Abstract
Within ovarian cancer research, patient-derived xenograft (PDX) models recapitulate histologic features and genomic aberrations found in original tumors. However, conflicting data from published studies have demonstrated significant transcriptional differences between PDXs and original tumors, challenging the fidelity of these models. We employed a quantitative mass spectrometry-based proteomic approach coupled with generation of patient-specific databases using RNA-seq data to investigate the proteogenomic landscape of serially-passaged PDX models established from two patients with distinct subtypes of ovarian cancer. We demonstrate that the utilization of patient-specific databases guided by transcriptional profiles increases the depth of human protein identification in PDX models. Our data show that human proteomes of serially passaged PDXs differ significantly from their patient-derived tumor of origin. Analysis of differentially abundant proteins revealed enrichment of distinct biological pathways with major downregulated processes including extracellular matrix organization and the immune system. Finally, we investigated the relative abundances of ovarian cancer-related proteins identified from the Cancer Gene Census across serially passaged PDXs, and found their protein levels to be unstable across PDX models. Our findings highlight features of distinct and dynamic proteomes of serially-passaged PDX models of ovarian cancer.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84874-3.
Keywords: Patient-derived xenograft models (PDX), Ovarian cancer, Proteogenomics, Mass spectrometry, Proteomics
Subject terms: Cancer, Proteomics, Cancer genomics
Introduction
Ovarian cancer encapsulates malignancies originating from ovarian, peritoneum, or fallopian tube cysts1,2. The most common type of ovarian cancer is epithelial ovarian cancer (EOC) which accounts for 90% of ovarian cancer diagnoses and consists of multiple subtypes. The high-grade serous carcinoma (HGSC) subtype is most frequently diagnosed (60%) with poor (30%) 5-year survival rates amongst women diagnosed at later stages3. Among the 70% of patients who respond positively to chemotherapy, approximately 80% eventually relapse and 50% develop treatment resistance1.
PDX models are frequently used within cancer research to study tumorigenesis, metastasis, and drug response due to their general ability to retain the molecular characteristics, phenotype and morphology of their patient tumor of origin4–6. PDX models are generated through the implantation of a biopsy specimen, surgically resected tumor sample, or malignant ascites-derived tumor cells from a patient into an immunodeficient mouse6. Tumors implanted into PDX models are monitored for growth and they are subsequently implanted into secondary recipient mice via a process referred to as tumor passaging. PDX models retain key patient features including tumor heterogeneity, mutations, and the tumor microenvironment7.
Strong correlations between patient drug responses and PDX response to the same drug have been observed8,9. However, the use of PDX models within ovarian cancer research specifically has been challenging due to the heterogeneous nature of the disease3,10. Despite the widespread utilization of PDX models in pre-clinical research settings, these models present several key limitations. Interactions between the immune system and tumorigenesis cannot be studied within a PDX model due to its immunodeficient characteristic; therefore, the contribution of the host immune system to cancer treatment response cannot be assessed11. In addition, PDX models are not suitable for studying early-stage cancers due to the low take-rate of these tumors into mice5. These models are also generally used for drug screening after three to five tumor passages, during which time the human tumor-associated stroma have all been replaced by murine-derived fibroblasts and extracellular matrix (ECM)12. Replacement of human stroma with murine-equivalent counterparts influences paracrine signaling within the tumor. Although mouse stromal and human stromal cell components share similarities in function, the exact influence of murine stroma on human cancer cells in PDX models is not fully understood4.
The utility of PDX models in cancer research is conflicting, and key findings from experiments conducted using these models are highly dependent on the analytical methodology used to study them. An advantage of using a proteomic approach in the study of in vivo experimental models, including PDX models, is the ability to identify specific interactions between the mouse microenvironment and the surgically implanted patient-derived tumors. Furthermore, the proteomic landscape of tumors can be investigated across successive passaging of PDX models, thereby providing insights into the validity of using PDXs within translational ovarian cancer research. Additionally, the integration of genomic datatypes into mass spectrometry-based proteomics, known as proteogenomics, allows for patient-specific genetic features to be retained and studied through successive PDXs13.
To date, there have been few studies analyzing the limitations of PDXs as an optimal in vivo model for ovarian cancer research using a proteogenomic approach. Specifically, the influence of successive tumor passaging on the proteome landscape of PDX tissues has not been fully explored. There is a need to comprehensively characterize PDX models at the proteomic level to understand protein interactions that occur within the host environment. Considering that RNA levels are approximately 50% correlated with protein levels across the Cancer Cell Line Encyclopedia, proteomics provides a deeper level of understanding for biological processes14.
In this study, we utilized a quantitative mass spectrometry (MS)-based proteomic approach to establish similarities and differences between the proteomes of ovarian cancer patient tumors and their serially passaged PDX counterparts – addressing the underlying assumption that molecular phenotypes do not change in PDX passaging. We created a patient-specific database from bulk RNA-seq based transcriptional profiles to retain genetic characteristics of the implanted patient tumors, enabling the identification of protein signaling pathways that account for the unique proteomic divergence between human ovarian cancer tumors and PDX mouse models.
Results
Mass spectrometry-based proteomic and proteogenomic analyses enable robust correlation analysis of proteomes of patient-derived ovarian tumor tissues and serially passaged PDXs
To investigate differences in the proteomes of original patient-derived ovarian tumors and engrafted PDX counterparts, we processed two sets of patient-derived ovarian tumors and their corresponding PDX tumors. Tumors isolated from a de-identified patient (Patient 136) were of ovarian tissue origin and were engrafted into PDXs and passaged 3 times, while tumors isolated from another de-identified patient (Patient 110) were of peritoneal origin and passaged twice (Fig. 1A). Tumors were processed for quantitative mass spectrometry analysis using a standardized protocol developed by the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC), utilizing peptide labeling with tandem mass tags (TMT) as summarized in Fig. 1B. Samples isolated from patients and PDX models were processed for proteomic analysis in duplicate. Sample information, tissue preservation methods, and PDX naming schemes are outlined in Table 1.
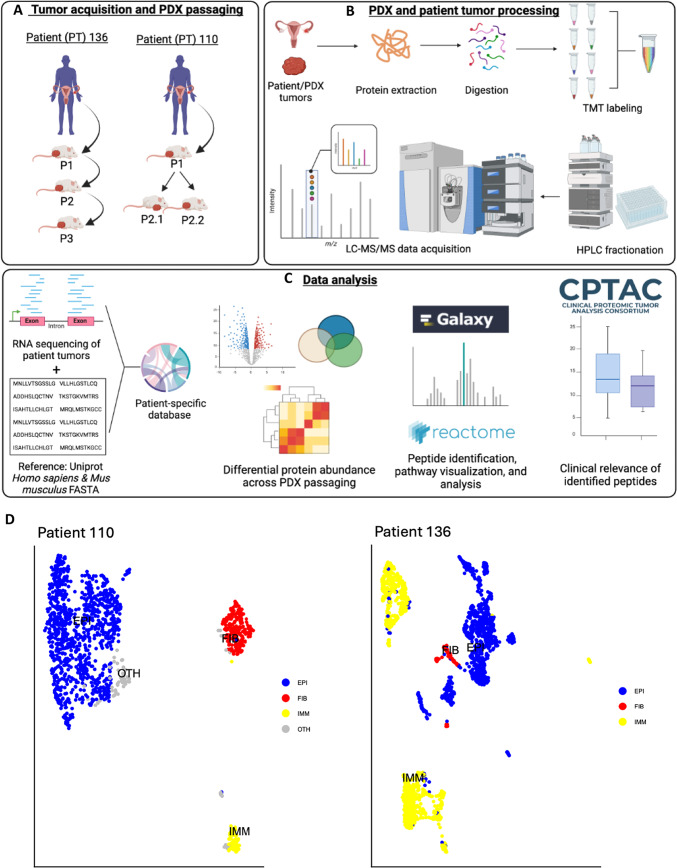
Fig. 1.
Experimental overview of the proteogenomic analysis of patient-specific ovarian cancer tumor characteristics in patient-derived xenograft (PDX) mouse models. (A) Patient-derived ovarian tumor tissues were isolated, subcutaneously implanted in PDXs, and allowed to grow to 2cm before being serially passaged. (B) Approximately 9-12mg of tissue were processed for protein extraction, protein digestion, and peptide labeling with isobaric tandem mass tags (TMT) prior to peptide fractionation and LC–MS/MS data acquisition. Samples were processed for proteomic analysis in duplicate. (C) Patient-specific databases were generated using a reference Homo sapiens proteome (source: UniProt) and bulk RNA-seq data of patient tumors. Downstream proteomic analysis was conducted with human proteins mapped from patient-specific RNA-seq data and UniProt database search results to increase the confidence of identified human proteins. (D) UMAP of single cells colored by cell type from scRNA sequencing of patient tumors including cancer epithelial (EPI), fibroblast (FIB), immune (IMM), or cells of unknown lineage (OTH).
Table 1.
Patient sample information, tissue preservation method, and PDX naming scheme.
| Patient | Disease | Passage | Tissue preservation method | Name |
|---|---|---|---|---|
| 110_Peritoneal nodule | High-grade serous ovarian cancer | P1 | Formalin fix | PDX110-P1 |
| P2 | Formalin fix | PDX110-P2.1 | ||
| P2 | Snap frozen | PDX110-P2.2 | ||
| 136_Ovarian tumor | Endometrioid adenocarcinoma of ovary | P1 | Snap frozen | PDX136-P1 |
| P2 | Snap frozen | PDX136-P2 | ||
| P3 | Snap frozen | PDX136-P3 |
To incorporate patient tumor-specific genomic mutations in our proteomic analysis, we created a patient-specific database containing predicted protein sequences generated from RNA-seq data from each patient (Fig. 1C). The incorporation of transcript-level data allows for the generation of a database containing protein sequences produced from genomic or transcriptomic mutations and aberrations from each patient. This database is then merged with human and mouse reference proteomes from the Universal Protein Resource (UniProt) reference database, allowing for the identification of proteins based on patient and taxonomy. The resulting database, containing 192,136 protein sequences (generated on 2024-01-17), was used to investigate differential protein abundances across PDX passaging. Furthermore, to characterize the level of intratumor heterogeneity, patient tumors were subjected to single cell RNA sequencing (scRNA seq) (Fig. 1D). Analysis of scRNA seq data identified three major cell populations with distinct relative proportions in both patient tumors: epithelial, fibroblast, and immune cells.
Following LC–MS/MS data acquisition and database search using MaxQuant, we compared the number of proteins quantified using our patient-specific protein sequence database with the number of proteins quantified from UniProt. A total of 7,384 proteins were quantified using the conventional UniProt reference database (containing human and mouse protein sequences), whereas 6,967 proteins were quantified using the patient-specific database. Considering that human tumor samples were grown in mice, we expected mouse proteins to be identified in addition to human proteins. We also expected to identify proteins identified from mouse and human taxonomy given that ~ 35% of peptides derived from enzymatic cleavage with trypsin are identical in their amino acid sequences between these two species15. Importantly, incorporation of the patient-specific database led to the identification of 14.6% more human proteins, 7.2% more mouse proteins, and 33.5% less human/mouse proteins (i.e. protein hits with high human and mouse sequence homology) compared to the UniProt reference database (Fig. 2A). Protein abundances were median-normalized, resembling an approximately normal distribution across samples (Supplementary Fig. 1A). Importantly, biological process replicates between samples were positively correlated, with a Pearson correlation coefficient > 0.9 for all pairwise comparisons (Supplementary Fig. 1B). These parameters highlight robust and correlative features of the generated proteogenomic dataset.
Fig. 2.
Utilization of patient-specific database increases human protein identifications in PDX models. (A) Total number of quantified proteins from the reference dataset utilizing only Homo sapiens UniProt database matches (“Reference”) or the patient-specific dataset utilizing UniProt and RNA-seq protein-specific matches (“Patient-specific”). (B) Protein match overlap between the Reference and Patient-specific dataset. (C) Welch’s T-test was conducted between the proteome of each patient tumor and its serially passaged PDX tumor to identify proteins with significantly different relative abundances [p < 0.05 and -1 < log2(fold change) > 1].
Although utilization of the patient-specific database resulted in a reduced number of proteins with ambiguous human/mouse species identification, downstream analysis was conducted on proteins: (1) with the highest confidence of originating from Homo sapiens, and (2) identified from one or both patients. For example, proteins could be identified from the human UniProt reference database and the mouse UniProt reference database; however, if these proteins were also identified from the patient-specific database with corresponding transcript sequences encoding these peptides generated from RNA-seq analysis, then they are considerably more likely to have originated from the patient tumor instead of being a byproduct of PDX implantation. The patient-specific database is, therefore, a useful feature that validates human protein identification in our experiment, thereby reducing false positive protein hits.
Protein identifications across the UniProt (i.e. reference) and patient-specific search databases in both patients had a high percent overlap: 96.86% and 97.65% of proteins were shared between the reference and patient-specific dataset for Patient 110 and Patient 136, respectively (Fig. 2B). Approximately 5.5% of proteins were only identified following the incorporation of patient-specific RNA-seq data. In addition, utilizing the patient-specific database enabled the identification of an increased number of human proteins with statistically significant differential abundances across all PDX passages compared to their respective patient tumor (Fig. 2C), as determined by Welch’s T-test (p value < 0.05) and a fold-change cutoff of 2. Overall, utilizing our patient-specific database increased the confidence of human-assigned proteins and revealed features unique to the incorporation of RNA-seq data into our bioinformatics pipeline.
The proteomic landscapes of PDX tumors are distinct from their patient tumors of origin
Given the utility of PDX models in cancer research, we hypothesized that the global protein abundance profiles compared between original patient tumors and PDX passages would cluster by patient. However, protein abundance profiles clustered independently from patient type, passaging, and taxonomy, emphasizing high levels of heterogeneity that develop with successive PDX passaging (Fig. 3A and Supplementary Fig. 2). To better discern the level of heterogeneity occurring across patient tumors and paired PDXs, we utilized the proteome of healthy ovarian tissue generated by NCI CPTAC16 as a reference (Fig. 3B). The CPTAC datasets were accessed through LinkedOmics (https://www.linkedomics.org/data_download/CPTAC-pancan-OV/). Here, protein abundances of healthy tissue were median normalized, and proteins identified from the reference proteome were compared to our dataset (n = 3077). The resulting heatmap not only highlights stark differences in protein abundances between the healthy and disease proteomes, but also further emphasizes differences between patient and corresponding PDX tumors.
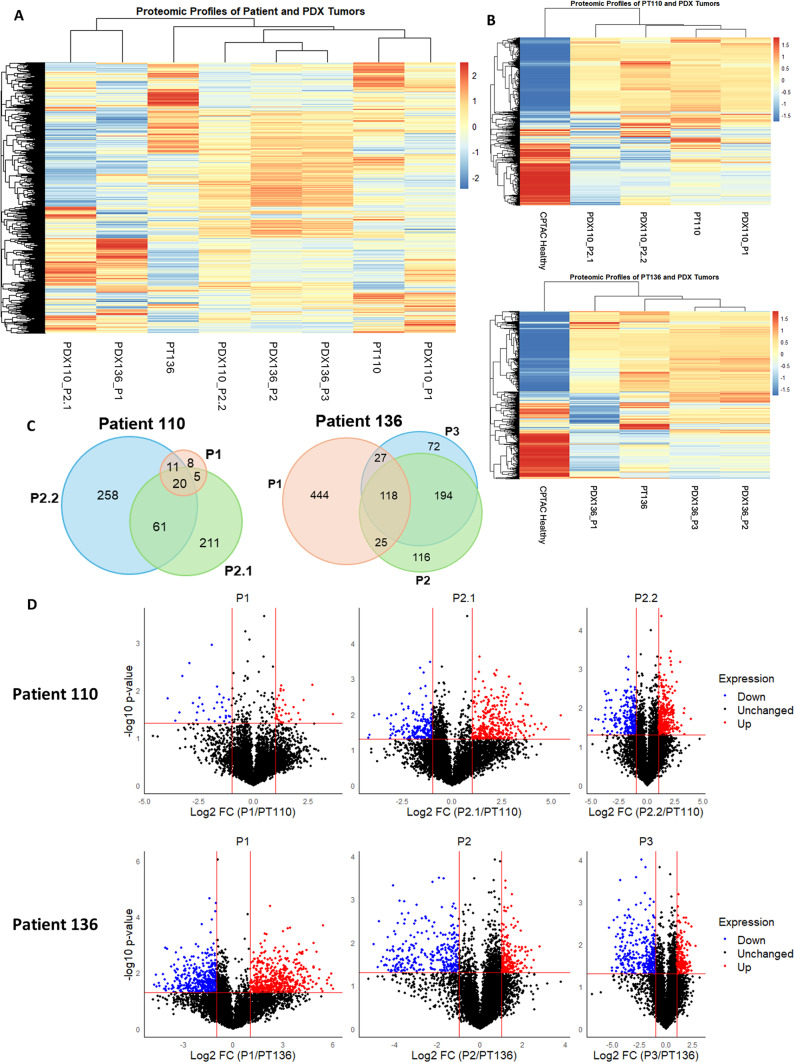
Fig. 3.
The human proteomes of serially passaged ovarian tumor PDXs differ significantly from their patient-derived tumor of origin. (A) Expression profiles of patient tumors and respective PDXs show dissimilar clustering of all proteins (n = 6967 proteins). (B) Expression profiles of human proteins compared with National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (NCI CPTAC)-derived healthy ovarian tissue highlights differences between a healthy reference proteome and patient tumors with corresponding PDXs (n = 3077 proteins). (C) Differentially abundant human proteins in PDX tumors compared to their patient tumors of origin are dissimilar across serial passages. (D) Distribution of increased and decreased protein abundances across each patient and PDX pair.
The concurrent passaging of tumors into multiple PDXs ensures statistical rigor for the analysis of in vivo models of cancer17,18. Given this framework, in addition to the specific immunodeficient mouse background, we expected a large overlap of the differentially abundant proteins among the PDX110-P2.1 and PDX110-P2.2 tumors. Examination of the differentially abundant proteins revealed 61 overlapping proteins between PDX110-2.1 and PDX110-2.2 in Patient 110 (Fig. 3C). A total of 473 differentially abundant proteins across passages in Patient 110 were unique. For PDX136, 194 proteins overlapped between passage two and passage three, while 444 proteins were uniquely identified to be differentially abundant in passage one compared to the original patient tumor. These data indicate that for PDX136, most proteome changes occurred during the first passage (Fig. 3C). The passage-specific changes in the significantly different protein abundances for Patient 110 and Patient 136 are shown in Fig. 3D. The tumor proteome of passage one from Patient 110 (PDX110-P1) exhibited fewer changes in differentially abundant proteins when compared to the original patient tumor. Upon serial passaging, however, PDX110-P2.1 and PDX110-P2.2 exhibited an increase in the number of differentially abundant proteins. Conversely, PDX136-P1 demonstrated an increased number of differentially abundant proteins compared with the original tumor. Serial passaging from PDX136-P2 to PDX136-P3 was also associated with proteomic changes that were significantly different from the original patient tumor. These results highlight the existence of a dynamic proteome that is sensitive to serial passaging within PDX models.
Distinct biological processes are enriched across serial passages of PDXs
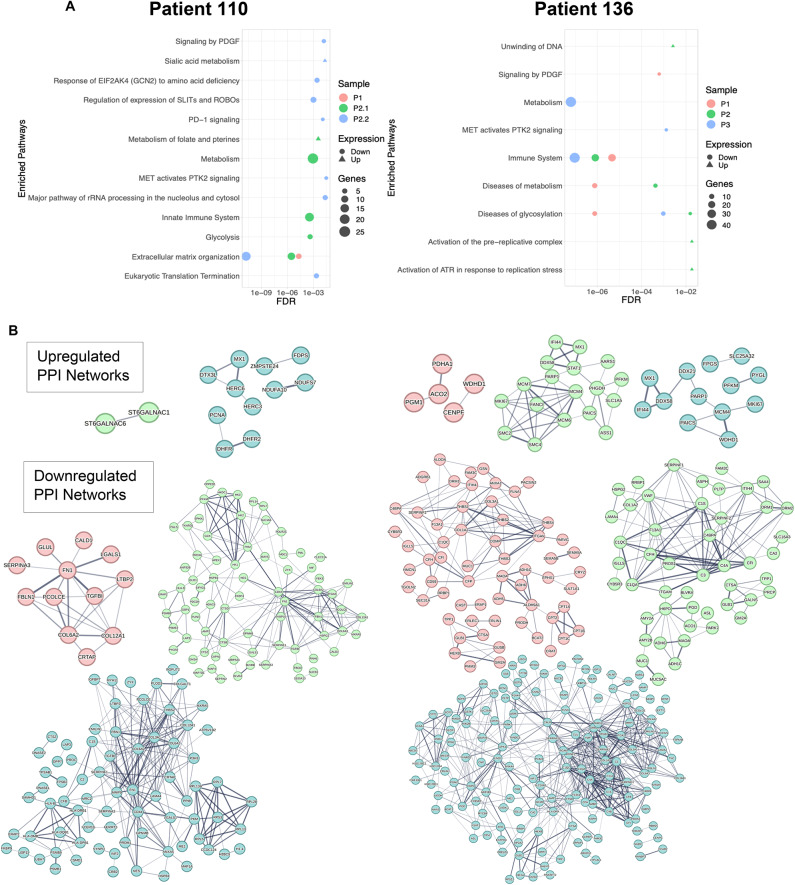
Since Euclidean distance clustering and Principal Component Analysis (Supplemental Fig. 2) of the human-specific proteins of all samples exhibited random clustering of PDX tumors and their patient-derived counterparts, we further examined the biological processes that exhibited significant differences between the patient-derived tumors and PDXs using the curated and peer-reviewed pathway database, Reactome19. The significance cutoff for enriched terms was an FDR-adjusted p value ≤ 0.05. Distinct biological pathways were identified among serially passaged PDXs for both patients (Fig. 4A). Many enriched pathways were associated with downregulated processes in both sets of PDX models and included changes in extracellular matrix organization, and significant decreases in the human innate immune system. Interestingly, only PDX110s demonstrated consistently downregulated extracellular matrix organization across successive passaging, while PDX136s showed consistent downregulation of the innate immune system. Biological pathways such as eukaryotic translation termination, and pathways associated with MET activation were downregulated in some passages (PDX110-P1), but not others (PDX110-P2.1 and PDX110-P2.2). These results suggest that the downregulation of some biological processes are conserved in PDXs; however, these features may be tumor specific.
Fig. 4.
Pathway analysis of differentially abundant human proteins in PDX models reveals enrichment of distinct biological pathways. (A) Pathway analysis using Reactome was conducted on each patient tumor and its corresponding PDX tumors. Biological pathways were considered enriched if the FDR was < 0.05. (B) Protein–protein interactions (PPIs) were determined using STRING for each serially passaged PDX tumor, demonstrating increased PPI connectivity after tumor passaging. PPIs are colored based on sample: PDX110-P1 (red), PDX110-P2.1 (green), PDX110-P2.2 (blue), PDX136-P1 (red), PDX136-P2 (green), PDX136-P3 (blue).
Our results also highlight the enrichment of distinct biological processes across tumors and PDX passages. “Sialic acid metabolism” and “metabolism of folate and pterines” were upregulated processes in PDX110-P2.1 and PDX110-P2.2. In addition, “unwinding of DNA” was a prominently upregulated feature in PDX136-P2, while the “diseases of glycosylation” biological process was enriched in PDX136-P3. These data highlight inherent differences in the biological processes of Patient 110 and Patient 136 tumors and their respective PDXs.
Next, we sought to examine the protein–protein interaction (PPI) networks of significantly differentially abundant proteins across PDXs. Downregulated PPI networks exhibited increased connectivity following serial passaging in the Patient 110 and Patient 136 tumors compared with upregulated PPI networks (Fig. 4B). These downregulated PPI networks represent protein abundances that were significantly decreased upon PDX passaging compared to patient tumors, which emphasizes the lack of stability among the proteomes of PDX models. Interestingly, we observed small changes in the protein interaction connectivity in PPI networks of upregulated proteins across serial passaging for both patient tumors. In addition, the upregulated proteins from PDX110-P1 did not meet the statistical threshold needed for the formation of a PPI network. Our data suggest that there are fewer upregulated processes occurring in serially passaged PDX models when compared to their original patient tumor.
Characterization of the proteome of epithelial cell clusters in tumors reveals perturbations in cancer-related processes across PDXs
One limitation of the proteomic analysis of bulk tumors is the inability to discern individual subtypes, as factors such as heterogeneity contribute to a wide range of proteomic profiles20. Essential biological signals related to cancer progression can be masked by highly abundant proteins broadly expressed in bulk samples. It is difficult to discern cell type from bulk proteomic data. The patient-specific proteins in our dataset could have been of epithelial, mesenchymal, or immune cell origin. Based on our scRNAseq data, proteins within the PDX tumors can belong to any of these cell types. In our study, we focused on proteins matched with epithelial cell markers identified through the scRNA seq data as these cell types are the main drivers of ovarian cancer. scRNA seq data from PT110 and PT136 original tumors were used to identify proteins highly expressed in epithelial cells. To accomplish this, mRNA associated with epithelial cell clusters was matched to corresponding proteins from the proteomic dataset, and significantly dysregulated proteins were identified. The mRNA transcripts associated with epithelial cells identified from scRNA seq data include 8,991 genes and are provided as a Supplementary File “master_gene_list”. This method resulted in 245 (PT110) and 521 (PT136) proteins uniquely identified from the epithelial cell cluster of both patient tumors (Supplementary Fig. 3). Next, the PPI networks of these differentially abundant proteins were generated using STRING, and pathway analysis was conducted to identify significantly enriched biological processes.
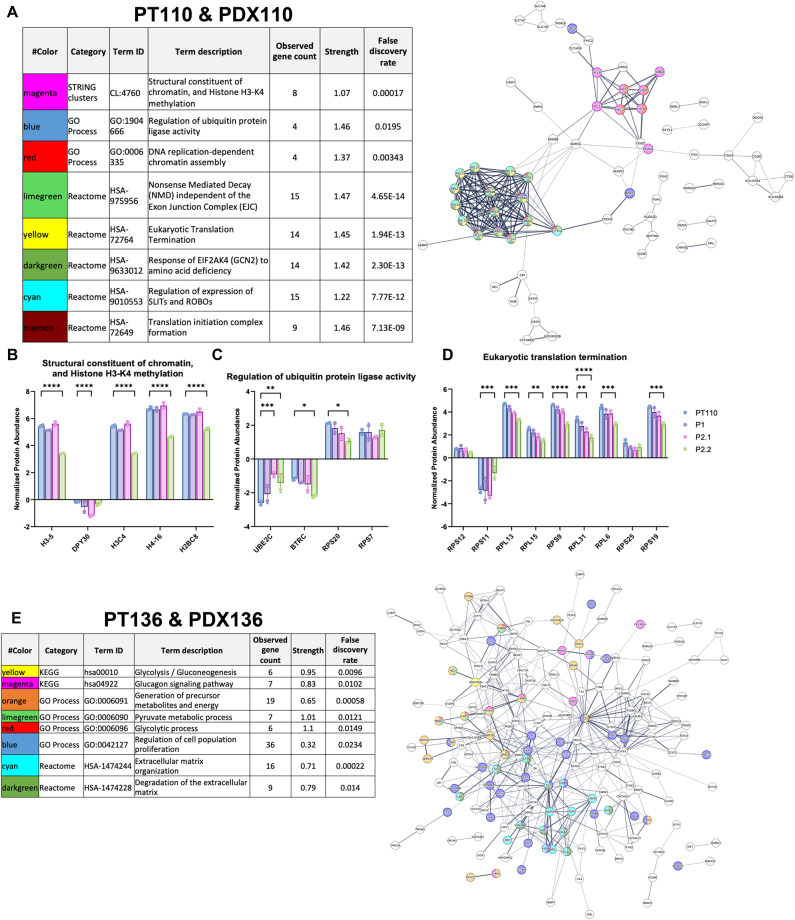
The PPI network of differentially abundant proteins in PDX110s highlighted two clusters of interacting proteins involved broadly in transcription and translation (Fig. 5A). Importantly, proteins involved in the structural constituent of chromatin and histone H3-K4 methylation showed a significant change in relative abundance compared to the original patient tumor (Fig. 5B). Several proteins involved in the regulation of protein ligase activity also show significant changes in relative abundance compared to patient tumors: notably UBE2C, BTRC, and RPS20. We also identified ribosome components involved in translation termination that had significant changes in abundance across PDX110 passages. The PPI network and biological pathway analysis of epithelial cell-associated proteins within PT136 and PDX136 was also assessed and revealed a network with strong connectivity (Fig. 5E). Interestingly, the PPI network for these proteins was highly interconnected with an enrichment of biological pathways involved in ECM, glycolytic processes, and regulation of cell population proliferation. The proteins involved in glycolysis/gluconeogenesis (Fig. 5F) and glucagon (Fig. 5G) processes had significantly different abundances in at least one PDX136 passage compared to the original patient tumor. Proteins involved in the regulation of cell population proliferation also demonstrated significantly different protein abundances across passages. Overall, our data highlights the presence of unstable epithelial cell-specific proteomes identified in PDX models.
Fig. 5.
Analysis of dysregulated proteins that constitute epithelial cells within patient and PDX tumors. Pathway analysis of PPI networks of proteins identified in epithelial cell clusters in (A) PT110/PDX110 and (E) PT136/PDX136 generated from the STRING database. Pathways enriched in PT110 demonstrate significant changes in proteins involved in (B) structural constituent of chromatin and histone H3-K4 methylation, (C) regulation of ubiquitin ligase activity, and (D) eukaryotic translation termination. Enriched pathways in PT136 demonstrate significant changes in proteins involved in (F) glycolysis/gluconeogenesis, (G) glucagon, and (H) regulation of cell population proliferation. Significance was determined following two-way ANOVA testing with multiple comparisons using Dunnett’s test. *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
Cancer gene consensus markers of ovarian cancer demonstrate unstable protein expression across serial passaging
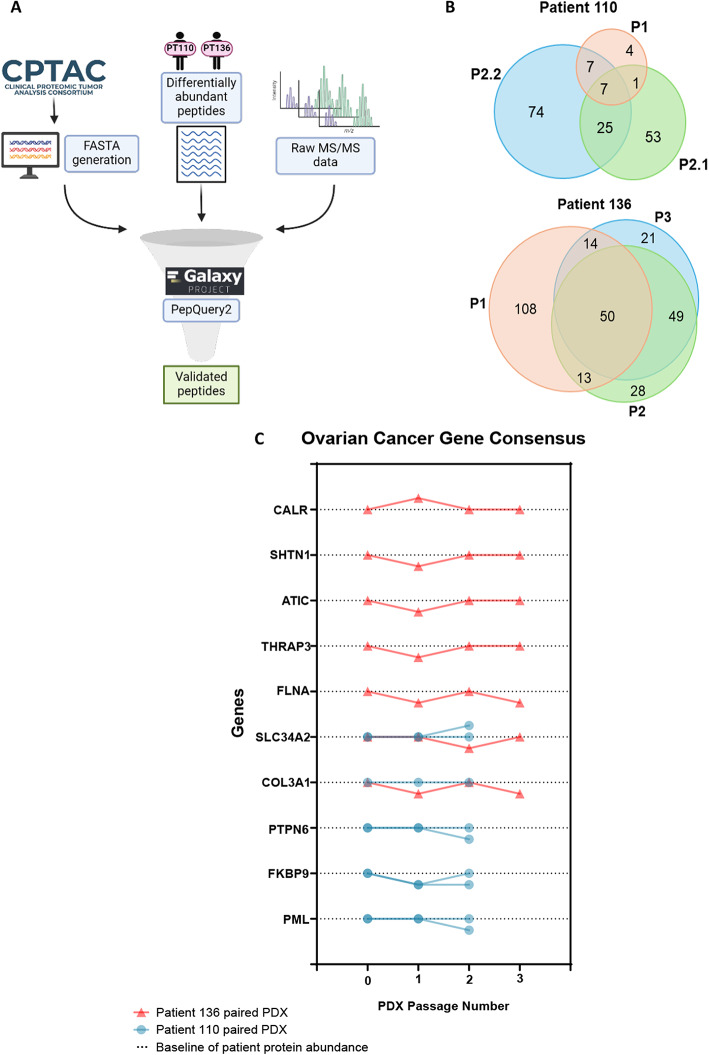
Following the analysis of enriched biological processes in patient tumors and their PDX models, we sought to examine the relative protein abundances of ovarian cancer-associated genes. To evaluate clinically relevant proteins, we generated a CPTAC-specific database containing proteins that were identified in high-grade serous ovarian cancer tumors processed for mass spectrometry-based proteomic analysis21. Our raw mass spectrometry data and the list of differentially abundant peptides were searched against the CPTAC-specific database using the targeted peptide search engine PepQuery222,23 within the Galaxy for Proteomics (Galaxy-P) platform24 (Fig. 6A). This resulted in detecting a list of verified peptides corresponding to novel proteoforms and mapped to proteins from the CPTAC-specific database: 16,099 dysregulated peptides were identified from the patient-specific dataset, and 5,488 of these peptides were validated using the PepQuery2 known mode (i.e. peptides shared between the patient-specific dataset and CPTAC HGSC dataset). The 5,488 validated peptides were mapped to 378 proteins in PT110 and 385 proteins in PT136 (Supplementary Table 1). Proteins identified from the CPTAC database originated from high-grade serous ovarian cancer tumors. There were no endometrioid ovarian cancer specimens in the CPTAC dataset, which precluded our ability to detect any endometrioid-specific cancer-associated proteins in the PDX models from PT136.
Fig. 6.
Proteins implicated in ovarian cancer progression have unstable abundance across PDX models. (A) Schematic outlining the identification and validation of clinically relevant peptides from CPTAC using PepQuery2 on GalaxyP. (B) Comparison of the number of significantly differentially abundant ovarian cancer disease-related proteins between PDXs. (C) Ovarian cancer disease-associated protein abundances across PDX passaging.
A total of 171 and 283 differentially abundant ovarian cancer-associated proteins were identified for PDX110 and PDX136, respectively. Of the 171 differentially abundant proteins in PDX110, 74 were uniquely identified in PDX110-P2.2, 53 were identified in PDX110-P2.1, and 4 distinct proteins were identified in PDX110-P1 (Fig. 6B). Likewise, PDX136-P1 had more ovarian cancer-associated proteins uniquely identified than in the subsequent passages: 108 proteins in PDX136-P1, 28 proteins in PDX136-P2, and 21 proteins in PDX136-P3. Overall, PDX136 had more overlap in the differentially abundant ovarian cancer-associated proteins across the passaged tumors. These results indicate that proteins implicated in ovarian cancer, as identified by CPTAC, undergo significant changes in relative abundance after serial passaging into PDXs.
To further investigate the impact of unstable protein expression in PDX models after serial passaging, we monitored trends in the relative protein abundances of genes belonging to the Cancer Gene Census25 on LinkedOmics26. Cancer-associated genes identified through the Catalogue of Somatic Mutations in Cancer (COSMIC) Cancer Gene Census25,27 were quantified in our proteomics dataset utilizing the patient-specific database. We identified 10 proteins associated with the Cancer Gene Census within our dataset that were previously shown to have significant differences in relative abundance in ovarian cancer patients compared with healthy patients from the CPTAC pan-cancer OV proteomics (Tumor) and proteomics (Normal) datasets accessed through LinkedOmics (https://www.linkedomics.org/data_download/CPTAC-pancan-OV/). Proteins such as CALR, SHTN1, ATIC, THRAP3, FLNA, and COL3A1 showed an immediate, significant change in relative protein abundance upon tumor implantation into PDX136-P1 (Fig. 6C). Protein abundances stabilized to baseline levels at PDX136-P2; however, the relative abundances of FLNA and COL3A1 decreased upon serial passaging into PDX136-P3. These results highlight that the abundances of disease-relevant proteins are unstable across serial passaging into PDXs. Interestingly, proteins identified in PDX110 demonstrate unchanging trends in protein abundance across the first passage except for FKBP9 (Fig. 6C). Of the 5 cancer gene census proteins identified in PDX110, only COL3A1 levels were consistent between PDX110-P2.1 and PDX110-P2.2. SLC34A2, PTPN6, FKBP9, and PML levels were different between PDX models of the same passage, indicating that there is a degree of inter-PDX model variability in disease-relevant proteins.
Discussion
Utilizing a proteogenomic approach involving generation of a customized protein sequence database from RNA-seq data28–30, we demonstrated that the proteome of PDX models differs from its patient tumor of origin. Although there were differences in tumor processing across samples (i.e. snap frozen and formalin fixation), the proteomes of snap frozen and formalin fixed samples have been shown to be highly correlative in several studies31–33. We highlighted the advantage of implementing a patient-specific database derived from RNA-seq data of patient tumors compared to conventional proteomic-based methods (Fig. 2). Using this approach, we identified and quantified more human proteins across samples compared to using the UniProt reference database containing only the Homo sapiens proteome. The increased number of human protein identifications using this approach stems from the identification of protein sequences belonging to unique proteoforms that are not part of the UniProt reference proteome.
However, one limitation of our study is the low number of serially passaged PDX tumors per patient. Our study utilized PDX models developed with subcutaneous injections of malignant ovarian cancer cells. Although this form of developing PDX models is the most common within in vivo cancer research, intra-bursal transplantation of cancer cells closely mimics the original tumor microenvironment which is highly vascularized. However, this method requires specialized training with tumor size being monitored with advanced imaging34.
In addition to processing two patient tumors and their respective PDXs (n = 3), another limitation of this study is that the original patient tumors originated from either a peritoneal nodule (PT110) or an endometrioid adenocarcinoma of an ovary (PT136). Due to this feature, and the small sample size, enriched biological pathways cannot be adequately compared between patients. Rather, patient features can be compared to their serially passaged PDX tumors. Future studies should consider the merit of using patient-specific databases from patients diagnosed with the same ovarian cancer disease subtype.
Our study highlights the dynamic proteomes of serially passaged PDX tumors (Fig. 3). Importantly, protein abundances cluster independently of the patient of origin and tumor passaging. Similar trends of protein characteristics in PDX models have been recently reported. One study investigating the proteomic characteristics of in vivo models of soft tissue sarcomas using 2D image-converted analysis of liquid chromatography and mass spectrometry (2DICAL) found strong heterogeneity among different PDX models that originated from the same patient tumor35. Other groups have investigated the genetic stability of PDX models. Ben-David et al. analyzed the accumulation of aneuploidy and large copy number alterations (CNAs) in 1,110 PDX samples from 24 cancer types using SNP arrays and comparative genomic hybridization arrays36. This study found that the CNA landscape of PDXs diverges from patient tumors during passaging. Approximately 12% of the genome in PDX tumors was affected by the fourth tumor passage. These studies provide evidence of genomic and proteomic perturbations associated with PDX models that differ from the patient tumor of origin.
We investigated the dynamic proteome of PDXs using biological pathway analysis and we identified distinct pathways that were enriched across PDXs (Fig. 4). Downregulated processes including extracellular matrix (ECM) organization and the immune system are pathways that are known to be perturbed in PDX studies6,37,38. The human ECM within a PDX tumor is replaced by murine-derived ECM and stromal cells after two to five passages39–41. Human immune processes become downregulated in immune-compromised PDX models due to species specificity leading to a lack of immune cell activation42. Upregulated processes such as the metabolism of folate and pterines were also observed; however, whether these are features of tumorigenesis or artifacts from PDX serial passaging cannot be discerned. Other studies investigating the use of PDX models in cancer have highlighted the same dysregulation in metabolic pathways such as glycolysis and metabolism of nucleotides in malignant pleural mesothelioma43. A proteomic study of non-small cell lung carcinoma PDX models identified distinct proteome subtypes in 137 models in passages two to five, highlighting tumor heterogeneity at the protein level44. Furthermore, another study discovered heterogeneity in PDX models derived from the same patient tumor in neuroblastomas45. Importantly, biological processes that were identified in one passage were not conserved upon serial passaging in PDXs.
In our study, major differences in the proteogenomic profiles of PDX110 and PDX136 could be explained by the loss of subclonal heterogeneity in passaged tumors resulting from experimental processing. Conversely, loss of subclonal heterogeneity in passaged tumors could also be due to the instability of the tumor microenvironment46. We addressed the high heterogeneity within bulk proteomic data through the identification of dysregulated proteins found in epithelial cell clusters in the scRNA seq data of patient tumors (Fig. 5).
Epithelial cell cluster-associated proteins in PT110 were enriched for several biological processes that are implicated in cancer. One vital process is histone methylation, which leads to alterations in gene expression and contributes to tumor progression in several cancers47,48. Histone 3 lysine 4 (H3K4) methylation is involved in ovarian cancer metastasis, and gene expression patterns of H3K4 can distinguish between sensitive and chemo-resistant tumors49,50. Ubiquitination is another biological process actively studied in ovarian cancer, specifically in its role in facilitating chemoresistance51,52. Similarly, pathway analysis of epithelial cell-associated proteins in PT136 revealed enrichment of biological processes that are heavily implicated in cancer formation and progression, namely glycolysis53,54. Our results highlight that the identification of epithelial cell-associated proteins enriches for pathways related to tumorigenesis, and differential protein abundances are observed across PDX passaging.
Proteins identified from the Cancer Gene Census have been shown to have significantly different abundances in tumor tissue compared with healthy tissue26. We are the first to demonstrate that proteins implicated in distinct aspects of ovarian cancer disease progression have unstable protein abundances across PDX models (Fig. 6). Calreticulin (CALR) protein expression, involved in modulating tumor cell death via the immune system, has prognostic value for high-grade serous ovarian cancer55,56. Other proteins such as Collagen type III (COL3A1) protein and promyelocytic leukemia (PML) protein, have also been investigated as prognostic biomarkers of ovarian cancer57–60. These proteins, whose expression levels serve as an indicator of disease, demonstrate unstable protein expression in serially passaged PDX models and intra-PDX samples based on data from our study. Furthermore, stabilization of protein abundances over serial passaging is not guaranteed as intratumoral heterogeneity can drive the propagation of subclones across PDXs as demonstrated in breast cancer PDX models61.
Overall, our study highlights distinct and dynamic proteomes of tumors within PDX models. The results from this study uncover features to consider when conducting proteomic or multi-omic analyses of PDX samples, namely the heterogenous protein landscape that exists between tumors of the same passage, and across tumors that are serially passaged over time.
Methods
All the experimental methods were carried out based on lab specific standard operating procedures.
Resource availability
Section 1: data
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD054649.
Section 2: code
This paper does not report original code.
Experimental model and subject details
This study utilized female Athymic Nude mice from Charles River Laboratories (strain-490) housed together in standard SPF cages (5 mice per cage). Mice were 6–8 weeks old at the time of tumor implantation.
Method details
Isolation of patient tumor tissue
Tumor tissues were obtained from chemo naïve patients undergoing debulking surgery for late-stage high-grade serous ovarian cancer. Tumor samples were confirmed malignant by pathology prior to lab collection. They were transported in cold DMEM or RPMI media and placed on ice during transportation. Tumor sample from Patient 110 originated from a peritoneal nodule while the tumor from Patient 136 originated from the ovary.
Implantation and isolation of tumor tissue from mice
Tumor implantation into each mouse was conducted within 60 min of the initial tumor collection from the patient. The tumor was separated from non-tumor tissue and necrotic tissue and cut into small 0.2–0.4 cm diameter pieces in a BSL-2 level hood under sterile conditions. Blood or other contaminants, if present, were removed by washing with ice-cold PBS before the implant. The tumors were again placed in ice-cold PBS on ice to be transported for implantation into nude mice. Tumors from each patient were typically implanted into five mice at two separate locations. The implants were also performed in a BSL-2 level hood under sterile conditions. The mice were anesthetized using isoflurane (5%) and kept under the effect of isoflurane throughout the surgery at a level of 3.5% isoflurane. The mice were constantly monitored (every 5 min) and half the cage was kept on a heating pad for the mouse to move away once not under the influence of isoflurane to the other side of the cage. During the surgery the mouse was placed on a heating pad to regulate body temperature. The depth of anesthesia was monitored by pinching the animal’s toe for any reflex, poking the eye, and heart rate was monitored at an ideal rate of 50–60 beats/min. Tumor pieces of 0.2–0.4 cm were subcutaneously implanted at the mouse flank and the base of the neck.
Tumor growth was monitored weekly, and once the size of the tumor reached ~ 2 cm or the mouse became morbid, the mouse was euthanized with 5% CO2 prior to harvesting tumors. Tumors were excised from the mouse and placed in a dish of ice-cold PBS, where the tumors were washed and separated from blood vessels or connective tissue. The tumor was then cut in small pieces to be stored in formalin, or snap frozen with liquid nitrogen. The Institutional Animal Care and Use Committee approved the animal studies. This study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Generation of tissue lysates from tumor samples
Snap frozen and formalin-fixed tumor tissues were utilized. The method used to process the snap frozen tissue was adapted from Mertins et al.15 Tumor tissue was weighed out and processed according to the final wet weight. Reagent volumes were adjusted accordingly: 200 µL of chilled urea lysis buffer (8 M urea, 75 mM NaCl, 50 mM Tris–HCl pH 8.0, 1 mM EDTA, 2 µg/mL aprotinin, 10 µg/mL leupeptin, 1 mM PMSF) was added to each sample per 50 mg of wet weight tissue. The tissue was vortexed for 10 s at the highest setting, then sonicated five times for 15 s at 20% output with resting on ice for 1 min in between each round of sonication. Following sonication, the tissue samples were placed on ice for 15 min, then five additional rounds of sonication were conducted. The samples were then centrifuged at 17,000 × g for 15 min at 4 °C. The supernatant was carefully removed and placed into a 2 mL conical tube, avoiding the layer of lipids that formed at the top. A BCA® Protein Assay (Thermo Fisher Scientific) was carried out to quantify the amount of protein in each sample. The protein concentration of each sample was adjusted to 8 µg/µL and the lysed samples were stored at –80 °C.
Formalin-fixed tissue processing was performed as described previously.62–64 Nine – 12 mg formalin-fixed tumor tissue was weighed out per sample. Reagent volumes were adjusted accordingly: the formalin-fixed tissue was minced into small pieces and placed into a microcentrifuge tube, and 200 µL of 0.2% RapiGest in 50 mM ammonium bicarbonate was added to each sample. The tissue samples were boiled at 95 °C for 30 min while shaking at 1000 rpm. Following the incubation, the samples were then rested on ice for 5 min and sonicated 5 times on ice for 30 s at 20% output. Following sonication, the samples were incubated at 80 °C for 120 min while shaking at 1000 rpm. After incubation, 200 µL of 50 mM ammonium bicarbonate was added to the samples to dilute the RapiGest to 0.1%. The samples were sonicated 5 times for 30 s on ice at a 20% output. The samples were then centrifuged at 13,000 rpm for 20 min at 4 °C. The supernatant was transferred to a new microcentrifuge tube. A BCA® Protein Assay was carried out to quantify the amount of protein in each sample. The sample concentration was adjusted to 0.5 µg/µL and the lysed samples were stored at –80 °C.
Sample preparation for mass spectrometry
Snap Frozen Tissue Processing: Protein disulfide bonds were reduced by adding 10 mM dithiothreitol (DTT) to 100 µg of protein to achieve a final concentration of 5 mM. Samples were incubated with DTT for 1 h at 37 °C. Sulfhydryl groups were then alkylated by adding 100 mM iodoacetamide (IAM) to achieve a final concentration of 10 mM. Samples were incubated for 45 min in the dark at room temperature. Following alkylation, samples were diluted 1:3 (vol/vol) with 50 mM Tris–HCl (pH 8.0) to decrease the urea concentration to < 2 M. Following dilution, 1 µg/µL of LysC was added at a 1:50 ratio (wt/wt) and incubated for 2 h at room temperature. Trypsin (1 µg/µL) was added to each sample at an enzyme/substrate ratio of 1:49 (wt/wt) for overnight digestion at room temperature with shaking. Following the overnight digestion (18 h), the trypsin was quenched by adding 100% formic acid (FA) to a final concentration of 1%. The digested samples were diluted in 0.1% FA (vol/vol) to a volume of 1.5 mL. This was followed by centrifugation at 1,500 × g at room temperature for 15 min. The supernatant was separated from the pellet and subjected to C18 Solid Phase Extraction (SPE).
The C18 SPE protocol was adapted from Mertins et al.15 Briefly, a 100 mg C18 SPE cartridge (SepPak, Waters) was conditioned with 1 mL of 100% ACN followed by 1 mL of 50% ACN/0.1% FA (vol/vol). The column was equilibrated four times with 0.1% TFA (vol/vol). The digested peptide samples were loaded onto their respective columns followed by washing three times with 1 mL of 0.1% TFA and then with 0.1% FA. Samples were eluted using 1 mL of 50% ACN/0.1% FA. The eluted peptides were dried using a speed vac and then stored at –80 °C.
Formalin-Fixed Tissue Processing: Disulfide bonds were reduced by adding 1 M DTT to 100 µg of protein to achieve a final concentration of 10 mM. Samples were incubated for 30 min at 60 °C. After sample reduction, 500 mM IAM was added to each sample for a final concentration of 40 mM and samples were incubated for 30 min in the dark at room temperature. After alkylation, the samples were quenched with 100 mM DTT to achieve a final concentration of 10 mM. LysC (1 µg/µL) was added to the sample at a ratio of 1:50 (wt/wt) for 2 h at 30 °C. The samples were further diluted in 50 mM ammonium bicarbonate at a 1:1 ratio (vol/vol). Trypsin was added to each sample (1 µg/µL) at a ratio of 1:50 (wt/wt) and incubated at 37 °C for 18 h. Samples were acidified with 20% TFA to achieve a final concentration of 0.5% TFA. Samples were incubated at 37 °C for 30 min, then centrifuged at 13,000 rpm for 10 min to remove any precipitate. The supernatant was removed and desalted using C18 SPE cartridges. The desalted peptides were dried using a speed vac and stored at –80 °C.
Tandem mass tag (TMT) labeling
Two sets of TMT 10-plex Isobaric Mass Tag labels (Thermo Fisher Scientific) were used, 1 set for each sample processing replicate. The following isobaric labels were used in this study: 126C, 127N, 127C, 128C, 129N, 129C, 130N, 130C, 131. The TMT labeling method was adapted from Zecha et al.65 The dried peptides were resuspended in 50 µL of 50 mM HEPES. For set 1:35 µg of peptide from each sample was aliquoted and diluted with 50 mM HEPES to a final volume of 100 µL. The TMT 10-plex labels (0.8 mg) were allowed to equilibrate to room temperature for 15 min then dissolved in 40 µL of anhydrous acetonitrile (ACN) for 5 min with vortexing. The TMT 10-plex labels were assigned to each sample in a randomized manner and 20 µL of the TMT label was added to its assigned sample. For set 2: 13.5 µg of peptide from each sample was aliquoted and diluted with 50 mM HEPES to a final volume of 46.55 µL, and 7.7 µL of TMT label was added to each sample. The volumes were adjusted to keep the ratio of peptide:TMT label the same as set 1. Each sample was incubated with its respective TMT label for 1 h at room temperature. To assess labeling efficiency, 1 µg of labeled peptide was aliquoted from each sample and quenched with 5% hydroxylamine to achieve a final concentration of 0.2%. The samples were quenched at room temperature for 15 min then dried using a speed vac. The remaining TMT-labeled peptides were stored at –80 °C.
Dried labeled peptides were desalted using C18 Stop and Go Extraction (STAGE) Tips using a method adapted from Mertins et al.15 and Rappsilber et al.66 Briefly, 200 μL C18 STAGE tips were prepared with two Empore C18 extraction disks. The STAGE tips were conditioned with 100 μL of ACN and then centrifuged at 3000 × g for 3 min at room temperature. The STAGE tips were washed with 100 μL of 50% ACN/0.1% formic acid (FA) (vol/vol). This was followed by equilibration twice with 100 μL of 0.1% FA. Samples were loaded onto the STAGE tips and washed twice with 100 μL of 0.1% FA. Then, the samples were eluted with 60 μL of 50% ACN/0.1% FA and fully dried using a speed vac. Dried peptides were then analyzed via liquid chromatography-tandem mass spectrometry (LC–MS/MS) as described in the following section.
After confirming that the TMT labeling efficiency of was > 99%, the remaining samples were quenched and pooled into their respective sets. The pooled peptides were dried and desalted using C18 SPE as described above. TMT-labeled peptides from Set 2 underwent a second round of desalting due to the presence of lipids.
LC–MS/MS
The TMT-labeled peptides were resuspended in 50 µL of Solvent A [2% ACN/4.5 mM ammonium formate pH 10 (28% (wt/vol) ammonium hydroxide), 10% FA] and fractionated using offline high-pH reversed-phase liquid chromatography (bRP-HPLC) where 96 fractions were prepared at a flow rate of 0.2 mL/min with the following gradient: 0% Solvent B for 7 min, 0–16% Solvent B at 7–13 min, 16-40% Solvent B at 13–73 min, 40–44% Solvent B at 73–77 min, 44-60% B at 77–96 min. Fractions were then pooled into 24 fractions using the pooling fraction scheme outlined in Mertins, et al.15 Twenty-four bRP-HPLC fractions from each sample set were subsequently dried and reconstituted in 5% ACN/0.1% FA and analyzed on an Orbitrap Q Exactive Plus mass spectrometer (Thermo Fisher Scientific) coupled with an Ultimate 3000 RSLCnano liquid chromatography system and interfaced with an EASY-Spray nanospray ionization source. 500 ng peptide from each fraction was injected into an EASY-Spray HPLC Column (75 µM × 150 mm with a 3 µM particle size) with the spray voltage set to 1.6 kV. Peptides were separated using an 85-min gradient with Solvent A (0.1% FA) and Solvent B (90% ACN/0.1% FA). The flow rate was set to 0.3 µL/min and the gradient was as follows: 0–5 min 3% B, 5–65 min 3%-40% B, 65–66 min 40%-90% B, 66–71 min 90% B, 71–72 min 90%-3% B, 72–85 min 3% B. Mass spectrometry data acquisition was carried out using a ddMS2 IT HCD top 10 method. The full MS scan range was 375–1400 m/z with a resolution of 70,000. Charge states 2 + to 7 + were included with a dynamic exclusion time of 30 s. HCD fragmentation was carried out using a fixed energy collision of 32% with an isolation window of 1.2 m/z, resolution of 35,000, AGC target of 1e5, and maximum IT of 120 ms. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE67 partner repository with the dataset identifier PXD054649.
Quantification and statistical analysis
LC–MS/MS data analysis
Raw mass spectrometry data were processed using MaxQuant version 2.1.468. Raw files were processed by setting group-specific parameters for enzymatic digestion, variable modifications, fixed modifications, and reporter ion MS2. Enzymatic digestion was set to specific with Trypsin/P and LysC with an allowable max number of missed cleavages set at 2. Oxidation of methionine and acetylation of protein N-terminus were set as variable modifications, and cysteine carbamidomethylation was set as a fixed modification. For TMT label protein quantification, reporter ion MS2 was selected and 9 isobaric labels with their correction factors were applied from a TMT 10-plex set. All other parameters within MaxQuant remained under default settings. Homo sapiens and Mus musculus UniProt/Swiss-Prot databases (downloaded on 2021-05-04) containing 17,121 of mouse proteins and 20,383 of human proteins were used for protein identification separately such that the resulting protein identifications were able to be sorted based on taxonomy ID this study. An additional protein identification search was conducted using a customized FASTA database containing patient-specific bulk RNA-seq data and used for downstream analysis; this merged FASTA file contained 192,136 unique entries with proteins from mouse, human, and protein isoforms identified from bulk RNA-seq patient data. All subsequent data analyses were performed using Perseus version 2.0, RStudio, Reactome, STRING, and PepQuery19,22,69–71.
Generation of patient-specific database for proteogenomic analysis
Bulk RNA sequencing data from Patient 110 and Patient 136 were. A custom protein database was generated from the patient RNA-seq data using the Proteogenomic 1: Database Creation workflow on Galaxy-P72. Briefly, Patient 110 and Patient 136 FASTQ files were aligned to the human genome (GRCh38) using HISAT2. Following sequence alignment, FreeBayes was used to detect genomic variants prior to variant annotation and genome mapping using CustomProDB. Next, StringTie was used to assemble RNA-seq alignments into potentially variant/unique transcripts and subsequently evaluated with GffCompare. Resulting transcripts from Patient 110 and 136 were translated and merged into a custom built FASTA file including Homo sapiens and Mus musculus (mm10_canonical) proteomes. The merged FASTA file was then used for protein identification and quantification on MaxQuant. Identified proteins were separated based on patient type (PT110 or PT136) and taxonomy (Homo and Mus).
Data preparation and quality control
Data from the ProteinGroups.txt file output from the database search conducted on MaxQuant was subsequently filtered and prepared using Perseus. Proteins only identified by a modification site, proteins identified as a potential contaminant from the CRAPome database73, and proteins identified by their reverse sequence (decoys) were removed from the dataset. Proteins identified in either PT110, PT136, or Homo sapiens were utilized for downstream analyses to minimize incorporation of mouse proteins and increase confidence for human-specific proteins within the dataset. Data were log2 transformed followed by median normalization, batch-effect correction using limma, and non-valid values were filtered out of the dataset. The resulting dataset included 4865 quantified human proteins (denoted as PT110, PT136, or Homo) identified across all samples and 2102 quantified mouse proteins (denoted as Mus) identified across all PDXs.
Bioinformatic analysis of proteomic data
To identify differentially abundant human proteins between each patient tumor and its paired PDX model, protein relative abundance values were analyzed using Welch’s T-test (p < 0.05) followed by assessing fold change (FC < -2 or FC > 2) between the patient tumor and corresponding PDX tumor. These analyses, followed by the assessment of Pearson’s correlation coefficient were conducted using RStudio. Pathway analysis of differentially abundant proteins was conducted using Reactome19. Pathways were designated as being significantly enriched based on p-value ≤ 0.05, adjusted via Benjamini–Hochberg for multiple corrections. STRING71 analysis was conducted on differentially abundant proteins with a medium confidence interaction score to assess protein–protein interaction networks. Proteome comparisons between our data and an ovarian cancer dataset generated from CPTAC were achieved using PepQuery2 using known mode with our patient-specific database with validated peptides present in both datasets. Proteins identified from the CPTAC database originated from high-grade serous ovarian cancer tumors. There were no endometrioid ovarian cancer specimens in the CPTAC dataset.
Sample processing for scRNAseq
Fat, fibrous and necrotic areas were removed from tumor sample with an ideal post-processing weight between 0.1 and 1.0 g. Single cell suspensions were created using the Miltenyi Biotech GentleMACs Tissue Dissociation Kit following protocol 2.2.1. Briefly, the tumor sample was minced and placed in a specialized conical tube containing a mixture of dissociation enzymes in media. The tube was placed on a mechanical rotator for 30 s followed by incubation on a rotator at 37 °C for 30 min. The process was repeated and then the media was poured through a 70-micron filter to remove clumps and debris. Cells were treated with red cell lysis buffer for 5–10 min at room temperature, centrifuged and resuspended in hypothermosol. An aliquot was removed for measuring cell viability using the Cell Countess (Life Sciences). The single cell solution was diluted to a concentration of 1,000 viable cells/µl and transported to the sequencing facility on ice.
scRNAseq sequencing
Samples were sequenced at the University of Minnesota’s Genomics Center using the 10 × Genomics Single Cell 3’ Protocol utilizing the ChromiumTM Single Cell 3’ Library & Gel Bead Kit and Chromium™ Single Cell A Chip Kit. Approximately 5000 cells were partitioned into nanoliter-scale Gel Bead-In-EMulsions (GEMs) with one cell per GEM. Within each GEM, cells were lysed, primers were released and mixed with cell lysate. Incubation of the GEMs produced barcoded, full-length cDNA from mRNA. The full-length, barcoded cDNA was then amplified by PCR prior to library construction. Sequencing was performed using an Illumina HiSeq 2500 or NovaSeq to a depth of at least 1 million paired-ends reads per cell.
scRNAseq analysis
Illumina raw sequencing output files were processed using Cell Ranger™ software to produce a filtered gene x cell matrix of UMI counts. The filtered matrix was used as input for the Seurat R software package.
Downstream analysis of UMI counts is performed using multiple R packages including Seurat74, scater 75, ccFindR76, scRAN77, CIDR78, SingleR79, and Bioconductor pipelines80.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
James Johnson developed customized tools on the Galaxy platform enabling the workflow used for proteogenomic database generation.
Author contributions
J.M.P. conducted experimental approaches, generated all data and wrote the manuscript. J.M.D. was involved in data generation, data analysis, and contributed to the manuscript. JR and SNT contributed to experimental design. J.R. generated preliminary data. S.N.T. contributed to the manuscript. M.S., B.W., and T.K.S. generated PDX models and provided PDX and patient tumors for the project. A.C.N. contributed to the pathology-based characterization of the patient tumors. S.M., P.D.J. and T.J.G. guided proteogenomic analysis using Galaxy for Proteomics Platform. All authors reviewed the manuscript.
Funding
Supported by the American Cancer Society (Institutional Research Grant #129819-IRG-21-049-61-IRG137 to SNT), V Foundation for Cancer Research (SNT), NCI Targets of Cancer Pre-doctoral Training Program (JMP; Grant #T32 CA009138), University of Minnesota Masonic Cancer Center, Adelson Medical Research Foundation (TKS, ACN, BW), University of Minnesota Department of Laboratory Medicine and Pathology, Proteogenomic Shared Resource in the University of Minnesota Masonic Cancer Center (National Cancer Institute Grant #P30CA077598; support for SM, PDJ, and TJG). The funders had no role in the design of the study, collection, analysis, interpretation of the data and writing of the manuscript.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD054649; additional information is available from the lead contact, Stefani N. Thomas (stefanit@umn.edu), upon request.
Declarations
Ethical statement
All authors certify that they comply with the ethical guidelines for authorship.
Ethics approval and consent to participate
Patients provided informed consent to research participation and sample collection under IRB approved protocols 1408M52905 and 1611M99903.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lisio, M. A., Fu, L., Goyeneche, A., Gao, Z. H. & Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci.10.3390/ijms20040952 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch.460(3), 237–249. 10.1007/s00428-012-1203-5 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Lheureux, S., Braunstein, M. & Oza, A. M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin.69(4), 280–304. 10.3322/caac.21559 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Yoshida, G. J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol.13(1), 4. 10.1186/s13045-019-0829-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdolahi, S. et al. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med.20(1), 206. 10.1186/s12967-022-03405-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi, J., Li, Y., Jia, R. & Fan, X. The fidelity of cancer cells in PDX models: Characteristics, mechanism and clinical significance. Int. J. Cancer146(8), 2078–2088. 10.1002/ijc.32662 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Jung, J., Seol, H. S. & Chang, S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res. Treat.50(1), 1–10. 10.4143/crt.2017.307 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zayed, A. A., Mandrekar, S. J. & Haluska, P. Molecular and clinical implementations of ovarian cancer mouse avatar models. Chin. Clin. Oncol.4(3), 30. 10.3978/j.issn.2304-3865.2015.04.01 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cybula, M. & Bieniasz, M. Patient-derived tumor models are attractive tools to repurpose drugs for ovarian cancer treatment: Pre-clinical updates. Oncotarget13, 553–575. 10.18632/oncotarget.28220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih, I. M., Wang, Y. & Wang, T. L. The origin of ovarian cancer species and precancerous landscape. Am. J. Pathol.191(1), 26–39. 10.1016/j.ajpath.2020.09.006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tudrej, P., Kujawa, K. A., Cortez, A. J. & Lisowska, K. M. Characteristics of in vivo model systems for ovarian cancer studies. Diagnostics (Basel)10.3390/diagnostics9030120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian, W. et al. Tumor purity in preclinical mouse tumor models. Cancer Res. Commun.2(5), 353–365. 10.1158/2767-9764.CRC-21-0126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, X. Y., Xu, Y. M. & Lau, A. T. Y. Proteogenomics in cancer: Then and now. J. Proteome Res.22(10), 3103–3122. 10.1021/acs.jproteome.3c00196 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Nusinow, D. P. et al. Quantitative proteomics of the cancer cell line Encyclopedia. Cell180(2), 387-402.e16. 10.1016/j.cell.2019.12.023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertins, P. et al. Reproducible workflow for multiplexed deep-scale proteome and phosphoproteome analysis of tumor tissues by liquid chromatography-mass spectrometry. Nat. Protoc.13(7), 1632–1661. 10.1038/s41596-018-0006-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang, H. et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer. Cell166(3), 755–765. 10.1016/j.cell.2016.05.069 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberg, A. L. et al. Statistical analysis of comparative tumor growth repeated measures experiments in the ovarian cancer patient derived xenograft (PDX) setting. Sci. Rep.11(1), 8076. 10.1038/s41598-021-87470-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunil, H. S. & O’Donnell, K. A. Capturing heterogeneity in PDX models: Representation matters. Nat. Commun.15(1), 4652. 10.1038/s41467-024-47607-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothfels, K. et al. Using the reactome database. Curr. Protoc.3(4), e722. 10.1002/cpz1.722 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paromov, V. et al. The proteomic analysis of cancer-related alterations in the human unfoldome. Int. J. Mol. Sci.10.3390/ijms25031552 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis, M. J. et al. CPTAC. Connecting genomic alterations to cancer biology with proteomics: The NCI Clinical Proteomic Tumor Analysis Consortium. Cancer Discov.3(10), 1108–1112. 10.1158/2159-8290.CD-13-0219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen, B., Wang, X. & Zhang, B. PepQuery enables fast, accurate, and convenient proteomic validation of novel genomic alterations. Genome Res.29(3), 485–493. 10.1101/gr.235028.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen, B., Li, K., Zhang, Y. & Zhang, B. Cancer neoantigen prioritization through sensitive and reliable proteogenomics analysis. Nat. Commun.11(1), 1759. 10.1038/s41467-020-15456-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta, S. et al. A Galaxy of informatics resources for MS-based proteomics. Expert Rev. Proteom.20(11), 251–266. 10.1080/14789450.2023.2265062 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Sondka, Z. et al. COSMIC: A curated database of somatic variants and clinical data for cancer. Nucleic Acids Res.52(D1), D1210–D1217. 10.1093/nar/gkad986 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao, Y. et al. A proteogenomics data-driven knowledge base of human cancer. Cell Syst.14(9), 777–87.e5. 10.1016/j.cels.2023.07.007 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondka, Z. et al. The COSMIC Cancer Gene Census: Describing genetic dysfunction across all human cancers. Nat. Rev. Cancer18(11), 696–705. 10.1038/s41568-018-0060-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mani, D. R. et al. Cancer proteogenomics: Current impact and future prospects. Nat. Rev. Cancer22(5), 298–313. 10.1038/s41568-022-00446-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menschaert, G. & Fenyö, D. Proteogenomics from a bioinformatics angle: A growing field. Mass Spectrom. Rev.36(5), 584–599. 10.1002/mas.21483 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nesvizhskii, A. I. Proteogenomics: Concepts, applications and computational strategies. Nat. Methods11(11), 1114–1125. 10.1038/nmeth.3144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craven, R. A. et al. Proteomic analysis of formalin-fixed paraffin-embedded renal tissue samples by label-free MS: Assessment of overall technical variability and the impact of block age. Proteom. Clin. Appl.7(3–4), 273–282. 10.1002/prca.201200065 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Ostasiewicz, P., Zielinska, D. F., Mann, M. & Wiśniewski, J. R. Proteome, phosphoproteome, and N-glycoproteome are quantitatively preserved in formalin-fixed paraffin-embedded tissue and analyzable by high-resolution mass spectrometry. J. Proteome Res.9(7), 3688–3700. 10.1021/pr100234w (2010). [DOI] [PubMed] [Google Scholar]
- 33.Sprung, R. W. et al. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol. Cell Proteom.8(8), 1988–1998. 10.1074/mcp.M800518-MCP200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran, T. M., Ho, G. Y. & Chu, S. Patient-derived xenograft models for ovarian cancer. Methods Mol. Biol.2806, 187–196. 10.1007/978-1-0716-3858-3_13 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Shiozawa, K. et al. Species-specific quantitative proteomics profiles of sarcoma patient-derived models closely reflect their primary tumors. Proteom. Clin. Appl.13(6), e1900054. 10.1002/prca.201900054 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet.49(11), 1567–1575. 10.1038/ng.3967 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz, L. et al. Differential transplantability of human endothelial cells in colorectal cancer and renal cell carcinoma primary xenografts. Lab. Invest.89(1), 91–97. 10.1038/labinvest.2008.108 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Villacorta-Martin, C., Craig, A. J. & Villanueva, A. Divergent evolutionary trajectories in transplanted tumor models. Nat. Genet.49(11), 1565–1566. 10.1038/ng.3983 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Hidalgo, M. et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov.4(9), 998–1013. 10.1158/2159-8290.CD-14-0001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julien, S. et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res.18(19), 5314–5328. 10.1158/1078-0432.CCR-12-0372 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Bergamaschi, A. et al. Molecular profiling and characterization of luminal-like and basal-like in vivo breast cancer xenograft models. Mol. Oncol.3(5–6), 469–482. 10.1016/j.molonc.2009.07.003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siolas, D. & Hannon, G. J. Patient-derived tumor xenografts: Transforming clinical samples into mouse models. Cancer Res.73(17), 5315–5319. 10.1158/0008-5472.CAN-13-1069 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, Z. et al. A novel PDX modeling strategy and its application in metabolomics study for malignant pleural mesothelioma. BMC Cancer21(1), 1235. 10.1186/s12885-021-08980-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirhadi, S. et al. Integrative analysis of non-small cell lung cancer patient-derived xenografts identifies distinct proteotypes associated with patient outcomes. Nat. Commun.13(1), 1811. 10.1038/s41467-022-29444-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braekeveldt, N. et al. Patient-derived xenograft models reveal intratumor heterogeneity and temporal stability in neuroblastoma. Cancer Res.78(20), 5958–5969. 10.1158/0008-5472.CAN-18-0527 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Liu, Y. et al. Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct. Target Ther.8(1), 160. 10.1038/s41392-023-01419-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell144(5), 646–674. 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Yang, Q. et al. Epigenetics in ovarian cancer: Premise, properties, and perspectives. Mol. Cancer17(1), 109. 10.1186/s12943-018-0855-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyu, T. et al. SMYD3 promotes implant metastasis of ovarian cancer via H3K4 trimethylation of integrin promoters. Int. J. Cancer146(6), 1553–1567. 10.1002/ijc.32673 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Chapman-Rothe, N. et al. Chromatin H3K27me3/H3K4me3 histone marks define gene sets in high-grade serous ovarian cancer that distinguish malignant, tumour-sustaining and chemo-resistant ovarian tumour cells. Oncogene32(38), 4586–4592. 10.1038/onc.2012.477 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Vriend, J. & Nachtigal, M. W. Ubiquitin proteasome pathway transcriptome in epithelial ovarian cancer. Cancers (Basel)10.3390/cancers13112659 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng, Y., Qiu, L., Zhang, S. & Han, J. The emerging roles of E3 ubiquitin ligases in ovarian cancer chemoresistance. Cancer Drug Resist.4(2), 365–381. 10.20517/cdr.2020.115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, C., Liu, F. Y., Shen, Y., Tian, Y. & Han, F. J. Research progress on the mechanism of glycolysis in ovarian cancer. Front. Immunol.14, 1284853. 10.3389/fimmu.2023.1284853 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xintaropoulou, C. et al. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC Cancer18(1), 636. 10.1186/s12885-018-4521-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaksman, O., Davidson, B., Tropé, C. & Reich, R. Calreticulin expression is reduced in high-grade ovarian serous carcinoma effusions compared with primary tumors and solid metastases. Hum. Pathol.44(12), 2677–2683. 10.1016/j.humpath.2013.07.009 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Kasikova, L. et al. Calreticulin exposure correlates with robust adaptive antitumor immunity and favorable prognosis in ovarian carcinoma patients. J. Immunother. Cancer7(1), 312. 10.1186/s40425-019-0781-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Januchowski, R. et al. Increased expression of several collagen genes is associated with drug resistance in ovarian cancer cell lines. J. Cancer7(10), 1295–1310. 10.7150/jca.15371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engqvist, H. et al. Immunohistochemical validation of COL3A1, GPR158 and PITHD1 as prognostic biomarkers in early-stage ovarian carcinomas. BMC Cancer19(1), 928. 10.1186/s12885-019-6084-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu, S. B., Shen, Z. F., Guo, Y. J., Cao, L. X. & Xu, Y. silencing inhibits cell proliferation and induces DNA damage in cultured ovarian cancer cells. Biomed. Rep.7(1), 29–35. 10.3892/br.2017.919 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gentric, G. et al. PML-regulated mitochondrial metabolism enhances chemosensitivity in human ovarian cancers. Cell Metab.29(1), 156–73.e10. 10.1016/j.cmet.2018.09.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landuzzi, L. et al. Early stability and late random tumor progression of a HER2-positive primary breast cancer patient-derived xenograft. Sci. Rep.11(1), 1563. 10.1038/s41598-021-81085-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennedy, J. J. et al. Optimized protocol for quantitative multiple reaction monitoring-based proteomic analysis of formalin-fixed, paraffin-embedded tissues. J. Proteome Res.15(8), 2717–2728. 10.1021/acs.jproteome.6b00245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griesser, E. et al. Quantitative profiling of the human substantia nigra proteome from laser-capture microdissected FFPE tissue. Mol. Cell Proteom.19(5), 839–851. 10.1074/mcp.RA119.001889 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Föll, M. C. et al. Reproducible proteomics sample preparation for single FFPE tissue slices using acid-labile surfactant and direct trypsinization. Clin. Proteom.15, 11. 10.1186/s12014-018-9188-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zecha, J. et al. TMT labeling for the masses: A robust and cost-efficient, in-solution labeling approach. Mol. Cell Proteom.18(7), 1468–1478. 10.1074/mcp.TIR119.001385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem.75(3), 663–670. 10.1021/ac026117i (2003). [DOI] [PubMed] [Google Scholar]
- 67.Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res.47(D1), D442–D450. 10.1093/nar/gky1106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc.11(12), 2301–2319. 10.1038/nprot.2016.136 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods13(9), 731–740. 10.1038/nmeth.3901 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Allaire, J. J. RStudio: Integrated Development Environment for R. RStudio, PBC (2020).
- 71.Szklarczyk, D. et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res.47(D1), D607–D613. 10.1093/nar/gky1131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Community G. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res.10.1093/nar/gkae410 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mellacheruvu, D. et al. The CRAPome: A contaminant repository for affinity purification-mass spectrometry data. Nat. Methods10(8), 730–736. 10.1038/nmeth.2557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol.33(5), 495–502. 10.1038/nbt.3192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: Pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics33(8), 1179–1186. 10.1093/bioinformatics/btw777 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo, J., Winterhoff, B. J., Starr, T. K., Aliferis, C. & Wang, J. De novo prediction of cell-type complexity in single-cell RNA-seq and tumor microenvironments. Life Sci. Alliance10.26508/lsa.201900443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lun, A. T., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with bioconductor. F1000Res5, 2122. 10.12688/f1000research.9501.2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin, P., Troup, M. & Ho, J. W. CIDR: Ultrafast and accurate clustering through imputation for single-cell RNA-seq data. Genome Biol.18(1), 59. 10.1186/s13059-017-1188-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol.20(2), 163–172. 10.1038/s41590-018-0276-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amezquita, R. A. et al. Orchestrating single-cell analysis with bioconductor. Nat. Methods17(2), 137–145. 10.1038/s41592-019-0654-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD054649; additional information is available from the lead contact, Stefani N. Thomas (stefanit@umn.edu), upon request.