Abstract
To develop an atrophic Meibomian Gland Dysfunction (MGD) animal model via liquid nitrogen cryotherapy, the eyelid edges of C57 mice exposure to liquid nitrogen for 30 s. Morphology of MG and ocular surface were assessed using stereomicroscopy and a slit lamp microscope at multiple time points post-injury. Acinar loss and atrophy were observed from day 7, with increased inflammation and apoptosis, and decreased proliferation in acinar cells. Corneal epithelial defects appeared after day 14. Liquid nitrogen induced selective damage to meibomian acinar cells, simulating MGD pathology effectively, with peak effects at day 21, providing a relevant model for atrophic MGD research.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84742-0.
Keywords: Meibomian gland dysfunction, Liquid nitrogen, Acinar atrophy, Model
Subject terms: Biological techniques, Biological models, Animal disease models
Introduction
Meibomian glands (MGs) are sebaceous glands located within the tarsal plates of the eyelids, responsible for secreting lipids that constitute the superficial layer of the tear film1,2. These lipids are critical for preventing tear evaporation and protecting the ocular surface from microbial and particulate matter. Meibomian gland dysfunction (MGD) is a prevalent chronic condition characterized by obstruction of the gland ducts and/or qualitative and quantitative alterations in lipid secretion3. The overall global prevalence rate is reported to be between 21.2% and 71.0%4. MGD is widely recognized as a leading cause of dry eye syndrome5. Based on the secretion status of the meibomian glands, Meibomian Gland Dysfunction (MGD) is divided into two major categories: hyposecretion type and hypersecretion type. The hyposecretion type is further subdivided into c type and obstructive type6.
Acini atrophy represents a degenerative change in the meibomian glands and is a significant pathological characteristic of Meibomian Gland Dysfunction (MGD), manifesting as a reduction in acinar cell volume and a decline in the secretion capacity of meibum6. The terminal ducts of the meibomian glands may not exhibit significant obstruction, whether this is accompanied by ductal atrophy requires further research. Atrophic type can be categorized into primary and secondary types7. The causes and mechanisms of primary meibomian gland atrophy are not well understood. Advanced age is a possible risk factor for primary gland atrophy. Type 2 diabetes, prolonged use of video display terminals, high sugar and high-fat diet, long-term use of retinoids, and long-term wear of contact lenses can lead to gland atrophy8–10. Additionally, this type of MGD can also be secondary to the increased glandular pressure caused by obstructive MGD. The ectodermal dysplasia syndrome can cause congenital developmental disorders of the meibomian gland acini, and such meibomian gland lesions do not belong to MGD7.
Despite the recognized importance of MGs in ocular health, the development of reliable animal models for atrophic MGD has been a subject of ongoing debate and refinement. Various knockout mice can cause changes in the structure and function of the meibomian glands11,12. MGD models were created by targeting the deletion of specific genes that encode enzymes or lipoproteins involved in lipid metabolism, such as High-fat diet (HFD) mice, Fatp4 − /−;Tg(IVLFatp4) mice, ELOVL3-ablated (E3hom) mice, ACAT-1-/- mice and so on13–15. Nevertheless, since the models were developed through systemic gene knockout, we cannot discount the potential for systemic effects on the anatomy and physiology of other ocular surface tissues. For example, ACAT-1-/- mice can lead to abnormalities in adrenal gland structure, atherosclerosis of the arteries, and severe alterations in other organs. If corneal epithelial damage occurs in a knockout animal model, it cannot be ruled out whether the gene defect itself affects the development of the cornea or whether it is a secondary change in the cornea caused by meibomian gland dysfunction. Moreover, the vast majority of patients with meibomian gland atrophy are not caused by genetic defects. Clinical studies report that during the aging process, meibomian glands (MG) undergo acinar atrophy, thickening of the basement membrane, and inflammatory changes16,17. Nien and colleagues examined the MG of mice at 2, 6, 12, and 24 months of age, finding that in older mice, there were alterations in the PPARγ receptor signaling, decreased cell proliferation, reduced lipid synthesis, and glandular atrophy18. These findings are consistent with age-related changes in human MG. The atrophy of MG in aged mice may be attributed to the loss of meibomian gland progenitor cells, rather than excessive ductal keratinization and gland obstruction. Therefore, aged mice can serve as a model for age-related MGD, with many similar age-related changes between mice and humans. However, the long breeding cycle of aged mice, high mortality rate, and the low rate of model development are need to considerate. The existing animal models of MGD induced by injury cause mechanical obstruction such as keratinization of the duct orifice, and do not represent the characteristics of atrophic MGD19. Animal models of primary meibomian gland atrophy remain missing.
Temperature is crucial to the functionality of the meibomian glands, particularly as it significantly influences the physicochemical properties of the meibum secreted by these glands20 Experiments conducted using a Langmuir trough have revealed that human meibomian lipids are characterized by their ability to form multilamellar liquid crystalline films that are highly compressible and resistant to collapse. These lipids possess intrinsic surfactant properties, enabling them to effectively spread across an aqueous substrate. However, temperature is a significant modulating factor; a decrease in temperature has been shown to augment the surfactant capabilities of these lipids21.The temperature at which phase transition occurs was markedly elevated by 4 degrees Celsius for the meibomian gland lipids in comparison to the mean values from age-matched controls without a history of dry-eye symptoms22. Patients with meibomian gland dysfunction exhibit a reduction in the surface temperature of the tarsal conjunctiva23. A study examines the influence of eyelid temperature on the secretion of oil from the meibomian glands. The study found that increasing the eyelid temperature enhanced oil secretion, while decreasing it reduced secretion. The results suggest that the viscosity of the meibomian oil is likely affected by temperature changes, which supports the use of warm compresses to improve oil delivery in cases of gland dysfunction24. Thermo-Transient Receptor Potential (TRP) channels are detectably expressed within meibomian glands (MGs) at both the genetic and protein levels. The potential functions of TRPV1 and TRPM8 may involve the regulation of lipid metabolism in the meibomian glands (MG)25.
In dermatology, cryotherapy is applied to specifically target and destroy sebaceous glands in the skin as a treatment for seborrheic dermatitis. Solid carbon dioxide (at − 78 °C) and liquid nitrogen (at − 195 °C) have long been utilized for the nonspecific ablation of epidermal lesions, including conditions like actinic keratosis and common warts26. They were examined in both murine and porcine models across a range of low temperatures, with subsequent trials on human participants. In the murine model, the apex of histological damage to the ears was observed at 72 h post-cooling application. It was characterized by eosinophilic necrosis within the sebaceous glands and a sustained reduction in gland count for up to seven days after treatment. The cooling process led to the disruption of Sebaceous gland cell membranes, a decrease in alkaline phosphatase activity, Inflammatory cell infiltration, and a marked reduction in the lipid content of sebaceous glands. In the human trials, controlled cooling resulted in sebaceous gland damage and a diminished sebum level for two weeks, with minimal adverse effects on the surrounding tissue27. It is widely acknowledged that thermal therapy and massage have therapeutic effects on meibomian glands. Hence, it is questioned whether cryogenic freezing can cause similar damage to the meibomian glands as it does to the skin, and whether such damage can simulate the pathophysiological process of atrophic meibomian gland dysfunction (MGD). What molecular mechanism changes can temperature or hypothermia induce in the meibomian glands? The following experiments were conducted to investigate these questions.
In this research, we introduce a murine model of meibomian gland dysfunction (MGD) that is induced by a targeted application of liquid nitrogen to the eyelid margin. This model is characterized by significant loss of meibomian gland acini and ductal structures, as well as decreasing secretion of meibomian glands. No damage to other tissues. The development of this model is expected to advance our understanding of the pathophysiological alterations within meibomian glands subjected to cold-induced injury. Furthermore, it provides a platform for the exploration of novel therapeutic approaches for MGD and the potential for regenerative medicine strategies targeting the meibomian glands.
Results
Assessment of meibomian glands and other ocular surface tissues following liquid nitrogen injury to the eyelid margin
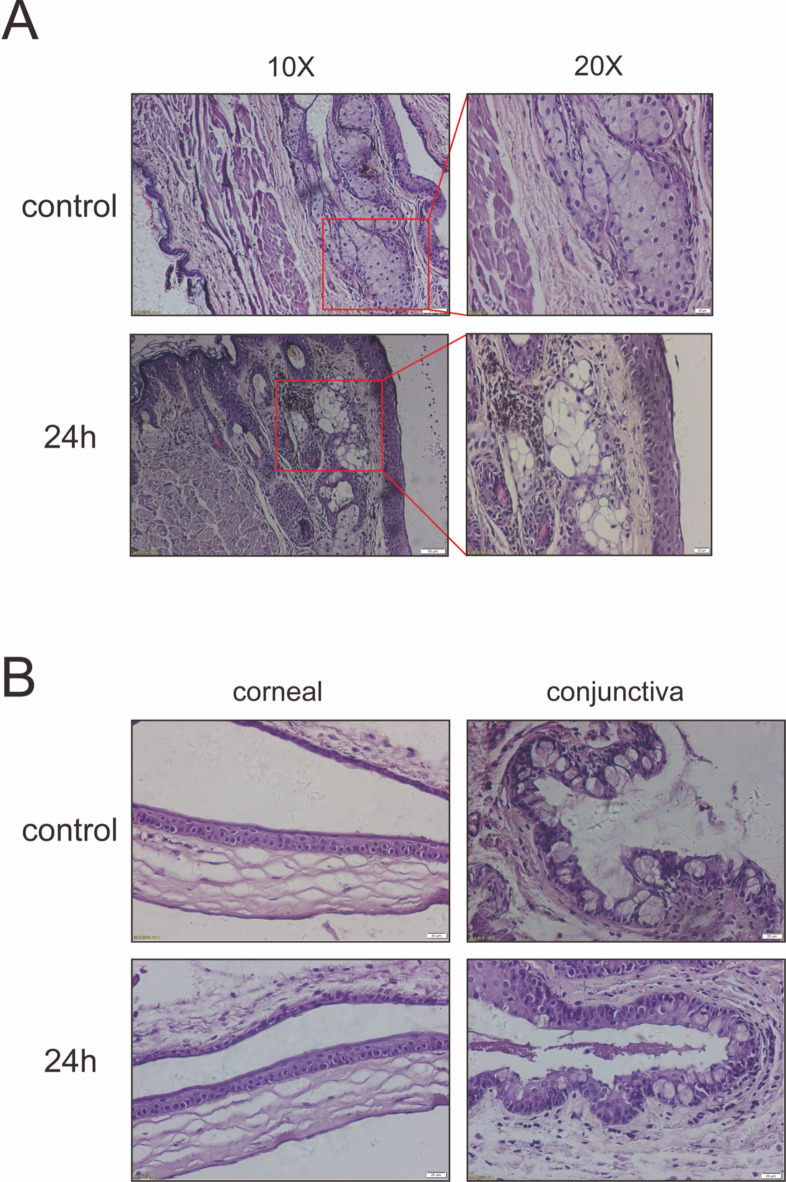
Twenty-four hours after cryotherapy of the eyelid margin with liquid nitrogen, meibomian gland tissue from the mice was obtained for hematoxylin and eosin (H&E) staining and observation. Only the structure of the meibomian glands was found to be disrupted, with no changes observed in other structures. This indicates that the application of liquid nitrogen to the eyelid margin can specifically damage the acinar cells of the meibomian glands without affecting other tissue cells (as shown in Fig. 1).
Fig. 1.
Assessment of Meibomian acinar cells and Cornea within 24 h following liquid nitrogen Injury to the Eyelid Margin. (A) Representative H&E staining showing the eyelids of uninjured, 24 h post liquid nitrogen injury mouse. Panes indicate meibomian acinar cells, specifically ruptured meibomian acinar cells after liquid nitrogen treatment. Scale bars represent 50 μm and 20 μm (n = 3). (B) Representative H&E staining of corneas and conjunctiva show no significant damage and no increase of cell infiltration in the corneal stroma after the eyelid margin liquid nitrogen injury. Scale bars represent 20 μm (n = 3).
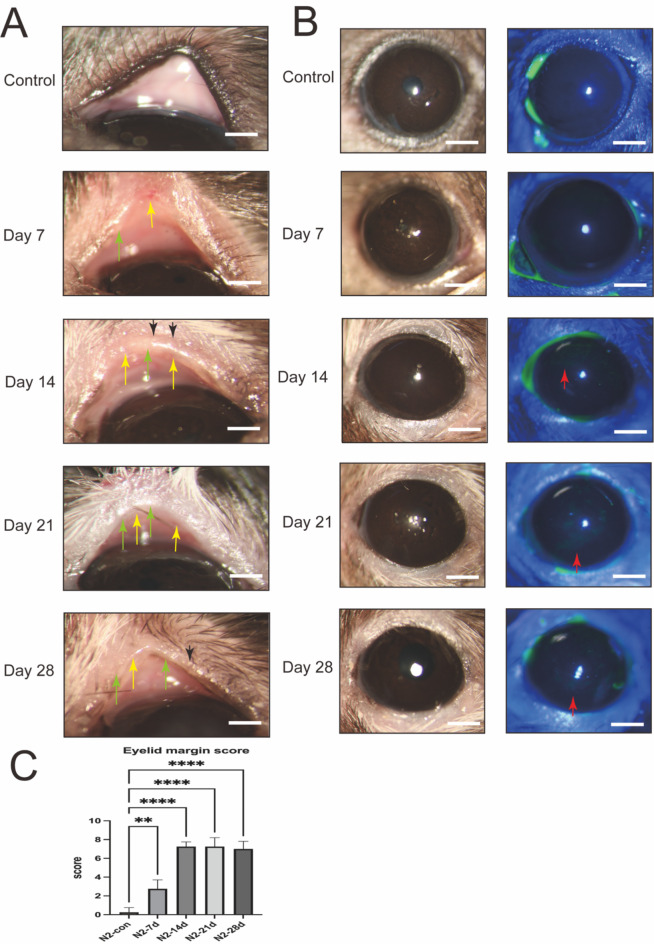
Mice were evaluated at specific intervals of 7-, 14-, 21-, and 28-days post-application of liquid nitrogen cryotherapy to the eyelid margin. In comparison to the control group that received no treatment, there were observable changes in the eyelid margin’s smoothness and thickness, with a progressive increase in the visibility of corneal fluorescence over time (as depicted in Fig. 2A, B). The group observed at 21 days post-treatment demonstrated the most significant alterations, with the eyelid margin being the roughest and the corneal fluorescence staining being the most intense. The meibomian gland scoring criteria indicate pathological changes in the morphology of the eyelid margin following cryogenic injury28 (as depicted in Fig. 2C).
Fig. 2.
Assessment of MGs and cornea following liquid nitrogen injury of the eyelid margin. (A) Representative slit-lamp images showing the eyelids of uninjured mouse (Control) and those of day 7, day 14, day 21 and day 28 post liquid nitrogen injury mouse. The MG orifice plugging (black arrowheads), hypertrophic eyelid margin (green arrowheads) and telangiectasia (yellow arrowheads) are indicated. Scale bars represent 10 mm in ×16 images. (B) The fluorescein staining of the corneal surface indicates a healthy epithelium at days 7 post liquid nitrogen injury, with punctate damage seen at day 14, 21, 28 (red arrowheads). Scale bars represent 10 mm in ×16 images. (C) Meibomian Gland Scoring System42. Data are shown as mean ± SD. n = 5, **p < 0.01, ****p < 0.0001.
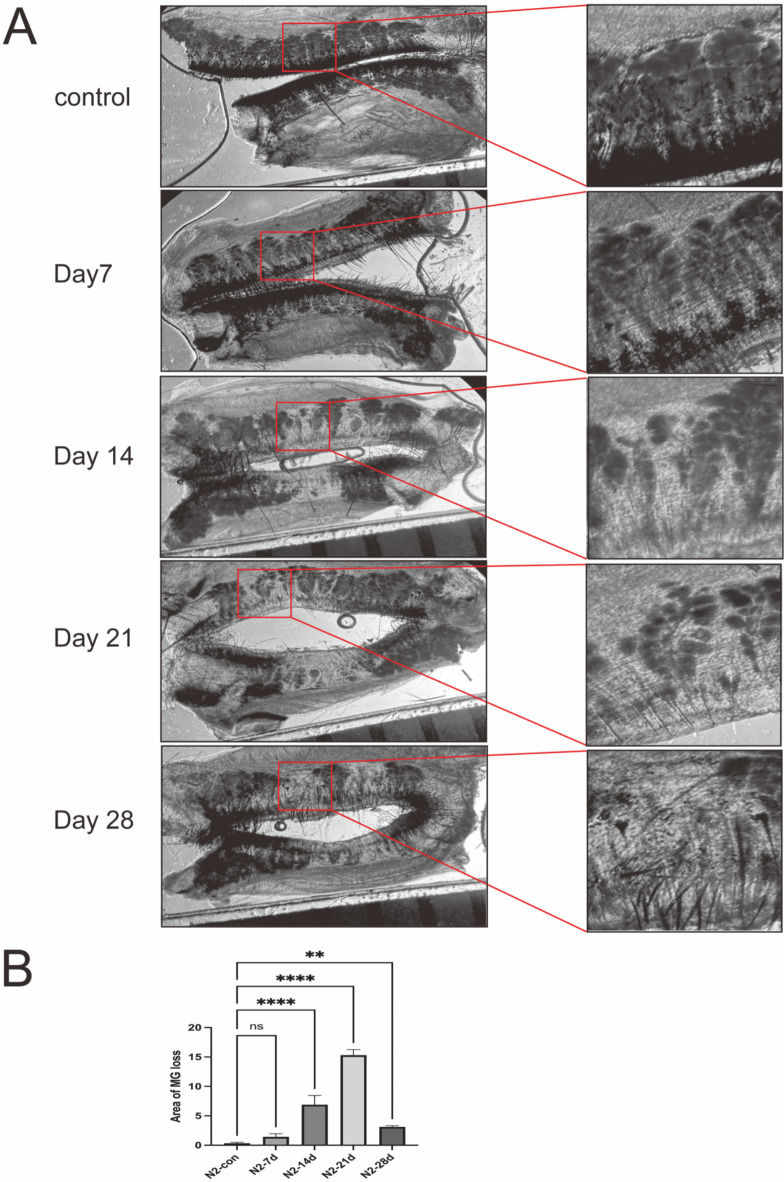
The loss of meibomian glands is a significant diagnostic indicator of meibomian gland dysfunction (MGD)29. Subsequent to the application of liquid nitrogen cryotherapy to the eyelid margin, progressive morphological alterations of the meibomian glands were observed, reflecting glandular atrophy and the loss of principal ducts with the passage of time (as shown in Fig. 3).
Fig. 3.
MG loss and morphological changes after liquid nitrogen. (A) Representative stereoscopic microscope images show MG loss and morphological changes of MGs after liquid nitrogen. The red rectangle represents the area of MG loss. Scale bars represent 1 mm (n = 3). (B) The area of meibomian gland loss gradually increases after liquid nitrogen freezes the meibomian margin. Data are shown as mean ± SD. n = 3, **p < 0.01. , ****p < 0.0001.
Changes of lipid synthesis after liquid nitrogen Injury to the eyelid margin
Oil Red O staining indicates that after liquid nitrogen cryopreservation, the staining intensity of the meibomian gland acinar cells has become lighter. The morphology of the meibomian gland acini exhibited varying degrees of atrophy with extended observation time. The Oil Red O staining within the ducts shows incomplete filling (as shown in Fig. 4A).
Fig. 4.
Histological changes and lipid synthesis of MGs after liquid nitrogen injury of the eyelid margin. (A) Oil red O staining showing the meibomian gland ducts are partially filling. at days 7, 14, 21 and 28 post liquid nitrogen injury (circle). Some oil red O-labeled droplets indicated atrophy acini (black star). Scale bars represent 200 μm (n = 3). (B, C) The expression of PPARγ in immunofluorescence significantly decreases on the 7th, 21st, and 28th days following liquid nitrogen cryopreservation. ****p < 0.0001. (D, E) Western blot analysis shows the decline in PPARγ after liquid nitrogen treatment. The expression reduction was most significant at 7 days and 21days. **p < 0.01, ns > 0.05.
PPAR-γ (Peroxisome Proliferator-Activated Receptor gamma) plays a significant role in maintaining the morphology of Meibomian glands (MGs) and the differentiation of acinar cells, and it is also a critical factor in the lipid synthesis of MGs30. Immunofluorescent staining of PPAR-γ and western blot analysis has shown that following cryotherapy with liquid nitrogen applied to the eyelid margin, there was a decrease in PPAR-γ expression, with the 7-day and 21-day post-treatment groups exhibiting particularly significant reductions. Although there was an observed increase in PPAR-γ expression in the 14-day and 28-day post-treatment groups, the levels remained below the normal range. This suggests that the application of liquid nitrogen to the eyelid margin has led to a diminished capacity for lipid synthesis in the MGs and indicates an impairment in the differentiation of the acinar cells (as shown in Fig. 4B–E). This suggests that cryogenic injury disrupts the structure of the cell membrane, thereby affecting the capacity for lipid synthesis.
Inflammation of MGs after liquid nitrogen injury of the eyelid margin
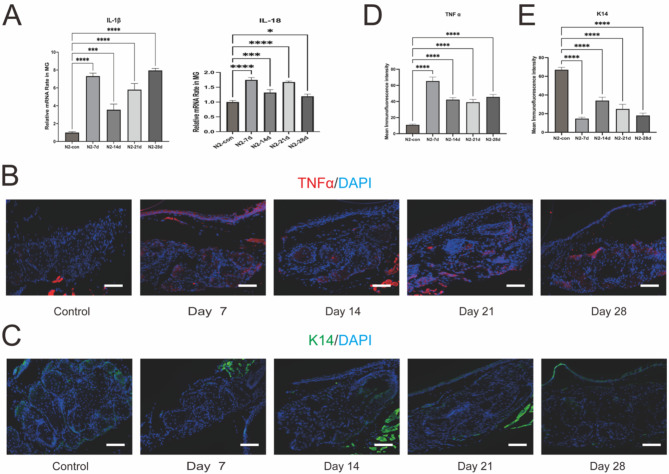
Inflammatory infiltration is a characteristic feature of Meibomian Gland Dysfunction (MGD)31. The expression levels of IL-1β and IL-18, as well as the immunofluorescence observation of TNFα, revealed that inflammatory cytokines peaked on the 7th day, likely due to the acute phase of injury. However, as the observation period of the experiment extended, inflammation did not show a significant decrease but rather remained at a higher level. Observations on the 21st and 28th days indicated that the inflammatory cytokines were still higher than those in the control group. We infer that this may be the reason for the further decline and deterioration of meibomian gland function (as shown in Fig. 5A, B, D).
Fig. 5.
Inflammation of MGs after liquid nitrogen injury of eyelid margin. (A) Gene expression levels of IL-1β, IL-18 were upregulated in the MG of mice post liquid nitrogen injury. ****p < 0.0001, ***p < 0.001. (B, D) Immunofluorescence staining of TNFα was upregulated in the MG post liquid nitrogen injury. Scale bars represent 50 μm (n = 3). ****p < 0.0001. (C, E) Immunofluorescence staining of K14 was downregulated in the meibomian gland acinar cells post liquid nitrogen injury. Scale bars represent 50 μm (n = 3). ****p < 0.0001.
We performed CD68 + iNOS double-labeling fluorescence staining to assess inflammatory cell infiltration in the meibomian glands of different groups. Observations revealed that CD68-positive cells were present at all stages following liquid nitrogen-induced eyelid margin injury, with iNOS-positive cells being more prominent in the early stages but decreasing at later stages (as shown in supplementary Figure).
At day 7, CD68(+) cells were predominantly located at the apex of the acini, with greater accumulation around the acini than at the eyelid margin. iNOS(+) cells were more abundant near the acini and fewer at the eyelid margin. By day 14, CD68(+) cells remained near the acini with minimal clustering at the eyelid margin, while iNOS(+) cells had significantly decreased compared to the day 7 group and were almost absent at the eyelid margin. At days 21 and 28, a small number of CD68(+) cells were observed near the acini, with no significant changes in their presence at the eyelid margin, and iNOS(+) cells were scarcely detected in either location. These changes are consistent with the pathological alterations reported in dermatology, where pro-inflammatory macrophages dominate in the early stages of cryotherapy-induced skin injury, and repair-oriented, anti-inflammatory cells become predominant at later stages32.
Cell proliferation and apoptosis in MGs after liquid nitrogen Injury to the eyelid margin
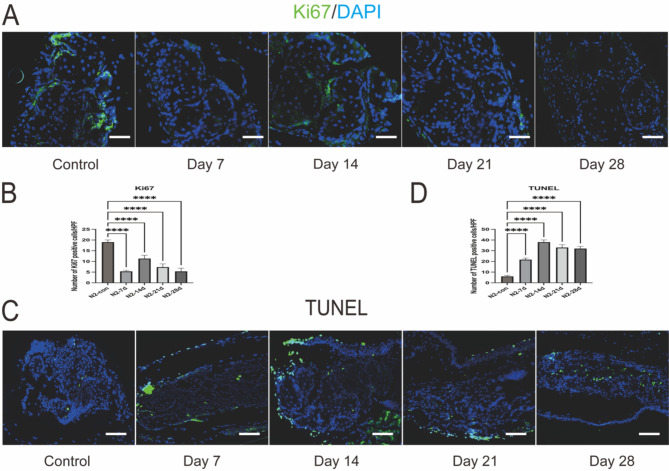
Ki67 serves as a marker for cells that are actively proliferating, including those within the meibomian glands and various other tissues29. Under normal physiological conditions, Ki67 is expressed in the basal and ductal cells of the meibomian gland acini, indicating cell proliferation. In this model, a decrease in Ki67 expression was observed. At 14 days post-treatment, expression was confined to partial basal areas of the acini. By day 21, Ki67 expression was significantly reduced, and minimal expression was detected at the 28-day assessment. This continuous decrease in Ki67 expression may reflect the acinar cells’ response to cold-induced injury, indicating a sustained decline in cell proliferative function within the meibomian glands and maintenance at a low level. The reduction in Ki67-positive cells may also suggest the presence of meibomian gland dysfunction and/or impaired regenerative capacity of the acinar cells (as shown in Fig. 6A, B).
Fig. 6.
Cell proliferation and apoptosis in MGs after liquid nitrogen injury. (A, B) Immunofluorescence staining of Ki67 and positive cell counting indicates a decreased in positive cells after liquid nitrogen injury, with labelled cells rarely detected at day 28. Scale bars represent 50 μm (n = 3). Data are shown as mean ± SD (n = 3), ****p < 0.0001. (C, D) TUNEL staining and positive cell counting shows abundant positive cells around MGs post liquid nitrogen injury Scale bars represent 50 μm (n = 3). ****p < 0.0001.
The TUNEL staining indicates that apoptosis is consistently present following liquid nitrogen injury, showing an upward trend from the 7th to the 14th day of observation. From day 14 to day 28, apoptosis can be essentially maintained at a relatively high level (as shown in Fig. 6C, D).
Basal epithelial cells displayed K14 staining, indicating the presence of intermediate filaments in the epithelial cell skeleton, which is one of the markers for basal cells33. Our data reveal a decrease in K14 staining following localized cryogenic injury, suggesting atrophy of the meibomian gland acini (as shown in Fig. 5C, E).
Keratinization of MG after liquid nitrogen Injury to the eyelid margin
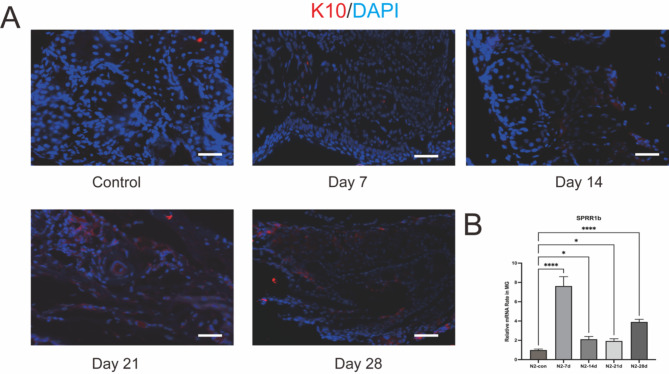
Hyper keratinization is a definitive pathological sign of obstructive Meibomian Gland Dysfunction (MGD)34, which can lead to ductal dilation and acinar atrophy. K10 (Keratin 10) is a biomarker for terminally differentiated keratinized epithelium, and its expression is relatively low in the central ducts of normal meibomian glands35. Beginning on the seventh day after cryotherapy of the eyelid margin with liquid nitrogen, immunofluorescence revealed the emergence of K10 expression in the acini, and an increase in K10 expression in the ducts with the extension of the observation period. Additionally, areas of meibomian gland atrophy and loss exhibited high levels of K10 expression. This indicates a significant increase in keratinization following the application of liquid nitrogen to the eyelid margin, and that this hyper-keratinized state persists over time (as shown in Fig. 7A).
Fig. 7.
keratinization of MGs after liquid nitrogen injury of eyelid margin. (A) Immunofluorescence staining of K10 revealing hyper-keratinization and thickening of the MG duct at days 21 and 28 post liquid nitrogen injury. Scale bars represent 50 μm (n = 3). (B) Gene expression levels of Sprr1β was upregulated in the MG of mice post liquid nitrogen injury. ****p < 0.001, *p < 0.05.
Sprr1b (Small Proline-Rich Protein 1B) is recognized as one of the markers for keratinization36. To further validate the occurrence of keratinization, we assessed the mRNA expression levels of Sprr1b and observed an increase in its expression following treatment with liquid nitrogen. Notably, the 7-day and 30-day post-treatment groups exhibited particularly significant elevations in Sprr1b expression. Although there was a slight decrease in the 14-day and 21-day groups, the levels remained higher than those of the control group (as shown in Fig. 7B).
Discussion
We have delineated a mouse model of atrophic meibomian Gland Dysfunction (MGD). In our study, we found that liquid nitrogen can specifically disrupt the cell membranes of acinar cells in the meibomian glands in the acute post-injury phase (within 24 h), without damaging adjacent tissues such as the orbicularis oculi muscle or the cornea. And then, there is a significant decrease in lipid content following injury. Concurrently, the meibomian glands have shown varying degrees of atrophy and loss, the eyelid margins have become thickened and rounded, and there is neovascularization. Commencing on day 14, the cornea exhibited significant fluorescence staining.
Since the use of the liquid nitrogen freezing method to create a meibomian gland dysfunction (MGD) model is being applied for the first time, there are no ophthalmology-specific references to explain the underlying mechanisms. The inspiration for this model comes from dermatology, where the mechanisms of similar models have been better studied. Based on the existing literature and the current experimental results, the possible mechanisms can be summarized as follows:
First, local freezing leads to a decrease in the number of acinar cells capable of producing meibum. HE staining at 24 h reveals significant structural damage to acinar cells, with disrupted cell membranes and compromised cellular integrity. Cryotherapy selectively damages lipid-rich cells while minimizing injury to surrounding tissues27. Intracellular lipids crystallize at temperatures higher than the freezing point of tissue fluid37. Additionally, freezing disrupts cell membranes, reducing the number of functional acinar cells and their ability to produce lipids. The initiating factor in this model is acinar damage, differing from other MGD models where atrophy occurs secondary to mechanical obstruction. Dermatological studies have reported that cryotherapy affects the differentiation process of basal stem cells in the skin, which might explain why acinar cells in this model fail to regenerate in a timely manner38.
Second, following acinar damage, a series of inflammatory responses occur within the meibomian gland. At different time points after injury, inflammatory cell infiltration is observed, primarily around the acinar tissue, along with the accumulation of inflammatory mediators. This process resembles changes seen in subcutaneous fat tissue after cryotherapy. Dieter Manstein’s study demonstrated increasing infiltration of inflammatory cells and progressive destruction of lipid-rich cells up to 30 days after cryotherapy in porcine skin (2). Similarly, Mathew M observed comparable results in human skin following cryotherapy, paralleling the changes observed in this model39.
Third, reduced meibum secretion destabilizes the tear film and increases tear osmolarity, triggering ocular surface inflammation and forming a vicious cycle. This inflammation may impair the repair of meibomian gland tissue and contribute to keratinization of the gland, likely due to the persistent accumulation of inflammatory mediators40.
Lastly, freezing may lead to ischemia in meibomian gland tissue. By day 7 of the model, neovascularization was observed at the eyelid margin, possibly as a compensatory response to cryotherapy-induced ischemia. Freeze injury can cause indirect damage, such as vasoconstriction, endothelial damage, and thrombosis, leading to localized ischemia. However, experimental evidence supporting this mechanism is insufficient and requires further investigation41.
Our findings are in accord with recent studies indicating that these observations are in accordance with the clinical presentation of atrophic Meibomian Gland Dysfunction (MGD)42. The clinical manifestations of age-related MGD mice were also very similar to those of this model18. However, Knockout-targeted genes are often key to lipid metabolism or ectoderm development, such as SCD1-null (SCD1 −/−) mice, K5-GR mice, Krox20-cKO mice, and Tabby mice22. These models often exhibit dysplasia of the meibomian glands, a gene knockout mouse that not only alters the function of the meibomian glands, but also causes significant morphological changes in the meibomian glands. Although these gene knockout models can explain the effects of the gene on the development and function of meibomian glands at the molecular or pathway level from some perspectives, most of them cannot mimic the pathophysiological process of patients with meibomian gland dysfunction in clinical practice, because the vast majority of patients have meibomian gland dysplasia or even abnormal, and the changes in meibomian gland morphology and function are also caused by various acquired factors, and these gene knockout models do not explain how acquired factors affect the gene. Therefore, there are still limitations to the study of pathogenic mechanisms. At the same time, gene knockout mice are the knockout of whole body cells, and it is impossible to specifically knock out one or several genes of meibomian gland tissue, which will inevitably cause damage to the ocular surface and other tissues and organs of the body to varying degrees, such as corneal neovascularization, corneal epithelial keratosis, and even kidney function damage in mice, etc., these side injuries are not desirable to us, and are not conducive to observing the pathophysiological changes that should occur on the ocular surface under MGD. In addition, genetic selection in knockout mice is also limited. I have to admit that the knockout genes that exist at present do have an impact on meibomian gland function, but there is no definite conclusion that these genes must be key and necessary influencing factors, and a large part of the genes that affect meibomian gland function may not be able to construct such transgenic mice, because those mice cannot survive after the gene is deleted, so that their effect on meibomian glands cannot be observed and explored through animal experimental models. This also limits the understanding of the mechanisms of meibomian gland dysfunction at the genetic level. In addition, although the elderly animal model well simulates some ocular surface changes in patients with age-related MGD in clinical practice, the feeding cost of aging animals is high, and not all elderly animals will suffer from MGD, and the low mold rate is a factor that has to be considered.
The reduction in the number of meibomian gland acinar cells leads to decreased expression of PPARγ, which may also be the initiating cause of atrophic MGD. PPARγ has anti-inflammatory effects, and the expression of inflammatory factors can be regulated through the PPAR-γ signaling pathway itself, and its role is to inhibit the expression of inflammatory factors, such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), etc., and promote the production of anti-inflammatory cytokines43. Observations indicate that IL-1β, IL-18, and TNFα in this model accumulate and increase over time, particularly noticeable by the 21st and 28th days, which we believe contributes to the further deterioration of acinar cell function. The apoptosis of acinar cells becomes increasingly evident, and the expression of the basal cell marker K14 also gradually decreases. This suggests that in the advanced stages of atrophic MGD, the self-renewal capacity of the acini has declined, possibly due to issues with the multipotency of basal cells. However, the specific mechanisms still require further experimental research to be revealed, which will also be the direction of future research for our group.
Surprisingly, upon extending the observation period in this model, it was found that around 60 days post cryotherapy with liquid nitrogen, a majority of the model mice exhibited regenerative capabilities of the meibomian glands, with the post-repair conditions largely resembling those prior to treatment. The localization of meibomian gland stem/progenitor cells is a current focal point and a matter of debate in research, with the majority of studies concentrating on animal models of embryonic development44. However, these models do not elucidate the mechanisms and processes by which adult meibomian glands self-renew. This indicates that the meibomian glands indeed possess regenerative potential, and this model can also serve as an animal model for subsequent studies tracking the localization of meibomian gland stem/progenitor cells, the pathways of acinar regeneration, and the mechanisms underlying acinar regeneration.
Materials and methods
Materials
Rabbit anti-Cytokeratin 10 (K10, ab76318), anti-Cytokeratin 14 (K14, ab181595), anti-Ki67 (ab16667), anti-PPAR-γ (ab45036). Alexa Fluor 488-conjugated IgG (A11055, A21206) and Alexa Fluor 594conjugated IgG (A11058) were from Invitrogen (Eugene, OR, USA). 40,6-diamidino- 2-phenylindole (DAPI; H-1200).
Animals
Eight-week-old male C57/BL6 mouses were from Changchun-bio (China), n = 80. Experimental procedures were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and with approval of the Animal Ethical Committee of Harbin medical University. Animals were given free access to standard rodent chow and water and kept in a standard pathogen-free environment at 25 °C ± 1 °C, relative humidity 60%±10%, and alternating 12 h light-dark cycles (from 8:00 AM to 8:00 PM).
Animal treatment
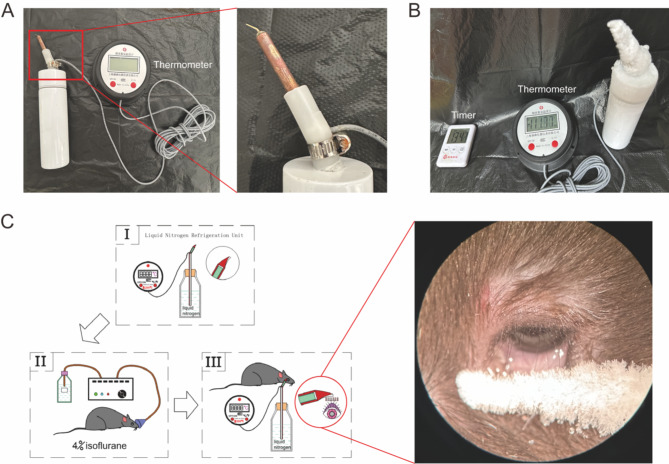
In this study, we administered liquid nitrogen cryotherapy to the eyelid margins of mouse in the treatment group. This procedure was carried out under general anesthesia induced by inhalation of 4% isoflurane (Sinopharm Chemical Reagent Co., Ltd., China) in oxygen, followed by topical application of 0.4% oxybuprocaine hydrochloride eye drops (Santen Pharmaceutical, Japan) for local anesthesia of the eyelid margins. Liquid nitrogen was placed in a thermos flask and then the temperature was conducted using a specially crafted pure copper device to apply cryotherapy to both the upper and lower eyelid margins. The temperature of the device that touches the eyelids can be maintained at minus 110 degrees Celsius.
degrees or so. The central two thirds of the upper eyelid margin of the selected eye was treated by applying the aforementioned device for 30 s (as shown in Fig. 8). In the control group, mice received no treatment and were housed in the same environment as those that underwent liquid nitrogen cryotherapy. Mice in the experimental group were sacrificed at 7, 14, 21, and 28 days following the eyelid margin cryotherapy. The control group mice were sacrificed after 28 days. At the end of the experiment, all animals were euthanized with a lethal dose of sodium pentobarbital (80 mg/kg BW), followed by cervical dislocation to ensure death.
Fig. 8.
Schematic illustration of the procedure to perform local Liquid nitrogen cryotherapy on mouse MG orifices. (A) Refrigeration units. (B) About 13 min after filling with liquid nitrogen, the temperature of the unit tends to stabilize at -100 degrees Celsius ± 15 degrees Celsius. (C) Flow diagram.
Slit-lamp microscopy examination
After the induction of general anesthesia, the patency of the meibomian gland (MG) orifices was evaluated utilizing a slit-lamp microscope. The state of the orifices was documented with a digital camera, and the quantity of obstructed orifices out of a total of ten MG orifices located centrally in the upper eyelid was enumerated. Orifices were categorized as obstructed if they exhibited opacity and swelling. To appraise the integrity of the corneal epithelium, one microliter of a 1% solution of liquid sodium fluorescein (Jingmingxin Co., Ltd., Tianjin, China) was instilled into the conjunctival sac. The corneal epithelium’s uptake of fluorescein was subsequently observed after a 90-second interval, employing a slit-lamp microscope equipped with a cobalt blue filter. A consistent ophthalmologist captured all images using an identical camera and settings throughout the examination process.
Histology
Post-injury collection of mouse eyelids was conducted at specific time points: 0, 1, 7, 14, 21, and 28 days. These eyelid samples were then embedded either in Optimal Cutting Temperature (OCT) medium or paraffin. From these blocks, sagittal sections of the tissue, measuring 6 micrometers in thickness, were prepared with three sections per slide, and three slides were created per animal, resulting in a total of 3 animals per experimental group. Hematoxylin and eosin (H&E) staining was applied to the paraffin-embedded sections to assess tissue morphology. Meanwhile, immunofluorescent staining and Oil Red O staining were conducted on the OCT-embedded frozen sections to further analyze cellular and lipid characteristics, respectively.
Oil red O staining
The accumulation of lipids in the meibomian glands (MGs) was determined by evaluating the intensity of the Oil Red O stain. The frozen sections of the eyelids were initially fixed in a 5% paraformaldehyde solution for a duration of 10 min, followed by a wash in phosphate-buffered saline (PBS) for 5 min. Subsequently, the sections were stained with a freshly prepared Oil Red O solution for 25 min. After staining, the sections were rinsed with PBS for another 5 min. Finally, the sections were mounted using a 90% glycerol solution and examined under a light microscope to visualize the lipid deposits.
Immunofluorescent staining
For the immunofluorescent staining procedure, cryosections of mouse eyelids, 6 micrometers in thickness, were treated by being fixed in cold acetone at -20 degrees Celsius for 10 min. This was followed by a series of three washes with phosphate-buffered saline (PBS), each lasting for 5 min. The sections were then permeabilized with a 0.3% Triton X-100 solution for 30 min and rinsed again with PBS in triplicate, with each wash being 5 min long.
Subsequently, the sections were blocked to reduce non-specific binding by incubating them with 2% bovine serum albumin (BSA) in PBS for 60 min at ambient temperature. The primary antibodies, K14, K10 (diluted 1:500) and Ki67, PPARγ (diluted 1:1000), were applied to the sections, which were then incubated for 16 h at 4 degrees Celsius. To ensure specificity, negative control sections were treated with a non-relevant isotype control antibody in place of the primary antibody.
Following the incubation with primary antibodies, the sections were washed extensively with PBS for 10 min per wash, three times in total. The sections were then incubated with the secondary antibodies, either Alexa Fluor 488-conjugated IgG (diluted 1:250) or Alexa Fluor 594-conjugated IgG (diluted 1:250), for 1 h at room temperature in a dark environment to protect the fluorescent labels from light-induced bleaching.
Finally, the sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei and then examined using a fluorescence microscope to capture images of the stained cells.
TUNEL assay
To assess cellular apoptosis, we employed the TUNEL assay kit from Elabscience One-step TUNEL In Situ Apoptosis Kit (E-CK-A320). Initially, paraffin-embedded tissue sections underwent rehydration and were then treated with a Proteinase-K solution in Tris/HCL buffer at a pH of 7.4, with a concentration of 10 mM, for a duration of 30 min at a controlled temperature of 37 °C. Subsequently, the sections were thoroughly rinsed with Phosphate-Buffered Saline (PBS), repeating the process three times for 5 min each to ensure complete removal of the Proteinase-K. Following the washes, 50 µl of the TUNEL reaction cocktail was applied to the sections, which were then incubated in the dark at 37 °C for a period of 1 h. Post-incubation, the sections were again subjected to three rounds of washing with PBS for 5 min each to eliminate any unbound reaction components. To visualize the nuclei, the sections were subsequently counterstained with 4’,6-diamidino-2-phenylindole (DAPI), a fluorescent stain that binds specifically to DNA. After the counterstaining procedure, the sections were mounted onto slides for preservation and clarity. Finally, the stained sections were examined and documented using a high-resolution microscope (model DM2500 from Leica Microsystems), capturing images that reveal the presence of apoptotic cells through the TUNEL assay.
Western blot analysis
Isolated meibomian gland (MG) samples were collected using a cold lysis buffer supplemented with a cocktail of protease and phosphatase inhibitors (Catalog No. 78440, Thermo Fisher Scientific). Protein content was determined utilizing a BCA Protein Assay Kit (Catalog No. 23225, Thermo Fisher Scientific). Each experimental set comprised three replicates, with each replicate being a composite of MGs harvested from both ocular glands of an individual mouse. A standardized quantity of protein lysate (20 µg) from each sample was loaded and resolved on 10% tricine gels, followed by transfer to a polyvinylidene fluoride (PVDF) membrane. The membranes were then blocked with 5% bovine serum albumin (BSA) for 60 min. Incubated with primary antibodies specific for PPAR-γ (dilution 1:1000) or β-actin (dilution 1:8000) after cropping at 4 °C overnight. After three rinses with Tris-buffered saline with 0.05% Tween 20 for 10-minute intervals, the membranes were treated with horseradish peroxidase (HRP)-tagged secondary antibodies directed against either mouse or rabbit IgG. For β-actin, an HRP-tagged anti-mouse antibody was applied as a loading control. Protein expression was visualized using an enhanced chemiluminescence substrate and documented with a ChemiDoc XRS imaging system.
RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Meibomian glands (MGs) were meticulously dissected under a microscope, with the surrounding skin, subcutaneous tissue, muscle, and palpebral conjunctiva being removed. Total RNA was then extracted from these MG samples utilizing the Invitrogen TRIzol reagent (catalog number 15596-018; Thermo Fisher Scientific). Each experimental group comprised three samples, with each sample being a composite of MG tissues harvested from both eyes of an individual mouse. A consistent quantity of RNA from each sample was converted into complementary DNA (cDNA) through a reverse transcription process, employing a commercially available kit (RR047A; TaKaRa, Shiga, Japan) and adhering to the provider’s guidelines. Subsequently, qRT-PCR was executed with the StepOne Real-Time PCR detection system and a SYBR Premix Ex Taq Kit (catalog number RR420A; TaKaRa), following the manufacturer’s prescribed protocol. The specific primer sequences for the targeted genes are outlined in the accompanying table. The qRT-PCR protocol involved an initial step of denaturation at 95 °C for 1 min, followed by 40 cycles of denaturation at 95 °C for 10 s and annealing/extension at 60 °C for 30 s. Post-amplification, a melt curve analysis was performed to verify the specificity of the amplification. The relative gene expression levels were determined using the comparative threshold cycle (Ct) method and were normalized against β-actin, which served as an endogenous control gene (Table 1).
Table 1.
Mouse primer sequences used for quantitative RT-PCR.
| Gene | Sense primer | Anti-sense primer |
|---|---|---|
| Mm IL-1β | GAGCATCCAGCTTCAAATCTCG | GGAACGTCACACACCAGCAG |
| Mm IL-18 | AGTGAACCCCAGACCAGACT | TGGCAAGCAAGAAAGTGTCC |
| Mm Sprr1β | GTTCCTGAGCCCTGCCTT | TTTGCTTTGTCTTCTGTTGGGCTA |
| Mm β-actin | CCTCTATGCCAACACAGT | AGCCACCAATCCACACAG |
Statistical analysis
Data were processed using GraphPad Prism 10.0 software (GraphPad Software Inc, San Diego, CA, USA). Statistical analysis was performed using a Mann-Whitney test with p < 0.05 considered as statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Author contributionsIn this study, the contributions of the authors were as follows:- Shu Wang, Yulin Lin were involved in the conception and design of the research.- Shu Wang, Yulin Lin, Jingfan Gao and Jia Lin conducted the experiments, data collection, and initial analysis.- Xin Jin were responsible for the statistical analysis and interpretation of the results.- Hong Zhang contributed to the acquisition of funding and supervision of the project.- Shu Wang drafted the initial manuscript and revised it critically for important intellectual content.- All authors read and approved the final manuscript.
Funding
National Natural Science Foundation of China (Grant No. U20A20363, 81970776, 82301173); The Natural Science Foundation of Heilongjiang Province, China (Grant No. LH2020H039); Heilongjiang Provincial Department of Education Heilongjiang Provincial Higher Education Fundamental Research Project (2021-KYYWF-0226); Heilongjiang Provincial key research and development plan guidance project (GZ20220125); The Science Fund for Excellent Young Scholars of First Affiliated Hospital of Harbin Medical University (Grant No.2024YQ04)
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
I can confirm that our study has been conducted in accordance with the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jester, J. V., Nicolaides, N. & Smith, R. E. Meibomian gland studies: Histologic and ultrastructural investigations. Invest. Ophthalmol. Vis. Sci.20, 537–547 (1981). [PubMed] [Google Scholar]
- 2.Driver, P. J. & Lemp, M. A. Meibomian gland dysfunction. Surv. Ophthalmol.40, 343–367. 10.1016/s0039-6257(96)80064-6 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Nelson, J. D. et al. The international workshop on meibomian gland dysfunction: Report of the definition and classification subcommittee. Invest. Ophthalmol. Vis. Sci.52, 1930–1937. 10.1167/iovs.10-6997b (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassanzadeh, S., Varmaghani, M., Zarei-Ghanavati, S., Heravian Shandiz, J. & Azimi Khorasani, A. Global prevalence of meibomian gland dysfunction: A systematic review and meta-analysis. Ocul. Immunol. Inflamm.29, 66–75. 10.1080/09273948.2020.1755441 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Bron, A. J. & Tiffany, J. M. The contribution of meibomian disease to dry eye. Ocul. Surf.2, 149–165. 10.1016/s1542-0124(12)70150-7 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Chinese Branch of the Asian Dry Eye, Ocular, Sarcoidosis, Tear Film Diseases Group of Ophthalmology Committee of Cross-Straits Medicine, Exchange, Association, Ocular, Sarcoidosis & Sarcoidosis, Dry Eye Group of Chinese Ophthalmologist, Association. Chinese expert consensus on meibomian gland dysfunction: Definition and classification (2023). Zhonghua Yan Ke Za Zhi59, 256–261. 10.3760/cma.j.cn112142-20230114-00023 (2023).
- 7.Schaumberg, D. A. et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest. Ophthalmol. Vis. Sci.52, 1994–2005 (2011). 10.1167/iovs.10-6997e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rueff, E. M., Tichenor, A. A., Ngo, W. & Pucker, A. D. A review of meibomian gland structure, function, and contact lens wear. Cont. Lens Anterior Eye45, 101560. 10.1016/j.clae.2021.101560 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Zhang, P. et al. Isotretinoin impairs the secretory function of meibomian gland via the PPARγ signaling pathway. Investig. Ophthalmol. Vis. Sci.63, 29. 10.1167/iovs.63.3.29 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremers, S. L. et al. New indicator of children’s excessive electronic screen use and factors in meibomian gland atrophy. Am. J. Ophthalmol.229, 63–70. 10.1016/j.ajo.2021.03.035 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Wang, Y. C. et al. Meibomian gland absence related dry eye in ectodysplasin a mutant mice. Am. J. Pathol.186, 32–42. 10.1016/j.ajpath.2015.09.019 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Jester, J. V., Rajagopalan, S. & Rodrigues, M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest. Ophthalmol. Vis. Sci.29, 1190–1194 (1988). [PubMed] [Google Scholar]
- 13.Bu, J. et al. High-fat diet induces inflammation of meibomian gland. Invest. Ophthalmol. Vis. Sci.62, 13. 10.1167/iovs.62.10.13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, M. H., Hsu, F. F. & Miner, J. H. Requirement of fatty acid transport protein 4 for development, maturation, and function of sebaceous glands in a mouse model of ichthyosis prematurity syndrome. J. Biol. Chem.288, 3964–3976. 10.1074/jbc.M112.416990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butovich, I. A., Wilkerson, A., Bhat, N., McMahon, A. & Yuksel, S. On the pivotal role of Elovl3/ELOVL3 in meibogenesis and ocular physiology of mice. FASEB J.33, 10034–10048. 10.1096/fj.201900725R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nien, C. J. et al. Effects of age and dysfunction on human meibomian glands. Arch. Ophthalmol.129, 462–469. 10.1001/archophthalmol.2011.69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obata, H. Anatomy and histopathology of human meibomian gland. Cornea21, S70–S74. 10.1097/01.ico.0000263122.45898.09 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Nien, C. J. et al. Age-related changes in the meibomian gland. Exp. Eye Res.89, 1021–1027. 10.1016/j.exer.2009.08.013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bu, J. et al. Transitory alkali exposure on meibomian gland orifices induces meibomian gland dysfunction. Ocul. Surf.29, 406–415. 10.1016/j.jtos.2023.06.007 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Butovich, I. A., Arciniega, J. C. & Wojtowicz, J. C. Meibomian lipid films and the impact of temperature. Invest. Ophthalmol. Vis. Sci.51, 5508–5518. 10.1167/iovs.10-5419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mudgil, P. & Millar, T. J. Surfactant properties of human meibomian lipids. Invest. Ophthalmol. Vis. Sci.52, 1661–1670. 10.1167/iovs.10-5445 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Borchman, D. et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest. Ophthalmol. Vis. Sci.52, 3805–3817. 10.1167/iovs.10-6514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arita, R. et al. Decreased surface temperature of tarsal conjunctiva in patients with meibomian gland dysfunction. JAMA Ophthalmol.131, 818–819. 10.1001/jamaophthalmol.2013.1895 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Nagymihalyi, A., Dikstein, S. & Tiffany, J. M. The influence of eyelid temperature on the delivery of meibomian oil. Exp. Eye Res.78, 367–370. 10.1016/s0014-4835(03)00197-0 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Keller, M. et al. Thermosensitive TRP channels are functionally expressed and influence the lipogenesis in human meibomian gland cells. Int. J. Mol. Sci.25. 10.3390/ijms25074043 (2024). [DOI] [PMC free article] [PubMed]
- 26.Kubyshkin, V. A., Ionkin, D. A., Kungurtsev, S. V. & Chzhao, A. V. History of cryosurgery. Khirurgiia (Mosk) 62–74. 10.17116/hirurgia2015562-74 (2015). [DOI] [PubMed]
- 27.Jalian, R. Selective cryolysis of sebaceous glands. J. Invest. Dermatol.135, 2173–2180. 10.1038/jid.2015.148 (2015). [DOI] [PubMed] [Google Scholar]
- 28.<中国睑板腺功能障碍专家共识.pdf>. 10.3760/cma.j.issn.0412-4081.2017.09.005 (2017).
- 29.Bu, J. et al. Hyperlipidemia induces meibomian gland dysfunction. Ocul. Surf.17, 777–786. 10.1016/j.jtos.2019.06.002 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Jester, J. V., Potma, E. & Brown, D. J. PPARgamma regulates mouse meibocyte differentiation and lipid synthesis. Ocul. Surf.14, 484–494. 10.1016/j.jtos.2016.08.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bu, J. et al. Models for meibomian gland dysfunction: In vivo and in vitro. Ocul. Surf.32, 154–165. 10.1016/j.jtos.2024.03.003 (2024). [DOI] [PubMed] [Google Scholar]
- 32.Friedman, N. B. & Kritzler, R. A. The pathology of high-altitude frostbite. Am. J. Pathol.23, 173–187 (1947). [PMC free article] [PubMed] [Google Scholar]
- 33.Call, M., Fischesser, K., Lunn, M. O. & Kao, W. W. A unique lineage gives rise to the meibomian gland. Mol. Vis.22, 168–176 (2016). [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki, T. Inflamed obstructive meibomian gland dysfunction causes ocular surface inflammation. Invest. Ophthalmol. Vis. Sci.59, DES94–DES101. 10.1167/iovs.17-23345 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Liu, Y. et al. Comparative characterization of human meibomian glands, free sebaceous glands, and hair-associated sebaceous glands based on biomarkers, analysis of secretion composition, and gland morphology. Int. J. Mol. Sci.2510.3390/ijms25063109 (2024). [DOI] [PMC free article] [PubMed]
- 36.Liu, R. et al. Oleic acid induces lipogenesis and NLRP3 inflammasome activation in organotypic mouse meibomian gland and human meibomian gland epithelial cells. Exp. Eye Res.241, 109851. 10.1016/j.exer.2024.109851 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Manstein, D. et al. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg. Med.40, 595–604. 10.1002/lsm.20719 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Wang, W. et al. Skin organoid transplantation promotes tissue repair with scarless in frostbite. Protein Cell.10.1093/procel/pwae055 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avram, M. M. & Harry, R. S. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg. Med.41, 703–708. 10.1002/lsm.20864 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Baudouin, C. et al. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol.100, 300–306. 10.1136/bjophthalmol-2015-307415 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheridan, R. L., Goldstein, M. A., Stoddard, F. J. Jr. & Walker, T. G. Case records of the Massachusetts General Hospital. Case 41-2009. A 16-year-old boy with hypothermia and frostbite. N. Engl. J. Med.361, 2654–2662. 10.1056/NEJMcpc0910088 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Chinese expert consensus on meibomian gland dysfunction: Diagnosis and management (2023). [Zhonghua Yan Ke Za Zhi] Chinese Journal of Ophthalmology59, 880–887. 10.3760/cma.j.cn112142-20230822-00054 (2023). [DOI] [PubMed]
- 43.Scirpo, R. et al. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology62, 1551–1562. 10.1002/hep.28000 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, X., Reneker, L. W., Zhong, X., Huang, A. J. W. & Jester, J. V. Meibomian gland stem/progenitor cells: The hunt for gland renewal. Ocul. Surf.29, 497–507. 10.1016/j.jtos.2023.07.004 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.