Abstract
China has implemented the “tiered medical services” policy since 2015, while there is a paucity of data evaluating the the current status of chronic obstructive pulmonary disease (COPD) management under the system. Characteristics and treatments from 11,905 COPD patients in 88 hospitals across different tiers in China were included and analyzed. We assessed the statistical significance of differences by one way analysis of variance (ANOVA) for continuous variables and with the chi-squared test for categorical variables. Patients in primary hospitals (Tier1) exhibited heightened exposure to risk factors including smoking, household biofuel, and family history of respiratory diseases, and displayed elevated COPD assessment test (CAT) and modified Medical Research Council (mMRC) dyspnea scale scores, and worse lung function, in comparison to tertiary (Tier3) hospitals (P < 0.001). However, the utilization of inhaled maintenance treatments in Tier1 hospitals is markedly lower than that in Tier3 hospitals (54.8% vs. 81.3%, P < 0.001). At odds with the patients with more severer symptoms (as indicated by CAT ≥ 10 or mMRC ≥ 2), a higher proportion relied exclusively on single bronchodilators in Tier1 hospitals was observed compared to secondary (Tier2) and Tier3 hospitals (37.7% vs. 32.1% vs. 26.3%, 40.0% vs. 29.8% vs. 25.6%, P<0.001). Dual bronchodilators (long-acting β2-agonists /long-acting muscarinic antagonist, LABA/LAMA) represented the least common medication regimen across all tiers of hospitals, albeit their usage rates increased in tandem with hospital tier (0.7% vs. 7.2% vs. 10.4%, P < 0.001). In addition, the use of inhalation therapies containing inhaled corticosteroids (ICS) in China’s primary care is notably lower (16.9%) than the United States, the United Kingdom, and other middle-to-high-income countries (29.5-57.0%). There was compelling evidence pointing to greater disease severity in Tier1 hospitals, attributable to the lower and inappropriate utilization of inhaled maintenance treatments. This underscores the necessity for enhanced availability of medications and educational initiatives aimed at both physicians and patients within Tier1 hospitals.
Keywords: Pulmonary Disease, Chronic Obstructive; Tiered Medical Services; Primary Care; COPD Management
Subject terms: Diseases, Health care, Medical research
Introduction
Chronic obstructive pulmonary disease (COPD) represents a significant public health challenge globally1,2, particularly affecting low- and middle-income countries where resources are often limited3–5. In China, COPD has emerged as a major cause of morbidity and mortality, with a disease burden of approximately 99.9 million6,7. And by 2060, it is estimated that over 5.4 million people will die annually from COPD and related illnesses8. Effective management of COPD involves long-term standardized treatments that can reduce symptoms, exacerbations, and mortality9,10. However, the implementation of these management strategies varies significantly across different regions and healthcare settings11–13. This disparity is largely influenced by variations in the availability of medical resources and healthcare infrastructure, which are more pronounced in resource-constrained settings14.
Recognizing the need to optimize healthcare resource allocation and improve patient outcomes, China implemented the tiered medical system in 201515. This system aims to streamline the management of chronic diseases such as COPD by enhancing primary care capabilities and improving the integration of healthcare services across different levels of medical institutions. Primary (Tier1) hospitals typically provide initial diagnosis, treatment, and ongoing management, secondary (Tier2) hospitals offer specialized care and may handle more severe cases or complications, and tertiary (Tier3) hospitals are equipped with advanced technologies and expertise for complex and critical cases, serving as medical centers16. However, compared to well-established referral systems in countries such as the United Kingdom (UK) and Canada, although China has proposed the concept of hierarchical medical services17, due to factors such as medications availability and patients’ medical habits, China still faces challenges in terms of primary care quality, resource distribution, and referral mechanisms.
There is a paucity of large-scale data evaluating the impact of the tiered medical system on COPD management in China. This gap hinders a comprehensive understanding of the effectiveness and quality of COPD care delivered in tertiary medical institutions. Our study seeks to analyze differences in clinical characteristics, risk factor distribution, and treatment patterns among COPD patients within this healthcare framework. By examining a large patient cohort, to provide a comprehensive overview of how China’s healthcare system operates in managing COPD.
Methods
Study design
This study was a cross-sectional analysis of the data from an ongoing multicentre prospective cohort study, the COPD Primary Healthcare cohort study (COPD-PHC), which was designed to explore the occurrence, characteristics, clinical features, and prognosis of COPD patients in real-world settings in China. Details of the COPD-PHC study protocol have been described elsewhere18. Briefly, We used a two-step screening strategy to identify Chinese adults with COPD, COPD Screening Questionnaire (COPD-SQ)19 and additional lnternet of things (IoT)-based spirometer were utilized to screen inhabitants among 18 cities in China. Individuals with COPD-SQ scores over 16 were defined as high-risk population, with the post-bronchodilator forced expiratory volume in one second and forced vital capacity ratio (FEV1: FVC) less than 0·70 were defined as COPD, according to 2021 global initiative for chronic obstructive lung disease (GOLD)20. High-risk population and COPD patients were included into the cohort for long-time follow-up.
Participants
The study population included both newly diagnosed COPD patients identified through screening strategy described above and those self-reporting a previous diagnosis and confirmed by this strategy during their clinic visit. 11,905 COPD patients aged over 40 confirmed between November 2021 and December 2023 were included for the study.
Data collection
For the study, data were extracted from the COPD-PHC cohort study and its corresponding database, involving demographic characteristics of the patients (such as age, gender, and address), lifestyle factors (smoking status and household biofuel exposure), and anthropometric measures (height, weight and body mass index, BMI). Additionally, clinical symptoms related to respiratory diseases and detailed family histories of respiratory conditions were gathered. Health condition (modified Medical Research Council (mMRC) dyspnea scale and COPD assessment test (CAT) ) and histories of acute exacerbations were also recorded, alongside data on the post-bronchodilator pulmonary function tests. Chronic bronchitis (CB) was defined as a combination of chronic cough and sputum scores ≥ 2 points from the CAT questionnaire as reported previously54.
In addition to the clinical data, we also obtained the comprehensive records of the pharmacological treatments administered to the patients from this COPD database. Both newly diagnosed COPD patients identified through screening strategy above and those self-reporting a previous diagnosis and confirmed during their clinic visit, were detailed documented about their medication regimens. This included detailed types of bronchodilators used (long-acting muscarinic antagonist (LAMA), inhaled corticosteroids (ICS)/long-acting β2-agonists (LABA); as well as the combinations LABA/LAMA and ICS/LABA/LAMA), oral medications prescribed, and the rehabilitation measures implemented. This comprehensive dataset allows for a multifaceted analysis of COPD management effectiveness in relation to various clinical factors and their treatment patterns.
Quality control
All professionals adopted a unified technical solution and operational process. The electronic structured questionnaire and pulmonary function results are transmitted automatically to the platform through the cloud database, and all inhaled medications related to COPD are provided in photo format in the application for doctors to select, so as to avoid errors caused by manual input. Project administrative meetings are held regularly to discuss and develop potential coping strategies for emerging problems.
Data security and privacy
For the purpose of data security and patients privacy, all data was stored on the government’s cloud server platform, every professional in this database system is assigned an exclusive account and all the operation logs are correspondingly preserved. Records of certain patients can only be accessed by relevant professional and clinicians. Technically speaking, data transmission is encrypted twice based on hypertext transfer protocol secure (HTTPS) encryption to protect patients’ privacy.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our study.
Statistical analysis
Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as number and percentage (%). We assessed the statistical significance of differences by one way analysis of variance (ANOVA) for continuous variables and with the chi-squared test for categorical variables. Two-tailed P value < 0.05 was considered statistically significant. Post hoc test was conducted by Bonferroni method. All analyses were conducted using SPSS (version 21; IBM Corporation, Armonk, NY, USA).
Results
Patients characteristics in different tiers of hospitals
Among 11,905 participants included in our study, 8,390 were from Tier3 hospitals, 2,767 were from Tier2 hospitals and 748 were from primary hospitals. The patients characteristics are shown in Table 1. The mean ± standard deviation age of the participants was 67.22 ± 11.74 years, and 75.87% (9,032/11,905) of the patients were males. The proportion of patients aged over 60 years is higher in Tier1 than Tier2 and Tier3 hospitals (84.6% vs. 78.1% vs. 76.1%, P < 0.001). Tier1 patients showed higher exposure to risk factors such as smoking, household biofuel, and family history of respiratory diseases (P < 0.001). The CAT and mMRC scores, as well as acute exacerbation rates, are higher in Tier1 and 2 compared to Tier3 hospitals (P < 0.001). Additionally, the proportions of patients with chronic bronchitis are higher in Tier1 than Tier3 hospitals (82.5% vs. 69.8%, P < 0.05). Among the patients in whom FEV1 was available (n = 6,901), the proportion of GOLD 3–4 patients in Tier1 hospitals is higher compared to Tier2 and Tier3 hospitals (70.4% vs. 60.9% vs. 63.5%, P < 0.001).
Table 1.
Patients characteristics among different tiers of hospitals in China.
| Tier1 (n = 748) | Tier2 (n = 2767) | Tier3 (n = 8390) | F/χ2 | P value | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 69.93 (10.21) | 67.74(11.08)* | 66.81 (12.03)† | 27.862 | <0.001 |
| Gender, No. (%) | 7.728 | 0.021 | |||
| Male | 536 (71.7) | 2106 (76.1)* | 6390 (76.2)† | ||
| Female | 212 (28.3) | 661 (23.9)* | 2000 (23.8)† | ||
| Smoking exposure, No. (%) | 66.259 | <0.001 | |||
| Yes | 494 (66.0) | 1793 (64.8) | 4787 (57.1)† | ||
| No | 254 (34.0) | 974 (35.2) | 3603 (42.9)† | ||
| BMIa, kg/m2, No. (%) | 79.595 | <0.001 | |||
| <18.5 | 44 (5.9) | 332 (12.0)* | 608 (7.2) | ||
| 18.5–23.9 | 410 (54.8) | 1497 (54.1) | 4476 (53.3) | ||
| ≥ 23.9 | 294 (39.3) | 938 (33.9)* | 3306 (39.4) | ||
| Chronic cough, No. (%) | 282.766 | <0.001 | |||
| Yes | 554 (74.1) | 2202 (79.6)* | 5269 (62.8)† | ||
| No | 194 (25.9) | 565 (20.4)* | 3121 (37.2)† | ||
| Dyspnoea, No. (%) | 320.190 | <0.001 | |||
| Yes | 689 (92.1) | 2623 (94.8)* | 6843 (81.6)† | ||
| No | 59 (7.9) | 144 (5.2)* | 1547 (18.4)† | ||
| Exposure to household biofuel, No. (%) | 945.535 | <0.001 | |||
| Yes | 416 (55.6) | 515 (18.6)* | 1034 (12.3)† | ||
| No | 332 (44.4) | 2252 (81.4)* | 7356 (87.7)† | ||
| Family history of respiratory diseases, No. (%) | 364.382 | <0.001 | |||
| Yes | 254 (34.0) | 702 (25.4)* | 1114 (13.3)† | ||
| No | 494 (66.0) | 2065 (74.6)* | 7276 (86.7)† | ||
| mMRCb, No. (%) | 336.994 | <0.001 | |||
| 0–1 | 298 (39.8) | 816 (29.5)* | 4136 (49.3)† | ||
| ≥ 2 | 450 (60.2) | 1951 (70.5)* | 4254 (50.7)† | ||
| CATc, No. (%) | 446.429 | <0.001 | |||
| <10 | 26 (3.5) | 205 (7.4)* | 1547 (18.4)† | ||
| 10–20 | 346 (46.3) | 991 (35.8)* | 3637 (43.3) | ||
| ≥ 20 | 376 (50.3) | 1571 (56.8)* | 3206 (38.2)† | ||
| Frequent acute exacerbation in previous 12 months, No. (%) | 388.336 | <0.001 | |||
| Yes | 533 (71.3) | 1574 (54.5)* | 3515 (44.0)† | ||
| No | 215 (28.7) | 1193 (45.5)* | 4875 (56.0)† | ||
| Chronic bronchitis, No. (%) | 195.179 | <0.001 | |||
| Yes | 617 (82.5) | 2272 (82.1) | 5856 (69.8)† | ||
| No | 131 (17.5) | 495 (17.9) | 2534 (30.2)† | ||
| Airway limitation, No. (%) | 68.303 | <0.001 | |||
| GOLDd 1 (n = 717) | 20 (8.6) | 235 (11.9) | 462 (9.8) | ||
| GOLD 2 (n = 1837) | 49 (21.0) | 535 (27.2) | 1253 (26.7) | ||
| GOLD 3 (n = 2850) | 136 (58.4) | 700 (35.6)* | 2014 (42.8)† | ||
| GOLD 4 (n = 1497) | 28 (12.0) | 497 (25.3)* | 972 (20.7)† |
Values are mean (SD) and No. (%).
a BMI, body mass index; b mMRC, modified British Medical Research Council Dyspnea Scale; c CAT, COPD Assessment Test; d GOLD, Global Initiative for Chronic Obstructive Lung Disease.
* Tier1 vs. Tier2 hospitals, P < 0.05; † Tier1 vs. Tier3 hospitals, P < 0.05.
Differences of inhaled maintenance treatment exist among different tiers of hospitals
Our study revealed significant disparities in the inhaled maintenance treatment for COPD across different tiers of hospitals (see Table 2; Fig. 1). In Tier1 hospitals, the rate of inhalation maintenance treatment was notably low, with 45.2% of patients not utilizing any inhalation medication (P < 0.001). Among those who did receive inhalation therapies, single bronchodilator was the most commonly prescribed treatment (37.3%), significantly more prevalent than in Tier2 (33.1%) and Tier3 hospitals (25.9%). This was followed by triple treatment (ICS/LABA/LAMA) and ICS/LABA regimens (9.1% and 7.8% respectively). While in Tier2 hospitals, the most frequent treatment was triple therapy, accounting for 33.9% of prescriptions, with ICS/LABA being the next most common (20.3%). Tier3 hospitals displayed a close usage rate between triple therapy and ICS/LABA, at 21.7% and 23.3% respectively. Notably, the use of dual bronchodilator (LABA/LAMA) was the least common in tertiary hospitals, although its prevalence increased with hospital tier (0.7% vs. 7.2% vs. 10.4%).
Table 2.
Distribution of inhaled bronchodilators among the three tiers of hospitals.
| Inhaled maintenance treatment | Tier1 (n = 748) |
Tier2 (n = 2767) |
Tier3 (n = 8390) |
χ2/F | P value |
|---|---|---|---|---|---|
| 999.509 | <0.001 | ||||
| Triple treatment (ICS/LABA/LAMA), No. (%) | 68(9.1) | 939(33.9)* | 1821(21.7)† | ||
| ICS/LABA, No. (%) | 58(7.8) | 562(20.3)* | 1954(23.3)† | ||
| Dual bronchodilator (LABA/LAMA), No. (%) | 5(0.7) | 200(7.2)* | 873(10.4)† | ||
| Single bronchodilator, No. (%) | 279(37.3) | 915(33.1)* | 2170(25.9)† | ||
| None inhalant, No. (%) | 338(45.2) | 151(5.5)* | 1572(18.7)† | ||
| Notes: | |||||
Values are No. (%) .
ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LAMA, Long-acting muscarinic antagonist. * Tier1 vs. Tier2 hospitals, P < 0.05; † Tier1 vs. Tier3 hospitals, P < 0.05.
Fig. 1.
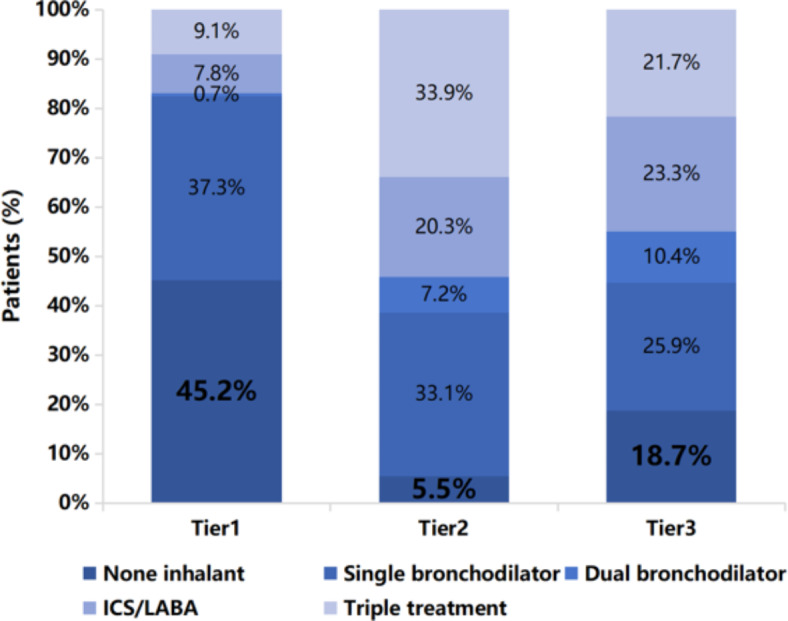
Distribution of inhaled bronchodilators among the three tiers of hospitals. Values are presented as percentage (%). LABA, long-acting β-agonists; ICS, inhaled corticosteroids.
Treatment patterns analysis based on the treatable characteristics of COPD
The utilization of different bronchodilator treatments and combinations vary across hospital tiers (See Fig. 2). At odds with the patients with more severe respiratory symptoms (as indicated by CAT scores of 10 or higher), a higher proportion of patients in tier1 hospitals relied exclusively on single bronchodilators compared to those in tier2 and tier3 hospitals (37.7% vs. 32.1% vs. 26.3%, P<0.001). This phenomenon is also observed in patients experiencing greater breathlessness (as indicated by mMRC scores of 2 or higher), The utilization rates of single bronchodilators in Tier1, 2 and 3 hospitals are 40.0%, 29.8% and 25.6%, respectively (P<0.001). Furthermore, although guidelines recommend the dual bronchodilators for this patient group, our research indicates that their utilization rates are consistently low (about 0.7-13.0%) across all tiers of hospitals. This may be related to the accessibility of these medications and health insurance policies, particularly impacting Tier1 hospitals.
Fig. 2.
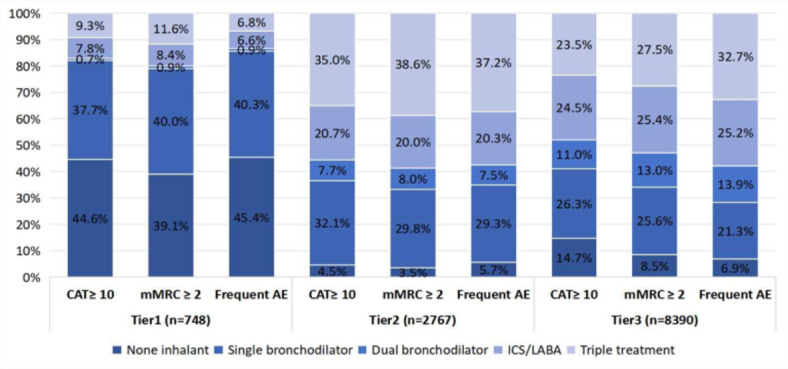
Distribution of treatments based on treatable characteristics of COPD among different tiers of hospitals. Values are presented as percentage (%). LABA, long-acting β-agonists; ICS, inhaled corticosteroids. mMRC, modified British Medical Research Council Dyspnea Scale; CAT, COPD Assessment Test; AE, acute exacerbation.
In addition, among patients experiencing frequent acute exacerbations, only 7.7% of patients in Tier1 hospitals met the GOLD guidelines recommendation for dual bronchodilators in combination with or without ICS, which much lower than in Tier2 (44.7%) and Tier3 hospitals (46.6%). Conversely, the disparity in the proportion of patients not using any inhalants was significantly more pronounced in tier1 hospitals compared to tier2 and tier3 hospitals (45.4% vs. 5.7% vs. 6.9%).
Other pharmacotherapies for COPD and differences among different tiers of hospitals
We observed a high utilization of theophylline across all hospital levels, with the highest usage noted in Tier1 hospitals, particularly among patients experiencing frequent acute exacerbations (55.9% vs. 26.7% vs. 27.9%, P < 0.001). Furthermore, when considering the administration of any oral medications, Tier1 hospitals had a notably higher rate among patients with frequent acute exacerbations compared to Tier2 and Tier3 hospitals (79.4% vs. 66.9% vs. 58.1%, P<0.001). This suggests a more aggressive pharmacological management in higher-tier hospitals, likely reflecting their capacity to implement more comprehensive treatment protocols.
COPD treatment patterns in primary care among China and other countries
In our analysis, we further compared the treatment patterns for COPD in Tier1 hospitals with previous study in China. We found an increase in the usage rates of inhaled maintenance treatment (54.8% vs. 49.7%). The usage rates of ICS/LABA dual therapy and ICS/LABA/LAMA triple therapy have slightly increased, although the utilization rate of dual bronchodilator was still very low (See Table 3).
Table 3.
COPD treatment patterns in primary care among China and other countries.
| Study | Country/region | Period | Sample/visit | Treatment patterns |
|---|---|---|---|---|
| This study | China | 2021–2023 | 748 | Single bronchodilator 37.3% Dual bronchodilator 0.7% ICS/LABA 7.8% Triple treatment 9.1% None inhalant 45.2% |
| Mao R, et al.30 | China | 2017–2018 | 197 | Single bronchodilator 7.1% Dual bronchodilator 0.5% ICS/LABA or ICS/LAMA 10.7% Triple treatment 4.0% None inhalant 50.3% Irregular treatments 28.5% |
| Pace WD, et al. 48 | the US | 2019–2020 | 17,192 | Single bronchodilator 12.7% Dual bronchodilator 13.2% ICS/LABA or ICS/LAMA 29.9% Triple treatment 27.1% Others 4.4% None inhalant 12.7% |
| Price D, et al. 49 | the UK | 2009–2013 | 24,957 | Single bronchodilator 20.8% Dual bronchodilator 2.2% ICS/LABA or ICS/LAMA 29.5% Triple treatment 24.2% Others 6.4% None inhalant 17.0% |
| Mangold V, et al. 50 | Swiss | 2015–2022 | 1,121 | Single bronchodilator 20.6% Dual bronchodilator 45.5% ICS/LABA or ICS/LAMA 11.7% Triple treatment 17.8% None inhalant 4.5% |
| Pirina P, et al.51 | Italy | 2015–2016 | 225 | Single bronchodilator 28.0% Dual bronchodilator 4% ICS/LABA 15.1% Triple treatment 36.4% Others 3.6% None inhalant 12.9% |
| Barrecheguren, Miriam, et al. 52 | Spain | 2007–2012 | 41,492 | Single bronchodilator 30.5% Dual bronchodilator 1.7% ICS/LABA 17.3% Triple treatment 12.2% Others 17.0% None inhalant 21.2% |
| Montes de Oca M, et al. 53 | Latin America countries (Argentina, Colombia, Venezuela and Uruguay) | 2012 | 309 | Single bronchodilator 25.0% ICS/LABA 13.6% Others 1.9% None inhalant 59.5% |
COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LAMA, Long-acting muscarinic antagonist.
We compared these findings with data from primary care settings in middle-to-high-income countries such as the United States (US), the UK, and other regions. Our analysis revealed that in China, the proportion of patients using inhaled maintenance treatments is lower compared to these countries, though higher than in some Latin American countries. Additionally, the utilization rate of single bronchodilators is highest in Chinese primary care settings (37.3%), compared to other countries (12.7–30.5%). Specifically, the use of inhalation therapies containing ICS in China’s primary care is notably lower (16.9%) than in other nations (29.5-57.0%). This discrepancy highlights a significant disparity in COPD management between China and these economically advanced nations.
Discussion
COPD presents a significant public health challenge in China21–23. In recent years, the Chinese government has implemented a series of reforms aimed at improving the management of COPD24, particularly through the widespread promotion of pulmonary function testing (PFT), accompanied by substantial educational and training resources for primary healthcare workers. Despite progress, gaps in COPD treatment capabilities between different tiers of hospitals remain. Primary hospitals face limitations in diagnostic capabilities and access to specialized care25,26. In contrast, Tier3 hospitals often deal with overcrowding and resource constraints. Data indicate that a mere 5.1% of Tier2 and Tier3 institutions for approximately half of all medical consultations27. In our study, we also noted that the majority of cases originated from Tier3 hospitals, and this distribution accurately represents the real-world patterns of COPD patients seeking care at different levels of healthcare institutions in China. We believe this distribution bias is closely related to the practical limitations of the hierarchical medical system in China and the behavior of patients seeking care, which highlights the insufficient in resource allocation and referral mechanisms within the hierarchical healthcare system.
Our study indicated that COPD patients in primary hospitals exhibit characteristics such as advanced age and exposure to multiple risk factors. Notably, 82.5% of these patients also suffer from chronic bronchitis, with the majority presenting symptoms like chronic cough and dyspnea. These patients experience a higher rate of acute exacerbations compared to those treated in Tier2 and Tier3 hospitals. This indicates that primary care settings bear a heavier disease burden, one reason may be limited disease awareness among this population and their reluctance to seek timely medical attention. In addition, due to the chronic and progressive nature of COPD, some patients with more severe diseases may choose primary care facilities for convenience, cost, or personal considerations. Third, severer disease burden suggests that these patients may not receive adequate disease management. Results from a study assessing primary care physicians’ knowledge of COPD in China revealed that 94.4% of the physicians lacked the knowledge of treating COPD with bronchodilators and 92% of physicians did not know the management for stable COPD28. This underscores the necessity for enhanced educational initiatives aimed at both physicians and patients within primary hospitals29.
Results of our study revealed primary hospitals maintaining a relatively low inhaled maintenance treatments rate of 54.8%—an improvement from previous study30 but still below the rates observed in most of the middle-to-high-income countries. This disparity may be attributed to a shortage of specialized staff, patient compliance, drug availability and affordability in primary care settings, which directly impacting the worse clinical outcomes for COPD patients. Studies indicates that both the direct and indirect medical expenses attributable to COPD are considerable31,32. In many countries, healthcare funding is predominantly government-provided, facilitating universal access. However, in lower-middle-income countries, a substantial portion of healthcare financing relies on out-of-pocket expenses, deterring individuals from seeking necessary treatment due to the financial strain it imposes. Given these challenges, policymakers may consider enhancing the availability and affordability of essential medications at the primary care by including basic COPD medications in the national health insurance reimbursement lists, fostering continuity of care and potentially alleviating the economic burden on patients3,33,34.
In our study, we found a misuse of ICS in all the tiers of hospitals. It appears that primary care physicians often rely solely on the tables and quick reference numbers provided in the guidelines, rather than the comprehensive document, leading to the lack of knowledge about the potential harms and availability of alternative therapies35.This issue may also attributed to the common practice of prescribing fixed-dose combinations of ICS with bronchodilators, such as the LABA/ICS combination, which has been popular for many years. Moreover, our study noted that with increasing disease severity, there is a general decrease in the prescription of standalone LABA and LAMA. Conversely, the use of combinations of these bronchodilators (LABA + LAMA), and their association with ICS, increases as COPD severity escalates. This to some extent reflects the effectiveness of the COPD management measures over the years in China. Future efforts should continue to advance this approach and strengthen primary care capacity in COPD prevention and management.
Under-diagnosis of COPD is a widespread problem worldwide36–38. Results from the international survey showed that 81.4% of COPD cases (as defined by spirometry) were undiagnosed39. Our study indicated that over 60% of diagnosed patients are already in the advanced stages (GOLD3-4) by the time they were diagnosed. Early diagnosis and intervention in COPD can significantly slow the progression of lung function decline and improve long-term outcomes40. Strengthening COPD prevention and management in primary care can significantly contribute to better health outcomes and more efficient use of healthcare resources41,42. Some actions need to be taken, such as enhance health education, raise disease awareness, and apply screening programs for high-risk subjects43, make use of diverse questionnaires44–46 and digital healthcare technologies47.
In this multicentre, population-based analysis, we examined clinical features, distribution of risk factors and treatment patterns in 11,905 COPD patients across 88 hospitals of varying tiers in China. To our knowledge, this study is notably the first of its scale to provide a comprehensive overview of the current state of COPD management within tiered medical system in China. By elucidating the disparities in COPD management within tiered healthcare environments, this study underscores the urgent need for policies that enhance resource allocation and training at primary care. These findings offer critical empirical evidence to understand the real-world outcomes and unique challenges faced by COPD patients within China’s tiered healthcare framework, and offers crucial insights for policymakers to optimize COPD management and enhance overall healthcare quality in similar settings worldwide.
The limitations of this study should also be acknowledged. First, there may have potential factors influencing health-seeking behaviors and prescription variations across different tiers of hospitals, such as health insurance coverage, economic level, geographic factors, medical leveland and etc. In the future, we will further collect relevant indicators to explore the underlying factors driving prescription variations across different tiers of hospital. Second, the history of exacerbations was self-reported from the patients, previous research has shown that exacerbation profiles vary between patients and that the patients themselves often have a poor understanding of what, exactly, the term exacerbation means.
Conclusions
The tiered medical system in China has demonstrated effectiveness in managing COPD. However, disparities in treatment patterns across hospital tiers highlight the critical need for better distribution of primary medical resources. Enhancing drug availability at the primary level and launching educational initiatives for both physicians and patients are essential steps.
Acknowledgements
This study is supported by multiple research groups under the Center of COPD Prevention and Control of Henan Province. We gratefully appreciate the contribution of staff in the 88 hospitals.
Author contributions
XLW and XRZ contributed to data collection and data interpretation. TQC and YY contributed to literature review. XRZ, ZWX and LJS contributed to data analytical strategy design. YXA and XJZ contributed to the study conception and design. ZQW and QCZ helped to data analysis. PJ provided invaluable guidance throughout the writing process. The original draft was written by XRZ, all authors read and approved the final manuscript. XLW and XRZ contributed equally to the work.
Funding
This work was supported by Henan Provincial Science and Technology Research Project [242102311142, 232102310145] and the “Health Central Plains” COPD Screening and Management Project [2021020].
Data availability
The datasets used and/or analysed during the current study available from the corresponding author ( Xiaoju Zhang [zhangxiaoju@zzu.edu.cn]) on reasonable request.
Declarations
Ethics approval and consent to participate
The project was approved by the Ethics Committee of Henan Provincial People’s hospital (No.2022042), and all patients in this study provided written informed consent prior to study initiation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoli Wang and Xingru Zhao contributed equally to the work.
Contributor Information
Yunxia An, Email: 23706216@qq.com.
Xiaoju Zhang, Email: zhangxiaoju@zzu.edu.cn.
References
- 1.Adeloye, D. et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir Med.10(5), 447–458. 10.1016/S2213-2600(21)00511-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stolz, D. et al. Towards the elimination of chronic obstructive pulmonary disease: A lancet commission. Lancet400(10356), 921–972. 10.1016/S0140-6736(22)01273-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolbrink, M. et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: A systematic review. Lancet Glob Health. 10(10), e1423–e1442. 10.1016/S2214-109X(22)00330-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabyshova, A. et al. Gaps in COPD guidelines of low- and middle-income countries. Chest159(2), 575–584. 10.1016/j.chest.2020.09.260 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou, J. et al. Distributions and trends of the global burden of COPD attributable to risk factors by SDI, age, and sex from 1990 to 2019: A systematic analysis of GBD 2019 data. Respir Res.23(1), 90. 10.1186/s12931-022-02011-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, C. et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet Lond. Engl.391(10131), 1706–1717. 10.1016/S0140-6736(18)30841-9 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Yin, P. et al. The burden of COPD in China and its provinces: findings from the global burden of disease study 2019. Front. Public. Health. 10, 859499. 10.3389/fpubh.2022.859499 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease - GOLD. Global Initiative for Chronic Obstructive Lung Disease & November GOLD 30, 2023. Accessed August 5, 2024. (2024). https://goldcopd.org/2024-gold-report/
- 9.The Lancet. Towards better management of COPD. Lancet385(9979), 1697. 10.1016/S0140-6736(15)60873-X (2015). [DOI] [PubMed] [Google Scholar]
- 10.Papaioannou, A. I., Hillas, G., Loukides, S. & Vassilakopoulos, T. Mortality prevention as the centre of COPD management. ERJ Open. Res.10(3), 850–2023. 10.1183/23120541.00850-2023 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins, C. Successes and challenges of COPD management in Australia: reflections on the past and future. Lancet Respir Med.4(6), 424–426. 10.1016/S2213-2600(16)30083-2 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Guan, W. J., Ran, P. X. & Zhong, N. S. Prevention and management of COPD in China: successes and major challenges. Lancet Respir Med.4(6), 428–430. 10.1016/S2213-2600(16)30092-3 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Gautam, R., Neupane, D., Karki, A. & Kallestrup, P. Community-based management of COPD in Nepal. Lancet Respir Med.5(1), e6. 10.1016/S2213-2600(16)30431-3 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Ghimire, S. et al. Guideline based knowledge and practice of physicians in the management of COPD in a low- to middle‐income country. Clin. Respir J.16(3), 190–199. 10.1111/crj.13468 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.General Office of the State Council of China. Guiding Opinions of the General Office of the State Council on Promoting the Construction of a Graded Diagnosis and Treatment System. September 11. Accessed August 5, 2024. (2015). http://www.nhc.gov.cn/yzygj/s3573/201509/9d657ca840ed4904ab02d954538dd475.shtml
- 16.Liu, X. et al. Management of acute exacerbation of chronic obstructive pulmonary disease under a tiered medical system in China. Ther. Adv. Respir Dis.16, 175346662210754. 10.1177/17534666221075499 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Bureau of Statistics of China. China Statistical Yearbook—2023. June 12. Accessed August 5, 2024. (2024). https://www.stats.gov.cn/sj/ndsj/2023/indexch.htm
- 18.Zhao, X. et al. Whole-course management of chronic obstructive pulmonary disease in primary healthcare: an internet of things-enabled prospective cohort study in China. BMJ Open. Respir Res.11(1), e001954. 10.1136/bmjresp-2023-001954 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou, Y. M. et al. Development and validation of a chronic obstructive pulmonary disease screening questionnaire in China. Int. J. Tuberc Lung Dis.17(12), 1645–1651. 10.5588/ijtld.12.0995 (2013). [DOI] [PubMed] [Google Scholar]
- 20.GOLD-2021. Accessed August 10. (2024). https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 21.Fang, L. et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med.6(6), 421–430. 10.1016/S2213-2600(18)30103-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J. et al. Solid fuel use and incident COPD in Chinese adults: findings from the China kadoorie biobank. Environ. Health Perspect.127(5), 57008. 10.1289/EHP2856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hüls, A. & Schikowski, T. Ambient particulate matter and COPD in China: a challenge for respiratory health research. Thorax72(9), 771–772. 10.1136/thoraxjnl-2016-209687 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Quan, Z. et al. Current status and preventive strategies of chronic obstructive pulmonary disease in China: a literature review. J. Thorac. Dis.13(6), 3865–3877. 10.21037/jtd-20-2051 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, X. et al. Quality of primary health care in China: challenges and recommendations. Lancet395(10239), 1802–1812. 10.1016/S0140-6736(20)30122-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossaki, F. M. et al. Strategies for the prevention, diagnosis and treatment of COPD in low- and middle- income countries: the importance of primary care. Expert Rev. Respir Med.15(12), 1563–1577. 10.1080/17476348.2021.1985762 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Yip, W. et al. Universal health coverage in China part 1: progress and gaps. Lancet Public. Health. 8(12), e1025–e1034. 10.1016/S2468-2667(23)00254-2 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Pan, Z. et al. An online survey of primary care physicians’ knowledge of common respiratory diseases in China. Npj Prim. Care Respir Med.32(1), 28. 10.1038/s41533-022-00289-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu, G., Fan, G. & Niu, W. COPD awareness and treatment in China. Lancet Respir Med.6(8), e38. 10.1016/S2213-2600(18)30200-5 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Mao, R. et al. Stable chronic obstructive pulmonary disease (COPD) management under a tiered medical system in China. Int. J. Chron. Obstruct Pulmon Dis.17, 181–194. 10.2147/COPD.S333274 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, S. et al. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020-50: a health-augmented macroeconomic modelling study. Lancet Glob Health. 11 (8), e1183–e1193. 10.1016/S2214-109X(23)00217-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foo, J. et al. Continuing to confront COPD international patient survey: Economic impact of COPD in 12 countries. Borrow R, ed. PLoS One;11(4):e0152618. (2016). 10.1371/journal.pone.0152618 [DOI] [PMC free article] [PubMed]
- 33.Robertson, N. M. et al. Urban-rural disparities in chronic obstructive pulmonary disease management and access in Uganda. Chronic Obstr. Pulm Dis. J. COPD Found.6(1), 17–28. 10.15326/jcopdf.6.1.2018.0143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurst, J. R. et al. Challenges in the implementation of chronic obstructive pulmonary disease guidelines in low- and middle-income countries: an official American thoracic society workshop report. Ann. Am. Thorac. Soc.18(8), 1269–1277. 10.1513/AnnalsATS.202103-284ST (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stryczek, K. et al. De-implementing inhaled corticosteroids to improve care and safety in COPD treatment: primary care providers’ perspectives. J. Gen. Intern. Med.35 (1), 51–56. 10.1007/s11606-019-05193-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casas Herrera, A. et al. COPD underdiagnosis and misdiagnosis in a high-risk primary care population in four latin american countries. A key to enhance disease diagnosis: the PUMA study. Chotirmall SH, ed. PLoS One11(4):e0152266. (2016). 10.1371/journal.pone.0152266 [DOI] [PMC free article] [PubMed]
- 37.Axelsson, M. et al. Underdiagnosis and misclassification of COPD in Sweden – a nordic epilung study. Respir Med.217, 107347. 10.1016/j.rmed.2023.107347 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Koga, Y. et al. Underdiagnosis of COPD: the Japan COPD real-world data epidemiological (CORE) study. Int. J. Chron. Obstruct Pulmon Dis.19, 1011–1019. 10.2147/COPD.S450270 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamprecht, B. et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest148(4), 971–985. 10.1378/chest.14-2535 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Aaron, S. D. et al. Early diagnosis and treatment of COPD and asthma — a randomized, controlled trial. N Engl. J. Med.390(22), 2061–2073. 10.1056/NEJMoa2401389 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Lambe, T. et al. Model-based evaluation of the long-term cost-effectiveness of systematic case-finding for COPD in primary care. Thorax74(8), 730–739. 10.1136/thoraxjnl-2018-212148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Licskai, C. et al. Quantifying sustained health system benefits of primary care-based integrated disease management for COPD: a 6-year interrupted time series study. Thorax79(8), 725–734. 10.1136/thorax-2023-221211 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, Q. et al. Cost-effectiveness of population-based screening for chronic obstructive pulmonary disease in China: a simulation modeling study. Lancet Reg. Health - West. Pac.46, 101065. 10.1016/j.lanwpc.2024.101065 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu, Y. et al. Performance of COPD population screener questionnaire in COPD screening: a validation study and meta-analysis. Ann. Med.53(1), 1199–1207. 10.1080/07853890.2021.1949486 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huynh, C. et al. Derivation and validation of the UCAP-Q case-finding questionnaire to detect undiagnosed asthma and COPD. Eur. Respir J.60(3), 2103243. 10.1183/13993003.03243-2021 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Su, K. C. et al. The accuracy of PUMA questionnaire in combination with peak expiratory flow rate to identify At-risk, undiagnosed COPD patients. Arch. Bronconeumol. Published Online June2024:S0300289624002345. 10.1016/j.arbres.2024.06.013 [DOI] [PubMed]
- 47.Watson, A. & Wilkinson, T. M. A. Digital healthcare in COPD management: a narrative review on the advantages, pitfalls, and need for further research. Ther. Adv. Respir Dis.16, 175346662210754. 10.1177/17534666221075493 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pace, W. D. et al. COPD population in US primary care: data from the optimum patient care DARTNet research database and the advancing the patient experience in COPD registry. Ann. Fam Med.20(4), 319–327. 10.1370/afm.2829 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price, D. et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int. J. Chron. Obstruct Pulmon Dis. Published Online August2014:889. 10.2147/COPD.S62750 [DOI] [PMC free article] [PubMed]
- 50.Mangold, V. et al. Adherence to the GOLD guidelines in primary care: data from the Swiss COPD cohort. J. Clin. Med.12(20), 6636. 10.3390/jcm12206636 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirina, P. et al. Treatment of COPD and COPD–heart failure comorbidity in primary care in different stages of the disease. Prim. Health Care Res. Dev.21, e16. 10.1017/S1463423620000079 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrecheguren, M. et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med.111, 47–53. 10.1016/j.rmed.2015.12.004 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Montes De Oca, M., Lopez Varela, M. V., Jardim, J., Stirvulov, R. & Surmont, F. Bronchodilator treatment for COPD in primary care of four latin America countries: The multinational, cross-sectional, non-interventional PUMA study. Pulm Pharmacol. Ther.38, 10–16. 10.1016/j.pupt.2016.04.002 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Stott-Miller, M. et al. Defining chronic mucus hypersecretion using the CAT in the SPIROMICS cohort. Int. J. Chron. Obstruct Pulmon Dis.15, 2467–2476. 10.2147/COPD.S267002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author ( Xiaoju Zhang [zhangxiaoju@zzu.edu.cn]) on reasonable request.


