Abstract
Fever is a complication after colorectal endoscopic submucosal dissection (ESD). The objective of this study was to explore the incidence and risk factors of fever after colorectal ESD and establish a predictive nomogram model. This retrospective analysis encompassed patients with colorectal lesions who underwent ESD between June 2008 and December 2021 in our center. Multivariate analyses were performed to identify the independent risk factors of fever after colorectal ESD based on univariate analysis, and derived predictive nomogram model was constructed. The performance of nomogram model was evaluated through the receiver operating characteristic curve, calibration curve, decision curve analysis (DCA) and clinical impact curve (CIC). Among the 1096 enrolled patients with colorectal lesions, fever after colorectal ESD occurred in 204 (18.6%) patients. Multivariate logistic regression revealed that tumor size (P < 0.001), ESD procedure time > 30 min (P < 0.001), injury to muscle layer (P < 0.001) and intraoperative perforation (P = 0.046) were estimated to be independent risk factors of fever after colorectal ESD. A predictive nomogram model, incorporating these four predictors, were established and performed well in both training and validation groups. Both DCA and CIC showed this nomogram model had a good potential for clinical practicability.
Keywords: Fever, Colorectal lesion, Endoscopic submucosal dissection, Risk factor, Nomogram
Subject terms: Colonoscopy, Risk factors, Oncology, Gastrointestinal system, Large intestine
Introduction
Endoscopic submucosal dissection (ESD), as a safe and effective endoscopic treatment technique, has gradually become a common endoscopic treatment for early colorectal tumors, which include lesions involving the mucosal and submucosal layers1–4. Compared with endoscopic mucosal resection (EMR), ESD achieves a high en bloc resection rate for lesions, which is beneficial for the overall pathological evaluation of the excised lesions and is associated with a low postoperative recurrence rate5. However, due to technical difficulties, colorectal ESD has a high incidence of postoperative complications such as perforation and delayed bleeding6,7.
Fever is another common adverse event that can occur after colorectal ESD8. Since different studies define the temperature of fever differently, previous studies have shown the different incidence rate of fever after colorectal ESD, which ranged widely from 12.4 to 46.7%9,10. Postoperative fever after colorectal ESD is believed to be associated with the exposure of feces and residues in the intestine, as they increase the risk of bacterial infection at exposed mucosal wounds by ESD10. Since fever after colorectal ESD generally has a good prognosis, it is easily neglected in clinical practice. Compared to the stomach and esophagus, the intestinal wall in the colon and rectum is thinner, and the intestinal tract harbors a more abundant microbiota. These anatomical and microbial characteristics result in a relatively higher risk of delayed perforation and postoperative infection11. Fever is often a clinical manifestation of delayed perforation following colorectal ESD, and it may even represent an early indicator of this complication, frequently presenting alongside abdominal pain12. Moreover, postoperative fever always induces discomfort and anxiety in patients and may potentially affect disease prognosis. Hence, exploring and identifying the risk factors for fever after colorectal ESD is significant for identifying individuals at greater risk of delayed perforation, enabling early intervention, as well as for timely predicting postoperative fever and informing patients in advance to reduce discomfort and anxiety.
Currently, the studies on the risk factors of fever after colorectal ESD are rare and their sample size was not large. Meanwhile, there is a lack of nomogram for the risk prediction of fever after colorectal ESD. Therefore, the aim of our study was to identify the predictors of fever after colorectal ESD based on a large sample size, and construct a nomogram to predict the probability of fever after colorectal ESD. Moreover, we also evaluated the predictive accuracy and clinical practicability of the established model.
Methods
Study design and participants
This retrospective study of clinical databases on ESD for colorectal lesions was conducted at the First Affiliated Hospital of Nanchang University in China. A total of 1134 patients who underwent colorectal ESD between June 2008 and December 2021 were enrolled. The exclusion criteria were as follows: (a) patients with fever (≥ 37.5 °C) before colorectal ESD; (b) patients treated with colorectal ESD for multiple lesions at the meantime; (c) patients with incomplete clinical information. Finally, 1096 patients were included in our study and were randomly divided into training group and verification group by a ratio of 8:2, and both groups were further divided into “Fever” group and “Non-fever” group according to whether fever occurred after colorectal ESD.
The information before, during and after ESD of the enrolled patients were collected based on the medical records and endoscopy databases at our center. All patients have written the informed consent for colorectal ESD before endoscopic procedure, and this study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. We confirm that all methods and procedures were performed in accordance with relevant guidelines and regulations.
Pre-ESD preparation
After admission, every patient underwent preoperative evaluation, including blood type, blood routine and biochemical examination, coagulation function, etc. Moreover, magnified chromoendoscopy, endoscopic ultrasound (EUS) and abdominal computer tomography (CT) were conducted as necessary to further evaluate the lesion type, depth of invasion and the possibility of metastases. The morphology of the lesions was decided and divided into three categories according to the Japanese classification of colorectal carcinoma, including 0-Is/Ip, LST-G and LST-NG13. All patients initiated a liquid diet for one day and fasted for eight hours before ESD procedure. In the meantime, bowel preparation was performed prior to the colorectal ESD using polyethylene glycol electrolyte solution. The quality of bowel preparation was evaluated by the Boston Bowel Preparation Scale (BBPS). Inadequate bowel preparation was defined as a total BBPS scores for all colon segments < 6 or a score of < 2 for any colon segments14.
Colorectal ESD procedure
All patients received general anesthesia with intravenous propofol by anesthesiologists. The colorectal ESD procedures were performed by 13 endoscopists, including 9 experts and 4 trainees. An expert endoscopist was defined as having at least 40 cases of experience with colorectal ESD15. The endoscopists performed all colorectal ESD procedures using a single-channel water-jet electric endoscope with a transparent cap and CO2 insufflation system. Briefly, colorectal ESD procedure was performed by the following specific steps16,17. First, using electrocautery or ESD knives to identify and mark the lesion, if necessary. After submucosal injection with lifting solution (epinephrine solution and methylene blue), a circumferential mucosal incision along the periphery of the electrocautery markers with one or two ESD knives was made. Then, the lesion was gradually separated from the submucosal layer. Complete en bloc resection was defined as a whole resection of specimen resected in a non-fragmenting piece18. Injury to muscle layer and non-lifting signs of the lesion were judged from endoscopic findings during the ESD procedure, and intraoperative bleeding and visible vessels were treated with knives or hemostatic forceps. Intraoperative bleeding was defined as the exudative or active bleeding that occurred during the ESD procedure19. Intraoperative perforation was defined as the appearance of a visible hole in the colorectal wall, exposing the pericolic fat or intra-abdominal organs during the ESD procedure11. After the lesion removal, the endoscopists will decide whether to close the ESD ulcer. The ESD procedure time was defined as the period from lesion marking or submucosal injection to the completion of ESD ulcer treatment20. The resected lesions were stretched and pinned on specimen fixed foam plates, fixed in formalin solution and sent for histopathological classification. The lesion size and pathological invasion depth were both judged from the final pathological findings, and histological assessment was judged based on the World Health Organization classification system21.
Post-ESD managements
The duration of the fast and the use of prophylactic antibiotics depends on the decision of endoscopists according to the patient’s intra-ESD situation. All patients were monitored routinely after ESD procedure for vital signs and symptoms such as fever, melena, hematochezia and abdominal pain. Delayed bleeding was defined as bleeding that manifested with clinical signs such as hematochezia or melena and necessitated endoscopic hemostasis within 1 week following ESD. Delayed perforation was defined as the detection of free air on radiographic imaging after the completion of colorectal ESD11. Postoperative fever was defined as the maximum body temperature ≥ 37.5 °C within 3 days after ESD. If patients suffered from fever, clinicians would decide on further treatment, including observation, physical cooling and antibiotics use. All patients were started on a liquid diet the day following the fasting period and were discharged when they resumed soft meals without experiencing any post-ESD complications.
Outcome assessment
The primary outcome was to identify the independent predictors of fever after colorectal ESD, and the secondary outcome was to construct and validate a novel predictive nomogram model aimed at predicting the probability of fever after colorectal ESD.
Statistical analysis
Categorical variables were presented as frequencies and percentages, and were assessed using the Chi-squared or Fisher’s exact test where appropriate. Normally distributed continuous variables were presented as mean ± standard deviation (SD), and compared using Student’s t test. Non-normally distributed continuous variables were presented as median and interquartile range (IQR) in parentheses, and analyzed using Mann–Whitney U test.
The total patients included in our study were randomly divided into a training group and a validation group by a ratio of 8:2 to ensure an adequate sample size for model development while maintaining sufficient data for independent validation22,23. The training group was used to explore the risk factors and build predictive models for fever after colorectal ESD, and the validation group was used to validate the model. Receiver operating characteristic (ROC) curve analysis was employed to determine the optimal cut-off values of age and ESD procedure time. Univariate analysis and multivariate logistic regression were conducted to identify the independent risk factors associated with fever after colorectal ESD. Variables associated with fever after colorectal ESD (P < 0.05) in the univariate analysis were included in the multivariate logistic regression analysis (backward stepwise) to identify the most accurate independent risk factors for predicting fever after colorectal ESD, and the outcomes were estimated by odds ratio (OR) and 95% confidence interval (CI) to illustrate the degree of risk associated with the risk factors. A predictive nomogram model was developed based on the final multivariate logistic regression analysis results (P < 0.05) in R software.
Subsequently, the performance of the nomogram was assessed by calibration curve with bootstrap resampling and ROC curve in both training and validation sets24. Furthermore, decision curve analysis (DCA) and clinical impact curve (CIC) was also performed in both datasets to assess the clinical usefulness and practicability of the nomogram model by calculating the net benefits at various realistic threshold probabilities25,26. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using the R software version 4.3.1 (www.r-project.org) and SPSS software version 28.0 (IBM; Chicago, IL, USA).
Results
Patient and lesion characteristics
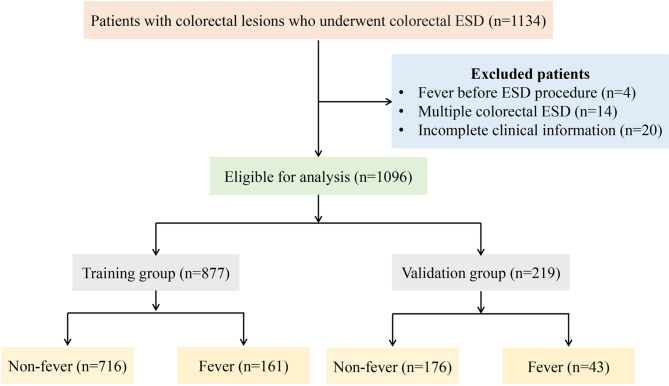
During the study period, the clinical data of 1134 patients with colorectal lesions who underwent ESD at our center were retrospectively analyzed. After applying the exclusion criteria mentioned above, 38 patients were excluded, resulting in a final total of 1096 patients for analysis (Fig. 1). After colorectal ESD, postoperative fever occurred in 204 (18.6%) patients. Among those with postoperative fever, the median (IQR) duration of fever was 1 (1–1) days, and the median (IQR) of highest body temperature was 38.1 (37.8–38.8) °C. Blood inflammatory response tests revealed elevated white blood cell count (WBC) count in 97 (47.6%) patients, and increased serum C-reactive protein (CRP) in 46 (22.6%) patients. Additionally, blood cultures were positive in 2 (0.1%) patients. Except for one patient who was transferred to surgical intervention for delayed perforation, all patients experienced favorable prognosis with fever recovered after conservative medical treatment. Among these, 45 (22.1%) patients were managed with clinical observation and physical cooling, and 159 (77.9%) patients received empirical antibiotic therapy (Table 1).
Fig. 1.
A flowchart of patients included in this study.
Table 1.
Clinical features of fever group.
| Variables | N = 204 |
|---|---|
| Inflammatory response | |
| Elevation of WBC count (n [%]) | 97 (47.6) |
| Elevation of Serum CRP (n [%]) | 46 (22.6) |
| Blood culture positive (n [%]) | 2 (0.1) |
| Treatment after fever | |
| Observation and physical cooling (n [%]) | 45 (22.1) |
| Antibiotics use (n [%]) | 159 (77.9) |
| Postoperative duration fever days, median (IQR) | 1 (1–1) |
| Highest body temperature (°C), median (IQR) | 38.1 (37.8–38.8) |
| Need for surgery (n [%]) | 1 (0.5) |
| Mortality (n [%]) | 0 (0) |
WBC, white blood cell count; CRP, C-reactive protein; IQR, interquartile range.
The baseline characteristics of the included patients are detailed in Table 2. No significant differences were observed in baseline characteristics between the training and validation group. Among the 1096 included patients, the median (IQR) age was 54.0 (46.0–64.0) years old, and 56.4% (618/1096) were male. A history of hypertension was present in 191 (17.4%) patients, abdominal operation in 119 (10.9%) patients, malignancy in 51 (4.7%) patients, and diabetes in 40 (3.6%) patients. Prior to undergoing colorectal ESD, 67 (6.1%) had inadequate bowel preparation. For endoscopic findings, the median (IQR) tumor size was 15.0 (10.0–23.0) mm, with the rectum being the most common site for colorectal lesions treated by ESD (70.3%), followed by the left and right-side colon. The morphology most frequently observed was laterally spreading tumors (LST). During the colorectal ESD procedures, en bloc resection was successfully achieved in 960 (87.6%), muscle layer injury occurred in 478 (43.6%), non-lifting sign was present in 192 (17.5%), electrocoagulation for ESD-induced ulcers was performed in 825 (75.3%), and complete closure of ESD ulcers was attained in 914 (83.4%). Furthermore, the median procedure time for ESD was 26.0 (17.0–40.0) minutes. Pathological examination revealed that the majority of lesions (54.1%) were confined to the mucosal layer, with adenomas being diagnosed in 441 patients (40.2%), encompassing both low-grade and high-grade intraepithelial neoplasia (LGIN and HGIN). Following colorectal ESD, prophylactic antibiotic therapy was administered to 195 patients (17.8%) at the discretion of the endoscopist. The median (IQR) duration of hospitalization was 7.0 (5.0–9.0) days, and the median (IQR) fasting period was 2.0 (2.0–3.0) days. Moreover, the total enrolled patients were stratified into fever and non-fever groups according to the presence or absence of postoperative fever. The baseline characteristics of the training cohort are summarized in Tables 3, 4, and 5.
Table 2.
Characteristics of patients and lesions in the training and validation group.
| Variables | All subjects (N = 1096) | Training group (N = 877) | Validation group (N = 219) | p value |
|---|---|---|---|---|
| Sex (n [%]) | 0.068 | |||
| Male | 618 (56.4) | 507 (57.8) | 111 (50.7) | |
| Female | 478 (43.6) | 370 (42.2) | 108 (49.3) | |
| Age (years), median (IQR) | 54.0 (46.0–64.0) | 54.0 (46.0–64.0) | 55.0 (46.0–63.0) | 0.860 |
| Medication history (n [%]) | ||||
| Use of Antithrombotics | 30 (2.7) | 26 (3.0) | 4 (1.8) | 0.489 |
| Use of Immunosuppressants | 3 (0.3) | 1 (0.1) | 2 (0.9) | 0.104 |
| Use of NSAIDs | 4 (0.4) | 3 (0.3) | 1 (0.5) | 1.000 |
| Comorbidity (n [%]) | ||||
| Hypertension | 191 (17.4) | 155 (17.7) | 36 (16.4) | 0.740 |
| Diabetes mellitus | 40 (3.6) | 33 (3.8) | 7 (3.2) | 0.843 |
| History of malignancy | 51 (4.7) | 46 (5.2) | 5 (2.3) | 0.093 |
| History of abdominal operation | 119 (10.9) | 97 (11.1) | 22 (10.0) | 0.756 |
| History of intestinal polyp removal | 31 (2.8) | 27 (3.1) | 4 (1.8) | 0.440 |
| Tumor size (mm), median (IQR) | 15.0 (10.0–23.0) | 15.0 (10.0–23.0) | 15.0 (10.0–23.0) | 0.768 |
| Tumor location (n [%]) | 0.099 | |||
| Right-side colon a | 148 (13.5) | 119 (13.6) | 29 (13.2) | |
| Left-side colon b | 178 (16.2) | 132 (15.1) | 46 (21.0) | |
| Rectum | 770 (70.3) | 626 (71.4) | 144 (65.8) | |
| Pathological invasion depth (n [%]) | 0.221 | |||
| Mucosal layer | 593 (54.1) | 463 (52.8) | 130 (59.4) | |
| Submucosal layer | 479 (43.7) | 394 (44.9) | 85 (38.8) | |
| Others | 24 (2.2) | 20 (2.3) | 4 (1.8) | |
| Histological findings (n [%]) | 0.475 | |||
| Adenoma (LGIN + HGIN) | 441 (40.2) | 347 (39.6) | 94 (42.9) | |
| Adenocarcinoma | 149 (13.6) | 119 (13.6) | 30 (13.7) | |
| Submucosal benign lesion | 412 (37.6) | 339 (38.7) | 73 (33.3) | |
| Others | 94 (8.6) | 72 (8.2) | 22 (10.0) | |
| Morphology (n [%]) | 0.304 | |||
| 0-Is/Ip | 99 (9.0) | 76 (8.7) | 23 (10.5) | |
| LST | 508 (46.4) | 400 (45.6) | 108 (49.3) | |
| SMT | 489 (44.6) | 401 (45.7) | 88 (40.2) | |
| Resection (n [%]) | 0.093 | |||
| Complete en bloc resection | 960 (87.6) | 776 (88.5) | 184 (84.0) | |
| Piecemeal resection | 136 (12.4) | 101 (11.5) | 35 (16.0) | |
| Injury to muscle layer (n [%]) | 478 (43.6) | 385 (43.9) | 93 (42.5) | 0.759 |
| Non-lifting sign (n [%]) | 192 (17.5) | 151 (17.2) | 41 (18.7) | 0.671 |
| Electrocoagulation for ESD ulcer (n [%]) | 825 (75.3) | 658 (75.0) | 167 (76.3) | 0.773 |
| Complete ESD ulcer closure (n [%]) | 914 (83.4) | 729 (83.1) | 185 (84.5) | 0.705 |
| Inadequate bowel preparation (n [%]) | 67 (6.1) | 53 (6.0) | 14 (6.4) | 0.972 |
| Intraoperative perforation (n [%]) | 77 (7.0) | 59 (6.7) | 18 (8.2) | 0.532 |
| Intraoperative bleeding (n [%]) | 206 (18.8) | 164 (18.7) | 42 (19.2) | 0.948 |
| ESD procedure time (min), median (IQR) | 26.0 (17.0–40.0) | 25.0 (16.0–40.0) | 28.0 (18.0–45.0) | 0.177 |
| Operator (n [%]) | 0.768 | |||
| Trainee | 210 (19.2) | 166 (18.9) | 44 (20.1) | |
| Expert | 886 (80.8) | 711 (81.1) | 175 (79.9) | |
| Immediate antibiotics therapy (n [%]) | 195 (17.8) | 149 (17.0) | 46 (21.0) | 0.197 |
| Hospitalization, median (IQR) | 7.0 (5.0–9.0) | 7.0 (5.0–8.0) | 7.0 (6.0–9.0) | 0.142 |
| Fasting period, median (IQR) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 0.910 |
| Fever | 204 (18.6) | 161 (18.4) | 43 (19.6) | 0.736 |
IQR, interquartile range; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs; LGIN, low-grade intraepithelial neoplasia; HGIN, high-grade intraepithelial neoplasia; Is, sessile configuration; Ip, pedunculated configuration; LST, laterally spreading tumor; SMT, submucosal tumor; ESD, endoscopic submucosal dissection.
aRight-side colon: cecum, ascending colon, and transverse colon.
bLeft-side colon: descending colon and sigmoid colon.
Table 3.
Clinical characteristics between non-fever and fever groups of the training cohort.
| Variables | All subjects (N = 877) | Non-fever (N = 716) | Fever (N = 161) | p value |
|---|---|---|---|---|
| Sex (n [%]) | 0.091 | |||
| Male | 507 (57.8) | 424 (59.2) | 83 (51.6) | |
| Female | 370 (42.2) | 292 (40.8) | 78 (48.4) | |
| Age (years), median (IQR) | 54.0 (46.0–64.0) | 54.0 (46.0–64.0) | 57.0 (50.0–66.0) | 0.023* |
| Age > 50 years (n [%]) | 541 (61.7) | 424 (59.2) | 117 (72.7) | 0.002* |
| Medication history (n [%]) | ||||
| Use of Antithrombotics | 26 (3.0) | 20 (2.8) | 6 (3.7) | 0.709 |
| Use of Immunosuppressants | 1 (0.1) | 0 (0.0) | 1 (0.6) | 0.413 |
| Use of NSAIDs | 3 (0.3) | 2 (0.3) | 1 (0.6) | 1.000 |
| Comorbidity (n [%]) | ||||
| Hypertension | 155 (17.7) | 125 (17.5) | 30 (18.6) | 0.811 |
| Diabetes mellitus | 33 (3.8) | 25 (3.5) | 8 (5.0) | 0.509 |
| History of malignancy | 46 (5.2) | 39 (5.4) | 7 (4.3) | 0.712 |
| History of abdominal operation | 97 (11.1) | 82 (11.5) | 15 (9.3) | 0.521 |
| History of intestinal polyp removal | 27 (3.1) | 25 (3.5) | 2 (1.2) | 0.215 |
IQR, interquartile range; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs. *P-value < 0.05.
Table 4.
Endoscopic and pathological findings between non-fever and fever groups of the training cohort.
| Variables | All subjects (N = 877) | Non-fever (N = 716) | Fever (N = 161) | p value |
|---|---|---|---|---|
| Tumor size (mm), median (IQR) | 1.5 (1.0–2.3) | 1.4 (1.0–2.1) | 2.0 (1.3–3.1) | < 0.001* |
| Tumor location (n [%]) | 0.417 | |||
| Right-side colon a | 119 (13.6) | 92 (12.8) | 27 (16.8) | |
| Left-side colon b | 132 (15.1) | 108 (15.1) | 24 (14.9) | |
| Rectum | 626 (71.4) | 516 (72.1) | 110 (68.3) | |
| Pathological invasion depth (n [%]) | 0.003* | |||
| Mucosal layer | 463 (52.8) | 364 (50.8) | 99 (61.5) | |
| Submucosal layer | 394 (44.9) | 339 (47.3) | 55 (34.2) | |
| Others | 20 (2.3) | 13 (1.8) | 7 (4.3) | |
| Histological findings (n [%]) | < 0.001* | |||
| Adenoma (LGIN + HGIN) | 347 (39.6) | 262 (36.6) | 85 (52.8) | |
| Adenocarcinoma | 119 (13.6) | 92 (12.8) | 27 (16.8) | |
| Submucosal benign lesion | 339 (38.7) | 300 (41.9) | 39 (24.2) | |
| Others | 72 (8.2) | 62 (8.7) | 10 (6.2) | |
| Morphology (n [%]) | < 0.001* | |||
| 0-Is/Ip | 76 (8.7) | 65 (9.1) | 11 (6.8) | |
| LST | 400 (45.6) | 299 (41.8) | 101 (62.7) | |
| SMT | 401 (45.7) | 352 (49.2) | 49 (30.4) | |
| Resection (n [%]) | 0.007* | |||
| Complete en bloc resection | 776 (88.5) | 644 (89.9) | 132 (82.0) | |
| Piecemeal resection | 101 (11.5) | 72 (10.1) | 29 (18.0) | |
| Injury to muscle layer (n [%]) | 385 (43.9) | 268 (37.4) | 117 (72.7) | < 0.001* |
| Non-lifting sign (n [%]) | 151 (17.2) | 96 (13.4) | 55 (34.2) | < 0.001* |
| Electrocoagulation for ESD ulcer (n [%]) | 658 (75.0) | 529 (73.9) | 129 (80.1) | 0.121 |
| Complete ESD ulcer closure (n [%]) | 729 (83.1) | 594 (83.0) | 135 (83.9) | 0.876 |
| Inadequate bowel preparation (n [%]) | 53 (6.0) | 47 (6.6) | 6 (3.7) | 0.237 |
| Intraoperative perforation (n [%]) | 59 (6.7) | 33 (4.6) | 26 (16.1) | < 0.001* |
| Intraoperative bleeding (n [%]) | 164 (18.7) | 124 (17.3) | 40 (24.8) | 0.036* |
| ESD procedure time (min), median (IQR) | 25 (16–40) | 23 (16–35) | 40 (24–57) | < 0.001* |
| ESD procedure time > 30 min (n [%]) | 321 (36.6) | 215 (30.0) | 106 (65.8) | < 0.001* |
| Operator (n [%]) | 1.000 | |||
| Trainee | 166 (18.9) | 136 (19.0) | 30 (18.6) | |
| Expert | 711 (81.1) | 580 (81.0) | 131 (81.4) |
IQR, interquartile range; LGIN, low-grade intraepithelial neoplasia; HGIN, high-grade intraepithelial neoplasia; Is, sessile configuration; Ip, pedunculated configuration; LST, laterally spreading tumor; SMT, submucosal tumor; ESD, endoscopic submucosal dissection. *P-value < 0.05.
aRight-side colon: cecum, ascending colon, and transverse colon.
bLeft-side colon: descending colon and sigmoid colon.
Table 5.
Clinical course after colorectal ESD between non-fever and fever groups of the training cohort.
| Variables | All subjects (N = 877) | Non-fever (N = 716) | Fever (N = 161) | p value |
|---|---|---|---|---|
| Immediate antibiotics therapy (n [%]) | 149 (17.0) | 115 (16.1) | 34 (21.1) | 0.153 |
| Hospitalization (d), median (IQR) | 7.0 (5.0–8.0) | 7.0 (5.0–8.0) | 7.0 (6.0–9.0) | < 0.001* |
| Fasting period (d), median (IQR) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 3.0 (2.0–3.5) | < 0.001* |
IQR, interquartile range; ESD, endoscopic submucosal dissection. *P-value < 0.05.
Risk factors of fever after colorectal ESD
To identify the potential risk factors for the occurrence of fever following colorectal ESD, a comparison between the fever and non-fever groups was conducted, as detailed in Tables 3 and 4. Univariate analysis revealed that age > 50 years (P = 0.002), tumor size (P < 0.001), pathological invasion depth (P = 0.003), histological findings (P < 0.001), morphology (P < 0.001), piecemeal resection (P = 0.007), injury to muscle layer (P < 0.001), non-lifting sign (P < 0.001), intraoperative perforation (P < 0.001), intraoperative bleeding (P = 0.036) and ESD procedure time > 30 min (P < 0.001) were statistically significantly associated with the development of fever after colorectal ESD.
Based on the identified potential risk factors mentioned above, these 11 variables were subsequently included in a multivariate logistic regression analysis using backward stepwise regression (Table 6). The analysis identified tumor size (OR: 1.34, 95% CI: 1.14–1.58, P < 0.001), ESD procedure time > 30 min (OR: 2.17, 95% CI: 1.41–3.35, P < 0.001), injury to muscle layer (OR: 2.64, 95% CI: 1.74–4.03, P < 0.001), and intraoperative perforation (OR: 1.84, 95% CI: 1.00–3.33, P = 0.046) as independent risk factors for the development of fever following colorectal ESD.
Table 6.
Risk factors associated with fever by multivariate logistic regression (backward stepwise).
| Variables | B | SE | Wald | OR (95% CI) | p value |
|---|---|---|---|---|---|
| Tumor size | 0.29 | 0.08 | 12.05 | 1.34 (1.14–1.58) | < 0.001 |
| ESD procedure time > 30 min | 0.79 | 0.22 | 12.37 | 2.17 (1.41–3.35) | < 0.001 |
| Injury to muscle layer | 0.97 | 0.21 | 20.44 | 2.64 (1.74–4.03) | < 0.001 |
| Intraoperative perforation | 0.60 | 0.30 | 3.99 | 1.84 (1.00–3.33) | 0.046 |
ESD, endoscopic submucosal dissection; OR, odds ratio; CI, confidence interval.
Establishment and validation of the nomogram model
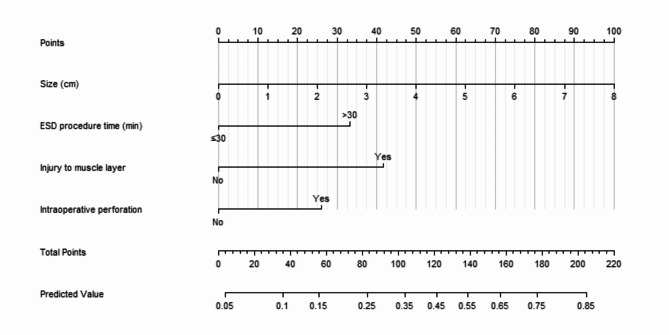
Utilizing the four independent risk factors identified through multivariate logistic regression, a predictive nomogram for the occurrence of fever following colorectal ESD was developed (Fig. 2). Each variable was assigned a specific score respectively on its corresponding axis, and the total score, calculated by summing the scores of all independent risk factors, corresponds to the risk of fever after colorectal ESD.
Fig. 2.
Nomogram for predicting the risk of fever after colorectal ESD.
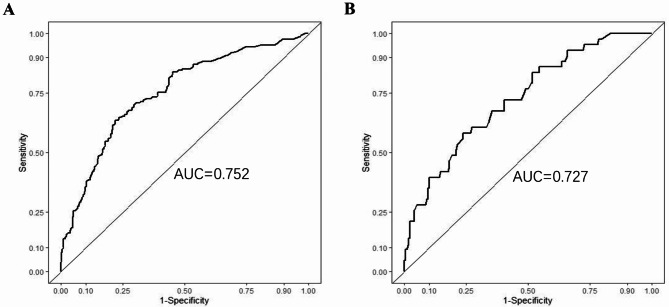
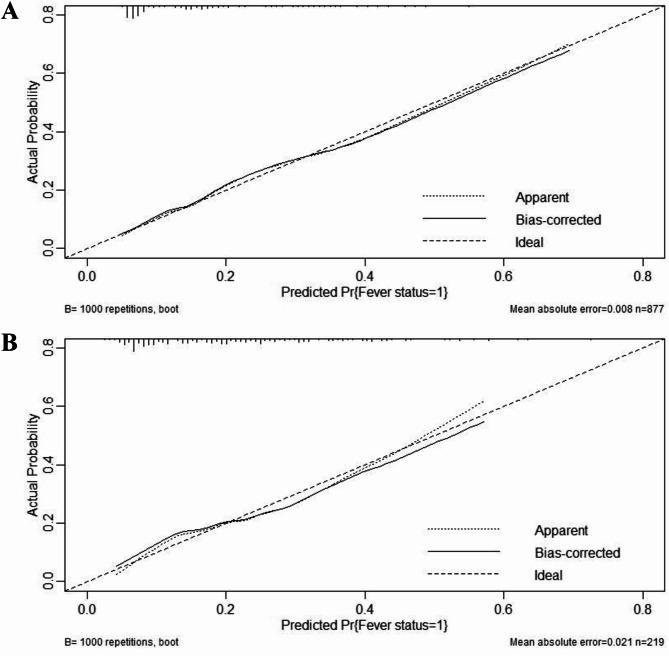
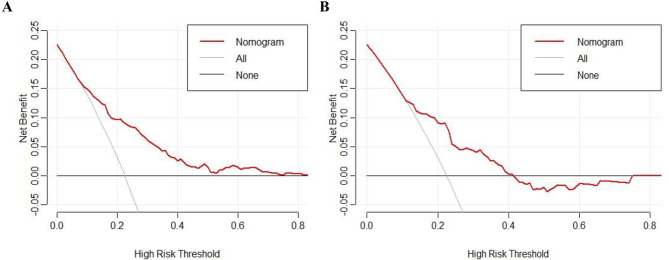
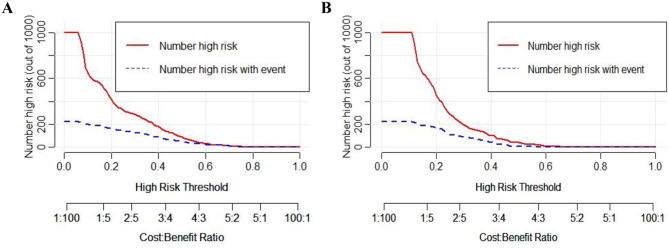
The predictive performance of this nomogram model was assessed using ROC curve, yielding an AUC of 0.752 (95% CI: 0.710–0.794) for the training group and 0.727 (95% CI: 0.645–0.810) for the validation group, indicating good discriminative ability (Fig. 3). The calibration curve for the predictive model exhibited strong consistency between predicted probabilities and actual observations in both the training and validation groups (Fig. 4). Furthermore, the DCA was performed for both groups to evaluate the clinical utility of the nomogram in improving clinical decision-making (Fig. 5). In addition, the CIC further demonstrated favorable concordance within a specific range between the estimated number of high-risk patients and the actual occurrence of fever following colorectal ESD across different risk thresholds in both training and validation group (Fig. 6).
Fig. 3.
ROC curve of prediction model for fever after colorectal ESD in the training (A) and validation (B) groups. ROC = receiver operating characteristic. AUC = area under the curve.
Fig. 4.
Calibration curves of the nomogram model in the training (A) and validation (B) groups. “Fever status = 1” means “Fever”, “Pr” means “Probability”.
Fig. 5.
DCA curves of the nomogram model in the training (A) and validation (B) groups. DCA = decision curve analysis.
Fig. 6.
CIC curves of the nomogram model in the training (A) and validation (B) groups. CIC = clinical impact curve.
Discussion
As a common postoperative adverse events of ESD, fever is somewhat neglected in clinical practice compared to other serious complications such as delayed bleeding and perforation8. Fever is frequently observed as a clinical manifestation of delayed perforation after colorectal ESD, and may serve as an early warning sign of this complication. Moreover, postoperative fever often increases anxiety and depression among patients and their families, increase the risk of complications, and adversely affects clinical outcomes27.
In our study, the incidence of fever after colorectal ESD was 18.6%, and the length of hospitalization and fasting in fever group was significantly longer than non-fever group patients. Although there was no patient died, one patient had delayed perforation following fever. Therefore, it is crucial to explore the risk factors for fever after ESD to identify the greater risk of delayed perforation, and reduce the anxiety and depression of patients and improve their clinical outcomes. However, compared to the stomach and esophagus, there are few large sample studies on the risk factors for fever after colorectal ESD, and no studies have established a predictive model for fever after colorectal ESD currently. Therefore, based on our center’s large sample size database, we screened out the risk factors and successfully constructed the first nomogram model to predict the occurrence of fever after colorectal ESD with a satisfactory predictive performance.
To facilitate the practical application of the developed nomogram in clinical settings, its use can be integrated into the preoperative and intraoperative procedures for patients undergoing colorectal ESD. The nomogram incorporates easily accessible clinical variables such as lesion size, ESD procedure time, injury to the muscle layer, and intraoperative perforation, which can be obtained intraoperatively or immediately after the procedure. By calculating the total score based on these variables, clinicians can determine the corresponding risk of postoperative fever using the predicted value scale provided. Furthermore, patients with a higher risk score may benefit from the enhanced postoperative observation and monitoring.
At present, the specific pathogenesis for the occurrence of fever after colorectal ESD is unclear yet. As an invasive procedure, the mucosal defect after ESD is exposed to a large number of indigenous bacterial flora or feces28. Postoperative fever is often associated with infection and increased inflammatory reaction in clinical practice29. In Kato et al.29 showed there was an increase in the plasma endotoxin levels and CRP after gastric ESD, while the risk of bacteremia was low. Moreover, Izumi et al.10 also indicated there was a low or very low probability of bacteremia after colorectal ESD by blood samples culture and 16S rRNA gene analysis. However, in 2021, Yamamoto et al.9 found the low number of bacteria after colorectal ESD by 16S rRNA gene analysis, but it did not associate with the occurrence of fever after colorectal ESD. Therefore, the evidence supporting the occurrence of bacteremia after ESD is limited currently, and the prophylactic antibiotics therapy on fever after colorectal ESD also remains controversial30,31. In our actual clinical practice, we usually perform blood culture for those with moderate fever after colorectal ESD. Consistent with the above previous studies, our study also indicated a low probability of bacteremia after colorectal ESD and prophylactic antibiotics therapy had no statistically significant between fever group and non-fever group.
Previous studies have suggested the risk factors for fever after colorectal ESD including elder, large lesion size, injury to muscle layer, intraoperative perforation and poor physical status and so on9,10, and the risk factors for fever after esophageal and gastric ESD including elder, large lesion size, long ESD operation time, postoperative nasogastric tube placement and intraoperative bleeding and so on32–35. In our study, the above findings were further confirmed. Large lesion size, ESD operation time > 30 min, injury to muscle layer and intraoperative perforation were identified as the independent risk factors for fever after colorectal ESD. Due to the low incidence of bacteremia after colorectal ESD, large lesion size, long ESD operation time and injury to muscle were likely to associated with more repetitive electrocoagulation during colorectal ESD, which may cause a temporary transmural burn and localized peritoneal inflammation at the resection site, resulting in an increased risk of postoperative fever9. Moreover, in our study, similar to the prophylactic antibiotics therapy, the prophylactic complete ESD ulcer closure also had no relationship with postoperative fever. Therefore, our study further confirmed that transmural damage by electrocoagulation during colorectal ESD was more likely to cause postoperative fever than bacteremia from mucosal defects. As a common complication of ESD procedure, intraoperative perforation was often associated with postoperative fever in previous studies9,33, and our study also confirmed this result. Therefore, in order to prevent occurrence of fever after colorectal ESD, large colorectal lesions should be paid more attention, and endoscopists should try to avoid muscle layer injury and intraoperative perforation as much as possible during colorectal ESD procedure.
To our best knowledge, this is the first study to establish a nomogram model for predicting fever after colorectal ESD, and it had a satisfactory predictive performance and clinical practicability. Our study has the following limitations. First, although our sample size was relatively large, this was a single-center retrospective study and had an inherent selective bias, rendering its generalizability to other countries and hospitals limited. Second, as a retrospective study, in addition to patients with moderate and high fever, patients with low fever may not had the records on blood culture and chest and abdominal computer tomography (CT) examination in our clinical practice, which makes it difficult to fully understand the cause of fever. Therefore, multi-center prospective clinical studies with large sample size are still needed in future.
Conclusion
In summary, present study found that large lesion size, ESD operation time > 30 min, injury to muscle layer and intraoperative perforation were independent risk factors of fever after colorectal ESD. Based on these factors, the proposed nomogram exhibited excellent performance and could accurately predict the risk of fever after colorectal ESD with a satisfactory clinical practicability. As a convenient and effective clinical tool, the nomogram could identify the high-risk patients with fever after colorectal ESD and help clinicians to timely inform patients in advance. This could allow patients benefit from identifying the greater risk of delayed perforation and avoiding anxiety and depression, and improve their clinical prognosis.
Acknowledgements
This work was supported by the Key Laboratory Project of Digestive Diseases in Jiangxi Province (2024SSY06101), and Jiangxi Clinical Research Center for Gastroenterology (20223BCG74011).
Author contributions
J.Q., Y.X. and Y.Z. collected data, analyzed relevant information, and drafted the manuscript. Q.O., L.W. and R.D. contributed to statistical analysis and manuscript revision. X.P. and X.S. clinically managed the patients. X.P. designed the study, critically revised the paper and approved the final submission. All authors contributed to the article and approved the submitted version.
Funding
This manuscript was supported by the Science and Technology Department of JiangxiProvince (No. 20192BAB215034); the Science and Technology Foundation of the Department of Education of Jiangxi Province (NO. GJJ210230); Scientific Research of Health Commission of Jiangxi Province (NO. 202310393).
Data availability
The clinical data were not made public to protect the privacy of the patients. However, the data can be made available upon reasonable request by emailing the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiayu Qiu, Yanhong Xia and Yanxia Zhang are equal primary authors.
References
- 1.Qi, Z. P. et al. Efficacy and safety of endoscopic submucosal dissection for submucosal tumors of the colon and rectum. Gastroint. Endosc.87, 540–548 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Colak, S., Gurbulak, B., Cakar, E. & Bektas, H. Evaluation of endoscopic mucosal resection and endoscopic submucosal dissection in submucosal lesions of the colon and rectum. Wideochir. Inne. Tech. Maloinwazyjne.13, 448–453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada, M. et al. Long-term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms in 423 cases: A retrospective study. Endoscopy.49, 233–242 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Shigita, K. et al. Long-term outcomes after endoscopic submucosal dissection for superficial colorectal tumors. Gastrointest. Endosc.85, 546–553 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Terasaki, M. et al. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J. Gastroenterol. Hepatol.27, 734–740 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi, Y. et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: Retrospective exploratory factor analysis of a multicenter prospective cohort. Int. J. Colorectal. Dis.29, 1275–1284 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Santos, J. B. et al. Risk factors for adverse events of colorectal endoscopic submucosal dissection: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol.33, e33–e41 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Sun, J. et al. Complications after endoscopic submucosal dissection for early colorectal cancer (Review). Oncol. Lett.25, 264 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto, S. et al. Fever and electrocoagulation syndrome after colorectal endoscopic submucosal dissection for patients with immunosuppressants and steroids. DEN. open.2, e83 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumi, K. et al. Frequent occurrence of fever in patients who have undergone endoscopic submucosal dissection for colorectal tumor, but bacteremia is not a significant cause. Surg. Endosc.28, 2899–2904 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Iwatsubo, T. et al. Differences in clinical course of intraprocedural and delayed perforation caused by endoscopic submucosal dissection for colorectal neoplasms: A retrospective study. Dig. Dis.37, 53–62 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa, K. et al. Coagulation syndrome: Delayed perforation after colorectal endoscopic treatments. World. J. Gastrointest. Endosc.7, 1055–1061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese Society for Cancer of the, C. & Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J. Anus. Rectum. Colon.3, 175–195 (2019). [DOI] [PMC free article] [PubMed]
- 14.Fuccio, L. et al. Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients. Clin. Gastroenterol. Hepatol.19, 339–348 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Hotta, K. et al. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig. Endosc.22, 302–306 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Fujishiro, M. Endoscopic submucosal dissection for colorectal neoplasms. World. J. Gastrointest. Endosc.1, 32–38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung, T. L. D., Chow, C. W. S., Chan, P. T. & Kwok, K. H. Review on colorectal endoscopic submucosal dissection focusing on the technical aspect. Surg. Endosc.34, 3766–3787 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Tanaka, S. et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc.32, 219–239 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Xu, S., Chai, N., Tang, X., Linghu, E. & Wang, S. Risk factors of major intraoperative bleeding and postoperative bleeding associated with endoscopic submucosal dissection for gastric neoplasms. Chin. Med. J.135, 309–316 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujishiro, M. et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin. Gastroenterol. Hepatol.5, 678–683 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Huang, S. F. The World Health Organization and the Vienna classification of gastrointestinal epithelial neoplasia. Zhonghua. Bing. Li. Xue. Za. Zhi.34, 540–541 (2005). [PubMed] [Google Scholar]
- 22.Mayourian, J. et al. Pediatric ECG-based deep learning to predict left ventricular dysfunction and remodeling. Circulation149, 917–931 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui, R. W. et al. Multimodal multiphasic pre-operative image-based deep-learning predicts hepatocellular carcinoma outcomes after curative surgery. Hepatology. (2024). [DOI] [PubMed]
- 24.Alba, A. C. et al. Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA.318, 1377–1384 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Vickers, A. J. & Elkin, E. B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Making.26, 565–574 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr, K. F., Brown, M. D., Zhu, K. & Janes, H. Assessing the clinical impact of risk prediction models with decision curves: Guidance for correct interpretation and appropriate use. J. Clin. Oncol.34, 2534–2540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dionigi, R., Dionigi, G., Rovera, F. & Boni, L. Postoperative fever. Surg. Infect.7(Suppl 2), S17-20 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Min, B. H. et al. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest. Endosc.68, 105–110 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Kato, M. et al. Bacteremia and endotoxemia after endoscopic submucosal dissection for gastric neoplasia: Pilot study. Gastric. cancer.15, 15–20 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Muro, T. et al. Post-operative infection of endoscopic submucosal dissection of early colorectal neoplasms: A case-controlled study using a Japanese database. J. Clin. Pharm. Ther.40, 573–577 (2015). [DOI] [PubMed] [Google Scholar]
- 31.La Regina, D. et al. Clinical adverse events after endoscopic resection for colorectal lesions: A meta-analysis on the antibiotic prophylaxis. Digest. Dis.38, 15–22 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Xu, Z., Zhuang, J., Zhu, X. & Yao, J. A nomogram for predicting the risk of postoperative fever in elderly patients undergoing endoscopic submucosal dissection of the upper gastrointestinal tract. Medicine102, e36438 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai, Y. et al. Incidence and risk factors for fever after endoscopic submucosal dissection and its derivative technology for gastric lesions. Heliyon10, e25748 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao, F. et al. Risk factors for fever after esophageal endoscopic submucosal dissection and its derived technique. Front. Med.9, 713211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi, T. et al. Risk factors for pyrexia after endoscopic submucosal dissection of gastric lesions. Endosc. Int. Open.2, E141-147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical data were not made public to protect the privacy of the patients. However, the data can be made available upon reasonable request by emailing the corresponding author.