Abstract
Recently, a new bacterial disease was detected on cucumber stalks. In order to study the pathogenesis of this disease, the pathogenic bacteria were isolated and identified on the basis of morphological and molecular characteristics, and further analyzed for pathogenicity and antagonistic evaluation. Pathogenicity analysis showed that HlJ-3 caused melting decay and cracking in cucumber stems, and the strain reisolated from re-infected cucumber stalks was morphologically identical to HlJ-3 colonies, which is consistent with the Koch’s postulates. The pathogenic strain HlJ-3 was identified as having similar morphological characteristics to Bacillus subtilis. Meanwhile, its internal transcribed spacer sequence (ITS) and DNA gyrase A subunit (gyrA) were both more than 99% homologous and clustered on the same branch with B. subtilis. Therefore, combined with morphological and molecular biological features, strain HlJ-3 was identified as B. subtilis. In addition, B. subtilis, which has a wide range of hosts, was able to infest other common crop species, including potato, tomato, pepper, melon, and radish. Furthermore, antagonistic evaluation confirmed that strain HlJ-3 strongly inhibited the mycelial growth of Colletotrichum coccodes and Alternaria tenuissima in vitro, with antagonistic effects of 69.92% and 68.08%, respectively. In conclusion, our results showed that strain HlJ-3 is B. subtilis, which is pathogenic to cucumber in vivo and can infect plants of Solanaceae, Cucurbitaceae and Brassicaceae with a wide range of hosts. In addition, this strain has good biocontrol effects against C. coccodes and A. tenuissima in vitro. The findings of this research will help to prevent and control the occurrence of this pathogen and regulate its use as a biocontrol agent.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84764-8.
Keywords: Cucumis sativus, Gummy stem blight disease, Bacillus subtilis, Isolation, Identification, Pathogenicity, Antagonistic function
Subject terms: Bacteria, Pathogens
Introduction
Cucumber (Cucumis sativus) is one of the world’s top ten vegetable crops, belonging to the Cucurbitaceae family of annual climbing herbaceous plants1, native to the Asian continent2. Due to its high nutritional and economic value3–5, it has been planted in large quantities in China and its production scale is the first in the world6. Unfortunately, pests and diseases that arise during cultivation have severely impacted the productivity and quality of cucumbers as well as their various growth phases, even while the area under cultivation keeps growing7. According to the report, the main diseases causing great losses in cucumber are downy mildew caused by Pseudoperonospora cubensis8, powdery mildew caused by Sphaerotheca fuliginea9, gray mold caused by Botrytis cinerea10, anthracnose caused by Colletotrichum spp11. and bacterial soft rot caused by Pectobacterium carotovorum12, among others, which leads to a serious reduction in cucumber yield, thus causing significant losses to the agricultural economy13.
Interestingly, Bacillus spp. is a common type of bacterium found in nature that has mostly been identified in earlier research as an excellent biocontrol agent that can control a wide range of plant fungi that cause disease14. Bacillus spp., however, is a plant pathogen that may also seriously harm a number of host plants, some of which have been recognized as phytopathogens. For example, B. pumilus causes the soft rot of potato tubers15, leaf blight of Mangifera indica16, and ginger rhizome rot17. Sagittaria sagittifolia black rot18 and Allium cepa bacterial rot caused by B. amyloliquefaciens19. B. megaterium caused leaf spots of Radermachera sinica20 and bacterial blight in Lupinis termis21. Bacillus subtilis is a species of Bacillus spp. commonus that has been shown in earlier research to be widely distributed in a range of different living environments, capable of producing endophytic spores, resilient to heat, and present on the surface of soils and plants in addition to being a common endophyte in plants22. As a result of these characteristics, B. subtilis is generally widely used as a biocontrol agent in production practice in most of the current studies, and previous antagonistic experiments have demonstrated that B. subtilis inhibited the development and colonization of Rhizoctonia cerealis23, Pythium spp., Cercospora spp., and Fusarium spp24,25. Furthermore, studies have shown that B. subtilis possesses strong biocontrol capabilities because of its capacity to generate a wide range of secondary metabolites, or compounds that resemble glycosinophilic acid that can stimulate the synthesis of endogenous hormones and cooperate with the up-regulation of genes related to defense enzymes to fend off plant disease infections26.
However, it has also been shown that B. subtilis is capable of resolving pectin and polysaccharides in plant tissues and that some strains of B. subtilis can infect potato tubers27, tomatoes28, sunflower29, Leucojum aestivum30, cabbage31 and grape32. Nonetheless, B. subtilis is not only effective in infecting crops, fruits and vegetables but also in causing diseases in humans33–35 and insects36,37. Hence, in the production process, B. subtilis does not only exist as a biocontrol microorganism but its pathogenic role should not be ignored.
Gummy stem blight disease in Cucumis sativus was recently discovered in a field in Baiyin City, Gansu Province, China. The symptoms were characterised by longitudinal cracks in the stems and loose whitish tissue. To identify the pathogen responsible for this disease, in this study, the collected diseased stalks were isolated and their pathogenicity was verified according to Koch’s postulates. The pathogen was identified based on morphological characteristics and gene sequence analysis. In addition, its biocontrol potential and host plant range were tested. All of this will help to concretely identify the causal agent of cucumber gummy stem blight, resulting in more effective diagnosis, control and reduced economic losses.
Materials and methods
Test plant materials
Test plant seeds: The seeds of Cucumis sativus Cucumis melo, Lycopersicon esculentum, Raphanus sativus and Capsicum annuum were purchased from Herun Wanjia Seed Company. Zea mays from ZhangYe City, Gansu Province and Solanum tuberosum (original seed ) from DingXi City, Gansu Province.
Pathogen isolation and purification
In October 2020, Cucumis sativus stems with typical bacterial disease symptoms were found in greenhouses in Baiyin, Gansu Province, China (maximum elevation 3321 m, minimum: 1275 m; geographic location. 103°33’-105°34’E, 35°33’-37°38’N) and were brought back to the laboratory for identification. Diseased and healthy stems were rinsed with sterile water and the tissue was cut with a sterilised knife and then sterilised with 75% alcohol for 30 s. The resulting stalk tissues were placed on beef extract peptone dextrose media (BPD), respectively. The inoculated plates were placed in an incubator at 28℃ and incubated under dark conditions for 48 h. After the colonies had grown, individual colonies of the same morphology, size and colour were separated by plate separation and numbered and transferred to test tubes and stored in a refrigerator at 4 °C for future use38.
Pathogenicity tests
When the cucumber grows to about 30 days, the isolates obtained in the previous step were transferred to BPD plates and cultured in the dark at 28℃ for 24–48 h, 5–6 single colonies were picked and added into 50 ml of BPD culture fluid, and cultured for 36 h under the condition of 28℃ and 180 rpm with shaking.
In vivo surface wound inoculation tests
Cucumber stalks used for pathogenicity test were wiped with 75% alcohol and then wounded with a sterile insect needle, and each treatment was repeated 6 times. The inoculated isolates were spread evenly on the wounded stalks with sterile cotton and then placed in a plastic bucket, sprayed with sterile water and incubated at room temperature in a moisturised environment for 48 h before being grown under normal conditions. Disease symptoms on stalks were continuously observed and captured with a digital camera. BPD culture fluid not inoculated with isolates were used as a control.
In vivo surface wound-free inoculation tests
The bacterial liquid was sprayed on healthy cucumber stalks wiped with 75% alcohol for a total of six treatments, with BPD cultures not inoculated with the isolate as control. The cucumbers were then moisturised and placed under normal growing conditions after 48 h. Observations were continued to record disease symptoms and photographs were taken with a digital camera.
Subsequently, diseased cucumber stems with similar disease symptoms to those in the field were reisolated at the disease-health junction according to Koch’s postulates to determine whether the inoculated isolates were pathogenic.
Identification with cultural and morphological characteristics
The pathogenic strains were inoculated onto BPD, Yeast Extract Tryptone culture Medium (LB) and beef extract peptone Nacl media (BPN) incubated at 28 ℃ in the dark for 48 h. The morphology of the colonies was observed, recorded and photographed. Then they were stained for Gram and Spore stain and 100 randomly selected single bacterium were measured for their size.
Identification with molecular characteristics
Ten single colonies of pathogenic bacteria were picked with a splice ring and incubated in 100 ml of BPD at 28°C and 180 r for 36 h. DNA was then extracted from the pathogenic bacteria using the TIANGEN Bacteria DNA Kit (TIANGEN BIOTECH (Bei Jing) CO, LTD). 16S ribosomal RNA (16S rRNA) (27F: 5’-AGAGTTTGATCCTGGCTCAG-3’)/1492R: 5’-GGTTACCTTGTTACGACTT-3’) and DNA gyrase A subunit (gyrA) (gyrA-bs1: 5’-GCGATCCTTGACATGAGGCT-3’ /gyrA-bs25’-AGACGCACACCTTGAGTAC-3’) 39 primers were used for amplification.
The reaction system for 16 S rRNA was 25 uL, including 12.5 uL PCR Mix, 1 uL DNA, 1 uL 1492R, 1 uL 27 F, 9.5 uL ddH2O; the reaction system for gyrA was 25 uL, including 12.5 uL Mix, 0.5 uL DNA, 1 uL gyrA-bs1, 1 uL gyrA-bs2, 10 uL ddH2O.
The amplified condition for the 16s rRNA region was 5 min initial denaturation at 94 °C, and denaturation at 94 °C for 1 min, annealing at 48 °C for 30 s, extending at 72 °C for 1 min, with 40 cycles, a final extension step of 1 min at 72 °C and the preservation at 4 °C; the condition for the gyrA region was 3 min initial denaturation at 95 °C, the denaturation at 95 °C for 30 s, the annealing at 58 °C for 30 s, the extend at 72 °C for 1 min, the cycles of 32, a final extension step of 5 min at 72 °C and the preservation at 4 °C。.
Finally, PCR products were detected by 1% agarose gel electrophoresis, and the PCR products with obvious bands were sent to Lanzhou Tianqi Gene Biotechnology LTD for sequencing. The multilocus sequences were compared with the sequences previously deposited in the GenBank Database using the BLAST, and both 16s rRNA and gyrA amplified sequences were found to be B. subtilis, and then the phylogenetic tree was constructed by MEGA 7.0 adopted with 1000 bootstrap replications to clarify the taxonomic status of pathogenic bacteria.
Host range tests
The inoculation methods are the same as for pathogenicity tests, and the cultured bacterial solution was inoculated onto melon (Cucumis melo), potato (Solanum tuberosum), tomato (Lycopersicon esculentum), radish (Raphanus sativus), chilli (Capsicum annuum), and corn (Zea mays). Using the BPD culture fluid without the pathogen as a control, and then placed in a sterilized plastic bucket to moisturize and incubate, disease symptoms were continuously observed and photographed and recorded for a total of 6 treatments, each replicated 6 times.
In vitro antagonistic activity tests
The in vitro antagonistic activity of B. subtilis was determined by an antagonistic culture method. The following pathogens were selected from the laboratory: Fusarium oxysporum, F. verticillioides, F. avenaceum, Alternaria tenuissima, A. solani, C. scovillei and C. coccodes were tested for antagonistic activity. The above pathogens were inoculated onto potato-dextrose agar (PDA) media in a 25 °C incubator for 7 d in the dark, and then perforated with a hole punch at the edge of the colony to form a 5 mm mycelial discs. It was placed in the center of the plate and four test strains were attached at equal distances on a circumference 2.5 cm from the center (i.e., standoff method) (Fig. 1). The plates were then incubated in the dark in an incubator at 25 °C. After the control dish was full, the fungal colony diameter was measured by the cross method, and each treatment was repeated seven times to calculate the inhibition rate. The mycelium at the edge of the colonies in the treatment and control groups was picked and the changes in mycelial morphology were observed under a microscope and photographed.
Fig. 1.

In vitro antagonistic activity test method. Gray represents the pathogenic fungi tested for antagonistic activity
Inhibition rate (%)=((diameter of control - diameter of treated pathogenic fungi)/(diameter of control − 0.5))×100, where 0.5 was the diameter of fungal plugs initially inoculated on the PDA plate.
Results
Field symptoms
During field investigations in Baiyin City, Gansu Province, China, it was found that cucumber gummy stem blight can occur both cucumber stems and fruits. Among them, the disease was most severe and obvious on the stalks, and the infested area was larger. The symptoms of old stalks are drying and cracking, with loose and whitish internal tissues (Fig. 2A and B). Newly grown stalks showed symptoms of wilting, yellowish-brown colour with a watery surface of the stalks (Fig. 2C, D and E). Cucumber fruits were cut open to a light yellowish-brown cracked appearance(Fig. 2F).
Fig. 2.
A gelatinous secretion produced on the stalks and fruits of cucumber, found in the field. A–E symptoms on the surface of cucumber stems (the stems were wilted, yellowed and split). F, symptoms on the fruit of cucumber
Isolation of pathogen and pathogenicity tests
Typical symptomatic and healthy stalks were brought back to the laboratory for pathogen isolation. Three isolates, named HlJ-1, HlJ-2 and HlJ-3, were obtained from diseased stalks and no strain was isolated from healthy stalks. The three isolates were then inoculated into cucumber stalks and only HlJ-3 was found to cause pathogenicity in cucumber stalks, and when the diseased stalks were reisolated, the reisolates were identical to the isolates in terms of morphological characteristics.
Symptoms of HlJ-3 inoculation were as follows: on the fifth day of inoculation with isolate HlJ-3 under needling conditions, small yellow-brown cracks appeared on the cucumber stalks (Fig. 3f, g, and h). As the disease expanded, the aboveground cucumber leaves turned yellow and withered (Fig. 3b, c, d, n, o and p), on the 10th day after inoculation, the stalks cracked and brown secretion appeared at the cracks (Fig. 3j, k, l, r, s t and supplementary Fig. 3), whereas the control did not develop the disease (Fig. 3a, e, i, m and q). Under non-needling conditions, the onset symptoms were consistent with the above description, with the difference that visible symptoms could be observed on day 15 after inoculation (Fig. 4). By reisolating the onset site, colonies consistent with isolate HlJ-3 were obtained (Fig. 5g and h). Thus, pathogenicity tests showed that isolate HlJ-3 is the pathogen responsible for cucumber stem disease.
Fig. 3.
Pathogenicity of isolate HlJ-3 in cucumber stalks with surface wound inoculation (vivo inoculation tests). a, e, i, m and q represent in vivo controls with surface wounds that showed no disease symptoms; b, c, d, n, o and p represent yellowing and wilting of foliage, splitting of stalks, and yellowish brown of cucumber when cucumber stalks were inoculated with HlJ-3 by pinprick inoculation 10 days after inoculation; and j, k, l, r, s and t represent localised symptoms of cucumber stalks inoculated with HlJ-3 10 days after inoculation, w stand for the third pathogenicity, the leftmost stalk is CK.
Fig. 4.
Pathogenicity of isolate HlJ-3 in cucumber stalks with surface non-wound inoculation (vivo inoculation tests). a and e, the control in vivo pathogenicity about surface non-wound inoculations, no disease symptoms appeared. b, c and d represent the disease status of the whole cucumber plant 15 days after inoculation with HlJ-3 under non-needling conditions; f, g and h represent the disease status of the inoculation site.
Fig. 5.
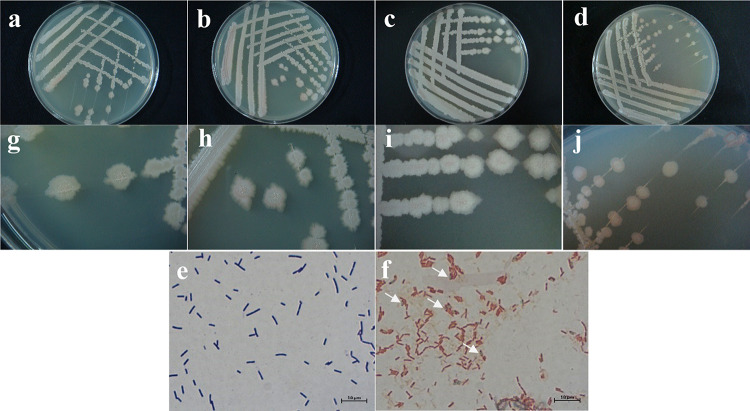
Morphological characteristics of strain HlJ-3. a and g, represent morphological characteristics of the proto-bacteria isolated from field samples and inoculated into NA medium. b and h, Morphological characteristics of the pathogenic bacteria obtained after isolate HlJ-3 was inoculated onto Cucumber stalks and caused disease in Cucumber and then re-inoculated into NA medium. c and i, represent the morphological characteristics of the colonies obtained after inoculating the pathogen on LB plates. d and j represent the morphological characteristics of colonies inoculated on NB plates. e represents the characteristics of the pathogenic bacteria HlJ-3 after Gram staining. f represents the characteristics of the pathogenic bacteria HlJ-3 after Spore stain, and the direction of the arrow is the stained bacteriophage.
Identification of morphological characteristics
The pathogenic bacterium HlJ-3 was inoculated onto BPD, BPN and LB media by plate scribing method, and incubated for about 48 h at 28 °C under dark conditions (Fig. 5a-d). Single colonies were found to be dirty white, rough and opaque on the surface, with central folds and spreading irregular edges, and a ring of light red pigment near the edge of the colony (Fig. 5g, h, i, and j). Single colonies were picked out for Gram staining and found to be Gram-positive with rod-shaped bodies, size (0.33–0.60) µm × (1.92–5.47) µm. It was found by Spore stain that bacteria were purplish red when stained, and endospores were colorless or light green (Fig. 5e and f). According to morphological observation, HlJ-3 colony morphology was found to be consistent with the morphology of B. subtilis in the Bergey’s manual of determinative bacteriology, which was initially identified as B. subtilis27.
16s rRNA and gyrA sequence analysis
16 S rRNA, 16 S ribosomal RNA gene; gyrA, DNA gyrase A subunit. HlJ-3 was the pathogen of the study.
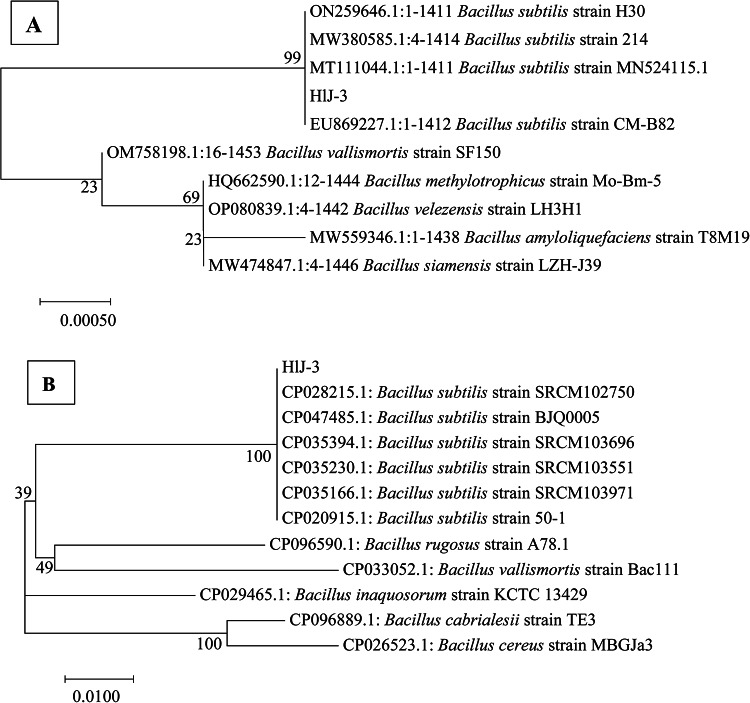
The DNA of strain HlJ-3 was amplified by 16 S rRNA and gyrA. The 1444 bp and 999 bp amplified fragments were obtained by 1% agarose gel electrophoresis, respectively. The 16 S rRNA (OP341449.1) and gyrA (OQ803359.1) sequences of strain HlJ-3 showed 100% homology with the B. subtilis sequences, respectively. The results of phylogenetic tree indicated that strain HlJ-3 clustered with B. subtilis with a bootstrap value of 99% and 100% (Fig. 6A and B). Based on the morphological and molecular characteristics, strain HlJ-3 was identified as B. subtilis.
Fig. 6.
Phylogenetic tree of B. subtilis strain HlJ-3 combined 16 S rRNA with gyrA. A amplifed by 16 S rRNA Primer; B amplifed by gyrA Primer.
Host range assays
In order to further investigate the pathogenicity of the HlJ-3 strain, the host range of the pathogen HlJ-3 was assessed by inoculating six common plants, namely Melon, Potato, Tomato, Corn, Radish and Chilli. Under pinprick conditions, the stems of all five plants, except maize, were infested (Table 1). Symptoms appeared on the 3rd day after inoculation in melon, radish and chilli, on the 7th day after inoculation in potato and on the 5th day after inoculation in tomato. We observed that the disease symptoms were evident on the 14th day after inoculation on the affected stalks (Fig. 7a to f). Cracking and brownish-yellow gelatinous secretion appeared on the surface of the stalks of melon and radish, whereas ulceration, melting and rotting appeared on the surface of the stalks of tomato, potato, and chilli, while none of the inoculated control plants developed the disease.
Fig. 7.
Lesions of infection by Bacillus subtilis HlJ-3 on melon, potato, tomato, corn, radish and chilli stems 14 days after surface wound and non-wound inoculation (a to f for needle inoculation, g to l for non-needle inoculation). m and n, models of different treatments for different crop species are in this figure. SW; Surface wound inoculation Bacillus subtilis HlJ-3. NW; Surface Non-wound inoculation Bacillus subtilis HlJ-3, CK (SW and NW) was inoculated with blank cultures.
Under non-needling conditions, 14 days after inoculation, stalks of maize, melon, and radish did not show any disease symptoms (Fig. 7g, j and k), whereas potato, tomato, and chilli were not as severe as under needling conditions, with only 1–2 plants showing localised tissue lysis on the surface of the stalks (Fig. 7h, i and l), and none of the inoculated control plants developed any disease. In the Fig. 7, m and n represent different treatment models for different crop varieties. Finally, reisolation of the above-diseased plants yielded strains consistent with the inoculated strain HlJ-3, indicating that HlJ-3 is the pathogen. The above results indicated that among these six hosts, maize plants were not infested under both needling and non- needling conditions, suggesting that the pathogen is not pathogenic. For the remaining five plants, on the other hand, it was strongly pathogenic under needling conditions.
Table 1.
The pathogenicity of B. subtilis HlJ-3 in 6 crops 14 days after surface wound and non-wound inoculation.
| Crop name | Scientific name | Family | Inoculation tissue | Degree of incidence g | |
|---|---|---|---|---|---|
| SW e | NW f | ||||
| Melon | Cucumis melo | Cucurbitaceae | Stem | +++ | - |
| Potato | Solanum tuberosum | Solanaceae Juss | Stem | +++ | + |
| Tomato | Lycopersicon esculentumMill. | Solanaceae Juss | Stem | +++ | + |
| Corn | Zea mays | Poaceae | Stem | - | - |
| Radish | Raphanus sativus | Brassicaceae | Stem | +++ | - |
| Chilli | Capsicum annuum | Solanaceae Juss | Stem | +++ | + |
e SW; Surface wound inoculation. f NW; Surface Non-wound inoculation. g Strain HlJ-3 infection host ability.
+ Represents 1–2 plants with disease, ++ represents 3–4 plants with disease, +++ represents 5–6 plants with disease, -represents no plant disease.
In vitro antagonistic activity test of HlJ-3
Since B. subtilis is a good biocontrol agent in the majority of studies, we tested the pathogen for antagonistic activity in vitro in order to verify whether it also functions as a biocontrol agent. The results of the plate standoff assay showed that strain HlJ-3 had different levels of antagonistic activity against the seven pathogenic fungi tested: F. oxysporum, A. tenuissima, A. solani, C. scovillei, F. verticillioides, F. avenaceum and C. coccodes.
Among them, the greatest inhibitory effect was observed on A. tenuissima and C. coccodes with obvious circles of inhibition (Fig. 8) whose inhibition rates were 69.92% and 68.08%, respectively; the inhibitory effect on C. scovillei and A. solani, the inhibitory effect was also more evident, with inhibition rates of 60.83% and 62.49%, respectively; while F. oxysporum, F. verticillioides and F. avenaceum had the worst inhibitory effect, with inhibition rates of 53.59%, 47.84%, and 44.29%, respectively (Table 2).
Fig. 8.
In vitro biocontrol of fungi by B. subtilis strain HlJ-3. The effects of HlJ-3 on C. coccodes (a1), A. tenuissima (a2), A. solani (a3), C. scovillei (a4), F. avenaceum (a5), F. verticillioides (a6), F. oxysporum (a7) for biological control. Pathogenic fungi and HlJ-3 were double-cultured on the same potato dextrose agar plate with a distance of 2.5 cm between them, and in the image, the pathogenic fungi are in the center, and HlJ-3 is around; a1 to a7 are negative controls, and the plates were cultured with a single pathogenic fungus. d1 to d2 are the effects of HlJ-3 on the mycelium of seven pathogenic fungi after standoff culture, respectively. c1 to c7 are mycelial controls, respectively.
Effect of strain HlJ-3 on mycelial growth of pathogenic fungi: Mycelia at the junction of strain HlJ-3 and pathogenic fungi were picked and observed under the microscope. As can be seen in Fig. 8, compared with the control, the mycelium of C. coccodes, A. tenuissima, A. solani and C. scovillei showed different degrees of breakage, disappearance of septa, curved expansion and protoplasmic spillage after treatment with HlJ-3, among which the mycelium of C. coccodes had the greatest degree of ring-breaking, and the vesicles appeared in the middle of the mycelium. The effects on the mycelium of F. oxysporum, F. verticillioides and F. avenaceum were not significant.
The above results showed that HlJ-3 had strong teratogenic and bacteriostatic effects on four fungi (i.e., C. coccodes, A. tenuissima, A. solani, and C. scovillei ), which suggests the potential biocontrol function of HlJ-3 (Fig. 8).
Table 2.
The inhibition rate of B. subtilis against pathogenic fungi (%).
| Pathogenic fungus | Strain HlJ-3 c | ||
|---|---|---|---|
| Diameter of CK colony(cm) | Diameter of treatment colony(cm) | Inhibition rate(%)l | |
|
C. scovillei (Capsicum annuum) d |
7.32 ± 0.08b | 3.17 ± 0.04c | 60.83 ± 0.52b |
|
A. solani (Solanum tuberosum) e |
8.08 ± 0.09a | 3.34 ± 0.03c | 62.49 ± 0.39b |
|
F. verticillioides (Zea mays) f |
8.27 ± 0.02a | 4.83 ± 0.20a | 44.29 ± 2.5e |
|
F. avenaceum (S. tuberosum) g |
8.18 ± 0.03a | 4.06 ± 0.03b | 53.59 ± 0.42c |
|
A. tenuissima (C. melo) h |
8.17 ± 0.08a | 2.81 ± 0.05d | 69.92 ± 0.64a |
|
C. coccodes (S. tuberosum) i |
7.37 ± 0.02b | 2.69 ± 0.03d | 68.08 ± 0.43a |
|
F. oxysporum (Allium tuberosum) j |
6.95 ± 0.24c | 3.86 ± 0.08b | 47.84 ± 1.19d |
| Average k | 7.76 | 3.54 | 58.15 |
c Strain HlJ-3 was the pathogen of the study.
k Average represents the overall antibacterial status of HlJ-3 against the 7 pathogenic fungi in the table.
d~j Host of pathogenic fungi.
l Data are presented as the means ± SE of seven replications. Different letters indicate the results of Duncan’s multiple range te (P < 0.05).
Discussion
A popular vegetable in China, cucumbers are produced for their anti-aging, diuretic, and oedema properties, as well as for their ability to dissipate heat and ease coughs. China is the world’s largest producer of cucumbers, with the largest planted area40. Although cucumbers are highly sought after in China, their quality and output have been negatively impacted by a number of diseases41. In this study, a cucumber disease was discovered in the field in Baiyin City, Gansu Province, China, which primarily infested cucumber stalks, its main symptoms were longitudinal surface cracking and drying, and its tissues were loose and whitish. The strain HlJ-3 was verified as the causative agent by Koch’s postulates, and was identified as B. subtilis. B. subtilis is generally considered non-pathogenic, and most of the laboratory cultures are considered contaminating or endophytic bacteria42. Since it is not generally pathogenic, three subsequent pathogenicity assays were performed in this study (The third pathogenicity results are shown in Supplementary Fig. 3), and all tests revealed that B. subtilis HlJ-3 was the pathogenic agent.
After inoculating cucumber stalks with HlJ-3, the disease firstly appeared as water-soaked, depressed onset site with a large amount of white to light yellow pus overflowing when humidity was high; later, the disease progressed to a middle stage where the stalks cracked and rotted longitudinally, leaving white scars at the onset site when humidity was lowered. Finally, the disease showed symptoms of soft-rotting and eventually caused the entire plant to wilt. The symptom was similar to the symptoms of the disease in the field while the control did not have the disease, which is similar to the symptoms of Melting Decay of ‘Red Globe’ Grapes32, and leaf mottle of sunflower reported in California and Northern Kazakhstan29, and the pathogen of both diseases is B. subtilis. However, compared with the symptoms in the field, the disease conditions were slightly different, which might be due to the different environmental conditions, host resistance, and the period when the pathogen infested the hosts, and the exact causes need to be further investigated.
Morphological culture and molecular identification are essential for differentiating complicated species within a genus for identifying diseases43,44. Since molecular biology technology has advanced recently, PCR and multigene identification techniques have become commonplace for species identification. These techniques allow for the quick identification of species based on their affinities45–47. However, it is hard to reliably discriminate between some subspecies and relatives of Bacillus spp. due to the variability of the 16s rRNA gene. To quickly identify species and analyze the evolutionary and genetic links between bacterial species, phylogenetic analyses have been used to 16s rRNA, gyrB, ropB, cheA, and gyrA gene sequences or gene fragments. The housekeeping genes gyrB, ropB, cheA, and gyrA, on the other hand, exhibit significantly higher genetic divergence48,49 because they are highly conserved and only exist in a single copy in all bacteria50. The gyrA gene may be utilized as a phylogenetic marker to differentiate between the B. subtilis group and related relatives because it encodes the DNA promoter enzyme A subunit, which regulates DNA superhelix and replication start51. This gene compensates for the limitations of the 16s rRNA gene. As a result, the gyrA gene gives a greater degree of differentiation compared to the gyrB, rpoB, and cheA genes 39. Following this, HlJ-3 morphology was found to be essentially comparable with that of B. subtilis, as documented in Berger’s manual of bacterial identification27,52. 16s rRNA and gyrA were utilized in this work to differentiate the taxonomic status of pathogenic bacteria to more precisely identify pathogenic bacteria. With bootstrap values greater than 99%, it was discovered that both gene sequences were grouped in the phylogenetic tree and were highly similar to B. subtilis, with more than 99% homology. Therefore, based on morphological characteristics and molecular biology, HlJ-3 was identified as B. subtilis causing cucumber gummy stem blight disease. To the best of our knowledge, this is the first report of B. subtilis causing cucumber gummy stem blight.
Six popular crops from four families—Cucurbitaceae, Solanaceae, Brassicaceae, and Poaceae—were chosen for the current study in order to further investigate the pathogenicity of HlJ-3 due to the paucity of references on the disease-causing potential of B. subtilis. Under needling conditions, HlJ-3 infected every inoculated crop in the research, except for maize. Three Solanaceae crop varieties—potato, tomato, and chilli—were shown to be infected in both wounding and non-wounding environments, suggesting that B. subtilis is a potent Solanaceae plant pathogen. HlJ-3 infested radish and melon under needling conditions, but strain HlJ-3 did not infest Brassicaceae radish and Cucurbitaceae melon under non-needling conditions, suggesting that strain HlJ-3 is an opportunistic pathogen. Among the six crops studied above, HlJ-3 (B. subtilis) infestation of potato and tomato has been reported, but not in chilli, radish and melon.
As Bacillus subtilis is often considered as in vitro biocontrol agent against a wide range of diseases including Colletotrichum spp53. , Fusarium spp54, Alternaria spp55 , Botrytis spp56. and Sclerotium spp57. Therefore, in the present study, strain HlJ-3 (B. subtilis) was evaluated using in vitro dual culture method against pathogenic fungi Fusarium oxysporum, F. verticillioides, venaceum, A. tenuissima, A. solani, C. scovillei and C. coccodes. The average antagonism value was greater than 58.15%, indicating that strain HlJ-3 possessed broad-spectrum antimicrobial activity, and HlJ-3 showed the greatest inhibition of A. tenuissima and C. coccodes, which was more than 68%. In contrast, lower inhibition rates were reported in the studies of Wu and Lee58,59. Meanwhile, we observed a significant effect of HlJ-3 on the mycelial growth of A. tenuissima and C. coccodes, as evidenced by mycelial rupture, vesicles, curling, and swelling (Fig. 8), which is in agreement with the effects of B. subtilis on the mycelium of pathogenic fungi reported by Khan and Zhang60,61. These results suggest that strain HlJ-3 has special biocontrol properties and broad-spectrum antimicrobial effects against various common pathogenic fungi.
Generally, this is the first report of B. subtilis induced cucumber gummy stem blight disease in China. It offers a theoretical foundation for the disease’s diagnosis and comprehensive control, but more research is still needed to determine the pathogenic mechanism. In the meantime, its virulence to host plants should not be disregarded when B. subtilis is employed as a biological microbe for agricultural development, based on the pathogenicity of B. subtilis on cucumber in this work. Therefore, in order to lessen the drop in agricultural output brought on by the employment of B. subtilis as a biological agent, it is imperative to further validate the pathogen’s host range. Additionally, owing to the broad-spectrum antimicrobial activity of B. subtilis, effective antagonistic substances can be extracted from it for further bioanalysis in crop production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the under Gansu Melon and Vegetable Industry System in China Grant GARS-GC-2.
Author contributions
CY, and MJ conceived and designed the study. YW performed most of the experiments.FC, TM, CW and NQ helped carry out experiments.CY and RO revised the manuscript and provided critical discussions.All authors contributed to the study and approved this submission.
Funding
This work was supported by the Gansu Melon and Vegetable Industry System in China, item No: GARS-GC-2.
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI repository with the GenBank accession numbers: 16 S rRNA, OP341449; gyrA, OQ803359.
Declarations
Competing interests
The authors declare no competing interests.
Research involving plants
The plant material collected for this test, as well as the plants for the test, complied with the relevant laws and regulations.
Reprints and permissions information
is available at www.nature.com/reprints. Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional afliations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bello, M. O., Owoeye, G., Abdulhammed, M. & Yekeen, T. A. Characterization of Gourd fruit (cucubitaceae) for dietary values and anti-nutrition constituent. Pharm. Biol. Chem. Sci.6, 7575–7585 (2014). [Google Scholar]
- 2.Abiodun, O. A. & Adeleke, R. O. Comparative studies on nutritional composition of four melon seeds varieties. Pakistan J. Nutr.9, 905–908. 10.3923/pjn.2010.905.908 (2010). [Google Scholar]
- 3.Aboloma, R. I., Onifade, A. K. & Adetuyi, F. C. Fungi associated with the deterioration of some fruits of the family Cucurbitaceae. Nigerian J. Mycolog. 2, 229–236 (2009). [Google Scholar]
- 4.Bates, D. M. & Richard, W. R. and Jeffrey C. Biology and utilization of the Curcurbitaceae. Cornell University Press. 44: 502. (1990). 10.5860/choice.28-0290
- 5.Mortimore, M. Dryland development: Success stories from West Africa environment. J. Biol. Sci.45, 10–21. 10.3200/ENVT.47.1.8-21 (2015). [Google Scholar]
- 6.Ishaya, M. et al. Isolation and identification of fungal pathogen associated with post harvest deterioration of cucumber (Cucumis sativus L.) fruits in three selected markets in Jos, Nigeria. Int. J. Plant. Soil. Sci.30, 1–8. 10.9734/ijpss/2019/v30i630196 (2019). [Google Scholar]
- 7.Luo, M. et al. Identification of a new Talaromyces strain DYM25 isolated from the Yap Trench as a biocontrol agent against Fusarium wilt of cucumber. Microbiol. Res.251, 126841. 10.1016/j.micres.2021.126841 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Rani, R., Negi, P., Sharma, S. & Jain, S. Occurrence of oosporic stage of Pseudoperonospora cubensis on cucumber, in Punjab, India: A first report. Crop Prot.155, 105939. 10.1016/j.cropro.2022.105939 (2022). [Google Scholar]
- 9.Wang, X. et al. Nucleotide-binding leucine-rich repeat genes CsRSF1 and CsRSF2 are positive modulators in the Cucumis sativus defense response to Sphaerotheca Fuliginea. Int. J. Mol. Sci.22, 3986. 10.3390/ijms22083986 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu, J. et al. The bioactivity and efficacy of benzovindiflupyr against Corynespora Cassiicola, the causal agent of cucumber corynespora leaf spot. Plant Dis.105, 3201–3207. 10.1094/PDIS-11-20-2334-RE (2021). [DOI] [PubMed] [Google Scholar]
- 11.Yousuf, S., Dar, G. H., Bhat, S. & Rasool, F. First report of Colletotrichum arbiculare causing anthracnose in cucumber. Int. J. Curr. Microbiol. App Sci.7, 857–859. 10.20546/ijcmas.2018.708.096 (2018). [Google Scholar]
- 12.Meng, X. et al. Emergence of bacterial soft rot in cucumber caused by Pectobacterium carotovorum subsp. brasiliense in China. Plant Dis.101, 279–287. 10.1094/PDIS-05-16-0763-RE (2017). [DOI] [PubMed] [Google Scholar]
- 13.Rishi, N. Significant plant virus diseases in India and a glimpse of modern disease management technology. J. Gen. Plant Pathol.75, 1–18. 10.1007/s10327-008-0139-8 (2009). [Google Scholar]
- 14.Ahmad, T. et al. Biocontrol potential of lipopeptides produced by the novel Bacillus subtilis strain Y17B against postharvest Alternaria fruit rot of cherry. Front. Microbiol.14, 1150217. 10.3389/fmicb.2023.1150217 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bathily, H., Babana, A. H. & Samaké, F. Bacillus pumilus, a new pathogen on potato tubers in storage in Mali. Afr. J. Microbiol. Res.4, 2067–2071 (2010). http://www.academicjournals.org/ajmr [Google Scholar]
- 16.Galal, A. A., El-Bana, A. A. & Janse, J. Bacillus pumilus, a new pathogen on mango plants. Egypt. J. Phytopathol.34, 17–29 (2006). [Google Scholar]
- 17.Peng, Q., Yuan, Y. & Gao, M. Bacillus pumilus, a novel ginger rhizome rot pathogen in China. Plant Dis.97, 1308–1315. 10.1094/PDIS-12-12-1178-RE (2013). [DOI] [PubMed] [Google Scholar]
- 18.Zhong, L. et al. First report of black rot of Sagittaria sagittifolia caused by Bacillus amyloliquefaciens in China. Plant Dis.99, 1270–1270. 10.1094/PDIS-02-15-0148-PDN (2015). [Google Scholar]
- 19.Hwang, S. K. et al. Occurrence of bacterial rot of onion caused by Bacillus amyloliquefaciens in Korea. J. Gen. Plant Pathol.78, 227–232. 10.1007/s10327-012-0376-8 (2012). [Google Scholar]
- 20.Li, Y. L., Zhou, Z., Yuan, Y. C. & Ye, J. R. First report of a leaf spot of Radermachera sinica in China caused by Bacillus megaterium. Plant Dis.98, 1425–1425. 10.1094/PDIS-05-14-0469-PDN (2014). [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Monaim, M. F., Gabr, M. R., El-Gantiry, S. M., Shaat, M. N. & El-Bana, A. A. Bacillus megaterium, a new pathogen on lupine plants in Egypt. J. Bacteriol. Res.4, 24–32. 10.5897/JBR12.022 (2012). [Google Scholar]
- 22.Jiang, L. L., Chen, Y. C. & Xin, M. X. Application and research advance in controlling plant fungous disease of Bacillus subtilis. Anhui Agricultural Sci. Bull.15, 37–39 (2009). [Google Scholar]
- 23.Yi, Y. et al. Efficacy of Bacillus subtilis XZ18-3 as a Biocontrol Agent against Rhizoctonia cerealis on wheat. Agriculture12, 258. 10.3390/agriculture12020258 (2022). [Google Scholar]
- 24.Priyanka, B. et al. Antifungal activity of lipopeptides from Bacillus subtilis isolates against Rhizome Rot of Ginger caused by Fusarium oxysporum and Pythium aphanidermatum. Mysore J. Agricultural Sci.56, 349–357 (2022). [Google Scholar]
- 25.Srimai, K. & Akarapisarn, A. Bacillus subtilis LBF02 as biocontrol agent against leaf spot diseases caused by Cercospora lactucae-sativae in lettuce. J. Agric. Sci.6, 151 (2014). 10.5539/jas.v6n3p151
- 26.Li, C. et al. Screening of Bacillus subtilis HAAS01 and its Biocontrol Effect on Fusarium wilt in Sweet Potato. Phyton91, 1780–1793. 10.32604/phyton.2022.020192 (2022). [Google Scholar]
- 27.Buchanan, R. E. & Gibbons, N. E. Bergey’s manual of determinative bacteriology. J. Protozoology. 10.1111/j.1550-7408.1975.tb00935.x (1974). 8th Ed. [Google Scholar]
- 28.Malcolmson, J. F. A disease of potatoes and tomatoes caused by Bacillus subtilis. Rep. Scot Soc. Res. Pl Breed. 24–28 (1960).
- 29.Kiyan, V. et al. Morphological and molecular characterization of bacterial pathogens associated with leaf mottle of sunflower in Northern Kazakhstan. Plant Dis.10.1094/PDIS-07-23-1352-SR (2023). [DOI] [PubMed] [Google Scholar]
- 30.Stoyanova, M. et al. New pathogens of Leucojum aestivum. pp. 728–734 in. Conference with International Participation THE MAN AND THE UNIVERSE, Smolyan, BULGARIA. (2011).
- 31.Qiu, W. F., Di, Y. B., Zhou, Y. Y. & Si, F. J. Studies on some spoilage bacteria in cabbage cellars. Acta Phytopathol. Sinica. 7, 127–128 (1964). [Google Scholar]
- 32.Morgan, D. P. & Michailides, T. J. First Report of Melting Decay of ‘Red Globe’ grapes in California. Plant Dis.88, 1407. 10.1094/PDIS.2004.88.9.1047C (2004). [DOI] [PubMed] [Google Scholar]
- 33.Bais, W. J. A case of pathogenicity of Bacillus Subtilis. J. Infect. Dis.40, 313–315 (1927). https://www.jstor.org/stable/30083295 [Google Scholar]
- 34.Greenspon, E. A. A. Pathogenic Bacillus Subtilis isolated from the Eye. Am. J. Ophthalmol.1, 316–318 (1918). [Google Scholar]
- 35.Sweany, H. C. A pathogenic Subtilis Bacillus from a patient with chronic tuberculosis. J. Infect. Dis.37, 340–343 (1925). [Google Scholar]
- 36.Srivastava, R., Nayak, P. & Naik, G. Pathogenicity of Bacillus subtilis (Ehrenberg) Cohn on Lepidopteran pests of rice. Abstract Biocontrol News Inform.11, 230 (1990). [Google Scholar]
- 37.Jacob, A., Philip, B. M. & Mathew, M. P. Bacillus subtilis as a pathogen on bhindi leaf roller, SyDerogatarogata (Pyralidae: Lepidoptera). J. Invertebr. Pathol.40, 301–302. 10.1016/0022-2011(82)90130-6 (1982). [Google Scholar]
- 39.Chun, J. & Bae, K. S. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek. 78, 123–127. 10.1023/a:1026555830014 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Li, X. et al. First report of Ceratocystis fimbriata causing postharvest fruit rot of cucumber in China. Plant Dis.103, 1432. 10.1094/PDIS-10-18-1904-PDN (2019). [Google Scholar]
- 38.Fang, Z. D. Phytophthora Research Methods 3rd Edn (China Agricultural, 1998).
- 41.Loredana, S. et al. First report of Pythium Spinosum as a causal agent of crown and root rot in greenhouse cucumber cultivation in Italy. Plant Dis.104, 3269. 10.1094/PDIS-02-20-0305-PDN (2020). [Google Scholar]
- 42.Sun, S. Y., Li, X., Zhang, T., Sheng, Y. M. & Yong, X. L. Clinical treatment practice of Bacillus subtilis infection after lumbar decompression and fusion. J. Clin. Ration. Use Drugs. 14, 171–172 (2021). [Google Scholar]
- 43.Zhao, P., Wang, Q. H., Tian, C. M. & Kakishima, M. Integrating a numerical taxonomic method and molecular phylogeny for species delimitation of Melampsora species (Melampsoraceae, Pucciniales) on willows in China. PLoS One. 10, e0144883. 10.1371/journal.pone.0144883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma, T., Yang, C., Cai, F. & Chen, Z. Morpho-cultural, physiological and molecular characterisation of Colletotrichum nymphaeae causing anthracnose disease of walnut in China. Microb. Pathog.166, 105537. 10.1016/j.micpath.2022.105537 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Hong, S. G., Maccaroni, M., Figuli, P. J., Pryor, B. M. & Belisario, A. Polyphasic classification of Alternaria isolated from hazelnut and walnut fruit in Europe. Mycol. Res.110, 1290–1300. 10.1016/j.mycres.2006.08.005 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Iwen, P. C., Hinrichs, S. H. & Rupp, M. E. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med. Mycol.40, 87–109. 10.1080/mmy.40.1.87.109 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Weir, B. S., Johnston, P. R. & Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol.73, 115–180. 10.3114/sim0011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim, B. J. et al. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol.37, 1714–1720. 10.1128/jcm.37.6.1714-1720.1999 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto, S., Bouvet, P. J. & Harayama, S. Phylogenetic structures of the Genus Acinetobacter based on gyrB sequences: Comparison with the grouping by DNA-DNA hybridization. Int. J. Syst. Evol. MicroBiol.49, 87–95. 10.1099/00207713-49-1-87 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Tayeb, L. A. et al. Comparative phylogenies of Burkholderia, Ralstonia, Comamonas, Brevundimonas and related organisms derived from rpoB, gyrB and rrs gene sequences. Res. Microbiol.159, 169–177. 10.1016/j.resmic.2007.12.005 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Barnes, M. H., LaMarr, W. A. & Foster, K. A. DNA gyrase and DNA topoisomerase of Bacillus subtilis: Expression and characterization of recombinant enzymes encoded by the gyrA, gyrB and parC, parE genes. Protein Exp. Purif.29, 259–264. 10.1016/s1046-5928(03)00068-8 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Pei, D., Zhang, Q., Zhu, X. & Han, S. Endophytic Bacillus subtilis P10 from Prunus cerasifera as a biocontrol agent against tomato verticillium wilt. Brazilian J. Biology. 83, e244261. 10.1590/1519-6984.244261 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Amaro, J. K. C., Vieira, B. S. & Sousa, L. A. Biological control of Colletotrichum gloeosporioides in Chilli with isolates of Bacillus subtilis. Brazilian J. Agric. - Revista De Agricultura. 93, 195–209 (2018). [Google Scholar]
- 54.Khedher, S. B., Mejdoub-Trabelsi, B. & Tounsi, S. Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol. Control. 152, 104444. 10.1016/j.biocontrol.2020.104444 (2021). [Google Scholar]
- 55.Awan, Z. A. et al. Antifungal potential of volatiles produced by Bacillus subtilis BS-01 against Alternaria Solani in Solanum lycopersicum. Front. Plant Sci.13, 1089562. 10.3389/fpls.2022.1089562 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bu, S. et al. Bacillus subtilis L1-21 as a biocontrol agent for postharvest gray mold of tomato caused by BotCinereainerea. Biol. Control. 157, 104568. 10.1016/j.biocontrol.2021.104568 (2021). [Google Scholar]
- 57.Ashok, V. G., Neha, A. M. & Pranita, G. A. Production of bioactive compound by Bacillus subtilis and its antagonistic activity against Sclerotium Rolfsii. Int. J. Life Sci.2, 127–133 (2014). [Google Scholar]
- 58.Lee, K. J., Kamala-Kannan, S., Sub, H. S., Seong, C. K. & Lee, G. W. Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillus subtilis. World J. Microbiol. Biotechnol.24, 1139–1145. 10.1007/s11274-007-9585-2 (2008). [Google Scholar]
- 59.Wu, Z., Ma, R., You, C. & Liang, Y. Identification of antagonistic bacteria against Alternaria tenuissima, and its effect on antagonism. Microbiol. China. 42, 1321–1330. 10.13344/j.microbiol.china.140809 (2015). [Google Scholar]
- 60.Khan, N. et al. Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol.9, 2363. 10.3389/fmicb.2018.02363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, D. et al. Biocontrol and action mechanism of Bacillus subtilis Lipopeptides’ fengycins against alternaria solani in potato as assessed by a transcriptome analysis. Front. Microbiol.13, 861113. 10.3389/fmicb.2022.861113 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data that support the findings of this study have been deposited in the NCBI repository with the GenBank accession numbers: 16 S rRNA, OP341449; gyrA, OQ803359.