Abstract
Background
Avelumab + axitinib was approved for advanced renal cell carcinoma (aRCC) in Japan in December 2019. We report long-term real-world outcomes with first-line avelumab + axitinib from the J-DART2 study in Japan.
Methods
J-DART2 was a multicenter, noninterventional, retrospective study examining clinical data from patients with curatively unresectable locally advanced or metastatic RCC who started treatment with first-line avelumab + axitinib in Japan between December 2019 and October 2022. Endpoints included patient characteristics, treatment patterns, and outcomes.
Results
Data from 150 patients across 19 sites were analyzed; median follow-up was 18.7 months (95% CI, 16.3–20.6 months). Median age was 70.5 years; 26.0% of patients were aged ≤64 years, 42.7% were aged 65–74 years, and 31.3% were aged ≥75 years. International Metastatic RCC Database Consortium risk was favorable in 26.0%, intermediate in 54.7% (1 risk factor in 30.7%; 2 risk factors in 24.0%), and poor in 19.3% of patients. Median progression-free survival (PFS) was 17.1 months, with 1- and 2-year PFS rates of 57.7% and 37.5%, respectively. Median overall survival (OS) was not reached, with 1- and 2-year OS rates of 90.6% and 84.7%, respectively. Objective response rate was 53.3%; disease control rate was 88.9%. Outcomes were similar across age groups, including patients aged ≥75 years.
Conclusions
J-DART2 is the largest retrospective study to report long-term real-world outcomes in patients with aRCC treated with avelumab + axitinib in Japan. Findings were similar to those observed in previous studies and support the benefit of avelumab + axitinib in clinical practice in Japan.
Keywords: Renal cell carcinoma, Avelumab, Axitinib, Real world, Retrospective, Japan
Introduction
The kidney and other urinary organs were the ninth most common cancer site in Japan in 2023, accounting for 31,500 new cases and 10,100 deaths [1]. Renal cell carcinoma (RCC) accounts for approximately 90% of kidney cancers [2]. Although the incidence of RCC is lower in Japan than in Western countries, it continues to increase annually [3, 4]. Based on results from several clinical trials, international guidelines recommend immune checkpoint inhibitor (ICI)-based regimens as the first-line (1L) standard-of-care treatment for advanced RCC (aRCC), including combination treatment with an ICI and a tyrosine kinase inhibitor (TKI) [2, 5–10].
Combination treatment with avelumab, an anti-PD-L1 monoclonal antibody, and axitinib, a multitargeted TKI that inhibits vascular endothelial growth factor receptors, is approved as a 1L treatment for patients with aRCC in various countries worldwide [11–15]. This approval was based on results from the phase 3 JAVELIN Renal 101 trial (NCT02684006), which demonstrated significantly longer median progression-free survival (PFS) and a higher objective response rate (ORR) with avelumab + axitinib vs. sunitinib, the prior standard of care (median PFS: 13.9 vs. 8.5 months, respectively; hazard ratio [HR], 0.67 [95% confidence interval (CI), 0.568–0.785], p < 0.0001; ORR, 59.3% vs. 31.8%) [16–18]. In a subgroup analysis of patients from JAVELIN Renal 101 who were enrolled in Japan (n = 67), avelumab + axitinib also showed longer PFS and a higher ORR vs. sunitinib (median PFS: 16.6 vs. 11.2 months, respectively; HR, 0.66 (95% CI, 0.296–1.464); ORR, 60.6% vs. 17.6%) [19]. Based on these findings, avelumab + axitinib was the first anti-PD-L1 ICI + TKI combination treatment approved for patients with aRCC in Japan in December 2019 [15]. The Japanese Urological Association clinical practice guidelines also recommended avelumab + axitinib as 1L treatment for patients with clear cell RCC in 2022 [9].
The incidence of cancer increases as populations age, with >50% of patients with cancer in high-income countries aged >70 years. Japan has a rapidly aging population, with a higher proportion of individuals aged ≥65 years compared with most countries [20]. It has been hypothesized that older patients may benefit less from immunotherapy because of immune senescence—a decline in immune activity that hinders the ability to combat carcinogenesis and promotes cancer development [21, 22]. A subgroup analysis from JAVELIN Renal 101 showed favorable efficacy and consistent tolerability with avelumab + axitinib vs. sunitinib across age groups, including patients aged ≥75 years [23]. Real-world data are needed to assess the effectiveness of 1L avelumab + axitinib in patients with aRCC receiving routine clinical care in Japan, including older patients.
In the real-world J-DART study (NCT05012865), a clinically meaningful benefit was observed in patients (N = 48) with aRCC treated with 1L avelumab + axitinib 1 year after its approval in Japan, including patients aged <75 years or ≥75 years. While J-DART contributed to the understanding of real-world treatment patterns of avelumab + axitinib soon after its approval, the study was limited by small patient numbers [24]. In addition, a post-marketing surveillance (PMS) study in Japan confirmed the acceptable safety and tolerability of avelumab + axitinib in patients with aRCC treated in clinical practice [25]. Here we report findings from the larger observational J-DART2 study (N = 150), which examined long-term, real-world baseline characteristics and treatment outcomes in patients with aRCC treated with 1L avelumab + axitinib and followed for ≥2 years in Japan. In addition, we report results from subgroup analyses in patients aged ≤64 years, 65–74 years, or ≥75 years.
Patients and methods
Study design
J-DART2 (NCT05650164) was a multicenter, observational, retrospective study performed at 19 sites in Japan. Clinical data from patients with aRCC who started treatment with 1L avelumab + axitinib between 20 December 2019 (approval date) and 17 October 2022 were analyzed. The observation period was from the date of the first prescription until 31 October 2022. Data were collected from patient medical records within the follow-up period. All decisions regarding the treatment and clinical management of patients were made by the investigator as part of standard clinical care in a real-world setting and irrespective of the patient’s participation in the study. Ethical review boards at all study sites approved the study protocol and related documentation. The study conduct complied with the Declaration of Helsinki and applicable local laws in Japan.
Patients
Patients were aged ≥18 years and received 1L avelumab + axitinib for curatively unresectable locally advanced or metastatic RCC (based on the General Rule for Clinical and Pathological Studies on Renal Cell Carcinoma [5th edition]). For surviving patients who had routine visits to the study site, a signed informed consent document was obtained. For surviving patients who had been transferred to another hospital, evidence of oral informed consent was obtained. Deceased patients who met the inclusion criteria were included unless the patient’s family opted out. Patients were ineligible if they were participating in a prospective interventional clinical trial during the follow-up period.
Objectives and assessments
The primary objective was to describe the demographic and clinical characteristics of patients with aRCC treated with 1L avelumab + axitinib in clinical practice in Japan. The secondary objective was to determine real-world treatment outcomes as measured by endpoints that included ORR and PFS per investigator assessment, overall survival (OS), best overall response, time to treatment discontinuation (TTD; defined as the time from start to end of 1L treatment with avelumab + axitinib for any cause except treatment effectiveness), treatment patterns, use of corticosteroids for immune-related adverse events (irAEs), and subsequent treatment patterns. We report the results of analyses in the overall population and in patients aged ≤64, 65–74, and ≥75 years.
Statistical analysis
The full analysis population included all enrolled patients at each site during the study period. Effectiveness was assessed in all patients from the full analysis population whose index date was prior to 30 April 2022 to ensure a 6-month follow-up period. Continuous variables were summarized using descriptive statistics. The duration of follow-up was calculated using the reverse Kaplan–Meier method (reversing censoring and event indicators). Qualitative variables were summarized as frequencies and percentages. Time-to-event endpoints (PFS, OS, TTD) were estimated using the Kaplan–Meier method, and corresponding CIs were calculated using the Brookmeyer-Crowley method. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc.).
Results
Patients and treatment
At data cutoff (31 October 2022), 150 patients from 19 sites were included in the effectiveness analysis population. The median observation period was 17.0 months (range, 0.5–32.7 months). Patient baseline characteristics are shown in Table 1. At baseline, the median age was 70.5 years (range, 33–87 years); 39 patients (26.0%) were aged ≤64 years, 64 (42.7%) were aged 65–74 years, and 47 (31.3%) were aged ≥75 years. Most patients were male (n = 110 [73.3%]) and had clear cell RCC (n = 134 [89.3%]); tumors had a sarcomatoid component in 10 patients (6.7%). The Eastern Cooperative Oncology Group performance status (ECOG PS) was 0 in 116 (77.3%), 1 in 23 (15.3%), and ≥2 in 10 (6.7%) patients. The International Metastatic RCC Database Consortium (IMDC) risk classification was favorable in 39 (26.0%), intermediate with 1 risk factor in 46 (30.7%), intermediate with 2 risk factors in 36 (24.0%), and poor in 29 (19.3%) patients.
Table 1.
Baseline characteristics in the overall population and in subgroups defined by age
| Overall population (N = 150) |
≤64 years (n = 39) |
65–74 years (n = 64) |
≥75 years (n = 47) |
|
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 110 (73.3) | 31 (79.5) | 45 (70.3) | 34 (72.3) |
| Female | 40 (26.7) | 8 (20.5) | 19 (29.7) | 13 (27.7) |
| Age, median (range), years | 70.5 (33–87) | 59 (33–64) | 70 (65–74) | 78 (75–87) |
| BMI, n (%) | ||||
| <25 kg/m2 | 110 (73.3) | 25 (64.1) | 49 (76.6) | 36 (76.6) |
| ≥25 kg/m2 | 39 (26.0) | 14 (35.9) | 14 (21.9) | 11 (23.4) |
| ECOG PS, n (%) | ||||
| 0 | 116 (77.3) | 37 (94.9) | 48 (75.0) | 31 (66.0) |
| 1 | 23 (15.3) | 1 (2.6) | 9 (14.1) | 13 (27.7) |
| ≥2 | 10 (6.7) | 1 (2.6) | 7 (10.9) | 2 (4.3) |
| CRP, n (%) | ||||
| <10 mg/L | 104 (69.3) | 30 (76.9) | 44 (68.8) | 30 (63.8) |
| ≥10 mg/L | 43 (28.7) | 9 (23.1) | 19 (29.7) | 15 (31.9) |
| eGFR, n (%) | ||||
| <60 mL/min | 109 (72.7) | 27 (69.2) | 45 (70.3) | 37 (78.7) |
| ≥60 mL/min | 40 (26.7) | 12 (30.8) | 18 (28.1) | 10 (21.3) |
| Pathological classification, n (%) | ||||
| Clear cell | 134 (89.3) | 34 (87.2) | 56 (87.5) | 44 (93.6) |
| Non-clear cell | 10 (6.7) | 2 (5.1) | 4 (6.3) | 0 |
| Unknown | 6 (4.0) | 3 (7.7) | 4 (6.3) | 3 (6.4) |
| Sarcomatoid, n (%) | 10 (6.7) | 3 (7.7) | 1 (1.6) | 6 (12.8) |
| No. of metastatic organs, n (%) | ||||
| 0 | 9 (6.0) | 2 (5.1) | 6 (9.4) | 1 (2.1) |
| 1 | 73 (48.7) | 20 (51.3) | 32 (50.0) | 21 (44.7) |
| ≥2 | 68 (45.3) | 17 (43.6) | 26 (40.6) | 25 (53.2) |
| Nephrectomy, n (%) | 114 (76.0) | 33 (84.6) | 48 (75.0) | 33 (70.2) |
| Clinically important comorbidities, n (%) | 101 (67.3) | 19 (48.7) | 46 (71.9) | 36 (76.6) |
| IMDC risk group, n (%) | ||||
| Favorable | 39 (26.0) | 14 (35.9) | 19 (29.7) | 6 (12.8) |
| Intermediate (1 risk) | 46 (30.7) | 9 (23.1) | 18 (28.1) | 19 (40.4) |
| Intermediate (2 risks) | 36 (24.0) | 9 (23.1) | 13 (20.3) | 14 (29.8) |
| Poor | 29 (19.3) | 7 (17.9) | 14 (21.9) | 8 (17.0) |
Patients with missing information in each category are not shown
BMI body mass index, CRP C-reactive protein, ECOG PS Eastern Cooperative Oncology Group performance status, eGFR estimated glomerular filtration rate, IMDC International mRCC Database Consortium, mRCC metastatic renal cell carcinoma
Compared with patients aged ≤64 years or 65–74 years, a lower proportion of patients aged ≥75 years had an ECOG PS of 0 (94.9% or 75.0% vs. 66.0%, respectively) and a higher proportion had an ECOG PS of 1 (2.6% or 14.1% vs. 27.7%, respectively) (Table 1). In addition, compared with patients aged ≤64 years or 65–74 years, a lower proportion of patients aged ≥75 years had favorable IMDC risk classification (35.9% or 29.7% vs. 12.8%, respectively), a higher proportion had intermediate IMDC with 1 risk factor (23.1% or 28.1% vs. 40.4%, respectively), and a higher proportion had RCC with sarcomatoid features (7.7% or 1.6% vs. 12.8%, respectively).
The median duration of avelumab + axitinib treatment was 10.7 months (interquartile range [IQR], 6.6–18.3 months), the median duration of avelumab treatment was 10.1 months (IQR, 6.0–17.0 months), and the median duration of axitinib treatment was 9.6 months (IQR, 4.9–16.0 months) (Table 2). The median relative dose intensity for avelumab was 100.0% in the overall population and in all age groups and for axitinib was 75.0% in the overall population and 80.0%, 70.0%, and 70.0% in patients aged ≤64 years, 65–74 years, and ≥75 years, respectively (Table 2).
Table 2.
Treatment exposure for avelumab and axitinib in the overall population and in subgroups defined by age
| Overall population (N = 150) | ≤64 years (n = 39) | 65–74 years (n = 64) | ≥75 years (n = 47) | |
|---|---|---|---|---|
| Duration of avelumab + axitinib treatment, median (IQR), months | 10.7 (6.6–18.3) | 11.5 (8.0–19.4) | 10.8 (6.7–19.0) | 10.3 (5.0–15.6) |
| Duration of avelumab treatment, median (IQR), months | 10.1 (6.0–17.0) | 10.8 (7.2–18.4) | 9.9 (6.5–17.9) | 9.0 (4.7–14.0) |
| Duration of axitinib treatment, median (IQR), months | 9.6 (4.9–16.0) | 10.1 (6.2–16.6) | 10.3 (5.8–18.0) | 8.3 (3.8–14.6) |
| Avelumab dose, median (IQR) | ||||
| Dose intensity, mg/kg/administration | 10.0 (8.2–10.0) | 10.0 (8.4–10.0) | 10.0 (8.3–10.0) | 10.0 (7.9–10.0) |
| Relative dose intensity, % | 100.0 (82.3–100.0) | 100.0 (84.0–100.0) | 100.0 (82.8–100.3) | 100.0 (78.5–100.0) |
| Axitinib dose, median (IQR) | ||||
| Dose intensity, mg/kg/administration | 5.2 (4.0–7.2) | 6.7 (4.4–9.6) | 5.6 (4.2–6.8) | 4.6 (3.6–6.7) |
| Relative dose intensity, % | 75.0 (60.0–100.0) | 80.0 (70.0–100.0) | 70.0 (60.0–100.0) | 70.0 (50.0–100.0) |
Clinical outcomes
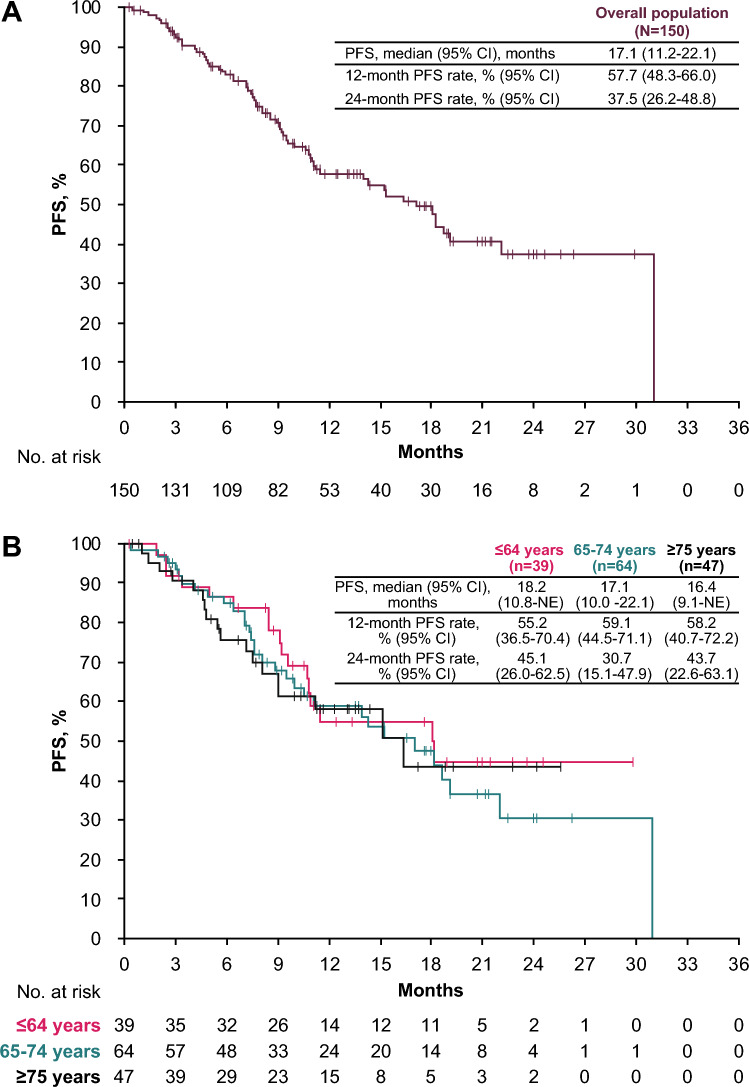
Median follow-up for PFS was 13.8 months (95% CI, 12.5–18.0 months). The median PFS in the overall population was 17.1 months (95% CI, 11.2–22.1 months), with 1- and 2-year PFS rates of 57.7% and 37.5%, respectively (Fig. 1A). In patients aged ≤64, 65–74, or ≥75 years, the median PFS was 18.2 months (95% CI, 10.8 months-not estimable [NE]), 17.1 months (95% CI, 10.0–22.1 months), and 16.4 months (95% CI, 9.1 months-NE); the 2-year PFS rates were 45.1%, 30.7%, and 43.7%, respectively (Fig. 1B).
Fig. 1.
Real-world PFS in A the overall population and B subgroups defined by age. CI confidence interval, NE not estimable, PFS progression-free survival
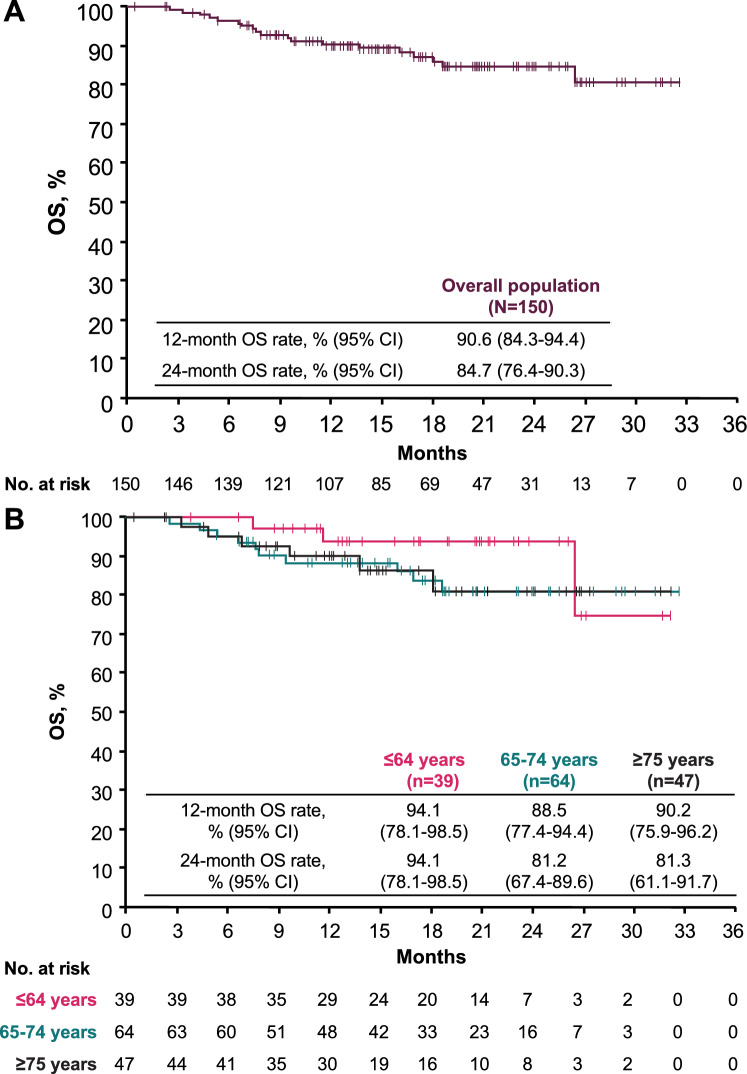
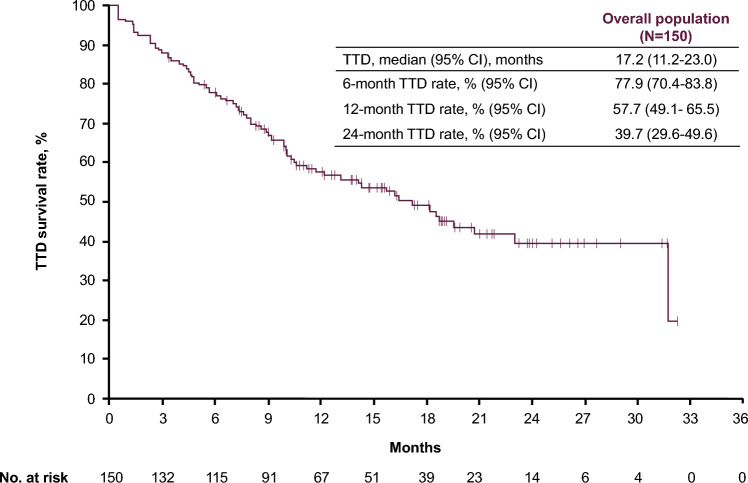
Median follow-up for OS was 18.7 months (95% CI, 16.3–20.6 months). The median OS was not reached in the overall population or in any of the subgroups defined by age (Fig. 2). In the overall population, 1- and 2-year OS rates were 90.6% and 84.7%, respectively (Fig. 2A). In patients aged ≤64, 65–74, or ≥75 years, 2-year OS rates were 94.1%, 81.2%, and 81.3%, respectively (Fig. 2B). The median TTD for avelumab + axitinib was 17.2 months (95% CI, 11.2–23.0 months), with 6-month, 1-year, and 2-year TTD rates of 77.9%, 57.7%, and 39.7%, respectively (Fig. 3).
Fig. 2.
Real-world OS in A the overall population and B subgroups defined by age. CI confidence interval, OS overall survival
Fig. 3.
Real-world TTD survival rate in the overall population. CI confidence interval, TTD time to treatment discontinuation
Among 135 patients assessed for best overall response, the ORR was 53.3% (95% CI, 44.6–62.0%) and the disease control rate (DCR) was 88.9% (95% CI, 82.3–93.6%) (Table 3). In patients aged ≤64, 65–74, or ≥75 years, ORRs were 55.3% (95% CI, 37.2–69.9%), 49.1% (95% CI, 29.9–55.2%), and 57.1% (95% CI, 41.0–72.3%) and DCRs were 84.2% (95% CI, 66.5–92.5%), 87.3% (95% CI, 62.8–85.0%), and 95.2% (95% CI, 83.8–99.4%), respectively.
Table 3.
Objective response in the overall population and in subgroups defined by age
| Overall population (n = 135)a |
≤64 years (n = 38) |
65–74 years (n = 55) |
≥75 years (n = 42) |
|
|---|---|---|---|---|
| BOR, n (%) | ||||
| Complete response | 12 (8.9) | 4 (10.5) | 6 (10.9) | 2 (4.8) |
| Partial response | 60 (44.4) | 17 (44.7) | 21 (38.2) | 22 (52.4) |
| Stable disease | 48 (35.6) | 11 (28.9) | 21 (38.2) | 16 (38.1) |
| Progressive disease | 14 (10.4) | 6 (15.8) | 6 (10.9) | 2 (4.8) |
| Not evaluable | 1 (0.7) | 0 | 1 (1.8) | 0 |
|
ORR, n (%) [95% CI] |
72 (53.3) [44.6–62.0] |
21 (55.3) [37.2–69.9] |
27 (49.1) [29.9–55.2] |
24 (57.1) [41.0–72.3] |
|
DCR, n (%) [95% CI] |
120 (88.9) [82.3–93.6] |
32 (84.2) [66.5–92.5] |
48 (87.3) [62.8–85.0] |
40 (95.2) [83.8–99.4] |
BOR best overall response, CI confidence internal, DCR disease control rate, ORR objective response rate
aBest overall response not reported in 15 patients (1 patient aged ≤64 years, 9 patients aged 65–74 years, and 5 patients aged ≥75 years)
Treatment discontinuations and subsequent treatment
At the end of the follow-up period, 75 patients (50.0%) were still receiving avelumab + axitinib treatment, including 22 patients (56.4%) aged ≤64 years, 30 (46.9%) aged 65–74 years, and 23 (48.9%) aged ≥75 years (Table 4). In patients who discontinued avelumab + axitinib (n = 75 [50.0%]), the most common reason for discontinuation was progressive disease (n = 37 [24.7%]), followed by occurrence of adverse events (n = 31 [20.7%]). In 54 patients (36.0%) who received a subsequent treatment, cabozantinib was most frequently administered followed by nivolumab (Table 4). Of patients who discontinued avelumab + axitinib treatment due to disease progression (n = 37) or adverse events (n = 31), 30 (81.1%) and 16 (51.6%) received a second-line treatment, respectively. Median time from avelumab + axitinib discontinuation to second-line treatment was 0.6 months (range, 0.03–5.6 months) in the overall population, 0.5 months (range, 0.03–2.8 months) in patients who discontinued due to disease progression, and 1.3 months (range, 0.1–5.6 months) in patients who discontinued due to adverse events. Rates of treatment discontinuation, reasons for treatment discontinuation, and subsequent treatments were generally similar across age groups.
Table 4.
Treatment discontinuation and subsequent treatments in the overall population and in subgroups defined by age
| Overall population (N = 150) |
≤64 years (n = 39) |
65–74 years (n = 64) |
≥75 years (n = 47) |
|
|---|---|---|---|---|
| Ongoing treatment at data cutoff, n (%) | 75 (50.0) | 22 (56.4) | 30 (46.9) | 23 (48.9) |
| Discontinued treatment, n (%) | 75 (50.0) | 17 (43.6) | 34 (53.1) | 24 (51.1) |
| Reasons for treatment discontinuation, n (%)a | ||||
| Progressive disease | 37 (24.7) | 9 (23.1) | 18 (28.1) | 10 (21.3) |
| Adverse event | 31 (20.7) | 6 (15.4) | 15 (23.4) | 10 (21.3) |
| Other | 12 (8.0) | 4 (10.3) | 4 (6.3) | 4 (8.5) |
| Received subsequent treatment, n (%) | 54 (36.0) | 16 (41.0) | 21 (32.8) | 17 (36.2) |
| Treatment regimen, n (%) | ||||
| Cabozantinib monotherapy | 38 (25.3) | 12 (30.8) | 16 (25.0) | 10 (21.3) |
| Nivolumab monotherapy | 9 (6.0) | 1 (2.6) | 3 (4.7) | 5 (10.6) |
| Nivolumab + cabozantinib | 2 (1.3) | 2 (5.1) | 0 | 0 |
| Axitinib monotherapy | 2 (1.3) | 0 | 1 (1.6) | 1 (2.1) |
| Pazopanib monotherapy | 2 (1.3) | 0 | 1 (1.6) | 1 (2.1) |
| Everolimus monotherapy | 1 (0.7) | 1 (2.6) | 0 | 0 |
aPatients with >1 reason for discontinuation are included in all relevant rows
Treatment for irAEs
Twenty-two patients (14.7%) received corticosteroid treatment at any dose for irAEs for a median of 2.7 months (range, 0.03–19.5 months) and 11 (7.3%) received high-dose corticosteroid treatment for a median of 2.8 months (range, 0.03–19.5 months) (Table 5). In patients aged ≤64 years, 65–74 years, or ≥75 years, 9 (23.1%), 5 (7.8%), and 8 (17.0%) patients received corticosteroid treatment at any dose and 5 (12.8%), 2 (3.1%), and 4 (8.5%) received high-dose corticosteroid treatment, respectively. The duration of corticosteroid treatment is shown in Table 5.
Table 5.
Use of corticosteroid for irAEs
| Overall population (N= 150) |
≤64 years (n = 39) |
65–74 years (n = 64) |
≥75 years (n = 47) |
|
|---|---|---|---|---|
| Corticosteroids, n (%) | 22 (14.7) | 9 (23.1) | 5 (7.8) | 8 (17.0) |
| Duration of treatment, median (range), months | 2.7 (0.03–19.5) | 2.1 (0.03–19.5) | 5.5 (0.1–16.1) | 1.5 (0.03–17.0) |
| High-dose corticosteroids, n (%)a | 11 (7.3) | 5 (12.8) | 2 (3.1) | 4 (8.5) |
| Duration of treatment, median (range), monthsb | 2.8 (0.03–19.5) | 2.8 (0.03–19.5) | 10.8 (5.5–16.1) | 0.1 (0.03–2.8) |
irAE immune-related adverse event
aHigh dose was defined as prednisolone-equivalent corticosteroid doses of ≥40 mg
bDuration period for ≥1 dose of high-dose corticosteroid
Discussion
J-DART2 represents the largest retrospective, observational study to provide real-world data on long-term outcomes in patients with aRCC treated with 1L avelumab + axitinib in Japan. Patient outcomes in the overall population of J-DART2 are consistent with those observed in the pivotal JAVELIN Renal 101 clinical trial (including subgroup analyses of patients enrolled in Japan and older patients), the smaller J-DART real-world study, and the PMS [16, 23–25].
Compared with the avelumab + axitinib arm of the JAVELIN Renal 101 trial, the patient population in J-DART2 had a higher proportion of older patients and patients who would not have been eligible for the clinical trial, including those with an ECOG PS ≥2 or non-clear cell RCC [16, 23]. Baseline characteristics in J-DART2 were generally consistent with those reported in J-DART [24] and PMS analyses [25].
The median PFS with 1L avelumab + axitinib in J-DART2 (17.1 months [95% CI, 11.2–22.1 months]) was similar to that reported in JAVELIN Renal 101, including the overall avelumab + axitinib arm and Japanese subgroup analyses (13.9 months [95% CI, 11.1–16.6 months] and 16.6 months [95% CI, 8.1 months-NE], respectively) and in J-DART (15.3 months [95% CI, 9.7 months-NE]) [18, 19, 24]. The ORR in J-DART2 (53.3%) was also similar to that in JAVELIN Renal 101, including the overall avelumab + axitinib arm and Japanese subgroup analysis (59.3% and 60.6%, respectively) and J-DART (48.8%) [18, 19, 24]. While OS was not reached by the data cutoff in J-DART2, the 12-month OS rate (90.6%) was similar to that in JAVELIN Renal 101 (86%) [26]. Although OS analyses did not reach statistical significance at the final analysis of JAVELIN Renal 101, results for PFS and ORR consistently favored avelumab + axitinib and support outcomes from the J-DART2 study in clinical practice in Japan [27].
Recent data from clinical trials and meta-analyses have suggested that older patients with aRCC can still benefit from ICI-based regimens [21–23, 28, 29]. A comprehensive review of ICI-based treatments in older patients with aRCC concluded that the available data do not suggest a lower efficacy compared with younger patients [21]. Because Japan has a rapidly aging population, J-DART2 examined long-term clinical outcomes in older patients with aRCC treated with 1L avelumab + axitinib. Compared with the avelumab + axitinib arm from the JAVELIN Renal 101 trial, the study population in J-DART2 had a higher proportion of older patients (aged ≤64 years, 61.3% vs. 26.0%; aged 65–74 years, 31.2% vs. 42.7%; aged ≥75 years, 7.5% vs. 31.3%, respectively) [23]. Baseline characteristics in J-DART2 were generally balanced across age groups, but the subgroup of patients aged ≥75 years had a higher proportion of patients with an ECOG PS of 1, tumors with sarcomatoid features, or intermediate IMDC risk classification (1 risk factor). PFS, OS, ORR and DCR were consistent across age groups in J-DART2, supporting the benefit of avelumab + axitinib treatment in patients with aRCC, including those aged ≥75 years.
In J-DART2, 7.3% of patients received high-dose corticosteroid treatment for irAEs, which is lower than in the JAVELIN Renal 101 trial (14.5%) but similar to that in subgroup analyses in Japan (9.1%) and in J-DART (6.3%) [18, 19, 24]. Rates of high-dose corticosteroid administration for irAEs were generally low across age subgroups, suggesting that avelumab + axitinib treatment has a manageable safety profile regardless of age.
Half of the patients enrolled in J-DART2 were still receiving 1L avelumab + axitinib at the end of the follow-up period. Of patients who discontinued treatment, 36% received a subsequent anticancer treatment, which is consistent with the JAVELIN Renal 101 trial (46.2%) [18]. Median time from avelumab + axitinib discontinuation to second-line treatment was longer in patients who discontinued due to adverse events vs. those who discontinued due to disease progression (1.3 vs. 0.5 months). However, >50% of patients who discontinued treatment due to adverse events received a second-line treatment, suggesting that appropriate management of adverse events may help the implementation of sequential treatment. Overall, the rates of treatment discontinuation and subsequent anticancer treatments were generally consistent between patients aged ≤64 years, 65–74 years, and ≥75 years.
Our study had some limitations. As a retrospective study, only existing data reported in patient records was available for analysis, and missing data may have affected the accuracy of estimations. The evaluation of disease response may have differed at each site, which might also have led to variations in estimated values. In addition, high-volume centers were preferentially selected for this study, which could have led to site selection and outcome reporting biases. Therefore, the study results may not accurately reflect clinical outcomes for all patients with aRCC in Japan. In addition, the patient population and methods of assessment in this study were different from those in the JAVELIN Renal 101 trial; thus, comparisons must be interpreted with caution. Lasty, J-DART2 did not collect data on adverse events to avoid overlap with a PMS study that has analyzed adverse events data for avelumab + axitinib in Japan [25].
Conclusion
J-DART2 provides real-world data on the long-term effectiveness of 1L avelumab + axitinib in patients with aRCC in clinical practice in Japan, including older patients. Avelumab + axitinib was associated with clinically meaningful benefits across patient age groups, and outcomes were generally consistent with those reported previously. J-DART2 further supports the continued use of avelumab + axitinib as a 1L standard of care for patients with aRCC.
Acknowledgements
The authors thank the patients and their families, investigators, coinvestigators, and supporting teams at each of the participating medical institutions. This study was sponsored by Pfizer and was previously conducted under an alliance between Merck (CrossRef Funder ID: 10.13039/100009945) and Pfizer. Medical writing support was provided by Katherine Quiroz-Figueroa of Nucleus Global and was funded by Merck. Statistical analysis support was provided by Miyoshi Sakai of EPS Corporation.
Author contributions
T. Kato: resources, investigation, visualization, writing—original draft, review, and editing. J. Furukawa, N. Hinata, K. Ueda, I. Hara, F. Hongo, R. Mizuno, T. Okamoto, H. Okuno: resources, investigation, writing—review and editing. T. Ito: conceptualization, visualization, validation funding acquisition, writing—review and editing. M. Kajita: data curation, formal analysis, writing—original draft, review, and editing. M. Oya, Y. Tomita, N. Shinohara: conceptualization, visualization, writin—review and editing. M. Eto: conceptualization, visualization, resources, writing—review and editing. H. Uemura: conceptualization, resources, visualization, supervision, project administration, writing—review and editing.
Data availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
Declarations
Conflict of interest
T. Kato has received honoraria for lectures from MSD. J. Furukawa has received honoraria for lectures from Merck and Ono Pharmaceutical. N. Hinata has received honoraria for lectures from AstraZeneca, Bayer Yakuhin, Bristol Myers Squibb, Kissei Pharmaceutical, MSD, Sanofi, Sysmex, and Takeda. K. Ueda has received honoraria for lectures from Merck and MSD. I. Hara has received honoraria for lectures from Astellas Pharma, Janssen, and Takeda and has received research funding from Bristol Myers Squibb. F. Hongo has received honoraria for lectures from Merck, MSD, Pfizer, and Takeda. R. Mizuno has received honoraria for lectures from Merck and Takeda. T. Okamoto and H. Okuno have nothing to disclose. T. Ito and M. Kajita report employment with Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA. M. Oya has received honoraria from Bayer, Bristol Myers Squibb, Eisai, Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA, MSD, Ono Pharmaceutical, Pfizer, and Takeda; and has received scholarship endowments from Bayer, Eisai, and Takeda. Y. Tomita has participated in consulting or advisory roles for Eisai, MSD, and Ono Pharmaceutical; has received honoraria from Astellas Pharma, Bristol Myers Squibb Japan, Chugai Pharma, Novartis, Ono Pharmaceutical, and Pfizer; and has received research funding from Astellas Pharma, AstraZeneca, Chugai Pharma, Eisai, MSD, Novartis, Ono Pharmaceutical, Pfizer, and Takeda. N. Shinohara has received grants or contracts from Bristol Myers Squibb, Ono Yakuhin, and Takeda; has received honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bristol Myers Squibb, MSD, Novartis, Ono Yakuhin, Pfizer, and Takeda; and is the president of the Japanese Society of Renal Cancer. M. Eto has received research funding from Takeda Pharmaceutical; and has received honoraria for lectures from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Bristol Myers Squibb, Eisai, Janssen Pharmaceutical, Merck Biopharma, MSD, Ono Pharmaceutical, Pfizer, and Takeda Pharmaceutical. H. Uemura has received research grants from Astellas, AstraZeneca, Janssen, Ono Pharmaceutical, Taiho, and Takeda; has received consulting fees from Ono Pharmaceutical and Sanofi; has received payment or honoraria for lectures and speakers’ bureaus from Bayer, Janssen, MSD, Pfizer, Sanofi, and Takeda; and has received payment for expert advisory testimony from AstraZeneca, Ono Pharmaceutical, and Sanofi.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer Information Service. National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare). Cancer statistics. https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.html. Accessed 28 May 2024
- 2.Powles T, Albiges L, Bex A et al (2024) Renal cell carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 35(8):692–706 [DOI] [PubMed] [Google Scholar]
- 3.Washio M, Mori M, Mikami K et al (2014) Risk factors for renal cell carcinoma in a Japanese population. Asian Pac J Cancer Prev 15(21):9065–9070 [DOI] [PubMed] [Google Scholar]
- 4.Hori M, Matsuoka J (2019) Kidney cancer incidence rates in the world from the Cancer Incidence in Five Continents XI. Jpn J Clin Oncol 49(3):295–296 [DOI] [PubMed] [Google Scholar]
- 5.Motzer R, Alekseev B, Rha SY et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384(14):1289–1300 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378(14):1277–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choueiri TK, Powles T, Burotto M et al (2021) Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384(9):829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini BI, Plimack ER, Stus V et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127 [DOI] [PubMed] [Google Scholar]
- 9.Japanese Urological Association. Supplement to clinical practice guidelines for renal cell carcinoma [2017 update] https://www.urol.or.jp/lib/files/other/guideline/33_renal_cancer_2017_rev2022_info.pdf. Accessed 28 May 2024
- 10.NCCN Clinical practice guidelines in oncology. Kidney cancer. Version 3.2024
- 11.Bavencio (avelumab). Prescribing information. EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA. 2024.
- 12.Bavencio (avelumab). Summary of product characteristics. Merck Europe B.V, Amsterdam, the Netherlands, an affiliate of Merck KGaA. 2024.
- 13.Inlyta (axitinib). Summary of product characteristics. Pfizer. 2021.
- 14.Inlyta (axitinib). Prescribing information. Pfizer. 2022.
- 15.Bavencio (avelumab). Prescribing information (Japan). Merck Biopharma Co., Ltd, Tokyo, Japan, affiliate of Merck KGaA. 2022.
- 16.Motzer RJ, Penkov K, Haanen J et al (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choueiri TK, Motzer RJ, Rini BI et al (2020) Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol 31(8):1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haanen JBAG, Larkin J, Choueiri TK et al (2023) Extended follow-up from JAVELIN Renal 101: subgroup analysis of avelumab plus axitinib vs. sunitinib by IMDC risk group in patients with advanced renal cell carcinoma. ESMO Open 8:101210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uemura M, Tomita Y, Miyake H et al (2020) Avelumab plus axitinib vs. sunitinib for advanced renal cell carcinoma: Japanese subgroup analysis from JAVELIN Renal 101. Cancer Sci 111(3):907–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagashima F, Furuse J (2022) Treatments for elderly cancer patients and reforms to social security systems in Japan. Int J Clin Oncol 27(2):310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lolli C, Altavilla A, Conteduca V et al (2020) A comprehensive review of the role of immune checkpoint inhibitors in elderly patients affected by renal cell carcinoma. Crit Rev Oncol Hematol 153:103036 [DOI] [PubMed] [Google Scholar]
- 22.Schulz GB, Rodler S, Szabados B et al (2020) Safety, efficacy and prognostic impact of immune checkpoint inhibitors in older patients with genitourinary cancers. J Geriatr Oncol 11(7):1061–1066 [DOI] [PubMed] [Google Scholar]
- 23.Tomita Y, Motzer RJ, Choueiri TK et al (2022) Efficacy and safety of avelumab plus axitinib in elderly patients with advanced renal cell carcinoma: extended follow-up results from JAVELIN Renal 101. ESMO Open 7(2):100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato T, Nakano Y, Hongo F et al (2023) Real-world outcomes of avelumab plus axitinib as first-line therapy in patients with advanced renal cell carcinoma in Japan: a multicenter, retrospective, observational study (J-DART). Int J Urol 31(3):265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oya M, Ito T, Sato M et al (2024) Subgroup analysis of postmarketing surveillance data for avelumab plus axitinib therapy in patients with advanced renal cell carcinoma in Japan by age group. Annual Meeting of the Japanese Urological Association, Yokohama, Japan [DOI] [PubMed]
- 26.National Institute for Health and Care Excellence. Single technology appraisal: avelumab with axitinib for untreated advanced or metastatic renal ccell carcinoma [ID1547]. Committee papers. https://www.nice.org.uk/guidance/ta645/documents/committee-papers. Accessed 28 May 2024
- 27.Motzer RJ, Penkov K, Uemura H et al (2024) Avelumab + axitinib vs sunitinib in patients (pts) with advanced renal cell carcinoma (aRCC): final overall survival (OS) analysis from the JAVELIN Renal 101 phase 3 trial. J Clin Oncol 42(16 Suppl). Abstract 4508 [Google Scholar]
- 28.Araujo DV, Wells JC, Hansen AR et al (2021) Efficacy of immune-checkpoint inhibitors (ICI) in the treatment of older adults with metastatic renal cell carcinoma (mRCC) an International mRCC Database Consortium (IMDC) analysis. J Geriatr Oncol 12(5):820–826 [DOI] [PubMed] [Google Scholar]
- 29.Yanagisawa T, Quhal F, Kawada T et al (2023) Association between age and efficacy of first-line immunotherapy-based combination therapies for mRCC: a meta-analysis. Immunotherapy 15(15):1309–1322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck’s Data Sharing Policy. All requests should be submitted in writing to Merck’s data sharing portal (https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.