Abstract
Real-world data on treatment outcomes or the quality of large-scale chronic hepatitis B (CHB) treatment programs in sub-Saharan Africa (SSA) is extremely difficult to obtain. In this study, we aimed to provide data on the prevalence and incidence of mortality, loss to follow-up (LFTU), and their associated factors in patients with CHB in three treatment centres in Eritrea. Additional information includes baseline clinical profiles of CHB patients initiated on nucleos(t)ide analogue (NUCs) along with a comparison of treatment with Tenofovir disoproxil fumarate (TDF) vs. TDF + Lamuvudine (LAM) using specific biochemical, haematological and virologic parameters. A multicenter retrospective cohort study was conducted on CHB patients in Asmara, Eritrea (2018–2021). Demographic, clinical, and laboratory information was collected from medical records using a structured checklist. Relevant parametric and nonparametric statistics were employed to explore treatment outcomes and to evaluate differences between groups. Where appropriate, Kaplan-Meier (KM) curves and univariate and multivariate Cox regression models were implemented. A two-sided p-value < 0.05 was considered significant. A total of 413 patients with HBV (median age (IQR) at diagnosis: 39 (IQR: 28–50 years; females: 118(28.6%); followed for a total of 22,921 person days) were studied. HBV/HIV co-infection was observed in 15(3.6%) and baseline ALT and AST were elevated in 99(31.2%) and 101(32.8%), respectively. The Fibrosis-4 (FIB-4) index estimates suggested that cirrhosis was highly likely in 33 (14%) patients with 49 (20.8%) patients in the indeterminate FIB-4 score category. During the follow-up period, 4.6% (95% CI: 2.5–6.6%) died, while 23.9% (95% CI: 19.8–28%) were LTFU. In the adjusted Cox proportional hazards model, LTFU were independently associated with baseline serum HBV DNA (IU/mL) (aHR = 1.3, 95% CI 1.04–1.7; p-value = 0.02); Not initiated on NUC (aHR = 3.9, 95% CI: 1.1–13.7, p-value = 0.02); and FIB-4 Score (aHR = 1.05, 95% CI: 1-1.1; p-value = 0.01). Of the 413 patients enrolled in the study, 98 cases (23.73%) were initiated on treatment. In the head-to-head comparison of the results in TDF and TDF + LAM after 12 weeks of treatment, VR was observed in 14(45.2%) vs. 17(54.8%), respectively, translating into an overall VR of 60.7% (95% CI 46.9–74.6). Furthermore, VR in TDF vs. TDF + LAM were similar, 14(45.2%) vs. 17(54.4%) respectively, p-value = 0.3). This study uncovered multiple systems- and patient-centered gaps in the three HBV treatment programs in Asmara, Eritrea. These include late presentation, high incidence of LTFU, inconsistencies in routine data, and poor data management. Interventions should target improvements in laboratory infrastructure, adherence to patient monitoring guidelines, HBV literacy, better tracking of patients, and documentation of patient’s information.
Keywords: Virologic response, TDF, Mortality, Loss to follow-up, Chronic Hepatitis B, Eritrea
Subject terms: Gastroenterology, Medical research
Introduction
Infection by Hepatitis B virus (HBV) is regarded as a major global public health concern1. The According to a Global Burden of Disease (GBD) report published in 2022, prevalence of chronic HBV infection in 2019 stood at 4.1% (95% uncertainty interval [UI] 3.7–4.5%), corresponding to 316 million (UI: 284 million – 351 million) infections2,3. More recently, the World Health Organization reports suggested a slightly lower figure for HBV – 254 million. According to the WHO report, the number of patients declined from 3 million in 2019 to 2.2 million in 2022 d1. The major complications/sequelae of chronic HBV (CHB) infections - liver cirrhosis and hepatocellular carcinoma (HCC) were associated with [331 000 (UI: 279 000-392 000)] and [192 000 (UI: 162 000-224000)] global deaths respectively1. This translates into 48·8% (44·6–52·7%) of all hepatitis-related mortalities worldwide. Importantly, primary epidemiological indices such as incidence, prevalence, mortality, and years of life lost (YLL) are worse in low-middle income countries (LMICs) – particularly in the WHO Western Pacific region and sub-Saharan Africa (SSA)4.
Recognizing the problem posed by viral hepatitis, a plenary convened by the World Health Assembly (WHA) in 2016 discussed and adopted the WHO Global Health Sector Strategy on Viral Hepatitis (WHO-GHSS) containment and subsequent elimination plan5. Key impact goals (with 2015 a baseline) by 2020 and 2030 included a 30% vs. 10% and 95% vs. 65% reduction in incidence rates and HBV-related mortality in respective time frames5. Although partial success has been achieved with regard to reduction in CHB, HCC, mortality due to cirrhosis, and other extrahepatic sequelae6; no country is on track to meet the 2030 WHO-GHSS targets7. Inadequate access to diagnostics, poor awareness of active infection, and inadequate treatment services - a problem compounded by limited access to risk assessment tools such as non-invasive tests for fibrosis (e.g. transient elastography (TE)/Fibroscan©) remains a problem in LMIC in SSA8. In 2021, for example, WHO estimates indicated that only 6.6 million (5.3–8.3 million) of the 30.4 million (24.3–38 million) diagnosed with chronic HBV infection were placed on treatment3,9.
Even though available treatment options (interferon preparations - standard interferon alfa-2b, peginterferon alfa 2a [peg-alfa 2a]); nucleos (t)ide analogues (NUC) - Tenofovir disoproxil fumarate (TDF); Tenofovir alafenamide fumarate (TAF), Entecavir (ETV); and Lamivudine (3TC), Adefovir dipivoxil (ADF) can stop or slow progression to hard endpoints such as HCC, cirrhosis and end-stage liver disease (ESLD); they are not sterilizing cures10. Instead, patients require lifelong suppressive therapy and robust follow-up11. In SSA, these prospects are undermined by a spectrum of factors related to patients and the system that present formidable barriers to the sustainability of HBV treatment programs and the long-term health of patients. Late presentation or inadvertent delays in diagnosis are common occurrences in the WHO-AFRO region. Therefore, the probability of progression to fatal complications remains high11.
Beyond the above-stated factors, researchers agree that impact targets should be aligned with local epidemiological circumstances, health systems capacity and population structures and dynamics12. Interestingly, this requires high-quality data in a variety of HBV-related parameters: vaccination coverage, availability of treatment, patient monitoring, treatment results, mortality, and LTFU, among others. Unfortunately, real-world data on clinical profiles of patients, treatment outcomes, or the quality of care is extremely difficult to obtain in countries in the WHO-AFRO region. In the absence of data on program quality or health-related quality of life (HRQOL), gaps in large-scale government-funded test-and-treat programs are difficult to uncover. Currently, we are unaware of any study that has reported these parameters for HBV patients in Eritrea, a country with an HBV seroprevalence of 2.3%13,14. Therefore, we set out to accomplish several goals. First, to provide a comprehensive description of baseline clinical (including fibrosis/or cirrhosis status) and demographic characteristics of patients with HBV infection in three treatment centres in Zoba Maekel - the Central region of Eritrea. Secondly, an analysis of the prevalence and incidence of mortality, LFTU, and their associated factors was attempted. In a subsequent analysis of patients on NUCs; we aimed to provide comprehensive information on the clinical profile of CHB patients initiated on NUCs and compare outcomes of treatment with TDF vs. TDF + LAM using specific biochemical, haematological and virologic parameters.
Methods
Study design and settings
This retrospective observational cohort study was conducted on CHB patients enrolled (period: 2016–2021) in three treatment programs in Asmara, Eritrea. The country is located in the upper part of the Horn of Africa and covers a surface area of ~ 124,000 Km2. It is bounded to the North West by Sudan; to the East by the Red Sea; to the South by Ethiopia and the South East by Djibouti. Administratively, the country is divided into six regions (Zobas): Zoba Maekel (Central Region); Anseba; Gash-Barka, Debub; Semanawi Keih Bahri (SKB) (Northern Red Sea); and Debubawi Keih Bahri (DKB) (Southern Red Sea). Healthcare service is delivered, almost exclusively, by the Eritrea Ministry of Health (EMoH). At the time this study was undertaken, treatment for hepatitis B or C patients in the country was undertaken at specific tertiary-level facilities - Orotta National Referral Hospital, Halibet National Referral Hospital and Haz-Haz Zonal Referral Hospital. Therefore, patients are pooled both from the Central Region and other regions of Eritrea. Accordingly, data from these centres can provide vital information on the quality of care for patients with CHB virus infection in Eritrea.
Upon enrollment in the respective follow-up centres, patients are monitored [as per the EMoH Guidelines for Chronic Hepatitis B and C infection (2018)13—A document that draws heavily from the WHO15 at a scheduled interval (3 months). Relevant parameters evaluated at these intervals include liver transaminases [Aspartate aminotransferase (ALT) and Alanine aminotransferase (AST)]; serum HBV-DNA; HBV serology; complete blood count (CBC); ALT/platelet (PLT) ratio (APRI); and Hepatitis B surface antigen (HBsAg). Notably, assessments/tests such as serum bilirubin (BIL); prothrombin time (PT); liver ultrasound, and international normalized ratio (INR) are performed at the discretion of physician/or clinician and the costs of treatment are covered by the government. Importantly, specific tests such as HBV-DNA, confirmatory tests for HBV infection; and biochemical tests are undertaken at the National Health Laboratory (NHL). The HBV DNA titer is generally evaluated using HBV DNA real-time PCR assay (COBAS® AmpliPrep/COBAS® TaqMan® system (Roche Molecular Systems Inc., Branchburg, NJ, USA).
According to the Eritrea MOH guidelines, treatment should be initiated under the following circumstances: clinical evidence of HBV-related cirrhosis or advanced fibrosis (APRI > 2); age > 30 and persistently elevated ALT (ALT > 2 x ULN); HBV DNA > 20,000 IU / ml16. Of note, HBeAg status evaluation is not part of routine care. Antiviral medicines used for the management of HBV infection in adults in these facilities include Tenofovir disoproxil fumarate (TDF) (300 mg/day) and TDF + Lamivudine (LAM) (300 mg/day + 245 mg/day). After initiation of NUC therapy, HBV-DNA titer is quantified on a quarterly basis until an undetectable status is achieved and subsequently every six months.
Participants
As previously noted, patients enrolled in the three treatment (Orotta National Referral Hospital, Halibet National Referral Hospital and Haz-Haz Zonal Referral Hospital) centres for HBV management are referred from other facilities in the country. In 2019, several patients were referred following screening campaigns among specific subgroups17. At enrollment, a file is commissioned for each patient. Variables captured at baseline, or at timed intervals, during follow-up include demographic (Age at enrollment, sex, enrollment year, and address) and clinical (treatment status, NUC regimen; HIV status, HCV antibody status, liver transaminases, HBV-DNA, and enrolment duration) information. Additional variables include outcomes such as LTFU, and death.
During the study period (2018–2022), a total of 413 were enrolled in these facilities: Orotta National Referral Hospital [279(71.9%)], Halibet National Referral Hospital [104 (25.2%)], and Haz-Haz Zonal Referral Hospital [12 (2.9%)]. See Fig. 1 for additional information.
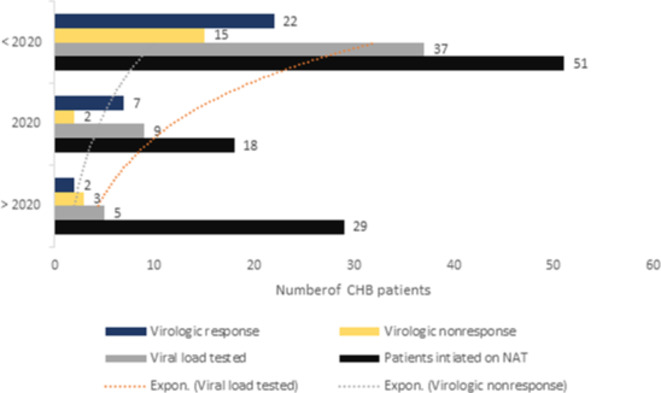
Fig. 6.
Relationship among viral load testing and virologic nonresponse detection.
Fig. 1.
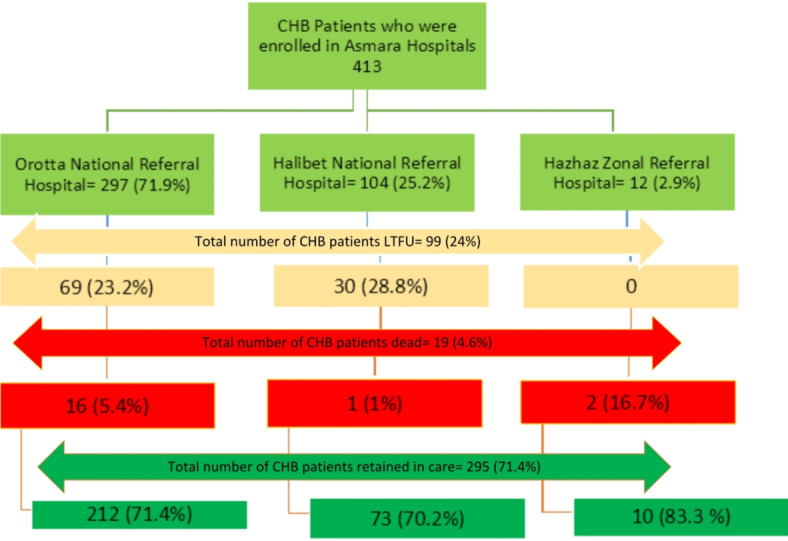
Flow diagram of chronic viral hepatitis B patients followed in three hospitals in Asmara, Eritrea.
Data collection tool
Data was collected via a structured checklist from each patient’s medical record. The card contains information on baseline status and information captured periodically during follow-up. Using this checklist, the following information was collected: sex, age at enrollment, address, enrollment year, marital status, treatment status, NUC regimen, HBV-DNA (baseline and follow-up), PLT, ALT, AST, BIL, HIV serostatus and HCV infection status. Follow-up outcomes (death and LTFU) were also collected. To meet specific ethical requirements, all participant data were de-identified.
Operational definition and study outcomes
Retention—Chronic HBV-infected patients who were alive and on follow-up in the clinics at the end of the study period.
The death events were defined as all-cause mortality occurring during the patient’s follow-up.
Loss-to-follow-up (LTFU) was defined as nonattendance of scheduled clinic appointments after enrollment into care.
The virologic response (VR) was defined as undetectable serum HBV DNA at least 12 weeks after initiation of treatment with NUCs18.
Virologic nonresponse was defined as HBV DNA levels > 1000 IU/ml at least 12 weeks following treatment initiation13.
Persistently elevated ALT levels (PNALT): 3 ALT evaluations > 40 U/L made at predefined intervals (12 months).
Cirrhosis probability was defined using FIB-4 (Non-invasive Fibrosis Evaluation in Hepatitis C) formula ([Age (years) × AST (U/L)] / [PLT (× 109/L) ×ALT1/2 (U/L)]. Computed scores are grouped as follows: Less likely (< 1.45 points), Indeterminate (1.45–3.25 points), and highly likely (> 3.25 points) [18].
In addition, the AST level to platelet ratio index (APRI) was also calculated: APRI = [(AST/upper limit of the normal AST level) × 100]/PLT (× 109/L). Computed scores were classified as follows: Cirrhosis unlikely (> 0.5 points), Cirrhosis possible (0.5-2 points), and Likely cirrhosis (> 2 points) [18].
Data processing and analysis
Analysis was conducted using IBM SPSS (version 26) and STATA version 12.0 (STATA Corporation, College Station, TX). FIB-4 and APRI scores were computed using relevant formula [18]. Descriptive statistics for categorical variables were analyzed using Chi-square (χ2)/Fisher’s exact test and summarized using proportions (percentages). For continuous variables, mean (± standard deviation (SD)) and median (interquartile range (IQR) were employed. Where appropriate, parametric (t-test) and nonparametric statistics (Mann-Whitney U and Kruskal Wallis) were used to evaluate differences. HBV DNA levels were logarithmically transformed for analysis. Kaplan-Meier (KM) curves were used to estimate survival rates and failure rates at different intervals of follow-up. The Univariate and Multivariate Cox regression model was implemented to assess the variables that predict LTFU. All predictors analyzed in bivariable Cox regression model were included in multivariable analysis. Adjustments were made using conditional: Log Rank (LR) approach. Model assumptions were evaluated using visual assessment of KM curves, log (-log) plots and testing of scaled Scheonfeld residuals. The goodness of fit was assessed using Cox-Snell residual analysis. The final results are presented as an adjusted or unadjusted hazard ratio with a 95% confidence interval (CI). A two-sided p-value < 0.05 was considered significant.
Ethical consideration
Ethical approval was obtained from the Ministry of Health (MOH) Research Ethics and protocol review committee with a letter of reference (Approval Number: Ref: 01/22). As the study also included data based on patients’ clinical cards, informed consent was waived by the Eritrean ministry of health research ethics and protocol review committee. All information gathered was DE identified, and at most confidentiality was upheld. All studies procedures also followed the recommendation of the Declaration of Helsinki Convention.
Results
A total of 413 patients were enrolled for care from 2016 to 2021 (149 (36.1%) < 2020; 104 (25.2%) = 2020; 160 (38.7%) > 2020). This translated into a total of 22,921 person-days of follow-up (PDFU) and a median duration of follow-up of 279 (IQR: 112–697) days. During this period, 19 (4.6%) died, 99 (24%) were LTFU and 295 (71.4%) were retained in care (Fig. 1).
Clinical and demographic characteristics of study participants
The median (IQR) age at diagnosis was 39 (IQR: 28–50) years and females accounted for 118 (28.6%) of the patient’s population. The majority of the participants were from the Central Zone, 246 (59.6%). HBV/HIV co-infection was observed in 15(3.6%). At enrollment, the median titer of HBV DNA (IQR), hemoglobin, PLT, ALT and AST were 971 (IQR: 109–288832) IU/mL, 15.3 g/dL (IQR: 14–16.3 g/dL); 216 (154–290) x 103UL; 28 IU/L (19–47 IU/L) and 30 IU/L (23–48 IU/L), respectively. Baseline ALT and AST were elevated in 99 (31.2%) and 101 (32.8%), respectively. See Table 1 for additional details.
Table 1.
Characteristics of study participants stratified by survival outcome.
| Characteristics | Population (%) | Retention n (%) | LTFU (%) | Dead (%) | P-value(χ2) |
|---|---|---|---|---|---|
| Total | 413 | 295 (71.4) | 99 (24) | 19 (4.6) | |
| Gender | |||||
| Male | 295 (71.4) | 207 (70.2) | 70 (70.7) | 18 (94.7) | 0.07 (5.3) |
| Female | 118 (28.6) | 88 (29.8) | 29 (29.3) | 1 (5.3) | |
| Age at enrollment in years, median (IQR) | 39 (28–50) | 40 (29–50) | 33 (24–52) | 41 (35–48) | 0.14a |
| < 21 | 22 (5.4) | 12 (4.1) | 10 (10.1) | 0 | 0.3 (6.7) |
| 21–50 | 293 (71.3) | 213 (72.4) | 67 (67.7) | 13 (72.2) | |
| 51–60 | 67 (16.3) | 48 (16.3) | 15 (15.2) | 4 (22.2) | |
| > 60 | 29 (7.1) | 21 (7.1) | 7 (7.1) | 1 (5.6) | |
| Address | |||||
| Central Zone | 246 (59.6) | 179 (60.7) | 61 (61.6) | 6 (31.6) | 0.03 (6.5) |
| Outside central zone | 167 (40.4) | 116 (39.3) | 38 (38.4) | 13 (68.4) | |
| Enrollment year, median (IQR) | 2020 (2019–2021) | 2020 (2018–2020) | 2020 (2019–2021) | < 0.001a | |
| < 2020 | 149 (36.1) | 73 (24.7) | 65 (65.7) | 11 (57.9) | < 0.001 (94.7) |
| 2020 | 104 (25.2) | 66 (22.4) | 33 (33.3) | 5 (26.3) | |
| > 2020 | 160 (38.7) | 156 (52.9) | 1 (1) | 3 (15.8) | |
| Marital status | |||||
| Married | 114 (77.6) | 96 (76.2) | 13 (86.7) | 5 (83.3) | 0.6 (0.9) |
| Single | 33 (22.4) | 30 (23.8) | 2 (13.3) | 1 (16.7) | |
| Treatment status | |||||
| On treatment | 98 (23.7) | 72 (24.4) | 22 (22.2) | 4 (21.1) | 0.8 (0.2) |
| Not on treatment | 315 (76.3) | 223 (75.6) | 77 (77.8) | 15 (78.9) | |
| NAT regimen | |||||
| TDF | 48 (49) | 30 (41.7) | 15 (68.2) | 3 (75) | 0.007 (13.9) |
| TDF + LAM | 47 (48) | 40 (55.5) | 7 (31.8) | 0 | |
| HIV status | |||||
| Positive | 15 (3.6) | 12 (4.1) | 2 (2) | 1 (5.3) | 0.5 (5.9) |
| Negative | 59 (14.3) | 49 (16.6) | 8 (8.1) | 2 (10.5) | |
| No data entry | 339 (82.1) | 234 (79.3) | 89 (89.9) | 16 (84.2) | |
| HCV antibody status | |||||
| Negative | 242 (58.6) | 176 (59.7) | 54 (54.5) | 12 (63.2) | 0.6 (0.9) |
| No data entry | 171 (41.4) | 119 (40.3) | 45 (45.5) | 7 (36.8) | |
| FIB-4 score, median (IQR) | 0.9 (0.6–1.9) | 1 (0.6–1.8) | 0.9 (0.5–2.3) | 1.6 (0.6–15.7) | 0.7a |
| Less likely (< 1.45) | 154 (65.3) | 126 (65.3) | 25 (67.6) | 3 (50) | 0.9 (0.7) |
| Indeterminate (1.45–3.25) | 49 (20.8) | 40 (20.7) | 7 (18.9) | 2 (33.3) | |
| Highly likely (> 3.25) | 33 (14) | 27 (14) | 5 (13.5) | 1 (16.7) | |
| APRI score, median (IQR) | 0.3 (0.2–0.7) | 0.3 (0.2–0.7) | 0.4 (0.2–1.2) | 0.3 (0.2–7.5) | 0.7a |
| Unlikely cirrhosis (> 0.5 ) | 155 (63) | 129 (64.5) | 21 (55.3) | 5 (62.5) | 0.5 (3.1) |
| Cirrhosis possible (0.5-2) | 65 (26.4) | 53 (26.5) | 10 (26.3) | 2 (25) | |
| Cirrhosis likely (> 2) | 26 (10.6) | 18 (9) | 7 (18.4) | 1 (12.5) | |
| Initial Viral load log10 HBV/DNA, median (IQR) | 3.1 (2.1–4.5) | 3.1 (2.1–4.6) | 3.1 (2-4.6) | 3.3 (2.3–4.4) | 0.9a |
| Baseline haematology | |||||
| Hemoglobin (g/dL), median(IQR) | 15.3 (14-16.3) | 15.3 (14-16.3) | 15.2 (14–16) | 15.8 (15-19.7) | 0.3a |
| Platelets (×109/µL), median (IQR) | 216 (154–290) | 209 (151–305) | 221 (141–283) | 143 (61–192) | 0.1a |
| Baseline ALT (IU/L), median (IQR) | 28 (19–47) | 28 (19–44) | 32.5 (19.7–65) | 36 (18–57) | 0.4a |
| ALT < 20 | 94 (29.7) | 70 (29) | 21 (32.3) | 3 (27.3) | 0.4 (6) |
| ALT 21–40 | 124 (39.1) | 101 (41.9) | 21 (32.3) | 2 (18.2) | |
| ALT 41–80 | 67 (21.1) | 49 (20.3) | 14 (21.5) | 4 (36.4) | |
| ALT > 80 | 32 (10.1) | 21 (8.7) | 9 (13.8) | 2 (18.2) | |
| Baseline AST (IU/L), median (IQR) | 30 (23–48) | 29 (23–48) | 30.5 (22.7–63.5) | 24 (17.5–73.5) | 0.8a |
| AST < 20 | 49 (15.9) | 35 (15.3) | 12 (18.5) | 2 (14.3) | 0.2 (8) |
| AST 21–40 | 158 (51.3) | 121 (52.8) | 29 (44.6) | 8 (57.1) | |
| AST 41–80 | 64 (20.8) | 49 (21.4) | 15 (23.1) | 0 | |
| AST > 80 | 37 (12) | 24 (10.5) | 9 (13.8) | 4 (28.6) | |
| Serum Bilirubin, median (IQR) | 1 (0.1-2) | 1 (0.1-2) | 2 (1–2) | 2 (0.1-2) | 0.9a |
| Bil < 1 mg/dL | 139 (33.7) | 114 (38.6) | 20 (20.2) | 5 (26.3) | < 0.001 (19.2) |
| Bil > 1 mg/dL | 77 (18.6) | 60 (20.3) | 14 (14.1) | 3 (15.8) | |
| Missing data | 197 (47.7) | 121 (41) | 65 (65.7) | 11 (57.9) | |
| Duration of follow-up in days, median (IQR) | 279 (112–697) | 477 (207–859) | 40.5 (4.7–384) | 11 (6–75) | < 0.001 a |
| Duration of treatment in days, median (IQR) | 348 (100–868) | 537(141–1086) | 224(0-388) | 24(7–41) | < 0.001 a |
TDF: Tenofovir; LAM: Lamivudine; FTC: Emtricitabine; EFV: Efavirenz; Anti-HCV: Anti-hepatitis C antibody; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; IQR: Interquartile range; SD: Standard deviation; NUCs: Nucleot(s)ide analogue therapy; HBV: Hepatitis B virus; DNA: Deoxyribonucleic acid; HIV: Human Immunodeficiency virus. Bil: Bilirubin.
a Kruskal-Wallis test.
Significant values are in bold.
Liver fibrosis/or cirrhosis status and its association with specific host factors
According to estimates of the FIB-4 score, cirrhosis was highly likely in 33 (14%), indeterminate in 154 (65.3%) and less likely in 49 (20.8%) of the patients. Compared to participants with FIB-4 > 3.25 points, participants with FIB-4 < 1.45 points were younger - median (IQR) age of 33 years (IQR: 25–42 years) vs. 48 (IQR: 33–55) years, p-value < 0.001. On the contrary, the median log 10VL (IQR) baseline was significantly lower in participants with FIB-4 < 1.45 (FIB-4 < 1.45: 3.1 IU/mL (2.1–4.3 IU/mL) vs. FIB-4 > 3.25: 5.6 IU/mL (4.3–6.5 IU/mL), p-value < 0.001). No significant differences were observed across sex and treatment regimen (See Table 2 for additional details).
Table 2.
Characteristics of the patients and stages of the Fib-4 score among the study participants.
| Characteristics | Cirrhosis less likely | Indeterminate | Cirrhosis highly likely | P-value |
|---|---|---|---|---|
| Female n (%) | 42 (72.4) | 9 (15.5) | 25 (12.1) | 0.41 |
| Age in years, median (IQR) | 33 (25–42) | 48 (33–55) | 48 (42–60) | < 0.001a |
| Baseline AST (IU/L), median (IQR) | 27 (21–33) | 45 (24–80) | 86 (65–167) | < 0.001a |
| Baseline ALT (IU/L), median (IQR) | 24 (18–36) | 35 (22–77) | 67 (50–112) | < 0.001a |
| PLT (×109/µL) count, mean (± SD) | 264 (± 84) | 172 (± 57) | 82 (± 35) | < 0.0001b |
| Baseline Log10VL (IU/mL), median (IQR) | 3.1 (2.1–4.3) | 2.6 (2.1-5) | 5.6 (4.3–6.5) | < 0.001a |
| HGB (g/dL), median (IQR) | 15.8 (14.9–16.7) | 15 (13.1–16.2) | 15 (13.1–15.4) | 0.021a |
| NUCs, TDF (%) | 9(34.6) | 7(26.9) | 10(38.5) | 0.935 |
| NUCs, TDF + LAM (%) | 9(31) | 9(31) | 11(37.9) |
Abbreviations: AST: aspartate aminotransferase; ALT: Alanine aminotransferase; IQR: interquartile range; PLT: platelet count; HGB: Hemoglobin; TDF: Tenofovir; LAM: Lamivudine. NUCs: Nucleotide analogue therapy.
aOne-way ANOVA, b Kruskal-Wallis test.
Significant values are in bold.
Loss to follow-up and Mortality in the HBV care cascade
In this study, the reported mortality and LTFU in the clinic were 19 cases, 4.6% (95% CI 2.5–6.6) and 67 cases (23.9%, 95% CI: 19.8–28.1), respectively. Figure 2 shows the distribution of LFTU and deaths along the HBV cascade of care. LFTU and death at the follow-up appointment after diagnosis, 31 (33.3%) and 3 (15.7%); before the initiation of treatment 51 (51.5%) and 12(63.1%); on treatment with NUC, 9 (9%) and 4(21%); and post VR, 8(8%) and 0 (0%).
Fig. 2.
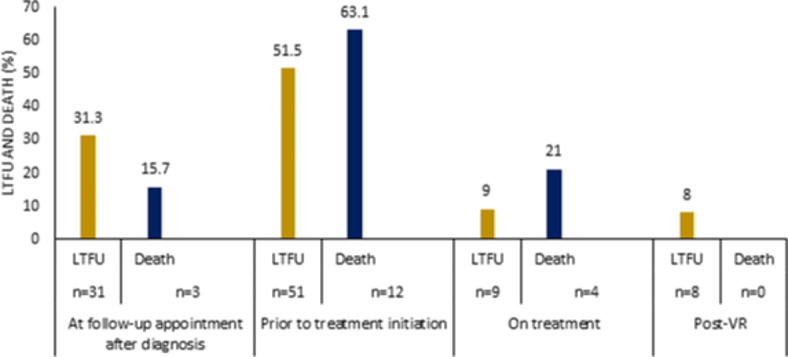
LTFU and death in chronic HBV care cascade.
Incidence rates, rate ratio, and Kaplan-Meier survival estimates for mortality
After 22, 921 (PDFU), the incidence rate of death was 0.82 (95% CI 0.52–1.3) per 1000 PDFU. In Kaplan-Meier survival analysis, male patients had a shorter mean survival duration (2,499 days (95% CI: 2452–2545) vs. 4667 days (95% CI: 4436–4897) and a higher relative risk (RR) of death, 7.3 (95% CI: 0.9–54.9) p-value log-rank 0.02) when compared to females. Further, a significantly shorter mean survival duration was observed in patient outside Central Zone (4461 days (95% CI: 4095–4826) and 4996 days (95% CI: 4885–5107), log-rank test p = 0.01) respectively. See Table 3for additional details.
Table 3.
Incidence rate and Kaplan-Meier survival estimates for mortality among chronic hepatitis B patients in Eritrea.
| Cohort characteristics | Person time (days) | death Events | Incidence of death per 1000 PDs (95% CI) | Relative risk rates (95% CI) | Mean duration of survival duration in days, 95% CI | P-value (log-rank) |
|---|---|---|---|---|---|---|
| Population | 229211 | 19 | 0.8 (0.5–1.3) | - | ||
| Gender | ||||||
| Female | 57523 | 1 | 0.1 (0.02–1.2) | 1 | 4667 (4436–4897) | 0.02 (5.1) |
| Male | 171688 | 18 | 1.04 (0.06–1.6) | 7.3 (0.9–54.9) | 2499 (2452–2545) | |
| Address | ||||||
| Central zone | 134618 | 6 | 0.4 (0.02–0.9) | 1 | 4996 (4885–5107) | 0.01 |
| Outside central zone | 94593 | 13 | 1.3 (0.7–2.3) | 3 (1-9.8) | 4461 (4095–4826) | |
| Enrollment year | ||||||
| ≤ 2019 | 161313 | 11 | 0.6 (0.03–1.2) | 1 | 4686 (4430–4942) | 0.2 (2.6) |
| 2020 | 39657 | 5 | 1.2 (0.05–3.02) | 0.8 (0.3–2.5) | 715 (679–751) | |
| ≥ 2021 | 28241 | 3 | 1.06 (0.03–3.2) | 0.3 (0.09–1.3) | 365 (357–372) | |
| Treatment status | ||||||
| Under treatment | 84194 | 4 | 0.4 (0.1–1.2) | 1 | 4892 (4682–5103) | 0.4 (0.4) |
| Not under treatment | 145017 | 15 | 1.03 (0.6–1.7) | 2.1 (0.6-9) | 4699 (4446–4952) | |
| AST | ||||||
| ≤ 40 U/L | 118503 | 10 | 0.8 (0.4–1.5) | 1 | 4717 (4436–4997) | 0.7 (0.1) |
| > 40 U/L | 70493 | 4 | 0.5 (0.2–1.5) | 0.6 (0.1–2.3) | 4862 (4622–5103) | |
| ALT | ||||||
| ≤ 40 U/L | 130614 | 5 | 0.3 (0.1–0.9) | 1 | 4954 (4778–5129) | 0.09 (2.8) |
| > 40 U/L | 63119 | 6 | 0.9 (0.4–2.1) | 2.4 (0.6–10.2) | 4752 (4472–5032) | |
| Non-invasive Fibrosis Evaluation in Hepatitis B (FIB-4) | ||||||
| Less likely (< 1.45) | 98142 | 3 | 0.3 (0.009-0.9) | 1 | 4697 (4507–4887) | 0.7 (0.5) |
| Indeterminate (1.45–3.25) | 41303 | 2 | 0.4 (0.01–1.9) | 1.9 (0.3–11.7) | 4907 (4595–5218) | |
| Highly likely (> 3.25) | 22027 | 1 | 0.4 (0.006-3.2) | 1.4 (0.1–14.1) | 4938 (4605–5272) | |
| Aspartate aminotransferase to platelet ratio index (APRI score) | ||||||
| Unlikely cirrhosis (> 0.5 ) | 88683 | 5 | 0.5 (0.02–1.3) | 1 | 4894 (4659–5129) | 0.9 (0.08) |
| Cirrhosis possible (0.5-2) | 56964 | 2 | 0.3 (0.008-1.4) | 0.8 (0.1–4.4) | 4673 (4433–4914) | |
| Cirrhosis likely (> 2) | 18597 | 1 | 0.5 (0.007-3.8) | 1.2 (0.1–10.4) | 4860 (4381–5340) | |
AST: aspartate aminotransferase; ALT: Alanine aminotransferase; CI: confidence interval; PDs: Person days of follow-up.
Incidence rates, rate ratio, and Kaplan–Meier survival estimates for LTFU
Table 4 displays Kaplan-Meier survival estimates. LTFU occurred in 99 patients, translating into a prevalence and incidence of 23.9% (95% CI 19.8–28.1) and 4.3 (95% CI: 3.5–5.2) per 1000 PDFU, respectively. In the Kaplan-Meier analysis of the relationship between the enrollment year and LTFU; the survival durations were as follows: ≤ 2019 = 2629 days (95% CI: 2151–3107); 2020 = 528 days (95% CI: 463–593); and 2021 = 369 days (95% CI: 365–374), log-rank test p < 0.001 (Fig. 3b). Similarly, patients not initiated on NUCs had shorter mean survival duration than those on treatment with NUCs, 2740 days (95% CI: 2039–3440) vs. 3217 days (95% CI: 2502–3932), log-rank test p < 0.07 (Fig. 3c).
Table 4.
Incidence rates, rate ratio and Kaplan-Meier survival estimates for LTFU.
| Cohort characteristics | Person time (days) | LTFU events | Incidence of LTFU per 1000 PDs (95% CI) | Relative risk rates (95% CI) | Mean duration of survival duration in days, 95% CI | P-value (log-rank) |
|---|---|---|---|---|---|---|
| Population | 229211 | 99 | 4.3 (3.5–5.2) | - | ||
| Gender | ||||||
| Male | 171688 | 70 | 4.07 (3.2–5.1) | 1 | 2966 (2460–3472) | 0.6 (0.1) |
| Female | 57523 | 29 | 5.04 (3.5–7.2) | 1.2 (0.7–1.9) | 1667 (1392–1942) | |
| Address | ||||||
| Central zone | 134618 | 61 | 4.5 (3.5–5.8) | 1 | 3164 (2631–3697) | 0.7 (0.1) |
| Outside central zone | 94593 | 38 | 4.01 (2.9–5.5) | 0.8 (0.5–1.3) | 2896 (2273–3520) | |
| Enrollment year | ||||||
| ≤ 2019 | 161313 | 65 | 4.02 (3.1–5.1) | 1 | 2629 (2151–3107) | < 0.001 (35.8) |
| 2020 | 39657 | 33 | 8.3 (5.9–11.7) | 1.5 (0.6–1.6) | 528 (463–593) | |
| ≥ 2021 | 28241 | 1 | 0.3 (0.04–2.5) | 0.02 (0.004-0.1) | 369 (365–374) | |
| Treatment status | ||||||
| Under treatment | 84194 | 22 | 2.6 (1.7–3.9) | 1 | 3217 (2502–3932) | 0.07 (3) |
| Not under treatment | 145017 | 77 | 5.3 (4.2–6.6) | 2 (1.2–3.4) | 2740 (2039–3440) | |
| AST | ||||||
| ≤ 40 U/L | 118503 | 41 | 3.4 (2.5–4.6) | 1 | 2952 (2175–3729) | 0.6 (0.2) |
| > 40 U/L | 70493 | 24 | 3.4 (2.2–5.07) | 0.9 (0.5–1.6) | 3352 (2729–3975) | |
| ALT | ||||||
| ≤ 40 U/L | 130614 | 42 | 3.2 (2.3–4.3) | 1 | 3143 (2450–3836) | 0.4 (0.5) |
| > 40 U/L | 63119 | 23 | 3.6 (2.4–5.4) | 1.1 (0.6–1.9) | 3310 (2640–3980) | |
| Non-invasive Fibrosis Evaluation in Hepatitis C (FIB-4) | ||||||
| Less likely (< 1.45) | 98142 | 25 | 2.5 (1.7–3.7) | 1 | 3592 (3027–4156) | 0.8 (0.2) |
| Indeterminate (1.45–3.25) | 41303 | 7 | 1.6 (0.8–3.5) | 0.7 (0.3–1.8) | 3995 (3103–4888) | |
| Highly likely (> 3.25) | 22027 | 5 | 2.2 (0.9–5.4) | 0.9 (0.3–2.4) | 3879 (2890–4868) | |
| Aspartate aminotransferase to platelet ratio index (APRI score) | ||||||
| Unlikely cirrhosis (> 0.5) | 88683 | 21 | 2.3 (15.4–36.3) | 1 | 3844 (3068–4620) | 0.2 (2.7) |
| Cirrhosis possible (0.5-2) | 56964 | 10 | 1.7(9.4–32.6) | 0.9 (0.4-2) | 3746 (3069–4423) | |
| Cirrhosis likely (> 2) | 18597 | 7 | 3.7 (17.9–78.9) | 1.9 (0.8–4.5) | 3175 (1961–4388) | |
AST: aspartate aminotransferase; ALT: Alanine aminotransferase; CI: confidence interval; PDs: Person days of follow-up.
Fig. 3.
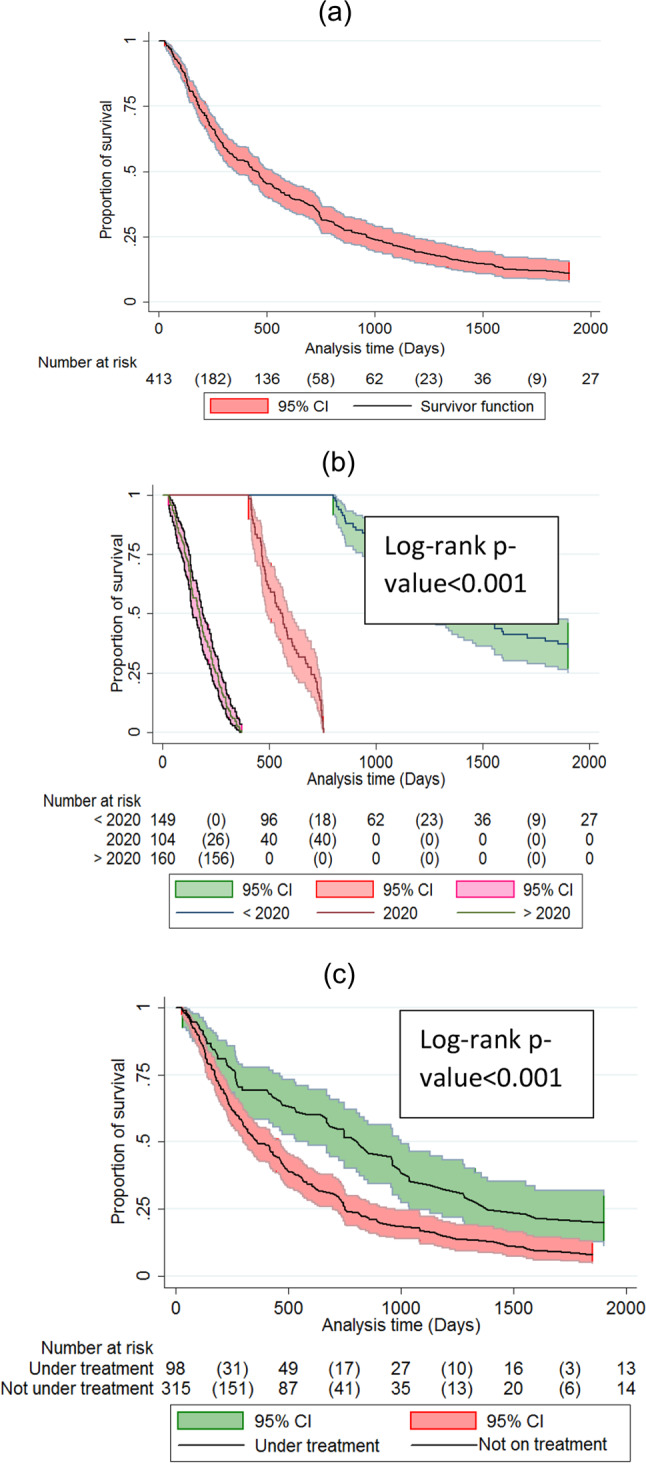
Kaplan-Meier curves for cumulative survival of chronic HBV patients followed in the three main treatment centres in Eritrea from 2018 to 2021. (a): overall cumulative proportion of survival; (b): overall cumulative proportion of survival by enrollment year; (c) cumulative proportion of survival by treatment status.
Univariate and multivariate analysis of independent predictors of LTFU in patients with CHB infections
In the adjusted Cox proportional hazards model, LTFU was independently associated with baseline serum HBV DNA (IU / ml) (aHR = 1.3, 95% CI: 1.04–1.7; p-value = 0.02); Not initiated in NUC (aHR = 3.9, 95% CI: 1.1–13.7, p-value = 0.02); and FIB-4 score (aHR = 1.05, 95% CI: 1-1.1; p-value = 0.01). See Table 5 for more details.
Table 5.
Cox proportional hazards of LTFU among chronic hepatitis B patients followed in the three treatment centres in Eritrea (2016–2021).
| Characteristics | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 1 (Ref) | 0.6 | ||
| Female | 1.09 (0.7–1.6) | |||
| Age at enrollment | 0.98 (0.97-1) | 0.1 | ||
| Address | ||||
| Central zone | 1 (Ref) | 0.7 | ||
| Outside central zone | 0.92 (0.6–1.3) | |||
| Enrollment year | 1.05 (0.9–1.1) | 0.2 | ||
| Baseline serum HBV DNA | 0.96 (0.8-1) | 0.5 | 1.3 (1.04–1.7) | 0.02 |
| Treatment status | ||||
| Under NAT | 1 (Ref) | 0.08 | 1 (Ref) | 0.02 |
| Not initiated on NAT | 1.5 (0.9–2.4) | 3.9 (1.1–13.7) | ||
| Baseline ALT | 0.9 | |||
| ≤ 40 U/L | 1 (Ref) | |||
| > 40 U/L | 1.2 (0.7-2) | 0.4 | ||
| Baseline AST | ||||
| ≤ 40 U/L | 1 (Ref) | 0.6 | ||
| > 40 U/L | 1.1 (0.6–1.8) | |||
| FIB score | 1.02 (0.9–1.07) | 0.2 | 1.05 (1-1.1) | 0.01 |
Abbreviations: ALT: alanine aminotransferase; AST: Aspartate Aminotransferase; CI: confidence interval; NAT: Nucleot(s)ide analogue therapy; HR: Hazards rate.
Clinical profile of CHB patients initiated on NUCS therapy
Of the 413 patients enrolled in the study, 98 cases (23.73%) were initiated on treatment. The median duration of treatment was 346 (IQR: 183–526) days. Reason for initiation of treatment included the following: APRI > 2, 18 (18.3%); HIV/HBV coinfection, 13 (13.2%); age > 30 and Persistently elevated ALT and HBV DNA > 20,000, 2 (2.1%); Persistently elevated ALT, 9 (19.3%); HBV DNA > 20,000 after 6 months of monitoring, 23 (23.4%) and clinically apparent presence of cirrhosis, 12 (12.2%). Furthermore, the patients in NUC (TDF and TDF + LAM) were significantly older (41 years (IQR: 24–53) and 40 years (IQR: 31–60) years vs. 39 years (IQR 27–48 years), p = 0.0.028); had a higher viral load (5.9 IU/mL (4.7-7) IU/mL and 6.4 IU/mL (4.5–7.3) IU/mL vs. 2.7 IU/mL (2-3.8) IU/mL; baseline ALT (49 IU / L (32 − 28) IU / L and 75 IU/L (47–145) vs. 23 IU/L17–35 IU/L). Furthermore, 18 CHB patients who were eligible for treatment were not on treatment [6 patients had APRI > 2, 6 cases had persistently elevated viral load and 6 more patients had persistently elevated ALT]. See Table 6 for additional details.
Table 6.
Clinical profile of CHB patients on NUCs.
| Variables | TDF | TDF + LAM | Not Initiated on NUCs | p-value |
|---|---|---|---|---|
| Age at enrollment in years, median (IQR) | 41 (24–53) | 40 (31–60) | 39 (27–48) | 0.028a |
| Initial viral load (IU/mL), median (IQR) | 5.9 (4.7-7) | 6.4 (4.5–7.3) | 2.7 (2-3.8) | < 0.001a |
| Baseline AST (IU/L), median (IQR) | 62 (43–135) | 65 (48–158) | 26 (21–34) | < 0.001a |
| Baseline ALT (IU/L), median (IQR) | 49 (32–78) | 75 (47–145) | 23 (18–36) | < 0.001a |
| Baseline Platelet count (×109/µL), median (IQR) | 118 (76–202) | 147 (76–187) | 221 (161–313) | < 0.001a |
| Baseline Hgb (g/dL ), median (IQR) | 14.8 (13–15) | 15.4 (14-16.3) | 15.7(14.9–16.5) | 0.46a |
| Baseline Bilirubin (mg/dL), median (IQR) | 1.2 (0.6–1.7) | 1 (0.8–1.4) | 0.7 (0.5–0.9) | < 0.001a |
| FIB-4 score, median (IQR) | 2.3 (1.1–10.3) | 1.8 (1.2–5.5) | 0.8 (0.6–1.2) | < 0.001a |
| APRI score, median (IQR) | 1.5 (0.7–5.3) | 1.3 (0.5–2.2) | 0.2 (0.2–0.4) | < 0.001a |
| Duration of follow-up (years), median (IQR) | 346 (71–880) | 991 (421–2361) | 433 (163–748) | < 0.001a |
Abbreviations: AST: aspartate aminotransferase; ALT: Alanine aminotransferase; IQR: interquartile range; PLT: platelet count; HGB: Hemoglobin; NAT: Nucleot(s)ide analogue.
a Kruskal-Wallis test.
The Impact of NUCs on selected laboratory parameters
In this analysis, both the TDF and TDF + LAM NA regimens resulted in a statistically significant improvement in laboratory parameters. ALT, AST, FIB-4, APRI, and platelet count decreased after treatment (Fig. 4).
Fig. 4.
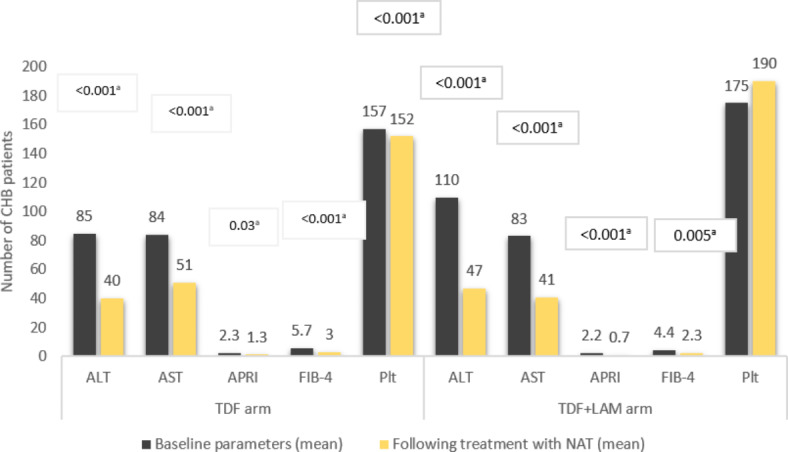
Alterations in laboratory parameters in response to NA therapy among chronic Hepatitis B infected individuals in Eritrea. Abbreviations: AST: Aspartate aminotransferase; ALT: Alanine aminotransferase. IQR: interquartile range; VL: Viral load.a Paired samples t-test p-value
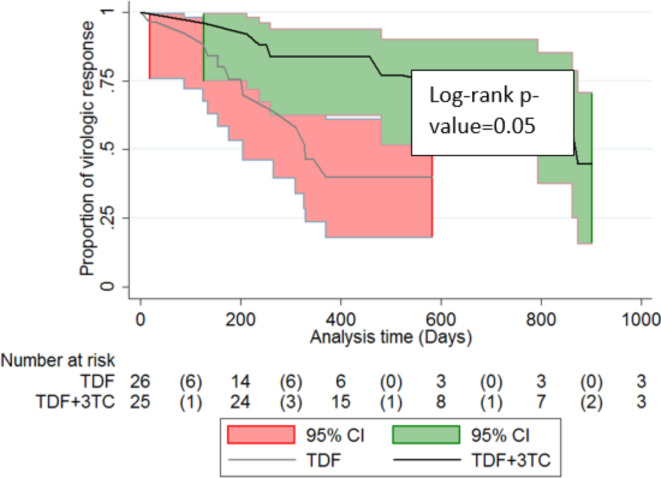
Survival function of virologic response (VR) in patients initiated on TDF monotherapy and TDF + LM regimen
In this analysis, 98 cases were followed for 26858 PD of treatment with NUCs. VR was observed in 31 cases, translating to an incidence rate of 11.5 (95% CI: 8.1–16.1) per 1000 PDs of treatment. Further, patients on TDF monotherapy had significantly shorter median survival duration as compared to those initiated on TDF + LM regimen, 874 (95% CI: 844–903) days vs. 330 (95% CI: 254–405) days, log-rank p-value = 0.05. See Fig. 5.
Fig. 5.
Kaplan-Meier curves for cumulative survival stratified by NUCs regimen.
Availability of viral load testing kits over time and its impact on treatment initiation
A strong time-dependent relationship was observed between the VL testing performed and VR and virologic nonresponse detection. We also found a potential connection between the availability of VL data and treatment initiation. See Figure Fig. 6 for more details.
Discussion
In response to the WHO-GHSS position that the elimination of HBV (in the long-term) will largely be determined by progress in achieving the HBV vaccination targets, early diagnosis, appropriate linkage to care, and subsequent retention in care19; Eritrea’s government established a government-funded test and treatment program for patients in 2018. Key study findings included the fact that the median (IQR) age at diagnosis in this cohort was 39 years (IQR: 28–50) years with a significantly lower proportion of females, 118(28.6%). Additional observations included a preponderance of patients from Central Zone. In contrast, LTFU was higher in patients in the central zone, while death rates were higher in patients outside the Central Zone. Other notable descriptors of the patients enrolled in the program include the high male-to-female ratio (2.5:1), the large number of patients from the central region, and the high number of patients with the APRI score [> 0.5 − 2 points 2 or > 2 or cirrhosis likely].
In general, a number of these observations are not unique to this setting. For example, the observed preponderance of younger individuals infected with HBV (28–50 years) has been observed in several LMICs: 43 years (IQR 34–48 years) in Gambia20; and 41.1 (± 21.05) years in Iran21. Remarkably, a higher median or mean age has been observed in treatment centres in some high-income countries (HICs) − 56 years (45–64 years) in Calgary, Canada22; and 55 years in Hong Kong23. A number of explanations can be invoked to explain the observed age disparity: mode of exposure, age at exposure, and access to diagnostic infrastructure, among others24. However, a prominent claim is that improvements in vaccine-induced immunity in the age range of 20–49 years in HIC may be responsible for the observed shift in median age24.
Although the lopsided male-to-female ratio appears to suggest differential exposure to HBV; the disparity is not fully understood. In Eritrea, context matters. First, mass testing of HBV or HCV has not been implemented in Eritrea. The nearest thing to mass testing of HBV in the country is the testing of blood donors. For a variety of reasons, blood donation was dominated by males (88.2%) in a study Gash-Barka25. In a subsequent within-sex analysis, the same study found a female vs. male HBV prevalence of 4.82% and 5.02%, respectively25. Aside from the higher likelihood of testing in males, the literature suggests that chronicity is likelier in males (male to female ratio 1.1:1 to 3:1) and natural progression of HBV to more severe complications (advanced fibrosis, cirrhosis, or symptomatic advanced stage HCC, and ESLD) is also faster26. Therefore, HBV-related morbidity and/or likelihood of hospitalization is higher in males. This possibility translates into higher hospitalization rates in higher-tier healthcare facilities located in the Central Region.
Another key finding was the significant number of mortality, 19 (4.6%) patients. A disproportional number of deaths occurred before treatment initiation - in the early period following program implementation (2019); and within a relatively short period of time after linkage to care, a median of 11 days (IQR: 6–75 days). Furthermore, most of the cases involved male patients and those with residences outside of the Central Region. There are many potential explanations for the observed outcome. First, the high death rate and (as we will highlight later) the high number of patients with liver cirrhosis highlights a late presentation problem. The higher death rate in the early period (< 2019) potentially reflects the cumulative effect of lack of treatment – note that the treatment program was initiated in 2018. Indeed, some reports suggest that lack of NUC treatment can increase the risk of death (weighted HR 3.05; 95% CI 2.70–3.44; p < 0.001) in some patients27. The subsequent decline in death in our cohort potentially reflects the positive impact of treatment and monitoring in subsequent years.
In many ways, these results are comparable to findings from the region. In a study conducted in Ethiopia, Desalegn et al. (2019) reported a slightly lower mortality rate of 2.68% over a two-year follow-up duration. The median duration (IQR) from initiation of treatment to death in this cohort was 110 days (IQR: 26–276) and most deaths were attributed to decompensated cirrhosis. The excess mortality in males is another recurring feature in HBV literature28. In a study conducted in Ethiopia, the likelihood of advanced liver disease (ALD) was higher in men28.These outcomes, experts agree; are largely driven by the asymptomatic nature of HBV - only presenting when advanced-stage sequelae emerge29. Importantly, the higher likelihood of ALD in males is potentially linked to differential exposure to hepatotoxins (hazardous alcohol consumption, aflatoxins (a toxin from Aspergillus flavus and Aspergillus parasiticus in locally brewed alcoholic drinks, and tobacco use, among others) or other high-risk behaviour24.
Data showing a higher probability of death in patients residing outside the Central Region are probably related to regional health disparities in access to advanced care. In other words, the underdiagnosis arising from limited healthcare access, lack of screening, and inadequate links to care in other administrative zones means that most patients seeking care in higher-tier facilities located in the central region will have fairly advanced diseases. The rural-urban disparity in access to health facilities is a well-documented problem in Africa11. Indeed, attempts at decentralization of services are often undermined by multiple structural problems: lack of hepatologists, problems with logistics, imaging equipment, distance facilities, and diagnostics. Interestingly, HBV care was decentralized in Eritrea in 2020; even then, we presume that the quality of care in facilities outside the Central Zone is encumbered by similar challenges. Ultimately, the unavoidable conclusion is that access to diagnosis and prompt linkage to care should be expanded in administrative zones outside the Central Region.
According to the treatment guidelines of the Asian Pacific Association for the Study of the Liver (APASL)30, European Association for the Study of the Liver (EASL)31, and the American Association for the Study of Liver Diseases (AASLD)16; A typical workup for treatment include several serological and biochemical assays [HBsAg, HBsAb, Hepatitis core antibody (HBcAb), hepatitis Be antigen (HBeAg), hepatitis Be antibody (HBeAb)]; HBV DNA PCR; liver function tests, CBC; α-fetoprotein (AFP), and imaging (transient elastography (TE) (Fibroscan [Echosens, Paris, France])30. Although liver biopsy has been described as a useful clinical tool by major international liver societies11,31; it is rarely utilized in clinics across the WHO-AFRO region32. In our cohort, no patient underwent the procedure. Furthermore, a significant fraction of the required tests were unavailable - HBeAg, HBeAb, and TE. As such, evaluation of HBeAg positivity (+ ve) or negativity (-ve) is not part of routine care in Eritrea. However, it is our presumption, based on a previous study in Eritrea; that most of the patients were HBeAg (-ve); Mohammed and colleagues reported that up to 93% of the patients in some of these clinics are HBeAg –ve33. Although HBsAg assays are widely available for use in the diagnosis of HBV in the country, their use in the assessment of treatment response was not evident in this cohort. Additional omissions included renal function test for patients on long-term TDF or TDF + LAM, AFP for assessment of HCC, poor documentation of patient history or details of physical examination – alcohol intake, family history of HCC, splenomegaly, and assessment of possible superinfection with other hepatitis viruses (HDV, HCV and/or HIV)11,30. Beyond these omissions, the lack of tests such as HBe-related assays and TE, two mainstays in treatment algorithms; meant that assessment of baseline disease activity (e.g., phase), and the risk of potentially fatal complications such as HCC or cirrhosis was inadequate. These observations, it must be emphasized; highlight the impact that the high cost of diagnostics and inadequate laboratory infrastructure is having on the treatment of CHB patients in the region.
Separately, we assessed that 98 (23.7%) of the patients in this cohort were in NUC (TDF, TDF + LAM). The preferred regimen was TDF, an oral phosphonoamidate prodrug. However, TDF + LAM), a regimen reserved for patients with HBV/HIV coinfection, was administered to a significant proportion of patients. On the whole, the proportion of patients on treatment was much lower than what has been reported in cohorts in HICs – 62% in the USA34. Predominant reasons for treatment initiation included cirrhosis (APRI > 2, 18 (18.3%) and HBV DNA > 20,000 after 6 months of monitoring, 23 (23.4%). In this regard, our data support the assertion that liver cirrhosis is the predominant reason for the initiation of treatment in SSA. At a pooled prevalence of 6.4% (95% CI 4.1-9.9, I2 = 87%), a recent meta-analytical report suggested that Cirrhosis is the most important driver of HBV-related morbidity and mortality in SSA34,35.
Although HBV-associated liver cirrhosis is considered one of the most important drivers of initiation and mortality in the WHO-AFRO region; underdiagnosis remains a problem. According to some estimates, only 10 − 15% of persons with liver cirrhosis in SSA are detected for linkage to appropriate treatment36,37. Recommended approaches for non-invasive diagnosis of cirrhosis such as APRI score and FIB-4 have not been validated for patients in the region. At present, experts agree that the APRI score cutoff of 2 that is used in the region underestimates advanced fibrosis38. In a study conducted in Gambia, 25% sensitivity was reported for an APRI cut-off of > 2 and an area under the receiver operating characteristic curve (AUROC) of 0.70 (CI 0.55–0.86)39. Similarly, a study in Ghana demonstrated a sensitivity of 45.4% when the cut-off was > 2. In these reports, the authors noted a significant increase in sensitivity when a cut-off of > 1 was used40. Highlighting this problem, they asserted that the current APRI threshold (> 2) is too high and should be revised to lower the cutoff with rule-in and rule-out criteria to improve the diagnosis of significant fibrosis (METAVIR score F2/F3), and possibly, METAVIR score F440. Further corroborations of these assertions have been demonstrated by investigators who have shown that at an APRI score of > 1.0 (low cut-off); the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the diagnosis of METAVIR F4 grade cirrhosis were 71–84%, 50–69%, 52–61% and 76–84% respectively and that the corresponding values at an APRI score of 2.0 (high cut-off) were 22–49%, 81–94%, 19–34% and 91–94%41. More recently, HEPSANET joint report suggested a new rule-in and rule-out threshold of 0.65 and 0.36, respectively, for improved cirrhosis detection (liver stiffness measurement (LSM) > 12.2 kPa)29.
The overriding implication of these findings is that a significant number of patients in this cohort who were in the grey zone (0.5-2 points); may have cirrhosis or significant fibrosis. To further extrapolate, the lower number of treatment-eligible patients in cohorts in the WHO-Afro region potentially reflects the underdiagnosis of significant fibrosis or cirrhosis42. Reiterating this problem, Dusheiko and Lamoine noted in an editorial letter that the use of the WHO guideline raises several major concerns – the poor concordance between the EASL and WHO criteria (Cohen’s kappa 0.518, p-value < 0.001) and its under-performance among young males and low overall sensitivity (49%). %). In their conclusion, they emphasized the need for SSA-specific or locally adopted treatment eligibility guidelines (more research on HBV disease) and low-cost diagnostics.
Another lingering concern pertains to appropriate cutoffs for ALT. In their latest report, the AASLD 2018 guidance update recommended male and female ALT cutoffs of 35 U/L and 25 U/L, respectively16. These recommendations are based on the observed association between ALT cutoff in the high-normal ALT range (> 40–70 IU/L) with liver necroinflammation, fibrosis, cirrhosis, HCC, and liver-related deaths43. Although the WHO guideline recommends the use of local ALT cut-offs of 30 U/L for men and 19 U/L for women44; we opted to use a cut-off of 40 U/L. This cut-off point is widely used in clinics in Eritrea33 and is currently recommended, with some reservations, in the EASL guidelines31. For decades, scientists have known that some patients with persistently normal ALT (PNALT) (e.g., sustained ALT levels < 40 U/L) have cirrhosis45. In one such study in HBeAg –ve patients, researchers demonstrated the presence of cirrhosis in patients with ALT [0.5–U/L upper limit of normal (ULN)46. Biopsy studies have uncovered similar results with some investigators concluding that ALT < 0.5 – U/L ULN levels are the most desirable47. Another extension to these disputations is the well-documented possibility that reference intervals (RIs) for ALT that are used across the WHO Afro region are locally inappropriate48,49. On this issue, it’s our opinion, that the ALT cut-off (40 U/L for males and females) used in Eritrea is based on limited evidence; therefore, it can potentially undermine the evaluation of treatment eligibility in patients. To remedy this concern, studies on appropriate ALT and AST cuff-offs are urgently needed in Eritrea. In particular, head-to-head comparison studies linking ALT (or APRI and FIB-4 score) levels with TE readings or biopsy findings should be considered. Overall, the lack of high-quality data on the burden of cirrhosis undermines treatment, elimination of HBV and reductions in mortality, DALY, or other quality-of-life indexes.
Similar to other real-world studies in the WHO-Afro region28, the limited range of tests performed in this cohort restricted our ability to undertake per-guideline evaluation of an ideal endpoint such as sustained off-therapy HBsAg loss (with or without seroconversion to anti-HBs), anti Be seroconversion, among others. This problem was further compounded by a lack of consistency in the use and/or reporting of available tests. For example, in our cohort, HBV DNA testing was delayed in multiple instances and 47(48%) patients had no viral load records after treatment initiation. We also demonstrated that access to HBV DNA testing has dwindled over time. The high point was in the pre-2020-year band. Thereafter, HBV DNA testing and uptake of therapy reached a nadir (from which it is yet to recover) in 2020. The reasons behind this sustained decline remain unknown.
In a separate analysis, we demonstrated that 18 treatment eligible individuals were not on treatment. Although some lag time between treatment-eligibility and treatment initiation is expected34; lengthy delays can be harmful to patients. Moreover, treatment delay is one of the biggest problems affecting patients with CHB in SSA – as previously noted, only 6.6 million (5.3–8.3 million) of the 30.4 million (24.3–38 million) diagnosed with CHB are on treatment [18]. Multiple drivers of suboptimal treatment of CHB patients in the region are known. These include suboptimal evaluation of newly diagnosed HBV patients due to the high cost of diagnostics, problems with evaluation of treatment eligibility, delays in treatment initiation, and suboptimal monitoring of patients in care or those receiving anti-HBV therapy.
In real-world studies, these concerns have been reinforced by a study which demonstrated that only 35% of untreated patients on follow-up received HBV DNA and ALT level testing as required50. Stockouts of reagents and knowledge gaps among caregivers with the most current management recommendations43 have also been implicated. On the whole, most of these barriers were identified in this setting. Priority areas in need of urgent redress include better training of personnel in HBV programs, and the need to reduce the cost of relevant laboratory assays. Importantly, data management should be optimized to support future research on the course of HBV disease, clinical decision-making; evaluation of program quality, among others.
Despite the shortcomings described above, it was possible to assess VR and biochemical response in a small subset of patients. In the head-to-head comparison of the results in TDF and TDF + LAM after 12 weeks of treatment, VR was observed in 14 (45.2%) vs. 17 (54.8%), respectively, translating into an overall VR of 60.7% (95% CI 46.9–74.6). The observed values for TDF or TDF + LAM appear to be lower compared to data from some landmark real-world studies. Lee and colleagues recently reported a VR of 91.6% after 12 months of treatment (TDF group, 88.6% vs. 92.6% in TDF + LAM group)51. The observed disparity in VR may be due to the heterogeneous nature of patients in the environment and the limited number of controls versus those in a trial environment. More importantly, VR is time-dependent with higher responses correlating strongly with the duration of treatment. For example, Park and colleagues reported a time-dependent VR at 3, 6, 12, 24, and 36 months of 20%, 54%, 79%, 86%, and 91%, respectively52. These results are relatively consistent with our results. In a related analysis, we noted a significant improvement in biochemical parameters in both groups. In most studies, the biochemical response in patients treated with TDF and TDF + LAM is similar53.
Finally, we were able to demonstrate that the prevalence and incidence of LTFU in this cohort were 23.9% (95% CI 19.8–28.1) and 4.3 (95% CI: 3.5–5.2) per 1000 PDFU, respectively. Independent predictors of LFTU included baseline serum HBV DNA, treatment status (not on treatment), and FIB-4 score. Much of this is supported by bivariate data demonstrating that a significant number of patients with LTFU were having ALT > 40 U/L (23[35.4%]) or were in the likely cirrhosis or unlikely cirrhosis category, 17(44.7%). The connection between the increasing levels of HBV DNA, the FIB score, and LTFU is hard to understand, in part because it runs counter to specific models of patient behaviour - the health belief model (HBM) posits that patients with more severe forms of disease tend to seek and stay in care. Furthermore, we believe that the high rate of LTFU before treatment initiation may, at least partially, be explained by unmet patient monitoring requirements. In other words, patients are rational actors. Therefore, when they fail to receive any testing, treatment or information on their status during scheduled visits; high attrition rates can be expected. In this regard, poor adherence to monitoring guidelines may act as a driver of attrition.
Apart from these concerns, we have to emphasize the fact that the outcome in patients who were LTFU remains unknown. This notwithstanding, we assume that the likelihood of death, flare-ups of necroinflammation and progression to more advanced HBV-sequelae is high. In addition to these detrimental effects, the financial costs associated with retreatment or re-entry into care represent unnecessary/or avoidable costs for programs. Another concerning finding was the fact that a large proportion of patients who were LTFU had viremia. Because viral suppression reduces a patient’s ability to transmit HBV; this outcome undermines the ultimate goal of eliminating HBV as a public health threat in Eritrea. Therefore, interventions designed to reduce LTFUs represent a powerful tool for HBV elimination. At a minimum, our finding reinforces the need for efforts directed at improvements in patient literacy and patient tracking (e.g., HBV registry, linked with outreach to patients).
Limitations of the study
Although our study adds important real-world evidence regarding the feasibility of large-scale government-sponsored treatment of HBV in emerging treatment programs in SSA; it has several limitations. Like many retrospective studies, this study had a missing data problem. As such, specific evaluations were only undertaken in a fraction of patients in multiple analyses. The consequent loss of power undermined our ability to undertake multivariable analysis. The missing data problem also contributed to the relatively short period of tracking of outcomes in VR. Furthermore, the descriptive nature of the study means that the direction of causality cannot be inferred. Therefore, none of the associations uncovered can be assumed to be causative.
Conclusions of the study
This study demonstrated that significant number of patients presented with active disease at baseline – APRI > 2, 18 (18.3%); persistently elevated ALT, 19 (19.3%); HBV DNA > 20,000 after 6 months of monitoring, 23 (23.4%) and clinically apparent presence of cirrhosis, 12 (12.2%). In spite of the significant number of cirrhosis (a marker of late presentation) in newly enrolled patients, potential underdiagnosis remains a concern. In many ways, our study provides a powerful illustration of the impact of inadequate diagnostic infrastructure (laboratory or imaging) on emerging HBV treatment programs in SSA. Note that in this program, it was associated with suboptimal evaluation of treatment eligibility; inability and /or delays in monitoring treatment response (serological, virological, and biochemical), poor adherence to the monitoring guideline; and inability to diagnose HBV-related sequelae such as HCC or specific health quality indexes. It may also contribute to high attrition rates. Despite a significant missing data problem, we demonstrated that the VR observed in this cohort are comparable to previous data from other real-world studies. Furthermore, VR after 12 weeks of follow-up was nearly similar in patients in the TDF and TDF + LAM treatment arms. Lastly, our study demonstrated that high LTFU occurred across the HBV care continuum, including prior to treatment initiation and the post treatment initiation period. On the whole, there are opportunities to achieve significant improvements in HBV management in Eritrea. Interventions include educational initiatives for caregivers/and or patients, improvements in laboratory infrastructure - to a level that can sustain mass HBV testing and per-guideline monitoring of patients; prompt linkage to care; treatment scale-ups, better tracking and documentation of patient’s data.
Acknowledgements
The authors would like to thank the clinical staff who supported this work at Orotta National Referral Hospital, Halibet National Referral Hospital, and Haz Haz zonal referral hospital.
Author contributions
GGG and MBS conceived the study and formulated the study design. G.G.G, MBS and ARM performed data collection. G.G.G, MBS, and O.O.A did the statistical analysis and data presentation. G.G.G, MBS, O.O.A, M.E.H, and A.B.M wrote/edited the manuscript. All authors read and approved the final manuscript.
Data availability
The data set supporting the conclusions of this article is available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Financial support
Data were collected from the Chronic Viral Hepatitis Care Center in ONRH and HNRH focal personnel with incentives and material support obtained from the Eritrean Ministry of Health, CDC division.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global National Burden of H–epatitis B. 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol. Hepatol.7, 796–829 (2022). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui, F. et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol. Hepatol.8, 332–342 (2023). (eng). [DOI] [PubMed] [Google Scholar]
- 3.Hepatitis, B. Accessed date: 2024 20 October. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- 4.Makokha, G. N., Zhang, P., Hayes, C. N., Songok, E. & Chayama, K. The burden of Hepatitis B virus infection in Kenya: A systematic review and meta-analysis. Front. Public. Health. 11, 986020 (2023). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayagam, S. & Thursz, M. Strategies for global elimination of chronic HBV infection: 2019 update. Curr. Hepatol. Rep.18, 300–309 (2019). [Google Scholar]
- 6.Torre, P., Aglitti, A., Masarone, M. & Persico, M. Viral hepatitis: Milestones, unresolved issues, and future goals. World J. Gastroenterol.27, 4603 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The, L. Viral hepatitis elimination: A challenge, but within reach. Lancet. 400, 251 (2022). (eng). [DOI] [PubMed] [Google Scholar]
- 8.Cooke, G. S. et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & hepatology commission. Lancet Gastroenterol. Hepatol.4, 135–184 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Wu, J., Wang, Y., Zhu, C. & Lin, W. Editorial: Diagnosis, treatment, and prognosis of viral Hepatitis. Front. Med. (Lausanne). 9, 882878 (2022). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcellin, P. et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet. 381, 468–475 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Spearman, C. W. et al. Hepatitis B in sub-saharan Africa: Strategies to achieve the 2030 elimination targets. Lancet Gastroenterol. Hepatol.2, 900–909 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Wang, M., Yan, L., Wang, J., Jin, Y. & Zheng, Z-J. Global burden of hepatitis B attributable to modifiable risk factors from 1990 to 2019: A growing contribution and its association with socioeconomic status. Globalization Health. 19, 23 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Division, C. D. C. in Guidelines for the care and Treatment of Persons Diagnosed with Chronic Hepatitis B and C Virus Infection in. (eds Division, C. D. C.) (Ministry of Health, 2018). [PubMed]
- 14.Hamida, M. E. et al. Prevalence of hepatitis B virus genotypes among patients with liver disease in Eritrea. Sci. Rep.11, 11323 (2021). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization, W. H. Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief (World Health Organization, 2016).
- 16.Terrault, N. A. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 67, 1560–1599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO welcomes Egypt. ’s support to 14 African countries in their fight against hepatitis C. Accessed date: 2024 20 October. https://www.afro.who.int/news/who-welcomes-egypts-support-14-african-countries-their-fight-against-hepatitis-c
- 18.Sarin, S. et al. Asian-pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hep. Intl.10, 1–98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amponsah-Dacosta, E. Hepatitis B virus infection and hepatocellular carcinoma in sub-saharan Africa: Implications for elimination of viral hepatitis by 2030? World J. Gastroenterol.27, 6025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vray, M. et al. Molecular epidemiology of hepatitis B virus in Dakar, Senegal. J. Med. Virol.78, 329–334 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Ghabeshi, S., Sharifi, Z., Hosseini, S. M. & Shooshtari, M. M. Correlation between viral load of HBV in chronic hepatitis B patients and precore and basal core promoter mutations. Hepat. Monthly ;13. (2013). [DOI] [PMC free article] [PubMed]
- 22.Azhari, H. et al. Real-world tertiary referral centre experience stopping nucleos (t) ide analogue therapy in patients with chronic hepatitis B. Can. Liver J.5, 453–465 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai, J. C. T. et al. Secular trend of treatment uptake in patients with chronic hepatitis B: a territory-wide study of 135 395 patients from 2000 to 2017. J. Gastroenterol. Hepatol.36, 3487–3499 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Gnyawali, B., Pusateri, A., Nickerson, A., Jalil, S. & Mumtaz, K. Epidemiologic and socioeconomic factors impacting hepatitis B virus and related hepatocellular carcinoma. World J. Gastroenterol.28, 3793 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keleta, Y. T. et al. Seroprevalence of transfusion transmitted infections among blood donors in Gash Barka Zonal Blood Transfusion Center, Barentu, Eritrea, 2014 through 2017. BMC Hematol.19, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zampino, R. et al. Hepatitis B virus burden in developing countries. World J. Gastroenterol.21, 11941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui, V. W. K. et al. Increasing antiviral treatment uptake improves survival in patients with HBV-related HCC. JHEP Rep.2, 100152 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aberra, H. et al. The WHO guidelines for chronic hepatitis B fail to detect half of the patients in need of treatment in Ethiopia. J. Hepatol.70, 1065–1071 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Johannessen, A. et al. Systematic review and individual-patient-data meta-analysis of non-invasive fibrosis markers for chronic hepatitis B in Africa. Nat. Commun.14, 45 (2023). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarin, S. K. et al. Asian-pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int.10, 1–98 (2016). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampertico, P. et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol.67, 370–398 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Vento, S., Dzudzor, B., Cainelli, F. & Tachi, K. Liver cirrhosis in sub-saharan Africa: Neglected, yet important. Lancet Glob Health. 6, e1060–e1061 (2018). (eng). [DOI] [PubMed] [Google Scholar]
- 33.Hamida, M. E., Raja, S. M., Seyoum, Y., Elkhidir, I. M. & Tekle, F. Prevalence of chronic hepatitis B phases in Eritrean patients: A laboratory-based cross-sectional study. BMC Gastroenterol.21, 198 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vu, V. D. et al. Long-term follow-up and suboptimal treatment rates of treatment-eligible chronic hepatitis B patients in diverse practice settings: A gap in linkage to care. BMJ Open. Gastroenterol.2, e000060 (2015). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surial, B. et al. Prevalence of liver cirrhosis in individuals with hepatitis B virus infection in sub-saharan Africa: Systematic review and meta-analysis. Liver Int.41, 710–719 (2021). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonderup, M. W. et al. Hepatitis B in sub-saharan Africa-How many patients need therapy? J. Viral Hepat.27, 560–567 (2020). (eng). [DOI] [PubMed] [Google Scholar]
- 37.Béguelin, C., Fall, F., Seydi, M. & Wandeler, G. The current situation and challenges of screening for and treating hepatitis B in sub-saharan Africa. Expert Rev. Gastroenterol. Hepatol.12, 537–546 (2018). (eng). [DOI] [PubMed] [Google Scholar]
- 38.Mitchell, T., Nayagam, J. S., Dusheiko, G. & Agarwal, K. Health inequalities in the management of chronic hepatitis B virus infection in patients from Sub-saharan Africa in high-income countries. JHEP Rep.5, 100623 (2023). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemoine, M. et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 65, 1369–1376 (2016). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nartey, Y. A. et al. Ambulatory end-stage liver disease in Ghana; patient profile and utility of alpha fetoprotein and aspartate aminotransferase: Platelet ratio index. BMC Gastroenterol.20, 428 (2020). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao, G., Yang, J. & Yan, L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: A systemic review and meta-analysis. Hepatology. 61, 292–302 (2015). (eng). [DOI] [PubMed] [Google Scholar]
- 42.Dusheiko, G. & Lemoine, M. An appraisal of the WHO Hepatitis B treatment guidelines applicability to africans. J. Hepatol.70, 1046–1048 (2019). (eng). [DOI] [PubMed] [Google Scholar]
- 43.Nguyen, M. H., Wong, G., Gane, E., Kao, J. H. & Dusheiko, G. Hepatitis B Virus: Advances in Prevention, diagnosis, and Therapy. Clin. Microbiol. Rev. 2020;33. (eng). [DOI] [PMC free article] [PubMed]
- 44.Vittal, A. & Ghany, M. G. WHO guidelines for prevention, care and treatment of individuals infected with HBV: A US perspective. Clin. Liver Dis.23, 417–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim, H. C. et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: Prospective cohort study. Bmj. 328, 983 (2004). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar, M. et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 134, 1376–1384 (2008). (eng). [DOI] [PubMed] [Google Scholar]
- 47.Lai, M., Hyatt, B. J., Nasser, I., Curry, M. & Afdhal, N. H. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J. Hepatol.47, 760–767 (2007). (eng). [DOI] [PubMed] [Google Scholar]
- 48.Odhiambo, C. et al. Evaluation of locally established reference intervals for hematology and biochemistry parameters in Western Kenya. PLoS One. 10, e0123140 (2015). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Achila, O. O. et al. Biochemistry reference intervals for healthy elderly population in Asmara, Eritrea. BMC Res. Notes. 10, 748 (2017). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juday, T. et al. Adherence to chronic hepatitis B treatment guideline recommendations for laboratory monitoring of patients who are not receiving antiviral treatment. J. Gen. Intern. Med.26, 239–244 (2011). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee, Y. B. et al. Tenofovir monotherapy versus tenofovir plus lamivudine or telbivudine combination therapy in treatment of lamivudine-resistant chronic hepatitis B. Antimicrob. Agents Chemother.59, 972–978 (2015). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park, J. et al. Effects of entecavir and tenofovir on renal function in patients with hepatitis B virus-related compensated and decompensated cirrhosis. Gut Liver. 11 (6), 828–834. 10.5009/gnl16484 (2017). PMID: 28651305; PMCID: PMC5669599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen, L. et al. Efficacy of tenofovir-based combination therapy versus tenofovir monotherapy in chronic hepatitis B patients presenting with suboptimal responses to pretreatment: A meta-analysis. Gastroenterol. Res. Pract.2016, 7214020 (2016). (eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting the conclusions of this article is available from the corresponding author on reasonable request.