPV infections can occur both as a result of contact with other OPV-infectious individuals and through receipt of vaccine according to the effective force-of-OPV vaccination, . For simplicity in Fig. 1(b) we characterize as a single quantity, but the model separately accounts for delivery of both routine and supplementary immunization doses and for the appropriate associated probability of “take” of the vaccine in any given context (see below).(19,40) Similarly, includes the effective force-of-IPV vaccination from any routine and supplemental IPV use.
All individuals infected with a LPV enter both the chain of “progression through oropharyngeal infection,” which leads to infectiousness to others via oropharyngeal poliovirus excretion, and the chain of “progression through intestinal infection,” which leads to infectiousness to others via fecal excretion (Fig. 1(b)). Thus, we assume that anyone with a fecal infection becomes an oropharyngeal excretor as well (i.e., we do not model relative susceptibility to oropharyngeal infection separately). However, we characterize the possibility of lower rates of oropharyngeal than fecal excretion and infectiousness in most immunity states by assuming shorter durations and lower relative infectiousness compared to fully susceptible individuals for oropharyngeal than fecal infections. These assumptions do not preclude the possibility that oropharyngeal transmission may dominate in some settings, which depends on the assumed situation-specific proportion of transmission occurring via the oropharyngeal route (), because the force-of-infection expression by excretion mode factors in differences in duration (see equations in Appendix A1) and because differences in relative infectiousness only account for disproportionate effects of immunity on each excretion mode (i.e., for fully susceptibles, relative infectiousness is 1 by definition for both fecal and oropharyngeal transmission). To preserve the correct population size, we model the oropharyngeal infection process as a “co-flow,”(33) (i.e., we do not take oropharyngeal infections out of the stock PIi or let them recover into the next LPV state, as indicated by the clouds in Fig. 1(b), and we do not double count individuals in these states in the population). We assume that individuals remain fully protected from homotypic reinfection while still fecally infectious to others, but that as they enter the next LPV immunity state after recovering from fecal infectiousness, they again become partially infectible according to the relative susceptibility of the next LPV state. Fig. 1(a) shows the next LPV state for each immunity state as a result of the arrows representing the epidemiological event “LPV infection” (e.g., previously “fully susceptible” individuals recover to “1 LPV infection”). To accommodate nonexponential distributions of the infectious period, we model the infection by dividing the infectious period into s equidistant stages and the latent period into r equidistant stages.(32) We characterize different levels of infectiousness for each infectious stage, including zero infectiousness for latent stages immediately following exposure.
In the model, vaccination occurs as a result of two different mechanisms (both included in and in Fig. 1(b) (i.e., the effective vaccination rate (evr) for SIAs, and the effective vaccination coverage (evc) for routine vaccination). The evr captures activities focused in time that target individuals in wide age groups, while the evc captures activities that occur continuously and target individuals as they reach specific ages according to an age-dependent immunization schedule.
We calculate evr for a given vaccine and age group at any given time point from the proportion of the population subject to vaccination that should remain not effectively vaccinated after applying evr for a given period of time. This proportion equals the product of the effective per-round impact of the SIA round and the average per-dose take rate . For example, for an SIA round conducted over days that leaves unvaccinated a proportion of a previously unvaccinated target population (i.e., equals 1 minus the product of coverage and ), the daily evr must satisfy:
Given that polio immunization strategies typically do not differentiate between fully susceptible individuals and immunes, other than by age or location, all immunity states in a given targeted age group get exposed to the same evr, but multiplied by relative susceptibility for OPV vaccination, to reflect the different probabilities of becoming LPV-infected by immunity state. In contrast, for IPV vaccination, we do not multiply by relative susceptibility because we assume that the vaccine takes in fully susceptible individuals at the same rate that it boosts already primed or immune individuals.(41) Given that our DEB model stratifies only by immunity state and not by dose history, the same evr applies to each individual regardless of dose history, which implies that the entire target population experiences an equal chance of receiving vaccine in any given round. This probably does not correspond to the reality in many countries facing continued indigenous poliovirus transmission or elevated WPV importation or cVDPV risks. In those settings, some children get chronically missed by repeated SIAs, while others may receive a very high number of doses, which implies a higher vaccination rate in already vaccinated children.(42) To account for this phenomenon in these settings, we assume much lower for SIA rounds than the actual reported coverage of individual SIA rounds in any setting in which multiple rounds occur within a short period of time. To verify whether these lower s produce a realistic cumulative effect of SIAs, we provide the annual cumulative percentage of missed children , calculated as:
where is the number of rounds in a given calendar year, and is the fraction of the target age groups in the population targeted by the SIA round. The latter equals 1 unless the geographic extent of the round does not include the entire model population. For example, if a country conducts five SIA rounds in a year with and in each round, then . In reality, the same result in terms of missed children may have occurred due to five rounds with 70% coverage, but that failed to reach the same 17% of children in each round. Thus, the model input represents a model construct that depends on the frequency of rounds and does not correspond to measured coverage in SIA rounds. While it allows us to characterize realistic cumulative percentages of missed children, it may underestimate the frequency of doses among well-reached children, which should have limited impact because of their relatively small impact on transmission regardless of how many doses they receive. Nevertheless, in some situations, we believe that the concentration of missed children who mix more intensely with each other than with the general population may play an important role in transmission, and in those situations we model separate subpopulations with entirely different values of to better capture the reality of chronically missed subgroups (i.e., the Netherlands, northern Nigeria, and western Uttar Pradesh (WUP)).
To characterize routine immunization, we assume vaccination occurs at fixed ages (e.g., at birth and at exactly 3 months). At each of these ages, we divert a fraction of the aging flow for all partially infectibles into the first latent OPV stage of the next age group and a fraction to the IPVE state of the next age group in Fig. 1(b). The IPVE state represents the brief period (i.e., with average duration ) after receipt of IPV, but before full protection from disease (in the case of previously fully susceptible individuals or maternally immunes) and acquisition of the relative susceptibility, duration of infectiousness, and relative infectiousness of the next IPV state. The remaining fraction (i.e., ) ages into the next age group of the same partially infectible state. The model assumes that any routinely immunized child either experiences an effective take with IPV or with OPV, but not with both at the same time, so that . The evc adjusts for take and in the case of OPV vaccination we also multiply by relative susceptibility for partially immunes. In many situations, we explicitly factor in the effect of coverage with fewer than the recommended doses on the effective coverage, which we shall refer to as partial coverage in the descriptions below of all situation-specific model inputs.
2.2. Model Calibration Process
We determined all model inputs through an extensive iterative process. Given the large space of model inputs, their complicated interdependence structure, and the multiple different objectives for the model calibration process (i.e., including reproducing cumulative cases, kinetics of the case incidence, age distributions of cases, times of WPV elimination or VDPV emergence, cumulative force of infection from OPV-related virus), we did not attempt to develop a formal fitting algorithm that would likely yield a local optimum or not meet all the requirements. We do not expect that our iterative process necessarily yielded a global optimum set of model inputs, but instead we focused on the key requirement that the model inputs produce behavior consistent with key features of poliovirus transmission across the nine situations. We started with plausible ranges for generic model inputs that we required to remain constant across the situations (Table I) and situation-specific model inputs. Within this space, we searched for combinations of generic model inputs that produced realistic behavior across all situations. After fixing the generic model inputs that produced realistic behavior across all situations, we varied situation-specific constants in conjunction with situation-specific inputs that change over time, such as the effective impact of individual SIA rounds, or cumulative coverage of campaigns on an annual basis, including time-dependent situation-specific inputs (e.g., ). For this last step, we constrained the space by requiring realistic percentages of annual cumulative missed children, and/or available information about the total annual IPV doses used in IPV (Salk) era for the USA. In the northern Nigeria and northern India models, we considered both the percentage of annual cumulative children missed by SIAs, and separately the annual cumulative percentage of children that did not receive OPV containing each serotype. Due to the interdependence of model inputs, this multi-step iterative process did not occur in a linear fashion, but involved multiple revisions and partial repeats of the process after making changes to the generic model inputs.
Table I.
Generic Model Inputs for an Expanded Poliovirus Transmission Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Characterization of recent immunity states: | |||
| Relative susceptibility (σ) of recent immunity states (for PV1;PV2;PV3) | 18,19 | “Probability of homotypic poliovirus infection in a recent immunity state divided by the probability of homotypic poliovirus infection in fully susceptible individuals, given identical exposure,”(19) based on means of 9 expert assessments(19) | |
| - Maternally immune | 0.78;0.79;0.77 | ||
| - 1 successful IPV | 0.91;0.92;0.90 | ||
| - 2 successful IPV | 0.80;0.80;0.79 | ||
| - ≥ 3 successful IPV | 0.72;0.72;0.71 | ||
| - 1 LPV infection | 0.42;0.43;0.41 | ||
| - ≥ 2 LPV infections | 0.21;0.22;0.20 | ||
| - IPV and LPV | 0.21;0.22;0.20 | ||
| Duration of latent period ( or , in days) | ∼ 3a | 18,19 | Average time between LPV exposure and becoming infectious, based on means of 9 expert assessments(19) and assumed equal for all waning stages |
| Duration of fecal infectiousness (, in days) of recent immunity states (for PV1;PV2;PV3) | 18,19 | “Average length of time of [fecal] excretion of sufficiently high concentrations of virus for infectiousness to others,”(19) based on means of 9 expert assessments(19) | |
| - Fully susceptible | 28.0;27.8;28.3 | ||
| - Maternally immune | 24.6;24.6;24.6 | ||
| - 1 successful IPV, | 24.5;24.4;24.7 | ||
| - 2 successful IPV | 21.1;20.8;21.3 | ||
| - ≥ 3 successful IPV | 18.0;17.7;18.2 | ||
| - 1 LPV infection | 11.6;10.5;10.5 | ||
| - ≥ 2 LPV infections | 10.1;8.9;8.9 | ||
| - IPV and LPV | 10.1;8.9;8.9 | ||
| Duration of oropharyngeal infectiousness (, in days) of recent immunity states (no serotype differences) | 18,19 | “Average length of time of [oropharyngeal] excretion of sufficiently high concentrations of virus for infectiousness to others,”(19) based on means of 9 expert assessments(19) | |
| - Fully susceptible | 13.4 | ||
| - Maternally immune | 11.9 | ||
| - 1 successful IPV | 9.9 | ||
| - 2 successful IPV | 6.6 | ||
| - ≥ 3 successful IPV | 6.1 | ||
| - 1 LPV infection | 5.0 | ||
| - ≥ 2 LPV infections | 3.7 | ||
| - IPV and LPV | 3.7 | ||
| Relative fecal infectiousness of recent immunity states (for PV1;PV2;PV3) | 18,19 | Computed as relative contribution to fecal transmission compared to fully susceptible individuals,(19) divided by relative duration of fecal infectiousness compared to fully susceptible individuals | |
| - Maternally immune | 0.96;0.96;0.95 | ||
| - 1 successful IPV | 0.92;0.92;0.91 | ||
| - 2 successful IPV | 0.70;0.69;0.68 | ||
| - ≥ 3 successful IPV | 0.61;0.59;0.59 | ||
| - 1 LPV infection | 0.39;0.43;0.43 | ||
| - ≥ 2 LPV infections | 0.20;0.23;0.23 | ||
| - IPV and LPV | 0.20;0.23;0.23 | ||
| Relative oropharyngeal infectiousness of recent immunity states (no serotype differences) | 18,19 | Computed as relative contribution to oropharyngeal transmission compared to fully susceptible individuals;(19) divided by relative duration of oropharyngeal infectiousness compared to fully susceptible individuals; for the IPV-only states, we divided the expert-based estimate by 2 to obtain roughly similar oropharyngeal infectiousness as LPV states after accounting for higher relative susceptibility in the IPV-only states | |
| - Maternally immune | 0.68 | ||
| - 1 successful IPV | 0.30 | ||
| - 2 successful IPV | 0.17 | ||
| - ≥ 3 successful IPV | 0.12 | ||
| - 1 LPV infection | 0.33 | ||
| - ≥ 2 LPV infections | 0.21 | ||
| - IPV and LPV | 0.21 | ||
| Number of infection stages | Fitted such that approximately 1:600 fully susceptible individuals remain fecally infectious after 90 days (see Appendix A2);(45) assumed equal for all immunity states and waning stages | ||
| - Latent period (r) | 2 | ||
| - Infectious period (s) | 4 | ||
| Relative weight of infection stages, compared to average weight over the infectious period | 18,19 | Values obtained by fitting to elicited relative contribution to transmission over time from experts for fecally infectious fully susceptible individuals (see text and Appendix A2) | |
| - Infection stage 0 and 1 (latent stages) | 0 | ||
| - Infectious stage 2 | 12/17 | ||
| - Infectious stage 3 | 40/17 | ||
| - Infectious stage 4 | 12/17 | ||
| - Infectious stage 5 | 4/17 | ||
| IPV immunity delay (, in days) | 7 | 13 | Average time between successful IPV administration and acquisition of properties of next IPV state |
| Characterization of waning of immunity to poliovirus transmission: | |||
| Number of waning stages () | 5 | Includes recent stage; model choice intended to reasonably represent continuous waning process | |
| Shape of waning function () | 5 | 18,19 | Shape parameter in waning function (see methods section and Appendix A2) |
| Average time to reach last waning stage (, in days) | 18,19 | Based on informed judgment and model calibration; only applies to active immunity (i.e., not to maternally immunes); fastest waning for type 3 given typically lower antibody titers over time after infection or vaccination(46–49) | |
| - Type 1&2 | 4 × 365 | ||
| - Type 3 | 3 × 365 | ||
| Average time for maternal immunes to wane to fully susceptible (, in days) | 0.25 × 365 | 18,19 | Model choice to approximate patterns elicited from 9 experts;(19) this value corresponds to the width of the first age group |
| Relative susceptibility () for last waning stage (no serotype differences) | 18,19 | Based on informed judgment and model calibration | |
| - 1 successful IPV | 1.0 | ||
| - 2 successful IPV | 1.0 | ||
| - ≥ 3 successful IPV | 1.0 | ||
| - 1 LPV infection | 0.8 | ||
| - ≥ 2 LPV infections | 0.7 | ||
| - IPV and LPV | 0.7 | ||
| Duration of fecal infectiousness (, in days) of last waning stage (for PV1;PV2;PV3) | 18,19 | Computed such that relative duration equals relative infectiousness for last waning stage compared to fully susceptible individuals; for “≥ 2 LPV infections” and “IPV and LPV”; this approach would imply shorter durations of infectiousness for the last waning stage than for the recent immunity state, and therefore we assigned duration values directly such that the product of relative infectiousness and relative duration equals 0.1225 ( = 0.352) based on informed judgment and model calibration | |
| - 1 successful IPV | 26.6;26.4;26.9 | ||
| - 2 successful IPV | 25.2;25.0;25.5 | ||
| - ≥ 3 successful IPV | 23.8;23.6;24.1 | ||
| - 1 LPV infection | 14.0;13.9;14.1 | ||
| - ≥ 2 LPV infections | 11.4;11.4;11.6 | ||
| - IPV and LPV | 11.4;11.4;11.6 | ||
| Duration of oropharyngeal infectiousness (, in days) of last waning stage (no serotype differences) | 18,19 | Computed such that relative duration equals relative infectiousness for last waning stage compared to fully susceptible individuals; for “≥ 3 successful IPV,” this approach would imply shorter durations of infectiousness for the last waning stage than for the recent immunity state, and therefore we assigned duration values directly such that the product of relative infectiousness and relative duration equals 0.1225 ( = 0.352) based on informed judgment and model calibration | |
| - 1 successful IPV | 11.4 | ||
| - 2 successful IPV | 6.7 | ||
| - ≥ 3 successful IPV | 6.6 | ||
| - 1 LPV infection | 6.7 | ||
| - ≥ 2 LPV infections | 4.0 | ||
| - IPV and LPV | 4.0 | ||
| Relative fecal infectiousness () of last waning stage (no serotype differences) | 18,19 | Based on informed judgment and model calibration | |
| - 1 successful IPV | 0.95 | ||
| - 2 successful IPV | 0.9 | ||
| - ≥ 3 successful IPV | 0.85 | ||
| - 1 LPV infection | 0.5 | ||
| - ≥ 2 LPV infections | 0.3 | ||
| - IPV and LPV | 0.3 | ||
| Relative oropharyngeal infectiousness () of last waning stage (no serotype differences) | 18,19 | Based on informed judgment and model calibration | |
| - 1 successful IPV | 0.43 | ||
| - 2 successful IPV | 0.25 | ||
| - ≥ 3 successful IPV | 0.13 | ||
| - 1 LPV infection | 0.5 | ||
| - ≥ 2 LPV infections | 0.3 | ||
| - IPV and LPV | 0.3 | ||
| Characterization of OPV evolution: | |||
| Number of reversion stages (h) | 20 | Stage 0 = OPV; stage h−1 = FRPV | |
| Shape of reversion function with respect to: | Shape parameter in reversion function that characterizes increase in R0 and ln(PIR) as a function of the reversion stage (see methods section and Appendix A2) | ||
| - R0 () | 1 | ||
| - ln(PIR) () | 2.5 | ||
| Average time to reach last reversion stage (, in days) (for PV1;PV2;PV3) | 547.5; 360; 547.5 | Based on assumption that OPV-related virus attains identical properties as typical homotypic WPVs after it reaches 1.5 times the GPLN threshold(4,17,121) of 10 (PV1&3) or 6 (PV2) nucleotide changes occurred in the VP1 region, with 10 assumed nucleotide changes per year(122) | |
| Paralysis-to-infection ratio for fully susceptible individuals infected with OPV (PIR0) (for PV1; PV2;PV3) | 0.26 × 10−6; 1.2 × 10−6 1.8 × 10−6 | Calibrated to USA VAPP data by dividing the estimated type-specific incidence of recipient VAPP 1980–1996 (CDC, unpublished data) by the total number of recipient OPV infections during the same time period | |
| Paralysis-to-infection ratio for fully susceptible individuals infected with FRPV (PIRh-1) (for PV1; PV2;PV3) | 0.005; 0.0005; 0.001 | 13,25 | Assumes similar PIRs for FRPVs as typical homotypic WPVs |
| Relative R0 of OPV vs. FRPV () (for PV1; PV2; PV3) | 0.37;0.56;0.25 | 18,19 | Based on literature review, expert elicitation, and model calibration |
| Other inputs: | |||
| Effective infectious proportion below which we assume 0 force-of-infection (transmission threshold EPI*) | 5/1,000,000 | Based on judgment and model calibration to produce approximately correct timing of die-out in the DEB model (see text); assumed equal for all reversion stages and mixing age groups | |
| Relative PIR for maternally immunes compared to fully susceptible individuals (RPIRMI) | 0.5 | Based on calibration of the USA model to the observed median age of 3 months for recipient VAPP during 1980–1996 (CDC, unpublished data) | |
| Ratio of R0 by serotype in the same setting (PV1:PV2:PV3) | 1:0.9:0.8 | Assumption based on relatively low frequency of WPV3 importations or cVDPV3 outbreaks despite generally low observed type 3 antibody levels,(46–49) and model calibration | |
| Average incubation period (, in days) | 10 | 13,123 | Average time between entering first latent stage and onset of paralysis for paralytic poliomyelitis patients |
| Demographics for all situations | Time series 1950–2100 | 61 | Death rates fitted to UN Population Division’s medium variant estimates of population by age group, using effective birth rates based on surviving infants (see Appendix A3) |
Acronyms: CDC = (U.S.) Centers for Disease Control and Prevention; cVDPV = circulating vaccine-derived poliovirus; DEB = differential equation-based; FRPV = fully-reverted poliovirus; GPLN = Global Polio Laboratory Network; IPV = inactivated poliovirus vaccine; LPV = live poliovirus; OPV = oral poliovirus vaccine; PIR = paralysis-to-infection ratio; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively); R0 = basic reproductive number; UN = United Nations; USA = United States of America; VAPP = vaccine-associated paralytic poliomyelitis; VP1 = viral protein 1; WPV(1,2,3) = wild poliovirus (type 1, 2, or 3, respectively)
Mean estimates obtained from experts and used in the model for the different immunity states, serotypes, and excretion modes vary between 2.85 and 3.37 days.
2.3. Generic Model Inputs
Table I shows the uncertain generic model inputs that we keep consistent across all settings. The first section of Table I shows the inputs that characterize the recent immunity states, fully susceptible individuals, and maternally immunes. We assume that these properties represent inherent, average properties of the immunity states, although we recognize that they may to some extent vary by setting and they certainly vary between individuals (i.e., we focus on population averages and assume that the use of relative values controls for any setting-specific differences). Unless otherwise noted, the best estimates for the recent immunity states reflect the means of the assessments from nine experts elicited during an extensive expert review process that involved elicitation of expert input based on a collective review of the literature, as described elsewhere.(18,19) Although very few experts expressed significant serotype differences on any elicited quantities,(19) we include their very small impact by using the means of the elicited values for each serotype in the model. We elicited relative susceptibility and durations of the latent and infectious periods with respect to both fecal and oropharyngeal infectiousness directly from the experts. We compute relative infectiousness as the relative contribution to transmission over the entire infectious period divided by the relative duration of infectiousness, compared to fully susceptible individuals. We used the contributions of transmission given infection as calculated separately for fecal and oropharyngeal infectiousness from the expert assessments from the probability of excretion over time, the concentration of excreted virus over time, and the relationship between excreted virus titers and infectiousness to others.(19) These calculations ignore the very small possible effect of differential mortality rates among infected people in different immunity states or settings on average durations of infectiousness. While some experts indicated some differences in the excretion pattern and infectiousness for OPV and WPV infections, these differences remain relatively small when considering the mean values. Given substantial uncertainty indicated by the experts related to these assessments and in the absence of assessments for each reversion stage between OPV and WPV,(19) we use the elicited values for WPV for both WPVs and all OPV-related infections in the model. We emphasize that the assumption of equal durations and relative infectiousness does not translate to equal transmissibility of WPV and OPV-related viruses, as we characterize the latter separately by the relative R0 for each reversion stage. Thus, we assume that inherent transmissibility represents at least to some degree a separate property from duration and relative infectiousness because it may relate to the human infectious dose and survival in the environment, which both probably differ for OPV compared to WPV.(18,19,43) The expert review process also revealed only very small differences between the immunity states “2 or more LPV infections” and “IPV and LPV,” and therefore we assume identical properties for both immunity states, although the model structure tracks them separately.
Both data from OPV challenge studies(18) and the assessments we elicited from experts(19) suggest that the duration of excretion does not follow the exponential distributions implied by a single-stage infectious process.(32) In particular, the exponential distribution produces a high fraction of infected individuals who recover almost immediately, as well as a high fraction that recovers much later than the average. While a few individuals with very rare immunodeficiencies may become chronically infected,(23) we treat these separately in modeling risks,(44) and therefore the long tail from the exponential distribution remains unrealistic. To better represent the infection process, we use two latent stages and four infectious stages, which matches the elicited distribution of excretion and satisfies the requirement that only about 1:600 previously fully susceptible individuals remain fecally infectious for longer than 90 days, based on the known prolonged but time-limited fecal excretion of individuals with certain types of antibody deficiencies that occur in roughly 1:600 people (see infection curves in Appendix A2).(45) Besides the nonexponential distribution of the excretion duration, we also obtained varying excreted virus concentrations over time from most experts, which imply changing levels of infectiousness to others over the excretion period. To reproduce this behavior we assigned different relative levels of infectiousness to each infectious stage and compared the resulting infectiousness to others over time with those computed from the expert assessments. We found that weighting infectiousness according to the ratios 0:0:3:10:3:1 by stage (i.e., the first two stages represent the latent stages) produced a good fit to the elicited expert assessment curves for fecal and oropharyngeal infectiousness in most recent immunity states (see Appendix A2). We did not attempt to mathematically derive best fits for each immunity state and transmission route given the substantial uncertainty expressed by the experts.(19) In addition, no data exist to support fitting each immunity state and attempting to fit these would add significant complexity to the model (i.e., possibly different numbers of stages and relative weights for each immunity state and transmission route). Assuming that both the distribution of the duration and the changing levels of infectiousness over the infectious period represent real phenomena that likely affect outbreak kinetics, we sought to include as realistic assumptions as possible in our model.
The expert review process identified very large uncertainties with respect to the impact of waning of immunity on the potential to contribute to transmission, sometimes with assessments of the long-term impact of waning varying between no effect and an eventual return to the same contribution to (asymptomatic) transmission as fully susceptible individuals.(19) Consequently, we characterize waning using a general function and we use the parameters of the waning function to fit the model to the set of historical experiences with poliovirus transmission covered by the diverse situations we modeled. We define the following functions to characterize a process that occurs over stages to change a given property :
| (1) |
where indicates the first stage, the last stage, and represents the shape parameter ( yields a linear, an exponential, and a logarithmic relationship). We apply this function to determine relative susceptibility, duration of infectiousness, and relative infectiousness for each waning stage, assuming waning stages, shape parameter , and average time of 4 (types 1 and 2) or 3 (type 3) years to reach the last stage with the assumed properties, as indicated in Table I (see Appendix A2 for the resulting waning curves we used for all of the situations). We assume that immunity wanes somewhat faster for type 3 than the other serotypes given the typically lower initial titers achieved with type 3 infection or vaccination and the frequently observed low antibody levels of type 3 in populations.(46–49) We assume that children born with maternal immunity become identical to fully susceptible individuals after 3 months on average, based on the pattern elicited during the expert review process.(19) We use the same function and parameters for all situations because we expect that waning in the absence of boosting infections or vaccinations represents a biological phenomenon that will not vary by situation (i.e., we assume similar waning behavior in populations in different situations, which represents an average of any differential waning occurring by individuals within the population).
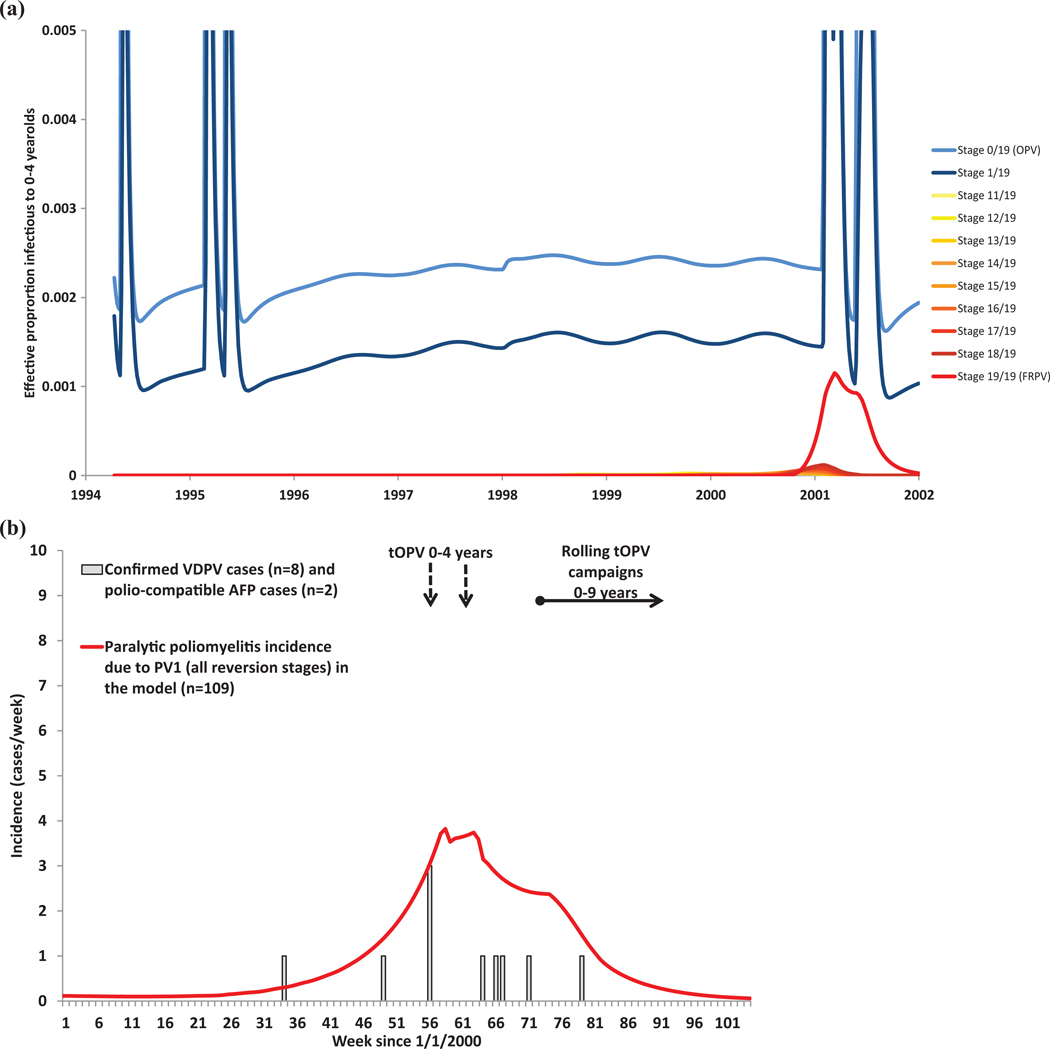
We characterize reversion using a function similar to Equation (1) to describe the increase in from stage 0 (i.e., OPV) to (i.e., FRPV). Although we assume a linear relationship between and the reversion stage (i.e., shape parameter ), this assumption implies that the average by age of virus increases logarithmically due to the exponential processes in the DEB model (see Appendix A2). The average time to reach the last reversion stage represents the most influential assumption related to reversion. Based on use of the model in settings in which cVDPV outbreaks did and did not occur, we assume that the time to exceed the threshold number of nucleotide changes in the VP1 region of the poliovirus genetic sequence used to classify VDPVs by the Global Polio Laboratory Network (GPLN) represents an adequate approximate estimate of the minimum time to reach the last reversion stage and observe transmissibility similar to typical homotypic WPVs.(17) Given that these observations represent the first observed instances of WPV-like behavior, we assume that the average time remains 1.5 longer than the minimum time to reach the genetic thresholds defined by the GPLN (i.e., given the structure of the model, this assumption produces observations of cVDPVs in the model consistent with real observations in the field). We also determined the relative of OPV vs. FRPVs and homotypic WPVs within the uncertainty range obtained by the expert review process,(18,19) the shape parameter , and the number of reversion stages by testing the model against actual experiences, again keeping these consistent across all situations. With respect to neurovirulence, animal studies suggest a very steep increase for OPV-related viruses initially that levels off to become similar to typical WPVs.(17,24) Therefore, we assume a logarithmic increase in PIR, with the shape parameter fit to yield nonrecipient VAPP numbers consistent with data, and Equation (1) applied to the natural logarithm of the PIR (i.e., ), because the scale of PIR runs from near 0 to 0.005 or less. This yielded a fitted value of (Table I). Appendix A2 includes plots of relative R0 and PIR as a result of reversion for each serotype.
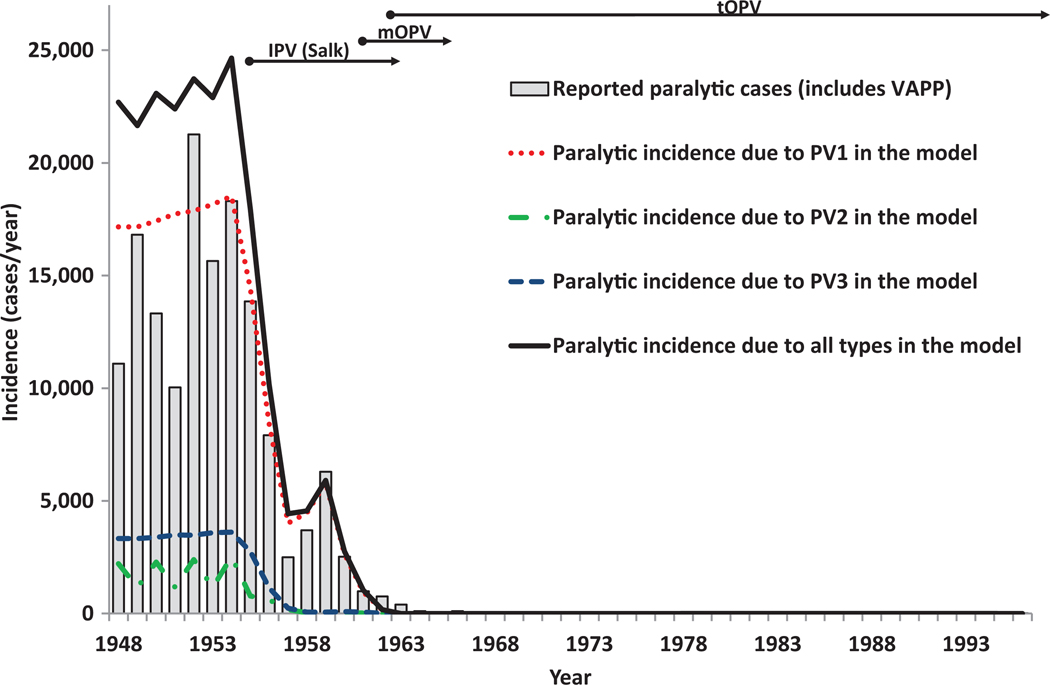
To estimate the PIR0 for OPV recipients, we derive values that reproduce the actual number of recipient VAPP cases reported during the routine tOPV and WPV-free period 1980–1996 in the USA model. Given that during this period the USA administered the first dose at 2 months of age, when most infants still reside in the maternally immune state in the model, the protection from paralysis provided by maternal immunity represents a critical assumption to calibrate PIR0. Table II shows the results of calibrating our assumptions regarding the PIRs for each reversion stage to the reported VAPP cases during 1980–1996. In this calibration we assume that: 1) our model adequately approximates the number of first OPV infections of each type among tOPV recipients, 2) our model adequately approximates the number of fully susceptible and maternally immune individuals infected with OPV-related viruses, and 3) a logarithmic relationship between PIR and reversion stage (i.e., age of virus) appropriately characterizes the behavior. The top section of Table II shows the estimated recipient and nonrecipient VAPP cases based on the total reported cases in each category multiplied by the distribution by serotype for those cases with an isolate of a single serotype (unpublished data from CDC; excluding immunodeficient VAPP cases and assuming 96% completeness of reporting(44,50)). Based on our take rate and coverage assumptions, we found that an assumed relative PIR of 50% for maternally immunes compared to fully susceptible individuals produced approximately the same median age of recipient VAPP of 3 months as observed in the USA during 1980–1996 (CDC, unpublished data). With this relative PIR, we then determined that PIRs for fully susceptible individuals of 0.26e−6 (PV1), 1.2e−6 (PV2), and 1.8e−6 (PV3) resulted in the same total number of recipient VAPP cases as estimated from the data (within rounding error).(44,50) Finally, a shape parameter of for the relationship between PIR and reversion stage produced the best fit for the total number of nonrecipient VAPP cases. Given that the incidence of nonrecipient VAPP depends strongly on model assumptions that we imposed to characterize the uncertain reversion process (i.e., number of reversion stages, elimination threshold, relative R0s by reversion stage, functional form of the relationship between PIR and reversion stage), we examine the VAPP numbers calculated by the model in other situations when appropriate (i.e., Albania, Cuba, Haiti, northern Nigeria, northern India) to determine whether they remained consistent with the expected true VAPP incidence.
Table II.
Calibration of the PIR of OPV and OPV-Related Viruses to the Observed VAPP Incidence After WPV Elimination and Before the Switch to the Sequential eIPV-tOPV Schedule in the USA (from CDC, unpublished data; excluding immunodeficient VAPP and assuming 96% completeness of reporting)(44,50)
| VAPP cases, 1980–1996 | Type 1 | Type 2 | Type 3 |
|---|---|---|---|
|
| |||
| Estimated actual VAPP casesa | |||
| - recipient VAPP | 2.4 | 19.2 | 43.2 |
| - nonrecipient VAPP | 4.7 | 20.4 | 28.2 |
| VAPP cases estimated by the USA model | |||
| - recipient VAPPb | 2.5 | 20.0 | 46.1 |
| - nonrecipient VAPPc | 6.0 | 26.9 | 25.5 |
Acronyms: CDC = (U.S.) Centers for Disease Control and Prevention; eIPV = enhanced-potency inactivated poliovirus vaccine; OPV = oral poliovirus vaccine; PIR = paralysis-to-infection ratio; tOPV = trivalent OPV; USA = United States of America; VAPP = vaccine-associated paralytic poliomyelitis; WPV = wild poliovirus
Numbers by serotype reflect relative frequency of each serotype isolated from VAPP cases with a single isolated serotype, multiplied by total estimated VAPP cases in the same category (i.e., recipient or nonrecipient).
Recipient VAPP incidence calibrated to match the estimated actual incidence by setting the PIR for OPV (PIR0; see Table I) equal to the estimated actual incidence divided by the total number of paralytic infections in OPV recipients in the model (small differences due to rounding of the PIR for OPV).
Nonrecipient VAPP incidence calibrated by finding the approximate shape parameter (zpir; see Table 1) that best matches the estimated actual nonrecipient VAPP cases for each serotype.
Finally, Table I includes several other inputs used to characterize various features of poliovirus transmission that we believe may significantly impact the model. First, we assume that the inherent transmissibility of WPV3 remains lower than that of WPV2 and even lower than that of WPV1 based on the relatively low frequency of WPV3 importations or cVDPV3 outbreaks(17) despite generally lower observed type 3 antibody levels.(46–49) Second, we adopt the same assumption about the incubation period of 10 days from our previous model.(13) Third, we include a threshold to force die-out of transmission in the model in order to partly overcome the well-known limitation of DEB models that they can maintain very small fractional numbers of infectious people when in fact the virus would die out (and a discrete stochastic or individual-based model would have 0 infectious people).(32,51) To do so, the model tracks the effective proportion of the population infectious with virus strain to age group a ( number of age groups −1; ) as the sum of the number of fecally- and oropharyngeally-infectious people from any immunity state and in any infectious stage, weighted by their relative infectiousness, the proportion of transmissions via the appropriate transmission route, and the relative weight of each age group to age group a according to the mixing matrix (see Appendix A1). We define as the transmission threshold. If , then we set the force of infection for age group a and virus strain to 0. This formulation implies that, with very low levels of transmission, the EPI may stay above the threshold in some age groups but die out earlier in others. In practice, we found that transmission may continue longest within the first (mixing) age group (i.e., children under 5 yrs), because it contains the most susceptibles due to inflow of births. The difference in timing of reaching the transmission threshold by age remains of little consequence for the overall model behavior because we cut off transmission at such a low level that any difference in the timing does not significantly influence population immunity. With a very high threshold, we found that WPVs die out too easily in the model, including during seasonal troughs even in geographic areas that could sustain indigenous transmission (e.g., northern Nigeria, northern India), or too soon after the introduction of vaccination campaigns (e.g., Haiti, Madura). With a very low threshold, we found that OPV-related viruses can sustain transmission even in places in which these viruses die out naturally. Given our other model input assumptions, we found only a small range of values for the transmission threshold EPI* that produced results consistent with the evidence for all modeled situations, and our value in Table I remains within this range and produces realistic elimination behavior across all of the situations.
2.4. Setting-Specific Model Inputs
Table III shows ranges of values we used for common model inputs across multiple situations that we believe should vary with specific situations (i.e., R0 and OPV take rates).(6,13,40,52–54) For each situation, we assess the development-hygiene tier the country falls into and use model input values consistent with the ranges in Table III. Only two situations (the Netherlands and USA, both in the highest tier) involve significant IPV use, and we ensure minimal differences in the average per-dose take rates for Salk IPV and enhanced-potency IPV (eIPV) between these situations. To mimic the effective cumulative take of multiple vaccine doses administered during routine immunization or successive SIAs, we base average effective per-dose take rates on the observed cumulative seroconversion(6) after multiple doses or on the observed efficacy of multiple doses as appropriate.(55) We define as the average probability that a dose of vaccine administered in field conditions to a fully susceptible recipient leads to infection (for OPV) or successful vaccination (for IPV) (i.e., it moves the recipient to the next LPV or IPV state). This may differ from the seroconversion observed in controlled studies with good delivery since the cold chain conditions may affect the effective take,(56,57) and therefore we adjust take rates where appropriate (e.g., in Albania). For estimated take after doses, the average per-dose take rate equals .(6) The use of the average per-dose take rate allows us to model the effect of vaccine given during a single SIA round, and in the case of tOPV averages out the effect of serotype interferences over multiple doses. Due to interference of the three Sabin strains in tOPV, individuals fully susceptible to all three types typically become infected with type 2 from the first dose and with types 1 and 3 from subsequent doses.(58,59) Consequently, calculating take based on observed seroconversion after the first tOPV dose for each serotype and applying these results to multiple doses would overestimate the cumulative take after multiple doses for type 2 and underestimate the cumulative take for the other two serotypes. We run our model for each serotype independently and account for the impact of interference by using appropriate type-specific take rates for tOPV. The DEB model does not track heterotypic immunity, which requires stratifying the population according to each possible combination of immunity states for the three serotypes (which would increase model complexity multiplicatively). The use of an individual-based model might allow better characterization of the timing of immunity by serotype for each individual, but it would do so at the expense of significantly increased assumptions about population structure and individual contact and mixing patterns.(60)
Table III.
Ranges for Common Situation-Specific Inputs by Setting Tiers, Showing Assumed Tiers for the Nine Modeled Situations
| Tier | Situations | a | Per-dose take rateb (tr) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tOPV1 | tOPV2 | tOPV3 | mOPV1 | mOPV2 | mOPV3 | bOPV1 | bOPV3 | |||
|
| ||||||||||
| High | The Netherlands, USA | 3–6 | 0.60–0.75 | 0.70–0.85 | 0.50–0.70 | 0.85–0.98 | 0.9–0.99 | 0.80–0.95 | 0.80–0.95 | 0.80–0.95 |
| Medium | Albania,c Cuba, Haiti, Madura, northern Nigeria, Tajikistan | 6–11 | 0.35–0.50 | 0.60–0.75 | 0.30–0.45 | 0.60–0.75 | 0.60–0.75 | 0.60–0.75 | 0.50–0.65 | 0.45–0.60 |
| Low | Northern India | 10–14 | 0.25–0.40 | 0.50–0.65 | 0.20–0.35 | 0.40–0.60 | 0.40–0.60 | 0.35–0.55 | 0.40–0.60 | 0.30–0.50 |
Acronyms: bOPV1,3 = type 1 or 3 component of bivalent oral poliovirus vaccine, respectively; mOPV1,2,3 = monovalent oral poliovirus vaccine type 1, 2, or 3, respectively; R0 = basic reproductive number; tOPV1,2,3 = type 1, 2, or 3 component of trivalent oral poliovirus vaccine, respectively; USA = United States of America
Based on existing estimates(9,10,13,26,31,53,54) and adjusted somewhat downward compared to prior work(9,10,13) due to updated characterization of immunity states and waning.
Use low-tier take rates during times of major cold chain issues.
We use “medium variant” estimates from the UN Population Division(61) available for all countries from 1950 forward to simulate the population by age in each situation. In the absence of mortality rates by age, we calculated mortality rates such that the model reproduces the reported population by age (see Appendix A3). Given that these data provide the age distribution by five-year age groups, we also calculate mortality rates by five-year age groups, thereby ignoring age differences when we use narrower age groups in the model. To partly overcome this limitation in the context of the known high mortality rates among infants (age 0) compared to 1–4 year olds, the model only adds the number of newborns that survive to age 1 (i.e., surviving infants) to the population at age 0, thus ignoring the impact on poliovirus transmission of the fraction of 0–11-month-old infants that die sometime during the first year of life.(61) In situations in which we model subnational regions (i.e., the Netherlands, Albania, Tajikistan, Madura, northern Nigeria, and northern India), we assume those populations follow the estimated national demographic multiplied by the relative size of these regions according to an appropriate census of subnational data.
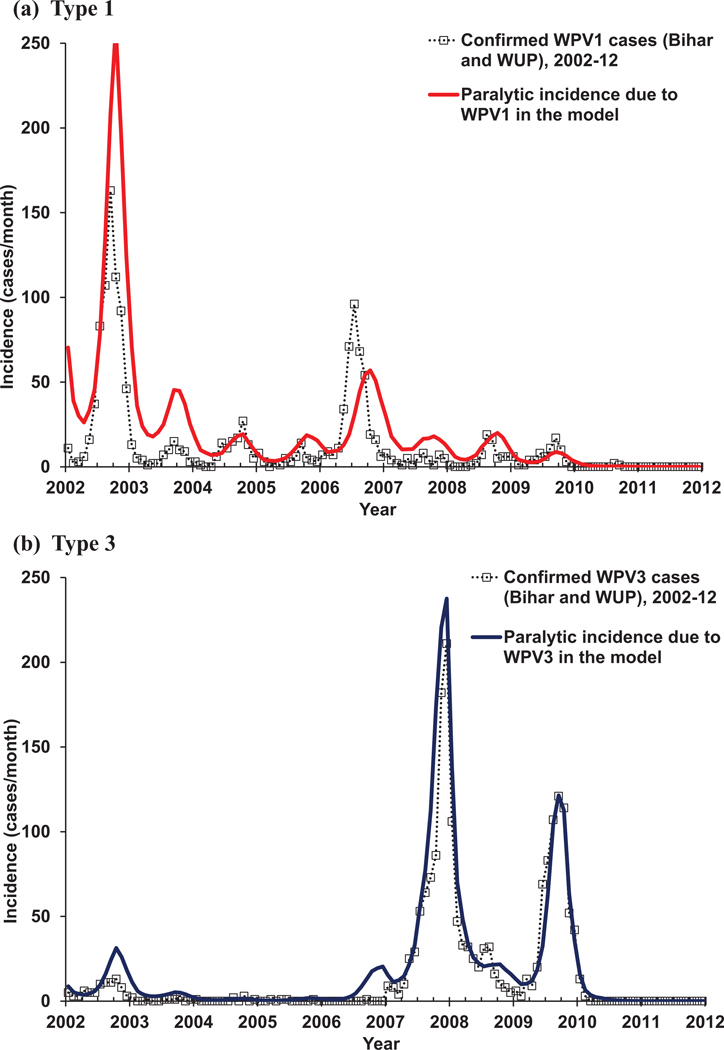
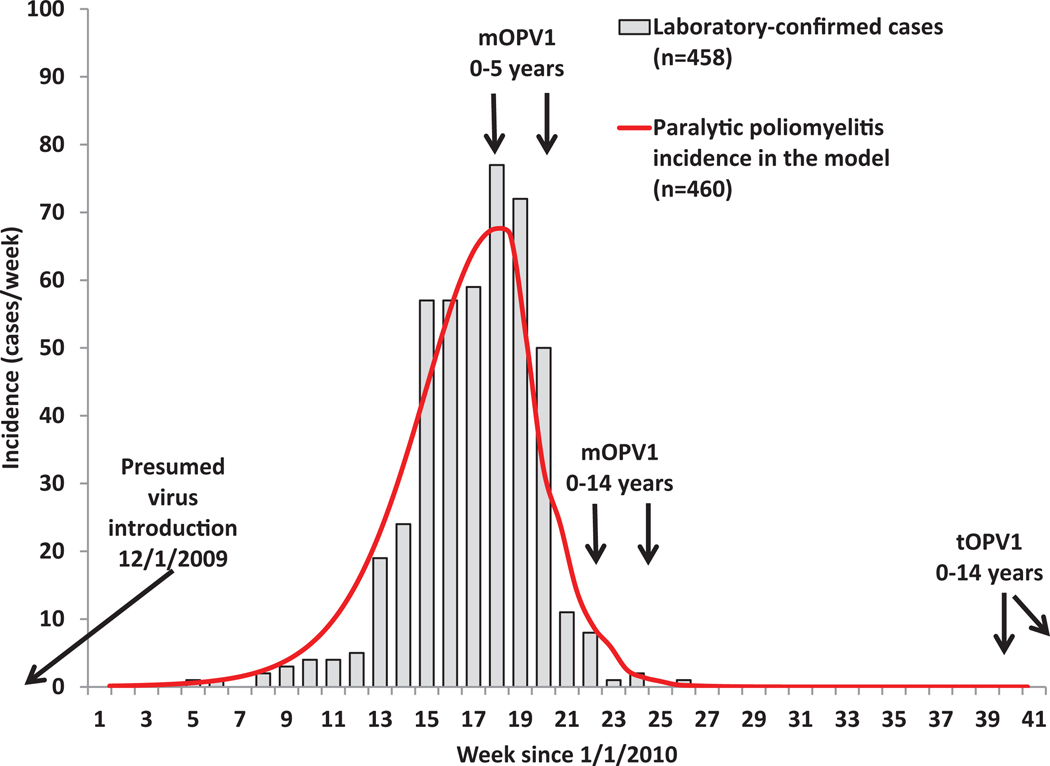
Tables IV–XII summarize our assumptions about setting-specific model inputs for each of the situations we modeled with time-dependent inputs provided in Appendix A4. The top of each table lists model input choices that directly relate to framing the model, including subpopulations, age groups, and the model time horizon. For each situation, we choose age groups that allow us to model different vaccination strategies used in that situation and/or to compare our results to age-specific data available for that situation (e.g., age distribution of cases). The width of the age groups affects the distributions implied by the aging process (e.g., multiple-stage processes like waning, reversion, and infection) and narrower age groups result in more realistic age distributions. In most situations, the most important changes related to poliovirus immunity occur in young children, and therefore we typically break the first five years into multiple smaller age groups. In some situations (i.e., the Netherlands, northern Nigeria, northern India) the available evidence motivates us to model important heterogeneity in mixing or vaccination by capturing multiple subpopulations. Our selection includes four situations (i.e., the USA, the Netherlands, Albania, northern India) that we previously modeled using our prior transmission model.(9,13) For those prior simulations, we used the available data to specify initial conditions in terms of the fraction of the population in each of the limited number of immunity states in that model.(13) With the expanded model, it becomes very challenging to estimate initial proportions in each of the immunity states from the data because the new model differentiates between varying numbers of successful doses and/or infections and multiple waning stages. Therefore, we determine the initial conditions from the model itself by calibrating the model based on assumptions leading up to the observed experiences. Thus, for each situation we begin the model well before routine or mass vaccination starts by introducing one infectious individual into an assumed entirely susceptible population. We then run the model so that it settles into an endemic equilibrium before we introduce vaccination, which typically requires going back relatively further in time for low-R0 situations. The approximate equilibrium may still involve some oscillations due to seasonality and/or changes in birth rates or other demographic model inputs. To speed up the process to reach the approximate pre-vaccine equilibrium, in some cases we run the model without seasonality and/or die-out for several years, depending on the situation, and then introduce these processes.
Table IV.
Model Inputs for the USA Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 1 | Model intended to reflect average conditions in the USA | |
| Number of age groups | 11 | 0–2; 3–5; 6–11 month; v1; 2; 3; 4; 5–9; 10–14; 15–19*; ≥ 20 years* | |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥15 years | |
| Year when model run-up starts | 1880 | ||
| Year when R0 seasonality starts | 1900 | ||
| Year when die-out first allowed | 1955 | ||
| Average basic reproductive number (R0) (PV1) | 5 | Fitted within range for highest tier (Table III); PV2&3 according to ratios in Table I | |
| Proportional change in R0 due to seasonality (α) | 0.05 | Lower value than previously assumed to avoid significant long-term oscillations that should cancel out for a large USA population | |
| Day of seasonal peak in R0 (pd) | 212 (July 31) | ||
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.35 | For simplicity, assume equal values for each mixing age group | |
| Proportion of transmissions via oropharyngeal route () | 0.8 | 19 | Fitted within range of expert assessments(19) and using value for the Netherlands as upper bound (Table V) |
| Per-dose take rate (tr) (PV1;PV2:PV3) | Fitted within range for highest tier (Table III); IPV take rates based loosely on field efficacy of IPV against poliomyelitis in the USA(55) in 1954 | ||
| - IPV (Salk) | 0.5;0.6;0.5 | ||
| - mOPV | 0.9;0.95;0.9 | ||
| - tOPV | 0.65;0.75;0.55 | ||
| Assumptions about IPV (Salk) campaigns 1955–1963 | 9,71,72 | Doses and coverage based on total IPV doses distributed by mid-1962 and immunization surveys by age; see Appendix A4 for | |
| - Annual cumulative coverage, age 0–19 years | Time series | coverage values used | |
| - Relative annual cumulative coverage, age ≥ 20 vs. 0–19 years Assumptions about mOPV campaigns 1962–1964 |
0.55 | 9,71,72 | Doses and coverage based on total mOPV doses distributed (16% wastage assumed) by 1965 and immunization surveys by age, adjusted for known underestimation; campaigns modeled as annual 1-day events on day 60 of each year, with each round effectively reaching 1/3rd of the eventually covered population |
| - Doses per covered person | 1 of each type | ||
| - Cumulative coverage, age 0–19 years | 0.8 | ||
| - Cumulative coverage, age ≥ 20 years | 0.4 | ||
| Routine coverage with 3 polio vaccine doses, and partial coverage | Time series 1965–96 | 9,71,72 | Partial coverage based on DTP1 and DTP3 data; see Appendix A4 for all values used |
| Characterization of routine IPV (Salk) (1958–1964) or tOPV (1965–1996) immunization | 73,74 | See methods for calculation of average effective vaccination coverage at each age | |
| - Cumulative effect of first 2 doses at age (months) | 3 | ||
| - Third dose at age (months) | 6 (<1967) or 12 (≥1967) | ||
| - Booster dose at age (years) | 5 | ||
| - Coverage of IPV (Salk) booster doses | 0.8 | ||
| - Coverage of booster tOPV doses | 0.9 | ||
Acronyms: DTP1(3) = coverage with 1 (3) dose(s) of difteria-tetanus-pertussis vaccine by age 1; IPV = inactivated poliovirus vaccine; mOPV = monovalent oral poliovirus vaccine; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively); tOPV = trivalent oral poliovirus vaccine; USA = United States of America
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns that receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
Table XII.
Model Inputs for the Northern India Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations (Bihar) | 1 | ||
| Number of subpopulations (WUP) | 2 | Subpopulation 1: undervaccinated subgroups (1/25th) of total WUP population; subpopulation 2: general population (24/25th) of total WUP population | |
| Relative population size compared to all of India | 125 | According to the 2011 census, approximately 1.2 billion live in all of India, 104 million in Bihar, and 71 million in included districts for WUPa | |
| - Bihar | 0.086 | ||
| - WUP | 0.059 | ||
| Number of age groups | 9 | 0–2; 3–11 months; 1; 2; 3; 4; 5–9; 10–14; ≥ 15* years | |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years | |
| Year when model run-up starts | 1950 | ||
| Year when R0 seasonality starts | 1965 | ||
| Year when die-out first allowed | 1965 | ||
| Average basic reproductive number (R0) (PV1) | 13 | Fitted within range for lowest tier (Table III); PV2&3 according to ratios in Table I | |
| Proportional change in R0 due to seasonality () | 0.25 | Seasonality modeled as step function | |
| Day of seasonal peak in R0(pd) | 195 (July 14) | ||
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.35 | For simplicity, assume equal values for each mixing age group | |
| Proportion of transmissions via oropharyngeal route () | 0.3 | Based on average of mean expert assessments for community and close contacts for type 1 in high R0 settings(19) | |
| Proportion of potentially infectious contacts of individuals in subpopulation 1 in WUP that are with other individuals in subpopulation 1 in WUP (pwithin) | 0.95 | Force of infection as a result of mixing between subpopulations modeled using previously described approach;(13) (see Appendix A1) model does not include mixing between Bihar and WUP | |
| Per-dose take rate (tr) (PV1;PV2;PV3) | |||
| - tOPV | 0.35;0.50;0.30 | ||
| - mOPV | 0.50;NA;0.45 | ||
| - bOPV | 0.45;NA;040 | ||
| Routine coverage with 3 or more doses by 1 year of age, birth dose coverage, and partial coverage | Time series 1980–2012 | 89 | |
| Characterization of routine tOPV vaccination, 1980–2012 | 89 | Primary nonbirth doses at 6–10–14 weeks modeled as the cumulative effect of all 3 nonbirth doses upon entering the 3–11-months-old age group, taking into account partial coverage | |
| - Doses at birth | 1 | ||
| - Cumulative effect of 3 primary nonbirth doses at ages (months) | 3 | ||
| Characterization of SIAs 1995–2012 | See Appendix A4 for effective per-round impact assumptions by year and subpopulation in the absence of good data and resulting annual cumulative percentages of missed children | ||
| - Dates, durations, and target population of SIAs | Time seriesb | ||
| - Effective per-round impact () | Varies | ||
| - Target age groups | 0–4 years | ||
| Date of introduction of WPV3 into Bihar for 2007 outbreak | January 1, 2007 | ||
Acronyms: bOPV = bivalent OPV; mOPV = monovalent OPV; OPV = oral poliovirus vaccine; PV1,2,3 = poliovirus type 1, 2, and 3, respectively; SIA = supplemental immunization activity; tOPV = trivalent OPV; WUP = Western Uttar Pradesh
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
We included the following districts: Agra, Aligarh, Baghpat, Bareilly, Bijnor, Budaun, Bulandshahr, Etah, Etawah, Farrukhabad, Firozabad, Gautam Buddha Nagar, Ghaziabad, Jyotiba Phule Nagar, Kanshiram Nagar, Mahamaya Nagar, Mainpuri, Mathura, Meerut, Moradabad, Muzaffarnagar, Pilibhit, Rampur, Saharanpur, Shahjahanpur.
The bottom parts of Tables IV–XII provide other setting-specific model inputs, including assumptions about R0 and heterogeneity in mixing between age groups, the relative importance of the two transmission routes, routine vaccination, regular SIAs, any outbreak response activities, and the assumed date of the virus introduction for the WPV importation outbreaks. When we introduce a single initiating infection in a large-population and WPV-free model, this does not lead to any transmission because at that point the prevalence remains below the threshold EPI* for transmission. Therefore, we instead create introductions by increasing the proportions of individuals in the first fecal and the first oropharyngeal infectious stage of each age group to EPI* and reducing the number of fully susceptible individuals by the corresponding number.
Very little empirical data exists about mixing patterns between age groups, particularly for fecal-oral transmission in developing countries. A survey designed to collect empirical data on contact patterns relevant to respiratory infection in a number of European countries suggests highly heterogeneous mixing between age groups, with highly preferential mixing between individuals of similar age and the highest mixing between different age groups occurring between young children and adults in their 30s.(62) Results of a similar approach applied to a nontemperate developing country (Viet Nam) reveals a similar overall pattern but with weaker preferential mixing.(63) In the absence of data for fecal-orally transmitted infections and specific to the situations and age groups we modeled, we assume highly simplified mixing matrices in an attempt to still reflect the possible impact of age-heterogeneity on transmission dynamics. We adopt the expression for preferential mixing proposed by Jacquez et al. (1988),(37) which assumes that for individuals in any given age group a a proportion of potentially infectious contacts remains reserved for individuals of the same age group, while the remainder occurs with any individual in the population (including from age group a) with equal chance (see Appendix A1). For simplicity, we consider such preferential mixing only for relatively wide mixing age groups (i.e., 0–4, 5–14, and 15 years) for all situations, unless we note specific reasons for different mixing age groups. While probably varies by age group, we do not know the directionality for fecal-oral transmission and we generally keep it equal for all mixing age groups. Given the uncertainty about mixing matrices for poliovirus, we determine partly based on fitting the situation-specific models. Specifically, we verified whether the mixing assumptions produce secondary OPV infection rates (USA, Cuba) or age distributions of cases (the Netherlands, Albania, Tajikistan, northern Nigeria, northern India) consistent with the data. For Albania, we encountered conflicts in the epidemiological evidence related to the historical experience and significant population changes (e.g., large net decreases in population due to emigration), which led us to explore different age-mixing inputs.
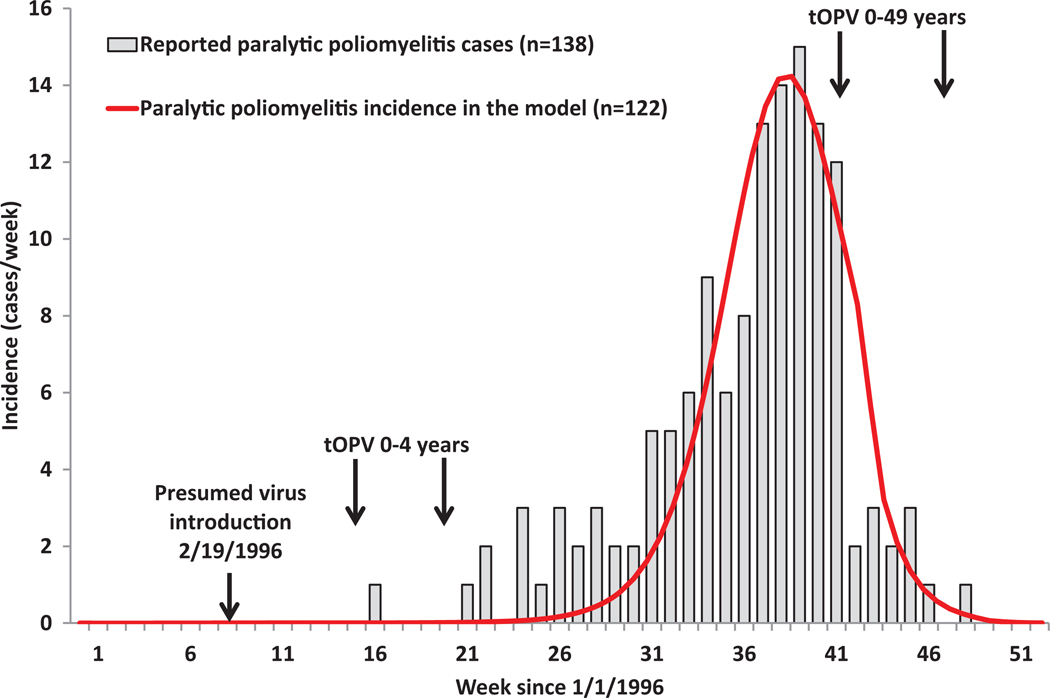
Our model for the USA (inputs in Table V) focuses on first reproducing the elimination of WPVs at a highly aggregate level and then verifying that the model correctly reproduces the occurrence of sporadic VAPP cases without any known cVDPV outbreaks in the general population during widespread OPV use from 1962–1996.(34) We also compare rates of secondary OPV spread with those obtained by serologic surveys among unvaccinated inner-city pre-school children in the early 1990s.(64) The USA became the first country to use poliovirus vaccines on a large scale with the licensing of Salk IPV in 1955, and the history of poliomyelitis in the USA remains very well-studied and documented.(34,65–67) We previously described the history in the context of a retrospective economic analysis of the changing vaccination programs over time,(9) which includes estimates of the national incidence of paralytic poliomyelitis, and vaccine coverage for each year between 1948–1996. In short, the massive campaigns with Salk IPV from 1955–1962 led to a dramatic reduction in the incidence, but outbreaks continued to occur, particularly affecting communities of lower socioeconomic status (SES). Researchers hypothesize that IPV effectively prevented oropharyngeal transmission of poliovirus, but that lower standards of hygiene in lower SES settings allowed the virus to spread by fecal-oral route even among successful IPV vaccinees to reach and cause paralysis in individuals not vaccinated or not successfully vaccinated with the Salk IPV of relatively low immunogenicity.(68–70)
Table V.
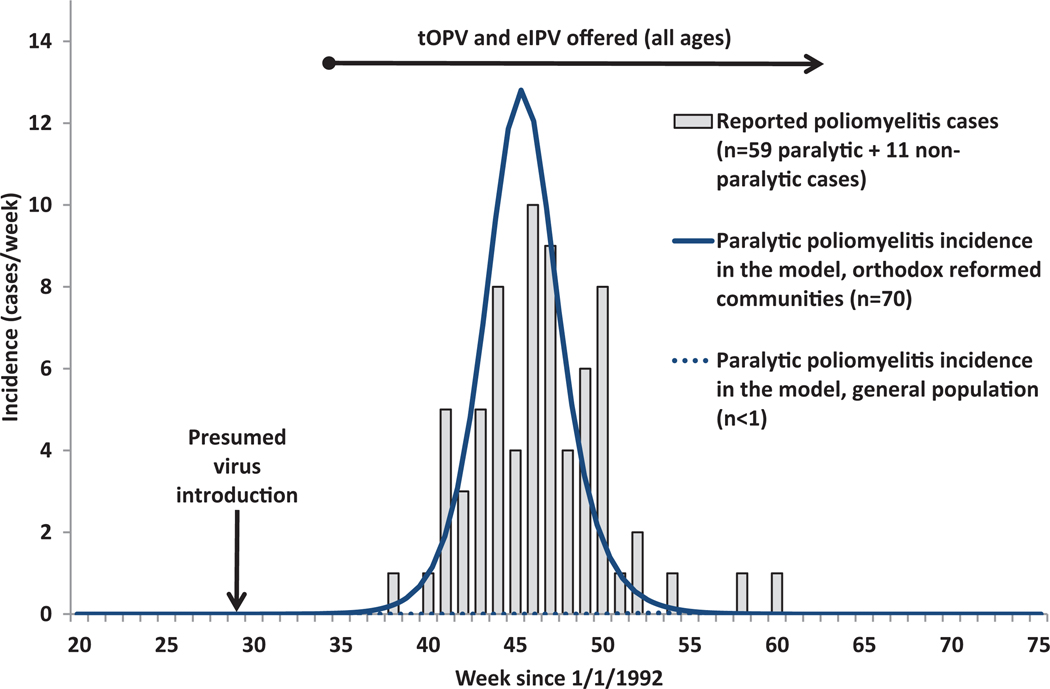
Model Inputs for the Outbreak Model for the Netherlands
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 2 | 13,79 | Subpopulation 1: orthodox reformed communities (1/50th of total Dutch population); subpopulation 2: general population (49/50th of total Dutch population) |
| Number of age groups | 12 | 0–2; 3–4; 5–11 months; 1–4; 5–9; 10–14; 15–19; 20–24*; 25–29*; 30–34*; 35–39*; ≥ 40 years | |
| Number of mixing age groups | 4 | 0–4; 5–14; 15–39; ≥ 40 years | |
| Year when model run-up starts | 1903 | Fitted to time peak WPV3 year before IPV introduction | |
| Year when R0 seasonality starts | 1920 | ||
| Year when die-out first allowed | 1950 | Circulation can continue after initial die-out due to assumed annual virus reintroductions | |
| Average basic reproductive number (R0) (PV3) | 4 | Fitted within range for highest tier (Table III) | |
| Proportional change in R0 due to seasonality (α) | 0.35 | 13 | Significant seasonality based on model calibration to contribute to natural die-out and observed outbreak kinetics |
| Day of seasonal peak in R(pd) | 270 (September 27) | 13 | Fitted within previously assumed range |
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.4 | For simplicity, assume equal values for each mixing age group | |
| Proportion of potentially infectious contacts of individuals in subpopulation 1 that are with individuals in subpopulation 1 (pwithin) | 0.99 | 13 | Force of infection as a result of mixing between subpopulations modeled using previously described approach(13) (see Appendix A1) |
| Characterization of regular WPV importations | Assume WPV importations reduced significantly after national control of WPV, with no significant WPV3 spread in the population since 1960 | ||
| - Frequency | Annual | ||
| -Day of introductions | 91 (April 1) | ||
| - First year without introductions | 1960 | ||
| Proportion of transmissions via oropharyngeal route () | 0.95 | 19 | Fitted within range of expert assessments(19) |
| Per-dose take rate (tr) (PV3) | Fitted within range for highest tier (Table III); IPV take rates based loosely on field efficacy of IPV against poliomyelitis in the USA in 1954;(55) better take rates after 1965(80) due to enhanced vaccine and known high seroconversion(6,41) | ||
| - IPV (Salk), 1957–1964 | 0.5a | ||
| - Improved IPV or eIPV, 1965–1994 | 0.9b | ||
| - tOPV | 0.55c | ||
| Assumptions about IPV campaigns 1957–1959 | 79,80 | Take rates based on field efficacy of IPV against type 1 poliomyelitis in the USA in 1954; assume catch-up campaigns target people born 1945–1957 and achieved ∼ 90% coverage(79,80) | |
| - Average cumulative number of doses per covered child aged up to 14 years | 3.0 | ||
| - Cumulative coverage of IPV campaigns | 0.9 | ||
| Nationwide coverage with recommended doses by age 1 | 76,79,80 | ||
| - Before 1963 | 0.9 | ||
| - 1963 | 0.92 | ||
| - 1964–1966 | 0.94 | ||
| - 1967–1974 | 0.95 | ||
| -1975–1994 | 0.97 | ||
| Coverage compared to national average | 13 | ||
| - Subpopulation 1, IPV campaigns 1957–1959 | 0.5 | ||
| - Subpopulation 1, routine IPV 1960–1992 | 0.2 | ||
| - Subpopulation 2 | 1.0 | ||
| Characterization of routine IPV immunization | 79,80 | Assume negligible effect of partial coverage of primary schedule given high coverage of complete schedule | |
| - Primary doses by age 3 months | 3 | ||
| - Primary doses at 12 months | 1 | ||
| - Coverage with 0, 1, 2, or 3 primary doses given < 4 doses by age 12 months | 0 | ||
| - Booster dose at ages (years) | 5,10 | ||
| - Relative coverage of booster vs. primary doses | 1.0 | ||
| Date of introduction for the 1992–1993 outbreak | July 18, 1992 | 13 | Fitted value within previously characterized range |
| Characterization of outbreak response, 1992 | 13 | Similar assumptions as in prior work(13) | |
| - First day of response (1992) | 266 (September 22) | ||
| - Duration of response (days) | 365 | ||
| - Average tOPV doses per covered person in subpopulation 1 | 3 | ||
| - Average eIPV doses per covered person in subpopulation 1 | 2 | ||
| - Coverage in subpopulation 1 | 0.30 | ||
| - Coverage in subpopulation 2 | 0.50 | ||
Acronyms: eIPV = enhanced-potency IPV; IPV = inactivated poliovirus vaccine; PV3 = poliovirus type 3; tOPV = trivalent oral poliovirus vaccine; USA = United States of America; WPV(3) = wild poliovirus (type 3)
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
For runs to verify behavior of other serotypes, we use take rates for IPV (Salk) of 0.5 and 0.6 for type 1 and 2, respectively.
For runs to verify behavior of other serotypes, we use take rates for eIPV of 0.9 for all types.
For runs to verify behavior of other serotypes, we use take rates for tOPV of 0.65 and 0.75 for type 1 and 2, respectively.
After the licensure of OPV, the USA conducted massive catch-up campaigns with mOPV1, mOPV2, and mOPV3 during 1962–1964 and then gradually replaced all IPV and mOPV use with tOPV.(71) The use of OPVs further dropped the incidence until only importations of WPV occurred, occasionally leading to outbreaks, and eventually VAPP became the main cause of paralytic poliomyelitis in the 1970s,(9) with 4–13 cases per year.(34) In the absence of much data on IPV campaigns by area or age and lacking reliable immunization coverage surveys,(72) we fit the IPV vaccination rates to the reported cases while approximately matching the total of 420 million IPV doses distributed during 1955–1963 (see Appendix A4).(71) We use the available coverage data only to estimate a relative coverage of 55% in adults compared to people under the age of 20 years, which represented the main focus of the IPV campaigns.(72) To model the mOPV campaigns that distributed just over 100 million mOPV doses of each type, we assume 1 dose of each type per covered person and differential coverage between the age groups similar to the IPV campaigns.(71) Based on assumed 16% wastage during the mOPV campaigns,(9) the approximately 100 million doses of each type translate into approximately 80% and 40% cumulative coverage with each type in people under and over 20 years old, respectively. A review of mOPV seroconversion studies(52) that served as the basis for our estimates in Table IV showed ranges and suggested approximately 90% or more take per dose depending on the conditions, with the highest take for type 2. While the routine tOPV immunization schedules probably varied with time and by state,(73–75) we simplify routine tOPV immunization in the model by combining the first two doses (consistently recommended at 2 and 4 months) as 1 dose with the cumulative effect of 2 doses at age 3 months. We model the third dose as an additional dose at 6 months until 1976 and at 12 months after 1976 based on changes in the recommended immunization schedule. We model the fourth dose as a preschool booster at age 5 years. USA coverage estimates with the recommended 3 primary doses dipped during the 1980s and early 1990s,(9,75,76) but population immunity nevertheless stayed high enough to prevent transmission due to the school-entry requirements and a high proportion of children who received 1 or 2 doses by age 12 months and the third and/or fourth dose at school entry (although not covered on time with the 3 primary doses).(64,75,77) To capture this, we assume 90% coverage with the booster dose regardless of the annually varying primary coverage estimates, which combined with the take rate for a single dose , leads to an evc at 5 years of 0.9×tr. We calculate the coverage with 1 or 2 doses by age 12 months for children who did not receive 3 or more doses by age 12 months from the reported difference between DTP1 and DTP3 coverage:(76)
where is the coverage by age 12 months with polio vaccine doses given receipt of fewer than 3 doses by age 12 months and the coverage by age 12 months with DTP doses. The formula assumes that among those children who receive a first dose but not a third dose, half receive a second dose. This leads to effective cumulative vaccination coverage by 12 months of age of:
where is the cumulative take rate for doses and POL3 the coverage with 3 or more polio doses by 12 months of age. We then use an average of for the cumulative effect of the first two doses, modeled to occur at age 3 months, and an average of for the third dose at age 6 (until 1976) or 12 months (from 1977). In reality, significant seasonality exists for poliovirus transmission in the USA,(67,78) which leads to inter-epidemic periods of multiple years. However, for the entire USA, different locations oscillate with different phases and this explains why the USA as whole did not experience long periods of near-zero incidence or transmission. To avoid very large differences between years in the immediate pre-vaccine era and thus better reproduce the reported annual national incidence pattern in this era, we assume very low seasonality in the USA model.
We previously described the outbreaks of WPV1 in Albania in 1996 and WPV3 in the Netherlands in 1992–1993 in the context of modeling those outbreaks.(13) Briefly, the outbreak in the Netherlands in 1992–1993 involved a WPV probably imported from India and caused 59 paralytic cases.(79) However, all but one of the cases during the outbreak remained limited to a socially well-connected subpopulation of members of orthodox reformed churches with a very low rate of vaccine uptake based on religious concerns. The Dutch outbreak involved predominantly older children and adults and unimmunized infants less than 1 year old.(79) The Netherlands relied on IPV exclusively for its immunization program that eliminated indigenous WPV transmission, although periodic outbreaks in vaccine-objector communities continued to occur until the large WPV1 outbreak in 1978.(80) In small, temperate-climate countries, strong seasonality possibly interrupted indigenous WPV transmission during the low season even in the pre-vaccine era,(81) with importations from endemic countries frequently reintroducing WPVs. We model this behavior assuming substantial seasonality and by introducing WPV (at the transmission threshold EPI* in each age group) in the spring of each year until 1960, after which, based on the age distribution of cases,(79) no widespread events of type 3 LPV exposure occurred. In response to the 1992–1993 outbreak, Dutch authorities offered tOPV to the affected communities, but they achieved only low uptake, and they offered eIPV to unvaccinated individuals in the general population with approximately 50% uptake.(13) Table V shows our assumptions for the Netherlands outbreak based on limited data on the IPV campaigns in the 1950s and previously established model inputs for the outbreak.(13) Notably, we model the orthodox reformed communities and the general population separately, with assumed significant interaction between them, to demonstrate that the model reproduces no cases in the general population due to IPV-induced herd immunity based on assumed predominant oropharyngeal transmission. Specifically, we assume that 1 in 100 potentially infectious contacts of people in the orthodox reformed communities of about 300,000 people occur with people in the general population. For routine vaccination, we assume no partial coverage with less than the full schedule given very high coverage with the full schedule. We otherwise follow a similar approach to the U.S. model, with the cumulative effect of the first 3 primary doses (scheduled at age 3, 4, and 5 months) modeled to occur at 3 months of age, the fourth primary dose (scheduled at age 11 or 14 months) modeled at 12 months, and two boosters (scheduled at age 4 and 9 years) modeled at 5 and 10 years with the slight differences due the age groups used in the model.(80) For the Dutch model, we include a fourth mixing age group of people aged 40 years or more because the age distribution of cases involving older adults motivated us to look more closely at the mixing for adults (i.e., in most other situations, we lump them together because we do not have data to compare to for adults of different ages).
The outbreak in Albania in 1996 involved widespread transmission of an imported WPV with 138 confirmed paralytic cases and documented exportations into neighboring countries.(57,82,83) The outbreak followed a preemptive national immunization day (NID) conducted due to concerns about vaccine failure in the past resulting from cold chain issues. The NID targeted children under the age of 5 years and reportedly provided relatively good protection to that age group, although the WPV1 continued to spread until after Albania conducted two rounds of response campaigns targeting all people under 50 years of age. Both the age distribution of cases and serologic results(47) among Albanian immigrants to Italy conducted prior to the outbreak suggest that suboptimal cold chain performance and disruptions in the supply of OPV contributed to a large immunity gap in adults. Until 1978, Albania relied only on annual mOPV campaigns with a poor cold chain and unstable vaccine supply, and the country sustained endemic transmission until probably around 1980.(57,82) However, from an epidemiological perspective, the large accumulation of susceptible individuals over 20 years of age by 1996 remains poorly understood in the context of presumed continued WPV circulation until ∼ 1980 (with a large outbreak in 1978), reported high coverage of OPV in most years that even under poor cold chain conditions would immunize some recipients and contacts, and no documented emergence of widespread cVDPV transmission. Table VI shows our assumptions for Albania, which include characterization of the changing vaccination strategies from the available data. Following similar calculations as in the USA model, we model the primary routine tOPV doses (recommended at ages 2, 4, and 6 months) as the cumulative effect of 3 doses at 3 months, and the 2 booster doses at 18 months and 5–6 years as separate booster doses at 18 months and 5 years, respectively. Given that DTP1 and DTP3 estimates for Albania remain very close,(76) we assume 0 partial coverage with 1 or 2 doses by age 1 year. We capture uncertainty about the actual routine immunization coverage (since 1978) by using an overall coverage correction factor. For the mOPV campaigns that occurred between 1960 and 1977, we remain highly uncertain about quality of the campaigns, which were “strictly dependent on vaccine supply and availability,” resulting in “long time intervals between immunization campaigns.”(82, p. 941) We capture the uncertainty about both the coverage and the effective take rate of the vaccine schedule (i.e., one monovalent dose of each type followed by a mix of all three monovalent vaccines one year later)(57) by adjusting the take rate below typical values for mOPV in the mid-tier (Table III) and by adjusting the coverage, which we assume became as low as 40–60% during the mid-1960s to late-1970s (see Appendix A4). Alternatively, assuming higher coverage, but much lower take rates would result in the same model behavior. Importantly, in an attempt to approximate both the age distribution of cases and the size and kinetics of the outbreak in 1996, we introduced several nonstandard assumptions. First, based on the geographic and age distribution of cases and serologic studies conducted before the outbreak,(47) we assume that the Albania outbreak affected primarily a relatively isolated subpopulation within the country in which indigenous WPV transmission stopped soon after the beginning of OPV campaigns in 1960. We assume that this subpopulation consists of half of the total population in Albania and that it did not experience any substantial WPV exposure until the 1996 outbreak, including no substantial exposure from the last prior WPV outbreak in 1978.(57,82) Second, we assume that the wave of emigration from Albania in the early 1990s disproportionately involved individuals from social classes that received better vaccination. We do so by reducing the emigration rate (captured by the age-specific net rate of population change (i.e., μ in Appendix A1)) for fully susceptible individuals to 5% of that for the rest of the population (i.e., the immunes) aged 10–49 years from 1990 forward. Third, we assume very strong preferential mixing once children reach the age of 5 given the evidence that children who escaped effective vaccination or secondary OPV infection in their early years did not get exposed to OPV as the immunization program improved between 1980 and 1996. We model narrower mixing age groups than for the other situations to characterize this behavior. Finally, we assume that the virus introduced in 1995 or 1996 represents a more transmissible strain (i.e., R0 = 8) than the endemic strains that previously circulated in the country (i.e., R0 = 7). The latter assumption may reflect different antigenic properties(83) and/or changing conditions in the country, as existing public health infrastructure declined. The higher R0 for the outbreak virus proved necessary to produce a large outbreak consistent with the evidence across a wide range of model assumptions that we explored during the course of our iterative validation process.
Table VI.
Model Inputs for the Albania Outbreak Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 1 | Focus model only on undervaccinated communities | |
| Relative population size compared to all of Albania | 0.5 | ||
| Number of age groups | 9 | 0–2; 3–17; 18–23 months; 2–4; 5–9; 10–19; 20–29*; 30–49*; ≥ 50 years | |
| Number of mixing age groups | 6 | 0–4; 5–9; 10–19; 20–29; 30–49; ≥ 50 years | |
| Year when model run-up starts | 1930 | ||
| Year when R0 seasonality starts | 1940 | ||
| Year when die-out first allowed | 1960 | ||
| Average basic reproductive number (PV1) (R0) | Fitted within range for middle tier (Table III); assume higher values during 1996 outbreak than endemic viruses | ||
| - During run-up | 7 | ||
| - During 1996 outbreak | 8 | ||
| Proportional change in R0 due to seasonality () | 0.4 | ||
| Day of seasonal peak in R(pd) | 200 (July 19) | 13 | |
| Relative weight of other age groups to force-of-infection () | Assume highly heterogeneous mixing to allow accumulation of fully susceptibility adults despite secondary OPV exposure | ||
| - 0–4 years | 0.5 | ||
| - 5–9 years | 0.97 | ||
| - 10–19 years | 0.97 | ||
| - 20–29 years | 0.95 | ||
| - 30–49 years | 0.7 | ||
| - ≥ 50 years | 0.7 | ||
| Proportion of transmissions via oropharyngeal route () | 0.8 | 19 | Fitted within range of expert assessments(19) and using Netherlands value as upper bound (Table V) |
| Per-dose take rate (tr)(PV1) | IPV take rates loosely based on field efficacy of IPV against type 1 poliomyelitis in the USA in 1954;(55) mOPV take rates based on assumed cumulative take after 1 mOPV1 dose and one tOPV dose a year later of 80% and 90% for poor and better cold chain conditions before and after 1970, respectively; increasing tOPV take rate reflects improving cold chain conditions | ||
| - IPV (Salk) | 0.50a | ||
| - mOPV1, 1960–1970 | 0.55b | ||
| - mOPV1, 19701–1977 | 0.68b | ||
| - tOPV, 1978–1991 | 0.30c | ||
| - tOPV, 1992–1996 | 0.45d | ||
| Assumptions about IPV use 1958–1959 | 57 | Coverage and schedule represent assumptions in the absence of details about IPV usage in Albania | |
| - Coverage with 3 doses by age 2 years | 0.72 | ||
| Coverage with 1 mOPV1 dose (1960–1977) or 3 tOPV doses (1978–1996) by age 1 year |
Time series | 57,76,82 | See Appendix A4 for values used |
| Characterization of annual mOPV campaigns 1960–1977 | 57 | ||
| - Date of round (day number) | 120 (April 30) | ||
| - Duration (days) | 30 | ||
| - Ages targeted | 0–18 months | ||
| Characterization of routine tOPV vaccination 1978–1996 | 57 | Coverage correction factor accounts for likely overreporting of coverage calculated from doses distributed;(82) booster dose at 5–6 years of age added to routine schedule in 1982(57) | |
| - Coverage correction factor | 0.9 | ||
| - Cumulative effect of 3 primary doses at ages (months) | 3 | ||
| - Booster doses at ages (years) | 1.5, 5 | ||
| - Relative coverage of booster dose | 0.5 | ||
| Characterization of 1983 tOPV campaign | 57 | Campaign targeted children born 1979–1981; assume NID dates as for mOPV campaigns | |
| - Number of doses, 2–4 years | 1 | ||
| - Effective impact () | 0.5 | ||
| Date of introduction for 1996 outbreak | February 19, 1996 | 13 | Fitted within previously characterized range |
| Characterization of spring’96 tOPV campaign | 13, 57 | Assume coverage significantly lower than official report of 98% coverage; campaigns targeted children ≥ 2 months of age, which represents one-third of the first age group in our model, leading to a relative coverage of one-third for this age group | |
| - Number of doses, 3 months–4 years | 2 | ||
| - First day of first round (1996) | 105 (April 8) | ||
| - First day of second round (1996) | 134 (May 13) | ||
| - Duration of both rounds (days) | 7 | ||
| - Effective per-round impact () | 0.6 | ||
| - Relative coverage for children aged 0–2 | 1/3 | ||
| Characterization of outbreak response campaign | 13, 57 | ||
| - Number of doses, 0–49 years | 2 | ||
| - First day of first round (1996) | 281 (October 7) | ||
| - First day of second round (1996) | 315 (November 11) | ||
| - Duration of both rounds (days) | 7 | ||
| - Effective per-round impact () (round 1; round 2) |
0.82; 0.88 | ||
Acronyms: IPV = inactivated poliovirus vaccine; mOPV(1) = monovalent oral poliovirus vaccine (type 1); NID = national immunization day; PV1 = poliovirus type 1; tOPV = trivalent oral poliovirus vaccine; USA = United States of America
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns that receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
For runs to verify behavior of other serotypes, we use take rates for IPV (Salk) of 0.6 and 0.5 for type 2 and 3, respectively.
For runs to verify behavior of other serotypes, we use the same take rates.
For runs to verify behavior of other serotypes, we use take rates for tOPV 1978–1991 of 0.55 and 0.27 for type 2 and 3, respectively.
For runs to verify behavior of other serotypes, we use take rates for tOPV 1992–1996 of 0.70 and 0.40 for type 2 and 3, respectively.
Tajikistan experienced an explosive outbreak due to a WPV1 importation in 2010, with 458 reported cases.(3) The majority of cases occurred in children younger than 5 years of age (65%), but the proportion of cases in children between 5–14 years of age (23% of cases) and over 15 years of age (12% of cases) increased as the outbreak neared its peak and continued after the first two mOPV1 response rounds that targeted only children younger than 6 years of age. Two more rounds targeting children younger than 15 years of age preceded the last case by less than four weeks. Tajikistan had not conducted SIAs since 2002 or 2003 (with the possible exception of a round with small geographic scope in 2007 targeting children younger than 15 years of age) and it experienced low routine coverage according to several surveys conducted between 2000 and 2007.(84–86) The country may have interrupted indigenous WPV transmission during the Soviet era, but it experienced significant numbers of WPV cases again in the 1990s during a period of civil unrest, until the reported incidence again dropped to 0 from 1995 on.(86) As in the Netherlands model, we introduce annual WPV1 importations into the model until 2000, after which we assume the country experienced no importations that established widespread transmission. Given that all but 1 case during the 2010 outbreak occurred in 3 of the 6 regions of the country (i.e., Dushanbe, Khatlon, and the Districts of Republican Subordination), we focus on these for the model (Table VII). We model routine vaccination based on available coverage surveys, which include polio vaccine coverage by dose, so that we do not need to approximate partial coverage from DTP1 and DTP3 data (as we did for the USA model). We estimate the coverage of 1 dose given fewer than 3 doses and 2 doses given fewer than 3 doses as:
where denotes the coverage with the nonbirth routine dose, measured at 12–23 months of age.(88–90) We model the cumulative effect of these 3 doses at 3 months by taking the aggregate effect of all 3 doses similar to the USA model. We assume coverage with the birth dose does not get recorded in the coverage survey results for POL1, POL2, and POL3, and approximate the coverage of an additional birth dose by the Bacille Calmette-Guérin (BCG) coverage (administered 3–5 days after birth). We further conservatively assume that the booster dose at 12 months of age gets included in the POL1, POL2, and POL3 coverage estimates (measured at 12–23 months of age), such that we do not add an additional dose at 12 months of age, and use the survey that reported the lowest coverage among 3 surveys during 2000–2007 as the basis for POL1, POL2, and POL3 coverage estimates.(84–86) We further assume no increase in routine coverage occurred since the 2007 survey despite the increase estimated by WHO/UNICEF based on administrative data.(91) We further apply a correction factor of 90% to all coverage values to account for unregistered children, which may represent an important group in the regions affected by the outbreak in Tajikistan.
Table VII.
Model Inputs for the Tajikistan Outbreak Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 1 | Includes 3 regions (of 6 total in the country) in which more than 1 case occurred (i.e., Dushanbe, Khatlon, Districts of Republican Subordination) | |
| Relative population size compared to all of Tajikistan | 0.67 | 85 | According to 2007 Living Standards Measurement Survey |
| Number of age groups | 11 | 0–2; 3–11 months; 1; 2; 3; 4; 5; 6–9; 10–14; 15–39*; ≥ 40* years | |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years | |
| Year when model run-up starts | 1930 | ||
| Year when R0 seasonality starts | 1935 | ||
| Year when die-out first allowed | 1960 | ||
| Average basic reproductive number (R0) (PV1) |
8 | Fitted within range for middle tier (Table III) | |
| Proportional change in R0 due to seasonality (α) | 0.5 | Assume substantial seasonality in mountainous, continental country | |
| Day of seasonal peak in R(pd) | 60 (March 1) | Month of maximum precipitation in Dushanbe (capital) | |
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.3 | For simplicity, assume equal values for each mixing age group | |
| Proportion of transmissions via oropharyngeal route () | 0.75 | 19 | Fitted within range of expert assessments(19) |
| Characterization of regular WPV importations | Assume no WPV importations established widespread transmission between the last SIAs in the early 2000s and the outbreak in 2010 | ||
| - Frequency | Annual | ||
| - Day of introductions | 91 (April 1) | ||
| - First year without introductions | 1995 | ||
| Per-dose take rate (tr) (PV1) | |||
| - tOPV | 0.4a | ||
| - mOPV1 | 0.65 | ||
| Routine coverage with 3 or more polio vaccine doses by age 1 year, partial coverage, and birth dose coverage | Time series 1960–2010 | 76, 85 | Coverage based on arbitrary assumption (of 90%) during Soviet era, then based on available surveys |
| Characterization of routine tOPV vaccination 1960–2010 | 84–86 | Assume birth dose not captured in survey results (i.e., model birth dose as a separate, additional dose), but booster dose (at 12 months) counted towards primary coverage by age 12 months (e.g., a child with a dose at birth and 2, 4, and 12 months gets counted towards coverage with 3 primary polio doses) | |
| - Doses at birth | 1 | ||
| - Cumulative effect of 3 primary nonbirth doses at ages (months) | 3 | ||
| - Relative coverage compared to most recent survey | 0.9 | ||
| Characterization of tOPV SIAs 1995–2002 | See Appendix A4 for effective per-round impact assumptions by year in the absence of good data | ||
| - Dates, durations, and target population of SIAs | Time seriesb | ||
| - Effective per-round impact () | Varies | ||
| Date of introduction for 2010 outbreak | November 1, 2009 | Based on model calibration within plausible range | |
| Characterization of outbreak response | 3 | Assume very high effective per-round impact given high coverage of response campaigns; ignore impact of mop-up rounds in some districts in September (after last case) | |
| - Per-dose take rate (PV1) | 0.4 | ||
| - First day (in 2010), by round (round 1; 2; 3; 4; 5; 6) | May 4; May 18; June 1; June 15; October 4; November 8 | ||
| - Target ages, by round (rounds 1–2; 3–6) | 0–5; 0–14 years | ||
| - Vaccine, by round (rounds 1–4; 5–6) | mOPV1, tOPV | ||
| - Effective impact () of each round | 0.8 | ||
| - Duration of each round (days) | 5 | ||
Acronyms: IPV = inactivated poliovirus vaccine; mOPV1 = monovalent oral poliovirus vaccine type 1; PV1 = poliovirus type 1; SIA = supplemental immunization activity; tOPV = trivalent oral poliovirus vaccine
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who received maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
For runs to verify behavior of other serotypes, we use take rates for tOPV of 0.65 and 0.35 for type 2 and 3, respectively.
Information about SIA history as reported to the World Health Organization.
Cuba probably became the first country to interrupt indigenous WPV transmission with a strategy of vaccinating young children exclusively during annual two-round campaigns with tOPV (except for two years of use of mOPV1 and bOPV types 2 and 3), with its apparent incidence dropping to 0 cases after the first two rounds in 1962.(92,93) Despite 18 reported VAPP cases during 1963–1996, no evidence exists of emergences of cVDPVs between campaigns, and several studies document the rapid disappearance of OPV-related viruses following campaigns.(48,94,95) We explored the situation in Cuba to verify that the campaigns in our model accomplish rapid WPV elimination and that OPV-related viruses also disappear quickly with no FRPV circulation between campaigns. We then use the same generic model assumptions about OPV evolution to simulate the cVDPV outbreaks that occurred in Haiti, Madura, and Nigeria. Our characterization in Table VIII of the vaccination history in Cuba draws directly from the overview by Más Lago. (92)
Table VIII.
Model Inputs for the Cuba Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 1 | Model intended to reflect average conditions in Cuba | |
| Number of age groups | 10 | 0–2; 3–11 months; 1; 2; 3; 4; 5–8; 9; 10–14; ≥ 15* years | |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years | |
| Year when model run-up starts | 1930 | ||
| Year when R0 seasonality starts | 1940 | ||
| Year when die-out first allowed | 1960 | ||
| Average basic reproductive number (R0) (PV1) | 8 | Fitted within range for middle tier (Table III); PV2&3 according to ratios in Table I | |
| Proportional change in R0 due to seasonality (α) | 0.05 | 13 | Comparatively less seasonality than in temperate climate countries |
| Day of seasonal peak in R(pd) | 182 (July 1) | 13 | Similar peak day as previously assumed for the Dominican Republic |
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.5 | For simplicity, assume equal values for each mixing age group | |
| Proportion of transmissions via oropharyngeal route () | 0.3 | 19 | Based on approximate average of mean expert assessments for community and close contacts for type 1 in high R0 settings(19) |
| Per-dose take rate (tr) (PV1;PV2;PV3) | 0.5;0.7;0.45 | Assume equivalent take for each serotype after bOPV23 then mOPV1 (in 1963 and 1968) and tOPV twice (all other years) | |
| Assumptions about OPV campaigns 1962–1996 | 92, 93 | Target age groups as described by Más Lago et al.(92) (for target age groups that partially overlap with situation-specific age groups (e.g. 5–6 year olds with 1 of 2 rounds in 1965), proportionately reduce effective vaccination rate according to width of targeted age group divided by width of situation-specific age group); average coverage per round based on total children targeted divided by 64 million doses administered 1962–1996(92) | |
| - Day of first round | 56 (February 25) | ||
| - Day of second round, 1962–1969 | 91 (April 1) | ||
| - Day of second round, 1970–1996 | 112 (April 22) | ||
| - Duration of each round (days) | 7 | ||
| - Effective per-round impact () | 0.94 | ||
| - Target age groups | Time series | ||
| - Relative coverage of booster doses at age 9 (starting 1970) | 1 | ||
Acronyms: bOPV23 = bivalent type 2 and 3 OPV; OPV = oral poliovirus vaccine; PV(1,2,3) = poliovirus (type 1, 2, or 3, respectively); tOPV = trivalent OPV
Note:
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1)
The type 1 cVDPV (cVDPV1) outbreak in Haiti during 2000–2001 represents the first cVDPV outbreak detected in real time (with a few other cVDPV outbreaks identified retrospectively).(23) While investigators found only 8 VDPV isolates from AFP cases and identified only 2 additional polio-compatible cases also probably caused by the cVDPV outbreak virus, poor surveillance probably led to approximately 80% underreporting(96,97) or even more given that not a single isolate from AFP cases exists for the years leading up to the outbreak. The virus spread to the Dominican Republic on the same island of Hispaniola to cause 13 more laboratory-confirmed cases and it continued to circulate in Haiti through two poor-quality response immunization campaigns until a rolling campaign targeting all children up to 10 years of age controlled the outbreak. We found very little available information about polio vaccination leading up to the outbreak in Haiti. Like all countries in the Western Hemisphere with ongoing indigenous WPV transmission near the end of the Pan American Health Organization’s campaign to eliminate polio from the region, Haiti implemented NIDs periodically, probably starting in the late 1980s.(98,99) The last WPV case occurred in 1989 and no evidence exists of any SIAs conducted after 1995.(96) Consistent with other cVDPV outbreaks,(23) Haiti experienced relatively low routine vaccination coverage, probably including large pockets of people with very low coverage.(76,90) We take the same approach as for Tajikistan to model routine vaccination and partial coverage, linearly interpolating between data points from Demographic and Health Surveys (DHS) conducted in 1994–1995, 2000, and 2005–2006.(90) The surveys report birth dose coverage, which we model as a separate dose at birth that occurs in addition to the effect of the 3 primary doses with cumulative effect at 3 months. Table IX summarizes the model inputs for Haiti.
Table IX.
Model Inputs for the Haiti Outbreak Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 1 | Model Haiti as one population in the absence of good data on heterogeneity | |
| Number of age groups | 9 | 0–2; 3–11 months; 1; 2; 3; 4; 5–9; 10–14; ≥ 15* years | |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years | |
| Year when model run-up starts | 1950 | ||
| Year when R0 seasonality starts | 1970 | ||
| Year when die-out first allowed | 1980 | ||
| Average basic reproductive number (R0) (PV1) | 9.5 | Fitted within range for middle tier (Table III) | |
| Proportional change in R0 due to seasonality () | 0.05 | 13 | Comparatively less seasonality than in temperate climate countries |
| Day of seasonal peak in R(pd) | 180 (June 29) | 13 | Similar peak day as previously assumed for the Dominican Republic |
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.3 | For simplicity, assume equal values for each mixing age group | |
| Proportion of transmissions via oropharyngeal route () | 0.3 | 19 | Based on approximate average of mean expert assessments for community and close contacts for type 1 in high R0 settings(19) |
| Per-dose take rate for tOPV (tr) (PV1) | 0.44a | ||
| Routine coverage with 3 or more doses by 1 year of age, birth dose coverage, and partial coverage | Time series 1979–2002 | 76, 90 | See Appendix A4 for values used |
| Characterization of routine tOPV vaccination, 1979–2002 | 90 | ||
| - Doses at birth | 1 | ||
| - Cumulative effect of 3 primary nonbirth doses at ages (months) | 3 | ||
| Characterization of tOPV campaigns 1986–1995 | Dates of rounds assumed similar to Cuba; see Appendix A4 for effective per-round impact assumptions by year in the absence of good data | ||
| - Day of first round | 50 (February 19) | ||
| - Day of second round | 120 (April 30) | ||
| - Duration of each round (days) | 7 | ||
| - Effective per-round impact () | Varies | ||
| - Target age groups | 0–4 years | ||
| Characterization of tOPV outbreak response, 2001 | 96, 97 | The third round was a rolling campaign that covered children under the age of 10 years over a span of 7 weeks(96) | |
| - Day of first round | 28 (January 28) | ||
| - Day of second round | 63 (March 4) | ||
| - Day of third round | 140 (May 20) | ||
| - Duration of rounds 1 and 2 (days) | 7 | ||
| - Duration of rounds 3 (days) | 56 | ||
| - Effective per-round impact () of rounds 1 and 2 | 0.5 | ||
| -Effective per-round impact () of round 3 | 0.9 | ||
| - Target age groups, rounds 1 and 2 | 0–4 years | ||
| - Target age groups, round 3 | 0–9 years | ||
Acronyms: PV1 = poliovirus type 1; tOPV = trivalent OPV
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
For runs to verify behavior of other serotypes, we use take rates for tOPV of 0.68 and 0.38 for type 2 and 3, respectively.
Madura, a small, densely populated island off the coast of Java in Indonesia, experienced a type 1 cVDPV outbreak in 2005 around the same time that it imported WPV1 from a large outbreak that occurred in other parts of Indonesia. Overall, Madura reported 45 laboratory-confirmed cVDPV1 cases, 8 laboratory-confirmed WPV1 cases, and 10 polio-compatible cases.(100) The cases occurred predominantly in rural areas of the island with routine vaccination coverage much below the averages for Indonesia, West Java, and Madura.(100) After an initial small-scale response in the cVDPV-affected villages, 3 national campaigns with tOPV in response to the WPV1 outbreak controlled both the cVDPV1 and the WPV1 outbreak. The WHO vaccine-preventable disease incidence series reports cases through 2000,(87) although Estívariz et al.(100) report no WPV circulation for Indonesia between 1995–2004. We assume that WPV circulation in Madura probably stopped well before 2000. The WHO also reports that Indonesia conducted 2 NID rounds targeting children under 5 years of age in 1995–1997 and 2002, mop-ups in 1999, and subnational NIDs in 2000–2001, with no SIAs between the NIDs in 2002 and the outbreak response in 2005 (Gacic-Dobo, 2009, personal communication). Table X shows our assumptions related to the Madura outbreak, with time series for effective perround impact documented in Appendix A4. Given the reported heterogeneity in coverage between rural and urban areas(100) and the possibility that the fixed-post campaign in 2002 missed entire rural villages, we focus on the rural population of approximately 900,000 people in 2005. Consistent with the progression of outbreak cases in time and space, we assume that the VDPV emerged from the rural areas although this remains uncertain. We emphasize our assumption of high R0 in the affected subdistricts given the reported “suboptimal hygienic conditions...observed in all households, with a lack of latrines in half of the villages visited and with boiling water for drinking reported by <40% of caregivers.”(100, p. 350) Similar to other situations, we model the cumulative effect of the primary doses as a single dose at age 3 months, taking into account estimates of the partial coverage of up to 4 doses in the affected population (Estívariz, 2012, personal communication).
Table X.
Model Inputs for the Madura Outbreak Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 1 | Focus on rural population of Madura | |
| Relative population size compared to all of Indonesia | 0.00405 | 61, 100 | Ratio of rural population of Madura (∼920,000) to all of Indonesia (∼227 million) in 2005 |
| Number of age groups | 9 | 0–2 months; 3–11 months; 1; 2; 3; 4; 5–9; 10–14; ≥ 15* years | |
| Number of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years | |
| Year when model run-up starts | 1950 | ||
| Year when R0 seasonality starts | 1970 | ||
| Year when die-out first allowed | 1980 | ||
| Average basic reproductive number (R0) (PV1) | 9 | Fitted within range for middle tier (Table III) | |
| Proportional change in R0 due to seasonality () | 0.2 | 13 | Somewhat less seasonality than in temperate climate countries |
| Day of seasonal peak in R0(pd) | 95 (April 5) | Based on model calibration | |
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.35 | For simplicity, assume equal values for each mixing age group | |
| Proportion of transmissions via oropharyngeal route () | 0.3 | 19 | Based on approximate average of mean expert assessments for community and close contacts for type 1 in high R0 settings(19) |
| Per-dose take rate (tr) (PV1) | |||
| - tOPV | 0.45a | ||
| - mOPV | 0.66 | ||
| Routine coverage with 3 or more doses by 1 year of age | Time series 1980–2007 | 76, 100 | See Appendix A4 for values used |
| Characterization of routine tOPV vaccination, 1981–2007 | 100 | Partial coverage based on convenience sample in rural areas; in the absence of separate estimates for the birth dose coverage, we model the cumulative effect of all four doses (scheduled at birth and 1, 2, and 3 months of age) at age 3 months | |
| - Cumulative effect of all 4 primary doses at age (months) | 3 | ||
| - Coverage with 1 dose given < 4 | 0.13 | ||
| - Coverage with 2 doses given < 4 | 0.13 | ||
| - Coverage with 3 doses given < 4 | 0.07 | ||
| Characterization of tOPV campaigns 1995–2002 | 100 | Dates of rounds reflect approximate average times of campaigns in Indonesia as reported to WHO; see Appendix A4 for effective per-round impact assumptions by year in the absence of good data | |
| - Day of first round | 240 (August 28) | ||
| - Day of second round | 280 (October 7) | ||
| - Duration of each round (day) | 7 | ||
| - Effective per-round impact () | Varies | ||
| - Target age groups | 0–4 years | ||
| Characterization of outbreak response, 2005 | 100 | ||
| - Vaccine, by round (rounds 1–2;3) | tOPV;mOPV1 | ||
| - Day of first round | 242 (August 30) | ||
| - Day of second round | 270 (September 27) | ||
| - Day of third round | 334 (November 30) | ||
| - Duration of each round (days) | 7 | ||
| - Effective per-round impact () (round 1;2;3) | 0.81;0.88;0.85 | ||
| - Target age groups for all rounds | 0–4 years | ||
Acronyms: mOPV1 = monovalent oral poliovirus vaccine type 1; PV1 = poliovirus type 1; tOPV = trivalent OPV; WHO = World Health Organization
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
For runs to verify behavior of other serotypes, we use take rates for tOPV of 0.7 and 0.4 for type 2 and 3, respectively.
Table XI lists the model inputs for Nigeria. Nigeria remains one of only 3 countries that have never interrupted indigenous transmission of WPV1 and WPV3 in all areas. Moreover, while Nigeria reported the last case of WPV2 in 1998,(2) it experienced sustained transmission of VDPV2 since 2005.(101) Nigeria conducted the first NID in late 1996. When Nigeria switched to a virological case definition in 2001 as surveillance improved, it reported only 56 WPV cases, but this probably still represents a low estimate.(102,103) In 2003–2004, some northern states suspended all immunizations, leading to exportations of WPV1 and WPV3 to previously polio-free states(103) (and to other countries).(6) Nigeria introduced mOPV1 for SIAs in 2006 and mOPV3 in 2007,(101) resulting in gaps of immunity to types 2 and 3. In 2010, Nigeria started using bOPV for most SIAs(101) while it continued to use tOPV in some NIDs and both mOPVs in some areas depending on the epidemiological situation. Continued circulation of all 3 poliovirus serotypes in Nigeria reflects chronic failure to vaccinate and to attain high coverage with routine immunization and SIAs in the northern states.(101) The northern Nigeria model (see Table XI for inputs) focuses on the northwestern (NW) zone, which accounts for ∼ 25% of the national population according to the 2006 census(104) and the majority of confirmed polio cases (WPV or cVDPV) during the last decade.(102,103,105–109) We assume that in this zone, a large fraction of the population gets chronically undervaccinated, while the general population receives most of the doses. Thus, we model two subpopulations (i.e., the general population that represents 90% of the total population and the remaining 10% an undervaccinated subgroup that mixes somewhat preferentially within itself). In reality, the undervaccinated children probably live scattered across the region and involve underserved urban and rural communities as well as migrant groups that remain poorly identified. Modeling these as one spatially homogeneous mixing subpopulation represents a simplification since scattered groups do not mix instantaneously with each other, but the inclusion of this subpopulation provides a better characterization of heterogeneity in coverage than including this group as part of one big population for the northwest. To characterize effective vaccination rates in these states, we use information on each SIA conducted since 1996 (Gacic-Dobo, 2009, personal communication). For SNIDs, we multiply the coverage by the fraction of the total population of the northwest using data on targeted states or districts when available and informed guesses otherwise. For routine immunization, we model the birth dose separately and the cumulative effect of the 3 primary, nonbirth doses at 3 months of age, taking into account data on coverage by dose and zone for surveys conducted periodically between 1999 and 2008 (see Appendix A4).(88)
Table XI.
Model Inputs for the Northern Nigeria Model
| Model input (symbol) | Best estimate | Source | Notes |
|---|---|---|---|
|
| |||
| Number of subpopulations | 2 | Subpopulation 1: undervaccinated subgroups (1/10th of total population); subpopulation 2: general population (9/10th of total population) | |
| Relative population size compared to all of Nigeria | 0.256 | 104 | Population of the 7 states in the Northwest Zone (i.e., Jigawa, Kaduna, Kano, Katsina, Kebbi, Sokoto, Zamfara) divided by total population of Nigeria, according to the 2006 census |
| Number of age groups | 10 | 0–2, 3–11 months; 1; 2; 3; 4; 5–9; 10–14; 15–39*; ≥ 40 years* | |
| umber of mixing age groups | 3 | 0–4; 5–14; ≥ 15 years | |
| Year when model run-up starts | 1970 | ||
| Year when R0 seasonality starts | 1980 | ||
| Year when die-out first allowed | 1980 | ||
| Average basic reproductive number (R0) (PV1) | 8 | Fitted within range for middle tier (Table III); PV2&3 according to ratios in Table I | |
| Proportional change in R0 due to seasonality () | 0.05 | Comparatively less seasonality than in temperate climate countries | |
| Day of seasonal peak in R0(pd) | 60 (March 1) | Most outbreak peaks occurred early in recent years | |
| Proportion of contacts reserved for individuals within the same mixing age group () | 0.4 | For simplicity, assume equal values for each mixing age group | |
| Proportion of potentially infectious contacts of individuals in subpopulation 1 that are with other individuals in subpopulation 1 (pwithin) | 0.88 | 13 | Force of infection as a result of mixing between subpopulations modeled using previously described approach(13) (see Appendix A1) |
| Proportion of transmissions via oropharyngeal route () | 0.3 | 19 | Based on approximate average of mean expert assessments for community and close contacts for type 1 in high R0 settings(19) |
| Per-dose take rate (tr) (PV1;PV2;PV3) | |||
| - tOPV | 0.45;0.7;0.4 | ||
| - mOPV | 0.66;NA;0.65 | ||
| - bOPV | 0.54;NA;0.52 | ||
| Routine coverage with 3 or more doses by 1 year of age, birth dose coverage, and partial coverage | Time series 1984–2012 | 76, 88 | See Appendix A4 for values used |
| Characterization of routine tOPV vaccination, 1984–2012 | 101 | ||
| - Doses at birth | 1 | ||
| - Cumulative effect of 3 primary nonbirth doses at ages (months) | 3 | ||
| Characterization of OPV campaigns 1996–2012 | See Appendix A4 for detailed assumptions about SIAs in each year and resulting annual cumulative percentages of missed children | ||
| - Dates, durations, and target population of SIAs | Time seriesa | ||
| - Effective per-round impact () | Varies | ||
| - Target age groups | 0–4 years | ||
Acronyms: bOPV = bivalent OPV; mOPV = monovalent OPV; NA = not applicable; OPV = oral poliovirus vaccine; PV1,2,3 = poliovirus type 1, 2, and 3, respectively; SIA = supplemental immunization activity; tOPV = trivalent OPV
Age groups marked with an asterisk indicate age groups that count towards determining the fraction of newborns who receive maternal antibodies, based on the immune fraction in those age groups (see Appendix A1).
Table XII lists the model inputs for northern India. India reported the last WPV2 case in 1999, within a few years after introducing NIDs in 1995. SIAs with tOPV continued to intensify, but failed to interrupt transmission of WPV1 and WPV3 in the northern states of Uttar Pradesh and Bihar, probably due to a combination of poor take of tOPV and failure to reach the last pockets of susceptibles. India first started using mOPV1 in January 2006 (following small field trials that began in April 2005(110)), mOPV3 in December 2006, and bOPV in January 2010 (Gacic-Dobo, 2009, personal communication). After concerted efforts to reach the last remaining reservoirs of WPV transmission, India reported the apparent last case of WPV in January 2011.(111) To model poliovirus transmission in northern India, we separately consider Bihar and those districts (listed in Table XII) in WUP that reported the majority (i.e., 69%) of cases from Uttar Pradesh.(112) Similar to the northern Nigeria model, we further divided the population of WUP into a general population (96% of the total population considered) and a small reservoir of chronically undervaccinated subgroups. We follow the same approach as Nigeria to model routine vaccination using linear interpolation of data from available surveys.(89) However, given the very large difference between estimated DTP3 versus POL3 coverage, we suspect that doses administered during SIAs may inflate the OPV3 coverage, while the relatively lower focus on DTP than polio as well as possible DTP shortages may contribute to lower DTP3 coverage. Consequently, we assume for our coverage estimates the midpoints between DTP and POL coverage. We use information on actual SIAs conducted in India (Gacic-Dobo, 2009, personal communication) about dates, vaccine used, and target population (for SNIDs) as well as fitted estimates of effective per-round impacts to characterize the effective vaccination rates due to SIAs in the model (see Appendix A4).
3. RESULTS
We present the results for each of the modeled situations separately. Tables XIII–XIV and Figs. 2–10 provide the main results. Appendix A5 includes further results for each situation, including figures that show the run-ups and specific comparisons with data.
Table XIII.
Comparison of Modeled and Observed Age-Specific Paralytic Incidence for Situations that Allow Meaningful Comparisona
| Paralytic cases (percentage of total cases) |
||
|---|---|---|
| Age group | Reported | Model |
|
| ||
| The Netherlands, 1992–1993 (79)b | ||
| 0–4 years | 14 (20%) | 12 (17%) |
| 5–9 years | 8 (11%) | 8 (12%) |
| 10–19 years | 16 (23%) | 14 (20%) |
| 20–29 years | 16 (23%) | 15 (22%) |
| 30–39 years | 14 (20%) | 12 (17%) |
| ≥40 years | 3 (4%) | 9 (13%) |
| Albania, 1996 (57) | ||
| 0–9 years | 19 (14%) | 12 (10%) |
| 10–19 years | 42 (30%) | 62 (51%) |
| 20–39 years | 76 (55%) | 47 (39%) |
| ≥40 years | 1 (1%) | 1 (1%) |
| Tajikistan, 2010 (3) | ||
| <1 year | 90 (20%) | 104 (23%) |
| 1–4 years | 208 (45%) | 217 (47%) |
| 5–14 years | 107 (23%) | 108 (4%) |
| ≥15 years | 53 (12%) | 29 (6%) |
| Madura, 2005 (100)c | ||
| 0–4 years | 36 (80%) | 75 (90%) |
| ≥5 years | 9 (20%) | 8 (10%) |
| Northern Nigeria, 2003–2011 d | ||
| 0–4 years | Not available | 5,099 (94%) |
| ≥5 years | Not available | 282 (6%) |
| Northern India, 2002–2010 (112)e | ||
| 0–4 years | 3,286 (97%) | 4,784 (95%) |
| ≥5 years | 102 (3%) | 257 (5%) |
For Cuba and Haiti, the number of cases remains too low for meaningful comparison; for the USA, the model does not produces a realistic age distribution of cases because it averages out local inter-epidemic periods with low seasonality to yield relatively consistent national totals in the pre-vaccine era.
Reported numbers include 10 nonparalytic cases.
Cases from OPV-related type 1 virus in all reversion stages combined (excluding recipient vaccine-associated paralytic polio).
Cases from all types and all reversion stages combined (excluding recipient vaccine-associated paralytic polio).
Cases for Bihar and Western Uttar Pradesh combined.
Table XIV.
Comparison of Modeled and Observed Secondary OPV Infections Following NIDs in Cuba, Based on Cumulative Force of Infection and Percentage Seropositive Before the Next NID, Respectively
| Percentage secondarily infected (95% CI, if reported) |
|||
|---|---|---|---|
| Source | Type 1 | Type 2 | Type 3 |
|
| |||
| Más Lago et al.(48) | |||
| - Children born during first month after 1991 NID (n = 28) | 46.4% (27.6–65.2) | 46.4% (27.6–65.2) | 17.9% (3.4–32.4) |
| - Children born during second month after 1991 NID (n = 37) | 5.4% (0.0–12.8) | 13.5% (2.3–24.7) | 8.1% (0.0–17.1) |
| - Children born during third month after 1991 NID (n = 53) | 0.0% (0.0–0.0) | 1.9%a (0.0–5.7) | 0.0% (0.0–0.0) |
| Más Lago et al.(95) | |||
| - Children born during first or second month after 1997 NID (n = 14) | 0.0% | 0.0% | 0.0% |
| Model | |||
| - Fully susceptible children exposed from day 1 after 1991 NID | 14.0% | 16.3% | 8.4% |
| - Fully susceptible children exposed from day 16 after 1991 NID | 5.5% | 5.3% | 3.2% |
| - Fully susceptible exposed from day 31 after 1991 NID | 1.9% | 1.6% | 1.1% |
| - Fully susceptible exposed from day 46 after 1991 NID | < 1% | < 1% | < 1% |
Acronyms: CI = confidence interval; NID = national immunization day; OPV = oral poliovirus vaccine
Seropositivity in this age group may reflect residual maternal immunity given that maternal antibody levels were still at 24.5% in 1-month younger children at the time of serologic testing.(48)
Fig. 2.
Reported paralytic cases(9) and modeled paralytic incidence for the USA. Acronyms: IPV = inactivated poliovirus vaccine; mOPV = monovalent oral poliovirus vaccine; PV1,2,3 = poliovirus type 1, 2, and 3, respectively; tOPV = trivalent oral poliovirus vaccine; USA = Unites States of America; VAPP = vaccine-associated paralytic poliomyelitis.
Fig. 10.
Laboratory-confirmed wild poliovirus cases 2002–2012,(112) and modeled paralytic incidence for the combined results from Bihar and WUP. Acronyms: WPV1,3 = poliovirus type 1 or 3, respectively; WUP = Western Uttar Pradesh.
3.1. USA
Fig. 2 shows the results for the USA model using the best estimates for the generic model inputs in Table I and for the USA-specific inputs in Table IV. The model reproduces the general behavior observed in the USA, with a significant drop in incidence after the introduction of IPV in 1955, and behavior in the late 1950s reflecting the accumulation of susceptibles and continued outbreaks leading to a resurgence of cases. The assumption that the majority (80%) of transmissions comes from oropharyngeal infections (Table IV) represents a major determinant of the impact of IPV. For example, if we decrease this proportion to 50%, then the incidence of type 1 poliovirus in 1961 (i.e., the year before OPV use starts in the model) is 3.5 times higher than for the run shown in Fig. 2, and the cumulative incidence during 1955–1961 1.4 times higher. WPV elimination occurs soon after the start of the mOPV campaigns, which the model begins in 1962. Given the uncertainties and the oversimplification of one large homogenous population with perfect mixing, we observe some differences. First, the model estimates higher incidence than the reported estimates of paralytic cases in the pre-vaccine era. Underreporting(70) may account for some of the difference, and the PIRs, which directly impact the absolute numbers of cases in the model and that we based on typical estimates,(25,70,113) may slightly overestimate the true values. Second, once we uniformly introduce mOPVs, WPV elimination occurs in the same year as the effective proportion infectious (EPI) drops below the transmission threshold EPI* = 5 × 10−6 (Table I). Continued WPV transmission beyond 1962 in reality(67,70,71) likely involved pockets of unvaccinated populations with relatively higher R0 and/or lower coverage that sustained some local WPV transmission naturally until seasonality and vaccination led virus transmission to completely die out.(67,70)
Despite the above limitations, our model provides an opportunity to compare the more stable situation after the last known WPV transmission in 1979(34) with data obtained during this era of routine tOPV use in the USA. First, we found no emergences of FRPVs (i.e., defined as OPV-related viruses with the same PIR and R0 as typical homotypic WPVs) of any serotype exceeding our transmission threshold EPI* (Table I). Thus, although very low prevalence of FRPV occurs due to fractional reversion from more attenuated states, our model remains consistent with the lack of evidence of cVDPVs in the USA during the OPV period from 1980–1996. This result depends on the assumed elimination thresholds and number of reversion stages, which we fitted to produce cVDPV outbreaks where they occurred (i.e., Madura, Haiti, northern Nigeria) and no cVDPV outbreaks where none occurred despite OPV use (e.g., Albania, Tajikistan, Cuba).
Second, we compared the average forces of infection generated by all OPV-related viruses (including OPV itself) in the model with the average force of infection needed to produce the observed seropositivity levels among unvaccinated children measured by Chen et al.(64) during 1990–1991 (see Appendix A5). Both the model and the data remain consistent with substantial exposure to OPV viruses, particularly for type 2, and the model produces values within the reported confidence intervals (except for type 1 in one of the two study sites, but not in the other).(64)
The assumed weak seasonality in the model to compensate for the averaging out of local periodicity at the aggregate level reduces the time between peaks in the model, and therefore we cannot make meaningful comparisons between data and model for the USA as it relates to the age distribution of cases, although good information exists about the age distribution of cases in the USA in the pre-vaccine era.
3.2. The Netherlands
Fig. 3 shows the results of the model for the type 3 WPV outbreak in the Netherlands in 1992–1993, which involves the two subpopulations described in the methods section. Due to strong seasonality coupled with a low R0, the model produces WPV3 elimination in the pre-vaccine era. However, we reintroduce virus annually from importations, so that significant outbreaks continue to occur. Following large-scale campaigns with IPV during 1957–1959 and subsequent high routine coverage, WPV3 introductions no longer take off in the general population, and we assume no WPV3 introduction established circulation in the religious communities from 1960 on (Table V). As in the USA, the assumption that oropharyngeal spread dominates transmission plays an important role in IPVs ability to provide herd immunity in the Netherlands because we assume IPV-induced immunity does not reduce participation in fecal-oral transmission by as much as it reduced participation in oropharyngeal transmission. Although we do not have data by type during the WPV elimination phase in the Netherlands, occasional WPV outbreaks continued to occur through the 1970s, including one large WPV1 outbreak in 1978 in the same subpopulation of religious vaccine-objector communities.(80) With the introduction of the WPV3 in late 1992, the large numbers of susceptibles that had accumulated in the low-coverage orthodox communities led to the outbreak shown in Fig. 3, which corresponds well with respect to the timing and size of the reported outbreak due to calibration of the date of introduction and peak of seasonal transmission (Table V).(79) The small difference in total number of cases in the model compared to the data may reflect variation in the true PIR for the WPV3 of the outbreak compared to the average value of 1:1000 that we assumed (Table I). Alternatively, different assumptions about R0 (including seasonality), coverage in the religious communities, date of introduction, outbreak response, and relative importance of oropharyngeal transmission all affect the case total, but unlike the PIR they also affect the timing of the outbreak. Importantly, our model remains consistent with the evidence of significant herd immunity in the general Dutch population in that it does not produce any cases outside of the religious communities.(79) This remains true even if we assume no vaccination response in the general population and/or if we increase the proportion of contacts with the general population for individuals in the orthodox communities to as much as 50%.
Fig. 3.
Reported poliomyelitis cases(79) and modeled paralytic incidence for the Netherlands. Acronyms: eIPV = enhanced-potency inactivated poliovirus vaccine; tOPV = trivalent oral poliovirus vaccine.
Table XIII compares the age distribution of cases for the Netherlands model with the data, showing a consistent pattern. This suggests adequate characterization of the run-up in the model, as well as a realistic aging process in the model owing to the large number of age groups. The higher frequency of cases in adults of 40 years of age in the model probably still relates to the exponential aging process in the model, which allows some fraction of individuals to escape WPV exposure as they age into the next age group. Alternatively, older adults may in reality have participated less in transmission compared to school-aged children,(114) or some underreporting among older adults occurred. Uncertainty remains about the possibility that the PIR increases by age.(25) Given the exponential aging processes in our model, an increasing PIR by age, while supported by some evidence,(25) would further shift the age distribution towards older adults in the Netherlands. The use of tOPV for the response in the religious communities did not produce any spread of FRPVs of any type.
3.3. Albania
Fig. 4 shows the results for the WPV1 outbreak in Albania in 1996. Unlike our prior model for this outbreak, which assumed the initial values immediately before the outbreak,(13) we generated the initial conditions for the model by running it from a pre-vaccine time up to the outbreak. This proved challenging given very limited data on the vaccination history, in particular during the 1960s and 1970s when Albania relied solely on OPV campaigns for which timing depended on vaccine supply,(82) and complicated population dynamics that led to a net decline in the population of adults around the time of the outbreak.(61) The Appendix shows the run-up of the model to 1996, with die-out in the modeled subpopulation in 1960 as a result of strong seasonality and the onset of campaigns with mOPV. Due to the assumed heterogeneity in mixing between age groups (Table VI), parts of cohorts that escaped OPV vaccination remain relatively unaffected by secondary OPV spread, leading to accumulation of fully susceptible adults until 1996, which we assume did not emigrate at the same rate as immunes (see methods section). The model does not generate FRPVs because OPV-related viruses primarily transmit between younger children and die out before they reach the last reversion stage or transmit within the more susceptible older age cohorts. The introduction in early 1996 of a WPV1 causes an outbreak roughly similar to the observed outbreak (Fig. 4). The low population immunity over many years leading up to the outbreak suggests the possibility that Albania faced a potentially high risk of a cVDPV outbreak and this appears consistent with the relatively high rates of VAPP it experienced for types 2 and 3.(82) Table XIII includes the age distribution of cases for Albania and suggests some differences between the model and the data. While the reported data(57) suggest most cases occurred in people over 20 years of age, the model yields similar numbers more cases in the 10–19 than the over 20 year olds. While we found no conclusive evidence to suggest that the PIR changes as a function of age,(25,67) strong evidence exists that the severity of paralysis and case fatality rates increase significantly with age,(25,67) which would explain a higher probability of detection for the older age groups. Our model for the Albanian outbreak assumes both constant PIR and constant probability of detection with age (i.e., of 100%) while in reality undetected paralytic cases in children probably occur more often than in adults. We emphasize that the epidemiology of the 1996 outbreak remains a puzzle in the context of the reported history of polio in the country and high quality of immunization leading up to the outbreak that remains uncharacteristic,(47,57,82,83) and that modeling this outbreak required non-standard assumptions about the transmissibility of the outbreak virus compared to earlier viruses in the same population, the strongly age-heterogeneous nature of mixing, and disproportionately low rates of emigration for fully susceptibles.
Fig. 4.
Reported paralytic poliomyelitis cases(57,83) and modeled paralytic incidence for Albania. Acronyms: tOPV = trivalent oral poliovirus vaccine.
3.4. Tajikistan
Fig. 5 shows the results from the Tajikistan model. The run-up yields elimination as soon as we allow it in 1960 with the onset of routine OPV immunization (Table VII), owing to the assumed strong seasonality (see Appendix A5). The WPV1 importations introduced into the model do not take off until outbreaks in the early 1990s, when routine immunization coverage decreases. The SIAs during the mid-1990s to early 2000s again raise population immunity. With conservative assumptions about routine vaccination since the last SIAs and fitted data of introduction and peak of seasonality (Table VII), the model of the three regions affected by the outbreak produces a good fit to the reported data.(3) The model results suggest that within the assumed closed population of the three affected regions, the outbreak nearly reached its natural peak by the time the outbreak response campaigns started. Table XIII includes the comparison of age distributions of model vs. reported data,(3) which also shows a good match. Unlike the Netherlands model, slightly increasing the PIR by age would improve the fit of the proportion of cases over 15 years of age.
Fig. 5.
Wild poliovirus type 1-confirmed poliomyelitis cases(3) and modeled paralytic incidence for Tajikistan. Acronyms: mOPV1 = monovalent oral poliovirus vaccine type 1; tOPV = trivalent oral poliovirus vaccine.
3.5. Cuba
Due to the absence of cases since the early 1960s, Fig. 6 shows the behavior of the persistence of OPV-related viruses for Cuba during 2 years (1990–1991), rather than the incidence of WPV as shown in the previous result figures. Although the pattern shown in Fig. 6 looks very similar to the pattern in other years, we focus on 1991 because we can compare the model results to data reported by a seroprevalence study conducted following the NIDs in 1991.(48) Consistent with the evidence,(92) the first campaigns in 1962 led to WPV elimination of all three poliovirus serotypes in the model within a year (see run-up shown in Appendix A5). Fig. 6 shows the EPI to 0–4 year olds for all three serotypes and broken down by reversion stage. Not surprisingly, administration of OPV to children under 5 years of age with high coverage and relatively high take rates (Table VIII) leads to a high prevalence of OPV viruses (i.e., reversion stage 0) during and immediately after NIDs. This keeps population immunity high enough to prevent sustained transmission of all three types of WPV, FRPV, or OPV-related virus. At this level of population immunity, each infection generates less than 1 new infection, but more than 0 new infections. The number of new infections generated per infection increases with the reversion stage due to increasing R0, but remains below 1 even for the R0 of WPVs and FRPVs. A fraction of OPV infections generated during the NID (from recipients and contacts) reaches the next reversion stage (stage 1), which also generates some transmission, but not as much as the OPV virus. Similarly, we find some EPI for subsequent stages, but the proportion rapidly decreases with the reversion stage. Fig. 6 only shows those reversion stages for which the EPI exceeds the transmission threshold EPI* at any time during the time period shown. Because of the high level of population immunity, none of the viruses sustain transmission following the initial pulse of OPV viruses during the NID, such that the EPI for each reversion stage rapidly drops below the threshold for transmission. Type 2 viruses achieve the highest reversion stage (i.e., stage 9) that exceeds the transmission thresholds following the NID, consistent with the assumption of faster reversion and higher relative R0 for type 2 (Table I). Although we see very low prevalence of FRPVs due to reversion from infections that started in lower stages of reversion, our model does not record any emergence or spread of FRPVs of any type over the full time period 1962–1997.
Fig. 6.
Behavior OPV-related virus in the model following bi-annual NIDs in Cuba, 1991–1992. Acronyms: NID = national immunization day; OPV = oral poliovirus vaccine; PV 1,2,3 = poliovirus type 1, 2, or 3, respectively.
The model for Cuba yields approximately 18 total VAPP cases (60% recipient VAPP) for all serotypes combined during 1963–1996, compared to 18 total reported VAPP cases.(92) As a direct result of the serotype distribution for recipient VAPP in the USA, our model yields mostly type 3 VAPP cases (59%) while investigators in Cuba reported the isolation of type 2 in 12 (71%) of the 17 VAPP cases with a single serotype isolated.(92) Table XIV compares the cumulative secondary OPV infection rates in the model based on the cumulative force of infection from all OPV-related viruses after the second NID round in 1991 with reported seropositivity levels among unvaccinated infants born after the second round of NIDs and tested before the next round.(48,95) Remarkably, the study conducted after the 1991 NID(48) found much higher seropositivity levels than the study conducted after the 1997 NID.(95) The fact that the 1991 NID targeted children 0–3 and 9 years of age and the 1997 NID targeted only children 0–2 and 9 years of age(92) may partly explain this difference. Moreover, the number of children in the study born immediately after the 1997 NID remained very small (n = 14) and only 3 (21.4%) had older siblings in the household.(95) We requested and failed to obtain information about siblings for the study conducted after the 1991 NID.(48) In any case, both studies found that secondary OPV spread ceases by 3 months after the second NID round. Our model, which does not track siblings or households but reflects the average force-of-infection for the entire mixing age group, also yields a very rapid drop in the force-of-infection of OPV-related viruses (see Appendix A5) and therefore in the cumulative exposure to those viruses over time after the second NID round. Transmission of all OPV-related viruses stops 115 days after the second NID round in 1991 (August 22), a little after the period when laboratories typically stop isolating OPV-related viruses from environmental samples in Cuba.(92) We did not obtain such high rates of secondary infection immediately after the second NID round as those reported by Más Lago et al.,(48) and we found that even with higher R0s and/or take rates, our model would not produce such high rates. We also did not obtain the contrasting relatively lower rates implied by Más Lago et al.,(95) and thus our model remains within the combined range of the divergent findings reported by these two studies. Given limitations in the data, we believe that intense household transmission due to a high proportion of infants in the study population with older siblings vaccinated during the NID may offer the most likely explanation for the 1991 data(48,95) and the discrepancy in the results for our model that does not explicitly differentiate household and community transmission but only reflects an average-based effect based on the age-heterogeneous mixing matrix.
3.6. Haiti
Fig. 7 shows the results for the cVDPV outbreak in Haiti in 2000–2001. The run-up to the outbreak includes the beginning of routine immunization in 1980 and NIDs in 1986, which leads to WPV elimination in 1989, consistent with the evidence (see Appendix A5).(96) As in most settings without active, systematic surveillance, the model produces much higher paralytic poliomyelitis incidence in the pre-eradication era than the reported numbers, but this remains consistent with the difference between the reported numbers of paralytic poliomyelitis cases and findings from lameness surveys before the GPEI organized active AFP surveillance in all countries.(16,115) Replicating this outbreak with the model proved challenging due to limited data on the true size of the outbreak and vaccination activities between the last reported WPV case in 1989 and the response to the cVDPV outbreak in 2000. Haiti reported no AFP cases at all in the years leading up to the outbreak and the 10 reported virologically-confirmed and polio-compatible outbreak cases may represent only 20% or less of true paralytic incidence for the outbreak.(97) Fig. 7(a) shows the prevalence of OPV-related viruses assuming significant partial coverage despite low coverage with the third dose in Haiti.(76,90) Unlike the results from the Cuba model (Fig. 6), we can discern continuous prevalence of OPV virus and viruses in early stages of reversion due to routine immunization and increasing prevalence of OPV-related viruses in high reversion stages as time since the last assumed NIDs in 1995 passes. The model then produces a relatively gradual outbreak, which gets curtailed by the response efforts initiated before the outbreak reaches its natural peak (Fig. 7(b)). The first FRPVs emerge in the model in the summer of 2000, and viruses in earlier reversion stages, but with elevated PIRs, already lead to an increasing number of nonrecipient type 1 VAPP cases (i.e., 1 in 1998, 3 in 1999, and 8 in 2000 before the FRPV emergence). The occurrence of cases before the first detected case remains likely for the actual outbreak.(97) The inclusion of much more heterogeneity would slow down the propagation of the outbreak and could accommodate earlier FRPV emergences without a very large undetected outbreak, but this requires better data on the population structure and immunization coverage in Haiti. The model produces no FRPV transmission for types 2 and 3. Given the low numbers of reported cases, we did not compare the reported age distribution of cases to that obtained by the model (Table XIII), which includes approximately 75% of cases in children younger than 5 years of age.
Fig. 7.
Results for the type 1 circulating VDPV emergence and outbreak in Haiti. (a) Behavior of OPV-related viruses. (b) Outbreak curve showing laboratory-confirmed VDPV and polio-compatible cases,(96) and modeled paralytic incidence for Haiti. Acronyms: AFP = acute flaccid paralysis; FRPV = fully-reverted poliovirus; OPV = oral poliovirus vaccine; PV1 = poliovirus type 1; tOPV = trivalent OPV; VDPV = vaccine-derived poliovirus.
3.7. Madura Island
Fig. 8 shows the results of the model for the 2005 type 1 cVDPV outbreak in Madura. Unlike Haiti, Madura conducted SIAs up to as recently as 3 years before the outbreak and nationwide coverage estimates remained higher than those in Haiti in the years leading up to the outbreak.(76,100) However, the outbreak primarily affected and probably emerged from a small number of rural subdistricts in Madura with very poor hygiene and very low coverage, based on convenience surveys conducted during outbreak investigations.(100) Consequently, our model focuses on the rural population of Madura and assumes: 1) a relatively high average R0 of 9 for a country with otherwise relatively good sanitary conditions, and 2) very low routine coverage in recent years of 15% with 4 doses and some additional partial coverage with fewer than 4 doses (Table X). With these assumptions, type 1 FRPVs emerge in the model in early 2005 and lead to a rapid initial growth of paralytic poliomyelitis incidence consistent with the data (Fig. 8). Several factors may explain the differences in total cases between our model and the reported data. First, the outbreak involved 10 polio-compatible AFP cases probably caused by the cVDPV,(100) and intensive outbreak investigation possibly missed some paralytic cases in isolated communities altogether. Second, health authorities conducted small-scale vaccination efforts in affected villages early during the outbreak (covering approximately 19,000 children) and several weeks before the NID, which probably slowed down the outbreak effectively before the NID.(100) Third, significant heterogeneity in the population of relatively isolated villages potentially slowed down the spread across the island or spared areas with better coverage altogether, while our model assumes uniformly low coverage for the entire rural population. Nevertheless, even without spatially homogeneous mixing, our model for OPV evolution appears to correctly reproduce the emergence of cVDPVs on the island of Madura in 2005 and the approximate outbreak curve. The model produces a total of 1.4 FRPVs for type 2 between 1981 and 1987 during times of very low routine coverage and a FRPV type 2 emergence in 2005 that translates into less than a case (i.e., cumulative paralytic incidence of 0.05). The model produces no FRPV transmission for type 3. Given that no surveillance for VDPVs existed in the 1980s, we cannot verify the correctness of this model behavior.
Fig. 8.
Laboratory-confirmed VDPV cases,(100) and modeled paralytic incidence for Madura. Acronyms: mOPV1 = monovalent OPV type 1; OPV = oral poliovirus vaccine; PV1 = poliovirus type 1; tOPV = trivalent OPV; VDPV = vaccine-derived poliovirus.
3.8. Northern Nigeria
Fig. 9 shows the behavior of all three types in the model of the NW zone of Nigeria during 2003–2011. In the absence of data by type and region, Appendix A5 includes the run-up, comparing the models with the approximate annual totals by type for the entire country.(42,101–103,105–109,116,117) Given the frequent isolation of orphan viruses from AFP cases (i.e., polioviruse isolations in the absence of isolations of genetically close progenies from other AFP cases) in parts of northern Nigeria, we suspect that the reported cases may represent as little as 50% of true cases in the early 2000s although with improvements in surveillance we believe reported cases probably represent a much higher percentage of actual cases since 2007 or later. Thus the overall level of incidence in the model appears consistent with the likely true level. To fit the model to produce plausible kinetics, we used detailed data about SIAs (Gacic-Dobo, 2009, personal communication) and varied the effective per-round impact () of rounds, as detailed in Appendix A4. This process produced estimates of the annual cumulative percent of missed children consistent with our understanding of the situation in northern Nigeria (Appendix A4). Overall, the model produced fits for types 1 and 3 that appear reasonable compared to annual averages and reproduced WPV2 elimination in 1999(2,118) (in the undervaccinated subpopulation) and subsequent emergence of FRPV2s in 2004. However, given that we do not characterize the geographic heterogeneity in the undervaccinated subpopulation but instead lump them all as one instantly mixing population (see methods section), we do not get the same extent of local VDPV2 outbreaks during 2006–2008 that probably occurred in reality.(101) With the absence of tOPV SIAs for an extended period of time during 2007–2009, the model accumulates sufficient susceptibles to allow an explosive outbreak in 2009. The more explosive nature of the outbreak results from the assumption of instantaneous mixing in each subpopulation (i.e., a more geographically scattered subpopulation would produce a more gradual outbreak). Nevertheless, the model adequately reproduces the cumulative total number of cases and occurrence of major peaks and troughs in incidence for each type.(42,106,108,111,116) This suggests that the model captures the overall impact of changing vaccination strategies (i.e., timing and vaccine choices for SIAs) on the average population immunity, and that the OPV evolution process approximates the behavior for type 2. The inclusion of at least 1 undervaccinated subpopulation proved important to reproduce the behavior of polioviruses in northern Nigeria.
Fig. 9.
Modeled paralytic incidence for northern Nigeria, 2003–2011. Acronyms: PV2 = poliovirus type 2; WPV1,3 = wild poliovirus type 1 or 3, respectively.
3.9. Northern India
Fig. 10 shows the results for the northern India model for 2002–2012 compared with the incidence of laboratory-confirmed cases.(112) Detailed data on WPV cases(112) allowed us to focus on the key WPV reservoirs of Bihar and WUP as separate subpopulations (see methods section) and to compare cases at a better resolution than annual aggregate case counts. In calibrating the model, we separately varied for each type and subpopulation the implied cumulative missed children with all SIA doses containing the given type. Appendix A5 shows the impact of the start of the routine immunization and SIAs on the reported incidence (for all of India) and on the modeled incidence (for Bihar and WUP only), showing overall similar patterns. For types 1 and 3, the model calibration process led to a good correspondence with the observed incidence. In Bihar, the model yields WPV3 elimination by 2004, as observed in reality, and after we reintroduce WPV3 in January 2007, we obtain an outbreak consistent with the reported outbreak.(112) WUP sustains indigenous WPV1 and WPV3 transmission through 2010, with the last WPV infections occurring in the undervaccinated subpopulation in November 2010, soon after the introduction of bOPV and assumed further improvements in reducing the percentage of missed children in the chronically undervaccinated subpopulation (see Appendix A4). Discrepancies in the size of outbreak peaks (e.g., WPV1 in 2002 and 2006) reflect the assumption of spatially homogeneous mixing within Bihar and within WUP, which clearly represents a simplification for the very large populations modeled for this situation. WUP and to some extent Bihar experienced a limited cVDPV2 outbreak during 2009–2010, although the origin of the VDPV emergence from within or outside these areas remains uncertain. Our model does not reproduce an indigenous emergence of FRPVs in either of these areas, consistent with the self-limiting nature of the observed VDPV2 event due to the uninterrupted policy of two annual tOPV NIDs.
4. DISCUSSION
Despite decades of intense study of polio, many aspects of poliovirus transmission and evolution remain highly uncertain and variable,(18,19) mainly due to the high fraction of asymptomatic infections, which makes it very challenging to observe transmissions. In addition, the inability to conduct ethical experiments with neurovirulent polioviruses in humans further limits observations. The detection of cVDPV outbreaks remains relatively recent and we do not know how well in vitro studies of vaccinederived polioviruses translate to neurovirulence and more importantly to transmissibility in humans.(17) Given the high number of uncertain model inputs related to poliovirus evolution and transmission, our iterative process to validate the model suggested the need to model as many situations as we did. Specifically, we did not obtain confidence in the model’s ability to adequately represent the behavior of OPV-related viruses in large populations until we were able to simultaneously generate cVDPV outbreaks in Haiti, Madura Island, and northern Nigeria while not seeing transmission of highly evolved viruses in the USA, the Netherlands, and Cuba.
We developed an expanded differential-equation-based (DEB) model for poliovirus transmission that we believe captures the most important features of poliovirus immunity and transmission to model the average behavior of poliovirus spread in large populations. Despite the uncertainty in model inputs and the simplifying assumptions of space-homogeneous mixing and mixing patterns between very wide age groups, the model appears to produce realistic behavior for endemic WPV transmission before vaccination, for the impact of OPV and IPV vaccination, and for WPV importations into populations with low immunity. With respect to the impact of IPV on transmission, we found more impact as the role of oropharyngeal transmission increases, and we expect that a better understanding of the importance of oropharyngeal transmission will help assess the potential impact of IPV in developing countries. The model also does not produce emergences of cVDPVs in situations in which they did not emerge and generates cVDPVs in situations of very low population immunity in which they did emerge. However, our model does not capture potentially important micro-dynamics that may affect the emergence and kinetics of VDPVs, in particular as it relates to the dichotomy between household and community transmission.(17) The process of calibrating our model indirectly accounts for these dynamics at the average level by adjusting average-based model inputs such as the relative R0 and timing of different reversion stages, which for individuals might occur faster, but for populations might not progress as fast because most viruses die out before gaining increased transmissibility. Interpretation of results from this DEB model must consider the limitations associated with the assumption of homogeneous, instantaneous mixing within subpopulations, which can imply rapid transmission across large populations. As seen in the context of modeling the Netherlands, northern Nigeria, and northern India, potential chronically undervaccinated subpopulations can play an important role in both outbreaks and sustained endemic transmission. For one of the nine situations (i.e., Albania), we faced very significant challenges to reproduce the run-up to the outbreak and could only approximate some of its features by making questionable assumptions about R0 differences, more heterogeneous age-mixing than in the other situations, and a disproportionately low immigration rate for fully susceptibles. The epidemiology for this event remains not fully understood,(47,57,82,83) and we could not fully resolve this situation in the model either. Possibly, some highly isolated communities may have missed vaccination and WPV exposure altogether for long periods of time, and some fraction did not get exposed to OPV in households when coverage improved in the 1980s. However, our model does not provide this level of granularity and data to support such hypothesis do not exist.
Our assumptions about the highly uncertain OPV evolution process might also significantly affect performance of the model. In our DEB model, we included multiple stages of reversion such that the implied distribution of the duration until full reversion remains centered around the assumed mean reversion times, but some fraction of OPV infections still revert to FRPV virtually immediately. If a true minimum time exists before an OPV virus can acquire the R0 of FRPV or typical homotypic WPVs, then a more discrete reversion process that one cannot easily implement in a strict DEB model may provide a more realistic approximation. In constructing the model, we considered and tested fixed-delay processes(33) for reversion, but for short reversion times this still did not produce results consistent with the evidence (e.g., emergence of VDPVs in places where they did not). Moreover, in reality, distributions exist for the time it takes for individual viruses to gain neurovirulence and transmissibility, and in this sense the high-order exponential delay process that we assumed provides a more realistic model than a fixed-delay process, even if we remain uncertain about the true distributional forms. Randomness both in the reversion process and contacts encountered by any individual OPV-related virus in a population or other unknown factors (e.g., recombination with nonpolio enteroviruses) may explain why cVDPV outbreaks did not occur in some places where we might expect them to occur based on population immunity alone (e.g., Albania, Haiti before 2000 or with type 2).
Individual-based, stochastic, and discrete poliovirus transmission models may draw from the model inputs and structure of immunity states developed for this DEB model given that it performs relatively well in several diverse situations. However, individual-based models face significant challenges with respect to specification of assumptions about the population structure, mixing, and transmission dynamics.(60,119) Thus, while individual-based models might yield important insights about the risk of cVDPV emergence in particular, they will face the same significant limitations in data and knowledge that arise from our inability to observe OPV evolution as it occurs, due in large part to the spread of OPV through asymptomatic infections.(17) On top of this, they also face challenges associated with specifying the appropriate population network structures and mixing rates, which depend on both the specific population and the nature of viral transmission. For large policy decisions, we believe that the average-based DEB approach offers sufficiently accurate results to assess the impact of the decisions on population immunity and expected poliomyelitis cases. Although the model reproduced poliovirus behavior in a large number of situations, this validation does not prove the correctness of all model inputs. Most inputs remain interdependent, and thus other combinations of inputs may lead to similar or better fits. For example, largely due to the absence of good data, we assumed no differences in duration and level of infectiousness between OPV and WPV. If important differences exist, then this means that the model requires a different relative R0 of OPV compared to WPV or other input to produce similar behavior. Thus, significant uncertainty remains related to poliovirus transmission and evolution and therefore uncertainty and sensitivity analyses of policy models remain critical to interpret results and identify drivers of uncertainty.(14,120)
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the U.S. Centers for Disease Control and Prevention (CDC) for supporting the development of an expanded poliovirus transmission model under Cooperative Agreement U66IP000519-01. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. The authors thank Drs. Gregory Armstrong, Concepción Estívariz, Johannes Everts, Marta Gacic-Dobo, Alex Gasasira, Olen Kew, Benjamin Lopman, Patrick O’Connor, Becky Prevots, Roland Sutter, Rudi Tangermann, Iris Tetford, Gregory Wallace, Chris Wolff, and the Global Polio Laboratory Network for information.
REFERENCES
- 1.World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000 (Resolution 41.28). Geneva: World Health Organization, 1988. Available at: http://www.who.int/csr/ihr/polioresolution4128en.pdf, accessed June 12, 2012. [Google Scholar]
- 2.World Health Organization. Transmission of wild poliovirus type 2 – apparent global interruption. Weekly Epidemiological Record, 2001; 76:95–97. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Outbreaks following wild poliovirus importations—Europe, Africa, and Asia, January 2009–September 2010. Morbidity and Mortality Weekly Report, 2010; 59(43):1393–1399. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses–worldwide, July 2009–March 2011. MMWR Morb Mortal Wkly Rep, 2011; 60(25):846–850. [PubMed] [Google Scholar]
- 5.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Cochi SL. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Analysis, 2013; 33(4):647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SG, Kim J-H, Cochi SL. Pre-eradication vaccine policy options for poliovirus infection and disease control. Risk Analysis, 2013; 33(4):516–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson KM, Duintjer Tebbens RJ. Current polio global eradication and control policy options: Perspectives from modeling and prerequisites for OPV cessation. Expert Reviews of Vaccines, 2012; 11(4):449–459. [DOI] [PubMed] [Google Scholar]
- 8.Sangrujee N, Duintjer Tebbens RJ, Cáceres VM, Thompson KM. Policy decision options during the first 5 years following certification of polio eradication. Medscape General Medicine, 2003; 5(4):35. [PubMed] [Google Scholar]
- 9.Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Analysis, 2006; 26(6):1423–1440. [DOI] [PubMed] [Google Scholar]
- 10.Thompson KM, Duintjer Tebbens RJ. Eradication versus control for poliomyelitis: An economic analysis. Lancet, 2007; 369(9570):1363–1371. [DOI] [PubMed] [Google Scholar]
- 11.Thompson KM, Duintjer Tebbens RJ, Pallansch MA, Kew OM, Sutter RW, Aylward RB, Watkins M, Gary HE Jr., Alexander JP Jr., Jafari H, Cochi SL. The risks, costs, and benefits of possible future global policies for managing polioviruses. American Journal of Public Health, 2008; 98(7):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson KM, Duintjer Tebbens RJ. Using system dynamics to develop policies that matter: Global management of poliomyelitis and beyond. System Dynamics Review, 2008; 24(4):433–449. [Google Scholar]
- 13.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Sutter RW, Thompson KM. A dynamic model of poliomyelitis outbreaks: Learning from the past to help inform the future. American Journal of Epidemiology, 2005; 162(4):358–372. [DOI] [PubMed] [Google Scholar]
- 14.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Sutter RW, Aylward RB, Watkins M, Gary HE Jr., Alexander JP Jr., Jafari H, Cochi SL, Thompson KM. Uncertainty and sensitivity analyses of a decision analytic model for post-eradication polio risk management. Risk Analysis, 2008; 28(4):855–876. [DOI] [PubMed] [Google Scholar]
- 15.Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Analysis, 2006; 26(6):1541–1556. [DOI] [PubMed] [Google Scholar]
- 16.Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Linkins J, Sutter RW, Aylward RB, Thompson KM. Economic analysis of the Global Polio Eradication Initiative. Vaccine, 2011; 29(2):334–343. [DOI] [PubMed] [Google Scholar]
- 17.Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste MS, Diop OM, Wassilak SGF, Cochi SL, Thompson KM. Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Analysis, 2013; 33(4):680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Expert review on poliovirus immunity and transmission. Risk Analysis, 2013; 33(4):544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Analysis, 2013; 33(4):606–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine—Live. Pp. 631–686 in Plotkin SA, Orenstein WA, Offit PA (eds). Vaccines. 5th ed. Philadelphia: Saunders Elsevier, 2008. [Google Scholar]
- 21.Melnick JL. Poliovirus and other enteroviruses. Pp. 583–663 in Evans AS, Kaslow RA (eds). Viral Infections of Humans: Epidemiology and Control. 4th ed. New York: Plenum Medical, 1997. [Google Scholar]
- 22.Minor PD. The molecular biology of poliovaccines. Journal of General Virology, 1992; 73(pt. 12):3065–3077. [DOI] [PubMed] [Google Scholar]
- 23.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annual Review of Microbiology, 2005; 59:587–635. [DOI] [PubMed] [Google Scholar]
- 24.Chumakov K, Ehrenfeld E, Wimmer E, Agol VI. Vaccination against polio should not be stopped. Nature Reviews Microbiology, 2007; 5(12):952–958. [DOI] [PubMed] [Google Scholar]
- 25.Nathanson N, Kew OM. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. American Journal of Epidemiology, 2010; 172(11):1213–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichner M, Dietz K. Eradication of poliomyelitis: When can one be sure that polio virus transmission has been terminated? American Journal of Epidemiology, 1996; 143(8):816–822. [DOI] [PubMed] [Google Scholar]
- 27.Eichner M, Hadeler KP. Deterministic models for the eradication of poliomyelitis: Vaccination with the inactivated (IPV) and attenuated (OPV) polio virus vaccine. Mathematical Biosciences, 1995; 127(2):149–166. [DOI] [PubMed] [Google Scholar]
- 28.Elveback LR, Ackerman E, Gatewood L, Fox JP. Stochastic two-agent epidemic simulation models for a community of families. American Journal of Epidemiology, 1971; 93(4):267–280. [DOI] [PubMed] [Google Scholar]
- 29.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: Implications for the global poliomyelitis eradication initiative. American Journal of Epidemiology, 1999; 150(10):1001–1021. [DOI] [PubMed] [Google Scholar]
- 30.Agol VI. Vaccine-derived polioviruses. Biologicals 2006; 34(2):103–108. [DOI] [PubMed] [Google Scholar]
- 31.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. New York: Oxford University Press, 1991. [Google Scholar]
- 32.Lloyd AL. Realistic distributions of infectious periods in epidemic models: Changing patterns of persistence and dynamics. Theoretical Population Biology 2001; 60(1):59–71. [DOI] [PubMed] [Google Scholar]
- 33.Sterman J Business Dynamics: Systems Thinking and Modeling for a Complex World. Boston, MA: McGraw-Hill, 2001. [Google Scholar]
- 34.Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA, Sutter RW. Vaccine policy changes and epidemiology of poliomyelitis in the United States. Journal of the American Medical Association, 2004; 292(14): 1696–1701. [DOI] [PubMed] [Google Scholar]
- 35.Edmunds WJ, Gay NJ, Kretzschmar M, Pebody RG, Wachmann H. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: Implications for modelling studies. Epidemiology and Infection, 2000; 125(3):635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diekmann O, Heesterbeek JA, Metz JA. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology, 1990; 28(4):365–382. [DOI] [PubMed] [Google Scholar]
- 37.Jacquez JA, Simon CP, Koopman J, Sattenspiel L, Perry T. Modeling and analyzing HIV transmission: The effect of contact patterns. Mathematical Biosciences, 1988; 92:119–99. [Google Scholar]
- 38.Glasser J, Feng Z, Moylan A, Del Valle S, Castillo-Chavez C. Mixing in age-structured population models of infectious diseases. Mathematical Biosciences, 2012; 235(1):1–7. [DOI] [PubMed] [Google Scholar]
- 39.Towers S, Feng Z. Social contact patterns and control strategies for influenza in the elderly. Mathematical Biosciences, 2012; 240(2):241–249. [DOI] [PubMed] [Google Scholar]
- 40.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: Review. Reviews of Infectious Diseases, 1991; 13:926–939. [DOI] [PubMed] [Google Scholar]
- 41.Plotkin SA, Vidor E. Poliovirus vaccine—Inactivated. Pp. 605–630 in Plotkin SA, Orenstein WA, Offit PA (eds). Vaccines. 5th ed. Philadelphia: Saunders Elsevier, 2008. [Google Scholar]
- 42.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2011–September 2012. Morbidity and Mortality Weekly Report, 2012; 61(44):899–904. [PubMed] [Google Scholar]
- 43.Dowdle WR, van der Avoort HGAM, de Gourville EM, Delpeyroux F, Deshpande JM, Hovi T, Martín J, Pallansch MA, Kew OM, Wolff C Containment of polioviruses after eradication: Characterizing risk to improve management. Risk Analysis, 2006; 26(6):1449–1469. [DOI] [PubMed] [Google Scholar]
- 44.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, Aylward RB, Thompson KM. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Analysis, 2006; 26(6):1471–1505. [DOI] [PubMed] [Google Scholar]
- 45.Savilahti E, Klemola T, Carlsson B, Mellander L, Stenvik M, Hovi T. Inadequacy of mucosal IgM antibodies in selective IgA deficiency: Excretion of attenuated polio viruses is prolonged. Journal of Clinical Microbiology, 1988; 8(2): 89–94. [DOI] [PubMed] [Google Scholar]
- 46.Böttiger M Polio immunity to killed vaccine: An 18-year follow-up. Vaccine, 1990; 8(5):443–445. [DOI] [PubMed] [Google Scholar]
- 47.Squarcione S, Germinario C, Iandolo E, Lo Caputo S, Bergamini F, Profeta M, Greco D, Quarto M, Barbuti S. Seroimmunity to poliomyelitis in an Albanian immigrant population. Vaccine, 1992; 10(12):853–856. [DOI] [PubMed] [Google Scholar]
- 48.Más Lago P, Bravo JR, Andrus JK, Comellas MM, Galindo MA, de Quadros CA, Bell E. Lesson from Cuba: Mass campaign administration of trivalent oral poliovirus vaccine and seroprevalence of poliovirus neutralizing antibodies. Bulletin of the World Health Organization, 1994; 72(2):221–225 [PMC free article] [PubMed] [Google Scholar]
- 49.Pirez MC, Olivera I, Diabarboure H, Montano A, Baranano R, Badia F, Bonnet MC. Seroprevalence of anti-polio antibodies in a population 7 months to 39 years of age in Uruguay: Implications for future polio vaccination strategies. Vaccine, 2009; 27(20):2689–2694. [DOI] [PubMed] [Google Scholar]
- 50.Prevots DR, Sutter RW, Strebel PM, Weibel RE, Cochi SL. Completeness of reporting for paralytic poliomyelitis, United States, 1980 through 1991. Archives of Pediatrics and Adolescent Medicine, 1994; 148:479–185. [DOI] [PubMed] [Google Scholar]
- 51.Koopman J Modeling infection transmission. Annual Reviews of Public Health, 2004; 25:303–326. [DOI] [PubMed] [Google Scholar]
- 52.Cáceres VM, Sutter RW. Sabin monovalent oral polio vaccines: Review of past experiences and their potential use after polio eradication. Clinical Infectious Diseases, 2001; 33(4):531–541. [DOI] [PubMed] [Google Scholar]
- 53.Fine PEM, Carneiro IAM. Transmissibility and Persistence of Oral Poliovirus Vaccine Viruses: Implications for the Global Poliomyelitis Eradication Initiative. London: Infectious Disease Epidemiology Unit, Department of Infectious and Tropical Diseases, London School of Hygiene, 1998. [Google Scholar]
- 54.Patriarca PA, Sutter RW, Oostvogel PM. Outbreaks of paralytic poliomyelitis, 1976–1995. Journal of Infectious Diseases, 1997; 175(Suppl 1):S165–S172. [DOI] [PubMed] [Google Scholar]
- 55.Francis T Jr., Korns RF, Voight RB, Boisen M, Hemphill FM, Napier JA, Tolchinsky E. An evaluation of the 1954 poliomyelitis vaccine trials. Summary report. American Journal of Public Health, 1955; 45 (Part II, May Supplement):1–51. [PMC free article] [PubMed] [Google Scholar]
- 56.Forbes C, Fernando N, de la Motte P, Mendis N. A survey to determine the immunity status and antibody creating effect of oral polio vaccine. Journal of Tropical Medicine and Hygiene, 1980; 83(3):109–113. [PubMed] [Google Scholar]
- 57.Prevots DR, Ciofi degli Atti ML, Sallabanda A, Diamanti E, Aylward RB, Kakariqqi E, Fiore L, Ylli A, van der Avoort HG, Sutter RW, Tozzi AE, Panei P, Schinaia N, Genovese D, Oblapenko G, Greco D, Wassilak SG. Outbreak of paralytic poliomyelitis in Albania, 1996: High attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clinical Infectious Diseases, 1998; 26(2):419–425. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine. Factors affecting the immunogenicity of oral poliovirus vaccine: A prospective evaluation in Brazil and the Gambia. World Health Organization collaborative study group on oral poliovirus vaccine. Journal of Infectious Diseases, 1995; 171(5):1097–1106. [DOI] [PubMed] [Google Scholar]
- 59.Triki H, Abdallah M, Ben Aissa R, Bouratbine A, Kacem MBA, Bouraoui S, Koubaa C, Zouari S, Mohsni E, Crainic R, Dellagi K. Influence of host related factors on the antibody response to trivalent oral polio vaccine in Tunisian infants. Vaccine, 1997; 15(10):1123–1129. [DOI] [PubMed] [Google Scholar]
- 60.Rahmandad H, Hu K, Duintjer Tebbens RJ, Thompson KM. Development of an individual-based model for polioviruses: Implications of the selection of network type and outcome metrics. Epidemiology and Infection, 2010; 139(6):836–848. [DOI] [PubMed] [Google Scholar]
- 61.UN Population Division. World population prospects population database: The 2010 revision population database. 2012: http://esa.un.org/wpp/unpp/panelindicators.htm, accessed April 2, 2012.
- 62.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J, Heijne J, Sadkowska-Todys M, Rosinska M, Edmunds WJ. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Medecine, 2008; 5(3):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horby P, Pham QT, Hens N, Nguyen TT, Le QM, Dang DT, Nguyen ML, Nguyen TH, Alexander N, Edmunds WJ, Tran ND, Fox A. Social contact patterns in Vietnam and implications for the control of infectious diseases. PLoS One, 2011; 6(2):e16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen RT, Hausinger S, Dajani AS, Hanfling M, Baughman AL, Pallansch MA, Patriarca PA. Seroprevalence of antibody against poliovirus in inner-city preschool children. Journal of the American Medical Association, 1996; 275(21):1639–1645. [PubMed] [Google Scholar]
- 65.Offit PA. The Cutter Incident: How America’s First Polio Vaccine Led to the Growing Vaccine Crisis. New Haven: Yale University Press, 2005. [Google Scholar]
- 66.Oshinsky DM. Polio. An American Story. New York: Oxford University Press; 2005. [Google Scholar]
- 67.Nathanson N, Martin JR. The epidemiology of poliomyelitis: Enigmas surrounding its appearance, epidemicity, and disappearance. American Journal of Epidemiology, 1979; 110(6):672–692. [DOI] [PubMed] [Google Scholar]
- 68.Chin TD, Marine WM, Hall EC, Gravelle CR, Speers JF. Poliomyelitis in Des Moines, Iowa, 1959. The influence of Salk vaccination on the epidemic pattern and the spread of the virus in the community. American Journal of Hygiene, 1961; 74:67–94. [DOI] [PubMed] [Google Scholar]
- 69.Marine WM, Chin TD, Gravelle CR. Limitation of fecal and pharyngeal poliovirus excretion in Salk-vaccinated children. A family study during a type 1 poliomyelitis epidemic. American Journal of Hygiene, 1962;76:173–195. [DOI] [PubMed] [Google Scholar]
- 70.Nathanson N Eradication of poliomyelitis in the United States. Reviews of Infectious Diseases, 1982; 4(5):940–950. [DOI] [PubMed] [Google Scholar]
- 71.Morris L, Witte JJ, Gardner P, Miller G, Henderson DA. Surveillance of poliomyelitis in the United States, 1962–65. Public Health Reports, 1967; 82(5):417–427. [PMC free article] [PubMed] [Google Scholar]
- 72.Simpson DM, Ezzati-Rice TM, Zell ER. Forty years and four surveys: How does our measuring measure up? American Journal of Preventive Medicine, 2001; 20(Suppl. 4):6–14. [DOI] [PubMed] [Google Scholar]
- 73.National Communicable Disease Center. Recommendation of the Public Health Advisory Committee on Immunization Practices—Poliomyelitis Vaccines. Morbidity and Mortality Weekly Report, 1967; 16(33):278–279. [Google Scholar]
- 74.Centers for Disease Control and Prevention. Past immunization schedules, 2012. Available at: http://www.cdc.gov/vaccines/schedules/past.html, accessed August 24, 2012.
- 75.Zell ER, Dietz V, Stevenson JM, Cochi SL, Bruce RH. Low vaccination levels of US preschool and school-age children: Retrospective assessments of vaccination coverage, 1991–1992. Journal of the American Medical Association, 1994; 271(11):833–839. [PubMed] [Google Scholar]
- 76.World Health Organization. WHO/UNICEF estimated coverage time series. WHO and UNICEF, 2012. Available at: http://www.who.int/entity/immunization monitoring/data/coverageestimatesseries.xls, accessed April 4, 2012. [Google Scholar]
- 77.Hatzandrieu EJ, Palmer CS, Halpern MT. A Cost Benefit Analysis of the OPV Vaccine. Atlanta: Batelle; Centers for Disease Control and Prevention, 1994. [Google Scholar]
- 78.Hall WJ, Nathanson N, Langmuir AD. The age distribution of poliomyelitis in the United States in 1955. American Journal of Hygiene, 1957; 66(214–34). [DOI] [PubMed] [Google Scholar]
- 79.Oostvogel P, van Wijngaarden J, van der Avoort HG, Mulders MN, Conyn-van Spaendonck MA, Rümke H, van Steenis G, van Loon AM. Poliomyelitis outbreak in an unvaccinated community in the Netherlands, 1992–3. Lancet, 1994; 344(8923): 665–670. [DOI] [PubMed] [Google Scholar]
- 80.Bijkerk H. Surveillance and control of poliomyelitis in the Netherlands. Reviews of Infectious Diseases, 1984; 6(Suppl. 2):S451–S456. [DOI] [PubMed] [Google Scholar]
- 81.von Magnus H, Petersen I. Vaccination with inactivated poliovirus vaccine and oral poliovirus vaccine in Denmark. Reviews of Infectious Diseases, 1984; 6 (Suppl 2):S471–S474. [DOI] [PubMed] [Google Scholar]
- 82.Diamanti E, Ibrahimi B, Tafaj F, Mezini E, Dodbiba A, Dobi V, Catone S, Genovese D, Simeoni P, Fiore L. Surveillance of suspected poliomyelitis in Albania, 1980–1995: Suggestion of increased risk of vaccine associated poliomyelitis. Vaccine 1998; 16(9):940–948. [DOI] [PubMed] [Google Scholar]
- 83.Fiore L, Genovese D, Diamanti E, Catone S, Ridolfi B, Ibrahimi B, Konomi R, van der Avoort HG, Hovi T, Crainic R, Simeoni P, Amato C. Antigenic and molecular characterization of wild type 1 poliovirus causing outbreaks of poliomyelitis in Albania and neighboring countries in 1996. Journal of Clinical Microbiology, 1998; 36(7):1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.State Committee on Statistics of the Repubic of Tajikistan. Multiple Indicator Cluster Survey 2005, Final Report. Dushanbe, Tajikistan: State Committee on Statistics of the Repubic of Tajikistan, 2007.
- 85.State Committee on Statistics of the Repubic of Tajikistan. Tajikistan Living Standards Measurement Survey 2007. Dushanbe, Tajikistan: State Committee on Statistics of the Repubic of Tajikistan, 2009.
- 86.UNICEF. Multiple indicator cluster survey, Tajikistan, April 2000.2000. Available at: http://www.childinfo.org/files/tajikistan.pdf, accessed February 11, 2013. [Google Scholar]
- 87.World Health Organization. Incidence series .2012. Available at: http://www.who.int/entity/immunization monitoring/data/incidence series.xls, accessed April 6, 2012.
- 88.Measure DHS. Demographic and health surveys, Nigeria 1990, 1999, 2003, and 2008, 2012. Available at: http://measuredhs.com/, accessed August 20, 2012.
- 89.Measure DHS. Demographic and health surveys, India, 1992–93, 1998–99, 2005–06, 2013. Available at: http://measuredhs.com/, accessed February 11, 2013. [Google Scholar]
- 90.Measure DHS. Demographic and health surveys, Haiti, 1994–95, 2000, and 2005–06, 2013. Available at: http://measuredhs.com/, accessed February 11, 2013.
- 91.World Health Organization and UNICEF. Tajikistan: WHO and UNICEF estimates of immunization coverage: 2011 revision, 2012. Available at: http://www.who.int/immunization monitoring/data/tjk.pdf, accessed February 11, 2013.
- 92.Más Lago P Eradication of poliomyelitis in Cuba: A historical perspective. Bulletin of the World Health Organization, 1999; 77(8):681–687. [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez Cruz R Cuba: Mass polio vaccination program, 1962–1982. Reviews of Infectious Diseases, 1984; 6(Suppl 2):S408–S412. [PubMed] [Google Scholar]
- 94.Más Lago P, Gary HE Jr., Pérez LS, Cáceres VM, Olivera, Puentes RP, Corredor MB, Jímenez P, Pallansch MA, González Cruz R. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. International Journal of Epidemiology, 2003; 32:772–777. [DOI] [PubMed] [Google Scholar]
- 95.Más Lago P, Cáceres VM, Galindo MA, Gary HE Jr., Valcarcel M, Barrios J, Sarmiento L, Avalos I, Bravo JA, Palomera R, Bello M, Sutter RW, Pallansch MA, de Quadros CA. Persistence of vaccine-derived poliovirus following a mass vaccination campaign in Cuba: Implications for stopping polio vaccination after global eradication. International Journal of Epidemiology, 2001; 30:1029–1034. [DOI] [PubMed] [Google Scholar]
- 96.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Garib Z, Andre J, Blackman E, Freeman CJ, Jorba J, Sutter R, Tambini G, Venczel L, Pedreira C, Laender F, Shimizu H, Yoneyama T, Miyamura T, van Der Avoort H, Oberste MS, Kilpatrick D, Cochi S, Pallansch M, de Quadros C. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science, 2002; 296(5566):356–359. [DOI] [PubMed] [Google Scholar]
- 97.Wringe A, Fine PE, Sutter RW, Kew OM. Estimating the extent of vaccine-derived poliovirus infection. PLoS One, 2008; 3(10):e3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olive JM, Risi JB Jr., de Quadros CA. National immunization days: Experience in Latin America. Journal of Infectious Diseases, 1997; 175 (Suppl 1):S189–S193. [DOI] [PubMed] [Google Scholar]
- 99.de Quadros CA, Andrus JK, Olive J-M, Guerra de Macedo C, Henderson DA. Polio eradication from the Western Hemisphere. Annual Reviews of Public Health, 1992; 13:239–252. [DOI] [PubMed] [Google Scholar]
- 100.Estívariz CF, Watkins M, Handoko D, Rusipah R, Deshpande J, Rana BJ, Irawan E, Widhiastuti D, Pallansch MA, Thapa A, Imari S. A large vaccine-derived poliovirus outbreak on Madura Island—Indonesia, 2005. Journal of Infectious Diseases, 2008; 197(3):347–354. [DOI] [PubMed] [Google Scholar]
- 101.Wassilak SGF, Pate MA, Wannemuehler K, Jenks J, Burns C, Chenoweth P, Abanida EA, Adu F, Baba M, Gasasira A, Iber J, Mkanda P, Williams AJ, Shaw J, Pallansch MA, Kew OM. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: Emergence and widespread circulation in an underimmunized population. Journal of Infectious Diseases, 2011; 203(7):898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2002–March 2003. Morbidity and Mortality Weekly Report, 2003; 52(24):567–570. [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2003–March 2004. Morbidity and Mortality Weekly Report, 2004; 53(16):343–346. [PubMed] [Google Scholar]
- 104.Nigeria/Africa Masterweb. Nigeria 2006 census figures, 2013. Available at: http://www.nigeriamasterweb.com/Nigeria06CensusFigs.html, accessed February 11, 2013.
- 105.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2004–July 2005. Morbidity and Mortality Weekly Report, 2005; 54(35):873–877. [PubMed] [Google Scholar]
- 106.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, 2005–2006. Morbidity and Mortality Weekly Report 2007; 56(12):278–281. [PubMed] [Google Scholar]
- 107.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2007–August 12, 2008. Morbidity and Mortality Weekly Report, 2008; 57(34):942–946. [PubMed] [Google Scholar]
- 108.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2008 – July, 2009. Morbidity and Mortality Weekly Report, 2009; 58(41):1150–1154. [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2009–June 2010. Morbidity and Mortality Weekly Report, 2010; 59(26):802–807. [PubMed] [Google Scholar]
- 110.Grassly NC, Wenger J, Durrani S, Bahl S, Deshpande JM, Sutter RW, Heymann DL, Aylward RB. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: A case-control study. Lancet, 2007; 369(9570):1356–1362. [DOI] [PubMed] [Google Scholar]
- 111.World Health Organization. Global Polio Eradication Initiative—Cases of wild poliovirus by country and by year 2000–2011, 2012. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpolioviruslist.aspx, accessed May 13, 2012.
- 112.South East Asia Regional WHO Office. Immunization and vaccine development—Surveillance, 2011. Available at: http://www.searo.who.int/EN/Section1226/Section1635.htm, accessed January 17, 2011.
- 113.Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine—Live. Pp. 651–705 in Plotkin SA, Orenstein WA (eds). Vaccines. 4th ed. Philadelphia: W.B. Saunders, 2004. [Google Scholar]
- 114.Oostvogel P, Rumke H, Conyn-Van Spaendonck MA, van der Avoort HG, Leeuwenburg J, van Loon AM. Poliovirus circulation among schoolchildren during the early phase of the 1992–1993 poliomyelitis outbreak in the Netherlands. Journal of Infectious Diseases, 2001; 184(11):1451–1455. [DOI] [PubMed] [Google Scholar]
- 115.Bernier RH. Some observations on poliomyelitis lameness surveys. Reviews of Infectious Diseases, 1984; 6 (Suppl 2):S371–S375. [DOI] [PubMed] [Google Scholar]
- 116.World Health Organization. Circulating vaccine-derived poliovirus (cVDPV) 2000–2012, 2012. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Circulatingvaccinederivedpoliovirus.aspx, accessed August 22, 2012.
- 117.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, 1996–1998. Morbidity and Mortality Weekly Report, 1999; 48(15):312–316. [PubMed] [Google Scholar]
- 118.World Health Organization. Progress towards global poliomyelitis eradication—The approaching extinction of wild poliovirus type 2. Weekly Epidemiological Record, 1999; 74(34):286–288. [PubMed] [Google Scholar]
- 119.Rahmandad H, Sterman J. Heterogeneity and network structure in the dynamics of diffusion: Comparing agent-based and differential equation models. Management Science, 2008; 54(5):998–1014. [Google Scholar]
- 120.Duintjer Tebbens RJ, Thompson KM, Hunink M, Mazzuchi TM, Lewandowski D, Kurowicka D, Cooke RM. Uncertainty and sensitivity analyses of a dynamic economic evaluation model for vaccination programs. Medical Decision Making, 2008; 28(2):182–200. [DOI] [PubMed] [Google Scholar]
- 121.World Health Organization. Summary of Discussions and Recommendations, 16th Informal Consultation of the Global Polio Laboratory Network, 22–23 September 2010, WHO/HQ. Geneva: World Health Organization, 2010. [Google Scholar]
- 122.Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. Journal of Virology, 2008; 82(9):4429–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Horstmann DM, Paul JR. The incubation period in human poliomyelitis and its implications. Journal of the American Medical Association, 1947; 135(1):11–14. [DOI] [PubMed] [Google Scholar]
- 124.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Angola, Democratic Republic of Congo, Ethiopia, and Nigeria, January 2000–July 2001. Morbidity and Mortality Weekly Report, 2001; 50(38): 826–829. [PubMed] [Google Scholar]
- 125.Government of India. 2011 census data. Office of the General & Census Commission, Ministry of Home Affairs, Governement of India, 2013. Available at: http://censusindia.gov.in/, accessed February 11, 2013. [Google Scholar]
- 126.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—India, January 2007–May 2009. Morbidity and Mortality Weekly Report, 2010; 58(26):718–723. [PubMed] [Google Scholar]
- 127.World Health Organization. Progress towards poliomyelitis eradication in India, January 2005 to June 2006. Weekly Epidemiological Record, 2006; 81(29):286–291. [PubMed] [Google Scholar]
- 128.World Health Organization. Progress towards poliomyelitis eradication in India, January 2007–May 2009. Weekly Epidemiological Record, 2009; 84(28):281–287. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.